Abstract
Icetexane diterpenoids are a diverse family of natural products sourced from several species of terrestrial plants. Icetexanes exhibit a broad array of biological activities and together with their complex 6-7-6 tricyclic scaffolds, they have piqued the interest of synthetic organic chemists, natural products chemists, and biological investigators over the past four decades and were reviewed 13 years ago. This review summarizes icetexane natural products isolated since 2009, provides an overview of new synthetic approaches to the icetexane problem, and proposes an additional classification of icetexanes based on novel structures that are unlike previously isolated materials.
Keywords: icetexanes, abietanes, terpenoids, bioactive natural products, synthesis
1. Introduction
Icetexanes[1] are a family of diterpenoid natural products sourced from terrestrial plants with a fascinating 6-7-6 tricyclic framework that exhibit a wide array of biological activity ranging from antimicrobial activity (lanigerol, 1)[2] and anti-inflammatory activity (salviasperanol, 2)[3] to trypanocidal ((+)-komaroviquinone, 3)[4] and anticancer activity (4,[5] and salviadenone A, 5[6]) and thus are inspiring scaffolds for new drug leads (Figure 1). The first example of these natural products (icetexone, 6) was discovered in 1976 in the extracts of aerial parts of Salvia ballotaeflora[7] and its absolute stereochemical configuration was unambiguously proven in 2017.[8] In the 46 years since the discovery of icetexone, more than 100 novel icetexanes have been isolated and described. Icetexane natural products were the subject of a 2009 review[1] (including a proposed classification system for this family of natural products) and since that time alone >50 novel icetexanes have been discovered and several new synthetic approaches to the characteristic 6-7-6 scaffold have been reported. The synthetic relationship between abietanes and icetexanes and approaches to both have recently been explored.[9] The focus of this review includes a summary of these newly described natural products and any known biological activity, an overview of novel approaches to icetexanes since 2009, as well as a proposed new descriptive class of icetexane natural products that falls outside the realm of previously reported structures.
Figure 1.

Selected examples of biologically active icetexanes isolated between 1976 and 2022.
2. Early Synthetic Milestones
Matsumoto and coworkers reported the first total synthesis of an icetexane in 1986,[10] ten years after the first appearance of these important natural products in the literature.[7] This racemic approach toward pisiferin (15) was initiated by a Wittig reaction[11,12] joining the phosphonium ylide 7 and the racemic aldehyde 8 followed by a selective hydrogenation of the resulting styrene to produce the trisubstituted aromatic 9. Epoxidation of 9 with m-chloroperbenzoic acid (mCPBA) followed by epoxide opening under the action of lithium N,N-diethylamide (LiNEt2) resulted in the alcohol 10.[13] Oxidation of the alcohol 10 with pyridinium chlorochromate (PCC) provided the corresponding enone which underwent an intramolecular cyclization upon heating at 80–85°C with polyphosphoric acid to generate a mixture of the epimeric ketones 11 and 12. The ketone 11 was then reduced to the corresponding secondary alcohol upon treatment with lithium aluminum hydride (LiAlH4), and subsequent demethylation of the phenyl methyl ether afforded the alcohol 13. Regioselective dehydration of 13 was achieved through bismesylation followed by heating in 2,4-lutidine to give the trisubstituted alkene 14, which was then demesylated under the action of LiAlH4 to generate racemic pisiferin 15 (Scheme 1).
Scheme 1.

Total synthesis of (±)-pisiferin (15).
The first asymmetric total synthesis of an icetexane was described in 2007 by Majetich and his group.[14,15] The synthesis began with an eight-step preparation of the benzyl bromide 17 (inset, Scheme 2) from 3,4,5-trimethoxybenzoic acid. Deprotonation of the highly acidic diketone 16 with sodium hydride and alkylation of the resultant enolate with the bromide 17 was followed by a second enolization event and O-methylation with dimethyl sulfate to give the enone 18. The enone 18 then underwent a tandem Isler alkynylation[16] – Stork–Danheiser transposition[17] to generate the enynone 19, thus incorporating all the carbon atoms necessary to close the central seven-membered ring of the icetexane core. Lindlar reduction[18] of 19 then generated the conjugated dienone 20, which was cyclized under the action of Lewis acid to afford the tricyclic product 21. Bromination of 21 with N-bromosuccinimide (NBS) in acetic acid followed by a radical dehalogenation set the stage for the key stereodefining event – reduction of the enone under the action of borane and the Corey-Bakshi-Shibata catalyst[19] afforded the acetates 22 as a 1:1 diastereoisomeric mixture but establishing absolute configuration at C(1). Relay of configuration within the acetates 22 proceeds via a Myers allylic transposition;[20] a two-step acetate cleavage-oxidation sequence afforded the ketoalkene 24, thus ablating inconsequential configuration at C(7). Introducing NBS in wet acetone to the ketoalkene 24 followed by radical dehalogenation generated 25 which upon oxidation under the action of silver(II) oxide in 7N nitric acid afforded (+)-komaroviquinone 3.[4]
Scheme 2.

Enantioselective total synthesis of (+)-komaroviquinone (3).
3. Biosynthetic Origins
Icetexane natural products are most commonly extracted from terrestrial plants that also produce abietane natural products (comprised of a 6-6-6 tricyclic framework, e. g., 26, Scheme 3) as secondary metabolites.[1] Consequently, it is believed that icetexanes are products of an enzyme-mediated rearrangement in the skeleton of abietanes and hence the formal name 9(10→20)-abeo-abietane with the name ‘icetexane’ was proposed by Rodriguez–Hahn and coworkers in 1989.[21]
Scheme 3.

Proposed biosynthetic origins of icetexanes from abietanes.
Gonzalez and coworkers proposed a general biosynthetic pathway from abietanes to icetexanes.[22] Beginning from 26, it was proposed that an enzymatic protonation-dehydration of C(20) furnishes the intermediate primary cation 27 which undergoes a Wagner–Meerwein rearrangement[23,24] to produce the central seven-membered ring of the icetexanes. Trapping of the carbocation 28 by water generates 29. It has also been proposed that the C(20) activated abietane 27 could result from enzymatic hydride abstraction from the C(20) methyl group of the abietane 30.[25]
In 1983, the first laboratory synthetic link between an icetexane and an abietane was observed by Kelecom during the structural elucidation of barbatusol 36 (inset, Scheme 4).[26] It was observed that treating the abietane 31 with potassium carbonate and iodomethane in wet acetone results in the opening of the lactone moiety and formation of a C(6)–C(7) double bond to generate the ester 33. Sequential hydrogenation and reduction furnished the primary alcohol 34, which upon treatment with an excess amount of p-toluenesulfonyl chloride in pyridine generated barbatusol dimethyl ether 35 (Scheme 4).
Scheme 4.

Synthesis of a barbatusol icetexane (35) from an abietane (31).
4. Classification of Icetexanes
Icetexanes vary widely in their degree and regiochemistry of oxygenation in both the aromatic and aliphatic moieties. Simmons and Sarpong proposed a classification system for icetexanes based upon the presence or absence of oxygenations about the scaffold, specifically at C(3), C(11), C(12), C(14), and C(19).[1] Herein we propose a sixth class member in order to represent novel icetexanes isolated since 2009 that do not fit within the previously defined five classes (Figure 2).
Figure 2.

Classification of icetexanes.
Icetexane class 1 (37) is oxygenated at C(12) and lacks oxygenation at the other positions. The parent member of this class is the natural product pisiferin 15 (Scheme 1 and Figure 3), which was first extracted from leaves of Chamaecyparis pisifera.[27] As an important historical aside, the structure of this compound was originally proposed as the 7-6-6 tricyclic framework 43 and was later revised to 15 after a second independent isolation from the seeds of Chamaecyparis pisifera.[28]
Figure 3.

Parents of icetexane classes.
Icetexane class 2 (38) is oxygenated at both C(11) and C(12) and lacks oxygenations at other positions. The parent member of this class is the natural product barbatusol 36 (Scheme 4), which was first extracted from the bark and heartwood of Coleus barbatus.[26] As previously noted, the structural elucidation of barbatusol was one of the first chemical experiments demonstrating the relationship between icetexanes and abietanes.[26]
Icetexane class 3 (39) is oxygenated at C(3), C(11) and C(12) and lacks oxygenations at other positions. The parent members of this class are the natural products taxamairin A (44) and taxamairin B (45), both of which were isolated from the bark of Taxus mairei (Figure 3).[29]
Icetexane class 4 (40) is oxygenated at C(11), C(12) and C(14) and lacks oxygenations at other positions. The parent member of this class is the natural product coulterone (46), which was first isolated from roots of Salvia coulteri (Figure 3).[30]
Icetexane class 5 (41) is oxygenated at C(11), C(12), C(14) and C(19) and lacks oxygenation at C(3). The parent member of this class is icetexone (6), the first icetexane to be discovered.[7]
Finally, we propose the addition of icetexane class 6 (42) which is oxygenated at C(11) and thus cannot be considered as a member of the pisiferin or barbatusol families. (e. g., 4 Figure 1).[5] We propose a new classification designated deoxo-barbatusols for novel natural products with this oxygenation pattern (vide infra, section 7).
Importantly, in addition to the key distinguishing oxygen features, members of each of these icetexane classes can bear further oxygenation at non-specified positions; for example, oxygenation at C(1), C(10) or C(16) are very common (e. g., 1–5, Figure 1). In the sections that follow, we aimed to organize newly isolated natural products and highlighted new synthetic efforts chronologically within these distinct classifications.
5. Newly Discovered Pisiferins and Barbatusols
Zhao and coworkers extracted fokihodgin J 47 from twigs and leaves of Fokienia hodginsii along with nine other novel diterpenoids in 2013 (Figure 4).[31] Fokihodgin J was expected to exhibit some activity against human cancer cell lines based on other similar members of this icetexane family and was screened against a panel of five representative cancer cell lines – human myeloid leukemia (HL-60), hepatocellular carcinoma (SMMC-7721), lung cancer (A-549), breast cancer (MCF-7), and colon cancer (SW-480). Unfortunately, the natural product was found to be inactive against all of them (IC50 >40 μM, cisplatin as positive control).
Figure 4.

Novel pisiferins.
Yue and coworkers isolated 3-oxopisiferanol (48) from powdered twigs of Podocarpus imbricatus along with 13 other novel diterpenoids (Figure 4).[32] As part of this study, four of these diterpenoids were screened for activity relevant to Zika virus, however 48 was not among those selected for detailed testing.
The ethanolic extract of the perennial shrub Perovskia atriplicifolia yielded one new pisiferin – 1 α-hydroxypisiferanol (49, Figure 4) – and four new barbatusols – perovskatone B 50, 1α-hydroxybrussonol 51, perovskatone C 52, and perovskatone D 53 in a study described by Jiang and coworkers (Figure 5).[33] Demethylsalvicanol quinone 54 was also isolated for the first time from Perovskia atriplicifolia by the Jiang group but prior to this isolation, 54 was a known synthetic intermediate generated during the course of several syntheses.[34–37] Brussonol (56)[25] and przewalskin E (55)[38] were isolated in this study as well but were previously known natural products. Jiang screened 49–56 for their activities toward hepatitis-B virus in the HepG 2.2.15 cell lines, with 50 and 51 exhibiting modest activity in vitro.[33] Other biological testing of synthetically prepared 54 was completed in 1996 by the Moujir group; 54 was found to have modest anticancer activity (HeLa IC50=1.3 μg/mL) and was found to be inactive against Staphylococcus aureus, Staphylococcus albus, Staphylococcus epidermidis, Micrococcus luteus, Bacillus pumilus, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans at a level of 20 μg/mL.[37] Further, in 2021 icetexane 54 was isolated again from Perovskia scrophulariifolia and tested for inhibitory activity (44.8% at 25 μM with no cytotoxicity) on 1L-1β production from LPS-simulated microglial cells.[39]
Figure 5.

Barbatusols from Pervoskia atriplicifolia.
Zhao and coworkers discovered przewalskin C (57) and przewalskin D (58) (Figure 6), two new barbatusol icetexanes from an acetone extract of the Chinese plant Salvia przewalskii, however no biological activity was reported at that time.[40]
Figure 6.

Barbatusols from Salvia przewalskii.
Karalai and coworkers isolated the novel barbatusol type icetexane 59 from twigs and roots of Premna obtusifolia[3] alongside three previously known icetexanes; 60 and 2[41] were previously isolated from Salvia aspera and 61[42] was previously isolated from Rosmarinus officinalis (Table 1). The antibacterial activity of compounds 59, 60, and 2 was evaluated against five Gram-positive and three Gram-negative bacterial cell lines[3] and it was determined that 2 is significantly active against S. sonei and moderately active against B. subtilis, E. faecalis, MRSA, and VRE and 60 is moderately active against MRSA. In addition, the inhibitory effects upon NO production by icetexanes 59–61 and 2 was also evaluated (IC50=27.6–35 μg/mL) which is suggestive that these compounds may have promising anti-inflammatory potential.[3]
Table 1.
Barbatusol type icetexanes from Premna obtusifolia and antibacterial activities.

|
||||||||
|---|---|---|---|---|---|---|---|---|
| B. subtilis [a] | S. aureus [a] | E. faecalis [a] | MRSA[a] | VRE[a] | S. typhi [b] | S. sonei [b] | P. aeruginosa [b] | |
| 59 | 75 | 75 | 75 | 75 | 75 | 75 | 37.5 | 150 |
| 60 | 37.5 | 75 | 75 | 9.37 | 75 | 75 | 18.75 | >300 |
| 2 | 9.37 | 18.75 | 9.37 | 9.37 | 9.37 | 37.5 | 2.34 | >300 |
| Vancomycin | <2.34 | <2.34 | <2.34 | <2.34 | <2.34 | <2.34 | <2.34 | <2.34 |
Antibacterial activity (MIC, μg/mL).
Gram-positive bacteria.
Gram-negative bacteria.
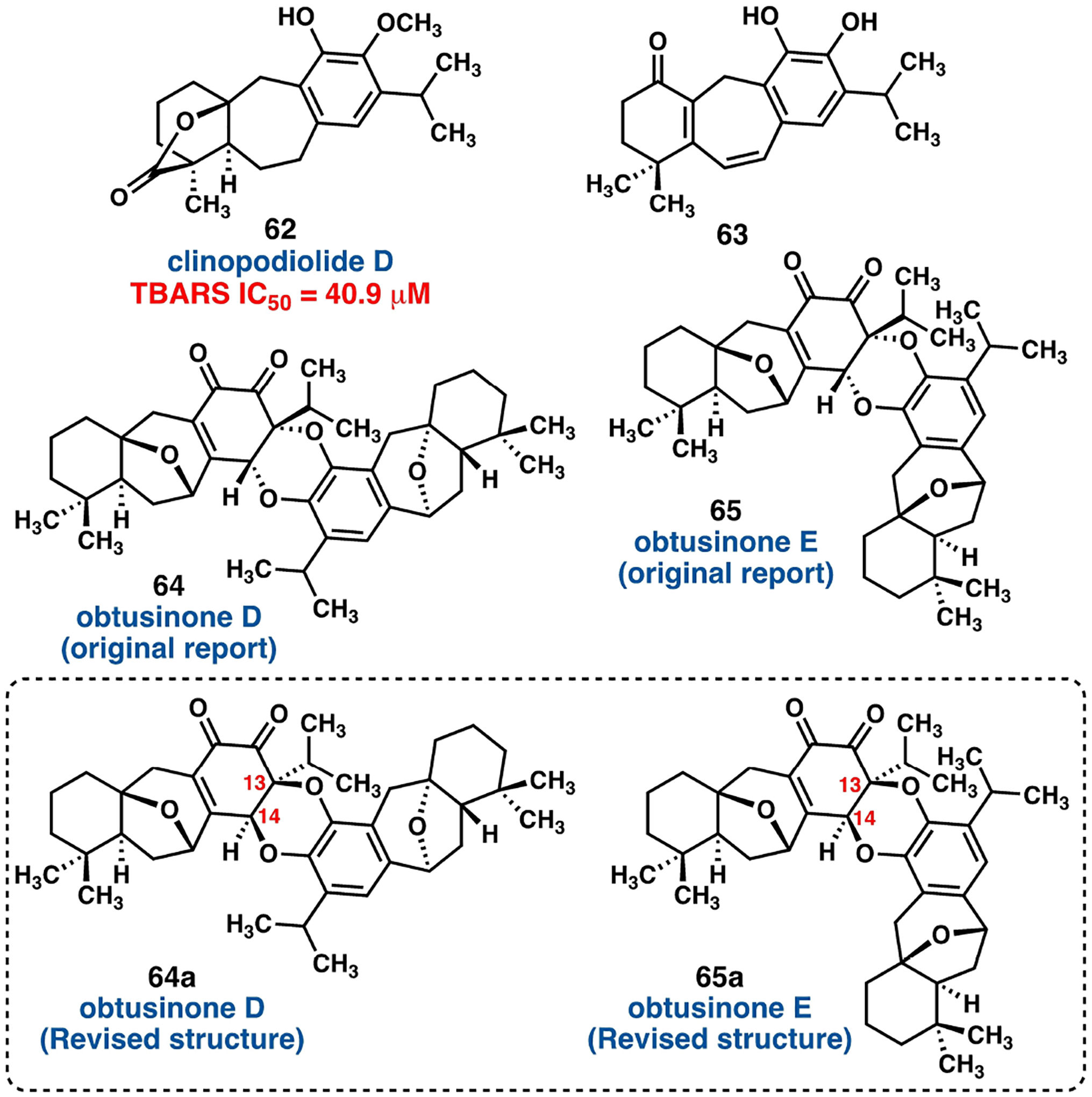
Quijano and coworkers discovered clinopodiolide D (62, Figure 7) from extracts of the leaves of Salvia clinopodioides.[43] As part of their study investigating novel radical scavengers and antioxidants, clinopodiolide D was evaluated in several assays including a thiobarbituric acid-reactive substances screen (TBARS) with modest results. Fun and coworkers extracted the barbatusol type icetexane (63, Figure 7) from the roots of Premna obtusifolia and elucidated its structure by X-ray crystallographic techniques.[44]
Figure 7.
Novel icetexanes isolated from Salvia clinopodioide and Premna obtusifolia.
Salae and Boonnak reported the discovery of obtusinone D (64) and obtusinone E (65) from the root extracts of Premna obtusifolia.[45] Obtusinones D (64) and E (65) are constitutional hetero-dimeric isomers of each other joining two monomeric units of przewalskin E (55), fused by a putative hetero-Diels–Alder event in either a linear or angular manner, respectively (Figure 7. It is important to note that the structures of the obtusinones were revised during the course of synthetic studies (vide infra). Jiang and coworkers described a similar dimeric material, biperovskatone B (66) alongside 1α-hydroxydemethylsalvicanolquinine (67) from ethanolic extract of cultured Perovskia atriplicifolia (Table 2).[46] Biperovskatone B (66) is a hetero-dimeric barbatusol type icetexane that alongside 67 exhibits noteworthy activity against the hepatitis B virus (HBV) by inhibiting replication of HBV DNA.[46]
Table 2.
Icetexanes 66 and 67 from Perovskia atriplicifolia and their activities against hepatitis B cell lines in vitro.

|
|||||||
|---|---|---|---|---|---|---|---|
| HBsAg | HBeAg | Inhibitory HBV DNA replication | |||||
| CC50 (mM) | IC50 (mM) | SI | IC50 (mM) | SI | IC50 (mM) | SI | |
| 66 | >2.08 | 1.36 | >1.53 | 1.85 | >1.12 | 10.78 | 192.95 |
| 67 | 1.15 | 0.84 | 1.35 | >2.08 | – | 8.61 | 133.57 |
| 3TC[a] | 28.32 | 22.62 | 1.25 | 28.17 | 1.01 | 1.15 | 24626.09 |
All values are mean of two independent experiments; SI=CC50/IC50.
3TC: Lamivudine, positive control.
Li and coworkers reported salprzeside A (68) and salprzeside B (69), two new barbatusol type icetexanes from extracts of Salvia przewalskii (Figure 8).[47] Both 68 and 69 exhibited anti-angiogenic activity against human umbilical vascular endothelial cells (HUVECs).
Figure 8.

Anti-angiogenic barbatusols 68 and 69.
Aisa and coworkers reported the isolation of four new icetexanes from root extracts of Salvia deserta, salviadenones A–D (5, 70–72, Figure 9).[6] Interestingly, three of these new icetexanes (70–72) have a C(20) carbonyl function which has been observed previously only in the class 3 icetexane taxamairin H (73).[48]
Figure 9.

Salviadenones A–D (5, 70–72) from Salvia deserta and taxamairin H (73).
The salviadenones were evaluated for cytotoxicity against five different cancer cell lines – human myeloid leukemia (HL-60), hepatocellular carcinoma (SMMC-7721), lung cancer (A-549), breast cancer (MCF-7), and colon cancer (SW-480) – and a noncancerous cell lines (BEAS-2B); only salviadenone A (5) showed significant activity in this study (IC50 values ranged from 17.70–31.98 μM in sensitive cell lines).[6]
Xu and coworkers described przewalskone (74) from root extracts of Salvia przewalskii (Table 3).[49] Przewalskone (74) is an especially interesting terpenoid natural product as it may constitute the result of a putative hetero-Diels–Alder event between the icetexane przewalskin E (55) and a danshenol-type C23 terpenoid. Danshenol A (75) is the parent member of this family of natural products.[50]
Table 3.
Przewalskone (74) isolated from Salvia przewalskii and danshenol A 75.

| ||
|---|---|---|
| Cell lines (IC50, μM) | Przewalskone | Cisplatin |
| HL-60 | 0.69 | 1.10 |
| SMMC-7721 | 2.35 | 14.75 |
| A-549 | 1.82 | 13.39 |
| MCF-7 | 0.90 | 12.99 |
| SW-480 | 0.72 | 12.61 |
| BEAS-2B | 21.26 | 14.84 |
Cisplatin: positive control.
Przewalskone (74) was evaluated for cytotoxicity against five different cancer cell lines – human myeloid leukemia (HL-60), hepatocellular carcinoma (SMMC-7721), lung cancer (A-549), breast cancer (MCF-7), and colon cancer (SW-480) – and a noncancerous cell lines (BEAS-2B), with the natural product exhibiting significant activity against all five cell lines in the study and outperforming cisplatin as the positive control (Table 3).[49]
Zhang and coworkers isolated a compound (76) that they described as ‘phyllane A’ from twigs and leaves of Isodon phyllopodus along with another novel diterpenoid in 2021 (Table 4).[51] It is interesting to note the C(19) oxygenation of this barbatusol type icetexane which has been previously encountered only in the class 5 icetexone family of icetexanes. The compound described as ‘phyllane A’ (76) showed anti-HIV activity with an IC50 of 15.7 μM employing an assay described by the same group in 2017.[52] While the icetexane 76 is indeed a novel compound, it is important to note that the name ‘phyllane A’ had been used previously in 2017 to describe a different diterpenoid compound isolated from Phyllanthus acidus.[53] Hamburger and coworkers discovered 12-methoxybarbatusol (77) from extracts of aerial parts of Perovskia abrotanoides,[54] which exhibited some antiprotozoal activity in multiple assays.
Table 4.
Phyllane A (76) and 12-methoxybarbatusol (77).

| ||
|---|---|---|
| Cell lines (IC50, μM)[a] | 77 | Positive control |
| T. b. rhodesiense | 45.8 (43.6, 48.0); 1.7[b] | 0.04[c] |
| T. cruzi | 111.5 (134.8, 88.2); 0.7[b] | 5.7[d] |
| L. donovani | 34.8 (17.8, 51.9); 2.3[b] | 0.9[e] |
| P. falciparum | 10.7 (9.8, 11.6); 7.4[b] | 0.01[f] |
| L6 cells | 79.4 (54.0, 104.8) | 0.009[g] |
Each value corresponds to the mean of two independent assays, with individual values indicated in parentheses.
Selectivity index,
Melarsoprol,
Benznidazole,
Miltefosine,
Chloroquine,
Phodophyllotoxin.
Rao and coworkers reported the isolation of four new icetexanes from the stem-bark of Premna tomentosa,[55] two of which are barbatusol type icetexanes-icetexanes (78 and 79) (Figure 10); the remaining two structures will be discussed in the context of the proposed new class of deoxybarbatusol class 6 icetexanes (vide infra). It is noteworthy that 78 and 79 are among the first known icetexanes that are oxygenated at C(16) and to date, icetexanes oxygenated at C(16) have been isolated from organisms exclusively within the genus Premna including Premna tomentosa and Premna latifolia. Rao and coworkers evaluated the cytotoxicity of (78) against five different cancer cell lines (Table 8).
Figure 10.

Novel barbatusols icetexane (78) and icetexane (79) isolated from Premna tomentosa.
Table 8.
Novel deoxo-barbatusols isoloated from Premna tomentosa.

|
|||||
|---|---|---|---|---|---|
| IC50 (μg/mL) | HT-29 | MCF-7 | Hep-G2 | A-549 | A-431 |
| Hexane extract | 41.01±6.08 | 75.77±1.61 | 45.01±0.60 | 61.65±0.04 | 123.1±14.7 |
| 78 | 16.21±0.00 | 15.96±0.21 | 18.63±0.73 | 18.62±0.02 | NA |
| 4 | NA | 80.72±4.65 | NA | 43.65±0.32 | NA |
| 110 | 14.57±0.69 | 15.84±0.37 | 34.41±0.46 | 21.37±0.10 | NA |
NA=not active.
In 2011, Babu and coworkers reported the isolation of four new barbatusol type icetexanes – latifolionol (80), dihydrolatifolionol (81), latiferanol (82), and premnalatifolin A (83) (Table 5).[56,57] These four icetexanes are the first known examples that introduce a dihydrobenzofuran cycle to the family. Moreover, premnalatifolin A is an especially interesting hetero-dimeric icetexane as it is the only example of such a dimer wherein the monomers are joined through a C−O−C diaryl ether bond linkage. This group of icetexanes was isolated as part of a search for anticancer natural products and all were found to have some cytotoxicity against eight different cancer lines.
Table 5.
Novel icetexanes from Premna latifolin and in vitro cytotoxicity against human cancer.

|
||||||||
|---|---|---|---|---|---|---|---|---|
| IC50 (μg/mL) | HT-29 | MCF-7 | Hep-G2 | A-549 | A-431 | PC-3 | B-16F10 | ACHN |
| 80 | 0.04±0.02 | 1.11±0.23 | 2.13±1.92 | 6.05±0.71 | 33.40±1.95 | 3.24±0.38 | 6.41±5.47 | 4.83±0.32 |
| 81 | 2.17±0.71 | 6.02+0.00 | 0.18±0.06 | 58.24±6.8 | 10.14±1.39 | 3.77±0.18 | 5.71±0.28 | 0.40±0.04 |
| 82 | 15.25±0.54 | 11.49±0.53 | 18.92±0.99 | 9.85±0.54 | 21.22±0.74 | 9.27±0.67 | 19.65±0.69 | 24.50±0.9 |
| 83 | 12.15±2.29 | 1.11±0.23 | 13.29±0.64 | 12.21±0.32 | 22.55±0.30 | 17.38±0.4 | 22.91±0.53 | 1.40±0.13 |
| Doxorubicin | 21.54±0.29 | 2.01±0.03 | 1.63±0.04 | 2.68±0.28 | 4.23±0.20 | 1.71±0.11 | 21.22±0.74 | 1.29±0.02 |
Doxorubicin: positive control.
Ayinampudi and coworkers reported icetexatriene (84) and icetexatriene (85), two new barbatusol type icetexanes from the extracts of the dried roots of Premna tomentosa (Figure 11), though the C(15) configuration of 84 was not determined at the time of disclosure.[58] Both icetexatriene (84) and icetexatriene (85) were screened for rat intestinal α-glucosidase inhibitory and free radical scavenging potentials with both compounds showing some activity (IC50=7–25 μg/mL).
Figure 11.

Novel barbatusols isolated from Premna tomentosa.
In 2022, Nian and coworkers described a number of novel salpratins (86–95), new icetexanes from the extracts of the dried roots of Salvia prattii (Figure 12).[59] These natural products and synthetic derivatives are thought to be promising analgesic compounds, owing to their inhibitory activity toward CaV3.2 T-type calcium ion channels with 86, 92, 94 and 95 showing the most promise with IC50 values of 2.9, 5.1, 2.3, and 3.2 μM, respectively. Salpratin E (86) is especially noteworthy as a 19(4→3)-abeo-icetexane.
Figure 12.

Novel barbatusols from Salvia prattii.
6. Newly Discovered Taxamairins and Icetexones
In 2011, Gan and coworkers reported amentonone (96), a new taxamairin type icetexane from the barks of Amentotaxus formosana,[60] alongside brevitaxin[61] (97), a previously known taxamairin (Table 6); amentonone (96) and brevitaxin (97) were evaluated for cytotoxicity against four different cancer lines as part of this isolation work.[60]
Table 6.
Amentonone (96) and brevitaxin (97) and their cytotoxicity against various cancer cell lines in vitro.

|
||||
|---|---|---|---|---|
| ED50 (μg/mL) | A-549 | Hep 3B | HT-29 | MCF-7 |
| 96 | 19.1±2.9 | 15±2.3 | ||
| 97 | 5.1±0.9 | 6.1±0.6 | 2.72±0.1 | 0.08±0.05 |
| 5-Fluorouracil | 3.1±0.2 | 0.6±0.3 | 0.6±0.1 | 1.5±0.1 |
5-Fluorouracil: positive control.
Hu and coworkers reported the isolation of three new taxamairin type icetexanes from the leaves and twigs of Amentotaxus argotaenia (Figure 13) – amentotaxin N (98), amentotaxin O (99), and amentotaxin P (100)[62] – alongside amentonone (96)[60] and the previously described demethylsalvicanol (54)[33,39] (Figure 5).
Figure 13.

Novel taxamairins isolated from Amentotaxus argotaenia.
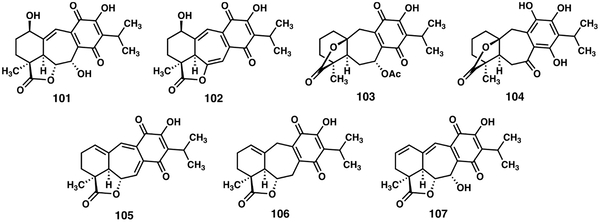
The newest members of the icetexone class of icetexanes were extracted from the aerial parts of Salvia ballotiflora and described by Quijano and coworkers.[63] Novel icetexanes ballotiquinone (101), 6,7-anhydroballotiquinone (102), 7α-acetoxy-6,7-dhydroicetexone (103), and 6,7,11,14-tetrahydro-7-oxo-icetexone (104) were accompanied by four previously known materials, icetexone (6),[7] anastomosine (105),[21] 7,20-dihydroanastomosine (106),[64] and the as yet unnamed icetexane 107[65] (Table 7). The icetexone 6, 7α-acetoxy-6,7-dhydroicetexone 103, anastomosine 105, and 7,20-dihydroanastomosine 106 were evaluated for cytotoxicity against four cancer cell lines in vitro.[63]
Table 7.
Novel and known icetexones isolated from Salvia ballotiflora.
|
|||||
|---|---|---|---|---|---|
| IC50 (μM) (SI) | U251 | SKLU-1 | COS-7 | K562 | MCF-7 |
| 6 | ND | ND | ND | 17.0±1.4 | 28.7±1.6 |
| 103 | 1.4±0.03 (1.2) | 0.82±0.06 (2.0) | 1.62±0.1 | ND | ND |
| 105 | 0.27±0.08 (2.3) | 0.46±0.05 (1.3) | 0.61±0.007 | ND | ND |
| 106 | ND | ND | ND | 31.2±1.1 | 33.24±1.2 |
| Adriamycin | 0.08±0.003 (3.1) | 0.05±0.003 (5.0) | 0.25±0.009 | 0.20±0.02 | 0.23±0.02 |
Results represent the mean±SD of ≥3 experiments, ND=not determined. SI=selective index calculated at the quotient of IC50 of COS-7/IC50 of cancer cell lines. For 103 and 105, IC50 was determined at four concentrations in a range of 1.0–0.18 μM, for 106 in a range of 75.0–12.5 μM, and for 6 a range of 50.0–6.25 μM. Adriamycin: positive control.
In addition, 7α-acetoxy-6,7-dihydroicetexone (103), anastomosine (105), and 7,20-dihydroanastomosine (106) were evaluated on the TPA model[66] of induced acute inflammation in mice with only 7α-acetoxy-6,7-dihydroicetexone (103) having any measureable effect (~37% inhibition of edema).[63]
Von Poser and coworkers reported isolation of a new class 5 icetexane, isoicetexone 108, in addition to two known compounds, the icetexone 6,[7] and 7α-acetoxy-6,7-dihydroicetexone 103,[63] from aerial extracts of Salvia uliginosa (Figure 14);[67] isoicetexone 108 and icetexone 6 were evaluated for potential anti-inflammatory activity as judged by inhibition of PMNs migration in vitro with complete inhibition of migration at 0.29 and 2.92 μM, respectively.
Figure 14.

A novel icetexone isolated from Salvia uliginosa and a known icetexone isolated from Salvia ballotaeflora.
In 2013, the biological activity of 19-deoxyicetexone (109) was reinvestigated and it was found that this natural product exhibited some promising anti-diarrheal activity in mice and rats.[68] This new activity is in addition to that mentioned upon its original isolation from Salvia ballotaeflora in 1997.[64]
7. Newly Discovered Deoxo-barbatusol Icetexanes
As part of the same work that revealed barbatusol icetexanes 78 and 79, Rao and coworkers[55] isolated two other icetexanes from stem bark of Premna tomentosa that do not match the oxygenation pattern of any of the previously proposed classes. Icetexane (4) and icetexane (110) (Table 8) are both oxygenated at C(11) thus they fall outside the pisiferin and barbatusol families and here we propose the natural products 4 and 110 be considered the first members icetexane class 6; deoxo-barbatusols (42, Figure 2).
As part of this bioassay guided fractionation, Rao and coworkers found icetexane (78), icetexane (4), and icetexane (110) to exhibit cytotoxic activity against five different cancer cell lines in vitro.[55] It is noteworthy that the hexane extract has dramatically different activity than the pure materials; interestingly, no pure compound is active against A-431 despite the extract itself exhibiting measurable cytotoxicity, suggesting there may be compounds within the extract that were not characterized in this study.
8. Highlighted Recent Synthetic Efforts Toward Icetexanes
In 2010, Brutoloso and coworkers described their synthetic efforts toward the core structure of (±)-brussonol (56) that features an epoxide ring-opening approach to forge the first key carbon-carbon bond (Scheme 5).[69] The epoxide 112 was synthesized by way of a Corey–Chaykovsky epoxidation event[70] upon the ketone 111. The key epoxide ring-opening was initiated by treatment of 112 with the lithiated aromatic 113; trapping of the resultant alkoxide with chlorotrimethylsilane (TMSCl) generated 114. The aldehyde 115 was generated through a carbon-carbon double bond cleavage using the Lemieux–Johnson oxidation protocol[71] and upon treatment with a Lewis acid, an efficient cascade of events initiated by cleavage of the trimethylsilyl group and terminated by a Marson type Friedel–Crafts cyclization reaction[72] afforded the desired scaffold 117.
Scheme 5.

Brutoloso’s and Jennings’ efforts toward (±)-brussonol analogs.
Brutoloso and coworkers indeed tried to utilize their approach toward a synthesis of (±)-brussonol (56),[73] but were unable to achieve the desired sequence of transformations with substrates more closely related to the natural product. Unfortunately, this difficulty was independently confirmed by Jennings and coworkers when they did not achieve a key epoxide ring-opening reaction that was intended to join 118 and 119; ultimately, the desired product 120 was not formed (Scheme 5).[74]
Although the epoxide ring-opening under the action of a tetrasubstituted lithiated aromatic nucleophile was unsuccessful, the strategic bond formation was achieved by a reductive cross-coupling reaction that joined the epoxide 121 with the aryl halide 122 in a nickel-catalyzed process that afforded the hemiacetal 124 in 40% isolated yield (77% yield brsm, Scheme 6). A Friedel–Crafts cyclization event under the action of boron trifluoride diethyl ether complex (BF3·OEt2) that closely mimics the original strategy furnished 125, which after global deprotection of the aryl methyl ethers successfully afforded (±)-brussonol (56).
Scheme 6.

Brutoloso’s synthesis of (±)-brussonol (56).
The Brutoloso group successfully applied the same cross-coupling strategy and epoxide 121 starting material to the natural product komaroviquinone (3) by way of the aryl halide 126 (Scheme 7).[73] The resultant lactol 127 was further oxidized to the corresponding lactone 128 under the action of the Fetizon reagent.[75] Iodination of 128 resulted in the fully substituted aromatic substrate 129; the central seven-membered ring was forged via an intramolecular nucleophilic cyclization[76] to afford the icetexane 25. Finally, oxidation of 25 under the action of silver(II) oxide in nitric acid afforded (±)-komaroviquinone 3.
Scheme 7.

Brutoloso’s synthesis of (±)-komaroviquinone (3).
In 2010, Suto and coworkers reported an asymmetric approach to (+)-komaroviquinone (3) by intercepting the same intermediate lactone 128 in optically enriched form (Scheme 8).[76] In their work, joining the optically enriched ester 130 with the benzyl bromide 131 under the Negishi protocol[77] afforded the alkylated ester 132. Upon treatment with aqueous acid, the lactone 128 in optically enriched form was obtained in 93% yield. Transformation of the lactone 128 into (+)-komaroviquinone (3) was achieved in three steps as previously described.[73]
Scheme 8.

Suto’s asymmetric approach to (+)-komaroviquinone (3).
In 2010, Sarpong and coworkers reported a formal synthesis of (±)-icetexone 6 (Figure 1) featuring a Ga(III)-catalyzed cyclo-isomerization reaction as their key step to form the seven-membered ring (Scheme 9).[78] Claisen condensation[79] of the indanone 133 and dimethyl carbonate generated a transient β-ketoester function which facilitates the critical alkylation reaction with the alkyl iodide 134, thus efficiently affording the alkyne 135 in 86% yield over two steps. Following the key alkylation event, the β-ketoester function was excised from the alkyne 135, which upon redox adjustment and elimination afforded the key intermediate 137 in 63% yield over those two steps. Cycloisomerization of the alkyne 126 was ultimately successful under the action of GaCl3 to form 138 in 91% isolated yield.
Scheme 9.

Sarpong’s approach in a formal synthesis of (±)-5-epi-icetexone and (±)-icetexone (6).
With the icetexane core structure 138 in hand, hydration of the nitrile under the action of Ghaffar and Parkins’ phosphonito complex resulted in primary amide 139,[80] which led to 140 upon subsequent diastereoselective (and directed) epoxidation with mCPBA (Scheme 10). The fate of 140 toward the diastereoisomeric icetexones 141 and 142 was highly dependent upon the conditions employed for a diazene rearrangement. Treatment of 140 with camphorsulfonic acid and tosylhydrazide in benzene generated a 1:2.5 mixture of 141 and 142 while treating 140 with camphorsulfonic acid in wet dichloromethane followed by subsequent treatment with camphorsulfonic acid and tosylhydrazide in benzene favored 141 over icetexone 142 (>10:1 dr). Both 141 and 142 were previously employed by Majetich and Grove to complete 5-epi-icetexone and icetexone (6).[81] In 2013, Sarpong and coworkers adapted this strategy into an enantioselective formal synthesis of 5-epi-icetexone and icetexone 6 that relied upon optically enriched 134 generated in a process that exploited rhodium catalysis.[82]
Scheme 10.

Endgame of Sarpong’s formal synthesis of (±)-5-epi-icetexone and (±)-icetexone 6.
In 2010, Shia and coworkers reported an asymmetric approach to (−)-isopisiferin (150) that served to confirm the absolute configuration of the natural product (Scheme 11).[83] Starting from the optically enriched aldehyde 143, a 1,2-addition of the aryl lithium derived from the aryl bromide 144 and subsequent acid-mediated elimination of the resultant alcohol afforded the styrene 145 in 75% over two steps. Conversion of the vinyl iodide moiety to a carboxylic acid and removal of the styrene unsaturation to give 146 was achieved in three steps in 85% overall yield, which set the stage for the key ring-closing event. Treatment of the acid 146 with trifluoroacetic anhydride (TFAA) and trifluoroacetic acid (TFA) induced a Friedel–Crafts type electrophilic aromatic acylation event that forged the central seven-membered ring to give the ketone 147 in 52% yield. Reductive excision of the ketone under the action of sodium borohydride (NaBH4) was followed by immediate treatment with methanesulfonyl chloride in order to facilitate an elimination reaction that afforded a mixture of alkenes 148 and 149 which were not isolated but rather treated under reductive conditions (hydrogen gas, palladium on carbon) that smoothly transformed the mixture into the alkene 148 exclusively in 79% yield over three steps from 147. Nucleophilic deprotection of the phenol with sodium ethanethiolate generated (−)-isopisiferin (150) in 62% yield.
Scheme 11.

Shia’s asymmetric approach to (−)-isopisiferin (150).
In 2011, Wang and coworkers reported a racemic synthesis of the icetexane core 156 (Scheme 12).[84] Starting from the tetra-substituted aromatic ring 151, a three-step sequence furnished a key electrophile; ozonolysis was followed by a reduction to generate the corresponding alcohol, and iodination by the Appel protocol[85] gave 152 in 40% overall yield. The primary iodide was engaged by the metalloenamine 153; hydrolysis of the resultant alkylation product under acidic conditions afforded the ketone 154. The ketone 154 was homologated to the corresponding aldehyde 155 as a mixture of stereoisomers in 87% overall yield by Wittig olefination[12] with methoxymethylenetriphenylphosphine and subsequent hydrolysis. Treating 155 with sodium methoxide enhanced the trans/cis ratio of the 1,2-disubstituted cyclohexane unit, which improved the efficiency of the subsequent manipulations; 155 was later treated with hydrochloric acid to induce a Friedel–Crafts type electrophilic aromatic substitution process to complete the synthesis of the icetexane core 156.
Scheme 12.

Wang’s synthesis of (±)-icetexane core 156.
In 2011, Green and coworkers reported the use of the Nicholas reaction[86] in synthesizing the tricyclic core of icetexanes (Scheme 13).[87] Five examples of the allylic acetate complexes 157 were treated with boron trifluoride diethyl etherate complex (BF3·OEt2) to furnish the icetexane core structures 158 in 40–90% yields. The Co2(CO)6 protective group can be released by treating 158a with conditions described by Isobe followed by in situ protodesilylation with TFA generating 159.[88]
Scheme 13.

Green’s synthesis of icetexane core 159.
In 2015, Green and coworkers described more successful examples employing their approach and a new method for cleaving the cobalt complex (Scheme 14).[89] They discovered that a stepwise hydro-sylilation-protodesilylation sequence rather than the previously described one-pot protocol furnishes 160a without overreduction. Alternatively, positive results were obtained by the use of NaH2PO2·H2O and 2-methoxyethanol to give 161 in 76% yield directly.
Scheme 14.

Alternative deprotections of the cobalt complex.
Green and coworkers also reported synthesis of an unnatural pisiferin (163) employing their cobalt methodology (Scheme 15). The cobalt complex of 162 proved to be unstable; a one-pot complexation-Nicholas reaction and decomplexation tactic was developed which resulted in 163 in 28% overall yield for the sequence.
Scheme 15.

Synthesis of an unnatural pisiferin 163.
In 2015, Xie and coworkers described a synthesis of (±)-pisiferin (15) that was reminiscent of the first synthesis of the natural product reported by Matsumoto in 1986[10] but was shortened by virtue of advances in cross-coupling chemistries (Scheme 16).[90] In their work, Xie and coworkers treated the diene 164 with 9-borabicyclo[3.3.1]nonane (9-BBN) and subjected the alkylborane so obtained to the action of palladium tetrakis(triphenylphosphine) and the aryl bromide 165 to give the coupled product 166 directly in 58% yield. Epoxidation and isomerization to the corresponding allylic alcohol 167 proceeded in a manner analogous to the Matsumoto synthesis, and this allylic alcohol was cyclized directly to (±)-pisiferin (15) in 60% yield by treatment with BF3·OEt2 in dichloroethane (DCE) in a Friedel–Crafts alkylation process. The overall sequence is remarkably shorter than the Matsumoto synthesis, a testament to 30 years of methodological improvements.
Scheme 16.

Synthesis of (±)-pisiferin (15) by Xie and coworkers.
In 2015, Jiao and coworkers described an asymmetric approach to (−)-brussonol (56) and (−)-przewalskine E (55) that exploited a dihydroxylation reaction as the key stereodefining event and a tandem C−H oxidation/cyclization/rearrangement sequence (Scheme 17).[91] The benzyl bromide 168 was joined with the vinyl triflate 169 via a Negishi protocol[77] similar to that executed above by Suto[76] and following acidic deprotection the aldehyde 170 was obtained in 69% overall yield. Redox adjustment of 170 under the action of diisobutylaluminum hydride (DIBAL-H) gave the diol 171 in 93% yield, setting stage for the critical stereodefining event. The diol 171 was treated with (−)-diethyltartrate, titanium(IV) tetraisopropoxide, and t-butylhydroperoxide to afford the spiroether 172 in 90% yield and 83% ee, thus establishing a critical oxygen-bearing stereogenic center that was subsequently elaborated to the core of the natural products. Conversion of the spiroether 172 to the rearrangement substrate was achieved in three steps to give the alkene 173. Treatment of 173 with indium(III) chloride (InCl3) and dichlorodicyanoquinone (DDQ) induced a tandem C−H oxidation/cyclization/rearrangement sequence that established the familiar icetexane 6-7-6 scaffold 174 that was then transformed into (−)-brussonol (56) in just four more steps. Oxidative conversion of (−)-brussonol (56) to (−)-przewalskine E (55) was executed as previously described under the action of silver(I) oxide.
Scheme 17.

Synthesis of (−)-brussonol (56) and (−)-przewalskine E (55) by Jiao and coworkers.
In 2016, Matsushita and coworkers reported synthesis of three different barbatusol type icetexanes-(−)-barbatusol (36), (+)-demethylsalvicanol (29), (+)-rosmaridiphenol (59) – from their corresponding pisiferin type icetexanes via an ortho-selective oxygenation reaction (Schemes 18 and 19).[92] Synthesis of MOM-protected pisiferin 177 was accomplished by a modified reaction condition previously described by Kametani and coworkers starting from protected abietane type material 176 (Scheme 18).[93] Conversion to three pisiferins from this single platform was achieved by distinct sequences to access 15, 1, and 178.
Scheme 18.

Asymmetric synthesis of pisiferins from 176.
Scheme 19.

Synthesis of (−)-barbatusol (36), (+)-demethylsalvicanol (29), (+)-rosmaridiphenol (59).
Selective oxygenation of C(11) using SIBX (a mixture of IBX, benzoic acid and isophthalic acid) or pre-MIBS (5-methyl-2-iodobenzenesulfonic acid) and Oxone® was successful in generating (−)-barbatusol (36), (+)-demethylsalvicanol (29), and (+)-rosmaridiphenol (59) in 35–59% yields under conditions developed by Tada and coworkers (Scheme 19).[94]
In 2016, Oh and workers described a formal synthesis of (±)-komaroviquinone (3) by a strategy in which a functionalized aromatic precursor was cyclized under the action of a platinum catalyst (Scheme 20).[95] In their work, after extensive optimization of the reaction conditions, the fully substituted aromatic precursor 179 was treated with platinum(II) chloride in warm toluene in the presence of water and a hydrative cyclization process afforded the icetexane scaffold 180 in 54% yield. A two-step sequence featuring PCC oxidation and the Meerwein–Ponndork–Verley (MPV) reduction resulted in transposition of the ketal function to give 181 in 56% overall yield. Elimination and ring-opening of the ketal function intercepted the known intermediate 24[14,15] which can be elaborated to (±)-komaroviquinone (3) in three known steps.
Scheme 20.

A formal approach to (±)-komaroviquinone (3) by Oh and coworkers.
In 2017, Gademann and coworkers showed the first experimental support for the non-enzymatic mechanism for the attack of a water molecule upon the cationic intermediate 183, and moreover they demonstrated that no enzymatic assistance was required for the β-face attack selectivity, thus generating the trans stereochemical outcome that corresponded to the saturated icetexane core (Scheme 21).[22,96] A selective demethylation of 184 furnished (+)-salvicanol 185, a barbatusol type icetexane.
Scheme 21.

Gademann’s approach to (+)-salvicanol 185.
The acetate 186 was oxidized at C(7) under a modified Hirao protocol using RuCl3 to give the icetexane 187 (Scheme 22) which was then deacetylated and further oxidized to produce (+)-komaroviquinone (3).[97] (+)-Komaroviquinone (3) was then reduced in an aqueous ethereal solution of sodium dithionite to furnish (−)-coulterone (46). Additionally, (+)-komaroviquinone (3) was subjected to photolysis to furnish (−)-cyclocoulterone (188) and the rearranged icetexane komarovispirone (189).
Scheme 22.

Synthesis of icetexanes 3, 46, 188 and rearranged icetexane 189.
(+)-Salvicanol (185) was oxidized with DDQ in acetone to obtain demethylsalvicanol quinone (54, Scheme 23). Gademann and his group realized that prolonged exposure of 54 to silica gel yielded a mixture of (−)-przewalskin E (55) and (−)-brussonol (56). Interestingly enough, adhering 54 to silica gel open to air with frequent mixing resulted in (−)-przewalskin E (55) in 50% yield without any detectable formation of (−)-brussonol (56). (−)-Przewalskin E (55) and (−)-brussonol (56) are also inter-convertible with discreet chemical oxidizing and reducing events. Importantly however, it must be noted that the spectral data exhibited by synthetic (−)-brussonol (56) is in agreement with those collected with the naturally obtained material, however, the spectral data exhibited by synthetic (−)-przewalskin E (55) and the natural material deviate from each other with respect to 1H-NMR chemical shifts and FTIR absorption bands.[34]
Scheme 23.

Synthesis of icetexanes 54, 55, 56, 65, and 66.
With (−)-przewalskin E (55) in hand, Gademann and his group utilized modified Takeya conditions to generate both the dimeric (−)-obtusinone D (64) and (−)-obtusinone E (66).[35] The spectral data of synthetic (−)-obtusinone D (64) and (−)-obtusinone E (65) were in good agreement with that of the natural products however X-ray crystallographic studies of synthetic (−)-obtusinone D (64) by Gademann and his group showed that the configurations at C(13) and C(14) are different from those of reported for the natural material by Salae and Boonnak – those assignments were inferred on the basis of a NOESY experiment (Scheme 24).[45] Consequently, Gademann and coworkers suggested that the configuration of C(13) and C(14) for (−)-obtusinone E (65) should be revised as well.
Scheme 24.

Revised structures of (−)-obtusinone D (64) and (−)-obtusinone E (65).
In 2021, Deng and coworkers reported the synthesis of a group of icetexanes by a biomimetic approach (Scheme 25).[98] The abietane alcohol 26 was treated with triphenylphosphine and diisopropylazodicarboxylate to construct the rearranged core structure of (−)-barbatusol (36). The sequence relies upon a gentle Mitsunobu type activation[99] of the alcohol which facilitates the biomimetic rearrangement without capture of the putative cationic intermediate by moisture.
Scheme 25.

Deng and coworkers biomimetic approach to generate icetexanes from an abietane.
Having barbatusol (36) in hand afforded a springboard for the synthesis of other icetexanes (Scheme 26). (−)-Barbatusol (36) was globally acetylated in 92% yield and then was treated with mCPBA to generate the epoxide 191 in 87% isolated yield; subsequent exposure to lithium aluminum hydride (LiAlH4) resulted in regioselective reductive epoxide fracture and cleavage of the acetate groups to afford demethylsalvicanol (29) in 78% yield. Epoxide-diene conversion of 191 afforded 192, which upon reductive cleavage of the acetates generated (−)-przewalskin D 58.[100]
Scheme 26.

Deng’s synthesis of (−)-demethylsalvicanol (29) and (−)-przewalskin D (58).
Further, (−)-demethylsalvicanol (29) was oxidized under the action of silver(I) oxide to construct demethylsalvicanol quinone (54) which was dimerized upon heating at 100°C to furnish (+)-grandione (193) (accompanied by (−)-brussonol (56) as a minor product, Scheme 27).[101,102] Additionally, exposure of demethylsalvicanol quinone (54) to silica gel can generate (−)-przewalskin E (55) but accompanied by 56 as a minor product and 193 as the major product.
Scheme 27.

Access to more icetexanes from (−)-demethylsalvicanol (29) including the dimeric icetexane (+)-grandione (193).
Nine of these synthetic icetexanes were investigated in the context of human colorectal cancer and could be promising lead compounds toward novel therapeutics (Table 9).[98]
Table 9.
Anticancer studies of synthetic icetexanes reported by Deng and coworkers.
| IC50 (μM)[a] | HCT-116 | COLO-205 | Caco-2 |
|---|---|---|---|
| 36 | >20.87 | >20.87 | 13.71±1.36 |
| 29 | 18.77±1.39 | 11.15±1.07 | 3.07±1.52 |
| 54 | 2.93±1.08 | 3.18±0.95 | 2.71±1.10 |
| 55 | 13.33±1.57 | 10.42±1.38 | 3.86±0.98 |
| 56 | >20.23 | >20.23 | >20.23 |
| 58 | 10.58±2.01 | 7.81±1.74 | 7.20±1.56 |
| 190 | 18.55±2.33 | 18.52±2.52 | 10.42±2.64 |
| 191 | 11.07±1.92 | 7.52±1.19 | 13.81±1.82 |
| 193 | 2.70±0.73 | 3.39±1.45 | 2.69±1.08 |
| 5-FU [b] | 7.38 + 0.83 | 5.29±0.32 | 7.77±1.24 |
5-Fluorouracil (5-FU): positive control.
An average of three determinations.
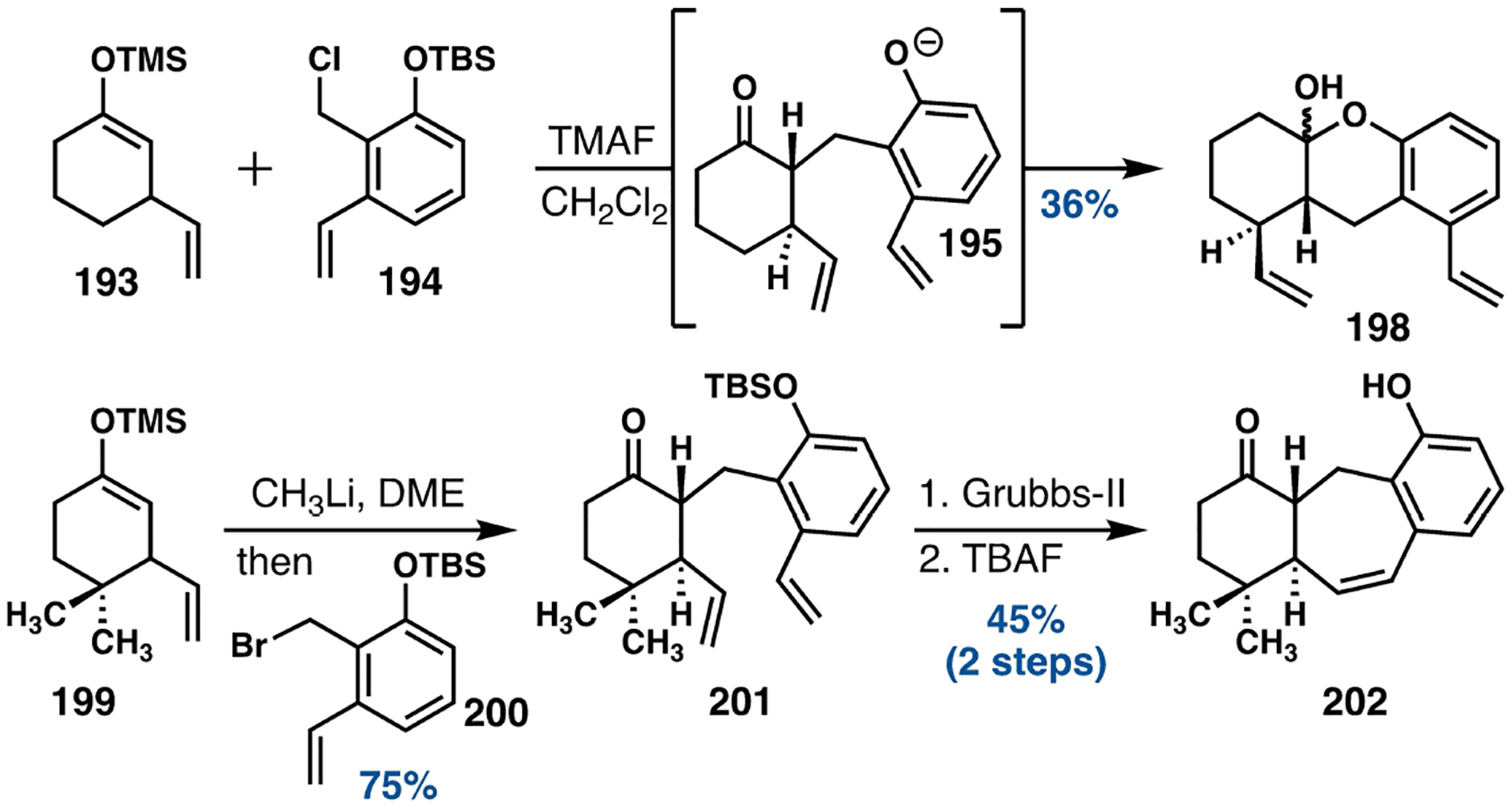
In 2018, Chain and coworkers attempted to employ their ortho-quinone methide-based methodology to construct the core structure of icetexanes (Scheme 28).[103,104] Exposure of a mixture of the silyl enol ether 193 and the silyloxybenzyl chloride 194 to tetramethylammonium fluoride was successful in inducing a diastereoselective alkylation reaction to give the intermediate 195, however subsequent condensation of the resultant phenol or phenoxide upon the carbonyl function generated the robust hemiacetal 198; neither acidic nor basic reaction conditions could facilitate the isolation of 198 in its open form. Chain and his group addressed this problem by employing a more conventional alkylation reaction strategy. Treatment of the silyl enol ether 199 with methyl lithium revealed the corresponding lithium enolate, which upon exposure to the electrophile 200 generated 201. The silyl group prevented any unproductive acetal formation, and the icetexane core 202 was then completed upon treatment of 201 with the 2nd generation Grubbs catalyst[105] followed by a desilylation reaction.
Scheme 28.
An enolate-based approach to the icetexane core by Chain and coworkers.
In 2019, Oh and coworkers reported a Heck reaction-based strategy for the synthesis of taxamairin B (45, Scheme 29).[106] The cyclohexanone 203 was converted to the corresponding lithium enolate upon treatment with lithium N,N-diisopropylamide (LDA), which underwent smooth alkylation with the o-bromobenzyl bromide 204 to furnish the benzylated cyclohexanone 205. A 1,2-addition of an allyl group under Barbier conditions generated a mixture of diastereoisomeric tertiary alcohols 206, the configuration of which is inconsequential.[107] The intramolecular Heck reaction[108] forged the seven-membered ring to generate 207, which was then subjected to an oxidative cleavage of the exocyclic alkene using a modified OsO4-NaIO4 protocol.[109] Dehydration of 208 and deletion of the only stereogenic center by exposure to 6 N HCl in acetone afforded 209 which gave rise to taxamairin B (45) under the oxidative action of DDQ.
Scheme 29.
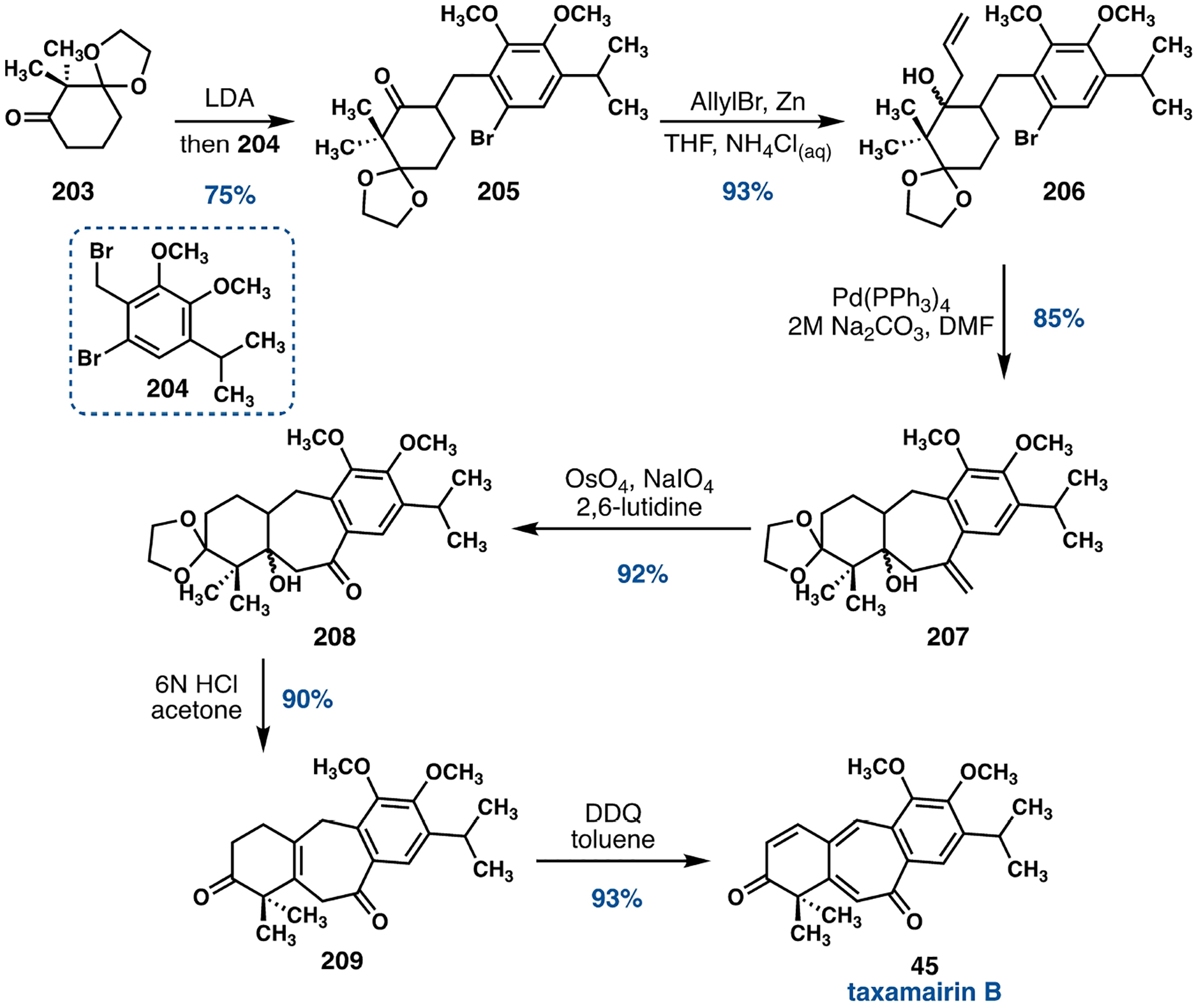
Heck strategy developed by Oh and coworkers for the synthesis of taxamairin B (45).
In 2020 Oh and coworkers reported cycloisomerization-based approaches to the syntheses of taxamairin B (45) and (±)-rosmaridiphenol (61).[110] They employed a gold-catalyzed cycloisomerization of diynals and enynals toward complex 6–7-n tricyclic systems.[111] After testing different reaction conditions, it was discovered that treating compound 210 with AuBr3 in 1,2-dichloroethane (DCE) as the solvent would result in the tricyclic structure of icetexane 211 (Scheme 30) which is an intermediate toward the synthesis of rosmaridiphenol (61). Hydrogenation of 211 generated a diastereoisomeric mixture of cis and trans ketones 212; the thermodynamically-favored trans 212 product could be obtained exclusively by prolonged exposure of the mixture to potassium tbutoxide in t-butanol to give the desired stereoisomer in 74% overall yield from 211. Demethylation of trans 211 so obtained produced (±)-rosmaridiphenol (61).
Scheme 30.

Cycloisomerization-based approach to rosmaridiphenol (61) by Oh and coworkers.
Similarly, treating the regioisomeric ketone 213 with AuCOCl furnished 214, which has the 6-7-6 core structure of taxamairin B (45, Scheme 31). A mild oxidation of 214 using IBX resulted in diketone 215 which was converted to 216 upon treating with DDQ. Prolonged treatment of 216 with DDQ generated taxamairin B (45).
Scheme 31.

Cycloisomerization-based approach to taxamairin B (45) by Oh and coworkers.
In 2020, Qiu and coworkers reported a cycloaddition-based approach toward (±)-euolutchuol E (188), (±)-przewalskin E (55), and (±)-brussonol (56, Scheme 32).[112] A tandem oxopyrylium ion [5+2]/Diels–Alder[113–115] cycloaddition sequence joined 217 and 218 to afford the polycylic icetexane scaffold 219 in a single laboratory operation in 64% isolated yield. A hydroboration-oxidation sequence followed by a base-mediated elimination furnished 220 in 62% yield over three steps, which was aromatized upon treatment with selenium dioxide in aqueous dioxane. Protection of the phenol 221 as the corresponding methyl ether by treatment with iodomethane and reduction of the ketone function under the action of sodium borohydride (NaBH4) generated 222. The natural product (±)-euolutchuol E (223) was prepared after a radical-mediated excision of the secondary alcohol followed by deprotection of the phenols with nucleophilic thiol (83 %, three steps).[116] Ortho-selective oxygenation of 223 furnished (±)-przewalskin E (55), which could be gently reduced to (±)-brussonol (56) in 94% isolated yield under the action of sodium thionite (Na2S2O4).
Scheme 32.

Cycloaddition-based approach to (±)-euolutchuol E (223), (±)-przewalskin E (55), and (±)-brussonol (56) developed by Qiu and coworkers.
In 2021, Gao and coworkers reported a photochemical approach to construct the icetexane core reliant upon a chiral TADDOL type ligand (225) to affect asymmetric induction in a key cycloaddition event (Scheme 33).[117] An asymmetric photoenolization/Diels–Alder reaction[118] between the fully substituted aromatic aldehyde 224 and the enone 223 furnished the tricyclic system 226 in 94% yield and 20:1 dr. Oxidization to the corresponding ketone 227 was followed by a three-step sequence wherein the benzyl protecting group was removed followed by selective reduction of the aldehyde function conversion of the resultant alcohol to the iodide 228 by an Appel reaction[85] in 84% overall yield. The final step of constructing the icetexane core was a radical-mediated ring expansion reaction reminiscent of biomimetic approaches to generate 229 in 68% isolated yield.
Scheme 33.

A photochemical approach to the icetexane core developed by Gao and coworkers.
In 2022, Oh and coworkers described a streamlined racemic approach to the icetexane core by transforming a more elaborated abietane scaffold by a ring expansion strategy (Scheme 34).[119] Exposure of the abietane 230 to trifluoromethanesulfonic anhydride (Tf2O) with pyridine as base successfully facilitated the cationic ring-expansion process to afford the 6-7-6 scaffold 231. Hydrogenative reduction of the alkene function under the action of palladium on carbon and hydrogen gas followed by deprotection of the phenol with boron tribromide (BBr3) the known intermediate 178 (Scheme 18, vide infra) in 80% yield over two steps. During the reduction-deprotection process, the cis fused ring system within 231 is epimerized to the more stable trans fused isomer, setting the stage for the elaboration of 178 into (±)-rosmaridiphenol (61), (±)-barbatusol (36), and (±)-pisiferin (15) in processes very similar to those previously described.
Scheme 34.

An approach to racemic icetexanes from an abietane by Oh and coworkers.
9. Conclusion
Over the last 13 years, over 50 novel icetexanes have been reported and more than a dozen new synthetic strategies have been described for their laboratory preparation. These structurally interesting terrestrial natural products exhibit promising biological activities and are inspiring new leads for novel therapeutics in the context of human cancer, pain and inflammation management, and antimicrobial agents. They will no doubt continue to pique the interest of synthetic chemists in the coming years and will inspire new approaches and fruitful methodological developments.
Acknowledgements
Financial support from the National Institutes of Health (P20GM104316) is gratefully acknowledged.
Biographies
Ali Amiri Naeini was born and grew up in Iran. He received his BS in Chemistry from Sharif University of Technology in 2015, and then moved to the United States of America to begin his doctoral studies at the University of Delaware under the guidance of Prof. William Chain studying the synthesis of monomeric and dimeric icetexane natural products. After defending his thesis in 2022, Ali begun his postdoctoral studies in organic synthesis with Prof. Mohammad Movassaghi at the Massachusetts Institute of Technology.

Alexandre Ziegelmeier was born in Delaware and raised in the central Pennsylvania area. He received his BS in Chemistry from West Virginia University in 2020 and is currently pursuing his Ph.D. from the University of Delaware under the direction of Prof. William Chain studying the synthesis of monomeric and dimeric icetexane natural products.

William Chain received his BS in Chemistry from Pennsylvania State University and his Ph.D. in Chemistry from Harvard University. After a postdoctoral appointment at Princeton University, he was appointed Assistant Professor at the University of Hawaii in 2009. In 2015, he was appointed Associate Professor at the University of Delaware. His current research interests are natural products total synthesis, methods development based around latent aromatic systems and carbonyl chemistry.

Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
References
- [1].Simmons EM, Sarpong R, ‘Structure, biosynthetic relationships and chemical synthesis of the icetexane diterpenoids’, Nat. Prod. Rep 2009, 26, 1195–1217. [DOI] [PubMed] [Google Scholar]
- [2].El-Lakany AM, Abdel-Kader MS, Sabri NN, Stermitz FR, ‘Lanigerol: a new antimicrobial icetexane diterpene from Salvia lanigera’, Planta Med 1995, 61, 559–560. [DOI] [PubMed] [Google Scholar]
- [3].Salae A-W, Rodjun A, Karalai C, Ponglimanont C, Chantrapromma S, Kanjana-Opas A, Tewtrakul S, Fun H-K, ‘Potential anti-inflammatory diterpenes from Premna obtusifolia’, Tetrahedron 2012, 68, 819–829. [Google Scholar]
- [4].Uchiyama N, Kiuchi F, Ito M, Honda G, Takeda Y, Khodzhimatov OK, Ashurmetov OA, ‘New icetexane and 20 norabietane diterpenes with trypanocidal activity from Dracocephalum komarovi’, J. Nat. Prod 2003, 66, 128–131. [DOI] [PubMed] [Google Scholar]
- [5].N. VGM, Atmakur H, Katragadda SB, Devabakthuni B, Kota A, C. K. S, Kuncha M, V. PSVM, Kulkarni P, Janaswamy MR, Sistla R, ‘Antioxidant, hepatoprotective and cytotoxic effects of icetexanes isolated from stem-bark of Premna tomentosa’, Phytomedicine 2014, 21, 497–505. [DOI] [PubMed] [Google Scholar]
- [6].Zheng G, Kadir A, Zheng X, Jin P, Liu J, Maiwulanjiang M, Yao G, Aisa HA, ‘Spirodesertols A and B, two highly modified spirocyclic diterpenoids with an unprecedented 6-isopropyl-3H-spiro[benzofuran-2,1′-cyclohexane] motif from Salvia deserta’, Org. Chem. Front 2020, 7, 3137–3145. [Google Scholar]
- [7].Watson WH, Taira Z, Dominguez XA, Gonzales H, Guiterrez M, Aragon R, ‘Isolation and structure of two diterpene quinones from Salvia Ballotaeflora Benth (Labiatae)’, Tetrahedron Lett 1976, 17, 2501–2502. [Google Scholar]
- [8].Esquivel B, Burgueno-Tapia E, Bustos-Brito C, Perez-Hernandez N, Quijano L, Joseph-Nathan P, ‘Absolute configuration of the diterpenoids icetexone and conacytone from Salvia ballotaeflora’, Chirality 2018, 30, 177–188. [DOI] [PubMed] [Google Scholar]
- [9].Kang J, Quynh Le T, Oh CH, ‘Recent advances in abietane/icetexane synthesis’, Tetrahedron Lett 2022, 108, 154133. [Google Scholar]
- [10].Matsumoto T, Imai S, Yoshinari T, Matsuno S, ‘The Total Synthesis of (±)-Pisiferin’, Bull. Chem. Soc. Jpn 1986, 59, 3103–3108. [Google Scholar]
- [11].Matsumoto T, Usui S, ‘A Simple Total Synthesis of (+)-Ferruginol, (+)-Sempervirol, and (+)-Podocarpa-8(14)-en-13-one’, Bull. Chem. Soc. Jpn 1979, 52, 212–215. [Google Scholar]
- [12].Schöllkopf U, ‘Neuere präparative Methoden der organ-ischen Chemie II 13. Carbonyl-Olefinierung mit Triphenylphosphin-methylenen Wittig-Reaktion’, Angew. Chem 1959, 71, 260–273. [Google Scholar]
- [13].Crandall J, Apparu M, Org. React 1983, 29, 345–443. [Google Scholar]
- [14].Majetich G, Li Y, Zou G, ‘Total Synthesis of (±)-Komaroviquinone’, Heterocycles 2007, 73, 227–235. [Google Scholar]
- [15].Majetich G, Yu J, Li Y, ‘Total Synthesis of (+)-Komaroviquinone’, Heterocycles 2007, 73, 217–225. [Google Scholar]
- [16].Isler O, Montavon M, Rüegg R, Zeller P, ‘Synthesen in der Carotinoid-Reihe. 2. Mitteilung. Über den Aufbau der Ringkomponenten’, Helv. Chim. Acta 1956, 39, 259–273. [Google Scholar]
- [17].Stork G, Danheiser RL, ‘Regiospecific alkylation of cyclic.beta.-diketone enol ethers. General synthesis of 4-alkylcyclohexenones’, J. Org. Chem 2002, 38, 1775–1776. [Google Scholar]
- [18].Lindlar H, ‘Ein neuer Katalysator für selektive Hydrierungen’, Helv. Chim. Acta 1952, 35, 446–450. [Google Scholar]
- [19].Corey EJ, Bakshi RK, Shibata S, ‘Highly enantioselective borane reduction of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications’, J. Am. Chem. Soc 1987, 109, 5551–5553. [Google Scholar]
- [20].Myers AG, Zheng B, ‘An efficient method for the reductive transposition of allylic alcohols’, Tetrahedron Lett 1996, 37, 4841–4844. [Google Scholar]
- [21].Sánchez C, Cárdenas J, Rodríguez-Hahn L, Ramamoorthy TP, ‘Abietane diterpenoids of Salvia anastomosans’, Phytochemistry 1989, 28, 1681–1684. [Google Scholar]
- [22].González AG, Andrés LS, Luis JG, Brito I, Rodríguez ML, ‘Diterpenes from Salvia mellifera’, Phytochemistry 1991, 30, 4067–4070. [Google Scholar]
- [23].Meerwein H, van Emster K, ‘Über die Gleichgewichts-Isomerie zwischen Bornylchlorid, Isobornylchlorid und Camphen-chlorhydrat’, Ber. Dtsch. Chem. Ges. (A and B Series) 1922, 55, 2500–2528. [Google Scholar]
- [24].Wagner G, Brickner W, ‘Ueber die Beziehung der Pinenhaloïdhydrate zu den Haloïdanhydriden des Borneols’, Ber. Dtsch. Chem. Ges 1899, 32, 2302–2325. [Google Scholar]
- [25].Fraga BM, Diaz CE, Guadano A, Gonzalez-Coloma A, ‘Diterpenes from Salvia broussonetii transformed roots and their insecticidal activity’, J. Agric. Food Chem 2005, 53, 5200–5206. [DOI] [PubMed] [Google Scholar]
- [26].Kelecom A, ‘Isolation, structure determination, and absolute configuration of barbatusol, a new bioactive diterpene with a rearranged abietane skeleton from the labiate coleus barbatus’, Tetrahedron 1983, 39, 3603–3608. [Google Scholar]
- [27].Yatagai M, Takahashi T, ‘New diterpenes from Chamaecyparis pisifera’, Phytochemistry 1980, 19, 1149–1151. [Google Scholar]
- [28].Hasegawa S, Hirose Y, Yatagai M, Takahashi T, ‘On the Structures of Pisiferin and Isopisiferin’, Chem. Lett 1984, 13, 1837–1838. [Google Scholar]
- [29].Liang JY, Min ZD, Iinuma M, Tanaka T, Mizuno M, ‘Two new antineoplastic diterpenes from Taxus mairei’, Chem. Pharm. Bull 1987, 35, 2613–2614. [DOI] [PubMed] [Google Scholar]
- [30].Frontana B, Cárdenas J, Rodríguez-Hahn L, ‘Diterpenoids from Salvia coulteri’, Phytochemistry 1994, 36, 739–741. [Google Scholar]
- [31].Wu XD, He J, Li XY, Dong LB, Gong X, Song LD, Li Y, Peng LY, Zhao QS, ‘Diterpenoids from the twigs and leaves of Fokienia hodginsii’, J. Nat. Prod 2013, 76, 1032–1038. [DOI] [PubMed] [Google Scholar]
- [32].Zhou B, Ren YH, Han YS, Mesplede T, Yue JM, ‘Diverse Types of Diterpenoids with an Aromatized C Ring from the Twigs of Podocarpus imbricatus’, J. Nat. Prod 2020, 83, 2416–2424. [DOI] [PubMed] [Google Scholar]
- [33].Jiang ZY, Yu YJ, Huang CG, Huang XZ, Hu QF, Yang GY, Wang HB, Zhang XY, Li GP, ‘Icetexane diterpenoids from Perovskia atriplicifolia’, Planta Med 2015, 81, 241–246. [DOI] [PubMed] [Google Scholar]
- [34].Aoyagi Y, Takahashi Y, Fukaya H, Takeya K, Aiyama R, Matsuzaki T, Hashimoto S, Kurihara T, ‘Semisynthesis of isetexane diterpenoid analogs and their cytotoxic activity’, Chem. Pharm. Bull 2006, 54, 1602–1604. [DOI] [PubMed] [Google Scholar]
- [35].Aoyagi Y, Takahashi Y, Satake Y, Fukaya H, Takeya K, Aiyama R, Matsuzaki T, Hashimoto S, Shiina T, Kurihara T, ‘Biomimetic synthesis of grandione from demethylsalvicanol via hetero-Diels–Alder type dimerization and structure revision of grandione’, Tetrahedron Lett 2005, 46, 7885–7887. [Google Scholar]
- [36].Majetich G, Zou G, ‘Total synthesis of (−)-barbatusol, (+)-demethylsalvicanol, (−)-brussonol, and (+)-grandione’, Org. Lett 2008, 10, 81–83. [DOI] [PubMed] [Google Scholar]
- [37].Moujir L, Gutiérrez-Navarro AM, San Andrés L, Luis JG, ‘Bioactive Diterpenoids Isolated from Salvia mellifera’, Phytother. Res 1996, 10, 172–174. [Google Scholar]
- [38].Xu G, Peng L-Y, Tu L, Li X-L, Zhao Y, Zhang P-T, Zhao Q-S, ‘Three New Diterpenoids from Salvia przewalskii Maxim’, Helv. Chim. Acta 2009, 92, 409–413. [Google Scholar]
- [39].Tanaka N, Takahashi S, Kajihara S, Tsuji D, Itoh K, Mamadalieva NZ, Kashiwada Y, ‘Diterpenes from an Uzbek medicinal plant Perovskia scrophulariifolia: Their structures and anti-neuroinflammatory activity’, Fitoterapia 2021, 149, 104826. [DOI] [PubMed] [Google Scholar]
- [40].Xu G, Peng LY, Zhao Y, Li XL, Tu L, Zhao QS, Sun HD, ‘Two new icetexane diterpenoids from Salvia przewalskii’, Chem. Pharm. Bull 2005, 53, 1575–1576. [DOI] [PubMed] [Google Scholar]
- [41].Esquivel B, Flores M, Hernández-Ortega S, Toscano RA, Ramamoorthy TP, ‘Abietane and icetexane diterpenoids from the roots of Salvia aspera’, Phytochemistry 1995, 39, 139–143. [Google Scholar]
- [42].Pertino MW, Schmeda-Hirschmann G, ‘The corrected structure of rosmaridiphenol, a bioactive diterpene from Rosmarinus officinalis’, Planta Med 2010, 76, 629–632. [DOI] [PubMed] [Google Scholar]
- [43].Bustos-Brito C, Joseph-Nathan P, Burgueno-Tapia E, Martinez-Otero D, Nieto-Camacho A, Calzada F, Yepez-Mulia L, Esquivel B, Quijano L, ‘Structure and Absolute Configuration of Abietane Diterpenoids from Salvia clinopodioides: Antioxidant, Antiprotozoal, and Antipropulsive Activities’, J. Nat. Prod 2019, 82, 1207–1216. [DOI] [PubMed] [Google Scholar]
- [44].Razak IA, Chantrapromma S, Salae AW, Fun HK, ‘11,12-Dihy-droxy-10,6,8,11,13-icetexapentan-1-one’, Acta Crystallogr 2011, 67, o256–o257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Salae A-W, Boonnak N, ‘Obtusinones D and E, linear and angular fused dimeric icetexane diterpenoids from Premna obtusifolia roots’, Tetrahedron Lett 2013, 54, 1356–1359. [Google Scholar]
- [46].Liu WX, Zhao JW, Zuo AX, Yang Z, Gao L, Zhou M, Jiang ZY, ‘Two novel terpenoids from the cultured Perovskia atriplicifolia’, Fitoterapia 2018, 130, 152–155. [DOI] [PubMed] [Google Scholar]
- [47].Qiu BM, Wang P, Li J, ‘Salprzesides A and B: two novel icetexane diterpenes with antiangiogenic activity from Salvia przewalskii Maxim’, Nat. Prod. Res 2022, 36, 2479–2485. [DOI] [PubMed] [Google Scholar]
- [48].Yang S-J, Fang J-M, Cheng Y-S, ‘Diterpenes from Taxus mairei’, Phytochemistry 1998, 49, 2037–2043. [Google Scholar]
- [49].Xu G, Yang XW, Wu CY, Li XN, Su J, Deng X, Li Y, Qin HB, Yang LX, Zhao QS, ‘Przewalskone: a cytotoxic adduct of a danshenol type terpenoid and an icetexane diterpenoid via hetero-Diels–Alder reaction from Salvia przewalskii’, Chem. Commun 2012, 48, 4438–4440. [DOI] [PubMed] [Google Scholar]
- [50].Tezuka Y, Kasimu R, Basnet P, Namba T, Kadota S, ‘Aldose reductase inhibitory constituents of the root of Salvia miltiorhiza Bunge’, Chem. Pharm. Bull 1997, 45, 1306–1311. [DOI] [PubMed] [Google Scholar]
- [51].Deng R, Zou J, Zhao CL, Yang YM, Ku CF, Zhu Y, Liang ZM, Ye JH, Pan LT, Zhang HJ, ‘Bioactive icetexane and abietane diterpenes from Isodon phyllopodus’, Nat. Prod. Res 2021, 1–9, DOI: 10.1080/14786419.2021.1950716. [DOI] [PubMed] [Google Scholar]
- [52].Zhang HJ, Rumschlag-Booms E, Guan YF, Wang DY, Liu KL, Li WF, Nguyen VH, Cuong NM, Soejarto DD, Fong HHS, Rong L, ‘Potent Inhibitor of Drug-Resistant HIV-1 Strains Identified from the Medicinal Plant Justicia gendarussa’, J. Nat. Prod 2017, 80, 1798–1807. [DOI] [PubMed] [Google Scholar]
- [53].Duong T-H, Bui X-H, Le Pogam P, Nguyen H-H, Tran T-T, Nguyen T-A-T, Chavasiri W, Boustie J, Nguyen K-P-P, ‘Two novel diterpenes from the roots of Phyllanthus acidus (L.) Skeel’, Tetrahedron 2017, 73, 5634–5638. [Google Scholar]
- [54].Tabefam M, Farimani MM, Danton O, Ramseyer J, Kaiser M, Ebrahimi SN, Salehi P, Batooli H, Potterat O, Hamburger M, ‘Antiprotozoal Diterpenes from Perovskia abrotanoides’, Planta Med 2018, 84, 913–919. [DOI] [PubMed] [Google Scholar]
- [55].Hymavathi A, Suresh Babu K, Naidu VG, Rama Krishna S, Diwan PV, Madhusudana Rao J, ‘Bioactivity-guided isolation of cytotoxic constituents from stem-bark of Premna tomentosa’, Bioorg. Med. Chem. Lett 2009, 19, 5727–5731. [DOI] [PubMed] [Google Scholar]
- [56].Suresh G, Babu KS, Rao VRS, Rao MSA, Nayak VL, Ramakrishna S, ‘Novel cytotoxic icetexane diterpenes from Premna latifolia Roxb’, Tetrahedron Lett 2011, 52, 1273–1276. [Google Scholar]
- [57].Suresh G, Suresh Babu K, Suri Appa Rao M, Rama Subba Rao V, Ashok Yadav P, Lakshma Nayak V, Ramakrishna S, ‘Premnalatifolin A, a novel dimeric diterpene from Premna latifolia Roxb’, Tetrahedron Lett 2011, 52, 5016–5019. [Google Scholar]
- [58].Ayinampudi SR, Domala R, Merugu R, Bathula S, Janaswamy MR, ‘New icetexane diterpenes with intestinal alpha-glucosidase inhibitory and free-radical scavenging activity isolated from Premna tomentosa roots’, Fitoterapia 2012, 83, 88–92. [DOI] [PubMed] [Google Scholar]
- [59].Xia F, Du SZ, Wu MK, Liu R, Ye YS, Yang J, Xu G, Nian Y, ‘Icetexane diterpenoids as Cav3.2 T-type calcium channel inhibitors from Salvia prattii and analgesic effect of their Semi-synthesized derivatives’, Bioorg. Chem 2022, 128, 106059. [DOI] [PubMed] [Google Scholar]
- [60].Chen HL, Lin KW, Gan KH, Wang JP, Won SJ, Lin CN, ‘New diterpenoids and cytotoxic and anti-inflammatory diterpenoids from Amentotaxus formosana’, Fitoterapia 2011, 82, 219–224. [DOI] [PubMed] [Google Scholar]
- [61].Arslanian RL, Bailey DT, Kent MC, Richheimer SL, Thornburg KR, Timmons DW, Zheng QY, ‘Brevitaxin, a new diterpenolignan from the bark of Taxus brevifolia’, J. Nat. Prod 1995, 58, 583–585. [DOI] [PubMed] [Google Scholar]
- [62].Li H, Liang YR, Chen SX, Wang WX, Zou Y, Nuryyeva S, Houk KN, Xiong J, Hu JF, ‘Amentotaxins C−V, Structurally Diverse Diterpenoids from the Leaves and Twigs of the Vulnerable Conifer Amentotaxus argotaenia and Their Cytotoxic Effects’, J. Nat. Prod 2020, 83, 2129–2144. [DOI] [PubMed] [Google Scholar]
- [63].Esquivel B, Bustos-Brito C, Sanchez-Castellanos M, Nieto-Camacho A, Ramirez-Apan T, Joseph-Nathan P, Quijano L, ‘Structure, Absolute Configuration, and Antiproliferative Activity of Abietane and Icetexane Diterpenoids from Salvia ballotiflora’, Molecules 2017, 22, 1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Esquivel B, Calderón J, Flores E, Sánchez A-A, Rosas Rivera R, ‘Abietane and icetexane diterpenoids from Salvia ballotaeflora and Salvia axillaris’, Phytochemistry 1997, 46, 531–534. [Google Scholar]
- [65].Cárdenas J, Rodríguez-Hahn L, ‘Abietane and icetexane diterpenoids from Salvia candicans’, Phytochemistry 1995, 38, 199–204. [Google Scholar]
- [66].Carlson RP, O’Neill-Davis L, Chang J, Lewis AJ, ‘Modulation of mouse ear edema by cyclooxygenase and lipoxygenase inhibitors and other pharmacologic agents’, Agents Actions 1985, 17, 197–204. [DOI] [PubMed] [Google Scholar]
- [67].Cezarotto CS, Dorneles A, Baldissera FG, da Silva MB, Markoski MM, Junior LCR, Peres A, Fazolo T, Bordignon SAL, Apel MA, Romao PRT, von Poser GL, ‘Leishmanicidal and antichemotactic activities of icetexanes from Salvia uliginosa Benth’, Phytomedicine 2019, 58, 152748. [DOI] [PubMed] [Google Scholar]
- [68].Perez-Gutierrez S, Zavala-Mendoza D, Hernandez-Munive A, Mendoza-Martinez A, Perez-Gonzalez C, Sanchez-Mendoza E, ‘Antidiarrheal activity of 19-deoxyicetexone isolated from Salvia ballotiflora Benth in mice and rats’, Molecules 2013, 18, 8895–8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Carita A, Burtoloso ACB, ‘An epoxide ring-opening approach for a short and stereoselective synthesis of icetexane diterpenoids’, Tetrahedron Lett 2010, 51, 686–688. [Google Scholar]
- [70].Corey EJ, Chaykovsky M, ‘Dimethylsulfoxonium Methylide’, J. Am. Chem. Soc 1962, 84, 867–868. [Google Scholar]
- [71].Pappo R, Allen JD, Lemieux R, Johnson W, ‘Notes – Osmium Tetroxide-Catalyzed Periodate Oxidation of Olefinic Bonds’, J. Org. Chem 1956, 21, 478–479. [Google Scholar]
- [72].Marson CM, Campbell J, Hursthouse MB, Malik KMA, ‘Stereocontrolled Routes to Bridged Ethers by Tandem Cyclizations’, Angew. Chem. Int. Ed 1998, 37, 1122–1124; [DOI] [PubMed] [Google Scholar]; Angew. Chem 1998, 110, 1170–1172. [Google Scholar]
- [73].Ahmad A, Burtoloso ACB, ‘Total Synthesis of (+/−)-Brussonol and (+/−)-Komaroviquinone via a Regioselective Cross-Electrophile Coupling of Aryl Bromides and Epoxides’, Org. Lett 2019, 21, 6079–6083. [DOI] [PubMed] [Google Scholar]
- [74].Martinez-Solorio D, Jennings MP, ‘Convergent formal syntheses of (+/−)-Brussonol and (+/−)-Abrotanone via an Intramolecular Marson-type cyclization’, Org. Lett 2009, 11, 189–192. [DOI] [PubMed] [Google Scholar]
- [75].Fetizon M, Balogh V, Golfier M, ‘Oxidations with silver carbonate/celite. V. Oxidations of phenols and related compounds’, J. Org. Chem 1971, 36, 1339–1341. [Google Scholar]
- [76].Suto Y, Kaneko K, Yamagiwa N, Iwasaki G, ‘A short and efficient asymmetric synthesis of komaroviquinone’, Tetrahedron Lett 2010, 51, 6329–6330. [Google Scholar]
- [77].Negishi E.-i., Tan Z, Liou S-Y, Liao B, ‘Strictly Regiocontrolled α-Monosubstitution of Cyclic Carbonyl Compounds with Alkynyl and Alkyl Groups via Pd-Catalyzed Coupling of Cyclic α-Iodoenones with Organozincs’, Tetrahedron 2000, 56, 10197–10207. [Google Scholar]
- [78].Cortez Fde J, Sarpong R, ‘Ga(III)-catalyzed cycloisomerization approach to (+/−)-icetexone and (+/−)-epi-icetexone’, Org. Lett 2010, 12, 1428–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Claisen L, Lowman O, ‘Ueber eine neue Bildungsweise des Benzoylessigäthers’, Ber. Dtsch. Chem. Ges 1887, 20, 651–654. [Google Scholar]
- [80].Ghaffar T, Parkins AW, ‘The catalytic hydration of nitriles to amides using a homogeneous platinum phosphinito catalyst’, J. Mol. Catal. A 2000, 160, 249–261. [Google Scholar]
- [81].Majetich G, Grove JL, ‘Total synthesis of (+)-19-deoxyicetexone, (−)-icetexone, and (+)-5-epi-icetexone’, Org. Lett 2009, 11, 2904–2907. [DOI] [PubMed] [Google Scholar]
- [82].de Jesus Cortez F, Lapointe D, Hamlin AM, Simmons EM, Sarpong R, ‘Synthetic studies on the icetexones: enantioselective formal syntheses of icetexone and epi-icetexone’, Tetrahedron 2013, 69, 5665–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Jan N-W, Liu H-J, Hsieh M-T, Shia K-S, ‘Total Synthesis of (−)-Isopisiferin: Confirmation of Absolute Configuration’, Eur. J. Org. Chem 2010, 2010, 4271–4275. [Google Scholar]
- [84].Chen DL, Liu XY, Cheng H, Wang FP, ‘Rapid construction of the tricyclic cores of the abietane- and icetexane-type diterpenoids’, Chin. Chem. Lett 2011, 22, 774–776. [Google Scholar]
- [85].Appel R, ‘Tertiary Phosphane/Tetrachloromethane, a Versatile Reagent for Chlorination, Dehydration, and P?N Linkage’, Angew. Chem. Int. Ed 1975, 14, 801–811; [Google Scholar]; Angew. Chem 1975, 87, 863–874. [Google Scholar]
- [86].Nicholas KM, Pettit R, ‘An alkyne protecting group’, Tetrahedron Lett 1971, 12, 3475–3478. [Google Scholar]
- [87].Green J, Kolodziej I, ‘Vinylogous Nicholas Reactions in the Synthesis of Icetexane, Faveline, and Related Ring Systems’, Synlett 2011, 16, 2397–2401. [Google Scholar]
- [88].Kira K, Tanda H, Hamajima A, Baba T, Takai S, Isobe M, ‘Mechanistic studies on the hydrosilylation of an acetylene cobalt complex; trapping an active catalyst Co2(CO)6 causing olefin-isomerization and O-silylation’, Tetrahedron 2002, 58, 6485–6492. [Google Scholar]
- [89].Kolodziej I, Green JR, ‘Vinylogous Nicholas reactions in the synthesis of bi- and tricyclic cycloheptynedicobalt complexes’, Org. Biomol. Chem 2015, 13, 10852–10864. [DOI] [PubMed] [Google Scholar]
- [90].Li Y, Li L, Guo Y, Xie Z, ‘Concise total synthesis of (±)-pisiferin’, Tetrahedron 2015, 71, 9282–9286. [Google Scholar]
- [91].Jiao ZW, Tu YQ, Zhang Q, Liu WX, Zhang SY, Wang SH, Zhang FM, Jiang S, ‘Tandem C−H oxidation/cyclization/rearrangement and its application to asymmetric syntheses of (−)-brussonol and (−)-przewalskine E’, Nat. Commun 2015, 6, 7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Nisigaki E, Sugamoto K, Nishida M, Matsushita Y-I, ‘Semisynthesis of (−)-Barbatusol, (+)-Demethylsalvicanol, and (+)-Rosmaridiphenol from (+)-Pisiferic Acid’, Chem. Lett 2016, 45, 746–748. [Google Scholar]
- [93].Kametani T, Kondoh H, Tsubuki M, Honda T, ‘Synthesis of (±)-pisiferin, (±)-pisiferol, and related compounds by intramolecular [4+2]cycloaddition’, J. Chem. Soc. Perkin Trans 1 1990, 5–10. [Google Scholar]
- [94].Tada M, Ohkanda T, Kurabe J, ‘Syntheses of carnosic acid and carnosol, anti-oxidants in Rosemary, from pisiferic acid, the major constituent of Sawara’, Chem. Pharm. Bull 2010, 58, 27–29. [DOI] [PubMed] [Google Scholar]
- [95].Oh CH, Piao L, Jung J, Kim J, ‘A Formal Synthesis of Komaroviquinone: Use of a Pt-Catalyzed Hydrative Cyclization Reaction’, Asian J. Org. Chem 2016, 5, 1237–1241. [Google Scholar]
- [96].Thommen C, Neuburger M, Gademann K, ‘Collective Syntheses of Icetexane Natural Products Based on Biogenetic Hypotheses’, Chem. Eur. J 2017, 23, 120–127. [DOI] [PubMed] [Google Scholar]
- [97].Amaya T, Hifumi M, Okada M, Shimizu Y, Moriuchi T, Segawa K, Ando Y, Hirao T, ‘Synthesis of oxosumanenes through benzylic oxidation’, J. Org. Chem 2011, 76, 8049–8052. [DOI] [PubMed] [Google Scholar]
- [98].Cao W, Liu T, Yang S, Liu M, Pan Z, Zhou Y, Deng X, ‘Efficient Synthesis of Icetexane Diterpenes and Apoptosis Inducing Effect by Upregulating BiP-ATF4-CHOP Axis in Colorectal Cells’, J. Nat. Prod 2021, 84, 2012–2019. [DOI] [PubMed] [Google Scholar]
- [99].Mitsunobu O, Yamada M, ‘Preparation of Esters of Carboxylic and Phosphoric AcidviaQuaternary Phosphonium Salts’, Bull. Chem. Soc. Jpn 1967, 40, 2380–2382. [Google Scholar]
- [100].Demir AS, ‘Tetramethyldiamidophosphoric acid chloride mediated epoxide-diene conversion and steroidal aromatization’, Tetrahedron 2001, 57, 227–233. [Google Scholar]
- [101].Galli B, Gasparrini F, Lanzotti V, Misiti D, Riccio R, Villani C, Guan-fu H, Zhong-wu M, Wan-fen Y, ‘Grandione, a new heptacyclic dimeric diterpene from Torreya grandis Fort’, Tetrahedron 1999, 55, 11385–11394. [Google Scholar]
- [102].Quijano-Quinones RF, Castro-Segura CS, Mena-Rejon GJ, Quesadas-Rojas M, Caceres-Castillo D, ‘Biosynthesis of Grandione: An Example of Tandem Hetero Diels–Alder/Retro-Claisen Rearrangement Reaction?’, Molecules 2018, 23, 2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Lewis RS, Garza CJ, Dang AT, Pedro TK, Chain WJ, ‘Michael Additions of Highly Basic Enolates to ortho-Quinone Methides’, Org. Lett 2015, 17, 2278–2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Moon DJ, Al-Amin M, Lewis RS, Arnold KM, Yap GPA, Sims-Mourtada J, Chain WJ, ‘A Strategy toward Icetexane Natural Products’, Eur. J. Org. Chem 2018, 2018, 3348–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Scholl M, Ding S, Lee CW, Grubbs RH, ‘Synthesis and activity of a new generation of ruthenium-based olefin metathesis catalysts coordinated with 1,3-dimesityl-4,5-dihydroimidazol-2-ylidene ligands’, Org. Lett 1999, 1, 953–956. [DOI] [PubMed] [Google Scholar]
- [106].Le TQ, Karmakar S, Lee S, Chai U, Le MH, Oh CH, ‘Generation of the Icetexane Core by Use of a Heck Strategy: Total Synthesis of Taxamairin B’, ChemistrySelect 2019, 4, 11926–11929. [Google Scholar]
- [107].Li C-J, ‘Aqueous Barbier-Grignard type reaction: Scope, mechanism, and synthetic applications’, Tetrahedron 1996, 52, 5643–5668. [Google Scholar]
- [108].Heck RF, ‘Acylation, methylation, and carboxyalkylation of olefins by Group VIII metal derivatives’, J. Am. Chem. Soc 1968, 90, 5518–5526. [Google Scholar]
- [109].Yu W, Mei Y, Kang Y, Hua Z, Jin Z, ‘Improved procedure for the oxidative cleavage of olefins by OsO4-NaIO4’, Org. Lett 2004, 6, 3217–3219. [DOI] [PubMed] [Google Scholar]
- [110].Le QT, Guo L, Lee SL, Lee J, Oh CH, ‘Gold-Catalyzed Synthesis of Icetexane Cores: Short Synthesis of Taxamairin B and Rosmaridiphenol’, Org. Lett 2020, 22, 9225–9228. [DOI] [PubMed] [Google Scholar]
- [111].Kim N, Kim Y, Park W, Sung D, Gupta AK, Oh CH, ‘Gold-catalyzed cycloisomerization of o-alkynylbenzaldehydes with a pendant unsaturated bond: [3+2] cycloaddition of gold-bound 1,3-dipolar species with dipolarophiles’, Org. Lett 2005, 7, 5289–5291. [DOI] [PubMed] [Google Scholar]
- [112].Zhang J, Jin Y, Qiu FG, ‘Tandem [5+2]/[4+2] Cycloadditions To Construct the [6-7-6] Tricyclic Skeleton of Icetexane Diterpenes: Total Synthesis of Euolutchuol E, Przewalskine E and Brussonol’, Org. Lett 2020, 22, 7415–7418. [DOI] [PubMed] [Google Scholar]
- [113].Burns NZ, Witten MR, Jacobsen EN, ‘Dual catalysis in enantioselective oxidopyrylium-based [5+2] cycloadditions’, J. Am. Chem. Soc 2011, 133, 14578–14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Diels O, Alder K, ‘Über die Ursachen der Azoesterreaktion’, Justus Liebigs Ann. Chem 1926, 450, 237–254. [Google Scholar]
- [115].Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G, ‘The Diels–Alder Reaction in Total Synthesis’, Angew. Chem. Int. Ed 2002, 41, 1668–1698; [DOI] [PubMed] [Google Scholar]
- Angew. Chem 2002, 114, 1742–1773. [Google Scholar]
- [116].Inaba Y, Hasuda T, Hitotsuyanagi Y, Aoyagi Y, Fujikawa N, Onozaki A, Watanabe A, Kinoshita T, Takeya K, ‘Abietane diterpenoids and a sesquiterpene pyridine alkaloid from Euonymus lutchuensis’, J. Nat. Prod 2013, 76, 1085–1090. [DOI] [PubMed] [Google Scholar]
- [117].Yang B, Wen G, Zhang Q, Hou M, He H, Gao S, ‘Asymmetric Total Synthesis and Biosynthetic Implications of Perovskones, Hydrangenone, and Hydrangenone B’, J. Am. Chem. Soc 2021, 143, 6370–6375. [DOI] [PubMed] [Google Scholar]
- [118].Cuadros S, Melchiorre P, ‘Organocatalytic Strategies to Stereoselectively Trap Photochemically Generated Hydroxy-o-quinodimethanes’, Eur. J. Org. Chem 2018, 2018, 2884–2891. [Google Scholar]
- [119].Oh CH, Le TQ, Lee J, ‘Synthesis of the Icetexane Diterpenoids (±)-Rosmaridiphenol, (±)-Pisiferin, and (±)-Barbatusol from Abietane’, Synlett 2022, 33, 983–987. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


