Abstract
Evidence from animal models suggests that dietary fatty acids have both anticancer and tumor-promoting effects. Whether dietary fatty acids are associated with colorectal cancer (CRC) in humans remains inconclusive. We investigated associations between dietary fatty acids and risk of CRC among 59,986 men who participated in the Shanghai Men’s Health Study (SMHS), an ongoing population-based prospective cohort study. We identified 876 incident CRC cases in the SMHS during a mean follow-up of 9.8 years. Associations between dietary fatty acid intake and CRC risk were evaluated by Cox proportional hazard regression analyses. Consumption of saturated (SFA), monounsaturated (MUFA) and polyunsaturated (PUFA) fatty acids was not significantly associated with CRC risk. Multivariate hazard ratios (HR) and respective 95% confidence intervals (CI) for quartile 4 vs. quartile 1 were 0.92 (0.74–1.14; Ptrend=0.47) for SFA, 0.95 (0.79–1.16; Ptrend=0.74) for MUFA and 1.18 (0.95–1.46; Ptrend=0.21) for PUFA. No significant association was found for total n-6 PUFA and total n-3 PUFA. Additionally, we performed a meta-analysis to summarize results from the present study and 28 reports from 26 additional cohorts, which supported the overall null association between dietary fatty acid intake and CRC risk among men. Docosahexanoic acid (DHA) and eicosapentaenoic acid (EPA) were associated with 11–12% reduced risk, and linoleic acid (LA) a 19% increased risk, of CRC in the meta-analysis of combined sexes. In conclusion, this population-based prospective study and meta-analysis of cohort studies found little evidence that dietary fatty acid intake was associated with risk of CRC in men.
Keywords: Colorectal cancer, Dietary fatty acid, Prospective cohort study, Meta-analysis
INTRODUCTION
Colorectal cancer (CRC) is the third most commonly diagnosed cancer among men globally, with an estimated 1.03 million incident cases worldwide in 2018.1 In the United States, CRC accounts for 8.8% of all incident cancers among men, with approximately 78,300 new CRC cases projected for 2020.2 Over the past two decades, the CRC incidence rate has been substantially reduced in the United States,3 but has increased rapidly in China and many other developing countries in Asia.4 In 2018, CRC was the third most common cancer and fifth leading cause of all cancer-related deaths among men in China, with an estimated 303,853 incident CRC cases and 142,476 CRC deaths.1 Adoption of a “Westernized” diet, including a high content of protein and saturated fat (derived from animal-sourced foods), refined grain, sugar and sugar-sweetened beverages and alcohol, and low intake of fiber, fruits and vegetables, has been suggested as a major contributor to the increasing trend of CRC in China.5
The impact of high dietary fatty acids on the risk of CRC currently remains inconclusive. In 2011, a meta-analysis of 13 prospective cohort studies involving 3,635 CRC cases found no effect of total fatty acids (TFA) and specific fatty acids on the risk of CRC.6 No significant association was observed among men or women in most prospective cohort studies, with exception to the Nurses’ Health Study (NHS)7 and Singapore Chinese Health Study (SCHS).8 High intakes of fatty acids, especially saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA) from animals, were associated with an increased risk of CRC among women in the NHS.7 Similarly, a positive association between TFA and SFA and localized CRC (Dukes A or B) was found among women in the SCHS.8 A recent meta-analysis conducted in 2018, which included 18 prospective cohorts, however, reported no effect of TFA, SFA, MUFA, and a combination of total polyunsaturated fatty acids (PUFA), n-3 PUFA and n-6 PUFA on the risk of CRC, overall and in subgroup analyses by sex or continent.9 Among specific types of PUFA, the potentially preventive effects of total n-3 PUFA and marine-derived n-3 PUFA on CRC risk have been investigated in several epidemiological studies.10 A 2015 meta-analysis of 14 prospective cohorts with 8,775 cancer cases revealed no association between total n-3 PUFA and marine-derived n-3 PUFA intake and risk of CRC, but a significantly positive association was found between marine-derived n-3 PUFA intake and risk of CRC among Asian men.10
In this study, we evaluated the association of dietary fatty acid intake with the risk of CRC among Chinese men using data from a population-based cohort study, the Shanghai Men’s Health Study (SMHS). Additionally, we carried out a meta-analysis to summarize the results from the most up-to-date literature and the current study.
METHODS
Study Population
The SMHS is an ongoing population-based cohort study conducted in eight communities of urban Shanghai, China. Detailed information on the study design and methods are described elsewhere.11 Briefly, between January 2002 and September 2006, a total 61,480 men aged 40 to 74 years who were free of any cancer were recruited for the SMHS, with an overall participation rate of 74.1%. At baseline recruitment, information on socio-demographic characteristics, medical history, dietary habits, physical activity and lifestyle factors was collected through in-person interviews, and anthropometric measurements were taken.11
Dietary and Fatty Acids Intake Assessment
Information on dietary intake at study enrollment and the first follow-up survey was collected using a validated semi-quantitative food-frequency questionnaire (FFQ), which included 87 food items and food groups commonly consumed in urban Shanghai. This FFQ showed high validity and reproducibility when compared with multiple 24 hour dietary recalls.12 Individual nutrient intakes including SFA, MUFA, PUFA and specific fatty acids were calculated as the sum of products of individual amounts of foods consumed, with nutrient contents based on the 2002 Chinese Food Composition Table. To improve the validity of assessing usual dietary intakes, the mean value of individual dietary intakes for fatty acids, based on baseline FFQ and first follow-up FFQ, was used in the analysis for participants who did not report any diagnosis of cancer, myocardial infarction, stroke or diabetes mellitus during the period between the baseline FFQ and the first follow-up FFQ. For participants who were diagnosed with any of these conditions between the baseline FFQ and the second follow-up FFQ, only baseline-reported fatty acid intake was included in the current study.
TFA were calculated by combining SFA, MUFA and PUFA intake. Total n-6 PUFA intake was calculated by combining linoleic acid (LA, C18:2) and arachidonic acid (AA, C20:4), and total n-3 PUFA intake combined alpha-linolenic acid (ALA, C18:3), eicosapentaenoic acid (EPA, C20:5), docosapentaenoic acid (DPA, C22:5) and docosahexanoic acid (DHA, C22:6). Total n-3 highly unsaturated fatty acids (HUFA, which are fatty acids with ≥20 carbon molecules) were calculated by combining EPA, DHA and DPA; marine-derived n-3 PUFA intake (including EPA and DHA) was also calculated. The ratios between total dietary n-6 PUFA to total dietary n-3 PUFA, PUFA and MUFA to SFA were also included in the study.
Outcome Ascertainment
Participants were followed-up through a combination of annual record linkages to the Shanghai Cancer Registry and Shanghai Vital Statistics Registry and via in-person interviews every two to four years. Respective response rates for the first, second and third in-person follow-up surveys were 97.6%, 91.9% and 93.6%. Linkage identified or self-reported CRC cases, as defined by the 9th version of the International Classification of Diseases, codes 153 to 154, were verified through a review of medical charts from the diagnostic hospitals.
Statistical Analysis
For the current analysis, we excluded 404 participants who reported extreme total energy intake (i.e., either >4200 Kcal/day or <800 Kcal/day).13 To minimize the influence of preclinical cancer-related dietary pattern changes, we further excluded any cancer case diagnosed within the first two years after study enrollment (n=99), and those with less than two years of follow-up (n=991). For the current analysis, the final sample size was 59,986, with 876 incident CRC cases, diagnosed from time of enrollment through December 31, 2016.
Continuous variables and categorical variables were described as mean ± standard deviation (SD) and percentages, respectively. Age-adjusted baseline characteristics were compared using analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. The crude relationship between dietary fatty acids intake and food groups were tested with Spearman rank correlations. Dietary intake of fatty acids was adjusted for total energy intake using the residual method to control for confounding by total energy intake and to reduce extraneous variations.13 Participants were then categorized into quartile distributions of energy-adjusted residual intakes of fatty acids in the SMHS cohort. Cox proportional hazard models, with age as the time metric, were used to evaluate the associations between dietary intake of fatty acids and CRC risk. The follow-up time in the SMHS was calculated beginning two years after the date of study enrollment (i.e., excluding the first two years of cohort observation) and ending at the date of cancer diagnosis, death, loss to follow-up, or December 31, 2016, whichever came first. Hazard ratios (HRs) and respective 95% confidence intervals (CIs) were calculated and adjusted for potential confounders using the lowest quartile as the reference group.
The following covariates were considered as potential confounders and were included in the Cox proportional hazard regression models: age at cohort entry (continuous), educational level (i.e., elementary or less, middle school, high school or less, and professional/college or higher), income (i.e., low, lower middle, upper middle, and high), cigarette smoking (i.e., never smoking, pack-years <20, and pack-years ≥20), alcohol consumption (continuous, drinks/day; one drink equaled 14.18 g of alcohol; non-regular alcohol consumption was defined as alcohol consumption of <3 times per week for six months or longer), family history of CRC (yes/no), body mass index (BMI, kg/m2, continuous), physical activity in metabolic equivalent task score per hour per week (continuous), and fiber and calcium intake (Kcal/day, continuous). The proportional hazard assumption for the multivariable Cox model was evaluated by plots of scaled Schoenfeld residuals versus the ranking of time. Due to observed assumption violations, Cox proportional hazard regression models were adjusted for aforementioned potential confounders and stratified by cigarette smoking and income level. A linear trend was tested by treating the median value of each quartile as a continuous variable. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute, Cary, NC, USA), and a two-sided p<0.05 was considered statistically significant.
Meta-analysis
We systematically searched the PubMed database through May 20, 2020 using Medical Subject Heading (MESH) terms to identify published prospective cohort studies that evaluated associations of specific dietary fatty acid intakes with the risk of CRC (search strategy provided in Supplementary Materials and Methods). Additionally, we reviewed the reference lists from retrieved articles to identify potentially missed publications. We limited our selection of articles to (1) prospective study design; (2) total fat, subtypes of fat and specific fatty acids as the exposures of interest; (3) CRC and its subtypes as the outcomes of interest; (4) estimates including relative risk (RR), hazard ratios (HRs) or odds ratio (OR, for nested case-control studies), with corresponding 95% CIs; and (5) in the English language. Multiple publications from the same study were carefully reviewed and selected based on consideration of the number of events, duration of follow-up, and information on reported fatty acids.
A total 278 articles were identified through a MESH search strategy on the PubMed database. After excluding irrelevant studies by reviewing titles and abstracts, and adding 7 articles from the reference lists, 32 articles7, 8, 14–43 were identified to be potentially appropriate for the meta-analysis. Six publications17, 25, 28, 36, 38, 39 resulted from the same two cohort studies; the two publications25, 36 with the largest events and longest duration of follow-up time were included in the current analysis. Four reports14, 18, 31, 32 that were based on the same study population but evaluated different types of dietary fat intake with CRC risk were included in the meta-analysis. Overall, we selected 28 studies,7, 8, 15, 16, 18–27, 29–37, 41–43 derived from 26 population-based prospective cohorts, for this meta-analysis (Supplemental Figure 1). Including our current study, the number of studies was 16 for TFA,7, 8, 15, 16, 18–22, 24–27, 32, 40 18 for SFA,7, 8, 14–16, 19–27, 32, 37, 4114 for MUFA,7, 8, 15, 16, 18, 19, 21, 22, 25–27, 32, 41 and 10 for PUFA.8, 15, 21, 22, 25, 27, 32, 37, 41 As most prior prospective studies derived total n-6 PUFA by the sum of LA and AA,33, 36 we applied this definition in the meta-analysis, resulting in 12 studies for total n-6 PUFA assessment.8, 26, 30, 31, 33, 36, 37, 40, 41, 43 However, discrepant findings were observed between studies regarding the method for choosing which fatty acids to include in the consumption summaries for total n-3 PUFA and subtype n-3 PUFA.10 Thus, the number of selected studies in the final meta-analysis were 13 for total n-3 PUFA (ALA+EPA+DHA+DPA or ALA+EPA+DHA),8, 15, 26, 29–31, 33, 37, 40, 41, 43 five for n-3 HUFA (EPA, DHA, and DPA),31, 33, 36, 42 and six for marine-derived PUFA (EPA and DHA).8, 21, 27, 30, 35, 40
We performed a meta-analysis applying the random effect model. To evaluate potential modification of sex, cancer sites and geographic region on the association, sex-specific, anatomic-specific and region-specific analyses were also performed. The heterogeneity across studies was examined by Q test and I2 statistics. For the Q test, a p value <0.1 was considered statistically significant. For I2 statistics, a value of <25% was considered as a low level of heterogeneity; 25–50% was considered as a moderate level, and >50% as a high level. Publication bias was assessed by applying the Begg rank correlation method and the Egger weighted regression method, and a p<0.1 was considered as a statistically significant publication bias. All statistical meta-analyses were carried out using Stata software, version 14.0 (StataCorp. College Station, TX, USA).
RESULTS
We identified 876 incident CRC cases (including 534 colon cancers and 342 rectal cancers) among a total 59,986 participants during a mean follow-up of 9.8 years in the SMHS. When compared with participants who did not develop CRC, those who developed CRC (cases) were approximately six years older (61.0 years vs. 55.1 years). Cases were more likely to drink alcohol, be obese and to have a family history of CRC than non-cases (Table 1). Cases and non-cases did not differ in educational attainment, income levels and smoking habits. The Spearman rank correlation (r) between dietary fatty acid intake and food groups are presented in Supplementary Table 4 (all P <0.001).
Table 1.
Characteristics of study participants by subsequent colorectal cancer diagnosis (n=59,986)
| Non-CRC | CRC | p-value * | |
|---|---|---|---|
|
|
|||
| No. of participants (number) | 59,110 | 876 | |
| Age (Years; Mean ± SD) | 55.1±9.7 | 61.0±9.3 | |
| Educational levels (%) | |||
| Elementary or less | 6.4 | 11.3 | 0.84 |
| Middle school | 33.3 | 31.5 | |
| High school | 36.2 | 31.1 | |
| Professional/college or higher | 24.1 | 26.1 | |
| Income levels (%) a | |||
| Low | 12.5 | 9.7 | 0.7 |
| Lower middle | 42.5 | 43.6 | |
| Upper middle | 35.2 | 36.9 | |
| High | 9.8 | 9.8 | |
| Lifetime smoking (pack-years; Mean ± SD) | 0.3±3.0 | 0.4±3.1 | 0.09 |
| Never smoking | 30.3 | 34.3 | 0.31 |
| pack-years <20 | 30.9 | 24.9 | |
| Pack-years ≥20 | 38.8 | 38.8 | |
| Alcohol consumption (drinks/day; Mean ± SD) b | 0.8±1.7 | 0.9±1.8 | 0.02 |
| Lifetime non-regular alcohol drinker | 66.5 | 64.7 | 0.02 |
| <1 drink/day | 7.1 | 7.3 | |
| ≥1 drink/day | 26.4 | 28.0 | |
| Family history of CRC (%) | 2.1 | 3.1 | 0.05 |
| History of other diseases (%) c | 20.8 | 21.9 | 0.06 |
| Body mass index (kg/m2; Mean ± SD) | 23.7±3.1 | 24.3±3.3 | <0.0001 |
| <22.9 | 40.8 | 34.6 | 0.0002 |
| 23.0–24.9 | 26.1 | 25.6 | |
| 25.0–27.9 | 24.9 | 27.1 | |
| ≥28.0 | 8.2 | 12.7 | |
| Physical activity (MET-h/week; Mean ± SD) | 59.5±34.2 | 66.7±35.6 | 0.1 |
Mean ± SD for continuous variables and % for categorical variables.
The differences across subgroups were compared using ANOVA tests for continuous variables and χ2 for categorical variables by adjusting for age.
Defined as low: < ¥500 person per month; Lower middle: ¥500-999 per person per month; Upper middle: ¥1000-1999 per person per month; High: ≥ ¥2000 per person per month.
One drink equaled 14.18 g of alcohol. Non-regular alcohol consumption was defined as alcohol consumption of <3 times per week for six months or longer.
Reported any diagnosis of cancer, myocardial infarction, stroke or diabetes mellitus during the period between the baseline FFQs and the second follow-up FFQ.
CRC: colorectal cancer.
Association of dietary fatty acid intake and CRC risk
TFA intake, and the ratio of PUFA and MUFA to SFA, were not significantly associated with the risk of CRC in the age- and energy-adjusted model, or in the multivariate adjusted model. SFA, MUFA and PUFA were not associated with the risk of CRC. The respective multivariable HRs and 95% CI, when comparing the 2nd, 3rd and 4th (highest) quartiles with the 1st (lowest) quartile, were 0.88 (0.73–1.07), 0.85 (0.69–1.04), 0.92 (0.74–1.14) and Ptrend=0.47 for SFA; 0.89 (0.74–1.08), 0.90 (0.75–1.10), 0.95 (0.79–1.16) and Ptrend=0.74 for MUFA; and 1.16 (0.95–1.41), 1.10 (0.90–1.35), 1.18 (0.95–1.46) and Ptrend=0.21 for PUFA (Table 2). There were also no significant associations found between TFA, SFA, MUFA or PUFA intake, or with the ratio of PUFA and MUFA to SFA and CRC risk, by anatomic site of CRC (Supplemental Table 2–3).
Table 2.
HRs (95% CIs) for colorectal cancer by quartile of total and subtypes of dietary fatty acid intakes (N=59,986)
| Q1 | Q2 | Q3 | Q4 | P trend | |
|---|---|---|---|---|---|
|
| |||||
| Total fatty acids (TFA) | |||||
| No. of cases/person-years | 213/176,584 | 233/176,611 | 202/177,836 | 228/179,7764 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.06 (0.88–1.27) | 0.90 (0.74–1.09) | 1,06 (0.88–1.28) | 0.82 |
| Multivariable HR b | 1 (ref.) | 1.08 (0.89–1.30) | 0.91 (0.74–1.12) | 1.04 (0.84–1.27) | 0.98 |
| Saturated fatty acids (SFA) | |||||
| No. of cases/person-years | 231/176,349 | 207/177,283 | 208/177,780 | 230/179,382 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 0.88 (0.73–1.06) | 0.86 (0.71–1.03) | 0.96 (0.80–1.16) | 0.72 |
| Multivariable HR b | 1 (ref.) | 0.88 (0.73–1.07) | 0.85 (0.69–1.04) | 0.92 (0.74–1.14) | 0.47 |
| Monounsaturated fatty acids (MUFA) | |||||
| No. of cases/person-years | 234/176,826 | 212/176,906 | 213/177,573 | 217/179,488 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 0.87 (0.73–1.05) | 0.90 (0.74–1.08) | 0.98 (0.82–1.18) | 0.95 |
| Multivariable HR b | 1 (ref.) | 0.89 (0.74–1.08) | 0.90 (0.75–1.10) | 0.95 (0.79–1.16) | 0.74 |
| Polyunsaturated fatty acids (PUFA) | |||||
| No. of cases/person-years | 193/176,746 | 222/176,487 | 218/177,729 | 243/179,832 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.11 (0.92–1.35) | 1.05 (0.86–1.27) | 1.14 (0.95–1.38) | 0.24 |
| Multivariable HR b | 1 (ref.) | 1.16 (0.95–1.41) | 1.10 (0.90–1.35) | 1.18 (0.95–1.46) | 0.21 |
| Ratio (PUFA + MUFA)/SFA | |||||
| No. of cases/person-years | 237/176,966 | 199/178,881 | 199/178,071 | 241/176,875 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 0.92 (0.76–1.11) | 0.96 (0.80–1.16) | 1.10 (0.92–1.32) | 0.21 |
| Multivariable HR b | 1 (ref.) | 0.94 (0.78–1.14) | 1.00 (0.81–1.22) | 1.14 (0.93–1.40) | 0.12 |
Model 1: Cox proportional hazards model was adjusted for age, and total energy intake (residual method).
Model 2: Cox proportional hazards model was adjusted for age, total energy intake (residual method), education levels, alcohol consumption (continuous - drinks/day), family history of CRC, history of other diseases (cancer, myocardial infarction, stroke or diabetes mellitus), BMI (continuous - kg/m2), physical activity (continuous - MET-hour/week), fiber intake, calcium intake (kcal/day, continous) and was stratified income levels and cigarette smoking.
Total fatty acids (TFA): SFA + MUFA + PUFA.
There were no significant associations between dietary intake of LA, AA, total n-6 PUFA, ALA, EPA, DHA, marine-derived n-3 PUFA, n-3 HUFA, or total n-3 PUFA and CRC risk. The respective multivariable HRs and 95% CI, when comparing the 2nd, 3rd and 4th (highest) quartiles with the 1st (lowest) quartile, were for total n-6 PUFA: 1.16 (0.95–1.41), 1.20 (0.98–1.47), 1.18 (0.95–1.47) and Ptrend =0.16; and for total n-3 PUFA: 1.08 (0.89–1.31), 1.16 (0.95–1.42), 1.09 (0.87–1.36) and Ptrend =0.42 (Table 3). Likewise, we did not find any significant trend or association between dietary intake of each specific fatty acid and CRC or its subsites, with one exception. In the multivariate adjusted model, the 4th quartile of ALA intake (C18:3, a n-3 PUFA) was associated with an increased risk of rectal cancer (HRQ4 vs Q1: 1.45 (1.03–2.05); Ptrend =0.01) (Supplemental Tables 1–3).
Table 3.
HRs (95% CIs) for colorectal cancer by quartiles of dietary intake of n-6 and n-3 PUFA (N=59,986)
| Q1 | Q2 | Q3 | Q4 | p trend | |
|---|---|---|---|---|---|
|
| |||||
| Total n-6PUFA | |||||
| No. of cases/person-years | 186/177,229 | 217/176,460 | 231/177,444 | 242/179,661 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.11 (0.91–1.35) | 1.14 (0.94–1.38) | 1.14 (0.94–1.38) | 0.21 |
| Multivariable HR b | 1 (ref.) | 1.16 (0.95–1.41) | 1.20 (0.98–1.47) | 1.18 (0.95–1.47) | 0.16 |
| Linoleic acid (LA) 18:2 | |||||
| No. of cases/person-years | 184/177,212 | 218/176,437 | 232/177,460 | 242/179,684 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.13 (0.93–1.37) | 1.15 (0.95–1.40) | 1.15 (0.95–1.39) | 0.19 |
| Multivariable HR b | 1 (ref.) | 1.18 (0.96–1.44) | 1.22 (0.99–1.49) | 1.19 (0.96–1.48) | 0.15 |
| Arachidonic acid (AA) 20:4 | |||||
| No. of cases/person-years | 246/175,841 | 212/176,601 | 212/178,158 | 206/180,195 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 0.87 (0.72–1.05) | 0.87 (0.73–1.05) | 0.91 (0.76–1.10) | 0.38 |
| Multivariable HR b | 1 (ref.) | 0.89 (0.74–1.07) | 0.89 (0.74–1.08) | 0.89 (0.73–1.09) | 0.30 |
|
| |||||
| Total n-3PUFA | |||||
| No. of cases/person-years | 210/175,390 | 219/176,252 | 233/178,258 | 214/180,894 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.03 (0.85–1.25) | 1.11 (0.92–1.34) | 1.07 (0.88–1.29) | 0.39 |
| Multivariable HR b | 1 (ref.) | 1.08 (0.89–1.31) | 1.16 (0.95–1.42) | 1.09 (0.87–1.36) | 0.42 |
| n-3HUFA | |||||
| No. of cases/person-years | 249/175,078 | 253/176,174 | 194/178,154 | 180/181,388 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.07 (0.90–1.27) | 0.93 (0.77–1.21) | 0.96 (0.79–1.17) | 0.44 |
| Multivariable HRb | 1 (ref.) | 1.08 (0.91–1.29) | 0.94 (0.78–1.14) | 0.94 (0.77–1.15) | 0.35 |
| Marine-derived PUFA | |||||
| No. of cases/person-years | 254/175,133 | 248/176,164 | 195/178,243 | 179/181,254 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.03 (0.86–1.22) | 0.92 (0.76–1.11) | 0.94 (0.77–1.14) | 0.36 |
| Multivariable HR b | 1 (ref.) | 1.04 (0.87–1.24) | 0.93 (0.77–1.12) | 0.92 (0.75–1.12) | 0.27 |
| Alpha linolenic acid (ALA) C18:3 | |||||
| No. of cases/person-years | 199/175,846 | 212/176,614 | 240/177,848 | 225/180,487 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.04 (0.86–1.26) | 1.17 (0.97–1.42) | 1.10 (0.91–1.33) | 0.22 |
| Multivariable HR b | 1 (ref.) | 1.09 (0.89–1.33) | 1.25 (1.02–1.52) | 1.15 (0.92–1.43) | 0.18 |
| Eicosapentaenoic acid (EPA) C20:5 | |||||
| No. of cases/person-years | 250/175,036 | 248/176,047 | 201/178,266 | 177/181,445 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.04 (0.87–1.23) | 0.95 (0.79–1.15) | 0.95 (0.78–1.15) | 0.47 |
| Multivariable HR b | 1 (ref.) | 1.05 (0.88–1.25) | 0.96 (0.79–1.16) | 0.93 (0.75–1.14) | 0.33 |
| Docosapentaenoic acid (DPA) C22:5 | |||||
| No. of cases/person-years | 241/174,933 | 252/176,140 | 201/178,019 | 182/181,702 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.10 (0.92–1.31) | 0.98 (0.81–1.18) | 1.00 (0.82–1.21) | 0.69 |
| Multivariable HR b | 1 (ref.) | 1.12 (0.93–1.33) | 1.00 (0.82–1.21) | 0.99 (0.81–1.21) | 0.63 |
| Docosahexanoic acid (DHA) C22:6 | |||||
| No. of cases/person-years | 254/175,152 | 243/176,161 | 198/178,225 | 181/181,256 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 1.01 (0.85–1.20) | 0.93 (0.77–1.12) | 0.94 (0.78–1.14) | 0.43 |
| Multivariable HR b | 1 (ref.) | 1.02 (0.86–1.22) | 0.95 (0.78–1.14) | 0.92 (0.76–1.13) | 0.34 |
|
| |||||
| Ratio n-6PUFA/n-3 PUFA | |||||
| No. of cases/person-years | 183/180,326 | 194/179,208 | 225/177,344 | 274/173,915 | |
| Age, energy intake adjusted HR a | 1 (ref.) | 0.94 (0.77–1.15) | 0.99 (0.81–1.20) | 1.08 (0.89–1.30) | 0.29 |
| Multivariable HR b | 1 (ref.) | 0.96 (0.78–1.18) | 1.02 (0.83–1.24) | 1.11 (0.91–1.36) | 0.22 |
Model 1: Cox proportional hazards model was adjusted for age, and total energy intake (residual method).
Model 2: Cox proportional hazards model was adjusted for age, total energy intake (residual method), education levels, alcohol consumption (continuous - drinks/day), family history of CRC, history of other diseases (cancer, myocardial infarction, stroke or diabetes mellitus), BMI (continuous - kg/m2), physical activity (continuous - MET-hour/week), fiber intake, calcium intake (kcal/day, continous) and was stratified income levels and cigarette smoking.
Total n-6 PUFA: LA + AA; Total n-3 PUFA: EPA + DHA + ALA + DPA or EPA + DHA + ALA; n-3 HUFA: EPA + DHA + DPA; Marine-derived PUFA: EPA + DHA.
Meta-analysis
In the overall meta-analysis, there were no significant associations between TFA, SFA, MUFA or PUFA, including total n-6 PUFA, total n-3 PUFA, n-3 HUFA, and marine-derived PUFA with CRC risk. The combined relative risk (RR) and 95% CIs were 1.01 (0.92–1.10) for TFA, 0.98 (0.90–1.07) for SFA, 1.04 (0.93–1.15) for MUFA, 1.04 (0.92–1.17) for PUFA, 1.02 (0.93–1.11) for total n-6 PUFA, 1.00 (0.91–1.10) for total n-3 PUFA, 0.94 (0.87–1.01) for n-3 HUFA, and 1.03 (0.92–1.13) for marine-derived PUFA (Figures 1–2, Supplemental Figure 2 & Table 4).
Figure 1:
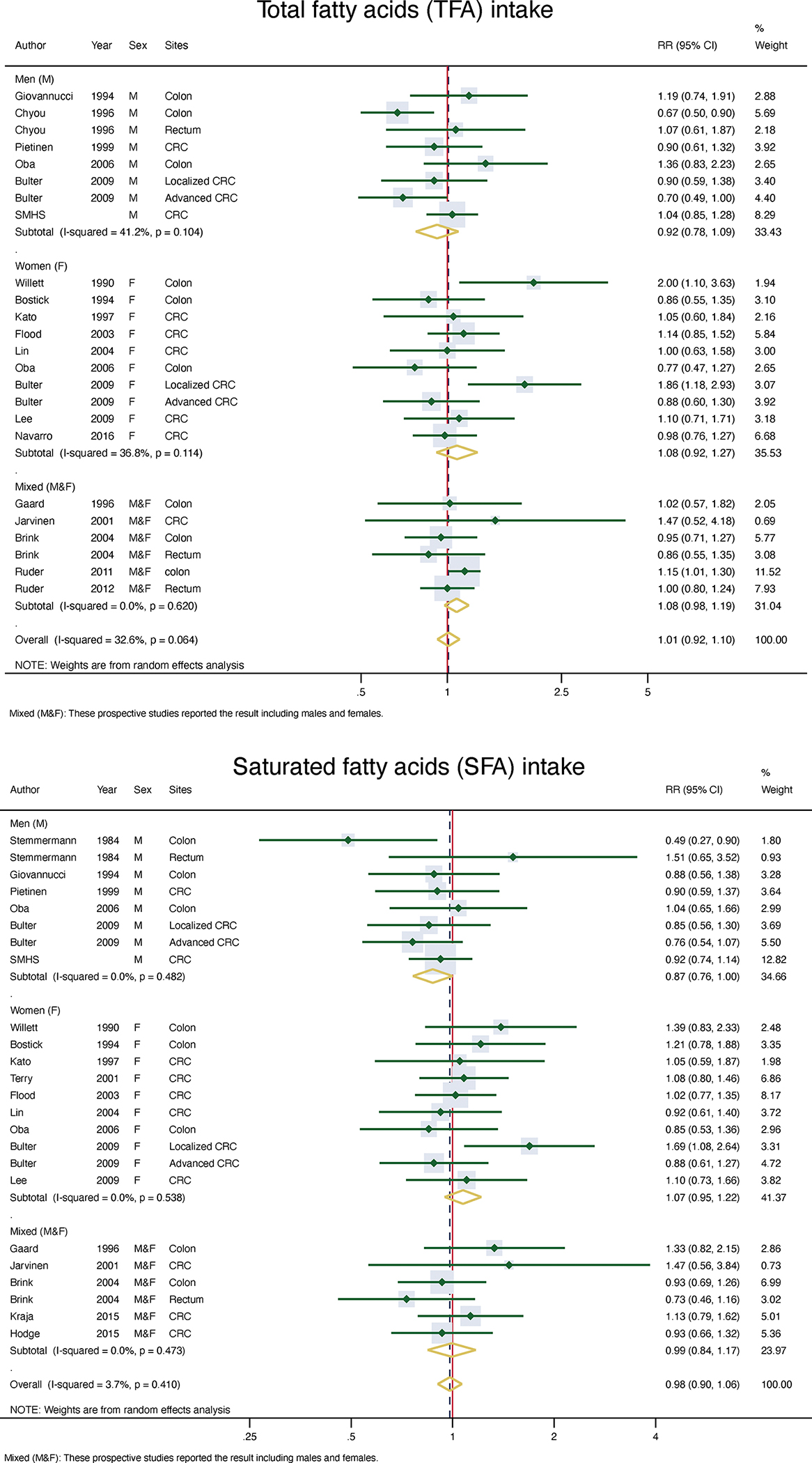
Meta-analysis of TFA and SFA intake and risk of CRC
Figure 2:
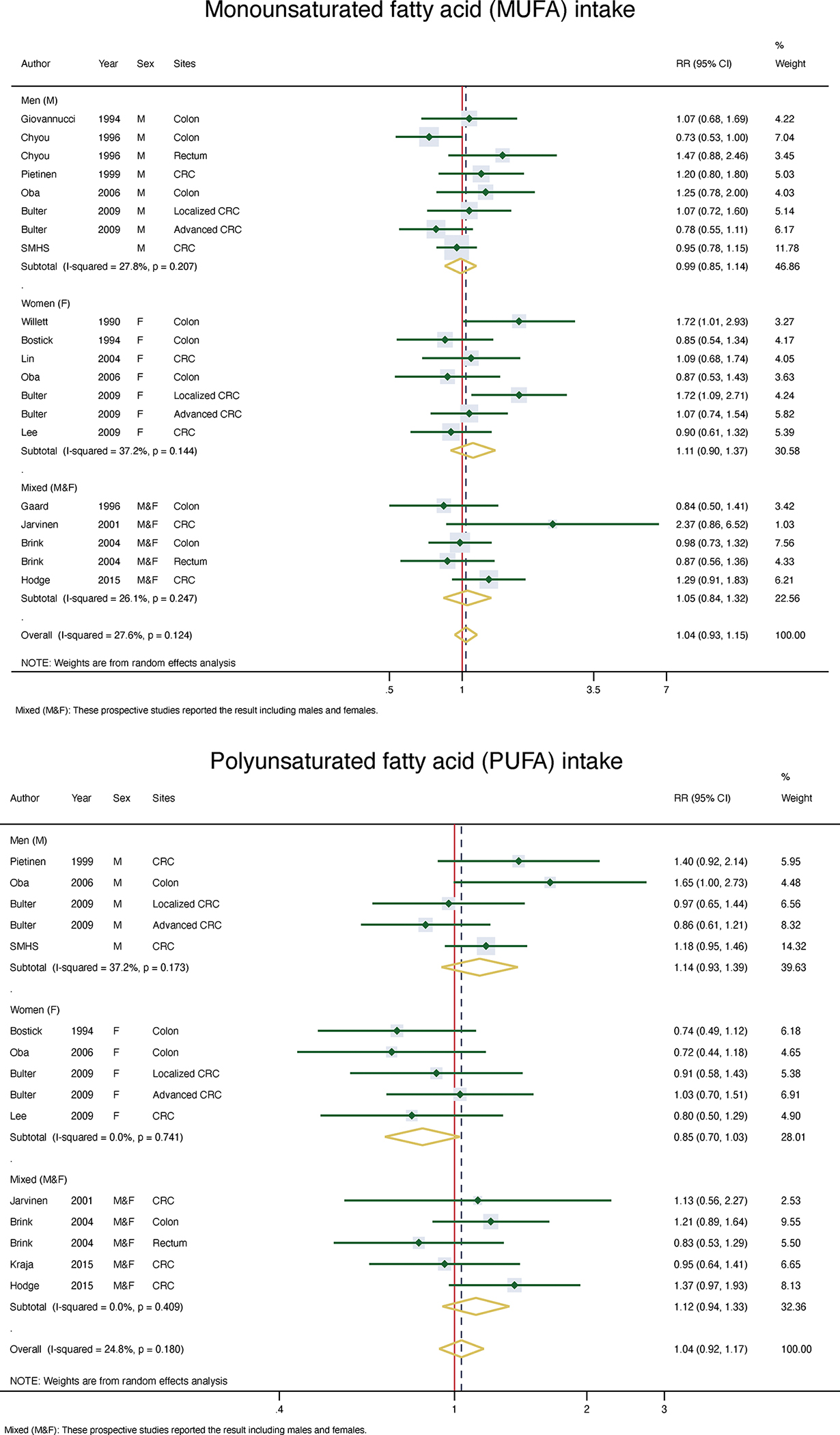
Meta-analysis of MUFA and PUFA in take and risk of CRC
Table 4.
Meta-analysis of the association between types of dietary fatty acid intake (highest vs lowest intake) and the risk of CRC by gender
| Test for heterogeneity | Test for publication bias | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Fatty acids | # of studies | RR (95%CI) | pa | I2(%) | p for Eggerb | p for Beggc | p for EMd |
|
| |||||||
| TFA | |||||||
| Women | 9 | 1.08 (0.92–1.28) | 0.11 | 36.8 | 0.49 | 0.72 | 0.11 |
| Men | 6 | 0.92 (0.78–1.09) | 0.10 | 41.2 | 0.72 | 0.17 | |
| Mixedǂ | 4 | 1.08 (0.98–1.19) | 0.62 | 0.0 | 0.39 | 1.0 | |
| Overall | 17 | 1.01 (0.92–1.10) | 0.06 | 32.6 | 0.77 | 0.32 | |
| SFA | |||||||
| Women | 9 | 1.07 (0.95–1.22) | 0.54 | 0.0 | 0.47 | 0.37 | 0.09 |
| Men | 6 | 0.87 (0.76–1.00) | 0.48 | 0.0 | 0.91 | 0.54 | |
| Mixedǂ | 5 | 0.99 (0.84–1.17) | 0.47 | 0.0 | 0.45 | 0.26 | |
| Overall | 18 | 0.98 (0.90–1.07) | 0.41 | 3.7 | 0.37 | 0.31 | |
| MUFA | |||||||
| Women | 6 | 1.10 (0.90–1.37) | 0.14 | 37.2 | 0.44 | 0.76 | 0.46 |
| Men | 6 | 0.99 (0.85–1.14) | 0.21 | 27.8 | 0.22 | 0.02 | |
| Mixedǂ | 4 | 1.05 (0.84–1.32) | 0.25 | 26.1 | 0.53 | 0.81 | |
| Overall | 14 | 1.04 (0.93–1.15) | 0.12 | 27.6 | 0.05 | 0.04 | |
| PUFA | |||||||
| Women | 4 | 0.85 (0.70–1.03) | 0.74 | 0.0 | 0.26 | 0.46 | 0.04 |
| Men | 4 | 1.14 (0.94–1.39) | 0.17 | 37.2 | 0.74 | 0.22 | |
| Mixedǂ | 4 | 1.12 (0.94–1.33) | 0.41 | 0.0 | 0.49 | 0.46 | |
| Overall | 10 | 1.04 (0.92–1.17) | 0.18 | 24.8 | 0.26 | 0.43 | |
| Total n-6PUFA | |||||||
| Women | 8 | 1.02 (0.89–1.17) | 0.16 | 31.5 | 0.42 | 0.37 | 0.70 |
| Men | 5 | 0.99 (0.86–1.13) | 0.20 | 29.8 | 0.06 | 0.76 | |
| Mixedǂ | 3 | 1.11 (0.85–1.44) | 0.15 | 46.7 | - | 1.0 | |
| Overall | 13 | 1.02 (0.93–1.11) | 0.13 | 27.3 | 0.84 | 0.72 | |
| Total n-3PUFA | |||||||
| Women | 8 | 1.03 (0.89–1.20) | 0.17 | 29.8 | 0.83 | 0.28 | 0.43 |
| Men | 6 | 0.94 (0.82–1.08) | 0.21 | 21.4 | 0.78 | 1.0 | |
| Mixedǂ | 3 | 1.08 (0.78–1.48) | 0.07 | 62.9 | 0.16 | 30 | |
| Overall | 14 | 1.00 (0.91–1.10) | 0.08 | 32.2 | 0.61 | 0.70 | |
| n-3 HUFA | |||||||
| Women | 3 | 0.99 (0.81–1.20) | 0.32 | 14.2 | 0.75 | 1.0 | 0.11 |
| Men | 3 | 0.99 (0.86–1.14) | 0.90 | 0.0 | 0.85 | 0.73 | |
| Mixedǂ | 1 | 0.86 (0.78–0.95) | - | - | - | - | |
| Overall | 6 | 0.94 (0.87–1.01) | 0.38 | 7.1 | 0.45 | 0.92 | |
| Marine-derived PUFA | |||||||
| Women | 4 | 0.98 (0.82–1.17) | 0.14 | 42.0 | 0.92 | 0.46 | 0.34 |
| Men | 5 | 1.09 (0.95–1.24) | 0.36 | 8.2 | 0.03 | 0.26 | |
| Mixedǂ | 1 | 0.88 (0.65–1.20) | - | - | - | - | |
| Overall | 7 | 1.03 (0.92–1.13) | 0.21 | 24.0 | 0.31 | 0.63 | |
| Ratio n-6/n-3 PUFA | |||||||
| Women | 2 | 1.24 (0.62–2.48) | 0.11 | 60.7 | - | 1.0 | 0.71 |
| Men | 2 | 1.09 (0.92–1.28) | 0.71 | 0.0 | - | 1.0 | |
| Mixedǂ | 1 | 1.22 (0.92–1.62) | - | - | - | - | |
| Overall | 4 | 1.10 (0.97–1.25) | 0.49 | 0.0 | 0.28 | 0.81 | |
| Ratio n-3/n-6 PUFA | |||||||
| Women | 2 | 1.01 (0.80–1.28) | 0.71 | 0.0 | 0.78 | 0.73 | 0.26 |
| Men | 2 | 1.21 (0.99–1.49) | 0.43 | 0.0 | 0.71 | 0.73 | |
| Mixedǂ | 0 | ||||||
| Overall | 2 | 1.12 (0.96–1.31) | 0.61 | 0.0 | 0.83 | 0.71 | |
| Type of fatty acids | |||||||
| LA (C18:2) | |||||||
| Women | 5 | 1.20 (1.00–1.44) | 0.68 | 0.0 | 0.85 | 0.81 | 0.65 |
| Men | 3 | 1.10 (0.85–1.41) | 0.21 | 36.8 | 0.71 | 1.0 | |
| Mixedǂ | 2 | 1.28 (1.04–1.57) | 0.54 | 0.0 | 0.43 | 1.0 | |
| Overall | 10 | 1.19 (1.07–1.33) | 0.62 | 0.0 | 0.52 | 0.76 | |
| AA (C20:4) | |||||||
| Women | 3 | 0.85 (0.69–1.06) | 0.75 | 0.0 | 0.09 | 0.30 | 0.42 |
| Men | 1 | 0.89 (0.72–1.09) | - | - | - | - | |
| Mixedǂ | 1 | 1.12 (0.79–1.58) | - | - | - | - | |
| Overall | 5 | 0.91 (0.79–1.04) | 0.68 | 0.0 | 0.62 | 0.46 | |
| ALA (C18:3) | |||||||
| Women | 6 | 1.10 (0.98–1.24) | 0.77 | 0.0 | 0.92 | 0.76 | 0.54 |
| Men | 5 | 1.00 (0.86–1.16) | 0.25 | 24.9 | 0.77 | 0.71 | |
| Mixedǂ | 2 | 1.02 (0.83–1.24) | 0.82 | 0.0 | 0.58 | 1.0 | |
| Overall | 11 | 1.05 (0.97–1.13) | 0.71 | 0.0 | 0.95 | 0.75 | |
| EPA (C20:5) | |||||||
| Women | 4 | 0.85 (0.70–1.03) | 0.35 | 9.7 | 0.14 | 0.22 | 0.87 |
| Men | 2 | 0.92 (0.76–1.11) | 0.80 | 0.0 | 0.99 | 1.0 | |
| Mixedǂ | 3 | 0.92 (0.78–1.08) | 0.21 | 36.7 | 0.37 | 0.30 | |
| Overall | 8 | 0.88 (0.82–0.95) | 0.60 | 0.0 | 0.96 | 0.53 | |
| DHA (C22:6) | |||||||
| Women | 4 | 0.89 (0.72–1.09) | 0.29 | 19.0 | 0.77 | 0.81 | 0.82 |
| Men | 2 | 0.94 (0.79–1.12) | 0.83 | 0.0 | 0.03 | 0.30 | |
| Mixedǂ | 3 | 0.88 (0.80–0.97) | 0.50 | 0.0 | 0.54 | 0.30 | |
| Overall | 8 | 0.89 (0.83–0.97) | 0.72 | 0.0 | 0.46 | 0.35 | |
| DPA (C22:5) | |||||||
| Women | 3 | 0.87 (0.54–1.39) | 0.13 | 50.7 | 0.94 | 1.0 | 0.29 |
| Men | 2 | 0.99 (0.83–1.18) | 0.59 | 0.0 | 0.79 | 1.0 | |
| Mixedǂ | 2 | 0.84 (0.76–0.94) | 0.31 | 2.1 | - | 1.0 | |
| Overall | 5 | 0.90 (0.80–1.01) | 0.28 | 18.5 | 0.31 | 0.71 | |
: These prospective studies reported the result including males and females.
: p value for heterogeneity
: p of Egger, p value of Egger rank correlation method for testing publication bias
: p of Begg, p value of Begg rank correlation method for testing publication bias
: p value for effect modification by gender (only women, only men, and men & women)
Total fatty acids (TFA): SFA + MUFA + PUFA; Total n-6 PUFA: LA + AA; Total n-3 PUFA: EPA + DHA + ALA + DPA or EPA + DHA + ALA; n-3 HUFA: EPA + DHA + DPA; Marine-derived PUFA: EPA + DHA.
No significant sex-specific, anatomic-specific or region-specific associations were found for TFA, SFA, MUFA or PUFA, including total n-6 PUFA, total n-3 PUFA, n-3 HUFA, and marine-derived PUFA on CRC risk in the stratified analyses. ALA intake was not significantly associated with CRC risk in overall, or sex-specific, anatomic-specific and region-specific analyses (Supplemental Table 3). There was an indication of possible modification by sex for PUFA (P=0.04).
In the meta-analysis of men only, the combined RR and 95% CIs were 0.92 (0.78–1.09) for TFA, 0.87 (0.76–1.00) for SFA, 0.99 (0.85–1.14) for MUFA, 1.14 (0.94–1.39) for PUFA, 0.99 (0.86–1.13) for total n-6 PUFA, 0.94 (0.83–1.08) for total n-3 PUFA, 0.99 (0.86–1.14) for n-3 HUFA, and 1.09 (0.95–1.24) for marine-derived PUFA. There was evidence of heterogeneity across studies for TFA and total n-3 PUFA (P <0.1), and a significant publication bias in the literature regarding CRC risk with MUFA (P for Begg = 0.04 in the analysis of combined sexes) and marine-derived PUFA (P for Egger = 0.03 in the male analysis) (Table 4 & Supplemental Tables 5–6).
Regarding the type of n-6 PUFA, LA was significantly associated with a 19% increased risk of CRC (combined RR= 1.19; 95% CI: 1.07–1.33) in the analysis of combined sexes, and a 25% increased risk of CRC among European and Australian populations (combined RR= 1.25; 95% CI: 1.09–1.45) in the region-specific analysis. An approximately 42% increased risk of rectal cancer was also found for LA in the analysis with combined sexes (combined RR= 1.42; 95% CI: 1.11–1.81) (Table 4 & Supplemental Tables 5–6).
The meta-analysis of combined sexes also showed an 11–12% reduced risk of CRC associated with intake of DHA (combined RR= 0.89; 95%CI: 0.83–0.97) or EPA (combined RR= 0.88; 95%CI: 0.82–0.95) (Table 4). Furthermore, EPA and DPA intake were associated with a 12–13% lowered risk of colon cancer, and DHA intake was inversely associated with a 13% reduced risk of rectal cancer (Supplemental Table 5). In the region-specific analysis, DHA and DPA intake were associated with an 11% and 15% lowered risk of CRC in studies conducted in Europe and Australia, respectively (Supplemental Table 6).
DISCUSSION
In our prospective population-based cohort study of 59,986 Chinese men in Shanghai with 876 incident CRC cases, and a meta-analysis with 28 reports from an additional 26 prospective cohorts, we found no evidence that TFA, SFA, MUFA or PUFA were significantly associated with the risk of CRC in overall, or sex-specific, anatomic-specific and region-specific analyses. In a meta-analysis of combined sexes, we found that higher EPA intake was significantly associated with an 11% lowered risk of CRC and 12% lowered risk of colon cancer, while a high DHA intake was associated with a respective 12% and 13% lowered risk of CRC and rectal cancer compared with those with low intakes of these fatty acids. Furthermore, significant positive associations were observed between LA intake and risk of CRC and rectal cancer. The significant associations between LA, EPA and DHA intakes with CRC risk were only significant among European and Australian populations.
Previous animal studies have suggested that dietary fatty acid intake may have both anticancer and tumor-promoting effects. Animal experiments have shown that high fat diets could lead to an increase in biliary secretion, which could damage the mucosal epithelium, induce frequent apoptosis and increase the risk of endogenous mutations and development of CRC.44 A high intake of SFA could increase free fatty acid and bile acid levels, which are metabolized by gut microbes to generate secondary bile acid. Secondary bile acid can promote colorectal carcinogenesis through an increase in pro-inflammatory effectors and oxidative stress mediated via engagement of the nuclear factor-kB (NFkB) and cyclooxygenase-2 pathways (COX-2/prostaglandin synthase-2).45 Among specific types of PUFA, the potential effects of dietary n-3 PUFA (in which marine fish oil is rich) and n-6 PUFA (in which vegetable oils, processed and fast foods, and red meats are rich) on carcinogenesis have been mostly investigated. In mouse models, n-3 PUFA, such as EPA (C20:5) and DHA (C22:6), have been consistently shown to have anti-inflammatory and antineoplastic effects.46 Conversely, n-6 PUFA, such as LA (C18:2) and AA (C20:4), have been found to have tumor-promoting effects.46
The associations of dietary fatty acids with CRC risk in humans have been inconclusive in epidemiological literature.6,10 The null associations for TFA, SFA, MUFA and PUFA, as well as for specific fatty acids among men, found in the current study, are in line with several previous studies conducted on both Caucasian and Asian male populations.8, 16, 18, 21, 30, 36 In 2014, Song et al. found no association between total n-6 PUFA or marine-derived PUFA with CRC risk among 76,386 American men.36 Null associations were also reported in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study (ATBC) and Cancer Prevention Study-II (CPS-II).30 Moreover, two prospective cohort studies involving 883 CRC cases among 55,276 Japanese male participants showed no significant associations between CRC and TFA, SFA, MUFA, PUFA or marine-derived PUFA (EPA+DHA), or total n-6 PUFA, total n-3 PUFA, or the subtype ratio of n-6 to n-3 PUFA with CRC risk.27, 33 Dietary intake of TFA, SFA and MUFA was also not significantly associated with risk of CRC in a prospective study among 27,293 Singaporean Chinese men.8 Our finding from the SMHS is also supported by the results of our meta-analysis.
A significant inverse association between total n-3 PUFA intake and CRC risk was reported in 2008 in the Physicians’ Health Study (PHS), which included 21,406 men.29 A significant inverse association between colon cancer and saturated fat intake was also reported in a Japan-Hawaii Cancer study among 7,074 men of Japanese ancestry during a 15-year follow-up.14 In the SMHS, we found that ALA intake was associated with an increased risk of rectal cancer. However, our meta-analysis showed null associations for ALA intake in overall, or sex-specific, anatomic-specific and region-specific analyses. Thus, this positive association needs to be interpreted with caution and requires future confirmation.
Meta-analysis of prospective studies, including our current cohort study and previous studies, found no associations between TFA, SFA, MUFA or PUFA, including total n-6 PUFA, total n-3 PUFA, n-3 HUFA and marine-derived PUFA in overall, or sex-specific, anatomic-specific and region-specific analyses. Our finding that EPA, DHA and DPA intakes were inversely associated with CRC is in line with the finding from a previous meta-analysis.47 However, the observed inverse associations were only significant among European and Australian populations. We also found that LA intake was positively associated with CRC risk among European and Australian populations. Further studies are needed to investigate the contributors to population-specific associations.
Our meta-analyses showed no heterogeneity in fatty acid and CRC associations by sex, with the exception of PUFA intake (P for interaction <0.05). We found that PUFA intake was associated with a reduced risk of CRC among women, and an increased risk of CRC in men. Estrogen level has been suggested to lead to changes in fatty acid utilization and oxidation.48 High estrogen levels may decrease the production of prostaglandin E2 (PGE2, a product of AA-derived eicosanoids), promoting tumor cell survival.49 In the Shanghai Women’s Health Study among 73,242 Chinese women, Murff et al. reported n-6 to n-3 PUFA intake ratios were significantly associated with an increased risk of CRC and increased production of prostaglandin E2 (PGE2, a product of AA-derived eicosanoids) in only CRC cases. A significant positive association between TFA and SFA and the risk of localized CRC (Dukes A or B) was observed among Singaporean Chinese women, but not among Singaporean Chinese men.8 Recently, the European Prospective Investigation into Cancer and Nutrition (EPIC) study, involving an evaluation of 6,291 CRC cases out of 521,324 cohort members, reported that high dietary intakes of n-3 HUFA (as long-chain n-3 PUFA), EPA, DPA and DHA were significantly associated with a reduced risk of CRC, and the n-6 to n-3 HUFA intake ratio was associated with significantly increased risk of CRC, colon and rectal cancers.42 These significant associations were only significant among women, although there was no heterogeneity by sex.42 More research is needed to further investigate the potential modification of sex on the dietary fatty acid intake and CRC association and its underlying biological mechanisms.
The most notable strength of our study is its prospective population-based cohort study design, high follow-up rates, repeated dietary assessments and detailed information on a wide array of potential confounders. The SMHS FFQ covered approximately 90% of commonly consumed foods in urban Shanghai and showed high validity and reproducibility, compared with multiple 24 hour dietary recalls, performed separately for men.12 In addition, 26 prospective cohort studies were included in our meta-analysis.
Our study also has some limitations. First, although dietary information was collected through in-person interviews using validated FFQs, potential measurement error is inevitable. We used repeated dietary assessments implemented at baseline and the first follow-up to improve dietary intake assessment. We further excluded the first two years of follow-up to minimize the influence of preclinical cancer-related dietary pattern changes and reduce potential influences of reverse causation and varied dietary measures. Nonetheless, measurement errors are unavoidable and may have biased our results towards the null. Second, we did not collect information on supplemental fish oil intake, which may confound the associations under study. Due to the lack of information on trans-fat in the Chinese Food Composition Table, trans-fat was not included in our analysis. Finally, the findings from our prospective cohort study may not be generalizable to all populations, such as people living in rural areas or with low socioeconomic status (SES), as all participants of the SMHS were recruited from urban areas, and most of the cohort studies included in the meta-analysis did not include sufficient participants with low SES.
In conclusion, we found that TFA, SFA, MUFA and PUFA, or specific PUFA subtypes or other specific fatty acid intake, were not associated with the risk of CRC among Chinese men in Shanghai. Meta-analysis findings from 26 prospective cohort studies and the current study confirmed the null association among men. However, the meta-analysis of men and women combined suggests that high EPA and DHA intakes were inversely associated, and high LA intake was positively associated, with CRC risk, particularly among European and Australian populations.
Supplementary Material
Novelty and impact.
Comprehensive analysis of data from a prospective cohort study and meta-analysis of data from 26 additional cohorts found little evidence that consumption of total, saturated, monounsaturated or polyunsaturated fatty acids, including specific fatty acids, were significantly associated with colorectal cancer (CRC) risk among men, overall or by anatomic site. Meta-analysis of men and women combined showed linoleic acid (LA) intake positively associated, and eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA) inversely associated with CRC risk.
ACKNOWLEDGMENTS
We would like to thank the participants and the research staff members of the Shanghai Men’s Health Study, without whom this study would not have been possible. The SMHS were supported by the U.S. National Institutes of Health [R01 HL079123 to Dr. X.O. Shu]. Yong-Bing Xiang was partially supported by the National Key Project of Research and Development Program of China (2016YFC1302503). Sang Nguyen was supported by a VECD Global Health Fellowship, funded by the National Cancer Institute (NCI) and the Fogarty International Center (FIC) of the NIH (D43 TW009337). We would like to thank Dr. Alicia Beeghly-Fadiel for her helpful comments. We also thank Dr. Mary Shannon Byers for editing this manuscript.
Abbreviations
- AA
arachidonic acid
- ALA
alpha-linolenic acid
- CI
confidence interval
- CRC
colorectal cancer
- DHA
docosahexanoic acid
- DPA
docosapentaenoic acid
- EPA
eicosapentaenoic acid
- EPIC
European Prospective Investigation into Cancer and Nutrition
- FFQ
food frequency questionnaire
- HR
hazard ratio
- HUFA
highly unsaturated fatty acids
- JPHC
Japan Public Health Center
- LA
linoleic acid
- MESH
Medical Subject Heading
- MUFA
monounsaturated fatty acids
- NHS
Nurses’ Health Study
- PHS
Physicians’ Health Study
- PUFA
polyunsaturated fatty acids
- RR
relative risk
- SFA
saturated fatty acids
- SMHS
Shanghai Men’s Health Study
- TFA
total fatty acids
Footnotes
CONFLICT OF INTEREST
None of the authors have a conflict of interest.
ETHICS STATEMENT
All SMHS participants provided their written consent to be studied, and the Institutional Review Boards of participating institutions approved the study protocols.
DATA ACCESSIBILITY
SMHS data used in this research project will be available following the study data sharing policy posted online at: https://swhs-smhs.app.vumc.org/smhs_index.php.
REFERENCE
- 1.Ferlay J, Colombet M, Soerjomataram I, Global and Regional Estimates of the Incidence and Mortality for 38 Cancers: GLOBOCAN 2018, 2018. [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020. [DOI] [PubMed] [Google Scholar]
- 3.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, et al. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 2010;116: 544–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Center MM, Jemal A, Ward E. International trends in colorectal cancer incidence rates. Cancer Epidemiol Biomarkers Prev 2009;18: 1688–94. [DOI] [PubMed] [Google Scholar]
- 5.Statovci D, Aguilera M, MacSharry J, Melgar S. The Impact of Western Diet and Nutrients on the Microbiota and Immune Response at Mucosal Interfaces. Front Immunol 2017;8: 838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L, Zhuang W, Wang RQ, Mukherjee R, Xiao SM, Chen Z, Wu XT, Zhou Y, Zhang HY. Is dietary fat associated with the risk of colorectal cancer? A meta-analysis of 13 prospective cohort studies. Eur J Nutr 2011;50: 173–84. [DOI] [PubMed] [Google Scholar]
- 7.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 1990;323: 1664–72. [DOI] [PubMed] [Google Scholar]
- 8.Butler LM, Wang R, Koh WP, Stern MC, Yuan JM, Yu MC. Marine n-3 and saturated fatty acids in relation to risk of colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer 2009;124: 678–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim M, Park K. Dietary Fat Intake and Risk of Colorectal Cancer: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen GC, Qin LQ, Lu DB, Han TM, Zheng Y, Xu GZ, Wang XH. N-3 polyunsaturated fatty acids intake and risk of colorectal cancer: meta-analysis of prospective studies. Cancer Causes Control 2015;26: 133–41. [DOI] [PubMed] [Google Scholar]
- 11.Shu XO, Li H, Yang G, Gao J, Cai H, Takata Y, Zheng W, Xiang YB. Cohort Profile: The Shanghai Men’s Health Study. Int J Epidemiol 2015;44: 810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villegas R, Yang G, Liu D, Xiang YB, Cai H, Zheng W, Shu XO. Validity and reproducibility of the food-frequency questionnaire used in the Shanghai men’s health study. Br J Nutr 2007;97: 993–1000. [DOI] [PubMed] [Google Scholar]
- 13.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124: 17–27. [DOI] [PubMed] [Google Scholar]
- 14.Stemmermann GN, Nomura AM, Heilbrun LK. Dietary fat and the risk of colorectal cancer. Cancer Res 1984;44: 4633–7. [PubMed] [Google Scholar]
- 15.Bostick RM, Potter JD, Kushi LH, Sellers TA, Steinmetz KA, McKenzie DR, Gapstur SM, Folsom AR. Sugar, meat, and fat intake, and non-dietary risk factors for colon cancer incidence in Iowa women (United States). Cancer Causes Control 1994;5: 38–52. [DOI] [PubMed] [Google Scholar]
- 16.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res 1994;54: 2390–7. [PubMed] [Google Scholar]
- 17.Goldbohm RA, van den Brandt PA, van ‘t Veer P, Brants HA, Dorant E, Sturmans F, Hermus RJ. A prospective cohort study on the relation between meat consumption and the risk of colon cancer. Cancer Res 1994;54: 718–23. [PubMed] [Google Scholar]
- 18.Chyou PH, Nomura AM, Stemmermann GN. A prospective study of colon and rectal cancer among Hawaii Japanese men. Ann Epidemiol 1996;6: 276–82. [DOI] [PubMed] [Google Scholar]
- 19.Gaard M, Tretli S, Loken EB. Dietary factors and risk of colon cancer: a prospective study of 50,535 young Norwegian men and women. Eur J Cancer Prev 1996;5: 445–54. [PubMed] [Google Scholar]
- 20.Kato I, Akhmedkhanov A, Koenig K, Toniolo PG, Shore RE, Riboli E. Prospective study of diet and female colorectal cancer: the New York University Women’s Health Study. Nutr Cancer 1997;28: 276–81. [DOI] [PubMed] [Google Scholar]
- 21.Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, Virtamo J. Diet and risk of colorectal cancer in a cohort of Finnish men. Cancer Causes Control 1999;10: 387–96. [DOI] [PubMed] [Google Scholar]
- 22.Jarvinen R, Knekt P, Hakulinen T, Rissanen H, Heliovaara M. Dietary fat, cholesterol and colorectal cancer in a prospective study. Br J Cancer 2001;85: 357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terry P, Bergkvist L, Holmberg L, Wolk A. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev 2001;10: 913–4. [PubMed] [Google Scholar]
- 24.Flood A, Velie EM, Sinha R, Chaterjee N, Lacey JV, Jr., Schairer C, Schatzkin A. Meat, fat, and their subtypes as risk factors for colorectal cancer in a prospective cohort of women. Am J Epidemiol 2003;158: 59–68. [DOI] [PubMed] [Google Scholar]
- 25.Brink M, Weijenberg MP, De Goeij AF, Schouten LJ, Koedijk FD, Roemen GM, Lentjes MH, De Bruine AP, Goldbohm RA, Van Den Brandt PA. Fat and K-ras mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis 2004;25: 1619–28. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Zhang SM, Cook NR, Lee IM, Buring JE. Dietary fat and fatty acids and risk of colorectal cancer in women. Am J Epidemiol 2004;160: 1011–22. [DOI] [PubMed] [Google Scholar]
- 27.Oba S, Shimizu N, Nagata C, Shimizu H, Kametani M, Takeyama N, Ohnuma T, Matsushita S. The relationship between the consumption of meat, fat, and coffee and the risk of colon cancer: a prospective study in Japan. Cancer Lett 2006;244: 260–7. [DOI] [PubMed] [Google Scholar]
- 28.Weijenberg MP, Luchtenborg M, de Goeij AF, Brink M, van Muijen GN, de Bruine AP, Goldbohm RA, van den Brandt PA. Dietary fat and risk of colon and rectal cancer with aberrant MLH1 expression, APC or KRAS genes. Cancer Causes Control 2007;18: 865–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MN, Chavarro JE, Lee IM, Willett WC, Ma J. A 22-year prospective study of fish, n-3 fatty acid intake, and colorectal cancer risk in men. Cancer Epidemiol Biomarkers Prev 2008;17: 1136–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniel CR, McCullough ML, Patel RC, Jacobs EJ, Flanders WD, Thun MJ, Calle EE. Dietary intake of omega-6 and omega-3 fatty acids and risk of colorectal cancer in a prospective cohort of U.S. men and women. Cancer Epidemiol Biomarkers Prev 2009;18: 516–25. [DOI] [PubMed] [Google Scholar]
- 31.Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, Yang G, Cai H, Wen W, Gao YT, Zheng W. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev 2009;18: 2283–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee SA, Shu XO, Yang G, Li H, Gao YT, Zheng W. Animal origin foods and colorectal cancer risk: a report from the Shanghai Women’s Health Study. Nutr Cancer 2009;61: 194–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, Yamaji T, Takachi R, Tsugane S, Japan Public Health Center-Based Prospective Study G. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer 2011;129: 1718–29. [DOI] [PubMed] [Google Scholar]
- 34.Key TJ, Appleby PN, Masset G, Brunner EJ, Cade JE, Greenwood DC, Stephen AM, Kuh D, Bhaniani A, Powell N, Khaw KT. Vitamins, minerals, essential fatty acids and colorectal cancer risk in the United Kingdom Dietary Cohort Consortium. Int J Cancer 2012;131: E320–5. [DOI] [PubMed] [Google Scholar]
- 35.Kantor ED, Lampe JW, Peters U, Vaughan TL, White E. Long-chain omega-3 polyunsaturated fatty acid intake and risk of colorectal cancer. Nutr Cancer 2014;66: 716–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song M, Chan AT, Fuchs CS, Ogino S, Hu FB, Mozaffarian D, Ma J, Willett WC, Giovannucci EL, Wu K. Dietary intake of fish, omega-3 and omega-6 fatty acids and risk of colorectal cancer: A prospective study in U.S. men and women. Int J Cancer 2014;135: 2413–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraja B, Muka T, Ruiter R, de Keyser CE, Hofman A, Franco OH, Stricker BH, Kiefte-de Jong JC. Dietary Fiber Intake Modifies the Positive Association between n-3 PUFA Intake and Colorectal Cancer Risk in a Caucasian Population. J Nutr 2015;145: 1709–16. [DOI] [PubMed] [Google Scholar]
- 38.Song M, Nishihara R, Wu K, Qian ZR, Kim SA, Sukawa Y, Mima K, Inamura K, Masuda A, Yang J, Fuchs CS, Giovannucci EL, et al. Marine omega-3 polyunsaturated fatty acids and risk of colorectal cancer according to microsatellite instability. J Natl Cancer Inst 2015;107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song M, Nishihara R, Cao Y, Chun E, Qian ZR, Mima K, Inamura K, Masugi Y, Nowak JA, Nosho K, Wu K, Wang M, et al. Marine omega-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA Oncol 2016;2: 1197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Navarro SL, Neuhouser ML, Cheng TD, Tinker LF, Shikany JM, Snetselaar L, Martinez JA, Kato I, Beresford SA, Chapkin RS, Lampe JW. The Interaction between Dietary Fiber and Fat and Risk of Colorectal Cancer in the Women’s Health Initiative. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodge AM, Williamson EJ, Bassett JK, MacInnis RJ, Giles GG, English DR. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int J Cancer 2015;137: 1224–34. [DOI] [PubMed] [Google Scholar]
- 42.Aglago EK, Huybrechts I, Murphy N, Casagrande C, Nicolas G, Pischon T, Fedirko V, Severi G, Boutron-Ruault MC, Fournier A, Katzke V, Kuhn T, et al. Consumption of Fish and Long-chain n-3 Polyunsaturated Fatty Acids Is Associated With Reduced Risk of Colorectal Cancer in a Large European Cohort. Clin Gastroenterol Hepatol 2020;18: 654–66 e6. [DOI] [PubMed] [Google Scholar]
- 43.Shin A, Cho S, Sandin S, Lof M, Oh MY, Weiderpass E. Omega-3 and −6 Fatty Acid Intake and Colorectal Cancer Risk in Swedish Women’s Lifestyle and Health Cohort. Cancer Res Treat 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernstein H, Bernstein C, Payne CM, Dvorakova K, Garewal H. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res 2005;589: 47–65. [DOI] [PubMed] [Google Scholar]
- 45.Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology 2015;148: 1244–60 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McEntee MF, Whelan J. Dietary polyunsaturated fatty acids and colorectal neoplasia. Biomed Pharmacother 2002;56: 380–7. [DOI] [PubMed] [Google Scholar]
- 47.Kim Y, Kim J. Intake or Blood Levels of n-3 Polyunsaturated Fatty Acids and Risk of Colorectal Cancer: A Systematic Review and Meta-analysis of Prospective Studies. Cancer Epidemiol Biomarkers Prev 2020;29: 288–99. [DOI] [PubMed] [Google Scholar]
- 48.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr 2004;79: 935–45. [DOI] [PubMed] [Google Scholar]
- 49.Chulada PC, Thompson MB, Mahler JF, Doyle CM, Gaul BW, Lee C, Tiano HF, Morham SG, Smithies O, Langenbach R. Genetic disruption of Ptgs-1, as well as Ptgs-2, reduces intestinal tumorigenesis in Min mice. Cancer Res 2000;60: 4705–8. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
SMHS data used in this research project will be available following the study data sharing policy posted online at: https://swhs-smhs.app.vumc.org/smhs_index.php.


