Abstract
Background
This joint expert review by the Obesity Medicine Association (OMA) and National Lipid Association (NLA) provides clinicians an overview of the pathophysiologic and clinical considerations regarding obesity, dyslipidemia, and cardiovascular disease (CVD) risk.
Methods
This joint expert review is based upon scientific evidence, clinical perspectives of the authors, and peer review by the OMA and NLA leadership.
Results
Among individuals with obesity, adipose tissue may store over 50% of the total body free cholesterol. Triglycerides may represent up to 99% of lipid species in adipose tissue. The potential for adipose tissue expansion accounts for the greatest weight variance among most individuals, with percent body fat ranging from less than 5% to over 60%. While population studies suggest a modest increase in blood low-density lipoprotein cholesterol (LDL-C) levels with excess adiposity, the adiposopathic dyslipidemia pattern most often described with an increase in adiposity includes elevated triglycerides, reduced high density lipoprotein cholesterol (HDL-C), increased non-HDL-C, elevated apolipoprotein B, increased LDL particle concentration, and increased small, dense LDL particles.
Conclusions
Obesity increases CVD risk, at least partially due to promotion of an adiposopathic, atherogenic lipid profile. Obesity also worsens other cardiometabolic risk factors. Among patients with obesity, interventions that reduce body weight and improve CVD outcomes are generally associated with improved lipid levels. Given the modest improvement in blood LDL-C with weight reduction in patients with overweight or obesity, early interventions to treat both excess adiposity and elevated atherogenic cholesterol (LDL-C and/or non-HDL-C) levels represent priorities in reducing the risk of CVD.
Keywords: Adiposopathy, Adiposopathic dyslipidemia, Cholesterol, Obesity, Triglycerides
Graphical abstract
1. Introduction
According to the Obesity Medicine Association (OMA), obesity can be defined as a: “serious, chronic, progressive, relapsing, and treatable multi-factorial, neurobehavioral disease, wherein an increase in adiposity promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences” [1]. Obesity is a chronic disease affecting over 40% of adults in the United States (U.S.) and nearly 2 billion adults worldwide. Cardiovascular disease (CVD) is a leading cause of death among individuals with obesity [2,3], and CVD among patients with increased adiposity is a major contributor to increased mortality and disability-adjusted life-years [4]. Disparities exist in obesity rates based on race/ethnicity, sex, gender and sexual identity, and socioeconomic status; however, such disparities may not be adequately explained by health behaviors, socioeconomic position, or cumulative stress [5]. Genetics, community, and environmental factors may also play a significant role [[5], [6], [7], [8], [9], [10]]. The total direct and indirect annual costs of obesity in the U.S. are estimated to be over $400 billion [11].
The metrics applicable to the diagnosis of obesity include [1]:
-
•
For the general population, body mass index (BMI) ≥25 kg/m2 is considered overweight; BMI ≥30 kg/m2 is considered obesity.
-
•
Central obesity is defined as waist circumference ≥40 inches (102 cm) for males and ≥35 inches (88 cm) for females (≥90 cm for Asian males; ≥80 cm for Asian females).
-
•
BMI and even waist circumference can sometimes be misleading. Assessment of body composition (i.e., percent body fat and body fat distribution) often reflects a more accurate representation of the degree of adiposity and associated health risks, especially among sex and race/ethnicity subgroups [[6], [7], [8], [9],12].
In 2016, the OMA, the National Lipid Association (NLA), and the American Society for Metabolic and Bariatric Surgery, crafted Scientific Statements regarding “Lipids and Bariatric Procedures” [[13], [14], [15]]. The NLA has also published articles on the relationship of obesity and blood lipid levels, including a 2013 Consensus Statement on obesity, adiposity, and dyslipidemia [16]. The OMA has published Clinical Practice Statements regarding obesity and CVD risk factors, such as diabetes mellitus [17], hypertension [18], and thrombosis [19], as well as reviews regarding obesity and CVD [20,21].
The purpose of this current joint expert review by the OMA and NLA is to provide clinicians with an overview of the pathophysiology and clinical considerations regarding obesity, dyslipidemia, and CVD risk. Obesity is associated with increased CVD risk, substantially due to worsening of CVD risk factors that, in addition to high blood pressure, high blood glucose, inflammation, and increased risk of thrombosis, includes an adiposopathic, atherogenic lipid profile. Among patients with obesity, interventions that reduce body weight and improve CVD outcomes are generally associated with improved lipid levels. Given the modest improvement in blood low-density lipoprotein cholesterol (LDL-C) levels with weight reduction in patients with, or at risk for atherosclerotic CVD (ASCVD), a dual priority is early intervention to prevent and/or treat both excess adiposity and elevated blood levels of atherogenic cholesterol (i.e., increased LDL-C and/or non-HDL-C). Box 1 provides a summary of 10 sentinel takeaway messages regarding the clinical management of increased adiposity and dyslipidemia.
Box 1. Summary of 10 sentinel takeaway messages regarding the clinical management of increased adiposity and dyslipidemia. Non‐HDL‐C sums the cholesterol content of all pro‐atherogenic particles that, in addition to LDL‐C, also includes VLDL-C, IDL-C, remnant lipoprotein cholesterol, and cholesterol carried by lipoprotein (a).
EXECUTIVE CLINICAL SUMMARY
Obesity and dyslipidemia.
-
1)
The Obesity Medicine Association has defined obesity as a: “serious, chronic, progressive, relapsing, and treatable multi-factorial, neurobehavioral disease, wherein an increase in adiposity promotes adipose tissue dysfunction and abnormal fat mass physical forces, resulting in adverse metabolic, biomechanical, and psychosocial health consequences.”
-
2)
In white adipocytes, triglycerides make up over 90% of adipocyte volume.
-
3)
Adipose tissue represents the largest body reservoir for free cholesterol.
-
4)
Most of the lipids in adipose tissue are derived from interactions with circulating lipoproteins.
-
5)
Among patients with increased adiposity, a commonly described lipid pattern is adiposopathic dyslipidemia (“atherogenic dyslipidemia”) that includes elevated blood triglyceride levels, reduced blood HDL-C levels, increased non-HDL-C, elevated apolipoprotein B, increased LDL particle number, and increased small dense LDL particles.
-
6)
The mild to modest increase in blood LDL-C levels in patients with obesity may not adequately characterize the increased ASCVD risk attributable to dyslipidemia.
-
7)
Among patients with increased adiposity, healthful nutrition and routine physical activity may help reduce body weight and improve lipid levels.
-
8)
Among patients with increased adiposity, weight reduction of ≥5% may reduce blood triglyceride levels, with greater weight reduction further reducing blood triglyceride levels and increasing blood HDL-C levels; even greater weight reduction of >10–15% has the potential to reduce CVD risk.
-
9)
Among patients with increased adiposity, weight reduction typically results in only mild to modest reductions in blood LDL-C levels.
-
10)
Among patients with increased adiposity, treatment strategies to reduce CVD risk include evidenced-based therapies that concurrently facilitate weight reduction and atherogenic lipid lowering.
Alt-text: Box 1
ASCVD: Atherosclerotic cardiovascular disease
HDL-C: High-density lipoprotein cholesterol
IDL-C: Intermediate density lipoprotein cholesterol
LDL-C: Low-density lipoprotein cholesterol
VLDL-C: Very low density lipoprotein cholesterol
2. What is the role of cholesterol and triglycerides in adipose tissue?
Lipids are hydrophobic organic compounds that include cholesterol, other sterols, fat-soluble vitamins, fats and oils (e.g., monoglycerides, diglycerides, triglycerides), and phospholipids (i.e., 3 carbon glycerol molecule backbone attached to a phosphate group and 2 fatty acids (e.g., examples include phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine). Sphingomyelin is similar in structure to phospholipids. However, sphingomyelin contains a sphingosine backbone (instead of a glycerol molecule) attached to a phosphocholine head group (which is also attached to a choline unit) and a fatty acid. Cholesterol is a waxy substance with the name derived from “chole,” which means bile, and “steros,” which means solid or three dimensional [14]. Cholesterol serves as a precursor to steroid hormone and vitamin D production, as well as a precursor to bile acid production for the purpose of digestion and absorption of dietary fats. The composition of most plasma membranes includes approximately 50% lipid and 50% protein by weight [22]. The carbohydrate portions of glycolipids and glycoproteins constitute 5–10% of the plasma membrane mass [22]. Cholesterol is the major sterol component of animal cell membranes, where it functions to stabilize cellular membranes and aid in cell signaling. Phospholipids are the most abundant lipid component of cell membranes, whose structure is composed of a hydrophilic outward face and a hydrophobic inner face (i.e., polar phosphate-linked head and two hydrophobic fatty acid tails), which helps form a phospholipid bilayer. While phospholipids contain fatty acids, and while free fatty acids are also found in plasma membranes (i.e., participating in cell membrane structure, signaling, and function) [23], triglycerides are not a significant contributor to, and are essentially absent from, cell membranes.
Triglycerides are lipid esters formed when three fatty acids are combined with a three-carbon glycerol, hence the alternative name of triacylglycerol. Triglycerides stored in cells such as adipocytes serve as a source of energy. When incorporated into adipose tissue, the accumulation of triglycerides in body fat provides insulation aiding in body temperature regulation and provides cushioning to protect body organs [24]. Adipocytes store cholesterol and triglycerides during times of positive energy (caloric) balance, manifest by increasing adipocyte number (i.e., hyperplasia), enlarging of adipocytes (i.e., hypertrophy), and expanding adipose tissue size. The profound potential for expansion accounts for why adipose tissue represents the body organ having the greatest potential weight variance among individuals, with percent body fat (i.e., total fat mass/total body mass) ranging from less than 5% to well over 60% [25]. Accompanying adipocyte hypertrophy, adipocyte proliferation, and adipose tissue expansion is an increase in lipid storage. Adipose tissue typically stores the greatest amount of body energy, mainly in the form of triglycerides [16].
2.1. What are the cellular components of adipose tissue?
Components of adipose tissue include adipocytes, pre-adipocytes, mesenchymal cells, endothelial precursor cells, fibroblasts, smooth muscle cells, collagen, nerve cells, blood vessels, blood cells, and immune cells, all whose biological processes are important for human health, and whose dysfunction may contribute to metabolic and immune disease. When assessed based upon cellular content, less than 50% of the cells in adipose tissue are adipocytes, with over 50% being other cells, such as those found in the stromal vascular fraction [26]. When assessed based upon the degree of occupied space, adipocytes make up over 80% of adipose tissue volume [26].
2.2. How are cholesterol and triglycerides stored in adipose tissue?
As with other living cells ranging from bacteria to mammals [27], white adipocytes contain cytosolic lipid droplets, which are energy storage organelles consisting of a hydrophobic core of neutral lipids (e.g., mainly esterified triglyceride with some esterified cholesterol), enclosed by a phospholipid monolayer that contains proteins [PAT family (perilipin, adipose differentiation-related protein, tail-interacting protein of 47 kD), lipid and energy-related proteins, signaling proteins, membrane tracking proteins, and other miscellaneous proteins] and free (unesterified) cholesterol [27,28]. Steroidogenic cells have a disproportionate amount of cholesterol (which is the backbone structure of hormones), while adipocytes have a disproportionate amount of triglycerides. In white adipocytes, triglycerides make up over 90% of adipocyte volume [29], such that the single lipid droplet within the adipocyte pushes other organelles, including the nucleus, to the cell's periphery [30]. Beyond storing the greatest amount of the body's energy in the form of triglycerides, over 90% of the cholesterol stored in adipocytes is in the amphiphilic (partially hydrophilic/partly lipophilic) unesterified form (i.e., free cholesterol) [31,32]. Adipose tissue represents the largest body reservoir for free cholesterol [33]. As adiposity increases with obesity, adipocyte hypertrophy often also occurs, accompanied by increased adipocyte cholesterol content [34].
For most cells, free cholesterol is biologically active (i.e., playing structural and signaling roles) and excessive free cholesterol may be toxic. Excessive free cholesterol impairs membrane fluidity (resulting in membrane protein dysfunction), disrupts membrane domains (resulting in impaired signaling), induces apoptosis (leading to cell death), crystalizes intracellular cholesterol (leading to organelle dysfunction), facilitates toxic oxysterols (leading to oxidative damage), and alters gene expression (leading to protein dysfunction) [35]. In non-adipose tissues, esterification of cholesterol is a process that mitigates the cellular toxicity of free cholesterol [34]. Cholesteryl esters are typically stored within cytosolic lipid droplets in most all cells and found in the core of lipoproteins, surrounded by free cholesterol, phospholipids, and apolipoproteins, for cholesterol transport in the blood [36].
Within adipocytes, nearly all the free cholesterol is distributed on the triglyceride droplet monolayer surface interface, with the remainder located in the plasma membrane [34]. In fact, cholesterol accumulation and redistribution from the plasma membrane to the lipid droplet monolayer is a crucial determinant of adipocyte function during hypertrophic expansion [34]. Adipocytes can accommodate high levels of free cholesterol that may be toxic to other cells [34]. However, a relative decrease in cholesterol in adipocyte plasma membranes may contribute to adipocyte dysfunction, potentially resulting in insulin resistance [34]. While free cholesterol associated with adipocyte lipid droplets may not be toxic, excess free cholesterol deposition can cause dysfunction of adipocyte organelles, such as increased endoplasmic reticulum “stress [37],” potentially resulting in adiposopathic immune and metabolic consequences [38].
3. What is the origin of cholesterol in adipose tissue?
The liver is the body organ most responsible for regulating circulating lipoprotein cholesterol, through hepatic lipoprotein secretion balanced against lipoprotein uptake, with elimination of cholesterol primarily via biliary excretion [44]. The role of circulating lipoproteins is to facilitate the delivery and/or uptake of cholesterol (and other lipids) to and from body tissues. Given that most body tissues synthesize cholesterol, some authors suggest that most of the de novo total body cholesterol synthesis occurs in non-hepatic body organs [45,46]. Body organs described as having among the highest concentration of cholesterol include the brain [47], liver [48], muscle [49], tendon [49], skin [49], intestine, steroidogenic organs (e.g., adrenal, gonadal organs) [50], and adipose tissue [49].
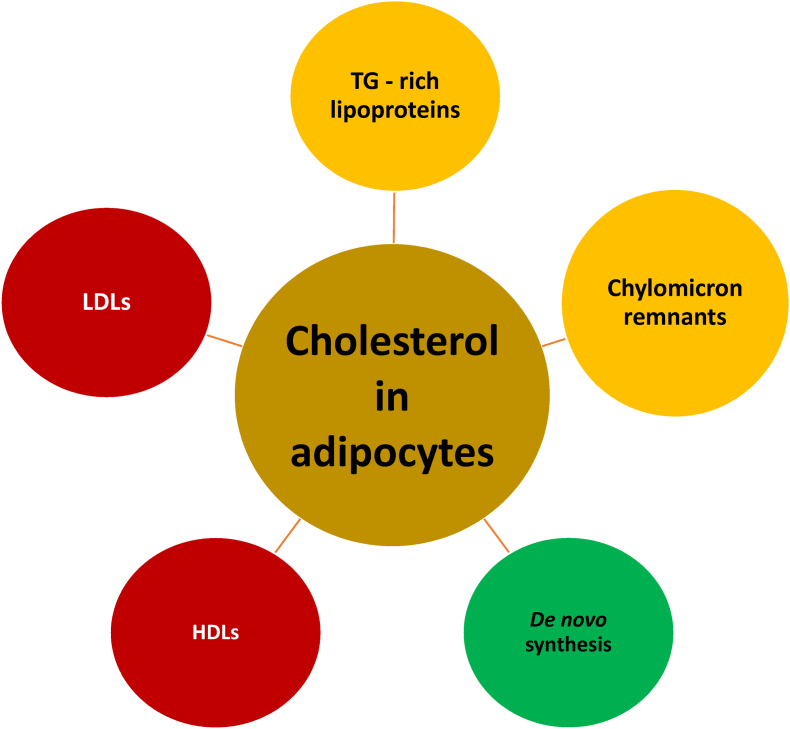
In addition to the limited amount of cholesterol synthesized by adipocytes [51], the origin of most cholesterol in adipocytes is derived from interactions with circulating lipoproteins (see Fig. 1). Because cholesteryl esters and triglycerides are insoluble in water, these hydrophobic lipids must be carried in the blood by water-soluble lipoproteins which, depending on their classification and stage of metabolism, contain varying amounts of cholesterol and triglycerides. An adverse cardiovascular consequence of increased circulating atherogenic lipoproteins [LDL, intermediate-density lipoprotein (IDL), very-low-density lipoprotein (VLDL), chylomicron remnants, and lipoprotein (a)], which all contain cholesterol, is the promotion of cholesterol plaques in the walls of arteries (e.g., coronary, cerebral, aortic, and peripheral vascular arteries). These atherosclerotic arterial lesions may lead to angina, myocardial infarction, claudication, limb amputation, revascularization, stroke, aneurysmal rupture, and ASCVD death [52].
Fig. 1.
Cholesterol influx within adipocytes. Adipocytes undergo limited de novo synthesis of cholesterol [29]. The origin of most cholesterol found in adipocytes is from interactions with lipoproteins. Hepatically secreted, circulating TG-rich lipoproteins such as VLDL and their lipoprotein remnants (i.e., small VLDL and intermediate density lipoproteins) interact with adipocyte membrane-anchored lipoprotein receptors such as the VLDL receptor (member of the LDL receptor family) [34,39]. Similarly, circulating LDL particles interact with adipocyte LDL receptors. Interaction of lipoproteins (along with their cholesterol) with their respective adipocyte receptors result in their endocytosis within adipocytes [40]. Postprandial cholesterol may be delivered to adipocytes from circulating remnants of chylomicrons (i.e., chylomicrons being large lipoproteins produced by the small intestine), via interactions with adipocyte LDL receptors and LDL receptor-related proteins that recognize and interact with apolipoprotein E containing lipoproteins [41,42]. Finally, adipocytes may obtain additional cholesterol from hepatic/intestinal-secreted HDL via bidirectional SR-B1 [43].
TG: triglycerides; VLDL: very-low-density lipoprotein; LDL: low-density lipoprotein; HDL: high-density lipoprotein; SR-B1: scavenger receptor type B1.
3.1. What is the origin of lipoproteins applicable to lipid transfer to adipose tissue?
Especially during times of positive caloric balance and overconsumption of unhealthful saturated fatty acids and refined sugars/simple carbohydrates (i.e., as often found in ultra-processed foods), excess fatty acids and glycerol accumulation may occur in the liver, increasing triglyceride synthesis and storage [53]. Increased hepatic fatty acid influx and increased triglyceride synthesis may enhance the secretion of VLDL particles from the liver into the circulation [54]. VLDLs are particles that have a core containing both hydrophobic/lipophilic triglycerides and cholesterol, mainly in the form of non-polar, hydrophobic/lipophilic cholesteryl esters, which are long-chain fatty acids esterified to the cholesterol hydroxyl group. Surrounding the hydrophobic/lipophilic core of the VLDL particle is a water-soluble outer shell of hydrophilic phospholipids and apolipoproteins, as well as partially hydrophilic “free cholesterol” (i.e., unesterified to fatty acids). It is the hydrophilic properties of the lipoprotein outer shell that help account for the water-soluble amphiphilic properties of lipoproteins, and thus allow for circulatory transport of otherwise insoluble hydrophobic/lipophilic lipids (i.e., triglycerides and cholesterol). Once in the circulation, the triglyceride mass in VLDL particles is hydrolyzed by body lipases to release free fatty acids for uptake by systemic tissues. As the VLDL particles are progressively hydrolyzed, some authors refer to these smaller VLDL particles as “remnant” or “IDL” with VLDL remnants or IDLs being an intermediate stage, both in size and lipid content, between VLDL and LDL [55]. When assessed by a simple calculation, the term “remnant cholesterol” is sometimes considered equivalent to VLDL-C [55]. Yet other authors refer to hydrolyzed VLDL remnants as apolipoprotein B (apoB)-containing triglyceride-rich lipoproteins found within the small VLDL and IDL size range [56].
Human genetic data coupled with epidemiologic, preclinical, and clinical trial data support a causal association between triglyceride-rich lipoproteins (i.e., VLDL and their remnants, chylomicrons and their remnants, and IDL) and the increased risk of myocardial infarction, ischemic stroke, and aortic valve stenosis. Increased triglyceride-rich lipoproteins represent increased residual cardiovascular risk in patients on optimized LDL-C lowering therapy [56]. Remnant lipoproteins may contain up to 4 times more cholesterol per particle than LDL particles, highlighting their atherogenic potential. Additionally, largely due to their small size, triglyceride-rich lipoprotein remnants (i.e., VLDL remnants, IDLs, and chylomicron remnants) [57] readily penetrate the arterial wall. As opposed to LDL particles that first undergo oxidation and/or other modification that initiate an inflammatory process (i.e., facilitating oxidized LDL uptake by subendothelial macrophages [58]), remnant lipoproteins can be taken up by scavenger receptors on macrophages directly without modification [59]. Irrespective of the terminology regarding lipoprotein “remnants,” smaller VLDL particles are further hydrolyzed to create LDL particles, mainly through the actions of lipoprotein lipase and hepatic lipase. LDL particles are the end-product of VLDL metabolism and are generally triglyceride poor and enriched with cholesteryl ester.
Adipose tissue lipases are further discussed in section 5.0. Examples of lipases most applicable to lipoprotein triglyceride lipolysis (i.e., conversion to fatty acids and glycerol) include [60]:
-
•
Lipoprotein lipase (LPL): Extracellular enzyme on the vascular endothelial surface of capillary walls of adipose tissue, muscle, and heart tissue (not liver).
-
•
Hepatic lipase (HL): Located on the cell surface of liver cells, with some expression in the adrenal glands and ovaries.
-
•
Endothelial lipase (EL): Expressed in a variety of tissues (e.g., placenta, thyroid, liver, lung, kidney, ovary, and testes), with high phospholipase activity. In addition to metabolism of HDLs, EL is a key collaborator with lipoprotein lipase in mediating the lipolysis of triglyceride-rich lipoproteins [61].
Finally, the above pathogenic processes describe the “inside-to-in” or “inside out” mechanism regarding the role of atherogenic lipoproteins in the pathogenesis of atherosclerosis. Traditionally, atherosclerosis is thought to involve endothelial dysfunction, insudation of the arterial intima by circulating lipoproteins, followed by inflammation, plaque lesion formation, and fibrous cap vulnerability. Disruption of the fibrous cap exposes the lipid core to clotting factors in the vessel lumen, promoting thrombosis, and clinically leading to acute ischemic events (i.e., myocardial infarction, stroke). Thus, atherogenic lipoprotein deposition in the intima is thought to be due to a movement of atherogenic lipoproteins from “in” the lumen of arterial vessels to the arterial intima.
However, another proposed mechanism of atherosclerosis is the “outside-to-in” process. In this model of atherosclerosis, adiposopathic inflammatory responses of cardiac and vascular adipose tissues (i.e., epicardial and perivascular adipose tissue) [62] not only increase the secretion of cytokines and adipokines, activate an immune response, and facilitate glucotoxicity [63], but also contribute to lipotoxicity and promote inflammatory macrophages that traverse the vascular adventitia, all potentially contributing to atherosclerosis [64].
3.2. What are the mechanisms of cholesterol influx from lipoproteins to adipose tissue?
Regarding cholesterol influx, cholesterol-enriched lipoproteins, such as LDL particles (especially if oxidized), IDL, VLDL remnants, and chylomicron remnants, interact with respective adipocyte receptors (e.g., LDL receptor, LDL receptor-related protein-1) where they undergo endocytosis within adipocytes [[65], [66], [67], [68], [69]]. Dietary cholesterol levels appear more strongly correlated with adipocyte cholesterol content than plasma cholesterol concentrations, presumably because of adipocyte uptake of cholesterol of dietary origin (i.e., via chylomicrons and chylomicron remnants) [34].
Once internalized, the lipoprotein cores containing cholesteryl esters undergo hydrolysis by lysosomal hydrolases into unesterified free cholesterol [65,69]. Adipocytes also express bidirectional facilitated scavenger receptor class B type 1 (SR-B1) transporters. Thus, adipocytes may also obtain cholesterol from the interaction of their SR-B1 receptors with hepatic/intestinal-secreted HDL particles [29]. The adipocyte hydrolysis of lipoproteins, coupled with the relative lack of adipocyte acyl-coenzyme A:cholesterol acetyltransferase (ACAT) enzyme activity [70], results in over 90% of the cholesterol in adipocytes being amphiphilic, non-esterified, “free cholesterol.” [51,71].
3.3. What are the mechanisms of cholesterol efflux from adipose tissue to lipoproteins?
Regarding cholesterol efflux, adipocytes express cholesterol transporters, such as the adenosine triphosphate (ATP)-binding cassette sub-family A member 1 (ABCA1) transporters, as well as bidirectional facilitated SR-B1 transporters, both of which may provide a gateway for cholesterol efflux to apoA-I or HDL particles [70].
Among individuals without obesity, adipose tissue generally stores about 25% of the body's free cholesterol [34,71]. Among individuals with obesity, it is reported that adipose tissue may store over 50% of the body's free cholesterol [31,33,34]. Adipose tissue is often described as the organ that stores the greatest total amount of free or non-esterified cholesterol [72]. Some authors have claimed that not only does adipose tissue contain the largest pool of free cholesterol, but in adult patients with obesity, adipose tissue contains over 50% of the total body cholesterol [73]. Other authors report that regarding rat tissue, adipose tissue contains more cholesterol than liver, muscle, or kidney when expressed on a mg per protein basis, and more than all other body organs except skeletal muscle when expressed on a whole organ basis [31].
That said, fat cells of rats reportedly contain an average of 0.5 mg of cholesterol per gram of triglyceride [51], with 90% of cholesterol being nonesterified and 80% located in the lipid storage compartment [51]. As adipocytes enlarge and as adipose tissue expands, the total amount of cholesterol and triglycerides in adipose tissue increases as well [74]. The marked increase in triglycerides relative to cholesterol helps account for why some authors report that while adipose tissue may represent the largest cholesterol pool of the body, the concentration of cholesterol in adipocytes may represent only about 0.5% of total lipids [75], with triglycerides making up to 99% of lipid species in adipose tissue [76].
4. How are fatty acids and triglycerides transported in the blood?
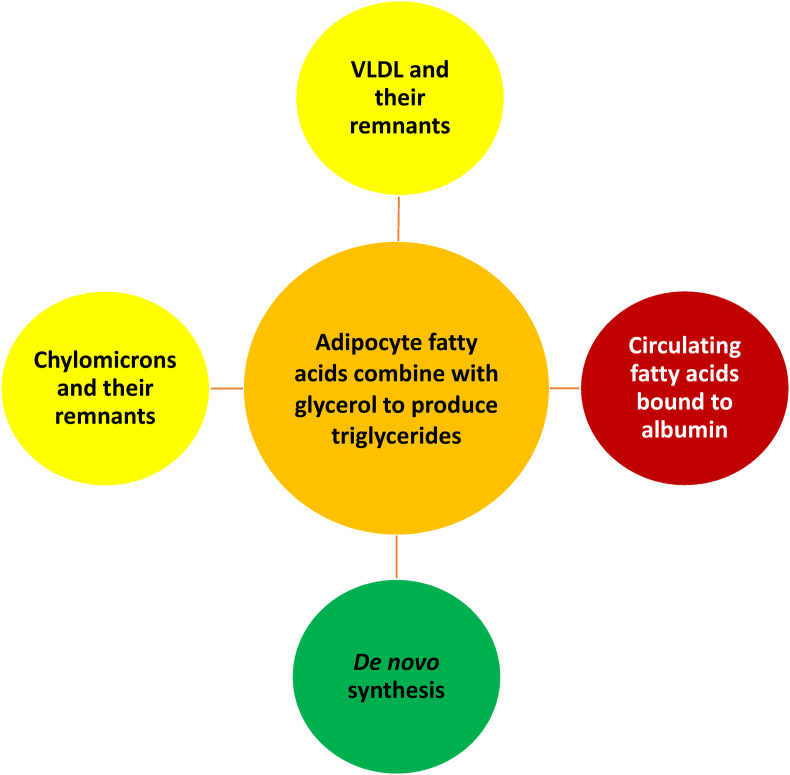
Most circulating non-esterified fatty acids are transported in the bloodstream bound by albumin. Dissociation of fatty acids from albumin may contribute to fatty acids that enter adipocytes [77]. As with cholesteryl esters, triglycerides are non-polar, and thus hydrophobic. Also as with cholesteryl esters, triglycerides circulate in the blood mainly within amphiphilic lipoproteins. Examples of triglyceride-rich lipoproteins include hepatically-secreted apoB-100 containing VLDL particles and VLDL remnants, as well as intestinally-secreted apoB-48 containing chylomicrons and chylomicron remnant particles [56] (see Fig. 2). About 80% of the rise in triglyceride levels after a fat-load meal is due to intestinally derived apoB-48-containing chylomicrons; however, about 80% of the rise in lipoprotein particle concentration is due to apoB-100-containing VLDL particles [56].
Fig. 2.
Origin of fatty acids and triglycerides within adipocytes. Adipocytes undergo limited de novo synthesis of triglycerides. Endogenous triglycerides synthesized by the liver circulate within hepatically-secreted triglyceride-rich lipoproteins, such as VLDL and their lipoprotein remnants (i.e., intermediate density lipoproteins). Dietary triglycerides are carried in the circulation by triglyceride-rich chylomicrons and their remnants (i.e., chylomicrons being large lipoproteins produced by the small intestine). LPL found on adipose tissue capillary endothelial cells hydrolyzes triglycerides from triglyceride-rich lipoproteins, generating fatty acids and glycerol. Fatty acids are generated when LPL acts upon triglyceride-rich lipoproteins. These fatty acids enter adipocytes, where they may be re-esterified with glycerol to (re)form triglycerides. In addition, fatty acids are carried in the circulation attached to albumin. Fatty acids entering adipocytes may also be derived from fatty acids that dissociate from albumin.
VLDL: very-low-density lipoprotein; LPL: lipoprotein lipase.
4.1. What is the origin of fatty acids in adipose tissue?
The types of fatty acids in adipose tissue largely reflect the types of fatty acids present in triglycerides in apoB-100-containing VLDL secreted from the liver (and subsequent VLDL remnants), as well as the fatty acids consumed in the diet, that are ultimately bound as triglycerides and carried and transported from the intestine to adipose tissue via apoB-48-containing chylomicrons and chylomicron remnants (see Fig. 3). A clinical application is that adipose tissue biopsies are sometimes used to roughly estimate specific fatty acids consumed in the diet [78]. That said, assessing the effects of dietary fatty acids (e.g., consumed omega-3 fatty acids) more often involves measurement of plasma and red blood cell fatty acids [79]. Patients with obesity may have greater concentrations of red blood cell saturated fatty acids (e.g., palmitate and stearate) compared to leaner individuals who may have greater concentrations of red blood cell unsaturated fatty acids (e.g., oleate, palmitoleate, and palmitelaidic acid, with the latter being a trans isomer of palmitoleic acid) [80]. Older data suggested that de novo lipogenesis was virtually nonexistent in the human adipocyte [81]. However, human adipocytes are capable of synthesis of fatty acids and triglycerides, with de novo lipogenesis of fatty acids in adipose tissue enhanced with consumption of a carbohydrate-rich diet [82].
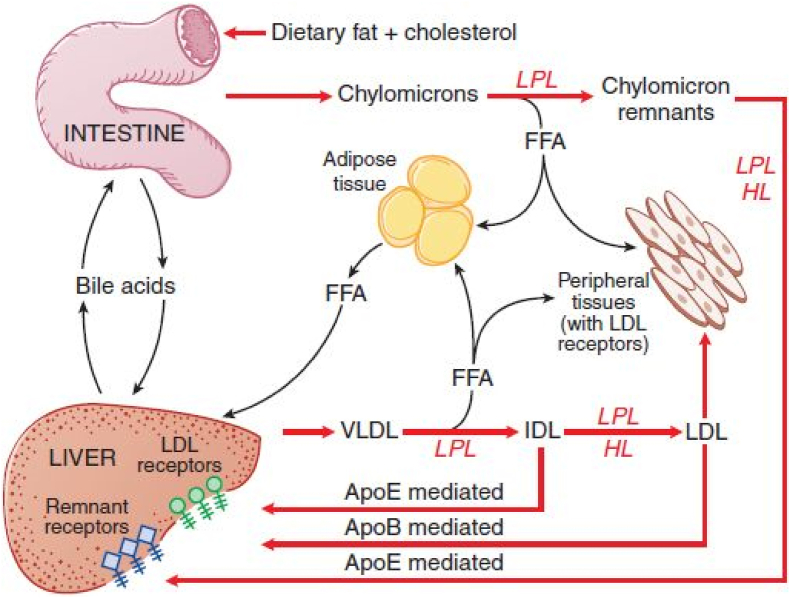
Fig. 3.
Key elements of triglyceride metabolism: This figure summarizes the major pathways for metabolism of chylomicrons synthesized in the intestine and VLDL synthesized in the liver.
ApoB: apolipoprotein B; ApoE: apolipoprotein E; FFA: free fatty acids; HL: hepatic lipase, IDL: intermediate-density lipoprotein; LDL: low-density lipoprotein; LPL: lipoprotein lipase; VLDL: very-low-density lipoprotein. (Source: Copied with permission from: Hall JE. Chapter 68: Lipid metabolism. In: Guyton and Hall Textbook of Medical Physiology. 12th ed. Philadelphia, PA: Saunders Elsevier; 2011:819–830.)
4.2. What is the origin of triglycerides in adipose tissue?
As with cholesterol, de novo synthesis of triglycerides in adipocytes is limited, albeit sufficient for maturation of differentiating adipocytes [81]. LPL is an enzyme on the intravascular surface of capillaries surrounding adipocytes (as well as other body tissues) that facilitates the hydrolysis of triglycerides from circulating lipoproteins into fatty acids. LPL is an extracellular enzyme that hydrolyzes the core triglycerides within circulating triglyceride-rich lipoproteins into monoglycerides and fatty acids; thus, increased LPL activity reduces circulating blood triglyceride levels [83].
Apolipoproteins are proteins found on lipoprotein carriers of otherwise insoluble lipids, which interact with receptors of body tissues. Apolipoproteins that affect LPL activity include apoC-I (inhibits LPL), apoC-II (activates LPL), apoC-III (inhibits LPL) and apoA-V (activates LPL) [84,85]. This is mechanistically important because in addition to apoB and apoE, triglyceride-rich lipoproteins (i.e., VLDL) also contain apoC-I, apoC-II, apoC-III, and apoA-V. For example, obesity is often associated with an increase in apoC-III levels, which would be expected to inhibit LPL, and impair the lipolysis of triglyceride-rich lipoproteins, and thus increase blood triglyceride levels [86]. This is therapeutically important because LPL is stimulated by physical exercise, insulin, fibrates, omega-3 fatty acids, angiopoietin-like protein 3 inhibitors (e.g., evinacumab) and apoC-III inhibitors (e.g., olezarsen), which explains why these interventions lower blood triglyceride levels [87].
Once the triglycerides within triglyceride-rich lipoproteins are hydrolyzed into fatty acids by adipose tissue capillary-bound LPL, fatty acids can be transported into adipocytes via diffusion or transporter proteins [88]. The uptake of fatty acids into adipocytes is rapid, with the movement of local fatty acids into adipocytes likely being a balance between diffusion-mediated and protein-mediated processes [77]. Examples of putative fatty acid transporters include fatty acid transport proteins (SLC27A1-6) [89], plasma membrane fatty acid binding protein (FABPpm), caveolin-1, fatty acyl CoA synthetases (FATP and ACSL), and fatty acid translocase (FAT/CD36) [77].
One of the more well-described transport proteins is CD36, which is a scavenger receptor found on the surface of many body cells, and which has different functions (e.g., neurologic functions), especially involving atherogenic processes [90], such as management of oxidized LDL particles, thrombosis, and immune responses. CD36 is a FAT that transports long-chain fatty acids and cholesterol across the plasma membrane of adipocytes [91]. CD36 is a marker of human adipocyte progenitors. Cells with a high CD36 level have pronounced adipogenicity and triglyceride accumulation potential with silencing of the CD36 gene attenuating adipocyte adipogenesis. CD36 deletion promotes triglyceride hydrolysis and an increase of circulating free fatty acids [90]. Thus, some authors suggest adipocyte CD36 may normally be metabolically protective, and its selective upregulation might have therapeutic potential in insulin resistance [92]. CD36 may be necessary for the maintenance of lipid homeostasis, with CD36 deficiency or CD36 gene polymorphism contributing to a preference for food with high-fat content, impairment of chylomicron formation and clearance, reduced lipid utilization and lipid storage, and increased lipolysis [90]. However, other authors suggest that, when its expression is increased with obesity [93], then CD36 in both macrophages and adipocytes plays an important contributory role in diet-induced adipose tissue inflammation and adipocyte cell death [94]. CD36 may facilitate an inflammatory paracrine loop between adipocytes and macrophages that facilitates chronic inflammation and contributes to insulin resistance commonly found in patients with obesity and dyslipidemia [95].
Once in adipocytes, fatty acids combine with glycerol [i.e., derived from glucose transport via the facilitative insulin sensitive glucose transporter (GLUT) 4] [17] to form adipocyte triglycerides. Specifically, once inside the adipocyte, glycerol 3-phosphate acyltransferase (GPAT) serves as the rate limiting enzyme in a series of reactions that facilitate the combination of fatty acids with glycerol to form triglycerides, with diacylglycerol acyltransferase 2 (DGAT-2) participating in the final step of triglyceride biosynthesis [96].
4.3. What is the role of insulin resistance regarding LPL activity?
Obesity is usually associated with increased total LPL activity. However, among those with obesity, the responsiveness of LPL to insulin and feeding may be relatively decreased [97]. Thus, even if total LPL activity is increased with obesity, it may be inadequate to fully hydrolyze the obesity-related increase in circulating triglyceride-rich lipoproteins (i.e., lower functional LPL activity per unit fat mass in patients with obesity [98]), contributing to the common clinical finding of elevated triglyceride levels in patients with obesity [86]. This is especially so given that insulin resistance is associated with increased activity of apoC-III (an inhibitor of LPL) [99], reduced activity of apoC-II (an activator of LPL) [100], and reduced activity of apoA-V (an activator of LPL) [101], all which would be expected to reduce LPL activity. Thus, while total LPL activity may be increased in patients with obesity and insulin resistance, its relative impairment per unit fat mass may contribute to LPL enzyme activity that is insufficient to prevent hypertriglyceridemia. An analogy is increased insulin levels that often occur early in the course of type 2 diabetes mellitus (T2DM) among patients with obesity and insulin resistance which, despite being elevated, may be insufficient to prevent hyperglycemia [17].
5. How are fatty acids released into the circulation from adipose tissue?
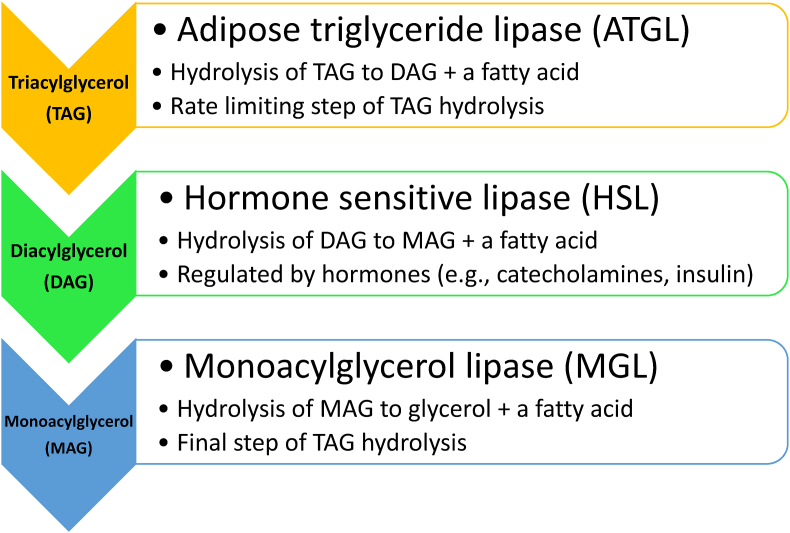
The efflux of fatty acids from adipocytes begins with intracellular hydrolysis of triglycerides (i.e., lipolysis of triglycerides in the adipocyte lipid droplet [27]), via consecutive hydrolysis of triglycerides by adipose triglyceride lipase (ATGL), hormone sensitive lipase (HSL), and monoacylglycerol lipase (MGL); this sequence ultimately generates free fatty acids (and glycerol) [103] (see Fig. 4). ATGL is an enzyme predominantly found in adipocytes and is the rate limiting enzyme for triglyceride lipolysis (i.e., hydrolysis of triacylglycerol to diacylglycerol) [104]. HSL is also an enzyme found in adipocytes that mediates triglyceride hydrolysis (e.g., diacylglycerol), generating free fatty acids and glycerol via hormonally-controlled lipolytic processes (i.e., activated by hormones, such as corticotropin-releasing hormone, epinephrine, norepinephrine, and glucagon and suppressed by insulin) [102]. Finally, MGL catalyzes the hydrolysis of monoacylglycerols to fatty acids and glycerol.
Fig. 4.
Lipolysis of adipocyte triglycerides. The intracellular lipolytic pathways in adipocytes involve: (a) hydrolysis of TAG to DAG and a fatty acid by ATGL, (b) hydrolysis of DAG to MAG and a fatty acid by HSL, and (c) hydrolysis of MAG to glycerol and a fatty acid by MGL [102]. HSL is a sentinel enzyme within adipocytes that is activated by hormones, such as corticotropin-releasing hormone, epinephrine, norepinephrine, and glucagon, and suppressed by insulin [102]. While this figure describes HSL activity on DAG, HSL also has hydrolytic activity regarding other substrates, such as TAG, cholesteryl esters, and retinyl esters [102]. Given that HSL is suppressed by insulin, insulin resistance may result in dysregulation of lipolysis, potentially contributing to enlargement of adipose tissue mass [102].
ATGL: adipose triglyceride lipase; DAG: diacylglycerol; HSL; hormone sensitive lipase; MAG: monoacylglycerol; MGL: monoglycerol lipase; TAG: triacylglycerol (triglyceride).
Fatty acids within triglycerides may be shuttled from adipocytes to adipose tissue macrophages via exosomes [17,103]. Otherwise, once triglycerides are metabolized in adipocytes into fatty acids and glycerol, the efflux of fatty acids from adipocytes into the circulation mainly occurs via diffusion [88]. Adipocyte fatty acid efflux may also be protein mediated [105]. Once fatty acids are released into the circulation, fatty acids are bound by albumin and may be transported to skeletal and cardiac muscle and oxidized to generate adenosine triphosphate via beta-oxidation. Collectively, circulating fatty acids that originate from adipocytes, diet, and hepatic secretion are transported to the liver bound to albumin or carried by triglyceride-rich lipoproteins where they may be converted to triglycerides and contribute to fatty liver disease [106] and the increased secretion of VLDL particles [107]. Fatty acids may also be used by the liver to generate ketone bodies as an alternative to glucose for the following: (a) for body fuel during fasting (i.e., times of fasting are often associated with the highest fatty acid levels); (b) for body fuel during low carbohydrate intake (i.e., a ketogenic diet); and/or (c) for ketone production, thus accounting for ketoacidosis among patients with type 1 diabetes mellitus during absence of insulin.
6. What is a lipid profile pattern often described in patients with obesity?
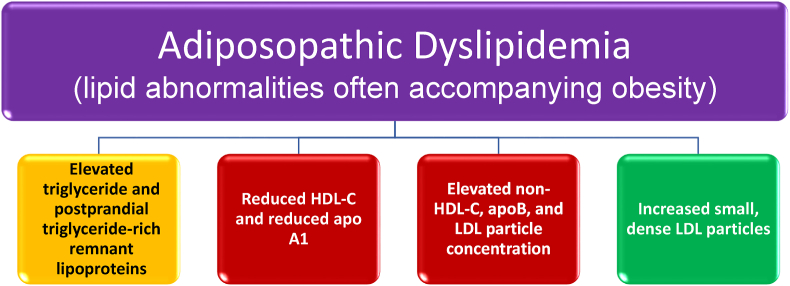
Increased adiposity alone (i.e., overweight or obesity) does not exclude such a patient from having primary (i.e., genetic) and/or other secondary causes of elevated blood LDL-C levels (e.g., diabetes, hypothyroidism, kidney disease, adverse side effect of concomitant medications). As with patients without overweight or obesity, an elevated blood LDL-C level in a patient with overweight or obesity is a risk factor for ASCVD that should be aggressively evaluated and managed [108]. That said, a common clinical lipid profile pattern (reflected by underlying abnormalities in lipoproteins) associated with obesity (especially in the presence of adiposopathy and insulin resistance) includes elevated blood triglyceride levels due to elevated triglyceride-rich lipoproteins, reduced HDL-C, reduced apoA-I (i.e., reflecting a decrease in HDL particle concentration), elevated non-HDL-C, elevated apoB, and increased proportion of small dense LDL particles with an increase in LDL particle concentration [86] (see Fig. 5). For most non-lipid specialists, the adiposopathic dyslipidemia found with obesity might be more simply described as high blood triglyceride levels and low blood HDL-C levels, even as blood LDL-C levels may be mildly increased.
Fig. 5.
Adiposopathic dyslipidemia (“atherogenic dyslipidemia”) [16]. A pattern of dyslipidemia described in patients with obesity often includes elevated triglycerides, reduced HDL-C, increased non-HDL-C, elevated apoB, increased LDL particle number, and increased small dense LDL particles. apoAI: apolipoprotein AI; apoB: apolipoprotein B; HDL-C: high-density lipoprotein cholesterol; LDL: low-density lipoprotein; Non-HDL-C: non-high-density lipoprotein cholesterol.
In addition to fasting dyslipidemia, postprandial hyperlipidemia with increases in triglyceride-rich lipoproteins is also associated with ASCVD [109]. Similar to fasting dyslipidemia, accumulation of postprandial triglyceride-rich lipoproteins is driven by overproduction and/or decreased catabolism of these particles, with predisposing factors being genetic variations and clinical conditions, such as obesity and insulin resistance [56]. Acute postprandial increases in triglyceride-rich lipoproteins and their remnants impair vasodilation, enhance endothelial dysfunction and inflammation, upregulate production of proinflammatory cytokines, upregulate expression of vascular cell adhesion molecule-1 and monocyte activation, facilitate production of reactive oxygen species, and contribute to plaque rupture and thrombus formation [56].
In other publications, the lipid pattern described with obesity is sometimes termed “atherogenic dyslipidemia” [110] or “diabetes dyslipidemia” [111]. However, an elevation in blood LDL-C level is also “atherogenic,” with an elevation in blood LDL-C level not considered part of “atherogenic dyslipidemia” described above. Also, this lipid pattern is not exclusively found in patients with diabetes; this lipid pattern is often found in patients with obesity who do not have diabetes mellitus. Given this lipid pattern is commonly described with obesity, an alternative description is termed “adiposopathic dyslipidemia” [8,9,16,17,112].
7. How does obesity affect blood LDL-C levels?
The results of population studies, such as the Framingham Offspring Study, suggest that while some variance may exist depending on age, the increase in blood LDL-C levels with obesity is typically modest [113]. One reason why blood LDL-C levels may not be substantially increased in patients with obesity is because of the capacity of expanding adipose tissue to store cholesterol. The static storage of cholesterol in adipose tissue presents the net result of obesity's dynamic effects on cholesterol influx (i.e., effects of obesity on various enzymes and transport) and efflux, with excess cholesterol either stored in cellular lipid droplets or exported via cholesterol transport [114]. Some authors note that “cholesterol cannot be degraded by mammalian cells” [114]. For example, when LDL particles are hydrolyzed within lysosomes, cholesterol is not broken down, but instead released as free cholesterol [115]. That said, cholesterol can be broken down into bile acids for excretion, mostly in the liver [116] (although some cholesterol breakdown into bile acids can occur in other tissues such as brain [117]).
Obesity increases cholesteryl ester transfer protein (CETP) levels. In fact, adipose tissue is one of the major sources of CETP production [118]. In addition to the transfer of triglycerides from apoB-containing particles to HDL particles, CETP also facilitates the transfer of cholesteryl esters from HDL particles to apoB-containing particles, such as LDL [119]. Thus, given sufficient cholesterol in HDL particles, an increase in CETP activity might be expected to transfer more cholesterol from HDL particles to LDL particles, potentially resulting in both an increase in blood LDL-C levels and a decrease in blood HDL-C levels. The converse is also true. Inhibition of CETP not only raises blood HDL-C levels, but may also decrease blood LDL-C levels, such as reported via the use of some CETP inhibitors [119]. Thus, one might expect obesity to increase blood LDL-C levels, perhaps profoundly so. Contrary to this expectation, obesity is usually associated with only mild increases in blood LDL-C levels.
It is possible that one of the mitigating factors affecting this potential cholesterol lipoprotein transfer is that, once stored, the efflux of cholesterol from adipose tissue to HDL particles is diminished due to downregulated ABCA1 and SR-B1 expression. Decreased cholesterol efflux to HDL particles may allow for less cholesterol to be available in HDL particles to exchange with LDL particles. When coupled with other causes for reduced blood HDL-C levels, the diminished transfer of cholesterol from HDL particles to LDL particles is another potential mechanism that might help mitigate the otherwise expected rise in blood LDL-C levels.
7.1. How does obesity affect intestinal cholesterol absorption?
Another factor that may limit the increase in blood LDL-C levels with obesity is that increased adiposity is associated with decreased intestinal cholesterol absorption, especially among patients with insulin resistance [120]. Among patients with obesity, weight reduction may improve intestinal cholesterol absorption [121]. This may help explain why weight reduction with very-low carbohydrate diets (i.e., ketogenic diets) may sometimes cause markedly increased blood LDL-C levels [122]. Usually, ketogenic diets modestly increase blood LDL-C levels [123]. However, some patients with obesity have marked increases in blood LDL-C levels with ketogenic diets [124]. It is proposed that the marked increase in blood LDL-C levels in some patients with obesity may represent the combined result of increased dietary saturated fat and cholesterol intakes (i.e., as may occur via very-low carbohydrate, ketogenic diet), coupled with the unmasking of genetic intestinal hyperabsorption (via weight reduction and reduction in insulin resistance). The variability of blood LDL-C levels with dietary cholesterol is not unlike what is found in patients with sterol hyperabsorption, as illustrated by sitosterolemia [122]. If a patient treated with a ketogenic diet develops moderate/marked increases in blood LDL-C levels, then recommendations include: (a) replacing dietary saturated fatty acids with polyunsaturated and monounsaturated fatty acids; (b) reducing dietary cholesterol; (c) consider a trial of ezetimibe; (d) consider additional cholesterol-lowering drug treatment (e.g., statin); and/or (e) temporarily or permanently discontinue the ketogenic diet to determine if elevated blood LDL-C levels resolve [122].
7.2. What is the clinical meaning of a lack of substantially increased blood LDL-C levels among some patients with obesity?
The lack of a substantially elevated blood LDL-C level in some patients with obesity may be misleading. Obesity may contribute to a reduced LDL particle size, with smaller more dense LDL particles potentially being more atherogenic because they may more easily cross the arterial intima, may more avidly bind to intra-arterial proteoglycans (trapping them within the arterial wall), and are more susceptible to oxidation, enhancing their uptake by local macrophages and facilitating a pro-inflammatory state [86]. Smaller sized LDL particles may also have conformational changes where smaller LDL particles may have a lower affinity for LDL receptors. Lower LDL receptor affinity may decrease LDL catabolism, increase the time LDL particles reside in the circulation, and thus may increase the number of circulating atherogenic LDL particles [125]. Because it is the cholesterol carried by LDL particles that is most often measured clinically (i.e., blood LDL-C levels), then, at the same level of LDL-C, the patient with smaller LDL particles may have an increase in atherogenic lipoprotein particle concentration. In other words, the patient with obesity often will have a predominance of small, dense LDL particles, and thus will have higher levels of apoB and LDL particle concentration for a given blood LDL-C level clinically reported on the lipid profile. This is supported by the increase in percent body fat being associated with an increase in apoB levels [126] (i.e., one molecule of apoB exists on each atherogenic lipoprotein) and an increase in LDL particle concentration [86]. The clinical relevance is that an increase in atherogenic particle number is an important risk factor for ASCVD. In fact, an increase in LDL particle concentration is more strongly related to incident CVD events compared to the cholesterol carried by LDL (i.e., blood LDL-C levels) [127].
7.3. What is the clinical relevance of increased blood non-HDL-C levels among patients with obesity?
In 2015, the NLA published its recommendations regarding patient-centered management of dyslipidemia [128]. The conclusions included: (1) “An elevated level of cholesterol carried by circulating apolipoprotein B-containing lipoproteins (non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol [LDL-C], termed atherogenic cholesterol) is a root cause of atherosclerosis, the key underlying process contributing to most clinical ASCVD events; (2) reducing elevated levels of atherogenic cholesterol will lower ASCVD risk in proportion to the extent that atherogenic cholesterol is reduced.”
The prioritization of the evaluation and treatment of blood non-HDL-C levels reflects the limitations of relying upon blood LDL-C levels alone to assess ASCVD risk. Non-HDL-C is calculated by total cholesterol less HDL-C, and therefore is a measure of the cholesterol carried by all atherogenic lipoproteins, including, but not exclusively blood LDL-C. As with apoB (where one molecule of apoB resides on each atherogenic lipoprotein), non‐HDL‐C is a better predictor of ASCVD risk than LDL-C alone. Non‐HDL‐C sums the cholesterol content of all pro‐atherogenic particles, which in addition to LDL‐C, also includes VLDL-C, IDL-C, remnant lipoprotein cholesterol, and cholesterol carried by lipoprotein (a). Thus, especially in patients with increased triglyceride-rich lipoproteins (as often occurs in patients with obesity), measurement and management of blood non-HDL-C levels is more inclusive than an isolated focus on the measurement and management of blood LDL-C levels. Organizations such as the NLA, as well as European guidelines, have emphasized the importance of blood non‐HDL‐C and apoB levels, along with blood LDL‐C levels, in the clinical management of the patient's lipid profile and lipoproteins [129,130].
8. How does obesity contribute to elevated blood triglyceride levels?
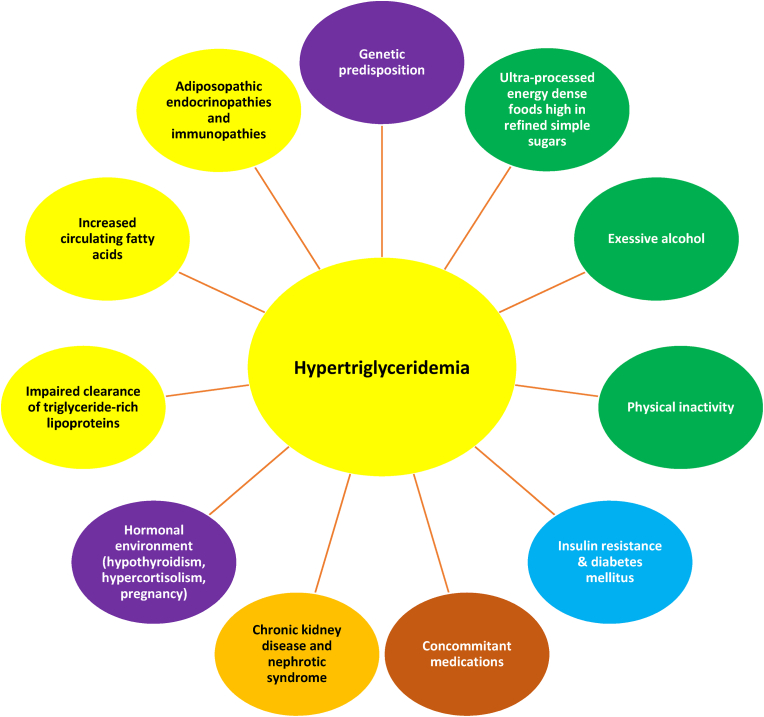
Fig. 5 illustrates a lipid pattern often described with the adiposopathic consequences of obesity. Fig. 6 describes common causes of elevated blood triglyceride levels, which may not be specific to patients with obesity. Especially in patients with marked increases in triglycerides, hypertriglyceridemia is often due to a “two hit” phenomenon of genetic predisposition accompanied by secondary causes that exacerbate the triglyceride elevation. Some of the contributors to hypertriglyceridemia include unhealthful dietary intake, excessive alcohol intake, physical inactivity, insulin resistance, diabetes mellitus, and concomitant medications (e.g., estrogens, corticosteroids, protease inhibitors, and antipsychotics) [[138], [139], [140], [141]], as well as chronic kidney disease, nephrotic syndrome, hypothyroidism, hypercortisolism, pregnancy, increased delivery of fatty acids to the liver, and the adiposopathic endocrinopathies and immunopathies that often accompany obesity.
Fig. 6.
Causes of elevated triglycerides in patients with obesity. Elevated blood triglyceride levels, especially very high triglyceride levels, are often the result of a “two hit” phenomenon of genetic predisposition, accompanied by secondary contributors [131].
Specifically, regarding obesity, adipose tissue endocrinopathies and immunopathies increase triglyceride-rich lipoprotein production and decrease triglyceride-rich lipoprotein lipolysis. The adiposopathic impairment of fat storage in dysfunctional adipocytes (i.e., often already hypertrophied in patients with obesity) during positive caloric balance increases circulating fatty acids that flux to the liver (i.e., energy overflow), leading to increased hepatic VLDL production, and thus increased circulating blood triglyceride levels. In an example of how adiposopathic impairment of triglyceride-rich lipoprotein lipolysis also contributes to increased circulating blood triglyceride levels [86], adiponectin decreases apoC-III (an LPL inhibitor) leading to the catabolism of triglyceride-rich lipoproteins and thus a decrease in blood triglyceride levels [86]. One of the endocrinopathies of obesity is a decrease in adiponectin levels, which would be expected to impair LPL activity, reduce catabolism of triglyceride-rich lipoproteins, and thus increase blood triglyceride levels. Regarding the adiposopathic immunopathies associated with obesity, an increase in pro-inflammatory cytokines secretion stimulates the production and delays the clearance of triglyceride-rich lipoproteins. For example, tumor necrosis factor and interleukin-1 are proinflammatory, stimulate lipolysis in adipocytes, and increase circulating free fatty acid levels, which increases the delivery of substrate for hepatic triglyceride synthesis [86]. In the liver, pro-inflammatory cytokines may also stimulate de novo fatty acid and triglyceride synthesis, leading to increased production and secretion of VLDL. Angiopoietin like protein 4, (an inhibitor of LPL) may also be increased, decreasing the expression of LPL, thus impairing the hydrolysis and clearance of triglyceride-rich lipoproteins [86].
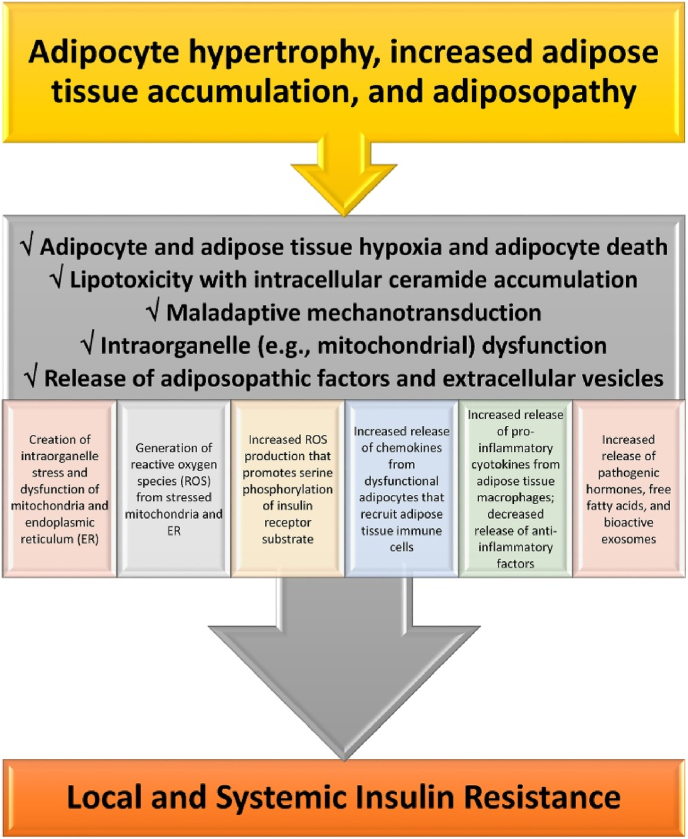
Adiposopathy is defined as pathogenic adipose tissue anatomic/functional derangements, promoted by positive energy balance in genetically and environmentally susceptible individuals, that result in adverse endocrine and immune responses that directly and/or indirectly contribute to metabolic diseases (e.g., T2DM, hypertension, dyslipidemia, CVD, and cancer) [1]. One of the most recognized adiposopathic consequences of obesity, and one of the most described contributors to hypertriglyceridemia, is insulin resistance [16]. Fig. 7 provides an overview of how the adiposopathic consequences of obesity lead to insulin resistance. Fig. 8 provides a more detailed discussion of how the adiposopathic endocrinopathies and immunopathies of obesity affect the insulin receptor, disrupt glucose transport, and contribute to insulin resistance. The main adverse clinical consequences of insulin resistance that contribute to hypertriglyceridemia include:
-
•
Impaired lipolysis coupled with increased delivery of fatty acids to adipose tissue increases fat mass, potentially further leading to adiposopathic endocrinopathies and immunopathies.
-
•
Impaired suppression of lipolysis in adipocytes [i.e., impaired suppression of HSL (see Fig. 4)] leads to increased release of fatty acids into the circulation that are delivered to the liver that, when coupled with impaired suppression of hepatic triglyceride production, all lead to fatty liver and increased VLDL secretion (see Fig. 3, Fig. 9).
-
•
Adiposopathic impairment of LPL activity due to: (a) competition for LPL hydrolysis due to increased concentration of triglyceride-rich lipoproteins [142]; (b) increased inflammation and oxidative stress that impairs LPL function [143,144]; (c) alterations in apolipoproteins that affect LDL, and (d) endocrinopathies such as insulin resistance which impairs insulin-mediated increases in LPL activity, decreased adiponectin levels which may reduce LPL activity [145], and reduced or insufficient leptin activity, which might otherwise have increased LPL activity [146].
Fig. 7.
Mechanisms detailing how adiposopathic processes lead to insulin resistance. If obesity-mediated adipocyte hypertrophy and adipose tissue accumulation outgrows its vascular supply, then the insufficient delivery of oxygen may contribute to adipocyte and adipose tissue hypoxia and increased adipocyte death. Adipocyte and adipose tissue hypoxia may adversely affect multiple metabolic processes regarding angiogenesis, adipocyte proliferation, adipocyte differentiation, reactive oxygen species generation, inflammation, and fibrosis. Beyond adipocyte and adipose tissue hypoxia, excessive intracellular lipids in the form of fatty acids may lead to ceramide (i.e., sphingolipid esterified to a fatty acid) and diacylglycerol (DAG) formation in adipocytes, where similar to the adverse effect of increased fatty acid influx and ceramide and DAG accumulation in liver and muscle, may cause lipotoxicity leading to adipocyte dysfunction, such as: (a) inhibiting AKT Protein Kinase B and thus decreasing glucose uptake via GLUT 4, (b) inhibiting hormone sensitive lipase and thus decreasing adrenergic-mediated lipolysis, and (c) impairing mitochondrial function, resulting in a pro-oxidative state from the increased production of oxygen free radicals and impairments in energy dynamics because of reduced adenosine triphosphate production, all contributing to insulin resistance. Mechanotransduction occurs when cells sense, integrate, and respond to mechanical stimuli via biologic signaling and adaptations. During healthful expansion, adipose tissue responds by adapting to its microenvironment (e.g., formation, dissolution, and reformation of extracellular matrix) via continuous remodeling to maintain its structural and functional integrity. During positive caloric balance, especially if proliferation is impaired, adipose tissue expansion is often accompanied by hypertrophy of existing adipocytes. Adipocyte hypertrophy, immune cells infiltration, fibrosis and changes in vascular architecture may generate mechanical stress on adipocytes, alter healthful adaptive mechanotransduction, and disrupt healthful adipose cell expansion physiology. Maladaptive mechanotransduction may promote obesity-associated dysfunction in adipose tissue (i.e., adiposopathy). Overall, contributors to mitochondrial dysfunction include adipocyte and adipose tissue hypoxia, lipotoxicity, maladaptive mechanotransduction, hyperglycemia, intake of certain unhealthful saturated fatty acids [132], and with specific regard to obesity, excess nutrient supply [133]. Adipocyte mitochondrial dysfunction is a potential primary cause of adipose tissue inflammation. Among the adverse consequences of adiposopathic mitochondrial (and endoplasmic reticulum) dysfunction is the generation of reactive oxygen species (ROS). ROS are unstable molecules containing oxygen that easily react with other cellular molecules, contributing to deoxynucleic acid damage, cancer, fibrosis, and aging. Other contributors to increased ROS production are hyperglycemia and adiposopathic increases in cytokines, such as tumor necrosis factor. Increased tumor necrosis factor-mediated mitochondrial ROS production may facilitate JNK activation, increase serine phosphorylation of insulin receptor substrate-1 (IRS-1), decrease insulin-stimulated tyrosine phosphorylation of IRS-1, and thus contribute to obesity-mediated insulin resistance. In summary, adipocyte hypertrophy leading to initial adipocyte dysfunction results in local proinflammatory effects that, in turn, further worsen adipocyte function, resulting in worsening adiposopathy and adipocyte insulin resistance. Systemically, adiposopathic proinflammatory factors, pathogenic hormones, and free fatty acids may be released into the circulation either directly from adipose tissue, or via adipocyte extracellular vesicles (e.g., bioactive molecules such as lipids, proteins, and nucleic acids that are packaged and transferred from adipocytes to other body tissues via exosomes, microvesicles, and apoptotic bodies formed as the result of adipocyte necroptosis or pyroptosis). The increase in pro-inflammatory factors (e.g., tumor necrosis factor and interleukins 1 beta and 6) and decrease secretion of anti-inflammatory factors (e.g., adiponectin) may promote insulin resistance (i.e., reduced cellular surface insulin receptors and post-insulin receptor defects) in susceptible non-adipose tissue peripheral organs, such as skeletal muscle and liver, contributing to “inflexibility” in managing, responding, or adapting to changes in metabolic substrates. (Adapted/copied with permission from: Bays HE, Obesity Pillars. 2023) [17].
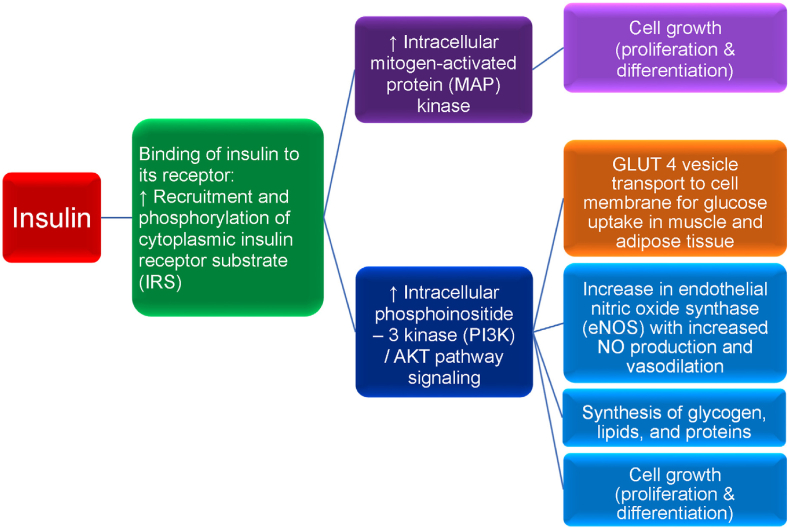
Fig. 8.
Insulin and insulin receptor functions. Diminished insulin activity can be due to an absolute or relative decrease in circulating insulin and/or impaired insulin signaling via a reduced number of insulin receptors and/or impaired post-receptor insulin signaling. Normoglycemia can be maintained in the early stages of insulin resistance by increased basal insulin (i.e., hyperinsulinemia), as often occurs in patients with obesity. However, over time, insulin secretion may no longer be sufficient to overcome insulin resistance, resulting in hyperglycemia. Obesity and the hyperglycemia of type 2 diabetes mellitus (T2DM) may result in a relative decrease in pancreatic insulin secretion, potentially due to elevations in leptin levels, as well as due to apoptosis with decreased pancreatic beta cell mass as the result of: (a) beta cell exhaustion/overload, (b) glucolipotoxicity, (c) increase in pro-inflammatory factors, and (d) decrease in anti-inflammatory factors (e.g., adiponectin). Insulin is a peptide hormone released by pancreatic beta cells in response to a rise in blood glucose (e.g., postprandial response to carbohydrate ingestion). Fructose, some amino acids, and fatty acids can also augment insulin release. Insulin binds to the extracellular alpha subunit portion of the transmembrane insulin cellular receptor of body tissues (e.g., liver, muscle, fat, brain). This activates a phosphorylation cascade involving transmembrane insulin receptor beta subunits that process tyrosine kinase activity, auto-phosphorylating insulin receptor tyrosines, and promoting the phosphorylation and activation of cytoplasmic insulin receptor substrate (IRS). Activated IRS stimulates intracellular mitogen-activated protein (MAP) kinase, which in turn, promotes cell growth (e.g., proliferation and differentiation of tissues, such as skeletal muscle cells and fat cells). While insulin mainly functions as a physiologic mitogenic facilitator, hyperinsulinemia may predispose to unregulated mitogenesis and cancer. Insulin-mediated phosphorylation of IRS also facilitates the phosphoinositide 3-kinase (PI3K)/AKT pathway (i.e., AKT is also known as protein kinase B), which is responsible for most of insulin's metabolic effects, such as the transport of glucose vesicle transporters (GLUT 4) to outer cellular membranes resulting in glucose uptake from the circulation into body tissues, thus lowering blood glucose. Insulin-dependent GLUT 4 is found in skeletal muscle and adipose tissue; insulin-independent GLUT 2 is found in the liver. Increased PI3K/AKT signaling also promotes (a) increase in endothelial nitric oxide synthase (eNOS) that facilitates increased nitric oxide production, increased vasodilation, and increased adipose tissue perfusion allowing for enhanced glucose and free fatty acids uptake in adipocytes for storage, (b) synthesis of glycogen, lipids, and proteins, and (c) cell growth (i.e., proliferation and differentiation). Dysregulation of the Ras/MAP kinase and/or PI3K/AKT pathways may adversely affect otherwise healthy cell growth (i.e., proliferation and differentiation), potentially leading to the development of human cancer [134,135], which has potential application regarding the increased risk of cancer among patient with obesity [136]. Among patients with obesity and T2DM, in addition to a reduced number of insulin receptors potentially as the result of impaired insulin receptor delivery to the cell surface due to endoplasmic reticulum stress, severe insulin resistance is mainly described as a post insulin receptor signaling defect, via disruption of the IR/IRS cascade. Specifically, obesity may result in adiposopathic increases in inflammatory factors (e.g., cytokines such as tumor necrosis factor and interleukins) and free fatty acids that may impair PI3K/AKT signaling while hyperinsulinemia continues to stimulate MAP kinase signaling, potentially contributing to post-receptor insulin resistance, prediabetes, T2DM, endothelial dysfunction, and/or cancer. (Adapted/copied with permission from: Bays HE, Obesity Pillars. 2023) [17].
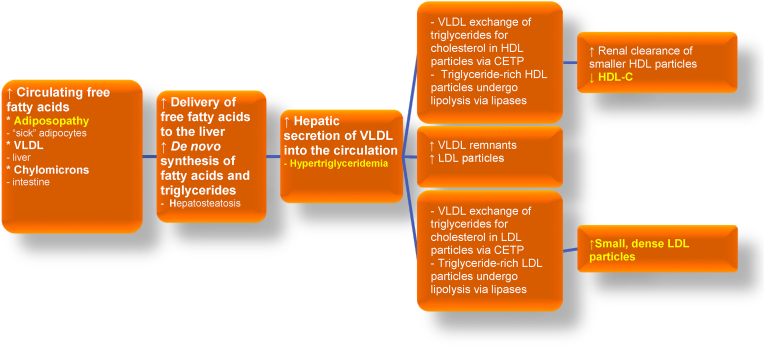
Fig. 9.
Adiposopathic dyslipidemia: Excess adiposity can contribute to hypertriglyceridemia, reduced blood HDL-C levels, and elevated smaller and more dense LDL particles. Terms that are similar to “adiposopathic dyslipidemia” include “atherogenic dyslipidemia” and/or “diabetes dyslipidemia,” all intended to reflect a lipid pattern found in many patients with obesity, as well as patients with type 2 diabetes mellitus and/or MASLD [137]. The origins of free fatty acid delivery to the liver include lipolysis of triglyceride-rich lipoproteins (e.g., VLDL originally from the liver and chylomicrons from the intestine), as well as the adiposopathic flux of fatty acids from adipose tissue as the result of insulin resistance. Finally, obesity can be associated with increased de novo synthesis of fatty acids [86]. A substantial contributor to adiposopathic dyslipidemia is CETP, which facilitates exchange of triglyceride and cholesteryl esters among circulating lipoproteins. CETP is increased with obesity, which may enhance the exchange of triglyceride from VLDL for cholesteryl esters from HDL. Similarly, CETP may facilitate the exchange of triglycerides from VLDL for cholesteryl esters in LDL. The action of body lipases on triglyceride-rich HDL particles results in smaller HDL particles that are more easily metabolized by the liver and kidney, resulting in reduced blood HDL-C levels. The action of body lipases on triglyceride-rich LDL particles results in smaller, more dense LDL particles. (Figure adapted with permission from Bays HE et al. Obesity Algorithm Slides, presented by the Obesity Medicine Association. www.obesityalgorithm.org. 2020). CETP: cholesteryl ester transfer protein; HDL-C: high-density lipoprotein cholesterol; LDL: low-density lipoprotein; MASLD: metabolic dysfunction-associated steatotic liver disease; VLDL: very-low-density lipoprotein.
9. How does obesity contribute to reduced blood HDL-C levels?
Low blood HDL-C levels are associated with increased CVD risk. This may be because a low blood HDL-C level is associated with CVD risk factors, such as obesity, T2DM, consumption of ultra-processed foods high in refined carbohydrates, physical inactivity, cigarette smoking, and increased triglyceride-rich lipoproteins. The challenge is that patients with very high blood HDL-C levels are not necessarily at low CVD risk and may sometimes be at increased risk for CVD, CVD death, and all-cause death [[154], [155], [156]]. Furthermore, treatments specifically directed towards increasing blood HDL-C levels have not yet proven to reduce the risk of CVD [155,157].
Fig. 10 illustrates obesity-related factors associated with reduced blood HDL-C levels. Blood levels of HDL-C are largely dependent on an individual's genetic predisposition [147], nutrition, and physical activity. Blood HDL-C levels are often lower in patients who consume ultra-processed foods [148] containing refined simple sugars [149]) and/or who are physically inactive [158]. Some or all of these factors may also contribute to obesity. Adiposopathic reduction in LPL activity may impair the metabolism of triglyceride-rich lipoproteins (i.e., VLDLs and chylomicrons) [118]. Furthermore, obesity facilitates greater CETP-mediated transfer of cholesterol from HDL particles to triglyceride-rich lipoproteins in exchange for triglycerides. Triglyceride-rich HDL particles may then undergo lipolysis from HL, creating smaller HDL particles that are more easily metabolized by the liver and kidneys [118,151], resulting in reduced blood HDL-C levels. Additionally, the HDL-C-lowering effect of CETP may be exacerbated in obesity due to adiposopathic endocrine increase in leptin levels, which increase CETP activity [118].
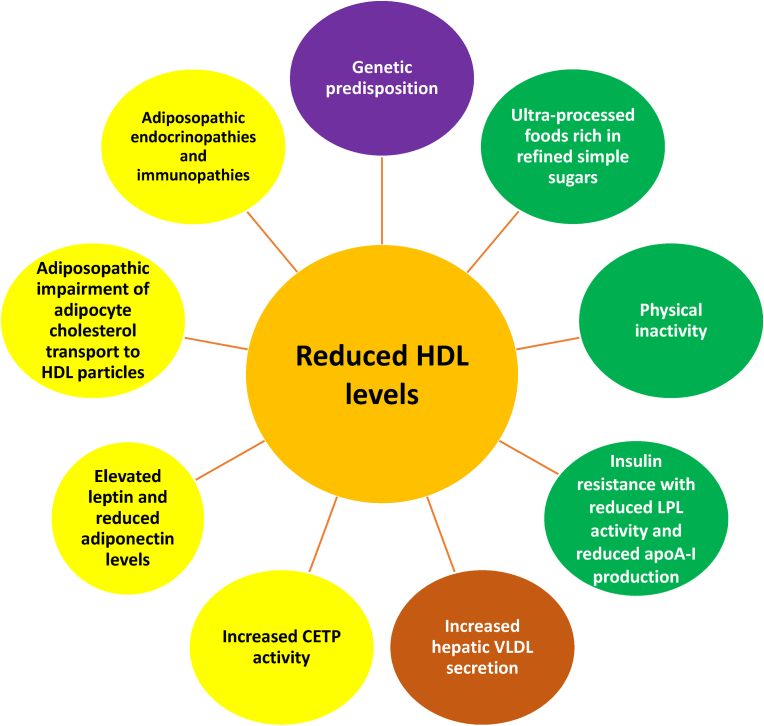
Fig. 10.
Relationship between reduced HDL-C and obesity. Blood levels of HDL-C are largely determined by individual genetics [147]. Among the more common causes of reduced blood HDL-C levels are ultra-processed foods [148] containing refined simple sugars [149]) and physical inactivity [150], both that may accompany obesity. The adiposopathic reduction in LPL activity found in patients with obesity impairs the metabolism of triglyceride-rich lipoproteins (i.e., VLDL and chylomicrons) [118]. Obesity may facilitate greater CETP mediated transfer of cholesterol from HDL particles to triglyceride-rich lipoproteins in exchange for triglycerides. Triglyceride-rich HDL particles may then undergo lipolysis from hepatic lipase, creating smaller HDL particles that are more easily metabolized by the liver and kidneys [151], resulting in reduced blood HDL-C levels. Additionally, the HDL-C lowering effect of CETP may be exacerbated in obesity due to adiposopathic endocrine increase in leptin levels, which increase CETP activity [118]. Another adiposopathic endocrine consequence of obesity is reduced adiponectin levels. Adiponectin increases the production of apoA-I (an apolipoprotein that is a major component of HDL particles) as well as hepatic ABCA1, which functions as a lipid transporter that mediates the transfer of cellular phospholipid and free (unesterified) cholesterol to apoA-I and related proteins [152], which would be expected to increase blood HDL-C levels. Conversely, the decrease in adiponectin commonly found with obesity would be expected to decrease blood HDL-C levels [118] Similarly, adiposopathic promotion of insulin resistance (see Fig. 7, Fig. 8) may also reduce apoA-I production [153]. Finally, an adiposopathic immune effect of obesity is adipocyte inflammation which may downregulate ABCA1 and SR-B1 expression and thus impair cholesterol efflux from adipocytes to HDL particles.
ABCA1: adenosine triphosphate-binding cassette sub-family member 1; apoA-I: apolipoprotein A–I: CETP: cholesteryl ester transfer protein; HDL-C: high-density lipoprotein cholesterol; LPL: lipoprotein lipase; SR-B1: Scavenger receptor class B type 1; VLDL: very-low-density lipoprotein.
Another adiposopathic endocrine consequence of obesity is decreased adiponectin levels. In addition to decreasing apoC-III (an LPL inhibitor) leading to a catabolism of triglyceride-rich lipoproteins and thus a decrease in blood triglyceride levels [86], adiponectin increases the production of apoA-I (an apolipoprotein that is a major component of HDL particles), as well as hepatic ABCA1 (which functions as a lipid transporter that mediates the transfer of cellular phospholipid and free unesterified cholesterol to apoA-I and related proteins [152]), which would be expected to increase blood HDL-C levels. Conversely, the decrease in adiponectin commonly found with obesity would be expected to decrease blood HDL-C levels [118]. Similarly, adiposopathic promotion of insulin resistance (see Fig. 8, Fig. 9) may also reduce apoA-I production [153]. Finally, an adiposopathic immune effect of obesity is adipocyte inflammation, which may downregulate ABCA1 and SR-B1 expression and thus impair cholesterol efflux from adipocytes to HDL particles. For example, normally, lipidation of apoA-I gradually creates discoidal particles enriched in unesterified cholesterol. Esterification of free cholesterol by lecithin cholesterol acyltransferase (LCAT) converts these disc-shaped particles into spherical HDL particles, which promote cellular cholesterol efflux through adenosine triphosphate-binding cassette transporter G1 (ABCG1) and SR-B1. Afterwards, as HDL particles acquire more cholesterol, they engage in the exchange with triglyceride-rich lipoproteins mediated by CETP [70].
10. What is the clinical relationship between obesity and lipoprotein (a) levels?
Fig. 5 illustrates how obesity affects various lipoprotein lipid parameters. Not yet discussed is lipoprotein (a), which is an LDL-like particle with an attached apolipoprotein (a) moiety. Increased lipoprotein (a) is an independent risk factor for CVD that is largely determined by genetics and not thought to be influenced by dietary intake or physical activity. However, both BMI and lipoprotein (a) are ASCVD risk factors, with increased lipoprotein (a) and increased BMI being additive in increasing the risk for ASCVD [159].
11. What is the clinical relationship between abdominal (visceral) obesity, blood lipid levels, and metabolic syndrome?
A misconception is that obesity is due to a genetically defined number of adipocytes, with the greater number of adipocytes resulting in obesity and increased complications, and a lower number of adipocytes resulting in leanness and decreased complications. However, adiposity reflects the mass of triglycerides in adipocytes, and not simply adipocyte number. Adipose tissue is a highly dynamic organ. It is estimated that approximately 10% of adipocytes undergo turnover annually throughout adulthood and all ranges of BMI [160]. When body weight is stable, the total number of adipocytes may remain relatively constant. At least over the timeframes currently studied, during fat weight gain, the number and size of adipocytes may increase; during fat weight reduction, the size of adipocytes may decrease with limited change in adipocyte number [161]. It is estimated that lean individuals may typically have 25–35 billion adipocytes, while patients with obesity may typically have 100–150 billion adipocytes [24]. In a study of patients without obesity, the estimated number of adipocytes was 41–65 billion (variation of 60%) while among patients with obesity, the estimated number of adipocytes was 128–237 billion (variation of 85%) [162].
While true that the increased number and size of adipocytes alone can contribute to biomechanical complications of obesity (i.e., “fat mass disease”), it is the functionality of adipocytes that determines the predisposition to metabolic diseases (i.e., “sick fat disease”) [1,163]. Although it may seem paradoxical, an increase in the number of functional adipocytes may sometimes mitigate metabolic disease, while a decrease in the number of functional adipocytes may predispose to metabolic disease. For example, patients with benign multiple symmetrical lipomatosis have increased proliferation of smaller, more functional adipocytes (and increased secretion of anti-inflammatory adipokines, such as adiponectin) within subcutaneous adipose tissue. Despite an increase in regional adiposity, patients with benign multiple symmetrical lipomatosis are not at increased risk for hyperglycemia or dyslipidemia [164]. In another illustrative example, while T2DM is often considered a complication of too much body fat, one of the treatments of T2DM includes the use of thiazolidinediones (i.e., peroxisome proliferator activated receptor gamma agonists, such as pioglitazone). An important mechanism of action of thiazolidinediones is the increase in the proliferation of functional adipocytes, which in addition to fluid retention, helps account for the increase in body weight with thiazolidinediones. Improved adipose tissue function via the increased number of functional adipocytes helps explain why thiazolidinediones improve both glycemia and hypertriglyceridemia [165]. Some have suggested that: “Strategies to increase the recruitment of adipocyte progenitor cells to expand adipose tissue by increasing adipose cell numbers could be protective against the metabolic consequences of obesity” [166].
Conversely, patients with inherited lipodystrophy have a variable lack of adipose tissue, which may contribute to a genetic lack of adipocyte function, resulting in hypertriglyceridemia and hyperglycemia [164]. In patients with human immunodeficiency virus (HIV) treated with some antiretroviral agents (e.g., nucleoside reverse transcriptase inhibitors and protease inhibitors), adipocyte function may be adversely affected [167], potentially leading to HIV lipodystrophy, and likewise development of metabolic diseases [164], such as the metabolic profile found with adiposopathic dyslipidemia. Adipose hypertrophy with HIV lipodystrophy is often accompanied by increased visceral adipose stores, increased blood triglyceride levels, decreased blood HDL-C levels, adipocyte insulin resistance, as well as hypertension and a tendency to develop T2DM [168].
Even without a defined primary or secondary cause, if a patient's subcutaneous adipose tissue is unable to adequately proliferate and differentiate during positive energy balance (sometimes described as a type of “acquired lipodystrophy” [169]), then the energy overflow via increased circulating free fatty acids locate to other fat depots, such as visceral adipose tissue, pericardial fat, and perivascular fat [164]. Additionally, the adiposopathic increase in circulating free fatty acids may promote “lipotoxicity” [164] in non-adipose tissue, such as the liver, skeletal muscle, heart, endothelia, pancreas, and kidney, contributing to insulin resistance [17], hyperglycemia [17], fatty liver disease [170], metabolic syndrome/dyslipidemia [171,172], hypertension [18], sarcopenia [173], atherosclerosis [174], cardiomyopathy [173], endothelial dysfunction [175], diminished insulin secretion [176], and renal apoptosis with proteinuria [173].
Due to confounders related to race and sex, central obesity may not always correlate with visceral adiposity [7,177]. However, the presence of central obesity may reflect an adiposopathic inability to accumulate adipose tissue in peripheral subcutaneous adipose tissue, while visceral adiposity is reasonably considered a marker for global adipose tissue dysfunction and increased CVD risk [178]. From a clinical perspective, all these factors help explain the diagnostic criteria for “metabolic syndrome,” which includes central obesity, increased triglycerides, low HDL-C, increased blood glucose, and increased blood pressures. While visceral fat may correlate to blood LDL-C levels in some populations [179], the dyslipidemias most often described with adiposopathic dyslipidemia are hypertriglyceridemia and reduced blood HDL-C levels [164]. In short, metabolic syndrome is not a “disease.” No medication has a Food and Drug Administration approved indication to treat the metabolic syndrome. However, metabolic syndrome is a common clustering of cardiovascular risk factors (i.e., including lipid abnormalities), with increased waist circumference as the only anatomic diagnostic criterion [164].
12. What is the effect of dietary intake on the clinical lipid profile and blood lipoprotein levels?
Nutritional intake affects both body weight as well as blood lipoprotein levels and common clinical lipid profiles. A thorough discussion of medical nutrition therapy/dietary patterns and the lipid effects of dietary macronutrients is beyond the scope of this expert review, and reviewed elsewhere [122,203,204]. Table 1 shows the general effects of dietary macronutrient substitutions on common lipid profile and lipoprotein levels. However, the lipid profile, lipoprotein, and cardiovascular effects of nutritional intake may vary within an individual patient depending on factors such as genetic background, age, race, sex, concomitant metabolic diseases, concomitant medications, and environment, as well as the dynamics of weight changes (i.e., weight increases or weight decreases), whether the macronutrients are added or substituted for other macronutrients, and the subtypes of macronutrients. For example, while both are carbohydrates, consumption of complex carbohydrates may have different effects on blood lipid levels compared to consumption of refined/simple carbohydrates (See Table 1). Similarly, stearic acid (C18:0) (i.e., about 25% of the saturated fatty acids in the U.S. adult diet) has minimal effects upon blood LDL-C levels, while palmitic acid (C16:0) (i.e., 50% of the saturated fatty acids in the U.S. adult diet) increases blood LDL-C levels [122]. Also, while dietary fats and dietary carbohydrates provide a potential source of fatty acids via de novo lipogenesis in fasting healthy individuals, factors such as obesity, insulin resistance, and fatty liver may contribute to marked de novo alterations in fatty acid composition, affecting tissue content of fatty acids, such as palmitic acid [205]. Table 2 describes some sentinel publications regarding nutritional intervention, weight reduction, and lipid effects, with the general clinical message being that, across a spectrum of nutritional interventions, lipid levels typically improve with weight reduction, especially blood triglyceride and HDL-C levels.
Table 1.
Summary of effects of isocaloric replacement of selected macronutrients on circulating concentrations of LDL-C, triglycerides, and HDL-C from intervention trials [[180], [181], [182], [183], [184], [185]]. If refined starches and added sugars, alcohol, and/or some unhealthful saturated fatty acids are substituted by more healthful macronutrients, then this may potentially improve lipid and lipoprotein levels. Macronutrient quality and quantity consumption may have variable effects on the lipid profile within an individual patient depending on factors such as genetic background, age, race, sex, concomitant metabolic diseases, concomitant medications, and environment, as well as the dynamics of weight changes (i.e., weight increases or weight decreases) [86,[186], [187], [188], [189]].
| Macronutrient | LDL-C | Triglycerides | HDL-C |
|---|---|---|---|
| SFA replaced with carbohydrate | ↓ | ↑ | ↓ |
| SFA replaced with UFA | ↓ | ↓ | ↓ |
| SFA replaced with protein | ↓ | ↓ | ↓ |
| Carbohydrate replaced with UFA | ↓ | ↓ | ↑ |
| Carbohydrate replaced with protein | ↓ | ↓ | ↓ |
| Alcohol replaced with carbohydrate | ↔ | ↓ ↔ | ↓ |
| Alcohol replaced with UFA | ↓ | ↓ | ↓ |
↓ indicates decrease; ↔ indicates no change; ↑ indicates increase
HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; SFAs: saturated fatty acids; UFA: unsaturated fatty acids (e.g., mono- and/or polyunsaturated fatty acids)
Table 2.
Effect of weight reduction on lipid levels. Variance exists in the degree of lipid effects of weight reduction, depending on the studies selected for meta-analyses. The overall message is that weight reduction generally reduces blood LDL-C and triglycerides, and raises HDL-C, with the effects dependent on the amount of weight reduction.
| Study | Weight & lipid effects | Type of study (reference) |
|---|---|---|
| Dattilo, Kris-Etherton (1992) | For every 1-kg decrease in body weight:
|
Meta-analysis [190] |
| Zomer (2016) | Overall lipid effects regarding interventions that promote weight reduction:
|
Meta-analysis [191] |
| Hasan (2020) | For every 1 kg decrease in body weight with lifestyle interventions (“diet, exercise, or both”):
|
Meta-analysis [192] |
HDL-C: High-density lipoprotein cholesterol
LDL-C: Low-density lipoprotein cholesterol
TG: Triglycerides
↓: Decreases
↑: Increases
↔: Neutral effect
13. What is the effect of physical activity on the clinical lipid profile and blood lipid levels?
Increased physical activity is a priority towards managing CVD risk, which includes clinical recommendations regarding both dynamic (“aerobic”) physical exercise [122,206] and resistance training (“anaerobic”) [207]. Lipid profile changes with increased dynamic physical activity generally include an increase in blood HDL-C levels, a reduction in blood triglyceride levels, and variable effects on blood LDL-C levels [208,209]. Resistance training (“anaerobic”) similarly may result in an increase in blood HDL-C levels, a reduction in blood triglyceride levels, and (again) variable effects on blood LDL-C levels [209]. It is unclear whether time engaged in the combination of dynamic and resistance training has any greater effect on the lipid profile and lipoprotein levels compared to the same time engaged in either form of exercise alone [209].
14. In patients with obesity, what is the effect of weight reduction on the lipid profile, blood lipoprotein levels, and CVD risk?
Table 2 summarizes the effects of weight reduction on lipid profile and lipoprotein levels among patients with increased adiposity. Intentional weight reduction among patients with obesity can improve lipid levels, especially in reducing blood triglyceride levels. The data are more scarce and more variable in support of reduction of blood LDL-C levels with weight reduction. Some publications report that rapid weight reduction can sometimes result in a transient increase in LDL-C levels, with a potential disproportionate increase in larger LDL particles [210]. Results of other publications suggest that rapid weight reduction may initially reduce LDL-C levels, followed by a transient rise in LDL-C, possibly due to mobilization of adipose cholesterol stores, which may resolve upon stabilization of body weight [211]. Other observational trials suggest that a weight reduction of 5–10% or more is required for longer-term, meaningful reductions in blood LDL-C levels [212]. The type of nutritional intervention promoting weight reduction influences the effect on LDL-C levels, with lower dietary fat intake decreasing LDL-C levels relative to higher (saturated) dietary fat intake which may increase LDL-C levels [213].
The effects of weight reduction on blood HDL-C levels may be time dependent, with no change or reduction in blood HDL-C levels during active weight reduction and return to baseline or increase in blood HDL-C levels once weight stabilizes [214,215]. In general, the greater the weight reduction, the greater the improvement in lipid levels.
The Look AHEAD (Action for Health in Diabetes) study was a multicenter, randomized clinical trial examining the long-term effects of lifestyle interventions on cardiovascular morbidity and mortality in 5145 patients with overweight or obesity and T2DM who were randomized to intensive lifestyle intervention or usual care. After a median follow-up of 9.6 years, the study was stopped early due to futility in reducing CVD events [216]. An observational analysis of the Look AHEAD study based upon the magnitude of weight reduction supported the principle that a modest weight reduction of 5–10% was associated with improvements in CVD risk factors at 1 year, but larger weight reduction had greater benefits [217]. Specifically, HbA1c and blood pressure (especially systolic blood pressure) were further reduced with each increment of weight reduction (≥ 2% to < 5%, ≥ 5% to < 10%, ≥ 10 to < 15%, and ≥ 15%). Proportional to the amount of weight reduction across these categorical ranges, blood triglyceride levels were progressively, and significantly reduced >60 mg/dL and blood HDL-C levels were progressively and significantly increased ∼4–5 mg/dL. In contrast, the effects on blood LDL-C levels did not achieve statistical significance, either in the overall patient population, or among patients on lipid-lowering medications [217]. Regarding other lipid parameters, weight reduction may reduce apoB levels (i.e., one molecule of apoB resides on each atherogenic lipoprotein) more consistently than a reduction in blood LDL-C levels [218].
Overall, among patients with increased adiposity, weight reduction of ≥2.5% can result in mild improvement in cardiometabolic parameters, such as a reduction in blood triglyceride levels and some improvement in glucose metabolism. Greater weight reduction of ≥5% is usually required for more clinically meaningful improvements in the multiple adiposopathic and fat mass complications of obesity, including further reduction in blood triglyceride levels and increases in blood HDL-C levels. Even greater reduction in body weight (e.g., >15%) has the potential to reduce cardiovascular and overall mortality [219,220].
15. In patients with obesity, what is the effect of anti-obesity medications on the lipid profile, lipoprotein levels, and cardiovascular risk?
Anti-obesity medications (AOMs) can reduce CVD risk factors [221]. Table 3 illustrates reported lipid effects of AOMs. Additionally, a ”real world” analysis of AOMs prior to the approval of highly-effective AOMs (defined as AOM's producing weight reduction ≥15% in a clinically meaningful percent of patients [222]) suggested that mild reduction in body weight was associated with marginal improvements in CVD risk factors (including blood lipids) with older and less effective AOMs [223].
Table 3.
Effects of anti-obesity medications (AOM) and bariatric surgery on lipid levels. These reported effects are not intended to reflect efficacy in patients with specific dyslipidemias. Also, because these are independent studies, of varying duration, with different protocol designs (i.e., some including patients with diabetes mellitus, others not), different baseline lipid levels, different dose ranges, and at different points in active weight reduction, the reported lipid effects of these interventions are not intended to reflect direct comparisons. See references for more information about the interventions, doses of AOMs, drug formulations, duration of studies, and other details of each study. While somewhat variable depending on the analysis, the overall message is that AOMs generally have the greatest effect on lowering blood triglyceride levels, with neutral to increases in HDL-C levels, and marginal decreases in blood LDL-C levels. The exception may be orlistat, that may have greater reductions in blood LDL-C levels due to mechanisms beyond weight reduction alone [201]. Bariatric surgery generally results in greater weight reduction and greater improvements in lipid profiles than current monotherapy AOMs.
| Intervention | Change TGa | Change HDL-Ca | Change LDL-Ca | Change weighta | Ref. |
|---|---|---|---|---|---|
| Anti-obesity medications | |||||
| Orlistat | −0.09 mmol (−8 mg/dL) |
−0.034 mmol/L (−1.3 mg/dL) |
−0.27 mmol/L (−10.44 mg/dL) |
−2.12 kg | [193] |
| Phentermine/topiramate | −13.38% | +4.62% | −0.96% | −7.73 kg | [194] |
| Naltrexone/bupropion | −11 to −15 mg/dL | +3 to +5 mg/dL | −1 to −4 mg/dL | [195,196] | |
| Liraglutide | −23 mg/dL | −0.4 mg/dL | −4.6 mg/dL | −2.38 kg | [195,196] |
| Semaglutide | −15.64% | +4.24% | −2.18% | −8.51% | [197] |
| Tirzepatide | −20.3 mg/dL | +8.8 mg/dL | −4.2% | −17.8% with 15-mg dose | [198] |
| Pharmacotherapy in general | −1.25 mg/dL per one kg weight reduction | +0.37 mg/dL per one kg weight reduction | −1.67 mg/dL per one kg weight reduction | NA | [192] |
| Bariatric surgery | |||||
| Gastric bypass | −36 to −63% | +23 to +39% | −17 to −31% | −35% | [199,200] |
| Sleeve gastrectomy | −35 to −42% | +19 to +28% | −12 to −23% | −34% | [199] [200] |
| Bariatric surgery in general | −2.47 mg/dL per one kg weight reduction | +0.42 mg/dL per one kg weight reduction | −0.33 mg/dL per one kg weight reduction | NA | [192] |
HDL-C: High-density lipoprotein cholesterol
LDL-C: Low-density lipoprotein cholesterol
NA: Not applicable
TG: Triglycerides
Some of the chosen cited references were sentinel clinical trials, while others were meta-analyses. The reported data reflect variances in how the data were reported (i.e., percent versus mean reductions). Also, while the bariatric literature mostly reports “excessive weight loss,” this is a different calculation than total weight loss, and makes it challenging to compare the bariatric literature to the medical literature (i.e., publications regarding AOMs [202]). The values given here are “total weight loss” and not “excessive weight loss.”
Conversely, results from clinical trials support that, among patients with obesity, some highly effective AOM's that reduce body weight and improve CVD outcomes, also improve the lipid profile and blood lipoproteins levels. An illustrative example is the Semaglutide and Cardiovascular Outcomes in Obesity without Diabetes (SELECT) Trial. SELECT was an integral part of the semaglutide development program for treatment of obesity [220]. SELECT was a placebo-controlled cardiovascular outcomes trial of semaglutide 2.4 mg subcutaneously once weekly involving patients with overweight or obesity with CVD but without diabetes mellitus [197]. In this cardiovascular outcome study of 17,604 patients treated with semaglutide or placebo for 34.2 ± 13.7 months (and the mean duration of follow-up was 39.8 ± 9.4 months), the semaglutide group experienced a 20% reduction in major adverse cardiac events. The baseline lipid values in the semaglutide group included total cholesterol 153 mg/dL, LDL-C 78 mg/dL, triglycerides 134 mg/dL, and HDL-C 44 mg/dL. The mean change in body weight over the 104 weeks after randomization was −9.4% with semaglutide and −0.88% with placebo (estimated treatment difference, −8.5% points). At study end, semaglutide produced a reduction in total cholesterol of 4.6%, reduction in LDL-C of 5.3%, reduction in triglycerides of 18.3%, and increase in HDL-C of 4.9%. While the lipid effects were generally favorable, reduction in LDL-C levels were modest. Furthermore, this study did not evaluate the degree that the CVD risk reduction was due to improvement in lipid levels, nor did this study specifically evaluate the degree the CVD risk reduction (or the reduction in C-reactive protein) was due to weight reduction alone.
16. In patients with obesity, what is the effect of bariatric surgery on the lipid profile, blood lipoprotein levels, and cardiovascular risk?
Bariatric surgery can reduce CVD risk factors [221]. Table 3 illustrates reported lipid effects of bariatric surgery. The effects of bariatric procedures/surgery on the lipid profile and lipoprotein levels can vary, depending on the types of bariatric procedures/surgery. Compared to the more rarely performed laparoscopic banding, sleeve gastrectomy, Roux-en-Y gastric bypass, and biliopancreatic diversion/duodenal switch have directionally similar, but more profound effects in reducing blood LDL-C, reducing blood triglyceride levels, increasing blood HDL-C levels, as well as decreasing non-HDL-C, apoB, lipoprotein particle number, and lipoprotein remnants [15]. Results from some studies suggest bariatric surgery produces no change in lipoprotein (a) [15], while the results from other studies suggest bariatric surgery may increase lipoprotein (a) [224]. Yet the results of other studies suggest bariatric surgery may decrease lipoprotein (a) [225], independent of weight reduction [226], with some indication that gastric bypass may lower lipoprotein (a) more than sleeve gastrectomy [227].
Similar to the SELECT trial evaluating the cardiovascular outcomes, lipid profile, and lipoprotein effects of AOMs, bariatric surgery may have cardiovascular outcome benefits. A supporting clinical trial would be the Swedish Obese Subjects (SOS) study, which was a prospective, controlled, 10.9-year study that included 4047 participants with obesity who were treated with bariatric surgery (i.e., gastric bypass, 32%; vertical-banded gastroplasty, 25%; and banding, 20%) or conventional treatment (matched control group). Bariatric surgery resulted in a relative risk reduction in mortality of 24% with 25 deaths due to myocardial infarction in the control group and 13 subjects in the bariatric surgery group. Death due to cancers were 47 in the control group and 29 in the surgery group [228]. By study end, the incidence of study participants diagnosed with hypertriglyceridemia was 27% in the control group and 17% in the surgery group, while the incidence of low HDL-C was 6% in the control group and 3% in the surgery group [229]. The results of other analyses also support that bariatric surgery reduces blood LDL-C and triglyceride levels, and increases blood HDL-C [230] levels, with the most consistent lipid profile and lipoprotein effects being a decrease in triglycerides and increase in blood HDL-C levels [231].
17. Among patients with increased adiposity, what are the relative benefits of weight reduction versus blood LDL-C level lowering in reducing ASCVD risk?
Without head-to-head comparative data derived from specifically designed clinical outcomes trials, it is challenging to compare the CVD benefits of reducing body weight versus lowering blood LDL-C levels among patients with increased adiposity. Management of both increased body fat and increased blood atherogenic lipids are important components of a global strategy to reduce CVD risk.
Reduction in blood LDL-C levels primarily reduces the risk of ASCVD. Among patients with increased adiposity, weight reduction may not only improve blood lipid levels, but also may improve other major ASCVD risk factors, such as blood glucose, blood pressure, as well as inflammation, thrombosis, and ability to engage in routine physical activity. Reducing body weight in patients with overweight or obesity may also improve both “sick fat disease” and “fat mass disease” (see Section 11), with potential improvements in ASCVD (as well as other CVDs such as heart failure and atrial fibrillation), as well as improvements in sleep apnea [232]).
Regarding weight reduction, prior to the favorable CVD findings in the SELECT study of the AOM semaglutide [197] (previously discussed in Section 15.0), little evidence supported that weight reduction with AOM use resulted in a clinically meaningful reduction in CVD risk. Furthermore, as previously noted, the SELECT study did not evaluate the degree that the CVD risk reduction was due to improvement in lipid levels, nor did the SELECT study specifically evaluate the degree the CVD risk reduction was due to weight reduction alone. The same might be said regarding bariatric surgery. Overall, among patients with obesity, bariatric surgery has the potential to reduce all-cause mortality, cardiovascular mortality, heart failure, myocardial infarction, and stroke by ∼40–50% [233]. It is uncertain how much of the benefit of bariatric surgery in patients with obesity is due to weight reduction alone and how much is due to improvement in cardiometabolic risk factors (e.g., improvement in blood lipid levels, blood pressure, blood glucose), improvement in heart failure, improvement in sleep apnea, or improvement in other metabolic, inflammatory, or fat mass factors that contribute to CVD.
Regarding blood lipid levels, among patients with LDL-C levels ∼90 mg/dL at baseline, analyses from ODYSSEY OUTCOMES (i.e., alirocumab) and FOURIER (i.e., evolocumab) supported that regarding patients at high CVD risk, lowering blood LDL-C levels over 50% with proprotein convertase subtilisin kexin-9 inhibitors resulted in about a 15% relative risk reduction of CVD [234,235]. Of note, patients in these studies were treated with maximally tolerated or high dose statins and underwent follow-up for a median of only 2–3 years.
Perhaps a more clinically relevant analysis is the Cholesterol Treatment Trialists Collaboration (CTT). The CTT evaluated a broader patient population treated with various statins, studied in multiple clinical trials, over a variable amount of time (i.e., often longer follow-up than the ODYSSEY OUTCOMES and FOURIER trials). The CTT authors concluded in their meta-analyses, that among patients with varying baseline blood LDL-C levels, the risk of an ASCVD event was reduced by 22% per 38.7 mg/dL reduction in blood LDL-C levels [236].
In short, in the SELECT trial, semaglutide reduced body weight by 8.5% and reduced CVD events by 20% [197]. When calculated as percent weight loss (and not “percent excess body mass index loss,”) bariatric surgery may reduce body weight approximately 30–40% [202] As previously noted, among patients with obesity, bariatric surgery has the potential to reduce all-cause mortality, cardiovascular mortality, heart failure, myocardial infarction, and stroke by ∼40–50%. Thus, among patients with increased adiposity, when the evidence of weight reduction is compared to the evidence of blood LDL-C lowering (i.e., utilizing CTT data), then current evidence provides little insight as to which is more effective in reducing CVD risk or events. Rather, the totality of evidence suggests that such a comparison is both unhelpful and unnecessary. Depending on the intervention, both weight reduction and blood LDL-C level lowering represent evidenced-based priorities in the management of patient with increased adiposity and increased atherogenic blood lipids.
The potential benefits of attaining optimal body weight and optimal blood lipid levels might be illustrated by human hunter-gatherer populations. Historically, the mean BMI of hunter-gatherers (e.g., Australian aborigines) has been reported as being <20 kg/m2 [237]. Reports have also recorded hunter-gatherer populations as having a total cholesterol level of ∼100 mg/dL [238]. Regarding hunter-gatherer populations, “coronary heart disease “is virtually absent and is rarely seen in patients admitted to rural hospitals” [239]. Conversely, over 2/3 of U.S. adults have overweight or obesity, with rates of obesity being higher among certain races and ethnicities (i.e., Blacks and Hispanics) [240] The average total blood cholesterol levels in U.S. adults in years from 1999 to 2000 was 188 mg/dL and the average blood LDL-C for the same time period was 112 mg/dL, which included blood lipid values among those using cholesterol lowering medications, such as statins [241]. Cardiovascular disease is the most common cause of mortality among U.S. adults [7].
The reduced risk for CVD among hunter-gatherers is despite whether the nutritional intake is higher or lower in fat [239,242], despite variances in plant vs meat intake [239,242], and despite weight-adjusted total energy expenditure among rural hunter-gathers being similar to some Western populations (i.e., European or US cities), which have high rates of obesity [243]. This suggests that lifelong avoidance of increased adiposity has the potential to substantially reduce the risk ASCVD. Furthermore, from a lipid standpoint, the CVD beneficial effects of lifelong limited exposure to adiposopathic CVD risk factors would be consistent with findings that incident CVD event risk is dependent on the cumulative time exposure to increased blood LDL-C levels. Collectively, this emphasizes the importance of optimal body weight and optimal blood LDL-C level control, starting early in life [244].
18. Among children with obesity, what is the effect of weight reduction on lipid profile, blood lipoprotein levels, and cardiovascular risk factors?
Regarding children, a systematic review and meta-regression evaluated 42 studies conducted through February 2015 of interventions to treat pediatric obesity (medication, surgery, lifestyle, and community-based interventions), all having a 6-month follow-up. The baseline characteristics of the participants included a mean age of 12.2 years, a mean weight of 74.7 kg, and a mean BMI of 31.7 kg/m2. The results of this analysis revealed that a 0.74-kg decrease in weight was associated with a 1-mg/dL increase in blood HDL-C levels and a 0.1-kg decrease in weight was associated with a 1-mg/dL decrease in blood triglyceride levels [245]. The conclusion of the authors was that weight reduction in children was associated with significant changes in measures of cardiometabolic risk, including an increase in blood HDL-C levels and a decrease in blood triglyceride levels (as well as systolic blood pressure), with the degree of anticipated improvement helping to inform shared decision-making and counseling [245].
19. What is the role of dyslipidemia management in patients with obesity?
Patients with acute adiposopathic metabolic consequences of obesity (i.e., severely elevated blood glucose, blood pressures, CVD, thrombosis, or cancer) require concomitant treatment of their acute metabolic diseases, as well as concomitant treatment of obesity. For patients with obesity without acute metabolic disease (i.e., those with modest elevations in blood glucose, blood pressure, or blood triglyceride levels), and at lower CVD risk, a Clinical Practice Statement from the OMA has suggested that some patients may benefit from a “Treat Obesity First” approach [17]. Such an approach may be especially considered with implementation of evidenced-based nutritional intervention, physical activity, AOMs, and bariatric procedures with proven effectiveness in improving these CVD risk factors (and cardiovascular outcomes), and in patients where anti-diabetes medications may increase the risk of hypoglycemia and antihypertensive medications may increase the risk of hypotension, if administered concurrently during active weight reduction. The recognition of the potential effectiveness of weight reduction to reduce blood glucose levels [17] and blood pressures [18] is reflected in the common medical practice of reducing (or discontinuing) anti-diabetes and anti-hypertensive medications before bariatric surgery, and the substantial rate of remission of diabetes mellitus and hypertension that directly correlates to the amount of post operative weight reduction [24,202,[246], [247], [248]], with a directionally similar need to adjust anti-diabetes and antihypertension medications with very low-calorie diets [249,250].
A potential exception to this “Treat Obesity First” paradigm would be patients with obesity with, or who are at high risk for, ASCVD and who have elevated blood LDL-C levels. Given the limited blood LDL-C level reduction anticipated with most treatments for obesity, and given improved CVD outcomes with earlier lipid-lowering therapy administration [112], patients with, or at high risk for, ASCVD should have management priorities that include both a reduction in excess adiposity and appropriate management of atherogenic lipoproteins [108]. Similarly, both management of obesity and management of dyslipidemia may be optimally achieved via use of a guideline-directed and multidisciplinary team approach [108,251,252].
20. Conclusion
The adiposopathic dyslipidemia pattern most described in patients with increased adiposity includes elevated blood triglyceride levels, reduced blood HDL-C levels, increased non-HDL-C levels, elevated apoB, increased LDL particle concentration, and increased small dense LDL particles. Population studies suggest blood LDL-C levels are modestly increased with increased adiposity. The clinical takeaways from this joint expert review by the OMA and NLA are that.
-
•
An increase in adiposity is generally associated with an atherogenic lipid profile, as well as other cardiometabolic risk factors, all that likely contribute to increased CVD risk.
-
•
Among patients with obesity, interventions that reduce body weight and improve CVD outcomes are generally associated with reduced blood triglyceride levels and increased blood HDL-C levels, often with only mild to modest improvements in blood LDL-C levels.
-
•
In patients with, or at risk for ASCVD, a dual priority includes early lifestyle and/or pharmacologic intervention to treat both excess adiposity and elevated levels of atherogenic cholesterol (i.e., LDL-C and/or non-HDL-C).
Transparency and group composition [253]
The authors reflect a multidisciplinary and balanced group of experts in obesity science, patient evaluation, and clinical treatment.
Author Contributions
HEB contributed the conceptualization, methodology, visualization, review, editing, and writing the original draft. Each of the remaining authors engaged in review and editing. All authors approved the final draft.
Managing disclosures and dualities of interest
Authors of this joint expert review received no payment for their writing, editing, and publishing work. While Editors of journals who are listed as authors received payment for their roles as Editors, they did not receive payment for their participation as authors.
Individual disclosures
HEB's research site institution has received research grants from 89Bio, Allergan, Alon Medtech/Epitomee, Altimmune, Amgen, Anji Pharma, AstraZeneca, Bionime, Boehringer Ingelheim, Eli Lilly, Esperion, Evidera, Fractyl, GlaxoSmithKline, HighTide, Home Access, Ionis, Kallyope, LG-Chem, Madrigal, Merck, New Amsterdam, Novartis, NovoNordisk, Pfizer, Satsuma, Selecta, Shionogi, TIMI, Viking, and Vivus. HEB has served as a consultant/advisor for 89Bio, Altimmune, Amgen, Boehringer Ingelheim, HighTide, and Lilly. CFK is an employee of Midwest Biomedical Research and has no additional conflicts of interest to report beyond those disclosed for KCM. During the last 36 months, KCM has received research funding and/or consulting fees from 89Bio, Acasti Pharmaceuticals, Beren Therapeutics, Bragg Live Products, Campbell's, Cargill, Eli Lilly and Co., General Mills, Greenyn Biotechnology, Hass Avocado Board, Indiana University Foundation, Lonza, Matinas Biopharma, Medifast, National Cattlemen's Beef Association/Beef Checkoff, National Dairy Council, Naturmega, New Amsterdam Pharmaceuticals, North Sea Therapeutics, Novo Nordisk, Pepsico, Pharmavite, and Seed, Inc. PPT is a speaker for Amgen, Lilly, and Novo-Nordisk; consultant to Lilly and Novartis. RTM is a faculty member and speaker for Rhythm Pharmaceuticals. JT reports no disclosures. SMC is a speaker for Novo Nordisk, Eli Lilly; advisor for Abbott Nutrition. DD has received research funding from Boehringer Ingelheim, Inc. TJ reports no disclosures.
Funding
Preparation of this manuscript received no funding.
Evidence
The content of this manuscript is supported by citations, which are listed in the References section.
Ethics review
This submission did not involve experimentation of human test subjects or volunteers. Authors who concomitantly served as journal Editors for Obesity Pillars or the Journal of Clinical Lipidology were not involved in editorial decisions or the peer review process. Journal editorial decisions and peer review management was delegated to non-author society members or non-author journal Editors.
Peer review
This joint expert review underwent peer review according to the policies of the participating organizations.
Conclusions and recommendations
This joint expert review is intended to be an educational tool that incorporates the current medical science and the clinical experiences of obesity specialists, lipidologists, and/or those engaged in preventive cardiology. The intent is to better facilitate and improve the clinical care and management of patients with obesity. This joint expert review should not be interpreted as “rules” and/or directives regarding the medical care of an individual patient. The decision regarding the optimal care of the patient with pre-obesity and obesity is best reliant upon a patient-centered approach, managed by the clinician tasked with directing an individual treatment plan that is in the best interest of the individual patient.
Updating
This joint expert review may require future updates. The timing of such an update will be determined by the respective societies authoring this document.
Disclaimer and limitations
This joint expert review was developed to assist health care professionals in providing care for patients with pre-obesity and obesity based upon the best available evidence. In areas regarding inconclusive or insufficient scientific evidence, the authors used their professional judgment. This joint expert review is intended to represent the state of obesity medicine at the time of publication. Thus, this joint expert review is not a substitute for maintaining awareness of emerging new science. Finally, decisions by clinicians and healthcare professionals to apply the principles in this joint expert review are best made by considering local resources, individual patient circumstances, patient agreement, and knowledge of federal, state, and local laws and guidance.
Declaration of Artificial intelligence (AI) and AI-assisted technologies in the writing process
Artificial intelligence was not used in the writing of the text nor creation of its figures; however, AI was used for suggestions regarding topic headings.
Contributor Information
Harold Edward Bays, Email: hbaysmd@outlook.com.
Carol Kirkpatrick, Email: ckirkpatrick@mbclinicalresearch.com.
Kevin C. Maki, Email: kmaki@mbclinicalresearch.com.
Peter P. Toth, Email: Peter.Toth@cghmc.com.
Ryan T. Morgan, Email: Ryan.Morgan@VitalisMetabolic.com.
Justin Tondt, Email: justintondt@gmail.com.
Sandra Michelle Christensen, Email: sam.chris@im-wm.com.
Dave Dixon, Email: dldixon@vcu.edu.
Terry A. Jacobson, Email: tjaco02@emory.edu.
References
- 1.Fitch A.K., Bays H.E. Obesity definition, diagnosis, bias, standard operating procedures (SOPs), and telehealth: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization . World Health Organization; 2023. Obesity and overweight.https://wwwwhoint/news-room/fact-sheets/detail/obesity-and-overweight.2021 [Updated 2021, June 9; Cited 2024, Jan 3] Available from: [Google Scholar]
- 3.Centers for Disease Control and Prevention . 2022. https://www.cdc.gov/obesity/data/adult.html (Overweight and obesity. Adult obesity facts). [Google Scholar]
- 4.Powell-Wiley T.M., Poirier P., Burke L.E., Despres J.P., Gordon-Larsen P., Lavie C.J., et al. Obesity and cardiovascular disease: a scientific statement from the American heart association. Circulation. 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anekwe C.V., Jarrell A.R., Townsend M.J., Gaudier G.I., Hiserodt J.M., Stanford F.C. Socioeconomics of obesity. Curr Obes Rep. 2020;9:272–279. doi: 10.1007/s13679-020-00398-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bays H.E., Munoz-Mantilla D.X., Morgan R., Nwizu C., Garcia T.T. Obesity pillars roundtable: obesity and diversity. Obes Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bays H.E., Gonsahn-Bollie S., Younglove C., Wharton S. Obesity Pillars Roundtable: body mass index and body composition in Black and Female individuals. Race-relevant or racist? Sex-relevant or sexist? Obes Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bays H.E., Shrestha A., Niranjan V., Khanna M., Kambhamettu L. Obesity pillars roundtable: obesity and South Asians. Obes Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bays H.E., Ng J., Sicat J., Look M. Obesity pillars roundtable: obesity and East Asians. Obes Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bays H.E., Antoun J., Censani M., Bailony R., Alexander L. Obesity pillars roundtable: obesity and individuals from the Mediterranean region and Middle East. Obes Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woods T., Miljkovic T. Modeling the Economic cost of obesity risk and its relation to the health Insurance Premium in the United States: a state level analysis. Risks. 2022;10:197. [Google Scholar]
- 12.Burridge K., Christensen S.M., Golden A., Ingersoll A.B., Tondt J., Bays H.E. Obesity history, physical exam, laboratory, body composition, and energy expenditure: an Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bays H.E., Jones P.H., Jacobson T.A., Cohen D.E., Orringer C.E., Kothari S., et al. Lipids and bariatric procedures part 1 of 2: scientific statement from the National lipid association, American society for metabolic and bariatric surgery, and obesity medicine association: EXECUTIVE summary. Journal of clinical lipidology. 2016;10:15–32. doi: 10.1016/j.jacl.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Bays H.E., Jones P.H., Jacobson T.A., Cohen D.E., Orringer C.E., Kothari S., et al. Lipids and bariatric procedures part 1 of 2: scientific statement from the National lipid association, American society for metabolic and bariatric surgery, and obesity medicine association: full report. Journal of clinical lipidology. 2016;10:33–57. doi: 10.1016/j.jacl.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Bays H., Kothari S.N., Azagury D.E., Morton J.M., Nguyen N.T., Jones P.H., et al. Lipids and bariatric procedures Part 2 of 2: scientific statement from the American society for metabolic and bariatric surgery (ASMBS), the National lipid association (NLA), and obesity medicine association (OMA). Surgery for obesity and related diseases. official journal of the American Society for Bariatric Surgery. 2016;12:468–495. doi: 10.1016/j.soard.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Bays H.E., Toth P.P., Kris-Etherton P.M., Abate N., Aronne L.J., Brown W.V., et al. Obesity, adiposity, and dyslipidemia: a consensus statement from the National Lipid Association. Journal of clinical lipidology. 2013;7:304–383. doi: 10.1016/j.jacl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Bays H.E., Bindlish S., Clayton T.L. Obesity, diabetes mellitus, and cardiometabolic risk: an obesity medicine association (OMA) clinical practice statement (CPS) 2023. Obes Pillars. 2023;5 doi: 10.1016/j.obpill.2023.100056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clayton T.L., Fitch A., Bays H.E. Obesity and hypertension: obesity medicine association (OMA) clinical practice statement (CPS) 2023. Obesity Pillars. 2023;8 doi: 10.1016/j.obpill.2023.100083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindlish S., Ng J., Ghusn W., Fitch A., Bays H.E. Obesity, thrombosis, venous disease, lymphatic disease, and lipedema: an obesity medicine association (OMA) clinical practice statement (CPS) 2023. Obesity Pillars. 2023;8 doi: 10.1016/j.obpill.2023.100092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abdul Wahab R., le Roux C.W. A review of the evidence on cardiovascular outcomes from obesity treatment. Obesity Pillars. 2023;7 doi: 10.1016/j.obpill.2023.100071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hritani R., Al Rifai M., Mehta A., German C. Obesity management for cardiovascular disease prevention. Obesity Pillars. 2023;7 doi: 10.1016/j.obpill.2023.100069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper GM. The Cell: A Molecular Approach. second ed.; Structure of the Plasma Membrane. Sinauer Associates; Available from: https://wwwncbinlmnihgov/books/NBK9898/.2000..
- 23.de Carvalho C., Caramujo M.J. The various roles of fatty acids. Molecules. 2018;23 doi: 10.3390/molecules23102583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bays H.E., Golden A., Tondt J. Thirty obesity Myths, Misunderstandings, and/or Oversimplifications: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry R., Jeffery E., Rodeheffer M.S. Weighing in on adipocyte precursors. Cell Metabol. 2014;19:8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corvera S. Cellular Heterogeneity in adipose tissues. Annu Rev Physiol. 2021;83:257–278. doi: 10.1146/annurev-physiol-031620-095446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Onal G., Kutlu O., Gozuacik D., Dokmeci Emre S. Lipid droplets in health and disease. Lipids Health Dis. 2017;16:128. doi: 10.1186/s12944-017-0521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olzmann J.A., Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J., Gong L., Liu S., Zhang Y., Zhang C., Tian M., et al. Adipose HuR protects against diet-induced obesity and insulin resistance. Nat Commun. 2019;10:2375. doi: 10.1038/s41467-019-10348-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richard A.J., White U., Elks C.M., Stephens J.M. In: Endotext. Feingold K.R., Anawalt B., Blackman M.R., Boyce A., Chrousos G., Corpas E., et al., editors. 2000. Adipose tissue: physiology to metabolic dysfunction. South Dartmouth (MA) [Google Scholar]
- 31.Krause B.R., Hartman A.D. Adipose tissue and cholesterol metabolism. Journal of lipid research. 1984;25:97–110. [PubMed] [Google Scholar]
- 32.Chung S., Cuffe H., Marshall S.M., McDaniel A.L., Ha J.H., Kavanagh K., et al. Dietary cholesterol promotes adipocyte hypertrophy and adipose tissue inflammation in visceral, but not in subcutaneous, fat in monkeys. Arterioscler Thromb Vasc Biol. 2014;34:1880–1887. doi: 10.1161/ATVBAHA.114.303896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rose AMl, Bazioti V., Westerterp M. Adipocyte membrane cholesterol regulates obesity. Arterioscler Thromb Vasc Biol. 2018;38:687–689. doi: 10.1161/ATVBAHA.118.310768. [DOI] [PubMed] [Google Scholar]
- 34.Chung S., Parks J.S. Dietary cholesterol effects on adipose tissue inflammation. Curr Opin Lipidol. 2016;27:19–25. doi: 10.1097/MOL.0000000000000260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabas I. Consequences of cellular cholesterol accumulation: basic concepts and physiological implications. The Journal of clinical investigation. 2002;110:905–911. doi: 10.1172/JCI16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feingold K.R. In: Endotext. South Dartmouth (MA): MDText.com, Inc. Copyright © 2000-2024. Feingold K.R., Anawalt B., Blackman M.R., Boyce A., Chrousos G., Corpas E., et al., editors. MDText.com, Inc.; 2000. Introduction to lipids and lipoproteins. [Google Scholar]
- 37.Chen Y., Wu Z., Zhao S., Xiang R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci Rep. 2016;6 doi: 10.1038/srep27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menikdiwela K.R., Tôrres Guimarães J.P., Ramalingam L., Kalupahana N.S., Dufour J.M., Washburn R.L., et al. Mechanisms linking endoplasmic reticulum (ER) stress and microRNAs to adipose tissue dysfunction in obesity. Crit Rev Biochem Mol Biol. 2021;56:455–481. doi: 10.1080/10409238.2021.1925219. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S., Sakai J., Fujino T., Hattori H., Zenimaru Y., Suzuki J., et al. The very low-density lipoprotein (VLDL) receptor: characterization and functions as a peripheral lipoprotein receptor. J Atherosclerosis Thromb. 2004;11:200–208. doi: 10.5551/jat.11.200. [DOI] [PubMed] [Google Scholar]
- 40.Kraemer F.B., Sather S.A., Park B., Sztalryd C., Natu V., May K., et al. Low density lipoprotein receptors in rat adipose cells: subcellular localization and regulation by insulin. Journal of lipid research. 1994;35:1760–1772. [PubMed] [Google Scholar]
- 41.Descamps O., Bilheimer D., Herz J. Insulin stimulates receptor-mediated uptake of apoE-enriched lipoproteins and activated alpha 2-macroglobulin in adipocytes. J Biol Chem. 1993;268:974–981. [PubMed] [Google Scholar]
- 42.Hofmann S.M., Zhou L., Perez-Tilve D., Greer T., Grant E., Wancata L., et al. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. The Journal of clinical investigation. 2007;117:3271–3282. doi: 10.1172/JCI31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fazio S., Linton M.F. Unique pathway for cholesterol uptake in fat cells. Arterioscler Thromb Vasc Biol. 2004;24:1538–1539. doi: 10.1161/01.ATV.0000140821.25572.1b. [DOI] [PubMed] [Google Scholar]
- 44.Nunes V.S., da Silva Ferreira G., Quintão E.C.R. Cholesterol metabolism in aging simultaneously altered in liver and nervous system. Aging (Albany NY) 2022;14:1549–1561. doi: 10.18632/aging.203880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dietschy J.M., Turley S.D., Spady D.K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. Journal of lipid research. 1993;34:1637–1659. [PubMed] [Google Scholar]
- 46.Dietschy J.M. Theoretical considerations of what regulates low-density-lipoprotein and high-density-lipoprotein cholesterol. The American journal of clinical nutrition. 1997;65 doi: 10.1093/ajcn/65.5.1581S. 1581s-9s. [DOI] [PubMed] [Google Scholar]
- 47.Jin U., Park S.J., Park S.M. Cholesterol metabolism in the brain and its association with Parkinson's disease. Exp Neurobiol. 2019;28:554–567. doi: 10.5607/en.2019.28.5.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berger J.O., Wang X., Shen L. A Bayesian approach to subgroup identification. J Biopharm Stat. 2014;24:110–129. doi: 10.1080/10543406.2013.856026. [DOI] [PubMed] [Google Scholar]
- 49.Crouse J.R., Grundy S.M., Ahrens E.H., Jr. Cholesterol distribution in the bulk tissues of man: variation with age. The Journal of clinical investigation. 1972;51:1292–1296. doi: 10.1172/JCI106924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hu J., Zhang Z., Shen W.J., Azhar S. Cellular cholesterol delivery, intracellular processing and utilization for biosynthesis of steroid hormones. Nutrition & metabolism. 2010;7:47. doi: 10.1186/1743-7075-7-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovanen P.T., Nikkila E.A., Miettinen T.A. Regulation of cholesterol synthesis and storage in fat cells. Journal of lipid research. 1975;16:211–223. [PubMed] [Google Scholar]
- 52.Grundy S.M. Statins: definitive translational research. Molecular medicine. 2014;20(Suppl 1):S20–S23. doi: 10.2119/molmed.2014.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henney A.E., Gillespie C.S., Alam U., Hydes T.J., Cuthbertson D.J. Ultra-processed food intake is associated with non-alcoholic fatty liver disease in adults: a systematic review and meta-analysis. Nutrients. 2023:15. doi: 10.3390/nu15102266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fon Tacer K., Rozman D. Nonalcoholic fatty liver disease: focus on lipoprotein and lipid Deregulation. Journal of Lipids. 2011;2011 doi: 10.1155/2011/783976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferrer R., Mansilla A., Tondo M., Blanco-Vaca F. Remnant cholesterol: new outcomes highlight its potential as a clinically useful cardiovascular risk factor. Journal of Laboratory and Precision Medicine. 2023;8 [Google Scholar]
- 56.Ginsberg H.N., Packard C.J., Chapman M.J., Borén J., Aguilar-Salinas C.A., Averna M., et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies—a consensus statement from the European Atherosclerosis Society. Eur Heart J. 2021;42:4791–4806. doi: 10.1093/eurheartj/ehab551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Baratta F., Cocomello N., Coronati M., Ferro D., Pastori D., Angelico F., et al. Cholesterol remnants, triglyceride-rich lipoproteins and cardiovascular risk. Int J Mol Sci. 2023:24. doi: 10.3390/ijms24054268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J., et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang K., Wang R., Yang J., Liu X., Shen H., Sun Y., et al. Remnant cholesterol and atherosclerotic cardiovascular disease: metabolism, mechanism, evidence, and treatment. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.913869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lim S.Y., Steiner J.M., Cridge H. Lipases: it's not just pancreatic lipase. American Journal of Veterinary Research. 2022;83 doi: 10.2460/ajvr.22.03.0048. ajvr.22.03.0048. [DOI] [PubMed] [Google Scholar]
- 61.Khetarpal S.A., Vitali C., Levin M.G., Klarin D., Park J., Pampana A., et al. Endothelial lipase mediates efficient lipolysis of triglyceride-rich lipoproteins. PLoS Genet. 2021;17 doi: 10.1371/journal.pgen.1009802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Packer M. Epicardial adipose tissue may mediate Deleterious effects of obesity and inflammation on the Myocardium. J Am Coll Cardiol. 2018;71:2360–2372. doi: 10.1016/j.jacc.2018.03.509. [DOI] [PubMed] [Google Scholar]
- 63.Iacobellis G. Epicardial adipose tissue in contemporary cardiology. Nat Rev Cardiol. 2022;19:593–606. doi: 10.1038/s41569-022-00679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim H.W., Shi H., Winkler M.A., Lee R., Weintraub N.L. Perivascular adipose tissue and vascular Perturbation/atherosclerosis. Arterioscler Thromb Vasc Biol. 2020;40:2569–2576. doi: 10.1161/ATVBAHA.120.312470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kuniyasu A., Hayashi S., Nakayama H. Adipocytes recognize and degrade oxidized low density lipoprotein through CD36. Biochem Biophys Res Commun. 2002;295:319–323. doi: 10.1016/s0006-291x(02)00666-6. [DOI] [PubMed] [Google Scholar]
- 66.Actis Dato V., Chiabrando G.A. The role of low-density lipoprotein receptor-related protein 1 in lipid metabolism, glucose homeostasis and inflammation. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19061780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen L., Peng H., Zhao S., Xu D. A potent soluble epoxide hydrolase inhibitor, t-AUCB, modulates cholesterol balance and oxidized low density lipoprotein metabolism in adipocytes in vitro. Biol Chem. 2014;395:443–451. doi: 10.1515/hsz-2013-0251. [DOI] [PubMed] [Google Scholar]
- 68.Lillis A.P., Van Duyn L.B., Murphy-Ullrich J.E., Strickland D.K. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zanoni P., Velagapudi S., Yalcinkaya M., Rohrer L., von Eckardstein A. Endocytosis of lipoproteins. Atherosclerosis. 2018;275:273–295. doi: 10.1016/j.atherosclerosis.2018.06.881. [DOI] [PubMed] [Google Scholar]
- 70.Zhang T., Chen J., Tang X., Luo Q., Xu D., Yu B. Interaction between adipocytes and high-density lipoprotein:new insights into the mechanism of obesity-induced dyslipidemia and atherosclerosis. Lipids Health Dis. 2019;18:223. doi: 10.1186/s12944-019-1170-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy A.J., Yvan-Charvet L. Adipose modulation of ABCG1 uncovers an intimate link between sphingomyelin and triglyceride storage. Diabetes. 2015;64:689–692. doi: 10.2337/db14-1553. [DOI] [PubMed] [Google Scholar]
- 72.Prattes S., Horl G., Hammer A., Blaschitz A., Graier W.F., Sattler W., et al. Intracellular distribution and mobilization of unesterified cholesterol in adipocytes: triglyceride droplets are surrounded by cholesterol-rich ER-like surface layer structures. J Cell Sci. 2000;113(Pt 17):2977–2989. doi: 10.1242/jcs.113.17.2977. [DOI] [PubMed] [Google Scholar]
- 73.Wang H., Peng D.-Q. New insights into the mechanism of low high-density lipoprotein cholesterol in obesity. Lipids Health Dis. 2011;10:176. doi: 10.1186/1476-511X-10-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schreibman P.H., Dell R.B. Human adipocyte cholesterol. Concentration, localization, synthesis, and turnover. The Journal of clinical investigation. 1975;55:986–993. doi: 10.1172/JCI108028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aguilar D., Fernandez M.L. Hypercholesterolemia induces adipose dysfunction in conditions of obesity and nonobesity. Adv Nutr. 2014;5:497–502. doi: 10.3945/an.114.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Sulaiti H., Diboun I., Banu S., Al-Emadi M., Amani P., Harvey T.M., et al. Triglyceride profiling in adipose tissues from obese insulin sensitive, insulin resistant and type 2 diabetes mellitus individuals. J Transl Med. 2018;16:175. doi: 10.1186/s12967-018-1548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson B.R., Lobo S., Bernlohr D.A. Fatty acid flux in adipocytes: the in's and out's of fat cell lipid trafficking. Mol Cell Endocrinol. 2010;318:24–33. doi: 10.1016/j.mce.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seidelin K.N., Myrup B., Fischer-Hansen B. n−3 fatty acids in adipose tissue and coronary artery disease are inversely related. The American journal of clinical nutrition. 1992;55:1117–1119. doi: 10.1093/ajcn/55.6.1117. [DOI] [PubMed] [Google Scholar]
- 79.Braeckman R.A., Manku M.S., Bays H.E., Stirtan W.G., Soni P.N. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study) Prostaglandins Leukot Essent Fatty Acids. 2013;89:195–201. doi: 10.1016/j.plefa.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 80.Song Y., Jensen M.D. Red blood cell triglycerides-a unique pool that incorporates plasma-free fatty acids and relates to metabolic health. Journal of lipid research. 2021;62 doi: 10.1016/j.jlr.2021.100131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins J.M., Neville M.J., Pinnick K.E., Hodson L., Ruyter B., van Dijk T.H., et al. De novo lipogenesis in the differentiating human adipocyte can provide all fatty acids necessary for maturation. Journal of lipid research. 2011;52:1683–1692. doi: 10.1194/jlr.M012195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Song Z., Xiaoli A.M., Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 2018;10 doi: 10.3390/nu10101383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Basu D., Goldberg I.J. Regulation of lipoprotein lipase-mediated lipolysis of triglycerides. Curr Opin Lipidol. 2020;31:154–160. doi: 10.1097/MOL.0000000000000676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wolska A., Dunbar R.L., Freeman L.A., Ueda M., Amar M.J., Sviridov D.O., et al. Apolipoprotein C-II: new findings related to genetics, biochemistry, and role in triglyceride metabolism. Atherosclerosis. 2017;267:49–60. doi: 10.1016/j.atherosclerosis.2017.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fuior E.V., Gafencu A.V. Apolipoprotein C1: its Pleiotropic effects in lipid metabolism and beyond. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20235939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feingold K.R. In: Endotext. Feingold K.R., Anawalt B., Blackman M.R., Boyce A., Chrousos G., Corpas E., et al., editors. 2000. Obesity and dyslipidemia. South Dartmouth (MA) [Google Scholar]
- 87.Moon J.H., Kim K., Choi S.H. Lipoprotein lipase: is it a magic Target for the treatment of hypertriglyceridemia. Endocrinol Metab (Seoul) 2022;37:575–586. doi: 10.3803/EnM.2022.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hasbargen K.B., Shen W.J., Zhang Y., Hou X., Wang W., Shuo Q., et al. Slc43a3 is a regulator of free fatty acid flux. Journal of lipid research. 2020;61:734–745. doi: 10.1194/jlr.RA119000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kazantzis M., Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim Biophys Acta. 2012;1821:852–857. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhao L., Varghese Z., Moorhead J.F., Chen Y., Ruan X.Z. CD36 and lipid metabolism in the evolution of atherosclerosis. Br Med Bull. 2018;126:101–112. doi: 10.1093/bmb/ldy006. [DOI] [PubMed] [Google Scholar]
- 91.Rupert J.E., Kolonin M.G. Fatty acid translocase: a culprit of lipid metabolism dysfunction in disease. Immunometabolism. 2022;4 doi: 10.1097/IN9.0000000000000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pietka T.A., Schappe T., Conte C., Fabbrini E., Patterson B.W., Klein S., et al. Adipose and muscle tissue profile of CD36 Transcripts in obese subjects Highlights the role of CD36 in fatty acid homeostasis and insulin resistance. Diabetes Care. 2014;37:1990–1997. doi: 10.2337/dc13-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo X., Li Y., Yang P., Chen Y., Wei L., Yu T., et al. Obesity induces preadipocyte CD36 expression promoting inflammation via the disruption of lysosomal calcium homeostasis and lysosome function. EBioMedicine. 2020;56 doi: 10.1016/j.ebiom.2020.102797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cai L., Wang Z., Ji A., Meyer J.M., van der Westhuyzen D.R. Scavenger receptor CD36 expression contributes to adipose tissue inflammation and cell death in diet-induced obesity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0036785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kennedy D.J., Kuchibhotla S., Westfall K.M., Silverstein R.L., Morton R.E., Febbraio M. A CD36-dependent pathway enhances macrophage and adipose tissue inflammation and impairs insulin signalling. Cardiovasc Res. 2010;89:604–613. doi: 10.1093/cvr/cvq360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takeuchi K., Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. American journal of physiology Endocrinology and metabolism. 2009;296:E1195–E1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang H., Eckel R.H. Lipoprotein lipase: from gene to obesity. American journal of physiology Endocrinology and metabolism. 2009;297:E271–E288. doi: 10.1152/ajpendo.90920.2008. [DOI] [PubMed] [Google Scholar]
- 98.McQuaid S.E., Hodson L., Neville M.J., Dennis A.L., Cheeseman J., Humphreys S.M., et al. Downregulation of adipose tissue fatty acid trafficking in obesity: a driver for ectopic fat deposition? Diabetes. 2011;60:47–55. doi: 10.2337/db10-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Aguilar-Recarte D., Palomer X., Vázquez-Carrera M. Uncovering the role of apolipoprotein C-III in insulin resistance. Clín Invest Arterioscler. 2021;33:108–115. doi: 10.1016/j.arteri.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 100.Béliard S., Nogueira J.-P., Maraninchi M., Lairon D., Nicolay A., Giral P., et al. Parallel increase of plasma apoproteins C-II and C-III in Type 2 diabetic patients. Diabet Med : a journal of the British Diabetic Association. 2009;26:736–739. doi: 10.1111/j.1464-5491.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 101.Huang X.S., Zhao S.P., Hu M., Bai L., Zhang Q., Zhao W. Decreased apolipoprotein A5 is implicated in insulin resistance-related hypertriglyceridemia in obesity. Atherosclerosis. 2010;210:563–568. doi: 10.1016/j.atherosclerosis.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 102.Althaher A.R. An overview of hormone-sensitive lipase (HSL) Sci World J. 2022;2022 doi: 10.1155/2022/1964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grabner G.F., Xie H., Schweiger M., Zechner R. Lipolysis: cellular mechanisms for lipid mobilization from fat stores. Nat Metab. 2021;3:1445–1465. doi: 10.1038/s42255-021-00493-6. [DOI] [PubMed] [Google Scholar]
- 104.Yang A., Mottillo E.P. Adipocyte lipolysis: from molecular mechanisms of regulation to disease and therapeutics. Biochem J. 2020;477:985–1008. doi: 10.1042/BCJ20190468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Henkin A.H., Ortegon A.M., Cho S., Shen W.J., Falcon A., Kraemer F.B., et al. Evidence for protein-mediated fatty acid efflux by adipocytes. Acta Physiol. 2012;204:562–570. doi: 10.1111/j.1748-1716.2011.02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fujii H., Kawada N., Japan Study Group Of Nafld J-N The role of insulin resistance and diabetes in Nonalcoholic fatty liver disease. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21113863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tiwari S., Siddiqi S.A. Intracellular trafficking and secretion of VLDL. Arterioscler Thromb Vasc Biol. 2012;32:1079–1086. doi: 10.1161/ATVBAHA.111.241471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., et al. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of cardiology/American heart association Task force on clinical practice guidelines. J Am Coll Cardiol. 2018 2018. [Google Scholar]
- 109.Yanai H., Adachi H., Hakoshima M., Katsuyama H. Postprandial hyperlipidemia: its pathophysiology, diagnosis, Atherogenesis, and treatments. Int J Mol Sci. 2023:24. doi: 10.3390/ijms241813942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Manjunath C.N., Rawal J.R., Irani P.M., Madhu K. Atherogenic dyslipidemia. Indian journal of endocrinology and metabolism. 2013;17:969–976. doi: 10.4103/2230-8210.122600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldberg I.J. Diabetic dyslipidemia: causes and consequences. The Journal of Clinical Endocrinology & Metabolism. 2001;86:965–971. doi: 10.1210/jcem.86.3.7304. [DOI] [PubMed] [Google Scholar]
- 112.Bays H.E., Kulkarni A., German C., Satish P., Iluyomade A., Dudum R., et al. Ten things to know about ten cardiovascular disease risk factors - 2022. Am J Prev Cardiol. 2022;10 doi: 10.1016/j.ajpc.2022.100342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garrison R.J., Wilson P.W., Castelli W.P., Feinleib M., Kannel W.B., McNamara P.M. Obesity and lipoprotein cholesterol in the Framingham offspring study. Metabolism: clinical and experimental. 1980;29:1053–1060. doi: 10.1016/0026-0495(80)90216-4. [DOI] [PubMed] [Google Scholar]
- 114.Lee M.-S., Bensinger S.J. Reprogramming cholesterol metabolism in macrophages and its role in host defense against cholesterol-dependent cytolysins. Cell Mol Immunol. 2022;19:327–336. doi: 10.1038/s41423-021-00827-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Altuzar J., Notbohm J., Stein F., Haberkant P., Hempelmann P., Heybrock S., et al. Lysosome-targeted multifunctional lipid probes reveal the sterol transporter NPC1 as a sphingosine interactor. Proc Natl Acad Sci USA. 2023;120 doi: 10.1073/pnas.2213886120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Staels B., Fonseca V.A. Bile acids and metabolic regulation: mechanisms and clinical responses to bile acid sequestration. Diabetes Care. 2009;32(Suppl 2):S237–S245. doi: 10.2337/dc09-S355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Weng Z.B., Chen Y.R., Lv J.T., Wang M.X., Chen Z.Y., Zhou W., et al. A review of bile acid metabolism and signaling in Cognitive dysfunction-related diseases. Oxid Med Cell Longev. 2022;2022 doi: 10.1155/2022/4289383. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 118.Stadler J.T., Marsche G. Obesity-related changes in high-density lipoprotein metabolism and function. Int J Mol Sci. 2020:21. doi: 10.3390/ijms21238985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nurmohamed N.S., Ditmarsch M., Kastelein J.J.P. Cholesteryl ester transfer protein inhibitors: from high-density lipoprotein cholesterol to low-density lipoprotein cholesterol lowering agents? Cardiovasc Res. 2022;118:2919–2931. doi: 10.1093/cvr/cvab350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Simonen P., Gylling H., Howard A.N., Miettinen T.A. Introducing a new component of the metabolic syndrome: low cholesterol absorption. The American journal of clinical nutrition. 2000;72:82–88. doi: 10.1093/ajcn/72.1.82. [DOI] [PubMed] [Google Scholar]
- 121.Flock M.R., Green M.H., Kris-Etherton P.M. Effects of adiposity on plasma lipid response to reductions in dietary saturated fatty acids and cholesterol. Adv Nutr. 2011;2:261–274. doi: 10.3945/an.111.000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alexander L., Christensen S.M., Richardson L., Ingersoll A.B., Burridge K., Golden A., et al. Nutrition and physical activity: an obesity medicine association (OMA) clinical practice statement 2022. Obesity Pillars. 2022;1 doi: 10.1016/j.obpill.2021.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kirkpatrick C.F., Bolick J.P., Kris-Etherton P.M., Sikand G., Aspry K.E., Soffer D.E., et al. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: a scientific statement from the National Lipid Association Nutrition and Lifestyle Task Force. Journal of clinical lipidology. 2019;13:689–711 e1. doi: 10.1016/j.jacl.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 124.Goldberg I.J., Ibrahim N., Bredefeld C., Foo S., Lim V., Gutman D., et al. Ketogenic diets, not for everyone. Journal of clinical lipidology. 2021;15:61–67. doi: 10.1016/j.jacl.2020.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rajman I., Maxwell S., Cramb R., Kendall M. Particle size: the key to the atherogenic lipoprotein? QJM : monthly journal of the Association of Physicians. 1994;87:709–720. doi: 10.1093/oxfordjournals.qjmed.a068888. [DOI] [PubMed] [Google Scholar]
- 126.Manapurath R.M., Hadaye R., Gadapani B. Normal weight obesity: role of apoB and insulin Sensitivity in predicting future cardiovascular risk. Int J Prev Med. 2022;13:31. doi: 10.4103/ijpvm.IJPVM_139_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cromwell W.C., Otvos J.D., Keyes M.J., Pencina M.J., Sullivan L., Vasan R.S., et al. LDL particle number and risk of future cardiovascular disease in the Framingham offspring study - implications for LDL management. Journal of clinical lipidology. 2007;1:583–592. doi: 10.1016/j.jacl.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jacobson T.A., Ito M.K., Maki K.C., Orringer C.E., Bays H.E., Jones P.H., et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 1--full report. Journal of clinical lipidology. 2015;9:129–169. doi: 10.1016/j.jacl.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 129.Bays H.E., Jones P.H., Orringer C.E., Brown W.V., Jacobson T.A. National lipid association annual summary of clinical Lipidology 2016. Journal of clinical lipidology. 2016;10:S1–S43. doi: 10.1016/j.jacl.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 130.Catapano A.L., Graham I., De Backer G., Wiklund O., Chapman M.J., Drexel H., et al. ESC/EAS guidelines for the management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. 2016. [DOI] [PubMed] [Google Scholar]
- 131.Bays H.E., Jones P.H., Brown W.V., Jacobson T.A. National lipid A. National lipid association annual summary of clinical Lipidology 2015. Journal of clinical lipidology. 2014;8 doi: 10.1016/j.jacl.2014.10.002. S1-36. [DOI] [PubMed] [Google Scholar]
- 132.Yu L., Fink B.D., Herlein J.A., Oltman C.L., Lamping K.G., Sivitz W.I. Dietary fat, fatty acid saturation and mitochondrial bioenergetics. J Bioenerg Biomembr. 2014;46:33–44. doi: 10.1007/s10863-013-9530-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.de Mello A.H., Costa A.B., Engel J.D.G., Rezin G.T. Mitochondrial dysfunction in obesity. Life Sci. 2018;192:26–32. doi: 10.1016/j.lfs.2017.11.019. [DOI] [PubMed] [Google Scholar]
- 134.Rodgers S.J., Ferguson D.T., Mitchell C.A., Ooms L.M. Regulation of PI3K effector signalling in cancer by the phosphoinositide phosphatases. Biosci Rep. 2017;37 doi: 10.1042/BSR20160432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Prior I.A., Hood F.E., Hartley J.L. The Frequency of Ras Mutations in cancer. Cancer Res. 2020;80:2969–2974. doi: 10.1158/0008-5472.CAN-19-3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lazarus E., Bays H.E. Cancer and obesity: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chan W.K., Chuah K.H., Rajaram R.B., Lim L.L., Ratnasingam J., Vethakkan S.R. Metabolic dysfunction-associated steatotic liver disease (MASLD): a state-of-the-Art review. J Obes Metab Syndr. 2023;32:197–213. doi: 10.7570/jomes23052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tondt J., Bays H.E. Concomitant medications, functional foods, and supplements: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Christensen S.M., Varney C., Gupta V., Wenz L., Bays H.E. Stress, psychiatric disease, and obesity: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ghusn W., Hurtado M.D., Acosta A. Weight-centric treatment of type 2 diabetes mellitus. Obesity Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghusn W., Bouchard C., Frye M.A., Acosta A. Weight-centric treatment of depression and chronic pain. Obesity Pillars. 2022;3 doi: 10.1016/j.obpill.2022.100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Klop B., Elte J.W., Cabezas M.C. Dyslipidemia in obesity: mechanisms and potential targets. Nutrients. 2013;5:1218–1240. doi: 10.3390/nu5041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yu Y.-H., Ginsberg H.N. Adipocyte signaling and lipid homeostasis. Circ Res. 2005;96:1042–1052. doi: 10.1161/01.RES.0000165803.47776.38. [DOI] [PubMed] [Google Scholar]
- 144.Beutler B., Greenwald D., Hulmes J.D., Chang M., Pan Y.C., Mathison J., et al. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 145.Yanai H., Yoshida H. Beneficial effects of adiponectin on glucose and lipid metabolism and atherosclerotic progression: mechanisms and perspectives. Int J Mol Sci. 2019;20:1190. doi: 10.3390/ijms20051190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Maingrette F., Gv Renier. Leptin increases lipoprotein lipase secretion by macrophages: Involvement of oxidative stress and protein kinase C. Diabetes. 2003;52:2121–2128. doi: 10.2337/diabetes.52.8.2121. [DOI] [PubMed] [Google Scholar]
- 147.Kjeldsen E.W., Thomassen J.Q., Frikke-Schmidt R. HDL cholesterol concentrations and risk of atherosclerotic cardiovascular disease - insights from randomized clinical trials and human genetics. Biochim Biophys Acta Mol Cell Biol Lipids. 2022;1867 doi: 10.1016/j.bbalip.2021.159063. [DOI] [PubMed] [Google Scholar]
- 148.Pagliai G., Dinu M., Madarena M.P., Bonaccio M., Iacoviello L., Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. The British journal of nutrition. 2021;125:308–318. doi: 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Siri-Tarino P.W., Sun Q., Hu F.B., Krauss R.M. Saturated fat, carbohydrate, and cardiovascular disease. The American journal of clinical nutrition. 2010;91:502–509. doi: 10.3945/ajcn.2008.26285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.O'Donovan G., Stensel D., Hamer M., Stamatakis E. The association between leisure-time physical activity, low HDL-cholesterol and mortality in a pooled analysis of nine population-based cohorts. Eur J Epidemiol. 2017;32:559–566. doi: 10.1007/s10654-017-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yang H., Fogo A.B., Kon V. Kidneys: key modulators of high-density lipoprotein levels and function. Curr Opin Nephrol Hypertens. 2016;25:174–179. doi: 10.1097/MNH.0000000000000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phillips M.C. Is ABCA1 a lipid transfer protein? Journal of lipid research. 2018;59:749–763. doi: 10.1194/jlr.R082313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mooradian A.D., Haas M.J., Wong N.C.W. Transcriptional control of apolipoprotein A-I gene expression in diabetes. Diabetes. 2004;53:513–520. doi: 10.2337/diabetes.53.3.513. [DOI] [PubMed] [Google Scholar]
- 154.Liu C., Dhindsa D., Almuwaqqat Z., Sun Y.V., Quyyumi A.A. Very high high-density lipoprotein cholesterol levels and cardiovascular mortality. Am J Cardiol. 2022;167:43–53. doi: 10.1016/j.amjcard.2021.11.041. [DOI] [PubMed] [Google Scholar]
- 155.Kim H.J., Jeong S., Oh Y.H., Park S.J., Cho Y., Park S.M. Changes in high-density lipoprotein cholesterol with risk of Cardiovascular Disease among initially high-density lipoprotein-high participants. Cardiovasc Diabetol. 2023;22:71. doi: 10.1186/s12933-023-01805-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu C., Dhindsa D., Almuwaqqat Z., Ko Y.-A., Mehta A., Alkhoder A.A., et al. Association between high-density lipoprotein cholesterol levels and adverse cardiovascular outcomes in high-risk populations. JAMA Cardiology. 2022;7:672–680. doi: 10.1001/jamacardio.2022.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Madsen C.M., Varbo A., Nordestgaard B.G. Novel insights from human studies on the role of high-density lipoprotein in mortality and Noncardiovascular disease. Arterioscler Thromb Vasc Biol. 2021;41:128–140. doi: 10.1161/ATVBAHA.120.314050. [DOI] [PubMed] [Google Scholar]
- 158.Braga P.G.S., Freitas F.R., Bachi A.L.L., Amirato G.R., Baroni R.V., Alves M., et al. Regular practice of physical activity improves cholesterol transfers to high-density lipoprotein (HDL) and other HDL metabolic parameters in older adults. Nutrients. 2023;15 doi: 10.3390/nu15234871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Teng R.-L., Wang H., Sun B.-C., Cai D.-P., He Y.-M. Interaction between lipoprotein (a) levels and body mass index in first incident acute myocardial infarction. BMC Cardiovasc Disord. 2020;20:350. doi: 10.1186/s12872-020-01626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Spalding K.L., Arner E., Westermark P.O., Bernard S., Buchholz B.A., Bergmann O., et al. Dynamics of fat cell turnover in humans. Nature. 2008;453:783–787. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 161.Arner P. Fat tissue growth and development in humans. Nestle Nutr Inst Workshop Ser. 2018;89:37–45. doi: 10.1159/000486491. [DOI] [PubMed] [Google Scholar]
- 162.Salans L.B., Cushman S.W., Weismann R.E. Studies of human adipose tissue. Adipose cell size and number in nonobese and obese patients. The Journal of clinical investigation. 1973;52:929–941. doi: 10.1172/JCI107258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lopez-Jimenez F., Almahmeed W., Bays H., Cuevas A., Di Angelantonio E., le Roux C.W., et al. Obesity and cardiovascular disease: mechanistic insights and management strategies. A joint position paper by the World Heart Federation and World Obesity Federation. European journal of preventive cardiology. 2022;29:2218–2237. doi: 10.1093/eurjpc/zwac187. [DOI] [PubMed] [Google Scholar]
- 164.Bays H. Adiposopathy, "sick fat," Ockham's razor, and resolution of the obesity paradox. Curr Atherosclerosis Rep. 2014;16:409. doi: 10.1007/s11883-014-0409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bays H.E. Adiposopathy is "sick fat" a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–2473. doi: 10.1016/j.jacc.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 166.Chait A., den Hartigh L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med. 2020;7:22. doi: 10.3389/fcvm.2020.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guzman N., Vijayan V. StatPearls. Treasure Island (FL; 2023. HIV-associated lipodystrophy. [PubMed] [Google Scholar]
- 168.Troll J.G. Approach to dyslipidemia, lipodystrophy, and cardiovascular risk in patients with HIV infection. Curr Atherosclerosis Rep. 2011;13:51–56. doi: 10.1007/s11883-010-0152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Heilbronn L., Smith S.R., Ravussin E. Failure of fat cell proliferation, mitochondrial function and fat oxidation results in ectopic fat storage, insulin resistance and type II diabetes mellitus. Int J Obes Relat Metab Disord : journal of the International Association for the Study of Obesity. 2004;28(Suppl 4):S12–S21. doi: 10.1038/sj.ijo.0802853. [DOI] [PubMed] [Google Scholar]
- 170.Wasilewska N., Lebensztejn D.M. Non-alcoholic fatty liver disease and lipotoxicity. Clin Exp Hepatol. 2021;7:1–6. doi: 10.5114/ceh.2021.104441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Unger R.H., Clark G.O., Scherer P.E., Orci L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim Biophys Acta Mol Cell Biol Lipids. 2010;1801:209–214. doi: 10.1016/j.bbalip.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 172.Lipke K., Kubis-Kubiak A., Piwowar A. Molecular mechanism of lipotoxicity as an interesting Aspect in the development of Pathological States-current View of knowledge. Cells. 2022;11 doi: 10.3390/cells11050844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Nishi H., Higashihara T., Inagi R. Lipotoxicity in kidney, heart, and skeletal muscle dysfunction. Nutrients. 2019;11 doi: 10.3390/nu11071664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ertunc M.E., Hotamisligil G.S. Lipid signaling and lipotoxicity in metaflammation: indications for metabolic disease pathogenesis and treatment. Journal of lipid research. 2016;57:2099–2114. doi: 10.1194/jlr.R066514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kim J.A., Montagnani M., Chandrasekran S., Quon M.J. Role of lipotoxicity in endothelial dysfunction. Heart Fail Clin. 2012;8:589–607. doi: 10.1016/j.hfc.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ye R., Onodera T., Scherer P.E. Lipotoxicity and β cell maintenance in obesity and type 2 diabetes. Journal of the Endocrine Society. 2019;3:617–631. doi: 10.1210/js.2018-00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Grundy S.M., Neeland I.J., Turer A.T., Vega G.L. Waist circumference as measure of abdominal fat compartments. Journal of obesity. 2013;2013 doi: 10.1155/2013/454285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bays H. Central obesity as a clinical marker of adiposopathy; increased visceral adiposity as a surrogate marker for global fat dysfunction. Curr Opin Endocrinol Diabetes Obes. 2014;21:345–351. doi: 10.1097/MED.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Luo Y., Ma X., Shen Y., Hao Y., Hu Y., Xiao Y., et al. Positive relationship between serum low-density lipoprotein cholesterol levels and visceral fat in a Chinese nondiabetic population. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Appel L.J., Sacks F.M., Carey V.J., Obarzanek E., Swain J.F., Miller E.R., 3rd, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA, J Am Med Assoc. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 181.Brien S.E., Ronksley P.E., Turner B.J., Mukamal K.J., Ghali W.A. Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. Bmj. 2011;342:d636. doi: 10.1136/bmj.d636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Contaldo F., D'Arrigo E., Carandente V., Cortese C., Coltorti A., Mancini M., et al. Short-term effects of moderate alcohol consumption on lipid metabolism and energy balance in normal men. Metabolism: clinical and experimental. 1989;38:166–171. doi: 10.1016/0026-0495(89)90257-6. [DOI] [PubMed] [Google Scholar]
- 183.Crouse J.R., Grundy S.M. Effects of alcohol on plasma lipoproteins and cholesterol and triglyceride metabolism in man. Journal of lipid research. 1984;25:486–496. [PubMed] [Google Scholar]
- 184.Maki K.C., Palacios O.M., Lindner E., Nieman K.M., Bell M., Sorce J. Replacement of refined starches and added sugars with Egg protein and unsaturated fats increases insulin Sensitivity and lowers triglycerides in overweight or obese adults with elevated triglycerides. The Journal of nutrition. 2017;147:1267–1274. doi: 10.3945/jn.117.248641. [DOI] [PubMed] [Google Scholar]
- 185.Mensink R.P., Zock P.L., Kester A.D., Katan M.B. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. The American journal of clinical nutrition. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 186.Belardo D., Michos E.D., Blankstein R., Blumenthal R.S., Ferdinand K.C., Hall K., et al. Practical, evidence-based Approaches to nutritional modifications to reduce atherosclerotic cardiovascular disease: an American society for preventive cardiology clinical practice statement. American Journal of Preventive Cardiology. 2022;10 doi: 10.1016/j.ajpc.2022.100323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Trautwein E.A., McKay S. The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients. 2020;12 doi: 10.3390/nu12092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Ma Y., Li Y., Chiriboga D.E., Olendzki B.C., Hebert J.R., Li W., et al. Association between carbohydrate intake and serum lipids. J Am Coll Nutr. 2006;25:155–163. doi: 10.1080/07315724.2006.10719527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ginsberg H.N., Karmally W., Barr S.L., Johnson C., Holleran S., Ramakrishnan R. Effects of increasing dietary polyunsaturated fatty acids within the guidelines of the AHA step 1 diet on plasma lipid and lipoprotein levels in normal males. Arterioscler Thromb. 1994;14:892–901. doi: 10.1161/01.atv.14.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dattilo A.M., Kris-Etherton P.M. Effects of weight reduction on blood lipids and lipoproteins: a meta-analysis. The American journal of clinical nutrition. 1992;56:320–328. doi: 10.1093/ajcn/56.2.320. [DOI] [PubMed] [Google Scholar]
- 191.Zomer E., Gurusamy K., Leach R., Trimmer C., Lobstein T., Morris S., et al. Interventions that cause weight loss and the impact on cardiovascular risk factors: a systematic review and meta-analysis. Obes Rev : an official journal of the International Association for the Study of Obesity. 2016;17:1001–1011. doi: 10.1111/obr.12433. [DOI] [PubMed] [Google Scholar]
- 192.Hasan B., Nayfeh T., Alzuabi M., Wang Z., Kuchkuntla A.R., Prokop L.J., et al. Weight loss and serum lipids in overweight and obese adults: a systematic review and meta-analysis. The Journal of clinical endocrinology and metabolism. 2020:105. doi: 10.1210/clinem/dgaa673. [DOI] [PubMed] [Google Scholar]
- 193.Sahebkar A., Simental-Mendía L.E., Reiner Ž., Kovanen P.T., Simental-Mendía M., Bianconi V., et al. Effect of orlistat on plasma lipids and body weight: a systematic review and meta-analysis of 33 randomized controlled trials. Pharmacol Res. 2017;122:53–65. doi: 10.1016/j.phrs.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 194.Lei X.-G., Ruan J.-Q., Lai C., Sun Z., Yang X. Efficacy and safety of Phentermine/Topiramate in adults with overweight or obesity: a systematic review and meta-analysis. Obesity. 2021;29:985–994. doi: 10.1002/oby.23152. [DOI] [PubMed] [Google Scholar]
- 195.Vorsanger M.H., Subramanyam P., Weintraub H.S., Lamm S.H., Underberg J.A., Gianos E., et al. Cardiovascular effects of the new weight loss agents. J Am Coll Cardiol. 2016;68:849–859. doi: 10.1016/j.jacc.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 196.Sun F., Chai S., Li L., Yu K., Yang Z., Wu S., et al. Effects of glucagon-like peptide-1 receptor agonists on weight loss in patients with type 2 diabetes: a systematic review and network meta-analysis. J Diabetes Res. 2015;2015 doi: 10.1155/2015/157201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lincoff A.M., Brown-Frandsen K., Colhoun H.M., Deanfield J., Emerson S.S., Esbjerg S., et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023 doi: 10.1056/NEJMoa2307563. [DOI] [PubMed] [Google Scholar]
- 198.Jastreboff A.M., Aronne L.J., Ahmad N.N., Wharton S., Connery L., Alves B., et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387:205–216. doi: 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 199.Piche M.E., Tardif I., Auclair A., Poirier P. Effects of bariatric surgery on lipid-lipoprotein profile. Metabolism: clinical and experimental. 2021;115 doi: 10.1016/j.metabol.2020.154441. [DOI] [PubMed] [Google Scholar]
- 200.Palumbo P., Banchelli F., Miloro C., Toschi P.F., Mecheri F., Gabriele S., et al. Weight loss trend after bariatric surgery in a population of obese patients. Clinical Nutrition ESPEN. 2023;57:58–64. doi: 10.1016/j.clnesp.2023.06.015. [DOI] [PubMed] [Google Scholar]
- 201.Erdmann J., Lippl F., Klose G., Schusdziarra V. Cholesterol lowering effect of dietary weight loss and orlistat treatment--efficacy and limitations. Alimentary pharmacology & therapeutics. 2004;19:1173–1179. doi: 10.1111/j.1365-2036.2004.01966.x. [DOI] [PubMed] [Google Scholar]
- 202.Shetye B., Hamilton F.R., Bays H.E. Bariatric surgery, gastrointestinal hormones, and the microbiome: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sikand G., Severson T. Top 10 dietary strategies for atherosclerotic cardiovascular risk reduction. Am J Prev Cardiol. 2020;4 doi: 10.1016/j.ajpc.2020.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kirkpatrick C.F., Sikand G., Petersen K.S., Anderson C.A.M., Aspry K.E., Bolick J.P., et al. Nutrition interventions for adults with dyslipidemia: a clinical perspective from the National lipid association. Journal of clinical lipidology. 2023;17:428–451. doi: 10.1016/j.jacl.2023.05.099. [DOI] [PubMed] [Google Scholar]
- 205.Carta G., Murru E., Banni S., Manca C. Palmitic acid: physiological role, metabolism and nutritional implications. Front Physiol. 2017;8 doi: 10.3389/fphys.2017.00902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Piercy K.L., Troiano R.P., Ballard R.M., Carlson S.A., Fulton J.E., Galuska D.A., et al. The physical activity guidelines for Americans. JAMA, J Am Med Assoc. 2018;320:2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Paluch A.E., Boyer W.R., Franklin B.A., Laddu D., Lobelo F., Lee D.C., et al. Resistance exercise training in individuals with and without cardiovascular disease: 2023 update: a scientific statement from the American heart association. Circulation. 2023 doi: 10.1161/CIR.0000000000001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Wang Y., Xu D. Effects of aerobic exercise on lipids and lipoproteins. Lipids Health Dis. 2017;16:132. doi: 10.1186/s12944-017-0515-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mann S., Beedie C., Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: review, synthesis and recommendations. Sports Med. 2014;44:211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Surampudi V., Biggs K., Zhaoping L. Weight loss and transient LDL increase. Curr Dev Nutr. 2019;3 [Google Scholar]
- 211.Phinney S.D., Tang A.B., Waggoner C.R., Tezanos-Pinto R.G., Davis P.A. The transient hypercholesterolemia of major weight loss. The American journal of clinical nutrition. 1991;53:1404–1410. doi: 10.1093/ajcn/53.6.1404. [DOI] [PubMed] [Google Scholar]
- 212.Brown J.D., Buscemi J., Milsom V., Malcolm R., O'Neil P.M. Effects on cardiovascular risk factors of weight losses limited to 5-10. Translational behavioral medicine. 2016;6:339–346. doi: 10.1007/s13142-015-0353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Fleming R.M. The effect of high-, moderate-, and low-fat diets on weight loss and cardiovascular disease risk factors. Prev Cardiol. 2002;5:110–118. doi: 10.1111/j.1520-037x.2002.01231.x. [DOI] [PubMed] [Google Scholar]
- 214.Follick M.J., Abrams D.B., Smith T.W., Henderson L.O., Herbert P.N. Contrasting short- and long-term effects of weight loss on lipoprotein levels. Arch Intern Med. 1984;144:1571–1574. [PubMed] [Google Scholar]
- 215.Moradi M., Mahmoudi M., Saedisomeolia A., Zahirihashemi R., Koohdani F. The effect of weight loss on HDL subfractions and LCAT activity in two genotypes of APOA-II -265T>C polymorphism. Nutr J. 2017;16:34. doi: 10.1186/s12937-017-0255-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Look A.R.G., Wing R.R., Bolin P., Brancati F.L., Bray G.A., Clark J.M., et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Wing R.R., Lang W., Wadden T.A., Safford M., Knowler W.C., Bertoni A.G., et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dansinger M.L., Williams P.T., Superko H.R., Schaefer E.J. Effects of weight change on apolipoprotein B-containing emerging atherosclerotic cardiovascular disease (ASCVD) risk factors. Lipids Health Dis. 2019;18:154. doi: 10.1186/s12944-019-1094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ryan D.H., Yockey S.R. Weight loss and improvement in Comorbidity: Differences at 5%, 10%, 15%, and over. Current obesity reports. 2017;6:187–194. doi: 10.1007/s13679-017-0262-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bays H.E., Fitch A., Christensen S., Burridge K., Tondt J. Anti-obesity medications and Investigational agents: an obesity medicine association (OMA) clinical practice statement (CPS) 2022. Obes Pillars. 2022;2 doi: 10.1016/j.obpill.2022.100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Maki K.C., Kirkpatrick C.F., Allison D.B., Gadde K.M. Pharmacotherapy for obesity: recent evolution and implications for cardiovascular risk reduction. Expert Rev Endocrinol Metab. 2023;18:307–319. doi: 10.1080/17446651.2023.2209176. [DOI] [PubMed] [Google Scholar]
- 222.Bays H.E., Burridge K., Richards J., Fitch A. Obesity Pillars roundtable: excessive weight reduction with highly effective anti-obesity medications (heAOMs) Obes Pillars. 2022;4 doi: 10.1016/j.obpill.2022.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ahmad N.N., Robinson S., Kennedy-Martin T., Poon J.L., Kan H. Clinical outcomes associated with anti-obesity medications in real-world practice: a systematic literature review. Obes Rev : an official journal of the International Association for the Study of Obesity. 2021;22 doi: 10.1111/obr.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ho J.H., Adam S., Liu Y., Azmi S., Dhage S., Syed A.A., et al. Effect of bariatric surgery on plasma levels of oxidised phospholipids, biomarkers of oxidised LDL and lipoprotein(a) Journal of clinical lipidology. 2021;15:320–331. doi: 10.1016/j.jacl.2020.12.002. [DOI] [PubMed] [Google Scholar]
- 225.Jamialahmadi T., Reiner Ž., Alidadi M., Kroh M., Almahmeed W., Ruscica M., et al. The effect of bariatric surgery on circulating levels of lipoprotein (a): a meta-analysis. BioMed Res Int. 2022;2022 doi: 10.1155/2022/8435133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Berk K.A., Borgeraas H., Narverud I., Mulder M.T., Øyri L.K.L., Verhoeven A.J.M., et al. Differential effects of bariatric surgery and lifestyle interventions on plasma levels of Lp(a) and fatty acids. Lipids Health Dis. 2022;21:145. doi: 10.1186/s12944-022-01756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lin B.X., Weiss M.C., Parikh M., Berger J.S., Fisher E.A., Heffron S.P. Changes in lipoprotein(a) following bariatric surgery. Am Heart J. 2018;197:175–176. doi: 10.1016/j.ahj.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Sjostrom L., Narbro K., Sjostrom C.D., Karason K., Larsson B., Wedel H., et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. doi: 10.1056/NEJMoa066254. [DOI] [PubMed] [Google Scholar]
- 229.Sjostrom L., Lindroos A.K., Peltonen M., Torgerson J., Bouchard C., Carlsson B., et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med. 2004;351:2683–2693. doi: 10.1056/NEJMoa035622. [DOI] [PubMed] [Google Scholar]
- 230.Srinivasan M., Thangaraj S.R., Arzoun H., Thomas S.S., Mohammed L. The impact of bariatric surgery on cardiovascular risk factors and outcomes: a systematic review. Cureus. 2022;14 doi: 10.7759/cureus.23340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Poirier P., Cornier M.-A., Mazzone T., Stiles S., Cummings S., Klein S., et al. Bariatric surgery and cardiovascular risk factors. Circulation. 2011;123:1683–1701. doi: 10.1161/CIR.0b013e3182149099. [DOI] [PubMed] [Google Scholar]
- 232.Ahammed M.R., Ananya F.N. Impact of weight loss on atrial fibrillation. Cureus. 2023;15 doi: 10.7759/cureus.46232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.van Veldhuisen S.L., Gorter T.M., van Woerden G., de Boer R.A., Rienstra M., Hazebroek E.J., et al. Bariatric surgery and cardiovascular disease: a systematic review and meta-analysis. Eur Heart J. 2022 doi: 10.1093/eurheartj/ehac071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sabatine M.S., Giugliano R.P., Keech A.C., Honarpour N., Wiviott S.D., Murphy S.A., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 235.Schwartz G.G., Steg P.G., Szarek M., Bhatt D.L., Bittner V.A., Diaz R., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]
- 236.Cholesterol Treatment Trialists C., Baigent C., Blackwell L., Emberson J., Holland L.E., Reith C., et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.O'Dea K. Cardiovascular disease risk factors in Australian aborigines. Clinical and experimental pharmacology & physiology. 1991;18:85–88. doi: 10.1111/j.1440-1681.1991.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 238.O'Keefe J.H., Jr., Cordain L., Harris W.H., Moe R.M., Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43:2142–2146. doi: 10.1016/j.jacc.2004.03.046. [DOI] [PubMed] [Google Scholar]
- 239.Walker A.R. Are health and ill-health lessons from hunter-gatherers currently relevant? The American journal of clinical nutrition. 2001;73:353–356. doi: 10.1093/ajcn/73.2.353. [DOI] [PubMed] [Google Scholar]
- 240.Henning R.J. Obesity and obesity-induced inflammatory disease contribute to atherosclerosis: a review of the pathophysiology and treatment of obesity. Am J Cardiovasc Dis. 2021;11:504–529. [PMC free article] [PubMed] [Google Scholar]
- 241.Gao Y., Shah L.M., Ding J., Martin S.S. US Trends in cholesterol Screening, lipid levels, and lipid‐lowering medication Use in US adults, 1999 to 2018. J Am Heart Assoc. 2023;12 doi: 10.1161/JAHA.122.028205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Pontzer H., Wood B.M. Effects of evolution, Ecology, and Economy on human diet: insights from hunter-gatherers and other small-Scale societies. Annu Rev Nutr. 2021;41:363–385. doi: 10.1146/annurev-nutr-111120-105520. [DOI] [PubMed] [Google Scholar]
- 243.Pontzer H., Raichlen D.A., Wood B.M., Mabulla A.Z., Racette S.B., Marlowe F.W. Hunter-gatherer energetics and human obesity. PLoS One. 2012;7 doi: 10.1371/journal.pone.0040503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Domanski M.J., Tian X., Wu C.O., Reis J.P., Dey A.K., Gu Y., et al. Time course of LDL cholesterol exposure and cardiovascular disease event risk. J Am Coll Cardiol. 2020;76:1507–1516. doi: 10.1016/j.jacc.2020.07.059. [DOI] [PubMed] [Google Scholar]
- 245.Rajjo T., Almasri J., Al Nofal A., Farah W., Alsawas M., Ahmed A.T., et al. The association of weight loss and cardiometabolic outcomes in obese children: systematic review and meta-regression. The Journal of Clinical Endocrinology & Metabolism. 2016;102:758–762. doi: 10.1210/jc.2016-2575. [DOI] [PubMed] [Google Scholar]
- 246.Tajeu G.S., Johnson E., Buccilla M., Gadegbeku C.A., Janick S., Rubin D., et al. Changes in antihypertensive medication following bariatric surgery. Obes Surg. 2022;32:1312–1324. doi: 10.1007/s11695-022-05893-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chumakova-Orin M., Vanetta C., Moris D.P., Guerron A.D. Diabetes remission after bariatric surgery. World J Diabetes. 2021;12:1093–1101. doi: 10.4239/wjd.v12.i7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Howard R., Chao G.F., Yang J., Thumma J.R., Arterburn D.E., Telem D.A., et al. Medication Use for obesity-related Comorbidities after sleeve gastrectomy or gastric bypass. JAMA surgery. 2022;157:248–256. doi: 10.1001/jamasurg.2021.6898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Juray S., Axen K.V., Trasino S.E. Remission of type 2 diabetes with very low-calorie diets-A Narrative review. Nutrients. 2021;13 doi: 10.3390/nu13062086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Leslie W.S., Ali E., Harris L., Messow C.M., Brosnahan N.T., Thom G., et al. Antihypertensive medication needs and blood pressure control with weight loss in the Diabetes Remission Clinical Trial (DiRECT) Diabetologia. 2021;64:1927–1938. doi: 10.1007/s00125-021-05471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Ramos Salas X., Saquimux Contreras M.A., Breen C., Preiss Y., Hussey B., Forhan M., et al. Review of an international pilot project to adapt the Canadian adult obesity clinical practice guideline. Obesity Pillars. 2023;8 doi: 10.1016/j.obpill.2023.100090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sikand G., Cole R.E., Handu D., deWaal D., Christaldi J., Johnson E.Q., et al. Clinical and cost benefits of medical nutrition therapy by registered dietitian nutritionists for management of dyslipidemia: a systematic review and meta-analysis. Journal of clinical lipidology. 2018;12:1113–1122. doi: 10.1016/j.jacl.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 253.Graham R., Mancher M., Miller Wolman D., Greenfield S., Steinberg E. National Academies Press; Washington (DC: 2011. Clinical practice guidelines We can Trust. [PubMed] [Google Scholar]