Abstract
Many protein kinases are regulated by phosphorylation in the activation loop, which is required for enzymatic activity. Glutamic acid can substitute for phosphothreonine in some proteins activated by phosphorylation, but this substitution (T169E) at the site of activation loop phosphorylation in the Saccharomyces cerevisiae cyclin-dependent kinase (Cdk) Cdc28p blocks biological function and protein kinase activity. Using cycles of error-prone DNA amplification followed by selection for successively higher levels of function, we identified mutant versions of Cdc28p-T169E with high biological activity. The enzymatic and biological activity of the mutant Cdc28p was essentially normally regulated by cyclin, and the mutants supported normal cell cycle progression and regulation. Therefore, it is not a requirement for control of the yeast cell cycle that Cdc28p be cyclically phosphorylated and dephosphorylated. These CDC28 mutants allow viability in the absence of Cak1p, the essential kinase that phosphorylates Cdc28p-T169, demonstrating that T169 phosphorylation is the only essential function of Cak1p. Some growth defects remain in suppressed cak1 cdc28 strains carrying the mutant CDC28 genes, consistent with additional nonessential roles for CAK1.
Cyclin-dependent kinase (Cdk) activation loop phosphorylation is required for Cdk activity (9, 20, 39) and may be an important part of the kinase activity cycle. A glutamic acid substitution for the phosphorylated threonine in fission yeast Cdc2 has partial biological activity consistent with failure to exit from mitosis (14). In the budding yeast Cdk Cdc28p, the same mutation (T169E) resulted in a low level of Cdc28p kinase activity, correlated with a limited ability to complement the cdc28-1N allele (an allele with a G2-M-phase-specific defect) and no ability to complement the G1-S and G2-M-defective cdc28-4 allele (27). Although the inactivity of cdc28-T169E could indicate a requirement for a cycle of T169 phosphorylation and dephosphorylation for cell cycle control (27), the glutamic acid substitution might simply be unable to fully substitute chemically for phosphothreonine in supporting active kinase architecture (20).
Here, we have used molecular evolution starting with the inactive cdc28-T169E to address two questions. First, is the combination of activation loop phosphorylation and dephosphorylation in Cdc28p of regulatory significance (as with mitogen-activated protein [MAP] kinases, for example [1, 20]), or is it only a precondition for enzymatic activity that is not subject to regulatory input? If phosphorylation or dephosphorylation of this site controls Cdc28p activity in some regulatory context, then a version of Cdc28p not subject to this control should abrogate this regulation.
Second, the essential gene CAK1/CIV1 encodes a kinase required for Cdc28p-T169 phosphorylation (12, 21, 47). Does Cak1p/Civ1p have other essential roles in addition to Cdc28p activation, such as the activation of other essential Cdk’s such as Kin28 (2, 47)?
MATERIALS AND METHODS
CDC28 mutagenesis.
Plasmid SF19 (CEN-ARS-TRP1-CDC28-HA [10]) and RD47 (without the hemagglutinin (HA) epitope tag) (from Peter Sorger) contain CDC28 under its own promoter. The T169E and the T169E,E171A mutations were introduced into pSF19 by splice-overlap extension PCR (18). Error-prone PCR amplification was performed as described previously (23) without MnCl2, by using 10 to 300 ng of plasmid template, with oligonucleotides priming 40 nucleotides 5′ to the CDC28 initiation codon and 3′ to the coding sequence for amino acid 267. Under these conditions, an error rate of about 0.1% is observed (reference 23 and data not shown). KL050-1 (SF19 with the AflII-ClaI fragment coding for amino acids 28 to 219 in CDC28 replaced with the chloramphenicol resistance gene) was digested with AflII and ClaI. Strain FC23-8 (cdc28::HIS3 pGAL1::CDC2-hs/URA3 leu2::LEU2::GAL1::CLN2 trp1) was transformed with this digest mixed with the PCR products. The human CDC2-hs gene substitutes for the homologous yeast gene CDC28 (51) in order to restrict recombinational repair of the gapped CDC28 plasmid (34) to the PCR product. The AflII-ClaI region was deleted in cdc28::HIS3. Transformants on galactose-tryptophan (ScGal-trp) medium (approximately 5,000/plate, with two or three plates screened for most experiments) were replica plated to yeast extract-peptone-dextrose (YEPD) for overnight growth at 30°C to allow depletion of Cdc2-hs. They were replica-plated to dextrose–fluoro-orotic acid (ScD-FOA) to select for growth of plasmid-loss segregants that lacked the CDC2-hs plasmid (42). They were then tested by replica plating for the ability to grow on YEPD at 30 or 38°C and for the ability to grow on yeast extract-peptone-galactose (YEPGal) (inducing GAL1::CLN2 expression, which causes α-factor resistance [33]) containing 0.3 μM α-factor at 30 or 38°C. The number of colonies screened was sufficient to examine a high proportion of the available single mutants based on the estimated 0.1% error rate. The CDC28 plasmid from the strongest positive colonies was isolated by transformation of Escherichia coli and retested. The complete region of DNA mutagenized by PCR amplification was sequenced for each mutant with an ABI sequencing machine by using oligonucleotide primers priming outside of the PCR-mutagenized regions.
To remove the HA epitope tag, the same gap repair procedure was used, except that an AflII-ClaI-gapped RD47 (untagged) derivative and a KpnI fragment (from 5′ polylinker to a CDC28 site downstream of all mutations but upstream of the HA epitope tag) from the mutant genes were cotransformed. The untagged mutants were confirmed to have similar biological activity to the tagged versions in the GAL1::CLN2 cdc28::HIS3 strain FC23-8 (described above). These untagged constructs were not sequenced, but two of each were tested in parallel in all experiments.
The β strand 4 (β4) mutant pool was constructed by splice-overlap extension PCR with high-fidelity thermostable DNA polymerase (Vent; NEB) in which the first amplification product was primed with an antisense oligonucleotide spanning the predicted β4 coding sequence (residues 72 to 79), in which each position that could change the coding sequence was synthesized with 91% wild-type nucleotide and 3% each of the other three nucleotides. This oligonucleotide had 20 nucleotides of exact complementarity at its 5′ end to the coding sequence 3′ to β4, allowing splice-overlap extension to a 3′ fragment containing T169E, T169A, or T169E,E171A DNA.
T169T revertants of -4324 and -5331 were constructed by SOE with a 5′ fragment containing the -4324 and -5331 mutations and a 3′ fragment containing T169T. Because of the method of construction, the final T169T version derived from -4324 had lost the A234V mutation (because mutations C terminal to 169 in -4324 were lost in recombination) so -4325 (identical to -4324, except for the absence of A234V) was used as a control for this derivative. The -4324 and -4325 mutants have similar biological activity (Table 1).
TABLE 1.
Sequence changes and biological activity of CDC28 mutantsa
| CDC28 gene assayed | Mutation | Result for growth on:
|
|||
|---|---|---|---|---|---|
| YEPD
|
YEPGal + α-factor
|
||||
| 30°C | 38°C | 30°C | 38°C | ||
| CDC28 parents | |||||
| CDC28 (wild type) | None | +++ | +++ | +++ | +++ |
| CDC28-T169E | T169E | − | − | − | − |
| PCR-generated mutants (generation) | |||||
| -2 (1st) | T169E, L44P, D75G | +/− | − | − | − |
| -21 (2nd) | -2 + K83E, R97G, I172V | + | +/− | +/− | − |
| -4 (1st) | T169E, H78R, K96E | +/− | − | − | − |
| -41 (2nd) | -4 + K83R | + | +/− | +/− | − |
| -43 (2nd) | -4 + A125E | + | + | +/− | − |
| -432 (3rd) | -43 + K83R | ++ | + | ++ | − |
| -4321 (4th) | -432 + D238E | ++ | ++ | ++ | + |
| -4323 (4th) | -432 + T18S | ++ | ++ | NDb | +/− |
| -4324 (4th) | -432 + L61I, A234V | ++ | ++ | ++ | + |
| -4325 (4th) | -432 + L61I | ++ | ++ | ND | + |
| -433 (3rd) | -43 + T18S | ++ | + | + | − |
| -434 (3rd) | -43 + F88L | + | + | + | + |
| -44 (2nd) | -4 + N69S, S216C | + | + | ++ | − |
| -443 (3rd) | -44 + V77A, I124V | ++ | ++ | ++ | +/− |
| -444 (3rd) | -44 + K83E, K146R | ++ | ++ | ++ | − |
| -5 (1st) | T169E, V77D | +/− | − | − | − |
| -52 (2nd) | -5 + T18A | + | − | +/− | − |
| -53 (2nd) | -5 + K96E | + | − | + | − |
| -531 (3rd) | -53 + I124V | ++ | +/− | ++ | − |
| -5311 (4th) | -531 + K83E | ++ | ++ | ND | +/− |
| -5313 (4th) | -531 + I172V | ++ | ++ | ND | +/− |
| -5315 (4th) | -531 + S213C | ++ | ++ | ND | +/− |
| -532 (3rd) | -53 + I124V | ++ | +/− | ++ | − |
| -533 (3rd) | -53 + K83E | ++ | +/− | ++ | − |
| -5331 (4th) | -533 + I124V | ++ | ++ | ND | +/− |
| -5333 (4th) | -533 + I124V | ++ | ++ | ND | +/− |
| -5335 (4th) | -533 + L93M | ++ | ++ | ND | +/− |
| β4 mutant pool | |||||
| 3a1, 3a2 | T169E, I76F, V77D | +/− | ND | ND | ND |
| 3a3 | T169E, D75G, V77A, H78R | +/− | ND | ND | ND |
| 3a4 | T169E, L73I, I76T | +/− | ND | ND | ND |
| 3a5 | T169E, R72K, I76S, V77A | +/− | ND | ND | ND |
| K96E, T169E derivatives | |||||
| K96E, T169E | K96E, T169E | − | ND | ND | ND |
| 96/169-1, 4 | K96E, T169E, V77A | + | − | +/− | − |
| 96/169-2 | K96E, T169E L44P | +/− | − | +/− | − |
| 96/169-3 | K96E, T169E K9T | +/− | − | ND | ND |
Strain FC23-8 (cdc28::HIS3 pGAL1::CDC2-hs/URA3 leu2::LEU2::GAL1::CLN2 trp1) was transformed with wild-type CDC28 on a TRP1/CEN-ARS plasmid, with the same plasmid containing the T169E mutation, or with the same plasmid containing the T169E mutation as well as additional suppressor mutation(s). Transformants on ScGal-trp medium were pooled (20 to 50/pool, 4 pools/mutant) and assayed as described in Materials and Methods. The table presents a qualitative scoring of activity in the replica-plating assays, compiled from different experiments; see Fig. 1 for results of serial dilution plating assays with selected mutants. The altered amino acids caused by the mutations are indicated. All mutants contained the mutations found in their parents plus the new mutations indicated. All amino acid positions mutated in more than one independent lineage are underlined (“independent” here means that the parents differed in at least one position, not that the lineages are completely independent from the starting T169E mutant cdc28). All mutations that were single-additional mutations that improved function in some context are indicated in boldface type. See Materials and Methods for construction of the β4 mutant pool and the K96E mutant derivatives.
ND, not determined.
Yeast strain construction.
Yeast strains were constructed by standard methods (16). Strain FC23-8 was congenic with BF264-15D (37). The cdc28::HIS3 construct was made by substituting the HIS3-kanr cassette in JA50-delP (6) for the AflII-ClaI region of CDC28 and was introduced into yeast cells carrying pURA3-CDC28 plasmid by one-step gene disruption (38). HA-tagged Clb2p was expressed from plasmid p143 (GAL1 promoter driving HA-CLB2) provided by R. Deshaies. HA-tagged Cln2p (also from the GAL1 promoter) was described previously (48) and was crossed into the BF264-15D background and then combined with the CDC28-csr1 mutation (25) that eliminates detectable interaction of Cdc28p-csr1 with Cln2p (see Fig. 3; compare lanes 2 and 3).
FIG. 3.
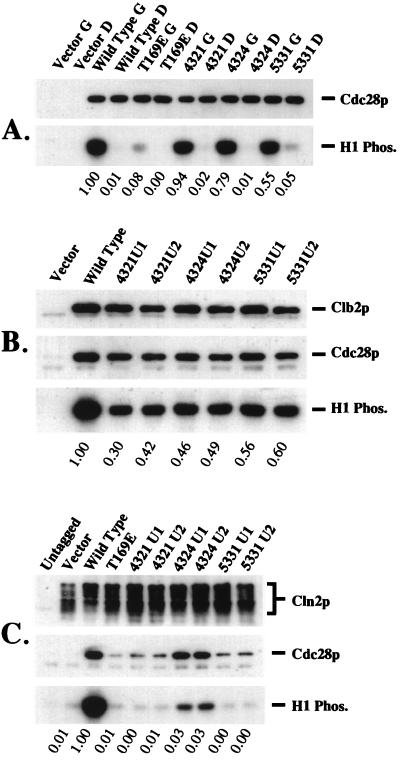
Kinase activity of mutant Cdc28p. (A) Protein kinase activity associated with mutant Cdc28p is dependent on G1 cyclin function. A cln1Δ cln2Δ cln3Δ leu2::LEU2::GAL1::CLN3 strain transformed with epitope-tagged CDC28 genes was grown to log phase in galactose medium, and a portion was blocked by cln deprivation by growth in glucose medium to shut off GAL1::CLN3 (4, 37). Cdc28p immunoprecipitates from cycling and blocked cultures were assayed for histone H1 kinase activity and for Cdc28p protein by immunoblotting. G, galactose medium (GAL1::CLN3 on; cycling cells); D, dextrose medium (GAL1::CLN3 off; blocked cells). (B and C) Cyclin binding and cyclin-associated kinase activity of mutant Cdc28p. Wild-type and mutant CDC28 genes lacking the epitope tag (two clones each) were introduced into strains expressing HA-tagged Clb2p or Cln2p. Immunoprecipitated cyclin, cyclin-associated Cdc28p, and histone H1 kinase activity were assayed. (B) Clb2p-bound Cdc28p. The first lane contains vector rather than the GAL-HA-CLB2 plasmid. (C) Cln2p-bound Cdc28p. The first lane is a strain lacking the integrated GAL-CLN2-HA construct. The second lane lacks exogenous introduced CDC28. In panel C, the endogenous CDC28 is defective for Cln2p binding (25). Phos., phosphorylated.
Strains used for the analysis of the CAK1 requirement were isogenic with W303 (provided by Ann Sutton [21]). The ability of CDC28-T169E-4321, -4324, and -5331, -4325, and the T169T versions of -4325 and -5331 to rescue cak1, cdc28, and cak1 cdc28 strains was determined by tetrad analysis as described in the legend to Table 2. For analysis of the cak1-22 temperature-sensitive allele, strains SY132 (cak1::HIS3 pLEU2-cak1-22) or SY227-1 (cak1::HIS3 clb2::LEU2 pURA3-cak1-22) (from Ann Sutton) were used.
TABLE 2.
Rescue of inviability due to cak1 deletiona
| CDC28 gene on plasmid | No. of segregants containing CDC28 plasmid and lacking CAK1 plasmid
|
|||
|---|---|---|---|---|
| CAK1 CDC28 | CAK1 cdc28Δ | cak1Δ CDC28 | cak1Δ cdc28Δ | |
| CDC28 (wild type) | 21 | 19 | 0 | 0 |
| CDC28-4321 | 9 | 14 | 13 | 8 |
| CDC28-4324 | 10 | 9 | 10 | 10 |
| CDC28-4325 | 12 | 17 | 18 | 11 |
| CDC28-4325-169T | 8 | 12 | 0 | 0 |
| CDC28-5331 | 28 | 17 | 17 | 25 |
| CDC28-5331-169T | 14 | 13 | 0 | 0 |
Strains of genotype cak1::LEU2 pURA3-CAK1 were mated to isogenic (W303 background) strains of genotype cdc28::HIS3 pTRP1-CDC28 (either wild-type, or 4th generation CDC28 mutant strain). In two cases (CDC28-4325-169T and CDC28-5331-169T), the 4th generation CDC28 mutant had been reverted to contain the wild-type T169T sequence instead of T169E. Following tetrad dissection, viable spores were replica plated to determine auxotrophy for Leu, His, Trp, and Ura. The estimated spore viability of CAK1 CDC28 segregants was greater than 90% in most crosses (the lowest was 83% for CDC28-4324-169T crosses) (determined by comparing the recovery of Leu− His− spores to the expected average of 1 per tetrad dissected). Trp+ Ura− segregants from the crosses (containing the CDC28 plasmid and lacking the CAK1 plasmid) were scored for cak1Δ and cdc28Δ by the Leu+ and His+ phenotypes, respectively. Replicate crosses gave similar results, so data from up to three isogenic crosses were pooled. The numbers are the total recovered spores of each genotype. Only spores from tetrads in which genotypes of all inviable spores could be deduced, assuming 2:2 segregation of all markers (excluding plasmids), are included in the tabulation. A 2:2 segregation of nonplasmid markers was observed in all four-viable-spore tetrads. Due to 3:1 and 1:3 segregation of the plasmid observable in some four-viable-spore tetrads, the plasmid content of inviable spores could not be determined reliably.
Protein analysis.
Extraction, immunoprecipitation, protein kinase assays, and immunoblotting for protein analysis were performed as described previously (24, 25). Immunoprecipitation was done with the 12CA5 anti-HA monoclonal antibody (Babco), and immunoblots used the polyclonal anti-HA.11 antibody (Babco). To analyze G1 cyclin dependence of mutant Cdc28p kinase activity, strain 1607-2D (MATa cln1Δ cln2Δ cln3Δ leu2::LEU2::GAL1::CLN3) was transformed with vector or with CDC28 plasmids containing wild-type CDC28, T169E, or CDC28-169-4321, -4324, or -5331 (all C-terminally tagged with the 12CA5 HA epitope tag). All transformants were completely dependent on galactose for viability, and upon switch to glucose medium, they arrested as large unbudded cells as described previously (4, 37); thus the mutants do not bypass the G1 cyclin requirement. Transformants were grown at 30°C in ScGal-trp medium and shifted for 3.5 h into ScDex-trp medium to shut off GAL1::CLN3, or were left in ScGal-trp medium. Cultures were extracted, extracts were immunoprecipitated with 12CA5, and immunoprecipitates were assayed for histone H1 kinase activity and for Cdc28p protein by immunoblotting. Cell cycle arrest by CLN deprivation (4, 37) was monitored by accumulation of unbudded cells; in galactose cultures, this value ranged from 56 to 66%, and in the dextrose cultures, this value ranged from 94 to 98%.
RESULTS
Mutations in β strand 4 are second-site suppressors of the cdc28-T169E defect.
Although negatively charged residues substitute effectively for phosphorylated residues in some proteins (for example, in the Cdk7 activation loop [29]) the substitution of glutamic acid for the site of activation loop phosphorylation (T169) in the Cdk Cdc28p results in very low protein kinase and biological activity (24). Previous studies with this mutant (27) were limited by the inability of the mutant to function as the sole CDC28 gene in the cell. We therefore performed error-prone PCR amplification of cdc28-T169E, and identified CDC28 plasmids that retained the T169E mutation but rescued viability in a cdc28::HIS3 deletion strain (Fig. 1 and Table 1). Three of these mutant genes were sequenced: CDC28-169-2 (L44P,D75G,T169E), -4 (H78R,K96E,T169E); and -5 (V77D,T169E). D75G, H78R and V77D are predicted to alter β strand 4, based on alignment of Cdc28p to the crystal structure of Cdk2 (8, 16).
FIG. 1.
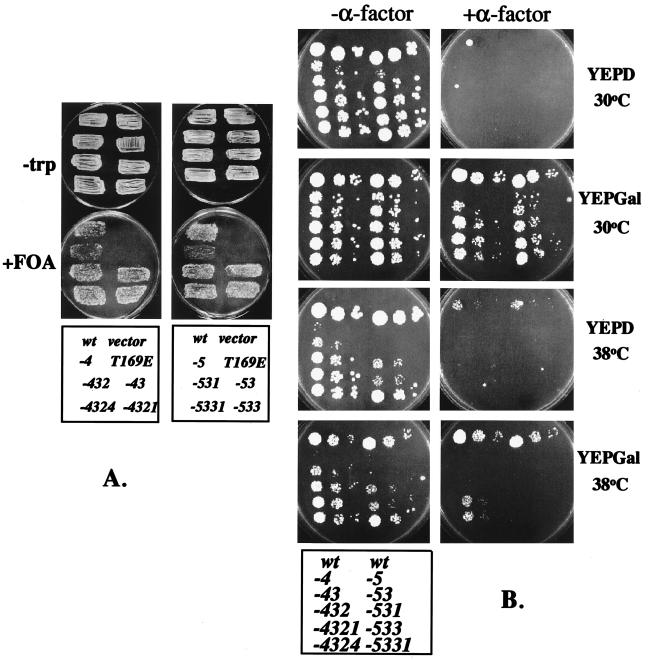
Functional CDC28 containing the T169E mutation. (A) Strain FC23-8 (cdc28::HIS3 pGAL1::CDC2-hs/URA3 leu2::LEU2::GAL1::CLN2) was transformed with vector, with the wild-type (wt) CDC28-containing plasmid, or with mutated CDC28 plasmids: CDC28-T169E: T169E; see Table 1 for other sequences. Twenty to 50 pooled transformants were tested for complementation of cdc28::HIS3 by selecting for growth of segregants that had lost the CDC2-hs/URA3 plasmid by using FOA (42). The plasmids transformed in each patch are indicated in the key at the bottom of the figure. (B) Stationary-phase cultures of FOA-resistant strains shown in panel A were suspended in water to an optical density at 660 nm of 0.7 to 1.2 and diluted 1:100, 1:1,000, and 1:10,000, and then 4 μl of each dilution was spotted on YEPD or YEPGal, with or without 0.3 μM α-factor (medium, temperature, and presence or absence of α-factor are indicated). Plates were incubated for 3 days at 30°C or for 4 days at 38°C. The plasmids transformed in each patch are indicated in the key at the bottom of the figure. The strains are arranged in two columns (three 10-fold serial dilutions per column) with the wild type given at the top in each column. The left column contains members of the lineages leading to the 4th generation −4321 and −4324 mutants; the right column contains the -5331 lineage. See Table 1 for sequences.
To confirm that β4 mutations were sufficient to suppress the T169E defect, we constructed an oligonucleotide designed to yield all single-nucleotide substitutions that change the β4 coding sequence (3% substitution for each change). This oligonucleotide was recombined with the remainder of the coding sequence, containing T169E CDC28, T169A CDC28, or T169E,E171A CDC28, to test the requirement for negative charges at 169 and 171. (In the unphosphorylated Cdk2-cyclin A complex, the E171 side chain occupies the positively charged binding pocket occupied by phosphorylated T169 [19, 39].) The β4 mutant pool yielded biologically active CDC28 (at a frequency of approximately 10%) when recombined with T169E DNA (data not shown). The β4 regions of five of these mutants contained multiple mutations, including the three β4 mutations recovered in the initial random mutagenesis (Table 1). When the β4 mutant pool was recombined with T169E,E171A or T169A DNA, no active clones were recovered (all apparent positives contained reversions of T169A back to T or of E171A back to E). Thus suppression of the T169E defect by the β4 mutation may require carboxylate groups at both nucleotides 169 and 171.
Evolving better suppressors of cdc28-T169E.
The initial cdc28-T169E pseudorevertants had significant defects (Fig. 1). We used these mutants as templates for additional rounds of random mutagenesis. We screened for better growth at 30 or 38°C or for mating factor resistance when the G1 cyclin CLN2 was overexpressed (mating factor resistance in this assay requires CDC28 function in addition to CLN2 overexpression [25, 33]). After four cycles of mutation and selection, we isolated mutants with high (although still less than wild-type) biological activity (Fig. 1 and Table 1).
Multiple mutation may be required for high activity.
In the course of sequential mutagenesis, some amino acid residues were mutated independently in different contexts (T18, L44, D75, V77, H78, K83, K96, I124, and I172 [Table 1]), suggesting that mutations of these residues may act semi-independently to improve function of Cdc28p-T169E. The K96E mutation (in α helix 2) was one of two mutations in 169-4 (in addition to the H78R β4 mutation) and also was the sole mutation responsible for the improvement of 169-5 to 169-53 (Table 1 and Fig. 1). Therefore, we asked if it had the ability to suppress the T169E mutation by itself. A cdc28::HIS3 GAL1::CDC2-hs strain transformed by gap repair of CDC28 with amplified CDC28 DNA containing this mutation and T169E was essentially negative for viability on glucose (CDC2 off) (Table 1); rare transformants that were viable on glucose had additional mutations (Table 1) probably generated during amplification. Two mutants had picked up a β4 mutation (V77A) in addition to K96E; two weaker ones had picked up mutations N terminal to β4 (K9T or L44P) (Table 1). β4 mutations are thus confirmed to be an efficient route to T169E suppression without being absolutely required, at least in the presence of the independently activating K96E mutation.
Close examination of the data in Table 1 suggests that multiple mutations accumulated in successive rounds of mutagenesis are in many cases all important for the final phenotype. In one test of the idea that the mutations identified in these experiments can have semiadditive effects on rescue (see Discussion), we introduced the T18S mutation (recovered independently twice in the −4 lineage [Table 1]) into the CDC28-T169-4324 and −5331 genes. We found that this additional mutation significantly improved rescue of the T169E defect according to the assays in Fig. 1 (data not shown).
Phosphorylation-dephosphorylation cycles on T169 are not required for cell cycle regulation.
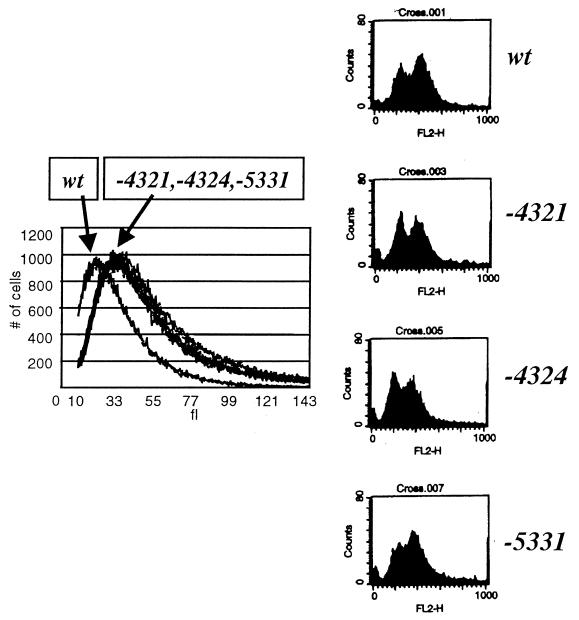
Three of the 4th generation mutants (Fig. 1B) were chosen for further characterization. These mutants supported doubling times of 1.1 to 1.2 times that of the wild type, with cell volumes 1.5 times that of the wild type (Fig. 2). Increased cell size is a sensitive indicator of slowing cell cycle progression (3). Thus, this result suggests only minor defects in the speed of completion of some cell cycle event(s). Cell morphology was fairly normal, though some cells had misshapen buds (data not shown). The percentage of unbudded cells in log-phase cultures was 30 to 40% (wild-type value, 35%); thus the duration of the budded phase of the cell cycle, which is regulated by cyclin-Cdc28p activity (26, 36), was similar to that of the wild type. DNA flow cytometric analysis (Fig. 2) showed only minor changes in the proportions of 1n and 2n cells in log phase compared to those of the wild type, with no accumulation of aneuploids. Stationary-phase cultures of mutant and wild-type cells accumulated greater than 90% unbudded cells with high cell viability, indicating normal regulation of the cell cycle by nutrient deprivation. The mutant strains were sensitive to mating factor, provided CLN2 was not overexpressed (Fig. 1B, YEPD+α-factor). To test the sensitivity of the CDC28 mutants to the Sic1 inhibitor of B-type cyclin-Cdc28p kinase (11, 30, 32, 40), the mutants were introduced (in addition to resident wild-type CDC28) into a strain containing multiple copies of a GAL-SIC1 construct (7) and also introduced into a cln1 cln2 CLN3 CDC28 strain containing a single copy of GAL1::SIC1 (increasing SIC1 expression is highly lethal in a cln1 cln2 background [49]). The mutant CDC28 genes did not prevent SIC1-induced arrest (data not shown). Thus the CDC28 mutants did not significantly alter cell cycle regulation by extrinsic (mating factor, nutrient deprivation) or intrinsic (Sic1, S/M alternation) factors. The CDC28 mutants should abrogate any form of regulation that requires Cdc28p-T169 phosphorylation or dephosphorylation. Therefore, cyclic phosphorylation of the Cdc28p activation loop may not be required for cell cycle regulation.
FIG. 2.
Characterization of 4th generation CDC28 mutants. Strain FC23-8 (cdc28::HIS3 leu2::LEU2::GAL1::CLN2 trp1) carrying the indicated CDC28 genes on plasmids was grown to log phase in YEPD medium at 30°C. Samples were prepared for analysis of cell volume with a Coulter Channelyzer (left) and for DNA flow cytometry (right). wt, wild type.
Cyclin requirements for genetic function of CDC28-T169E suppressor mutants.
The CDC28 mutants do not bypass the requirement for a G1 cyclin (4, 37), because they were unable to rescue a cln1Δ cln2Δ cln3Δ GAL1::CLN3 strain on glucose medium (in the absence of CLN G1 cyclin expression) (data not shown) (Fig. 3). Deletion of the major G1 CLN1 and CLN2 (5) cyclins or the major mitotic CLB2 (15, 31, 44) cyclin almost eliminated the ability of the CDC28 mutants to rescue a cdc28::HIS3 strain, while wild-type CDC28 was unaffected (data not shown); thus the CDC28 mutants are cyclin dependent in vivo.
Cyclin dependence of Cdc28p kinase activity in the absence of the ability to dephosphorylate the activation loop.
Cdk7 phosphorylated in its activation loop is partially active in the absence of cyclin H (29). Although Cdc28p can be phosphorylated on T169 by Cak1p without activating its kinase activity, this does not address the question of what happens if phosphorylated and cyclin-bound active Cdc28p undergoes cyclin degradation in the absence of dephosphorylation. “Protein memory” (41) could then allow persistence of the active conformation. Since the T169E substitution is presumably acting as a phosphothreonine mimic that cannot be dephosphorylated, it was thus possible that the Cdc28p-T169E mutants might retain kinase activity after cyclin degradation. To test this, we wanted to prepare cyclin-free Cdc28p and measure its associated kinase activity.
cln1 cln2 cln3 GAL1::CLN3 cells incubated in glucose medium are cyclin deficient: the G1 CLN cyclins have been removed genetically, and a combination of transcriptional control and proteolytic control effectively eliminates B-type cyclins (reviewed in references 5 and 31). The CDC28 mutants (epitope tagged) were introduced into a cln1 cln2 cln3 GAL1::CLN3 CDC28 strain. Essentially normal levels of histone H1 kinase activity were observed in immunoprecipitates of epitope-tagged Cdc28p-4321, -4324, and -5331 from extracts made from galactose-grown (asynchronous) cultures, while Cdc28p-T169E without additional mutations had very low protein kinase activity (Fig. 3A). Protein kinase activity associated with mutant Cdc28p decreased 10- to 80-fold in cln1 cln2 cln3 GAL1::CLN3 strains upon inactivation of GAL1::CLN3 transcription (the wild-type value decreased 100-fold), suggesting that kinase activity associated with the mutant Cdc28p is largely cyclin dependent (Fig. 3A). (This does not imply that the CLN3-dependent kinase activity observed is directly due to Cln3p-Cdc28p complexes; it is much more likely to be due to complexes of Cdc28p with various B-type cyclins that are activated by Cln3p-Cdc28p [5, 15, 31].)
To show that the low recovery of kinase activity of Cdc28p immunoprecipitated from these cyclin-deficient cells was specifically due to the lack of cyclin, we supplemented these kinase reactions with 300 ng of recombinant cyclin A (a gift from A. Koff). Kinase activity of wild-type Cdc28p extracted from cyclin-deficient cells was stimulated over 200-fold by cyclin A, and kinase activities of Cdc28p-4324 and Cdc28p-5331 were stimulated 40- and 20-fold respectively. Cdc28p-4321 was poorly (fivefold) activated by cyclin A (data not shown). The comparison between the mutant and wild type is somewhat difficult in that no exogenous Cak1p (12, 18, 47) was included in the assay. The wild-type but not the mutant Cdc28p may have a significant requirement for in vitro Cak1p phosphorylation of T169, and this could improve recovery of activity in the mutants. On the other hand, Cak1p copurifies with Cdc28p from yeast extracts (47), so its exogenous addition may not be necessary. Despite these concerns, it is clear that these Cdc28p preparations from the blocked cultures are defective at least in large part because of the lack of cyclin, for both mutant and wild-type Cdc28p.
Coimmunoprecipitation of the mutant Cdc28p with epitope-tagged Clb2p (the major mitotic B-type cyclin [15, 44]) and the kinase activity of Clb2p-associated mutant Cdc28p were close to those of the wild type (Fig. 3B). Taken together with the low level of kinase activity associated with wild-type and mutant Cdc28p from cyclin-deficient cells, this result indicates that the kinase activity of the mutant Cdc28p is stimulated by Clb2p binding almost as well as the kinase activity of wild-type Cdc28p.
Thus, the T169E-suppressing mutations bypass the requirement for T169 phosphorylation for kinase activity, but still show strong cyclin dependence for function in vivo and in vitro. The moderate decrease in regulation of kinase activity by cyclin deprivation of the mutants compared to that of the wild type (Fig. 3A) could suggest decreased cyclin dependence for either activation or maintenance of kinase activity of these mutants. Further work and additional mutational analysis will be required to clarify this possibility.
The Cdc28p-T169E mutants retain a strong defect in Cln2p-associated kinase activity.
Despite CLN2-dependent genetic function of the 4th generation Cdc28p mutants (Fig. 2), they were surprisingly defective in Cln2p-associated kinase. Cdc28p-4324 bound to Cln2p with about 50% wild-type efficiency, but gave only 2 to 3% of wild-type Cln2p-associated kinase activity; −4321 and −5331 were about as defective as Cdc28p-T169E (27) at Cln2p binding and Cln2p-associated kinase (Fig. 3C). Nevertheless, these mutants complemented the viability of a cln1 CLN2 cln3 cdc28-13 (temperature sensitive) strain at 38°C, indicating effective CDC28 function with CLN2 as the sole G1 cyclin (data not shown). We lack an easy explanation of this discrepancy. It may be that the in vitro Cln2p-associated kinase activity is a poor guide to the in vivo activity, especially since these mutants are all significantly temperature sensitive for Cln2p-dependent mating factor resistance (Fig. 1B). Temperature-sensitive mutants are commonly unconditionally defective in vitro. We also recently observed a lack of correlation between Cln2p-dependent biological activity and Cln2p-associated histone H1 kinase activity among Cdc28p mutants, so it is possible that this assay is not completely informative for all aspects of Cln2p-Cdc28p biological function (25).
The CDC28-T169E mutants bypass the requirement for CAK1 for viability: T169 phosphorylation is the only essential role for Cak1p.
The essential Cak1p/Civ1p kinase carries out activation loop phosphorylation of Cdc28p (12, 18, 45, 47). It has been suggested that Cak1p has other essential roles, including, possibly, activation of the essential Kin28 Cdk (2, 47).
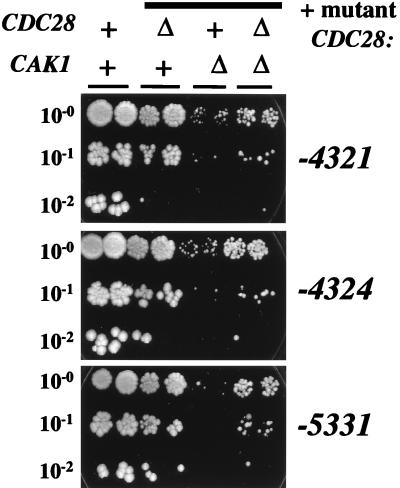
These CDC28 mutants should relieve the requirement for Cak1p for Cdc28p activation, since T169, the sole known Cak1p site in Cdc28p (12, 18, 47), is removed by the T169E mutation. They therefore allow a direct test of the idea that Cak1p has other essential roles in addition to Cdc28p activation. By tetrad analysis, we determined that the 4th generation CDC28 mutants on plasmids rescued viability in cdc28Δ, cak1Δ, and cdc28Δ cak1Δ strains with essentially complete recovery of the expected singly or doubly mutant spores as viable colonies (Table 2), while a wild-type CDC28 plasmid rescued only cdc28Δ and a CAK1 plasmid rescued only cak1Δ (Table 2 and data not shown). cdc28-T169E did not rescue either cdc28Δ or cak1Δ (Fig. 1 and data not shown). Thus, the mutant CDC28 genes bypass the Cak1p requirement. The rescued cak1Δ CDC28 strains grew significantly more slowly than the rescued cak1Δ cdc28Δ strains (Fig. 4, compare third and fourth columns), suggesting that unphosphorylated wild-type Cdc28p may interfere with function of the mutant Cdc28p.
FIG. 4.
Mutant CDC28 genes bypass the CAK1 requirement. Stationary-phase YEPD cultures of segregants from the crosses described in Table 2 were suspended in water at an optical density of 660 nm of 1.2 to 1.4. A 1:200 dilution of this suspension was made in water, and 5 μl of this suspension, or a 1:10 or a 1:100 dilution (as indicated), was spotted on a YEPD plate. The plate was incubated at 30°C for 3 days. Two segregants of each genotype were tested. ++, wild-type strains (one cak1::LEU2 CDC28 strain carrying the pCAK1 plasmid and one CAK1 cdc28::HIS3 strain carrying the wild-type CDC28 plasmid; Δ+, CAK1 cdc28::HIS3 strains carrying the indicated mutant CDC28 plasmids; +Δ, cak1::LEU2 CDC28 strains carrying the indicated mutant CDC28 plasmids; ΔΔ, cak1::LEU2 cdc28::HIS3 strains carrying the indicated mutant CDC28 plasmids.
Other nonessential roles for Cak1p are suggested by the slow growth of the rescued cak1Δ cdc28Δ strains compared to that of the rescued CAK1 cdc28Δ strains (Fig. 4, compare second and fourth columns). (Note that this is the correct comparison for determining the role of Cak1p, since only this comparison eliminates the competitive effect of unphosphorylated Cdc28p discussed in the last paragraph.) The doubling time in rich medium of CAK1 cdc28::HIS3 pCDC28-T169-4325 strains was about 90 min, which increased to 120 min for cak1::LEU2 cdc28::HIS3 pCDC28-T169-4325 strains. The corresponding numbers for -5331 were about 120 and 225 min respectively (data not shown).
Still further improvement of rescue of cdc28Δ cak1Δ inviability was attained by introduction of the T18S mutation (Table 1) into the CDC28-T169-4324 and -5331 genes. This additional mutation improved rescue of both the cdc28Δ and cdc28Δ cak1Δ backgrounds. Doubling times in rich medium for cdc28Δ CAK1 and cdc28Δ cak1Δ strains for these two plasmids were 78 and 110 min for -4324/T18S and 90 and 118 min for -5331/T18S. Thus, the growth defect due to deletion of CAK1 can be significantly reduced but probably not eliminated by improving the rescuing CDC28 mutant.
These results indicate that Cak1p has other nonessential roles in addition to Cdc28p-T169 phosphorylation and that even highly active Cdc28p cannot compensate fully for the absence of Cak1p.
Reversion of T169E to T169T in the CDC28-169-4325 and -5331 mutants prevented rescue of the cdc28Δ cak1Δ and CDC28 cak1Δ strains (Table 2). In contrast, the reverted mutants rescued cdc28Δ CAK1 strains better than the T169E versions (data not shown). Thus, the suppressor mutations did not bypass the requirement for a negative charge at position 169.
The cak1-22 temperature-sensitive allele (18) is also rescued by these mutant CDC28 genes, as expected (data not shown). The cak1-22 temperature sensitivity is strongly enhanced by deletion of CLB2, encoding the major mitotic B-type cyclin (18). Deletion of CLB2 eliminated rescue of cak1-22 by the mutant CDC28 genes, even at 30°C (data not shown). This result confirms that the mutants are strongly dependent on Clb2p, as described above.
DISCUSSION
Molecular evolution: multiple mutants are a necessary evil.
The cycles of mutagenesis and selection carried out here yielded multiply mutant CDC28-T169E derivatives with high biological activity. It may be impossible to isolate single mutants with activity close to that detected in the final products, at least by PCR mutagenesis, since a large proportion of the available single mutants were screened at each step (see Materials and Methods). In one case examined in detail, the K96E mutation clearly enhances function of the -5 V77D mutant (-53 mutant) (Table 1 and Fig. 1B), but the K96E mutation by itself lacks T169E-suppressing activity (Table 1). Also, the -531 (V77D, K96E, I124V) and -533 (V77D, K83E, K96E) triple mutants derived from the -53 mutant are significantly less active than the -5331 (V77D, K83E, K96E, I124V) quadruple mutant (Table 1 and Fig. 1B). Thus, the full set of four suppressor mutations is clearly required for the efficient suppression of the T169E defect in mutant -5331. Therefore, the use of the molecular evolution method may be a requirement for generation of highly active mutants, since such a quadruple mutant would be impossible to isolate in a single step of mutagenesis and screening.
It appears likely that the multiple mutations in our T169E suppressors have positive effects in a semi-context-independent fashion, since the same mutations were isolated multiple times in different backgrounds of other suppressor mutations (see Results). The idea that mutants with independent weakly positive effects can interact positively may explain the success of the DNA shuffling method, in which weak mutants are recombined with each other to yield highly active products (43). This idea was confirmed directly by the observation that recombining the T18S mutation (Table 1) with the already highly active 4th generation -4324 and -5331 mutants resulted in significant improvement in biological activity (described above).
In most cases, the mutations alter residues that are not close to the location of the phosphorylated threonine in the cognate Cdk2 structure (39), so an overall conformational alteration may be responsible rather than a precise remodeling of the phosphothreonine binding pocket. The mammalian Cdk7-cyclin H complex requires activation loop phosphorylation, but this requirement can be bypassed by the presence of the Mat1 protein, which forms a heterotrimeric complex with Cdk7 and cyclin H (13, 46). Presumably Mat1 induces folding in a Cdk7-cyclin H complex that compensates for the absence of phosphorylation. We may have induced similar folding alterations by mutagenesis of Cdc28p to allow acceptance of the glutamic acid substitution for phosphothreonine.
Cdc28p mutants that cannot be regulated by activation loop phosphorylation and dephosphorylation remain cyclin dependent in vivo and in vitro.
The CDC28-T169E mutants are dependent on cyclin binding for enzymatic activity. Correlated to this, they are genetically dependent on both G1 cyclins and on mitotic cyclins. These observations suggest that Cdc28p-T169-phosphate complexed to cyclin requires cyclin for maintenance of the active conformation and enzymatic activity. The observation that Cdc28p can be phosphorylated by Cak1p in the absence of cyclin, without activating its kinase activity (12, 18, 47), is consistent with this conclusion. The Cdc28p-T169E suppressor mutants are not fully wild type for function, however, and this may generate a requirement for cyclin binding for maintenance of activity that might not be present in authentic Cdc28p-T169-phosphate. There is a suggestion in the data that some of these mutants may be less cyclin dependent than the wild type. (The -5331 mutant reproducibly shows a higher background signal in the cln-blocked culture [Fig. 3], and all of the mutants are significantly less well activated by cyclin A than the wild type.) It is possible that selection for the ability to tolerate the T169E substitution entails coselection of reduced cyclin dependence.
Why is there CAK?
These experiments demonstrate that cyclical phosphorylation and dephosphorylation of T169 are not required for effective cyclin-dependent cell cycle control. Cyclic phosphorylation of T169 might be involved in cell cycle regulation, but redundant with other controls. If T169 dephosphorylation were regulated during exit from mitosis (28), the effect of blocking this control could be masked by the redundant controls on mitotic exit due to control of cyclin proteolysis (22, 31) or the inhibitor Sic1 (9, 30, 32, 40). The 4th generation CDC28-4324 and -5331 mutants were able to rescue a sic1::LEU2 cdc28::HIS3 strain, however, (data not shown), suggesting that T169 phosphorylation-dephosphorylation cycles are not required, even in the absence of negative control of Cdc28p by Sic1 inhibition.
The observation that the Cak1p kinase exhibits no cell cycle variation in its in vitro activity (12, 45) is consistent with the idea that activation loop phosphorylation may be constitutive, but does not in itself prove it: Cak1p could be regulated in vivo, or else a phosphatase that dephosphorylates Cdc28p could be regulated. The mammalian Kap1 phosphatase dephosphorylates the Cdk2 activation loop only upon cyclin degradation (35). The present results suggest, though, that activation loop dephosphorylation is not obligatory for any cell cycle regulatory event.
The reduced growth rate of cak1Δ cdc28Δ strains rescued by the CDC28-T169 mutants (Fig. 4) is evidence of nonessential roles for Cak1p that are independent of T169 phosphorylation. Although a role for Cak1p in spore wall morphogenesis was reported (50), we do not have clues to the nature of the nonessential roles of Cak1p in vegetative growth. In metazoans, Cdk activation is most likely carried out by the Cdk7-cyclin H complex, which is also required for transcription (reviewed in reference 17). Cdk7 is not at all homologous to Cak1; the transcriptional role carried out by Cdk7 in metazoans may be carried out in budding yeast by the essential Cdk7 homolog Kin28 (2, 17).
There are at least three ways to explain the evolutionary persistence of the Cak1p-Cdc28p activation loop phosphorylation system in the face of its apparent dispensability. One possibility is that Cak1p phosphorylation of Cdc28p may be required for some form of regulation that we have not tested for, or it may be an important but redundant aspect of control of mitotic exit. Another possibility is that activation loop phosphorylation may stabilize and reinforce cyclin activation of the Cdk, especially in case phosphorylation is dependent on cyclin binding (as for Cdc2-cyclin B [9]) or in case dephosphorylation is dependent on cyclin removal (35). A final possibility is that CAK phosphorylation fulfills a simple structural requirement for the Cdk: the role of Cdk activation loop phosphorylation may be solely to provide a dianionic group (not available in the genetic code) for nucleating conformational changes leading to maximal catalytic activity (1, 20, 39). While the present results show that there are evolutionary alternatives to phosphorylated threonine for Cdc28p, these alternatives require multiple amino acid substitutions, and even so, the resulting Cdc28p is not fully wild type for function. The persistence of a requirement for Cdk activation loop phosphorylation by CAK may be a consequence of the difficulty of achieving these alternatives by natural evolution: if the T169E substitution occurred first, this would be lethal, while in the presence of T169, there is no evident selection for the suppressor mutations. If the suggestion of incomplete cyclin regulation of the mutant Cdc28p (see Fig. 3A and Results) is correct, then this could imply that mutations that effectively bypass the Cak1p requirement might partially disable cyclin regulation of the kinase activity, thus imposing a cost on accumulation of the T169E suppressor mutations. The simultaneous presence of Cak1p and a phosphorylatable activation loop residue may therefore represent a stable adaptive peak, especially given that Cak1p has other nonessential roles that should select for its continued maintenance.
ACKNOWLEDGMENTS
Thanks go to Ray Deshaies, Peter Sorger, and Ann Sutton for materials and to Rob Fisher, John Kuriyan, Nikola Pavletich, Mark Solomon, and Ann Sutton for helpful discussion.
This work was supported by PHS grant GM47238. K.L. is a Howard Hughes Medical Institute predoctoral fellow.
REFERENCES
- 1.Canagarajah B J, Khokhlatchev A, Cobb M H, Goldsmith E J. Activation mechanism of the MAP kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/s0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- 2.Cismowski M J, Laff G M, Solomon M J, Reed S I. KIN28 encodes a C-terminal domain kinase that controls mRNA transcription in Saccharomyces cerevisiae but lacks cyclin-dependent kinase-activating kinase (CAK) activity. Mol Cell Biol. 1995;15:2983–2992. doi: 10.1128/mcb.15.6.2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross F, Roberts J, Weintraub H. Simple and complex cell cycles. Annu Rev Cell Biol. 1989;5:341–395. doi: 10.1146/annurev.cb.05.110189.002013. [DOI] [PubMed] [Google Scholar]
- 4.Cross F R. Cell cycle arrest caused by CLN gene deficiency in Saccharomyces cerevisiae resembles START-I arrest and is independent of the mating-pheromone signalling pathway. Mol Cell Biol. 1990;10:6482–6490. doi: 10.1128/mcb.10.12.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross F R. Starting the cell cycle: what’s the point? Curr Opin Cell Biol. 1995;7:790–797. doi: 10.1016/0955-0674(95)80062-x. [DOI] [PubMed] [Google Scholar]
- 6.Cross F R. ’Marker swap’ plasmids: convenient tools for budding yeast molecular genetics. Yeast. 1997;13:647–653. doi: 10.1002/(SICI)1097-0061(19970615)13:7<647::AID-YEA115>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Dahmann C, Diffley J F X, Nasmyth K A. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 8.DeBondt H L, Rosenblatt J, Jancarik J, Jones H D, Morgan D O, Kim S H. Crystal structure of cyclin-dependent kinase 2. Nature. 1996;363:595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- 9.Desai D, Wessling H C, Fisher R P, Morgan D O. Effects of phosphorylation by CAK on cyclin binding by CDC2 and CDK2. Mol Cell Biol. 1995;15:345–350. doi: 10.1128/mcb.15.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshaies R J, Kirschner M. G1 cyclin-dependent activation of p34CDC28 (Cdc28p) in vitro. Proc Natl Acad Sci USA. 1995;92:1182–1186. doi: 10.1073/pnas.92.4.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donovan J D, Toyn J H, Johnson A L, Johnston L H. P40SDB25, a putative CDK inhibitor, has a role in the M/G1 transition in Saccharomyces cerevisiae. Genes Dev. 1994;8:1640–1653. doi: 10.1101/gad.8.14.1640. [DOI] [PubMed] [Google Scholar]
- 12.Espinoza F H, Farrell A, Erdjument-Bromage H, Tempst P, Morgan D O. A cyclin-dependent kinase-activating kinase (CAK) in budding yeast unrelated to vertebrate CAK. Science. 1996;273:1714–1717. doi: 10.1126/science.273.5282.1714. [DOI] [PubMed] [Google Scholar]
- 13.Fisher R P, Jin P, Chamberlin H M, Morgan D O. Alternative mechanisms of CAK assembly require an assembly factor or an activating kinase. Cell. 1995;83:47–57. doi: 10.1016/0092-8674(95)90233-3. [DOI] [PubMed] [Google Scholar]
- 14.Gould K L, Moreno S, Owen D J, Sazer S, Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991;10:3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grandin N, Reed S I. Differential function and expression of Saccharomyces cerevisiae B-type cyclins in mitosis and meiosis. Mol Cell Biol. 1993;13:2113–2125. doi: 10.1128/mcb.13.4.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guthrie C, Fink G R. Guide to yeast genetics and molecular biology. Methods in enzymology. Vol. 194. New York, N.Y: Academic Press, Inc.; 1991. [PubMed] [Google Scholar]
- 17.Harper J W, Elledge S J. The role of Cdk7 in CAK function, a retro-retrospective. Genes Dev. 1998;12:285–289. doi: 10.1101/gad.12.3.285. [DOI] [PubMed] [Google Scholar]
- 18.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 19.Jeffrey P D, Russo A A, Polyak K, Gibbs E, Hurwitz J, Massagu J, Pavletich N P. Mechanism of CDK activation revealed by the structure of a cyclin A-CDK2 complex. Nature. 1995;376:313–320. doi: 10.1038/376313a0. [DOI] [PubMed] [Google Scholar]
- 20.Johnson L N, Noble M E M, Owen D J. Active and inactive protein kinases: structural basis for regulation. Cell. 1996;85:149–158. doi: 10.1016/s0092-8674(00)81092-2. [DOI] [PubMed] [Google Scholar]
- 21.Kaldis P, Sutton A, Solomon M J. The Cdk-activating kinase (CAK) from budding yeast. Cell. 1996;86:553–564. doi: 10.1016/s0092-8674(00)80129-4. [DOI] [PubMed] [Google Scholar]
- 22.King R W, Deshaies R J, Peters J M, Kirschner M W. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 23.Leung D W, Chen E, Goeddel D V. A method for random mutagenesis of a defined DNA segment using a modified polymerase chain reaction. Technique. 1989;1:11–15. [Google Scholar]
- 24.Levine K, Huang K, Cross F R. Saccharomyces cerevisiae G1 cyclins differ in their intrinsic functional specificities. Mol Cell Biol. 1996;16:6794–6803. doi: 10.1128/mcb.16.12.6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine K, Oehlen L J W M, Cross F R. Isolation and characterization of new alleles of the cyclin-dependent kinase gene CDC28 with cyclin-specific functional and biochemical defects. Mol Cell Biol. 1998;18:290–302. doi: 10.1128/mcb.18.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lew D J, Reed S I. Morphogenesis in the yeast cell cycle: regulation by Cdc28 and cyclins. J Cell Biol. 1993;120:1305–1320. doi: 10.1083/jcb.120.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim H H, Loy C J, Zaman S, Surana U. Dephosphorylation of threonine 169 of Cdc28 is not required for exit from mitosis but may be necessary for start in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:4573–4583. doi: 10.1128/mcb.16.8.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorca T, Labbe J-C, Devault A, Fesquet D, Capony J-P, Cavadore J-C, le Bouffant F, Doree M. Dephosphorylation of cdc2 on threonine 161 is required for cdc2 kinase inactivation and normal anaphase. EMBO J. 1992;11:2381–2390. doi: 10.1002/j.1460-2075.1992.tb05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez A-M, Afshar M, Martin F, Cavadore J-C, Labbe J-C, Doree M. Dual phosphorylation of the T-loop in cdk7: its role in controlling cyclin H binding and CAK activity. EMBO J. 1997;16:343–354. doi: 10.1093/emboj/16.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mendenhall M D. An inhibitor of p34CDC28 protein kinase activity from Saccharomyces cerevisiae. Science. 1993;259:216–219. doi: 10.1126/science.8421781. [DOI] [PubMed] [Google Scholar]
- 31.Nasmyth K. At the heart of the budding yeast cell cycle. Trends Genet. 1996;12:405–412. doi: 10.1016/0168-9525(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 32.Nugroho T T, Mendenhall M D. An inhibitor of yeast cyclin-dependent protein kinase plays an important role in ensuring the genomic integrity of daughter cells. Mol Cell Biol. 1994;14:3320–3328. doi: 10.1128/mcb.14.5.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oehlen L J W M, Cross F R. G1 cyclins CLN1 and CLN2 repress the mating factor response pathway at Start in the yeast cell cycle. Genes Dev. 1994;8:1058–1070. doi: 10.1101/gad.8.9.1058. [DOI] [PubMed] [Google Scholar]
- 34.Orr-Weaver T L, Szostak J W, Rothstein R J. Genetic applications of yeast transformation with linear and gapped plasmids. Methods Enzymol. 1983;101:228–245. doi: 10.1016/0076-6879(83)01017-4. [DOI] [PubMed] [Google Scholar]
- 35.Poon R Y C, Hunter T. Dephosphorylation of Cdk2 Thr160 by the cyclin-dependent kinase-interacting phosphatase KAP in the absence of cyclin. Science. 1995;270:90–93. doi: 10.1126/science.270.5233.90. [DOI] [PubMed] [Google Scholar]
- 36.Pringle J R, Hartwell L H. The Saccharomyces cerevisiae cell cycle. In: Strathern J N, Jones E W, Broach J R, editors. The molecular biology of the yeast Saccharomyces: life cycle and inheritance. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1981. pp. 97–142. [Google Scholar]
- 37.Richardson H E, Wittenberg C, Cross F, Reed S I. An essential G1 function for cyclin-like proteins in yeast. Cell. 1989;59:1127–1133. doi: 10.1016/0092-8674(89)90768-x. [DOI] [PubMed] [Google Scholar]
- 38.Rothstein R. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- 39.Russo A A, Jeffrey P D, Pavletich N P. Structural basis of cyclin-dependent kinase activation by phosphorylation. Nature Struct Biol. 1996;3:696–700. doi: 10.1038/nsb0896-696. [DOI] [PubMed] [Google Scholar]
- 40.Schwob E, Bohm T, Mendenhall M D, Nasmyth K. The B-type cyclin kinase inhibitor p40SIC1 controls the G1 to S transition in S. cerevisiae. Cell. 1994;79:233–244. doi: 10.1016/0092-8674(94)90193-7. [DOI] [PubMed] [Google Scholar]
- 41.Shinde U P, Liu J J, Inouye M. Protein memory through altered folding mediated by intramolecular chaperones. Nature. 1997;389:520–522. doi: 10.1038/39097. [DOI] [PubMed] [Google Scholar]
- 42.Sikorski R S, Boeke J D. In vitro mutagenesis and plasmid shuffling: from cloned gene to mutant yeast. Methods Enzymol. 1990;194:302–318. doi: 10.1016/0076-6879(91)94023-6. [DOI] [PubMed] [Google Scholar]
- 43.Stimmer W P C. Rapid evolution of a protein in vitro by DNA shuffling. Nature. 1994;370:389–391. doi: 10.1038/370389a0. [DOI] [PubMed] [Google Scholar]
- 44.Surana U, Robitsch H, Price C, Schuster T, Fitch I, Futcher A B, Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991;65:145–161. doi: 10.1016/0092-8674(91)90416-v. [DOI] [PubMed] [Google Scholar]
- 45.Sutton A, Freiman R. The Cak1p protein kinase is required at G1/S and G2/M in the budding yeast cell cycle. Genetics. 1997;147:57–71. doi: 10.1093/genetics/147.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tassan J P, Jaquenoud M, Fry A M, Frutiger S, Hughes G J, Nigg E A. In vitro assembly of a functional human CDK7-cyclin H complex requires MAT1, a novel 36 kDa RING finger protein. EMBO J. 1995;14:5608–5617. doi: 10.1002/j.1460-2075.1995.tb00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thuret J Y, Valay J G, Faye G, Mann C. Civ1 (CAK in vivo), a novel Cdk-activating kinase. Cell. 1996;86:565–576. doi: 10.1016/s0092-8674(00)80130-0. [DOI] [PubMed] [Google Scholar]
- 48.Tyers M, Tokiwa G, Futcher B. Comparison of the Saccharomyces cerevisiae G1 cyclins: Cln3 may be an upstream activator of Cln1, Cln2 and other cyclins. EMBO J. 1993;12:1955–1968. doi: 10.1002/j.1460-2075.1993.tb05845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tyers M. The cyclin-dependent kinase inhibitor p40SIC1 imposes the requirement for Cln G1 cyclin function at start. Proc Natl Acad Sci USA. 1996;93:7772–7776. doi: 10.1073/pnas.93.15.7772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wagner M, Pierce M, Winter E. The CDK-activating kinase CAK1 can dosage suppress sporulation defects of smk1 MAP kinase mutants and is required for spore wall morphogenesis in Saccharomyces cerevisiae. EMBO J. 1997;16:1305–1317. doi: 10.1093/emboj/16.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wittenberg C, Reed S I. Conservation of function and regulation within the Cdc28/cdc2 protein kinase family: characterization of the human Cdc2Hs protein kinase in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:4064–4068. doi: 10.1128/mcb.9.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]