Abstract
Physiological cell deaths occur ubiquitously throughout biology and have common attributes, including apoptotic morphology with mitosis-like chromatin condensation and prelytic genome digestion. The fundamental question is whether a common mechanism of dying underlies these common hallmarks of death. Here we describe evidence of such a conserved mechanism in different cells induced by distinct stimuli to undergo physiological cell death. Our genetic and quantitative biochemical analyses of T- and B-cell deaths reveal a conserved pattern of requisite components. We have dissected the role of cysteine proteases (caspases) in cell death to reflect two obligate classes of cytoplasmic activities functioning in an amplifying cascade, with upstream interleukin-1β-converting enzyme-like proteases activating downstream caspase 3-like caspases. Bcl-2 spares cells from death by punctuating this cascade, preventing the activation of downstream caspases while leaving upstream activity undisturbed. This observation permits an operational definition of the stages of the cell death process. Upstream steps, which are necessary but not themselves lethal, are modulators of the death process. Downstream steps are effectors of, and not dissociable from, actual death; the irreversible commitment to cell death reflects the initiation of this downstream phase. In addition to caspase 3-like proteases, the effector phase of death involves the activation in the nucleus of cell cycle kinases of the cyclin-dependent kinase (Cdk) family. Nuclear recruitment and activation of Cdk components is dependent on the caspase cascade, suggesting that catastrophic Cdk activity may be the actual effector of cell death. The conservation of the cell death mechanism is not reflected in the molecular identity of its individual components, however. For example, we have detected different cyclin-Cdk pairs in different instances of cell death. The ordered course of events that we have observed in distinct cases reflects essential thematic elements of a conserved sequence of modulatory and effector activities comprising a common pathway of physiological cell death.
Although interest in the process of physiological cell death has grown enormously in recent years, the mechanism of death has remained enigmatic. While the induction of physiological death in diverse cell types is effected by a wide variety of stimuli, a common morphology, described as apoptosis, ensues in all cases. The commonality of morphology has led to the belief that disparate inducers trigger distinct signaling events which ultimately converge in a common biochemical pathway of death. This hypothesis suggests a division of the biochemical process into upstream events that are specific for individual inducers and downstream steps, comprising the common pathway, which bring about the actual demise of the cell.
Since most cell deaths in the nematode Caenorhabditis elegans are induced in a lineage-determined program, the simple pathway of death elucidated in that species (17) is likely to be revealing of downstream steps. Cell death in C. elegans is dependent on the activation of Ced3, a cysteine protease (77, 79), and is inhibited by Ced9 (27). In mammalian cells, a group of Ced3 homologs, termed caspases (1), appears to play a role in virtually all of the physiological cell deaths studied to date. These enzymes cleave on the carboxyl-terminal side of aspartate residues within distinct recognition motifs. Each caspase is synthesized as a proenzyme and activated by cleavage at internal sites, potentially by the same or another caspase class (66, 77). This leads to the notion that caspases function in an ordered cascade, with members of one family activating members of the next. Data consistent with this pattern have been obtained from studies in vitro (41, 60, 65).
Of the large family of mammalian caspases, caspase 3 is closely homologous to Ced3 and appears to be involved widely in cell deaths (50, 65). Nonetheless, specific caspases seem not to be associated uniquely with distinct cases of death, and gene-targeting experiments reveal that the absence of a single caspase has extremely limited consequences for cell death responsiveness (38, 39).
Similarly, a family of ced9-related death response modulatory genes exists in mammals; the most closely related homolog, bcl-2, is functionally interchangeable with ced9 in the worm (28, 73). These gene products do not function in all mammalian cell deaths (61, 72). Moreover, while the products of some bcl-2 gene family members have death-sparing activity (6, 7), others exert the opposite effect (52, 78).
Several cellular proteins, among them poly(ADP-ribose) polymerase (PARP), nuclear lamins, fodrin, and DNA-dependent protein kinase (10, 16, 34), are targets for cleavage by various caspases. In cells spared from death, for example by Bcl-2, these proteolytic events do not occur (9, 13, 18). Still, the cleavage of none of these proteins has been shown to be essential for the cell death response (42, 54, 74). The specific consequences of caspase activation which are lethal are unknown.
It may be that the consequence of protease activity is the specific activation of distinct death effectors. We have proposed that essential genes involved in cell division may be critically involved in cell death as well and that the difficulty in identifying distal effector steps genetically reflects the indispensable function of those gene products in cell life (67). Data from several groups have shown that cell cycle catastrophes, the precocious expression of mitosis-like cyclin-dependent histone kinases (Cdks), are associated with a variety of physiological cell deaths and that the inhibition of death by Bcl-2 is associated with alterations in the expression and localization of these Cdk proteins (22, 23, 29, 36, 40, 46, 47, 58, 59, 70).
We have taken advantage of the death-sparing activities of Bcl-2 and two viral caspase inhibitors, CrmA and p35 (64, 77), to dissect the mechanism of cell death in two separate cellular paradigms. These studies allow us to draw a generalized skeletal pathway of the death-associated biochemical activities discussed above and demonstrate the requisite involvement of these different classes of activities in a conserved and ordered pathway by which cells die physiologically.
MATERIALS AND METHODS
Cell culture.
Freshly cloned cells were grown in RPMI 1640 medium (Whittaker Bioproducts, Walkersville, Md.) supplemented with glutamine (2 mM), 2-mercaptoethanol (50 μM), and heat-inactivated fetal bovine serum (10% [vol/vol]; Tissue Culture Biologicals, Tulare, Calif.). Cell death was assessed routinely as trypan blue dye uptake.
Transfections.
Transfectant clones were selected in 1.5 mg of G418 sulfate (Gibco BRL, Grand Island, N.Y.) per ml added 24 h after electroporation (250 V and 960 μF) of 107 cells with 20 μg of linearized pSFFV-Neo/Bcl-2 (30) or constructs of CrmA, CrmAmut, or p35 in pcDNA3 vector (64). For each construct, at least 10 independent transfectant clones were isolated; in each case, the cell death responses of these clones were similar. Expression of transfected Bcl-2 and CrmA proteins was confirmed by Western blot analysis (data not shown) with antibodies specific for Bcl-2 (Santa Cruz Biotechnology; Santa Cruz, Calif.) and CrmA (Pharmingen, San Diego, Calif.). The cell death responses of vector-only controls were unaltered from those of untransfected parental cells.
Cell cycle analysis.
Cell cycle analysis was performed by staining with propidium iodide (37). Briefly, 106 cells were washed twice with phosphate-buffered saline (PBS) and fixed in 1 ml of 50% ethanol for 30 min on ice. The cells were pelleted and treated in the dark with 1 mg of RNase A per ml and 50 μg of propidium iodide per ml in 400 μl of PBS for 30 min at room temperature. Stained cells were analyzed cytofluorimetrically on an EPICS 753 or Elite ESP sorter (Coulter, Hialeah, Fla.). Cell cycle distributions were quantified with Multicycle AV software (Phoenix Flow Systems, Inc., San Diego, Calif.). The fraction of cells with subdiploid DNA content was consistent with that found by other measures of cell death.
Extract preparation.
Crude cytoplasmic and nuclear extracts were prepared from cells after two washes in PBS and one in buffer HKEB (100 mM HEPES, 10 mM MgCl2, 5 mM EGTA, 100 μM phenylmethylsulfonyl fluoride [PMSF], 1 mM dithiothreitol, 50 μM γ-S-ATP). The cells then were incubated (10 min at 25°C) in buffer HKEB with 50 μg of digitonin per ml (35). Following centrifugation (20 min at 13,000 × g), the resulting cytoplasmic supernatants were frozen at −70°C. Nuclear supernatants were prepared by dispersing the pellets in the same buffer with three 1-s 50-W pulses of a Vibra-cell sonicator (Sonics and Materials, Danbury, Conn.) and centrifuging the suspension (10 min at 13,000 × g). Protein concentrations were determined by the Bradford (Bio-Rad, Richmond, Calif.) or bicinchoninic acid (55) microtiter plate assay. Preferential extraction of dying cells can lead to an augmentation of apparent specific enzyme activity; we have avoided this artifact by monitoring the extent of extraction as indicated by trypan blue dye inclusion during digitonin treatment. Typically, complete permeabilization was achieved with 300 μl of HKEB-digitonin buffer per 5 × 106 cells. The efficacy of cellular fractionation was monitored as well. The activity of the cytoplasmic enzyme l-lactate dehydrogenase (Sigma Chemical Co., St. Louis, Mo.) was assayed in cellular fractions. We generally recovered 80% of lactate dehydrogenase activity (approximately 250 pmol of β-NADH/min/105 cells) in cytoplasmic fractions and the remainder (approximately 60 pmol of β-NADH/min/105 cells) in nuclear fractions. The total protein content of cytoplasmic extracts was typically threefold greater than that of nuclear extracts from both dying and viable populations.
Caspase assay and reagents.
Interleukin-1β-converting enzyme (ICE)-like and caspase 3-like activities were assayed in 50-μl reaction mixtures with fluorogenic reporter substrate peptides specific for ICE (acetyl-Tyr-Val-Ala-Asp-4-methylcoumaryl-7-amide [YVAD-MCA; Peninsula Laboratories, Belmont, Calif.]) (66) and the mammalian Ced3 homolog caspase 3 (acetyl-Asp-Glu-Val-Asp-4-methylcoumaryl-7-amide [DEVD-MCA; Peptides International, Louisville, Ky.]). The substrate peptide (200 μM) was incubated at 37°C with cytoplasmic extract (5 μg of extract for the DEVD-MCA reaction and 20 μg of extract for the YVAD-MCA assay) in 100 mM HEPES–10% sucrose–10 mM dithiothreitol–0.1% 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate (CHAPS). Fluorescence was measured after 120 min (excitation wavelength, 360 nm; emission wavelength, 460 nm) with a Cytofluor 2350 fluorescence plate reader (Millipore, Marlborough, Mass.). A standard curve was prepared with 7-amino-4-methyl-coumarin (AMC; Peninsula Laboratories). Stock solutions of peptide reagents, including aldehyde-derivatized inhibitors (Peptides International), were prepared in anhydrous dimethyl sulfoxide.
Histone kinase assay.
Histone (Sigma; type VS) was modified by addition of 2-iminobiotin as described previously (31). Nuclear extracts (5 μg diluted to 10 μl in 80 mM sodium β-glycerophosphate, 10 mM MgCl2, 5 mM EGTA [pH 7.5] [EB]) were mixed with 10 μl of assay mix consisting of 40 mM HEPES (pH 7.3), 10 mM EGTA (pH 8.0), 20 mM MgCl2, 400 μl of [γ-32P]ATP (5 μCi/mmol), and 150 μg of 2-iminobiotinyl histone per ml. After incubation (30 min at 25°C), the reactions were stopped by the addition of 1 ml of 50 mM NaCO3 (pH 10.5)–0.5 M NaCl–5 mM EDTA–50 μl of avidin-agarose, and the mixtures were rotated for 60 min at 4°C. Protein A-agarose (Sigma) was used as a negative control. Agarose pellets were washed three times with 1 ml of the same buffer and once with 1 ml of PBS and were eluted twice with 100 μl of 100 mM sodium acetate (pH 4.0)–0.5 M NaCl. The eluates were pooled, and 32P-incorporation was determined by scintillation analysis.
In addition, Cdk activity was assayed with a synthetic substrate peptide derived from the site of H1 phosphorylation mediated by Cdk1 (Cdc2). In these assays, 440 μM histone H1 peptide (Upstate Biotechnology, Inc., Lake Placid, N.Y.) was substituted for 2-iminobiotinyl histone. The reactions were stopped after 10 min by the addition of 5 μl of 5% H3PO4, and the mixtures were spotted on duplicate 1.5- by 1.5-cm squares of P-81 paper, washed three times for 15 min in 1% H3PO4, dried, and subjected to scintillation analysis. Purified p34cdk1 complex (from starfish oocytes [Promega, Madison, Wis.]) was used as a positive control.
Western immunoblot analysis.
Nuclear and cytoplasmic extract proteins (100 μg/well) were run on 10% polyacrylamide gels and transferred to Immobilon P (Millipore, Bedford, Mass.). The blots were probed with rabbit antisera raised against either a C-terminal peptide of cyclin A or a peptide corresponding to the conserved PSTAIRE domain of Cdk1 and Cdk2 (Santa Cruz Biotechnology). The bands were visualized by the luminol reaction (Renaissance; DuPont NEN, Boston, Mass.).
Cdk analysis by p9cksHs2 adsorption and by immunodepletion.
p9cksHs2 agarose (20 μl; Promega) was incubated at 4°C for 60 min with 40 μg of nuclear extract in 100 μl of EB supplemented with 10 mg of bovine serum albumin per ml. The p9cksHs2 agarose was pelleted, washed three times with the same buffer, and analyzed for histone activity with biotinylated H1 substrate. Supernatants also were analyzed for activity. For immunodepletions, 100 μg of nuclear extract in 200 μl of buffer HKEB was incubated overnight at 4°C with 1 μg of specific antibody (Santa Cruz Biotechnology). Immune complexes were cleared by incubation for 2 h with 20 μl of protein A-agarose (Sigma); the kinase activity remaining in the supernatant was assayed. Immunodepletion of Cdk components was confirmed by Western blot analysis of both immune complex pellets and supernatants.
RESULTS
In distinct cases, Bcl-2 sparing of cell death is independent of the associated G1 arrest.
In an effort to test the hypothesis that diverse cases of cell death have a common effector pathway, we sought to identify general features by correlating results from two independent cases of physiological cell death. One is the classical model of glucocorticoid-mediated death of T cells, represented here by the DO11.10 T-cell hybridoma. The second, in cells of the B-lymphocyte lineage, reflects a connection between cell death and enforced cell cycle progression. For this model, we used DE, a pre-B-cell line transformed by a temperature-sensitive v-Abl oncoprotein (12).
DE cells proliferate at the permissive temperature (34°C), but at the restrictive temperature (39.5°C) they spontaneously arrest in the G1 phase of the cell cycle and undergo physiological cell death (Table 1; Fig. 1A) (12). Arrest in a postmitotic (especially G1) compartment of the cell cycle (2, 26) is a common feature of physiological cell death (67). The glucocorticoid-mediated death response of the T hybridoma DO11.10 also involves a G1 arrest (Table 1). Figure 2A reveals the kinetics of death in DO11.10 cells treated with the glucocorticoid dexamethasone.
TABLE 1.
The cell death inhibitors Bcl-2 and CrmA do not relieve death-associated G1 arresta
| Cell line | Treatment | Cell cycle distribution (% of population)
|
|||
|---|---|---|---|---|---|
| Subdiploidb | G1 | S | G2/M | ||
| DE | 34°C | 5 | 56 | 35 | 2 |
| 39.5°C | 47 | 41 | 6 | 6 | |
| DE/Bcl-2 | 34°C | 1 | 66 | 28 | 2 |
| 39.5°C | 1 | 93 | 3 | 3 | |
| DO11.10 | None | 0 | 35 | 56 | 9 |
| Dexc | 35 | 42 | 11 | 12 | |
| DO11.10/Bcl-2 | None | 0 | 43 | 49 | 8 |
| Dex | 1 | 58 | 14 | 28 | |
| DO11.10/CrmA | None | 0 | 43 | 52 | 4 |
| Dex | 1 | 73 | 8 | 18 | |
Cell cycle distributions of DE cells and DE/Bcl-2 cells were analyzed at the permissive temperature (34°C) and 9 h after a shift to the restrictive temperature (39.5°C). Cell cycle distributions also were determined in untreated cultures of DO11.10 cells and representative clones of DO11.10 cells transfected with Bcl-2 and with CrmA and following treatment with 10−6 M dexamethasone for 18 h.
The subdiploid fraction represents dead cells that have lost DNA solubilized as a result of genome digestion.
Dex, dexamethasone.
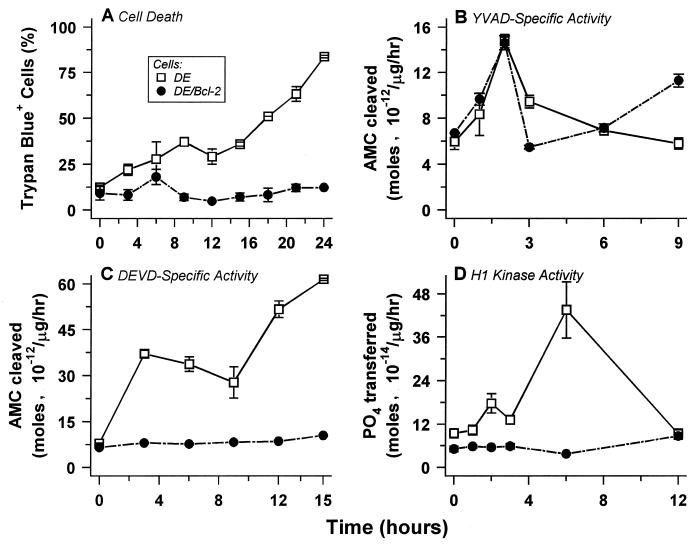
FIG. 1.
In B cells, physiological cell death and associated caspase 3-like protease and Cdk activities are inhibited by Bcl-2, but upstream ICE-like protease activity is not. The kinetics of induction of death (A), cytoplasmic YVAD-specific ICE-like (B) and DEVD-specific caspase 3-like (C) activities, and nuclear H1-specific Cdk activity (D) upon shift to the nonpermissive temperature were monitored in DE cells and DE/Bcl-2 cells. Cysteine protease activities were not detectable in nuclear extracts, and only basal levels of H1 kinase activity were present in cytoplasmic extracts of dying cells. The data presented are derived from one experiment in which a single set of extracts was used for all activity determinations. These data are representative of three separate experiments. In other experiments, elevated DEVD-specific caspase activity was not detectable at time points before 3 h of temperature shift.
FIG. 2.
Glucocorticoid-mediated cell death of T cells and associated caspase 3-like protease and Cdk activities are inhibited by Bcl-2, but upstream ICE-like protease activity is not. The kinetics of induction of death (A), cytoplasmic YVAD-specific ICE-like (B) and DEVD-specific caspase 3-like (C) activities, and nuclear H1-specific Cdk activity (D) were monitored after treatment with 10−6 M dexamethasone in DO11.10 cells and in representative clones of DO11.10 transfected with bcl-2 and crmA. The behaviors of DO11.10 cells transfected with p35 and with CrmA were identical; data for the DO11.10/p35 transfectants is omitted for clarity of presentation. Note that histone kinase activity in panel D was measured with histone H1 substrate peptide (see Materials and Methods). Comparable kinetics of appearance of kinase activity, although with lower apparent specific activity, were observed with biotinylated H1 substrate (see Fig. 6 for a comparison). The data presented are derived from one experiment in which a single set of extracts was used for all activity determinations. These data are representative of three separate experiments.
Bcl-2 expression does not relieve the G1 arrest associated with the induction of death in DE cells, but it significantly inhibits the actual cell death response upon temperature shift (Table 1; Fig. 1A). Bcl-2 also inhibits the death of DO11.10 cells (Fig. 2A) but has no effect on the associated G1 arrest (Table 1). These data map critical effector steps of cell death sensitive to Bcl-2 inhibition downstream of cell cycle arrest.
ICE-like protease activity is necessary for cell death but is not itself lethal.
We characterized quantitatively the appearance of caspase activities in the cell death responses of these lymphocyte cell lines. Among caspases, those most closely related to ICE generally exhibit activity directed to the tetrapeptide sequence YVAD, modified from ICE cleavage site 2 in the interleukin-1β precursor (43, 63, 66). We used a fluorogenic substrate derived from this tetrapeptide, YVAD-MCA, to assay ICE-like activity.
A transient twofold induction of ICE-like activity in DE cells occurs within 2 h of the death-inducing shift to the restrictive temperature and precedes death by several hours (Fig. 1A and B). Permeabilization with digitonin (see Materials and Methods) revealed that virtually all of the death-associated ICE-like activity is soluble and cytoplasmic (data not shown). Strikingly, although it spares DE cells from death upon temperature shift, Bcl-2 exerts no effect on the appearance (Fig. 1A and B) or localization (data not shown) of ICE-like protease activity.
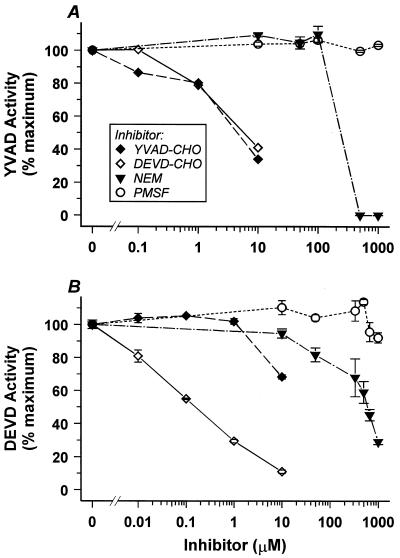
We obtained similar results when examining DO11.10 cells treated with the glucocorticoid dexamethasone (Fig. 2B). A modest twofold induced peak of ICE-like activity appears early in the DO11.10 death response, roughly following the period of required macromolecular synthesis (5, 11, 69). The YVAD-specific activity in DO11.10 cells, like that in DE cells, represents an authentic caspase by several criteria. It is inactivated in vitro by the cysteine-reactive reagent N-ethylmaleimide (NEM) and is relatively insensitive to inhibition by PMSF, a preferential inhibitor of serine proteases (Fig. 3A). Moreover, two viral gene products which have been shown to inhibit ICE-like proteases, the poxvirus serpin CrmA and the baculovirus p35 protein (76) (see below), inhibit this YVAD-specific activity in vivo (Fig. 2B) and in vitro (data not shown). Consistent with a previous characterization of ICE-like protease inhibition profiles (43), the aldehyde-derivatized YVAD tetrapeptide (YVAD-CHO), as well as the aldehyde-derivatized DEVD tetrapeptide (DEVD-CHO), inhibits the death-associated ICE-like activities we detected (Fig. 3A) (see below). The biphasic nature of the YVAD-CHO inhibition curve suggests that this activity may comprise two (or more) individual caspases; for brevity, we refer to YVAD-specific caspase activity below as class I activity.
FIG. 3.
Characterization in vitro of cell death-associated cysteine protease activities. YVAD-specific ICE-like (A) and DEVD-specific caspase 3-like (B) activities were assayed in the presence of the protease inhibitors NEM and PMSF and the specific aldehyde-derivatized tetrapetide inhibitors YVAD-CHO and DEVD-CHO.
In addition to inhibiting class I activity, expression of CrmA and p35 inhibit glucocorticoid-mediated death in transfected DO11.10 cells (Fig. 2A) (also see reference 56). As with Bcl-2, inhibition of death is not associated with relief from G1 arrest (Table 1). A mutant of the poxvirus crmA gene that lacks protease-inhibiting activity (65) fails to spare transfected cells from cell death (data not shown). These data implicate class I caspase activity as being critically involved in the cell death response. In contrast to CrmA and p35, the death-sparing action of Bcl-2 has no effect on the appearance of the class I activity in DO11.10 cells (Fig. 2B). These results suggest that Bcl-2 acts to interfere with cell death at a step(s) distinct from cell cycle arrest and CrmA-inhibitable class I protease activation. Most simply, this is consistent with the biochemical action of Bcl-2 occurring downstream of class I activity.
Induction of caspase 3-like activity occurs downstream of the action of Bcl-2.
We next asked where caspase 3-like activity, representing the other major caspase family, mapped in the cell death process. The protease activity of many members of this family of caspases is directed to the peptide sequence DEVD, representing the sequence of the caspase 3 cleavage site of PARP. We found that caspase 3-like protease activity, detected quantitatively with the fluorogenic peptide substrate, DEVD-MCA, also was activated in the cytoplasm of lymphocytes induced to die. Again for ease, we refer to this DEVD-specific caspase activity as class III activity.
In contrast to the early peak of class I activity following a death-inducing temperature shift, cytoplasmic class III activity increases eightfold over a more prolonged course and is sustained at that higher level in dying DE cells (Fig. 1B and C). Still, class III activity appears early in the cell death process, well before the terminal manifestations of death such as chromatin condensation, genome digestion, and loss of plasma membrane integrity (Fig. 1A). The pattern we observe, in which the rise of class III activity follows the class I protease peak in vivo, is consistent with previous work in vitro demonstrating that caspase 3-like proteases can be activated by ICE-like proteases (41, 60, 65). In cells spared from death by expression of Bcl-2, class III activity is not induced (Fig. 1C). This is in striking contrast to the ability of Bcl-2 to dissociate class I activity and cell death (Fig. 1B).
A similar pattern is seen in the death response of DO11.10 T cells (Fig. 2B and C). A sustained 20-fold induction of class III activity follows the earlier induction of class I activity triggered by glucocorticoid treatment. By the criteria outlined above for class I activity, the class III activity detected is the product of one or more authentic caspases (Fig. 3B). Specifically, class III activity in vitro is inactivated by NEM but not by PMSF, and it is preferentially inhibited by the aldehyde-derivatized DEVD tetrapeptide inhibitor (DEVD-CHO) and not by the aldehyde-derivatized YVAD-tetrapeptide inhibitor (YVAD-CHO).
As in the case of DE cells induced to die, the death-sparing expression of Bcl-2 in DO11.10 cells precludes the induction of class III protease activity (Fig. 2C). That class I activity but not class III activity is induced in the presence of death-sparing Bcl-2 reveals that the upstream activity itself is not lethal and implies that downstream class III activity must be necessary for cell death.
Class III activity is necessary for death and is dependent on upstream class I activity.
Class I and class III activities are absent in cells spared from death by virtue of expression of the transfected baculovirus protease inhibitor p35 (data not shown), which interferes directly with class I and class III proteases (76). Both classes of activities also are absent in transfectants spared from death by the expression of CrmA (Fig. 2B and C). Because CrmA is a specific inhibitor of class I proteases and a very poor inhibitor of class III proteases (50), these data are consistent with the notion, founded on in vitro studies (13, 20, 41, 60), that the appearance of class III protease activity is dependent on upstream activation of class I proteases.
To explore further the dependence of class III activity on upstream class I activity and the necessity of class III activity, distinct from class I activity, for cell death, we used the specific tetrapeptide inhibitors in vivo. Although these pharmacologic agents are only partially effective at inhibiting death, the involvement of class I and class III proteases in several cases of physiological cell death has been inferred from the limited death-sparing effects afforded by in vivo treatment (15, 33, 49). Simultaneous treatment of DO11.10 cells with the DEVD-CHO inhibitor and death-inducing glucocorticoid hormone inhibited class III activity and spared cells from glucocorticoid-mediated death (Fig. 4). Surprisingly, the YVAD-CHO inhibitor was much less effective in blocking the appearance of class III activity and cell death (Fig. 4) when added at low (≤1 μM) concentrations, where its unique specificity for class I proteases is manifest (Fig. 3). YVAD-CHO inhibition at high concentrations (≥10 μM) may be due to cross-reactive inhibition of the DEVD-specific activity (43).
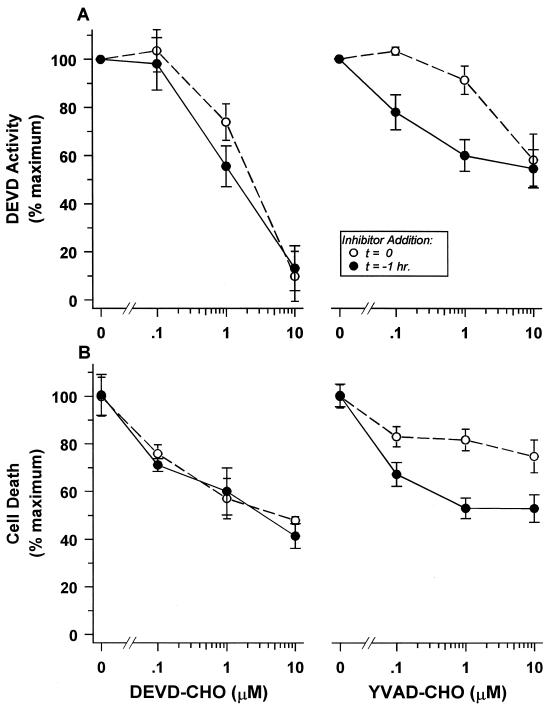
FIG. 4.
The appearance of class III caspase activity and cell death is dependent on upstream class I activity. The ability of aldehyde-derivatized tetrapeptide protease inhibitors to block in vivo DEVD-specific class III caspase activity (A) and cell death (B) induced by glucocorticoid treatment was monitored in DO11.10 cells. The derivatized DEVD tetrapeptide itself (left) or the derivatized YVAD tetrapeptide specific for class I proteases (right) was added at the indicated concentrations simultaneously with or 1 h before the addition of 10−6 M dexamethasone. DEVD-specific activity was assayed 12 h after glucocorticoid addition. Cell death was quantified by measurement of trypan blue inclusion 24 h after hormone addition.
Our kinetic data prompted us to explore whether the early induction of class I activity precludes its effective inhibition by added YVAD-CHO, while the consequent and more delayed appearance of class III activity allows the more effective action of its cognate inhibitor. Indeed, a 1-h pretreatment of cells with YVAD-CHO before the application of the death-inducing stimulus significantly enhanced the effectiveness of the class I inhibitor at low concentrations to avert class III activity and death (Fig. 4). Pretreatment had little effect on the efficacy of the DEVD-CHO inhibitor (Fig. 4). A similar pattern of inhibition was seen with DE cells (data not shown). We interpret these results to suggest that pharmacologic inhibition of the protease cascade and actual death demand that sufficient YVAD-CHO inhibitor be present in the cell at the time of class I protease activation to prevent subsequent class III protease activation and its lethal consequences.
Together, these data indicate that both class I and class III protease activities are necessary for cell death. However, class I protease activity is not sufficient for cell death and is not itself lethal. The upstream, transient class I activity appears to function to activate the downstream class III proteases; Bcl-2 acts to interfere with the cell death response by punctuating this caspase cascade. The appearance of class III protease activity downstream of Bcl-2 and operationally not dissociable from actual death in these cases is consistent with a role within the effector phase of the cell death process.
Induction of death-associated nuclear cyclin-dependent kinase activity is dependent on the cytoplasmic caspase cascade.
Having characterized class I caspase activity as an activator of class III caspases and class III activity as a downstream effector, we sought to determine where Cdks might act within the cell death process. Cdk activity was quantified by a novel assay which employs biotinylated histone H1 as the substrate, allowing for rapid and quantitative retrieval of the 32P-phosphorylated product with avidin-agarose. Since many different Cdks phosphorylate histone H1, this assay allows the most inclusive assessment of Cdk activities while effectively eliminating the experimental background of phosphorylation of endogenous proteins. Because significant quantities of endogenous H1 are liberated with chromatin condensation and genome digestion in dying cells (3), solubilized H1 substrate may mask death-associated Cdk activity.
Histone kinase activity appeared transiently in the nuclei of DE cells, with kinetics of induction similar to the cytoplasmic class III protease activity (Fig. 1D). The appearance of nuclear histone kinase also was associated with the induction of cell death in DO11.10 cells and was indistinguishable kinetically from that of class III activity (Fig. 2D). The presence of this three- to fourfold-elevated level of H1 kinase activity in cells arrested in the G1 compartment is closely linked to ultimate death. Proliferating cells traversing G1 exhibit only a basal level of nuclear activity, and the induction of kinase activity does not ensue when cells refractile to death by virtue of Bcl-2 expression are subjected to treatments that induce death in susceptible cells (Fig. 1D and 2D). Furthermore, inhibitors of the caspase cascade, including CrmA (Fig. 2D) and p35 (data not shown), also prevent the appearance of nuclear kinase. These data suggest that death-associated H1 kinase activity maps to the effector phase of the cell death process.
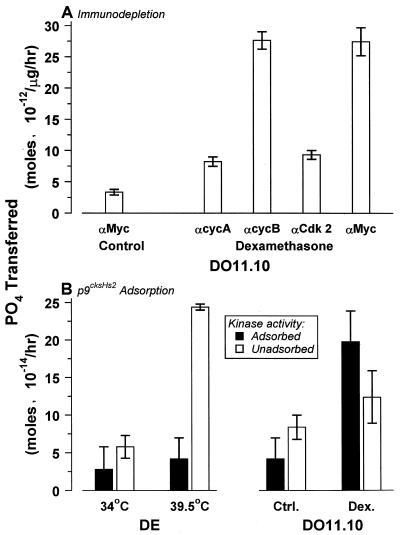
Immunoprecipitation and immunoblot analyses reveal the death-associated nuclear H1 kinase activities to represent authentic cyclin-dependent kinases. The H1 kinase activities in both DE B cells and DO11.10 T cells are composed of 60-kDa cyclin A protein (Fig. 5A) complexed with ca. 34-kDa polypeptides reactive with antibodies specific for the conserved PSTAIRE motif of the Cdk family (Fig. 5B). In these lymphocyte cell lines, all H1 kinase activity appears to be dependent on cyclin A exclusively. No other cyclin species are detectable in nuclear extracts containing death-associated H1 kinase activity (data not shown), and virtually all death kinase activity is specifically and quantitatively depleted by antibody to cyclin A (Fig. 6A).
FIG. 5.
Immunoblot analysis of cell death-associated nuclear cyclin A-dependent kinases. Cyclin A (A) and Cdk (B) components were identified by immunoblot analysis. Cytoplasmic and nuclear extracts were prepared from DE and DE/Bcl-2 cells (upper panels) incubated at the permissive temperature (34°C; −) and at the restrictive temperature (39.5°C; +) for 4 h and from DO11.10 cells and representative clones of DO11.10 transfected with bcl-2 (DO/Bcl-2) and crmA (DO/CrmA) (lower panels) that were untreated (−) or after treatment with 10−6 M dexamethasone for 6 h (+). Analysis of a nuclear extract from DO11.10 cells treated for 12 h with 2 μg of aphidicolin per ml (Aph.) is included for comparison in panel A. The densitometric quantitation of nuclear cyclin A expression is given in Table 2.
FIG. 6.
Cell death-associated histone H1 kinase activities represent cyclin A-dependent Cdks. (A) Death kinase activity was analyzed by immunodepletion analysis. Nuclear extracts from untreated DO11.10 cells or cells treated for 12 h with 10−6 M dexamethasone were analyzed for histone kinase activity following depletion with antibody specific for the indicated cyclin (αcyc) or Cdk (αCdk 2) or with irrelevant anti-Myc (αMyc) antibody. (B) Nuclear extracts from DE cells incubated at the restrictive temperature for 6 h and from DO11.10 cells treated with dexamethasone (Dex.) as above, as well as viable control (Ctrl.) cells, were treated with p9cksHs2 agarose to adsorb nuclear Cdk activity. Adsorbed and unadsorbed Cdk activities from 1 μg of nuclear extract were quantified. Note that for the experiments in panel A, H1 substrate peptide was used, while the activity in panel B was assayed with biotinylated H1. Comparison of DO11.10 activity in the two panels demonstrates the difference in apparent specific activity obtained with the different substrates.
By comparison, the identities of the multiple Cdk components are more ambiguous. Although antigenically similar, subtle structural differences among the death-associated Cdks exist and are manifest as differences in substrate specificities and accessory molecule interactions. A peptide sequence that is phosphorylated by the mitotic kinase complex of cyclin B and Cdk1 serves as a substrate for the death kinase from DO11.10 cells but not that from DE cells (Fig. 6). The death kinase of DE cells is not adsorbed by p9cksHs2, the mammalian homolog of p13suc1, which has been shown to interact specifically with Cdk1, Cdk2, and Cdk3 (48). On the other hand, about half of the DO11.10 kinase activity is adsorbed by p9cksHs2 (Fig. 6B). Antibody to Cdk2 similarly immunodepletes about half of the total H1 kinase activity (data not shown) and all of the peptide-specific activity (Fig. 6A). These results reveal that different Cdks contribute to death kinases in different cells and that death kinase activity may be composed of more than one Cdk in the same cell.
Death-associated Cdk activity results from the recruitment of cyclin molecules to the nucleus.
The appearance of cyclin A-dependent death kinase in the nucleus correlates with elevated nuclear cyclin A levels. In cells spared from death by virtue of Bcl-2 or viral protease inhibitor expression, nuclear cyclin A levels (Fig. 5A; Table 1), like cyclin A-dependent kinase activity (Fig. 1D and 2D), do not accumulate. The approximately twofold increase evident in dying DO11.10 cells and the more subtle change in DE cells reveal the specificity of this death-associated nuclear cyclin A recruitment (Fig. 5A; Table 2). Analysis of whole-cell and cytoplasmic cyclin A levels (Fig. 5A) suggests that these death-specific changes are not a consequence of the relatively small changes in gross levels of total cellular cyclin A.
TABLE 2.
Densitometric quantitation of nuclear cyclin A expressiona
| Cell line | Treatmentb | Nuclear cyclin A (%)c |
|---|---|---|
| DE | 34°C | 29 |
| 39.5°C | 30 | |
| Aph | 100 | |
| DE/Bcl-2 | 34°C | 26 |
| 39.5°C | 2 | |
| DO11.10 | None | 41 |
| Dex | 79 | |
| Aph | 100 | |
| DO11.10/Bcl-2 | None | 28 |
| Dex | 38 | |
| DO11.10/CrmA | None | 21 |
| Dex | 23 |
Cyclin A immunoblot bands were scanned and quantified by densitiometry.
Aph, aphidicolin (2 μg/ml); Dex, dexamethasone (10−6 M).
Relative to that for aphidicolin-treated cells, which was set at 100%.
DE cells exhibit a substantial basal level of nuclear cyclin A, which appears to change little with the induction of cell death. This basal level of nuclear cyclin A expression reflects the steady-state fraction (35%) of S-phase cells (or cells at the G1/S transition) within the DE cell culture (Table 1); cyclin A expression is maximal within that compartment of the cell cycle (53). When the entire population resides within the S phase, due to arrest induced by aphidicolin treatment, cyclin A levels are elevated proportionately (threefold greater by densitometric analysis [Table 2]). Bcl-2 expression causes DE cells to transit the cell cycle more slowly (also see references 8 and 44), and as a consequence, the fraction of cells in the S phase (80% of control cells [Table 1]) and the levels of nuclear cyclin A (90% of control [Table 2]) both are diminished. The presence of cyclin A in the nucleus of viable cells therefore reflects the extent of productive cell cycle transit.
Under conditions of death induction, the presence of cyclin A in the nucleus instead appears to reflect the extent to which cells execute the death process. In dying DE cells, nuclear cyclin A levels appear similar to those seen in proliferating cells (Fig. 5A; Table 2); however, when DE cells spared from death by Bcl-2 are arrested in G1 upon temperature shift, nuclear cyclin A expression is lost (Fig. 5A; Table 2). Thus, the termination of cycle transit with stable G1 arrest uncovers the apparently rapid turnover to which nuclear cyclin A is subject. The sustained level of death-associated nuclear cyclin A represents the active recruitment of cyclin A from the cytoplasm above turnover. Death-specific nuclear Cdk activity appears to depend on this active import process.
It is worth noting that the death-specific nuclear cyclin is present in cells before the execution of the death process as a function of normal cell cycle transit and growth arrest. Indeed, total cellular levels of cyclin A protein (Fig. 5A), as well as cyclin A mRNA (11), change little (less than 20%) during the cell death response. These data, together with those for class III activity, suggest that posttranslational activation of resident products of the caspase 3 and Cdk families is a common theme in the effector phase of cell death.
DISCUSSION
While late events, such as the digestion of genomic DNA and the loss of plasma membrane integrity, unambiguously mark a cell as dead, the process by which a cell reaches that state physiologically has not been well characterized. Consequently, it has not been possible to define precisely the steps of dying or to identify the point at which a cell is irreversibly committed to death. Our studies have sought to address these issues by examining quantitatively the activities of families of gene products with respect to both their requisite function and their order of action during distinct physiological cell death responses.
The data reported here define and map a conserved process comprising the physiological cell death pathway. Death effector activities, including cytoplasmic class III (DEVD-specific caspase 3-like) caspases and nuclear Cdks, act in an ordered sequence between upstream modulatory steps, including the activation of class I (YVAD-specific ICE-like) caspase activity, and downstream posteffector events.
An amplifying cytoplasmic caspase cascade, punctuated by Bcl-2, is necessary for cell death.
Studies in vitro have led to the notion that a cascade of caspases is involved in the physiological cell death process. The pattern of appearance of caspase activities observed in cells induced to die via the CD95 death-signaling receptor (20) also supports this view; however, the functional demonstration of such a cascade in vivo has been lacking.
On the basis of several criteria, including the use of death-inhibitory gene products, such as the viral serpin CrmA, as well as pharmacologic protease inhibitors, we found that both class I and class III caspases are required for these cell death responses. The most striking conclusion from our characterization of caspase activities in these cells is that the early transient class I activity is necessary but not sufficient for cell death. Because class I activity is induced normally in cells spared from death by Bcl-2 expression, the presence of activated class I protease cannot itself be lethal. Its function as the activator of downstream class III activity in cell death identifies the class I activity as serving a requisite regulatory function. The amplified activation of caspase 3-like activity in response to a death-inducing stimulus is the obligate function of the caspase cascade; our results extend more general observations that terminal proteolytic events attributed to class III proteases ensue only in dying cells and not in cells spared from death by Bcl-2 family members (7, 9, 13, 18).
The ability of Bcl-2 and CrmA to spare cells from glucocorticoid-mediated death rebuts proposed models which invoke alternate pathways of death either sensitive to Bcl-2 inhibition or dependent on ICE-like caspase activation (32, 62). Even in cases where pharmacologic inhibitors are inactive (45, 75), we have found that the viral inhibitors are effective at sparing cells from death and reveal the same pattern of activities (5). The caspase activities we detect appear to reflect the products of multiple genes; we have yet to identify the specific caspases involved. The aldehyde-derivatized tetrapeptide inhibition profiles for ICE-like activity both in T cells (Fig. 3A) and in B cells (data not shown) are consistent at least with ICE itself and caspase 4 (43).
An operational definition of commitment leads to the discrimination of modulatory and effector phases of the cell death process.
Steps of the cell death process up to and including class I activity are not lethal and cannot themselves be effectors of death. The commitment of a cell to die must succeed class I activation. We define precommitment events as modulatory steps within the death response. On the other hand, we have not been able to dissociate class III activity from cell death. This implies that the irreversible commitment to die, at least operationally, is equivalent (or closely linked) to class III activation. More precisely, at our current level of discrimination, we can define the step inhibitable by Bcl-2 to represent the point of cell death commitment.
Commitment signifies the point of initiation of the essential lethal steps of cell death. We define this stage as the effector phase of cell death. Our previous work identified genome digestion as a common and dispensable consequence of physiological cell death (51, 71). We can describe the effector phase as lying between such downstream consequences and the upstream point of commitment. By this mapping analysis, class III caspase activity functions within the effector phase of cell death.
The lethal effector phase of cell death involves nuclear Cdk activity.
Our results with nuclear Cdks map Cdk activity as another component of the effector phase of physiological cell death. The appearance of effector-phase nuclear Cdk activity is kinetically indistinguishable from the appearance of cytoplasmic class III activity in our experiments. However, the CrmA and p35 transfectants reveal that the caspase cascade is requisite for Cdk induction, suggesting that Cdk activity acts downstream of the caspases within a sequential and linear pathway.
That Cdks play an essential role in the cell death process has been inferred from genetic studies with transiently expressed antisense and dominant negative Cdk mutants (22, 47) and a somatic cell mutant conditionally defective in Cdk1 (59). However, those studies did not allow the function of Cdk activities identified to be mapped with respect to the effector phase of the cell death pathway. Cdks certainly can play a role in the modulation of a death response; this seems to be the case, for example, with myc-transfected cells subjected to serum deprivation (24). Independent of such a particular function in signaling, our findings demonstrate that Cdk components play a role generally within the effector phase of cell death. We imagine that the link between cell cycle arrest and cell death serves to ensure the presence in dying cells of specific Cdk components, especially cyclins. It is notable that our transfectant studies do not exclude cycle arrest as a requisite step in the cell death pathway but do reveal that cell cycle arrest occurs by a signaling process that is independent of caspases and not inhibitable by Bcl-2.
The manner in which resident Cdk components are recruited and activated within the nucleus during the effector phase of cell death remains to be understood. We find that constitutive levels of PSTAIRE-reactive nuclear Cdks are unaltered as a function of the cell death response, in contrast to other reports (46). On the other hand, a significant augmentation in nuclear cyclin A levels precedes the appearance of Cdk activity and death. That cyclin A recruitment to the nucleus does not occur in cells spared from death by Bcl-2 or caspase inhibitors suggests the possibility that cyclin recruitment occurs via a caspase-dependent process. The caspase-specific proteolytic activation of other kinases, such as protein kinase C-δ (25) and the Cdk-related PITSLRE kinase (4, 40), during cell death also presents an attractive precedent for an alternative mode of kinase activation.
Physiological cell death occurs by a thematically conserved and ordered pathway.
The data reported here support a model for the cell death process in which diverse stimuli act afferently to modulate a common effector pathway of death. That components of the effector phase, caspase 3-like caspases (13, 19, 21, 57) and Cdks (this work), appear to be resident in cells independent of new gene expression (11, 68) suggests that any requirement for macromolecular synthesis in cell death must be limited to the modulatory phase of death signaling.
While previous studies have suggested that specific caspases can function in an ordered manner (13, 20, 60), the variety of interactions among individual caspases, especially differences in the abilities of caspase inhibitors to interfere with particular cell death responses (14, 62), has obscured the generality of this pattern. Our data reveal that in different cases of cell death, a variety of biochemical activities, including but not limited to caspases, function in a conserved and requisite order. Differences in caspases as well as effector Cdks in disparate cases of cell death inform our view of the cell death pathway (68) as conserved in theme but not in the molecular identity of individual components.
ACKNOWLEDGMENTS
We are grateful to Naomi Rosenberg for DE and DE/Bcl-2 cells and to Stan Korsmeyer and Vishva Dixit for bcl-2 and viral inhibitor clones, respectively. We thank our colleagues Raj Belani, Sandra Chang, Bill Hendrickson, Phil Matsumura, and William Walden for constructive comments.
This work was supported by grants to D.S.U. from the National Institutes of Health and as a Scholar of the Leukemia Society of America.
REFERENCES
- 1.Alnemri E S, Livingston D J, Nicholson D W, Salvesen G, Thornberry N A, Wong W W, Yuan J. Human ICE/Ced-3 protease nomenclature. Cell. 1996;87:171. doi: 10.1016/s0092-8674(00)81334-3. [DOI] [PubMed] [Google Scholar]
- 2.Ashwell J D, Cunningham R E, Noguchi P D, Hernandez D. Cell growth cycle block of T cell hybridomas upon activation with antigen. J Exp Med. 1987;165:173–194. doi: 10.1084/jem.165.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter G D, Smith P J, Lavin M F. Molecular changes associated with induction of cell death in a human T-cell leukemia line: putative nucleases identified as histones. Biochem Biophys Res Commun. 1989;162:30–37. doi: 10.1016/0006-291x(89)91957-8. [DOI] [PubMed] [Google Scholar]
- 4.Beyaert R, Kidd V J, Cornelis S, Van de Craen M, Denecker G, Lahti J M, Gururajan R, Vandenabeele P, Fiers W. Cleavage of PITSLRE kinases by ICE/CASP-1 and CPP32/CASP-3 during apoptosis induced by tumor necrosis factor. J Biol Chem. 1997;272:11694–11697. doi: 10.1074/jbc.272.18.11694. [DOI] [PubMed] [Google Scholar]
- 5.Blomquist, J. F. Unpublished data.
- 6.Boise L H, Gonzalez-Garcia M, Postema C E, Ding L, Lindsten T, Turka L A, Mao X, Nuñez G, Thompson C B. bcl-x, a bcl-2 related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 7.Boise L H, Thompson C B. Bcl-xL can inhibit apoptosis in cells that have undergone Fas-induced protease activation. Proc Natl Acad Sci USA. 1997;94:3759–3764. doi: 10.1073/pnas.94.8.3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borner C. Diminished cell proliferation associated with the death-protective activity of Bcl-2. J Biol Chem. 1996;271:12695–12698. doi: 10.1074/jbc.271.22.12695. [DOI] [PubMed] [Google Scholar]
- 9.Boulakia C A, Chen C, Ng F W H, Teodoro J G, Branton P E, Nicholson D W, Poirier G G, Shore G C. Bcl-2 and adenovirus E1B 19 kDa protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymerase. Oncogene. 1996;12:529–535. [PubMed] [Google Scholar]
- 10.Casciola-Rosen L, Nicholson D W, Chong T, Rowan K R, Thornberry N A, Miller D K, Rosen A. Apopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic death. J Exp Med. 1996;183:1957–1964. doi: 10.1084/jem.183.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang, S. H., and D. S. Ucker. Unpublished data.
- 12.Chen Y-Y, Rosenberg N. Lymphoid cells transformed by Abelson virus require the v-abl protein-tyrosine kinase only during early G1. Proc Natl Acad Sci USA. 1992;89:6683–6687. doi: 10.1073/pnas.89.15.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinnaiyan A M, Orth K, O’Rourke K, Duan H, Poirier G G, Dixit V M. Molecular ordering of the cell death pathway: Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J Biol Chem. 1996;271:4573–4576. doi: 10.1074/jbc.271.9.4573. [DOI] [PubMed] [Google Scholar]
- 14.Datta R, Kojima H, Banach D, Bump N J, Talanian R V, Alnemri E S, Weichselbaum R R, Wong W W, Kufe D W. Activation of a CrmA-insensitive, p35-sensitive pathway in ionizing radiation-induced apoptosis. J Biol Chem. 1997;272:1965–1969. doi: 10.1074/jbc.272.3.1965. [DOI] [PubMed] [Google Scholar]
- 15.Deshmukh M, Vasilakos J, Deckwerth T L, Lampe P A, Shivers B D, Johnson E M., Jr Genetic and metabolic status of NGF-deprived sympathetic neurons saved by an inhibitor of ICE family proteases. J Cell Biol. 1996;135:1341–1354. doi: 10.1083/jcb.135.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Stasi A M M, Gallo V, Ceccarini M, Petrucci T C. Neuronal fodrin proteolysis occurs independently of excitatory amino acid-induced neurotoxicity. Neuron. 1991;6:445–454. doi: 10.1016/0896-6273(91)90252-u. [DOI] [PubMed] [Google Scholar]
- 17.Ellis H M, Horvitz H R. Genetic control of programmed cell death in the nematode C. elegans. Cell. 1986;44:817–829. doi: 10.1016/0092-8674(86)90004-8. [DOI] [PubMed] [Google Scholar]
- 18.Emoto Y, Manome Y, Meinhardt G, Kisaki H, Kharbanda S, Robertson M, Ghayur T, Wong W W, Kamen R, Weichselbaum R, Kufe D. Proteolytic activation of protein kinase C δ by an ICE-like protease in apoptotic cells. EMBO J. 1995;14:6148–6156. doi: 10.1002/j.1460-2075.1995.tb00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature. 1995;375:78–81. doi: 10.1038/375078a0. [DOI] [PubMed] [Google Scholar]
- 20.Enari M, Talanian R V, Wong W W, Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 21.Fernandes-Alnemri T, Litwack G, Alnemri E S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1β-converting enzyme. J Biol Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 22.Fotedar R, Flatt J, Gupta S, Margolis R L, Fitzgerald P, Messier H, Fotedar A. Activation-induced T-cell death is cell cycle dependent and regulated by cyclin B. Mol Cell Biol. 1995;15:932–942. doi: 10.1128/mcb.15.2.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Freeman R S, Estus S, Johnson E M., Jr Analysis of cell cycle-related gene expression in postmitotic neurons: selective induction of Cyclin D1 during programmed cell death. Neuron. 1994;12:343–355. doi: 10.1016/0896-6273(94)90276-3. [DOI] [PubMed] [Google Scholar]
- 24.Galaktionov K, Chen X, Beach D. Cdc25 cell-cycle phosphatase as a target of c-myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 25.Ghayur T, Hugunin M, Talanian R V, Ratnofsky S, Quinlan C, Emoto Y, Pandey P, Datta R, Huang Y, Kharbanda S, Allen H, Kamen R, Wong W, Kufe D. Proteolytic activation of protein kinase C δ by an ICE/CED 3-like protease induces characteristics of apoptosis. J Exp Med. 1996;184:2399–2404. doi: 10.1084/jem.184.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmon J M, Norman M R, Fowlkes B J, Thompson E B. Dexamethasone induces irreversible G1 arrest and death of a human lymphoid cell line. J Cell Physiol. 1979;98:267–278. doi: 10.1002/jcp.1040980203. [DOI] [PubMed] [Google Scholar]
- 27.Hengartner M O, Ellis R E, Horvitz H R. Caenorhabditis elegans gene ced-9 protects cells from programmed cell death. Nature. 1992;356:494–499. doi: 10.1038/356494a0. [DOI] [PubMed] [Google Scholar]
- 28.Hengartner M O, Horvitz H R. C. elegans cell survival gene ced-9 encodes a functional homolog of the mammalian proto-oncogene bcl-2. Cell. 1994;76:665–675. doi: 10.1016/0092-8674(94)90506-1. [DOI] [PubMed] [Google Scholar]
- 29.Hoang A T, Cohen K J, Barrett J F, Bergstrom D A, Dang C V. Participation of cyclin A in Myc-induced apoptosis. Proc Natl Acad Sci USA. 1994;91:6875–6879. doi: 10.1073/pnas.91.15.6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hockenbery D, Nuñez G, Milliman C, Schreiber R D, Korsmeyer S J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 31.Hooper L V, Baenziger J U. Sulfotransferase and glycosyltransferase analyses using a 96-well filtration plate. Anal Biochem. 1993;212:128–133. doi: 10.1006/abio.1993.1301. [DOI] [PubMed] [Google Scholar]
- 32.Huang D C S, Cory S, Strasser A. Bcl-2, Bcl-xL and adenovirus protein E1B19kD are functionally equivalent in their ability to inhibit cell death. Oncogene. 1997;14:405–414. doi: 10.1038/sj.onc.1200848. [DOI] [PubMed] [Google Scholar]
- 33.Jacobson M D, Weil M, Raff M C. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol. 1996;133:1041–1051. doi: 10.1083/jcb.133.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufmann S H. Induction of endonucleolytic DNA cleavage in human acute myelogenous leukemia cells by etoposide, camptothecin, and other cytotoxic anticancer drugs: a cautionary note. Cancer Res. 1989;49:5870–5878. [PubMed] [Google Scholar]
- 35.Kearse K P, Hart G W. Lymphocyte activation induces rapid changes in nuclear and cytoplasmic glycoproteins. Proc Natl Acad Sci USA. 1991;88:1701–1705. doi: 10.1073/pnas.88.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kranenburg O, van der Eb A J, Zantema A. Cyclin D1 is an essential mediator of apoptotic neuronal cell death. EMBO J. 1996;15:46–54. [PMC free article] [PubMed] [Google Scholar]
- 37.Krishan A. Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining. J Cell Biol. 1975;66:188–193. doi: 10.1083/jcb.66.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuida K, Lippke J A, Ku G, Harding M W, Livingston D J, Su M S-S, Flavell R A. Altered cytokine export and apoptosis in mice deficient in interleukin-1β converting enzyme. Science. 1995;267:2000–2006. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 39.Kuida K, Zheng T S, Na S, Kuan C-Y, Yang D, Karasuyama H, Rakic P, Flavell R A. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–372. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- 40.Lahti J M, Xiang J, Heath L S, Campana D, Kidd V J. PITSLRE protein kinase activity is associated with apoptosis. Mol Cell Biol. 1995;15:1–11. doi: 10.1128/mcb.15.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X, Kim C N, Pohl J, Wang X. Purification and characterization of an interleukin-1β-converting enzyme family protease that activates cysteine protease p32 (CPP32) J Biol Chem. 1996;271:13371–13376. [PubMed] [Google Scholar]
- 42.MacFarlane M, Cain K, Sun X-M, Alnemri E S, Cohen G M. Processing/activation of at least four interleukin-1β converting enzyme-like proteases occurs during the execution phase of apoptosis in human monocytic tumor cells. J Cell Biol. 1997;137:469–479. doi: 10.1083/jcb.137.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Margolin N, Raybuck S A, Wilson K P, Chen W, Fox T, Gu Y, Livingston D J. Substrate and inhibitor specificity of interleukin-1β-converting enzyme and related caspases. J Biol Chem. 1997;272:7223–7228. doi: 10.1074/jbc.272.11.7223. [DOI] [PubMed] [Google Scholar]
- 44.Mazel S, Burtrum D, Petrie H T. Regulation of cell division cycle progression by bcl-2 expression: a potential mechanism for inhibition of programmed cell death. J Exp Med. 1996;183:2219–2226. doi: 10.1084/jem.183.5.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McCarthy N J, Whyte M K B, Gilbert C S, Evan G I. Inhibition of Ced-3/ICE-related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meikrantz W, Gisselbrecht S, Tam S W, Schlegel R. Activation of cyclin A-dependent protein kinases during apoptosis. Proc Natl Acad Sci USA. 1994;91:3754–3758. doi: 10.1073/pnas.91.9.3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meikrantz W, Schlegel R. Suppression of apoptosis by dominant negative mutants of cyclin-dependent protein kinases. J Biol Chem. 1996;271:10205–10209. doi: 10.1074/jbc.271.17.10205. [DOI] [PubMed] [Google Scholar]
- 48.Meyerson M, Enders G H, Wu C-L, Su L-K, Gorka C, Nelson C, Harlow E, Tsai L-H. A family of human cdc2-related protein kinases. EMBO J. 1992;11:2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milligan C E, Prevette D, Yaginuma H, Homma S, Cardwell C, Fritz L C, Tomaselli K J, Oppenheim R W, Schwartz L M. Peptide inhibitors of the ICE protease family arrest programmed cell death of motoneurons in vivo and in vitro. Neuron. 1995;15:385–393. doi: 10.1016/0896-6273(95)90042-x. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson D W, Ali A, Thornberry N A, Vaillancourt J P, Ding C K, Gallant M, Gareau Y, Griffin P R, Labelle M, Lazebnik Y A, Munday N A, Raju S M, Smulson M E, Yamin T-T, Yu V L, Miller D K. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. doi: 10.1038/376037a0. [DOI] [PubMed] [Google Scholar]
- 51.Odaka C, Ucker D S. Apoptotic morphology reflects mitotic-like aspects of physiological cell death and is independent of genome digestion. Microsc Res Tech. 1996;34:267–271. doi: 10.1002/(SICI)1097-0029(19960615)34:3<267::AID-JEMT9>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 52.Oltvai Z N, Milliman C L, Korsmeyer S J. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 53.Pines J, Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991;115:1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rao L, Perez D, White E. Lamin proteolysis facilitates nuclear events during apoptosis. J Cell Biol. 1996;135:1441–1455. doi: 10.1083/jcb.135.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Redinbaugh M G, Turley R B. Adaptation of the bicinchoninic acid protein assay for use with micotiter plates and sucrose gradient fractions. Anal Biochem. 1986;153:267–271. doi: 10.1016/0003-2697(86)90091-6. [DOI] [PubMed] [Google Scholar]
- 56.Robertson N M, Zangrilli J, Fernandes-Alnemri T, Friesen P D, Litwack G, Alnemri E S. Baculovirus P35 inhibits the glucocorticoid-mediated pathway of cell death. Cancer Res. 1997;57:43–47. [PubMed] [Google Scholar]
- 57.Schlegel J, Peters I, Orrenius S, Miller D K, Thornberry N A, Yamin T-T, Nicholson D W. CPP32/Apopain is a key interleukin 1β converting enzyme-like protease involved in Fas-mediated apoptosis. J Biol Chem. 1996;271:1841–1844. doi: 10.1074/jbc.271.4.1841. [DOI] [PubMed] [Google Scholar]
- 58.Shi L, Chen G, He D, Bosc D G, Litchfield D W, Greenberg A H. Granzyme B induces apoptosis and cyclin A-associated cyclin-dependent kinase activity in all stages of the cell cycle. J Immunol. 1996;157:2381–2385. [PubMed] [Google Scholar]
- 59.Shi L, Nishioka W K, Th’ng J, Bradbury E M, Litchfield D W, Greenberg A H. Premature p34cdc2 activation required for apoptosis. Science. 1994;263:1143–1145. doi: 10.1126/science.8108732. [DOI] [PubMed] [Google Scholar]
- 60.Srinivasula S M, Ahmad M, Fernandes-Alnemri T, Litwack G, Alnemri E S. Molecular ordering of the Fas-apoptotic pathway: the Fas/APO-1 protease Mch5 is a CrmA-inhibitable protease that activates multiple Ced-3/ICE-like cysteine proteases. Proc Natl Acad Sci USA. 1996;93:14486–14491. doi: 10.1073/pnas.93.25.14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Strasser A, Harris A W, Huang D C S, Krammer P H, Cory S. Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J. 1995;14:6136–6147. doi: 10.1002/j.1460-2075.1995.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Susin S A, Zamzami N, Castedo M, Daugas E, Wang H-G, Geley S, Fassy F, Reed J C, Kroemer G. The central executioner of apoptosis: multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talanian R V, Quinian C, Trautz S, Hackett M C, Mankovich J A, Banach D, Ghayur T, Brady K D, Wong W W. Substrate specificities of caspase family proteases. J Biol Chem. 1997;272:9677–9682. doi: 10.1074/jbc.272.15.9677. [DOI] [PubMed] [Google Scholar]
- 64.Tewari M, Dixit V M. Fas- and TNF-induced apoptosis is inhibited by the poxvirus crmA gene product. J Biol Chem. 1995;270:3255–3360. doi: 10.1074/jbc.270.7.3255. [DOI] [PubMed] [Google Scholar]
- 65.Tewari M, Quan L T, O’Rourke K, Desnoyers S, Zeng Z, Beidler D R, Poirier G G, Salvesen G S, Dixit V M. Yama/CPP32β, a mammalian homolog of CED-3, is a CrmA-inhibitable protease that cleaves the death substrate poly(ADP-ribose) polymerase. Cell. 1995;81:801–809. doi: 10.1016/0092-8674(95)90541-3. [DOI] [PubMed] [Google Scholar]
- 66.Thornberry N A, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostura M J, Miller D K, Molineaux S M, Weidner J R, Aunins J, Elliston K O, Ayala J M, Casano F J, Chin J, Ding G J-F, Egger L A, Gaffney E P, Limjuco G, Palyha O C, Raju S M, Rolando A M, Salley J P, Yamin T-T, Lee T D, Shively J E, MacCross M, Mumford R A, Schmidt J A, Tocci M J. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 67.Ucker D S. Death by suicide: one way to go in mammalian cellular development? New Biol. 1991;3:103–109. [PubMed] [Google Scholar]
- 68.Ucker D S. Death and dying in the immune system. Adv Pharmacol. 1997;41:179–218. doi: 10.1016/s1054-3589(08)61059-5. [DOI] [PubMed] [Google Scholar]
- 69.Ucker D S, Ashwell J D, Nickas G. Activation-driven T cell death I. Requirements for de novo transcription and translation and association with genome fragmentation. J Immunol. 1989;143:3461–3469. [PubMed] [Google Scholar]
- 70.Ucker D S, Hebshi L D, Blomquist J F, Torbett B E. Physiological T cell death: susceptibility is modulated by activation, aging, and transformation, but the mechanism is constant. Immunol Rev. 1994;142:273–299. doi: 10.1111/j.1600-065x.1994.tb00893.x. [DOI] [PubMed] [Google Scholar]
- 71.Ucker D S, Obermiller P S, Eckhart W, Apgar J R, Berger N A, Meyers J. Genome digestion is a dispensable consequence of physiological cell death mediated by cytotoxic T lymphocytes. Mol Cell Biol. 1992;12:3060–3069. doi: 10.1128/mcb.12.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vaux D L, Aguila H L, Weissman I L. Bcl-2 prevents death of factor-deprived cells but fails to prevent apoptosis in targets of cell mediated killing. Int Immunol. 1992;4:821–824. doi: 10.1093/intimm/4.7.821. [DOI] [PubMed] [Google Scholar]
- 73.Vaux D L, Weissman I L, Kim S K. Prevention of programmed cell death in Caenorhabditis elegans by human bcl-2. Science. 1992;258:1955–1957. doi: 10.1126/science.1470921. [DOI] [PubMed] [Google Scholar]
- 74.Wang Z-Q, Auer B, Stingl L, Berghammer H, Haidacher D, Schweiger M, Wagner E F. Mice lacking ADPRT and poly(ADP-ribosyl)ation develop normally but are susceptible to skin disease. Genes Dev. 1995;9:509–520. doi: 10.1101/gad.9.5.509. [DOI] [PubMed] [Google Scholar]
- 75.Xiang J, Chao D T, Korsmeyer S J. Bax-induced cell death may not require interleukin 1β-converting enzyme-like proteases. Proc Natl Acad Sci USA. 1996;93:14559–14563. doi: 10.1073/pnas.93.25.14559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xue D, Horvitz H R. Inhibition of the Caenorhabditis elegans cell-death protease CED-3 by a CED-3 cleavage site in baculovirus p35 protein. Nature. 1995;377:248–251. doi: 10.1038/377248a0. [DOI] [PubMed] [Google Scholar]
- 77.Xue D, Shaham S, Horvitz H R. The Caenorhabditis elegans cell-death protein CED-3 is a cysteine protease with substrate specificities similar to those of the human CPP32 protease. Genes Dev. 1996;10:1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- 78.Yang E, Zha J, Jockel J, Boise L H, Thompson C B, Korsmeyer S J. Bad, a heterodimeric partner for Bcl-xL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 79.Yuan J, Shaham S, Ledoux S, Ellis H M, Horvitz H R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1β-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]