Abstract
Background
H3 K27M-mutant diffuse glioma primarily affects children and young adults, is associated with a poor prognosis, and no effective systemic therapy is currently available. ONC201 (dordaviprone) has previously demonstrated efficacy in patients with recurrent disease. This phase 3 trial evaluates ONC201 in patients with newly diagnosed H3 K27M-mutant glioma.
Methods
ACTION (NCT05580562) is a randomized, double-blind, placebo-controlled, parallel-group, international phase 3 study of ONC201 in newly diagnosed H3 K27M-mutant diffuse glioma. Patients who have completed standard frontline radiotherapy are randomized 1:1:1 to receive placebo, once-weekly dordaviprone, or twice-weekly dordaviprone on 2 consecutive days. Primary efficacy endpoints are overall survival (OS) and progression-free survival (PFS); PFS is assessed by response assessment in neuro-oncology high-grade glioma criteria (RANO-HGG) by blind independent central review. Secondary objectives include safety, additional efficacy endpoints, clinical benefit, and quality of life. Eligible patients have histologically confirmed H3 K27M-mutant diffuse glioma, a Karnofsky/Lansky performance status ≥70, and completed first-line radiotherapy. Eligibility is not restricted by age; however, patients must be ≥10 kg at time of randomization. Patients with a primary spinal tumor, diffuse intrinsic pontine glioma, leptomeningeal disease, or cerebrospinal fluid dissemination are not eligible. ACTION is currently enrolling in multiple international sites.
Keywords: diffuse glioma, dordaviprone, H3 K27M, ONC201, Phase 3
Key Points.
H3 K27M-mutant diffuse glioma has a poor prognosis and no effective, systemic treatment options.
ACTION is an international phase 3 trial evaluating the imipridone ONC201 in patients with H3 K27M-mutant diffuse glioma.
Importance of the Study.
With no effective treatments other than radiation and a median overall survival of approximately 1 year, H3 K27M-mutant diffuse glioma requires novel, effective therapies. ONC201 (dordaviprone) is an oral antagonist of dopamine receptor D2 and agonist of ClpP that has previously demonstrated safety and efficacy in open-label trials of patients with recurrent H3 K27M-mutant diffuse glioma. ACTION (NCT05580562) is an international, randomized, double-blind, placebo-controlled, phase 3 trial of ONC201 in adult and pediatric patients with H3 K27M-mutant diffuse glioma, and was designed to serve as the basis for potential regulatory approval.
The H3 K27M mutation is a histone mutation observed in up to 70% of pediatric cases of diffuse intrinsic pontine glioma (DIPG) and 60% of adult patients with diffuse midline glioma (DMG), where it is associated with a poor prognosis and aggressive disease course.1–3 Overall survival (OS) for patients with H3 K27M-mutant glioma is approximately 1 year, with a 1-year OS rate of 55.9%.3,4 In 2016, the World Health Organization classified H3 K27M-mutant DMG as a distinct form of Grade IV glioma, regardless of histological features, which is characterized by poor prognosis and global loss of H3 K27 trimethylation (H3 K27me3-loss).5 In the 2021 update of this criterion, the definition was further refined and extended to “diffuse midline glioma, H3 K27-altered” to be inclusive of DMGs that exhibit H3 K27me3-loss due to the H3 K27M mutation or other molecular culprits such as EZHIP.6 Despite the appreciation of H3 K27M status as an important diagnostic factor with a dismal prognosis,7,8 the standard of care for these patients remains radiation followed by monitoring; no effective, systemic therapies are currently available.
ONC201 (dordaviprone) has demonstrated antitumor efficacy in preclinical and clinical evaluations of H3 K27M-mutant glioma.9,10 ONC201 is a first-in-class small-molecule, bitopic antagonist of dopamine receptor D2/3 (DRD2/3) and allosteric agonist of the mitochondrial protease caseinolytic mitochondrial matrix peptidase proteolytic subunit (ClpP).11–15 In the clinic, early clinical trials established therapeutic intratumoral concentrations were achieved in recurrent glioblastoma patients following oral administration of ONC201, without any reports of dose-limiting toxicities. In a phase 2 study of molecularly unselected patients with glioblastoma, the only patient to achieve a durable, radiographic response to monotherapy ONC201 incidentally harbored the H3 K27M-mutation.
Subsequently, an integrated efficacy and safety analysis of 50 patients from 5 open-label clinical studies was designed with input from regulatory authorities to evaluate the safety and efficacy of ONC201 in patients with recurrent H3 K27M-mutant DMG, with a primary endpoint of overall response rate (ORR) by response assessment in neuro-oncology (RANO) high-grade glioma (HGG) criteria.16 The eligibility criteria for the integrated analysis were designed to isolate the single-agent activity of ONC201 in this patient population (eg, patients were treated at least 90 days from prior radiotherapy). The ORR by RANO-HGG criteria was 20.0% (95% confidence interval [CI], 10.0–-33.7); ORR using best response by either HGG or low-grade glioma RANO criteria was 30.0% (95% CI, 17.9–44.6). Duration of response by RANO-HGG was 11.2 months (95% CI, 3.8–not reached) and median time to response was 8.3 months (range, 1.9–15.9). Additionally, 46.7% of patients on corticosteroids at baseline had >50% decreases in steroid dose and 20.6% of patients with performance status deficiencies at baseline demonstrated an increase in performance status score. A subgroup analysis suggested that responders tended to have a higher baseline performance score and a single target lesion. Both of these characteristics may be enriched in the frontline setting relative to the recurrent setting17 Recently, an analysis of H3 K27M DMG patients treated with ONC201 following frontline radiation reported a 21.7-month median OS from diagnosis compared to 12 months for historical control patients.18
Based on these findings, the phase 3 ACTION trial (NCT05580562) was developed to assess the safety and efficacy of once- or twice-weekly ONC201 in a double-blind, placebo-controlled randomized clinical trial in pediatric and adult patients with newly diagnosed H3 K27M-mutant diffuse glioma.
Methods
Study Design
ACTION (ONC201-108, NCT05580562) is a phase 3, randomized, double-blind, placebo-controlled international trial that is evaluating ONC201 following standard-of-care radiotherapy in pediatric and adult patients with newly diagnosed, H3 K27M-mutant diffuse glioma. The protocol, any amendments, informed consent forms, investigators brochure, and other relevant materials must be reviewed and approved by each site’s institutional review board.
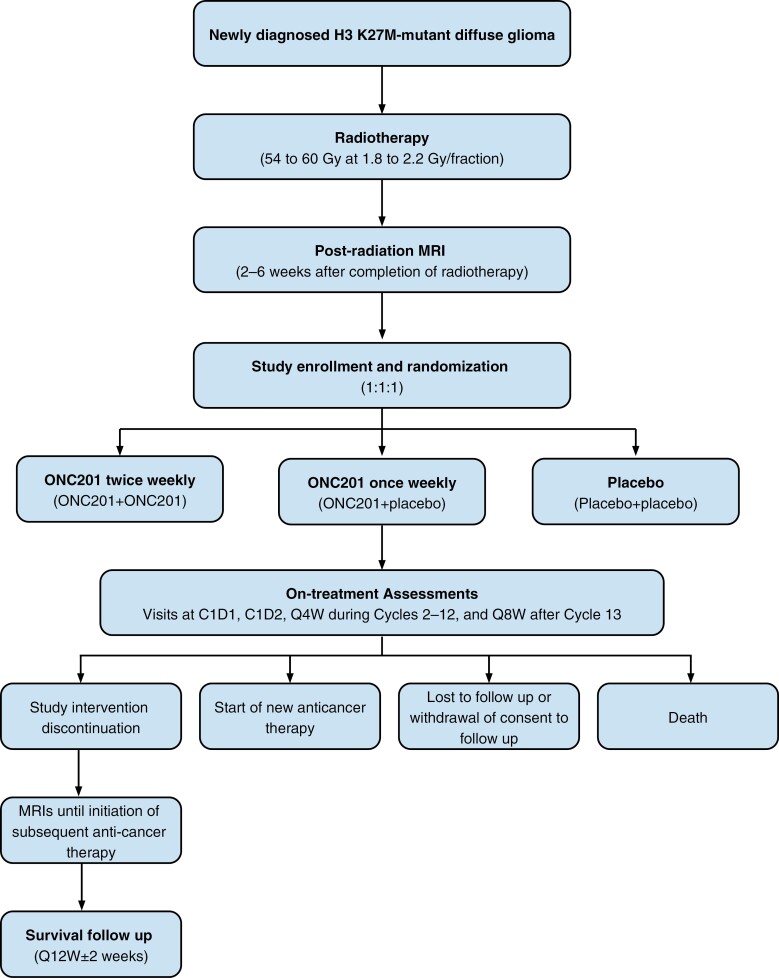
Study eligibility requires known evidence of the H3 K27M-mutation by immunohistochemistry or gene sequencing of the tumor in a CLIA or equivalent setting. Patients must be randomized 2–6 weeks following completion of radiotherapy and must be randomized within 21 days of their post-radiotherapy MRI. Patients are randomized 1:1:1 to receive twice-weekly ONC201, once-weekly ONC201, or placebo (Figure 1). Stratification factors include age (<21 years; ≥21 years) and a risk category based on the presence of enhancing tumors≥10 cm2, multifocal lesions, and/or brainstem tumor location. All patients receive ONC201 or placebo capsules on two consecutive days (day 1 and day 2) each week. In the twice-weekly treatment arm, ONC201 is administered on both day 1 and day 2. In the once-weekly treatment cohort, ONC201 is administered on day 1, followed by a placebo dose on day 2. The placebo arm receives placebo on both days. Treatment and study assessments are based on 28-day cycles.
Figure 1.
Study schematic. C1D1, Cycle 1 Day1; C1D2, Cycle 1 Day 2; MRI, magnetic resonance imaging, Q4W, every 4 weeks; Q8W, every 8 weeks; Q12W, every 12 weeks.
ONC201 is administered at 625 mg per dose to patients weighing at least 52.5 kg; patients weighing less than 52.5 kg receive a dose scaled by body weight and rounded to the nearest 125 mg capsule or matching placebo (Supplementary Table 1). For patients unable to swallow capsules, ONC201 can be extemporaneously dissolved in approved diluents.
Treatment continues at least until confirmed disease progression, unacceptable adverse event, withdrawal of consent, or change in the patient’s condition judged by the treating physician to render the patient ineligible for further treatment (complete reasons for discontinuation are shown in Supplementary Table 2). Among patients with confirmed progression, treatment beyond the first progression is permitted at the investigator’s discretion and may be administered with concomitant bevacizumab or re-irradiation. Gadolinium-enhanced MRI is performed approximately every 8 weeks. Treatment with ONC201 beyond first progression discontinues if patients initiate an anticancer therapy other than bevacizumab and/or re-irradiation. Patients who discontinue treatment can continue to be followed for safety assessment until death, withdrawal of consent to follow-up, or study completion. Dose modifications such as treatment discontinuation, treatment interruption, and dose reduction may be implemented in the event of grade 3/4 treatment-related, treatment-emergent adverse events or grade 4 any-cause adverse events of special interest (Supplementary Table 3).
Objectives
The primary objective of the study is to evaluate the efficacy of ONC201; primary endpoints are OS and progression-free survival (PFS) by RANO-HGG criteria. Secondary objectives include safety, additionally efficacy assessments, clinical benefit, and quality of life. A summary of primary and secondary objectives and endpoints is available in Table 1.
Table 1.
Objectives and endpoints
| Objectives | Endpoints |
|---|---|
| Primary | |
| To evaluate the efficacy of ONC201 administered following radiotherapy in participants with H3 K27M-mutant diffuse glioma | • OS • PFS using RANO-HGG criteria |
| Secondary | |
| To evaluate the safety and tolerability of ONC201 versus placebo | • Incidence of AEs: overall, treatment-related, Grade 3 or higher in severity, serious, fatal, those resulting in treatment discontinuation, and events of special interest • Change from baseline in clinical laboratory parameters • Distribution of graded clinical laboratory parameters |
| To evaluate the efficacy of ONC201 administered following radiotherapy using RANO-HGG criteria in participants with H3 K27M-mutant diffuse glioma | • PFS using RANO-HGG criteria for participants with measurable contrast-enhancing disease |
| To evaluate clinical benefits of treatment with ONC201 | • Corticosteroid response • Time to first corticosteroid response • Duration of first corticosteroid response • Cumulative duration of corticosteroid responses • Corticosteroid dose and change from baseline over time • Time to corticosteroid use deterioration • Performance status response • Time to first performance status response • Duration of first performance status response • Cumulative duration of performance status responses • Performance status and change from baseline over time • Time to performance status deterioration |
| To evaluate the impact of ONC201 on health-related QoL and neurological function | • Change from baseline in QoL assessments ◦ ≥18 years of age: EORTC-QLQ-C30, QLQ-BN20, and MDASI-BT ◦ 2 to <18 years of age: PedsQL Brain Tumor Module • Change from baseline NANO results |
| Exploratory | |
| To assess plasma concentrations and estimate PK parameters for ONC201 | • Plasma concentrations of ONC201 • PK parameters for ONC201 |
| To evaluate the exposure-response relationship | • Correlation between extent of exposure to ONC201 and select efficacy and safety endpoints |
| To evaluate the efficacy of ONC201 using RANO-LGG criteria | • PFS using RANO-LGG criteria |
| To evaluate the impact of molecular profile on outcomes | • Correlation between the molecular profile of the tumor and efficacy (OS and PFS) |
| To compare outcomes among selected subgroups | • OS, PFS • Incidence of AEs |
| To evaluate the impact of ONC201 treatment on health care resource utilization | • Health care resource utilization |
Abbreviations: AE, adverse events; HGG, high-grade glioma; LGG, low-grade glioma; NANO, Neurologic Assessment in Neuro-Oncology; OS, overall survival; RANO, response assessment in neuro-oncology; PFS, progression-free survival; PK, pharmacokinetic.
Patients
Eligible patients must weigh at least 10 kg, have been diagnosed with H3 K27M-mutant diffuse glioma, and have completed standard radiotherapy (54–60 Gy at 1.8–2.2 Gy/fraction) within 2–6 weeks of randomization. H3 K27M mutational status must be identified using immunohistochemistry or gene sequencing of the tumor in a clinical laboratory improvement amendments (CLIA)-approved laboratory or equivalent setting. Eligible patients must have a KPS/LPS of at least 70, be receiving a stable or decreasing dose of corticosteroids and anti-seizure medications for 7 days prior to initiation of study drug, and have a sufficient washout from temozolomide, DRD2 antagonists, strong CYP3A4/5 inhibitors and inducers, and investigational agents. Patients with spinal tumors, DIPG, concurrent malignancy, or prior receipt of whole-brain radiotherapy, proton radiotherapy, ONC201, ONC206, bevacizumab, or tumor-treating fields are not eligible. Complete eligibility criteria are shown in Table 2.
Table 2.
Eligibility criteria
| Inclusion criteria |
|---|
| Able to understand the study procedures and agree to participate in the study by providing written informed consent (by participant or legally authorized representative), and assent when applicable. |
| Body weight ≥10 kg at time of randomization. |
| Histologically diagnosed H3 K27M-mutant diffuse glioma (new diagnosis, nonrecurrent). Detection of a missense K27M mutation in any histone H3-encoding gene detected by local testing of tumor tissue (IHC or NGS in a CLIA-certified or equivalent laboratory). Site to provide (as available): ≥ 10 unstained FFPE slides from tumor tissue |
| Completed standard frontline radiotherapy (54 to 60 Gy at 1.8 to 2.2 Gy/fraction) ≤ 6 weeks prior to randomization. |
| At least 1, high-quality, contrast-enhanced MRI of the brain obtained prior to starting radiotherapy for submission to sponsor’s imaging vendor for central read. For participants who had a surgical resection, this scan must be post-resection; for participants who did not have a resection, this scan may be pre- or post-biopsy. |
| At least 1 high-quality, contrast-enhanced MRI of the brain obtained within 2–6 weeks after completion of frontline radiotherapy. Site to also provide (if available): pre-surgery/biopsy, post-surgery, and radiation planning MRIs. |
| KPS/LPS ≥70 at time of randomization |
| Stable or decreasing dose of corticosteroids and anti-seizure medications for 7 days prior to randomization, if applicable. Stable steroid dose is defined as ≤2 mg/day increase (based on dexamethasone dose or equivalent dose of alternative steroid). |
| Exclusion criteria |
| Primary spinal tumor. |
| DIPG, defined as tumors with a pontine epicenter and diffuse involvement of the pons. |
| Evidence of leptomeningeal spread of disease or CSF dissemination. |
| Any known concurrent malignancy. |
| New lesion(s) outside of the radiation field. |
| Received whole-brain radiotherapy. |
| Received proton therapy for glioma. |
| Use of any of the following treatments within the specified time periods prior to randomization: • ONC201 or ONC206 at any time. • Bevacizumab (includes biosimilars) at any time. • Temozolomide within past 3 weeks. • Tumor-treating fields at any time. • DRD2 antagonist within past 2 weeks. • Any investigational therapy within past 4 weeks. • Strong CYP3A4/5 inhibitors (see Appendix 8) within 3 days. • Strong CYP3A4/5 inducers (includes enzyme-inducing antiepileptic drugs; see Appendix 8) within 2 weeks. |
| Laboratory test results meeting any of the following parameters within 2 weeks prior to randomization: • ANC < 1.0 × 109/L or platelets < 75 × 109/L. • Total bilirubin > 1.5 × ULN (participants with Gilbert’s syndrome may be included with total bilirubin > 1.5 × ULN if direct bilirubin is ≤ 1.5 × ULN). • AST or ALT > 2.5 × ULN. • Creatinine clearance ≤ 60 mL/min as calculated by the Cockcroft Gault equation (or estimated glomerular filtration rate < 60 mL/min/1.73 m2. |
| QTc > 480 ms (based on mean from triplicate ECGs) during screening |
| Known hypersensitivity to any excipients used in the study intervention formulation. |
| Pregnant, breastfeeding, or planning to become pregnant while receiving study intervention or within 3 months after the last dose. Participants of childbearing potential must have a negative serum pregnancy test within 72 h prior to receiving the first dose of study intervention. |
| Uncontrolled intercurrent illness including, but not limited to, ongoing or active infection requiring systemic therapy or psychiatric illness/social situations that would limit compliance with study requirements. |
| Any other condition (eg, medical, psychiatric, or social) that, in the opinion of the investigator, may interfere with participant safety or the ability to complete the study according to the protocol. |
ANC, Absolute neutrophil count; ALT, alanine aminotransferase alanine aminotransferase; AST, aspartate aminotransferase; CLIA, clinical laboratory improvement amendments; CSF, cerebrospinal fluid; DIPG, diffuse intrinsic pontine glioma; DRD2, dopamine receptor D2; ECG, electrocardiogram; FFPE, formalin-fixed paraffin-embedded; ICH, immunohistochemistry; KPS, Karnofsky Performance Status; LPS, Lansky Performance Status NGS, next-generation sequencing; MRI, magnetic resonance imaging; ULN, upper limit of normal.
Assessments
Patients have study visits every 4 weeks during cycles 1–12; subsequent visits occur every 8 weeks. Where permitted, home health visits may replace an office visit for even numbered cycles through cycle 12. Disease assessment via contrast-enhanced MRI scans of the brain occurs approximately every 8 weeks (±2 weeks) until death, initiation of subsequent anticancer therapy, or withdrawal of consent. PFS endpoints are assessed by blinded, independent central review using a dual reader with adjudication paradigm. Where available, patients provide MRIs conducted prior to radiotherapy. A summary of select study visit assessments is shown in Table 3.
Table 3.
Study visit summary of select assessments
| Screening (≤ Day 28) | Cycle 1, Day 1 | Cycle 1, Day 2 | Cycle 2, Day 1 | Cycle 3–12 (Q4W) | Cycle ≥13 | |
|---|---|---|---|---|---|---|
| Weight and height | X | X | X | X | ||
| Physical exam/vital signs | X | Cycle 3, Day 1 + Q8W | ||||
| 12-lead electrocardiograma | X | X | X | Cycle 3, Day 1 + Q8W | ||
| Quality of life questionnaires | X | Cycle 3, Day 1 + Q8W | ||||
| Performance status (KPS/LPS) | X | X | Cycle 3, Day 1 + Q8W | |||
| Neurologic exam | X | X | Cycle 3, Day 1 + Q8W | |||
| MRIb | X | Cycle 3, Day 1 + Q8W | ||||
| Blood collection | ||||||
| Hematology | X | X | X | X | X | |
| Serum chemistries | X | X | X | X | X | |
| Plasma for exploratory biomarkers/biobanking | X | Cycle 3, Day 1 + Q8W | ||||
| Plasma pharmacokinetics | X | X | Cycle 3, Day 1 + Q8W | |||
Abbreviations: KPS/LPS, Karnofsky performance score/Lansky performance score; MRI, magnetic resonance imaging.
aScreening electrocardiogram, collect in triplicate (≥1 min intervals); cycle 1, day 1 and cycle 1 day 2, single tracings predose and between 1 and 2 h post dose (before pharmacokinetic blood draws); other time points, single tracings, predose when applicable. If a tracing indicates QTc prolongation >500 ms, repeat to confirm, then determine if participant requires treatment interruption or discontinuation.
bFirst on-treatment MRI will be collected at C3D1 (±7 days). Subsequent MRIs will be collected every 8 weeks (±14 days) while the participant is receiving study intervention and through the time of initiation of a subsequent anticancer therapy. Upon determination of possible radiographic progression per the investigator, a confirmatory scan is required 4–10 weeks later.
Patient tissue samples may be used for correlative biomarker analyses. Blood samples are collected for biobanking and future analysis on day 1 (predose), C3D1 (±7 days), and every 8 weeks (±7 days) thereafter, ideally within 5 days of each scheduled MRI (Table 3). When available, formalin-fixed paraffin-embedded slides are collected to enable biomarker analyses (Table 2).
Statistical Considerations
Approximately 530 patients will be screened and approximately 450 patients will be randomized to achieve 327 OS events and 286 PFS events at each final analysis. The expected true hazard ratio is 0.65 for OS and 0.60 for PFS, with a minimum desired statistical power of 80%. Each primary analysis will independently compare each of the 2 ONC201 dosing arms with the placebo arm.
Two interim OS analyses are planned following 164 OS events (0.00098 nominal 2-sided alpha level) and 246 OS events (0.01064 nominal 2-sided alpha level). The final OS analysis, conducted once 327 OS events have been observed, will be performed at a 0.0346 nominal 2-sided alpha level. The final PFS analysis will be performed once 286 PFS events have occurred (0.012 alpha level); no interim PFS analysis is planned. For both PFS and OS analyses, the Hochberg method will be used to account for the 2-dose group comparison.
Study Sites
More than 120 international study sites are targeted in at least 15 countries globally, including centers in North America, Europe, Israel, and Asia Pacific. Currently, open sites can be found at https://clinicaltrials.gov/ct2/show/NCT05580562.
Discussion
With no known effective treatments other than radiation, H3 K27M-mutant diffuse glioma has a dire unmet need for new therapies. ACTION is the largest interventional clinical trial in patients with H3 K27M-mutant diffuse gliomas, and was designed to serve as the basis for regulatory approval of ONC201. The planned enrollment of 450 patients requires international collaboration and support from treating physicians. Transparency for the rationale for the scientific design of clinical trials is vital to collaboration and, as such, we outline the rationale for key aspects of ACTION trial design below.
Inclusion of Only Patients With H3 K27M-Mutant Disease
The ACTION trial only enrolls patients who have H3 K27M-mutant diffuse glioma as confirmed by IHC or NGS. To date, responses to ONC201 monotherapy among patients with glioma have been limited to those with the H3 K27M mutation and therefore the ACTION trial is limited to that population.19 The definition of H3 K27M-mutant glioma in this protocol reflects guidance from the 2016 WHO classification of CNS tumors.5 As ACTION is intended as a global registrational trial, it is beneficial to specifically define the patient population, where the mutation has been well characterized and is part of the standard diagnostic criteria. H3 K27M mutational status is often determined as part of diagnosis, either by IHC or NGS, and there is a high level of concordance between these 2 testing strategies.20–25
The 2021 version of the WHO classification of solid tumors expanded the definition to H3 K27-altered tumors, which is inclusive of other tumors that result in a global loss of H3 K27 trimethylation due to the H3 K27M mutation or other molecular mechanisms.6 It is important to note that the H3 K27M mutation accounts for the majority of thalamic and spinal cases of pediatric HGG.26,27 Furthermore, although one could argue that treatment with ONC201 could benefit these patients by the reversal of H3 K27 trimethyl loss,18 this activity has not yet been evaluated in patients.
Tumor Location
Tumor location is not restricted to the midline for ACTION eligibility, as ONC201 has demonstrated responses in both midline16 and non-midline diffuse glioma.28 Primary spinal or pontine tumors are excluded due to competing pediatric studies that were ongoing prior to ACTION. One such trial is PNOC022, a phase 2 trial evaluating ONC201 in combination with other anticancer compounds,29 and the second is the phase 3 Biological Medicine for DIPG Eradication 2.0 (BIOMEDE2.0) trial, which is evaluating the efficacy of ONC201 relative to everolimus.30 As such, excluding DIPG from ACTION ensures a more homogeneous patient population for this potentially registrational trial while also avoiding competition for patients with other important trials in DIPG.
Definition of Standard of Care
Currently, the standard of care for H3 K27M-mutant diffuse glioma is radiotherapy followed by supportive care. Some physicians treat patients with temozolomide due to its activity in molecularly unselected glioblastoma. Additionally, patients treated with temozolomide are permitted to enroll on trial, with a required 3-week washout. However, temozolomide has failed to demonstrate efficacy in patients with H3 K27M-mutant or MGMT-unmethylated HGG.8,31–34
ACTION requires that all enrolled patients receive frontline radiotherapy. At first progression and at the physician’s discretion, patients may continue on treatment and additionally receive re-irradiation and bevacizumab. Alternatively, any patients may discontinue treatment and pursue other treatment options. This is in line with the current standard of care, in which patients are monitored for progression following radiation, after which other treatment options may be considered.
Inclusion of Placebo
ACTION is a placebo-controlled trial that is designed to be a global registrational trial and facilitate regulatory approval. Placebo-controlled studies are the most robust clinical trial design methodology, as it minimizes bias from multiple sources. Placebo ensures compliance within the study, as otherwise an open-label design would be expected to have increased study dropout rates and/or lost-followed that would preclude evaluation.35
Inclusion of Two Active Dose Levels
ACTION evaluates 2 ONC201 dose schedules. The decision to include 2 dose schedules of ONC201 was based on FDA feedback related to Project Optimus.36 Furthermore, the randomization that includes treatment arms ensures that two-thirds of enrolled patients receive ONC201.
Cross-over
Due to the requirement of overall survival as the key regulatory endpoint of ACTION, cross-over is not permitted. This decision was made due to the confounding effects cross-over may have on OS. If an effect on OS becomes apparent only later in disease course, following the time at which most placebo-treated patients cross-over, efficacy analyses could be confounded by treatment with the active compound and affect the validity of results.37 In a review of cross-over and non-cross-over oncology studies, OS improvement was observed in 70.2% (33/47) non-cross-over trials, compared with only 25.0% (4/16) of cross-over designs.38 There have been examples of late-stage trials in HGGs where cross-over has confounded the evaluation of OS.39,40 The omission of cross-over from ACTION’s design provides the best opportunity for an unconfounded trial and, if successful, ultimately regulatory approval of ONC201. A decision to unblind participants may be considered at the time of a positive planned interim analysis, in conjunction with guidance from regulatory authorities.
Conclusions
Participation in rigorously designed clinical trials is essential for the proper evaluation of novel therapeutics for underserved indications and is required for regulatory authorization. The ACTION trial will assess the safety and efficacy of ONC201 in newly diagnosed patients with H3 K27M-mutant diffuse glioma following radiotherapy and is intended to support regulatory approval in multiple countries if proven effective.
Supplementary Material
Acknowledgments
This article appears as part of the supplement “H3 K27M-mutant Glioma: Disease State Overview,” sponsored by Chimerix, Inc. Medical writing support was provided by Meghan Sullivan, PhD, CMPP, an employee of Chimerix, Inc.
Contributor Information
Isabel Arrillaga-Romany, Mass General Cancer Center, Neuro-Oncology, Massachusetts General Hospital, Boston, Massachusetts, USA.
Andrew Lassman, Columbia University Vagelos College of Physicians and Surgeons, Herbert Irving Comprehensive Cancer Center, New York-Presbyterian Hospital, New York City, New York, USA.
Susan L McGovern, Department of Radiation Oncology, University of Texas MD Anderson Cancer Center, Houston, Texas, USA.
Sabine Mueller, Helen Diller Family Comprehensive Cancer Center, University of California, San Francisco, San Francisco, California, USA.
Burt Nabors, Department of Neuro-Oncology, University of Alabama at Birmingham, Birmingham, Alabama, USA.
Martin van den Bent, Brain Tumor Center at Erasmus MC Cancer Institute, University Medical Center Rotterdam, Rotterdam, The Netherlands.
Michael A Vogelbaum, Moffitt Cancer Center, Tampa, Florida, USA.
Joshua E Allen, Chimerix, Inc., Durham, North Carolina, USA.
Allen S Melemed, Chimerix, Inc., Durham, North Carolina, USA.
Rohinton S Tarapore, Chimerix, Inc., Durham, North Carolina, USA.
Patrick Y Wen, Center For Neuro-Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts, USA.
Timothy Cloughesy, Bowyer Oncology Center, University of California Los Angeles, Los Angeles, California, USA.
Funding
Chimerix, Inc.
Conflict of Interest
I.A.-R. has received research funding Chimerix, Inc. A.L. has served on advisory board for Chimerix, Inc. and received equipment, materials, or medical writing support from Chimerix, Inc. S.L.M. has served on an advisory board for Chimerix, Inc. S.M. has no relevant conflicts of interest to declare. B.N. has served on an advisory board for Chimerix, Inc. M.v.d.B. has no relevant conflicts of interest to declare. M.V. has served on an advisory board for Chimerix, Inc. J.E.A., A.S.M., and R.S.T. are employees of and have stock ownership in Chimerix, Inc. J.E.A. has patents related to ONC201. P.Y.W. has received research funding from Chimerix, Inc., served as a consultant for Chimerix, Inc., and served on an advisory board for Chimerix, Inc. T.C. has received research funding from Chimerix, Inc, owns stock in Chimerix, Inc, and receives milestone payments and possible future royalties from Chimerix, Inc.
Supplement sponsorship
This article appears as part of the supplement “H3 K27M-mutant Glioma: Disease State Overview,” sponsored by Chimerix, Inc.
Author Contributions
Study Design: I.A.-R., A.L., S.L.M., S.M., B.N., M.v.d.B., M.V., J.E.A., A.S.M., R.S.T., P.Y.W., T.C. Data Collection: non-applicable. Statistical Analyses: non-applicable. Manuscript Preparation: I.A.-R., J.E.A., A.S.M., R.S.T. Manuscript Review: I.A.R., A.L., S.L.M., S.M., B.N., M.v.d.B., M.V., J.E.A., A.S.M., R.S.T., P.Y.W., T.C.
References
- 1. Di Nunno V, Franceschi E, Gatto L, et al. How to treat histone 3 altered gliomas: molecular landscape and therapeutic developments. Expert Rev Clin Pharmacol. 2023;16(1):17–26. [DOI] [PubMed] [Google Scholar]
- 2. Feng J, Hao S, Pan C, et al. The H3.3 K27M mutation results in a poorer prognosis in brainstem gliomas than thalamic gliomas in adults. Hum Pathol. 2015;46(11):1626–1632. [DOI] [PubMed] [Google Scholar]
- 3. Khuong-Quang DA, Buczkowicz P, Rakopoulos P, et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124(3):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ostrom QT, Shoaf ML, Cioffi G, et al. National-level overall survival patterns for molecularly-defined diffuse glioma types in the United States. Neuro Oncol. 2023;25(4):799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 6. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13(4):410–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abe H, Natsumeda M, Kanemaru Y, et al. MGMT expression contributes to temozolomide resistance in H3K27M-mutant diffuse midline gliomas and MGMT silencing to temozolomide sensitivity in IDH-mutant gliomas. Neurol Med Chir (Tokyo). 2018;58(7):290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allen JE, Kline CL, Prabhu VV, et al. Discovery and clinical introduction of first-in-class imipridone ONC201. Oncotarget. 2016;7(45):74380–74392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Prabhu VV, Morrow S, Rahman Kawakibi A, et al. ONC201 and imipridones: anti-cancer compounds with clinical efficacy. Neoplasia. 2020;22(12):725–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Allen JE, Krigsfeld G, Mayes PA, et al. Dual inactivation of Akt and ERK by TIC10 signals Foxo3a nuclear translocation, TRAIL gene induction, and potent antitumor effects. Sci Transl Med. 2013;5(171):171ra–17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Free RB, Cuoco CA, Xie B, et al. Pharmacological Characterization of the imipridone anticancer drug ONC201 reveals a negative allosteric mechanism of action at the D2 dopamine receptor. Mol Pharmacol. 2021;100(4):372–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madhukar NS, Khade PK, Huang L, et al. A Bayesian machine learning approach for drug target identification using diverse data types. Nat Commun. 2019;10(1):5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ishizawa J, Zarabi SF, Davis RE, et al. Mitochondrial ClpP-mediated proteolysis induces selective cancer cell lethality. Cancer Cell. 2019;35(5):721–737.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graves PR, Aponte-Collazo LJ, Fennell EMJ, et al. Mitochondrial protease ClpP is a target for the anticancer compounds ONC201 and related analogues. ACS Chem Biol. 2019;14(5):1020–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arrillaga-Romany I, Gardner S, Odia Y, et al. ONC201 (Dordaviprone) in recurrent H3 K27M-mutant diffuse midline glioma [accepted, pending publication]. J Clin Oncol. 2024; ePub ahead of print. doi: 10.1200/JCO.23.01134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arrillaga-Romany I, Kurz S, Tarapore R, et al. LTBK-05. Clinical efficacy of ONC201 in recurrent H3 K27M-mutant diffuse midline glioma patients. Neuro Oncol. 2021;23(Supplement_6):vi230–vi230. [Google Scholar]
- 18. Venneti S, Kawakibi AR, Ji S, et al. Clinical efficacy of ONC201 in H3K27M-mutant diffuse midline gliomas is driven by disruption of integrated metabolic and epigenetic pathways. Cancer Discov. 2023;13(11):2370–2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arrillaga-Romany I, Chi AS, Allen JE, et al. A phase 2 study of the first imipridone ONC201, a selective DRD2 antagonist for oncology, administered every three weeks in recurrent glioblastoma. Oncotarget. 2017;8(45):79298–79304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tarapore RS, Arain S, Blaine E, et al. Immunohistochemistry detection of histone H3 K27M mutation in human glioma tissue. Appl Immunohistochem Mol Morphol. 2023;32(2):96–101. [DOI] [PubMed] [Google Scholar]
- 21. Bechet D, Gielen GG, Korshunov A, et al. Specific detection of methionine 27 mutation in histone 3 variants (H3K27M) in fixed tissue from high-grade astrocytomas. Acta Neuropathol. 2014;128(5):733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang T, Garcia R, Qi J, et al. Detection of histone H3 K27M mutation and post-translational modifications in pediatric diffuse midline glioma via tissue immunohistochemistry informs diagnosis and clinical outcomes. Oncotarget. 2018;9(98):37112–37124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Venneti S, Santi M, Felicella MM, et al. A sensitive and specific histopathologic prognostic marker for H3F3A K27M mutant pediatric glioblastomas. Acta Neuropathol. 2014;128(5):743–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao H, Fang X, Xue B.. Four methods to analyze H3K27M mutation in diffuse midline gliomas. Pathol Res Pract. 2020;216(9):153065. [DOI] [PubMed] [Google Scholar]
- 25. Manjunath N, Jha P, Singh J, et al. Clinico-pathological and molecular characterization of diffuse midline gliomas: is there a prognostic significance? Neurol Sci. 2021;42(3):925–934. [DOI] [PubMed] [Google Scholar]
- 26. Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sturm D, Witt H, Hovestadt V, et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell. 2012;22(4):425–437. [DOI] [PubMed] [Google Scholar]
- 28. Odia Y, Sumrall AL, Cloughesy TF, et al. Single agent activity of ONC201 in non-midline H3 K27M-mutant diffuse gliomas. J Clin Oncol. 2021;39(15_suppl):e14037–e14037. [Google Scholar]
- 29. ONC201 in H3 K27M-mutant Diffuse Glioma Following Radiotherapy (the ACTION Study). ClinicalTrials.gov identifier: NCT05580562. Updated April 10, 2023. Accessed April 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05580562 [Google Scholar]
- 30. Biological Medicine for Diffuse Intrinsic Pontine Glioma (DIPG) Eradication 2.0. ClinicalTrials.gov identifier: NCT05476939. Updated January 30, 2023. Accessed April 18, 2023. https://clinicaltrials.gov/ct2/show/NCT05476939 [Google Scholar]
- 31. Stupp R, Hegi ME, Mason WP, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. [DOI] [PubMed] [Google Scholar]
- 32. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 33. Cohen KJ, Heideman RL, Zhou T, et al.. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children's Oncology Group, Neuro-Oncol. 2011;13(4):410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chassot A, Canale S, Varlet P, et al. Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J Neuro-Oncol. 2011;106(2):399–407. [DOI] [PubMed] [Google Scholar]
- 35. Monaghan TF, Agudelo CW, Rahman SN, et al. Blinding in clinical trials: seeing the big picture. Medicina (Kaunas). 2021;57(7):647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Research FaDACfDEa. Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases: Guidance for Industry. MD: FDA; 2023. [Google Scholar]
- 37. Prasad V, Grady C.. The misguided ethics of crossover trials. Contemp Clin Trials. 2014;37(2):167–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen EY, Prasad V.. Crossover is not associated with faster trial accrual. Ann Oncol. 2018;29(3):776–777. [DOI] [PubMed] [Google Scholar]
- 39. Liau LM, Ashkan K, Tran DD, et al. First results on survival from a large phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wick W, van den Bent MJ.. First results on the DCVax phase III trial: raising more questions than providing answers. Neuro Oncol. 2018;20(10):1283–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.