Abstract
The HXK2 gene is required for a variety of regulatory effects leading to an adaptation for fermentative metabolism in Saccharomyces cerevisiae. However, the molecular basis of the specific role of Hxk2p in these effects is still unclear. One important feature in order to understand the physiological function of hexokinase PII is that it is a phosphoprotein, since protein phosphorylation is essential in most metabolic signal transductions in eukaryotic cells. Here we show that Hxk2p exists in vivo in a dimeric-monomeric equilibrium which is affected by phosphorylation. Only the monomeric form appears phosphorylated, whereas the dimer does not. The reversible phosphorylation of Hxk2p is carbon source dependent, being more extensive on poor carbon sources such as galactose, raffinose, and ethanol. In vivo dephosphorylation of Hxk2p is promoted after addition of glucose. This effect is absent in glucose repression mutants cat80/grr1, hex2/reg1, and cid1/glc7. Treatment of a glucose crude extract from cid1-226 (glc7-T152K) mutant cells with λ-phosphatase drastically reduces the presence of phosphoprotein, suggesting that CID1/GLC7 phosphatase together with its regulatory HEX2/REG1 subunit are involved in the dephosphorylation of the Hxk2p monomer. An HXK2 mutation encoding a serine-to-alanine change at position 15 [HXK2 (S15A)] was to clarify the in vivo function of the phosphorylation of hexokinase PII. In this mutant, where the Hxk2 protein is unable to undergo phosphorylation, the cells could not provide glucose repression of invertase. Glucose induction of HXT gene expression is also affected in cells expressing the mutated enzyme. Although we cannot rule out a defect in the metabolic state of the cell as the origin of these phenomena, our results suggest that the phosphorylation of hexokinase is essential in vivo for glucose signal transduction.
Glucose repression is a transcriptional regulatory mechanism in Saccharomyces cerevisiae which permits the cell to adapt its metabolism to the availability of glucose or other fermentable carbon sources (for reviews, see references 14, 21, and 36). Genetic analysis has identified several transcription factors and transcriptional regulators of glucose-repressible genes (22, 25, 31, 35, 44). Furthermore, it has been shown that glucose transport and phosphorylation are involved in triggering glucose signaling (21, 34, 35, 37, 42). However, the mechanism of glucose signaling and the links between the signal and the elements implied in the glucose repression response have not been elucidated yet.
S. cerevisiae contains three glucose-phosphorylating enzymes, hexokinase PI (HXK1), hexokinase PII (HXK2), and glucokinase (GLK1). The HXK2 gene product appears to play an important role in glucose-mediated response (8, 26, 37). Mutations in HXK2 abolish catabolite repression of invertase (8) and other glucose-regulated genes (35), whereas the deletion of the other sugar kinases does not have this effect (37). HXK2 is also required for generation of the induction signal for expression of the HXT gene encoding for hexose transporters (35). The molecular basis of the specific role of Hxk2p in glucose signaling is still unclear. Initially, glucose repression was inversely correlated to the sugar-phosphorylating activity of Hxk2p (26, 37). Nevertheless, overexpression of HXK1, but not of GLK1, restored glucose repression in an hxk2 mutant (37). Recently, it has been shown that the glucose repression of the SUC2 gene may be resolved into two steps: an early repression response which is mediated through any of the three hexose-phosphorylating enzymes present in S. cerevisiae and a long-term response which requires Hxk2p on glucose (40) and either Hxk1p or Hxk2p on fructose (6). Hence, hexokinases should exhibit any property, not shared by glucokinase, that relates them to the mechanism of glucose repression.
Hexokinases can exist in vitro either as dimers or as monomers depending on the binding of glucose and nucleotides (13, 19, 30, 51). In vitro assays also indicate that the hexokinase PII monomer shows a higher Km for glucose than does the dimer (50). The relevance of the Hxk2p dimerization for glucose repression has been questioned since the repression of invertase by glucose occurs in the presence of a truncated Hxk2p derivative (lacking the 15 N-terminal amino acids) that is unable to form a dimer (27).
It has also been reported that both Hxk1p and Hxk2p are phosphoproteins which are predominantly phosphorylated in conditions of derepression (23, 48). The in vivo phosphorylation site has been identified as serine-15, located in a protein kinase A consensus phosphorylation sequence (23). This residue was phosphorylated in vitro by protein kinase A, whereas an alanine-15 mutant protein (S15A) was not. Attempts to correlate this modification with glucose repression of invertase failed (23). However, these experiments were carried out with multicopy plasmids resulting in an unusually high level of hexokinase activity, as was remarked by Kriegel et al. (23). Neither the physiological significance of the in vivo phosphorylation nor the implied protein kinase and protein phosphatase are known.
The reversible phosphorylation of proteins depends on the activities of either protein kinases or protein phosphatases. Both kinases and phosphatases have been shown to play critical roles in regulating glucose repression (3, 45). It is known that protein phosphatase type 1 (PP1) of S. cerevisiae, encoded by the GLC7/CID1 gene (10, 33), is required to maintain glucose repression (45). Cid1p appears to function antagonistically to the kinase Cat1p/Snf1p. Genetic and molecular approaches have led to the identification of PP1 regulatory subunits (12, 46). Hex2p/Reg1p has been demonstrated to bind Cid1p, and it is proposed to direct the activity of Cid1p to the glucose repression pathway (46), although the target of Cid1p activity in the signaling cascade has not been identified yet.
We demonstrate in this work that phosphorylation of Hxk2p is involved in the mechanism underlying the function of hexokinase in glucose response. Mutants containing the S15A substitution prevent phosphorylation in vivo of Hxk2p, lead to derepression of the SUC2 gene in glucose-growing cells, and abolish the glucose-induced HXT gene expression. We also report here genetic evidence that Hxk2p is a target of the protein phosphatase Cid1p. The physiological implications of these results in respect to glucose signaling are discussed.
MATERIALS AND METHODS
Strains and culture conditions.
The yeast strains used in this work are listed in Table 1. Yeast cells were grown in YP rich medium (1% yeast extract, 2% peptone) containing 2% glucose, 2% galactose, 2% maltose, 2% raffinose, 2% sucrose, or 3% ethanol as carbon source. In some experiments yeast cells were grown in minimal medium (0.67% yeast nitrogen base without amino acids [Difco] plus the corresponding carbon source) supplemented with the appropriate concentrations of histidine, tryptophan, and uracil as described by Sherman et al. (41).
TABLE 1.
S. cerevisiae strains used in this study
| Strain | Genotype |
|---|---|
| ENY.WA-1A | MATα ura 3-52 his3-Δ1 leu2-3,112 trp1-289 MAL2-8c MAL3 SUC3 |
| ENY.hex2-3A | MATα ura 3-52 leu2-3,112 hex2-3 MAL2-8c MAL3 SUC3 |
| ENY.cyc8-2D | MATα ura 3-52 his3-Δ1 leu2-3,112 cyc8-20 MAL2-8c MAL3 SUC3 |
| JS88.3-1A | MATα ura 3-52 his3-Δ1 cat4-1 MAL2-8c MAL3 SUC3 |
| WAY.JF1 | MATα ura 3-52 his3-Δ1 cat80::HIS3 MAL2-8c MAL3 SUC3 |
| ENY.cid1-9C | MATα ura 3-52 leu2-3,112 ade2 cid1-226 MAL2-8c MAL3 SUC3 |
| WAY.14-1A | MATα ura 3-52 his3-Δ1 HXK1 hxk2::LEU2 glk1::LEU2 MAL2-8c MAL3 SUC3 |
| WAY.glk1-5C | MATα leu2-3,112 trp1-289 hxk1::HIS3 HXK2 glk1::LEU2 MAL2-8c MAL3 SUC3 |
| WAY.78-1 | MATα ura3-52 leu2-3,112 trp1-289 hxk1::HIS3 hxk2::LEU2 glk1::LEU2 MAL2-8c MAL3 SUC3 |
| W303-1A | MATa ade2-1 his3-11,15 leu-2-3,112 trp1-1 ura3-1 can1-100 GAL mal SUC2 |
| YSH7.4-11A | MATa ade2-1 his3-11,15 leu-2-3,112 trp1-1 ura3-1 can1-100 hxk1::HIS3 glk1::LEU2 GAL mal SUC2 |
| YSH7.4-3C | MATa ade2-1 his3-11,15 leu-2-3,112 trp1-1 ura3-1 can1-100 hxk1::HIS3 hxk2::LEU2 glk1::LEU2 GAL mal SUC2 |
| CEN.PK130-7B | MATα ura3-52 his3-Δ1 leu2-3,112 trp1-289 cat1::HIS3 SUC2 |
Escherichia coli DH10B was grown in Luria Bertani medium (1% peptone, 0.5% yeast extract, 0.5% NaCl).
Preparation of yeast extracts.
Cells were harvested, washed in cold homogenization buffer (10 mM triethanolamine, 0.2 mM EDTA [pH 7.6]), and transferred into a tube containing 0.3 ml of the same buffer and 1.0 g of glass beads (acid washed, 0.4-mm diameter). The protease inhibitors pepstatin (1 μg/ml), leupeptin (0.5 μg/ml), aprotinin (2 μg/ml), and phenylmethylsulfonyl fluoride (1 mM) were added, and the mixture was vortexed 3 times for 1 min each time with 1-min intervals of resting on ice between each mixing. Finally, the crude extract was centrifuged at 18,000 × g (4°C) for 10 min, and the supernatant was used for further analysis.
Electrophoretic analyses.
Polyacrylamide gel electrophoresis (PAGE) of native proteins and sodium dodecyl sulfate (SDS)-PAGE were performed on 7.5 and 10% polyacrylamide gels, respectively, with the buffer systems described by Davis (5) and Laemmli (24). Two-dimensional electrophoresis was carried out by running the samples on a polyacrylamide gel as the first dimension. Then, a gel strip was cut and packed with 1% agarose on the top of an SDS-PAGE stacking gel as the second dimension.
Gel filtration.
Gel filtration was carried out with a Superdex 200 pg column (300 ml; Pharmacia) equilibrated with 50 mM Tris-HCl (pH 7.3). Cells were grown in galactose medium to mid-log phase, and crude extracts were prepared as described above. Samples were further clarified by centrifugation at 10,000 × g (4°C) for 10 min and applied (3.5 ml) onto the column. Fractions (4.8 ml) were collected and analyzed for PAGE mobility and hexokinase activity. A molecular weight marker mixture containing cytochrome c (12.3 kDa), ovalbumin (66 kDa), β-amylase (200 kDa), and apoferritin (443 kDa) was used to generate a standard curve. Chromatography fractions were subjected to hexokinase test as described below.
Immunoblotting and antibodies.
Transfer of proteins to a nitrocellulose membrane was carried out by Western blotting as described by Towbin et al. (43). Hxk2p was detected by sequential incubation with crude polyclonal antibody (1:1,500 dilution) and goat peroxidase-coupled anti-rabbit immunoglobulin G (1:3,000 dilution) or by enhanced chemiluminescence (ECL). ECL detection was performed by probing the membrane with biotinylated anti-Hxk2p (1:1,500 dilution) and subsequent screening with horseradish peroxidase-labeled streptavidin (1:2,000 dilution).
Specific anti-Hxk2p serum was raised in rabbits by sequential immunization with a purified fraction of hexokinase PII. The crude serum was purified by standard protocols (17), and antibodies were biotinylated according to the protein biotinylation module instructions from Amersham (catalog no. RPN2203). Mouse antiphosphoserine monoclonal antibody was from Sigma.
λ-Phosphatase treatment.
Crude extracts obtained as described above were treated with λ-phosphatase (200 U; New England Biolabs) with or without the addition of a phosphatase inhibitor cocktail (5 mM sodium fluoride, 5 mM sodium phosphate [pH 8.0], 10 mM sodium pyrophosphate, 5 mM EGTA, 5 mM EDTA, 0.1 mM sodium orthovanadate). Protein samples (30 μg) were incubated in a final volume of 20 μl for 1 h at 30°C. Incubations at 4°C were carried out in parallel as control assays.
DNA manipulations.
Standard DNA manipulation techniques were carried out as described by Sambrook et al. (38). Restriction enzymes were from Boehringer GmbH (Mannheim, Germany). The Sure Clone ligation kit was from Pharmacia Biotech. Probes for Southern blot analyses were radiolabeled with the random primer labeling kit Ready to Go (Pharmacia Biotech) and [α-32P]dCTP (Amersham).
Transformants.
Yeast cells were transformed by the lithium acetate method (20). E. coli was transformed by electroporation following the manufacturer’s instructions (Eppendorf).
Constructions of plasmids and integrative mutants.
The HXK2 coding region with its corresponding promoter (+1 to −505) was amplified by PCR with S. cerevisiae genomic DNA and the oligonucleotides Hxk2-1 5′CACATTGGATCCTAGAAATGG3′ (BamHI site underlined) and Hxk2-2 5′GATCATAGAATTCATGTTCAC3′ (EcoRI site underlined). The amplified 2.0-kb fragment was subcloned into the pUC18-SmaI plasmid, resulting in plasmid pUC-HXK2(S15).
HXK2 point mutation changing serine to alanine at position 15 (S15A) was achieved by overlap extension (18) with PCR and oligonucleotide primers Hxk2-3 5′CCACAAGCCAGAAAGGGTGCCATGGC3′, including the desired change of T to G (in boldface), and Hxk2-4 (reverse sequence to Hxk2-3). Oligonucleotides Hxk2-1 and Hxk2-2 were used as primers for PCR amplifications with pUC-HXK2(S15) as template in the first step.
The mutated HXK2 PCR product was subsequently cloned into the pUC18-SmaI plasmid, generating the construct pUC-HXK2(S15A). The YIplac204 plasmid (16) was treated with EcoRI and BamHI in order to accommodate the EcoRI-BamHI fragments from plasmids pUC-HXK2(S15) and pUC-HXK2(S15A), resulting in plasmids YIpHXK2(S15) and YIpHXK2(S15A), respectively. Integrative plasmids were digested with Bsu36I or BanII in order to direct the integration to the TRP1 locus of the triple kinase mutant strain WAY.78-1 (ENY.WA background) or YSH7.4-3C (W303 background), respectively. Integrative transformants were selected in glucose minimal medium lacking tryptophan and confirmed by Southern blot analysis.
DNA sequencing.
Inserts present in plasmids YIpHXK2(S15) and YIpHXK2(S15A) were sequenced by the dideoxy nucleotide chain termination procedure (39).
Southern blot analysis.
A TRP1 fragment (EcoRI-PstI) from the YRp7 plasmid was radiolabeled and used as a probe to hybridize genomic DNAs from WAY.78-1 and YSH7.4-3c cells transformed with the integrative plasmids after digestion with XmnI or HindIII.
Enzyme determinations.
External invertase was assayed as described by Gascon and Lampen (15). One unit is defined as the amount of enzyme that is able to release 1 nmol of glucose per min under the assay conditions.
Hexokinase activity was carried out as described by Bergmeyer (2) with fructose as substrate. One unit is defined as the amount of enzyme that is able to produce 1 μmol of NADPH per min under the assay conditions.
β-Galactosidase activity was determined in permeabilized cells grown to mid-log phase as described previously (35).
RESULTS
The dimer-monomer equilibrium of Hxk2p is affected by phosphorylation.
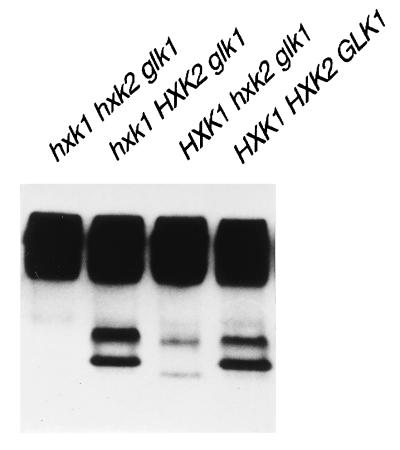
In order to investigate the putative interactions of Hxk2p with other proteins, we analyzed by native gel electrophoresis crude extracts from different glucose phosphorylation mutants grown in galactose medium. Immunoblotting analyses using biotinylated anti-Hxk2p polyclonal antibody allowed the detection of two well-defined specific bands (Fig. 1). In addition, a smear at the top of the blot also appeared, but as this was the only signal present in a triple kinase mutant, hxk1 hxk2 glk1, we assumed that this corresponded to unspecific cross-reaction of the antibody or to biotinylated material present in the crude extract. The polyclonal antibody was also able to recognize Hxk1p. Cells harboring Hxk1p as single hexokinase exhibited two faint bands differing slightly in relative mobility to those observed for Hxk2p. In a wild-type strain the predominant protein detected in galactose medium corresponded to Hxk2p, as based on the relative mobility of the bands.
FIG. 1.
Immunoblot analysis of hexokinases from galactose-grown cells. Protein extracts (30 μg) were separated by 7.5% PAGE, subjected to immunoblotting with biotinylated anti-Hxk2p antibody and detected by using the ECL detection system. Relevant genotypes of the strains are indicated.
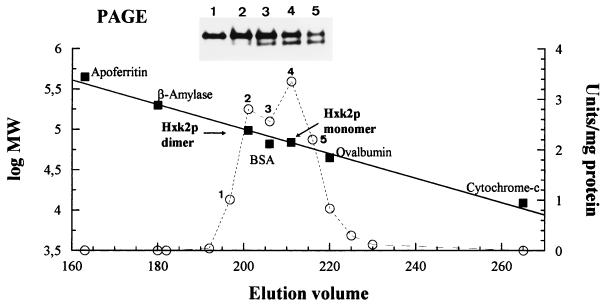
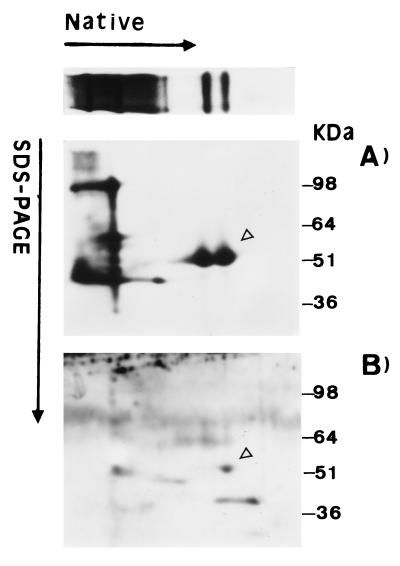
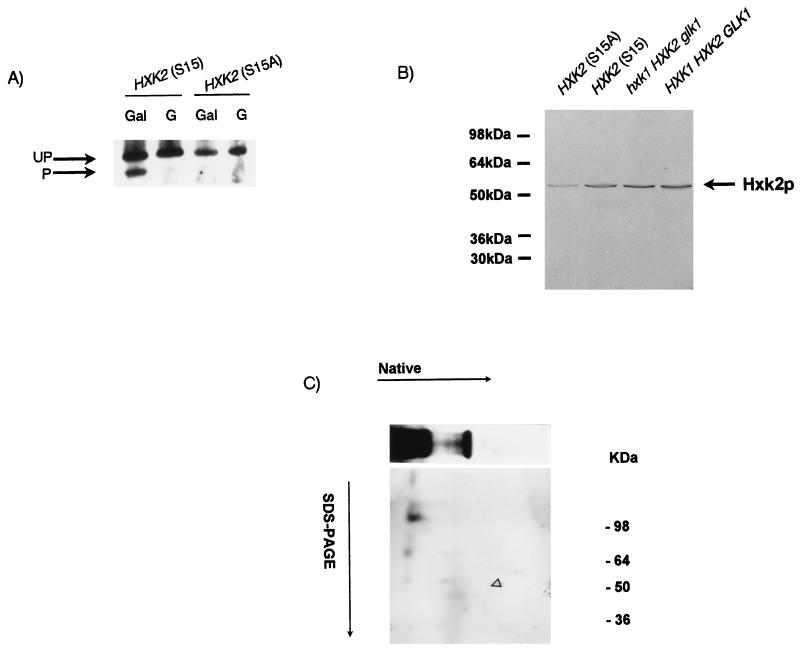
As has been previously reported, Hxk2p exists in vitro as two isoforms, a monomer and a dimer (7, 13). To clarify if the bands observed by immunoblot analysis could be assigned to Hxk2p isoforms, we carried out gel filtration chromatography of a galactose-grown crude extract from WAY.glk1-5C (glk1 hxk1 HXK2) cells, and the corresponding fractions were assayed by PAGE and hexokinase activity. The faster-migrating band in the native blot corresponded to a protein with an apparent size of 65 kDa (Fig. 2, fraction 4), whereas the low-mobility band had an apparent molecular weight of 98 kDa (Fig. 2, fraction 2), although in this case, significant levels of material were also observed in later fractions (e.g., fraction 5). These values were in agreement with the putative presence of a monomer and dimer forms of hexokinase and suggested that the upper band on the PAGE gel could contain a mixture of the dimer and monomer of Hxk2p but that only a monomer would be present in the faster-migrating band. In coincidence with this, hexokinase activity fractionated in a broad peak corresponding to the elution of catalytically active Hxk2p. Furthermore, crude protein extracts from galactose-grown cells were first separated in native gels and then subjected to an SDS-PAGE analysis in the second dimension (Fig. 3A). The immunoblot confirmed that the two defined bands in the native first-dimension analysis each corresponded with Hxk2p subunits. All these results suggested that Hxk2p existed in vivo in a dimer-monomer equilibrium represented in the upper band on the PAGE gel. As mobility in native gels is dependent on both size and charge, the presence of a lower band on the PAGE gel could be attributed to differences in charge between Hxk2p isoforms.
FIG. 2.
Gel filtration chromatography. Results of native gel analysis (PAGE) and corresponding profiles of hexokinase activities of crude extracts from WAY.glk1-5C cells cultivated on galactose are shown. The molecular weights (MW) of the Hxk2p isoforms were calculated from the standard curve. BSA, bovine serum albumin.
FIG. 3.
Identification of Hxk2p isoforms by two-dimensional electrophoresis. A protein crude extract from galactose-grown cells was separated in the first dimension by 7.5% native PAGE. A slice from this gel was electrophoresed in the second dimension on an SDS–10% PAGE gel and immunodetected with biotinylated anti-Hxk2p (A) or antiphosphoserine (B) antibodies. The phosphorylated monomer is indicated by the open arrows.
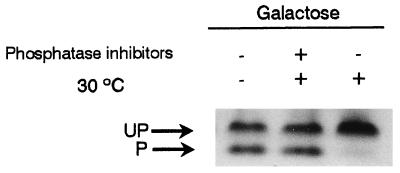
It is known that Hxk2p is a phosphoprotein (23, 48). Accordingly, we investigated by immunodetection if the two PAGE forms of Hxk2p also displayed different phosphorylation states. Western blot analyses, using antiphosphoserine antibody after a two-dimensional electrophoresis, showed a clear spot corresponding with the lower band on the PAGE gel (Fig. 3B). Additionally, incubation at 30°C of a crude protein extract resulted in a drastic change in the relative abundance of the forms of Hxk2p (Fig. 4). The lower band disappeared, whereas a parallel increase of the upper band was observed. The use of phosphatase inhibitors blocked this transition, indicating that it was due to the activity of endogenous protein phosphatases. These results indicated that the lower band was a monomeric phosphorylated isoform of Hxk2p, whereas the upper band could be a mixture of the dimer and monomer but was unphosphorylated.
FIG. 4.
The Hxk2p monomer is a phosphoprotein. Protein extracts (30 μg) from WAY.glk1-5C cells grown in galactose were incubated for 1 h at 30°C in the presence (+) or absence (−) of phosphatase inhibitors. As a control, samples were kept on ice. Proteins were separated and analyzed as described in the legend for Fig. 1. UP, unphosphorylated forms; P, phosphorylated form.
Glucose promotes in vivo dephosphorylation of Hxk2p.
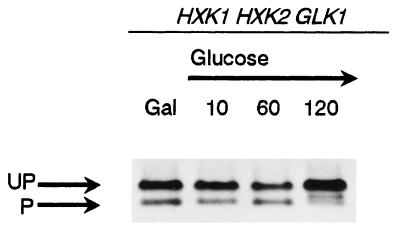
To study if the relative abundance of Hxk2p isoforms was affected by the addition of glucose, wild-type cells grown exponentially in galactose medium, were transferred to glucose-containing medium, and samples were collected at the times indicated (Fig. 5). After 120 min of incubation, the presence of glucose reduced the abundance of the phosphorylated monomer and increased that of the unphosphorylated forms. Similar results were also found when cells were grown on raffinose or ethanol medium and then transferred to glucose-containing medium (data not shown). In order to determine if this change was exclusively carbon source dependent, wild-type cells growing in galactose were subjected to different stress conditions. Neither heat shock nor oxidative or osmotic stress induced a change in the relative abundance of the two bands of Hxk2p (data not shown).
FIG. 5.
Modification of the phosphorylated state of hexokinase upon glucose addition. Wild-type cells (ENY.WA-1A) were grown to mid-log phase on 2% galactose (Gal) medium and then transferred to 2% glucose medium. Samples were taken at the indicated times (min). Proteins (30 μg per lane) were separated and analyzed as indicated in the legend for Fig. 1. UP, unphosphorylated forms; P, phosphorylated form.
Therefore, the in vivo Hxk2p isoforms’ equilibrium was carbon source dependent. Cells growing with less easily fermentable carbon sources showed similar amounts of the unphosphorylated and phosphorylated isoforms, but in the presence of glucose the unphosphorylated Hxk2p isoforms were predominant.
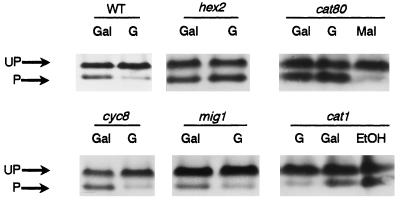
The phosphorylation of Hxk2p is affected by Cid1p/Glc7p but not by Cat1p/Snf1p.
To further investigate the dependency of the Hxk2p isoform changes on glucose, we analyzed this process in different glucose repression mutants (Fig. 6 and 7). When mig1/cat4 or cyc8/ssn6 mutant cells were shifted from galactose to glucose medium, the unphosphorylated forms of Hxk2p were predominant, as in the wild-type strain. However, the transition of the phosphomonomer to the unphosphorylated dimer-monomer forms was abolished in cat80/grr1, hex2/reg1, and cid1/glc7-T152K mutants. It has been reported that in the cat80/grr1 mutant glucose uptake is affected due to a decrease in the expression of various HXT genes encoding for glucose transporters (35, 47). Therefore, the glucose repression defect in this mutant might be a mere corollary of reduced glucose uptake and glycolytic flux. To test this possibility, the transfer experiment was carried out with maltose as inducer of the Hxk2p changes. Indeed, the transition of Hxk2p forms was observed upon maltose addition to galactose-growing wild-type (Fig. 7A) and cat80 (Fig. 6) cells. However, under the same conditions the transition remained absent in cid1/glc7 mutants (Fig. 7A).
FIG. 6.
Hxk2p dephosphorylation in different catabolite repression mutants. Cells grown on galactose (Gal) medium were shifted for 3 h to glucose (G)- or maltose (Mal)-containing medium, as indicated. In the case of the cat1 mutant, cells were cultivated on glucose and then transferred for 4 h to Gal or ethanol (EtOH) medium. Protein samples from wild-type (WT; ENY.WA-1A), mig1/cat4 (JS88.3-1A), cyc8/ssn6 (ENY/cyc8-2D), cat80/grr1 (WAY.JF1), hex2/reg1 (ENY.hex2-3A), and cat1/snf1 (CEN.PK130-7B) cells were treated as described in the legend to Fig. 1. Protein samples were treated as described in the legend for Fig. 1. UP, unphosphorylated forms; P, phosphorylated form.
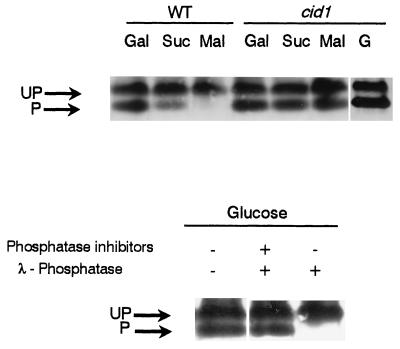
FIG. 7.
Effects of different carbon sources on the Hxk2p phosphorylation in wild-type and cid1 mutant cells (top) and λ-phosphatase treatment of a cid1 protein extract (bottom). Cultures were grown in galactose (Gal) medium and then transferred for 3 h to 2% glucose (G), 2% sucrose (Suc), or 2% maltose (Mal) medium as indicated. (Lower) Protein extracts (30 μg) from cid1 mutant cells grown in glucose were incubated for 1 h at 30°C in the absence (−) or presence (+) of λ-phosphatase and phosphatase inhibitors. Proteins were separated and analyzed as described in the legend for Figure 1. UP, unphosphorylated forms; P, phosphorylated form.
Since maltose promoted the dephosphorylation of Hxk2p in a wild-type strain we tested if this result could be extended to other rapidly fermentable carbon sources. In fact, similar results were found in wild-type cells transferred to sucrose medium. Nevertheless, no change was observed in a cid1 mutant strain (Fig. 7A).
As an independent approach to determining if the results observed in a cid1 mutant were a consequence of the absence of the PP1 function, we treated crude extracts from glucose-grown cid1 cells with λ-phosphatase in the presence or absence of phosphatase inhibitors. Only under the latter condition was the transition of Hxk2p forms observed (Fig. 7B).
These results suggested that Cid1p was involved (directly or indirectly) in the dephosphorylation of Hxk2p. Consistent with this conclusion was the fact that a mutant lacking Hex2p/Reg1p, known to be a regulatory subunit of Cid1p, showed the same Hxk2p isoform pattern as did cid1 mutant cells (Fig. 6 and 7). Since it has been proposed that Hex2p directs the Cid1p activity towards substrates in the glucose repression pathway that are phosphorylated by Cat1p/Snf1p (46), we examined whether the deletion of CAT1/SNF1 affects the Hxk2p monomer-dimer equilibrium. We found that when cat1/snf1 cells were transferred from glucose to galactose or ethanol medium, the phosphorylated monomer of hexokinase appeared (Fig. 6), so CAT1/SNF1 was not involved in the phosphorylation of Hxk2p.
The phosphorylation of hexokinase PII is required for glucose signaling.
Since both Hxk2p and the Cid1p-Hex2p complex are implicated in the glucose response, and in turn the PP1 function was involved in the phosphorylation state of Hxk2p, we tried to clarify the physiological significance of the Hxk2p dephosphorylation in the in vivo function of hexokinase PII.
It is known that the in vivo phosphorylation site of Hxk2p is S15 (23, 48). We constructed integrative plasmids carrying a wild-type copy of the HXK2 gene or a mutant copy encoding a change at residue 15 from serine to alanine (S15A) to avoid phosphorylation. In both cases the protein was expressed under its own promoter in order to keep the same gene regulation conditions. Plasmids were transformed into the triple kinase mutant strains WAY.78-1 (ENY.WA background) and YSH7.4-3c (W303 background), and we examined the effects of this point mutation on in vivo Hxk2p phosphorylation. Figure 8A shows the PAGE pattern of Hxk2p from HXK2(S15) and HXK2(S15A) cells grown on galactose and transferred to glucose. As we expected, cells containing a mutated enzyme, unable to undergo phosphorylation, revealed a single band corresponding with unphosphorylated Hxk2p forms. In contrast, samples from the triple mutant strain WAY.78-1 transformed with the wild-type gene HXK2(S15) exhibited the same bands as we described above (Fig. 6). The absence of phosphorylation in the mutated enzyme was also confirmed by immunodetection with antiphosphoserine antibody after a two-dimensional electrophoresis (Fig. 8C). Furthermore, crude protein extracts from these transformant cells were compared to their wild-type counterparts by Western blot analysis after SDS-PAGE (Fig. 8B). The S15A mutant protein showed the same relative mobility as wild-type Hxk2p.
FIG. 8.
Hxk2p(S15A) is unable to undergo phosphorylation. Triple null hexokinase mutants (WAY.78-1) carrying a single copy of HXK2(S15) or HXK2(S15A) were grown on 2% galactose (Gal) medium and then transferred to 2% glucose (G) medium for 3 h. A total of 30 μg of crude protein extracts was separated by PAGE (A) or by SDS-PAGE (B) and immunodetected with ECL (A) or peroxidase-conjugated antibody (B). (C), Crude protein extracts from galactose-grown transformants [HXK2(S15A)] were separated by two-dimensional electrophoresis and immunodetected with antiphosphoserine antibody. The open arrow indicates the position in which the upper band on the PAGE gel migrates in the second dimension (see also Fig. 3). UP, unphosphorylated forms; P, phosphorylated form.
Since both the wild-type and the mutated enzyme were properly expressed, we further examined the level of invertase activity in these transformants and in different sugar kinase mutants grown on glucose and raffinose (Table 2). Interestingly, when Hxk2p was unable to undergo phosphorylation (S15A), the cells could not provide glucose repression, as showed by the high invertase values obtained under repressing conditions (Table 2). In light of these results, we tested if this repression defect could be extended to other glucose-induced phenomena.
TABLE 2.
Effect of HXK2(S15A) mutation on glucose repressiona
| Relevant genotype | Mean (±SE) invertase activityb
|
|||
|---|---|---|---|---|
| ENY.WA
|
W303
|
|||
| 4% Glucose | 2% Raffinose | 4% Glucose | 2% Raffinose | |
| Wild type | 3.1 ± 1.5 | 399 ± 18 | 3.5 ± 0.9 | 336 ± 12 |
| glk1 hxk1 HXK2 | 4.0 ± 1.9 | 415 ± 10 | 3.3 ± 0.6 | 299 ± 9.8 |
| GLK1 HXK1 hxk2 | 414 ± 25 | 395 ± 9.2 | 436 ± 17 | 413 ± 26 |
| YIpHXK2(S15)c | 3.1 ± 0.2 | 375 ± 30 | 9.4 ± 2.7 | 343 ± 25 |
| YIpHXK2(S15A)c | 203 ± 21 | 372 ± 12 | 258 ± 26 | 280 ± 21 |
Cells were grown on YP medium supplemented with glucose or raffinose as indicated and harvested at the mid-log phase (A600, 1.0 to 1.5).
Values are means of at least three determinations and are expressed as nanomoles of glucose released from sucrose/min/mg (dry weight) of cells.
Integrations were carried out in the triple kinase mutant (glk1 hxk1 hxk2) WAY.78-1 (ENY.WA background) and YSH7.4-3C (W303 background).
HXK2 is required for full induction of HXT genes by both high and low levels of glucose (35). Therefore, we studied the glucose-induced expression of the hexose transporters HXT1, HXT2, and HXT4 in our HXK2(S15) and HXK2(S15A) strains (Table 3). We transformed YIpHXK2(S15) and YIpHXK2(S15A) cells with plasmids pBM2636, pBM2717, and pBM2800 (35), containing the lacZ gene fused to the promoters of the HXT1, HXT2, and HXT4 genes, respectively. Consistent with the high invertase levels showed above, expression of HXT2 and HXT4 in HXK2(S15A) transformants was about fourfold higher in cells grown on 4% glucose in comparison with their HXK2(S15) counterparts, while induction on raffinose was reduced. Induction of the HXT1 gene by high levels of glucose was also abolished in cells with a mutated enzyme (Table 3). Thus, the in vivo phosphorylation of Hxk2p appeared to be required both in the induction and repression mechanisms of hexose transporter genes.
TABLE 3.
Expression of the HXT genes in HXK2 transformantsa
| lacZ reporter | Mean (±SE) β-galactosidase activity (U)b
|
|||||
|---|---|---|---|---|---|---|
| YIpHXK2(S15)
|
YIpHXK2(S15A)
|
|||||
| Gal | Glu | Raf | Gal | Glu | Raf | |
| pHXT1 | 4.8 ± 1.4 | 225 ± 59 | 4.2 ± 1.4 | 1.0 ± 0.6 | 3.4 ± 2.6 | 2.0 ± 1.2 |
| pHXT2 | 19.5 ± 4.9 | 29.8 ± 8.9 | 101 ± 10 | 25.7 ± 7.3 | 123 ± 11 | 80 ± 18 |
| pHXT4 | 15 ± 6.5 | 9.2 ± 5.8 | 81 ± 9.7 | 14.2 ± 6.7 | 36 ± 6.9 | 29.6 ± 11.8 |
Cells were grown on minimal medium (2% galactose), collected, and then transferred to the same medium containing 2% galactose (Gal), 4% glucose (Glu), or 2% raffinose plus 0.1% glucose (Raf). After 4 h of incubation, the expression of HXT genes was measured by determination of β-galactosidase activity.
Values are means of at least three assays of four independent transformants. Activities are expressed in Miller units. Integrations for both YIpHXK2(S15) and YIpHXK2(S15A) were carried out in the same strain (WAY.78-1).
Since the correlation between the sugar-phosphorylating activity of hexokinase II and glucose repression is well documented (26, 37), we were interested in determining the catalytic activity on different carbon sources for wild-type and mutant enzymes. As shown in Table 4, crude extracts from HXK2(S15A) cells grown on glucose exhibited a lower in vitro hexokinase activity than strains expressing the wild-type enzyme. This was also true for cells grown on raffinose or galactose, although the particular level of activity varied for each carbon source and strain tested (Table 4).
TABLE 4.
Hexokinase activity in different hexose kinase mutantsa
| Strain | Relevant genotype | Mean (±SE) sp act (U/mg)b
|
||
|---|---|---|---|---|
| 2% Galactose | 4% Glucose | 2% Raffinose | ||
| ENY.WA-1A | GLK1 HXK1 HXK2 | 2.43 ± 0.31 | 1.38 ± 0.01 | 2.61 ± 0.96 |
| WAY.glk1-5C | glk1 hxk1 HXK2 | 0.94 ± 0.07 | 0.87 ± 0.09 | 1.62 ± 0.47 |
| WAY.78-1 | glk1 hxk1 hxk2 | 0.00 | NDd | ND |
| WAY.78-1 | YIpHXK2(S15)c | 1.15 ± 0.08 | 1.15 ± 0.04 | 2.01 ± 0.36 |
| WAY.78-1 | YIpHXK2(S15A)c | 0.47 ± 0.06 | 0.49 ± 0.05 | 0.63 ± 0.06 |
| W303-1A | GLK1 HXK1 HXK2 | 1.45 ± 0.02 | 1.34 ± 0.15 | 1.43 ± 0.34 |
| YSH7.4-11A | glk1 hxk1 HXK2 | 0.80 ± 0.09 | 0.98 ± 0.10 | 1.45 ± 0.09 |
| YSH7.4-3C | glk1 hxk1 hxk2 | 0.00 | ND | ND |
| YSH7.4-3C | YIpHXK2(S15)e | 0.63 ± 0.06 | 1.39 ± 0.40 | 1.47 ± 0.19 |
| YSH7.4-3C | YIpHXK2(S15A)e | 0.43 ± 0.10 | 0.67 ± 0.09 | 0.86 ± 0.08 |
Cells were grown on YP medium supplemented with galactose, glucose, or raffinose as indicated and harvested at the mid-log phase (A600, 1.0 to 1.5).
Activity in the crude extracts was assayed using fructose as substrate. One unit of activity corresponds to the amount of enzyme that produces 1 μmol of NADPH/min/mg of protein. Values are means of at least three determinations.
Integrations were carried out in the triple kinase mutant (glk1 hxk1 hxk2) WAY.78-1.
ND, not determined (due to the lack of growth of the strain).
Integrations were carried out in the triple kinase mutant (glk1 hxk1 hxk2) YSH7.4-3C.
These results raised the possibility that the glucose-signaling defects observed in the HXK2(S15A) transformant could be the result of a less enzymatically active form of Hxk2p. However, Km determinations for the Hxk2(S15) and Hxk2(S15A) enzymes from glucose extracts did not reveal large differences i.e. 2.1 ± 0.1 mM and 2.7 ± 0.3 mM, respectively. On galactose, the Km value for the wild-type enzyme increased (to 3.7 ± 0.2 mM), probably as a consequence of the variations in Hxk2p isoforms showed above. In consonance with this, no differences were found in the kinetic constants for the mutated enzyme in all media tested. Growing rates for both transformants were also very similar [0.361 and 0.357 h−1 on glucose and 0.272 and 0.277 h−1 on raffinose for HXK2(S15) and HXK2(S15A) transformants, respectively]. Therefore, both forms of Hxk2p displayed similar affinities for their substrate and provided enough sugar-phosphorylating activity to support growth at similar rates.
DISCUSSION
We show in this work that the association-dissociation equilibrium of yeast hexokinase existed in vivo. This equilibrium was affected by phosphorylation-dephosphorylation of the protein in response to carbon source. Phosphorylation of hexokinases had been shown previously (23, 48), but no relationship between this modification and the conformational state of Hxk2p had been established yet. We also show that the Hxk2p phosphoprotein was a monomer that underwent dephosphorylation triggered by glucose addition.
Easily fermentable carbon sources such as glucose, maltose, and sucrose were able to trigger the dephosphorylation of Hxk2p. Conversely, when cells were grown with poorly fermentable or nonfermentable carbon sources like raffinose, galactose, and ethanol both phosphorylated and dephosphorylated forms appeared in similar amounts. These results were consistent with the in vivo 32P-labeling experiments of hexokinases which showed the most extensive phosphorylation in galactose or low-glucose medium (23, 48).
The phosphorylated state of hexokinase was affected by the carbon source, but this dependence was not regulated by the general mechanism of glucose repression. As in wild-type cells, glucose-induced Hxk2p transition was observed in mutants with defects in transcription factors or effectors involved in glucose repression (cyc8/ssn6 [22, 49] and mig1/cat4 [31]), indicating that this effect was not a final result of the glucose repression pathway. However, in mutants with defects affecting the primary steps of the pathway (cat80/grr1 [1, 4, 9], hex2/reg1 [9, 29], and cid1/glc7 [32]), the ratio of Hxk2p phosphorylated forms was not altered by the presence of glucose, indicating that the transition was a specific response to glucose sensing, as we confirmed in a cat80/grr1 mutant using maltose as the inductor of Hxk2p transition. On maltose the dephosphorylation of hexokinases occurred in this mutant, which confirmed that on glucose, cat80/grr1 mutants did not provide sufficient glucose to trigger dephosphorylation because of a reduced glucose uptake.
An important finding was that in cid1/glc7 and hex2/reg1 mutants the dephosphorylation of hexokinase did not take place under any condition studied, suggesting that Hxk2p was a direct or indirect target of the CID1/GLC7 protein phosphatase.
Conversely to the results of previous studies on the functional role of the phosphorylation of Hxk2p (23, 27), our experiments with single-copy plasmids of the HXK2 gene controlled under its own promoter showed that this modification is key in the glucose-signaling pathways. Both glucose repression and glucose-induced expression of hexose transporters and glucose repression of invertase were affected in cells expressing a Hxk2(S15A)-mutated protein, in a similar manner to the defects reported for a null hxk2 mutant (8, 35). This observation was confirmed in different genetic backgrounds.
In vitro hexokinase activity data showed less sugar phosphorylation in crude extracts from HXK2(S15A) cells, raising the possibility that those regulatory defects could be the result of a reduced glucose metabolism. However, growth rate measurements indicated that the in vivo catalytic activity of the Hxk2(S15A) protein was very similar to that of the wild type. It is worth pointing out that previous studies have shown an inverse correlation between growth rate and level of glucose repression, supporting the hypothesis that the catalytic activity of hexokinase PII was correlated to its function in glucose repression (26). Kinetic assessment results also make it unlikely that large differences exist between wild-type and mutant enzymes. The lower in vitro sugar-phosphorylating activity found in HXK2(S15A) strains could be explained at least in part by shelf regulatory defects of the HXK2 gene. Expression studies of hexose kinases have shown that HXK2 is induced by glucose (6, 28). This response could be abolished in HXK2(S15A) transformants in the same way as we demonstrated for HXT genes. In the present study, this possibility was well correlated with some differences observed in the amount of Hxk2p detectable by PAGE and SDS-PAGE in extracts from cells of wild-type and mutant enzyme (Fig. 8). Hence, the phenotype presented in this work for the HXK2(S15A) transformants could be ascribed to a glucose-signaling defect. Although, we cannot rule out completely a metabolic problem as the origin of this phenotype, the evidence presented here leads us to suggest that the lower hexokinase activity values found are a secondary consequence of this signaling defect and not the origin of it.
In contrast to an earlier hypothesis placing Hxk2p as a part of a putative glucose sensor (for review, see reference 42), our results suggest that hexokinase PII is involved in the transduction of glucose signals. In this role, the phosphorylated monomer of Hxk2p would be a key element, being able to receive the signal and to transmit it. How the phosphorylation state of Hxk2p controls the on-off switching of the signal is still an open question. The genetic evidence presented in this work shows that Hxk2p is a direct or indirect target of the protein phosphatase Cid1p-Hex2p complex, suggesting a putative interaction between the Hxk2p phosphorylated monomer and this protein complex. As a result of this interaction downstream elements would be activated in the transmission of the glucose signal. In this model, the phosphate group would likely be critical for controlling the affinity of the interaction site of Hxk2p with the Cid1p-Hex2p complex. By removing the phosphate, the strength of the interaction would be decreased, making the signal transduction weaker. Thus, the Hxk2(S15A) mutant protein would still be able to interact in some manner with such a complex, giving a certain grade of glucose sensitivity (Table 2). By overexpressing the Hxk2(S15A) protein, the interaction could be improved and the glucose repression signal transmitted as previously reported (23, 27).
To make the phosphorylation reversible there clearly must be a specific protein kinase. Our results exclude the possibility that Cat1p/Snf1p could be the kinase of Hxk2p. Phosphorylation of hexokinase by protein kinase A has also been dismissed (48) even though the phosphorylation site (Ser 15) has been located in a protein kinase A consensus phosphorylation sequence. It remains possible that in vivo phosphorylation of hexokinase could be the result of substrate-induced autophosphorylation, as has been investigated previously (11).
We hypothesize that the specific role of hexokinase PII in the glucose response is mediated by phosphorylation-dephosphorylation. Our future efforts will seek to identify downstream targets of Hxk2p and to further clarify the connections between Cid1p-Hex2p and Hxk2p in glucose signal transduction. Experiments to further investigate these issues are now in progress.
ACKNOWLEDGMENTS
We are very grateful to M. Johnston for providing the HXT::lacZ plasmids and to J. M. Thevelein for the yeast strains W303-1A, YSH7.4-11A, and YSH7.4-3C. We thank the members of the K.-D.E. laboratory for helpful discussion.
This work was supported by Conselleria de Educacion y Ciencia, Generalitat Valenciana Project GV-3125/95. F.R.G. is supported by a fellowship from the MEC of Spain.
REFERENCES
- 1.Bailey R B, Woodward A. Isolation and characterization of a pleiotropic glucose repression resistant mutant of Saccharomyces cerevisiae. Mol Gen Genet. 1984;193:507–512. doi: 10.1007/BF00382091. [DOI] [PubMed] [Google Scholar]
- 2.Bergmeyer H U. Methods of enzymatic analysis. 2nd ed. Weinheim, Germany: Verlag Chemie GmbH; 1974. p. 473. [Google Scholar]
- 3.Celenza J L, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. doi: 10.1126/science.3526554. [DOI] [PubMed] [Google Scholar]
- 4.Conklin D S, Kung C, Culbertson M R. The COT2 gene is required for glucose-dependent divalent cation transport in Saccharomyces cerevisiae. Mol Cell Biol. 1993;13:2041–2049. doi: 10.1128/mcb.13.4.2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis B J. Disc electrophoresis. II. Method and application to human serum proteins. Ann NY Acad Sci. 1964;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- 6.De Winde J H, Crauwels M, Hohmann S, Thevelein J M, Winderickx J. Differential requirements of the yeast sugar kinases for sugar sensing in establishing the catabolite-repressed state. Eur J Biochem. 1996;241:633–643. doi: 10.1111/j.1432-1033.1996.00633.x. [DOI] [PubMed] [Google Scholar]
- 7.Easterby J S, Rosemeyer M A. Purification and subunit interactions of yeast hexokinase. Eur J Biochem. 1972;28:241–252. doi: 10.1111/j.1432-1033.1972.tb01907.x. [DOI] [PubMed] [Google Scholar]
- 8.Entian K-D. Genetic and biochemical evidence for hexokinase PII as a key enzyme involved in catabolite repression in yeast. Mol Gen Genet. 1980;178:633–637. doi: 10.1007/BF00337871. [DOI] [PubMed] [Google Scholar]
- 9.Entian K-D, Zimmermann F K. Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet. 1980;177:345–350. doi: 10.1007/BF00267449. [DOI] [PubMed] [Google Scholar]
- 10.Feng Z, Wilson S E, Peng Z Y, Schlender K K, Reiman E M, Trumbly R J. The yeast GLC7 gene required for glycogen accumulation encodes a type 1 protein phosphatase. J Biol Chem. 1991;266:23796–23801. [PubMed] [Google Scholar]
- 11.Fernández R, Herrero P, Fernández E, Fernández M T, López-Boado Y S, Moreno F. Autophosphorylation of yeast hexokinase PII. J Gen Microbiol. 1988;134:2493–2498. doi: 10.1099/00221287-134-9-2493. [DOI] [PubMed] [Google Scholar]
- 12.Frederick D L, Tatchell K. The REG2 gene of Saccharomyces cerevisiae encodes a type 1 protein phosphatase-binding protein that functions with Reg1p and the Snf1 protein kinase to regulate growth. Mol Cell Biol. 1996;16:2922–2931. doi: 10.1128/mcb.16.6.2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furman T C, Neet K E. Association equilibria and reacting enzyme gel filtration of yeast hexokinase. J Biol Chem. 1983;258:4930–4936. [PubMed] [Google Scholar]
- 14.Gancedo J M. Carbon catabolite repression in yeast. Eur J Biochem. 1992;206:297–313. doi: 10.1111/j.1432-1033.1992.tb16928.x. [DOI] [PubMed] [Google Scholar]
- 15.Gascon S, Lampen J O. Purification of the internal invertase of yeast. J Biol Chem. 1968;243:1573–1577. [PubMed] [Google Scholar]
- 16.Gietz R D, Sugino A. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene. 1988;74:527–534. doi: 10.1016/0378-1119(88)90185-0. [DOI] [PubMed] [Google Scholar]
- 17.Goding J W. Monoclonal antibodies: principles and practice. 2nd ed. New York, N.Y: Academic Press; 1986. [Google Scholar]
- 18.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 19.Hoggett G, Kellett G L. Kinetics of the monomer-dimer reaction of yeast hexokinase PI. Biochem J. 1992;287:567–572. doi: 10.1042/bj2870567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ito H, Jukuda K, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnston M, Carlson M. Regulation of carbon and phosphate utilization. In: Brosch J, et al., editors. Gene expression: the molecular and cellular biology of the yeast Saccharomyces. Vol. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 193–28. [Google Scholar]
- 22.Keleher C A, Redd M J, Schultz J, Carlson M, Johnson A D. Ssn6-Tup1 is a general repressor of transcription in yeast. Cell. 1992;68:708–719. doi: 10.1016/0092-8674(92)90146-4. [DOI] [PubMed] [Google Scholar]
- 23.Kriegel T M, Rush J, Vojtek A B, Clifton D, Fraenkel D G. In vivo phosphorylation site of hexokinase 2 in Saccharomyces cerevisiae. Biochemistry. 1994;33:148–152. doi: 10.1021/bi00167a019. [DOI] [PubMed] [Google Scholar]
- 24.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 25.Lutfiyya L L, Johnston M. Two zinc-finger-containing repressors are responsible for glucose repression of SUC2 expression. Mol Cell Biol. 1996;16:4790–4797. doi: 10.1128/mcb.16.9.4790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, Bloom L M, Walsh C T, Botstein D. The residual enzymatic phosphorylation activity of hexokinase II mutants is correlated with glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1989;9:5643–5649. doi: 10.1128/mcb.9.12.5643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma H, Bloom L M, Dakin S, Walsh C T, Botstein D. The 15 N-terminal amino acids of hexokinase II are not required for in vivo function: analysis of a truncated form of hexokinase II in Saccharomyces cerevisiae. Proteins. 1989;5:218–223. doi: 10.1002/prot.340050305. [DOI] [PubMed] [Google Scholar]
- 28.Martínez-Campa C, Herrero P, Ramírez M, Moreno F. Molecular analysis of the promoter region of the hexokinase 2 gene of Saccharomyces cerevisiae. FEMS Lett. 1996;137:69–74. doi: 10.1111/j.1574-6968.1996.tb08084.x. [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto K, Yoshimatsu T, Oshima Y. Recessive mutations conferring resistance to carbon catabolite repression of galactokinase synthesis in Saccharomyces cerevisiae. J Bacteriol. 1983;153:1405–1414. doi: 10.1128/jb.153.3.1405-1414.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayes E L, Hoggett J G, Kellett G L. The binding of glucose to native and proteolytically modified yeast hexokinase PI. Eur J Biochem. 1983;133:127–134. doi: 10.1111/j.1432-1033.1983.tb07437.x. [DOI] [PubMed] [Google Scholar]
- 31.Nehlin J O, Ronne H. Yeast MIG1 repressor is related to the mammalian early growth response and Wilm’s tumour finger proteins. EMBO J. 1990;9:2891–2898. doi: 10.1002/j.1460-2075.1990.tb07479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neigeborn L, Carlson M. Mutations causing constitutive invertase synthesis in yeast: genetic interactions with snf mutations. Genetics. 1987;115:247–253. doi: 10.1093/genetics/115.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohkura H, Kinoshita N, Minatani S, Toda S, Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989;57:997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- 34.Özcan S, Freidel K, Leuker A, Ciriacy M. Glucose uptake and catabolite repression in dominant HTR1 mutants of Saccharomyces cerevisiae. J Bacteriol. 1993;175:5520–5528. doi: 10.1128/jb.175.17.5520-5528.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Özcan S, Johnston M. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol Cell Biol. 1995;15:1564–1572. doi: 10.1128/mcb.15.3.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ronne H. Glucose repression in fungi. Trends Genet. 1995;11:12–17. doi: 10.1016/s0168-9525(00)88980-5. [DOI] [PubMed] [Google Scholar]
- 37.Rose M, Albig W, Entian K-D. Glucose repression in Saccharomyces cerevisiae is directly associated with hexose phosphorylation by hexokinases PI and PII. Eur J Biochem. 1991;199:511–518. doi: 10.1111/j.1432-1033.1991.tb16149.x. [DOI] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanz P, Nieto A, Prieto J A. Glucose repression may involve processes with different sugar kinase requirements. J Bacteriol. 1996;178:4721–4723. doi: 10.1128/jb.178.15.4721-4723.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 42.Thevelein J M, Hohmann S. Trehalose synthase: guard to the gate of glycolysis in yeast. Trends Biochem Sci. 1995;20:3–10. doi: 10.1016/s0968-0004(00)88938-0. [DOI] [PubMed] [Google Scholar]
- 43.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Treitel M A, Carlson M. Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc Natl Acad Sci USA. 1995;92:3132–3136. doi: 10.1073/pnas.92.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tu J, Carlson M. The GLC7 type 1 protein phosphatase is required for glucose repression in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:6789–6796. doi: 10.1128/mcb.14.10.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tu J, Carlson M. REG1 binds to protein phosphatase type 1 and regulates glucose repression in Saccharomyces cerevisiae. EMBO J. 1995;14:5939–5946. doi: 10.1002/j.1460-2075.1995.tb00282.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vallier L G, Coons D, Bisson L F, Carlson M. Altered regulatory responses to glucose are associated with a glucose transport defect in grr1 mutants of Saccharomyces cerevisiae. Genetics. 1994;136:1279–1285. doi: 10.1093/genetics/136.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vojtek A B, Fraenkel D G. Phosphorylation of yeast hexokinases. Eur J Biochem. 1990;190:371–375. doi: 10.1111/j.1432-1033.1990.tb15585.x. [DOI] [PubMed] [Google Scholar]
- 49.Williams F E, Varanasi U, Trumbly R J. The CYC8 and TUP1 proteins involved in glucose repression in Saccharomyces cerevisiae are associated with a protein complex. Mol Cell Biol. 1991;11:3307–3316. doi: 10.1128/mcb.11.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Womack F, Colowick S P. Catalytic activity with associated and dissociated forms of the yeast hexokinases. Arch Biochem Biophys. 1978;191:742–747. doi: 10.1016/0003-9861(78)90415-0. [DOI] [PubMed] [Google Scholar]
- 51.Woolfitt A R, Kellett G L, Hoggett J G. The binding of glucose and nucleotides to hexokinase from Saccharomyces cerevisiae. Biochim Biophys Acta. 1988;952:238–243. doi: 10.1016/0167-4838(88)90121-5. [DOI] [PubMed] [Google Scholar]