Abstract
This study primarily aimed to explore the capabilities of digitalisation in the healthcare context, focusing on a specific disease. In this case, the study examined the potential of remote monitoring of gait to address the sensitivity of multiple sclerosis progression to gait characteristics by adopting a non-invasive approach to remotely quantify gait disturbances in a patient's daily life. To better understand the managerial aspects associated with this approach, the researchers conducted a literature review along with a set of semi-structured interviews. The target population included MS patients as well as the key agents involved in their care: patients' family members, neurologists, MS nurses, physiotherapists, medical directors, and pharmacist. The study identifies the perceived barriers and drivers that could contribute to the successful deployment of PSS remote gait monitoring as a healthcare service: i) At mega-level governance. Implications on privacy and security data are notable barriers missing on the speech. ii) At macro level, funding is highlighted as main barrier. The cost and lack of health system subsidies may render initiatives unsustainable, as emphasised by the interviewees. iii) At meso level, useable data is recognised as a driver. The data collection process can align with diverse interests to create value and business opportunities for the ecosystem actors, enhance care, attract stakeholders, such as insurers and pharma, and form partnerships. iv) At micro-level processes, we find two potential barriers: wearable device and app usability (comfort, navigation, efficiency) and organisational/behavioural aspects (training, digital affinity, skills), which are crucial for value creation in innovation ecosystems among patients and healthcare professionals. Finally, we find an interesting gap in the literature and interviews. Stakeholders' limited awareness of technological demands, especially from information technologies, for a successful long-term service, can be consider two key barriers for PSS.
Keywords: Healthcare, Product service system, Digital transformation
Graphical abstract
1. Introduction
One of the main areas of focus in digital transformation is health system theory and practice [[1], [2], [3]]. For example, the European Union (EU) is committed to enhancing citizens' well-being [4]. Achieving these goals requires member states' collaborative actions driven by EU directives, research and development (R&D) investments, and electronic health (eHealth) technology adoption [5].
EHealth holds promise in healthcare, but it faces challenges in integrating research into clinical practice [6]. Diaz-Skeete et al. [7] proposed a holistic implementation approach that considers technology, infrastructure, management, economics, societal impacts, usability, evaluation and evidence, legislation, governance, policies, and procedures.
Wearable technologies offer solutions. Piwek et al. [8] discussed the growing adoption of wearables, driven by the overall digitization trend. Patients and physicians already employ digital tools (e.g. thermometers and glucose monitors) to identify and discuss symptoms [9] However, challenges arise when the interpretation of longitudinal data is necessary.
Aditionally, wearables can provide patients with personal analytic tools, enhancing health and preventive care for ongoing conditions. Physicians can monitor patients' well-being using wearables to guide treatment [10]. This transition to data-driven healthcare can empower patients and support informed medical decisions.
Today, the core function of healthcare systems is to provide cost-effective services that are efficient and serve the needs and preferences of society as a whole [11] from different observation levels (micro, meso, macro, and mega) [5,12].
Kraus et al. [3] highlighted ongoing research on digital healthcare transformation, primarily addressing initial aspects (micro level) rather than strategic or structural viewpoints. Hines et al. [13] explores health professionals’ experiences of, and attitudes towards eHealth technologies to support interdisciplinary practice and point out there has been minimal attention in the literature to organisational, policy, and clinical leadership factors that facilitate the implementation of eHealth across various organizations and throughout the continuum of care. Tran et al. [14] reported a substantial gap in existing literature regarding the barriers and enablers which characterize a successful interdisciplinary eHealth model for people with traumatic brain injury.
On the one hand, despite of the growing evidence of the positive effects of eHealth interventions, Varsi et al. review [15] points to the lack of eHealth studies that report implementation strategies in a comprehensive way and highlights the need to design robust studies focusing on implementation strategies. Numerous pilot projects have emerged, often concluding without continuity owing to cost, efficiency, technology support, gaps, or security [16]). But while technical aspects receive considerable focus [17], managing the transformation is crucial. Shahabad and Al-Hussein [18]) highlight for efficient utilisation of IoT technologies in any field like healthcare, it is important to address the functional requirements just as important as the non-functional one, in order to gain personal and social profits. Wentink et al. [19] was the first study that prioritized different requirements for e-rehabilitation amongst multiple subgroups (patients, informal caregivers, and health professionals) and in which informal caregivers were treated as a separate group of end users, noting that the literature had not previously identified such variations in the importance of requirements for comprehensive e-health interventions.
Owing to diverse agent levels and conceptual complexities, we adopted a case study focused on acting on a common degenerative neurological disease affecting a relatively young population to explore the significance of digital transformation. The interest lies in understanding gait disorders in patients with multiple sclerosis (MS), aiding analysis and conceptualisation across different aspects of digitalisation.
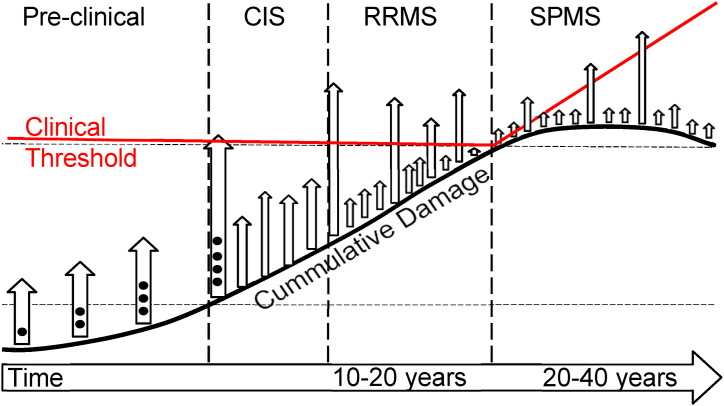
Multiple sclerosis is a chronic inflammatory and degenerative central nervous system disorder that mainly affects a relatively young population, with a higher prevalence in women (>60 %). It can be highly incapacitating, greatly impacting the patient's quality of life [20]. Fig. 1 shows the disease stages.
Fig. 1.
General picture of MS progression (Courtesy of Karolinska Institute). In the preclinical phase disease activity is detected by MRI. CIS (clinically isolated syndrome) is the first symptom, RRMS (relapsing-remitting MS) is characterized by relapses with or without complete recovery. SPMS (secondary-progressive MS) is defined by continuous progression of disability.
Recent evidence suggests that disability progression in MS occurs from the onset of the disease [21], with gait disturbances being the main cause of MS progression. To date, reliable clinical measures and definitive biomarkers to detect and monitor progression are lacking [22]. Given the increasing number of therapeutic options, monitoring progression with reliable and sensitive tools is of paramount importance.
The study of gait with wearable sensors in MS is currently the subject of research, with a specific focus on a technological approach, generally studies in the clinical setting to compare sensor metrics with clinical scales [[23], [24], [25], [26], [27]]. After a careful analysis of the published reviews and the existing literature [10,[13], [14], [15],28,29] we were unable to identify significant research on barriers and facilitators from the different perspectives and agents involved to implement these wearable devices in the MS clinical routine. Therefore, we believed it made sense to explore the perspective of different agents and dimensions in close relationship with the specific pieces of knowledge found, and to propose recommendations for improving deployments.
The remainder of this paper is organised as follows. Section 2 reviews the relevant information from the current literature. Section 3 describes the adopted methodology and justification for its adoption. Section 4 presents the main findings after the application of the methodology, and Section 5 elaborates on the interpretation of the findings and the reasons for the lack of contribution in some of the dimensions. Finally, Section 6 summarises the relevant aspects, justifies the highlights, and provides additional perspectives on managerial and scientific dimensions, limitations, and potential research areas.
2. Literature review
There has been a long debate in public administration theory and practice on how to improve public services, particularly health, through active citizen participation [30,31] The profound impact of digitalisation on healthcare is acknowledged and anticipated to grow further. Through this, patients gain empowerment, access to records, symptom tracking, and engagement with healthcare providers, enhancing adherence, self-efficacy, and health outcomes [32]. It also aids disease management by offering understanding, progress tracking, informed lifestyle choices, and community support [33].
Healthcare digitalisation encompasses diverse dimensions, such as telemedicine, which enables virtual consultations for timely medical guidance and treatment adjustments [34]. This aids clinical trials by improving patient recruitment and data collection, hastening research and providing innovative interventions [35]). Wearable technology is another dimension that enables the observation in a natural environment [36], which is a crucial part of mobile health (mHealth) and technology-enabled care (TEC) [37,38].
Although digitalisation technologies contribute to equitable patient support for those affected by MS and simplified access to services, regardless of socioeconomic factors, ensuring equitable care for effective MS management [39], the ecosystem is complex. The change of paradigm embraces fairness and inclusivity, assuring that all individuals have equal opportunities for support and care, fostering better health management. However, it also demands specific relationships between different agents in the solution ecosystem, from the different types of solution providers to the final users and relatives.
Therefore, privacy and data security require careful attention. Decision-makers must prioritise patient privacy, confidentiality, and data protection. Assessing risks and making ethical judgments safeguard patient information [40], along with data protection regulations, such as the General Data Protection Regulation (GDPR) in Europe [[41], [42], [43]] This entails the availability of wearable sensors, an IT platform for relevant stakeholders, and GDPR compliance [44]. The envisioned operation requires a more intricate system than the current healthcare approach, which requires other agents, such as patient associations and pharmaceutical companies, to increase system resilience. Additionally, the original equipment manufacturer (OEM) and value-added resellers (VAR) are required to help with the daily development of the solution, providing guidance, sensor maintenance, platform updates, and algorithm improvements [45,46].
Gait impairment is the main cause of disability progression in more than 90 % of people with MS [20], in the absence of validated biomarkers of progression, this is difficult to identify and monitor, leading to a delay in diagnosis of 2 or more years [47]. In MS patients, digitization adds value to the analysis of gait disturbances and disease progression. Quantitative monitoring using wearable sensors provides more objective and ecological measurements obtained in a clinical [25,26,48] and in a real-life setting, as well as early detection of qualitative changes in gait, that are not visible with current tools. Features that would allow earlier diagnosis, better symptom management and treatment optimization.
Gait speed is typically measured by timing people as they walk a known distance, such as a hallway [49]. This enables frequent assessment and remote monitoring of gait speed over time, offering a cost-effective alternative to lab-based motion capture systems. Raw data allow semantic step extraction from both legs, offering insights into step execution. High-frequency data enable detailed step analysis, leading to higher-level key performance indicators (KPIs). Longitudinal KPI analysis and cross-comparisons provide updated information to physicians in clinical practice, helping characterize the progression process. However, most previous studies have validated gait speed estimation algorithms in clinical settings, which can differ from home assessments in which patients demonstrate their actual performance [50].
Wearable inertial sensors (e.g. accelerometers and gyroscopes) integrated into devices, such as smartwatches or specialised systems, facilitate gait analysis (Fig. 2), capture body segment movements during walking or running, and enable continuous monitoring to analyse gait patterns in MS patients. These systems minimise intrusion and provide valuable insights into patients' gait patterns and speed [36,48]. The major challenge is obtaining and securely uploading high-frequency data (>50 Hz) to a remote database with sufficient granularity to support frequentist gait analysis.
Fig. 2.

Example from the sensorised socks in use for the selected use case, where sampling frequency is higher than 50Hz. (Courtesy of Sensoria Inc.).
To increase the probability of success of any digitalisation initiative in the healthcare context, the management dimension is extremely important because such a digitalisation effort and personalised healthcare approach will dramatically disrupt the current healthcare system in most developed countries [51]. Healthcare providers are undergoing a substantial shift from competency and resource holders to skills, resources, and technology integrators for intricate value-creation processes [52,53]. This transformation, called servitisation, marks a departure from traditional product-based business models towards customer-centric strategies that emphasize performance-based sales. Product-Service Systems (PSS) offer a potential avenue for this shift, comprising products, services, player networks, and infrastructures that prioritise competitiveness, customer satisfaction, and environmental responsibility [[54], [55], [56]].
Owing to advancements within and beyond the healthcare sector, trade-offs between (possibly competing) goals, such as price and quality, are often found where moral judgments are required [57] (White, 2015), which must balance various ethical considerations, such as affordability and accessibility, to ensure that PSS solutions are reasonably priced and available to those who need them [58] Other judgments must ensure that the chosen PSS contributes to positive health outcomes, improves the quality of care provided [59] promotes patient autonomy, and empowers individuals to make decisions that align with their values and preferences [60] Furthermore, the value proposal for the PSS must be described for the entire ecosystem and, in this case, it is connected with a quantitative monitoring of the gait disturbance to help in the diagnosis of the progress of the disease, making it possible to apply convenient treatment. Its inherent value lies in remote monitoring, which enables healthcare providers to track symptoms, disease progression, and treatment using wearables and applications [23]. Additionally, it offers a longitudinal view of patients' lives, thereby enhancing comprehensive patient understanding. Integrating advanced analytics and artificial intelligence techniques can provide insights from diverse datasets and facilitate personalised treatment strategies [61]. Furthermore, predictive models for disease progression can be derived from an integrated perspective of different patients over time.
Existing literature underscores the significance of emerging technology success, involving both the development and substitution of established practices [62,63]. However, limited attention has been paid to how entrenched practices and their rules influence novelty creation [64]. Moreover, studies investigating PSS in the healthcare industry are limited [12,65,66]. The Technological Innovation System (TIS) approach posits that innovations exist within socio-technical systems (STS) and are enabled by policies and legitimacy [62,67]. Diverse actors contribute to innovation, with each playing a unique role. For example.
-
−
Physicians and technicians work together for knowledge development.
-
−
Healthcare equipment providers develop either technological or service capabilities to offer the PSS solution.
-
−
General and medical directors integrate PSS into clinical workflows and additional value-added services, such as consulting, training, and education.
-
−
Policy makers as financial providers redesign the supply chain for a whole PSS package.
-
−
Pharmaceutical industry.
-
−
Solution providers and integrators.
However, these actors are constrained and empowered in their actions by the structure of the healthcare ecosystem, and because it is highly regulated to ensure patient and staff safety, all technical, social, and organisational approaches are necessary [68] These categorical approaches reveal diverse barriers, facilitators, and risks, as reported in the literature. Javaid et al. [10] highlight that real-time health monitoring and reliable data collection are technical facilitators. Internet-of-Things (IoT) devices enable healthcare operations and yield crucial insights for swifter decisions. This study recognises a barrier: healthcare service providers must meet new demands to successfully provide outstanding medical and health programs.
Complex technical systems can be analysed using STS. The socio-technical perspective seeks to understand how human social dynamics influence the functioning of technical systems. The analysis of STS offers a robust framework for examining the underlying factors responsible for the limited acceptance, adoption, and effectiveness of various information and communication technology interventions. Owing to their heavy reliance on intricate human organisational frameworks, healthcare systems are exceptionally well suited to STS [69]. Technical systems have social consequences, and social systems have technical consequences [13]. Therefore, the analysis of systems from different agents, represented by layers, can provide a useful technique for barrier identification [10]. From an organisational perspective, the PSS element presents further advantages, particularly concerning IoT integration in healthcare. This integration enhances user-experience consistency and reduces the time burden on clinics, physicians, and healthcare providers. Patient charting, which is a time-intensive task, benefits from IoT-enabled high-frequency sensors that transmit vital health data in real time. This enables prompt disease detection and intervention.
The proliferation of consumer-oriented health technologies for managing chronic diseases is on the rise [15]; however, the majority face the challenge of low adoption rates or adherence [33,70]. From the analysis of the literature, there is a limited exploration of potential barriers in disease-specific digitalisation deployment within healthcare. The present article addresses this gap by examining the situation from the selected use case of gait disorders in patients with MS, where the specific dimension provides a context for limitations.
3. Methodology
3.1. Research design
As highlighted in the Introduction and revised in the Literature Review, current research on healthcare's digital transformation lacks a comprehensive consideration of patient support for enhancing traditional processes alongside new value creation. Additionally, it does not thoroughly investigate the structural changes within healthcare organizations resulting from technologically driven advancements [71].
Given that the success of complex interventions is significantly influenced by the perspectives of various stakeholders, and that these perspectives are closely tied to the healthcare system's environment, our team opted for an interview-based inductive qualitative study involving participants from different stakeholder groups. Interviews are widely employed in qualitative research because they provide a platform for individuals to articulate their experiences and insights into the phenomena being investigated [[72], [73], [74]].
Semi-structured interviews prove suitable when: (a) there is insufficient knowledge about the topic, making it difficult to develop comprehensive survey questions; (b) there is a need to comprehend interviewee perspectives; (c) there is an opportunity to engage with experts, such as key informants [75].
Reliability and validity are often intertwined; however, semi-structured interviews enhance validity by capturing rich insights from a limited number of knowledgeable participants [75]. This approach ensures familiarity with the perspectives of health services and organisational environments, and it maintains a comparative view across stakeholder groups.
The interview results were harmonised with the findings of the literature review, incorporating diverse perspectives (technical, social, and organisational), to identify barriers and opportunities. This methodological strategy fortified robustness and broad analytical generalisation through theoretical elaboration, while allowing us to investigate complicated contextual quirks associated with various stakeholders, facilitating the exploration of intricate contextual intricacies within stakeholder dynamics [76].
Furthermore, the analysis and discussion delve into the transformations and strategies pertinent to the healthcare ecosystem when confronted with the integration of digital information.
3.2. Participant recruitment
Ethical approval was obtained from the university's ethics committee (project number: DYCDVEESSE-MG-DATOS-20230301). Consent forms were collected and signed by the participants. The selection criteria included essential actors in the ecosystem, such as hospital managers, doctors, nurses, patients, pharmaceutical professionals, and patients' relatives.
Two critical considerations guided the profile selection. First, the insights gained from classifying the agents at the ecosystem level enabled the identification of those with the greatest relevance or influence on the disease, whether at the diagnostic or economic level. Second, input from an expert neurologist in MS played a crucial role in seeking a diverse range of interviewees. This approach aimed to mitigate potential biases in the results by incorporating the perspectives of various stakeholders.
A total of 25 interviews were conducted involving various stakeholders: patients with MS, patients’ family members, neurologists (specialists and general practitioners), MS nurses, physiotherapists, medical directors, and pharmacists (see Table 1). To streamline the focus, a specific type of physical therapist was selected based on the high demand for rehabilitation therapy [77]. The inclusion of family members recognised their influential role and dependency as the patients' conditions progressed.
Table 1.
Summary of the people interviewed.
| Role | Men | Women |
|---|---|---|
| Physician | 1 | 6 |
| Physiotherapist | 0 | 2 |
| Patients | 2 | 7 |
| Nurse | 0 | 2 |
| Relatives | 0 | 2 |
| Pharmacists | 1 | 0 |
| Hospital Managers | 1 | 1 |
Neurologist selection aimed for diversity by including physicians from various hospitals, reflecting Spain's varied MS approach due to categorisation. Eight top-tier hospitals were chosen, mostly in Madrid: (i) three high-tech third-level hospitals and specialised institutes; (ii) 4 second-level reference hospitals of intermediate complexity; (iii) one first-level hospital. One neurologist practised across two hospitals (third and second levels). Neurologists played a vital role in patient selection, considering the clinical evaluation, age, and sex ratio (three times more common in women). They also facilitated connections with professionals, such as pharmacists and healthcare staff.
3.3. Data collection
The interviews, conducted in 2023, were facilitated through online platforms. The participants were invited to the sessions scheduled for their convenience. Each interview lasted approximately 50 min on average for a total of 21 h and 30 min. These interviews were exploratory and semi-structured, incorporating aim-oriented questions, with room for exploration and unexpected insights.
Open-ended lead questions tailored slightly for each target group provided a framework for the interviews, allowing participants to share their expertise on the topic and context of the PSS project. Participants’ roles as experts eliminated the need for complicated sampling techniques in study participation [75].
The sessions were led by researchers who received support from a neurologist, with expertise in MS, covering aspects ranging from the disease process and diagnostic tests to the clinical procedures and various agents involved. This collaboration ensured that researchers had sufficient background information on MS to assess the impact of PSS and develop a semi-structured interview schedule for data collection and discussion initiation [75]. Additionally, a pilot test of the interview model was conducted to ensure accurate formulation of the interviews.
The interview process commenced with an introduction to the PSS configuration and outlined the technology to be used and its associated objectives. Subsequently, the researcher posed a series of questions categorised into two key blocks of interest. The first block pertained to enquiries about the interview participants, including roles, responsibilities, and experiences with MS, among other relevant aspects. The second block focused on questions related to the PSS implementation process, specifically addressing barriers and facilitators to the deployment of digitalisation.
The interviews and their recordings were conducted using Microsoft Teams [78] and occasionally using Google Meet. However, because of the limitations of the basic package of the latter, which does not offer voice and image recording, OBS Studio [79] was utilised.
3.4. Data coding
Interviews were transcribed using Sonix software to prepare the corpus. Data categorisation employed two multivariate techniques: descending hierarchical classification (DHC), which confirmed thematic categories, and a dendrogram displaying stable word classes with similar vocabulary.
The variables considered were.
-
•
Scope and role: Health (hospital manager, doctor, nurse, physiotherapist, or pharmacist) or No Health (patient or relative).
-
•
Gender.
-
•
Comments on aspects related to the PSS implementation.
3.5. Data analysis
The analysis software supports quantitative analysis, enabling the identification of agents, delving into their understanding, examining the relationships among them, and analysing the perceived barriers and facilitators. The software IRAMUTEQ version 0.7 alpha 2 http://www.iramuteq.org/was used to analyse the interviews, as suggested by other authors [[80], [81], [82], [83], [84]].
IRaMuTeQ, an R interface for multidimensional analysis of texts and questionnaires, is freely accessible software based on the R and Python languages. Python, from the perspective of lexical analysis, handles text processing and the generation of cloned tables, while R is the source of all statistical analyses and the production of the graphics provided by the interface.
This software is highly useful, particularly for analysing extensive sets of texts. In cases where the volume of information is substantial and challenging to cover manually, IRaMuTeQ is a valuable tool [82]. Notably, this tool provides a dual analysis. On the one hand, it performs quantitative analysis by conducting statistical analyses, comparative calculations, and, in some analyses, by considering the absence or presence of chart results. On the other hand, it offers a qualitative approach, as the user is responsible for interpreting and contextualising the data to provide meaning to the obtained results.
Before analysing the texts, it is essential to prepare them.
-
−
Documents must be in plain text format (.txt), which is preferably saved in UTF-8.
-
−
Each analysis should consolidate all the texts into a single text file, working with as many files as the analyses.
-
−
The start of each text in the corpus should be marked with four asterisks (****) followed by variables, each preceded by an asterisk (*), an underscore, and its modality.
-
−
The grammar restrictions include not using certain special characters, indentations, margins, tabulations, or text justifications.
-
−
For terms written separately but used together (e.g. eHealth 4.0), an underscore should be used to ensure that the software interprets it as a single word (eHealth_40).
The analytical approach parallels some studies [83,84], meeting the recommended 20 to 30 text minimum [85].
Among the analyses provided by IRaMuTeQ, the following were utilised for the current project.
-
⁃
Word Cloud: This initial analysis organises active forms of words into a cloud based on their frequency in the input texts. In this configuration, the tool limited the number of words to 100 for a clear and representative graphic. Some word groups, such as verbs and their complementary forms, or adjectives like ‘good’, ‘both’, or ‘much’, were excluded from the analysis as they did not contribute significantly to the meaningful content. This analysis served as the starting point for a more in-depth examination.
-
⁃
The Descending Hierarchical Classification (DHC) is a dendrogram illustrating classes of words with a similar vocabulary, enabling the observation of similarities within these classes and their distinctions from others. It provides insights into the associations or disparities between texts and words, aiding in the identification of explanatory factors for the groups or variables utilised. This analysis also offers profiles that provide a detailed view of the lexicon within each class, including information, such as position, grammatical category, and form itself. For effective classification, text fragments must retain over 75 % [85]; our study exceeded 80 %.
-
⁃
The Correspondence Factor Analysis (CFA), represented in a Cartesian plane, should be interpreted together with DHC. This demonstrates the association and opposition between words and texts. The two factors on the x and y axes show the percentages that explain the differences between classes, words, and variables. Additionally, it enabled the identification of the association between the classes obtained in the DHC and the variables defined in the analysis. Factors are latent dimensions (constructs) that summarise or explain a set of observed variables [86].
Finally, a complementary option to all the analyses provided by IRaMuTeQ is that, for each form, two visualisations are available: concordance and associated forms. The associated forms display, for that word, forms that have been lemmatised (e.g. doctor and doctors). This concordance allows us to see the text segments of the class in which the chosen form appears in our corpus. This was useful for complementing and guiding the analyses described previously.
4. Results. Enablers and barriers analysis
The analysed corpus only included the answers to the following questions for the interviewee.
-
•
Do you think the implementation of PSS is viable?
-
•
What barriers or facilitators do you identify in its implementation?
-
•
What strategies do you think could help with its implementation?
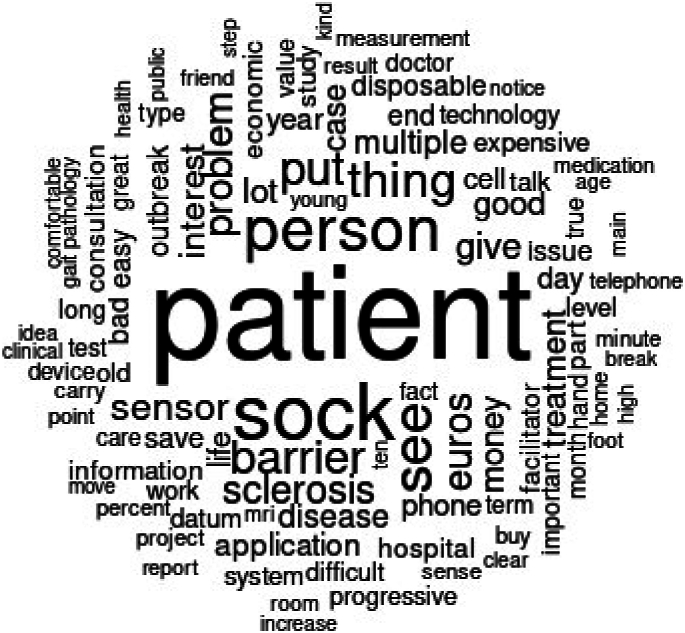
This study aimed to capture actors' perceptions of barriers and enablers. All ecosystem participants expressed a person-centred care approach (see Fig. 3) using words such as ‘sock’, ‘the PSS’, and ‘euro, cost, money’, identifying the key barrier as PSS funding. Given escalating healthcare costs, some authors deem the current care paradigm unsustainable [87,88] The potential of digitalisation to delay disease progression and ease the societal economic burden has been acknowledged [42]. However, this hinges on disruptive technological changes in healthcare, organisational structures, and information systems [89].
Fig. 3.
Word cloud of enablers and barriers analysis.
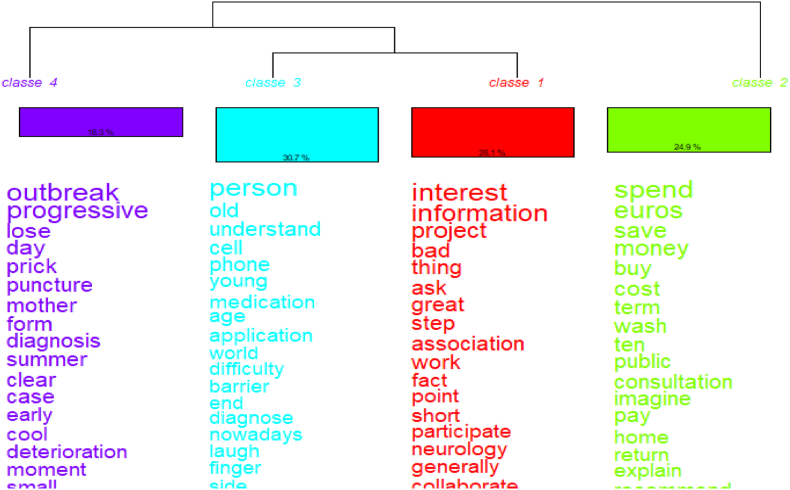
The DHC (see Fig. 4) generated four classes, representing 28.1 %, 24.9 %, 30.7 %, and 16.3 % of the analysed content, respectively. An analysis of 81.67 % of the global text was achieved. Class 3 (blue) represented the approaches of patients, nurses, and relatives. We named this the ‘impact on the person's life’ (see Table 2). Some words in this class were ‘barriers’, ‘phone’, ‘old’, and ‘age’, which were the main barriers identified by all actors, that is, limited financial resources and technical gadget problems. However, patients' attitude regarding smartphone use was positive.
Fig. 4.
Descending Hierarchical Classification (DHC) of the enablers and barriers answers.
Table 2.
Class 3 (blue) Patients', relatives', and nurses’ approaches.
| Words | Interviews | Issue | Attribute | References | |
|---|---|---|---|---|---|
| Impact on person's life | barrier, phone, old, age | Relative 2: ‘How are you going to be connected to the phone, if you have any problems walking?’ | Barriers | Limited financial resources and troubles with technical gadgets | [10,42] |
| Patient 3: ‘People closest to the progressive forms are older. … Handling of a smartphone is more complicated for them.’ | |||||
| Nurse 2: ‘Patients may not like it so much, not by age. Some older patients handle their smartphones phenomenally.’ | |||||
| application, medication, diagnosis | Patient 7: ‘The application, having to connect it to the socks and so on, wouldn't affect me either.’ | Drivers | Proactive attitude | [90] | |
| Nurse 1: ‘All of this will help us to know how patients are really doing and, based on that, we can give them a medication.’ |
These findings align with those of Griffin and Kehoe [90], highlighting the strong digital affinity and competence of younger patients with MS, boding well for PSS success. However, disease progression and MS-related impairments (visual, concentration, and motor skills) may impede their use. Voigt et al. [42] suggested alternative functionalities, such as print, voice control, and scalable interfaces to enhance adherence and promote patient empowerment and co-creation [91]. Minimal concerns emerged in all the cases. Given the vast volume of data that may complicate care decisions, the focal point was data privacy and security [10].
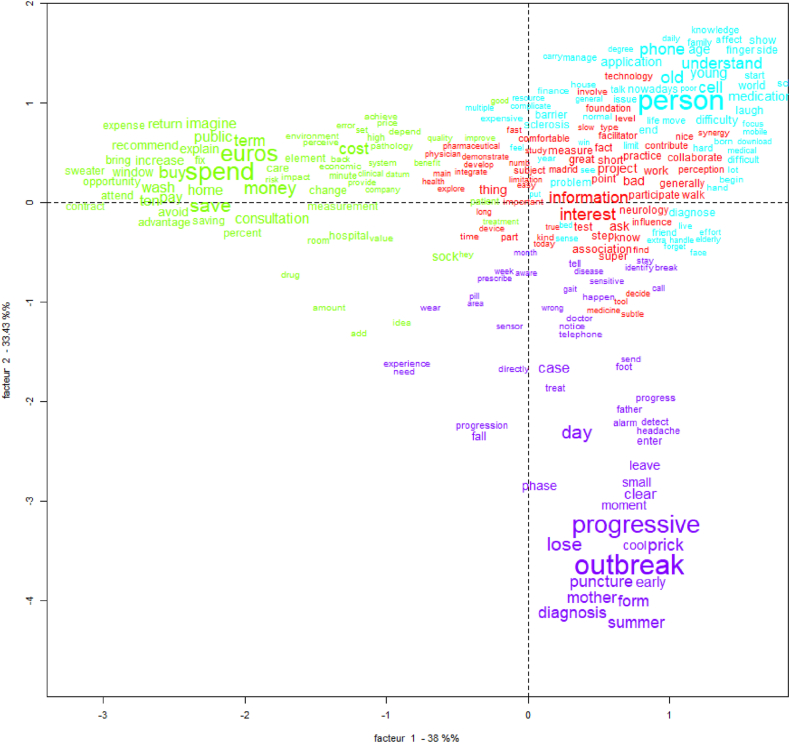
In the next analysis, the CFA graph describes a factorial plan (see Fig. 5), where Factor 1 (x-axis) explains 38 % of the difference between variables and Factor 2 (y-axis) explains 33.43 % and shows the proximity of Class 1 (red) and Class 3 (blue), with both connected to Class 4 and disease-related terms. Class 1 represents the approaches of physicians and pharmaceutical companies. We named this the ‘impact on diagnosis’ (Table 3). Some words in these classes are ‘project’, ‘short’, and ‘step’, linked to the financing and cost trade-offs, and ‘information’ and ‘time’, linked to the PSS information generated; both refer to PSS implementation barriers [92]. Other words are ‘interest’ and ‘participate’, which refer to the facilitators identified. The PSS technology is not something they consider complicated to implement a priori [93].
Fig. 5.
Correspondence Factor Analysis (CFA) of the enablers and barriers answers.
Table 3.
Class 1 (red) doctors and pharmaceuticals’ approach.
| Words | Interviews | Issue | Attribute | References | |
|---|---|---|---|---|---|
| Impact on MS diagnosis | project, short, step | Doctor 1: ‘The main barrier of the project is economical.’ | Barriers | Financing and cost trade-offs | [94,95] |
| Doctor 2: ‘More than giving more life expectancy is trying to shorten the progression of disability as much as possible.’ | |||||
| information, time | Doctor 1: ‘That it does not make me waste time in the consultation and that it does not mess us up.’ | Challenges about skills for digitalisation | [32,42] | ||
| Doctor 6: ‘We also need information useful and understandable by the neurologist.’ | |||||
| interest, participate | Doctor 3: ‘It will give them information on how their disease is controlled. They will want to participate.’ | Drivers | Adherence of MS patients to PSS | [42,91] |
These findings are consistent with those of the literature review. The PSS integration depends on technological and organisational factors. It necessitates seamless integration with medical documentation and resources for healthcare professionals, and it addresses shifted skills due to new technology. The skills and knowledge requirements are altered, and systematic monitoring in clinical practice is often incompatible with the limited time available for consultation [32,42].
The PSS incurs substantial costs, but long-term savings can offset these costs, reducing the economic impact of MS [94,95]. Security and privacy data issues were overlooked, requiring the rethinking of established processes, behaviours, and cultures. Institutional and competitive elements influence technology adoption decisions across micro, meso, and macro levels. Standardisation and regulation are necessary to foster ecosystem collaboration [10,13].
Fig. 5 representing the CFA graph additionally shows Class 2 (green). It represents hospital managers and physiotherapists' approach. We call it Governance’ (see Table 4). Some words in this class are ‘euros’, ‘money’, ‘cost’, ‘return’, and ‘wash’, linked to the delivery of cost-effective healthcare. This class unveils distinct perspectives: the physiotherapist embodies professional logic, while hospital managers adhere to managerial logic [96].
Table 4.
Class 2 (green) hospital managers and physiotherapists’ approach.
| Words | Interviews | Issue | Attribute | References | |
|---|---|---|---|---|---|
| Governance | euros, money, cost, return, wash | Director_1: ‘If I am going to spend two more euros on MS, it has to be because it will produce a return.’ | Barriers | Efficacy, safety, quality, cost-effective. | [97,98,100] |
| Physio 1: ‘Also the cost, because they need many treatments. Although they have many aids, they are also very expensive.’ | |||||
| Director_2: ‘Because of course, I understand that those socks have to be washed and the sensor deteriorates.’ | |||||
| Director 1: ‘It seems to me it is a facilitating element. But be careful, we return to the same thing, cost efficiency.’ | Drivers |
In this study, individual actors co-opted strategic elements from opposing institutional logics attuned to both resource allocation and costs. The imperative for PSS in MS healthcare aligns with the demand for enhanced collaborative information and performance management, crucial for curative, preventive, rehabilitative, and palliative care [87,93]. Improved patient outcomes yield increased social and economic values across the healthcare continuum. The PSS, which focuses on the early diagnosis of chronic and costly MS conditions, can potentially mitigate disease progression to severe disabilities. It has a direct positive impact on ecosystem actors: (i) streamlined health system operations; (ii) improved treatment planning by clinicians; (iii) most significantly, early diagnosis and healthier lives for patients.
However, the introduction of this novel technology faces three pivotal barriers: proving its effectiveness, safety, and quality. Moreover, regulatory bodies and public entities increasingly consider cost-effectiveness to be a fourth barrier [97]. Hospital managers note that evaluations of healthcare digitalisation, although limited, often indicate feasibility and user acceptability. Although evidence of its impact on health outcomes is scant, most studies have found it to be cost-effective [98].
In the PSS context, relevance surpasses costs and demands a comprehensive assessment of both expenses and effects. A cost-utility analysis assessing the value proposition [99] becomes vital. In light of this, MS introduces complications and life changes that affect independence, potentially leading to physical, psychological, social, or economic issues that must be addressed.
Finally, the analysis highlights the pivotal managerial role of eHealth service requirements. The implementation of PSS necessitates [101]: (i) IoT-based solutions, new IT roles, and data management; (ii) empowering all involved in value co-creation; (iii) data-sharing policies to legitimise value creation among ecosystem actors.
5. Discussion
As mentioned in the introduction section, the core function of healthcare systems is to provide cost-effective services that are efficient and serve the needs and preferences of society from micro, meso, macro, and mega observation levels, and PSS can play a significant role in the tracking and management of MS. However, PSS applications may vary in their specific features and capabilities depending on the specific value proposal. At the micro level, individual health-service interactions occur among nurses, doctors, and patients. The meso-level encompasses the activities of interest groups, such as patient associations or medical services within or across hospitals. The macro-level pertains to regional and national healthcare systems. Finally, the mega level represents the EU dimension, requiring the interoperability of national systems to ensure seamless citizen mobility and business relationships while assessing the adopted principles.
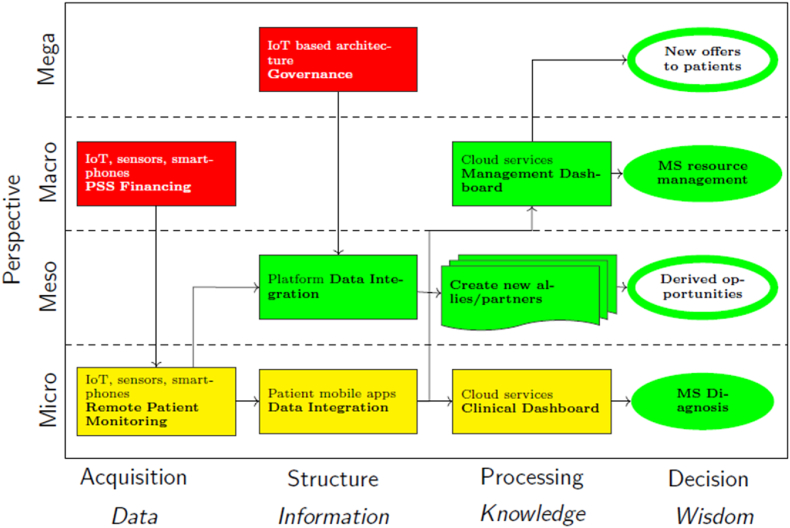
The potential impact of integrating remote patient-monitoring PSS for patients with MS is presented in Fig. 6 to identify the barriers (red), facilitators (green), and risks (orange). Focusing on one or more levels, interests can be seen as business interactions within a participant–population business ecosystem [102].
Fig. 6.
Explanation of the PSS business model for the OEM.
Therefore, these relationships should be analysed at different levels of the ecosystem (vertical axis) and at different stages of information management (horizontal axis).
-
•
On the one hand, a PSS operates at the micro level (green) to aid neurologists in disease monitoring, achieving early detection that impacts care quality, resources, and costs at the macro level (green). At the micro level, the process begins with wearable devices (smart bracelets, accelerometers, insoles/socks) for assessing gait disturbances. A digital platform involving mobile applications gathers data from wearables to analyse and identify key performance indicators (KPIs) for effective gait disturbance detection and monitoring.
Barriers, particularly funding, condition PSS as highlighted by all interviewees. Macro-level initiation involves Health System approval and financing (red), followed by hospital financing (red). The cost and lack of health system subsidies [88] may render initiatives unsustainable, as emphasised by the interviewees. Healthcare systems worldwide grapple with escalating costs and a worsening imbalance, which is particularly evident in countries, such as Spain, where healthcare relies on universal coverage and funding via general taxation. In this context, hospital administrators emphasize several points concerning payer approval and reimbursement for PSS healthcare providers to facilitate their adoption.
It is important to bear in mind that the cost of MS depends on the degree of disability, that MS affects people between the ages of 20 and 40, that it is a disabling disease and that its treatment is costly. In Europe, the cost of MS has recently been estimated, taking into account only the pharmacological and other costs, but excluding those derived from loss of productivity. In Spain, the annual cost of a MS patient with mild disability was estimated in €20,600, rising to €48,500 and €68,700 for moderate and severe disability, respectively. In addition, more than half are unemployed and work productivity is affected in 72 % of people with MS [103].
Furthermore, governments and healthcare entities urging the substantiation of eHealth investments through rigorous, relevant, and pragmatic means and adopting a multistakeholder perspective [99] can enhance this process. Factors related to person-centred care could also be factors in evaluating the PSS and its potential expansion to other patient groups, such as post-recovery stroke patients and individuals with Parkinson's disease, as highlighted in interviews with neurologists.
Micro-level processes are influenced (orange) by wearable device and app usability (comfort, navigation, efficiency) and organisational/behavioural aspects (training, digital affinity, skills). Both impact and actor engagement, are crucial for value creation in innovation ecosystems [51] among patients and healthcare professionals.
Attaining this level of engagement requires careful consideration. While the study highlights the shared adoption of a Person-Centred Care approach among all interviewed ecosystem stakeholders, it remains crucial to comprehend the broader social context and navigate conflicting interests. Different actor groups may have different motivations and expectations [104,105] Integrating PSS into healthcare has several implications. This necessitates an overhaul of diagnostic processes, training of healthcare professionals in its application, and logistical adjustments for its dissemination, among other facets. In general, as digital health resources become more widespread, it is essential to assess individuals’ ability to interact with these materials or technologies, their digital health literacy, or electronic health (eHealth) literacy [106], to ensure that they are appropriate for the target audiences [107].
Establishing a stable network of actors and sustaining collaboration entails addressing cultural, value-based, and belief-driven aspects. This helps to forestall individualistic or opportunistic actions and potential friction among stakeholders [108]. In this context, the concern arises that the implementation of a PSS might result in a reduction in consultation time for physicians, a concern shared by both physicians and patients, given the already tight schedules [109].
Second, the lack of end-user engagement may be linked to technical components encompassing patient-related artefacts, such as socks and phone interfaces. The importance of comfort and user-friendliness is consistently underscored by all stakeholders, especially when considering the challenges faced by some patients with MS in advanced disease stages, as a high percentage of them have cognitive impairment. Additionally, healthcare professionals expressed reservations about cloud software, encompassing interface usability, customisation, implementation, and integration. Several studies have shown that many eHealth technologies fail to take up because health professionals are not interested in change and want to maintain the status quo [110]. However, the availability of the technical infrastructure in the facility can influence the poor perception that health professionals might have, building their confidence that when they transition to eHealth, it will be successful [111]. Schmidt et al. [56] suggest that enhancing customer acceptance of PSS aligns with a pull strategy that identifies barriers to patient or healthcare professional acceptance, and they examine how PSS can influence these factors. Shifting from a product-centric to a user-centric approach necessitates the development of customised apps or platforms for stakeholders [33], further away from those devoted to patient data collection. However, this transition also brings forth new challenges for the PSS socio-technical ecosystem [93] These challenges extend beyond technological considerations, involving the comprehensive integration of IoT architecture, and encompass human factors, including the delicate balance between adaptability and resistance to change. Additionally, there is a need to address shifts in processes, moving from a reactive to a more proactive and predictive approach [44].
-
•
By contrast, PSS enables the sharing of structured information via a meso-level (green) platform. This information is used to create value and business opportunities for the ecosystem actors (green), enhance care, attract stakeholders, such as insurers and pharma, and form partnerships. We recognise useable data as a valuable asset [112], and the data collection process can align with diverse interests. In the context of health data within the MS PSS ecosystem, stakeholders pursue two primary intentions, leading to distinct approaches to data utilisation: (i) commercial use, inherently seeking economic advantages and adhering to economic principles; (ii) a non-profit commitment, aligning with broader societal goals. For instance, pharmacists and health insurance companies may identify cost reduction in R&D and medical services, respectively, as a key driver, reflecting economic principles in their commercial utilisation of data. The Public Health System may recognise the value of data as an opportunity to invest in its utilisation to prioritise the well-being of patients with MS [113,114].
The PSS in healthcare initially hinges on data security and privacy, which are key barriers missing in speech. Data-sharing policies to promote and legitimise (red) this value co-creation process within the ecosystem actors are needed. To achieve an effective legal framework, each country requires the adaptation of PSS to its national legislation and with consideration of the country's legal framework [115]. The GDPR (EU 2016/679) states that the responsibility for any data collected lies with the institution that collects and processes them. The ramifications of this legislation are far-reaching as it places the institution responsible for gathering and managing all data via remote monitoring in a position of accountability. Therefore, stakeholders involved in PSS should establish protective measures to guarantee the security and confidentiality of personal medical information. Furthermore, when it is advantageous to generalise access to such data, appropriate access protocols should be ensured.
In addition, an interesting gap in the literature and interviews is stakeholders' limited awareness of technological demands, especially from information technologies, for a successful long-term service. Health-related data have significant potential, but face challenges in their effective use. Despite these challenges, these PSS data in MS will provide research knowledge on the behaviour of progression and, importantly, contribute to the development of treatments aimed at controlling progression by improving the outcome measures in clinical trials, especially in progressive phenotypes of MS where more therapies are needed [116]. Currently, outcome measures to assess progression are based on less sensitive and reliable measures such as the ordinal scales Expanded Disability Status Scale [117]. In clinical practice, it will allow the impact of gait impairment to be assessed in a more realistic context, its early detection and monitoring, and thus to optimize and personalize therapeutic decisions. Additionally, it can reduce healthcare spending by tapping into the underutilised potential of data. Voigt et al. [118] discussed the use of digital twins for MS as a revolutionary tool to improve diagnosis, monitoring, and therapy to improve patient well-being, reducing economic costs and preventing disease progression. However, owing to the intricate and prolonged nature of MS, a notably extensive and multidimensional dataset must be gathered and structured for development. Ensuring high-quality data is crucial; they should be collected accurately to appropriately represent the patient [119]. However, each stakeholder develops a perspective based on their own short-term expectations, making it difficult to obtain a long-term overview. The collected data are typically stored across diverse repositories in various formats owned by different data providers. Four key challenges impede consistent and seamless data evaluation [120]: i) data heterogeneity: additional processing steps are necessary before conducting a comparative analysis; ii) distributed storage: data are dispersed across different repositories, including cloud storage, servers, and private storage locations for patients or healthcare professionals; iii) the need for collaborative communication: open and cooperative communication among ecosystem stakeholders is essential; iv) data quality and interoperability: ensuring high-quality data and compatibility across different systems is crucial. Therefore, a recommendation for future interview planning processes is to include potential technological stakeholders who can provide a longitudinal overview of potential constraints.
6. Conclusions
Globally, healthcare is facing growing demand for personalised care within budget constraints. The PSS applications hold immense potential to enable innovative technologies and practices across providers, professionals, and patients in a coordinated healthcare ecosystem. The need for a PSS in healthcare has been shown to be key to supporting the requirements for better and more collaborative information and performance management, which are critical for treating patients with MS with preventive and palliative care. Wearable sensors integrate technology with advanced disease monitoring, assessment of gait disorder progression, and individual patient performance tracking. Combining services, products, and technologies suits healthcare needs, enhances system efficiency, optimises MS treatment strategies, and facilitates early diagnosis of progression in MS for healthier lives.
The PSS introduces opportunities and challenges across social, institutional, and organisational domains. Therefore, focusing on the combined effects of PSS on practices, structures, and values, an in-depth interview study was conducted with essential ecosystem actors–hospital managers, doctors, nurses, patients, pharmaceuticals, and patient relatives–in the paradigm centres on data. As presented in the discussion, data empowers value co-creation, yet also poses a key barrier.
This study's exploration of healthcare as a complex socio-technical system offers actionable insights for healthcare providers. This aids in identifying areas of focus and challenges related to barriers, drivers, and risks. Adapting PSS for patients with MS involves shifting to a user-centric, service-dominant approach, addressing.
-
⋅
Micro-level needs regarding technical gadgets applications involving patients and healthcare professionals. So, two potential barriers are identified: usability issues with wearable devices and apps (related to comfort, navigation, and efficiency), and organizational/behavioral factors (including training, digital literacy, and skills).
-
⋅
Meso-level alliances within healthcare ecosystems. The availability of useable data is recognised as a driver, suggesting that aligning the data collection process with diverse interests could generate value and business opportunities.
-
⋅
Macro-level funding impact. All interviewees emphasize the main obstacle being the cost and lack of subsidies within the health system, potentially leading to unsustainable initiatives.
-
⋅
Mega-level governance implications on privacy and security data. Two notable barriers missing on the speech.
7. Managerial implications and future research
Contributions from this research stream are primarily directed at suggesting proper or more sustainable strategies within the healthcare ecosystem, including the ecosystem building process itself. The case study provided throughout this paper should offer some guidance on the main drivers and barriers for PSS development and implementation in the healthcare context, resulting in several key issues to consider for different actors to reap tangible benefits over time. In this sense, a strong recommendation is to explore the potential impact of integrating a remote patient monitoring PSS for patients with MS, not only at the ecosystem level, but also at the ecosystem stakeholder level, because it brings an extended understanding of facilitators and limitations at different aggregation levels, which can significantly increase the effectiveness of the PSS analysed in the STS.
The current research highlights different aspects, which require further research. It seems that different stakeholders have different and polysemic definitions for concepts, such as ‘perceived quality of service’ in healthcare. Another relevant topic requiring further explanation is ‘value creation’ and its relationship with other factors, such as ecosystems and digital innovation. In addition, there is a need to explore ways to enhance patient engagement and user experience in digital PSS solutions by considering factors, such as usability, accessibility, and patient preferences. From a technical point of view, an indirect value of the PSS is its capability to develop predictive models using data from digital PSS systems to forecast disease progression in patients with MS, helping healthcare providers make informed decisions.
Another relevant aspect to be considered in the PSS is seamless integration with electronic health records to provide a comprehensive view of the patient's health. Finally, there is an important need to investigate how digital PSS can address health disparities in MS care, ensuring equitable access to resources and support for all patients, regardless of their location or socioeconomic status, at the macro and mega levels of analysis, thereby enhancing the sustainable progress of the ecosystem and service levels. Therefore, there are significant aspects to be investigated and clarified to build an integrated management view that efficiently serves the needs and preferences of society as a whole.
8. Limitations and suggestions
The specific dimension of the research provides a context for limitations. Besides, a deeper study is necessary to confirm the different research findings on interviews and include the opinion of the second level of agents (operators such as IT, maintenance, sock sterilization, etc.). Besides, another limitation is the difficulty in estimating a quantitative metric for the contribution of value to the Health system.
Moreover, this integrated framework can guide ecosystem creation and benefit cases, such as post-stroke rehabilitation and Parkinson's disease monitoring. Suggestions for improving the set of stakeholders have been provided because of the long-term limitations arising from the complexities of the PSS itself.
Data availability statement
The data that has been used is confidential.
Ethics declarations
This study was reviewed and approved by Ethics Committee of the Polytechnic University of Madrid, with the approval number: DYCDVEESSE-MG-HUMANOS-20230307.
All participants/patients (or their proxies/legal guardians) provided informed consent to participate in the study.
All participants/patients (or their proxies/legal guardians) provided informed consent for the publication of their anonymized case details and images.
CRediT authorship contribution statement
Mercedes Grijalvo: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Joaquín Ordieres-Meré: Writing – review & editing, Writing – original draft, Validation, Methodology, Investigation, Formal analysis, Conceptualization. Javier Villalba-Díez: Investigation, Conceptualization. Yolanda Aladro-Benito: Writing – original draft, Validation, Methodology, Investigation, Conceptualization. Guillermo Martín-Ávila: Methodology, Investigation, Conceptualization. Arancha Simón-Hurtado: Investigation, Conceptualization. Carlos Vivaracho-Pascual: Investigation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was partially supported by the Spanish “Agencia Estatal de Investigación” through the grant PID2022-137748OB-C31 funded by MCIN/AEI/10.13039/501100011033 and ”ERDF A way of making Europe”. We want to recognise the preliminary work carried out by MsC Andrea Herrero García in her master's thesis for the Industrial Engineering Degree at the Technical University of Madrid.
References
- 1.Agarwal R., Gao G.G., DesRoches C., Jha A.K. Research commentary: the digital transformation of healthcare: current status and the road ahead. Inf. Syst. Res. 2010;21:796–809. [Google Scholar]
- 2.ao Reis J., Amorim M., ao N.M., Matos P. Advances in Intelligent Systems and Computing. Springer International Publishing; 2018. Digital transformation: a literature review and guidelines for future research; pp. 411–421. [Google Scholar]
- 3.Kraus S., Schiavone F., Pluzhnikova A., Invernizzi A.C. Digital transformation in healthcare: analyzing the current state-of-research. J. Bus. Res. 2021;123:557–567. [Google Scholar]
- 4.European Union, Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients’ rights in cross-border healthcare, Off. J. Eur. Union (2011), 4.4.2011, L 88/45–L 88/64.
- 5.Schiza E.C., Kyprianou T.C., Petkov N., Schizas C.N. Proposal for an ehealth based ecosystem serving national healthcare. IEEE Journal of Biomedical and Health Informatics. 2019;23:1346–1357. doi: 10.1109/JBHI.2018.2834230. [DOI] [PubMed] [Google Scholar]
- 6.Nittas V., Zecca C., Kamm C.P., Kuhle J., Chan A., von Wyl V. Digital health for chronic disease management: an exploratory method to investigating technology adoption potential. PLoS One. 2023;18(4) doi: 10.1371/journal.pone.0284477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diaz-Skeete Y., Giggins O.M., McQuaid D., Beaney P. Enablers and obstacles to implementing remote monitoring technology in cardiac care: a report from an interactive workshop. Health Inf. J. 2020;26:2280–2288. doi: 10.1177/1460458219892175. [DOI] [PubMed] [Google Scholar]
- 8.Piwek L., Ellis D.A., Andrews S., Joinson A. The rise of consumer health wearables: promises and barriers. PLoS Med. 2016;13 doi: 10.1371/journal.pmed.1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siala H., Wang Y. SHIFTing artificial intelligence to be responsible in healthcare: a systematic review. Soc. Sci. Med. 2022;296 doi: 10.1016/j.socscimed.2022.114782. [DOI] [PubMed] [Google Scholar]
- 10.Javaid M., Haleem A., Singh R.P., Rab S., Haq M.I.U., Raina A. Internet of things in the global healthcare sector: significance, applications, and barriers. International Journal of Intelligent Networks. 2022;3:165–175. [Google Scholar]
- 11.Hu Y., Bai G., A systematic literature review of cloud computing in ehealth, arXiv preprint arXiv:1412.2494. Health Inform. Int. J. (HIIJ) 3 (4) (2014) 11–20.
- 12.Alenoghena C.O., Onumanyi A.J., Ohize H.O., Adejo A.O., Oligbi M., Ali S.I., Okoh S. Aehealth: a survey of architectures, developments in mhealth, security concerns and solutions. Int. J. Environ. Res. Publ. Health. 2022;19(20) doi: 10.3390/ijerph192013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hines M., Brunner M., Poon S., Lam M., Tran V., Yu D., Togher L., Shaw T., Power E. Tribes and tribulations: interdisciplinary ehealth in providing services for people with a traumatic brain injury (tbi) BMC Health Serv. Res. 2017;17:1–13. doi: 10.1186/s12913-017-2721-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran V., Lam M.K., Amon K.L., Brunner M., Hines M., Penman M., Lowe R., Togher L. Interdisciplinary ehealth for the care of people living with traumatic brain injury: a systematic review. Brain Inj. 2017;31:1701–1710. doi: 10.1080/02699052.2017.1387932. [DOI] [PubMed] [Google Scholar]
- 15.Varsi C., Solberg Nes L., Kristjansdottir O., Kelders S., Stenberg U., Zangi H., Børøsund E., Weiss K., Stubhaug A., Asbjørnsen R., Westeng M., Ødegaard M., Eide H. Implementation strategies to enhance the implementation of eHealth programs for patients with chronic illnesses: realist systematic. Review J Med Internet Res. 2019;21(9) doi: 10.2196/14255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hidalgo A., Pérez N., Lemus-Aguilar I. Factors determining the success of eHealth innovation projects. Int. J. Software Sci. Comput. Intell. 2022;14(1):1–22. [Google Scholar]
- 17.Dhanvijay M.M., Patil S.C. Internet of Things: a survey of enabling technologies in healthcare and its applications. Comput. Network. 2019;153:113–131. [Google Scholar]
- 18.Shehabat I.M., Al-Hussein N. Deploying internet of things in healthcare: benefits, requirements, challenges and applications. J. Commun. 2018;13(10):574–580. [Google Scholar]
- 19.Wentink M.M., van Bodegom-Vos L., Brouns B., Arwert H.J., Vlieland T.P.V., De Kloet A.J., Meesters J.J. What is important in E-health interventions for stroke rehabilitation? A survey study among patients, informal caregivers and health professionals. Int. J. Telerehabilitation. 2018;10(1):15. doi: 10.5195/ijt.2018.6247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobson R., Giovannoni G. Multiple sclerosis – a review. Eur. J. Neurol. 2019;26:27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 21.Portaccio E., Bellinvia A., Fonderico M., Pastò L., Razzolini L., Totaro R., Spitaleri D., Lugaresi A., Cocco E., Onofrj M., Di Palma F., Patti F., Maimone D., Valentino P., Confalonieri P., Protti A., Sola P., Lus G., Maniscalco G.T., Brescia Morra V., Salemi G., Granella F., Pesci I., Bergamaschi R., Aguglia U., Vianello M., Simone M., Lepore V., Iaffaldano P., Filippi M., Trojano M., Amato M.P. Progression is independent of relapse activity in early multiple sclerosis: a real-life cohort study. Brain. 2022;145:2796–2805. doi: 10.1093/brain/awac111. [DOI] [PubMed] [Google Scholar]
- 22.Pardo G., Coates S., Okuda D.T. Outcome measures assisting treatment optimization in multiple sclerosis. J. Neurol. 2022;269:1282–1297. doi: 10.1007/s00415-021-10674-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexander S., Peryer G., Gray E., Barkhof F., Chataway J. Wearable technologies to measure clinical outcomes in multiple sclerosis: a scoping review. Mult. Scler. J. 2021;27:1643–1656. doi: 10.1177/1352458520946005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motti Ader L.G., Greene B.R., McManus K., Tubridy N., Caulfield B. Short bouts of gait data and body-worn inertial sensors can provide reliable measures of spatiotemporal gait parameters from bilateral gait data for persons with multiple sclerosis. Biosensors. 2020;10:128. doi: 10.3390/bios10090128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angelini L., Buckley E., Bonci T., Radford A., Sharrack B., Paling D., et al. A multifactorial model of multiple sclerosis gait and its changes across different disability levels. Trans Biomed Eng. 2021;68:3196–3204. doi: 10.1109/TBME.2021.3061998. [DOI] [PubMed] [Google Scholar]
- 26.Flachenecker F., Gaßner H., Hannik J., Lee D.H., Flachenecker P., Winkler J., et al. Objective sensor-based gait measures reflect motor impairment in multiple sclerosis patients: reliability and clinical validation of a wearable sensor device. Mult Scler Relat Disord. 2020;30 doi: 10.1016/j.msard.2019.101903. [DOI] [PubMed] [Google Scholar]
- 27.Woelfle T., Bourguignon L., Lorscheider J., Kappos L., Naegelin Y., Jutzeler C.R. Wearable sensor technologies to assess motor functions in people with multiple sclerosis: systematic scoping review and perspective. J. Med. Internet Res. 2023;25 doi: 10.2196/44428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reitzel S.B., Lynning M., Skovgaard L. Neurologists' views on patient reported outcomes in multiple sclerosis care. Heliyon. 2022;8(6) doi: 10.1016/j.heliyon.2022.e09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouwman E., Pluijm S.M., Stollman I., Araujo-Soares V., Blijlevens N.M., Follin C., Falck Winther J., Hjorth L., Kepak T., Kepakova K., et al. Healthcare professionals' perceived barriers and facilitators of health behavior support provision: a qualitative study. Cancer Med. 2023;12:7414–7426. doi: 10.1002/cam4.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osborne S.P., Radnor Z., Nasi G. A new theory for public service management? toward a (public) service-dominant approach. Am. Rev. Publ. Adm. 2013;43:135–158. [Google Scholar]
- 31.Pestoff V. Collective action and the sustainability of co-production. Publ. Manag. Rev. 2014;16:383–401. [Google Scholar]
- 32.Allen-Philbey K., Middleton R., Tuite-Dalton K., Baker E., Stennett A., Albor C., Schmierer K. Can we improve the monitoring of people with multiple sclerosis using simple tools, data sharing, and patient engagement? Front. Neurol. 2020;11:464. doi: 10.3389/fneur.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salimzadeh Z., Damanabi S., Kalankesh L.R., Ferdousi R. Mobile applications for multiple sclerosis: a focus on self-management. Acta Inf. Med. 2019;27:12. doi: 10.5455/aim.2019.27.12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeroushalmi S., Maloni H., Costello K., Wallin M.T. Telemedicine and multiple sclerosis: a comprehensive literature review. J. Telemed. Telecare. 2020;26:400–413. doi: 10.1177/1357633X19840097. [DOI] [PubMed] [Google Scholar]
- 35.Schepici G., Silvestro S., Bramanti P., Mazzon E. The gut microbiota in multiple sclerosis: an overview of clinical trials. Cell Transplant. 2019;28:1507–1527. doi: 10.1177/0963689719873890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilty D.M., Armstrong C.M., Luxton D.D., Gentry M.T., Krupinski E.A. A scoping review of sensors, wearables, and remote monitoring for behavioral health: uses, outcomes, clinical competencies, and research directions. J. Appl. Behav. Sci. 2021;6:278–313. doi: 10.1007/s41347-020-00190-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kvedar J., Coye M.J., Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff. 2014;33:194–199. doi: 10.1377/hlthaff.2013.0992. [DOI] [PubMed] [Google Scholar]
- 38.Greenes R.A., Bates D.W., Kawamoto K., Middleton B., Osheroff J., Shahar Y. Clinical decision support models and frameworks: seeking to address research issues underlying implementation successes and failures. J. Biomed. Inf. 2018;78:134–143. doi: 10.1016/j.jbi.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 39.Bacanoiu M.V., Danoiu M. New strategies to improve the quality of life for normal aging versus pathological aging. J. Clin. Med. 2022;11(14):4207. doi: 10.3390/jcm11144207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novitzky P., Janssen J., Kokkeler B. A systematic review of ethical challenges and opportunities of addressing domestic violence with AI-technologies and online tools. Heliyon. 2023;9(6) doi: 10.1016/j.heliyon.2023.e17140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larrucea X., Moffie M., Asaf S., Santamaria I. Towards a gdpr compliant way to secure european cross border healthcare industry 4.0. Comput. Stand. Interfac. 2020;69 [Google Scholar]
- 42.Voigt I., Benedict M., Susky M., Scheplitz T., Frankowitz S., Kern R., Müller O., Schlieter H., Ziemssen T. A digital patient portal for patients with multiple sclerosis. Front. Neurol. 2020;11:400. doi: 10.3389/fneur.2020.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broer T. The googlization of health: invasiveness and corporate responsibility in media discourses on facebook's algorithmic programme for suicide prevention. Soc. Sci. Med. 2022;306 doi: 10.1016/j.socscimed.2022.115131. [DOI] [PubMed] [Google Scholar]
- 44.Reis J., Melão N. Digital transformation: a meta-review and guidelines for future research. Heliyon. 2023;9 (1) doi: 10.1016/j.heliyon.2023.e12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rayes A., Salam S., Rayes A., Salam S. IoT vertical markets and connected ecosystems. Internet of Things From Hype to Reality: The Road to Digitization. 2019:239–268. [Google Scholar]
- 46.Hughes-Lartey K., Li M., Botchey F.E., Qin Z. Human factor, a critical weak point in the information security of an organization’s Internet of things. Heliyon. 2021;7(3) doi: 10.1016/j.heliyon.2021.e06522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cree B., Hollenbach J., Bove R., Kirkish G., Sacco S., Caverzasi E., et al. Silent progression in disease activity-free relapsing multiple sclerosis. Ann. Neurol. 2019;85:653–666. doi: 10.1002/ana.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Angelini L., Hodgkinson W., Smith C., Dodd J.M., Sharrack B., Mazzà C., Paling D. Weaeable sensors can reliably quantify alterations associated with disability in people with progressive multiple sclerosis in a clinical setting. J. Neurol. 2020;267:2897–2909. doi: 10.1007/s00415-020-09928-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Visch L., Oudenhoven L.M., Timmermans S.T., Beckerman H., Rietberg M.B., de Groot V., van der Krogt M.M. The relationship between energy cost of walking, ankle push-off and walking speed in persons with multiple sclerosis. Gait Posture. 2022;98:160–166. doi: 10.1016/j.gaitpost.2022.08.015. [DOI] [PubMed] [Google Scholar]
- 50.Atesaei A., Dadashi F., Mariani B., Gonzenbach R., Aminian K. Toward a remote assessment of walking bout and speed: application in patients with multiple sclerosis. IEEE J Biomed Health Inform. 2021;5:4217–4228. doi: 10.1109/JBHI.2021.3076707. [DOI] [PubMed] [Google Scholar]
- 51.Marent B., Henwood F., Darking M., Consortium E., et al. Ambivalence in digital health: Co-designing an mhealth platform for HIV care. Soc. Sci. Med. 2018;215:133–141. doi: 10.1016/j.socscimed.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 52.Albert-Cromarias A., Santos C.D. Coopetition in healthcare: heresy or reality? an exploration of felt outcomes at an intra-organizational level. Soc. Sci. Med. 2020;252 doi: 10.1016/j.socscimed.2020.112938. [DOI] [PubMed] [Google Scholar]
- 53.Cherif E., Bezaz N., Mzoughi M. Do personal health concerns and trust in healthcare providers mitigate privacy concerns? effects on patients' intention to share personal health data on electronic health records. Soc. Sci. Med. 2021;283 doi: 10.1016/j.socscimed.2021.114146. [DOI] [PubMed] [Google Scholar]
- 54.Mont O. Clarifying the concept of product–service system. J. Clean. Prod. 2002;10:237–245. [Google Scholar]
- 55.Gaiardelli P., Pezzotta G., Rondini A., Romero D., Jarrahi F., Bertoni M., Wiesner S., Wuest T., Larsson T., Zaki M., Jussen P., Boucher X., Bigdeli A.Z., Cavalieri S. Product-service systems evolution in the era of industry 4.0. Service Business. 2021;15:177–207. [Google Scholar]
- 56.Schmidt D.M., Bauer P., Mörtl M. PSS for influencing customer barriers and acceptance. Journal of Economics, Business and Management. 2015;3(10):990–993. [Google Scholar]
- 57.White F. Primary health care and public health: foundations of universal health systems. Med. Princ. Pract. 2015;24:103–116. doi: 10.1159/000370197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Roth E.G., Minden S.L., Maloni H.W., Miles Z.J., Wallin M.T. A qualitative, multiperspective inquiry of multiple sclerosis telemedicine in the United States. Int J MS Care. 2022;24:275–281. doi: 10.7224/1537-2073.2021-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kever A., Buyukturkoglu K., Riley C.S., De Jager P.L., Leavitt V.M. Social support is linked to mental health, quality of life, and motor function in multiple sclerosis. J. Neurol. 2021;268:1827–1836. doi: 10.1007/s00415-020-10330-7. [DOI] [PubMed] [Google Scholar]
- 60.Bayas A., Berthele A., Hemmer B., Warnke C., Wildemann B. Controversy on the treatment of multiple sclerosis and related disorders: positional statement of the expert panel in charge of the 2021 dgn guideline on diagnosis and treatment of multiple sclerosis, neuromyelitis optical spectrum diseases and mogigg-associated disorders. Neurol. Res. Pract. 2021;3:1–7. doi: 10.1186/s42466-021-00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Petrova-Antonova D., Spasov I., Krasteva I., Manova I., Ilieva S. Computational Science and its Applications–ICCSA 2020: 20th International Conference, Cagliari, Italy, July 1–4, 2020, Proceedings, Part I 20. Springer; 2020. A digital twin platform for diagnostics and rehabilitation of multiple sclerosis; pp. 503–518. [Google Scholar]
- 62.Kooijman M., Hekkert M.P., van Meer P.J., Moors E.H., Schellekens H. How institutional logics hamper innovation: the case of animal testing. Technol. Forecast. Soc. Change. 2017;118:70–79. [Google Scholar]
- 63.Haland E. Introducing the electronic patient record (epr) in a hospital setting: boundary work and shifting constructions of professional identities. Sociol. Health Illness. 2012;34:761–775. doi: 10.1111/j.1467-9566.2011.01413.x. [DOI] [PubMed] [Google Scholar]
- 64.Carboni C., Wehrens R., van der Veen R., de Bont A. Conceptualizing the digitalization of healthcare work: a metaphor-based critical interpretive synthesis. Soc. Sci. Med. 2022;292 doi: 10.1016/j.socscimed.2021.114572. [DOI] [PubMed] [Google Scholar]
- 65.Xing K., Rapaccini M., Visintin F. PSS in healthcare: an under- explored field. Procedia CIRP. 2017;64:241–246. [Google Scholar]
- 66.Gonzalez E., Peña R., Avila A., Vargas-Rosales C., Munoz-Rodriguez D., et al. A systematic review on recent advances in mhealth systems: deployment architecture for emergency response. J Healthc Eng. 2017;2017 doi: 10.1155/2017/9186270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marent B., Henwood F., Consortium E. Platform encounters: a study of digitised patient follow-up in hiv care. Sociol. Health Illness. 2021;43:1117–1135. doi: 10.1111/1467-9566.13274. [DOI] [PubMed] [Google Scholar]
- 68.Mair F.S., May C., O'Donnell C., Finch T., Sullivan F., Murray E. Factors that promote or inhibit the implementation of e-health systems: an explanatory systematic review. Bull. World Health Organ. 2012;90:357–364. doi: 10.2471/BLT.11.099424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coiera E. Putting the technical back into socio-technical systems research. Int. J. Med. Inf. 2007;76:S98–S103. doi: 10.1016/j.ijmedinf.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 70.Gromisch E.S., Turner A.P., Haselkorn J.K., Lo A.C., Agresta T. Mobile health (mHealth) usage, barriers, and technological considerations in persons with multiple sclerosis: a literature review. JAMIA open. 2021;4(3):ooaa067. doi: 10.1093/jamiaopen/ooaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ghosh K., Dohan M.S., Veldandi H., Garfield M. Digital transformation in healthcare: insights on value creation. J. Comput. Inf. Syst. 2022;63:449–459. [Google Scholar]
- 72.DiCicco-Bloom B., Crabtree B.F. The qualitative research interview. Med. Educ. 2006;40:314–321. doi: 10.1111/j.1365-2929.2006.02418.x. [DOI] [PubMed] [Google Scholar]
- 73.Al-Busaidi Z.Q. Qualitative research and its uses in health care. Sultan Qaboos University Medical Journal. 2008;8:11. [PMC free article] [PubMed] [Google Scholar]
- 74.Janghorban R., Roudsari R.L., Taghipour A. Skype interviewing: the new generation of online synchronous interview in qualitative research. Int. J. Qual. Stud. Health Well-Being. 2014;9 doi: 10.3402/qhw.v9.24152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahlin E. SAGE Publications Ltd; 2019. Semi-Structured Interviews with Expert Practitioners: Their Validity and Significant Contribution to Translational Research. [Google Scholar]
- 76.Ketokivi M., Choi T. Renaissance of case research as a scientific method. J. Oper. Manag. 2014;32:232–240. [Google Scholar]
- 77.Forbes A., While A., Taylor M. What people with multiple sclerosis perceive to be important to meeting their needs. J. Adv. Nurs. 2007;58:11–22. doi: 10.1111/j.1365-2648.2007.04219.x. [DOI] [PubMed] [Google Scholar]
- 78.Singh R., Awasthi S. Updated comparative analysis on video conferencing platforms -zoom, Google meet, Microsoft teams, WebEx teams and GoToMeetings. EasyChair Preprint. 2020;4026:1–9. [Google Scholar]
- 79.Kristandl G. “All the world’sa stage”–the Open Broadcaster Software (OBS) as enabling technology to overcome restrictions in online teaching. Compass: The Journal of Learning and Teaching at the University of Greenwich. 2021;14(2):1–16. [Google Scholar]
- 80.Rizzoli V. Histories of Social Psychology in Europe and North America, as Seen from Research Topics in Two Key Journals. Tracing the Life Cycle of Ideas in the Humanities and Social Sciences. 2018:65–86. [Google Scholar]
- 81.Chaves M.M.N., dos Santos A.P.R., dos Santosa N.P., Larocca L.M. In: Computer Supported Qualitative Research. Costa A.P., Reis L.P., Neri de Sousa F., Moreira A., Lamas D., editors. Springer International Publishing; Cham: 2017. Use of the software iramuteq in qualitative research: an experience report; pp. 39–48. [Google Scholar]
- 82.Ramos M.G., do Rosário Lima V.M., Amaral-Rosa M.P. In: Computer Supported Qualitative Research. Costa A.P., Reis L.P., Moreira A., editors. Springer International Publishing; Cham: 2019. Iramuteq software and discursive textual analysis: interpretive possibilities; pp. 58–72. [Google Scholar]
- 83.Sabeh A.C.B., Cecilio H.P.M., Campos C.J.G., Reis H.F.T., Wysocki A.D., Santos E.M.d. Social representations of nurses of the emergency care unit towards people with mental disorder. Rev. Esc. Enferm. USP. 2023;57 [Google Scholar]
- 84.Costa F.G., de Lima Coutinho M.D.P., dos Santos Cipriano J.P., Araújo J.M.G., de Carvalho C.F.D.C., Patrício J.M. Representações sociais sobre diabetes mellitus e tratamento: uma pesquisa psicossociológica. Revista de Psicologia da IMED. 2018;10(2):36–53. [Google Scholar]
- 85.Camargo B.V., Justo A.M. Iramuteq: um software gratuito para análise de dados textuais. Temas em psicologia. 2013;21:513–518. [Google Scholar]
- 86.Hair J.F., Black W.C., Babin B.J., Anderson R.E., Tatham R.L. Pearson Prentice Hall; 2010. Multivariate Data Analysis. [Google Scholar]
- 87.Jayadevappa R., Chhatre S. Patient centered care-a conceptual model and review of the state of the art. Open Health Serv. Pol. J. 2011;4(1) [Google Scholar]
- 88.Davenport T.H., Glaser J.P. Factors governing the adoption of artificial intelligence in healthcare providers. Discover Health Systems. 2022;1:4. doi: 10.1007/s44250-022-00004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.López-Valcárcel B.G., Ortún V. Reconstrucción del sistema sanitario: gobernanza, organización y digitalización. Informe SESPAS 2022. Gac. Sanit. 2022;36(1):44–50. doi: 10.1016/j.gaceta.2022.02.010. [DOI] [PubMed] [Google Scholar]
- 90.Griffin N., Kehoe M. A questionnaire study to explore the views of people with multiple sclerosis of using smartphone technology for health care purposes. Disabil. Rehabil. 2018;40:1434–1442. doi: 10.1080/09638288.2017.1300332. [DOI] [PubMed] [Google Scholar]
- 91.McColl-Kennedy J.R., Vargo S.L., Dagger T.S., Sweeney J.C., Kasteren Y.v. Health care customer value cocreation practice styles. J. Serv. Res. 2012;15:370–389. [Google Scholar]
- 92.Rodriguez N.M., Burleson G., Linnes J.C., Sienko K.H. Thinking beyond the device: an overview of human-and equity-centered approaches for health technology design. Annu. Rev. Biomed. Eng. 2023;25:257–280. doi: 10.1146/annurev-bioeng-081922-024834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Negash Y.T., Sarmiento L.S.C. Smart product-service systems in the healthcare industry: intelligent connected products and stakeholder communication drive digital health service adoption. Heliyon. 2023;9(2) doi: 10.1016/j.heliyon.2023.e13137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nicholas J., Zhou H., Deshpande C. Annual cost burden by level of relapse severity in patients with multiple sclerosis. Adv. Ther. 2021;38:758–771. doi: 10.1007/s12325-020-01570-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ness N.H., Schriefer D., Haase R., Ettle B., Cornelissen C., Ziemssen T. Differentiating societal costs of disability worsening in multiple sclerosis. J. Neurol. 2020;267:1035–1042. doi: 10.1007/s00415-019-09676-4. [DOI] [PubMed] [Google Scholar]
- 96.Andersson T., Liff R. Co-optation as a response to competing institutional logics: professionals and managers in healthcare. J. Prof. Organ. 2018;5:71–87. [Google Scholar]
- 97.López-Valcárcel B.G., Torres L.V., Domínguez J.P. La transformación digital del sector sanitario. Economistas. 2021;174:75–84. [Google Scholar]
- 98.Hewitt S., Sephton R., Yeowell G. The effectiveness of digital health interventions in the management of musculoskeletal conditions: systematic literature review. J. Med. Internet Res. 2020;22 doi: 10.2196/15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lau F., Kuziemsky C. University of Victoria; Victoria (BC): 2016. Handbook of eHealth Evaluation: an Evidence-Based Approach.https://openlibrary-repo.ecampusontario.ca/jspui/handle/123456789/346 [PubMed] [Google Scholar]
- 100.Triantafyllidis A.K., Tsanas A. Applications of machine learning in real-life digital health interventions: review of the literature. J. Med. Internet Res. 2019;21(4) doi: 10.2196/12286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Balta M., Valsecchi R., Papadopoulos T., Bourne D.J. Digitalization and co-creation of healthcare value: a case study in occupational health. Technol. Forecast. Soc. Change. 2021;168 [Google Scholar]
- 102.Chang H.H., Chou P.B., Ramakrishnan S. 2009 IEEE International Conference on E-Business Engineering. IEEE; 2009. An ecosystem approach for healthcare services cloud; pp. 608–612. [Google Scholar]
- 103.Oreja-Guevara C., Kobelt G., Berg J., Capsa D., Eriksson J., European Multiple Sclerosis Platform New insights into de burden and costs of multiple sclerosis in Europe: results for Spain. Mult. Scler. 2017;23:166–178. doi: 10.1177/1352458517708672. [DOI] [PubMed] [Google Scholar]
- 104.Dos Santos D.A.G., Zen A., Bittencourt B.A. From governance to choreography: coordination of innovation ecosystems. Innovation & Management Review. 2021;19:26–38. [Google Scholar]
- 105.Oskam I., Bossink B., de Man A.P. Valuing value in innovation ecosystems: how cross-sector actors overcome tensions in collaborative sustainable business model development. Bus. Soc. 2021;60:1059–1091. [Google Scholar]
- 106.Griebel L., Enwald H., Gilstad H., Pohl A.L., Moreland J., Sedlmayr M. eHealth literacy research—quo vadis? Inf. Health Soc. Care. 2018;43(4):427–442. doi: 10.1080/17538157.2017.1364247. [DOI] [PubMed] [Google Scholar]
- 107.Faux-Nightingale A., Philp F., Chadwick D., Singh B., Pandyan A. Available tools to evaluate digital health literacy and engagement with eHealth resources: A scoping review. Heliyon. 2022;8(8) doi: 10.1016/j.heliyon.2022.e10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mignoni J., Bittencourt B.A., da Silva S.B., Zen A.C. Orchestrators of innovation networks in the city level: the case of Pacto Alegre. Innovation & Management Review. 2023;20(3):194–210. [Google Scholar]
- 109.Berman A.E., Miller D.D. Measuring physician efficiency using data envelopment analysis: lessons and practical applications. J. Med. Pract. Manag.: J. Med. Pract. Manag. 2020;36(3):121–124. [Google Scholar]
- 110.Kim E., Gellis Z.D., Bradway C., Kenaley B. Key determinants to using telehealth technology to serve medically ill and depressed homebound older adults. J. Gerontol. Soc. Work. 2019;62(4):451–474. doi: 10.1080/01634372.2018.1499575. [DOI] [PubMed] [Google Scholar]
- 111.Mensah N.K., Adzakpah G., Kissi J., Boadu R.O., Lasim O.U., Oyenike M.K., Sukums F. Health professional’s readiness and factors associated with telemedicine implementation and use in selected health facilities in Ghana. Heliyon. 2023;9(3) doi: 10.1016/j.heliyon.2023.e14501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Corrado C., Haskel J., Iommi M., Jona-Lasinio C. OECD Economics Department Working Papers 1731. OECD Publising; Paris: 2022. Measuring data as an asset: framework, methods and preliminary estimates. [DOI] [Google Scholar]
- 113.Dalla Costa G., Leocani L., Montalban X., Guerrero A.I., Sørensen P.S., Magyari M., Dobson R.J., Cummins N., Narayan V.A., Hotopf M., et al. Real-time assessment of covid-19 prevalence among multiple sclerosis patients: a multicenter european study. Neurol. Sci. 2020;41:1647–1650. doi: 10.1007/s10072-020-04519-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee B., Chan F. The development and psychometric validation of the Brief Disability-Related Stress Scale in individuals with multiple sclerosis. Rehabil. Counsel. Bull. 2023;67(1):24–33. [Google Scholar]
- 115.Sarabdeen J., Chikhaoui E., Ishak M.M.M. Creating standards for Canadian health data protection during health emergency–An analysis of privacy regulations and laws. Heliyon. 2022;8(5) doi: 10.1016/j.heliyon.2022.e09458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dangond F., Donnelly A., Hohlfeld R., Lubetzki C., Kohlhaas S., Leocani L., Ciccarelli O., Stankoff B., Sormani M.P., Chataway J., Bozzoli F., Cucca F., Melton L., Coetzee T., Salvetti M. Facing the urgency of therapies for progressive MS – a progressive MS alliance proposal. Nat. Rev. Neurol. 2021;17:185–192. doi: 10.1038/s41582-020-00446-9. [DOI] [PubMed] [Google Scholar]
- 117.Koch M.W., Mostert J., Repovic P., Bowen J.D., Uitdehaag B., Cutter G. Reliability of outcome measures in clinical trials in secondary progressive multiple sclerosis. Neurology. 2021;96:e111–e120. doi: 10.1212/WNL.0000000000011123. [DOI] [PubMed] [Google Scholar]
- 118.Voigt I., Inojosa H., Dillenseger A., Haase R., Akgün K., Ziemssen T. Digital twins for multiple sclerosis. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.669811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Machado T.M., Berssaneti F.T. Literature review of digital twin in healthcare. Heliyon. 2023;9(9) doi: 10.1016/j.heliyon.2023.e19390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sterkenburgh T.R., Villalba-Diez J., Ordieres-Meré J. Socio-technical analysis of the benefits and barriers to using a digital representation of the global horse population in equine veterinary medicine. Animals. 2023;13(22):3557. doi: 10.3390/ani13223557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.