Abstract
Background
‘Definite Neuroborreliosis (NB)’ is diagnosed with the presence of NB-specific symptoms, cerebrospinal fluid (CSF) pleocytosis and an elevated Borrelia Burgdorferi antibody index. However, some diagnostic uncertainties exist. The B-cell chemokine CXCL13 represents an emerging biomarker for the diagnosis and treatment of NB because its intrathecal concentration rises prior to the Borrelia antibody index and drops rapidly after antibiotic therapy. Nevertheless, due to lacking prospective data, a definite CXCL13 cut-off for the diagnosis of NB is still pending.
Objective
Definition of a CSF CXCL13 cut-off for the diagnosis of acute and untreated NB in a prospective study setting.
Design and methods
This multicentre prospective study involved 6 neurological departments treating patients in the Lower Austria district (1.7 million inhabitants). The controls were patients scheduled for a spinal tap but not clinically diagnosed with NB. Demographic data, clinical characteristics and blood counts, as well as inflammatory CSF values and CSF CXCL13-concentration were analysed.
Results
We recruited 440 adult patients, of whom 42 have been diagnosed as having an acute and untreated ‘definite NB’. Three hundred ninety-eight patients were assigned to the control group. The median intrathecal CXCL13 concentration was 2384 pg/ml for patients with NB and 0 pg/ml for controls. The difference was highly statistically significant (P ≤ .001). A CSF CXCL13 cut-off of 271 pg/ml resulted in a sensitivity of 95.2% and a specificity of 97.2% for the confirmation or exclusion of NB.
Conclusion
Based on our results, we propose a CSF CXCL13 cut-off of 271 pg/ml with Euroimmun-Elisa for the diagnosis of acute and untreated NB. Due to its high sensitivity and specificity, CXCL13 is a strong candidate biomarker for routine NB assessment, especially in clinically unclear cases.
Keywords: CXCL13, neuroborreliosis, cut-off, prospective multicentre study
Introduction
Neuroborreliosis (NB) is the most common neuroinfectious arthropod-borne disease in Europe and Northern America 1 and is caused by the spirochaete Borrelia burgdorferi. 2 Weeks or months after the appearance of the pathognomonic erythema chronicum migrans, up to 15% of patients develop infection of the nervous system, typically presenting with meningopolyradiculitis or peripheral nerve palsy. 3
Diagnostics includes the patient history, clinical neurological examination and laboratory tests. ‘Definite NB’ is diagnosed by the presence of NB-specific neurological signs and symptoms, cerebrospinal fluid pleocytosis and an elevated CSF serum antibody index ≥1.6, indicating intrathecal production of Borrelia antibodies. 4 Patients with ‘probable NB’ show NB-specific symptoms, CSF pleocytosis and serum Borrelia antibody production, but intrathecal Borrelia antibody production is absent. 4 However, routinely applied CSF and serum tests can have some diagnostic uncertainties: At the beginning of the disease, the Borrelia antibody index may be falsely negative,1,5-10 whereas CSF pleocytosis1,5 and/or a positive Borrelia antibody index are not specific for acute NB. Moreover, several difficulties arise in the diagnosis of NB, as an intrathecal Borrelia-specific antibody production can persist for years after acute infection,1,5-8,10 chronic inflammatory diseases can lead to false-positive pathogen-specific antibody indices11-13 and unspecific test reactions can occur during an infection with Treponema pallidum.14,15 Thus, there is a need for biomarkers that are highly specific to acute and untreated NB.
One interesting biomarker is CXCL13, a B-cell chemoattracting chemokine that is secreted in response to the interaction of surface proteins of Borrelia burgdorferi with toll-like receptor 2 of monocytes. 16 The increased intrathecal concentration of CXCL13 then triggers the migration of B-cells into the central nervous system and the induction of an immune response. 17 CXCL13, as a biomarker, is particularly promising for the early diagnosis of NB because its intrathecal concentration rises prior to the Borrelia antibody index.6,8,10 In addition, the CXCL13 concentration decreases rapidly after antibiotic therapy,1,5,7,9,10,17 making it also a suitable biomarker for monitoring responses to antibiotics. The sensitivity of CXCL13 for the diagnosis of NB varies, depending on the study, between 75% and 100%, and the specificity varies between 63% and 99.7%.1,5,7-10,17-27
Elevated CXCL13 concentrations have been also found in patients with HIV,1,28 CNS lymphoma,8,29 chronic inflammatory demyelination9,28 and other infectious CNS diseases.8-10,28 However, due to a lack of prospective data, a definitive CXCL13 cut-off with the best sensitivity and specificity for the exclusion or confirmation of untreated NB is still pending. The aim of the present large-scale multicentre prospective study is to provide evidence that CXCL13 is a sensitive and specific biomarker for the diagnosis of acute and untreated NB in adults.
Material and methods
We conducted a multicentre prospective study in Austria, specifically in the Lower Austria district (1.7 million inhabitants). All 6 neurological departments within the district participated in the study. The recruitment phase lasted between January 2019 and January 2022. The study included adult patients with acute and untreated ‘definite NB’ and adult control group participants: ‘Definite NB’ was diagnosed by the presence of NB-specific neurological symptoms, cerebrospinal fluid pleocytosis >5/mcl and an elevated CSF to serum antibody index ≥1.6 (according to criteria of the German Society of Neurology [DGN] 4 ). The controls consisted of patients in whom a spinal tap was indicated and who have not been clinically diagnosed with NB. Table 1 shows the inclusion and exclusion criteria for NB- and control-patients. The DGN-criteria for diagnosis of NB were chosen, as they are commonly used in Austria. NB-guidelines from Europe and North America are quite similar regarding clinical features and diagnostic requirements, 30 which facilitates the translation of our results into other guidelines.
Table 1.
Inclusion and exclusion criteria for NB and control patients; NB = neuroborreliosis.
| Inclusion criteria for Patients with NB | Exclusion criteria Patients with NB |
| 1. Clinical symptoms of NB 2. Ability to consent 3. A signed consent form |
1. Spinal tap contraindicated 2. Antibiotic therapy within the last month (as it can lead to a rapid reduction in CXCL13 concentration) 3. Inability to consent |
| Inclusion criteria for control subjects | Exclusion criteria for control subjects |
| 1. All other diseases or conditions, which clinically indicate spinal tap 2. Ability to consent 3. A signed consent form |
1. Spinal tap contraindicated 2. Antibiotic therapy within the last month 3. Inability to consent |
In addition to demographic data and clinical characteristics, blood count as well as inflammatory cerebrospinal fluid (CSF) values and intrathecal CXCL13-concentration were collected for patients with NB and control subjects. The Numeric Pain Rating Scale (NRS) and Borrelia antibody index were only determined in patients with NB. The NRS ranges from 0 to 10, with no pain at 0 and maximum pain at 10 points.
The intrathecal CXCL13 concentration was centrally evaluated at the Clinical Institute for Hygiene and Microbiology at University Hospital St. Poelten. CSF samples from other locations were sent to St. Poelten for this purpose. The CXCL13 concentration was measured using the CXCL13 enzyme-linked immunosorbent assay (ELISA) kit from EUROIMMUN AG (Luebeck, Germany). Depending on the kit, the highest values to be measured varied between 470 and 500 pg/ml. Concentrations above these cut-off levels were measured again by diluting samples by 1:10 or 1:100 to get precise results. EUROIMMUN AG recommends the interpretation of CXCL13 results as follows: Normal range: <20 pg/ml, borderline range: ≥20 to <30 pg/ml, increased: ≥30 - ≤100 pg/ml and strongly increased: >100 pg/ml.
The IgG-Borrelia antibody index was tested via Elisa from Siemens Healthcare Diagnostics GmbH.
Due to the prospective setting of our study, potential sources of error (e.g. interruption of the cold chain, long storage periods, previous antibiotic therapy) leading to adulterated results have been minimized.
Statistics
An estimate of the required sample size was made prior to the start of the study. A preliminary study showed that about one-sixth of the patients screened in a hospital in Lower Austria actually have definite NB. 31 A conservative assumption of 10% prevalence was used to estimate the sample size. With a cut-off of 200 pg/ml, we cautiously assumed a sensitivity of 90% and a specificity of 80% to account for the variability observed in the preliminary study with 100% sensitivity and 92.4% specificity with a small sample size. 31 The maximum acceptable width of the 95% confidence interval for the sample size estimate was set to 10%. Under these conditions, the minimum sample size required was estimated to be 346. 32 A total of 440 patients were included in the study.
Statistical analyses of the study data were performed using the free software environment for statistical computing (Gnu R, version 4.2.1). The Shapiro-Wilk test was used to test the normal distribution of the underlying sampled populations. As the underlying data did not follow a normal distribution, the differences in the distributions of CXCL13 concentrations between NB and control group were verified using a two-sided Wilcoxon Rank Sum test. An optimal cut-off point to distinguish between the two groups, based on CSF CXCL13 concentrations, was determined by using the receiver operator characteristic (ROC) curve. The optimal cut-off point was estimated by maximising the Youden Index (hereafter referred to as J for the sake of compact notation), where J = sensitivity + specificity – 1. 33 The values of J range between 0 and 1. A value of J = 1 means that the procedure is perfectly specific and sensitive. A value of J = 0 means that the probability of correctly classifying the two groups is equivalent to a coin toss. The ROC analysis was performed using the Gnu R package ‘cutpointr’ (version 1.1.2).
As all the data samples analysed contained outliers and were not normally distributed, as verified by the Shapiro-Wilk test, thus the hypothesis tests in Table 3 were performed using the Wilcoxon rank sum test. Because of the multiple testing, the significance level was adjusted using Benjamini & Hochberg correction. Relationships between two interval-scaled data samples were quantized using Pearson’s correlation, and the correlation ratio was used for relationships between a nominally scaled and an interval-scaled data sample, cf. Table 4.
Table 3.
Intrathecal CXCL13 concentration, CSF cell count, Borrelia antibody index and cerebrospinal fluid protein in NB and control group patients; IQR = interquartile range; Wilcoxon rank sum test, adjustment of significance level for multiple testing by Benjamini & Hochberg correction. Correlation of intrathecal CXCL13 concentration with other parameters.
| Neuroborreliosis,= N = 42 | Control, N = 398 | Overall, N = 440 | P-value | ||
|---|---|---|---|---|---|
| CXCL13 concentration pg/ml | Median (IQR) | 2384 (795, 9010) | 0 (0, 8) | 0 (0, 16) | (P ≤ .001) |
| Range | 35, 53000 | 0, 4822 | 0, 53000 | ||
| CSF cell count/µl | Median (IQR) | 118 (53, 258) | 2 (1, 6) | 3 (1, 11) | (P ≤ .001) |
| Range | 10, 523 | 0, 31861 | 0, 31861 | ||
| Borrelia antibody index | Median (IQR) | 12 (6, 40) | 0 (0, 0) | 0 (0, 0) | (P ≤ .001) |
| Range | 2, 413 | 0, 107 | 0, 413 | ||
| Unknown | 4 | 111 | 115 | ||
| CSF protein mg/dl | Median (IQR) | 105 (78, 136) | 41 (32, 56) | 43 (32, 63) | (P ≤ .001) |
| Range | 38, 1282 | 0, 600 | 0, 1282 | ||
| Unknown | 0 | 1 | 1 |
Table 4.
Correlation of intrathecal CXCL13 concentration with other parameters in NB patients: Gender to CXCL13 by correlation ratioꜛ, the others by pearson correlation coefficient* (strong correlation >.8).
| CXCL13 | |
|---|---|
| Age* | .194 |
| Time between symptom onset and spinal tap (days)* | −.041 |
| CSF cell count* | .295 |
| Borrelia antibody index* | −.056 |
| CSF-protein count* | .715 |
| Genderꜛ | .106 |
Results
Demographic data and final diagnoses
Overall, 440 adult patients were included in our study: 42 had a final diagnosis of acute and untreated ‘definite NB’ and 398 were assigned to the control group (Table 2). The mean age of patients with NB was 58 years vs 51 years in the control group. Fifty-seven percent of the patients with NB were male, compared to 44% in the control group (Table 2).
Table 2.
Demographic data and final diagnoses; age: mean, standard deviation; AIDP = acute inflammatory demyelinating disease, CIDP = chronic inflammatory demyelinating disease, CIS = clinical isolated syndrome, NB = Neuroborreliosis.
| N | Age (mean +/− SD) | Male | Female | ||
|---|---|---|---|---|---|
| Neuroborreliosis | 42 | 58 ± 17 | 24 (57%) | 18 (43%) | |
| Control | 398 | 51 ± 18 | 175 (44%) | 223 (56%) | |
| Other, including epilepsy | 85 | 52 ± 16 | 30 (35%) | 55 (65%) | |
| Headache, other than meningitis | 61 | 48 ± 18 | 20 (33%) | 41 (67%) | |
| Idiopathic facial nerve palsy | 53 | 47 ± 20 | 35 (66%) | 18 (34%) | |
| Multiple Sclerosis/CIS | 49 | 38 ± 13 | 14 (29%) | 35 (71%) | |
| Peripheral nerve disorders, other than AIDP/CIDP | 27 | 55 ± 15 | 16 (59%) | 11 (41%) | |
| Meningitis/Encephalitis | 26 | 47 ± 19 | 13 (50%) | 13 (50%) | |
| Other cranial nerve disorders | 23 | 64 ± 18 | 13 (57%) | 10 (43%) | |
| Cerebrovascular diseases | 20 | 63 ± 18 | 10 (50%) | 10 (50%) | |
| Musculoskeletal disorders | 19 | 58 ± 20 | 6 (32%) | 13 (68%) | |
| Dementia | 14 | 65 ± 11 | 6 (43%) | 8 (57%) | |
| AIDP/CIDP | 13 | 60 ± 20 | 8 (62%) | 5 (38%) | |
| Probable NB | 6 | 63 ± 16 | 3 (50%) | 3 (50%) | |
| B-cell lymphoma | 2 | 63 ± 23 | 1 (50%) | 1 (50%) | |
| All | 440 | 51.8 ± 18 | 199 (45%) | 241 (55%) |
In the control group (n = 398), the most common final diagnoses beside ‘others’ were ‘headache, other than meningitis’ (n = 61), ‘idiopathic facial nerve palsy’ (n = 53) and ‘multiple sclerosis’ (n = 49) (Table 2). Detailed information regarding the final diagnoses and corresponding CSF results is provided in Table 6 (Supplementary Material).
Presenting neurological signs and symptoms of patients with NB
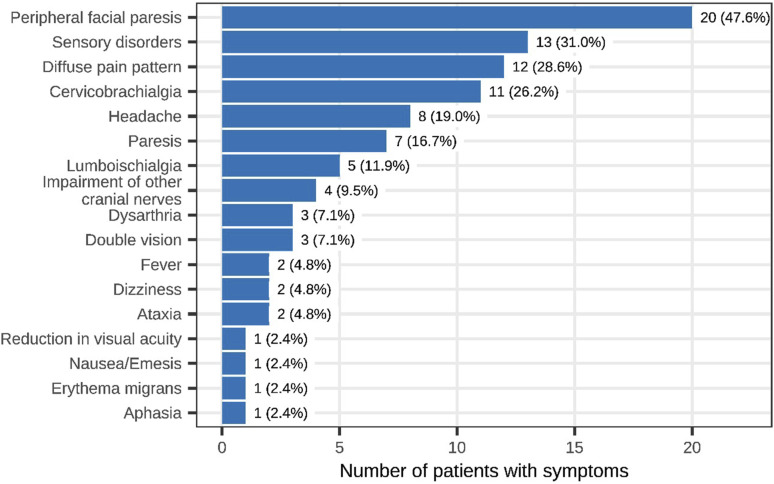
Presenting signs and symptoms included facial nerve palsy in 20 of the 42 patients with NB (48%), sensory deficits in 13 (31%), diffuse pain in 12 (29%) and cervicobrachialgia in 11 patients (26%) (Figure 1). Data regarding pain was collected from 15 patients with NB was collected. The median NRS score was 3 points, with a minimum of 0 and a maximum of 10 points. Six patients (40%) had no pain at all, while 6 other patients reported pain scores greater than 5 points on the NRS.
Figure 1.
Clinical signs and symptoms of NB patients (n = 42).
CSF laboratory findings for NB vs controls
The median intrathecal CXCL13 concentration of patients with NB was 2384 pg/ml, with a minimum of 35 and a maximum of 53000 pg/ml. In the control group, the median CXCL13 concentration was 0 pg/ml (min. 0, max. 4822 pg/ml, Table 3). CXCL13-concentrations, CSF cell counts, Borrelia antibody-indices and cerebrospinal fluid protein levels between the NB and control groups were significantly different(P ≤ .001, Table 3).
Correlation of intrathecal CXCL13 concentration with other parameters
The Pearson correlation coefficient was estimated between CXCL13 and the parameters age, time between symptom onset and spinal tap, CSF cell count, Borrelia antibody index and CSF-protein count as well as the correlation ratio between CXCL13 and gender. We assume a strong correlation from a value >.8. No strong correlations were observed between intrathecal CXCL13-concentration and age (.194), gender (.106), time between symptom onset and spinal tap (−.041), CSF cell count (.295), Borrelia antibody index (−.056) or CSF-protein count (.715) (Table 4).
CXCL13 cut-off for the diagnosis of NB
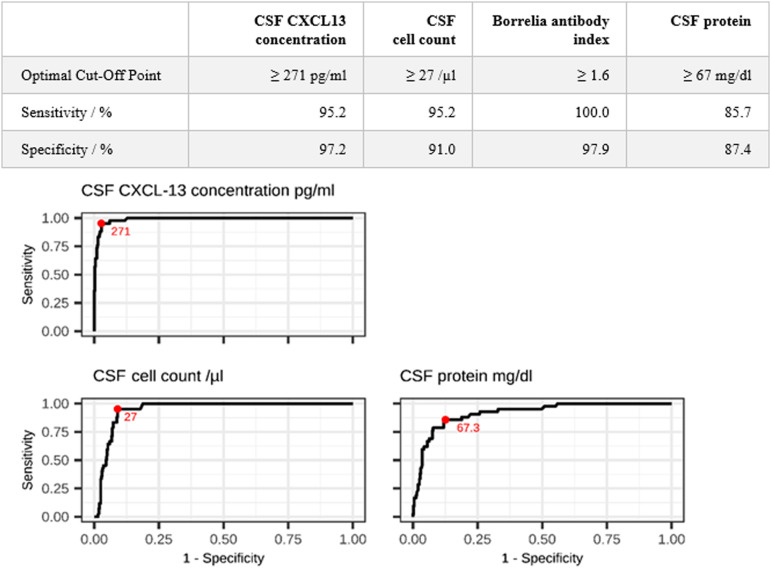
Using a CSF CXCL13 cut-off of 271 pg/ml for the confirmation or exclusion of NB resulted in a sensitivity of 95.2% and a specificity of 97.2% (Figure 2). We achieved 100% sensitivity with a CXCL13 cut-off of 35 pg/ml and 100% specificity with a cut-off of 1900 pg/ml (with a loss of either specificity or sensitivity, respectively).
Figure 2.
Cut-offs and Receiver operating curves (CSF CXCL13 concentration, CSF cell count, Borrelia antibody index, CSF protein) for the diagnosis of NB; optimal cut-off highlighted in red.
A cell count cut-off of 27 cells/µl resulted in a similar sensitivity to that achieved using a CXCL13 cut-off of 271 pg/ml, but the specificity was lower (91% vs 97.2%).
The Borrelia antibody index cut-off of 1.6 yielded a 100% sensitivity and a 97.9% specificity. 100% sensitivity is required, as an antibody index >1.5 is the key criterion for definite NB in our study.
A CSF protein cut-off of 67 mg/dl resulted in a sensitivity of 85.7% and a specificity of 87.4% for confirmation or exclusion of NB (Figure 2).
CSF Laboratory results in Control Group
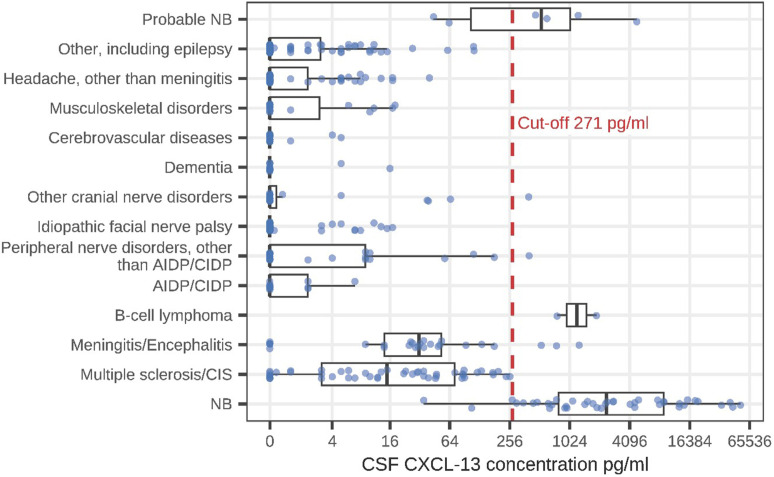
The highest intrathecal CXCL13 concentrations were detected in patients with definite NB (median 2384 pg/ml; Figure 3).
Figure 3.
Comparison of intrathecal CXCL13 concentrations between NB and other diagnoses (boxplots: median, Q1 =25th percentile, Q3=75th percentile, minimum =Q1-1.5*IQR, maximum =Q3 +1.5*IQR).
Patients with multiple sclerosis had a median CXCL13 concentration of 15 pg/ml. Their highest CXCL13 concentration (257 pg/ml) was below our CXCL13 cut-off of 271 pg/ml. The difference in the CXCL13-concentrations between patients with NB and those with multiple sclerosis was highly statistically significant (P < .001).
Apart from the patients with NB, only 7 patients with other diagnoses had an intrathecal CXCL13 concentration above our cut-off of 271 pg/ml; these were 2 patients with B-cell lymphoma (CXCL13 concentrations 1898 pg/ml and 764 pg/ml), 1 patient with neurosarcoidosis (1272 pg/ml), 1 patient with IgG-4 autoimmune encephalitis (753 pg/ml), 1 patient with viral meningitis (530 pg/ml), 1 patient with neuritis of the brachial plexus (400 pg/ml) and 1 patient with idiopathic abducens paresis (396 pg/ml) (Figure 3).
Discussion
Based on the results of this prospective multicentre study, we propose a CSF CXCL13 cut-off of 271 pg/ml using the Elisa kit of Euroimmun, which yields a sensitivity of 95.2% and a specificity of 97.2% for the diagnosis of acute and untreated NB. The high sensitivity and specificity of CXCL13 make this biomarker a prime candidate for the routine assessment of acute NB, especially in clinically unclear situations.
A Borrelia antibody index higher than 1.5, combined with typical neurological signs and symptoms and an elevated CSF cell count, represents the current diagnostic criteria for NB, according to the DGN. 4 However, diagnostic uncertainties arise in particular cases. For example, at the beginning of the disease, the Borrelia antibody index may be a false negative,1,5-10 which could lead to a mistaken exclusion of NB. Similarly, a positive Borrelia antibody index can persist for years after recovery from NB.1,5-8,10 Therefore, a positive Borrelia antibody index does not automatically indicate acute NB. In addition, certain chronic inflammatory diseases, such as multiple sclerosis, can lead to false positive results for pathogen-specific antibody indices.11-13 To overcome these potential uncertainties the value of CXCL13 as an additional biomarker for the diagnosis of acute and untreated NB was investigated.
Previous studies have already shown a high sensitivity for CXCL13 of 75%-100% and a specificity of 63%-99.7% for the diagnosis of NB.1,5,7-10,17-27 However, the quality of these studies is limited due to their primarily small sample sizes and their predominantly retrospective study design. Moreover, the results of these studies also show a broad range of CXCL13 levels, which could possibly reflect that the measured CXCL13 concentration depends on storage duration, 8 antibiotic use prior to the spinal tap 1,5,7,9,10,17 and an uninterrupted cold processing chain. 26 These are all features that are difficult to control in a retrospective setting.
A high statistically significant difference was detected in the CXCL13 concentration between patients with NB and the control group. However, a total of 7 patients with elevated CXCL13 levels above the cut-off of 271 pg/ml in the control group were identified (Figure 3, Table 5): Two patients with B-cell Lymphoma had highly elevated CXCL13 concentrations (1330 pg/ml), which has been reported in earlier studies.8,29 Our study adds new data to elevated intrathecal CXCL13 concentration in 1 patient with Neurosarcoidosis (1272 pg/ml) and 1 patient with IgG-4 autoimmune encephalitis (753 pg/ml). One patient with suspected viral meningitis and one with brachial plexus neuritis also had elevated CXCL13 concentration and elevated CSF cell count, but a negative Borrelia antibody index (Figure 3, Table 5). Another patient with idiopathic abducens paresis had a normal CSF cell count, but an elevated CXCL13 concentration and a Borrelia antibody index of 1.08 (Figure 3, Table 5). In the latter 3 cases, as the Borrelia antibody index as well as CSF cell count can give false negatives in early NB,1,5-10,34 a second spinal tap test may have revealed a rising antibody index in order to confirm or exclude NB. However, a second spinal tap test was not performed in these 3 patients.
Table 5.
CXCL13-concentration in the cohorts; 1 = neuroborreliosis, 2 = multiple sclerosis, 3 = meningitis/encephalitis, 4 = b-cell lymphoma, 5 = AIDP/CIDP, 6 = peripheral nerve disorders, other than AIDP/CIDP, 7 = idiopathic facial nerve palsy, 8 = other cranial nerve disorders, 9 = dementia, 10 = cerebrovascular diseases, 11 = musculoskeletal disorders, 12 = headache, other than meningitis, 13 = other, including epilepsy, 14 = probable neuroborreliosis.
| 1 (NB) | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (N = 42) | (N = 49) | (N = 26) | (N = 2) | (N = 13) | (N = 27) | (N = 53) | (N = 23) | (N = 14) | (N = 20) | (N = 19) | (N = 61) | (N = 85) | (N = 6) | ||
| CSF CXCL13 concentrat. Pg/ml | Mean (SD) | 8270 (12800) | 47 (65) | 132 (289) | 1330 (802) | 1 (2) | 30 (84) | 2 (4) | 24 (83) | 2 (4) | .5 (1) | 3 (6) | 3 (6) | 5 (18) | 1210 (1820) |
| Median [Q1, Q3] | 2380 [795, 9010] | 15 [3, 72] | 32 [14, 53] | 1330 [1050, 1610] | 0 [0, 2] | 0 [0, 9] | 0 [0, 0] | 0 [0, .3] | 0 [0, 0] | 0 [0, 0] | 0 [0, 4] | 0 [0, 2] | 0 [0, 3] | 535 [163, 1090] | |
| Min, max | 35, 53000 | 0, 257 | 0, 1270 | 764, 1900 | 0, 7 | 0, 400 | 0, 17 | 0, 396 | 0, 16 | 0, 5 | 0, 18 | 0, 40 | 0, 112 | 44, 4820 | |
| CSF cell count/µl | Mean (SD) | 167 (136) | 10 (13) | 1440 (6210) | 19 (8) | 5 (13) | 15 (38) | 4 (11) | 16 (46) | 2 (1) | 2 (2) | 2 (2) | 4 (11) | 4 (5) | 155 (249) |
| Median [Q1, Q3] | 118 [52, 258] | 8 [3, 13] | 142 [37, 386] | 19 [15, 21] | 1 [1, 2] | 2 [2, 4] | 2 [1, 4] | 2 [1, 10] | 2 [1, 2] | 2 [1, 2] | 2 [1, 3] | 2 [1, 4] | 2 [1, 4] | 53 [21, 122] | |
| Min, max | 10, 523 | 0, 86 | 3, 31900 | 13, 24 | 0, 49 | 0, 163 | 0, 77 | 1, 222 | 0, 4 | 0, 9 | 0, 7 | 0, 81 | 0, 40 | 13, 654 | |
| Borrelia antibody index | Mean (SD) | 37 (71) | .2 (.8) | .1 (.3) | 0 (0) | .2 (.4) | .1 (.3) | .5 (2) | .3 (.6) | 0 (0) | .2 (.5) | .3 (.5) | 0 (0) | 2 (13) | .7 (.2) |
| Median [Q1, Q3] | 12 [6, 40] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, 0] | 0 [0, .4] | 0 [0, 0] | 0 [0, 0] | 0 [0, .2] | 0 [0, 0] | 0 [0, 0] | .7 [.5, 1] | |
| Min, max | 2, 413 | 0, 5 | 0, 1 | 0, 0 | 0, 1 | 0, 1 | 0, 9 | 0, 2 | 0, 0 | 0, 2 | 0, 1 | 0, 0 | 0, 107 | .4, 1 | |
| Missing | 4 (10%) | 7 (14%) | 5 (19%) | 0 (0%) | 2 (15%) | 4 (15%) | 20 (38%) | 4 (17%) | 5 (36%) | 4 (20%) | 3 (16%) | 40 (66%) | 17 (20%) | 0 (0%) | |
| CSF protein level mg/dl | Mean (SD) | 146 (192) | 35 (14) | 93 (110) | 72 (12) | 51 (20) | 55 (29) | 44 (15) | 56 (21) | 37 (10) | 48 (24) | 44 (21) | 43 (20) | 42 (26) | 91 (64) |
| Median [Q1, Q3] | 105 [78, 136] | 36 [26, 43] | 71 [48, 85] | 72 [68, 76] | 42 [36, 66] | 51 [37, 62] | 40 [33, 51] | 57 [42, 67] | 34 [32, 43] | 42 [32, 56] | 46 [26, 58] | 42 [32, 49] | 36 [27, 49] | 72 [51, 105] | |
| Min, max | 38, 1280 | 0, 74 | 19, 600 | 63, 80 | 28, 97 | 25, 140 | 19, 95 | 15, 101 | 18, 56 | 24, 133 | 19, 92 | 12, 122 | 15, 178 | 32, 208 | |
| Missing | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |
Apart from the diagnostic value of elevated CXCL13 concentrations, negative CXCL13 levels are also valuable diagnostic indicators (sensitivity 95.2%, specificity 97.2%). Two of the patients presented with peripheral facial nerve paresis and an elevated Borrelia antibody index (2.55 and 8.9) but had a normal CSF cell count and a CXCL13 concentration of 0 pg/ml. A normal CSF cell count is a strong parameter for the exclusion of active NB, but in early cases, the CSF cell count can be false negative. 34 The negative CXCL13 concentration helps in these cases to support the exclusion of acute and untreated NB.
Two out of the 42 patients with NB had CXCL13 concentrations below our suggested cut-off of 271 pg/ml (Figure 3). Possible explanations could be 1) the pathophysiology of NB, which may lead to low CXCL13 concentrations in selected cases, 2) immunological circumstances, such as specific viral infections, which may have an inhibitory effect on the production of CXCL13, 35 or 3) despite its prospective study setting, our study may have had technical or logistical problems, such as an interrupted cold chain, which cannot always be ruled out and could cause adulterated CXCL13 concentrations. 26
Patients with ’probable NB’ show NB-specific symptoms, CSF pleocytosis and serum Borrelia antibody production but lack intrathecal Borrelia antibody production (according to the DGN criteria 4 ). We identified a total of 6 patients with “probable” NB. Four patients had CXCL13 concentrations above and 2 had CXCL13 levels below the cut-off of 271 pg/ml (Figure 3). For the 2 patients with CXCL13 below the cut-off, the same arguments as mentioned above may be considered (pathophysiology of NB, immunological circumstances, technical/logistical problems). The other 4 patients with CXCL13 above the cut-off are very likely to be true NB.
Patients with multiple sclerosis, who may show Borrelia antibody indices >1.5,11-13 had CXCL13 concentrations below our suggested cut-off of 271 pg/ml, with a median CXCL13 concentration of 15 pg/ml (min. 0, max. 257 pg/ml). The difference in the CXCL13 concentrations in patients with NB and multiple sclerosis was highly statistically significant (P < .001). CXCL13 seems to differ consistently between multiple sclerosis and NB.
Data regarding neurological signs and symptoms, CSF cell count, Borrelia antibody index and CSF protein levels of NB patients were in line with literature (Figure 1, Table 3).36-38
In order to examine the reliability of CXCL13 in NB-diagnostics, we tested for possible correlations between the intrathecal CXCL13 and other parameters (Table 4): We could not find a strong correlation in any of the tested parameters, such as CSF cell count, Borrelia antibody index, CSF protein count or time between symptom onset and the spinal tap. These results support the early rise in intrathecal CXCL13 in acute NB, despite still low CSF cell count. The CXCL13 concentration does not differ between patients with short- or long-term NB, supporting CXCL13 as a reliable parameter for NB diagnostics from the very early stage to the subacute and chronic stages of the disease.
Limitations of our study: The recruitment phase was affected in 2020 and 2021 by the covid pandemic, which slowed recruiting and led to a lower number of participants as initially expected. Also a 14 day follow-up of patients with NB after antibiotic treatment, including a symptom questionnaire and collection of blood and cerebrospinal fluid samples, had been planned. However, due to the pandemic, human resources were limited, and only interviews to check for symptom changes in 12 patients with NB were conducted. Furthermore, accelerated by the pandemic most of the patients with NB were discharged from hospital in the first 14 days. Consequently, blood samples were retrieved from only 5 patients with NB and CSF-samples from only 4 patients with NB 14 days after the first spinal tap. This low number of cases caused us to abandon follow-up analysis.
Conclusion
The diagnosis of NB can be challenging, as several pitfalls are encountered in CSF analysis and interpretation, along with a broad spectrum of clinical signs and symptoms. To overcome the uncertainties of ‘borderline’ cases, we propose the use of the CSF biomarker CXCL13 for the diagnosis of acute and untreated NB. An early and accurate diagnosis of NB with rapid start of antibiotic treatment prevents patient suffering.
Supplemental Material
Supplemental Material for CXCL13 as a biomarker in the diagnostics of European lyme Neuroborreliosis - A prospective multicentre study in Austria by Christoph Waiß, Barbara Ströbele, Uwe Graichen, Sascha Klee, Joshua Gartlehner, Estelle Sonntagbauer, Stephanie Hirschbichler, Alexander Tinchon, Emrah Kacar, Bianca Wuchty, Bianka Novotna, Zofia Kühn, Johann Sellner, Walter Struhal, Christian Bancher, Peter Schnider, Susanne Asenbaum-Nan, and Stefan Oberndorfer in Journal of Central Nervous System Disease
Author contributions: All authors contributed to the study conception and design. The first draft of the manuscript was written by Christoph Waiß and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Forschungsimpulse [project ID: RTO_0009], a program of Karl Landsteiner University of Health Sciences funded by the Federal Government of Lower Austria.
Informed consent: Written informed consent for publication was obtained from all individual participants included in the study.
Supplemental Material: Supplemental material for this article is available online.
Ethical statement
Ethical approval
All procedures performed in studies involving human participants are in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Lower Austria (No. GS1-EK-1/185-2018). Informed consent was obtained from all individual participants included in the study.
ORCID iDs
Christoph Waiß https://orcid.org/0009-0004-0809-3720
Uwe Graichen https://orcid.org/0000-0003-2144-4682
Johann Sellner https://orcid.org/0000-0001-8749-5533
Data Availability Statement
We provide our statistical data file on request. Contact information: christoph.waiss@stpoelten.lknoe.at
References
- 1.Bremell D, Mattsson N, Edsbagge M, et al. Cerebrospinal fluid CXCL13 in Lyme neuroborreliosis and asymptomatic HIV infection. BMC Neurol. 2013;13:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skogman BH, Sjöwall J, Lindgren PE. The NeBoP score - a clinical prediction test for evaluation of children with Lyme Neuroborreliosis in Europe. BMC Pediatr. 2015;15:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kindler W, Wolf H, Their K, et al. Peripheral facial palsy as an initial symptom of Lyme neuroborreliosis in an Austrian endemic area. Wien Klin Wochenschr. 2015;28(21-22):837-840. [DOI] [PubMed] [Google Scholar]
- 4.Rauer SKSea, Neuroborreliose, S3-Leitlinie. Deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie, 2018. [Google Scholar]
- 5.Senel M, Rupprecht TA, Tumani H, Pfister HW, Ludolph AC, Brettschneider J. The chemokine CXCL13 in acute neuroborreliosis. J Neurol Neurosurg Psychiatr. 2010;81(8):929-933. [DOI] [PubMed] [Google Scholar]
- 6.Cerar T, Ogrinc K, Lotrič-Furlan S, et al. Diagnostic value of cytokines and chemokines in lyme neuroborreliosis. Clin Vaccine Immunol. 2013;20(10):1578-1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ljostad U, Mygland A. CSF B--lymphocyte chemoattractant (CXCL13) in the early diagnosis of acute Lyme neuroborreliosis. J Neurol. 2008;255(5):732-737 [DOI] [PubMed] [Google Scholar]
- 8.Schmidt C, Plate A, Angele B, et al. A prospective study on the role of CXCL13 in Lyme neuroborreliosis. Neurology. 2011;76(12):1051-1058. [DOI] [PubMed] [Google Scholar]
- 9.van Burgel ND, Bakels F, Kroes ACM, van Dam AP. Discriminating Lyme neuroborreliosis from other neuroinflammatory diseases by levels of CXCL13 in cerebrospinal fluid. J Clin Microbiol. 2011;49(5):2027-2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hytönen J, Kortela E, Waris M, Puustinen J, Salo J, Oksi J. CXCL13 and neopterin concentrations in cerebrospinal fluid of patients with Lyme neuroborreliosis and other diseases that cause neuroinflammation. J Neuroinflammation. 2014;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keymeulen B, Somers G, Naessens A, Verbruggen LA. False positive ELISA serologic test for Lyme borreliosis in patients with connective tissue diseases. Clin Rheumatol. 1993;12(4):526-528. [DOI] [PubMed] [Google Scholar]
- 12.Koike T, Sueishi M, Funaki H, Tomioka H, Yoshida S. Anti-phospholipid antibodies and biological false positive serological test for syphilis in patients with systemic lupus erythematosus. Clin Exp Immunol. 1984;56(1):193-199. [PMC free article] [PubMed] [Google Scholar]
- 13.Rensch MJ, Szyjkowski R, Shaffer RT, et al. The prevalence of celiac disease autoantibodies in patients with systemic lupus erythematosus. Am J Gastroenterol. 2001;96(4):1113-1115. [DOI] [PubMed] [Google Scholar]
- 14.Naesens R, Vermeiren S, Van Schaeren J, Jeurissen A. False positive Lyme serology due to syphilis: report of 6 cases and review of the literature. Acta Clin Belg. 2011;66(1):58-59. [DOI] [PubMed] [Google Scholar]
- 15.Magnarelli LA, Anderson JF, Johnson RC. Cross-reactivity in serological tests for Lyme disease and other spirochetal infections. JID (J Infect Dis). 1987;156(1):183-188. [DOI] [PubMed] [Google Scholar]
- 16.Bernardino AL, Myers TA, Alvarez X, Hasegawa A, Philipp MT. Toll-like receptors: insights into their possible role in the pathogenesis of lyme neuroborreliosis. Infect Immun. 2008;76(10):4385-4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rupprecht TA, Lechner C, Tumani H, Fingerle V. CXCL13 als Biomarker der akuten Neuroborreliose. Nervenarzt. 2014;85(4):459-464. [DOI] [PubMed] [Google Scholar]
- 18.Rupprecht TA, Manz KM, Fingerle V, et al. Diagnostic value of cerebrospinal fluid CXCL13 for acute Lyme neuroborreliosis. A systematic review and meta-analysis. Clin Microbiol Infection. 2018;24(12):1234-1240. [DOI] [PubMed] [Google Scholar]
- 19.van Gorkom T, van Arkel GHJ, Heron M, Voet W, Thijsen SFT, Kremer K. The usefulness of two CXCL13 assays on cerebrospinal fluid for the diagnosis of lyme neuroborreliosis: a retrospective study in a routine clinical setting. J Clin Microbiol. 2021;59(9):e0025521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckman EA, Clausen DM, Herdt AR, Pacheco-Quinto J, Halperin JJ. Specificity and diagnostic utility of cerebrospinal fluid CXCL13 in lyme neuroborreliosis. Clin Infect Dis. 2021;72(10):1719-1726. [DOI] [PubMed] [Google Scholar]
- 21.Knudtzen FC, Nilsson AC, Hovius JW, Skarphedinsson S. The predictive value of CXCL13 in suspected Lyme neuroborreliosis: a retrospective cross-sectional study. Eur J Clin Microbiol Infect Dis. 2020;39(8):1461-1470. [DOI] [PubMed] [Google Scholar]
- 22.Lintner H, Hochgatterer-Rechberger P, Pischinger B, et al. Sensitivity and specificity of cerebrospinal fluid CXCL13 for diagnosing Lyme neuroborreliosis - a study on 1410 patients and review of the literature. J Neurol Sci. 2020;414:116843. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler K, Rath A, Schoerner C, et al. Comparative analysis of the Euroimmun CXCL13 enzyme-linked immunosorbent assay and the ReaScan lateral flow immunoassay for diagnosis of lyme neuroborreliosis. J Clin Microbiol. 2020;58(9):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindström J, Bremell D, Grahn A, et al. CXCL13 in patients with facial palsy caused by varicella zoster virus and Borrelia burgdorferi: a comparative study. Diagn Microbiol Infect Dis. 2020;98(1):115095. [DOI] [PubMed] [Google Scholar]
- 25.Markowicz M, Schötta A-M, Kundi M, et al. CXCL13 concentrations in cerebrospinal fluid of patients with Lyme neuroborreliosis and other neurological disorders determined by Luminex and ELISA. Ticks and Tick-borne Diseases. 2018;9(5):1137-1142. [DOI] [PubMed] [Google Scholar]
- 26.Haglund S, Lager M, Gyllemark P, et al. CXCL13 in laboratory diagnosis of Lyme neuroborreliosis-the performance of the recomBead and ReaScan CXCL13 assays in human cerebrospinal fluid samples. Eur J Clin Microbiol Infect Dis. 2022;41(1):175-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leth TA, Dessau RB, Møller JK. Discriminating between Lyme neuroborreliosis and other central nervous system infections by use of biomarkers CXCL13 and IL-6. Ticks and Tick-borne Diseases. 2022;13(5):101984. [DOI] [PubMed] [Google Scholar]
- 28.Kowarik MC, Cepok S, Sellner J, et al. CXCL13 is the major determinant for B cell recruitment to the CSF during neuroinflammation. J Neuroinflammation. 2012;9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer L, Korfel A, Pfeiffer S, et al. CXCL13 and CXCL12 in central nervous system lymphoma patients. Clin Cancer Res. 2009;15(19):5968-5973. [DOI] [PubMed] [Google Scholar]
- 30.Eldin C, Raffetin A, Bouiller K, et al. Review of European and American guidelines for the diagnosis of Lyme borreliosis. Med Maladies Infect. 2018;49:121-132. [DOI] [PubMed] [Google Scholar]
- 31.Waiß C, Kindler W, Ströbele B, et al. [CXCL-13 as a biomarker in the diagnostics of neuroborreliosis]. Nervenarzt. 2017;88(6):635-641. [DOI] [PubMed] [Google Scholar]
- 32.Buderer NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med. 1996;3(9):895-900. [DOI] [PubMed] [Google Scholar]
- 33.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32-35. [DOI] [PubMed] [Google Scholar]
- 34.Hufschmidt A RS, Glocker F, Neurologie compact, 9. Thieme; 2022. [Google Scholar]
- 35.Takatani A, Nakamura H, Furukawa K, et al. Inhibitory effect of HTLV-1 infection on the production of B-cell activating factors in established follicular dendritic cell-like cells. Immunity, Inflammation and Disease. 2021;9(3):777-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rauer S, Kastenbauer S, Fingerle V, Hunfeld K-P, Huppertz H-I, Dersch R. Lyme neuroborreliosis. Deutsches Ärzteblatt international. 2018;115(45):751-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koedel U, Fingerle V, Pfister HW. Lyme neuroborreliosis-epidemiology, diagnosis and management. Nat Rev Neurol. 2015;11(8):446-456. [DOI] [PubMed] [Google Scholar]
- 38.Djukic M, Schmidt-Samoa C, Lange P, et al. Cerebrospinal fluid findings in adults with acute Lyme neuroborreliosis. J Neurol. 2012;259(4):630-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for CXCL13 as a biomarker in the diagnostics of European lyme Neuroborreliosis - A prospective multicentre study in Austria by Christoph Waiß, Barbara Ströbele, Uwe Graichen, Sascha Klee, Joshua Gartlehner, Estelle Sonntagbauer, Stephanie Hirschbichler, Alexander Tinchon, Emrah Kacar, Bianca Wuchty, Bianka Novotna, Zofia Kühn, Johann Sellner, Walter Struhal, Christian Bancher, Peter Schnider, Susanne Asenbaum-Nan, and Stefan Oberndorfer in Journal of Central Nervous System Disease
Data Availability Statement
We provide our statistical data file on request. Contact information: christoph.waiss@stpoelten.lknoe.at