Key Points
Question
Is the efficacy of dequalinium chloride comparable to that of oral metronidazole for the treatment of bacterial vaginosis?
Findings
In this randomized clinical trial of 147 premenopausal women with bacterial vaginosis, the difference in the clinical cure rate between dequalinium chloride and metronidazole was −0.5. The results are statistically significant and indicate the noninferiority of dequalinium chloride.
Meaning
These findings indicate that treatment with dequalinium chloride is as effective as oral metronidazole for curing bacterial vaginosis, thus showing similar efficacy to first-line antibiotic treatments.
This randomized clinical trial examines the comparability of dequalinium chloride with metronidazole in the treatment of bacterial vaginosis among premenopausal women.
Abstract
Importance
Bacterial vaginosis (BV) is a common cause of vaginal infection. First-line treatments of BV are metronidazole and clindamycin. Due to the increase in antibiotic resistance, effective nonantibiotic treatments for BV are needed.
Objective
To examine whether dequalinium chloride, a broad-spectrum antiseptic, is noninferior to oral metronidazole for the treatment of BV.
Design, Setting, and Participants
This phase 4, multicenter, triple-blind, double-dummy, parallel, noninferiority randomized clinical trial was conducted from July 29, 2021, to August 25, 2022, with a 1-month follow-up. Participants were premenopausal women 18 years or older with BV from 11 gynecologic practices and 1 hospital in Poland, Slovakia, and the Czech.
Intervention
Patients were randomized to treatment with dequalinium chloride vaginal tablets (10 mg once daily for 6 days) or oral metronidazole (500 mg twice daily for 7 days). Double-dummy medication kits contained vaginal and oral tablets with placebo and active medication.
Main Outcomes and Measures
The main outcome was the noninferiority margin (of 15 percentage points) in the absolute difference in clinical cure rates between dequalinium chloride and metronidazole 7 to 11 days after start of treatment (visit 1). Noninferiority was met if the lower 95% CI for the difference in clinical cure rate was less than 15 percentage points at visit 1.
Results
A total of 147 women (mean [SD] age, 36.7 [9.0] years) were treated with dequalinium chloride (n = 72) or metronidazole (n = 75). The clinical cure rates at visit 1 were 64 of 69 (92.8%) for dequalinium chloride vs 69 of 74 (93.2%) for metronidazole in the intention-to-treat population, whereas in the per-protocol population, cure rates were 54 of 58 (93.1%) for dequalinium chloride vs 48 of 53 (90.6%) for metronidazole. The treatment differences of −0.5 percentage points (95% CI, −10.8 to 9.8 percentage points; P = .002) in the intention-to-treat population and 2.5 percentage points (95% CI, −9.4 to 14.4 percentage points; P = .001) in the per-protocol population confirmed the noninferiority of dequalinium chloride. The tolerability of dequalinium chloride was rated as very good by 30 of 50 patients (60.0%) but only by 21 of 54 (38.9%) for metronidazole. Three patients in the metronidazole group suspended treatment due to an adverse event.
Conclusions and Relevance
This randomized clinical trial showed that dequalinium chloride was not inferior to metronidazole for the treatment of BV. Dequalinium chloride had a similarly high cure rate but with better tolerability and fewer adverse events. With a similar efficacy to metronidazole and clindamycin, dequalinium chloride warrants consideration as first-line treatment for BV to help reduce antibiotic consumption.
Trial Registration
EudraCT: 2020-002489-15
Introduction
Bacterial vaginosis (BV) remains a common and recurrent cause of vaginal infection in women of reproductive age,1,2 with substantial impact in quality of life and health care costs.2,3,4 The clinical diagnosis of BV is based on the presence of at least 3 Amsel criteria: characteristic vaginal discharge; “fishy” smell, clue cells; and vaginal pH greater than 4.5.5 Bacterial vaginosis can also be diagnosed using the Nugent score, a microscopic assessment of a Gram staining of the vaginal discharge, rarely performed at the point of care because it requires more time, trained personnel, and specialized equipment.6,7
First-line treatments for BV are metronidazole and clindamycin.8,9,10,11 Their 1- to 4-week cure rate is 55% to 90%,12,13,14,15 but the recurrence rate within 6 months is 30% to 70%.13,16 This high recurrence has been attributed to the bacterial biofilm, a protective bacterial reservoir that neither treatment is able to eradicate.17,18,19 Moreover, antibiotics have frequent adverse effects and carry the risk of development of resistance17; thus, effective nonantibiotic treatments for BV are needed.
One such alternative is dequalinium chloride, a broad-spectrum antiseptic effective against gram-positive and gram-negative bacteria, fungi, and protozoa, used since the 1950s.20,21,22,23,24,25 Several clinical studies25,26,27,28,29,30 have reported the efficacy and safety of dequalinium chloride against vaginal infections. Dequalinium chloride is recommended by several international and European guidelines as alternative treatment for BV or inflammatory vaginitis.8,10,11,31,32,33 Dequalinium chloride adsorbs into the cell surface, altering its permeability; disrupts protein synthesis; and induces protein denaturation and precipitation of nucleic acids.25 Due to these multiple modes of action, the development of resistance is unlikely. Furthermore, in vitro experiments showed recently that dequalinium chloride disrupts the bacterial biofilm and impairs metabolic activity.34 Dequalinium chloride was shown to be noninferior to clindamycin for treatment of BV28; thus, the primary objective of this study was to assess the noninferiority of dequalinium chloride compared with oral metronidazole, the other first-line treatment.
Methods
Trial Design and Participants
In this phase 4, multicenter, triple-blind, parallel, double-dummy, noninferiority randomized clinical trial, patients with symptomatic BV were recruited from July 29, 2021, to August 25, 2022, in Poland, the Czech Republic, and Slovakia from 11 gynecology practices and 1 hospital. The study was approved by the local ethics committees and competent authorities. It was conducted in accordance with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use Guideline for Good Clinical Practice and the Declaration of Helsinki.35 The original study design was based on the 2019 US Food and Drug Administration (FDA) guidance for industry for BV.36 This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.37
Premenopausal women 18 years or older with BV (defined as 4 positive Amsel criteria) who signed informed consent forms were eligible to participate. Exclusion criteria were as follows: uterine bleeding (including menstruation) or vaginal bleeding of unknown origin, ulcerations or erosions of the vaginal mucosa or cervix, candidiasis, aerobic vaginitis, sexually transmitted infections, severe systemic diseases, hypersensitivity to one of the study medications, pregnancy, lactation, vaginal medication or douching in the previous 7 days, recent participation in another study with an investigational drug, refusal to refrain from alcohol consumption, unable to follow the procedures of the study, and being related to or working for the investigator. An additional inclusion criterion for the main efficacy assessment was a Nugent score of 7 or higher,36 but due to a higher-than-expected screening failure rate, the protocol was amended to allow the inclusion of all patients in the main analysis. The trial protocol can be found in Supplement 1.
Interventions and Treatments
Potentially eligible patients signed the informed consent forms before any study-specific procedures were performed. These procedures included a pregnancy test and a gynecologic examination to collect samples for evaluation of clue cells in saline wet mount, whiff test, and pH. A pseudonymized sample was sent to an independent central laboratory to assess the Nugent score. Eligible patients were then randomized and handed a medication kit to start treatment the same day. Demographics, somatometrics, medical history, medications, and vital signs were recorded. Information on self-reported race and ethnicity was collected as part of the demographic data of the study participants. A link to an electronic patient diary was provided to document adherence to study medication, symptoms, efficacy, tolerability, and adverse events. Patients were instructed to use contraception methods, abstain from using intravaginal products or douching, and return the medication kit at their next visit. Follow-up visits were performed on days 7 to 11 (visit 1) and 20 to 40 (visit 2) after the start of treatment. Vaginal samples were taken and the occurrence of adverse events recorded at each visit.
The investigational drug was dequalinium chloride, 10-mg vaginal tablets (Fluomizin, Medinova AG). The comparator was metronidazole, 500-mg oral tablets (Arilin, Dr August Wolff GmbH & Co.KG Arzneimittel). Double-dummy medication kits prepared by an independent contractor according to the randomization list contained vaginal and oral tablets, of which one was placebo and the other active medication. Vaginal tablets (containing either dequalinium chloride or placebo) were applied once a day for 6 days. Oral tablets (containing metronidazole or placebo) were taken twice a day for 7 days.
Main Outcomes
The outcome measure of the primary objective was a noninferiority margin of 15 percentage points in the absolute difference in clinical cure rates between dequalinium chloride and metronidazole 7 to 11 days after start of treatment. The secondary objectives were to assess the clinical cure rate, bacteriologic cure rate, therapeutic cure rate, clinical improvement, time to resolution of clinical symptoms, subjective efficacy and tolerability, recurrence, and safety within 1 month (±10 days) of treatment.
Following FDA guidance, BV was defined as the presence of all 4 Amsel criteria: greyish-white homogenous vaginal discharge, more than 20% clue cells on saline wet mount microscopy, fishy amine odor on the vaginal discharge with 10% potassium hydroxide (whiff test), and vaginal pH greater than 4.5.5,36 Clinical cure was defined as the resolution of abnormal vaginal discharge, whiff test, and clue cells (ie, all 3 criteria must be negative).36 Bacteriologic cure was defined as a Nugent score of 3 or less. The Nugent score uses a 0 to 10 scale, with 0 to 3 indicating normal flora, 4 to 6 indicating intermediate flora, and 7 to 10 indicating BV.6 Therapeutic cure was a combination of clinical and bacteriologic cure (ie, patient must meet the criteria of both). The standard Amsel criteria define BV as the presence of 3 or more criteria.5 Conversely, 2 or more negative criteria are considered BV negative. Clinical improvement was defined as 2 or more negative Amsel criteria at both visits. The time to resolution of clinical symptoms was calculated from the day the patients reported no symptoms. Subjective efficacy and tolerability to the treatment were rated independently by patients and investigators as very good, good, moderate, or poor. Adverse events within 24 days of randomization were defined as treatment emergent. Their severity, seriousness, and relationship to study medication were assessed at every visit. Treatment adherence was calculated from the number of medications returned.
Statistical Analysis
Cure rates with metronidazole range from 66% to 90% using diverse combinations of Amsel criteria.38,39,40,41,42 Because the definition of clinical cure for this study was more restrictive, the cure rate was assumed to be 80% and the noninferiority margin was set at 15 percentage points.28,41 A sample size of 236 patients (118 per group) was calculated assuming a 1-sided α = .025, 80% power, and 5% dropout rate with nQuery Statsols, version 8.5.2.0 (Statsols) (the statistical analysis plan can be found in Supplement 1). The planned interim analysis to adjust the sample size was performed by a blinded independent statistician after approximately 50% had completed the study.
The randomization sequence was prepared by an independent statistician using block randomization with variable block sizes stratified by center in a 1:1 ratio. An interactive randomization tool in the electronic case report form provided the medication kit number, ensuring allocation concealment. Patients, investigators, and outcome assessors at the central laboratory were blinded to the allocation treatment.
Demographic and baseline data were summarized using mean (SD) or median (IQR) for continuous variables and number (percentage) for categorical variables. The difference in proportions between groups (with 95% CIs) for clinical cure, bacteriologic cure, and therapeutic cure were analyzed using a Farrington-Manning test with a 1-sided significance level of α = .025 and a noninferiority margin of 15 percentage points. A post hoc analysis using the standard Amsel criteria was performed as above. Multivariable logistic regressions to adjust for variables likely to influence the cure rate, such as treatment, timing of the visit, Nugent score at baseline, and previous BV, were performed using the intention-to-treat (ITT) and per-protocol (PP) populations. Subjective assessment of treatment efficacy and tolerability was performed using a 2-sided Wilcoxon-Mann-Whitney U test. All statistical analyses were performed with SAS software, version 9.4 (SAS Institute Inc).
The ITT population included all randomized and treated patients allocated according to their randomization. The PP population included patients without major protocol violations. The safety population included patients who took at least 1 dose of study medication. Originally, the main efficacy analysis was planned to be performed using the modified ITT population, a subset of the ITT population that excluded patients with a Nugent score less than 7. The protocol was amended to change the main study population to the ITT. The primary efficacy analysis was performed on the ITT and PP populations. Secondary outcomes are presented using the ITT population.
Results
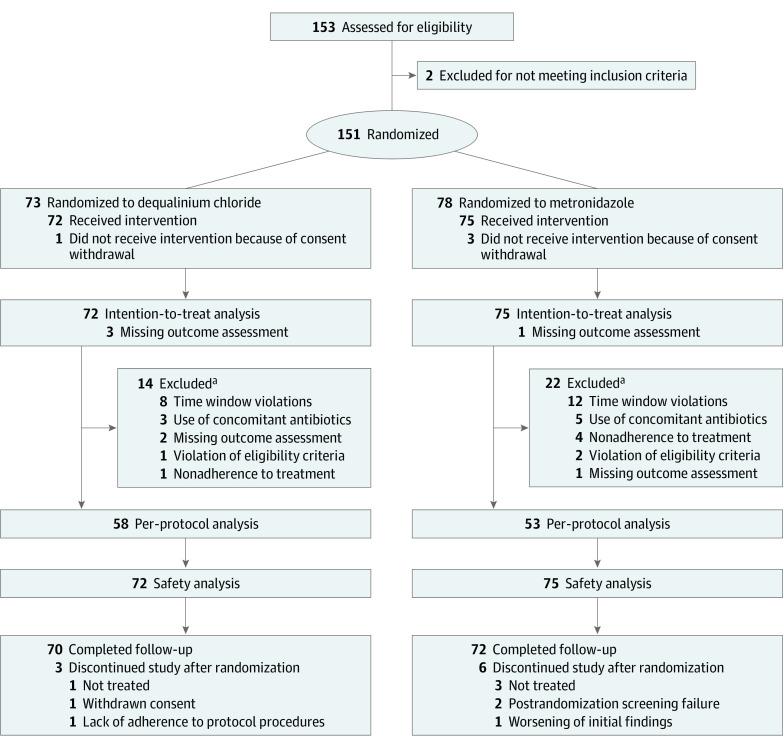
A total of 151 patients were recruited and randomly assigned to receive dequalinium chloride (n = 73) or metronidazole (n = 78). The baseline characteristics were similar between the groups (Table 1). The number of patients with BV by Nugent score was 39 of 72 (54.2%) in the dequalinium chloride group and 41 of 75 (54.7%) in the metronidazole group. The dequalinium chloride group had a lower proportion of patients with a normal Nugent score compared with the metronidazole group (Table 1; eTable in Supplement 2). The conditional power of the interim analysis confirmed the sample size. Unfortunately, the trial was terminated early due to recruitment delays caused mostly by the COVID-19 pandemic. The ITT population consisted of 147 White women (145 non-Hispanic or Latino and 2 Hispanic or Latino; mean [SD] age, 36.7 [9.0] years) treated with dequalinium chloride (n = 72) or metronidazole (n = 75), and 142 patients completed the study (Figure 1). There were no treatment crossovers. The PP population included 58 in the dequalinium chloride group and 53 in the metronidazole group. Reasons for exclusion are listed in Figure 1. A total of 70 of 72 patients (97.2%) in the dequalinium chloride group and 71 of 75 (94.7%) in the metronidazole group had 100% adherence to treatment.
Table 1. Baseline Characteristics of the Participants in the Intention-to-Treat Analysisa.
| Characteristic | Dequalinium chloride (n = 72) | Metronidazole (n = 75) | Total (N = 147) |
|---|---|---|---|
| Age, mean (SD), y | 36.0 (9.2) | 37.5 (8.9) | 36.7 (9.0) |
| Ethnicity | |||
| Not Hispanic or Latino | 71 (98.6) | 74 (98.7) | 145 (98.6) |
| Hispanic or Latino | 1 (1.4) | 1 (1.3) | 2 (1.4) |
| White race | 72 (100) | 75 (100) | 147 (100) |
| Nugent score | |||
| Normal | 18 (25.0) | 23 (30.7) | 41 (27.9) |
| Intermediate | 15 (20.8) | 10 (13.3) | 25 (17.0) |
| Bacterial vaginosis | 39 (54.2) | 41 (54.7) | 80 (54.4) |
| Missing score | 0 | 1 (1.3) | 1 (0.7) |
| Gynecologic history, median (IQR) | |||
| No. of pregnancies | 1.5 (0.0-2.0) | 1.0 (0.0-2.0) | 1 (0.0-2.0) |
| No. of vaginal infections in the last 12 mo | 1.0 (0.0-3.0) | 0.0 (0.0-3.0) | 1 (0.0-3.0) |
Data are presented as number (percentage) of patients unless otherwise indicated.
Figure 1. Consort Flow Diagram.
aSome patients had more than 1 major protocol violation.
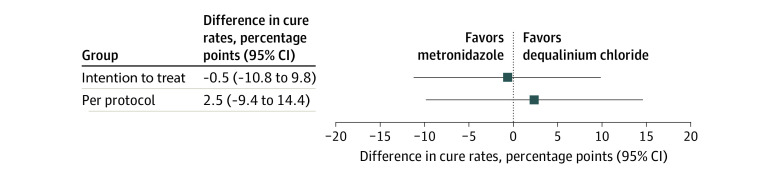
The clinical cure rate at visit 1 in the ITT population was 64 of 69 (92.8%) for the dequalinium chloride group and 69 of 74 (93.2%) for the metronidazole group (Table 2), with a difference in cure rates between groups of −0.5 percentage points (95% CI, −10.8 to 9.8 percentage points; P = .002). In the PP population, the cure rates were 54 of 58 (93.1%) for the dequalinium chloride group vs 48 of 53 (90.6%) for the metronidazole group, with a difference of 2.5 percentage points (95% CI −9.4 to 14.4 percentage points; P = .001). These results confirm the noninferiority of dequalinium chloride (Figure 2 and Table 2).
Table 2. Clinical, Bacteriologic, Therapeutic, and Standard Amsel Criteria Cure Ratesa.
| Outcome | No. of cured patients/total No. with nonmissing data (%) | Treatment difference (95% CI) | P value | |
|---|---|---|---|---|
| Dequalinium chloride | Metronidazole | |||
| Primary outcome | ||||
| Clinical cure rate at visit 1 | ||||
| Intention-to-treat population | 64/69 (92.8) | 69/74 (93.2) | −0.5 (−10.8 to 9.8) | .002 |
| Per-protocol population | 54/58 (93.1) | 48/53 (90.6) | 2.5 (−9.4 to 14.4) | .001 |
| Secondary outcomes (intention to treat) | ||||
| Clinical cure rate | ||||
| Visit 2 | 55/69 (79.7) | 62/71 (87.3) | −7.6 (−20.1 to 4.8) | .12 |
| Bacteriologic cure rate | ||||
| Visit 1 | 35/69 (50.7) | 51/74 (68.9) | −18.2 (−34.1 to −2.3) | .65 |
| Visit 2 | 28/69 (40.6) | 38/71 (53.5) | −12.9 (−29.3 to 3.4) | .40 |
| Therapeutic cure rate | ||||
| Visit 1 | 34/69 (49.3) | 48/74 (64.9) | −15.6 (−31.6 to 0.5) | .53 |
| Visit 2 | 26/69 (37.7) | 33/71 (46.5) | −8.8 (−25 to 7.4) | .23 |
| Standard Amsel criteria cure rate | ||||
| Visit 1 | 68/69 (98.6) | 73/74 (98.6) | −0.1 (−8.9 to 8.7) | <.001 |
| Visit 2 | 61/69 (88.4) | 67/71 (94.4) | −6.0 (−16.2 to 4.3) | .04 |
Findings of the noninferiority analyses for the primary and secondary outcomes are given. The primary outcome was analyzed using the intention-to-treat and per-protocol populations. The secondary outcomes were analyzed using the intention-to-treat population. Visit 1 was 7 to 11 days after treatment start; visit 2 was 20 to 40 days after treatment start. The 95% CIs and P values are based on the Farrington-Manning test with a 1-sided significance level of α = .025 and a noninferiority margin of 15 percentage points.
Figure 2. Clinical Cure Rate at Visit 1.
Primary objective analyses using the intention-to-treat and per-protocol populations are shown. The lower limits of the 95% CIs of the difference in cure rate (dequalinium chloride minus metronidazole) are above the noninferiority margin (−15 percentage points) in both populations, confirming the noninferiority of dequalinium chloride compared with metronidazole. Visit 1 was 7 to 11 days after treatment start.
The cure rate using the standard Amsel criteria also showed the noninferiority of dequalinium chloride (difference, −0.1 percentage points; 95% CI, −8.9 to 8.7 percentage points; P < .001)] at visit 1 (Table 2). At visit 2, the results were inconclusive, including the clinical cure rate at visit 2 and the bacteriologic and therapeutic cure rates at both visits. Overall, the cure rates at visit 2 were lower than at visit 1 (Table 2). There was no significant effect of any of the covariates studied in the logistic regression models using the ITT or PP populations.
The clinical improvement rate was 59 of 67 (88.1%) for the dequalinium chloride group and 65 of 70 (92.9%) for the metronidazole group. The mean (SD) time to resolution of clinical symptoms was 6.7 (2.6) days in the dequalinium chloride group (n = 38) vs 6.5 (2.7) days in the metronidazole group (n = 38).
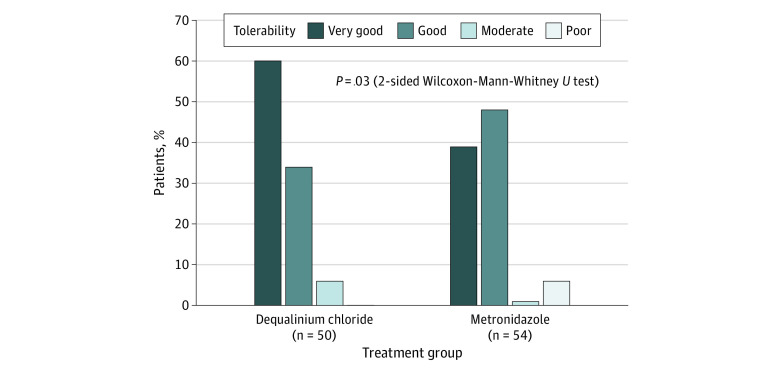
The subjective efficacy to dequalinium chloride treatment was rated as good or very good by 45 of 50 patients (90.0%) and 64 of 69 investigators (92.8%). Metronidazole was rated as good or very good by 46 of 54 patients (85.2%) and 69 of 74 investigators (93.2%). The tolerability of dequalinium chloride was rated as very good by 30 of 50 patients (60.0%) but by only 21 of 54 (38.9%) for metronidazole (Figure 3). Conversely, tolerability was considered poor by 3 of 54 patients (5.6%) treated with metronidazole but by none treated with dequalinium chloride. The difference between groups in the tolerability assessment was statistically significant (P = .03).
Figure 3. Tolerability Assessment by the Patients.
A total of 28 treatment-emergent adverse events were reported by 8 patients treated with dequalinium chloride (n = 11) and by 15 patients treated with metronidazole (n = 17). None of the adverse events were serious, and 18 were considered related to the treatment. The dequalinium chloride group reported 7 related adverse events in 4 patients: candidiasis (n = 2), vaginal infection (n = 1), dysgeusia (n = 1), pruritus (n = 1), pharyngeal swelling (n = 2), and headache (n = 1). The metronidazole group reported 11 related adverse events in 10 patients: candidiasis (n = 4), burning sensation (n = 2), vulvovaginal swelling (n = 1), dysgeusia (n = 1), dyspepsia (n = 1), abdominal pain (n = 1), and fatigue (n = 1). Three patients treated with metronidazole suspended use of the medication due to an adverse event, 1 of whom had worsening of the initial findings and discontinued the study.
Discussion
The results of this randomized clinical trial confirmed that dequalinium chloride is not inferior to metronidazole for the treatment of BV. More than 90% of the patients had resolution of the clinical signs and were considered cured at visit 1, showing constancy and neither loss of effect nor biocreep.43,44 In a noninferiority trial, a type 1 error concludes noninferiority when the new treatment is in fact inferior (false positive), whereas a type 2 error concludes inferiority in a treatment that is noninferior (false negative).44 Small sample sizes tend to lack power to detect an effect when there is one; however, because this study had a higher cure rate and a difference between groups far smaller than anticipated (even favoring dequalinium chloride in the PP analysis), the reduced sample size was enough to demonstrate the noninferiority of dequalinium chloride.43 Having similar results in the PP and ITT analyses strengthens this conclusion.43,44,45 The low P values indicate an extremely low likelihood of a false positive, whereas the careful design and conduct of the study minimized deficiencies that could make treatment groups appear more similar, increasing the certainty that the results are real.43,45
The original study design and primary end point were in line with FDA guidance but do not reflect standard clinical practice. The protocol amendment to analyze all patients regardless of their Nugent score and the post hoc analysis using the standard Amsel criteria are closer to reality. The latter also confirmed the noninferiority of dequalinium chloride (difference, −0.1 percentage points; 95% CI, −8.9 to 8.7 percentage points; P < .001). In this study, the clinical cure rates for both treatments are higher than those found in previous reports.12,13,15,27,28 This finding could be because most patients had 3 or fewer vaginal infections in the previous year, so pathogens were largely unexposed to previous treatments and potentially more susceptible. The validity of the study results, however, is supported by the trial design and execution, comparable baseline characteristics, and few missing data.
The bacteriologic cure rates based on Nugent score were considerably lower than the clinical cure rates, but similar results were seen in a recent study.14 The first assessment was performed immediately after the end of treatment, and the lactobacillary flora may have still not had enough time to recover. However, like the clinical cure rate, at visit 2 the bacteriologic cure rate worsened in both groups, suggesting that some patients either never reached a normal Nugent score or relapsed shortly afterward. The noninferiority analyses of these exploratory secondary outcomes were inconclusive. Factors contributing to relapses, such as intercourse, hormones, and hygiene, should be taken into consideration in future trials.
The low correlation between the Nugent score and the Amsel criteria has been reported before,46,47 but it is still surprising because the Nugent score assesses the flora composition, whereas the Amsel criteria assess the clinical effects of bacterial overgrowth. Despite having 4 Amsel criteria at entry, approximately 45% had a Nugent score of 6 or less (ie, BV negative). This rate is higher than the 19% to 26% reported by a recent study with a similar design and patient population14 but lower than the 75% reported elsewhere.48 Clinicians rely almost exclusively on the Amsel criteria to diagnose BV and prescribe treatment, and this empirical treatment approach for vaginal infections is unlikely to change anytime soon. Although clindamycin and metronidazole are effective in the short term, they have several disadvantages that warrant further consideration of the first-line treatment options. For example, the spectrum of each antibiotic does not fully cover all the pathogens typically present in BV,17,49,50 and combinations with both antibiotics have not significantly improved the efficacy.16 Successive rounds of treatment rapidly increase the resistance to antibiotics,17 and neither metronidazole nor clindamycin seems capable of completely eliminating the biofilm.18,19,49 Prolonged antibiotic treatment is temporarily effective to reduce the relapse rate but potentially damages the normal flora.16,17,51,52,53,54 The risk of subsequent vulvovaginal candidiasis is 10% to 25% of the treated patients,40,52,55 likely due to an overgrowth of Candida or a misdiagnosed mixed infection. Finally, adherence to the treatment is crucial for efficacy and to prevent the development of resistance, but the frequent adverse effects and personal preferences can reduce patient adherence. Adverse effects can be as high as 32% with metronidazole13 and 20% with clindamycin,56 with nausea, vomiting, and abdominal pain among the most frequent. Some patients reportedly dislike antibiotics and prefer other treatments even if perceived as less effective.13
The cure rate for BV is similar to that of clindamycin and metronidazole, but unlike them, the spectrum of dequalinium chloride covers pathogens associated with BV, aerobic vaginitis, and candidiasis.20,21,22 Accordingly, treatment with dequalinium chloride had a lower rate of vulvovaginal candidiasis than metronidazole and clindamycin.28 Despite its 30 years in the market, there are no reports of clinically relevant resistance to dequalinium chloride. The tolerability and safety profile may increase adherence to the treatment: most patients rated the tolerability of dequalinium chloride as very good, and the overall rate of adverse effects with dequalinium chloride is very low. Because there is no systemic absorption, most adverse effects are local and mild, making dequalinium chloride safe enough to use during pregnancy. Therefore, dequalinium chloride warrants consideration as first-line empirical treatment for BV due to its similar efficacy to metronidazole and clindamycin, broad spectrum, lack of resistance, tolerability, and safety. Unfortunately, the recurrence rate with dequalinium chloride was similar to that of antibiotics, likely due to a residual dysbiosis. Restoring the vaginal flora using probiotics after any anti-infective treatment for BV could help reduce recurrences.15,57,58
Strengths and Limitations
The strengths of the study are the randomized noninferiority study design with a well-defined noninferiority margin and parallel allocation. Potential biases were minimized to increase the internal validity: the double-dummy medication and triple-blinding ensured the allocation concealment, whereas the high adherence and retention rates reduced the attrition bias. The multicenter setting and broad inclusion criteria increase the generalizability. The limitations of the study are the short follow-up; the reduced sample size, which hindered subgroup analyses; and the patient population limited to White European individuals.
Conclusions
This randomized clinical trial confirmed that dequalinium chloride is not inferior to metronidazole in the treatment of BV, displaying a similarly high cure rate but with better tolerability and fewer adverse events. Dequalinium chloride could help reduce antibiotic consumption and thus warrants consideration as first-line treatment for BV due to its broad spectrum, efficacy, safety, tolerability, and less likelihood of resistance.
Trial Protocol and Statistical Analysis Plan
eTable. Subgroup Analyses Stratified by Nugent Score
Nonauthor Collaborators. Fluomizin Study Group
Data Sharing Statement
References
- 1.Kenyon C, Colebunders R, Crucitti T. The global epidemiology of bacterial vaginosis: a systematic review. Am J Obstet Gynecol. 2013;209(6):505-523. doi: 10.1016/j.ajog.2013.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Peebles K, Velloza J, Balkus JE, McClelland RS, Barnabas RV. High global burden and costs of bacterial vaginosis: a systematic review and meta-analysis. Sex Transm Dis. 2019;46(5):304-311. doi: 10.1097/OLQ.0000000000000972 [DOI] [PubMed] [Google Scholar]
- 3.Chow K, Wooten D, Annepally S, Burke L, Edi R, Morris SR. Impact of (recurrent) bacterial vaginosis on quality of life and the need for accessible alternative treatments. BMC Womens Health. 2023;23(1):112. doi: 10.1186/s12905-023-02236-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bilardi JE, Walker S, Temple-Smith M, et al. The burden of bacterial vaginosis: women’s experience of the physical, emotional, sexual and social impact of living with recurrent bacterial vaginosis. PLoS One. 2013;8(9):e74378. doi: 10.1371/journal.pone.0074378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis: diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983;74(1):14-22. doi: 10.1016/0002-9343(83)91112-9 [DOI] [PubMed] [Google Scholar]
- 6.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J Clin Microbiol. 1991;29(2):297-301. doi: 10.1128/jcm.29.2.297-301.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muzny CA, Cerca N, Elnaggar JH, Taylor CM, Sobel JD, Van Der Pol B. State of the art for diagnosis of bacterial vaginosis. J Clin Microbiol. 2023;61(8):e0083722. doi: 10.1128/jcm.00837-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sherrard J, Wilson J, Donders G, Mendling W, Jensen JS. 2018 European (IUSTI/WHO) International Union Against Sexually Transmitted Infections (IUSTI) World Health Organisation (WHO) guideline on the management of vaginal discharge. Int J STD AIDS. 2018;29(13):1258-1272. doi: 10.1177/0956462418785451 [DOI] [PubMed] [Google Scholar]
- 9.Workowski KA, Bachmann LH, Chan PA, et al. Sexually transmitted infections treatment guidelines, 2021. MMWR Recomm Rep. 2021;70(4):1-187. doi: 10.15585/mmwr.rr7004a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farr A, Swidsinski S, Surbek D, et al. Bacterial vaginosis: guideline of the DGGG, OEGGG and SGGG (S2k-level, AWMF registry No. 015/028, June 2023). Geburtshilfe Frauenheilkd. 2023;83(11):1331-1349. doi: 10.1055/a-2169-8539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vieira-Baptista P, Stockdale CK, Sobel J. International Society for the Study of Vulvovaginal Disease Recommendations for the Diagnosis and Treatment of Vaginitis. Admedic; 2023:198. [Google Scholar]
- 12.Verwijs MC, Agaba SK, Darby AC, van de Wijgert JHHM. Impact of oral metronidazole treatment on the vaginal microbiota and correlates of treatment failure. Am J Obstet Gynecol. 2020;222(2):157.e1-157.e13. doi: 10.1016/j.ajog.2019.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armstrong-Buisseret L, Brittain C, Kai J, et al. Lactic acid gel versus metronidazole for recurrent bacterial vaginosis in women aged 16 years and over: the VITA RCT. Health Technol Assess. 2022;26(2):1-170. doi: 10.3310/ZZKH4176 [DOI] [PubMed] [Google Scholar]
- 14.Mauck C, Hillier SL, Gendreau J, et al. Single-dose, bioadhesive clindamycin 2% gel for bacterial vaginosis: a randomized controlled trial. Obstet Gynecol. 2022;139(6):1092-1102. doi: 10.1097/AOG.0000000000004805 [DOI] [PubMed] [Google Scholar]
- 15.Oduyebo OO, Anorlu RI, Ogunsola FT. The effects of antimicrobial therapy on bacterial vaginosis in non-pregnant women. Cochrane Database Syst Rev. 2009;(3):CD006055. doi: 10.1002/14651858.CD006055.pub2 [DOI] [PubMed] [Google Scholar]
- 16.Bradshaw CS, Pirotta M, De Guingand D, et al. Efficacy of oral metronidazole with vaginal clindamycin or vaginal probiotic for bacterial vaginosis: randomised placebo-controlled double-blind trial. PLoS One. 2012;7(4):e34540. doi: 10.1371/journal.pone.0034540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muzny CA, Sobel JD. The role of antimicrobial resistance in refractory and recurrent bacterial vaginosis and current recommendations for treatment. Antibiotics (Basel). 2022;11(4):500. doi: 10.3390/antibiotics11040500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swidsinski A, Mendling W, Loening-Baucke V, et al. An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am J Obstet Gynecol. 2008;198(1):97.e1-97.e6. doi: 10.1016/j.ajog.2007.06.039 [DOI] [PubMed] [Google Scholar]
- 19.Rosca AS, Castro J, França Â, Vaneechoutte M, Cerca N. Gardnerella vaginalis dominates multi-species biofilms in both pre-conditioned and competitive in vitro biofilm formation models. Microb Ecol. 2022;84(4):1278-1287. doi: 10.1007/s00248-021-01917-2 [DOI] [PubMed] [Google Scholar]
- 20.Della Casa V, Noll H, Gonser S, Grob P, Graf F, Pohlig G. Antimicrobial activity of dequalinium chloride against leading germs of vaginal infections. Arzneimittelforschung. 2002;52(9):699-705. [DOI] [PubMed] [Google Scholar]
- 21.D’Auria FD, Simonetti G, Strippoli V. Antimicrobial characteristics of a tincture of dequalinium chloride. Article in Italian. Ann Ig. 1989;1(5):1227-1241. [PubMed] [Google Scholar]
- 22.Lopes dos Santos Santiago G, Grob P, Verstraelen H, Waser F, Vaneechoutte M. Susceptibility testing of Atopobium vaginae for dequalinium chloride. BMC Res Notes. 2012;5:151. doi: 10.1186/1756-0500-5-151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babbs M, Collier HO, Austin WC, Potter MD, Taylor EP. Salts of decamethylene-bis-4-aminoquinaldinium (dequadin); a new antimicrobial agent. J Pharm Pharmacol. 1956;8(2):110-119. [DOI] [PubMed] [Google Scholar]
- 24.Roddie TW. Clinical evaluation of dequadin in the treatment of vaginal infections & infestations. Med J Malaya. 1958;13(2):171-172. [PubMed] [Google Scholar]
- 25.Mendling W, Weissenbacher ER, Gerber S, Prasauskas V, Grob P. Use of locally delivered dequalinium chloride in the treatment of vaginal infections: a review. Arch Gynecol Obstet. 2016;293(3):469-484. doi: 10.1007/s00404-015-3914-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donders G, Bellen G, Donders F, et al. Improvement of abnormal vaginal flora in Ugandan women by self-testing and short use of intravaginal antimicrobials. Eur J Clin Microbiol Infect Dis. 2017;36(4):731-738. doi: 10.1007/s10096-016-2856-9 [DOI] [PubMed] [Google Scholar]
- 27.Antoni Vives J, Cancelo MJ, Losada MA, Doménech A. Dequalinium chloride use in adult Spanish women with bacterial vaginosis: an observational study. J Obstet Gynaecol. 2022;42(1):103-109. doi: 10.1080/01443615.2020.1867966 [DOI] [PubMed] [Google Scholar]
- 28.Weissenbacher ER, Donders G, Unzeitig V, et al. ; Fluomizin Study Group . A comparison of dequalinium chloride vaginal tablets (Fluomizin®) and clindamycin vaginal cream in the treatment of bacterial vaginosis: a single-blind, randomized clinical trial of efficacy and safety. Gynecol Obstet Invest. 2012;73(1):8-15. doi: 10.1159/000332398 [DOI] [PubMed] [Google Scholar]
- 29.Petersen EE, Weissenbacher ER, Hengst P, et al. Local treatment of vaginal infections of varying etiology with dequalinium chloride or povidone iodine. A randomised, double-blind, active-controlled, multicentric clinical study. Arzneimittelforschung. 2002;52(9):706-715. [DOI] [PubMed] [Google Scholar]
- 30.Eckel F, Farr A, Deinsberger J, Kernmayer-Farr K, Foessleitner P. Dequalinium chloride for the treatment of vulvovaginal infections: a systematic review and meta-analysis. J Low Genit Tract Dis. 2024;28(1):76-83. doi: 10.1097/LGT.0000000000000790 [DOI] [PubMed] [Google Scholar]
- 31.Portuguese Society of Gynecology (SPG) . Revisão dos Consensos em Infecções Vulvovaginais [Guidelines on the Treatment of Vaginal Infections]. SPG; 2012.
- 32.Sociedad Española de Ginecología y Obstetricia (SEGO) . Diagnóstico y tratamiento de las infecciones vulvovaginales: actualizado 2016. Prog Obstet Ginecol. 2016;59(5):350-362. [Google Scholar]
- 33.Zimmer M, Huras H, Kaminski P, et al. Polish Society of Gynecologists and Obstetricians recommendation on the use of antiseptics for treatment of inflammatory vaginitis. Ginekol Pol. 2020;91(7):432-437. doi: 10.5603/GP.2020.0104 [DOI] [PubMed] [Google Scholar]
- 34.Gaspar C, Rolo J, Cerca N, Palmeira-de-Oliveira R, Martinez-de-Oliveira J, Palmeira-de-Oliveira A. Dequalinium chloride effectively disrupts bacterial vaginosis (BV) Gardnerella spp. biofilms. Pathogens. 2021;10(3):261. doi: 10.3390/pathogens10030261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 36.US Food and Drug Administration. Bacterial vaginosis: developing drugs for treatment guidance for industry. US Food and Drug Admnistration. Accessed April 12, 2024. August 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bacterial-vaginosis-developing-drugs-treatment-guidance-industry
- 37.Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group . Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA. 2012;308(24):2594-2604. doi: 10.1001/jama.2012.87802 [DOI] [PubMed] [Google Scholar]
- 38.Lugo-Miro VI, Green M, Mazur L. Comparison of different metronidazole therapeutic regimens for bacterial vaginosis: a meta-analysis. JAMA. 1992;268(1):92-95. doi: 10.1001/jama.1992.03490010094036 [DOI] [PubMed] [Google Scholar]
- 39.Hanson JM, McGregor JA, Hillier SL, et al. Metronidazole for bacterial vaginosis: a comparison of vaginal gel vs. oral therapy. J Reprod Med. 2000;45(11):889-896. [PubMed] [Google Scholar]
- 40.Schmitt C, Sobel JD, Meriwether C. Bacterial vaginosis: treatment with clindamycin cream versus oral metronidazole. Obstet Gynecol. 1992;79(6):1020-1023. [PubMed] [Google Scholar]
- 41.Paavonen J, Mangioni C, Martin MA, Wajszczuk CP. Vaginal clindamycin and oral metronidazole for bacterial vaginosis: a randomized trial. Obstet Gynecol. 2000;96(2):256-260. doi: 10.1097/00006250-200008000-00019 [DOI] [PubMed] [Google Scholar]
- 42.Larsson PG, Forsum U. Bacterial vaginosis: a disturbed bacterial flora and treatment enigma. APMIS. 2005;113(5):305-316. doi: 10.1111/j.1600-0463.2005.apm_113501.x [DOI] [PubMed] [Google Scholar]
- 43.US Food and Drug Administration. Non-Inferiority Clinical Trials to Establish Effectiveness: Guidance for Industry. US Food and Drug Administration; 2016. Accessed March 14, 2024. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/non-inferiority-clinical-trials [Google Scholar]
- 44.Walker J. Non-inferiority statistics and equivalence studies. BJA Educ. 2019;19(8):267-271. doi: 10.1016/j.bjae.2019.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim K, Zeraatkar D, Pitre TS, et al. ; Retina Evidence and Trials INternational Alliance (R.E.T.I.N.A.) Study Group . Noninferiority randomised trials in ophthalmology. Eye (Lond). 2023;37(15):3059-3060. doi: 10.1038/s41433-023-02465-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hillier SL, Austin M, Macio I, Meyn LA, Badway D, Beigi R. Diagnosis and treatment of vaginal discharge syndromes in community practice settings. Clin Infect Dis. 2021;72(9):1538-1543. doi: 10.1093/cid/ciaa260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sha BE, Chen HY, Wang QJ, Zariffard MR, Cohen MH, Spear GT. Utility of Amsel criteria, Nugent score, and quantitative PCR for Gardnerella vaginalis, Mycoplasma hominis, and Lactobacillus spp. for diagnosis of bacterial vaginosis in human immunodeficiency virus-infected women. J Clin Microbiol. 2005;43(9):4607-4612. doi: 10.1128/JCM.43.9.4607-4612.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen HM, Chang TH, Lin FM, et al. Vaginal microbiome variances in sample groups categorized by clinical criteria of bacterial vaginosis. BMC Genomics. 2018;19(suppl 10):876. doi: 10.1186/s12864-018-5284-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li T, Zhang Z, Wang F, et al. Antimicrobial susceptibility testing of metronidazole and clindamycin against Gardnerella vaginalis in planktonic and biofilm formation. Can J Infect Dis Med Microbiol. 2020;2020:1361825. doi: 10.1155/2020/1361825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nyirjesy P, McIntosh MJ, Steinmetz JI, Schumacher RJ, Joffrion JL. The effects of intravaginal clindamycin and metronidazole therapy on vaginal Mobiluncus morphotypes in patients with bacterial vaginosis. Sex Transm Dis. 2007;34(4):197-202. doi: 10.1097/01.olq.0000235152.98601.d7 [DOI] [PubMed] [Google Scholar]
- 51.Reichman O, Akins R, Sobel JD. Boric acid addition to suppressive antimicrobial therapy for recurrent bacterial vaginosis. Sex Transm Dis. 2009;36(11):732-734. doi: 10.1097/OLQ.0b013e3181b08456 [DOI] [PubMed] [Google Scholar]
- 52.Surapaneni S, Akins R, Sobel JD. Recurrent bacterial vaginosis: an unmet therapeutic challenge. experience with a combination pharmacotherapy long-term suppressive regimen. Sex Transm Dis. 2021;48(10):761-765. doi: 10.1097/OLQ.0000000000001420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Balkus JE, Carter KA, McClelland RS. Lessons from suppressive therapy and periodic presumptive treatment for bacterial vaginosis. Curr Infect Dis Rep. 2019;21(10):34. doi: 10.1007/s11908-019-0688-3 [DOI] [PubMed] [Google Scholar]
- 54.Balkus JE, Srinivasan S, Anzala O, et al. Impact of periodic presumptive treatment for bacterial vaginosis on the vaginal microbiome among women participating in the preventing vaginal infections trial. J Infect Dis. 2017;215(5):723-731. doi: 10.1093/infdis/jiw622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ferris DG, Litaker MS, Woodward L, Mathis D, Hendrich J. Treatment of bacterial vaginosis: a comparison of oral metronidazole, metronidazole vaginal gel, and clindamycin vaginal cream. J Fam Pract. 1995;41(5):443-449. [PubMed] [Google Scholar]
- 56.Johnson M. Clindamycin: An Overview. UpToDate. Accessed April 4, 2023. https://www.uptodate.com/contents/clindamycin-an-overview?search=clindamycin&source=search_result&selectedTitle=2~145&usage_type=default&display_rank=1
- 57.Jeng HS, Yan TR, Chen JY. Treating vaginitis with probiotics in non-pregnant females: a systematic review and meta-analysis. Exp Ther Med. 2020;20(4):3749-3765. doi: 10.3892/etm.2020.9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chieng WK, Abdul Jalal MI, Bedi JS, et al. Probiotics, a promising therapy to reduce the recurrence of bacterial vaginosis in women? a systematic review and meta-analysis of randomized controlled trials. Front Nutr. 2022;9:938838. doi: 10.3389/fnut.2022.938838 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eTable. Subgroup Analyses Stratified by Nugent Score
Nonauthor Collaborators. Fluomizin Study Group
Data Sharing Statement