Abstract
Pancreatic β-cell-type-specific and glucose-inducible transcription of the insulin gene is mediated by the basic helix-loop-helix factors that bind to and activate expression from an E-box element within its enhancer. The E-box activator is a heteromeric complex composed of a β-cell-enriched factor, BETA2/NeuroD, and ubiquitously distributed proteins encoded by the E2A and HEB genes. Previously, we demonstrated that the adenovirus type 5 E1A proteins repressed stimulation by the E-box activator in β cells. In this study, our objective was to determine how E1A repressed activator function. The results indicate that E1A reduces activation by binding to and sequestering the p300 cellular coactivator protein. Thus, we show that expression of p300 in β cells can relieve inhibition by E1A, as well as potentiate activation by the endogenous insulin E-box transcription factors. p300 stimulated activation from GAL4 (amino acids 1 to 147) fusion constructs of either BETA2/NeuroD or the E2A-encoded E47 protein. The sequences spanning the activation domains of BETA2/NeuroD (amino acids 156 to 355) and E47 (amino acids 1 to 99 and 325 to 432) were required for this response. The same region of BETA2/NeuroD was shown to be important for binding to p300 in vitro. The sequences of p300 involved in E47 and BETA2/NeuroD association resided between amino acids 1 and 1257 and 1945 and 2377, respectively. A mutation in p300 that abolished binding to BETA2/NeuroD also destroyed the ability of p300 to activate insulin E-box-directed transcription in β cells. Our results indicate that physical and functional interactions between p300 and the E-box activator factors play an important role in insulin gene transcription.
Insulin plays an essential role in mammals in the regulation of carbohydrate, protein, and fatty acid metabolism. In adults, insulin is expressed exclusively in pancreatic β cells, the major endocrine cell type in the islet of Langerhans which are distributed throughout the pancreas. A deficiency in the production of insulin causes diabetes mellitus, a complex and debilitating disease that if untreated is eventually fatal. Pancreatic β cell-type-specific transcription of the insulin gene appears to be principally controlled by the trans-acting factors that act upon its enhancer, which is located between nucleotides −340 to −91 relative to the transcription start site (reviewed in references 59 and 70). Detailed mutagenesis studies of this region indicates that selective expression is predominantly mediated by the A3 (−201 to −196 bp) (18, 52, 54), C1 (−115 to −107 bp) (64), and E-box (−100 to −91 bp) (9, 28, 72) elements. (These insulin cis elements are labeled in accordance with the nomenclature proposed by German et al. [20].) The factors that act on these sites also control glucose-inducible transcription (19, 37, 39, 54, 61), the primary metabolic regulator of insulin expression in vivo.
The activators that regulate A3 and E-box element-directed transcription have been isolated, although the C1 activator gene (cDNA) has not. The A3 element is regulated by PDX-1 (48, 51, 52, 54), a homeoprotein that is selectively expressed in the pancreas and duodenum (23). (This factor has also been referred to as IPF-1 [48], STF-1 [33], and IDX-1 [40] but was renamed PDX-1 [for pancreas and duodenal homeobox gene-1] by the International Nomenclature Committee of Standardized Genetic Nomenclature in Mice and will be referred to as such here.) The positive regulator of E-directed transcription is composed BETA2/NeuroD (44) and the E2A- and HEB-encoded proteins (3, 8, 17, 59, 64) of the basic helix-loop-helix (bHLH) family. This activator is typical of other tissue-specific activators of the bHLH class, the best characterized of which are the myogenic bHLH activators (i.e., MyoD, myogenin, myf-5, and MRF-4) (reviewed in reference 41), as it functions in a heteromeric complex of ubiquitously distributed and tissue-enriched proteins (44). The E2A (3, 8, 17)- and HEB (55)-encoded gene products represent the generally distributed bHLH proteins present in the E-box activator, and BETA2/NeuroD (44) represents the more selectively distributed protein. BETA2/NeuroD is expressed in pancreatic islet endocrine cells (42, 43), the intestine (42), and a subset of neurons in the central and peripheral nervous system (30). Interestingly, this factor was independently isolated and characterized by its ability to activate insulin gene transcription (termed BETA2 [43]) and neurite formation upon ectopic expression in Xenopus embryos (termed NeuroD [30]). BETA2/NeuroD also appears to be important for secretin gene transcription in the intestine (42).
Gene targeting experiments have also established an important role for PDX-1 and BETA2/NeuroD in pancreatic development. Thus, homozygous pdx-1−/− mutant mice are apancreatic (26, 46), whereas the absence of BETA2/NeuroD results in a block in islet morphogenesis and the loss of secretin- and cholecystokinin-producing enteroendocrine cells in the intestine (43). In both cases, insulin gene expression was severely reduced in the homozygous mutant animals, which develop severe diabetes and die within a few days of birth. Collectively, these studies clearly demonstrated that BETA2/NeuroD and PDX-1 played a key role during pancreatic development and in islet-specific gene expression, although the molecular mechanisms that are important in these events are poorly understood.
Insight into the cellular proteins that mediate insulin E-activator stimulation has been provided in studies with the adenovirus type 5 E1A oncoprotein. E1A represses the activation directed by the insulin enhancer in β cells, and a specific target is the E-box element (68). In addition, E1A mutants that were unable to bind to the p300 family of cellular transcriptional coactivators, which includes p300 and the closely related CREB-binding protein (CBP) (36), were unable to repress insulin enhancer activation (69). In contrast, mutants defective in binding to the retinoblastoma (Rb) family of pocket proteins were effective repressors. These results suggested that E1A inhibited insulin transcription by sequestering the p300/CBP coactivator required for E-activator function.
Recent studies have demonstrated that p300/CBP modulates the activity of a number of key activators, including those involved in regulating cellular proliferation and differentiation (reviewed in references 15 and 65). p300/CBP appears to function as a transcriptional coactivator by bridging, through direct interactions, the activator to the basal transcriptional machinery (15, 65). In addition, these proteins possess intrinsic histone acetyltransferase activity (6, 47, 73), which could modify chromatin structure by promoting a locally open and transcriptionally active configuration. Here we demonstrate that p300 interacts with BETA2/NeuroD and the HEB- and E2A-encoded proteins of the insulin E-box activator to potentiate their activity. Sequences spanned by the activation domain in these E-box regulatory proteins were found to functionally interact with the p300 protein. These results suggest that the ability of this insulin activator to mediate important functions during development and in the adult may be linked to the transcriptional signaling properties of p300/CBP.
MATERIALS AND METHODS
Cell culture and transfections.
The hamster insulinoma tumor cell line HIT T-15 2.2.2 (16) was grown in Dulbecco’s modified Eagle’s medium supplemented with 15% (vol/vol) horse serum, 2.5% (vol/vol) fetal bovine serum, and 50 μg each of streptomycin and penicillin per ml. Approximately 18 h before transfection, 2.5 × 106 cells were plated onto 100-mm2 plates. The luciferase (LUC) and chloramphenicol acetyltransferase (CAT) expression plasmids were introduced into HIT T-15 cells as calcium phosphate coprecipitates as detailed by Whelan et al. (72). The activity from a cotransfected simian virus 40 enhancer-driven CAT or LUC construct served as a recovery marker. Cells were harvested 40 to 48 h after transfection. LUC and CAT enzymatic assays were performed as described by De Wet et al. (12) and Nordeen et al. (45), respectively. The LUC activity is defined in arbitrary relative light units. Each experiment was repeated several times with at least two different plasmid preparations.
DNA constructs.
Construction of the RIPE3:LUC wild-type, C1, and E expression plasmids was previously described (24). The RIPE3 (−126 to −86 bp), C1 (−125 to −101 bp), and E (−110 to −86 bp) constructs contain three copies of rat insulin II gene sequence inserted in its normal orientation directly upstream of the ovalbumin TATA box in a LUC expression plasmid. The E-box:LUC expression plasmid contains three copies of the −102 to −87 bp region from the rat insulin II gene inserted just upstream of the rabbit β-globin promoter fused to LUC (24). The adenovirus type 5 E1A expression plasmids encode for the wild type (E1A), a p300/CBP-binding mutant (E1A Δ2-36; internal deletion of amino acids 2 to 36), and an Rb pocket protein-binding mutant (E1A 928; cysteine 124-to-glycine mutation) of the 243-amino-acid protein (69). GAL4:E47(1-99) (38, 63), GAL4:E47(325-432) (57), and GAL4:E47(325-432)m (57) have been described elsewhere. Lysine 403 was changed to glutamic acid and aspartic acid 404 was changed to arginine in GAL4:E47(325-432)m (57), which reduces activity 17-fold in HIT T-15 cells (data not shown) and 35-fold in COS-7 cells (57) relative to GAL4:E47(325-432). [The GAL4:E47(325-432) constructs span amino acids 325 to 432 and 259 to 366 of E47 and E2/5, respectively; previously they were referred to as GAL4:E2A(259-366) (57).] (GAL4)5E1bCAT contains five GAL4-binding sites inserted upstream of the minimal adenovirus E1B promoter linked to the CAT gene (35). The p300 expression plasmids were constructed in the cytomegalovirus (CMV) enhancer-driven vector described by Eckner et al. (13). The wild-type and p300 dl10 (deletion of amino acids 1680 to 1811) mutant have been described previously (31). Amino acid 1945 to 2377 were removed from p300 in p300ΔQ. To construct the herpesvirus acidic activation region (VP16) fusion constructs, p300 cDNA sequences were fused in frame to VP16 as follows: Np300:VP16 (spans amino acid sequences 1 to 1257 [31]), Cp300:VP16 (amino acids 871 to 2378 [31]); Cp300 Br:VP16 (amino acids 871 to 1134), Cp300ΔQ:VP16 (amino acids 871 to 1945), Q:VP16 (amino acids 1945 to 2377), Cp300ΔCH2:VP16 (amino acids 871 to 2378 with an internal deletion of amino acids 1139 to 1393), and Cp300ΔCH2/3:VP16 (amino acids 871 to 2378 with internal deletion of amino acids 1139 to 1944). The GAL4:BETA2 and GST (glutathione S-transferase):BETA2 plasmids were constructed from the hamster BETA2 cDNA (44) by cloning nucleotide sequences spanning amino acids 1 to 355, 1 to 99, 1 to 155, and 156 to 355 into a GAL4 (pGAL4(1-147) (35)] and/or GST (pGEX2TK; Pharmacia, Piscataway, N.J.) expression vector to create in-frame fusion proteins. Each newly constructed p300 and BETA2 construct was verified by restriction enzyme and partial DNA sequencing analyses.
In vitro translation and GST binding assay.
GST:BETA2(1-155) and GST:BETA2(156-355) fusion proteins were prepared as specified by the manufacturer (Pharmacia). Translation reactions were performed in vitro with the Cp300 and Cp300ΔQ plasmids, using a TNT kit (Promega, Madison, Wis.) with l-[35S]methionine (10 mCi/ml; Amersham, Arlington Heights, Ill.). Labeled proteins were incubated for 1 h in EBC buffer (50 mM Tris-HCl [pH 8.0], 140 mM NaCl, 0.5% Nonidet P-40, 100 mM NaF, 200 μM Na3VO4) with GST:BETA2 fusion proteins coupled to glutathione-Sepharose beads (Pharmacia). The beads were then washed three times with EBC buffer, and the bound protein complexes were eluted with 1× gel loading buffer (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 2% sodium dodecyl sulfate [SDS], 0.1% bromophenol blue, 10% glycerol), resolved by SDS–10% polyacrylamide gel electrophoresis (PAGE), and visualized by autoradiography.
RESULTS
p300 can potentiate E-box activation.
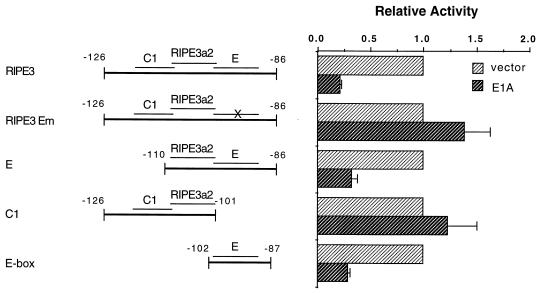
Previous work had demonstrated that E1A repression was mediated through at least two independently acting insulin control elements, the E-box element at −100 to −91 bp and one internal to nucleotides −340 to −101 bp (68). To determine whether the C1 enhancer element at −115 to −107 bp was also a target of E1A inhibition, we analyzed repression of rat insulin II gene minienhancer constructs containing sequences from −126 to −86 bp in HIT T15 β cells. The bp −126 to −86 region (termed RIPE3 [25]) contains three mutationally sensitive elements: C1, RIPE3a2, and E (Fig. 1) (64). The factors which bind to these elements activate RIPE3 minienhancer unit transcription to approximately the same level as the intact insulin enhancer (25), and the region from −126 to −86 bp is sufficient to direct expression to the islet in transgenic animals (71).
FIG. 1.
The E box in the bp −126 and −86 region is a specific E1A target. Schematic representations of the insulin:LUC constructs with the C1 (−115 to −107 bp), RIPE3a2 (−108 to −99 bp), and E (−100 to −91 bp) factor binding sites (64). HIT T-15 cells were transfected with 2.5 μg of insulin:LUC and 2.5 μg of either E1A or the CMV4 vector. The activity ± standard error of the mean from three to five independent experiments is presented relative to the insulin:LUC construct alone. The activity of each of the insulin-driven constructs was similar to that previously reported (58, 63); values for RIPE3, RIPE3 Em, E, C1, and E box were 150,473, 45,667, 9,753, 8,942, and 4,000 relative light units, respectively.
The level of E1A-mediated repression from RIPE3 wild type (RIPE3) was compared to those for E mutant RIPE3 (RIPE3 Em)-, C1 (C1, −125 to −101 bp)-, and E (E, −110 to −86 bp; E box, −102 to −87 bp)-driven constructs (Fig. 1). Expression from all of the E-box-containing constructs was repressed by E1A. In contrast, the activities of the constructs driven by the C1 and RIPE3a2 elements were not affected by E1A (compare E1A plus RIPE3 Em, or C1, to RIPE3 in Fig. 1). The most straightforward interpretation of these results is that E1A disrupts the function of the RIPE3 region activator which mediates E- but not C1-stimulated expression.
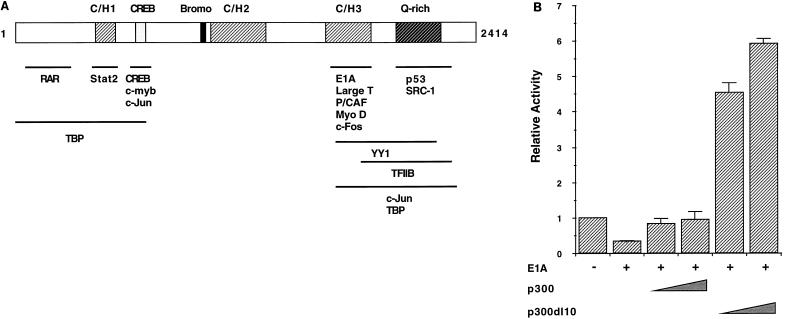
Given that insulin enhancer activation was repressed by E1A as a result of binding to the p300/CBP coregulator (69), we investigated whether inhibition of insulin E-box activation was regulated by the same mechanism. In contrast to wild-type E1A, the E1A Δ2-36 mutant failed to repress RIPE3-dependent transcription (Fig. 2). This mutant binds to phosphorylated Rb but not to p300/CBP (69). The E1A mutant, E1A 928, which binds to p300/CBP but not to proteins of the Rb pocket family (69), fully repressed activity (Fig. 2). In addition, overproduction of wild-type p300 overrode E1A-imposed repression (Fig. 3B). It was also found that the p300 mutant p300 dl10, which cannot bind to E1A because it lacks the cysteine/histidine-rich region (termed C/H3) between amino acids 1680 to 1811 (31), stimulated RIPE3 activity (Fig. 3). These data strongly implicate p300/CBP in E-box-dependent transcription. In addition, they suggest that the E1A-binding region of p300 does not contribute in coactivation.
FIG. 2.
The p300/CBP binding region of E1A mediates repression. (A) The diagram shows the domain organization of the 243-amino-acid E1A protein, indicating conserved regions (CR1 and CR2). The N-terminal region and CR1 bind to the p300/CBP family. The integrity of CR1 (amino acids 40 to 80) and CR2 (amino acids 120 to 140) is essential for E1A to associate with the Rb family, which includes p107 and p130 (reviewed in reference 65). The asterisk indicates the site of a Cys-to-Gly change at amino acid 124 in the E1A 928 mutant. (B) HIT T-15 cells were transfected with 2.5 μg of RIPE3:LUC with or without 2.5 μg of E1A, E1A Δ2-36, E1A 928, or CMV4. The activity ± standard error of the mean is presented relative to RIPE3:LUC alone.
FIG. 3.
p300 relieves E1A-mediated repression. (A) Schematic representation of p300/CBP with the cysteine/histidine-rich (CH/1, CH/2, and CH/3), bromo, and glutamine-rich (Q-rich) domains. The acetyltransferase activity of p300 is mediated by association with a cellular p300/CBP-associated factor (P/CAF [73]), and/or by an inherent activity catalyzed by the sequences between positions 1135 and 1810 (6, 47). The numbers correspond to the amino acid residues in human p300; CBP has a similar organization (2,440 residues) (reviewed in references 15 and 65). The regions in p300/CBP required for binding to various target proteins are shown (15, 65). RAR, retinoic acid receptor; TBP, TATA-binding protein. (B) HIT T-15 cells were transfected with RIPE3:LUC (2.5 μg), E1A (0.25 μg), and 5 or 10 μg of pCMVβ-p300 or p300 dl10. The sequences in p300 required for E1A association (amino acids 1680 to 1811) are missing in p300 dl10. The activity ± standard error of the mean is presented relative to RIPE3:LUC alone.
p300 stimulates BETA2/NeuroD and E2A activities.
The ability of p300 to specifically stimulate E-box-directed transcription prompted us to test in vivo whether this factor acted as a coactivator of the ubiquitously distributed (E2A and HEB) and/or islet-enriched (BETA2/NeuroD) proteins of the activator. To address this question, we analyzed in HIT T-15 cells how p300 affected the transactivation ability of fusion proteins between these bHLH factors and the GAL4 DNA-binding domain. The E2A and HEB studies were conducted with activation domain sequences from the E2A-encoded protein E47, as these sequences are closely related to HEB and are conserved functionally (38, 57). GAL4:BETA2/NeuroD constructs spanned the entire protein. The activation domain of BETA2/NeuroD is located within the C-terminal region, between positions 189 and 355 (63a).
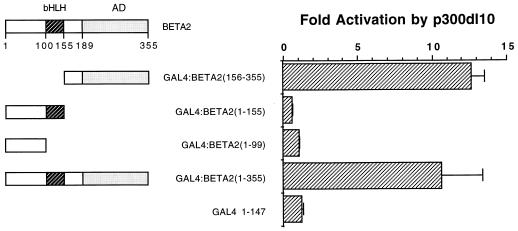
p300 stimulated the activity of GAL4 fusion constructs spanning the activation domains in E2A and HEB (Fig. 4). GAL4:BETA2 activity was potentiated by both p300 and p300 dl10 (Fig. 5). Similar activation properties were observed with p300 in assays using the insulin RIPE3 enhancer constructs (Fig. 3). It is unclear why p300 dl10 activated GAL4:BETA2 (Fig. 5) and GAL4:E47 (data not shown) expression more effectively than p300. p300 dl10 (Fig. 6) and p300 (data not shown) selectively potentiated the activity of C-terminal GAL4:BETA2/NeuroD constructs containing the BETA2/NeuroD activation domain and not fusions of the N-terminal and bHLH sequences alone. In addition, GAL4:BETA2(156-355) and GAL4:E47(325-432) DNA-binding activity was unchanged in extracts prepared from control and p300-transfected cells (data not shown). These results strongly indicate that p300 activation of insulin E-box-directed transcription is mediated through interactions with the BETA2/NeuroD and E2A/HEB proteins.
FIG. 4.
p300 stimulates E2A-mediated activity. The schematic shows the activation (AD1 and AD2) and bHLH domains of E47. HIT T-15 cells were transfected with 1 μg of a GAL4:E47 construct, 10 μg of pCMVβ-p300, and 1 μg of (GAL4)5E1bCAT. The amino acids of E47 present in the GAL4 fusion are in parentheses. All data are presented as fold activation relative to the GAL4:E47 construct alone. Fold activation is expressed as the ratio of CAT activity in the presence of p300 DNA divided by the level of CAT activity in its absence.
FIG. 5.
BETA2/NeuroD activation is potentiated by either p300 or p300 dl10. HIT T-15 cells were transfected with 1 μg of GAL4:BETA2(1-355), 0, 5, 10, or 20 μg of pCMVβ-p300 or pCMVβ-p300 dl10, and 1 μg of (GAL4)5E1bCAT. Fold activation is expressed as the ratio of CAT activity in the presence of p300 DNA divided by the level of CAT activity in its absence.
FIG. 6.
Carboxy-terminal sequences in BETA2/NeuroD are required in p300 activation. The diagram shows the activation domain (AD; amino acids 189 to 355) and bHLH domain (amino acids 100 to 155) of BETA2/NeuroD. HIT T-15 cells were transfected with 1 μg of a GAL4:BETA2 construct, 10 μg of pCMVβ-p300 dl10, and 1 μg (GAL4)5E1bCAT. The amino acids of BETA2/NeuroD present in the GAL4 fusion are in parentheses. Fold activation is expressed as the ratio of CAT activity in the presence of p300 dl10 DNA divided by the level of CAT activity in its absence.
p300 functionally interacts with BETA2/NeuroD and E2A.
We next wanted to identify the regions of p300 that were involved in binding to BETA2/NeuroD and E2A/HEB. p300 has at least three domains that bind transcription factors: a region near the amino terminus which is required for interactions with the retinoic acid receptor (27, 74), TATA-binding protein (1, 11), CREB (7, 29, 49), c-Jun (5, 32), and c-Myb (10); a region necessary for binding to E1A (13), c-Fos (4), P/CAF (73), MyoD (14, 60, 75), and TFIIB (73); and a carboxy-terminal region which is important for interactions with SRC-1 (66, 74) and p53 (21, 34) (Fig. 3A).
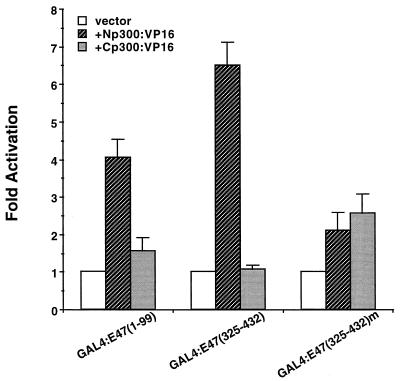
To test in vivo for interaction domains within p300 for E47 and BETA2/NeuroD, we constructed a series of fusion proteins between p300 and the VP16 activation domain and examined whether they could stimulate GAL4:BETA2 and GAL4:E47 activity in HIT T-15 cells. We initially found that a p300:VP16 construct spanning amino acids 1 to 1257 (termed Np300) potentiated GAL4:BETA2 activity poorly compared to a p300 expression plasmid spanning carboxy-terminal amino acids 871 to 2378 (Cp300) (Fig. 7B). Mutagenesis of the Cp300 region was then undertaken to more precisely identify p300-interacting sequences. These results indicated that the glutamine-rich region found in the C-terminal portion of p300 was sufficient for BETA2/NeuroD binding in vivo (compare Q:VP16 to Cp300:VP16 in Fig. 7B and C).
FIG. 7.
BETA2/NeuroD associates with the C-terminal glutamine-rich domain of p300 in vivo. (A) Representation of p300/CBP with the CH/1, CH/2, and CH/3, bromo (Br), and glutamine-rich (Q-rich) domains (see Fig. 3A). (B) One microgram of GAL4:BETA2(1-355) or (C) GAL4:BETA2(156-355) and VP16 fused to different domains of p300 (10 μg) were cotransfected with (GAL4)5E1bCAT (1.0 μg) into HIT T-15 cells. Activation of the CAT reporter due to interaction of the p300 domain with BETA2 is shown as fold activation (± standard error of the mean).
The p300 sequences important for E2A/HEB activation in vivo appear to be distinct from those for BETA2/NeuroD, as GAL4:E47(1-99) and GAL4:E47(325-432) expression was specifically stimulated by Np300 (amino acids 1 to 1257 of p300 [Fig. 8]). Np300 potentiation was reduced in an activation-defective mutant of GAL4:E47(325-432) [Fig. 8; compare p300 stimulation in GAL4:E47(325-432) to that in GAL4:E47(325-432)m], which is the activation domain of E47/HEB that is preferentially active in β cells (3, 58). These results indicate that sequences within the N-terminal region of p300 are essential for E2A/HEB binding and activation.
FIG. 8.
N-terminal sequences in p300 functionally interact with the E47/HEB activation domains in vivo. The GAL4:E47 activation domain fusion constructs (1.0 μg) and VP16 fused to p300 sequences (10 μg) from amino acids 1 to 1257 (Np300) and 871 to 2378 (Cp300) were cotransfected with (GAL4)5E1bCAT (2.5 μg) into HIT T-15 cells. Activation of the CAT reporter due to interaction with p300 is shown as fold activation ± standard error of the mean.
p300 binds directly to BETA2/NeuroD.
The interaction between the glutamine-rich region of p300 and BETA2/NeuroD was corroborated in vitro upon investigating the binding properties of Cp300 (amino acids 871 to 2378) and a glutamine-rich region Cp300 deletion mutant (termed Cp300ΔQ) to GST:BETA2 affinity columns in which the regions from amino acids 1 to 155 and 156 to 355 of BETA2/NeuroD served as the ligands (Fig. 9). Cp300 bound effectively to the GST:BETA2(156-355) column, whereas little or no p300 binding was detected in the eluates from the GST or GST:BETA2(1-155) column (Fig. 9A). This result is also consistent with the results of in vivo experiments shown in Fig. 6. In contrast, p300ΔQ binding was barely detected with the GST:BETA2(156-355) column (Fig. 9B). E2A/HEB was also shown to bind in vitro to N-terminal p300 sequences (amino acids 1 to 596 [data not shown]). Together, these results demonstrated that BETA2/NeuroD interacted in vitro and in vivo with the C-terminal region of p300.
FIG. 9.
p300 and BETA2/NeuroD interact in vitro. Radiolabeled Cp300 (A) and Cp300ΔQ (B) were incubated with a purified GST control and GST-BETA2 fusion proteins bound to glutathione-Sepharose beads. Bound p300 was then eluted, separated by SDS-PAGE, and detected by autoradiography. The input lane represents 10% of the total volume used in the binding assay.
The glutamine-rich region of p300 is required in insulin enhancer-mediated activation in vivo.
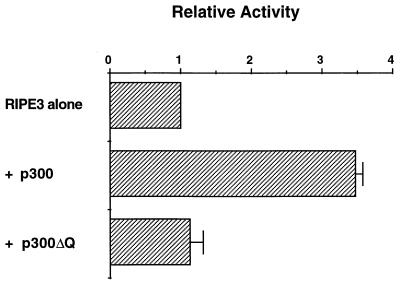
The functional consequence of the interaction between BETA2/NeuroD and the glutamine-rich region of p300 was investigated by cotransfecting HIT T-15 cells with p300, p300ΔQ, and the insulin minienhancer construct, RIPE3. p300 increased the level of RIPE3 activation approximately 3.5-fold, whereas p300ΔQ had no effect (Fig. 10). These results strongly suggest that the glutamine-rich region of p300 is not only important for BETA2/NeuroD binding in vitro but also necessary for insulin enhancer activation in β cells.
FIG. 10.
Removing the C-terminal glutamine-rich domain inhibits the ability of p300 to stimulate insulin enhancer-driven activity. HIT T-15 cells were transfected with 2.5 μg of RIPE3:LUC and 20 μg of either pCMVβ-p300 or pCMVβ-p300ΔQ. The activity ± standard error of the mean) is presented relative to RIPE3:LUC alone.
DISCUSSION
The results of this study suggest an important role for p300 in the biological activities of the insulin E-box activator factors. Thus, p300 was shown to selectively stimulate E-box-dependent transcription in β cells. Activation was mediated through association of p300 with the BETA2/NeuroD and E2A/HEB proteins. Removing the C-terminal glutamine-rich sequences of p300 that are required for BETA2/NeuroD binding prevented E-dependent activation, establishing that p300 is an important regulator of BETA2/NeuroD-directed transcription in vivo. The p300 binding site within the BETA2/NeuroD and E2A proteins spanned their activation domain region sequences. Taken together, these results strongly imply that p300 acts as a coactivator by interacting and potentiating the activation domain function of the insulin bHLH regulators.
p300 and CBP are highly homologous generally distributed nuclear proteins originally identified for their ability to interact with the adenovirus E1A protein (13) and the cellular transcription factor CREB (29). Our studies suggest that the ability of E1A to repress insulin E-element-mediated transcription results from its binding to p300/CBP. E1A inhibition presumably results from the recruitment of p300/CBP away from BETA2/NeuroD and E2A/HEB. Previous results had demonstrated that E-driven transcription was not the only insulin enhancer region target for E1A (68). The results here indicate that insulin C1 element-associated transcription, which is essential in both β-cell-specific- and glucose-regulated expression, is not affected by E1A. As recent experiments have demonstrated that the insulin A3 activator, PDX-1, functions synergistically with E2A/HEB to stimulate insulin enhancer activation (50, 53), this factor may be a target for E1A inhibition.
p300/CBP has emerged as a transcriptional coactivator for a broad group of cellular DNA-binding transcriptional activators involved in proliferation, signaling, and differentiation (15, 65). Although the precise mechanisms by which activators stimulate the transcriptional machinery through p300/CBP are unclear, BETA2/NeuroD- and E2A/HEB-mediated activation appears to involve specific protein-protein interactions between activation domain region sequences and p300. Recent studies indicate that p300 may also interact with the bHLH region of the E2A-encoded proteins (14). The myogenic bHLH activator, MyoD, also appears to have binding sites for p300/CBP within its activation domain (60, 75) and bHLH regions (14).
Importantly, our observations demonstrate that p300/CBP can interact with each of the proteins in the insulin E-box activator. p300/CBP also appears to bind independently to the AP-1 activator factors, c-Jun, and c-Fos (4, 5). The regions of p300/CBP important for association with BETA2/NeuroD (amino acids 1945 to 2377) and E2A/HEB (amino acids 1 to 1257) appear to be distinct from those necessary for binding to the general transcription factors (i.e., TATA-binding protein and TFIIB) or its histone acetyltransferase activity (Fig. 3A). This finding implies that gene activation may result from cooperative interactions between p300, BETA2/NeuroD, E2A/HEB, and these distinct factors to provide a concerted regulatory effect. However, we also found that insulin enhancer activation by p300 was compromised in the BETA2/NeuroD-binding-region mutant p300ΔQ (Fig. 10), which may indicate that interactions between p300 and BETA2/NeuroD are more critical to E-activator function than those with E2A/HEB.
In addition to being involved in insulin gene transcription in islet β cells, BETA2/NeuroD plays a major role during pancreatic islet differentiation (43) and neuronal development (30). The target genes that operate in these other responses of BETA2/NeuroD are unknown. However, the recruitment by BETA2/NeuroD of p300, with its inherent acetyltransferase activity, to these genes could induce local nucleosome modifications, facilitating the binding of the other activators and general transcription factors required for initiation. p300/CBP has also recently been shown to stimulate p53 tumor suppressor transcription factor activity by direct acetylation (22). As a consequence, activation of BETA2/NeuroD-driven transcription by p300 may result from acetylation of the activators themselves. In this regard, it may be noteworthy that p300 dl10 was a more effective coactivator of BETA2/NeuroD than the wild-type protein (Fig. 3B and 5). The amino acids deleted from p300 dl10 (1680 to 1811) lie near those removed in mutants (1452 to 1522 or 1603 to 1653) lacking intrinsic histone acetyltransferase activity (56), which were also more effective coactivators than the normal protein (Fig. 5) (56). We are currently determining if histone acetyltransferase activity is reduced in p300 dl10. If so, this indicates that this acetyltransferase domain reduces the coactivation properties of p300 with BETA2/NeuroD, at least under these set of conditions. It will also be interesting to determine whether p300/CBP also directly mediates BETA2/NeuroD signaling during islet differentiation and neuronal development and, if so, the mechanisms important in these transcriptional responses.
ACKNOWLEDGMENTS
We thank Susan Samaras, Mina Peshavaria, and Kevin Gerrish for constructive criticism of the manuscript and Ming-Jer Tsai for generously providing the hamster BETA2 cDNA.
This work was supported by National Institutes of Health grants NIH RO1 DK49852 (to R.S.). Partial support was also derived from the Vanderbilt University Diabetes Research and Training Center Molecular Biology Core Laboratory (Public Health Service grant P60 DK20593 from the National Institutes of Health).
REFERENCES
- 1.Abraham S E, Lobo S, Yaciuk P, Wang H-G H, Moran E. p300, and p300-associated proteins, are components of TATA-binding protein (TBP) complexes. Oncogene. 1993;8:1639–1647. [PubMed] [Google Scholar]
- 2.Arany Z, Newsome D, Oldread E, Livingston D M, Eckner R. A family of transcriptional adapter proteins targeted by the E1A protein. Nature. 1995;374:81–84. doi: 10.1038/374081a0. [DOI] [PubMed] [Google Scholar]
- 3.Aronheim A, Ohlsson H, Park C W, Edlund T, Walker M D. Distribution and characterization of helix-loop-helix enhancer-binding proteins from pancreatic beta cells and lymphocytes. Nucleic Acids Res. 1991;19:3893–3899. doi: 10.1093/nar/19.14.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannister A J, Kouzarides T. CBP-induced stimulation of c-Fos activity is abrogated by E1A. EMBO J. 1995;14:4758–4762. doi: 10.1002/j.1460-2075.1995.tb00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannister A J, Oehler T, Wilhelm D, Angel P, Kouzarides T. Stimulation of c-Jun activity by CBP: c-Jun residues Ser63/73 are required for CBP induced stimulation in vivo and CBP binding in vitro. Oncogene. 1995;11:2509–2514. [PubMed] [Google Scholar]
- 6.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 7.Chrivia J C, Kwok R P S, Lamb N, Hagiwara M, Montminy M R, Goodman R H. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855–859. doi: 10.1038/365855a0. [DOI] [PubMed] [Google Scholar]
- 8.Cordle S R, Henderson E, Masuoks H, Weil A P, Stein R. Pancreatic β-cell-type-specific transcription of the insulin gene is mediated by basic helix-loop-helix DNA-binding proteins. Mol Cell Biol. 1991;11:1734–1738. doi: 10.1128/mcb.11.3.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowe D T, Tsai M-J. Mutagenesis of the rat insulin II 5′-flanking region defines sequences important for expression in HIT cells. Mol Cell Biol. 1989;9:1784–1789. doi: 10.1128/mcb.9.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai P, Akimaru H, Tanaka Y, Hou D-X, Yasukawa T, Kanei-Ishii C, Talahashi T, Ishii S. CBP as a transcriptional coactivator of c-Myb. Genes Dev. 1996;10:528–540. doi: 10.1101/gad.10.5.528. [DOI] [PubMed] [Google Scholar]
- 11.Dallas P B, Yaciuk P, Moran E. Characterization of monoclonal antibodies raised against p300: both p300 and CBP are present in intracellular TBP complexes. J Virol. 1997;71:1726–1731. doi: 10.1128/jvi.71.2.1726-1731.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Wet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckner R, Ewen M E, Newsine D, Gerdes M, DeCaprio J A, Lawrence J B, Livingston D M. Molecular cloning and functional analysis of the adenovirus E1A-associated 300-kD protein (p300) reveals a protein with properties of a transcriptional adapter. Genes Dev. 1994;8:869–884. doi: 10.1101/gad.8.8.869. [DOI] [PubMed] [Google Scholar]
- 14.Eckner R, Yao T-P, Oldread E, Livingston D M. Interaction and functional collaboration of p300/CBP and bHLH proteins in muscle and B-cell differentiation. Genes Dev. 1996;10:2478–2490. doi: 10.1101/gad.10.19.2478. [DOI] [PubMed] [Google Scholar]
- 15.Eckner R. p300 and CBP as transcriptional regulators and targets of oncogenic. Biol Chem. 1997;377:685–688. [PubMed] [Google Scholar]
- 16.Edlund T, Walker M D, Barr P J, Rutter W J. Cell-specific expression of the rat insulin gene: evidence for role of two distinct 5′-flanking sequences. Science. 1985;230:912–916. doi: 10.1126/science.3904002. [DOI] [PubMed] [Google Scholar]
- 17.German M S, Blanar M A, Nelson C, Moss L G, Rutter W J. Two related helix-loop-helix proteins participate in separate cell-specific complexes that bind the insulin enhancer. Mol Endocrinol. 1991;5:292–299. doi: 10.1210/mend-5-2-292. [DOI] [PubMed] [Google Scholar]
- 18.German M S, Moss L G, Wang J, Rutter W J. The insulin and islet amyloid polypeptide genes contain similar cell-specific promoter elements that bind identical β-cell nuclear complexes. Mol Cell Biol. 1992;12:1777–1788. doi: 10.1128/mcb.12.4.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.German M S, Wang J. The insulin gene contains multiple transcriptional elements that response to glucose. Mol Cell Biol. 1994;14:4067–4075. doi: 10.1128/mcb.14.6.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.German M S, Ashcroft S, Docherty K, Edlund H, Edlund T, Goodison S, Imura H, Kennedy G, Madsen O, Melloul D, Moss L, Olson K, Permutt M A, Philippe J, Robertson R P, Rutter W J, Serup P, Stein R, Steiner D, Tsai M-J, Walker M D. The insulin promoter: a simplified nomenclature. Diabetes. 1995;44:1002–1004. doi: 10.2337/diab.44.8.1002. [DOI] [PubMed] [Google Scholar]
- 21.Gu W, Roeder R C. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 22.Gu W, Shi X-L, Roeder R C. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–822. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 23.Guz Y, Montminy M R, Stein R, Leonard J, Gamer L W, Wright C V E, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in β-cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitor during ontogeny. Development. 1995;121:11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 24.Henderson E, Stein R. c-jun inhibits transcriptional activation by the insulin enhancer, and the insulin control element is the target of control. Mol Cell Biol. 1994;14:655–662. doi: 10.1128/mcb.14.1.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hwung Y-P, Gu Y-Z, Tsai M-J. Cooperativity of sequence elements mediates tissue specificity of the rat insulin II gene. Mol Cell Biol. 1990;10:1784–1788. doi: 10.1128/mcb.10.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 27.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokave R, Gloss B, Lin S-C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson O, Edlund T, Moss J B, Rutter W J, Walker M D. A mutational analysis of the insulin gene transcription control region: expression in beta cells is dependent on two related sequences within the enhancer. Proc Natl Acad Sci USA. 1987;84:8819–8823. doi: 10.1073/pnas.84.24.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwok R P S, Lundblad J R, Chrivia J C, Richards J P, Bachinger H P, Brennan R G, Roberts S G E, Green M R, Goodman R H. Nuclear protein CBP is a coactivator for the transcription factor CREB. Nature. 1994;370:223–226. doi: 10.1038/370223a0. [DOI] [PubMed] [Google Scholar]
- 30.Lee J E, Hollenberg S M, Snider L, Turner D L, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–843. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- 31.Lee J-S, Galvin K M, See R H, Eckner R, Livingston D, Moran E, Shi Y. Relief of YY1 transcriptional repression by adenovirus E1A is mediated by E1A-associated protein p300. Genes Dev. 1995;9:1188–1198. doi: 10.1101/gad.9.10.1188. [DOI] [PubMed] [Google Scholar]
- 32.Lee J-S, See R H, Deng T, Shi Y. Adenovirus E1A downregulates c-Jun- and JunB-mediated transcription by targeting their coactivator p300. Mol Cell Biol. 1996;16:4312–4326. doi: 10.1128/mcb.16.8.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonard J, Peers B, Johnson T, Ferrere K, Lee S, Montminy M. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cell. Mol Endocrinol. 1993;7:1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- 34.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 35.Lillie J C, Green M R. Transcription activation by adenovirus E1A protein. Nature. 1989;338:39–44. doi: 10.1038/338039a0. [DOI] [PubMed] [Google Scholar]
- 36.Lundblad J R, Kwok R P S, Laurance M E, Harter M L, Goodman R H. Adenoviral E1A-associated protein p300 as a functional homologue of the transcriptional co-activator CBP. Nature. 1995;374:85–88. doi: 10.1038/374085a0. [DOI] [PubMed] [Google Scholar]
- 37.Macfarlane W M, Read M L, Gilligan M, Bujalska I, Docherty K. Glucose modulates the binding activity of the β-cell transcription factor IUF1 in a phosphorylation-dependent manner. Biochem J. 1994;303:625–631. doi: 10.1042/bj3030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massari M E, Jennings P A, Murre C. The AD1 transactivation domain of E2A contains a highly conserved helix which is required for its activity in both Saccharomyces cerevisiae and mammalian cells. Mol Cell Biol. 1996;16:121–129. doi: 10.1128/mcb.16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Melloul D, Ben-Neriah Y, Cerasi E. Glucose regulates the binding of an islet-specific factor to a conserved sequence within the rat and the human insulin promoter. Proc Natl Acad Sci USA. 1993;90:3865–3869. doi: 10.1073/pnas.90.9.3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller C P, McGhee R E, Jr, Habener J F. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 1994;13:1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murre C, Baltimore D. The helix-loop-helix motif: structure and function. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 861–879. [Google Scholar]
- 42.Mutoh H, Fung B P, Naya F, Tsai M-J, Nishitani J, Leiter A B. The basic helix-loop-helix transcription factor BETA2/NeuroD is expressed in mammalian enteroendocrine cells and activates secretin gene expression. Proc Natl Acad Sci USA. 1997;94:3560–3564. doi: 10.1073/pnas.94.8.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Naya F J, Huang H-P, Qiu Y, Mouth H, DeMayo F J, Leiter A B, Tsai M-J. Diabetes, defective pancreatic morphogenesis, and abnormal enteroendocrine differentiation in BETA2/NeuroD-deficient mice. Genes Dev. 1997;11:2323–2334. doi: 10.1101/gad.11.18.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naya F J, Stellrecht C M M, Tsai M-J. Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev. 1995;9:1009–1019. doi: 10.1101/gad.9.8.1009. [DOI] [PubMed] [Google Scholar]
- 45.Nordeen S K, Green III P P, Fowles D M. Laboratory methods. A rapid, sensitive, and inexpensive assay for chloramphenicol acetyltransferase. DNA. 1987;6:173–178. doi: 10.1089/dna.1987.6.173. [DOI] [PubMed] [Google Scholar]
- 46.Offield M F, Jetton T L, Stein R, Labosky T, Ray R, Magnuson M, Hogan B, Wright C V E. PDX-1 is required for development of the pancreas and differentiation of the rostral duodenum. Development. 1996;122:983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 47.Ogryzko V V, Schiltz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 48.Ohlsson H, Karlsson K, Edlund T. IPF-1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12:4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parker D, Ferreri K, Nakajima T, Lamorte V J, Evans R, Koerber S C, Hoeger C, Montminy M R. Phosphorylation of CREB at Ser-133 induces complex formation with CREB-binding protein via a direct mechanism. Mol Cell Biol. 1996;16:694–703. doi: 10.1128/mcb.16.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peers B, Leonard J, Sharma S, Teitelman G, Montminy M R. Insulin expression in pancreatic islet cells relies on cooperative interactions between the helix loop helix factor E47 and the homeobox factor STF-1. Mol Endocrinol. 1994;8:1798–1806. doi: 10.1210/mend.8.12.7708065. [DOI] [PubMed] [Google Scholar]
- 51.Peers B, Sharma S, Johnson T, Kamps M, Montminy M R. The pancreatic islet factor STF-1 binds cooperatively with Pbx to a regulatory element in the somatostatin promoter: importance of the FPWMK motif and of the homeodomain. Mol Cell Biol. 1995;15:7091–7097. doi: 10.1128/mcb.15.12.7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright C V E, Stein R. XIHbox8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol Endocrinol. 1994;8:806–816. doi: 10.1210/mend.8.6.7935494. [DOI] [PubMed] [Google Scholar]
- 53.Peshavaria M, Henderson E, Sharma A, Wright C V E, Stein R. Functional characterization of the transactivation properties of the PDX-1 homeodomain protein. Mol Cell Biol. 1997;17:3987–3996. doi: 10.1128/mcb.17.7.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Petersen H V, Serup P, Leonard J, Michelsen B K, Madsen O D. Transcriptional regulation of the human insulin gene is dependent of the homeodomain proteins STF1/IPF1 acting through the CT boxes. Proc Natl Acad Sci USA. 1994;91:10465–10469. doi: 10.1073/pnas.91.22.10465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peyton M, Moss L, Tsai M-J. Two distinct class A helix-loop-helix transcription factors, E2A and BETA1, form separate DNA-binding complexes on the insulin E-box. J Biol Chem. 1994;269:25936–25941. [PubMed] [Google Scholar]
- 56.Puri P L, Sarorelli V, Yang X-J, Hamamori Y, Ogryzko V V, Howard B H, Kedes L, Wang Y Y Y, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 57.Quong M W, Massari M E, Zwart R, Murre C. A new transcriptional-activation motif restricted to a class of helix-loop-helix proteins is functionally conserved in both yeast and mammalian cells. Mol Cell Biol. 1993;13:792–800. doi: 10.1128/mcb.13.2.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Robinson G L W G, Henderson E, Massari M E, Murre C, Stein R. c-jun inhibits insulin control element-mediated transcription by affecting the transactivation potential of the E2A gene products. Mol Cell Biol. 1995;15:1398–1404. doi: 10.1128/mcb.15.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sander M, German M S. The beta cell transcription factors and development of the pancreas. J Mol Med. 1997;75:327–340. doi: 10.1007/s001090050118. [DOI] [PubMed] [Google Scholar]
- 60.Sartorelli V, Huang J, Hamamori Y, Kedes L. Molecular mechanisms of myogenic coactivation by p300: direct interaction with the activation domain of MyoD and with the MADS box of MEF2C. Mol Cell Biol. 1997;17:1010–1026. doi: 10.1128/mcb.17.2.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sharma A, Stein R. Glucose-induced transcription of the insulin gene is mediated by factors required for β-cell-type-specific expression. Mol Cell Biol. 1994;14:871–879. doi: 10.1128/mcb.14.2.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sharma A, Olson L K, Robertson R P, Stein R. The reduction of insulin gene transcription in HIT-T15 β cells chronically exposed to high glucose concentration is associated with the loss of RIPE3b1 and STF-1 transcription factor expression. Mol Endocrinol. 1995;9:1127–1134. doi: 10.1210/mend.9.9.7491105. [DOI] [PubMed] [Google Scholar]
- 63.Sharma A, Henderson E, Gamer L, Zhuang Y, Stein R. Analysis of the role of E2A-encoded proteins in insulin gene transcription. Mol Endocrinol. 1997;11:1608–1617. doi: 10.1210/mend.11.11.0004. [DOI] [PubMed] [Google Scholar]
- 63a.Sharma, A., and R. Stein. Unpublished data.
- 64.Shieh S-Y, Tsai M-J. Cell-specific and ubiquitous factors are responsible for the enhancer activity of the rat insulin II gene. J Biol Chem. 1991;266:16708–16714. [PubMed] [Google Scholar]
- 65.Shikama N, Lyon J, La Thangue N B. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 66.Smith C L, Onate S A, Tsai M-J, O’Malley B. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Biochemistry. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Song C-Z, Loewenstein P M, Toth K, Tang Q, Nishikawa A, Green M. The adenovirus E1A repression domain disrupts the interaction between the TATA binding protein and the TATA box in a manner reversible by TFIIB. Mol Cell Biol. 1997;17:2186–2193. doi: 10.1128/mcb.17.4.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stein R W, Whelan J. Insulin gene enhancer activity is inhibited by adenovirus 5 E1a gene products. Mol Cell Biol. 1989;9:4531–4534. doi: 10.1128/mcb.9.10.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stein R W, Corrigan M, Yaciuk P, Whelan J, Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990;64:4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stein R. Regulation of insulin gene transcription. Trends Endocrinol Metab. 1993;4:96–101. doi: 10.1016/1043-2760(93)90086-t. [DOI] [PubMed] [Google Scholar]
- 71.Stellrecht C M M, DeMayo F J, Finegold M J, Tsai M J. Tissue-specific and developmental regulation of the rat insulin II gene enhancer, RIPE3, in transgenic mice. J Biol Chem. 1997;272:3567–3572. doi: 10.1074/jbc.272.6.3567. [DOI] [PubMed] [Google Scholar]
- 72.Whelan J, Poon D, Weil P A, Stein R. Pancreatic β-cell-type-specific expression of the rat insulin II gene is controlled by positive and negative transcriptional elements. Mol Cell Biol. 1989;9:3253–3259. doi: 10.1128/mcb.9.8.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nalatani Y. A p300/CBP-associated factor that competes with the adenoviral oncoprotein E1A. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 74.Yao T-P, Ku G, Zhou N, Scully R, Livingston D M. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci USA. 1996;93:10626–10631. doi: 10.1073/pnas.93.20.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yuan W, Condorelli G, Caruso M, Felsani A, Giordano A. Human p300 protein is a coactivator for the transcription factor MyoD. J Biol Chem. 1996;271:9009–9013. doi: 10.1074/jbc.271.15.9009. [DOI] [PubMed] [Google Scholar]