Abstract
Background and Objectives
Comorbidity is common in multiple sclerosis (MS) with the most prevalent conditions being depression, anxiety, hypertension, and hyperlipidemia. Limited information regarding the representation of comorbidity status is available from phase III clinical trials in MS leading to concern about the potential underrepresentation of individuals with comorbidity in clinical trials. The objective was to estimate the prevalence of comorbidities in MS clinical trial populations.
Methods
Individual-level data from multiple sponsors were requested for a 2-stage meta-analysis of phase III clinical trials of MS disease-modifying therapies. To ensure consistency of our approach across trials, we followed the Maelstrom retrospective harmonization guidelines. Chronic comorbidities at clinical trial enrollment recommended by the International Advisory Committee on Clinical Trials in MS were considered (depression, anxiety, hypertension, hyperlipidemia, migraine, diabetes, chronic lung disease). Additional comorbidities were also classified. Classification was based on medical history data. Individual comorbidities were summed and categorized as 0, 1, 2, or ≥3. We report the pooled prevalence (95% confidence interval [95% CI]) of comorbidity. The pooled prevalence and prevalence ratios across age, sex, race, disability level, and treatment were also reported. Heterogeneity was assessed using the I2 statistic.
Results
Seventeen trials involving 17,926 participants were included. Fourteen trials enrolled participants with relapsing MS (RMS) while 3 enrolled participants with progressive MS (PMS). The distributions of sex, age, and disability level were generally consistent within RMS and PMS trials. When pooled, almost half of trial participants (46.5%) had ≥1 comorbidity (1: 25.0%, 95% CI 23.0–27.0, I2 = 89.9; 2: 11.4% [9.3–14.0], I2 = 96.3; ≥3: 6.0% [4.2–8.4], I2 = 97.7). Depression (16.45% [12.96–20.88], I2 = 98.3) was the most prevalent comorbidity reported, followed by hypertension (10.16% [8.61–11.98], I2 = 93.2). Heterogeneity was high across trials. Older age and female participants were associated with increased number of comorbidities. Older individuals and male participants had a higher prevalence of hyperlipidemia, while older individuals and female participants had a higher prevalence of depression and anxiety.
Discussion
Individuals with comorbidities are included in clinical trials, although they may still be underrepresented compared with the general MS population. Given the comorbidity prevalence in the trial populations and studies suggesting an association of comorbidities with disease activity, comorbidity may influence outcomes in clinical trials.
Background
Comorbidity is common in multiple sclerosis (MS) throughout the disease course. Comorbidity is defined as the “total burden of illness other than the disease of interest.”1 In prevalent MS cohorts, the most common comorbidities are depression (23.7%), anxiety (21.9%), hypertension (18.6%), hyperlipidemia (10.9%), and chronic lung disease (10.0%).2 The prevalence of comorbidity increases with age, but even at the time of MS diagnosis, comorbidity is common.3
Increasingly, concern has been raised about the potential underrepresentation of individuals with comorbidities in clinical trials because of restrictive inclusion and exclusion criteria related to comorbidity and age.4,5 Exclusion of these individuals leads to reduced applicability of the findings to typical clinical real-world populations.4 Despite such inclusion and exclusion criteria, we found that in the CombiRx clinical trial, which was conducted 2006–2013, the prevalence of comorbidity was relatively high with over 50% of the participants having at least one comorbidity and largely consistent with the reported prevalence of comorbidity in the general MS population.6 In addition, the burden of comorbidity has been increasing in the general population.7 This suggests that clinical trial populations in the modern era may not differ as much from typical clinical population with respect to the prevalence of comorbidity as is widely believed. However, whether this observation applies more broadly in other trials is largely unexplored.
The International Advisory Committee on Clinical Trials in MS highlighted knowledge gaps with respect to comorbidities in MS clinical trials and outlined recommendations to address these gaps.5 One recommendation was to clearly and consistently describe the comorbidity status of enrolled clinical trial populations for common comorbidities (e.g., depression, anxiety, hypertension, hyperlipidemia, diabetes, chronic lung disease, and migraine). Many trials have not reported comorbidity status, and there is a need to understand the applicability of clinical trial findings to those in clinical practice. These practices are aligned with recent guidance by the Food and Drug Administration (FDA) to broaden eligibility criteria in phase III clinical trial populations to include a wide range of baseline characteristics to increase the understanding of a treatment's benefits and risks in the population that is likely to use the treatment in clinical practice.4
Thus, we aimed to comprehensively establish the prevalence of comorbidities in MS disease-modifying therapy (DMT) phase III clinical trial populations. We hypothesized that the most prevalent comorbidities would be depression, anxiety, hypertension, dyslipidemia, and migraine and that the prevalence of comorbidity at enrollment would be higher in more recently conducted clinical trials.
Methods
Study Design
We conducted a 2-stage meta-analysis of individual participant data from phase III clinical trials of MS disease-modifying therapies. Individual participant data were obtained from the multiple industry sponsors. Because of differences in participant-level data accessibility between sponsors (e.g., access for some trials was only available through specific secure analytic platforms in which data must remain), a 2-stage meta-analysis approach was required. We consistently applied the same analytical approach across all trials and pooled the prevalence and prevalence ratios across clinical trials using random-effects meta-analysis.
Standard Protocol Approvals, Registrations, and Patient Consents
This study was designated as nonhuman participant research by the UT Southwestern institutional review board. Trial sponsors obtained study consents and reviewed the data access requests to ensure they were consistent with consents obtained for the trials.
Trials and Populations
Data were requested through multiple sponsor-dependent platforms. Eighteen trials were requested and 17 trials approved for the proposed research. One trial (PROMISE, sponsor: TEVA) request was not approved because the trial was not approved in the United States or European Union for the requested indication per their data sharing policy implemented in 2020. The 17 phase III MS DMT clinical trial data sets obtained for this study are described in Table 1. We used the intent-to-treat population from each trial for this study.
Table 1.
Clinical Trials Included
| Study acronym | ClinicalTrials.gov number | Year | Indication | Test substance | Comparator | Sample size, n | Age criteria, y | EDSS criteria |
| AFFIRM8 | NCT00027300 | 2006 | RRMS | Natalizumab | Placebo | 942 | 18–50 | 0–5.0 |
| SENTINEL9 | NCT00030966 | 2006 | RRMS | Natalizumab | Interferon β-1a | 1,171 | 18–55 | 0–5.0 |
| COMBIRX10 | NCT00211887 | 2006 | RRMS | Interferon β-1a and glatiramer Acetate | Interferon β-1a, glatiramer Acetate | 1,008 | 18–60 | 0–5.5 |
| FREEDOMS11 | NCT00289978 | 2006 | RRMS | Fingolimod | Placebo | 1,272 | 18–55 | 0–5.5 |
| FREEDOMS II12 | NCT00355134 | 2006 | RRMS | Fingolimod | Placebo | 1,083 | 18–55 | 0–5.5 |
| CLARITY13 | NCT00213135 | 2010 | RRMS | Cladribine | Placebo | 1,326 | 18–65 | 0–5.5 |
| TRANSFORMS14 | NCT00340834 | 2010 | RRMS | Fingolimod | Interferon β-1a | 1,292 | 18–55 | 0–5.0 |
| CARE-MS I15 | NCT00530348 | 2012 | RRMS | Alemtuzumab | Interferon β-1a | 563 | 18–50 | 0–3.0 |
| CARE-MS II16 | NCT00548405 | 2012 | RRMS | Alemtuzumab | Interferon β-1a | 798 | 18–55 | 0–5.0 |
| CONFIRM17 | NCT00451451 | 2012 | RRMS | Dimethyl fumarate | Placebo, glatiramer acetate | 1,417 | 18–55 | 0–5.0 |
| DEFINE18 | NCT00420212 | 2012 | RRMS | Dimethyl fumarate | Placebo | 1,234 | 18–55 | 0–5.0 |
| ADVANCE19 | NCT00906399 | 2014 | RRMS | PEG interferon β-1a | Placebo | 1,512 | 18–65 | 0–5.0 |
| INFORMS20 | NCT00731692 | 2016 | PPMS | Fingolimod | Placebo | 823 | 25–65 | 3.5–6.0 |
| OPERA I21 | NCT01247324 | 2016 | RMS | Ocrelizumab | Interferon β-1a | 821 | 18–55 | 0–5.5 |
| OPERA II22 | NCT01412333 | 2016 | RMS | Ocrelizumab | Interferon β-1a | 835 | 18–55 | 0–5.5 |
| ORATORIO23 | NCT01194570 | 2016 | PPMS | Ocrelizumab | Placebo | 732 | 18–55 | 3.0–6.5 |
| ASCEND24 | NCT01416181 | 2018 | SPMS | Natalizumab | Placebo | 887 | 18–58 | 3.0–6.5 |
Abbreviations: EDSS = Expanded Disability Status Scale; PPMS = primary progressive multiple sclerosis; RMS = relapsing multiple sclerosis; RRMS = relapsing-remitting multiple sclerosis; SPMS = secondary progressive multiple sclerosis.
Harmonization
The Maelstrom retrospective harmonization guidelines were used to ensure consistency of our approach across studies and to allow pooling across studies.25 Following these steps, initially we gathered the study data and documentation (protocol, case report forms, when provided) and identified the availability of data and variables of interest to create harmonized individual comorbidities in each data set. On review, we determined it was feasible to identify comorbidities and harmonize the data in the trials according to a protocol and data schema. Multiple trial data sets were already mapped to the current Clinical Data Interchange Standard Consortium foundational standard (SDTMIG v3.2) which facilitated harmonization. Patient or trial characteristics underwent a similar process to determine categories to use in the analysis for these factors.
Comorbidities
We concentrated our classification on chronic conditions and did not include transient conditions or complications or symptoms of MS (e.g., urinary tract infections, trigeminal neuralgia) to focus the number of conditions examined. Of primary interest were a focused group of conditions recommended by the International Advisory Committee on Clinical Trials in MS, which included depression, anxiety, hypertension, hyperlipidemia, migraine, diabetes, and chronic lung disease (including asthma and chronic obstructive pulmonary disease).5 Additional comorbidities classified included autoimmune thyroid disease, other psychological disorders, cerebrovascular, other cardiovascular diseases, peripheral vascular disease (PVD), other miscellaneous autoimmune conditions, and skin conditions (eTable 1, links.lww.com/WNL/D409).5 Comorbidities at trial enrollment were based on medical history data reported at baseline. We did not use data on medications to avoid misclassification of comorbidities due to off-label uses of a medication. Keywords for each comorbidity were identified based on prior work in rheumatoid arthritis in which terms were mapped to specific conditions through MeDRA coding (a widely applied standardized medical terminology system); the physician (RAM) on our study team provided guidance on additional terms for applicability to the specific conditions of interest.26 Medical history data were then searched to identify these keywords and classify each comorbidity. Any remaining data not classified were manually reviewed to capture all occurrences of the comorbidities. The presence of each comorbid condition was indicated if any of the associated terms were present for an individual.
Covariates
Following a similar harmonization process, additional data on demographic and clinical characteristics were considered in this analysis for stratification of prevalence estimates. Data included sex, age, race, clinical course (clinically isolated syndrome, relapsing-remitting MS, secondary progressive MS, primary progressive MS), and disability level, measured using the Expanded Disability Status Scale (EDSS), at enrollment. Age had to be treated as categorical because of several trials11,12,14,15 reporting age in 5-year increments. Race was inconsistently categorized across studies and collapsed to White and other. The year the study was initiated was also identified and categorized as 2006 or earlier, 2007–2012, 2013 or later based on years when major revisions in the diagnostic criteria were made. The test substance for the trial was another factor considered and grouped into low-efficacy (dimethyl fumarate, fingolimod, glatiramer acetate, interferon β-1a, pegylated interferon) and high-efficacy (natalizumab, alemtuzumab, ocrelizumab, cladribine) test substance categories.27
Analysis
Our primary analysis considered the count of comorbidities, and secondary analyses examined individual comorbidities. Comorbidity count summed the individual comorbidities within an individual participant for the set of comorbidities noted in eTable 1 (links.lww.com/WNL/D409) and categorized as 0, 1, 2, or 3 or more (3+). The total number of comorbidities was 15 and included hypertension, hyperlipidemia, heart (functional), ischemic, cerebrovascular, PVD, diabetes, autoimmune thyroid, miscellaneous autoimmune conditions, migraine, lung, skin conditions, depression, anxiety, and other psychological disorders. For those with 2 and 3 comorbidities, we examined the pooled frequency of the individual comorbidities in these dyads and triads, respectively.
We report the pooled prevalence (95% CI) of comorbidity at enrollment and stratified at the participant level by sex (male vs female [reference]), age group (>18–30, >30–40 [reference], >40–50, >50), race (White [reference], other), EDSS at enrollment (0–3.5 [reference] vs 4+), treatment (placebo [reference], active comparator, intervention), and at the trial level by MS clinical course (relapsing MS [RMS], progressive MS [PMS]), year the study was initiated (2006 or earlier [reference], 2007–2012, 2013 or later) and by test substance categories (low efficacy [reference], high efficacy). The 95% CI were based on the Agresti-Coull approximation of the binomial distribution for each individual trial. In addition, we report the pooled prevalence ratios (PR) and 95% CI for the comparison of comorbidity status across sex, age, race, EDSS, and treatment groups with the same categories used for the pooled prevalence.
Findings were pooled across clinical trials using random-effects meta-analysis using the Der-Simonian and Laird estimator. Prediction intervals and outlier and influence diagnostics were reported for the meta-analyses.28 Influence diagnostics examined studentized deleted residuals and effects on parameter estimates and standard error for mixed models.28 The heterogeneity of findings across studies was assessed using the I2 statistic. I2 values were interpreted as 0%–50% indicating low heterogeneity and 51%–100% indicating high heterogeneity.29 In addition, a metaregression was conducted to examine the effect of age (median for each trial) on the heterogeneity across trials for the prevalence of an individual comorbidity. For the trials that only provided age as categorical values, we estimated the median age from the cumulative frequency distribution. Based on the cumulative frequency distribution, we identified the category at the 50th percentile and took the midpoint of the range. As a sensitivity analysis, we also examined the results excluding the trials where we estimated a median age. We compared the pooled prevalence with published systematic reviews using a 2 independent sample one-sided t-test on the log-transformed prevalence estimates to evaluate equivalence with a 5% margin between the clinical trial prevalence and the systematic reviews (see eMethods, http://links.lww.com/WNL/D409).30 Data analysis was conducted in SAS v9.4 (SAS Institute; Cary, NC) and R for Statistical Computing (metagen package for meta-analysis and diagnostics31 and ggplot2 for figure generation).
Data Availability
Investigators may request access to anonymized individual patient data and redacted trial documents from each sponsor based on their individual processes. Before use of the data, proposals need to be approved by the sponsor or an independent review panel, as appropriate, and a signed data sharing agreement be put in place. The investigators will provide the code for replication of results, on request.
Results
Trials
The analysis included 17,926 individual participants from 17 phase III clinical trials of disease-modifying therapies in MS. eTable 2 (links.lww.com/WNL/D409) summarizes the frequencies of the harmonized participant characteristics considered for the analysis. Of these 17 trials, 14 trials were relapsing MS trials while 3 trials were progressive MS trials. The frequencies of sex, age, and EDSS level are generally consistent within the RMS and PMS trials (eTable 2). Representation of races other than White participants was generally low across trials and was not reported/missing for INFORMS and not reported in 72.8% of participants in ASCEND.
Comorbidity Prevalence
The pooled prevalence of participants with no comorbidity was 53.5% (95% CI 48.5–59.0, I2 = 98.5, Table 2). About one-quarter of the trial participants had 1 comorbidity in the pooled data (25.0%) while 11.4% had 2 comorbidities and 6.0% had 3 or more comorbidities. However, there was high heterogeneity across trials. The FREEDOMS II trial had the lowest prevalence of participants with no comorbidity (22.7% [20.3–25.3]) while CLARITY and ADVANCE had a high prevalence of participants with no comorbidity (74.2% [71.7–76.4] and 71.8% [69.5–74.0], respectively). Across trials in those with 2 comorbidities (11.4%), the most frequent dyad was cardiometabolic and mental comorbidities (20.7% [17.6–24.4]), followed by 2 mental conditions (14.8% [11.6, 18.8]), migraine and mental comorbidities (12.9% [9.8–16.9]) and 2 cardiometabolic conditions (11.2% [7.3–17.1]; eFigure 1A, links.lww.com/WNL/D409). Those with 3 comorbidities had a pooled frequency of 4.1%. The most frequent triad was 3 cardiometabolic conditions (41.9% [35.5–49.5]) and 3 mental conditions (19.5% [15.8–24.0], eFigure 1B).
Table 2.
Prevalence by Trial and Pooled for Comorbid Conditions Count
| Comorbidity count | ||||
| Trial | 0 | 1 | 2 | 3+ |
| AFFIRM | 65.1 (62.0, 68.1) | 23.7 (21.1, 26.5) | 8.6 (6.96, 10.6) | 2.7 (1.79, 3.91) |
| SENTINEL | 40.2 (37.5, 43.0) | 31.6 (29.0, 34.3) | 17.5 (15.4, 19.7) | 10.7 (9.07, 12.6) |
| COMBI | 37.7 (34.8, 40.7) | 32.6 (29.8, 35.6) | 18.5 (16.2, 21.0) | 11.2 (9.40, 13.3) |
| FREEDOMS | 60.1 (57.3, 62.7) | 27.1 (24.8, 29.6) | 9.5 (8.02, 11.3) | 3.3 (2.44, 4.44) |
| FREEDOMS II | 22.7 (20.3, 25.3) | 27.4 (24.8, 30.2) | 25.8 (23.2, 28.5) | 24.1 (21.6, 26.7) |
| CLARITY | 74.2 (71.7, 76.4) | 19.0 (17.0, 21.2) | 5.0 (3.92, 6.29) | 1.9 (1.27, 2.78) |
| TRANSFORMS | 59.0 (56.3, 61.6) | 26.0 (23.7, 28.5) | 10.5 (8.96, 12.3) | 4.5 (3.48, 5.77) |
| CARE-MS I | 68.0 (64.1, 71.7) | 17.4 (14.5, 20.8) | 8.5 (6.47, 11.1) | 6.0 (4.33, 8.34) |
| CARE-MS II | 48.0 (44.5, 51.5) | 24.3 (21.5, 27.4) | 14.9 (12.6, 17.6) | 12.8 (10.6, 15.3) |
| CONFIRM | 62.4 (59.8, 64.8) | 22.7 (20.6, 24.9) | 9.1 (7.71, 10.7) | 5.9 (4.77, 7.23) |
| DEFINE | 56.2 (53.4, 58.9) | 24.4 (22.1, 26.9) | 12.4 (10.7, 14.4) | 7.0 (5.66, 8.51) |
| ADVANCE | 71.8 (69.5, 74.0) | 19.7 (17.8, 21.8) | 6.1 (5.04, 7.47) | 2.4 (1.71, 3.28) |
| OPERA I | 59.1 (55.7, 62.4) | 26.1 (23.2, 29.2) | 10.6 (8.66, 12.9) | 4.3 (3.06, 5.89) |
| OPERA II | 57.1 (53.7, 60.4) | 25.6 (22.8, 28.7) | 10.7 (8.74, 12.9) | 6.6 (5.08, 8.49) |
| INFORMS | 45.5 (42.4, 48.6) | 31.4 (28.6, 34.4) | 16.5 (14.3, 19.0) | 6.6 (5.19, 8.35) |
| ORATORIO | 54.0 (50.3, 57.5) | 26.1 (23.0, 29.4) | 12.0 (9.85, 14.6) | 7.9 (6.17, 10.1) |
| ASCEND | 58.6 (55.3, 61.8) | 23.7 (21.1, 26.6) | 11.9 (9.95, 14.2) | 5.7 (4.38, 7.48) |
| Pooled Prevalence | 53.5 (48.5, 59.0) | 25.0 (23.1, 27.0) | 11.4 (9.3, 14.0) | 6.0 (4.2, 8.4) |
| I2 | 98.5 (97.2, 99.7) | 89.9 (82.7, 97.1) | 96.3 (93.5, 99.1) | 97.7 (95.7, 99.7) |
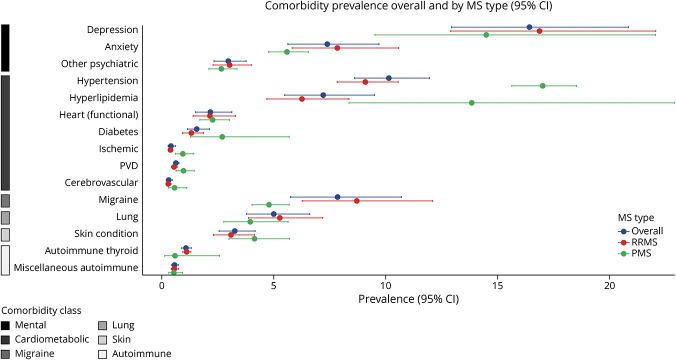
The individual comorbidities where at least 10% of the trial participants were identified to have the condition were depression (16.5% [12.96–20.88], I2 = 98.3) and hypertension (10.2% [8.61–11.98], I2 = 93.2, Figure 1). Comorbidities where less than 1% of the trial participants reported the condition were cerebrovascular, ischemic heart conditions, PVD, and miscellaneous autoimmune conditions. The meta-analyses for the individual comorbidities are provided in eFigure 2 (links.lww.com/WNL/D409). The heterogeneity was found to be generally high, although low heterogeneity was observed for autoimmune thyroid, cerebrovascular, ischemic heart conditions, PVD, and miscellaneous autoimmune comorbidities. The influence of a specific trial on the estimated prevalence for individual comorbidities showed that for 4 comorbidities (cerebrovascular, functional and ischemic heart conditions, and miscellaneous autoimmune conditions), a trial was influential in the meta-analysis (eFigure 3). In general, the influence analysis showed the estimated pooled prevalence was consistent among the remaining 16 trials when an individual trial was omitted (eFigure 3).
Figure 1. Pooled Prevalence Estimates for Individual Comorbidities Overall and by MS Type.
The prevalence of individual conditions by MS clinical course was generally consistent; however, the prevalence of hypertension and hyperlipidemia was higher in PMS trials while migraine was more prevalent in the RMS trials (Figure 1). Over calendar time, the number of comorbidities and most individual comorbid conditions generally were consistent or decreased in prevalence (eFigure 4, links.lww.com/WNL/D409). Individual comorbidities that increased in prevalence over calendar time were diabetes (2006 or earlier, 0.73 [0.30–1.78], 2007–2012, 1.97 [1.67–2.33], 2013 or later, 2.70 [1.28–5.69]) and hypertension (2006 or earlier, 8.61 [6.40–11.58], 2007–2012, 9.72 [9.02–10.48], 2013 or later, 17.01 [15.63–18.52]). Hyperlipidemia prevalence was similar during 2006 or earlier and 2007–2012 calendar periods but doubled in the 2013 or later period (2006 or earlier, 6.47 [3.99–10.50], 2007–2012, 6.11 [4.57–8.17], 2013 or later, 13.84 [8.36–22.91]).
Prevalence Ratios
Comorbidity Count
Sex, age, and disability level were associated with the number of comorbidities. Female participants had higher odds of having more comorbidities compared with male participants (pooled PR [95% CI]: 1.49 [1.32–1.69], I2 = 68.0). As compared with those in the older than 30–40-year age group, older age groups had higher odds of having a greater number of comorbidities (older than 18–30: 0.65 [0.60, 0.71], I2 = 0.0; older than 40–50: 1.64 [1.49–1.82], I2 = 45.5; older than 50: 2.73 [2.35–3.18], I2 = 47.8). Greater disability was also associated with more comorbidities (1.217 [1.02–1.34], I2 = 63.6). No associations between number of comorbidities and treatment group (placebo: 0.98 [0.91–1.06], I2 = 0.0; active comparator: 0.97 [0.85–1.10], I2 = 39.0) and racial groups (0.97 [0.79–1.18], I2 = 73.1) were observed.
Individual Comorbidities
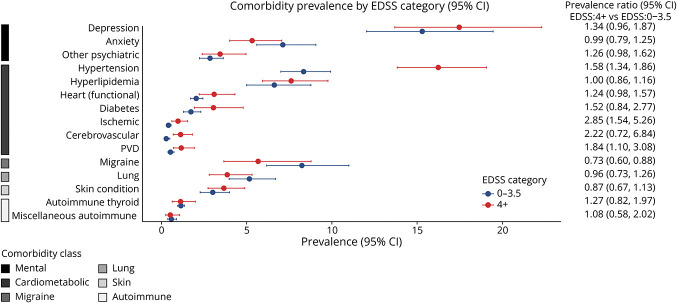
Sex, age, and disability level were associated with differences in the prevalence of specific comorbidities, but treatment arms were not (eFigure 5, links.lww.com/WNL/D409). Notable differences are described below, and the prevalence for each category and the PRs are shown in Figures 2–5.
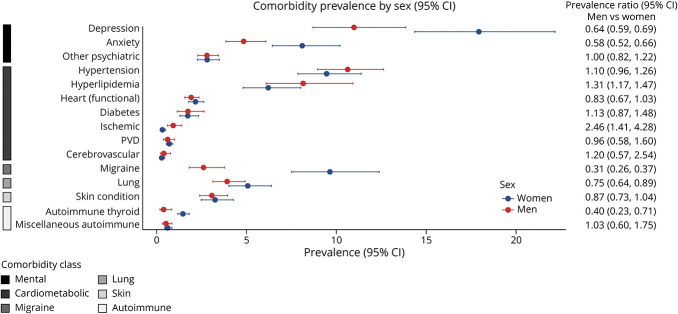
Figure 2. Pooled Prevalence Ratios for Sex.
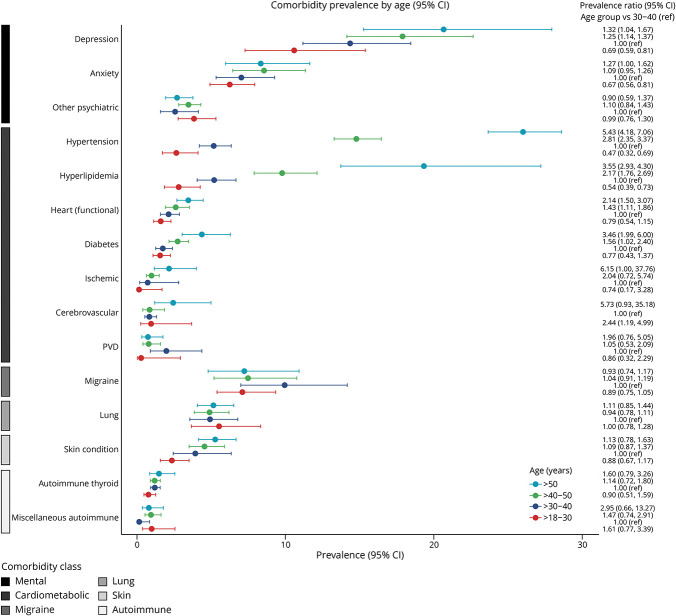
Figure 3. Pooled Prevalence Ratios for Age Groups.
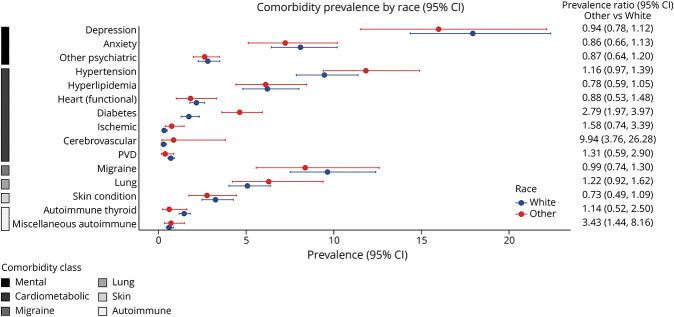
Figure 4. Pooled Prevalence Ratios for Race.
Figure 5. Pooled Prevalence Ratios for Disability Level.
Among cardiometabolic conditions, we noted that male participants had a higher prevalence of hyperlipidemia and ischemic heart conditions than female participants. Older as compared with younger age was associated with an increased prevalence of hypertension, hyperlipidemia, and diabetes. Compared with the White racial group, other racial groups had a higher prevalence of diabetes and cerebrovascular disease. Greater disability was associated with an increased prevalence of hypertension, ischemic heart conditions, and PVD. For other comorbidities, female participants and participants with lower disability levels had a higher prevalence of migraine. Female participants also had a higher prevalence of autoimmune thyroid disease and chronic lung conditions. A higher prevalence of other autoimmune disease was observed for other racial groups compared with White racial groups. For mental health conditions, female participants and those in older age groups had a higher prevalence of depression and anxiety.
The metaregression examining the effect of age on the heterogeneity of the prevalence showed a statistically significant moderator effect for hypertension (p < 0.0001), hyperlipidemia (p < 0.0001), ischemic heart conditions (p = 0.019), and PVD (p = 0.035; eFigure 6, eTable 3, links.lww.com/WNL/D409). No difference in the prevalence of individual comorbidities by the test substance category was identified (eFigure 7). Results for the sensitivity analysis excluding those with the calculated age were consistent (data not shown). Comparison of the prevalence in the clinical trials with those from systematic reviews, depression, other psychiatric conditions, hypertension, hyperlipidemia, and diabetes were found to be equivalent with 5% percent margin (eFigure 8).
Discussion
We examined the comorbidity status of nearly 18,000 participants with MS enrolled in 17 phase III clinical trials. When pooled, 46.5% of trial participants had one or more comorbid conditions. In addition, we identified depression, hypertension, migraine, anxiety, and dyslipidemia as being the most prevalent comorbidities in these trials. We observed an increase in the prevalence of diabetes and hypertension in the trials over a fifteen-year period, yet the prevalence of most comorbid conditions decreased or remained constant over time. The prevalence of hypertension hyperlipidemia and diabetes were higher in older age groups, and migraine was more prevalent in female participants and younger age groups. This is consistent with the expectations for these conditions and reflects differences in PMS and RMS where PMS trial populations tend to include older persons with MS and a higher proportion of male participants compared with RMS trial populations.
The most common individual conditions in the clinical trials were also the most common conditions in a 2015 systematic review of observational studies.5 The high prevalence of depression mirrors the general MS population.2 The estimated pooled prevalence of these conditions for these clinical trials was similar to what was reported in the systematic review for some of the conditions. Comorbidities, such as depression, other psychiatric conditions, hypertension, hyperlipidemia, diabetes, and other autoimmune conditions, had a similar prevalence compared with the systematic review prevalence, and other conditions fell within the reported 95% confidence intervals for most conditions reported (i.e., chronic lung disease (10.0% [0.0–20.9]), autoimmune thyroid (6.4% [0.19–12.7]), cerebrovascular (3.3% [0.0–9.0]), ischemic heart conditions (2.5% [0.0–5.8]), PVD (2.4% [0.0–5.1]), functional heart conditions (0.9% [0.1–6.4])).5 As the systematic review notes,2 few studies included in the review were population-based or standardized to a common population, study designs were heterogeneous, and the validity of the some data sources not well-established. In addition, while we harmonized the systematic review data to our comorbidity categories, there was still some variability in how the conditions considered for some categories. However, the true prevalence of these comorbidities is not known, and the systematic reviews provide the most comprehensive source for comparison. In addition, factors such as study design, demographic, and clinical characteristics were more homogeneous in this meta-analysis.
Multiple chronic conditions were observed in approximately 17% of trial participants. The most frequent dyad was cardiometabolic and mental conditions, and 3 cardiometabolic conditions were the most frequent triad. Little is known about the patterns of comorbidity in MS as much of the research in comorbidities focuses on a single disease. Additional investigation into prevalence of these dyads and triads are needed to understand how representative these may be to the general MS population and the potential impact these comorbidity combinations may have on clinical management in those with multimorbidity.
Heterogeneity was high for most comorbidities examined, although the I2 statistic should be interpreted cautiously because it has been shown to have limited utility in meta-analyses of prevalence estimates.29 In the metaregression, age had a significant effect on the heterogeneity in the prevalence for only a few individual cardiometabolic comorbidities. In addition, no single trial seemed to drive the heterogeneity. FREEDOMS II had a higher prevalence of individual comorbidities compared with other trials in this analysis. Yet, it was considered influential for only functional heart conditions, and the estimated pooled prevalence for the comorbidity was slightly lower when omitting the trial. The differences of FREEDOMS II may be due to true differences in the enrolled trial population or differences in the ascertainment of medical history and oversight associated with the conduct of the trial. For example, reports from FREEDOMS II noted the differences in the participant characteristics in this trial population, specifically compared with the related FREEDOMS trial, or there may have been more intense review of cardiovascular comorbidities with the potential for cardiac risks at treatment initiation that were being observed.12
In contrast to our hypothesis, we did not observe increases over time in most of the comorbid conditions examined. We observed an increase in diabetes, hypertension and hyperlipidemia prevalence, possibly driven by trials in the latter period being conducted in progressive MS populations and the difference in the mean age for PMS compared with RMS trials, as shown in the metaregression results for hypertension and hyperlipidemia. In addition, the RMS trials conducted before 2013 may be too similar and the span of time too short to detect any difference among the trials. In an analysis of a US commercial claims data, the prevalence of common comorbidities varied minimally from 2006 to 2014.32 Data on the prevalence of these comorbidities beyond 2014 are not available to understand whether these trends continue. Altogether, this may suggest that there was little change in inclusion practices in trials during this period. As more data becomes available, it may be possible to examine this in the future.
The burden of comorbidity was higher at older ages, consistent with observations in the general population.7,33 Comorbidities, such as hypertension, hyperlipidemia and diabetes, are associated with increasing age in the general population and in other MS populations.32,34,35 Studies examining the prevalence of these comorbidities compared with a comparator or general population have mixed findings and quality.36 There is no consistent evidence regarding MS being an additional risk factor for these comorbidities. Our ability to investigate racial and ethnic differences in the prevalence of comorbidities was limited by the ability to harmonize data across trials. There was variability in the trials with respect to whether race information was collected (e.g., self-report vs physician report), the granularity in how it was collected, and by the generally low diversity in the trials.37 A recent systematic review highlighted the underreporting and underrepresentation of non-White participants with MS in clinical trials.37 From the general population, there are expected differences in the prevalence of comorbid conditions by race, which have implications for trial eligibility criteria and generalizability of trial results.33-35 The FDA recommends consideration of broadening enrollment practices for both demographic and nondemographic factors, such as race and comorbid conditions, to better reflect the population likely to use the therapy, if approved.4
This study has limitations. Because the trials considered in this analysis were conducted before the International Advisory Committee on Clinical Trials in MS recommendations regarding comorbidity,5 the eligibility criteria among trials related to comorbidity were generally vague, and it was unclear how they were operationalized for inclusion into the trials. We relied on medical history data collected at enrollment for each trial to ascertain comorbidity status, and this may have contributed to the high heterogeneity in estimated prevalence observed in this study. While we consistently used the same methodology and definitions across trials, there is likely some variability in ascertainment of these conditions for each trial. Some trial sites may have verified the condition using the medical records while others may have relied on self-report at the time of trial enrollment. In addition, we were not able to assess the severity and timing of the comorbidities with the data available for these trials. While the severity of comorbidity may have implications regarding evaluation of their effect on outcomes, this may not be as critical when examining the prevalence of comorbidity. There is a need for more structured and validated approaches to assess comorbidity status and severity in clinical trials to reduce variability and improve the reporting of comorbidity status in future trials. In addition, more standardized reporting of comorbid status, at least for the most common conditions, would allow clinicians to assess the generalizability of trial results to the general MS population where comorbidity is common. For common mental comorbidities (e.g., depression, anxiety), standardization of instruments used to assess the comorbidity at enrollment would allow for more consistent characterization and comparisons across trials. We cannot exclude that depression and anxiety may have arose due to MS or its treatments. However, these comorbidities have been found to precede a diagnosis of MS in some individuals, and a systematic review of adverse events reported in MS trials for more recent disease-modifying therapies found no increased risk of psychiatric adverse events associated with these therapies.38,39 We were not able to obtain all phase III clinical trials for MS disease-modifying therapies and were limited in the number of PMS trials and more current RMS trials with accessible data. There is an increasing awareness of data sharing in clinical trials in the pharmaceutical industry and principles to guide responsible data sharing.40 However, there is variability in the specific details (e.g., adoption, embargo period, process, etc.) by company, and older trials may lack the proper consents for data sharing. Nevertheless, this study was able to analyze individual level data from 17 clinical trials spanning over 15 years.
Our study shows individuals with comorbidities are present in clinical trials, although potentially underrepresented compared with the general MS population, and the prevalence varies by demographic and clinical characteristics. Although the estimates varied, when pooled, almost half of participants in these trials had at least one of 15 specific comorbidities; this may be higher than expected for clinical trial populations. Generally, individuals with comorbidities are believed to be excluded from clinical trials. Studies suggest that the presence of comorbidities are associated with disability progression and relapse rates, both common clinical trial outcomes. The effects of anxiety and migraine on relapses suggest that comorbidity may be an unmeasured factor that has the potential to influence study design through trial event rates and thus statistical power.6 Given the relatively high prevalence of comorbidity in these trial populations and previous studies suggesting it affects outcomes, it will be important to further understand the influence of comorbidity on outcomes in the clinical trial setting. Future work investigating potential differences in treatment response according to comorbidity status are warranted.
Acknowledgment
This publication is based on research using data from Biogen provided by Biogen, Cambridge, USA, and the health care business of Merck KGaA provided by the health care business of Merck KGaA, Darmstadt, Germany (CrossRef Funder ID: 10.13039/100009945). This publication is based on research using data from Novartis Pharma AG and Sanofi-Aventis Groupe that has been made available through ClinicalStudyDataRequest.com. This publication is based on research using data from Roche that has been made available through Vivli, Inc. Vivli has not contributed to or approved and is not in any way responsible for the contents of this publication. CombiRx (NCT00211887) was funded by the NIH, National Institute of Neurologic Disorders and Stroke (phase III study: UO1NS045719, Planning Grant R21NS41986), and the study agents and matched placebo were kindly provided by their manufacturers, Biogen and Teva Pharmaceutical.
Glossary
- CI
confidence interval
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- FDA
Food and Drug Administration
- MS
multiple sclerosis
- PMS
progressive MS
- PR
prevalence ratios
- PVD
peripheral vascular disease
- RMS
relapsing MS
Appendix. Authors
| Name | Location | Contribution |
| Amber Salter, PhD | Department of Neurology, Section on Statistical Planning and Analysis, UT Southwestern Medical Center, Dallas, TX | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Samantha Lancia, MS | Department of Neurology, Section on Statistical Planning and Analysis, UT Southwestern Medical Center, Dallas, TX | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Kaarina Kowalec, PhD | College of Pharmacy, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada; Department of Medical Epidemiology & Biostatistics, Karolinska Institutet, Sweden | Analysis or interpretation of data |
| Kathryn C. Fitzgerald, ScD | Department of Neurology, Johns Hopkins School of Medicine, Baltimore, MD | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Ruth Ann Marrie, MD, PhD | Departments of Internal Medicine and Community Health Sciences, Max Rady College of Medicine, Rady Faculty of Health Sciences, University of Manitoba, Winnipeg, Canada | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
This work is supported by the Department of Defense through the Multiple Sclerosis Research Program under Award No. W81XWH-21-1-0721. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. Amber Salter is supported (in part) by a Biostatistics/Informatics Junior Faculty Award (BI-2105-37656) from the National Multiple Sclerosis Society.
Disclosure
A. Salter receives research funding from Multiple Sclerosis Society of Canada, National Multiple Sclerosis Society, CMSC, and the Department of Defense Congressionally Directed Medical Research Program and is a member of editorial board for Neurology. She serves as a consultant for Gryphon Bio, LLC. She is a member of the Data and Safety Monitoring Board for Premature Infants Receiving Milking or Delayed Cord Clamping (PREMOD2), Central Vein Sign: A Diagnostic Biomarker in Multiple Sclerosis (CAVS-MS), and Ocrelizumab for Preventing Clinical Multiple Sclerosis in Individuals With Radiologically Isolated Disease (CELLO). She holds the Kenney Marie Dixon‐Pickens Distinguished Professorship in Multiple Sclerosis Research; S. Lancia has nothing to disclose; K.C. Fitzgerald received research funding from the NIH, National Multiple Sclerosis Society, the Department of Defense Congressionally Directed Medical Research Program, and the International Progressive Multiple Sclerosis Alliance. She is a member of the Data and Safety Monitoring Board for A Trial of Bile Acid Supplementation in Patients With Multiple Sclerosis, Comparative Effectiveness Trial of COVID-19 Testing Modalities (C-FORWARD), and VIRTual vs UsuAL in-office care for MS (VIRTUAL-MS); K. Kowalec receives research funding from the CMSC, the University of Manitoba, the Department of Defense Congressionally Directed Medical Research Program, through the Multiple Sclerosis Research Program (Award No. W81XWH2010566) and NIMH; R.A. Marrie receives research funding from: CIHR, Research Manitoba, Multiple Sclerosis Society of Canada, Multiple Sclerosis Scientific Foundation, Crohn's and Colitis Canada, National Multiple Sclerosis Society, CMSC, the Arthritis Society and the US Department of Defense, and is a coinvestigator on studies receiving funding from Biogen Idec and Roche Canada. She holds the Waugh Family Chair in Multiple Sclerosis and serves on the editorial board of Neurology. Go to Neurology.org/N for full disclosures.
References
- 1.Gijsen R, Hoeymans N, Schellevis FG, Ruwaard D, Satariano WA, van den Bos GA. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001;54(7):661-674. doi: 10.1016/s0895-4356(00)00363-2 [DOI] [PubMed] [Google Scholar]
- 2.Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: overview. Mult Scler. 2015;21(3):263-281. doi: 10.1177/1352458514564491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marrie RA, Patten SB, Tremlett H, et al. Sex differences in comorbidity at diagnosis of multiple sclerosis: a population-based study. Neurology. 2016;86(14):1279-1286. doi: 10.1212/WNL.0000000000002481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. Enhancing the Diversity of Clinical Trial Populations—Eligibility Criteria, Enrollment Practices, and Trial Designs Guidance for Industry. Silver Spring, MD; 2020. Accessed December 20, 2020. fda.gov/regulatory-information/search-fda-guidance-documents/enhancing-diversity-clinical-trial-populations-eligibility-criteria-enrollment-practices-and-trial [Google Scholar]
- 5.Marrie RA, Miller A, Sormani MP, et al. The challenge of comorbidity in clinical trials for multiple sclerosis. Neurology. 2016;86(15):1437-1445. doi: 10.1212/WNL.0000000000002471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salter A, Kowalec K, Fitzgerald K, Cutter G, Marrie RA. Comorbidity is associated with disease activity in MS: findings from the CombiRx trial. Neurology. 2020;95(5):e446-e456. doi: 10.1212/WNL.0000000000010024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boersma P, Black LI, Ward BW. Prevalence of multiple chronic conditions among US adults, 2018. Prev Chronic Dis. 2020;17:E106. doi: 10.5888/PCD17.200130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):899-910. doi: 10.1056/NEJMoa044397 [DOI] [PubMed] [Google Scholar]
- 9.Rudick RA, Stuart WH, Calabresi PA, et al. Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med. 2006;354(9):911-923. doi: 10.1056/NEJMoa044396 [DOI] [PubMed] [Google Scholar]
- 10.Lublin FD, Cofield SS, Cutter GR, et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013;73(3):327-340. doi: 10.1002/ana.23863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387-401. doi: 10.1056/NEJMoa0909494 [DOI] [PubMed] [Google Scholar]
- 12.Calabresi PA, Radue EW, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545-556. doi: 10.1016/S1474-4422(14)70049-3 [DOI] [PubMed] [Google Scholar]
- 13.Giovannoni G, Comi G, Cook S, et al. A placebo-controlled trial of oral cladribine for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):416-426. doi: 10.1056/NEJMoa0902533 [DOI] [PubMed] [Google Scholar]
- 14.Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402-415. doi: 10.1056/NEJMoa0907839 [DOI] [PubMed] [Google Scholar]
- 15.Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1819-1828. doi: 10.1016/S0140-6736(12)61769-3 [DOI] [PubMed] [Google Scholar]
- 16.Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012;380(9856):1829-1839. doi: 10.1016/S0140-6736(12)61768-1 [DOI] [PubMed] [Google Scholar]
- 17.Fox RJ, Miller DH, Phillips JT, et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. N Engl J Med. 2012;367(12):1087-1097. doi: 10.1056/NEJMoa1206328 [DOI] [PubMed] [Google Scholar]
- 18.Gold R, Kappos L, Arnold DL, et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. N Engl J Med. 2012;367(12):1098-1107. doi: 10.1056/NEJMoa1114287 [DOI] [PubMed] [Google Scholar]
- 19.Calabresi PA, Kieseier BC, Arnold DL, et al. Pegylated interferon β-1a for relapsing-remitting multiple sclerosis (ADVANCE): a randomised, phase 3, double-blind study. Lancet Neurol. 2014;13(7):657-665. doi: 10.1016/S1474-4422(14)70068-7 [DOI] [PubMed] [Google Scholar]
- 20.Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075-1084. doi: 10.1016/S0140-6736(15)01314-8 [DOI] [PubMed] [Google Scholar]
- 21.Roche A. Randomized, Double-Blind, Double-Dummy, Parallel-Group Study to Evaluate the Efficacy and Safety of Ocrelizumab in Comparison to Interferon Beta-1a (Rebif®) in Patients with Relapsing Multiple Sclerosis; 2020. Accessed June 27, 2022. search.vivli.org/doiLanding/studies/00005773/isLanding [Google Scholar]
- 22.Roche A. Randomized, Double-Blind, Double-Dummy, Parallel-Group Study to Evaluate the Efficacy and Safety of Ocrelizumab in Comparison to Interferon Beta-1a (Rebif) in Patients with Relapsing Multiple Sclerosis; 2020. Accessed June 27, 2022. search.vivli.org/doiLanding/studies/00005784/isLanding [Google Scholar]
- 23.Roche A. Phase III, Multicentre, Randomized, Parallel-Group, Double-Blind, Placebo Controlled Study to Evaluate the Efficacy and Safety of Ocrelizumab in Adults with Primary Progressive Multiple Sclerosis; 2020. Accessed June 27, 2022. search.vivli.org/doiLanding/studies/00005767/isLanding [Google Scholar]
- 24.Kapoor R, Ho PR, Campbell N, et al. Effect of natalizumab on disease progression in secondary progressive multiple sclerosis (ASCEND): a phase 3, randomised, double-blind, placebo-controlled trial with an open-label extension. Lancet Neurol. 2018;17(5):405-415. doi: 10.1016/S1474-4422(18)30069-3 [DOI] [PubMed] [Google Scholar]
- 25.Fortier I, Raina P, Van den Heuvel ER, et al. Maelstrom Research guidelines for rigorous retrospective data harmonization. Int J Epidemiol. 2017;46(1):103-105. doi: 10.1093/ije/dyw075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Putrik P, Ramiro S, Lie E, et al. Deriving common comorbidity indices from the MedDRA classification and exploring their performance on key outcomes in patients with rheumatoid arthritis. Rheumatology. 2018;57(3):548-554. doi: 10.1093/rheumatology/kex440 [DOI] [PubMed] [Google Scholar]
- 27.Ontaneda D, Tallantyre EC, Raza PC, et al. Determining the effectiveness of early intensive versus escalation approaches for the treatment of relapsing-remitting multiple sclerosis: the DELIVER-MS study protocol. Contemp Clin Trials. 2020;95:106009. doi: 10.1016/j.cct.2020.106009 [DOI] [PubMed] [Google Scholar]
- 28.Viechtbauer W, Cheung MW-L. Outlier and influence diagnostics for meta-analysis. Res Synth Methods. 2010;1(2):112-125. doi: 10.1002/jrsm.11 [DOI] [PubMed] [Google Scholar]
- 29.Migliavaca CB, Stein C, Colpani V, et al. Meta-analysis of prevalence: I2 statistic and how to deal with heterogeneity. Res Synth Methods. 2022;13(3):363-367. doi: 10.1002/jrsm.1547 [DOI] [PubMed] [Google Scholar]
- 30.Tatem K, Romo M, McVeigh K, et al. Comparing prevalence estimates from population-based surveys to inform surveillance using electronic health records. Prev Chronic Dis. 2017;14:E44. doi: 10.5888/pcd14.160516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1-48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 32.Edwards NC, Munsell M, Menzin J, Phillips AL. Comorbidity in US patients with multiple sclerosis. Patient Relat Outcome Meas. 2018;9:97-102. doi: 10.2147/PROM.S148387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward BW, Schiller JS, Goodman RA. Multiple chronic conditions among US adults: a 2012 update. Prev Chronic Dis. 2014;11:E62. doi: 10.5888/PCD11.130389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benoit SR, Hora I, Albright AL, Gregg EW. New directions in incidence and prevalence of diagnosed diabetes in the USA. BMJ Open Diabetes Res Care. 2019;7(1):e000657. doi: 10.1136/bmjdrc-2019-000657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chobufo MD, Gayam V, Soluny J, et al. Prevalence and control rates of hypertension in the USA: 2017-2018. Int J Cardiol Hypertens. 2020;6:100044. doi: 10.1016/J.IJCHY.2020.100044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of cardiac, cerebrovascular, and peripheral vascular disease in multiple sclerosis. Mult Scler. 2015;21(3):318-331. doi: 10.1177/1352458514564485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onuorah HM, Charron O, Meltzer E, et al. Enrollment of non-white participants and reporting of race and ethnicity in phase III trials of multiple sclerosis DMTs: a systematic review. Neurology. 2022;98(9):E880-E892. doi: 10.1212/WNL.0000000000013230 [DOI] [PubMed] [Google Scholar]
- 38.Marrie RA, Walld R, Bolton JM, et al. Rising incidence of psychiatric disorders before diagnosis of immune-mediated inflammatory disease. Epidemiol Psychiatr Sci. 2019;28(3):333-342. doi: 10.1017/S2045796017000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gasim M, Bernstein CN, Graff LA, et al. Adverse psychiatric effects of disease-modifying therapies in multiple Sclerosis: a systematic review. Mult Scler Relat Disord. 2018;26:124-156. doi: 10.1016/j.msard.2018.09.008 [DOI] [PubMed] [Google Scholar]
- 40.PhRMA Principles for Clinical Trial Data Sharing. PhRMA. Accessed March 16, 2023. phrma.org/resource-center/Topics/Clinical-Trials/PhRMA-Principles-for-Clinical-Trial-Data-Sharing [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Investigators may request access to anonymized individual patient data and redacted trial documents from each sponsor based on their individual processes. Before use of the data, proposals need to be approved by the sponsor or an independent review panel, as appropriate, and a signed data sharing agreement be put in place. The investigators will provide the code for replication of results, on request.