Abstract
This parallel‐arm, phase I study investigated the potential cytochrome P450 (CYP)3A induction effect of NBI‐1065845 (TAK‐653), an investigational α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor potentiator in phase II development for major depressive disorder. The midazolam treatment arm received the sensitive CYP3A substrate midazolam on Day 1, followed by NBI‐1065845 alone on Days 5–13; on Day 14, NBI‐1065845 was administered with midazolam, then NBI‐1065845 alone on Day 15. The oral contraceptive treatment arm received ethinyl estradiol–levonorgestrel on Day 1, then NBI‐1065845 alone on Days 5–13; on Day 14, NBI‐1065845 was administered with ethinyl estradiol–levonorgestrel, then NBI‐1065845 alone on Days 15–17. Blood samples were collected for pharmacokinetic analyses. The midazolam treatment arm comprised 14 men and 4 women, of whom 16 completed the study. Sixteen of the 17 healthy women completed the oral contraceptive treatment arm. After multiple daily doses of NBI‐1065845, the geometric mean ratios (GMRs) (90% confidence interval) for maximum observed concentration were: midazolam, 0.94 (0.79–1.13); ethinyl estradiol, 1.00 (0.87–1.15); and levonorgestrel, 0.99 (0.87–1.13). For area under the plasma concentration–time curve (AUC) from time 0 to infinity, the GMRs were as follows: midazolam, 0.88 (0.78–0.98); and ethinyl estradiol, 1.01 (0.88–1.15). For levonorgestrel, the GMR for AUC from time 0 to the last quantifiable concentration was 0.87 (0.78–0.96). These findings indicate that NBI‐1065845 is not a CYP3A inducer and support its administration with CYP3A substrates. NBI‐1065845 was generally well tolerated, with no new safety signals observed after coadministration of midazolam, ethinyl estradiol, or levonorgestrel.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
NBI‐1065845 (TAK‐653) is an investigational α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid receptor potentiator in phase II development for the treatment of the major depressive disorder (MDD). Investigation of the potential for interaction of cytochrome P450 (CYP)3A with NBI‐1065845 is necessary to guide concomitant medication use.
WHAT QUESTION DID THIS STUDY ADDRESS?
This drug–drug interaction study investigated the CYP3A induction effect of NBI‐1065845 on the pharmacokinetics of the sensitive index substrate midazolam and oral contraceptives ethinyl estradiol and levonorgestrel.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
NBI‐1065845 did not meet the criteria to be defined as a CYP3A inducer, and the potential for drug–drug interactions between NBI‐1065845 and CYP3A substrates is considered low. No new safety findings for NBI‐1065845 were observed.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The results indicate that NBI‐1065845 can be administered in future studies in individuals with MDD who are taking combined oral contraceptives or other CYP3A substrates for coexisting health conditions (including some antidepressants, anxiety medications, and statins) without requiring modified dosing of NBI‐1065845 or CYP3A substrates.
INTRODUCTION
Major depressive disorder (MDD) is characterized by depressed mood and loss of interest or pleasure. More than 60% of patients with MDD do not benefit from the first antidepressant they are prescribed, and most patients will subsequently undergo a trial‐and‐error sequential treatment strategy. 1 Given that antidepressants can take several weeks to be fully effective, this approach can lead to significant delays before symptoms improve. 1 Furthermore, the STAR*D study estimated that one‐third of patients do not achieve complete remission after multiple courses of antidepressants. 1 Compared with individuals who remit, those who do not achieve remission with treatment have a lower quality of life, functional status, and work productivity, and also incur higher health care costs. 2
Drugs that activate α‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazolepropionic acid (AMPA) receptors (AMPA‐R) are emerging as promising candidates for new antidepressants. 3 , 4 For example, ketamine and its (S)‐enantiomer esketamine have been shown to produce rapid and sustained antidepressant effects in phase III studies in patients with treatment‐resistant depression (i.e., those with no response to at least two different antidepressants). 3 , 5 , 6 The antidepressant effects of ketamine result from blockade of N‐methyl‐d‐aspartate receptors, disinhibition of γ‐aminobutyric acid‐ergic interneurons, and downstream AMPA‐R activation. 3 Preclinical studies in rats have shown that AMPA‐R activation is associated with increased expression of brain‐derived neurotrophic factor (BDNF) and activation of mammalian target of rapamycin (mTOR) in the hippocampus and prefrontal cortex. 3 , 7 , 8 These AMPA‐R‐induced changes trigger synaptogenesis, an effect that is thought to underlie the clinical efficacy of ketamine. 9 However, ketamine and esketamine have been associated with psychiatric (dissociation), neurological, cardiovascular, and genitourinary adverse events (AEs), as well as a risk of abuse. 3 , 10 , 11 Consequently, there is an unmet medical need for rapid‐onset antidepressants with similar efficacy profiles to those of existing antidepressants and with superior safety and tolerability for patients with MDD who have experienced an inadequate response to antidepressant treatment.
The importance of AMPA‐R in the mechanism of action of ketamine has led to interest in the development of drugs that act on AMPA‐R directly, including positive allosteric modulators, several of which are in development and have shown antidepressant efficacy in preclinical studies. 4 NBI‐1065845 (also known as TAK‐653) is a potent investigational AMPA‐R positive allosteric modulator under investigation in the phase II SAVITRI study in adults with MDD in whom at least one antidepressant treatment had previously had an inadequate response (ClinicalTrials.gov identifier: NCT05203341). The agonistic profiles of some AMPA‐R potentiators may be associated with the increased risk of seizures due to nonspecific activation of resting AMPA‐Rs in the brain. In rodents, NBI‐1065845 showed virtually no agonistic activity and a wide safety margin against seizure of >1000‐fold for plasma area under the concentration–time curve (AUC) and >400‐fold for plasma maximum plasma concentration (C max) following acute dosing. 3 , 12 Furthermore, in rat primary cortical neurons, NBI‐1065845 was associated with increased BDNF production (AMPA‐mediated and dose‐dependent) and mTOR signaling. 7 , 12 In a rat model of depression, NBI‐1065845 demonstrated rapid, robust and sustained antidepressant‐like effects. 7 In a pharmacodynamic study using transcranial magnetic stimulation in healthy individuals, NBI‐1065845 increased the amplitude of motor‐evoked potentials, consistent with increased AMPA‐R‐mediated cortical excitability and neuroplasticity (ClinicalTrials.gov identifier: NCT03792672). 13 , 14 Based on the preclinical and clinical results, NBI‐1065845 may have the potential to overcome the limitations of known AMPA‐R potentiators and provide an effective therapeutic approach for MDD.
In a phase I study that investigated the pharmacokinetics (PK) of NBI‐1065845 in healthy adults, NBI‐1065845 was rapidly absorbed in plasma after single doses of 0.3–18 mg and multiple doses of 0.3–9 mg, reaching a mean C max of 4–126 ng/mL (depending on dose) 1–5 h after dosing (median time to C max (t max)) (ClinicalTrials.gov identifier: NCT02561156). Overall exposure was approximately proportional to the dose received. 15 Given its long half‐life of 33–48 hours, three‐fold to four‐fold accumulation of NBI‐1065845 was observed after repeated administration. 15
Given that NBI‐1065845 has been shown in vitro to increase cytochrome P450 (CYP)2B6 and CYP3A4 messenger RNA (mRNA) levels in a concentration‐dependent manner (unpublished data), we sought to investigate the potential of NBI‐1065845 to induce CYP3A metabolism in healthy adults. Midazolam is a sensitive index substrate of CYP3A and is often used for drug–drug interaction (DDI) studies. Ethinyl estradiol–levonorgestrel represents a typical combination oral contraceptive and CYP3A substrate that is widely used in the general population. Time‐dependent clearance of ethinyl estradiol and levonorgestrel is not apparent from published investigations. 16 , 17 Here, we report the results of a phase I DDI study that evaluated the effects of NBI‐1065845 on the PK of midazolam and ethinyl estradiol–levonorgestrel.
METHODS
Ethics statement
This study was performed in full compliance with the International Conference on Harmonisation Good Clinical Practice guidelines and the relevant laws and regulations of the USA. The protocol and informed consent form were approved by the institutional review board before study initiation. All participants provided written informed consent.
Study design
This open‐label, parallel‐arm, phase I DDI study was conducted at a single site in the USA between November 2021 and April 2022. The maximum daily dose of NBI‐1065845 under evaluation in the phase II SAVITRI study was selected as an appropriate dose to investigate any potential DDIs. The single daily doses of midazolam (4 mg), ethinyl estradiol (0.03 mg), and levonorgestrel (0.15 mg) are standard doses used for DDI studies.
The study consisted of two treatment arms. In the midazolam treatment arm, men and women received oral midazolam 4 mg on Day 1, followed by oral NBI‐1065845 alone on Days 5–13 to ensure maximal induction potential of CYP3A. On Day 14, NBI‐1065845 was administered with midazolam 4 mg and then alone on Day 15 to ensure the continued steady‐state influence of NBI‐1065845 through the PK sampling of midazolam (Figure 1).
FIGURE 1.
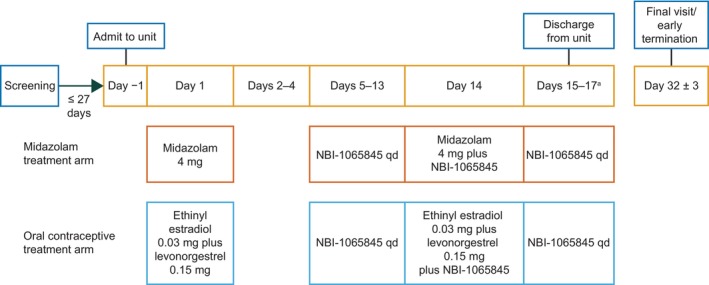
Study design. aParticipants in the combined oral contraceptive treatment arm were discharged from the unit in the morning of Day 17 after all study assessments were completed, whereas participants in the midazolam treatment arm were discharged from the unit in the morning of Day 15 after all study assessments were completed. qd, once a day.
In the combined oral contraceptive treatment arm, women received combination oral contraceptives (ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg) on Day 1, followed by oral NBI‐1065845 alone on Days 5–13 to ensure maximal induction potential of CYP3A at steady state. On Day 14, NBI‐1065845 was administered with ethinyl estradiol–levonorgestrel and then alone on Days 15–17 to ensure the continued steady‐state influence of NBI‐1065845 through the PK sampling of the oral contraceptive (Figure 1).
Participants were required to fast for at least 10 h before study drug administration and for 2 h after study drug administration on Days 1, 5, and 14, at which timepoints serial PK samples were collected. Participants were confined to the study center until all study assessments were completed (Day 15 for the midazolam treatment arm and Day 17 for the combined oral contraceptive arm) and returned to the study center as outpatients on Day 32 (±3 days) for a safety follow‐up visit.
Study participants
Eligible participants were healthy men and women aged 18–55 years (inclusive) with a body mass index (BMI) of 18–32 kg/m.2 Only women were allowed in the combined oral contraceptive treatment arm. Key exclusion criteria included as follows: pregnancy or lactating; evidence of chronic renal or liver disease; a history of epilepsy, seizures, or convulsions other than uncomplicated febrile convulsions with no sequelae; and imminent risk of suicide per the Columbia‐Suicide Severity Rating Scale (C‐SSRS). Full inclusion and exclusion criteria are reported in the Supplementary Materials S1.
Sample collection
Blood samples for PK analysis of midazolam and the combined oral contraceptives (ethinyl estradiol–levonorgestrel) were collected at prescheduled timepoints on Days 1 and 14; for NBI‐1065845, samples were collected on Days 5 and 14 (see Supplementary Materials S1).
Bioanalytical methods
Plasma samples were analyzed and concentrations of NBI‐1065845, ethinyl estradiol, levonorgestrel, and midazolam were quantified using a validated method by PPD, Inc. (Wisconsin, USA). The method was validated for the analysis of NBI‐1065845 (0.100–200 ng/mL) in 100 μL human plasma (EDTA) samples, ethinyl estradiol (2.00–500 pg/mL), and levonorgestrel (50.0–25,000 pg/mL) in 500 μL human plasma (potassium oxalate/sodium fluoride and cross‐validated to EDTA) samples and midazolam (0.100–100 ng/mL) in 150 μL human plasma (EDTA) samples. Plasma samples were extracted either by solid phase extraction (NBI‐1065845) or liquid/liquid extraction in which the organic solvent residues containing the analytes were derivatized and subsequently subjected to a second liquid/liquid extraction procedure (ethinyl estradiol and levonorgestrel) or supported liquid extraction (midazolam). Isolation and quantification of the analytes and internal standards were achieved using reversed‐phase liquid chromatography coupled with tandem mass spectrometry in electrospray positive mode.
Study parameters
The primary PK parameters were AUC from time 0 to infinity (AUC0–∞), C max of midazolam and ethinyl estradiol, and C max and AUC from time 0 to last measurable concentration (AUC0–tlast) for levonorgestrel (replacing AUC0–∞ as a primary PK parameter), in the presence and absence of NBI‐1065845.
Secondary PK parameters for midazolam, the metabolite of midazolam, ethinyl estradiol, and levonorgestrel with and without administration of NBI‐1065845 were AUC0–tlast, t max, apparent systemic clearance after oral administration, apparent terminal half‐life (t ½), apparent volume of distribution during terminal phase after oral administration, and AUC0–∞ and C max for the metabolite of midazolam. Secondary PK parameters for NBI‐1065845 included AUC for a dosing interval on Day 14, AUC from time 0–24 h (AUC0–24) on Day 5, C max and t max.
Data analysis
The study population sample size was not based on a statistical power calculation. The PK analysis set included all participants who received a dose of the study drug from either treatment arm and had at least one post‐dose plasma concentration quantified and one PK parameter available.
Descriptive statistics, including coefficient of variation (CV (%)), geometric mean, and geometric CV (%), were used to summarize the plasma concentrations by treatment and timepoint, and plasma PK parameters by analyte and treatment (except for t max, which was summarized using median, minimum, and maximum values). For each analyte, plasma concentration–time curves represented arithmetic mean (± standard deviation (SD)) by treatment on logarithmic scales. Noncompartmental analysis was performed in Phoenix WinNonlin version 8.3.4 to determine plasma PK parameters.
To compare the AUC0–tlast, AUC0–∞, and C max for midazolam or ethinyl estradiol and levonorgestrel in the presence and absence of NBI‐1065845, a mixed‐effect analysis of variance model was performed using the MIXED procedure of SAS v9.4 with treatment as a fixed effect and participants as a random effect. The estimate of the mean difference in the presence and absence of NBI 1065845 along with the two‐sided 90% confidence intervals (CIs) associated with the difference was calculated. Geometric mean ratios (GMRs) were calculated by exponentiating the geometric least‐squares mean difference in AUC0–tlast, AUC0–∞, and C max. Two‐sided 90% CIs of the GMRs were also calculated. A no‐effect range of 0.8–1.25 was used as an initial benchmark.
One of the original primary PK parameters for levonorgestrel (in combination with ethinyl estradiol) was AUC0–∞ of levonorgestrel in the presence and absence of NBI‐1065845; however, the statistical analysis plan was updated after several participants (Day 1, n = 7; Day 14, n = 9) had a percentage of AUC0–∞ extrapolated to infinity observed from t last to infinity of >20%. Therefore, AUC0–tlast replaced AUC0–∞ as the primary PK parameter for levonorgestrel. For ethinyl estradiol, AUC0–tlast was based on the 24‐h timepoint because concentrations were below the limit of quantification after 24 h.
Safety
Safety measures were assessment of AEs, clinical laboratory tests, vital signs, physical examinations, electrocardiograms, and results from the C‐SSRS. The safety analysis set included all treatment‐assigned participants who received a dose of the study drug from either treatment arm from Day 1 onward and was used for all summaries of safety, demographic, and baseline characteristics data.
RESULTS
Participant demographics and disposition
Of the 14 men and 4 women assigned to the midazolam treatment arm, 16 completed the study. One participant withdrew and one discontinued owing to an AE of tonsillitis considered unrelated to treatment (Table S1). The mean (SD) age and BMI of participants were 38.2 (9.1) years and 26.6 (3.6) kg/m,2 respectively. Nine participants (50.0%) were White, eight (44.4%) were Black or African American, and one (5.6%) was Asian; six (33.3%) participants were Hispanic or Latino (Table 1). All participants who completed the study complied with the full dosing regimen.
TABLE 1.
Baseline demographics and characteristics.
| Demographic or characteristic | Midazolam treatment arm (N = 18) | Oral contraceptive treatment arm (N = 17) |
|---|---|---|
| Age, years | ||
| Mean (SD) | 38.2 (9.1) | 41.5 (7.7) |
| Median (min–max) | 36.5 (22–55) | 39.0 (29–53) |
| Sex, n (%) | ||
| Male | 14 (77.8) | 0 |
| Female | 4 (22.2) | 17 (100) |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 6 (33.3) | 7 (41.2) |
| Not Hispanic or Latino | 12 (66.7) | 10 (58.8) |
| Race, n (%) | ||
| Asian | 1 (5.6) | 2 (11.8) |
| Black or African American | 8 (44.4) | 5 (29.4) |
| White | 9 (50.0) | 10 (58.8) |
| Height at screening, cm | ||
| Mean (SD) | 177.5 (9.2) | 161.5 (6.4) |
| Median (min–max) | 177.5 (161–196) | 161.0 (152–173) |
| Weight, kg | ||
| Mean (SD) | 84.17 (14.15) | 69.94 (11.46) |
| Median (min–max) | 83.10 (63.1–113.9) | 70.00 (49.5–93.5) |
| BMI, a kg/m2 | ||
| Mean (SD) | 26.64 (3.61) | 26.77 (3.87) |
| Median (min–max) | 26.05 (18.8–32.0) | 28.20 (20.3–32.8) |
Abbreviations: BMI, body mass index; SD, standard deviation.
BMI is calculated using height collected at the screening visit and the most recent result before dose administration for weight.
Of the 17 women assigned to the combined oral contraceptives treatment arm, 16 completed the study and one discontinued owing to an AE of COVID‐19 considered unrelated to treatment (Table 2, Table S1). The mean (SD) age and BMI of participants were 41.5 (7.7) years and 26.8 (3.9) kg/m,2 respectively. Ten participants (58.8%) were White, five (29.4%) were Black or African American, and two (11.8%) were Asian; seven (41.2%) participants were Hispanic or Latino (Table 1).
TABLE 2.
Summary of TEAEs (safety analysis set).
| TEAEs | Midazolam treatment arm | Oral contraceptive treatment arm | ||||
|---|---|---|---|---|---|---|
| Midazolam alone (N = 18), n (%) | NBI‐1065845 alone (N = 18), n (%) | Midazolam plus NBI‐1065845 (N = 16), n (%) | Ethinyl estradiol plus levonorgestrel (N = 17), n (%) | NBI‐1065845 alone (N = 17), n (%) | Ethinyl estradiol plus levonorgestrel plus NBI‐1065845, (N = 16), n (%) | |
| Participants with any TEAEs | 7 (38.9) | 7 (38.9) | 5 (31.3) | 5 (29.4) | 9 (52.9) | 0 |
| Participants with maximum TEAE severity of: a | ||||||
| Mild | 6 (33.3) | 1 (5.6) | 2 (12.5) | 4 (23.5) | 3 (17.6) | 0 |
| Moderate | 0 | 1 (5.6) | 1 (6.3) | 0 | 3 (17.6) | 0 |
| Severe | 0 | 0 | 0 | 0 | 0 | 0 |
| Most frequent TEAEs | ||||||
| Headache | 1 (5.6) | 1 (5.6) | 0 | 2 (11.8) | 6 (35.3) | 0 |
| Somnolence | 6 (33.3) | 1 (5.6) | 5 (31.3) | 0 | 0 | 0 |
| Dizziness | 2 (11.1) | 1 (5.6) | 1 (6.3) | 0 | 0 | 0 |
| COVID‐19 | 0 | 0 | 0 | 0 | 1 (5.9) | 0 |
| Serious AEs | 0 | 0 | 0 | 0 | 0 | 0 |
| TEAEs leading to study discontinuation | 1 (5.6) | 0 | 0 | 0 | 1 (5.9) | 0 |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: AE, adverse event; TEAE, treatment‐emergent AE.
Participants are included in the row representing the most severe intensity reported for any AE.
Pharmacokinetics
Midazolam
The mean plasma concentration–time profile for midazolam was similar in the presence or absence of NBI‐1065845 (Figure 2). Midazolam was rapidly absorbed (median t max of 0.5 hours), with a geometric mean (geometric CV (%)) C max of 19 ng/mL (27.5%) and AUC0‐∞ of 51 ng × h/mL (27.8%) in the absence of NBI‐1065845. Following multiple daily doses of NBI‐1065845, the geometric mean (geometric CV (%)) C max was 18 ng/mL (39.3%), and AUC0–∞ was 44 ng × h/mL (37.4%) (Table 3). Therefore, the PK parameters of midazolam were not appreciably affected by NBI‐1065845, with a GMR (90% confidence interval (CI)) for C max of 0.94 (0.79–1.13) and for AUC0–∞ of 0.88 (0.78–0.98) (Table 4).
FIGURE 2.
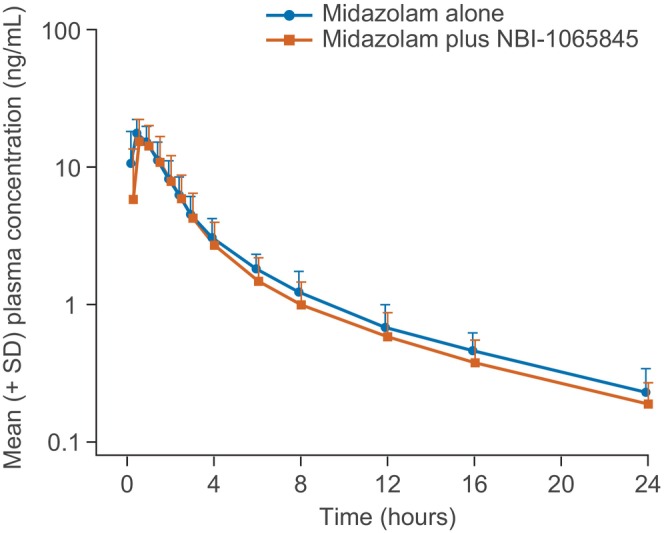
Midazolam plasma time–concentration in the presence or absence of NBI‐1065845 (logarithmic scale). SD, standard deviation.
TABLE 3.
PK parameters of midazolam, ethinyl estradiol, and levonorgestrel in the presence and absence of NBI‐1065845.
| Parameter | Midazolam treatment arm | Oral contraceptive treatment arm a | ||||
|---|---|---|---|---|---|---|
| Midazolam alone (N = 18) | Midazolam plus NBI‐1065845 (N = 16) | Ethinyl estradiol alone (N = 17) | Ethinyl estradiol plus NBI‐1065845 (N = 16) | Levonorgestrel alone (N = 17) | Levonorgestrel plus NBI‐1065845 (N = 16) | |
| C max, pg/mL b or ng/mL c | 18.9 (27.5%) | 17.5 (39.3%) | 69.3 (42.5%) | 68.1 (35.2%) | 2731 (49.5%) | 2631 (35.5%) |
| t max, hours | 0.50 (0.33–1.00) | 0.50 (0.25–1.45) | 1.48 (0.50–2.97) | 1.71 (0.95–3.03) | 1.58 (0.93–4.00) | 1.54 (0.92–2.97) |
| AUC0–∞, pg × h/mL b or ng × h/mL c | 50.7 (27.8%) | 43.9 (37.4%) | 688.1 (40.3%) d | 684.1 (32.3%) | 43,422 (54.1%) e | 36,240 (52.6%) f |
| AUC, g pg × h/mL b or ng × h/mL c | 49.0 (27.8%) | 42.4 (37.6%) | 494.9 (32.5%) | 495.5 (27.4%) | 32,639 (48.4%) | 27,434 (42.9%) |
| t ½, hours | 4.78 (37.9%) | 4.85 (45.2%) | 15.3 (34.2%) | 15.5 (32.6%) | 34.7 (53.0%) | 36.0 (50.7%) |
| Vz/F, a L | 544.0 (32.3%) | 637.7 (49.0%) | 960.4 (30.1%) | 980.8 (28.7%) | 172.9 (68.7%) | 214.7 (58.4%) |
| CL/F, L/h | 78.8 (27.8%) | 91.2 (37.4%) | 43.6 (40.3%) | 43.9 (32.3%) | 3.45 (54.1%) | 4.14 (52.6%) |
Note: All data are geometric mean (geometric CV (%)) except for t max, for which median (min–max) are reported.
Abbreviations: AUC, area under the concentration–time curve; AUC0–∞, AUC from time 0 to infinity; AUC%extrap, percentage of AUC0–∞ extrapolated to infinity observed from tlast to infinity; AUC0–24, AUC from time 0 to 24 h; AUC0–tlast, AUC from time 0 to last measurable concentration; CL/F, apparent oral clearance; C max, maximum plasma concentration; CV (%), coefficient of variation; PK, pharmacokinetics; t ½, half‐life; t max, time to C max; Vz/F, apparent volume of distribution.
Two participants in the midazolam treatment arm were included in the PK analysis set for Day 1 (midazolam alone) but excluded from the PK analysis set on Day 14 (midazolam and NBI‐1065845) owing to study drug discontinuation.
Units for ethinyl estradiol and levonorgestrel.
Units for midazolam.
Includes one participant whose AUC%extrap value was above 20%.
Includes seven participants whose AUC%extrap values were above 20%.
Includes nine participants whose AUC%extrap values were above 20%.
The following AUC values are reported: ethinyl estradiol, AUC0–24; levonorgestrel, AUC0–tlast (which was at 72 h except for one participant, whose AUC0–tlast was at 48 h for levonorgestrel alone); and midazolam, AUC0–tlast.
TABLE 4.
Geometric least‐squares means and GMRs for PK parameters of midazolam, ethinyl estradiol, or levonorgestrel.
| Midazolam treatment arm | ||||||
|---|---|---|---|---|---|---|
| Parameter | Midazolam alone (N = 18) | Midazolam plus NBI‐1065845 (N = 16) | Midazolam plus NBI‐1065845 vs. midazolam alone, GMR (90% CI) | |||
| C max, ng/mL | 18.93 | 17.89 | 0.94 (0.79–1.13) | |||
| AUC0–∞, ng × h/mL | 50.75 | 44.53 | 0.88 (0.78–0.98) | |||
| Oral contraceptive treatment arm | ||||||
| Parameter | Ethinyl estradiol alone ( N = 17) | Ethinyl estradiol plus NBI‐1065845 ( N = 16) | Ethinyl estradiol plus NBI‐1065845 vs. ethinyl estradiol alone, GMR (90% CI) | Levonorgestrel alone ( N = 17) | Levonorgestrel plus NBI‐1065845 ( N = 16) | Levonorgestrel plus NBI‐1065845 vs. levonorgestrel alone, GMR (90% CI) |
| C max, pg/mL | 69.3 | 69.4 | 1.00 (0.87–1.15) | 2731 | 2706 | 0.99 (0.87–1.13) |
| AUC0–∞, pg × h/mL | 688.1 | 692.4 | 1.01 (0.88–1.15) | – | – | – |
| AUC0–24, pg × h/mL | 494.9 | 500.8 | 1.01 (0.91–1.13) | – | – | – |
| AUC0–tlast, pg × h/mL | – | – | – | 32,639 | 28,295 | 0.87 (0.78–0.96) |
Abbreviations: AUC0–∞, area under the concentration–time curve from time 0 to infinity; AUC0–24, area under the concentration–time curve from time 0 to 24 h; AUC0–tlast, area under the concentration–time curve from time 0 to last measurable concentration; CI, confidence interval; C max, maximum plasma concentration; GMR, geometric mean ratio; PK, pharmacokinetics.
Oral contraceptives
The mean plasma concentration–time profiles for ethinyl estradiol and levonorgestrel were generally similar in the presence or absence of NBI‐1065845 (Figure 3). Ethinyl estradiol and levonorgestrel were rapidly absorbed (median t max of 1.5 h and 1.6 h, respectively), reaching geometric mean (geometric CV (%)) C max values of 69 pg/mL (42.5%) and 2731 pg/nL (49.5%), respectively, in the absence of NBI‐1065845 (Table 4). Furthermore, the geometric mean (geometric CV (%)) AUC0–∞ was 688 (40.3%) for ethinyl estradiol and AUC0–tlast was 32,639 (48.4%) for levonorgestrel.
FIGURE 3.
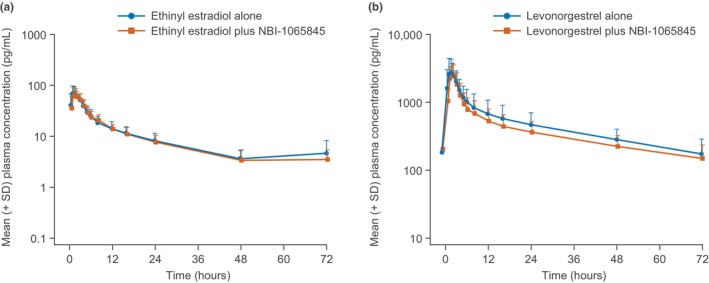
(a) Ethinyl estradiol plasma time–concentration and (b) levonorgestrel plasma time–concentration in the presence or absence of NBI‐1065845 (logarithmic scale). SD, standard deviation.
After multiple daily doses of NBI‐1065845, the geometric mean (geometric CV (%)) C max values were 68 pg/mL (35.2%) and 2631 pg/nL (35.5%) for ethinyl estradiol and levonorgestrel, respectively. The geometric mean (geometric CV (%)) AUC0–∞ was 684 (32.3%) for ethinyl estradiol, and AUC0–tlast was 27,434 (42.9%) for levonorgestrel (Table 3). As such, the key PK parameters of ethinyl estradiol were unaffected by NBI‐1065845, with GMRs (90% CI) for C max of 1.00 (0.87–1.15) and for AUC0–∞ of 1.01 (0.88–1.15) (Table 4). For levonorgestrel, PK parameters were not affected appreciably by multiple daily doses of NBI‐1065845, with GMRs (90% CI) for C max of 0.99 (0.87–1.13) and AUC0–tlast of 0.87 (0.78–0.96) (Table 4).
NBI‐1065845
Following multiple daily doses of NBI‐1065845, accumulation was observed, with approximately three‐fold to four‐fold accumulation increases in C max and AUC on Day 14 compared with Day 5 (Figure S1 and Table S1).
Safety
All AEs were mild or moderate in severity, with no deaths or serious AEs reported. One participant in each study arm discontinued due to AEs (COVID‐19 and tonsillitis); both AEs were considered unrelated to the study drug (Table 2). The most common AEs in the midazolam treatment arm were somnolence and dizziness (n = 9 (50.0%) and n = 3 (16.7%), respectively), whereas the most common AE in the oral contraceptive treatment arm was headache (n = 6 (35.3%)) (Table 2). During safety evaluations, one participant in the midazolam treatment arm and four women in the oral contraceptive treatment arm had potentially clinically significant blood pressure or heart rate measurements that were transient and not associated with AEs. Potentially clinically significant laboratory findings were observed in two participants in the midazolam treatment arm and were associated with AEs of moderately increased blood creatine phosphokinase and mildly decreased white blood cell count, both of which were considered unrelated to treatment. No tremors or seizures were observed in either treatment arm. No participants reported suicidal ideation or behavior during the study. No other notable physical examination, vital sign, or observation results were observed.
DISCUSSION
The US Food and Drug Administration's definition of weak CYP3A induction is a decrease of 20–<50% in exposure for the victim drug. 18 In this study, no statistically significant changes were observed for C max for midazolam, ethinyl estradiol, or levonorgestrel, given that the 90% CI of the GMR crossed 1. Additionally, no statistically significant change was observed for AUC0–∞ of ethinyl estradiol. Although the AUCs of midazolam and levonorgestrel were not within the no‐effect range, the magnitude of the GMRs did not meet the criteria for a DDI between NBI‐1065845 and the sensitive index substrate midazolam or levonorgestrel. Therefore, NBI‐1065845 is not considered a CYP3A inducer, and the potential for DDIs between NBI‐1065845 and CYP3A substrates is low.
These results indicate that NBI‐1065845 can be administered in future studies in individuals with MDD who are also taking combined oral contraceptives or other CYP3A substrates without modified dosing of CYP3A substrates. Commonly prescribed CYP3A substrates include some antidepressants (ketamine, selective serotonin reuptake inhibitors), anxiety medications, and statins, which individuals with MDD may be receiving for coexisting health conditions.
In vitro metabolic data found that NBI‐1065845 increased CYP3A4 mRNA levels (suggesting possible increased CYP3A4 activity), highlighting the importance of phase I clinical evaluation. The PK profile for NBI‐1065845 was in line with previous findings, such as the three‐fold to four‐fold increases in C max and AUC for NBI‐1065845 after repeated adminstration. 15 Although in vitro data suggested a potential interaction between CYP3A and NBI‐1065845, the findings from this study clearly conclude that NBI‐1065845 is not considered an inducer of CYP3A clinically, and administration of NBI‐1065845 with midazolam, combined oral contraceptives, or other CYP3A substrates is supported.
Safety results were consistent with those of previous studies, with all AEs being mild or moderate in severity. There were no serious AEs, and the potentially clinically significant laboratory findings (increased blood creatine phosphokinase and decreased white blood cell count) were not considered related to NBI‐1065845. In line with previous clinical studies of NBI‐1065845 in healthy individuals (NCT02561156) and individuals with MDD (unpublished data), no tremors or seizures nor suicidal ideation or behavior (based on the C‐SSRS and AE reporting) were reported in this DDI study. There were also no new safety signals observed for NBI‐1065845 in the presence of midazolam, ethinyl estradiol, or levonorgestrel. 13 , 14
CONCLUSIONS
The magnitude of the GMRs did not meet the criteria for a DDI between NBI‐1065845 and the sensitive index substrate midazolam or the oral contraceptives ethinyl estradiol–levonorgestrel. Therefore, NBI‐1065845 is not considered a CYP3A inducer, and NBI‐1065845 administration with combined oral contraceptives and other CYP3A substrates is supported. There were no new safety findings for NBI‐1065845 in the presence or absence of midazolam, ethinyl estradiol, or levonorgestrel in this DDI study. These findings support further investigation of NBI‐1065845 for the treatment of MDD.
AUTHOR CONTRIBUTIONS
A.I., D.P.W., J.B.S., J.M‐S., M.F., M.K., and S.L. wrote the manuscript; J.B.S. and S.L. designed the research; A.I., D.P.W., J.B.S., J.M‐S., M.F., M.K., and S.L. performed the research; A.I., D.P.W., J.B.S., and S.L. analyzed the data.
FUNDING INFORMATION
This study was funded by the sponsor, Neurocrine Biosciences, Inc.
CONFLICT OF INTEREST STATEMENT
S.L., A.I., J.M.‐S., M.K., M.F., and J.B.S. are employees of Neurocrine Biosciences, Inc. and own stock or share options. D.W. reports compensation for serving as a consultant or speaker, or research support, paid to the author or the institution they work for, from AbbVie, Acadia Pharmaceuticals, Alkermes, Allergan, Avanir Pharmaceuticals, Biogen, Boehringer Ingelheim, Cerevel Therapeutics, Indivior, Intra‐Cellular Therapies, Johnson & Johnson Pharmaceutical Research & Development, Janssen Pharmaceuticals, Karuna Therapeutics, Lundbeck, Lupin, Lyndra Therapeutics, Neurocrine Biosciences, Novartis, Noven Pharmaceuticals, Otsuka Pharmaceutical, Pfizer, Roche, Sunovion and Takeda.
Supporting information
Figure S1:
Data S1:
Table S1:
ACKNOWLEDGMENTS
The authors would like to thank the principal investigator, David Walling, PhD, and the study participants. Under the direction of the authors and funded by Neurocrine Biosciences, Inc., Dr R. Huntly and Dr A. Jones of Oxford PharmaGenesis, Oxford, UK, provided writing assistance for this publication, in accordance with Good Publication Practice 3 guidelines (http://www.ismpp.org/gpp3). Editorial assistance in formatting, proofreading, copy editing, and fact‐checking was also provided by Oxford PharmaGenesis.
Lin S, Ionescu A, Maynard‐Scott J, et al. Effects of the selective AMPA modulator NBI‐1065845 on the pharmacokinetics of midazolam or ethinyl estradiol–levonorgestrel in healthy adults. Clin Transl Sci. 2024;17:e13791. doi: 10.1111/cts.13791
DATA AVAILABILITY STATEMENT
The data are available following a request to the corresponding author, for researchers who provide a methodologically sound proposal. The data will be provided after its de‐identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.
REFERENCES
- 1. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer‐term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am. J. Psychiatry. 2006;163:1905‐1917. [DOI] [PubMed] [Google Scholar]
- 2. Mauskopf JA, Simon GE, Kalsekar A, Nimsch C, Dunayevich E, Cameron A. Nonresponse, partial response, and failure to achieve remission: humanistic and cost burden in major depressive disorder. Depress. Anxiety. 2009;26:83‐97. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki A, Hara H, Kimura H. Role of the AMPA receptor in antidepressant effects of ketamine and potential of AMPA receptor potentiators as a novel antidepressant. Neuropharmacology. 2023;222:109308. [DOI] [PubMed] [Google Scholar]
- 4. Kadriu B, Musazzi L, Johnston JN, et al. Positive AMPA receptor modulation in the treatment of neuropsychiatric disorders: a long and winding road. Drug Discov. Today. 2021;26:2816‐2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daly EJ, Trivedi MH, Janik A, et al. Efficacy of Esketamine nasal spray plus Oral antidepressant treatment for relapse prevention in patients with treatment‐resistant depression: a randomized clinical trial. JAMA Psychiatry. 2019;76:893‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Popova V, Daly EJ, Trivedi M, et al. Efficacy and safety of flexibly dosed Esketamine nasal spray combined with a newly initiated Oral antidepressant in treatment‐resistant depression: a randomized double‐blind active‐controlled study. Am. J. Psychiatry. 2019;176:428‐438. [DOI] [PubMed] [Google Scholar]
- 7. Hara H, Suzuki A, Kunugi A, Tajima Y, Yamada R, Kimura H. TAK‐653, an AMPA receptor potentiator with minimal agonistic activity, produces an antidepressant‐like effect with a favorable safety profile in rats. Pharmacol. Biochem. Behav. 2021;211:173289. [DOI] [PubMed] [Google Scholar]
- 8. Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine‐induced antidepressant effects are associated with AMPA receptors‐mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur. Psychiatry. 2014;29:419‐423. [DOI] [PubMed] [Google Scholar]
- 9. Zunszain PA, Horowitz MA, Cattaneo A, Lupi MM, Pariante CM. Ketamine: synaptogenesis, immunomodulation and glycogen synthase kinase‐3 as underlying mechanisms of its antidepressant properties. Mol. Psychiatry. 2013;18:1236‐1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McIntyre RS, Rosenblat JD, Nemeroff CB, et al. Synthesizing the evidence for ketamine and Esketamine in treatment‐resistant depression: an international expert opinion on the available evidence and implementation. Am. J. Psychiatry. 2021;178:383‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Short B, Fong J, Galvez V, Shelker W, Loo CK. Side‐effects associated with ketamine use in depression: a systematic review. Lancet Psychiatry. 2018;5:65‐78. [DOI] [PubMed] [Google Scholar]
- 12. Suzuki A, Kunugi A, Tajima Y, et al. Strictly regulated agonist‐dependent activation of AMPA‐R is the key characteristic of TAK‐653 for robust synaptic responses and cognitive improvement. Sci. Rep. 2021;11:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dijkstra F, O'Donnell P, Klaassen E, et al. Central nervous system effects of TAK‐653, an investigational alpha‐amino‐3‐hydroxy‐5‐methyl‐4‐isoxazole receptor (AMPAR) positive allosteric modulator in healthy volunteers. Transl. Psychiatry. 2022;12:408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. O'Donnell P, Dijkstra FM, Damar U, et al. Transcranial magnetic stimulation as a translational biomarker for AMPA receptor modulation. Transl. Psychiatry. 2021;11:325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Asgharnejad M, Zicha S, Xu L, et al. Pharmacokinetic and pharmacodynamic properties of the investigational AMPA receptor positive allosteric modulator TAK‐653 after single and multiple rising doses in healthy volunteers. Presented at the American College of Neuropsychopharmacology 57th Annual Meeting. Neuropsychopharmacology. 2018;43:77‐227. [Google Scholar]
- 16. Stanczyk FZ, Archer DF, Lohmer LRL, Pirone J, Previtera M, Korner P. Extended regimen of a levonorgestrel/ethinyl estradiol transdermal delivery system: predicted serum hormone levels using a population pharmacokinetic model. PLoS One. 2022;17:e0279640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reinecke I, Hofmann B, Mesic E, Drenth HJ, Garmann D. An integrated population pharmacokinetic analysis to characterize levonorgestrel pharmacokinetics after different administration routes. J. Clin. Pharmacol. 2018;58:1639‐1654. [DOI] [PubMed] [Google Scholar]
- 18. US Food and Drug Administration . Clinical drug interaction studies—cytochrome P450 enzyme‐ and transporter‐mediated drug interactions. Accessed November 10, 2022. https://www.fda.gov/media/134581/download 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Data S1:
Table S1:
Data Availability Statement
The data are available following a request to the corresponding author, for researchers who provide a methodologically sound proposal. The data will be provided after its de‐identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.


