Abstract
Laser Interstitial Thermotherapy is a minimally invasive treatment option in neurosurgery for intracranial tumors, including recurrent gliomas. The technique employs the thermal ablation of target tissue to achieve tumor control with real-time monitoring of the extent by magnetic resonance thermometry, allowing targeted thermal injury to the lesion. Laser Interstitial Thermotherapy has gained interest as a treatment option for recurrent gliomas due to its minimally invasive nature, shorter recovery times, ability to be used even in patients with numerous comorbidities, and potential to provide local tumor control. It can be used as a standalone treatment or combined with other therapies, such as chemotherapy or radiation therapy. We describe the most recent updates regarding several studies and case reports that have evaluated the efficacy and safety of Laser Interstitial Thermotherapy for recurrent gliomas. These studies have reported different outcomes, with some demonstrating promising results in terms of tumor control and patient survival, while others have shown mixed outcomes. The success of Laser Interstitial Thermotherapy depends on various factors, including tumor characteristics, patient selection, and the experience of the surgical team, but the future direction of treatment of recurrent gliomas will include a combined approach, comprising Laser Interstitial Thermotherapy, particularly in deep-seated brain regions. Well-designed prospective studies will be needed to establish with certainty the role of Laser Interstitial Thermotherapy in the treatment of recurrent glioma.
Keywords: brain tumor, glioblastoma, laser interstitial thermotherapy, glioma, radiation necrosis, stereotactic laser ablation, thermotherapy
Introduction
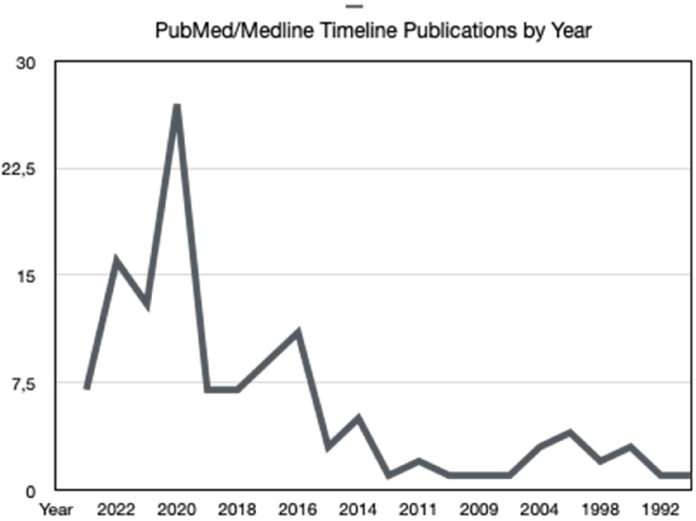
Laser Interstitial ThermoTherapy (LITT; also known as Stereotactic Laser Ablation) is a minimally invasive procedure that is recently gaining popularity in the treatment of malignant brain tumors. 1 Historically, the primary indication for LITT has been for the treatment of recurrent glioblastoma (GBM). However, while laser has long been utilized for open surgery, the earliest cases of LITT for brain tumors were reported in 1983. 2 After 2006, 3 technological innovations led to the widespread adoption of LITT which in turn led to increased use for tumors and epilepsy: the development of cooled probes suitable for MRI; MRI-compatible thermometry; and software that can integrate the results of repeated thermometry to identify cumulative thermal damage of several individual LITT treatments. Indeed, we can notice we can see the continued growth of LITT-related publications in Figure 1, especially since 2020.
Figure 1.
PubMed/Medline number of publications by year.
This technique, which has been gaining popularity in many hospitals over the past 5 years, allows the surgeon to precisely target the tumor and spare healthy brain tissue. Here, we provide a brief literature review of the various contemporary uses for LITT, particularly in recurrent glioma, and their reported outcomes and limitations (Table 1).
Table 1.
Details of Analyzed Studies.
| Study-type of study | Tumor grade (number of patients) | Mean age (age range) | Baseline KPS | Inoperable due to tumor location? (yes/no; cases) | Tumor area | Tumor volume median | GTA n (%) or mEOA % | Time procedure median (h) | Pre-LITT RT / CHT (cases) | Post LITT treatments (cases) | Complications (cases) | Median overall survival (months) | Discharged to home (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leuthardt et al
3
RCT |
rGBM 20 |
/ | / | / | 4 Te 6 Pa 1 In 1 Th 7 Fr 1 Pa/Oc |
/ | / | / | Yes 19 | CHT 20 | No | / | 14 (70) |
| Beaumont et al
4
Retrospective |
rGBM 6 nGBM 9 |
54.7 (36-69; M/F 9/6) | 81 | Yes | 7 Fr 2 Pa 6 CC |
18.67 cm3 | / | 7.7 | Yes 6 | CHT + RT 8 CHT 4 |
Edema 1 Hemiparesis 3 Ventriculitis 1 Hydrocephalus 1 Weakness 1 |
7.2 | 14 (93) |
| Kamath et al
5
Retrospective |
rGBM 41 nGBM 17 |
58.8 (35-78; M/F 37/17) | / | Yes; 19 |
14 Fr 9 Pa 8 Te 8 CC 8 Th 8 ML 2 In |
12.5 cm3 | / | 2.4 | Yes 40 | CHT 53 RT 15 Surgery 3 |
Edema 3 Seizures 3 Hydrocephalus 1 Hyponatremia 1 Infection 1 Mortalities 2 |
11.5 | / |
| Vega et al
6
Retrospective |
rGBM 4 |
54 (44-56; M/F 2/2) |
90 | / | 2 BG 1 Th 1Te |
10.15 cm3 | mEOA 90.4% | / | Yes 4 | CHT 1 RT 1 |
CSF leak 1 Transient hemiparesis and mild oculomotor nerve palsy 1 |
5 | 4 (100) |
| Shah et al
7
Retrospective |
rGBM 14 |
54 (29-73; M/F 7/7) |
91 | Yes | 4 Fr 2 Fr/Pa 1 Pa 6 Te 1 Oc |
3.8 cm3 | mEOA 87.5 | / | / | / | Wound infection 1 | 7.3 | 14 (100) |
| Traylor et al
8
Retrospective |
rGBM 49 nGBM 20 |
56 (15-77; M/F 43/26) | / | Yes | 27 DST 42 No DST |
10.4 cm3 | GTA 8 (40.6) No GTA 41 (59.4) |
/ | / | CHT 47 RT 19 |
Permanente neurological deficits 17 Seizures 4 Impaired cognition 3 Other medical complications 6 |
12 | / |
| De Groot et al
9
Prospective |
rGBM 60 |
59.0 (M/F 32/28) | 90 | Yes; 25 | 15 Th Others Unkn. |
8.5 cm3 | EOA * 18/58; ** 32/58; *** 8/58 |
3.29 | Yes 54 | / | Speech aphasia 1 Seizure 1 Edema 3 |
8.97 | 52 (87) |
| Muir et al
10
Retrospective |
rGBM 11 Others 2 |
56 (28-77; M/F 6/7) | 87 | / | 3 In 4 Te 1 Fr 1 Fr /Pa 1 Th 1 CC |
12.9 cm3 | GTA after: first LITT 7 (53.8) second LITT 2 (15.4) 3nd LITT 2 (66.7) |
/ | Yes 9 | Yes (8) | Weakness 2 Aphasia 2 |
first-LITT 6.0 second 3.2 3nd 2.1 |
13 (100) |
Abbreviations: RCT, randomized controlled trial; M/F, male/female; nGBM, newly diagnosed glioblastoma; rGBM, recurrent glioblastoma; Fr, frontal; Fr-Pa, frontal-parietal; Pa, parietal; Te, temporal; Oc, occipital; In, insular; Th, thalamic; BG, basal ganglial; CC, corpus callosum; ML, multiple lobes; Unkn, unknown; mEOA, median of extent of ablation, EOA* 100%, EOA ** 91%-100%, EOA*** 51%-90%; GTA, gross total ablation; Mo, months; DST, deep-seated tumor; CHT, chemotherapy; RT, radiotherapy; LITT, Laser Interstitial Thermotherapy.
Laser Interstitial Thermotherapy and GBM
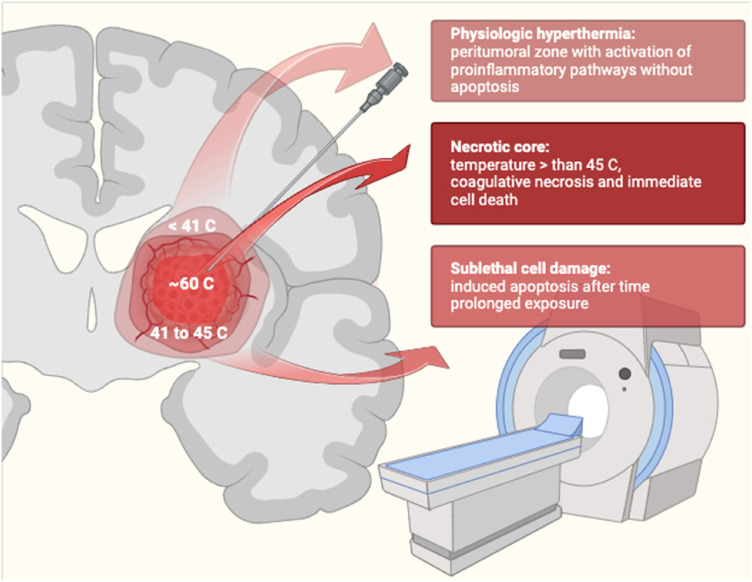
In glial disorders in which safe resection is impossible due to lesion site, location in eloquent or deep areas, or near white matter structures, the standard approach is nonoperative management with chemoradiotherapy and worse outcomes occur as a result.11,12 Considering that nowadays, according to the literature, less than a third of GBMs can be removed with a gross total resection, it is necessary to evaluate other cytoreductive methods.6,8,13,14 Furthermore, approximately 25% of GBM recurrences undergo surgical reoperation and 70% develop local tumor recurrence within a few months of multimodal approach.15,16 Nowadays surgery is the standard of care, since effective lesion debulking is critical to increase survival and ensure a good quality of life, the reason why in those who have no contraindications to surgery, subtotal resection is opted for when total resection is not possible, reducing perioperative morbidity and improving survival to optimize the survival benefit.17,18 Minimally invasive procedures are becoming increasingly popular in the treatment of hard-to-reach brain tumors. Of these, stereotactic radiosurgery (SRS) is still the main one; however, laser interstitial thermal therapy LITT is one of the possible cytoreductive methods of lesions 19 and according to the literature, LITT has a lower absolute risk of complications compared to SRS treatment. Laser Interstitial Thermotherapy consists of a laser probe that is placed stereotaxically to precisely provide warmth to adjacent brain tissue with magnetic resonance thermometry which enables real-time thermal reality and greater control of laser energy release, allowing to carefully monitor the ablation of diseased tissue, sparing the surrounding brain tissue, so as to reduce iatrogenic damage19,20 (Figure 2). This technique is based on the release of nonionizing radiation, in this case light, which is transformed into heat and spreads into the surrounding environment leading to cellular thermal damage and coagulative necrosis. In the case of tumors, the higher the levels of proteins and hemoglobin within them, the more it increases the assimilation of light over water in the surrounding edematous tissue and therefore makes it easier to facilitate heating of tumor tissue. 21 Over time, LITT is recognized as a valid treatment and in some pathologies, such as new-onset gliomas, recurrent gliomas, epileptic foci, recurrent brain metastases, and radiation necrosis,10,22 it is also proposed as a first-line choice, particularly in the case of relapsing gliomas,23,24 since recurrences are found in the peritumoral region and up to 90% grows within 2 to 3 cm around the primary site and are thought to develop from microscopic glioma cells infiltrating the peritumoral environment before resection of the primary tumor.3,25–27
Figure 2.
Schematic representation of Laser Interstitial Thermotherapy (LITT) mechanism of action.
Potential Advantages for Recurrent Gliomas
There is currently no consensus protocol for LITT. It remains a useful alternative for patients who are poor candidates for surgery. 9 Several factors, including the patient's comorbidities and the size and anatomic region of the lesion, remain critical when choosing LITT as the primary treatment for suspected high-grade gliomas and as such has been commonly applied in the following situations: postresection recurrence and maximal adjuvant therapy whereby considered refractory patients; high risk of postoperative complications due to concomitant comorbidities and/or localization; small tumor volume sizes up to 30 mm in diameter; patient preference; elderly patients who cannot tolerate prolonged anesthesia or blood loss.7,19,28
For lesions in deep-seated brain regions, gross-total or near-total resection cannot be achieved without significant morbidity and is therefore not commonly tried. In this scenario, LITT is a safe and effective treatment option for recurrent malignant gliomas that are difficult to access including lesions of the thalamus, insula, basal ganglia, and corpus callosum.5,29 Beaumont et al 4 demonstrated that LITT represents a safe and effective treatment option for GBM of the corpus callosum with limited complications after the procedure and a survival comparable to safe surgical resection with adjuvant chemoradiation therapy. According to Beaumont et al, 4 in deep-seated brain regions, LITT-associated complications are related to tumor volume and can be almost eliminated by limiting the procedure to tumors up to 15 cm3. 4
Furthermore, initial data of Kamath et al, 5 the addition of LITT at the time of recurrence resulted in an overall survival (OS) of 11.8 months. Although this is a prolongation of only 2 months, in the context of survival after GBM recurrence, this may represent a limited but significant survival benefit. Furthermore, this benefit is associated with significantly lower operative morbidity and recovery time compared to conventional craniotomy. On the other hand, while OS in GBM patients who undergo biopsy only followed by chemoradiation has been reported by Stupp et al to be 9.4 months, 30 Kamaht et al showed that the addition of first-line LITT did not demonstrate a survival benefit (OS 9.1 months) compared with these previous data. On the other hand, in recurrent GBM, there is no consensus on the role of repeat surgery. 31 Many patients with recurrent GBM do not undergo reresection but instead receive optimal medical therapy with a median postprogression survival of 7 to 10 months.32,33 For those who undergo repeat surgery, median postoperative survival ranges from 5 to 13 months. 34 Patients who are eligible for reresection may have a survival advantage due to their ability to tolerate surgery because of age, KPS or tumor size and location. When patients cannot be candidates for a second surgery, LITT remains an important treatment alternative, particularly when LITT can be followed with chemotherapy and radiotherapy.5,9
In view of the minimal invasiveness, current research focuses on this technique's ability to break up the blood–brain barrier BBB and elicit immunomodulatory effects.35,36 According to Leuthardt et al, 3 peritumoral hyperthermia causes localized and long-lasting disruption of the BBB which leads to changes in the perilesional vascular structures, and therefore, create a conduit for local therapeutic agents and thus allow for early administration of chemotherapy with minimal impact on wound healing. Indeed Butt et al 31 demonstrated that low-dose doxorubicin given after LITT is well tolerated and correlated with higher OS compared to historical controls treated with bevacizumab or LITT with standard salvage chemotherapy. Over to effects on the BBB and the delivery of chemotherapy, there are implications with radiotherapy as well, as shown by Man et al. In addition, hyperthermia has been found to enhance both innate and adaptive antitumor immune responses through different mechanisms, This involves the release of exosomes rich in tumor antigens, amplifying tumor antigen presentation; triggering the expression of immunostimulatory heat shock proteins; elevating cytokines and chemokines production to attract and activate antigen-presenting cells, cytotoxic T cells, and natural killer cells. Additionally, it induces vessel dilatation, disrupting the blood–brain barrier, enhancing perfusion, and enabling heightened immune surveillance. 35
Compared to the commonly used approach for the management of GBM, it is suggests that LITT has favorable outcomes in terms of outcomes, complication rates, maintenance of good quality of life both in the short and long term, and cost-effectiveness35,37 when adequate extent of ablation (EOA) is obtained. In fact, in the available literature, comparing LITT and standard craniotomy the results are in favor of the former, in terms of cost-effectiveness. According to Chen et al, 22 the hospital stay following the LITT procedure in recurrent GBM was shorter than after open surgery, the 2 largest case series using LITT in GBM also demonstrated improved survival in patients when no other treatment options were available to the patient except the best one palliative care.
Transient or permanent neurologic deficit in the perioperative period post LITT is given by the location of the tumor near noble structures of the white matter, rather than by the procedure itself 8 ; according to Jeffrey et al, however, there are 2 possibilities to reduce the rate of such complications: one option is to use diffusion tensor imaging maps during treatment to get a better idea about EOA, and another option is to start the ablation at a lower setting and observe the heat distribution in the lesion before increasing power. The most commonly reported serious complications include cerebral edema, hydrocephalus, possibility of leptomeningeal spread, and seizures15,22; the overall incidence of major and minor adverse events related to the LITT procedure was 31% for all intracranial lesions. 38
When comparing patients undergoing LITT and patients undergoing craniotomy, 21 the former tended to be older, had lower preoperative KPS, and they had recurrent gliomas rather than treated for newly diagnosed gliomas. 10 There were significantly lower rates of major complications in LITT compared to craniotomy with an absolute risk reduction of 10%. The average extent of resection (EOR) for LITT was 85.4%, which was significantly better than 77% for craniotomy. In addition, recurrence results in significant morbidity and may slow down adjuvant treatments. Barnett et al showed that their EOA was 85.4% with LITT compared to an EOR of 77% with open craniotomy.
Recraniotomy in patients with primary and metastatic brain tumors carries significant morbidity and can delay adjuvant treatments. Recurrent tumors, particularly GBM, when not amenable to surgery, can be safely treated using repeat LITT. Muir et al 10 showed that patients who underwent LITT 2 or 3 times, tolerate the procedure well and have a significant survival.
To data, there are insufficient studies to indicate LITT as a standard of care in treatment of recurrent glial pathologies. It is necessary to evaluate whether the cytoreduction obtained with LITT is equivalent to that obtained with open surgery. 20 According to Vega et al, concurrent surgical resection and LITT is an alternative minimally invasive technique to achieve sufficient EOR/ablation of deep GBM in good surgical candidates. Therefore, future prospective studies are needed to investigate the efficacy of this approach in prolonging overall and progression-free survival.6,13
Limitations and Future Perspectives
Laser Interstitial Thermotherapy is generally considered a safe procedure when performed by experienced neurosurgeons. However, as with any surgical procedure, there are potential risks and complications. Complications associated with LITT include edema, hemorrhage, infection, and damage to surrounding brain structures.22,39,40 In a 2020 systematic review conducted by of Montemurro et al, the prevalent common complications identified were as follows: seizures (2%), motor deficits (1.5%), wound infections (1.5%), transient hemiparesis (1%), and hemorrhage (0.5%). 24 Therefore, rigorous patient selection and careful monitoring is central to minimize the risk of adverse events.
In a retrospective multicenter study by Mohammadi et al in 2014, 40 encompassing 34 patients with high-grade gliomas treated with LITT, a complication rate of 37% was reported. Seventy-one percent of the lesions were situated in the frontal, temporal, or parietal lobes, with the remaining 29% in the insula, thalamus, or corpus callosum. The primary complication observed was the exacerbation of preoperative neurological deficits in 20% of cases, with the majority experiencing temporary declines. Risk factors for deficits were associated with the treatment of large, deep-seated, or eloquent lesions. Noteworthy complications included one case of new-onset seizures, one instance of postoperative hyponatremia, and one patient developing bilateral deep vein thrombosis (DVT). Infection occurred in 6% of cases, with one being a superficial wound infection in an elderly patient with a history of prior radiation, and the other being ventriculitis. Common treatment responses included subtle bleeding within the lased area on neuroimaging (hemorrhagic necrosis) and moderate perilesional edema. In 8% of cases, the volume of hemorrhage was characterized as moderate to large intracerebral hemorrhage in comparison to the initial tumor size.
In a meta-analysis examining the use of LITT as treatment strategy for high-grade gliomas, 24 which included 25 patients, no intraoperative mortality or complications were observed. Lesion localization was distributed as follows: 1 in the basal ganglia, 7 in the frontal lobe, 1 in the corpus callosum, 3 in the temporal lobe, 1 in the parietal lobe, 1 in the insula, 1 in the midbrain, and the remaining 10 in the thalamus. Two serious postoperative complications occurred: a central nervous system infection that led to death, and a significant ablation edema that developed in a patient with a large tumor lesion and needed decompressive hemicraniectomy. Additional postoperative complications included hyponatremia (4%), DVT (4%), and transient neurological deficits that recovered in 24 h (4%). Overall, length of hospital stay after LITT was only 1 to 2 days.
Literature findings suggest a correlation between the EOR and survival in patients dealing with recurrent high-grade gliomas.41,42 In this context, LITT, as a potential cytoreductive treatment may offer a promising strategy, alone or combined with open surgery, for an increased survival. Simultaneously, it holds the potential to reduce complications related to wound healing, cerebrospinal fluid fistulae, and overall recovery time. A review of LITT treatment outcomes for recurrent gliomas, encompassing 63 patients, revealed permanent neurological deficits in 12% cases, while wound infections were observed in 2%. 20 To the best of our knowledge, there is currently no published studies directly comparing LITT and open surgery for the treatment of recurrent high-grade glioma.
The size of tumor poses a constraint on the effectiveness of LITT. Generally, LITT achieves optimal outcomes in cases with smaller volumes, and the lesions with the most favorable results are typically those with diameters ranging from 2 to 3 cm, amenable to treatment with a single trajectory. In contrast, larger tumors may necessitate multiple laser insertions or sessions, resulting in prolonged operating times required for complete ablation. This characteristic may render LITT less appealing when compared to open surgery. 39 Additionally, larger tumors typically cause more swelling, which is poorly tolerated. On the other hand, very small or diffuse tumors may be challenging to target accurately with LITT.22,39
Histopathology provides important information about the tumor's molecular characteristics, which can guide treatment decisions, including targeted therapies. Laser Interstitial Thermotherapy present challenges in obtaining tissue samples for accurate histopathological diagnosis compared to surgical resection. However, if needed, stereotactic biopsy of the lesion can help guide biopsy sampling during or after LITT.
Laser Interstitial Thermotherapy requires specialized training and experience for an effective and safe treatment with optimal outcomes. Familiarity with image-guided techniques, including accurate trajectory planning, thermal monitoring, and treatment parameters, is essential for successful implementation. Surgeons and medical centers with expertise in LITT tend to achieve better outcomes, because experience with LITT allows for better patient selection, improved targeting, and reduction in complications.
The average procedure time is very variable, ranging from a minimum of 2.4 h to a maximum of 7.7 h. This depends very much on the hospital setting, the possibility of using the MRI and the experience of all healthcare personnel. The economic considerations of LITT can vary depending on factors such as the hospital, the surgical team, and additional treatments required. Compared to traditional open surgeries, LITT may have different cost implications that need to be considered. Although LITT may have higher upfront costs related to equipment and setup, it has the potential to reduce hospital and associated costs, potentially leading to overall cost-effectiveness. To date, only preliminary analyses of LITT for the treatment of high-grade gliomas have been conducted, revealing indications of cost-effectiveness, as reported by Voigt and Barnett. 41 Long-term analysis including recurrence rates and patient outcomes is necessary to evaluate the economic impact accurately.
Limitations in Molecular Subtypes
Glial disorders represent a highly heterogeneous group, with variable prognoses based on a wide range of factors, including performance status, number of localizations, primary tumor histology, systemic disease, and the presence/absence of targetable molecular alterations. Currently, there are no major studies on LITT analyzing the outcomes of high-grade gliomas on the basis of histological or molecular tumor heterogeneity. To properly compare efficacy of LITT against other local treatments, histologic types and molecular alterations should be matched in the setting of first-line therapy or salvage therapy at recurrence, either from retrospective studies or in new prospective trials (eg, presence of IDH wild-type or TERT promoter).
Laser Interstitial Thermotherapy in Other Brain Tumors
From a general perspective, the use of LITT has great benefits not only in glioma recurrences but also in metastases and partially in low-grade gliomas. Regarding metastatic brain tumors, LITT can be used both at first detection and in recurrence. 42 In a recent meta-analysis, the pooled 1-year PFS after LITT was 51.2% (95% CI, 36.7%-65.5%; I2 = 66.1%; P heterogeneity = 0.01; 7 studies) for patients with brain metastases. From this perspective, pooled PFS and OS were found to be not significantly inferior to outcomes reported in the literature. 38
Regarding LGGs, LITT is described in the literature as an alternative to surgery only in limited cases in surgically inaccessible or high-risk regions (hypothalamus or brainstem), and the subtypes described are mainly subependymal giant cell astrocytoma and glioneural tumors such as ganglioglioma.43,44
Conclusions
The success of LITT depends on various factors, including tumor characteristics, patient selection, and the experience of the surgical team, but the future direction of treatment of recurrent gliomas will include a combined approach, comprising LITT. When patients are not eligible for reresection due to the number of prior recurrences, lack of trial options, poor KPS, or other eligibility criteria, LITT remains an important treatment option, particularly when LITT can be followed with chemotherapy as also shown in prior reports. Well-designed prospective trials will be required to firmly establish the role of LITT in the treatment of recurrent glioma. Indeed, the literature is not rich in well-designed prospective studies. To provide further evidence, more studies should be conducted, for example, comparing LITT and SRS.
Abbreviations
- LITT
Laser Interstitial ThermoTherapy
- GBM
glioblastoma
- SRS
stereotactic radiosurgery
- OS
overall survival
- EOA
extent of ablation
- EOR
extent of resection
- DVT
deep vein thrombosis
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Andrea Bianconi https://orcid.org/0000-0002-1014-0731
References
- 1.Wright J, Chugh J, Wright CH, et al. Laser interstitial thermal therapy followed by minimal-access transsulcal resection for the treatment of large and difficult to access brain tumors. Neurosurg Focus. 2016;41(4):E14. doi: 10.3171/2016.8.FOCUS16233 [DOI] [PubMed] [Google Scholar]
- 2.Bown SG. Phototherapy in tumors. World J Surg. 1983;7(6):700-709. doi: 10.1007/BF01655209 [DOI] [PubMed] [Google Scholar]
- 3.Leuthardt EC, Duan C, Kim MJ, et al. Hyperthermic laser ablation of recurrent glioblastoma leads to temporary disruption of the peritumoral blood brain barrier. PLoS One. 2016;11(2):e0148613. doi: 10.1371/journal.pone.0148613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaumont TL, Mohammadi AM, Kim AH, Barnett GH, Leuthardt EC. Magnetic resonance imaging-guided laser interstitial thermal therapy for glioblastoma of the corpus callosum. Neurosurgery. 2018;83(3):556-565. doi: 10.1093/neuros/nyx518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kamath AA, Friedman DD, Akbari SHA, et al. Glioblastoma treated with magnetic resonance imaging-guided laser interstitial thermal therapy: safety, efficacy, and outcomes. Neurosurgery. 2019;84(4):836-843. doi: 10.1093/neuros/nyy375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega RA, Traylor JI, Patel R, Muir M, Bastos DCA, Prabhu SS. Combined surgical resection and laser interstitial thermal therapy for glioblastoma: technical note. J Neurol Surg A Cent Eur Neurosurg. 2020;81(4):348-354. doi: 10.1055/s-0040-1709163 [DOI] [PubMed] [Google Scholar]
- 7.Shah AH, Semonche A, Eichberg DG, et al. The role of laser interstitial thermal therapy in surgical neuro-oncology: series of 100 consecutive patients. Neurosurgery. 2020;87(2):266-275. doi: 10.1093/neuros/nyz424 [DOI] [PubMed] [Google Scholar]
- 8.Traylor JI, Patel R, Muir M, et al. Laser interstitial thermal therapy for glioblastoma: a single-center experience. World Neurosurg. 2021;149:e244-e252. doi: 10.1016/j.wneu.2021.02.044 [DOI] [PubMed] [Google Scholar]
- 9.de Groot JF, Kim AH, Prabhu S, et al. Efficacy of laser interstitial thermal therapy (LITT) for newly diagnosed and recurrent IDH wild-type glioblastoma. Neurooncol Adv. 2022;4(1):vdac040. doi: 10.1093/noajnl/vdac040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muir M, Traylor JI, Gadot R, Patel R, Prabhu SS. Repeat laser interstitial thermal therapy for recurrent primary and metastatic intracranial tumors. Surg Neurol Int. 2022;13:311. doi: 10.25259/SNI_418_2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Marco R, Pesaresi A, Bianconi A, et al. A systematic review of amino acid PET imaging in adult-type high-grade glioma surgery: a neurosurgeon’s perspective. Cancers (Basel). 2022;15(1):90. doi: 10.3390/CANCERS15010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianconi A, Bonada M, Zeppa P, et al. How reliable is fluorescence-guided surgery in low-grade gliomas? A systematic review concerning different fluorophores. Cancers (Basel). 2023;15(16):4130. doi: 10.3390/CANCERS15164130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vetkas A, Germann J, Boutet A, et al. Laser interstitial thermal therapy for the treatment of insular lesions: a systematic review. Front Neurol. 2022;13:1024075. doi: 10.3389/fneur.2022.1024075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiaschi P, Badaloni F, Cagetti B, et al. Disseminated oligodendroglial-like leptomeningeal tumor in the adult: case report and review of the literature. World Neurosurg. 2018;114:53-57. doi: 10.1016/J.WNEU.2018.02.160 [DOI] [PubMed] [Google Scholar]
- 15.Munoz-Casabella A, Alvi MA, Rahman M, Burns TC, Brown DA. Laser interstitial thermal therapy for recurrent glioblastoma: pooled analyses of available literature. World Neurosurg. 2021;153:91-97.e1. doi: 10.1016/j.wneu.2021.05.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saaid A, Monticelli M, Ricci AA, et al. Prognostic analysis of the IDH1 G105G (rs11554137) SNP in IDH-wildtype glioblastoma. Genes (Basel). 2022;13(8):1439. doi: 10.3390/GENES13081439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeppa P, De Marco R, Monticelli M, et al. Fluorescence-guided surgery in glioblastoma: 5-ALA, SF or both? Differences between fluorescent dyes in 99 consecutive cases. Brain Sci. 2022;12(5):555. doi: 10.3390/BRAINSCI12050555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Specchia FMC, Monticelli M, Zeppa P, et al. Let me see: correlation between 5-ALA fluorescence and molecular pathways in glioblastoma: A single center experience. Brain Sci. 2021;11(6):795. doi: 10.3390/BRAINSCI11060795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Franca SA, Tavares WM, Salinet ASM, Teixeira MJ, Paiva WS. Laser interstitial thermal therapy as an adjunct therapy in brain tumors: a meta-analysis and comparison with stereotactic radiotherapy. Surg Neurol Int. 2020;11:360. doi: 10.25259/SNI_152_2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I, Kalkanis S, Hadjipanayis CG. Stereotactic laser interstitial thermal therapy for recurrent high-grade gliomas. Neurosurgery. 2016;79(Suppl 1):S24-S34. doi: 10.1227/NEU.0000000000001443 [DOI] [PubMed] [Google Scholar]
- 21.Hong CS, Kundishora AJ, Elsamadicy AA, Chiang VL. Laser interstitial thermal therapy in neuro-oncology applications. Surg Neurol Int. 2020;11:231. doi: 10.25259/SNI_496_2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen C, Lee I, Tatsui C, Elder T, Sloan AE. Laser interstitial thermotherapy (LITT) for the treatment of tumors of the brain and spine: a brief review. J Neurooncol. 2021;151(3):429-442. doi: 10.1007/s11060-020-03652-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel PD, Patel NV, Danish SF. The evolution of laser-induced thermal therapy for the treatment of gliomas. Neurosurg Clin N Am. 2023;34(2):199-207. doi: 10.1016/j.nec.2022.12.004 [DOI] [PubMed] [Google Scholar]
- 24.Montemurro N, Anania Y, Cagnazzo F, Perrini P. Survival outcomes in patients with recurrent glioblastoma treated with Laser Interstitial Thermal Therapy (LITT): a systematic review. Clin Neurol Neurosurg. 2020;195:105942. doi: 10.1016/j.clineuro.2020.105942 [DOI] [PubMed] [Google Scholar]
- 25.Bianconi A, Aruta G, Rizzo F, et al. Systematic review on tumor microenvironment in glial neoplasm: from understanding pathogenesis to future therapeutic perspectives. Int J Mol Sci. 2022;23(8):4166. doi: 10.3390/IJMS23084166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianconi A, Palmieri G, Aruta G, et al. Updates in glioblastoma immunotherapy: an overview of the current clinical and translational scenario. Biomedicines. 2023;11(6):1520. doi: 10.3390/BIOMEDICINES11061520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Iturrioz-Rodríguez N, De Pasquale D, Fiaschi P, Ciofani G. Discrimination of glioma patient-derived cells from healthy astrocytes by exploiting Raman spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2022;269:120773. doi: 10.1016/J.SAA.2021.120773 [DOI] [PubMed] [Google Scholar]
- 28.Bruno F, Pellerino A, Pronello E, et al. Elderly gliobastoma patients: the impact of surgery and adjuvant treatments on survival: a single institution experience. Brain Sci. 2022;12(5):632. doi: 10.3390/BRAINSCI12050632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawasli AH, Bagade S, Shimony JS, Miller-Thomas M, Leuthardt EC. Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: Single-institution series. Neurosurgery. 2013;73(6):1007-1017. doi: 10.1227/NEU.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459-466. doi: 10.1016/S1470-2045(09)70025-7 [DOI] [PubMed] [Google Scholar]
- 31.Butt OH, Zhou AY, Huang J, et al. A phase II study of laser interstitial thermal therapy combined with doxorubicin in patients with recurrent glioblastoma. Neurooncol Adv. 2021;3(1):vdab164. doi: 10.1093/noajnl/vdab164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sastry RA, Shankar GM, Gerstner ER, Curry WT. The impact of surgery on survival after progression of glioblastoma: a retrospective cohort analysis of a contemporary patient population. J Clin Neurosci. 2018;53:41-47. doi: 10.1016/j.jocn.2018.04.004 [DOI] [PubMed] [Google Scholar]
- 33.Bianconi A, Rossi LF, Bonada M, et al. Deep learning-based algorithm for postoperative glioblastoma MRI segmentation: a promising new tool for tumor burden assessment. Brain Inform. 2023;10(1):26. doi: 10.1186/S40708-023-00207-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Birzu C, French P, Caccese M, et al. Recurrent glioblastoma: From molecular landscape to new treatment perspectives. Cancers (Basel). 2020;13(1):47. doi: 10.3390/cancers13010047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grabowski MM, Otvos B, Mohammadi AM. Stereotactic laser ablation of glioblastoma. Neurosurg Clin N Am. 2021;32(1):105-115. doi: 10.1016/j.nec.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 36.Barbieri F, Bosio AG, Pattarozzi A, et al. Chloride intracellular channel 1 activity is not required for glioblastoma development but its inhibition dictates glioma stem cell responsivity to novel biguanide derivatives. J Exp Clin Cancer Res. 2022;41(1). doi: 10.1186/S13046-021-02213-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhawan S, Bartek JJ, Chen CC. Cost-effectiveness of stereotactic laser ablation (SLA) for brain tumors. Int J Hyperthermia. 2020;37(2):61-67. doi: 10.1080/02656736.2020.1774084 [DOI] [PubMed] [Google Scholar]
- 38.Alkazemi M, Lo YT, Hussein H, et al. Laser interstitial thermal therapy for the treatment of primary and metastatic brain tumors: a systematic review and meta-analysis. World Neurosurg. 2023;171:e654-e671. doi: 10.1016/j.wneu.2022.12.079 [DOI] [PubMed] [Google Scholar]
- 39.Laurent D, Oliveria SF, Shang M, Bova F, Freedman R, Rahman M. Techniques to ensure accurate targeting for delivery of awake laser interstitial thermotherapy. Oper Neurosurg (Hagerstown). 2018;15(4):454-460. doi: 10.1093/ons/opx290 [DOI] [PubMed] [Google Scholar]
- 40.Elder JB, Huntoon K, Otero J, et al. Histologic findings associated with laser interstitial thermotherapy for glioblastoma multiforme. Diagn Pathol. 2019;14(1):19. doi: 10.1186/s13000-019-0794-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Voigt JD, Barnett G. The value of using a brain laser interstitial thermal therapy (LITT) system in patients presenting with high grade gliomas where maximal safe resection may not be feasible. Cost Eff Resour Alloc. 2016;14(1):6. doi: 10.1186/s12962-016-0055-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamba N, Wen PY, Aizer AA. Epidemiology of brain metastases and leptomeningeal disease. Neuro Oncol. 2021;23(9):1447-1456. doi: 10.1093/NEUONC/NOAB101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckley RT, Wang AC, Miller JW, Novotny EJ, Ojemann JG. Stereotactic laser ablation for hypothalamic and deep intraventricular lesions. Neurosurg Focus. 2016;41(4):E10. doi: 10.3171/2016.7.FOCUS16236 [DOI] [PubMed] [Google Scholar]
- 44.Dadey DYA, Kamath AA, Smyth MD, Chicoine MR, Leuthardt EC, Kim AH. Utilizing personalized stereotactic frames for laser interstitial thermal ablation of posterior fossa and mesiotemporal brain lesions: a single-institution series. Neurosurg Focus. 2016;41(4):E4. doi: 10.3171/2016.7.FOCUS16207 [DOI] [PubMed] [Google Scholar]