Abstract
Trypanosoma cruzi hosts can serve as a source of infection for animals, vectors, and humans, contributing to the establishment of Chagas disease (CD) in a given area. Traditionally, the Department of Córdoba has not been considered a transmission area for CD; however, the report of several acute cases of Chagas disease highlights the importance of studying the dynamics of disease transmission in this region. This study aimed to detect T. cruzi in domestic and wild mammals in the department of Córdoba. In 2017, a cross-sectional descriptive study was conducted in six villages in two municipalities in the department of Córdoba. Blood samples from dogs living in the zones were collected in EDTA vacutainer tubes for domestic mammals. Wild mammals were collected using Sherman and Tomahawk traps and mist nets in crops and peridomiciles. T. cruzi DNA was detected using the kinetoplast DNA (kDNA) variable region and the tandem repeat satellite region of T. cruzi as molecular targets. We sampled 168 dogs and 146 wild mammals. The detected prevalence of T. cruzi was 6.37%; the TcI lineage was found in D. marsupialis, H. anomalus, and one canine. A specimen of D. marsupialis with TcI and TcII lineages was also identified. T. cruzi DNA was detected in domestic and wild animals in the study area, indicating the circulation of the parasite in peridomestic environments. D. marsupialis may represent an important host in maintaining this region's wild and domestic cycle.
Keywords: Trypanosoma cruzi, Chagas disease, Dogs, Wild mammals, Colombia
Graphical abstract
Highlights
-
•
T. cruzi DNA was detected in domestic and wild animals in the studied area.
-
•
TcI is the most frequently identified T. cruzi discrete typing in domestic and wild mammals.
-
•
Mixed infection with TcI/TcII genotypes was detected in a specimen of D. marsupialis.
1. Introduction
Chagas disease (CD) is a zoonotic infection caused by the flagellate parasite Trypanosoma cruzi that affects approximately six million people and causes approximately 12,000 deaths annually (World Health Organization, 2020). T. cruzi is a multi-host parasite that can infect hundreds of mammalian hosts involving domestic and sylvatic habitats and cycles (Jansen et al., 2018; Roque et al., 2013). The domestic cycle involves the presence of domiciled triatomines, domesticated or synanthropic mammals, and humans, while the sylvatic cycle involves sylvatic triatomine species and their infected wild mammalian hosts (Jansen et al., 2015; Rodríguez-Monguí et al., 2019). T. cruzi has been detected in more than 150 species of mammals and marsupials. However, the dog (Canis lupus familiaris) is considered the most critical domestic host of CD (Cantillo-Barraza et al., 2015, 2020; Rodríguez-Monguí et al., 2019), while species such as Didelphis sp. are probably the most important in sylvatic cycles, serving as a link between domestic and sylvatic transmission cycles (Bezerra-Santos et al., 2021; Jansen et al., 2018; Rodríguez-Monguí et al., 2019).
Hosts act as sources of infection, thereby contributing to the establishment of the CD. Therefore, the identification of hosts is an essential topic in the epidemiology of the CD and a key issue in the design of surveillance efforts, especially for multi-host pathogens such as T. cruzi. (Roberts and Heesterbeek, 2003). T. cruzi has high genetic variability, and is currently, divided into seven discrete typing units (DTUs): T. cruzi I (TcI), T. cruzi II (TcII), T. cruzi III (TcIII), T. cruzi IV (TcIV), T. cruzi V (TcV) T. cruzi VI (TcVI) and TcBat. Some DTUs have a predilection for specific hosts and have been associated with specific clinical manifestations and geographic distributions of the disease (Velásquez-Ortiz et al., 2022; Zingales and Macedo, 2023)
In Colombia, T. cruzi infection has been detected mainly in endemic areas (Cantillo-Barraza et al., 2015, 2020, 2021, 2022; Díaz et al., 2015; Rodríguez-Monguí et al., 2019; Rueda et al., 2014). Approximately 2% of the Colombian population is infected with T. cruzi (Cantillo-Barraza et al., 2020).The department of Córdoba, located on the Colombian Caribbean coast, is not considered an endemic region for T. cruzi transmission; however, several acute cases of the disease have been reported by the regional health authorities, suggesting a possible scenario for the transmission of the parasite in rural areas of the department. In 2017, the first acute case of CD in Córdoba was described and diagnosed by microscopy and serologic tests, confirming the presence of autochthonous human cases in the area was confirmed (Tovar et al., 2017). The report of several acute cases of Chagas disease highlights the importance of studying the dynamics of disease transmission in this region. Therefore, this study aims to detect T. cruzi in domestic and wild mammals in the department Córdoba, Colombia. (Rodríguez-Monguí et al., 2019; Tovar et al., 2017).
2. Materials and methods
2.1. Study area
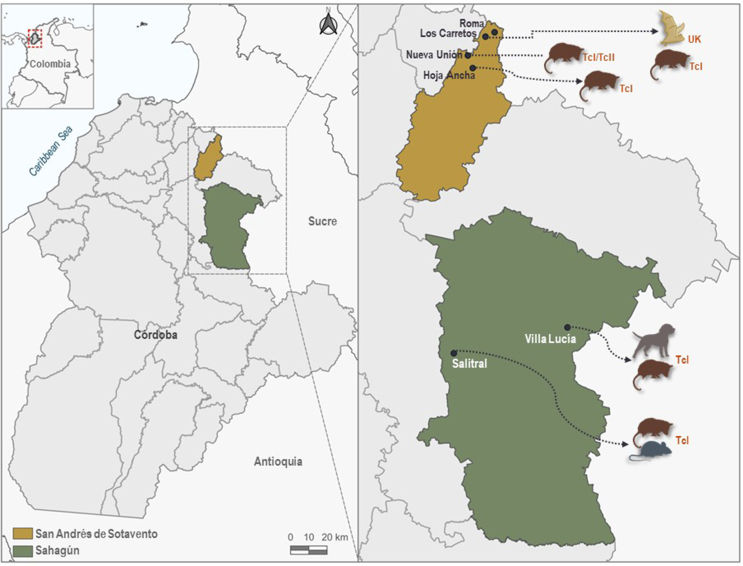
A cross-sectional descriptive study was conducted between October and November 2017 in four villages of the municipality of San Andrés de Sotavento (Hoja Ancha, Los Carretos, Nueva Unión and Roma) and two villages of the municipality of Sahagún (Salitral and Villa Lucía), located in the department of Córdoba on the Colombian Caribbean coast (Fig. 1), characterized by an ecological zone of tropical dry forest. The region has an average annual temperature of 28 °C and variable annual precipitation, averaging 1200 mm (Racero-Casarrubia et al., 2015).
Fig. 1.
Environmental characteristics of Villa Lucia village. a) Palms predominate in the vegetation; b) Crops and annexes adjacent to the house are present in the peridomicile.
These communities were selected because a previous study reported the presence of triatomine, domestic dogs infected with the parasite (Guhl et al., 2007a), and acute cases of Chagas disease were reported in the community of Sahagun (Tovar et al., 2017). The village's environmental characteristics, dominated by palm trees, crops, and houses built with adobe walls and palm roofs (Fig. 2), suggested an environment conducive to disease transmission. In addition, the villagers noted the presence of the disease vector and animals such as rodents and marsupials.
Fig. 2.
Study area and detection of T. cruzi in domestic and wild mammals. Discrete typing units (DTUs): TcI (T cruzi I genotype), TcII (T cruzi II genotype), UK (unknown genotype).
2.2. Domestic mammals and blood sampling
Blood samples were collected from the radial vein in Vacutainer tubes containing EDTA from 168 dogs in six villages, representing ∼36.7% of the total number of dogs (assuming the presence of at least one dog in each house). The collected blood was then mixed with one volume of guanidine hydrochloride EDTA. Dogs were selected at will, with the owner's permission, and after obtaining prior authorization through informed consent and completion of an epidemiologic record.
2.3. Wild animals capture and sample sampling
To capture small terrestrial mammals, 40 Sherman traps and 20 Tomahawk traps were set for three consecutive nights at each site for a trapping effort of 720 Sherman/night and 360 Tomahawk/night, respectively. These traps were strategically placed in grids measuring 20 × 10 m grids and positioned near streams, shrubs, within crops and peridomicile.
To capture bats at each site, five mist nets measuring 6 × 2.6 m were set up between 17:00 and 23:00 h for three consecutive nights at each site for a trapping effort of 540 net*hours/night. These nets were placed inside and at the edge of the forest fragment, along with a mist net near the houses. The trapping activities were carried out over five days in each site.
The terrestrial mammals and bats captured were identified using specialized taxonomic keys (Diaz et al., 2016; Emmons and Feer, 1997; Gardner, 2008; Lopez-Baucells et al., 2016; Patton et al., 2015). Captured animals were anesthetized with ketamine, considering the recommended doses for each taxonomic group, and blood samples were collected by heart puncture (Sikes and The Animal Care and Use Committee of the American Society of Mammalogists, 2018bib_sikes_2018). Blood collected in the EDTA tube was mixed with one volume of guanidine hydrochloride-EDTA. The amount of blood sampled varied according to the body size of each species. After blood collection, bats and small rodents were euthanized (Da Costa et al., 2015). Specimens were deposited at the Zoological Collection of the Universidad de Córdoba, Colombia (CZUC).
2.4. Blood DNA extraction and amplification
DNA extraction from blood and tissue was performed according to the protocol established by the High Pure PCR Template Preparation Kit (Roche Applied Sciences, IN, USA). The DNA solution was stored at −20 °C. Molecular detection of T. cruzi was performed by amplifying the variable region of the minicircle kinetoplast DNA using primers 121 and 122 (Jaimes-Dueñez et al., 2017; Ramirez et al., 2009) and the specific primers for the satellite nuclear repetitive DNA sequence, Cruzi1 and Cruzi2 (Piron et al., 2007). The mini-exon gene (SL-IR) intergenic region was used to distinguish TcI and TcII of DTU (Ramírez et al., 2010). PCR products were visualized by electrophoresis on 2% agarose gels and stained with ethidium bromide.
2.5. Ethical considerations
The Ethics Committee of the University of Sinú approved all the protocols described in this study (Act No 01 of 2017). The welfare of the animals was guaranteed by complying with the standards established for this purpose in Resolution No. 008430 of 1993, issued by the National Ministry of Health of Colombia. The collection of wild animals was supported by Resolution 00914, granted to the University of Cordoba by the National Authority of Environmental Licenses-ANLA. All researchers involved in the study declared their knowledge and understanding of the “Universal Declaration of Animal Rights” established by the International League for Animal Rights, the “Guide for the Care and Use of Laboratory Animals” (NRC, 2010).
3. Results
One hundred sixty-eight dogs were sampled in all six villages, with a mean age of 34.7 ± 28.6 months, a minimum of 3 months, and a maximum of 216 months. Of these, 28.57% were females, 71.43% were males, and 98.8% were of the Creole breed (Table 1). T. cruzi genotype TcI was detected in one dog from the village of Villa Lucía.
Table 1.
Main epidemiological characteristics of the analyzed dogs.
| Age (Months) |
Mean ± SD |
|
|---|---|---|
| 34.7 ± 28.6 | ||
| Age group | n | % |
| Puppy (1–11 mouths) | 21 | 12.50 |
| Adult (12–83 mouths) | 134 | 79.77 |
| Geriatric (≥84 mouths) | 13 | 7.73 |
| Sex | ||
| Female | 48 | 28.57 |
| Male | 120 | 71.43 |
| Breed | ||
| Creole | 166 | 98.80 |
| Mestizo | 2 | 1.20 |
| Villages | ||
| Hoja Ancha | 23 | 13.69 |
| Nueva Unión | 24 | 14.29 |
| Roma | 40 | 23.81 |
| Los carretos | 40 | 23.81 |
| Salitral | 27 | 16.07 |
| Villa Lucia | 14 | 8.33 |
One hundred and forty-six individuals of wild mammals belonging to 14 species of bats (n = 114), three species of rodents (n = 12), and two species of opossums (n = 20) were captured inside crops and peridomicile the houses. The most abundant species was Glossophaga soricina (17.12% of total captures), followed by Didelphis marsupialis (13.01%), Artibeus planirostris (10.95%), Artibeus lituratus (9.58%), Phyllostomus discolor (9.58%), Molossus molossus (8.21%), and Carollia castanea (4.79%). Other species accounted for the remaining 26.76% (Table 2). Overall, T. cruzi was detected in three wild mammal species, D. marsupialis (84.21%, 95% CI = 67.8%–100%), Heteromys anomalus (50%, 95% CI = 1.00%–99%) and Artibeus planirostris (6.25%, 95% CI = 0.00%–18.1%); the global prevalence was (6.37% 95% CI = 3.7%–9.1%). We detected the TcI genotype in 75% of the T. cruzi positive wild mammals: 11 individuals of D. marsupialis and two of H. anomalus. We also observed a mixed infection with TcI/TcII genotypes in one D. marsupialis specimen. Finally, the genotype of the positive bat was unknown (Fig. 1).
Table 2.
Trypanosoma cruzi detection in wilds mammals from Cordoba, Colombia. Data show the following: Positive T. cruzi/total number sampled.
| Taxa | Villages |
||||||
|---|---|---|---|---|---|---|---|
| Hoja Ancha | Los Carretos | Nueva Unión | Roma | Salitral | Villa Lucía | Total | |
| Order Chiroptera | |||||||
| Artibeus lituratus | 0/7 | 0/3 | 0/1 | 0/3 | – | – | 0/14 |
| Artibeus planirostris | 0/6 | 1/5 | 0/1 | – | 0/4 | – | 1/16 |
| Carollia castanea | – | 0/1 | – | – | 0/6 | – | 0/7 |
| Carollia perspicillata | – | 0/4 | – | – | 0/1 | – | 0/5 |
| Desmodus rotundus | – | – | – | – | 0/2 | – | 0/2 |
| Glossophaga soricina | 0/3 | 0/8 | 0/9 | 0/2 | 0/2 | 0/1 | 0/25 |
| Molossops temminekii | 0/2 | – | – | – | – | – | 0/2 |
| Molossus molossus | – | 0/12 | – | – | – | – | 0/12 |
| Myotis nigricans | – | – | – | – | 0/5 | – | 0/5 |
| Phyllostomus discolor | 0/5 | 0/3 | – | 0/5 | – | 0/1 | 0/14 |
| Phyllostomus hastatus | – | – | – | – | 0/3 | – | 0/3 |
| Sturnira sp. | 0/2 | – | – | – | – | 0/1 | 0/3 |
| Uroderma convexum | 0/2 | 0/1 | 0/2 | – | – | – | 0/5 |
| Uroderma magnirostrum | – | 0/1 | – | – | – | – | 0/1 |
| Order Didelphimorphia | |||||||
| Didelphis marsupialis | 2/2 | 1/2 | 5/6 | – | 2/3 | 6/6 | 16/19 |
| Marmosa sp. | – | – | – | 0/1 | – | – | 0/1 |
| Order Rodentia | |||||||
| Heteromys anomalus | 0/1 | 0/1 | – | – | 2/2 | – | 2/4 |
| Proechimys semispinosus | – | – | – | – | 0/1 | 0/1 | 0/2 |
| Zygodontomys brevicauda | – | – | 0/1 | – | – | 0/5 | 0/6 |
| Total | 2/30 | 2/41 | 5/20 | 0/11 | 4/29 | 6/15 | 19/146 |
4. Discussion
The domestic dog is considered an important host of T. cruzi, so in several countries of the Americas, they are used as sentinels of the risk of domestic and peridomestic infection (Ramírez et al., 2013). They are used to determine the exposure to different environmental risks due to their favorable characteristics, such as susceptibility to pathogens, detection of the infectious process, and accessibility for research studies like enumeration, capture, and monitoring allow representative sampling; on the other hand, these animals share habitats with humans and are helpful in detecting changes in prevalence and incidence of infection (Gürtler and Cardinal, 2015). In the present study, we detected the presence of T. cruzi in a dog from the village of Villa Lucía, the site of an acute case. The patient, 20 years old, was diagnosed postmortem and hospitalized with fever of unknown origin. In addition, Panstrongylus geniculatus infected with the parasite was found in the peridomicile of the patient, confirming the presence of all actors involved in the transmission cycle of CD for the department of Córdoba, as reported in the previous study in 2017 (Tovar et al., 2017). Other sampled places, like the municipality of San Andrés de Sotavento, also reported the presence of P. geniculatus and domestic dogs infected by the parasite (Guhl et al., 2007b).
In wild mammals, T. cruzi has been detected in D. marsupialis, H. anomalus and A. planirostris; D. marsupialis is one of the most critical hosts of T. cruzi in the world, especially in South America (Guhl and Ramírez, 2013), favored by its ability to eliminate the parasite in the urine and anal glands being and its highly anthropogenic habits (Bezerra-Santos et al., 2021; Rodríguez-Monguí et al., 2019). D. marsupialis used to invade dwellings and circulate in the peridomicile in close contact with the domestic and wild environment, facilitating the transmission of the parasite by the oral route when contaminating food and domestic utensils (Jansen et al., 2018; Rodríguez-Monguí et al., 2019). In Colombia, T. cruzi presents high infection rates in D. marsupialis with an estimated prevalence of 48.0% (Rodríguez-Monguí et al., 2019); in this study, it was obtained a prevalence by molecular tools of 84.21%, similar to the prevalence described for the species in the northern region of Colombia (85%) (Cantillo-Barraza et al., 2021); this mammal has been implicated in several outbreaks of acute Chagas with possible oral transmission (Rodríguez-Monguí et al., 2019; Rueda et al., 2014; Soto et al., 2014). For the department of Córdoba, D. marsupialis is distributed throughout the territory and associated with intervened and urbanized environments, reflecting the epidemiological importance of this species (Chacón et al., 2022; Rodríguez-Monguí et al., 2019).
In addition, other wild mammals, such as H. anomalus and A. planirostris, with a less known role in the transmission cycle, have also been reported infected with T. cruzi in Colombia and Brazil (Guhl and Ramírez, 2013; Mejía-Jaramillo et al., 2014). Rodents are relevant to public health because some species are host of microorganisms that cause various diseases in humans (Luis et al., 2013; Rodríguez-Monguí et al., 2019), these mammals have high species richness, and some species are widely distributed throughout the world (Patton et al., 2015). In Colombia, T. cruzi presents moderate infection rates in the order Rodentia, with an estimated prevalence of 6.0% (Rodríguez-Monguí et al., 2019).In this study, we also analyzed infections in P. semispinosus and Zygodontomys brevicauda species, and no infection was detected; however, here we report T. cruzi infection in H. anomalus species.
Bats can be an important host for the parasite because they are abundant, well adapted to human environments, and able to fly, leading to a high prevalence of infection in certain areas (Austen and Barbosa, 2021; Carbonara et al., 2023). T. cruzi has been detected in bats from Colombia and other neotropical countries (Lima et al., 2015a, 2015b). In Córdoba, there have been few epidemiologic studies on the relationship between bats and pathogenic microorganisms. Here, we report for the first time the circulation of T. cruzi in a bat species from Córdoba. A. planirostris is a species reported to be a host of parasites of the genus Trypanosoma and Leishmania; these parasites cause thousands of potentially fatal cases per year worldwide (de Oliveira et al., 2015).
The T. cruzi genotype with the highest prevalence and distribution in the Americas is TcI. In Colombia, this genotype circulates in wild and domestic animals and is the most dominant DTU associated with chronic Chagas cardiomyopathy (Díaz et al., 2015; Ramírez et al., 2013). On the other hand, no DTU has been identified in the bat positive. Nevertheless, six DTUs have been reported for Colombia, including the TcBat genotype in bats (Guhl and Ramírez, 2013; Lima et al., 2015a). We found a mixed infection with TcI and TcII in D. marsupialis. This situation has been previously described in wild animals by Jansen and collaborators in Brazil, where they detected mixed infections in 16% of the positive blood cultures and the most common combination was TcI with TcII; they also found mammals with a great diversity of DTUs such as Nasua sp. and Didelphis sp. They related the mixing of the infection to the generalist habits of these species and the inclusion of insects in their diet (Jansen et al., 2015).
Considering this, domestic and wild mammals should be monitored as possible host to prevent disease outbreaks in the context of the ecosystem changes occurring in the region. In Cordoba, some wild mammals are abundant and well-adapted to the anthropized environment. They often use residential areas as refuges in rural and urban zones (Benítez et al., 2021; Chacón et al., 2022).
TcI and TcII were detected in this study. It is possible that a bat with an unknown DTU has other Dtus circulating in Colombia that were not analyzed in this study, or it could even be another species of Trypanosoma, such as Trypanosoma rangeli (Ardila et al., 2022). Despite these limitations, the results of this study provide a valuable epidemiologic base that can serve as a starting point for future research in the region. In our study, T. cruzi was found in domestic and wild animals, suggesting that transmission occurs in the peridomicile. These results highlight the importance of further studies to determine the role of each mammalian species in the transmission cycle in this region. The wild and domestic hosts described here can be used in sentinel surveillance strategies and epidemiological and entomological monitoring to prevent potential disease outbreaks or to establish new transmission scenarios.
Research funding
The Government of Córdoba, the General System of Royalties (SGR), Colombia (Grant No. 754/2013), and the Universidad del Sinú —Elías Bechara Zainúm (UNISINÚ) funded this project.
CRediT authorship contribution statement
Dina Ricardo-Caldera: Writing – original draft, Visualization, Methodology, Conceptualization. Lyda Espitia-Pérez: Writing – review & editing, Writing – original draft, Conceptualization. Paula A. Avilés-Vergara: Writing – review & editing, Visualization, Methodology. Álvaro J. Benítez: Writing – review & editing, Methodology, Data curation. Julio Chacón-Pacheco: Writing – review & editing, Methodology, Data curation. Jesús Ballesteros-Correa: Writing – review & editing, Methodology, Data curation. Ana Negrette-Oquendo: Writing – review & editing, Data curation. Sara Soto-De León: Writing – review & editing, Methodology. Catalina Tovar-Acero: Writing – original draft, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no conflicts of interest, financial and personal, that could have appeared to influence the work reported in this article.
Acknowledgments
The authors would like to thank the Molecular Biology and Biomedical Science Laboratory of UNISINU for supporting the molecular protocols and the people who collaborated in the field phase.
References
- Ardila M.M., Herrera L., Zabala-Monterroza W., Bedoya-Polo A., Lozano-Arias D., García-Alzate Roberto, Pérez-Doria Alveiro. Molecular diagnosis of trypanosomatids in Didelphis marsupialis from Los Montes de María: a first report of Trypanosoma rangeli from Colombian Caribbean region. J. Parasit. Dis. 2022;46:323–327. doi: 10.1007/s12639-021-01459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austen J.M., Barbosa A.D. Diversity and epidemiology of bat trypanosomes: a one health perspective. Pathogens. 2021;10:1148. doi: 10.3390/pathogens10091148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez Á.J., Ricardo-Caldera D., Atencia-Pineda M., Ballesteros-Correa J., Chacón-Pacheco J., Hoyos-López R. DNA barcoding of bats (Chiroptera) from the Colombian northern region. Mammalia. 2021;85:462–470. doi: 10.1515/mammalia-2020-0138. [DOI] [Google Scholar]
- Bezerra-Santos M.A., Ramos R.A.N., Campos A.K., Dantas-Torres F., Otranto D. Didelphis spp. opossums and their parasites in the Americas: a One Health perspective. Parasitol. Res. 2021;120:4091–4111. doi: 10.1007/s00436-021-07072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantillo-Barraza O., Bedoya S.C., Xavier S.C.C., Zuluaga S., Salazar B., Vélez-Mira A., Carrillo L.M., Triana-Chávez O. Trypanosoma cruzi infection in domestic and synanthropic mammals such as potential risk of sylvatic transmission in a rural area from north of Antioquia, Colombia. Parasite Epidemiol Control. 2020;11 doi: 10.1016/j.parepi.2020.e00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantillo-Barraza O., Garcés E., Gómez-Palacio A., Cortés L.A., Pereira A., Marcet P.L., Jansen A.M., Triana-Chávez O. Eco-epidemiological study of an endemic Chagas disease region in northern Colombia reveals the importance of Triatoma maculata (Hemiptera: reduviidae), dogs and Didelphis marsupialis in Trypanosoma cruzi maintenance. Parasites Vectors. 2015;8:482. doi: 10.1186/s13071-015-1100-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantillo-Barraza O., Solis C., Zamora A., Herazo R., Osorio M.I., Garcés E., Xavier S., Mejía-Jaramillo A.M., Triana-Chávez O. Enzootic Trypanosoma cruzi infection by Rhodnius prolixus shows transmission to humans and dogs in Vichada, Colombia. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.999082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantillo-Barraza O., Torres J., Hernández C., Romero Y., Zuluaga S., Correa-Cárdenas C.A., Herrera G., Rodríguez O., Alvarado M.T., Ramírez J.D., Méndez C. The potential risk of enzootic Trypanosoma cruzi transmission inside four training and re-training military battalions (BITER) in Colombia. Parasites Vectors. 2021;14 doi: 10.1186/s13071-021-05018-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonara M., Mendonza-Roldan J.A., Perles L., Alfaro-Alarcon A., Romero L.M., Murillo D.B., Piche-Ovares M., Corrales-Aguilar E., Iatta R., Walochnik J., Santoro M., Otranto D. Parasitic fauna of bats from Costa Rica. Int. J. Parasitol Parasites Wildl. 2023;20:63–72. doi: 10.1016/j.ijppaw.2022.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacón J.J.P., Sánchez-Londoño J.D., Villada-Cadavid T., Ballesteros-Correa J. Update of the list of wild mammals of the department of Córdoba, Colombia. Biota Colomb. 2022;23:e966. doi: 10.21068/2539200X.966. [DOI] [Google Scholar]
- Da Costa A., Borges Costa F., Sousa Soares H., Garcia Ramirez D., De Carvalho Mesquita, Gennari S.M., Marcili A. Trypanosoma cruzi and Leishmania infantum chagasi Infection in Wild Mammals from Maranhao State, Brazil. Vector Borne Zoonotic Dis. 2015;15(11):656–666. doi: 10.1089/vbz.2015.1771. [DOI] [PubMed] [Google Scholar]
- de Oliveira F.M., Costa L.H.C., de Barros T.L., Ito P.K.R.K., Colombo F.A., Carvalho C. de, Pedro W.A., Queiroz L.H., Nunes C.M. First detection of Leishmania spp. DNA in Brazilian bats captured strictly in urban areas. Acta Trop. 2015;150:176–181. doi: 10.1016/j.actatropica.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Díaz M.L., Leal S., Mantilla J.C., Molina-Berríos A., López-Muñoz R., Solari A., Escobar P., González Rugeles C.I. Acute chagas outbreaks: molecular and biological features of Trypanosoma cruzi isolates, and clinical aspects of acute cases in Santander, Colombia. Parasites Vectors. 2015;8:608. doi: 10.1186/s13071-015-1218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M.M., Solari S., Aguirre L.F., Aguiar L.M.S., Barquez R.M. Publicación Especial N° 2, PCMA (Programa de Conservación de los Murciélagos de Argentina) 2016. Clave de identificación de los murciélagos de Sudamérica. [Google Scholar]
- Emmons L.H., Feer F. Neotropical rain forest mammals: a field guide. Second ed. University of Chicago Press; 1997. p. 396. [Google Scholar]
- Gardner A.L. University of Chicago Press; 2008. Mammals of South America, Volume 1: Marsupials, Xenarthrans, Shrews, and Bats. [Google Scholar]
- Guhl F., Aguilera G., Pinto N., Vergara D. Actualización de la distribución geográfica y ecoepidemiología de la fauna de triatominos (Reduviidae: Triatominae) en Colombia. Biomedica. 2007;27:143–162. [PubMed] [Google Scholar]
- Guhl F., Aguilera G., Pinto N., Vergara D. Actualización de la distribución geográfica y ecoepidemiología de la fauna de triatominos (Reduviidae: Triatominae) en Colombia. Biomedica. 2007;27:143–162. [PubMed] [Google Scholar]
- Guhl F., Ramírez J.D. Retrospective molecular integrated epidemiology of Chagas disease in Colombia. Infect. Genet. Evol. 2013;20:148–154. doi: 10.1016/J.MEEGID.2013.08.028. [DOI] [PubMed] [Google Scholar]
- Gürtler R.E., Cardinal M.V. Acta Tropica Reservoir host competence and the role of domestic and commensal hosts in the transmission of Trypanosoma cruzi. Acta Trop. 2015;151:32–50. doi: 10.1016/j.actatropica.2015.05.029. [DOI] [PubMed] [Google Scholar]
- Jaimes-Dueñez J., Triana-Chávez O., Cantillo-Barraza O., Hernández C., Ramírez J.D., Góngora-Orjuela A. Molecular and serological detection of Trypanosoma cruzi in dogs (Canis lupus familiaris) suggests potential transmission risk in areas of recent acute Chagas disease outbreaks in Colombia. Prev. Vet. Med. 2017;141:1–6. doi: 10.1016/j.prevetmed.2017.03.009. [DOI] [PubMed] [Google Scholar]
- Jansen A.M., Xavier S.C.C., Roque A.L.R. Trypanosoma cruzi transmission in the wild and its most important reservoir hosts in Brazil. Parasites Vectors. 2018;11:502. doi: 10.1186/s13071-018-3067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen A.M., Xavier S.C.C., Roque A.L.R. The multiple and complex and changeable scenarios of the Trypanosoma cruzi transmission cycle in the sylvatic environment. Acta Trop. 2015;151:1–15. doi: 10.1016/j.actatropica.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Lima L., Espinosa-Álvarez O., Ortiz P., Trejo-Varón J., Carranza J., Pinto C.M., Serrano M., Buck G., Camargo E., Teixeira M. Genetic diversity of Trypanosoma cruzi in bats, and multilocus phylogenetic and phylogeographical analyses supporting Tcbat as an independent DTU (discrete typing unit) Acta Trop. 2015;151:166–177. doi: 10.1016/j.actatropica.2015.07.015. [DOI] [PubMed] [Google Scholar]
- Lima L., Espinosa-álvarez O., Pinto C.M., Cavazzana M.J., Pavan A.C., Carranza J.C., Lim B.K., Campaner M., Takata C.S.A., Camargo E.P., Hamilton P.B., Teixeira M.M.G. New insights into the evolution of the Trypanosoma cruzi clade provided by a new trypanosome species tightly linked to Neotropical Pteronotus bats and related to an Australian lineage of trypanosomes. Parasites Vectors. 2015;8:1–18. doi: 10.1186/s13071-015-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Baucells A., Rocha R., Bobrowiec P.E.D., Palmeirim J.M., Meyer C.F.J. INPA; Manaus: 2016. Field Guide to Amazonian Bats. [Google Scholar]
- Luis A.D., Hayman D.T.S., O'Shea T.J., Cryan P.M., Gilbert A.T., Pulliam J.R.C., Mills J.N., Timonin M.E., Willis C.K.R., Cunningham A.A., Fooks R., Rupprecht C.E., Wood J.L.N., Webb C.T. A comparison of bats and rodents as reservoirs of zoonotic viruses: are bats special. Proc. R. Soc. A B. 2013;280 doi: 10.1098/rspb.2012.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejía-Jaramillo A., Agudelo-Uribe L., Dib J., Ortiz S., Solari A., Triana-Chávez O. Genotyping of Trypanosoma cruzi in a hyper-endemic area of Colombia reveals an overlap among domestic and sylvatic cycles of Chagas disease. Parasites Vectors. 2014;7:108. doi: 10.1186/1756-3305-7-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC . National Academies Press; Washington, DC: 2010. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- Patton J.L., Pardiñas U.F.J., D’Elía G. University of Chicago Press. The University Of Chicago Press Books; Chicago: 2015. Mammals of South America, Volume 2: Rodents. [DOI] [Google Scholar]
- Piron M., Fisa R., Casamitjana N., López-Chejade P., Puig L., Vergés M., Gascón J., Prat J.G. i, Portús M., Sauleda S. Development of a real-time PCR assay for Trypanosoma cruzi detection in blood samples. Acta Trop. 2007;103:195–200. doi: 10.1016/j.actatropica.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Racero-Casarrubia J., Ballesteros-Correa J., Perez-Torres J. Mamíferos del departamento de Córdoba-Colombia : historia y estado de conservación. Biota Colomb. 2015;16:128–148. [Google Scholar]
- Ramírez J.D., Guhl F., Rendón L.M., Rosas F., Marin J., Morillo C. Chagas cardiomyopathy manifestations and Trypanosoma cruzi genotypes circulating in chronic Chagasic patients. PLoS Neglected Trop. Dis. 2010;4:e899. doi: 10.1371/journal.pntd.0000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J.D., Guhl F., Umezawa E.S., Morillo C.A., Rosas F., Marin-Neto J.A., Restrepo S. Evaluation of adult chronic Chagas' heart disease diagnosis by molecular and serological methods. J. Clin. Microbiol. 2009;47:3945–3951. doi: 10.1128/JCM.01601-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez J.D., Turriago B., Tapia-Calle G., Guhl F. Understanding the role of dogs (Canis lupus familiaris) in the transmission dynamics of Trypanosoma cruzi genotypes in Colombia. Vet. Parasitol. 2013;196:216–219. doi: 10.1016/j.vetpar.2012.12.054. [DOI] [PubMed] [Google Scholar]
- Roberts M.G., Heesterbeek J.A.P. A new method for estimating the effort required to control an infectious disease. Proc. R. Soc. Lond. B Biol. Sci. 2003;270:1359–1364. doi: 10.1098/rspb.2003.2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Monguí E., Cantillo-Barraza O., Prieto-Alvarado F.E., Cucunubá Z.M. Heterogeneity of Trypanosoma cruzi infection rates in vectors and animal reservoirs in Colombia: a systematic review and meta-analysis. Parasites Vectors. 2019;12:1–19. doi: 10.1186/s13071-019-3541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roque A.L.R., Xavier S.C.C., Gerhardt M., Silva M.F.O., Lima V.S., D'Andrea P.S., Jansen A.M. Trypanosoma cruzi among wild and domestic mammals in different areas of the Abaetetuba municipality (Pará State, Brazil), an endemic Chagas disease transmission area. Vet. Parasitol. 2013;193:71–77. doi: 10.1016/j.vetpar.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Rueda K., Trujillo J.E., Carranza J.C., Vallejo G.A. Transmisión oral de Trypanosoma cruzi: una nueva situación epidemiológica de la enfermedad de Chagas en Colombia y otros países suramericanos. Biomedica. 2014;34:631–641. doi: 10.1590/S0120-41572014000400017. [DOI] [PubMed] [Google Scholar]
- Sikes R. The animal Care and use committee of the American society of Mammalogists. 2016 Guidelines Am. Soc. Mammal. Use. Wild Mammals Res. Edu. 2018;97:663–688. doi: 10.1093/jmammal/gyw078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto H., Tibaduiza T., Montilla M., Triana-Chávez O., Suárez D.C., Torres Torres M., Arias M.T., Lugo L. Investigación de vectores y reservorios en brote de Chagas agudo por posible transmisión oral en Aguachica, Cesar, Colombia. Cad. Saúde Pública. 2014;30:746–756. doi: 10.1590/0102-311X00024013. [DOI] [PubMed] [Google Scholar]
- Tovar Acero C., Negrete Peñata J., González C., León C., Ortiz M., Chacón Pacheco J., Monterrosa E., Luna A., Ricardo Caldera D., Espitia-Pérez L. New scenarios of chagas disease transmission in northern Colombia. J. Parasitol. Res. 2017;2017:1–5. doi: 10.1155/2017/3943215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar C., Negrete J., González C., León C., Ortiz M., Chacón J., Monterrosa E., Luna A., Ricardo D., Espitia-pérez L. New scenarios of chagas disease transmission in northern Colombia. J. Parasitol. Res. 2017:1–5. doi: 10.1155/2017/3943215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velásquez-Ortiz N., Herrera G., Hernández C., Muñoz M., David Ramírez J. Discrete typing units of Trypanosoma cruzi: geographical and biological distribution in the americas. Sci. Data. 2022;9 doi: 10.1038/s41597-022-01452-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Chagas Disease.https://www.who.int/health-topics/chagas-disease#tab=tab_1 (American trypanosomiasis) [WWW Document]. URL. [Google Scholar]
- Zingales B., Macedo A.M. Fifteen years after the definition of trypanosoma cruzi DTUs: what have we learned? Life. 2023;13:2339. doi: 10.3390/life13122339. [DOI] [PMC free article] [PubMed] [Google Scholar]