Abstract
Diffuse midline glioma (DMG) with H3K27M mutation is an aggressive and difficult to treat pediatric brain tumor. Recurrent gain of function mutations in H3.3 (H3.3A) and H3.1 (H3C2) at the 27th lysine to methionine (H3K27M) are seen in over 2/3 of DMGs, and are associated with a worse prognosis. Due to the anatomical location of DMG, traditional biopsy carries risk for neurologic injury as it requires penetration of vital midline structures. Further, radiographic (MRI) monitoring of DMG often shows nonspecific changes, which makes therapeutic monitoring difficult. This indicates a critical need for more minimally invasive methods, such as liquid biopsy, to understand, diagnose, and monitor H3K27M DMG. Here, we review the use of all modalities to date to detect biomarkers of H3K27M in cerebrospinal fluid (CSF), blood, and urine, and compare their effectiveness in detection, diagnosis, and monitoring treatment response. We provide specific detail of recent efforts to monitor CSF and plasma H3K27M cell-free DNA in patients undergoing therapy with the imipridone ONC201. Lastly, we discuss the future of therapeutic monitoring of H3K27M-DMG, including biomarkers such as mitochondrial DNA, mutant and modified histones, and novel sequencing-based approaches for improved detection methods.
Keywords: cell-free tumor DNA, diffuse midline glioma, H3K27M, liquid biopsy, ONC201
Key Points.
Liquid biopsy is proving valuable as an adjunct for disease monitoring and treatment stratification because there tends to be a correlation between biomarker levels in biofluids and tumor burden.
Based on existing literature, cerebrospinal fluid tends to contain higher concentrations of biomarkers than plasma to due closer proximity to disease and the blood-brain barrier, but plasma is easier to obtain.
Current best practices for liquid biopsy leverage droplet digital PCR or targeted sequencing techniques. Droplet digital PCR and rapid sequencing technologies are better for serial timepoint monitoring, while Next Generation Sequencing is better suited for periodic tumor evolution monitoring and screening.
H3K27-altered diffuse midline glioma (DMG), a recently defined entity within the 2021 World Health Organization Classification of Tumors of the Central Nervous System tumors (WHO CNS5), is a high-grade glioma most commonly occurring in midline structures such as the brainstem, thalamus, or spinal cord.1 These tumors—previously referred to as Diffuse Intrinsic Pontine Gliomas (DIPG) when originating in the pons—are aggressive and most frequently affect children, adolescents, and young adults.2 The H3K27 alteration occurs in ~80% of DMGs and is predictive of poor prognosis and worse clinical outcomes than wild-type DMGs, independent of location or histological grading.3,4 These tumors often involve vital structures and are locally invasive, which result in surgical difficulties to achieve biopsy and/or surgical resection. Unfortunately, chemotherapy has limited effectiveness in the treatment of DMGs, and standard adjuvant treatment is focal irradiation, which results in a median survival of less than 12 months.5,6 Fortunately, recent clinical trials have shown promising results for extensions of survival, including ONC201,7 an oncolytic viral therapy,8 and CAR-T therapy.9,10
Current standard-of-care disease monitoring approaches leverage radiographic imaging (eg, MRI) to identify if a tumor is responding to treatment. However, MRIs are expensive and may require general anesthesia in the pediatric use-case.11 Especially in the case of H3K27M DMGs, diffuse disease and radiation-induced swelling12 can increase the difficulty of image interpretation (known as “pseudoprogression”). Research has shown across different cancer types that levels of biomarkers in biofluids (eg, blood or cerebrospinal fluid [CSF]) correlate with disease burden, indicating that serial quantification of these biomarkers can help monitor disease progression during treatment13; this analysis of biofluids is commonly referred to as “liquid biopsy” and offers a promising approach to help improve DMG patient management.14,15
Liquid biopsy use in H3K27M DMG patients presents a unique challenge due to the location of the tumor and the selective filter of the blood-brain barrier.16 Cerebrospinal fluid has been shown to be a useful biomaterial for liquid biopsy. Due to its proximity to tumor tissue, CSF generally has a much higher concentration of biomarkers compared to patient plasma.17 Obtaining CSF, however, is more invasive than obtaining blood, especially in pediatric patients where general anesthesia is usually necessary to perform a lumbar puncture. Plasma is generally readily available due to routine blood draws in patients. However, due to selectivity of the blood-brain barrier, the concentration of DMG-derived biomarkers in plasma is much lower than in CSF.18 Thus, plasma assay sensitivity rates tend to be lower than CSF.19 As a result, researchers continue to work to develop higher-sensitivity assays and investigate monitoring of other, higher-concentration biomarkers to realize the potential utility of liquid biopsy for DMG management.
Mechanistic Overview of H3K27M
Recurrent gain of function mutation in H3.3 (H3.3A, previously H3F3A) and H3.1 (H3C2, previously HIST1H3B) at the 27th lysine to methionine (H3K27M) are seen in over 2/3 of DMGs. H3K27-altered DMGs are characterized by loss of trimethylation of histone H3 at lysine 27 (K27me3), further defined by the mechanism of loss of trimethylation: H3K27-mutant, H3-wildtype with overexpression of EZHIP, or H3-wildtype with EGFR alteration (either mutation or amplification).1,20 These H3 alterations result in epigenetic dysregulation due to inactivation or suppression of H3K27-specific histone methyltransferase enhancer of zeste homolog 2 (EZH2), a catalytic subunit of polycomb repressive complex 2 (PRC2), leading to further reduction in total chromatin H3 di- and trimethylation. These alterations ultimately inhibit cellular differentiation and promote gliomagenesis.21,22
Detecting Biomarkers of Pediatric Brain Tumors in Liquid Biopsy Specimens
The earliest investigation of liquid biopsy for DMG attempted to detect tumor-derived proteins in the CSF of DIPG patients.23 The study measured comprehensive protein profile of CSF from patients with DIPG and found 528 unique proteins, 71% of which were secreted proteins. Two secreted proteins, Cyclophilin A (CypA) and dimethylarginase 1 (DDAH1), were particularly upregulated in CSF samples from patients with DIPG compared to controls, including patients with lower-grade brain tumors or with other neurological disorders.23 Interestingly, immunohistochemical staining showed that the cytosolic forms of CypA and DDAH1 were not selectively upregulated in patients with DIPG. Given high detection of secreted CypA and DDAH1 in CSF, the researchers also analyzed expression levels of CypA and DDAH1 in blood and urine samples. Western blot showed that the characteristic upregulation of CypA and DDAH1 in DIPG can be detected in blood and urine samples as well, which require less invasive measures than obtaining CSF samples (Figure 1).23 Detectable in serum and urine, CypA levels may play a role in tumor diagnosis, subtype diagnosis, and treatment response. Additionally, patients with DDAH1 expression in CSF showed an aggressive clinical course and imaging results consistent with rapid tumor growth. Given that the differential expression of DDAH1 is detectable in CSF, it could have prognostic implications for patient stratification and assessment of treatment outcomes.
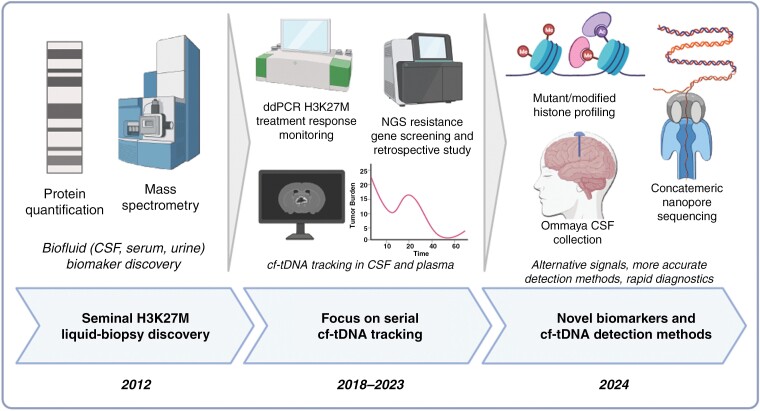
Figure 1.
Past, present, and future of H3K27M focused liquid biopsy. Seminal work identified disease-specific proteins in cerebrospinal fluid (CSF)—as well as patient serum and urine. The current state of the art is focused on serial quantification of H3K27M circulating tumor DNA in CSF and plasma using accurate droplet digital PCR assays and correlation with radiographic imaging to identify if liquid biopsy could be leveraged to predict response to treatment and help resolve pseudoprogression. Newer liquid biopsy approaches will leverage alternative and novel biomarkers such as mutant/modified histones derived from H3K27M mutant cells, routine, low-burden CSF collection via Ommaya reservoirs, as well as highly accurate and rapid sequencing approaches to simultaneously assay H3K27M along with other mutations (Created with BioRender.com).
Research then transitioned to detecting tumor-derived circulating tumor DNA (ctDNA). A tumor’s mutational profile is extremely valuable for proper diagnosis, and for devising targeted clinical treatment. A 2017 study was the first to report histone H3 mutation detection in ctDNA in CSF samples from children with DMG. Their 2 strategies, Sanger sequencing and nested PCR, both demonstrated feasibility in detecting H3 mutations in CSF.24 Sanger sequencing requires a large quantity of gene fragments, so nested-PCR was used when ctDNA yield was low. Nested-PCR helps to reduce nonspecific amplification of DNA and is used to help improve sensitivity of PCR amplification.24 Their technique was 100% specific for H3K27M detection in patients with DMG, with 87.5% sensitivity when matched tissue was available. Cerebrospinal fluid collection may thus be considered for H3 mutation analysis in patients with DMG for diagnosis and molecularly directed cancer therapy.
While this work demonstrated that it was possible to detect ctDNA in CSF, it was not able to accurately quantify ctDNA due to the limitations of Sanger sequencing. Droplet digital PCR (ddPCR) can be utilized for both detection of mutations and precise quantification (Figure 1).25 Droplet digital PCR works by fractionating a PCR reaction into tens of thousands of droplets such that each droplet contains approximately one template copy along with mutation-specific probes. Mutant ctDNA variant allele fraction (VAF) is derived from the ratio of mutant-positive to total mutant- and wild-type-positive droplets. A 2018 study found that the number of copies of H3K27M mutant DNA detected in patient CSF obtained from the lateral ventricle is twice as great as CSF obtained from a lumbar puncture.26 This indicates that proximity of CSF collection to the tumor may be important when measuring ctDNA. In vitro culture of DIPG cells showed that as the cells proliferate, more ctDNA is released into culture media, suggesting a correlation between ctDNA released into surrounding biofluids and tumor cell proliferation. When treated with radiation, total ctDNA, along with H3K27M copies, increases significantly 72–120 hours after radiation, after which the quantity levels off. These results indicate that we may be able to use ctDNA quantification from ddPCR to track tumor burden and response to treatment.26
Another 2018 study was the first to attempt serial timepoint collection and analysis of CSF samples to see if it was feasible to track treatment response in real patients using ddPCR.27 In the study, a total of 28 CSF samples were collected; histone H3 mutant and wild-type alleles were detected in 75% of CSF specimens collected at diagnosis (3/4), 67% of those collected during treatment (2/3), and 90% of those collected at postmortem (19/21).27 Again, location of CSF retrieval affected ctDNA abundance, as CSF samples obtained from areas in closer proximity to the tumor, such as the lateral ventricles, yielded more H3K27M copies than CSF from more distant locations. The study also sought to explore the possibility of using plasma for disease monitoring. H3K27M ctDNA was detected in plasma, but at much lower VAFs than CSF (P < .0001), perhaps as a result of the selective filtration by the blood-brain barrier. Lastly, this study also used liquid biopsy to measure treatment response. VAF of H3K27M ctDNA was measured at various time points during treatment and compared to radiographic response. In 83% of cases (10/12), a decrease in H3K27M ctDNA corresponded with tumor response to radiotherapy.27 A 2019 study explored detection of H3K27M mutations in known-positive patients undergoing radiotherapy.28 Plasma ctDNA analysis detected H3K27M ctDNA in 100% of subjects (6/6) postradiation, 100% of subjects (7/7) during treatment, 71% of subjects (5/7) at progression, and 100% of subjects (5/5) at the end-of-study visit. Taken together, these 2 studies demonstrate the translational relevance of using ctDNA to supplement MRIs during disease monitoring and the feasibility of using ctDNA in plasma as a diagnostic and prognostic tool.
While ddPCR is a powerful tool for detecting and quantifying small allele fractions for specific hotspot mutations (eg, H3K27M), it is not well suited to detect and monitor a wide variety of mutations. Next generation sequencing (NGS) is capable of sequencing gene fragments from an arbitrary number of loci with high accuracy, allowing cancer panels to both monitor multiple cancer-relevant genes for mutations and quantify VAF (Figure 1). A study in adult glioblastoma (GBM) in 2019 sequenced CSF ctDNA via NGS of a targeted cancer panel (MSK-IMPACT), allowing multigene assessment and tracking of tumor evolution.29 CSF was obtained via lumbar puncture, and ctDNA was detected in CSF in 49.4% (42/85) of patients. They found that the genomic alterations found in CSF resembled the genome in tumor biopsies. However, broad panel sequencing can require large amounts of CSF and ctDNA (>100 ng), which can be difficult or impossible to obtain from pediatric DMG patients due to their size and the higher risk of complications associated with midline tumor location.
While flexible and highly accurate, NGS panel sequencing can be expensive and have long turn-around times due to batch economics. This makes it a less practical option for treatment response monitoring of H3K27M-altered DMGs, as frequent, rapid results are desirable. To realize rapid, low-cost, and flexible panel sequencing, a 2020 study developed a protocol for “same-day” sequencing of amplicons from a small, in-house-designed panel using Oxford Nanopore sequencers. Results demonstrated high sensitivity with patient-specific mutations using small volume of CSF (0.5–2 mL) from pediatric patients with DMG and cortical high-grade glioma.30 In this study, targeted Nanopore amplicon sequencing was performed on a small number of H3K27M-DMG cases with serial CSF collected. In one patient treated with ONC201, an experimental impiridone,7 as the tumor size decreased in response to ONC201 treatment, VAF significantly decreased in parallel with multiple patient-specific alterations over first 6 months of treatment, correlating with longer-term response.30 However, high native error rates of Nanopore sequencing precluded meaningful analysis of allele fractions lower than about 2%, motivating methods to improve accuracy. While Nanopore sequencing requires further investigation, it has demonstrated enormous potential in diagnosis and monitoring in patients with DMG.
Liquid Biopsy for DMG Patients Treated With ONC201
Cantor et al. investigated the efficacy of tracking changes in ctDNA VAF as a prognostic tool for tumor progression in patients being treated with ONC201 after initial radiation. In a single arm of the phase 1 trial (NCT03416530), CSF was collected from 24 H3K27M DMG patients at baseline, 2 months, and 6 months of treatment at the recommended phase 2 dose. Patients were enrolled using the following criteria: H3K27M DMG diagnosis, at most one prior line of treatment, were willing to have CSF collected via lumbar puncture, and had no signs of elevated intracranial pressure (from clinical exam). To investigate the existence of a correlation between ctDNA VAF values and radiographic tumor area, MRI images obtained closest to the time of plasma or CSF sample collection were reviewed (Figure 1). Collection of CSF was demonstrated to be both feasible and safe.12 ddPCR was used to quantify H3K27M VAF in all samples. H3K27M ctDNA was detected in 53/62 plasma samples (sensitivity of 85.4%) and 28/29 CSF samples (sensitivity of 96.5%). No H3K27M-positive droplets were detected in controls, indicating 100% specificity.12Table 1 summarizes the findings from studies that utilized liquid biopsy methods.
Table 1.
A Summary of Liquid Biopsy Detection Methods for H3K27M. Studies That Use Liquid Biopsy to Detect and Monitor H3K27M Mutant Disease Along With Their Markers, Biofluids, Tumor Types, Targets, Sensitivity, and Specificity
| References | Marker | Detection Method | Biofluid Type | Tumor Type | Targets | Sensitivity | Specificity | Main Findings and Conclusions |
|---|---|---|---|---|---|---|---|---|
| Saratsis et al. (2012)23 | Protein | Proteomics | CSF, blood, urine | DIPG | CypA and DDAH1 levels | 100%—CypA and DDHA1 were upregulated in 10/10 DIPG samples. | N/A | Demonstrated that proteins characteristically upregulated in CSF, blood, and urine can be used for diagnosis and assessment of treatment outcome. |
| Huang et al. (2017)24 | ctDNA | Sanger sequencing | CSF | DIPG | H3K27M | 87.5% (7/8) | 100% (8/8) | Demonstrated that H3K27M can be detected in CSF. |
| Stallard et al. (2018)25 | ctDNA | ddPCR | CSF | DIPG | H3K27M | 100% (4/4) | N/A | Quantified ctDNA to monitor treatment response in vitro ctDNA levels change with radiation. Found that the number of copies of H3K27M mutant DNA detected in patient CSF obtained from different locations varied significantly. |
| Panditharatna et al. (2018)26 | ctDNA | ddPCR | CSF, plasma | DMG | H3K27M | 87% (20/23) | 100% (36/36) | Retrospective cohort demonstrating decreases in plasma ctDNA were noted after cycles of precision therapy, while increases in plasma ctDNA were noted with tumor growth in 1/1 CSF cases and 3/5 plasma cases with H3K27M-DMG when assessed serially. |
| Pan et al. (2018)27 | ctDNA | NGS panel sequencing | CSF, plasma | Brainstem glioma | Glioma associated genes | 82.5% (47/57) for any mutation | N/A | Indicated sequencing of CSF ctDNA can reliably detect mutations in brainstem tumors and that CSF has higher ctDNA levels than plasma. CSF analysis and biopsy sequencing can also identify more mutations than biopsy alone. |
| Mueller et al. (2019)28 | ctDNA | ddPCR | Plasma | DIPG | H3K27M | At diagnosis: 85% (11/13) During treatment: 100% (7/7) At progression: 71% (5/7) At end of study visit 100% (5/5) |
N/A | Prospective clinical trial cohort further demonstrating the feasibility of using ctDNA in plasma and CSF. |
| Bruzek et al. (2020)29 | ctDNA | Nanopore panel sequencing | CSF | DMG, pHHG | H3K27M | 67.9% (19/28) | 100% (28/28) | Manuscript demonstrated Nanopore sequencing of CSF ctDNA is feasible and efficient with low-input samples. Serial monitoring of H3K27M was performed in 2 cases. |
| Cantor et al. (2022)12 | ctDNA | ddPCR | CSF, plasma | DMG | H3K27M | CSF: 96.5% (28/29 samples); blood: 85.4% (53/62 samples) | 100% (70/70) | Manuscript demonstrated the utility of ctDNA in plasma and CSF to supplement radiographic monitoring in prospective clinical trial in 16 cases with serial CSF samples and 11 cases with serial plasma samples, and that increases in ctDNA of 25% predicted progression in 5/11 CSF cases and 8/16 plasma cases. |
Abbreviations: CSF= cerebrospinal fluid; ctDNA = circulating tumor DNA; ddPCR = droplet digital PCR; DIPG = diffuse intrinsic pontine glioma; DMG = diffuse midline glioma.
Assessment of the relationship between change in ctDNA VAF and progression-free survival (PFS) while on ONC201 demonstrated that a decrease in CSF ctDNA over time was associated with a longer PFS, and a trend toward this association was seen in plasma.12 Importantly, some patients displayed a “spike” in ctDNA VAF, defined as an increase of at least 25% from baseline, that preceded tumor progression, indicating that spikes in ctDNA VAF can predict disease progression.12 These spikes tended to occur 1 to 3 months before radiographic progression, potentially allowing for earlier detection. Several cases demonstrated the utility of plasma ctDNA VAF for differentiating between true and pseudoprogression. In one case, a 6-year-old female with a thalamic DMG showed an increase in tumor area on MRI after being treated with ONC201 and initial radiation, followed by an 85% regression of the tumor that persisted for 32 months on treatment.12 Her plasma VAF displayed a decrease in plasma ctDNA VAF, which more accurately reflected true response to treatment than concurrent imaging, indicating pseudoprogression. In contrast, in the case of a 14-year-old male with a spinal cord DMG, combination ONC201 and bevacizumab therapy resulted in a decrease of his spinal tumor area on T1 postcontrast MRI, which is expected with bevacizumab due to its effects on tumor perfusion. The patient, however, displayed a continued increase in his plasma VAF, which correctly predicted radiologic progression 8 weeks later.12
There was no direct correlation between tumor size by MRI and plasma or CSF VAF, VAF and tumor location, VAF and tumor size, or CSF or plasma ctDNA concentration and H3K27M VAF percentage.12 Interestingly, this may be explained by the deviations of individual VAF values from tumor area that resulted from phenomena such as the apparent decrease in tumor area or “pseudoresponse” that is expected as a result of bevacizumab treatment. In such cases, VAF values may be more clinically useful as discussed above in the case of a 14-year-old male with spinal cord DMG. Nevertheless, the cohort of this study was too heterogeneous to provide any statistical insight on such clinical situations.
This study demonstrated the utility of serial analysis of ctDNA from plasma and CSF as a complement to radiographic monitoring of DMG patients and showed that a liquid biopsy approach can provide early indication of disease progression or remission. Given the enrichment of glioma ctDNA in CSF, detection and monitoring of other tumor biomarkers present in CSF could also be achieved via ddPCR. While CSF VAF trends are a better predictor of clinical response compared to plasma, clinical acquisition of CSF poses more inherent clinical difficulty and risk.12 Plasma diagnostics require further optimization to aid or replace CSF analysis.
Ongoing Clinical Trials Leveraging Liquid Biopsy for DMG Monitoring
There are only a few clinical trials that are utilizing liquid biopsy biomarkers to measure outcomes. However, greater use of liquid biopsy biomarkers in clinical trials could help optimize liquid biopsy assays and allow for its comparison to traditional methods to measure outcomes.31
One ongoing clinical trial (NCT01106794) incorporates plasma and CSF collection for analysis. The study, composed of 100 pediatric patients with DIPG or brainstem glioma, includes genome-wide expression patterns of RNA in tumor samples, normal brain stem tissue, and CSF using Affymetrix gene expression profiling, as well as proteomic profiling of tumor, normal brainstem tissue, and CSF.
There are 4 CAR-T trials (NCT04196413, NCT04185038, NCT05768880, and NCT04099797) that are collecting CSF serially from Ommaya reservoirs. The ongoing trial (NCT04196413) at Stanford is testing whether GD2-CAR T cells can be made from immune cells collected from patient with H3K27M altered DMG. The researchers are utilizing ddPCR to detect the H3K27M mutation, and their findings demonstrate that ctDNA levels are elevated during peak inflammation posttreatment.10 In addition, they are tracking cytokine levels in CSF and blood to assess response to treatment. Though only 4 patients have been included in the publication as the clinical experience is early, the findings indicate the utility of liquid biopsy in clinical trials. Moreover, the 2 trials at Seattle Children’s Hospital (NCT04185038 and NCT05768880) are studying B7-H3-specific CAR T cell locoregional immunotherapy for patients with DMG. Lastly, researchers at Baylor College of Medicine are determining the largest safe dose of GD2-CAR T cells that can be administered intravenously.
An ongoing trial, titled “Combination Therapy for the Treatment of Diffuse Midline Gliomas” (NCT05009992), is studying if the combination of ONC201 with panobinostat or paxalisib is effective in treating patients with DMG. Similarly, another ongoing trial called “ONC206 for Treatment of Newly Diagnosed, or Recurrent Diffuse Midline Gliomas, and Other Recurrent Malignant CNS Tumors” (NCT04732065) is studying the effects and best dose of ONC206, with or without radiation, in treating patients with DMG. ONC206 is a recently discovered drug that has shown the potential to kill brain tumor cells by inducing a stress response in the tumor cells. Both trials seek to evaluate the correlation of biomarkers in CSF and clinical outcomes. The researchers have also employed a prospective program for both scheduled serial plasma and CSF collection for DMG patients enrolled at either up-front, postradiation, and recurrence.
Lastly, Memorial Sloan Kettering received approval in 2019 for their in-house liquid biopsy test called MSK-ACCESS (Analysis of Circulating cfDNA to Evaluate Somatic Status). MSK-ACCESS can detect the presence of genetic mutations in 129 cancer-associated genes using plasma. The team has published how the test was designed and validated, and their paper reported findings from the first 681 blood samples that were analyzed using MSK-ACCESS.32 The results demonstrate that somatic alterations were detected in 73% of samples, 56% of which have clinically actionable alterations, indicating the importance of ctDNA in plasma as a genomic profile source with clinical applications.
Current State-of-the-Art and Future Directions
Most ongoing work in liquid biopsy of H3K27M mutant tumors is continuing to evaluate the utility of detection and quantification of ctDNA to track disease and monitor for treatment response. However, accurate quantification of small concentrations of ctDNA, especially in patient plasma, remains technically challenging and error prone, and special care must be taken when choosing the appropriate assay. Table 2 summarizes the existing approaches to measuring ctDNA in biofluids, their pros and cons, and also the suggested use for each approach for liquid biopsy treatment-response monitoring.
Table 2.
ct-DNA Liquid Biopsy Methods, Their Pros and Cons, and the Applications in Liquid Biopsy for H3K27M Altered DMG
| Ct-DNA Liquid Biopsy Method | Pros | Cons | Best Suited for |
|---|---|---|---|
| Sanger Sequencing | Affordable, rapid, sequencing-based | Cannot detect lower than ~15% VAF | Generally not suitable for liquid biopsy |
| ddPCR | Affordable, rapid, precise, can detect VAFs down to ~0.05% | Generally limited to single allele assays; assay design and validation are arduous | Frequent monitoring of known hotspot mutations |
| Targeted NGS sequencing | Can survey a wide variety of mutations. Panel design is relatively low-effort | Expensive, long turnaround times | Disease screening, initial diagnostics, and tumor evolution monitoring |
| Targeted nanopore sequencing | Affordable assays and equipment costs. Rapid turn-around times | Requires concatemeric assays for error correction to increase sensitivity for low VAF samples | Frequent monitoring of a panel of genes or known resistance markers |
| NGS WES/WGS sequencing | Characterization of the entire tumor genome/exome. Unparalleled breadth of diagnostic information | Expensive, long turnaround times, lower depth per locus limiting sensitivity for low VAF samples | Limited utility due to current feasibility |
Abbreviations: CapEx = capital expenditure/equipment cost; ddPCR = droplet digital PCR; DMG= diffuse midline glioma; NGS = next generation sequencing; VAF = variant allele fraction; WES = whole exome sequencing; WGS = whole genome sequencing.
Regardless of detection method, spinal fluid has generally shown to be a superior analyte than plasma due to a higher concentration of tumor derived DNA33 but at the cost of increased patient burden. However, the recent increased use of Ommaya reservoirs has greatly improved ease of access to CSF (Figure 1). Ommaya reservoirs are small implants that are inserted under the scalp and connected to an intraventricular catheter. They are traditionally placed to improve administration of drugs directly to the brain, but also provide easy, routine access to CSF without a lumbar puncture and are well tolerated.34 While not currently standard of care for treatment of DMGs, increased usage would provide easy, and frequent access to CSF, and offer clear advantages over analysis of plasma.
Even with increased use of Ommaya reservoirs, plasma is still generally much easier to obtain. Routine blood draws could be sent to central testing facilities for analysis and would not require an expensive and disruptive site visit. Current best practices for liquid biopsy leverage ddPCR or error-corrected sequencing techniques such as Universal Molecular Identifiers as naive sequencing using current technologies has an error rate too high to resolve small ctDNA allele fractions. ddPCR offers clear advantages over sequencing, including much lower cost, increased turnaround time, and improved precision/reduced potential for amplification bias. However, assay design and validation is arduous, and the ability to track multiple mutations or screen for new mutations is limited as each assay must be carefully designed and validated for a particular point mutation. In the case of H3K27M altered DMGs, where 100% of cases are defined by 2 point mutations, this is not a problem. But ddPCR will most likely never be a practical method to monitor for the development of resistance markers, or other treatment-relevant mutations.
Next generation sequencing offers a much more general approach. Because NGS uses targeted enrichment via hybrid capture or targeted amplification via PCR, assays are much simpler to design and validate, and can survey mutations at hundreds of thousands of loci. However, sequencing is more expensive, and generally much slower, often taking weeks to return a result. While a 1-week turnaround time may not impact patient care, a shorter time-frame with same-visit results that coincide with other diagnostics is desirable; especially given the aggressive nature of H3K27M-altered DMGs. Large-panel sequencing will be most useful for screening or for tumor evolution monitoring during inflection points in patient care such as clinical progression.
In addition to tumor DNA, many other tumor-derived biomarkers are present in CSF and plasma of H3K27M mutant glioma patients, including mutant or modified histone proteins35 and nucleosome complexes.36 While one H3K27M mutant tumor cell contains one mutant nuclear DNA copy, each tumor cell contains tens of millions of mutant histone proteins. Thus, mutant histones and nucleosomes are present in a much higher concentration than ctDNA in patient samples. Monitoring for global aberrations in epigenetic histone modifications, such as methylation and acetylation, is shown to be useful in other cancers, and hence might also be used as a marker for H3K27M mutant DMGs. Tracking these proteins alongside ctDNA might allow increased precision and accuracy compared with ctDNA tracking, requires much less patient sample, and offers unique treatment-dependent kinetics that are different from that of nuclear ctDNA.
While detection of these alternate biomarkers might offer improved sensitivity compared to prior ctDNA monitoring techniques, ctDNA marker detection is still the most studied and standard method for monitoring treatment response and is immediately useful in clinic. This motivates the development of improved detection methods that are extremely sensitive, precise, and flexible in order to monitor for patient-specific markers. Targeted amplicon sequencing using real-time Nanopore sequencers offers an exciting alternative to ddPCR-based detection, given that it is low-cost, rapid, and allows for easier personalized assay design (Figure 1). Prior work leveraged this approach for CSF-based monitoring,30 but high error rates prevented use in plasma. Recent work has combined improvements in Nanopore sequencing chemistry and basecalling algorithms along with concatemeric assay consensus error correction37 to improve sensitivity. Regardless of the approach, detection methods should focus on maintaining a reasonable cost and a time-to-result of at most 3 days in order to improve the likelihood of implementation as a lab-designed test or in vitro diagnostic test.
While this review focuses on liquid biopsy use in H3K27M-altered DMGs, there are obviously many other cancers that would benefit from the lessons learned in this body of work. Many of the approaches and techniques described here can be applied to any cancer, given a set of mutations or genes that are commonly mutated in that disease. In particular, H3G34R-altered diffuse hemispheric gliomas (DHG) could benefit from these lessons. H3G34R-altered DHG is an emerging sub-class of pediatric diffuse gliomas, recently defined in the 2021 WHO CNS classification guide. Like the H3K27M altered DMG subclass, this disease is classified by a single point mutation and has important prognostic and therapeutic implications. Thus, ddPCR assays and inclusion of the H3G34 locus in targeted panels are desired.
Conclusion
Given the anatomic location and aggressive nature of H3K27M mutant DMG tumors, liquid biopsy is proving valuable as an adjunct for disease monitoring and treatment stratification. Multiple techniques have been developed over a short period of time for clinically relevant tumor liquid biopsy using CSF and plasma, and new technology holds future promise for detection of additional tumor biomarkers with increased sensitivity and specificity. An additional important hurdle will be to move these assays to CLIA certification for broad use in the clinic (outside of research testing). The processes for this transition are underway at University of Michigan, Tempus, and other academic and private companies. Ultimately, the ability to utilize serial liquid biopsy specimens for disease monitoring can decrease morbidity and increase treatment efficacy, with the potential to improve clinical outcomes for patients with this deadly disease.
Contributor Information
Jina Patel, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Rayan Aittaleb, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Robert Doherty, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Ananya Gera, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Benison Lau, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Dana Messinger, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Jack Wadden, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Andrea Franson, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Amanda Saratsis, Advocate Health Care, Chicago, Illinois, USA.
Carl Koschmann, Department of Pediatrics, Michigan Medicine, Ann Arbor, Michigan, USA.
Funding
This work was supported by the National Institutes of Health [grants R01-NS119231 and R01-NS124607], Department of Defense (DOD) [grant CA201129P1], University of Michigan Chad Carr Pediatric Brain Tumor Center, The Evans Family, ChadTough Defeat DIPG Foundation, Catching Up with Jack, Pediatric Brain Tumor Foundation, Michael Miller Memorial Foundation, Morgan Behen Golf Classic, The Musella Foundation, and Yuvaan Tiwari Foundation to C.K.
Conflict of interest statement
Amanda Saratsis is a consultant for Chimerix, Inc.
Supplement sponsorship
This article appears as part of the supplement “H3 K27M-mutant Glioma: Disease State Overview,” sponsored by Chimerix, Inc.
Authorship Statement
All authors have contributed in meaningful ways and reviewed the manuscript.
References
- 1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vallero SG, Bertero L, Morana G, et al. Pediatric diffuse midline glioma H3K27-altered: a complex clinical and biological landscape behind a neatly defined tumor type. Front Oncol. 2022;12:1082062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karremann M, Gielen GH, Hoffmann M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018;20(1):123–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mosaab A, El-Ayadi M, Khorshed EN, et al. Histone H3K27M mutation overrides histological grading in pediatric gliomas. Sci Rep. 2020;10(1):8368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffman LM, Veldhuijzen van Zanten SEM, Colditz N, et al. Clinical, radiologic, pathologic, and molecular characteristics of long-term survivors of Diffuse Intrinsic Pontine Glioma (DIPG): a collaborative report from the International and European Society for Pediatric Oncology DIPG Registries. J Clin Oncol. 2018;36(19):1963–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mackay A, Burford A, Carvalho D, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32(4):520–537.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Venneti S, Kawakibi AR, Ji S, et al. Clinical efficacy of ONC201 in H3K27M-mutant diffuse midline gliomas is driven by disruption of integrated metabolic and epigenetic pathways. Cancer Discov. 2023;13(11):2370–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gállego Pérez-Larraya J, Garcia-Moure M, Labiano S, et al. Oncolytic DNX-2401 virus for pediatric diffuse intrinsic pontine glioma. N Engl J Med. 2022;386(26):2471–2481. [DOI] [PubMed] [Google Scholar]
- 9. Vitanza NA, Johnson AJ, Wilson AL, et al. Locoregional infusion of HER2-specific CAR T cells in children and young adults with recurrent or refractory CNS tumors: an interim analysis. Nat Med. 2021;27(9):1544–1552. [DOI] [PubMed] [Google Scholar]
- 10. Majzner RG, Ramakrishna S, Yeom KW, et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature. 2022;603(7903):934–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alexiou GA, Argyropoulou MI.. Neuroimaging in childhood headache: a systematic review. Pediatr Radiol. 2013;43(7):777–784. [DOI] [PubMed] [Google Scholar]
- 12. Cantor E, Wierzbicki K, Tarapore RS, et al. Serial H3K27M cell-free tumor DNA (cf-tDNA) tracking predicts ONC201 treatment response and progression in diffuse midline glioma. Neuro Oncol. 2022;24(8):1366–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kilgour E, Rothwell DG, Brady G, Dive C.. Liquid biopsy-based biomarkers of treatment response and resistance. Cancer Cell. 2020;37(4):485–495. [DOI] [PubMed] [Google Scholar]
- 14. Yekula A, Muralidharan K, Rosh ZS, et al. Liquid biopsy strategies to distinguish progression from pseudoprogression and radiation necrosis in glioblastomas. Adv Biosyst. 2020;4(12):e2000029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jensen TJ, Goodman AM, Kato S, et al. Genome-wide sequencing of cell-free DNA identifies copy-number alterations that can be used for monitoring response to immunotherapy in cancer patients. Mol Cancer Ther. 2019;18(2):448–458. [DOI] [PubMed] [Google Scholar]
- 16. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Izquierdo E, Proszek P, Pericoli G, et al. Droplet digital PCR-based detection of circulating tumor DNA from pediatric high grade and diffuse midline glioma patients. Neurooncol Adv. 2021;3(1):vdab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Escudero L, Llort A, Arias A, et al. Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat Commun. 2020;11(1):5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. De Mattos-Arruda L, Mayor R, Ng CKY, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sievers P, Sill M, Schrimpf D, et al. A subset of pediatric-type thalamic gliomas share a distinct DNA methylation profile, H3K27me3 loss and frequent alteration of EGFR. Neuro Oncol. 2021;23(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gianno F, Giovannoni I, Cafferata B, et al. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO classification. Pathologica. 2022;114(6):422–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wierzbicki K, Ravi K, Franson A, et al. Targeting and therapeutic monitoring of H3K27M-mutant glioma. Curr Oncol Rep. 2020;22(2):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Saratsis AM, Yadavilli S, Magge S, et al. Insights into pediatric diffuse intrinsic pontine glioma through proteomic analysis of cerebrospinal fluid. Neuro Oncol. 2012;14(5):547–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang TY, Piunti A, Lulla RR, et al. Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun. 2017;5(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hindson BJ, Ness KD, Masquelier DA, et al. High-throughput droplet digital PCR system for absolute quantitation of DNA copy number. Anal Chem. 2011;83(22):8604–8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stallard S, Savelieff MG, Wierzbicki K, et al. CSF H3F3A K27M circulating tumor DNA copy number quantifies tumor growth and in vitro treatment response. Acta Neuropathol Commun. 2018;6(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panditharatna E, Kilburn LB, Aboian MS, et al. Clinically relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-derived liquid biopsy. Clin Cancer Res. 2018;24(23):5850–5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mueller S, Jain P, Liang WS, et al. A pilot precision medicine trial for children with diffuse intrinsic pontine glioma-PNOC003: a report from the Pacific Pediatric Neuro-Oncology Consortium. Int J Cancer. 2019;145(7):1889–1901. [DOI] [PubMed] [Google Scholar]
- 29. Miller AM, Shah RH, Pentsova EI, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565(7741):654–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bruzek AK, Ravi K, Muruganand A, et al. Electronic DNA analysis of CSF cell-free tumor DNA to quantify multi-gene molecular response in pediatric high-grade glioma. Clin Cancer Res. 2020;26(23):6266–6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soffietti R, Bettegowda C, Mellinghoff IK, et al. Liquid biopsy in gliomas: a RANO review and proposals for clinical applications. Neuro Oncol. 2022;24(6):855–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rose Brannon A, Jayakumaran G, Diosdado M, et al. Enhanced specificity of clinical high-sensitivity tumor mutation profiling in cell-free DNA via paired normal sequencing using MSK-ACCESS. Nat Commun. 2021;12(1):3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan C, Diplas BH, Chen X, et al. Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol. 2019;137(2):297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bin Nafisah S, Ahmad M.. Ommaya reservoir infection rate: a 6-year retrospective cohort study of Ommaya reservoir in pediatrics. Childs Nerv Syst. 2015;31(1):29–36. [DOI] [PubMed] [Google Scholar]
- 35. Tsoneva DK, Ivanov MN, Conev NV, et al. Circulating histones to detect and monitor the progression of cancer. Int J Mol Sci. 2023;24(2):942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fedyuk V, Erez N, Furth N, et al. Multiplexed, single-molecule, epigenetic analysis of plasma-isolated nucleosomes for cancer diagnostics. Nat Biotechnol. 2023;41(2):212–221. [DOI] [PubMed] [Google Scholar]
- 37. Wadden J, Newell BS, Bugbee J, et al. Ultra-rapid somatic variant detection via real-time targeted amplicon sequencing. Commun Biol. 2022;5(1):708. [DOI] [PMC free article] [PubMed] [Google Scholar]