Abstract
The mechanical properties of cells and tissues help determine their architecture, composition and function. Alterations to these properties are associated with many diseases, including cancer. Tensional, compressive, adhesive, elastic and viscous properties of individual cells and multicellular tissues are mostly regulated by reorganization of the actomyosin and microtubule cytoskeletons and extracellular glycocalyx, which in turn drive many pathophysiological processes, including cancer progression. This Review provides an in-depth collection of quantitative data on diverse mechanical properties of living human cancer cells and tissues. Additionally, the implications of mechanical property changes for cancer development are discussed. An increased knowledge of the mechanical properties of the tumour microenvironment, as collected using biomechanical approaches capable of multi-timescale and multiparametric analyses, will provide a better understanding of the complex mechanical determinants of cancer organization and progression. This information can lead to a further understanding of resistance mechanisms to chemotherapies and immunotherapies and the metastatic cascade.
Introduction
Metastatic cancer is one of the leading causes of death worldwide1,2. Fundamentally, cancer is a complex, multistage process that stems from dysregulation and uncontrolled cellular growth and proliferation leading to the formation of solid tumours3–5. Metastasis occurs when cancer cells escape the primary tumour and colonize new tissue3–5. For healthy cells and tissues to undergo this malignant transformation, numerous biological, chemical and physical alterations are required6,7. How cells enact (and react to) these processes is of great interest to cancer biologists, with a view to a more complete understanding of the process of oncogenesis. Critical processes that drive oncogenesis, tumour organization and overall disease progression include intracellular and intercellular reorganization within a tumour, as well as extracellular matrix (ECM) remodelling8. Research suggests that the study of mechanical properties of cells and tissues within a growing tumour is necessary to accurately understand how cancers regulate their molecular structure and organize9,10. Deformation of cancer cells by intracellular forces or intercellular forces within the tumour microenvironment (TME) can activate mechanosensitive biochemical signalling cascades that modify their molecular regulation and mechanical properties, further enhancing their metastatic potential10. Several mechanical properties have been shown to be modified by changes in cancer cells including cellular tension11–14, hydrostatic pressure12–14, adhesion force15,16, elasticity or Young’s modulus15,17–20 (denoted E) and viscosity17–20 (denoted η).
Understanding how modifications to these various mechanical properties, and how they impact biological systems, falls within the realm of mechanobiology, an emergent and multidisciplinary scientific field with the goal of integrating the principles of mechanical properties with biological sciences to improve understanding of morphogenesis and diseases at the cellular and tissue levels. Mechanobiology brings unique tools for investigating self-organization in cellular biology, including new biophysical tools to measure the mechanical properties of cells and tissues including tension21, pressure22, adhesion23, elasticity24 and viscosity25. As highlighted in the supplementary tables presented in this Review, various mechanobiological techniques can be exploited to analyse several mechanical properties under differing conditions, at either the cellular or the tissue level. Although these methods have greatly contributed to mechanobiological understanding in cells and tissues, current approaches have suboptimal spatiotemporal resolution for many fundamental biologically relevant mechanisms. Therefore, a degree of understanding of the biomechanical method resolution limits is required to properly use certain techniques for measuring critical mechanical properties under physiologically relevant conditions26,27. Improvements to current methods and the development of new methods are needed to help advance the field of mechanobiology and address unexplored scientific questions based on experimental demands.
Cancer tissues are intrinsically linked to the mechanical properties of their cellular subcomponents. Disruptions in the balance of these intracellular or intercellular mechanical forces and properties are associated with malignancies10,28,29, including cancer metastasis. Additionally, mutations in cancer cells are also involved in tumour progression, which can result in enhanced metastatic potential30,31. The actomyosin cytoskeleton has a critical role in tumorigenesis and progression of many cancers including breast, pancreatic and ovarian cancers9,32. In breast cancer, aberrations in actomyosin and microtubule cytoskeletons yield a highly disorganized network of structures associated with softer and more aggressive cancer cells32,33. In ovarian cancer, softening of malignant cancer cells owing to reduced actomyosin contractility leads to enhanced aggressiveness and increased migratory potential34. Additionally, malignant pancreatic cancer cells show a profound reduction in F-actin resulting in a marked reduction in cellular stiffness and increased invasive potential35. Therefore, understanding the mechanisms in which solid tumours and cancer cells undergo structural aberrations and their relation to changes in mechanical properties may reveal new therapeutic strategies to stop cancer progression and metastasis.
There is some debate among physical oncologists regarding whether a solid tumour can be universally defined across different cancers or whether the role of various mechanical properties varies depending on the tissue type. If these properties are to be better understood, they must be related to the underlying hallmarks of cancer3. One such hallmark, activating invasion and metastasis, is particularly relevant within the study of mechanobiology, as it is often associated with changes in the mechanical properties of cancer cells. The fundamental understanding of such properties and their implications in the development of metastasis is required to properly assess the malignant potential of cancerous tissues36. Most scientific literature indicates that cancer cells are softer than their normal counterparts, and factors that enhance aggressiveness further reduce this stiffness37. Cancer metastasis is initiated when cancer cells break free from the primary tumour and successfully colonize distant tissues. Several studies suggest a connection among fibrosis, tumour metastatic progression and enhanced aggression. In other words, ECM composition and structure can influence the ability of cancer cells to colonize an organ25,38–40 and ECM mechanical properties, particularly stiffness, are important for creating a pro-oncogenic TME38,41,42. For example, hypoxia and necrosis in the pro-metastatic TME substantially increase the internal hydrostatic and osmotic intratumoural pressures43, causing modifications to the internal tumour material properties. To date, the mechanobiological underpinnings of mechanoadaptive organ-specific programmes of the TME and organization are not well studied and remain poorly understood.
In this Review, we provide a comprehensive compilation of currently understood mechanisms contributing to the mechanical properties associated with malignant cancers. By integrating the molecular-mechanical properties at the cellular and tissue levels of different cancers, we can begin to focus on the applications of mechanobiology in the study of malignant disease states. Until recently, the study of cancer has been exclusive to biological and biochemical applications. We provide a comprehensive collection of quantitative mechanical-based data on diverse mechanical properties of living human cancer cells and tissues (Supplementary Tables 1 and 2). These tables were specifically designed to highlight research that quantitatively discusses the differences between normal and cancerous cells, deriving from the same human tissue (for instance, healthy against cancerous human breast cells or tissues). To this end, studies that do not include healthy, normal human cells or tissues (of the same origin as the cancer) as a control for direct mechanical comparison to human cancer are not discussed. Overall, we envision that more accurate knowledge of the mechanical properties of the intratumoural micro environment will eventually provide a general understanding of the complex mechanical determinants of cancer organization, progression, treatment resistance and metastasis.
Emergent molecular-dependent mechanical properties in cancer
The biological functions of cells are modulated by biochemical signalling cascades and molecular-mechanical properties. These in turn are regulated by cells as they sense mechanical and surface topographical stimuli, allowing them to transduce and respond to mechanical forces44,45. Consenquently, mechanical forces are relevant for the morphological features and processes of cells, and thus also tissues, organs and organisms46–48. A summary of some of the major mechanical properties and their effects on cancer cell progression is shown in Fig. 1. Of the multiple mechanical properties typically investigated for mechanobiological analyses, stiffness is perhaps the most widely used metric49. However, stiffness alone does not accurately represent the true mechanical behaviour of biological materials50. Given the highly heterogeneous structure of both cells and tissues (Fig. 1a), these structures tend to have a time-dependent response rather than a linear-elastic deformation when exposed to an external force25. The combined interactions of multiple subcellular and ECM components, and interconnected cells, often from different cell types, give these structures both an immediate elastic and time-dependent viscous response to external mechanical stressors25,51. For example, during tumour progression, malignant cells have reduced cell–cell adhesions, increased cell–ECM adhesions and a remodelled cytoskeleton, these factors all influencing how cells sense and respond to mechanical forces (Fig. 1a).
Fig. 1 |. Remodelling of the cellular cytoskeleton and extracellular matrix is a hallmark of cancer-causing alterations in cellular mechanical properties.

One hallmark of cancer is the excessive remodelling of the extracellular matrix (ECM) (part a). The marked increase in fibrillar collagen deposition within tumour microenvironments during cancer progression is often observed by alignment of the collagen fibrils and their decoration with adhesive ECM proteins including laminin and fibronectin. These ECM collagenous changes are known to markedly increase tumour tissue stiffness. Another critical hallmark of cancer centres around remodelling the cancer cell cytoskeleton. Progressive alterations to the cytoskeletal organization of cancer cells have been associated with numerous modified mechanical properties, including: cellular stiffness (denoted by k; part b), viscosity (denoted by η; part c), tension (denoted by T; part d), cell–ECM adhesion (denoted by Fadh; part e), hydrostatic and osmotic pressures (indicated by the pressure exerted by a fluid against the membrane; denoted by P; part f) and shear stress (indicated by changes in resistance to an applied shear force; denoted by Fs; part g). The individual schematics show the typically observed changes in mechanical behaviour of cells before and after transformation. An arrow with F (denoting force vector) indicates the direction of the applied external mechanical force. Most cancers demonstrate a substantial decrease in all mentioned mechanical properties, except for cellular adhesion. Note that there are conflicting reports showing different trends in mechanical properties for some cancer types and we briefly discuss some of them in this Review. Figure courtesy of Alan Hoofring.
Stiffness is usually expressed in the form of Young’s modulus or a linear spring constant52. In a linear-elastic material, Young’s modulus is defined as the quotient of the stress applied to a material (equivalent to the applied force over a given area) divided by the strain (the change in length compared with the length of the original material)53, and the spring constant (denoted by k) is the quotient between the applied force and the material deformation54,55. Measuring stiffness has been useful in differentiating various components within tissues from one another, such as cells and extracellular material56. In a diagnostic sense, the quantification of cellular stiffness has been of particular interest in cancer, because many studies showed that there is a clear distinction in the mechanical properties between cancer cells and the healthy tissue from which they arose24,57,58 (Fig. 1b). This difference is commonly seen as a reduction in the Young’s modulus of cancerous cells (Supplementary Table 1). The current consensus is that cancer cells are softer when they become more aggressive (Supplementary Table 1) and stiffer when their aggressiveness is reduced, as commonly seen with drug treatment or silencing various pro-oncogenic biomolecules in a multitude of different cancers59–61. However, some articles have challenged this notion, showing an increased stiffness with more aggressive cells in melanoma, pancreatic and prostate cancer35,62,63. In contrast to individual cells, cancerous tissues are stiffer at more advanced grades, in part due to the extensive changes to the ECM41,64,65. Data summarizing the mechanical changes in human tissue, including stiffness, are shown in Supplementary Table 2. Another highly related term used with certain measurement systems is the inverse of stiffness, also known as compliance (δ), referring to the flexibility of a material. One example highlighted in Supplementary Table 1 demonstrates the use of this parameter collected on oral cancer cells under an applied force by optical tweezers, indicating increased compliance compared to healthy cells from oral mucosa66.
Although not studied as extensively as stiffness, viscoelasticity may hold the key to more accurately assessing the mechanical behaviour of living cells and tissues. Many materials, including synthetic polymers, rubber and multiple biological tissues, are known to exhibit viscoelastic behaviour67. Viscoelastic materials exhibit both viscous (fluid) and elastic (solid) properties when subjected to external forces25,68 (Fig. 1b,c). The elastic and viscous components of a material are often defined as the storage modulus and loss modulus, respectively. A useful quantity used to determine the relative magnitude of these responses is the loss tangent, which is the ratio of the viscous to the elastic components of the material. The higher this ratio is, the more viscous the behaviour of the material is; conversely the lower, the more elastic19,69,70. There is an increased interest in quantitatively determining the viscoelastic properties of complex biological samples, with the hope that this information can be used as an enhanced mechanical biomarker of diseases17–20,25,71–75. For example, multiple types of cancer cells are reported to be softer and less viscous than healthy counterparts. Quantitative viscoelastic properties have been reported for different types of cancers including bladder76, breast77, liver78, prostate79 and thyroid cancers80, among others (Supplementary Table 1). In contrast to individual cells, cancerous tissues are stiffer and more viscous at more advanced grades64,81 (Supplementary Table 2).
Tensile forces are generated when a material is stretched and represent the inverse of compressive forces. Cancerous cells undergo changes that affect the structure of the cytoskeleton and plasma membrane, which are thought to be major determinants in the physical changes seen in cancer cells compared with healthy ones from a given tissue82,83. There is dysregulation in the structures of both the plasma membrane and actin cortex in cancerous cells, which is typically associated with an altered level of cortical cellular tension (denoted by T)84. Recent studies investigating changes in cortical tension in migratory human breast cancer and ovarian cancer cells observed an overall reduction in tension, thus indicating a correlation between decreased tension and malignant progression85,86 (Fig. 1d). Interestingly, other studies have shown an apparent increase in cortical tension in human melanoma and breast cancer cells owing to upregulation of ERK and Rho signalling substantially increasing actin cytoskeleton tension12,87.
Another emergent mechanical property that has been shown to change in cancer cells based on different degrees of malignancy and metastatic potential is the cellular surface adhesion (denoted by Fadh)39,88 (Fig. 1e). Adhesion force can be defined as the force required to detach two bodies in physical contact. For cells, surface adhesion is based on multiple transmembrane proteins expressed by cells to mediate cell–cell adhesion and cell–ECM interactions89,90 (Fig. 1e). These transmembrane proteins form part of an essential and complex adhesive structure on cells named the glycocalyx90,91. It has been shown that the adhesion force is substantially altered in multiple different cancers, with multiple reports detailing a considerably increased adhesion in cancer cells when compared with healthy counterparts (Supplementary Table 1), suggesting that it is an important indicator of malignancy and metastasis88,92,93. However, the role of adhesion in cancer tissues is somewhat complex, and how it changes can depend on the stage of disease. To our knowledge, no careful quantitative survey has been performed to measure the changes in adhesion forces between cancerous and healthy tissues. Another unique application of adhesion studies is in the detection of various surface molecules (Supplementary Table 1). A study in which a cholesterol-binding toxin molecule was conjugated to an atomic force microscopy (AFM) probe in an attempt to quantify membrane composition differences between normal and transformed cells observed that malignant-transformed cells, which contained an increased level of cholesterol within their membrane, show a higher frequency of binding events as determined by AFM pulling studies94. In another study, an antibody for N-cadherin (a transmembrane protein highly associated with epithelial-to-mesenchymal transition) was conjugated to an AFM probe, and when pulling studies were conducted on normal and cancerous cells, an increased separation force was noted on cancer cells, indicating a higher expression of this molecule95.
Pressure (denoted P), or the force applied over a given area, is another property that can be altered in cancerous cells and tissues96,97. Pressure acts normally to the surface and does not arise from within a material. Pressure stands in contrast to stress, a term used when referring to an internal response to external forces (Fig. 1f). At the cellular level, the upregulated proliferation of cancer cells increases the solid stress in tumours owing to increased crowding of cancer cells, infiltration of stromal cells and increased deposition of ECM98. At the tissue level, as tumours grow, cancer cells secrete molecules that promote the growth of new blood vessels to increase the uptake of various nutrients99. However, these tumour-associated vessels are often tortuous and leaky, which in turn leads to an increased hydrostatic pressure within the tumour100. This pressure increase in conjunction with a reduced lymphatic drainage leads to notably higher interstitial hydrostatic and osmotic pressures on cancerous tissue and is also responsible for the enhanced permeation and retention effect in cancer101. Pancreatic cancer has a substantially higher interstitial fluid pressure compared with healthy tissue, directly related to the highly stromal nature of this disease97.
Shear stress occurs when forces are applied in a transverse direction to a surface and material cross-section, causing a shear deformation9 (Fig. 1g). For example, in biology, shear is commonly associated with stress caused by fluid flow on the cell surface or tissue102. Cytoskeletal reorganization owing to shear stress has been studied for some time103. Given the considerable changes to cancer cell architecture, it is not surprising that research has also shown that cancerous cells have a lower shear modulus (denoted by G0) compared with their healthy counterparts, as one study highlights (MCF-10A G0 ≈ 3.8 Pa; MDA-MB-231 G0 ≈ 1.8 Pa)77. Furthermore, shear stress has been shown to have direct effects on metastasis based on its intensity — for instance, higher shear stress, typically associated with arteries, can lead to cell cycle arrest and is more likely to lead to cancer cell immune killing104,105.
Analysis of emergent mechanical properties of cells and tissues can give insights into how the modulation of various biomolecules, or how pharmacological treatments, can potentially increase or reduce cancer cell aggressiveness. The measurement methods, process scales and complex, dynamic response of biological systems represent a challenging set of conditions. However, the benefits associated with discovering new biomarkers for cancer, and obtaining a more thorough understanding of this disease cycle, outweigh the resources required for such research. Addressing the link between mechanical properties of cancerous samples and their pathology remains an active and promising area of research.
Measuring cellular-level and tissue-level mechanical properties
The most appropriate measurement technique for quantifying the mechanical properties of cells and tissues depends on the temporal and spatial scales of the sample and the required force sensitivity and throughput for the biological process being investigated. There are various approaches that can be used, but as suggested by the quantitative values shown in Supplementary Tables 1 and 2, the mechanical properties measured from live human cell and tissue specimens can vary by up to three orders of magnitude between different measurement systems. Besides assessing how closely new measurements compare with those reported in the literature, there is also little indication as to which approach results in the most accurate quantities for each case106. Considering this, researchers must appreciate the complex, highly heterogeneous mechanical landscape of cancer by evaluating the system through the lens of multiple properties. A more complete understanding of the cancer microenvironment could be achieved through the use and development of technologies capable of measuring cellular-level and tissue-level forces and mechanical properties over multiple length scales, timescales and dimensions.
Quantitative mechanical property measurements can be partially achieved with currently available technologies; however, each system has its own limitations that must be considered when using and interpreting the resulting data. Biomechanical techniques and instrumentation (Fig. 2), including micropipette aspiration (MPA), AFM, optical tweezers, microfluidic assays and traction force microscopy, help elucidate some of these properties11,107–112. We discuss each technique briefly, with emphasis on what insights it provides, its limitations and factors to consider when interpreting results.
Fig. 2 |. Mechanobiological techniques used to quantify multiple mechanical properties at both the cellular and tissue levels.
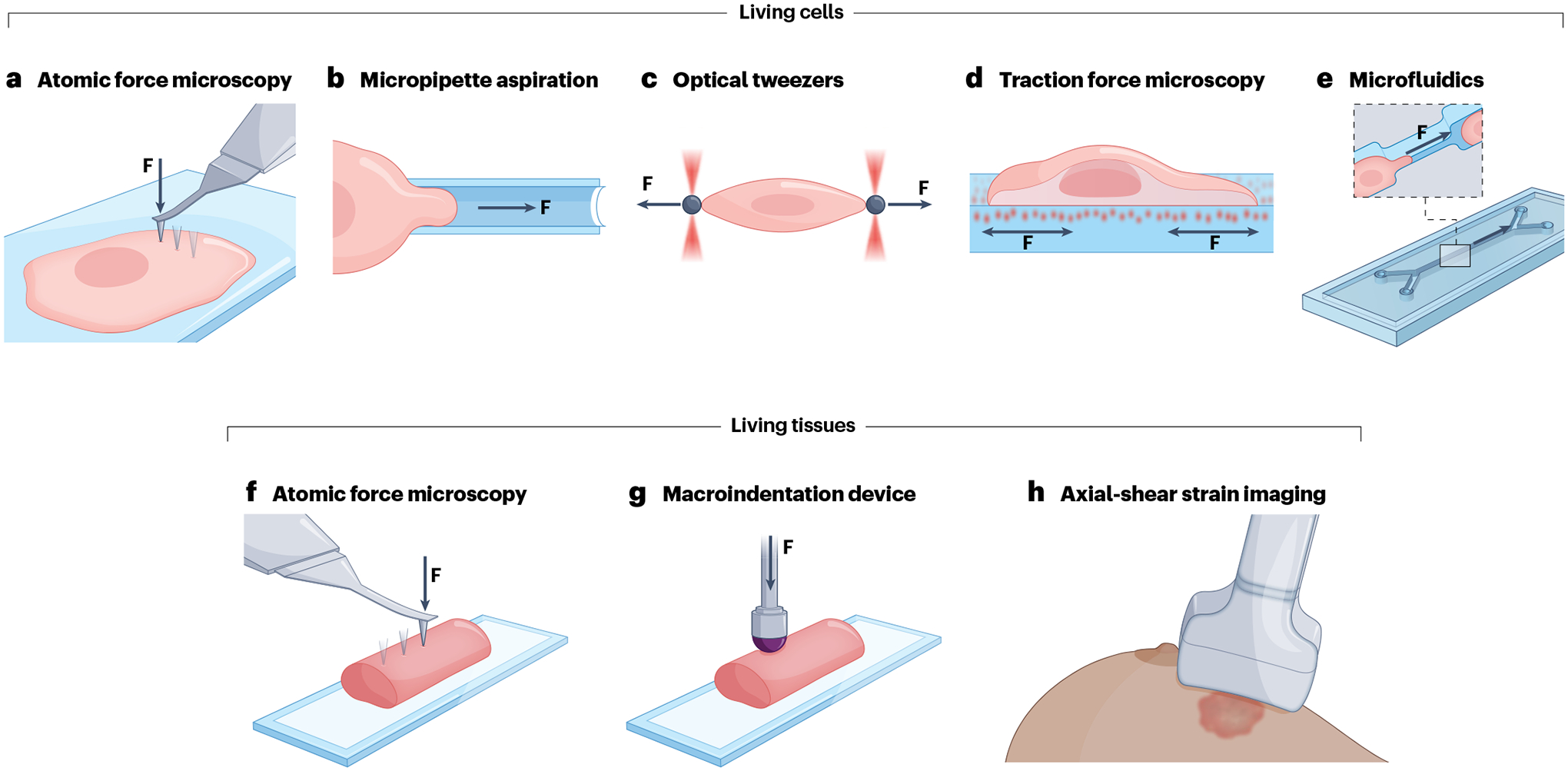
a, Atomic force microscopy has been used to determine the following mechanical properties: Young’s modulus, viscoelastic properties (storage and loss moduli), adhesion force, surface tension and hydrostatic pressure of single cancer cells. F denotes applied force. b, Micropipette aspiration has been used to measure the stiffness, viscoelastic properties (stiffness and viscosity), surface tension and hydrostatic pressure of individual cancer cells; it has not been used on tissues. c, Optical tweezers have only been used on single cancer cells to measure the stiffness, adhesion force and intracellular viscoelasticity (storage and loss moduli). d, Traction force microscopy has been used to measure the mechanical pulling and pushing forces that adherent single cancer cells exert on soft extracellular matrix-biomimetic hydrogels and has not been adapted on tissues. e, Microfluidic devices have been used to measure individual cancer cells compliance and stiffness. f, Atomic force microscopy has been used to measure only Young’s modulus on cancerous tissues. g, Macroindentation devices have been used to measure the stiffness and viscosity of cancerous tissues. h, Axial-shear strain imaging had been used to measure the stiffness of in vivo tumour lesions. Figure courtesy of Alan Hoofring.
AFM is a versatile type of scanning probe microscopy capable of nanometre spatial resolution113. This method works by bringing a flexible cantilever into contact with the surface of a sample114. AFM has been widely used for the analysis of multiple mechanical properties in various biological samples including cells (Fig. 2a) and tissues (Fig. 2f) across both normal and pathological states26,115–122. In certain ways, this technique represents a similar methodology as conducted by traditional macro-indentation analyses on tissues81 (Fig. 2g) but with a much higher spatial resolution. AFM instrumentation benefits from its ability to conduct scans with both nanometre resolution and ‘bulk’ micrometre-scale measurements, and the ability to perform measurements using indentations from hundreds of piconewtons to several micronewtons of force. The unique combination of high-resolution topographical imaging and force mapping makes AFM a useful tool for mechanobiological studies. Indeed, reports from the literature highlight that when measuring several mechanical properties (including Young’s modulus, viscosity, surface tension, hydrostatic pressure and adhesion forces) of cells or tissues, a large portion of all data collected was done using AFM, supporting the strength and versatility of this technique36,50,106. However, AFM is a low-throughput process that requires considerable technical skills from its users, and there exists an intrinsic tradeoff between image quality (measured in terms of spatial resolution) and scan acquisition time, which can complicate measurements for dynamic biological samples123. Given the experimental conditions, it is also crucial to understand how to carefully design the experiment, including proper probe selection and coating, scanning parameters and knowing the type of sample to be indented (and the region of interest on this cell or tissue).
Micropipette aspiration (Fig. 2b) is a common technique in the field of mechanobiology in which a cell is pulled into a glass micropipette by negative pressure while an optical system captures images of the interaction124,125. When the applied negative pressure is well characterized, the resulting optical images of the deformation are used to quantify how far the cell has been pulled into the pipette (known as the aspirating length)126. MPA data can be used to determine Young’s modulus by using a simple continuum model treating the biomaterial as a homogeneous, incompressible and linear-elastic half space126,127. In addition, surface tension and hydrostatic intracellular pressure can be measured by using Laplace’s law for spherical objects11. Although relatively cheap to perform, MPA is inherently limited owing to its low spatial and temporal resolutions and the resulting large deformations that are applied onto the cell128. In addition, the seal between the cell and micropipette is critical to acquiring reasonable and repeatable results, but there is no universal requirement for what seal quality is sufficient and the decision is left to the operator129.
Optical tweezers (Fig. 2c) use sharply focused laser beams to hold or apply forces to small objects130. The forces that arise due to photon scattering can be effectively used to manipulate small dielectric objects and generate forces in the femtonewton and piconewton range. These lower forces make optical tweezers ideal for molecular force analysis, with superior low force control to AFM. By calibrating the force applied to the bead in different ways, such as power spectrum analysis or ‘spring constant’ analysis of the optical trap, and by accurately tracking the position of the microbead, it is possible to determine the applied force on the bead. In turn, this information can be used to determine various mechanical properties (including mechanical compliance, adhesion force and surface tension) when various manipulations are carried out between the bead and a given target, such as a biomolecule or a cell131–133. Optical tweezer systems are inherently low throughput, with each bead being individually manipulated134.
Traction force microscopy (Fig. 2d) measures the forces objects exert on the surfaces they reside on or move across, typically a soft gel substrate135,136. This is accomplished by attaching the object of interest on an elastic surface and imaging the induced deformations — given an understanding of the mechanical properties of the substrate, traction forces can be calculated from these deflections. The technique can be done with no chemical perturbations, allowing for an efficient quantification of stresses exerted on the gel. After seeding the cells onto this substrate, images are taken while the cell is attached and after cell removal to visualize deformations in fluorescent beads embedded within the gel, allowing for the generation of a displacement field which can be converted into a traction force field using computational methods137–139.
Microfluidic assays (Fig. 2e) are versatile devices that can manipulate fluid flow in the micrometre range. They have been successfully applied in various biological studies140–144. Mechanical compliance, elastic and viscous properties can be measured, typically through optical analysis of cells; depending on the geometry of the microchannels, it may be possible to observe cells passing through narrow channels under various conditions, or to observe deformations under differing fluid flow rates or other phenomena145–148.
Although the aforementioned techniques have specific strengths for analysing various mechanical properties, one major caveat from a clinical standpoint is that physical contact is still required between a probing component and the cell or tissue being measured. To this end, several non-contact optical-based methods have been developed; one optical method that has recently gained interest is Brillouin microscopy, which makes use of the light scattering phenomenon of the same name. In brief, laser light is scattered from acoustic waves in a sample; components that are more solid produce higher frequency shifts149. In addition, clinicians have used ultrasound and magnetic resonance technologies to collect mechanical data non-invasively (Fig. 2h). These techniques work by emitting a pulse (either sonic or magnetic depending on the instrument), measuring the propagation of the waves in tissue and processing the acquired images to quantify various mechanical properties, typically in the form of an elastogram. Although completely non-invasive in comparison to the other methods listed in this Review, these techniques suffer from a comparatively lower spatial resolution — typically on the scale of millimetres to hundreds of micrometres, and thus certain cellular and subcellular information would be lost150,151.
Decoding mechanoregulation of cancer cells
When performing mechanobiological analyses, it is important to understand the role of cellular structures that can contribute to various mechanical properties. These can include structures such as the actin and microtubule cytoskeletons, plasma membrane and glycocalyx, among others; each of these play important roles in the integrity and mechanical properties of individual cells and, on a larger scale, tissues. To discern the relevance of certain biomechanical measurements, the corresponding molecular-mechanical properties must be investigated to paint a clearer picture. In this section, we briefly describe the complex relationship that exists between certain cellular structures and mechanical properties that promote transformation and metastasis. Note that changes in individual cellular material properties owing to remodelling of morphological-based structures is an active topic in cell biology, development and disease. By investigating specific cellular molecular and structural targets, scientists can better understand the implications of their mechanical measurements and hopefully better elucidate mechanisms for the targeted treatment of cancer.
Cellular surface
The cell surface is defined as the plasma membrane and associated structures: the extracellular glycocalyx and the intracellular actomyosin cortex. The plasma membrane is composed of multiple lipids that form a lipid bilayer, and cell surface receptors that sense mechanical signals from outside the cell and transduce the information to the intracellular cytoskeletal machinery. Forces sensed by the plasma membrane and glycocalyx are transmitted to the actomyosin cortex, which lies just beneath the plasma membrane to define cell shape and mechanical properties. The glycocalyx has also been shown to play a role in mechanosensation, cell–cell or cell–ECM adhesion, and membrane organization152. In cancer, various proteins and aberrant glycosylation can lead to changes in cell membrane structure, which in turn affects cellular mechanics, and has also been shown to affect adhesion kinetics153–155. One study showed that modulations to the composition of the glycocalyx can have direct physical effects on a cell — modulations to MUC13, a mucin known to be highly overexpressed in pancreatic cancer, have led to considerable reductions in Young’s modulus (Panc-1 control vector, E ≈ 8 kPa; Panc-1 MUC13 overexpressed, E ≈ 4 kPa) as well as a reduced cell–cell adhesion when overexpressed as evident by a reduced ability of cells to aggregate in vitro15. In addition, recent work has indicated that an overexpression of mucins can lead to an overall reduction in integrin binding and ECM–cell adhesion, yet drives clustering of integrins within the membrane, leading to the formation of mature adhesion complexes, which can enhance their function to foster signalling pathways that enhance metastasis156.
Cellular cytoskeleton
Transmission of mechanical forces from the cell surface to the inside of the cell leads to remodelling of the cytoskeleton, eventually determining cell fate, including polarization and migration. For contact-based mechanobiological measurements, perhaps the most critical part of the cytoskeleton is the actomyosin cortex, a filamentous actin meshwork connected to the membrane through several actin–membrane-binding molecules. The actin cortex has a critical role in regulating mechanotransduction and cell shape. Mechanotransduction is primarily achieved through the action of myosin, which pulls on actin filaments, generating tensile forces that in turn affect cell surface tension157,158. Previous studies have shown that softening of breast159,160, ovarian161,162, prostate24,163 and bladder cancer cells57,164 are due to dramatic remodelling of the actin and microtubule cytoskeletons of the cells. These findings are supported by other studies reporting that many features that are thought to be crucial for cellular homeostasis, including focal adhesions, actomyosin stress fibres, apical–basal polarization and cell–cell adhesion junctions, are either profoundly reduced in size and density or entirely missing from malignant and metastatic cancer cells165–168.
Together, these observations at the cellular level suggest that the molecular constituents of the cellular surface and cytoskeletal structures could be potential strategic targets for mechanical property-led targeted therapeutic interventions aimed at normalizing cellular structures, architectures and signalling.
Decoding mechanoregulation of cancer tissues
In cancer, tissue-level changes have been proposed as a novel ‘fingerprint’ for rapid diagnostic applications, with several studies indicating that cancerous tissue has substantially altered mechanical properties compared with its normal counterparts65,169–171. These mechanical changes are mainly a result of alterations in the TME. During tumour growth, individual cancer cells are exposed to several different types of mechanical forces such as tensile (contraction of actomyosin cortex owing to altered ECM stiffness), compressive (owing to cell growth in confined space) and shear (interstitial fluid pressure), all of which can affect signalling processes via mechanotransduction-based signalling36. The biomechanical, biochemical and structural properties of the TME vary depending on the histological differences between anatomical locations172. These variations in TME directly impact the mechanical properties and behaviour of individual cancer cells or the larger tumour173.
Of the different types of tissue within the human body, epithelial tissues are the most ubiquitous. Acting as a lining to organs, cavities and various ducts or glands, it can be found in nearly every part of the body and is the source of a vast majority of human cancers (carcinomas)2. Because of this, a considerable majority of the presented data in Supplementary Tables 1 and 2 detail changes in carcinoma-type cancers, highlighting that there is an absence of thorough data for other types, such as sarcoma, brain and spinal cord cancers, leukaemia and lymphoma. Thus, increased research is needed to better understand the role of mechanical properties in these varieties of cancers.
Given the diverse range of cancer cells and tissues listed in Supplementary Tables 1 and 2, a categorical overview of various systems in the human body is discussed in this section detailing cancers from various organs. Refer to the supplementary tables for all relevant data for each system.
Gastrointestinal cancers
Incorporating the stomach, intestines, liver, pancreas and gallbladder, the gastrointestinal system is involved in proper digestion, absorption of nutrients and the excretion of waste material from ingested food. Pancreatic cancer is one of the most aggressive gastrointestinal cancers and remains one of the highest causes of cancer-related death based on estimates from early 2024 (ref. 2). Pancreatic cancer has one of the lowest rates of survival, owing to clinical boundaries such as poor drug response and difficulty with early detection. Part of the reason for these factors is the highly desmoplastic nature of most pancreatic tumours174. Desmoplasia is a process whereby high levels of additional ECM proteins are deposited into the tumour, creating a dense barrier between the tumour and the rest of the organ, leading to a considerable stiffening of the cancerous tissue compared with normal adjacent (Fig. 3). This highly dense stromal microenvironment is a major reason pancreatic tumours are much stiffer than healthy tissue (healthy tissue range ~ 0.5–1.0 kPa; cancerous tissue range 0.75–5.5 kPa), or even cell-rich regions of tumours (cell-rich tumour, E ≈ 0.5 kPa; ECM-rich region, E ≈ 2 kPa)175, as reported in several recent studies81,176 (Supplementary Table 2). These changes have also been associated with profound changes in interstitial fluid pressure (healthy tissue P = 8–13 mmHg; cancer tissue P = 75–130 mmHg)97.
Fig. 3 |. Diverse changes in intratumoural microenvironmental architecture and mechanical properties for different cancers.

Tumours show different extracellular matrix (ECM) patterns, and cancer cells adopt differing morphologies owing to remodelling of their cytoskeletons, plasma membrane and glycocalyx and corresponding changes in mechanical properties. Summarized here are mechanical properties and structural changes associated with three malignant, highly desmoplastic cancers: breast ductal adenocarcinoma (part a), pancreatic ductal adenocarcinoma (part b) and ovarian cancer (part c). This illustration depicts the architectural, physical and mechanical complexities in each intratumoural microenvironment with progressively acquired changes in cancer cell behaviour and ECM architecture which can affect efficacy of chemotherapy and immunotherapy treatments, as well as enhance tumour progression, aggression and metastasis. Question marks denote that no quantitative results were found in the scientific literature; these are currently open questions. There are currently no comprehensive studies on the material properties of human ovarian tissue comparing normal and cancerous tissue sections. Figure courtesy of Alan Hoofring.
Reproductive cancers
Of note for this Review, the breasts are included in this section as they are commonly considered accessory organs to the female reproductive system for their role in milk production. In women, breast cancer is the most common and one of the most lethal malignancies2. Breast cancer, as with other cancers, undergoes dramatic structural changes during tumorigenesis (Fig. 3) and is one of the most highly studied cancers from a mechanobiological standpoint. As highlighted in Supplementary Table 1, breast cancer cells show softening with malignant transformation. For instance, one study took breast and ovarian cells from different patients, and with AFM analysis showed that their cancerous cells were considerably softer (0.4–0.5 kPa for ovarian; 0.2 ± 0.1 kPa for breast) compared with healthy cells extracted from the same patient (2.8 ± 1.7, 2.5 ± 1.1, 2.4 ± 1.0 and 2.4 ± 1.1 kPa, respectively, for ovarian; 1.7 ± 0.95 kPa for breast)161. At a tissue level, breast cancer tumour tissues at different stages were extracted from patients and subject to AFM biomechanical analyses. This work reported a bimodal distribution of stiffness in the cancerous tissue, with peaks at 0.47 ± 0.15 kPa and 1.54 ± 0.17 kPa compared with the healthy tissue average of 1.16 ± 0.2 kPa. These differing peaks were related to either a cell-rich (softer region) or an ECM-rich (stiffer region) area within the tumour tissue24. Similar results were observed from another independent work, showing that adjacent normal tissue had an average stiffness around 0.4 kPa, whereas tumour tissue was greater than 5 kPa (ref. 41).
A familiar trend emerges when observing the data for breast cancer at a cellular and tissue level. Even considering the various experimental parameters, methods used and cell lines being investigated (Supplementary Table 1), both the solid (normal E = 0.165–95 kPa) and viscous (normal η = 0.01–60 Pa s) components tend to be reduced at the cellular level (cancer E = 0.117–87.3 kPa; cancer η = 0.005–30 Pa s)24,33,94,159–161,177–186. At a tissue level (Supplementary Table 2), a stiffening of cancerous lesions is clear compared with healthy tissue (normal E = 0.4–57 kPa; cancer E = 0.47–490 kPa)24,41,64,187–190.
Urinary cancers
Incorporating the kidneys, ureters, bladder and urethra, the purpose of the urinary system is to filter blood via removing waste products and excess water, in turn regulating its volume, pressure and pH. On the basis of estimates as of early 2024, bladder cancer remains one of the most common and lethal malignancies, especially in the male population within the USA2. Bladder cancer is one of the most widely studied cancer models in the mechanobiological field. At a cellular level, cancerous cells are widely reported to be softer than their healthy counterparts; for example, one study using AFM reported that healthy Hu609 and HCV29 cells were considerably stiffer (12.9 ± 4.8 kPa and 10.0 ± 4.6 kPa, respectively) when compared with the cancerous BC3726, T24 and Hu456 cells (1.4 ± 0.7, 1.0 ± 0.5 and 0.4 ± 0.3 kPa, respectively)191. At a tissue level, softening of cancerous bladder tissues compared with healthy tissue has been reported, unlike nearly all other types of cancers reported in Supplementary Table 2. Using a nanoindenter, researchers measured a median stiffness of 33 kPa in the normal muscle tissue taken from a patient with cancer, with a median value of only 2 kPa with neoplastic infiltration of tumour cells in tissue from the same patient192.
Across the various studies conducted on bladder cancer, the stiffness data for healthy bladder cells were reported to be approximately between 0.5 kPa and 33 kPa, whereas cancerous cells were shown to be softer, with Young’s modulus values ranging from 0.36 kPa to 13 kPa57,76,95,164,191,193–195 (Supplementary Table 1). At a tissue level, healthy values ranged from 14.5 kPa to 33 kPa, whereas cancerous tissue fell to roughly 2–2.7 kPa (refs. 76,192) (Supplementary Table 2). This range can partly be explained by the fact that differing techniques (different types of contact mechanics via AFM analysis) were used to obtain these data.
Integumentary cancers
Acting as the outermost protective layer of the body, comprising skin, hair, nails, various glands and nerves, the integumentary system is a physical barrier between the rest of the body and the outside environment. One of the most common and lethal skin cancers is melanoma2. Metastatic melanoma has been demonstrated to have considerably softer cells in a cancerous state compared with its healthy counterparts. In one study, AFM measurements uncovered that healthy melanocytes had an average Young’s modulus of 14.3 ± 0.8 kPa, whereas there was a gradual decrease in stiffness from primary tumour (VGP, E = 9.91 ± 0.55 kPa), to localized skin metastasis (WM239, E = 7.8 ± 0.61 kPa), to a distant metastasis in lung tissue (A375P, E = 5.7 ± 0.61 kPa)196. Using a different technique based on optical tweezers, the compliance of oral squamous cells was measured. The study showed that healthy cells had a lower overall compliance (OKF-4: δ = 0.69 ± 0.46 × 10−3 Pa−1) when compared with squamous cell carcinoma cells (CAL-33: δ = 1.99 ± 1.63 × 10−3 Pa−1). This increased compliance in cancerous cells indicated that the cells were more easily deformed66 (Supplementary Table 1).
Neurological cancers
Comprising of the major nerves leading to the spinal cord and the brain, this system is responsible for cognition as well as controlling both active and autonomic processes in the body. Unfortunately, brain cancers still have comparatively low survival rates, with an estimated 34% 5-year survival based on data over the past few years2. Brain tissue is one of the softest tissues in the human body197. However, there is a heterogeneity in the mechanical changes that have been reported with various types of brain cancers. One study that used human tumour cells grown in a mouse model indicated that healthy brain tissue had a higher Young’s modulus (~0.4 kPa) compared with human cell line tumour models (U87: E ≈ 0.15 kPa; MGG8: E ≈ 0.1 kPa; BT475: E ≈ 0.1 kPa)198. However, a different study showed the opposite effect in glioblastomas: using freshly frozen human brain biopsies with AFM analysis, the researchers noted that non-malignant gliosis had the lowest overall ECM stiffness (E ≈ 10–180 Pa), with noticeable stiffening in low grade glioma (E ≈ 50–1,400 Pa) and glioblastoma (E ≈ 70–13,500 Pa)199 (Supplementary Table 2).
Endocrine cancers
The endocrine system is a series of glands and organs that release hormones (messenger molecules involved in numerous functions throughout the body). One of the most common endocrine cancers is thyroid cancer. Although survival rates are generally quite high for thyroid cancer, recent data suggest that the number of cases has increased in recent years2. The mechanobiology of endocrine cancer has been largely unstudied, with only a few investigations of changes in mechanical properties of thyroid cancer cells and thyroid tumour tissues. In one such study, the viscoelastic parameters of thyroid cancer cells were quantified using AFM indentations, which yielded a reduction in the viscous and elastic parameters for cancerous cells grown on Petri dishes (median values: normal cell EPetri = 1,190 Pa, ηPetri = 496 Pa s; cancer cell EPetri = 721 Pa, ηPetri = 357 Pa s)80. Another independent study reported similar results to cells being grown on Petri dishes, in which cancerous thyroid cells had a lower median range of stiffness compared with healthy cells (normal range 2,211–6,879 Pa, cancer range 1,189–1,365 Pa)200 (Supplementary Table 1). Tissue studies show a similar effect to other cancers: the cancerous thyroid stiffens compared with healthy (healthy tissue ~ 10–21.1 kPa; cancer tissue ~ 38–150 kPa)201,202 (Supplementary Table 2).
Outlook
An understanding of the relationship among mechanical properties, molecular interactions and dynamics of cancer will likely enhance understanding of its development, morphogenesis and proliferation. Through novel therapeutic interventions including pharmacological, immunological or a combination thereof, it is possible to directly destabilize the mechanical properties of the cell surface, cytoskeleton or the intratumoural microenvironment, which could help to halt metastatic progression. In comparison to the typical assessment of various biochemical assays for cancer diagnosis, analysing changes in the mechanical properties of cells and tissues can give a more rapid analysis without requiring chemical manipulation (fixation or labelling) of the altered phenotype, which in turn can yield information on biological processes, diagnostics or the effect of precision medicine more quickly.
As developmental and molecular cellular biologists generate more interesting data about the structure and composition of cells and tissues, new theoretical and experimental approaches combining biology, chemistry, mathematical modelling, physics and engineering will need to be developed to understand the complex 3D environment of healthy and disease-state tissue-level properties203. An important question to address is whether mechanical changes in the actomyosin cytoskeleton, a critical scaffold ordinarily regulated by molecular protein levels and dynamics of individual cells, are sufficient to fully explain the complexity of tissue structures on their own. For instance, one could use mechanical models for the force transmission and balance of the actomyosin cytoskeleton to simulate the combined effects of actomyosin protein dynamics and ask whether this yields the expected structure and architecture of an epithelium of interest. Another pressing question in cancer biology is whether tumour progression is driven only by changes in mechanical properties of cancer cells and the tumour ECM, or whether other key players in the TME such as infiltrating immune cells and transformed cancer-associated fibroblasts modify the overall intratumoural mechanical properties to drive tumour aggression204–207. For example, one could measure the mechanical properties of migrating immune cells intratumourally or in healthy tissues and determine whether these properties changed. Therefore, the intratumoural mechanical niche is one of the hallmarks of cancer that is poorly understood. Together, understanding how mechanobiological principles interplay with the various hallmarks of cancer may lead to the discovery and development of novel strategies to improve cancer therapies208. In conclusion, it is possible that expanding our study of mechanical properties to better appreciate the interplay between different properties with changes in cellular-level and tissue-level structure and composition will promote a higher level of understanding of the unique behaviours between cancer types.
Ultimately however, the problem of applying mechanobiological principles to the clinic remains a challenging obstacle for providing meaningful information in a timely manner. As the technical expertise required to use many of the currently available instruments and analysis systems remains complex, it creates a barrier for the clinic without expert assistance in the collection, analysis and interpretation of results to better aid in either diagnostic or therapeutic applications of mechanical properties to various disease states, especially cancer.
Supplementary Material
Key points.
Changes in molecular-level, cellular-level and tissue-level mechanical properties across multiple timescales and dimensions play a critical role in driving oncogenesis, tumour organization and disease progression.
An array of cellular and tissue mechanical properties, including surface tension, hydrostatic pressure, elasticity, viscosity and adhesion, can provide greater insights into distinguishing unique characteristics of different cancers.
Comprehensive supplementary tables gathering quantitative mechanical properties values of human cancer cells and tissues provide details of cancer development from a biomechanical perspective.
Quantification of multiple physical parameters of cells and tissues provides a multiscale, multidimensional and multiparametric understanding of physical oncology for the development of prognostic and diagnostic tools.
Acknowledgements
The authors acknowledge the help of A. Hoofring (Medical Arts Design Section, NIH) with preparation of the figures. The authors thank M. Mezher and A. Bluem for critical reading and thoughtful comments. The authors sincerely apologize to the many researchers whose relevant work we were unable to cite owing to space limitations. The authors acknowledge support by the intramural funding of the Division of Intramural Research Program at the National Institute of Biomedical Imaging and Bioengineering with grant ZIA-EB000094 and the NIH central funds for the NIH Distinguished Scholars Program award.
Glossary
- Actomyosin
Contractile filamentous actin network inside the cell that helps provide shape, motility and force generation for a cell. The actomyosin cytoskeleton consists of filamentous actin, non-muscle myosin II motor proteins and regulatory actin-binding proteins.
- Adhesion force
In biological terms, adhesion occurs directly between neighbouring cells via specialized proteins on the cell surface and indirectly via the extracellular matrix, both of which allow cells to communicate with one another and respond to their environment through processes such as signal transduction. In physics terms, adhesion is a type of attractive force that occurs between different objects through mechanical forces and electrostatic interactions.
- Cellular tension
The surface force needed to stretch the cell, which is dependent on its plasma membrane lipid composition, extracellular glycocalyx and the contractile forces of the intracellular actin cytoskeleton, all of which must be overcome to deform the cell.
- Cytoskeletons
Complex skeletal networks of proteins that provide structure to cells and play a major role in organization, motility and mechanotransduction. Several major components of this system include actin filaments, microtubules and intermediate filaments, which may be the stiffest structures in a cell.
- Glycocalyx
An extra-membranous coating rich with glycans and various transmembrane proteins, which typically act as a barrier against the environment.
- Intracellular forces
The different types of physical forces that exist within cells to maintain cellular homeostasis and cell-specific normal function. The major forces acting within a cell are tensional and compressive forces acting at the surface and cytoskeleton and traction forces at focal adhesions.
- Mechanosensation
The ability of a cell to sense and respond to mechanical stimuli in its microenvironment, including different types of stresses, strains and forces.
- Morphogenesis
The biological process that includes the development of cells, tissues or organs into a specified shape. This process is fundamental for developmental biology and tissue growth, both regulated and unregulated. Morphogenesis is also responsible for cellular differentiation.
- Tumour microenvironment
A complex, highly heterogeneous space consisting of a mixture of cancer cells, extracellular matrix, cancer-associated fibroblasts, immune cells and lymphatic vessels.
- Viscoelasticity
The mechanical behaviour of most soft ‘squishy’ materials exhibits both storage of elastic energy (solid behaviour) and dissipation of mechanical energy (fluid behaviour) when undergoing deformation. Viscoelasticity is a measurable retarded tendency of a material to return to its original shape after an applied force is removed.
- Viscosity
The resistance of a liquid to flow, the deformation of which is dependent on energy being dissipated or lost by its internal friction, or force per unit area and time (Pa s). More viscous liquids have a higher internal friction.
- Young’s modulus
A measure of tensile elasticity that indicates how much a material can deform for an applied force. It is defined as the ratio between stress, the force per unit area, and strain, extension per unit length (dimensionless). For soft materials such as living cells and tissues, it is applicable before the elastic region limit in which linearity breaks down and plastic deformation occurs. The higher the value is for Young’s modulus, the stiffer the material.
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information The online version contains supplementary material available at https://doi.org/10.1038/s42254-024-00707-2. References [209–232] are cited in the supplementary material.
References
- 1.Sung H et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin 71, 209–249 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Giaquinto AN & Jemal A Cancer Statistics, 2024. CA Cancer J. Clin 74, 12–49 (2024). [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D & Robert A Weinberg, the hallmarks of cancer. Cell 100, 57–70 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Hanahan D & Robert A Weinberg, hallmarks of cancer: the next generation. Cell 144, 646–674 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Hanahan D Hallmarks of cancer: new dimensions. Cancer Discov. 12, 31–46 (2022). [DOI] [PubMed] [Google Scholar]
- 6.Lu P, Weaver VM & Werb Z The extracellular matrix: a dynamic niche in cancer progression. J. Cell Biol 196, 395–406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langley RR & Fidler IJ Tumor cell–organ microenvironment interactions in the pathogenesis of cancer metastasis. Endocr. Rev 28, 297–321 (2007). [DOI] [PubMed] [Google Scholar]
- 8.Friedl P & Gilmour D Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol 10, 445–457 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Wirtz D, Konstantopoulos K & Searson PC The physics of cancer: the role of physical interactions and mechanical forces in metastasis. Nat. Rev. Cancer 11, 512–522 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar S & Weaver VM Mechanics, malignancy, and metastasis: the force journey of a tumor cell. Cancer Metastasis Rev 28, 113–127 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinevez J-Y et al. Role of cortical tension in bleb growth. Proc. Natl Acad. Sci. USA 106, 18581–18586 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logue JS et al. Erk regulation of actin capping and bundling by Eps8 promotes cortex tension and leader bleb-based migration. eLife 4, e08314 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Logue JS, Cartagena-Rivera AX & Chadwick RS c-Src activity is differentially required by cancer cell motility modes. Oncogene 37, 2104–2121 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams A Jr et al. Survey of cancer cell anatomy in nonadhesive confinement reveals a role for filamin-A and fascin-1 in leader bleb-based migration. Mol. Biol. Cell 32, 1772–1791 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massey AE et al. Biophysical changes caused by altered MUC13 expression in pancreatic cancer cells. Micron 130, 102822 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Z, Costell M & Fassler R Integrin activation by talin, kindlin and mechanical forces. Nat. Cell Biol 21, 25–31 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Krisenko MO et al. Nanomechanical property maps of breast cancer cells as determined by multiharmonic atomic force microscopy reveal Syk-dependent changes in microtubule stability mediated by MAP1B. Biochemistry 54, 60–68 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Efremov YM et al. Mapping heterogeneity of cellular mechanics by multi-harmonic atomic force microscopy. Nat. Protoc 13, 2200–2216 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Parvini CH, Cartagena-Rivera AX & Solares SD Viscoelastic parameterization of human skin cells characterize material behavior at multiple timescales. Commun. Biol 5, 17 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cartagena-Rivera AX et al. Fast, multi-frequency and quantitative nanomechanical mapping of live cells using the atomic force microscope. Sci. Rep 5, 11692 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang S & Ingber DE Cell tension, matrix mechanics, and cancer development. Cancer Cell 8, 175–176 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Purkayastha P, Jaiswal MK & Lele TP Molecular cancer cell responses to solid compressive stress and interstitial fluid pressure. Cytoskeleton 78, 312–322 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalili AA & Ahmad MR A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci 16, 18149–18184 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lekka M et al. Cancer cell detection in tissue sections using AFM. Arch. Biochem. Biophys 518, 151–156 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Chaudhuri O et al. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 584, 535–546 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dufrêne YF et al. Imaging modes of atomic force microscopy for application in molecular and cell biology. Nat. Nanotechnol 12, 295–307 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Mulligan JA et al. Emerging approaches for high-resolution imaging of tissue biomechanics with optical coherence elastography. IEEE J. Sel. Top. Quantum Electron 22, 246–265 (2016). [Google Scholar]
- 28.Isermann P & Lammerding J Nuclear mechanics and mechanotransduction in health and disease. Curr. Biol 23, R1113–R1121 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ayad NME, Kaushik S & Weaver VM Tissue mechanics, an important regulator of development and disease. Philos. Trans. R. Soc. B Biol. Sci 374, 20180215 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lambert AW, Pattabiraman DR & Weinberg RA Emerging biological principles of metastasis. Cell 168, 670–691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friedl P & Wolf K Tumour-cell invasion and migration: diversity and escape mechanisms. Nat. Rev. Cancer 3, 362–374 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Zhovmer AS et al. Mechanical counterbalance of kinesin and dynein motors in a microtubular network regulates cell mechanics, 3D architecture, and mechanosensing. ACS Nano 15, 17528–17548 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Calzado-Martín A et al. Effect of actin organization on the stiffness of living breast cancer cells revealed by peak-force modulation atomic force microscopy. ACS Nano 10, 3365–3374 (2016). [DOI] [PubMed] [Google Scholar]
- 34.Swaminathan V et al. Mechanical stiffness grades metastatic potential in patient tumor cells and in cancer cell lines. Cancer Res 71, 5075–5080 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen AV et al. Stiffness of pancreatic cancer cells is associated with increased invasive potential. Integr. Biol 8, 1232–1245 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suresh S Biomechanics and biophysics of cancer cells. Acta Biomater 3, 413–438 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alibert C, Goud B & Manneville JB Are cancer cells really softer than normal cells? Biol. Cell 109, 167–189 (2017). [DOI] [PubMed] [Google Scholar]
- 38.Mouw JK et al. Tissue mechanics modulate microRNA-dependent PTEN expression to regulate malignant progression. Nat. Med 20, 360–367 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gkretsi V & Stylianopoulos T Cell adhesion and matrix stiffness: coordinating cancer cell invasion and metastasis. Front. Oncol 8, 145–145 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGrail DJ, Kieu QM & Dawson MR The malignancy of metastatic ovarian cancer cells is increased on soft matrices through a mechanosensitive Rho–ROCK pathway. J. Cell Sci 127, 2621–2626 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Acerbi I et al. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. Quant. Biosci. Nano Macro 7, 1120–1134 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Przybyla L, Muncie JM & Weaver VM Mechanical control of epithelial-to-mesenchymal transitions in development and cancer. Annu. Rev. Cell Dev. Biol 32, 527–554 (2016). [DOI] [PubMed] [Google Scholar]
- 43.Jain RK Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 26, 605–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Discher DE, Janmey P & Wang Y-L Tissue cells feel and respond to the stiffness of their substrate. Science 310, 1139–1143 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Mammoto T, Mammoto A & Ingber DE Mechanobiology and developmental control. Annu. Rev. Cell Dev. Biol 29, 27–61 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Keller R Physical biology returns to morphogenesis. Science 338, 201–203 (2012). [DOI] [PubMed] [Google Scholar]
- 47.Heisenberg C-P & Bellaïche Y Forces in tissue morphogenesis and patterning. Cell 153, 948–962 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Burla F et al. From mechanical resilience to active material properties in biopolymer networks. Nat. Rev. Phys 1, 249–263 (2019). [Google Scholar]
- 49.Nia HT, Munn LL & Jain RK Physical traits of cancer. Science 370, eaaz0868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guimarães CF et al. The stiffness of living tissues and its implications for tissue engineering. Nat. Rev. Mater 5, 351–370 (2020). [Google Scholar]
- 51.Elosegui-Artola A The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr. Opin. Cell Biol 72, 10–18 (2021). [DOI] [PubMed] [Google Scholar]
- 52.Guz N et al. If cell mechanics can be described by elastic modulus: study of different models and probes used in indentation experiments. Biophys. J 107, 564–575 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ohring M Mechanical behavior of solids. in Engineering Materials Science Vol. 299 (ed. Ohring M) Ch. 7 (Academic Press, 1995). [Google Scholar]
- 54.Moeendarbary E & Harris AR Cell mechanics: principles, practices, and prospects. Wiley Interdiscip. Rev. Syst. Biol. Med 6, 371–388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gavara N A beginner’s guide to atomic force microscopy probing for cell mechanics. Microsc. Res. Tech 80, 75–84 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zemła J et al. Atomic force microscopy as a tool for assessing the cellular elasticity and adhesiveness to identify cancer cells and tissues. Semin. Cell Dev. Biol 73, 115–124 (2018). [DOI] [PubMed] [Google Scholar]
- 57.Canetta E et al. Discrimination of bladder cancer cells from normal urothelial cells with high specificity and sensitivity: combined application of atomic force microscopy and modulated Raman spectroscopy. Acta Biomater 10, 2043–2055 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Smolyakov G et al. Elasticity, adhesion, and tether extrusion on breast cancer cells provide a signature of their invasive potential. ACS Appl. Mater. Interfaces 8, 27426–27431 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Kubiak A et al. Nanomechanics in monitoring the effectiveness of drugs targeting the cancer cell cytoskeleton. Int. J. Mol. Sci 21, 8786 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Le Cigne A et al. Analysis of the effect of LRP-1 silencing on the invasive potential of cancer cells by nanomechanical probing and adhesion force measurements using atomic force microscopy. Nanoscale 8, 7144–7154 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Iturri J et al. Resveratrol-induced temporal variation in the mechanical properties of MCF-7 breast cancer cells investigated by atomic force microscopy. Int. J. Mol. Sci 20, 3275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park S & Lee YJ AFM-based dual nano-mechanical phenotypes for cancer metastasis. J. Biol. Phys 40, 413–419 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Weder G et al. Increased plasticity of the stiffness of melanoma cells correlates with their acquisition of metastatic properties. Nanomed. Nanotechnol. Biol. Med 10, 141–148 (2014). [DOI] [PubMed] [Google Scholar]
- 64.Plodinec M et al. The nanomechanical signature of breast cancer. Nat. Nanotechnol 7, 757–765 (2012). [DOI] [PubMed] [Google Scholar]
- 65.Stylianou A, Lekka M & Stylianopoulos T AFM assessing of nanomechanical fingerprints for cancer early diagnosis and classification: from single cell to tissue level. Nanoscale 10, 20930–20945 (2018). [DOI] [PubMed] [Google Scholar]
- 66.Remmerbach TW et al. Oral cancer diagnosis by mechanical phenotyping. Cancer Res 69, 1728–1732 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Corominas-Murtra B & Petridou NI Viscoelastic networks: forming cells and tissues. Front. Phys 9, 666916 (2021). [Google Scholar]
- 68.Efremov YM, Okajima T & Raman A Measuring viscoelasticity of soft biological samples using atomic force microscopy. Soft Matter 16, 64–81 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Amador C et al. Loss tangent and complex modulus estimated by acoustic radiation force creep and shear wave dispersion. Phys. Med. Biol 57, 1263–1282 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ferguson BG Calculation of the loss tangent for viscoelastic materials using the triple bar composite resonance technique. J. Acoust. Soc. Am 76, 1577–1579 (1984). [Google Scholar]
- 71.Mohammadalipour A, Burdick MM & Tees DFJ Viscoelasticity measurements reveal rheological differences between stem-like and non-stem-like breast cancer cells. Cell. Mol. Bioeng 10, 235–248 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie Y et al. The viscoelastic behaviors of several kinds of cancer cells and normal cells. J. Mech. Behav. Biomed. Mater 91, 54–58 (2019). [DOI] [PubMed] [Google Scholar]
- 73.Garcia PD, Guerrero CR & Garcia R Nanorheology of living cells measured by AFM-based force–distance curves. Nanoscale 12, 9133–9143 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Efremov YM et al. Measuring nanoscale viscoelastic parameters of cells directly from AFM force–displacement curves. Sci. Rep 7, 1541 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerrero CR, Garcia PD & Garcia R Subsurface imaging of cell organelles by force microscopy. ACS Nano 13, 9629–9637 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gnanachandran K et al. Discriminating bladder cancer cells through rheological mechanomarkers at cell and spheroid levels. J. Biomech 144, 111346 (2022). [DOI] [PubMed] [Google Scholar]
- 77.Mandal K et al. Mapping intracellular mechanics on micropatterned substrates. Proc. Natl Acad. Sci. USA 113, E7159–E7168 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang G et al. Mechanical properties of hepatocellular carcinoma cells. World J. Gastroenterol 8, 243–246 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang X et al. Effects of substrate stiffness on the viscoelasticity and migration of prostate cancer cells examined by atomic force microscopy. Beilstein J. Nanotechnol 13, 560–569 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rianna C & Radmacher M Comparison of viscoelastic properties of cancer and normal thyroid cells on different stiffness substrates. Eur. Biophys. J 46, 309–324 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Rubiano A et al. Viscoelastic properties of human pancreatic tumors and in vitro constructs to mimic mechanical properties. Acta Biomater 67, 331–340 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Martino F et al. Cellular mechanotransduction: from tension to function. Front. Physiol 9, 824 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Svitkina TM Actin cell cortex: structure and molecular organization. Trends Cell Biol 30, 556–565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sitarska E & Diz-Munoz A Pay attention to membrane tension: mechanobiology of the cell surface. Curr. Opin. Cell Biol 66, 11–18 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsujita K et al. Homeostatic membrane tension constrains cancer cell dissemination by counteracting BAR protein assembly. Nat. Commun 12, 5930 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hetmanski JHR et al. Membrane tension orchestrates rear retraction in matrix-directed cell migration. Dev. Cell 51, 460–475.e10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paszek MJ et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 8, 241–254 (2005). [DOI] [PubMed] [Google Scholar]
- 88.Janiszewska M, Primi MC & Izard T Cell adhesion in cancer: beyond the migration of single cells. J. Biol. Chem 295, 2495–2505 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cerutti C & Ridley AJ Endothelial cell–cell adhesion and signaling. Exp. Cell Res 358, 31–38 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Honig B & Shapiro L Adhesion protein structure, molecular affinities, and principles of cell–cell recognition. Cell 181, 520–535 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xu GK, Qian J & Hu J The glycocalyx promotes cooperative binding and clustering of adhesion receptors. Soft Matter 12, 4572–4583 (2016). [DOI] [PubMed] [Google Scholar]
- 92.Läubli H & Borsig L Altered cell adhesion and glycosylation promote cancer immune suppression and metastasis. Front. Immunol 10, 2120 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Makrilia N et al. Cell adhesion molecules: role and clinical significance in cancer. Cancer Invest 27, 1023–1037 (2009). [DOI] [PubMed] [Google Scholar]
- 94.Dumitru AC et al. Label-free imaging of cholesterol assemblies reveals hidden nanomechanics of breast cancer cells. Adv. Sci 7, 2002643 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lekka M et al. Characterization of N-cadherin unbinding properties in non-malignant (HCV29) and malignant (T24) bladder cells. J. Mol. Recognit 24, 833–842 (2011). [DOI] [PubMed] [Google Scholar]
- 96.Chugh M, Munjal A & Megason SG Hydrostatic pressure as a driver of cell and tissue morphogenesis. Semin. Cell Dev. Biol 131, 134–145 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Provenzano PP et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Stylianopoulos T et al. Coevolution of solid stress and interstitial fluid pressure in tumors during progression: implications for vascular collapse. Cancer Res 73, 3833–3841 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishida N et al. Angiogenesis in cancer. Vasc. Health Risk Manag 2, 213–219 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McDonald DM & Baluk P Significance of blood vessel leakiness in cancer. Cancer Res 62, 5381–5385 (2002). [PubMed] [Google Scholar]
- 101.Northcott JM et al. Feeling stress: the mechanics of cancer progression and aggression. Front. Cell Dev. Biol 6, 17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tzima E et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, 426–431 (2005). [DOI] [PubMed] [Google Scholar]
- 103.Janmey PA et al. Viscoelastic properties of vimentin compared with other filamentous biopolymer networks. J. Cell Biol 113, 155–160 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chang SF et al. Tumor cell cycle arrest induced by shear stress: roles of integrins and Smad. Proc. Natl Acad. Sci. USA 105, 3927–3932 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Huang Q et al. Fluid shear stress and tumor metastasis. Am. J. Cancer Res 8, 763–777 (2018). [PMC free article] [PubMed] [Google Scholar]
- 106.Wu PH et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 15, 491–498 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cartagena-Rivera AX et al. Actomyosin cortical mechanical properties in nonadherent cells determined by atomic force microscopy. Biophys. J 110, 2528–2539 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cartagena-Rivera AX et al. Apical surface supracellular mechanical properties in polarized epithelium using noninvasive acoustic force spectroscopy. Nat. Commun 8, 1030 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hénon S et al. A new determination of the shear modulus of the human erythrocyte membrane using optical tweezers. Biophys. J 76, 1145–1151 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lieber AD et al. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr. Biol 23, 1409–1417 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Lee HY et al. Noninvasive in vivo imaging reveals differences between tectorial membrane and basilar membrane traveling waves in the mouse cochlea. Proc. Natl Acad. Sci. USA 112, 3128–3133 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Koch TM et al. 3D traction forces in cancer cell invasion. PLoS ONE 7, e33476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Miller EJ et al. Sub-nanometer resolution imaging with amplitude–modulation atomic force microscopy in liquid. J. Vis. Exp 118, 54924 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Belec L & Joliff Y Mechanically affected zone in AFM force measurements — focus on actual probe tip geometry. Mater. Des 104, 217–226 (2016). [Google Scholar]
- 115.Lal R & John SA Biological applications of atomic force microscopy. Am. J. Physiol. Cell Physiol 266, C1–C21 (1994). [DOI] [PubMed] [Google Scholar]
- 116.Cho DH, Aguayo S & Cartagena-Rivera AX Atomic force microscopy-mediated mechanobiological profiling of complex human tissues. Biomaterials 303, 122389 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Raman A et al. Mapping nanomechanical properties of live cells using multi-harmonic atomic force microscopy. Nat. Nanotechnol 6, 809–814 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Cartagena A & Raman A Local viscoelastic properties of live cells investigated using dynamic and quasi-static atomic force microscopy methods. Biophys. J 106, 1033–1043 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Meister A et al. FluidFM: combining atomic force microscopy and nanofluidics in a universal liquid delivery system for single cell applications and beyond. Nano Lett 9, 2501–2507 (2009). [DOI] [PubMed] [Google Scholar]
- 120.Allison DP et al. Atomic force microscopy of biological samples. WIREs Nanomed. Nanobiotechnol 2, 618–634 (2010). [DOI] [PubMed] [Google Scholar]
- 121.Alsteens D et al. Force-induced formation and propagation of adhesion nanodomains in living fungal cells. Proc. Natl Acad. Sci. USA 107, 20744–20749 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pfreundschuh M, Hensen U & Müller DJ Quantitative imaging of the electrostatic field and potential generated by a transmembrane protein pore at subnanometer resolution. Nano Lett 13, 5585–5593 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Huang P & Andersson SB On detection and estimation in atomic force microscopy at different scan speeds*. IFAC Proc. Vol 46, 153–159 (2013). [Google Scholar]
- 124.Hochmuth RM Micropipette aspiration of living cells. J. Biomech 33, 15–22 (2000). [DOI] [PubMed] [Google Scholar]
- 125.Hogan B et al. Characterizing cell adhesion by using micropipette aspiration. Biophys. J 109, 209–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.González-Bermúdez B, Guinea GV & Plaza GR Advances in micropipette aspiration: applications in cell biomechanics, models, and extended studies. Biophys. J 116, 587–594 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Theret DP et al. The application of a homogeneous half-space model in the analysis of endothelial cell micropipette measurements. J. Biomech. Eng 110, 190–199 (1988). [DOI] [PubMed] [Google Scholar]
- 128.Lee LM & Liu AP The application of micropipette aspiration in molecular mechanics of single cells. J. Nanotechnol. Eng. Med 5, 0408011–0408016 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Oh M-J et al. Micropipette aspiration of substrate-attached cells to estimate cell stiffness. J. Vis. Exp 67, 3886 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choudhary D et al. Bio-molecular applications of recent developments in optical tweezers. Biomolecules 9, 23 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Evans E, Ritchie K & Merkel R Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophys. J 68, 2580–2587 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Heidarsson PO et al. Direct single-molecule observation of calcium-dependent misfolding in human neuronal calcium sensor-1. Proc. Natl Acad. Sci. USA 111, 13069–13074 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang H & Liu KK Optical tweezers for single cells. J. R. Soc. Interface 5, 671–690 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bustamante CJ et al. Optical tweezers in single-molecule biophysics. Nat. Rev. Methods Prim 1, 25 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mulligan JA et al. Traction force microscopy for noninvasive imaging of cell forces. Adv. Exp. Med. Biol 1092, 319–349 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hur SS et al. Traction force microscopy for understanding cellular mechanotransduction. BMB Rep 53, 74–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Plotnikov SV et al. High-resolution traction force microscopy. Methods Cell Biol 123, 367–394 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Huang Y et al. Traction force microscopy with optimized regularization and automated Bayesian parameter selection for comparing cells. Sci. Rep 9, 539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vorselen D et al. Microparticle traction force microscopy reveals subcellular force exertion patterns in immune cell–target interactions. Nat. Commun 11, 20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Köster S et al. Drop-based microfluidic devices for encapsulation of single cells. Lab Chip 8, 1110–1115 (2008). [DOI] [PubMed] [Google Scholar]
- 141.Jeon JS et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc. Natl Acad. Sci. USA 112, 214–219 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shin Y et al. Microfluidic assay for simultaneous culture of multiple cell types on surfaces or within hydrogels. Nat. Protoc 7, 1247–1259 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Liu X, Zheng W & Jiang X Cell-based assays on microfluidics for drug screening. ACS Sens 4, 1465–1475 (2019). [DOI] [PubMed] [Google Scholar]
- 144.Jaberi A et al. Microfluidic systems with embedded cell culture chambers for high-throughput biological assays. ACS Appl. Bio Mater 3, 6661–6671 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mietke A et al. Extracting cell stiffness from real-time deformability cytometry: theory and experiment. Biophys. J 109, 2023–2036 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mokbel M et al. Numerical simulation of real-time deformability cytometry to extract cell mechanical properties. ACS Biomater. Sci. Eng 3, 2962–2973 (2017). [DOI] [PubMed] [Google Scholar]
- 147.Fregin B et al. High-throughput single-cell rheology in complex samples by dynamic real-time deformability cytometry. Nat. Commun 10, 415 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gossett DR et al. Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc. Natl Acad. Sci. USA 109, 7630–7635 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Prevedel R et al. Brillouin microscopy: an emerging tool for mechanobiology. Nat. Methods 16, 969–977 (2019). [DOI] [PubMed] [Google Scholar]
- 150.Mariappan YK, Glaser KJ & Ehman RL Magnetic resonance elastography: a review. Clin. Anat 23, 497–511 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Youk JH, Gweon HM & Son EJ Shear-wave elastography in breast ultrasonography: the state of the art. Ultrasonography 36, 300–309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chighizola M et al. The glycocalyx affects the mechanotransductive perception of the topographical microenvironment. J. Nanobiotechnol 20, 418 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Buffone A & Weaver VM Don’t sugarcoat it: how glycocalyx composition influences cancer progression. J. Cell Biol 219, e201910070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kanyo N et al. Glycocalyx regulates the strength and kinetics of cancer cell adhesion revealed by biophysical models based on high resolution label-free optical data. Sci. Rep 10, 22422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Shurer CR et al. Physical principles of membrane shape regulation by the glycocalyx. Cell 177, 1757–1770.e21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Paszek MJ et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 511, 319–325 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chugh P & Paluch EK The actin cortex at a glance. J. Cell Sci 131, jcs186254 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haase K & Pelling AE The role of the actin cortex in maintaining cell shape. Commun. Integr. Biol 6, e26714 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Li QS et al. AFM indentation study of breast cancer cells. Biochem. Biophys. Res. Commun 374, 609–613 (2008). [DOI] [PubMed] [Google Scholar]
- 160.Wang Y et al. Quantitative analysis of the cell-surface roughness and viscoelasticity for breast cancer cells discrimination using atomic force microscopy. Scanning 38, 558–563 (2016). [DOI] [PubMed] [Google Scholar]
- 161.Cross SE et al. Green tea extract selectively targets nanomechanics of live metastatic cancer cells. Nanotechnology 22, 215101 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xu W et al. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS ONE 7, e46609 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Efremov YM et al. Distinct impact of targeted actin cytoskeleton reorganization on mechanical properties of normal and malignant cells. Biochim. Biophys. Acta 1853, 3117–3125 (2015). [DOI] [PubMed] [Google Scholar]
- 164.Ramos JR et al. The softening of human bladder cancer cells happens at an early stage of the malignancy process. Beilstein J. Nanotechnol 5, 447–457 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Fraley SI et al. A distinctive role for focal adhesion proteins in three-dimensional cell motility. Nat. Cell Biol 12, 598–604 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fischer RS et al. Contractility, focal adhesion orientation, and stress fiber orientation drive cancer cell polarity and migration along wavy ECM substrates. Proc. Natl Acad. Sci. USA 118, e2021135118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Halaoui R & McCaffrey L Rewiring cell polarity signaling in cancer. Oncogene 34, 939–950 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Brabletz T et al. EMT in cancer. Nat. Rev. Cancer 18, 128–134 (2018). [DOI] [PubMed] [Google Scholar]
- 169.Montagner M & Dupont S Mechanical forces as determinants of disseminated metastatic cell fate. Cells 9, 250 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Pepin KM & McGee KP Quantifying tumor stiffness with magnetic resonance elastography: the role of mechanical properties for detection, characterization, and treatment stratification in oncology. Top. Magn. Reson. Imaging 27, 353–362 (2018). [DOI] [PubMed] [Google Scholar]
- 171.Lyshchik A et al. Elastic moduli of thyroid tissues under compression. Ultrason. Imaging 27, 101–110 (2005). [DOI] [PubMed] [Google Scholar]
- 172.Frantz C, Stewart KM & Weaver VM The extracellular matrix at a glance. J. Cell Sci 123, 4195–4200 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Walker C, Mojares E & Del Río Hernández A Role of extracellular matrix in development and cancer progression. Int. J. Mol. Sci 19, 3028 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Whatcott CJ et al. Desmoplasia in primary tumors and metastatic lesions of pancreatic cancer. Clin. Cancer Res 21, 3561–3568 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Stylianou A et al. Pancreatic cancer presents distinct nanomechanical properties during progression. Ann. Biomed. Eng 51, 1602–1615 (2023). [DOI] [PubMed] [Google Scholar]
- 176.Rice AJ et al. Matrix stiffness induces epithelial–mesenchymal transition and promotes chemoresistance in pancreatic cancer cells. Oncogenesis 6, e352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cross SE et al. Nanomechanical analysis of cells from cancer patients. Nat. Nanotechnol 2, 780–783 (2007). [DOI] [PubMed] [Google Scholar]
- 178.Coceano G et al. Investigation into local cell mechanics by atomic force microscopy mapping and optical tweezer vertical indentation. Nanotechnology 27, 065102 (2016). [DOI] [PubMed] [Google Scholar]
- 179.Maja M et al. Surface cholesterol-enriched domains specifically promote invasion of breast cancer cell lines by controlling invadopodia and extracellular matrix degradation. Cell Mol. Life Sci 79, 417 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Corbin EA et al. Biophysical properties of human breast cancer cells measured using silicon MEMS resonators and atomic force microscopy. Lab. Chip 15, 839–847 (2015). [DOI] [PubMed] [Google Scholar]
- 181.Nematbakhsh Y, Pang KT & Lim CT Correlating the viscoelasticity of breast cancer cells with their malignancy. Converg. Sci. Phys. Oncol 3, 034003 (2017). [Google Scholar]
- 182.Fischer T, Hayn A & Mierke CT Effect of nuclear stiffness on cell mechanics and migration of human breast cancer cells. Front. Cell Dev. Biol 8, 393 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nikkhah M et al. Evaluation of the influence of growth medium composition on cell elasticity. J. Biomech 44, 762–766 (2011). [DOI] [PubMed] [Google Scholar]
- 184.Nikkhah M et al. The cytoskeletal organization of breast carcinoma and fibroblast cells inside three dimensional (3-D) isotropic silicon microstructures. Biomaterials 31, 4552–4561 (2010). [DOI] [PubMed] [Google Scholar]
- 185.Fischer T et al. Matrix and cellular mechanical properties are the driving factors for facilitating human cancer cell motility into 3D engineered matrices. Converg. Sci. Phys. Oncol 3, 044003 (2017). [Google Scholar]
- 186.Lee LM & Liu AP A microfluidic pipette array for mechanophenotyping of cancer cells and mechanical gating of mechanosensitive channels. Lab. Chip 15, 264–273 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Northey JJ et al. Stiff stroma increases breast cancer risk by inducing the oncogene ZNF217. J. Clin. Invest 130, 5721–5737 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zhang J et al. Rapid biomechanical imaging at low irradiation level via dual line-scanning Brillouin microscopy. Nat. Methods 20, 677–681 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Krouskop TA et al. Elastic moduli of breast and prostate tissues under compression. Ultrason. Imaging 20, 260–274 (1998). [DOI] [PubMed] [Google Scholar]
- 190.Patel BK et al. Association of breast cancer risk, density, and stiffness: global tissue stiffness on breast MR elastography (MRE). Breast Cancer Res. Treat 194, 79–89 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lekka M et al. Elasticity of normal and cancerous human bladder cells studied by scanning force microscopy. Eur. Biophys. J 28, 312–316 (1999). [DOI] [PubMed] [Google Scholar]
- 192.Martinez-Vidal L et al. Micro-mechanical fingerprints of the rat bladder change in actinic cystitis and tumor presence. Commun. Biol 6, 217 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Holuigue H et al. Force sensing on cells and tissues by atomic force microscopy. Sensors 22, 2197 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lekka M et al. Local elastic properties of cells studied by SFM. Appl. Surf. Sci 141, 345–349 (1999). [Google Scholar]
- 195.Lekka M et al. The effect of chitosan on stiffness and glycolytic activity of human bladder cells. Biochim. Biophys. Acta Mol. Cell Res 1540, 127–136 (2001). [DOI] [PubMed] [Google Scholar]
- 196.Bobrowska J et al. Biophysical and biochemical characteristics as complementary indicators of melanoma progression. Anal. Chem 91, 9885–9892 (2019). [DOI] [PubMed] [Google Scholar]
- 197.Metz H, McElhaney J & Ommaya AK A comparison of the elasticity of live, dead, and fixed brain tissue. J. Biomech 3, 453–458 (1970). [DOI] [PubMed] [Google Scholar]
- 198.Seano G et al. Solid stress in brain tumours causes neuronal loss and neurological dysfunction and can be reversed by lithium. Nat. Biomed. Eng 3, 230–245 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Miroshnikova YA et al. Tissue mechanics promote IDH1-dependent HIF1α-tenascin C feedback to regulate glioblastoma aggression. Nat. Cell Biol 18, 1336–1345 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Prabhune M et al. Comparison of mechanical properties of normal and malignant thyroid cells. Micron 43, 1267–1272 (2012). [DOI] [PubMed] [Google Scholar]
- 201.Lam AC et al. The influence of precompression on elasticity of thyroid nodules estimated by ultrasound shear wave elastography. Eur. Radiol 26, 2845–2852 (2016). [DOI] [PubMed] [Google Scholar]
- 202.Sebag F et al. Shear wave elastography: a new ultrasound imaging mode for the differential diagnosis of benign and malignant thyroid nodules. J. Clin. Endocrinol. Metab 95, 5281–5288 (2010). [DOI] [PubMed] [Google Scholar]
- 203.Wang S et al. Budding epithelial morphogenesis driven by cell–matrix versus cell–cell adhesion. Cell 184, 3702–3716.e30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tabdanov ED et al. Engineering T cells to enhance 3D migration through structurally and mechanically complex tumor microenvironments. Nat. Commun 12, 2815 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sahai E et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 20, 174–186 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zhovmer AS et al. Septins provide microenvironment sensing and cortical actomyosin partitioning in motile amoeboid T lymphocytes. Sci. Adv 10, eadi1788 (2024). [DOI] [PubMed] [Google Scholar]
- 207.Zhovmer AS et al. Septins enable T cell contact guidance via amoeboid–mesenchymal switch. Preprint at bioRxiv 10.1101/2023.09.26.559597 (2023). [DOI] [Google Scholar]
- 208.Solares SD & Cartagena-Rivera AX Frequency-dependent nanomechanical profiling for medical diagnosis. Beilstein J. Nanotechnol 13, 1483–1489 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mandal K et al. Role of a kinesin motor in cancer cell mechanics. Nano Lett 19, 7691–7702 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Rother J, Nöding H, Mey I & Janshoff A Atomic force microscopy-based microrheology reveals significant differences in the viscoelastic response between malign and benign cell lines. Open Biol 4, 140046 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hou HW et al. Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices 11, 557–564 (2009). [DOI] [PubMed] [Google Scholar]
- 212.Guck J et al. Optical deformability as an inherent cell marker for testing malignant transformation and metastatic competence. Biophys. J 88, 3689–3698 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lin HH et al. Mechanical phenotype of cancer cells: cell softening and loss of stiffness sensing. Oncotarget 6, 20946–20958 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Li Z et al. Cellular traction forces: a useful parameter in cancer research. Nanoscale 9, 19039–19044 (2017). [DOI] [PubMed] [Google Scholar]
- 215.Hayashi K & Iwata M Stiffness of cancer cells measured with an AFM indentation method. J. Mech. Behav. Biomed. Mater 49, 105–111 (2015). [DOI] [PubMed] [Google Scholar]
- 216.Zhao X, Zhong Y, Ye T, Wang D & Mao B Discrimination between cervical cancer cells and normal cervical cells based on longitudinal elasticity using atomic force microscopy. Nanoscale Res. Lett 10, 482 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Iyer S, Woodworth CD, Gaikwad RM, Kievsky YY & Sokolov I Towards nonspecific detection of malignant cervical cells with fluorescent silica beads. Small 5, 2277–2284 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Iyer S, Gaikwad RM, Subba-Rao V, Woodworth CD & Sokolov I Atomic force microscopy detects differences in the surface brush of normal and cancerous cells. Nat. Nanotechnol 4, 389–393 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Fuhrmann A et al. AFM stiffness nanotomography of normal, metaplastic and dysplastic human esophageal cells. Phys. Biol 8, 015007 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.van Helvert S & Friedl P Strain stiffening of fibrillar collagen during individual and collective cell migration identified by AFM nanoindentation. ACS Appl. Mater. Interfaces 8, 21946–21955 (2016). [DOI] [PubMed] [Google Scholar]
- 221.Rebelo LM, de Sousa JS, Mendes Filho J & Radmacher M Comparison of the viscoelastic properties of cells from different kidney cancer phenotypes measured with atomic force microscopy. Nanotechnology 24, 055102 (2013). [DOI] [PubMed] [Google Scholar]
- 222.Rianna C & Radmacher M Influence of microenvironment topography and stiffness on the mechanics and motility of normal and cancer renal cells. Nanoscale 9, 11222–11230 (2017). [DOI] [PubMed] [Google Scholar]
- 223.Cross SE et al. AFM-based analysis of human metastatic cancer cells. Nanotechnology 19, 384003 (2008). [DOI] [PubMed] [Google Scholar]
- 224.Faria EC, et al. Measurement of elastic properties of prostate cancer cells using AFM. Analyst 133, 1498–500 (2008). [DOI] [PubMed] [Google Scholar]
- 225.Erdogan B et al. Cancer-associated fibroblasts promote directional cancer cell migration by aligning fibronectin. J. Cell Biol 216, 3799–3816 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Xu H et al. Axial-shear strain imaging for differentiating benign and malignant breast masses. Ultrasound Med. Biol 36, 1813–1824 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lopez-Crapez E et al. Mechanical signatures of human colon cancers. Sci. Rep 12, 12475 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Brauchle E et al. Biomechanical and biomolecular characterization of extracellular matrix structures in human colon carcinomas. Matrix Biol 68–69, 180–193 (2018). [DOI] [PubMed] [Google Scholar]
- 229.Varinelli L et al. Decellularized extracellular matrix as scaffold for cancer organoid cultures of colorectal peritoneal metastases. J. Mol. Cell Biol 14, mjac064 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Ling W et al. Effects of vascularity and differentiation of hepatocellular carcinoma on tumor and liver stiffness: in vivo and in vitro studies. Ultrasound Med. Biol 40, 739–746 (2014). [DOI] [PubMed] [Google Scholar]
- 231.Rouvière O et al. Stiffness of benign and malignant prostate tissue measured by shear-wave elastography: a preliminary study. Eur. Radiol 27, 1858–1866 (2017). [DOI] [PubMed] [Google Scholar]
- 232.Hoyt K et al. Tissue elasticity properties as biomarkers for prostate cancer. Cancer Biomark 4, 213–225 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


