Abstract
The present investigation aimed to examine the impact of different dietary organic zinc nanoparticle (ZnNP) levels on gut bacteria, meat quality, growth performance, carcass traits, and blood indicators of broilers. A total of 180 unsexed one-wk broiler chicks (Cobb) were allotted to 3 experimental groups and received a basal diet supplemented with 0, 0.2, and 0.4 mg ZnNPs/Kg diet, respectively. The results showed that, after 38 d of age, the supplementary ZnNPs at a level of 0.4 mg/kg raised body weight and weight gain compared to the control and 0.2 mg ZnNPs/kg diet. The addition of ZnNPs improved the daily feed intake. Some of the carcass characteristics in ZnNPs groups excelled that of the control. ZnNPs treatments gave higher dressing % and decreased (P < 0.05) the cholesterol rates, LDL, and uric acid in the blood. In addition, it gave the best concentrations of ALT and AST. The ZnNPs groups exhibited substantially (P < 0.05) improved moisture and fat values in meat samples. The group given ZnNPs at a concentration of 0.4 mg/kg had a substantially (P < 0.05) lower count of TYMC and E. coli. In conclusion, the high level of ZnNPs (0.4 mg/kg) improved the broilers' performance and some of their carcass traits, enhancing their health and meat quality.
Key words: ZnNPs, broiler, growth, blood, gut microbiota
INTRODUCTION
The poultry industry, the most rapidly expanding farming industry and a significant economic player, is at the forefront of meeting the public's demand for meat and eggs and dealing with poultry items that improve the nutritional value of food for humans. The industry has gained popularity due to its rapid outcomes (Khan et al., 2012). Having high-quality, readily available elements in poultry feed that satisfy demands guarantees high animal productivity. Minerals such as can be provided to animals in a range of forms, such as chelates, inorganic and organic substances, and nanoparticles (Abd El-Hack et al., 2018; Khafaga et al., 2019; Ashour et al., 2020; Abd El-Hack et al., 2021; Hatab et al., 2022). To prevent the issue of antimicrobial resistance to antibiotics, the European Union outlawed the incorporation of antimicrobial agents into livestock nutrition on farms in 2006, according to the European Commission (2005). Many countries still use antibiotics due to a lack of planning and quantitative control over antibiotic growth stimuli (AGP), as stated by Jha & Berrocoso (2015) and Roth et al. (2019).
Numerous techniques exist for creating nanoparticles (NP), including biological, chemical, and physical approaches. Renewable, compatible with life, healthy, and secure is biosynthesis. In addition, while chemical or physical processes require dangerous solvents or a large amount of energy, they quickly reduce metal ions in ambient temperatures (Chokriwal et al., 2014). The size and form of biological NPs, which are resilient and eco-friendly nano-factories, can be regulated by microbes (Li et al., 2011). Strong capacity for adsorption, enhanced catalytic efficiency and an extensive surface area are just a few of the excellent qualities the NPs offer (Wijnhoven et al., 2009). There are indications that NPs can increase adsorption (Liao et al., 2010).
Zinc is a crucial microelement that impacts numerous biological functions in birds, including the immune system, production of hormones, synthesis of DNA and proteins, carbohydrate, fat, and protein digestion, and antioxidants (Hatab et al., 2022; Yusof et al., 2023). Poultry requires 40 ppm of zinc per day, according to the NRC (1994). Commercial feed producers add an extra 100–120 ppm of zinc to the diet to promote fast chick growth (Feng et al., 2010). This excess zinc leads to higher feed production expenses and elevated zinc elimination in the feces, resulting in contamination of the environment. It lowers vitamins and throws off the equilibrium between different microelements (Attia et al., 2013, 2016, 2019; Hassan et al., 2021).
Nonetheless, these issues might be resolved by making zinc more bioavailable (Feng et al., 2010). Zinc in nano form has greater bioavailability (Sagar et al., 2018). Nano zinc has positively impacted birds' digestion and wellness, so it has lately been used as a feed additive because its antibacterial properties strengthen the immune system (Sagar et al., 2018; Akhavan-Salamat and Ghasemi, 2019).
Numerous research studies have demonstrated that supplementing with zinc nanoparticle (ZnNP) improves body weight gain (BWG), feed conversion ratio (FCR) and quality of meat. Besides this, the gut microbiota and immune system were also boosted (Ahmadi et al., 2013; Zhao et al., 2014; Abd El-Hack et al., 2021). Therefore, the present study aimed to evaluate the effects of ZnNPs antimicrobial and antioxidant properties on the broiler chick's growth and carcass features.
MATERIALS AND METHODS
The Poultry Studies Farms, Department of Poultry, Faculty of Agriculture, Zagazig University, Zagazig, Egypt, was the site of this investigation. The protocols of a regional committee for testing the care of animals were followed in all procedures.
Biosynthesis of ZnNPs
Under ideal circumstances, ''10 mL of Bacillus subtilis AM12 the residue and 90 mL of zinc nitrate (1 mmol)'' were combined to create ZnNPs. The combination was allowed to incubate for 72 h at 30°C in a shaking incubator at 130 rpm. The colorless mixture visibly changes to white, indicating that Bacillus subtilis AM12 supernatant bio transforms zinc nitrate to ZnNPs (Gulluce et al., 2007). Zeta potential analysis revealed that the ZnNPs had a charge value of -28.7 mV and a spherical shape at an average diameter of 22 to 43 nm by TEM.
Birds, Design, and Nutrition
In a full randomization design test with three groups, 180 unsexed Cobb broilers aged 1 wk, weighing at the start 132.40 ± 0.12 g. Each treatment consisted of 60 birds divided into six replicates (3 × 6 × 10). We bought the chicks from an industrial incubator.
Ten chicks per floor pen were raised on a deep litter of wheat straw, measuring roughly (50 × 100 × 100 cm). The chicks were kept in a nearly constant daylight hour's length of 23L: 1D with rotation. "The Department of Agricultural Microbiology, Faculty of Agriculture, Zagazig University, Zagazig, Egypt, provided the ZnNPs (500 mg/L)". Birds were chosen at random, and they were divided into 3 experimental treatments. Zinc nanoparticles were added to the mixed ingredients in the mixer at concentrations of 0.0, 0.2, and 0.4 mg/kg before the pelleting process of mixed feed. The first treatment served as a control fed only the basal diet. ZnNPs 0.2 and 0.4 mg /kg feed were added to the base diet given to the other experimental treatments and fed to the first and second groups. The mixed ingredients were pelleted at a temperature of 82°C for 10 s in the presence of a moisture content of approximately 17% relative humidity. After pelleting, the feed was cooled, then the pelleted feed was crushed with a diameter of less than 1 mm for the starter stage from 7 to 21 d of age, then gradually moving to the finishing stage within 3 d before feeding on the finisher feed completely until the end of the experiment at 38 d of age. As stated by the NRC (1994), Table 1 showed that all chicks were given the main diet (especially in the form of pellets) for 7 to 38 d of age. There were two phases to the experimental diets: starter (7–21 d) and finisher (22–38 d). Every bird was housed in identical environmental, administrative, and sanitary conditions.
Table 1.
Chemical evaluation and composition of fed basal diets.
| Items | Basal diets |
|
|---|---|---|
| Starter (7–21 d) | Finisher (22–38 d) | |
| Components (g/kg diet) | ||
| Yellow corn | 57.00 | 58.70 |
| Soybean meal 44% | 30.6 | 26.1 |
| Concentrate 45%1 | 10.0 | 10.0 |
| Dicalcium phosphate | 0.4 | 0.4 |
| Limestone | 0.5 | 0.5 |
| DL-Methionine | 0.09 | 0.0 |
| L-Lysine HCl | 0.07 | 0.18 |
| Soybean oil | 1.34 | 4.30 |
| Total | 100 | 100 |
| Calculated analysis2: | ||
| Dry matter % | 91.72 | 90.43 |
| Metabolizable energy kcal. /kg diet | 2955.20 | 3149.10 |
| Calcium % | 1.00 | 0.99 |
| Phosphorous (Available) % | 0.43 | 0.42 |
| Zinc mg/kg diet | 81.8 | 80.4 |
| Lysine % | 1.3 | 1.10 |
| Methionine+ Cysteine % | 0.90 | 0.76 |
| Crude fiber % | 3.61 | 3.30 |
Added per kg Concentrate. 120,000 IU Vit. A, 35,000 IU Vit. D3, 400 mg Vit. E, 30 mg Vit. K3, 20 mg Vit. B1, 60 mg Vit. B2, 50 mg Vit. B6, 200 mic Vit. B12, 1 mg Cobalt, 750 mics. Biotin. 1,000 mg Mangan, 300 mg iron, 600 mg zinc, 100 mg copper, 20 mg folic acid, 450 mg niacin, 2 mg selenium, 10 mg iodine,120 mg pantothenic, 2,600 mg choline chloride (Added per Kg Conc.).
Protein concentrate (45%) its chemical analysis: Crude protein: 45%, ME: 2470 kcal /kg diet, Calcium: 6.13%, Phosphorus: 2.32%, Lysine: 2.67%, Methionine + cystine: 2.19% and fiber: 2.18%.
Calculated according to NRC (1994).
Measured Characteristics
Broiler Performance
Over the length of the study, which covered the period from 7 to 38 d of age, live body weight (LBW) was measured at 7, 21, and 38 d of age. Additionally, average BWG, daily feed intake (DFI) and FCR were measured during the intervals 7 to 21, 21 to 38 and 7 to 38 d of age.
Carcass Characteristics
Six birds were chosen at random from each pen at the age of 38 d, and they were slaughtered to end their lives. Following slaughter, the birds were scorched for 30 s at 55 to 60°C in a water tank before being taken out and their intestines removed along with the vent. After being removed and weighed separately, ''the heart, liver, gizzard, spleen, giblets, and abdominal fat'' were computed using body weight as a percentage. By splitting the destroyed weight—without the head and feet—by the preslaughter live weight, the destroyed yield percentage was calculated. Utilizing the edible portions of the dressed birds, the viscera and the eviscerated carcass, the edible yield percentage was computed similarly to a percentage of body weight:
Blood Sampling
Six birds from each group had blood samples taken via the slaughter process on the 38th d of their lives. Blood was collected using anticoagulant-free plane tubes. Serum specimens were stored at -20°C until needed for biochemical tests like liver enzyme activity of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the blood serum, in addition to total protein, total cholesterol, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) levels. Also, uric acid, creatinine, immune response such (IgG, IgM), and thyroid hormones (T3, T4). Appropriate personal protective equipment (PPE) was used during the sample gathering and sorting procedures.
Meat Quality
The main pectoral muscle of the broiler chest was subjected to sensory tests for pH evaluation and meat quality. The specimens of breast meat were chilled for 24 h at 4°C. The pH readings were then measured utilizing a pH meter (Romania, ADWA kft, AD12) by penetrating the muscle; according to (Selim et al., 2014), the minimum depth to use was 1 cm after the muscles were incised. A 5-point rating system was used to assess the general acceptance of chicken meat, considering the samples' texture, color, and Odor. Panelists assigned scores for a variety of sensory attributes, including texture (5, firm; 1, very soft); Odor (5, highly acceptable; 1, highly unacceptable/off-odors); and color discoloration (5, no discoloration; 1, extreme discoloration). Generally, acceptance (5, very desirable; 1, extremely unacceptable) is the average of these evaluations (Damaziak et al., 2019). Breast fillets were weighed again after being weighed and kept at 4°C for a full day. Weight variance between before and after chilling was divided to determine the drip loss %.
Caecal Microbiota
The cecum of the slaughtered birds was then taken for microbiological examination. Chickens' 1 g cecum was sterilized (from 2 birds per pen) to count the E. coli, Lactobacillus, and TBC and TYMC bacteria. The specimens were stored in cryovials at -80°C until analysis. Microbes were plated to a miniature size using a previously released method (Sieuwerts et al., 2008).
Statistical Analysis
A one-way ANOVA test and SPSS v 20 (IBM Corp., Armonk, NY) were utilized to analyze the replicated information utilizing GLM procedures. The model of statistics that was applied was:
Where ‘Yij = observed value; M = overall mean; Ti = treatment effect (control, and 2, 3); and eij = random error’. The student–Newman–Keuls test was utilized to estimate recorded mean variances. There were reports of the mean and SEM values. When p < 0.05, the group variations are deemed significant.
RESULTS
Growth Parameters
Table 2 displays the impact of ZnNPs on the LBW and BWG of chicks. According to the results of the test of variations, there was a substantial (p < 0.001) difference between the testing groups' BWG and LBW at the starter (7–21 d), finisher (21–38 d), and cycle (7–38 wk) in comparison to the control, except for the finisher's BWG (21–38 d), which was unaffected. The greatest LBW and BWG were obtained from T3 (0.4 mg ZnNPs/ kg diet) compared to the control and other groups (0.2 mg ZnNPs/ kg diet).
Table 2.
Live body weight and weight gain of broilers supplemented with varying amounts of dietary organic zinc nanoparticles.
| LBW (g) |
BWG (g) |
|||||
|---|---|---|---|---|---|---|
| Items | 7 D | 21 D | 38 D | 7–21 D | 21–38 D | 7–38 D |
| mg/kg diet | ||||||
| 0.0 | 132.17 | 774.44a | 1916.67b | 45.88a | 63.46 | 57.57a |
| 0.2 | 132.33 | 641.67c | 1816.67c | 36.38c | 65.28 | 54.33c |
| 0.4 | 132.50 | 726.67b | 1992.33a | 42.44b | 70.31 | 59.99b |
| SEM | 0.12 | 21.0 | 30.03 | 1.50 | 1.46 | 0.97 |
| P value | 0.579 | 0.003 | 0.023 | 0.003 | 0.128 | 0.022 |
D: days.
LBW: live body weight; BWG: body weight gain.
SEM: standard error mean.
Different letters within 1 column are substantially different (P < 0.05).
Feed intake was reduced computationally in T2 and T3 birds compared to control group birds at the finisher duration (21–38 d), and it lowered substantially in every group that was treated, contrasting to control birds at the starter duration (7–21 d). However, throughout the entire cycle (7–38 d), there were substantial variations in FI between T2 and T3 compared to the control group (Table 3).
Table 3.
Daily feed intake, feed conversion ratio and performance index of broilers fed different levels of dietary organic Zn nanoparticles supplementation.
| Items | DFI (g/d) |
FCR (g feed/ g gain) |
PI (%) | ||||
|---|---|---|---|---|---|---|---|
| 7–21 D | 21–38 D | 7–38 D | 7–21 D | 21–38 D | 7–38 D | ||
| mg/kg diet | |||||||
| 0.0 | 65.83a | 124.27 | 95.06a | 1.44c | 1.96 | 1.65a | 116.16b |
| 0.2 | 58.09c | 111.92 | 85.00c | 1.60a | 1.72 | 1.57b | 116.33b |
| 0.4 | 64.53b | 121.67 | 93.10b | 1.52b | 1.73 | 1.55c | 128.53a |
| SEM | 1.34 | 2.77 | 1.90 | 0.03 | 0.05 | 0.02 | 2.93 |
| P value | 0.009 | 0.157 | 0.041 | 0.005 | 0.058 | 0.229 | 0.033 |
D: days.
DFI: daily feed consumption; FCR: feed conversion ratio; PI: performance index.
SEM: standard error mean.
Different letters within 1 column are significantly different (P < 0.05).
Table 3 illustrates the effects of biological ZnNPs on FCR. ZnNP-supplemented diets typically produced a higher FCR than the control group. While T2 and T3 displayed the highest FCR during the final phase, the control demonstrated the best FCR during the initial period. T3 displayed the finest FCR throughout the entire cycle. Table 3 demonstrates the effects of biological ZnNPs on PI. Generally, the diets supplemented with ZnNPs gave a better PI than the control during the total period. The best PI was observed in the T3 group, which fed ZnNPs at a level 0.4 mg/kg diet, while the worst PI was given by the diet without additive (control). The second group gave the worst results compared to the third and the control group, so the high level of nano-zinc gave the best growth rates during the entire experiment period.
Carcass Traits
Table 4 lists the influence of nutritional treatment on the carcass traits of broiler chicks. The dressing yield of broiler groups supplementary with ZnNPs (0.2 and 0.4 mg ZnNPs/kg diet) raised considerably (P < 0.05) when compared to the control (T1). The dressing yield was higher than that of the other groups at a level of 0.4 mg ZnNPs/kg diet. The findings also showed that supplementing with ZnNPs through diet (0.2 and 0.4 mg ZnNPs/kg diet) greatly increased compared blood and feather percentages. On the contrary, other carcass traits studied were not affected.
Table 4.
Carcass characteristics of broilers fed different levels of dietary organic Zn nanoparticles supplementation.
| Items | Relative to pre-slaughter weight, % |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Blood | Feather | Carcass | Liver | Heart | Gizzard | Giblets | Dressing | Spleen | Abdominal fat | |
| mg/kg diet | ||||||||||
| 0.0 | 2.67c | 3.50c | 71.30 | 2.59 | 0.44 | 1.49 | 4.51 | 75.81c | 0.15 | 0.91 |
| 0.2 | 2.93b | 4.10b | 75.27 | 2.72 | 0.36 | 1.50 | 4.58 | 79.85b | 0.14 | 1.06 |
| 0.4 | 4.15a | 4.75a | 76.04 | 2.51 | 0.42 | 1.58 | 4.51 | 80.55a | 0.17 | 0.95 |
| SEM | 0.27 | 0.24 | 1.01 | 0.12 | 0.02 | 0.08 | 0.15 | 1.00 | 0.02 | 0.10 |
| P value | 0.026 | 0.044 | 0.103 | 0.823 | 0.216 | 0.907 | 0.980 | 0.043 | 0.777 | 0.865 |
SEM: standard error mean.
Different letters within one column are significantly different (P < 0.05).
Blood Parameters
The effects of feeding ZnNPs as an additive to the feed on the blood biochemical examination of broiler chicks are listed in Table 5. The study's findings demonstrated that when compared to the control, ZnNPs at each level dramatically decreased the cholesterol rates (P < 0.05), LDL, uric acid, and abdominal fat and raised HDL values. Also, each group of chicks given ZnNPs had the best concentrations of ALT and AST. The effects of dietary ZnNPs on T3, T4, and IgA and IgG levels were also most significant.
Table 5.
Effect of dietary ZnNPs treatments on broilers' serum biochemical parameters.
| Serum biochemical parameters | Zn nanoparticles (mg/kg) |
P value | ||
|---|---|---|---|---|
| Control | 0.2 | 0.4 | ||
| Uric acid (mg/dL) | 5.30a | 4.4b | 4.1b | 0.01 |
| Creatinine (mg/dL) | 0.32 | 0.30 | 0.29 | 0.88 |
| AST (U/I) | 245a | 208b | 188c | <0.0001 |
| ALT (U/I) | 6.1a | 4.8b | 3.5c | <0.0001 |
| Total cholesterol (mg/dL) | 136.1a | 118.4b | 102.8c | 0.001 |
| HDL (mg/dL) | 90.9b | 94.3ab | 96.4a | 0.015 |
| LDL (mg/dL) | 45.9a | 29.1b | 20.8c | 0.001 |
| Abdominal fat | 1.2a | 0.89b | 0.78c | 0.025 |
| T3 (ng/dL) | 2.5a | 2.4ab | 2.33b | 0.044 |
| T4 (ng/dL) | 131.2b | 134.6a | 132.6b | 0.045 |
| IgG (mg/dL) | 955.2c | 1072b | 1088a | 0.012 |
| IgA (mg/dL) | 179b | 198.3a | 199.9a | 0.015 |
n = 3 a-d Means within the same row with different superscripts differ significantly (P ≤ 0.05). ALT, Alanine aminotransferase; AST, aspartate aminotransferase; HDL, high-density lipoprotein; LDL, low-density lipoprotein; T3, triiodothyronine; T4, thyroxine; IgG, IgA immunoglobulins Isotypes G and A.
Different letters within one column are significantly different (P < 0.05).
Meat Quality
Table 6 displays an analysis of the carcass's traits. The findings indicate that the carcass's sensory attributes, including texture, aroma, tenderness, and juiciness, remained consistent despite the treatment. The ZnNPs groups exhibited substantially (P < 0.05) improved moisture and fat values compared to the control. Conversely, birds given diets with 0.2, 0.4 mg/kg of ZnNPs showed substantially (P < 0.05) fewer points for color and general acceptance than the control.
Table 6.
Effect of dietary ZnNPs treatments on meat quality of broilers.
| Parameters | Treatments (mg/kg) |
P value | ||
|---|---|---|---|---|
| Control | 0.2 | 0.4 | ||
| Chemical composition | ||||
| Moisture | 61.2c | 67.8a | 64.9b | 0.001 |
| Protein | 20.22c | 21.8a | 20.7b | 0.002 |
| Fat | 13.6a | 7.8c | 10.9b | 0.001 |
| Ash | 0.88a | 0.35c | 0.77b | 0.001 |
| pH | 5.7b | 6.5a | 5.9b | 0.04 |
| TVBN, mg/100g | 6.7a | 4.5c | 5.2b | 0.001 |
| TBA, mg malonaldehyde/kg | 0.75a | 0.31c | 0.45b | 0.0011 |
| Sensory properties | ||||
| Juiciness | 9a | 9a | 8.8b | 0.04 |
| Tenderness | 8.8a | 8.8a | 8.6b | 0.035 |
| Aroma | 8.4a | 8.3ab | 8.1b | 0.026 |
| Taste | 8.7ab | 8.8a | 8.2b | 0.013 |
| Color properties | ||||
| L* | 60.5b | 61.9a | 61.0b | 0.030 |
| a* | 5.9a | 5.3b | 5.4b | 0.042 |
| b* | 14.6b | 15.6a | 15.9a | 0.045 |
TVBN, total volatile basic nitrogen; TBA, thiobarbituric acid.
n = 3.
Means of rows that include various superscripts show a significant difference (P ≤ 0.05).
Cecal Microbiota
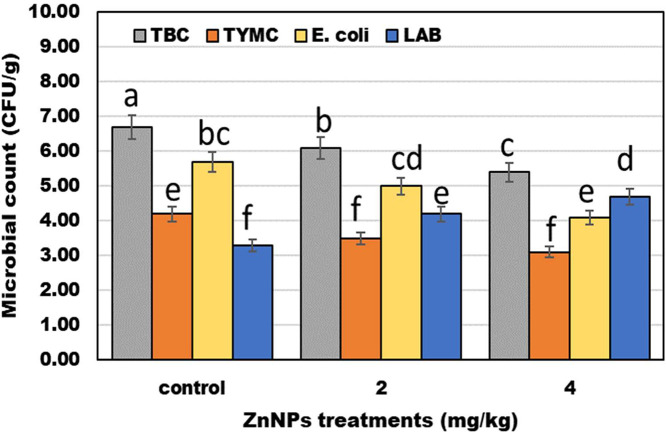
Figure 1 displays the findings for the microbiological counts of the groups' cecal inclusions that got varying doses of ZnNPs in addition to the control. Compared to other treatments, the group given ZnNPs at a concentration of 0.4 mg/kg had a substantially (P < 0.05) lower TYMC and E. coli count. Additionally, there were higher Lactobacillus counts in the groups fed ZnNPs at 0.2 and 0.4 mg/kg, according to the observations.
Figure 1.
Impact on diets ZnNPs treatments on the microbial count of broilers cecum.
DISCUSSION
ZnNPs' tiny dimensions and phenolic substances on their surface are responsible for their antimicrobial and antioxidant effects (NPs). The positive impact of NPs supplementary regarding the growth, features of the carcass, hematology, and quality of the meat, and bacterial condition of birds could be attributed to these active substances.
The application of biological additives to the diet in our study (ZnNPs), especially the high level (0.4 mg ZnNPs/kg diet), gave the best LBW and BWG compared to the control throughout the entire duration of the test (7–38 d of age) (Table 2). The results we obtained can be assigned to the beneficial impact result of ZnNPs' capacity to improve gastrointestinal absorption by boosting mucosal effectiveness, and this is the same result as those who indicated it (Abedini et al., 2017). Additionally, Zn bioavailability is enhanced by Zn nanoparticles' greater adsorption capacity (Zhao et al., 2014; Mahmoud et al., 2020). Zinc nanoparticles have been shown in earlier research to enhance LBW and FCR, lower populations of gut microbes, and strengthen the immune system (Ahmadi et al., 2013; Abd El-Hack et al., 2021). Additionally, Fatima et al. (2024) found that ZnONPs have efficient immuno-modulatory, growth-promoting, and antimicrobial properties in poultry birds, offering increased bioavailability, improved productivity, welfare, and reduced ecological harm.
According to the results we obtained, LBW and BWG of chicks were affected by adding 0.4 MG ZnNPs/KG Diet compared to other transactions, and these results are consistent with the results of Mahmoud et al. (2020), who found that broiler BWG and FCR were safely increased by ZnNPs (10 ppm). Furthermore, birds fed diets added with nano-ZnO showed lower FCR and greater (p < 0.05) BWG relative to the control group, according to Fathi et al. (2016). Numerous investigations confirmed that diets for broilers supplementary with zinc boosted growth rate and enhanced feed efficiency (Fazilati et al., 2013; Zhao et al., 2014). Because of their antibacterial and immune-modulating qualities, zinc oxide nanoparticles can affect birds' well-being and metabolic capacity (Akhavan-Salamat et al., 2019; Yusof et al., 2023). Additionally, several studies have shown that broiler performance can be improved by administering greater amounts of ZnNPs, such as 30 to 80 ppm (Ahmadi et al., 2013; Badawi et al., 2017; Mahmoud et al., 2020). This is because zinc is a crucial microelement that functions as a component of over 300 enzymes that regulate the metabolism of proteins and nucleic acids (Ibrahim et al., 2017; Jarosz et al., 2017). Moreover, ZnNPs can modify the metabolic rate of broilers by increasing the expression of genes related to growth hormone and insulin (El-Katcha et al., 2017).
According to El-Katcha et al. (2017), broiler BWG was increased when ZnNPs at a concentration of 15 ppm were added to the diet. Accordingly, Zhao et al. (2014) discovered that ZnNPs positively improve broiler efficiency. Hatab et al. (2022) partially corroborated our findings, demonstrating that the chicks fed nutrition ZnNPs supplemental intake at each level had the greatest LBW during the entire testing week compared to those given a control diet. They added that the findings indicated that diets impacted the quantity of FC by chicks weekly. The mechanism of ZnNPs in improving performance traits belongs to the indirect effects on stimulating the bird's immunity through immune cells and their functions. In addition, zinc is necessary for numerous tissues and cells to grow and function. It also has a role in lymphocyte gene expression, division, and apoptosis.
According to our findings, broiler chick dressing yield was considerably enhanced by nutritional treatment with ZnNPs, especially at a diet of 0.4 mg ZnNPs/kg. While it did not affect other carcass traits, this treatment also markedly increased the percentages of blood and feathers. These findings agree with those of Mahmoud et al. (2020), who discovered that adding 20 ppm ZnNPs to broilers’ diet enhanced gut health and FI compared to the control group. According to earlier studies, supplementing with dietary ZnNPs (40–90 ppm) raised dressing% and carcass yield (Chattopadhyay et al., 2004; Hani et al., 2014; Khafaga et al., 2019). This outcome could be explained by ZnNPs' antimicrobial capacity, which lowers the burden of pathogenic bacteria and enhances gut health (Sahoo et al., 2014; Wang et al., 2015; Ahmed et al., 2018).
Furthermore, it was verified by Mahmoud et al. (2020) that the spleen and bursa proportions of birds fed diets are supplementary with 10, 20, or 40 ppm of ZnNPs were greater (p < 0.05) than those of the neglected groups. It is possible to return this to the zinc, which significantly impacts immunomodulation by upregulating the manufacturing of interferons and thymocyte and peripheral T cell counts (Emadi and Kermanshahi, 2007). According to Hatab et al. (2022) discovered that broiler diets enriched with ZnONPs resulted in a noteworthy (P < 0.05) increase in carcass yield relative to the control. They added that ZnONPs at 40 mg/kg raised the yield of carcasses in contrast to other groups.
Based to our study findings, the ZnNP-based diet groups' blood variables for serum creatinine level, uric acid, HDL, LDL, ALT, and AST differed significantly from 1 another. According to Mahmoud et al. (2020), adding 20 ppm of ZnONPs to the diet significantly decreased the concentration of uric acid and serum TG. Contrasting to the broiler group under control, markedly increasing the HDL serum amount. Additionally, compared to control, ZnNPs at doses of 0.2 and 0.3 g/kg diet dramatically lowered serum levels of total cholesterol and LDL. They had the highest value for HDL in growing Japanese quail diets. Japanese quail given ZnONPs at 30 or 60 mg/kg showed a similar trend in their serum total cholesterol and TG levels, while the activity levels of AST and ALT did not change (Kidd et al., 1996). The current findings are also consistent with those of Attia, who found that enhancing with ZnONPs at a level of 20 or 40 mg/kg diet reduced cholesterol, TG, creatinine, uric acid, and raised HDL (El-Bahr et al., 2020). According to Bashar et al. (2023), adding dietary mineral nanoparticles and/or Spirulina platensis improved the feed conversion ratio and increased body weight. It lowered serum levels of uric acid, AST, ALT, and glycerides. Additionally, it improves NZW rabbits' heat tolerance and lessens the effects of summer stress.
The current study's notable rise in serum HDL and decrease in blood serum TG and cholesterol is consistent with 2 earlier studies. Attia (2020) used ZnONPs to treat laying hens and broilers. In the meantime, the reduction in cholesterol might be due to zinc's ability to stop cholesterol from being absorbed in the intestines and to encourage the development and endurance of lactic acid bacteria, which reduces cholesterol (Liao et al., 2020).
The results of this study made it clear that broilers were destroyed, and edible yield rose substantially when ZnNPs (0.2, 0.4 mg/kg) were included in their meals compared with the group under control. This result was in line with earlier studies that found that dietary ZnNPs substantially raised dressing %, carcass yield, and carcass weight at levels ranging from 40 to 90 ppm (Wang et al., 2011; El-Katcha et al., 2017). Additionally, the pH value of the bird's meat in this study, which is 5.9 to 6.5, is within the normal range (approximately 5.3–6.5) (Lina et al., 2009). Low muscle pH lengthens broiler meat's storage duration, whereas higher muscle pH raises the susceptibility of meat to bacterial deterioration (Allen et al., 1997). Zinc nanoparticles at a dosage of 0.2 mg/kg had a pH value that was noticeably greater than the value of the control. This was consistent with the findings of (Soeparno, 2009), who reported that broiler muscle pH values were substantially increased by additional zinc. Contrary to this, Selim et al. (2014) discovered that the pH of the thigh and breast muscles in broilers given ZONPs had decreased by 6.8%. Compared to the control, ZnNPs at 0.2 mg/kg had no discernible effect on color or overall acceptance. Similarly, ZONPs at 40 or 80 ppm were found to not affect the color, texture, aroma, or general acceptance of chicken meat, according to Selim et al. (2014).
However, Zinc oxide NPs have been shown in studies to be efficient against Gram-positive and Gram-negative bacteria (Azam et al., 2012). Compared to the overall aerobic bacterial count, ZONPs at lower concentrations (10 ppm) demonstrated the strongest antibacterial activity. According to certain investigators (Siddiqi et al., 2018), permeability—which has risen substantially and impacts proper transport all through the cell membrane—is the cause of bacterial cell death. The primary determinants of nanoparticles' antibacterial properties are their surface area and concentration Arabi et al. (2012). The surface area available for bacterial communication and the potent bactericidal effect increase with decreasing particle size (Adams et al., 2006).
According to Liu et al. (2011), ZONPs could effectively impede the growth of certain pathogens, like Salmonella enteritidis and Escherichia coli O157:H7. There is still much to learn about the precise method through which NP passes through the bacterial wall. ZONPs published ions cause bacterial cell division by combining with the thiol groups (eSH) in proteins, leading to protein inactivation and a decrease in membrane permeability (Rajendra et al., 2010). Arabi et al. (2012) proposed another potential mechanism for the antibiotic properties of ZONPs: ZONPs and MOS both carry +ve charges, which create an “electromagnetic” attraction that causes bacterial cell oxidation and death.
This research shows the effect of adding ZnNPs on increased lactobacilli count and reduced E. coli, especially 0.4 mg/kg ZnNPs, in contrast with the control group. Lin et al. (2013) noticed that adding antibiotic growth promoters to food led to a noteworthy decrease in Lactobacillus. Accordingly, a possible advantage of using ZONPs is that it causes the proportion of strains of the genus Lactobacillus in broilers to rise significantly (Song et al., 2018). Conversely, ZONPs significantly decreased the proportionate quantity of the Lactobacillus genus in the ileal digesta (Feng et al., 2017). Observations indicate that different Lactobacillus species responded differently to applying ZONPs in poultry diets, depending on which species predominated in the gut. As stated by Vahjen et al. (2011) reported that Lactobacillus spp. The abundances compared to ZONPs in food were markedly elevated, aside from L. amylovorus (not influenced) and Lactobacillus reuteri (reduced). Furthermore, 1% ZONPs inhibited Lactobacillus acidophilus in vitro. Also, According to Aldal’in et al. (2023), prodigiosin and ZnNPs can stop oxidative stress, DNA damage, inflammation, and endoplasmic reticulum stress signaling in stressed rabbits. This suggests that they may be useful in preventing the negative effects of heat stress. Additionally, El-Shobokshy et al. (2023) reported that the BW, weight gain and FCR of male rabbits were improved when Zn nanoparticles were substituted for inorganic zinc. Additionally, rabbits treated with ZnNP showed improvements in histopathologic findings, fertility, and oxidative parameters.
Thus, it is clear from our research that ZnNPs have exceptional antibacterial properties and can be included in chicken rations to promote growth or ward off illness.
CONCLUSIONS
The results of the present investigation demonstrated that supplementing ZnNPs had a significant antimicrobial effect on pathogenic bacteria and fungi. Besides, the use of ZnNPs improved broiler performance and some carcass traits. Also, ZnNPs significantly reduced chicks' cholesterol, LDL, uric acid, and abdominal fat concentrations and increased HDL values. Therefore, including ZnNPs at a level of 0.4 mg/kg diet was advised to maintain broiler chickens' dietary needs, health, and performance.
ACKNOWLEGMENTS
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for supporting this work through large groups under grant number RGP2/304/44.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Abd El-Hack M.E., Alaidaroos B.A., Farsi R.M., Abou-Kassem D.E., El-Saadony M.T., Saad A.M., Shafi M.E., Albaqami N.M., Taha A.E., Ashour E.R. Impacts of supplementing broiler diets with biological curcumin, zinc nanoparticles and Bacillus licheniformis on growth, carcass traits, blood indices, meat quality and cecal microbial load. Animals. 2021;11:1878. doi: 10.3390/ani11071878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd El-Hack M.E., Ashour E., Elaraby G.M., Osman A., Arif M. Influences of dietary supplementation of peanut skin powder (Arachis Hypogaea) on growth performance, carcass traits, blood chemistry, antioxidant activity and meat quality of broilers. Anim. Prod. Sci. 2018;58:965–972. [Google Scholar]
- Abedini M., Shariatmadari F., Torshizi M.K., Ahmadi H. Effects of a dietary supplementation with zinc oxide nanoparticles, compared to zinc oxide and zinc methionine, on performance, egg quality, and zinc status of laying hens. Livest. Sci. 2017;203:30–36. [Google Scholar]
- Adams L.K., Lyon D.Y., Alvarez P.J. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Res. 2006;40:3527–3532. doi: 10.1016/j.watres.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Ahmadi F., Ebrahimnezhad Y., Sis N.M., Ghiasi J. The effects of zinc oxide nanoparticles on performance, digestive organs and serum lipid concentrations in broiler chickens during starter period. Int. J. Biosci. 2013;3:23–29. [Google Scholar]
- Ahmed I., El-Rayes T., Ahmed A.I. Assessment of dietary supplementation of turmeric (Curcuma longa) as a phytobiotic on broiler performance and bacterial count. EJNF. 2018;21:519–528. [Google Scholar]
- Akhavan-Salamat H., Ghasemi H. Effect of different sources and contents of zinc on growth performance, carcass characteristics, humoral immunity and antioxidant status of broiler chickens exposed to high environmental temperatures. Livest. Sci. 2019;223:76–83. [Google Scholar]
- Aldal’in H.K., Al-Otaibi A.M., Alaryani F.S., Alsharif I., Alghamdi Y.S., Abd El-Hack M.E., Abdelnour S.A. Use of zinc nanoparticles and/or prodigiosin to mitigate heat stress in rabbits. Ann. Anim. Sci. 2023:1221–1230. [Google Scholar]
- Allen C.D., Russell S.M., Fletcher D.L. The relationship of broiler breast meat color and pH to shelf-life and odor development. Poult. Sci. 1997;76:1042–1046. doi: 10.1093/ps/76.7.1042. [DOI] [PubMed] [Google Scholar]
- Arabi F., Imandar M., Negahdary M., Imandar M., Noughabi M.T., Akbari-dastjerdi H., Fazilati M. Investigation anti-bacterial effect of zinc oxide nanoparticles upon life of listeria monocytogenes. Ann. Biol. Res. 2012;7:3679–3685. [Google Scholar]
- Ashour E.A., Abd El-Hack M.E., Swelum A.A., Osman A.O., Taha A.E., Alhimaidi A.R., Ismail I.E. Does the dietary graded levels of herbal mixture powder impact growth, carcass traits, blood indices and meat quality of the broilers? Ital. J. Anim. Sci. 2020;19:1226–1235. [Google Scholar]
- Attia F.M. Efficacy of dietary zinc oxide nanoparticles supplementation on serum biochemical, nutrients retention and chemical composition of meat and tibia in broiler chickens. Egypt. Poult. Sci. 2020;40:29–46. [Google Scholar]
- Attia Y.A., Bovera F., Abd El-Hamid A.E., Tag El-Din A.E., Al-Harthi M.A., El-Shafy A.S. Effect of zinc bacitracin and phytase on growth performance, nutrient digestibility, carcass and meat traits of broilers. J. Anim. Physiol. Anim. Nutr. 2016;100:485–491. doi: 10.1111/jpn.12397. [DOI] [PubMed] [Google Scholar]
- Attia Y.A., Addeo N.F., Abd Al-Hamid A.E., Bovera F. Effects of phytase supplementation to diets with or without zinc addition on growth performance and zinc utilization of white Pekin ducks. Animals 2019. 2019;9:280. doi: 10.3390/ani9050280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia Y.A., Abd El-Hamid A.E., Zeweil H.S., Qota E.M., Bovera F., Monastra M., Sahledom M.D. Effect of dietary amounts of organic and inorganic Zinc on productive and physiological traits of white peckin ducks. Animal. 2013;7:695–700. doi: 10.1017/S1751731113000050. [DOI] [PubMed] [Google Scholar]
- Azam A., Ahmed A.S., Oves M., Khan M.S., Habib S.S., Memic A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int. J. Nanomed. 2012;7:6003–6009. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badawi M., Ali M., Behairy A. Effects of zinc sources supplementation on performance of broiler chickens. J. Am. Sci. 2017;13:35–40. [Google Scholar]
- Bashar A.M., Abdelnour S.A., El-Darawany A.A., Sheiha A.M. dietary supplementation of microalgae and/or nanominerals mitigate the negative effects of heat stress in growing rabbits. Biol. Trace Elem. Res. 2023;1:1–14. doi: 10.1007/s12011-023-03953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattopadhyay I., Biswas K., Bandyopadhyay U., Banerjee R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004;87:44–53. [Google Scholar]
- Chokriwal A., Sharma M.M., Singh A. Biological synthesis of nanoparticles using bacteria and their applications. Am. j. PharmTech res. 2014;4:38–61. [Google Scholar]
- Damaziak K., Stelmasiak A., Riedel J., Zdanowska-Sąsiadek Z., Bucław M., Gozdowski D., Michalczuk M. Sensory evaluation of poultry meat: a comparative survey of results from normal sighted and blind people. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Bahr S.M., Shousha S., Albokhadaim I., Shehab A., Khattab W., Ahmed-Farid O., Shathele M. Impact of dietary zinc oxide nanoparticles on selected serum biomarkers, lipid peroxidation and tissue gene expression of antioxidant enzymes and cytokines in Japanese quail. BMC Vet. Res. 2020;16:1–12. doi: 10.1186/s12917-020-02482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Katcha M., Soltan M.A., El-Badry M. Effect of dietary replacement of inorganic zinc by organic or nanoparticles sources on growth performance, immune response and intestinal histopathology of broiler chicken. Alex. J. Vet. Sci. 2017;55:1–9. [Google Scholar]
- El-Shobokshy S.A., Abo-Samaha M.I., Sahwan F.M., El-Rheem A.M.A., Emam M., Khafaga A.F. Implication of apoptosis and oxidative stress in mitigation of ivermectin long-term hazards by zinc nanoparticles in male rabbits. Environ. Sci. Pollut. Res. 2023;30:26982–26997. doi: 10.1007/s11356-022-24095-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi M., Kermanshahi H. Effect of turmeric rhizome powder on the activity of some blood enzymes in broiler chickens. Int. J. Poult. Sci. 2007;6:48–51. [Google Scholar]
- European Commission . European Commision. Press Release; Brussels, Belgium: 2005. Ban on antibiotics as growth promoters in animal feed enters into effect, press release database. [Google Scholar]
- Fathi M., Haydari M., Tanha T. Effects of zinc oxide nanoparticles on antioxidant status, serum enzymes activities, biochemical parameters and performance in broiler chickens. J. livest. Sci. Technol. 2016;4:7–13. [Google Scholar]
- Fatima A., Zaheer T., Pal K., Abbas R.Z., Akhtar T., Ali S., Mahmood M.S. Zinc oxide nanoparticle's significant role in poultry and novel toxicological mechanisms. Biol. Trace Elem. Res. 2024;202:268–290. doi: 10.1007/s12011-023-03651-x. [DOI] [PubMed] [Google Scholar]
- Fazilati M. Investigation toxicity properties of zinc oxide nanoparticles on liver enzymes in male rat. Eur. J. Exp. Biol. 2013;3:97–103. [Google Scholar]
- Feng J., Ma W., Niu H., Wu X., Wang Y. Effects of zinc glycine chelate on growth, hematological, and immunological characteristics in broilers. Biol. Trace Elem. Res. 2010;133:203–211. doi: 10.1007/s12011-009-8431-9. [DOI] [PubMed] [Google Scholar]
- Feng Y., Min L., Zhang W., Liu J., Hou Z., Chu M., Zhang H. Zinc oxide nanoparticles influence microflora in ileal digesta and correlate well with blood metabolites. Front. Microbiol. 2017;8:992. doi: 10.3389/fmicb.2017.00992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulluce M., Sahin F., Sokmen M., Ozer H., Daferera D., Sokmen A., Polissiou M., Adiguzel A., Ozkan H. Antimicrobial and antioxidant properties of the essential oils and methanol extract from Mentha longifolia L. ssp. longifolia. Food Chem. 2007;103:1449–1456. [Google Scholar]
- Hani U., Shivakumar H. Solubility enhancement and delivery systems of curcumin a herbal medicine: a review. Curr. Drug Deliv. 2014;11:792–804. doi: 10.2174/1567201811666140825130003. [DOI] [PubMed] [Google Scholar]
- Hassan F., Mobarez S., Mohamed M., Attia Y.A., Mekawy A., Mahrose K. Zinc and/or selenium enriched Spirulina as antioxidants in growing rabbit diets to alleviate the deleterious impacts of heat stress during summer season. Animals 2021. 2021;11:756. doi: 10.3390/ani11030756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatab M.H., Rashad E., Hisham M., Saleh H.M., El‑Sayed E.R.S., Taleb A.A. Effects of dietary supplementation of myco‑fabricated zinc oxide nanoparticles on performance, histological changes, and tissues Zn concentration in broiler chicks. Scientific Reports. 2022;12:18791. doi: 10.1038/s41598-022-22836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D., Ali H.A., El-Mandrawy S.A. Effects of different zinc sources on performance, biodistribution of minerals and expression of genes related to metabolism of broiler chickens. Zagazig Vet. J. 2017;45:292–304. [Google Scholar]
- Jarosz M., Olbert M., Wyszogrodzka G., Młyniec K., Librowski T. Antioxidant and anti-inflammatory effects of zinc. Zinc-dependent NF-κB signaling. Inflammopharmacology. 2017;25:11–24. doi: 10.1007/s10787-017-0309-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha R., Berrocoso J. Dietary fiber utilization and its effects on physiological functions and gut health of swine. Animal. 2015;9:1441–1452. doi: 10.1017/S1751731115000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khafaga A.F., Abd El-Hack M.E., Taha A.E., Elnesr S.S., Alagawany M.M. The potential modulatory role of herbal additives against Cd toxicity in human, animal, and poultry: a review. Environ. Sci. Pollut. Res. 2019;26:4588–4604. doi: 10.1007/s11356-018-4037-0. [DOI] [PubMed] [Google Scholar]
- Khan R.U., Naz S., Javdani M., Nikousefat Z., Selvaggi M., Tufarelli V., Laudadio V. The use of turmeric (Curcuma longa) in poultry feed. Worlds Poult. Sci. J. 2012;68:97–103. [Google Scholar]
- Kidd M.T., Ferket P.R., Qureshi M.A. Zinc metabolism with special reference to its role in immunity. World's Poult. Sci. J. 1996;52:309–324. [Google Scholar]
- Li X., Xu H., Chen Z., Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011;1:1–16. [Google Scholar]
- Liao C.D., Hung W.L., Jan K.C., Yeh A.I., Ho C.T., Hwang L.S. Nano/sub-microsized lignan glycosides from sesame meal exhibit higher transport and absorption efficiency in Caco-2 cell monolayer. Food Chem. 2010;119:896–902. [Google Scholar]
- Liao C.D., Hung W.L., Jan K.C., Yeh A.I., Ho C.T., Hwang L.S. Nano/sub-microsized lignan glycosides from sesame meal exhibit higher transport and absorption efficiency in Caco-2 cell monolayer. Food Chem. 2020;119:896–902. [Google Scholar]
- Lin J., Hunkapiller A.A., Layton A.C., Chang Y.J., Robbins K.R. Response of intestinal microbiota to antibiotic growth promoters in chickens. Foodborne Pathog. Dis. 2013;10:331–337. doi: 10.1089/fpd.2012.1348. [DOI] [PubMed] [Google Scholar]
- Lina T., Jianyang J., Fenghua Z., Huiying R., Wenli L. Effect of nano-zinc oxide on the production and dressing performance of broiler. Chinese Agric. Sci. Bullet. 2009;2:318. [Google Scholar]
- Liu Z.H., Lu L., Li S.F., Zhang L.Y., Xi L., Zhang K.Y., Luo X.G. Effects of supplemental zinc source and level on growth performance, carcass traits, and meat quality of broilers. Poult. Sci. 2011;90:1782–1790. doi: 10.3382/ps.2010-01215. [DOI] [PubMed] [Google Scholar]
- Mahmoud U.T., Abdel-Mohsein H.S., Mahmoud M.A., Amen O.A., Hassan R.I., Abd-El-Malek A.M., Rageb S.M., Waly H.S., Othman A.A., Osman M.A. Effect of zinc oxide nanoparticles on broilers’ performance and health status. Trop. Anim. Health Prod. 2020;52:2043–2054. doi: 10.1007/s11250-020-02229-2. [DOI] [PubMed] [Google Scholar]
- NRC . 9th ed. National Academy Press; Washington, DC: 1994. National Research Council. Nutrient Requirements of Poultry. [Google Scholar]
- Rajendra R., Balakumar C., Ahammed H.A.M., Jayakumar S., Vaideki K., Rajesh E. Use of zinc oxide nanoparticles for production of antimicrobial textiles. Int. J. Eng. Sci. Technol. 2010;2:202–208. [Google Scholar]
- Roth N., Käsbohrer A., Mayrhofer S., Zitz U., Hofacre C., Domig K.J. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poul. Sci. 2019;98:1791–1804. doi: 10.3382/ps/pey539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagar P.D., Mandal A., Akbar N., Dinani O. Effect of different levels and sources of zinc on growth performance and immunity of broiler chicken during summer. Int. J. Curr. Microbiol. Appl. Sci. 2018;7:459–471. [Google Scholar]
- Sahoo A., Swain R., Mishra S.K. Effect of inorganic, organic and nano zinc supplemented diets on bioavailability and immunity status of broilers. Int. J. Adv. Res. 2014;2:828–837. [Google Scholar]
- Selim N.A., Amira M., Khosht A.R., Abd El-Hakim A.S. Effect of sources and inclusion levels of zinc in broiler diets containing different vegetable oils during summer season conditions on meat quality. Int. J. Poult. Sci. 2014;13:619. [Google Scholar]
- Siddiqi K.S., ur Rahman A., Tajuddin N., Husen A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018;13:1–13. doi: 10.1186/s11671-018-2532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieuwerts S., De Bok F.A., Mols E., De Vos W.M., van Hylckama Vlieg J.E.T.A. Simple and fast method for determining colony-forming units. Lett. Appl. Microbiol. 2008;47:275–278. doi: 10.1111/j.1472-765X.2008.02417.x. [DOI] [PubMed] [Google Scholar]
- Soeparno . Gadjah Mada University Press; Yogyakarta: 2009. Meat Science and Technology. Cetakan Kelima. [Google Scholar]
- Song R., Yao J., Shi Q., Wei R. Nanocomposite of half-fin anchovy hydrolysates/zinc oxide nanoparticles exhibits actual non-toxicity and regulates intestinal microbiota, short-chain fatty acids production and oxidative status in mice. Mar. Drugs. 2018;16:23. doi: 10.3390/md16010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahjen W., Pieper R., Zentek J. Increased dietary zinc oxide changes the bacterial core and enterobacterial composition in the ileum of piglets. Anim. Sci. J. 2011;89:2430–2439. doi: 10.2527/jas.2010-3270. [DOI] [PubMed] [Google Scholar]
- Wang D., Huang H., Zhou L., Li W., Zhou H., Hou G., Liu J., Hu L. Effects of dietary supplementation with turmeric rhizome extract on growth performance, carcass characteristics, antioxidant capability, and meat quality of Wenchang broiler chickens. Ital. J. Anim. Sci. 2015;14:3870. [Google Scholar]
- Wang J.H., Wu C.C., Feng J. Effect of dietary antibacterial peptide and zinc-methionine on performance and serum biochemical parameters in piglets. Czech J. Anim. Sci. 2011;56:30–36. [Google Scholar]
- Wijnhoven S.W., Peijnenburg W.J., Herberts C.A., Hagens W.I., Oomen A.G., Heugens E.H., Roszek B., Bisschops J., Gosens I., Van De Meent D. Nano-silver–a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3:109–138. [Google Scholar]
- Yusof H.M., Mohamad R., Zaidan U.H., Arshad M.A., Samsudin A.A. Effects of dietary zinc oxide nanoparticles supplementation on broiler growth performance, zinc retention, liver health status, and gastrointestinal microbial load. JTEMIN. 2023;4 [Google Scholar]
- Zhao C.Y., Tan S.X., Xiao X.Y., Qiu X.S., Pan J.Q., Tang Z.X. Effects of dietary zinc oxide nanoparticles on growth performance and antioxidative status in broilers. Biol. Trace Elem. Res. 2014;160:361–367. doi: 10.1007/s12011-014-0052-2. [DOI] [PubMed] [Google Scholar]