Abstract
Background
A history of pre-administration of immune checkpoint inhibitors has been reported to be associated with good outcomes of ramucirumab (RAM) plus docetaxel (DOC) combination therapy for advanced non-small-cell lung cancer (NSCLC). However, existing knowledge on the clinical significance of RAM and DOC following combined chemoimmunotherapy is limited. Therefore, we evaluated the efficacy and safety of RAM plus DOC therapy after combined chemoimmunotherapy and attempted to identify the predictors of its outcomes.
Patients and Methods
This multicenter, prospective study investigated the efficacy and safety of RAM plus DOC after combined chemoimmunotherapy. The primary endpoint was progression-free survival (PFS). Secondary endpoints were the objective response rate (ORR), disease control rate (DCR), overall survival (OS), and incidence of adverse events. An exploratory analysis measured serum cytokine levels at the start of treatment.
Results
Overall, 44 patients were enrolled from 10 Japanese institutions between April 2020 and June 2022. The median PFS and OS were 6.3 and 22.6 months, respectively. Furthermore, the ORR and DCR were 36.4% and 72.7%, respectively. The high vascular endothelial growth factor D (VEGF-D) group had a significantly shorter PFS and OS. A combination of high VEGF-A and low VEGF-D levels was associated with a longer PFS.
Conclusion
Our results showed that RAM plus DOC after combined chemoimmunotherapy might be an effective and relatively feasible second-line treatment for patients with advanced NSCLC in a real-world setting.
Keywords: ramucirumab, docetaxel, non-small-cell lung carcinoma, combined drug therapy, VEGF-A, VEGF-D
This study investigated the efficacy, safety, and predictive biomarkers of ramucirumab plus docetaxel as a second‐line therapy after combined chemoimmunotherapy in a real‐world setting.
Implications for Practice.
The application of combined chemoimmunotherapy exhibits improved outcomes for patients with advanced non-small-cell lung cancer (NSCLC); however, preferred options for subsequent treatment of patients with progressive disease remain to be established. In this prospective observational study, we show that ramucirumab (RAM) plus docetaxel (DOC) is an effective and relatively safe second-line treatment after combined chemoimmunotherapy for advanced NSCLC. Moreover, a combination of serum vascular endothelial growth factors A and D may be a potent biomarker for predicting the efficacy of RAM plus DOC.
Introduction
Lung cancer has become the leading cause of cancer-related deaths.1 The clinical development of immune checkpoint inhibitors (ICIs) has drastically improved the therapeutic outcome of advanced lung cancer.2-4 Moreover, novel combination therapies, such as combined chemoimmunotherapy, have recently emerged as standard treatments for patients with advanced non-small-cell lung cancer (NSCLC), replacing platinum-based chemotherapy.5-7 However, treatment options after combined chemoimmunotherapy pose major clinical challenges. Ramucirumab (RAM) is a human recombinant IgG1 monoclonal antibody that specifically targets the vascular endothelial growth factor (VEGF) receptor-2.8 In the REVEL phase III clinical trial, the effectiveness of RAM plus docetaxel (DOC) combination therapy was compared with that of DOC monotherapy in patients with advanced NSCLC who experienced disease progression after receiving platinum-based chemotherapy. The findings revealed that RAM plus DOC significantly improved progression-free survival (PFS), overall survival (OS), and objective response rate (ORR) compared to DOC monotherapy.9 RAM plus DOC is considered one of the standard second-line treatments for advanced NSCLC and has been approved in several countries, including the US and Japan.
VEGF modulates the tumor immune microenvironment by suppressing dendritic cell maturation and decreasing the number of CD4 + and CD8 + T cells.10,11 Alternatively, anti-angiogenic drugs reprogram the tumor milieu from an immunosuppressive to an immune permissive microenvironment.
Numerous clinical trials have demonstrated the potential for enhanced anticancer efficacy by combining anti-angiogenic agents with ICIs across various cancer types.10 The addition of atezolizumab to a combination of anti-VEGF antibody bevacizumab and chemotherapy is effective in NSCLC treatment, including epidermal growth factor receptor (EGFR)-positive lung cancer and liver metastases, owing to its synergy as a combined therapy of anti-angiogenic agents and ICIs.6
Previous studies have found that consecutive RAM plus DOC after ICI treatment is more effective than nonconsecutive RAM plus DOC after ICIs.12-15 Moreover, the measured half-life of anti-programmed cell death ligand 1 (PD-L1) antibody nivolumab has been reported to be 12-20 days, and the pharmacodynamic effects of programmed cell death protein 1 (PD-1) receptor occupancy were even more prolonged at 85 days.16 Therefore, a pseudo-combined effect of anti-angiogenic agents and PD-1 blockade by RAM plus DOC after ICI treatment may be expected.
Given that combined chemoimmunotherapy has become a standard first-line treatment for advanced NSCLC, validating the efficacy of RAM plus DOC after combined chemoimmunotherapy is crucial. Additionally, no promising predictive biomarkers currently exist for identifying patients with NSCLC who are more likely to benefit from RAM treatment. Therefore, to address these issues, this study investigated the efficacy, safety, and predictive biomarkers of RAM plus DOC as a second‐line therapy after combined chemoimmunotherapy in a real‐world setting.
Materials and Methods
Patients
We prospectively enrolled patients with advanced or recurrent NSCLC who provided written informed consent and were treated with combination chemoimmunotherapy at 8 institutions in Japan (the Kyoto Prefectural University of Medicine, Niigata University Graduate School of Medical and Dental Sciences, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Japanese Red Cross Kyoto Daini Hospital, Uji-Tokushukai Medical Center, Japanese Red Cross Kyoto Daiichi Hospital, Shonan Fujisawa Tokushukai Hospital, and Fukuchiyama City Hospital) between April 2020 and June 2022. The inclusion criteria were as follows: (i) histologically and cytologically confirmed unresectable advanced or recurrent NSCLC and (ii) previously treated with combined chemoimmunotherapy. Patients with systemic chemotherapy or immunotherapy history after combination chemoimmunotherapy were excluded. All patients were followed up from the start of treatment until November 2021. The study protocol was approved by the Ethics Committee of the University Hospital, Kyoto Prefectural University of Medicine (ERB-C-1649) and was conducted in accordance with the tenets of the Declaration of Helsinki. The study protocol was registered with the University Medical Hospital Information Network (UMIN) Clinical Trials Registry (UMIN 000044807). All patients provided written informed consent before enrollment.
Assessments of Serum VEGF-A, VEGF-D, and Soluble PD‐L1
Blood serum samples were collected from each participant at the start of DOC plus RAM and stored at –80 °C. Serum concentrations of VEGF-A and VEGF-D were measured using human VEGF-A or VEGF-D enzyme-linked immunosorbent assay (ELISA) kits (RayBiotech, Inc., Norcross, GA, USA). sPD-L1 serum concentrations were measured using a Human/Cynomolgus Monkey PD‐L1/B7‐H1 ELISA kit (RayBiotech, Inc.). The baseline cutoff values for VEGF-A and VEGF-D were set at the median values. We defined the sPD‐L1 cutoff value as 90 pg/mL based on previous studies.17
Sample Size Calculation
In the REVEL study, the median PFS was reported to be 4.5 months (95% CI: 4.2-5.4 months).9 In several retrospective studies comparing ICI pre-treatment followed by RAM plus DOC (ICI pre-treatment) with chemotherapy pre-treatment alone (ICI naïve), the median PFS for ICI pre-treatment was 5.1, 5.9, 5.7, and 5.7 months, while that for ICI naïve was 3.8, 2.6, 4.1, and 2.3 months.12-15 Based on these assumed PFS values and considering these data to be real-world data, including ineligible patients for clinical trials, such as patients with poor performance status (PS) and central nervous system metastases, PFS for RAM plus DOC after combined chemoimmunotherapy and RAM plus DOC with chemotherapy pre-treatment alone were assumed to be 6.0 and 3.0 months, respectively, with significance level and power of 5% (2 sided) and 80%, respectively, making the number of required patients 44.
Statistical Analysis
PFS and OS were calculated using the Kaplan-Meier method, and differences were compared using the log-rank test. When the lower limit of 95% confidence interval (CI) was above the threshold of PFS at 3.0 months, PFS was considered statistically significantly superior to the threshold value. The ORR values were presented with 95% CIs using the Wilson method. Regarding safety, adverse events (AEs) were summarized using the Common Terminology Criteria for Adverse Events version 5.0 grade. For univariate analysis, hazard ratios (HRs) and 95% CIs were estimated using Cox proportional hazards models; OS and PFS were censored at the date of last survival confirmation for patients who had no documented disease progression and were alive. Based on previous reports, the Eastern Cooperative Oncology Group performance status (ECOG-PS; ≥2), sex, and age (≥75 years) were selected as covariates.18 Statistical analyses were performed using EZR statistical software version 1.40.19
Results
Patient Characteristics
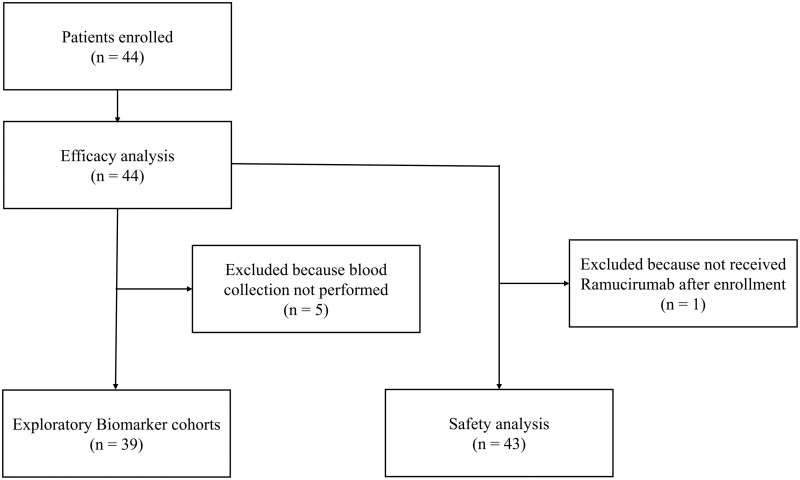
Forty-four patients were enrolled from 8 institutions in Japan from April 2020 to June 2022 in this study. One patient did not receive RAM owing to gastrointestinal hemorrhage (Fig. 1).
Figure 1.
CONSORT diagram of the study. CONSORT, Consolidated Standards of Reporting Trials.
The median (range) age was 69 (39-79) years; 30 (68.2%) patients were males, 34 (77.3%) had a smoking history, and most (93.2%) had an ECOG-PS of 0 or 1. The histological subtypes included adenocarcinoma (n = 31; 70.5%) and squamous cell carcinoma (n = 8; 18.2%). The PD-L1 tumor proportion score was ≥ 50%, 1%–49%, and < 1% in 9 (20.5%), 19 (43.2%), and 16 (36.4%) patients, respectively. Six (13.6%) patients had EGFR mutations, and none had anaplastic lymphoma kinase fusion. Except for one patient with EGFR exon20ins, 5 were treated with EGFR-TKIs before combined chemoimmunotherapy. The median time from the last dose of combined chemoimmunotherapy to the start of RAM plus DOC was 32.5 (14-505) days. The median PFS of previously combined chemoimmunotherapy was 8.0 months (95% CI: 6.2-9.7). Disease progression was the most common reason for discontinuing combined chemoimmunotherapy (86.4%; Table 1).
Table 1.
Patient characteristics.
| Characteristics | All patients |
|---|---|
| (n = 44) | |
| Age | |
| Median (range) | 69 (39-79) |
| Sex | |
| Male | 30 (68.2%) |
| Female | 14 (31.8%) |
| ECOG-performance status | |
| 0 | 17 (38.6%) |
| 1 | 24 (54.6%) |
| 2 | 3 (6.8%) |
| Stage | |
| III/IV | 41 (93.2%) |
| Recurrence | 3 (6.8%) |
| Oncogenic driver | |
| EGFR mutation positivity | 6 (13.6%) |
| ALK rearranged positivity | 0 (0.0%) |
| Smoking status | |
| Current/former | 34 (77.3%) |
| Never | 10 (22.7%) |
| Histology | |
| Adeno | 31 (70.5%) |
| Squamous | 8 (18.2%) |
| Others | 5 (11.4%) |
| PD-L1 status | |
| <1% | 16 (36.4%) |
| 1-49% | 19 (43.2%) |
| ≥50% | 9 (20.5%) |
| Metastasis sites | |
| Brain | 13 (19.6%) |
| Liver | 3 (6.8%) |
| Bone | 15 (34.1%) |
| Interval from combined chemoimmunotherapy to RAM + DOC, days | |
| Median (range) | 32.5 (14-505) |
| Previous combined chemoimmunotherapy regimen | |
| Platinum + pemetrexed + pembrolizumab | 22 (50.0%) |
| Platinum + paclitaxel/nab-paclitaxel + pembrolizumab | 8 (18.2%) |
| Platinum + paclitaxel/pemetrexed + bevacizumab + atezolizumab | 9 (20.5%) |
| Platinum + pemetrexed + atezolizumab | 2 (4.5%) |
| Platinum + paclitaxel/nab-paclitaxel + atezolizumab | 1 (2.3%) |
| Platinum + pemetrexed + ipilimumab + nivolumab | 2 (4.5%) |
| Reasons for discontinuation of combined chemoimmunotherapy | |
| Due to progression disease | 38 (86.4%) |
| Due to adverse event | 6 (13.6%) |
Efficacy of RAM Plus DOC After Combined Chemoimmunotherapy
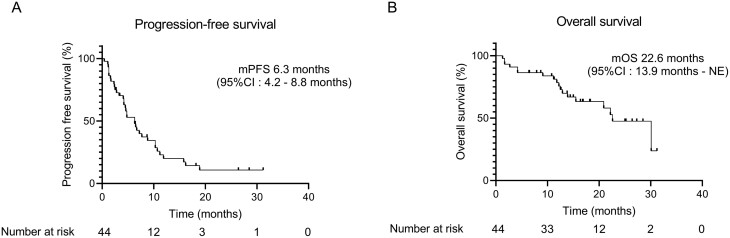
The median follow-up time was 13.9 months. Among the 44 patients with lung adenocarcinoma, 35 had disease progression, and 18 died by the cutoff date. The median PFS was 6.3 months (95% CI: 4.2-8.8). This treatment protocol surpassed the threshold value (≥3.0 months of the lower limit value of the 95% CIs), which met the primary endpoint (Fig. 2A).
Figure 2.
Progression-free survival (A) and overall survival (OS) (B) of patients treated with ramucirumab (RAM) plus docetaxel (DOC) after combined chemoimmunotherapy.
The median OS was 22.6 months (95% CI: 13.9-NE) (Fig. 2B). The ORR and disease control rate were 36.4% (95% CI: 22.4-52.2) and 72.7% (95% CI: 57.2-85.0), respectively. The overall responses were partial response, stable disease, progressive disease, and not assessable in 16 (36.4%), 16 (36.4%), 8 (18.2%), and 4 (9.1%) patients, respectively; no patients exhibited complete response (0.0%; Supplementary Table S1). The analysis excluding patients with EGFR-positive lung cancer also showed similar efficacy with PFS and OS of 6.6 (95% CI: 4.2-10.2) and 22.2 (95% CI: 12.7-NE) months, respectively (Supplementary Fig. S1).
Effect of Serum Biomarkers on Clinical Outcomes of RAM Plus DOC
Next, we investigated the effects of serum VEGF-A, VEGF-D, and sPD-L1 levels on the outcomes of RAM plus DOC combination therapy as an exploratory analysis. Among the 44 enrolled patients, serum VEGF-A, VEGF-D, and sPD-L1 levels were measured at the initiation of DOC plus RAM treatment in 39 patients (Fig. 1). The median (range) VEGF-A, VEGF-D, and sPD-L1 levels were 161 (14-710), 968 (194-2571), and 65 (37-2587) pg/mL, respectively. Regarding serum cytokines, the VEGF-D high group (n = 19) had significantly shorter PFS and OS (4.5 vs. 6.3 months; P = .04, 15.5 vs. 30.1 months; P = .002, respectively) than the VEGF-D low group (n = 20; Fig. 3).
Figure 3.
Progression-free survival (PFS) and overall survival (OS) based on the level of serum vascular endothelial growth factor (VEGF)-A, VEGF-D, and soluble programmed cell death ligand 1 (sPD-L1). PFS and OS analyses were performed by stratifying the VEGF-A, VEGF-D, and sPD-L1 levels. Comparison of Kaplan-Meier curves of PFS and OS between patients with VEGF-A (A, B), VEGF-D (C, D), and sPD-L1 (E, F).
Serum levels of VEGF-A and sPD-L1 showed no significant differences regarding PFS and OS. ORR was significantly higher in the VEGF-A high group than in the VEGF-A low group (47.4% [95% CI: 24.4%–71.1%] vs. 15.0% [95% CI: 3.2-37.9%], P = .04) (Supplementary Table S2).
In the univariate analysis, high VEGF-D levels were significantly correlated with shortened PFS and OS (HR, 2.17, 95% CI: 1.02-4.62, P = .04; and HR, 4.94, 95% CI: 1.60-15.3, P = .005, respectively). In the multivariate analysis, high VEGF-D levels were independently correlated with shortened PFS and OS (HR, 2.64, 95% CI: 1.15-6.03, P = .02; and HR, 7.25, 95% CI: 1.95-26.9, P = .003, respectively; Table 2).
Table 2.
Univariate and multivariate Cox hazard model for progression-free survival and overall survival of RAM + DOC.
| Items | Progression-free survival | Overall survival | ||
|---|---|---|---|---|
| (comparator) | Univariate | Multivariate | Univariate | Multivariate |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| P-value | P-value | P-value | P-value | |
| VEGF-A high | 0.68 (0.34-1.37) | 1.57 (0.59-4.16) | ||
| (vs. VEGF-A low) | P = .28 | P = .36 | ||
| VEGF-D high | 2.17 (1.02-4.62) | 2.64 (1.15-6.03) | 4.94 (1.60-15.3) | 7.25 (1.95-26.9) |
| (vs. VEGF-D low) | P = .04 | P = .02 | P = .005 | P = .003 |
| sPD-L1 high | 1.72 (0.84-3.50) | 1.83 (0.71-4.77) | ||
| (vs. sPD-L1 low) | P = .14 | P = .21 | ||
| Age ≥ 75 | 0.34 (0.05-2.51) | 0.21 (0.03-1.63) | 5.03 (0.99–25.3) | 4.69 (0.88-24.8) |
| (vs. < 75) | P = .29 | P = .14 | P = .05 | P = .07 |
| Male sex | 1.37 (0.62-3.01) | 1.53 (0.64-3.70) | 1.10 (0.40-3.06) | 0.50 (0.15-1.63) |
| (vs. female sex) | P = .43 | P = .34 | P = .85 | P = .25 |
| ECOG-PS 2 | 1.38 (0.32-5.89) | 2.50 (0.49-12.8) | 1.58 (0.32-7.90) | 2.61 (0.41-16.7) |
| (vs. 0,1) | P = .66 | P = .27 | P = .58 | P = .31 |
| Smoker | 1.01 (0.41-2.48) | 0.62 (0.20-1.92) | ||
| (vs. never smoker) | P = .99 | P = .41 | ||
| Adeno | 0.72 (0.30-1.73) | 1.10 (0.31-3.84) | ||
| vs. non-Adeno | P = .47 | P = .88 | ||
| PD-L1 ≥ 50% | 0.54 (0.19-1.56) | 0.31 (0.04-2.32) | ||
| (vs. < 50%) | P = .26 | P = .25 | ||
| EGFR mutation positive | 1.56 (0.59-4.12) | 0.86 (0.20-3.75) | ||
| (vs. all others) | P = .37 | P = .83 | ||
| Liver metastasis | 2.77 (0.63-12.2) | 2.78 (0.33-23.1) | ||
| (vs. non liver metastasis) | P = .18 | P = .34 | ||
| Brain metastasis | 1.09 (0.50-2.37) | 1.01 (0.32-3.13) | ||
| (vs. non brain metastasis) | P = .84 | P = .99 | ||
| Bone metastasis | 1.50 (0.73-3.01) | 1.02 (0.38-2.77) | ||
| (vs. non bone metastasis) | P = .37 | P = .96 | ||
| Bevacizumab administration | 1.49 (0.64-3.48) | 1.24 (0.40-3.80) | ||
| (vs. non Bevacizumab administration) | P = .36 | P = .71 | ||
| Interval from combined chemoimmunotherapy to RAM + DOC ≥ 60 days | 1.98 (0.83-4.69) | 0.73 (0.21-2.56) | ||
| (vs. < 60 days) | P = .12 | P = .62 | ||
| PFS of combined chemoimmunotherapy > 8.8 months | 0.58 (0.28–1.17) | 0.59 (0.23-1.53) | ||
| (vs. < 8.8 months) | P = .13 | P = .28 | ||
| discontinuation of combined chemoimmunotherapy due to progression disease | 1.14 (0.40-3.29) | 2.70 (0.36-20.5) | ||
| (vs. due to adverse event) | P = .80 | P = .34 | ||
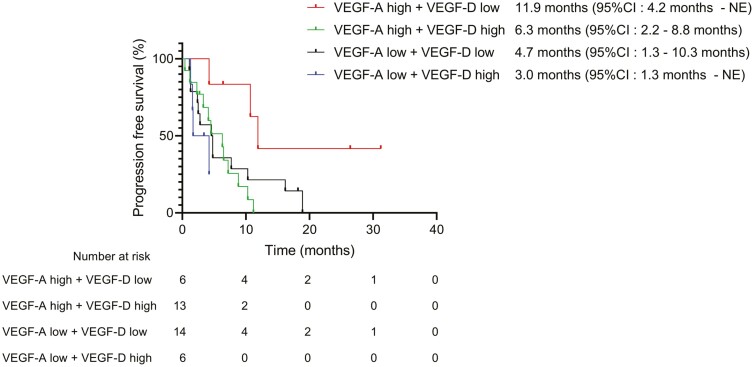
We also analyzed the relationship between VEGF-D levels and patient characteristics. The high VEGF-D group had a significantly higher incidence of high VEGF-A levels than the low VEGF-D group (P = .03; Supplementary Table S3). The levels of VEGF-D were positively correlated with those of VEGF-A (r = 0.41, P = .01; Supplementary Fig. S2). We further evaluated the predictive value of the combination of VEGF-A and VEGF-D levels. The PFS of patients in the high VEGF-A + low VEGF-D group was significantly longer than that of those in the other groups (high VEGF-A + low VEGF-D vs. low VEGF-A + high VEGF-D: P = .01; high VEGF-A + low VEGF-D vs. low VEGF-A + low VEGF-D: P = .04, high VEGF-A + low VEGF-D vs. high VEGF-A + high VEGF-D: P = .006; Fig. 4). These results suggest that a combination of pre-treatment serum levels of VEGF-A and VEGF-D is a predictive factor for identifying responders to RAM plus DOC treatment.
Figure 4.
Progression-free survival (PFS) based on the combination of vascular endothelial growth factor (VEGF)-A and VEGF-D.
Safety
A safety analysis was performed on 43 patients who received at least 1 dose of RAM plus DOC (Fig. 1). All AE grades and grades ≥ 3 were observed in 38 (88.4%) and 18 (41.9%) patients, respectively. Grades ≥ 3 neutropenia and febrile neutropenia were observed in 8 (18.6%) and 2 (4.7%) patients, respectively. All grades and grades ≥ 3 pneumonitis were observed in 4 (9.3%) and 3 (7.0%) patients, respectively. No grade 5 AE was observed (Table 3).
Table 3.
Safety analysis.
| All grade | Grade 3 or higher | |||
|---|---|---|---|---|
| Number of cases | % | Number of cases | % | |
| N | 43 | |||
| All adverse events | 38 | 88.4 | 18 | 41.9 |
| Neutropenia | 13 | 30.2 | 8 | 18.6 |
| Stomatitis | 5 | 11.6 | 0 | 0.0 |
| Thrombocytopenia | 5 | 11.6 | 0 | 0.0 |
| Interstitial lung disease | 4 | 9.3 | 3 | 7.0 |
| Leg edema | 4 | 9.3 | 0 | 0.0 |
| Paronychia | 3 | 4.7 | 1 | 2.3 |
| Febrile neutropenia | 2 | 4.7 | 2 | 4.7 |
| Intracranial hemorrhage | 2 | 4.7 | 2 | 4.7 |
| AST ALT increased | 2 | 4.7 | 1 | 2.3 |
| Hypertension | 2 | 4.7 | 0 | 0.0 |
| Nausea | 2 | 4.7 | 0 | 0.0 |
| Proteinuria | 2 | 4.7 | 0 | 0.0 |
| Dysgeusia | 2 | 4.7 | 0 | 0.0 |
| Alopecia | 2 | 4.7 | 0 | 0.0 |
| Peripheral neuropathy | 2 | 4.7 | 0 | 0.0 |
| Pneumothorax | 1 | 2.3 | 1 | 2.3 |
| General edema | 1 | 2.3 | 1 | 2.3 |
| Hyponatraemia | 1 | 2.3 | 1 | 2.3 |
| Fracture | 1 | 2.3 | 1 | 2.3 |
| Anorexia | 1 | 2.3 | 1 | 2.3 |
| Malaise | 1 | 2.3 | 1 | 2.3 |
| Gastrointestinal hemorrhage | 1 | 2.3 | 1 | 2.3 |
| Epistaxis | 1 | 2.3 | 0 | 0.0 |
| Anaemia | 1 | 2.3 | 0 | 0.0 |
| Bloody stools | 1 | 2.3 | 0 | 0.0 |
| Constipation | 1 | 2.3 | 0 | 0.0 |
| Dyspnoea | 1 | 2.3 | 0 | 0.0 |
Discussion
This prospective observational study demonstrated that combined chemoimmunotherapy with RAM plus DOC was effective for patients with NSCLC. Further, the PFS of RAM plus DOC after combined chemoimmunotherapy was 6.3 months (95% CI: 4.2-8.8), which met the primary endpoint. ORR for RAM plus DOC after combined chemoimmunotherapy was 36.4% (95% CI: 22.4-52.2). A recently reported multicenter phase II prospective study on RAM plus DOC after combined immunotherapy showed similar results with PFS and ORR of 6.5 months and 34.4%, respectively.20 These results were considered better than those of the REVEL trial and the phase II study of RAM plus DOC in Japanese patients (with PFS and ORR of 4.5 months and 22.9%, and 5.22 months and 28.9%, respectively),9,21 suggesting that the synergistic effects of RAM and history of ICIs pre-administration are the basis for the high efficacy of RAM plus DOC after combined immunotherapy. However, a previous retrospective study that evaluated RAM plus DOC immediately after PD-1 blockade plus platinum-based chemotherapy reported a median PFS and ORR of 4.1 months and 28.8%, respectively, which was inferior to our results.18 A possible explanation for this result could be the inclusion of many vulnerable patients with poor PS. In safety assessment, interstitial pneumonia and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) levels increased for grades ≥ 3 (7.0% and 2.3%, respectively) tended to be more frequent than those reported to have occurred in a RAM plus DOC prospective trial conducted in Japan (2.6% and 1.3%, respectively). A retrospective study of RAM plus DOC after combined chemoimmunotherapy found that pneumonitis and AST/ALT levels increased for grades ≥ 3 in 4.9% and 3.1% of the cases, respectively, consistent with the findings of this study.18 The delayed appearance of immune-related AEs due to previously combined chemoimmunotherapy may lead to more interstitial pneumonia and an increase in AST/ALT levels. Previous retrospective studies have reported that patients treated with RAM plus DOC after ICI had more AEs, particularly diarrhea, peripheral neuropathy, fever, myalgia, arthritis, pleural effusions, and pneumonia, than those treated with RAM plus DOC ICI naïve.13 Therefore, it may be necessary to exercise caution regarding AEs, such as pneumonitis, associated with the combination of DOC and RAM, particularly in patients with prior ICI treatment in a real-world setting. Further large-scale investigations of the safety of this approach are warranted. Overall, based on these results and our findings, RAM plus DOC may be an effective and relatively feasible second-line treatment for patients with advanced NSCLC who received combined chemoimmunotherapy.
The VEGF protein family is the most critical factor in the induction of neovascularization. Additionally, VEGF has been identified as a prognostic factor in various types of cancers. VEGF-A and its receptor VEGFR2 have major angiogenic effects. VEGF-D contributes to tumor angiogenesis and lymphangiogenesis by binding to VEGFR2 and VEGFR3.8 Elevated levels of VEGF-A and VEGF-D are associated with poor prognosis in cases of lung, colorectal, breast, and ovarian cancers.22-25 Furthermore, numerous blood cytokines have been documented as potent predictors of ICIs or angiogenesis inhibitors.26,27 Serum VEGF-A levels are reported as biomarkers of bevacizumab efficacy in breast cancer metastatic duodenal and jejunal adenocarcinoma.28,29 The RAISE study evaluating colorectal cancer showed that high plasma VEGF-D levels were associated with improved PFS and OS in patients with colorectal cancer treated with RAM + FOLFIRI.30 However, no predictive biomarkers currently exist for identifying patients with NSCLC who are more likely to benefit from RAM-containing treatments. Therefore, this study evaluated baseline serum VEGF-A and VEGF-D levels to identify potential RAM biomarkers. The serum-soluble form of PD‐L1 (sPD‐L1), which can bind to PD‐1 receptors and may play an important role in immunoregulation,17 was also evaluated. To the best of our knowledge, this is the first prospective real-world study to assess pre-treatment biomarkers of RAM plus DOC in patients with NSCLC after treatment with combined chemoimmunotherapy. Therefore, clinical research efforts aimed at identifying biomarkers that can predict treatment outcomes are crucial, given their potential to evolve into the basis of personalized medicine in the future.
In this study, the combination of high VEGF-A and low VEGF-D levels served as a predictive marker of the efficacy of RAM plus DOC. Notably, despite the positive correlation between VEGF-A and VEGF-D levels, VEGF-A was positively correlated with RAM plus DOC efficacy, whereas VEGF-D was negatively correlated. This discrepancy may be attributed to the different binding receptors of VEGF-A and VEGF-D. VEGF-A primarily binds to VEGFR2, promoting angiogenesis; in contrast, VEGF-D binds to both VEGFR2 and VEGFR3, exerting various tumor-promoting effects, such as tumor growth, angiogenesis, lymphangiogenesis, metastasis, and immunosuppression.31 Furthermore, the affinity of VEGF-D for VEGFR3 is reportedly higher than that for VEGFR2.32 Although RAM suppresses VEGFR-2 signaling activated by both VEGF-A and VEGF-D, VEGF-D may contribute to a poor prognosis, mainly by binding to VEGFR3 and inducing lymphangiogenesis and immunosuppression. These findings contradict the results of the RAISE trial, which might have been influenced by the fact that all patients in that study received bevacizumab as a first-line therapy. Therefore, further studies are needed to validate these hypotheses and determine the impact of VEGF-A and VEGF-D on the efficacy of angiogenesis inhibitors in advanced NSCLC.
This study has some limitations. First, the sample size was small. Second, we included only Japanese patients. Third, the follow-up period was relatively short and insufficient to evaluate the OS data. Fourth, the biomarker analyses were performed as exploratory studies. Some of the analyses, including univariate and multivariate analyses, were performed retrospectively; therefore, further prospective investigations are required to validate these findings. Finally, a selection bias may have occurred because only patients with NSCLC were treated with DOC plus RAM after first-line chemotherapy.
Conclusion
Our prospective study demonstrated that RAM plus DOC combined therapy might be a useful and relatively safe second-line treatment after combined chemoimmunotherapy for advanced NSCLC in the real-world setting. The combination of serum VEGF-A and VEGF-D may be a biomarker for predicting the efficacy of RAM plus DOC.
Supplementary Material
Supplementary material is available at The Oncologist online.
Acknowledgments
We thank the patients, their families, and all the investigators involved in this study. We also thank Editage (www.editage.com) for their help with English language editing. This work was supported by research grants from the Research Grant of the Princess Takamatsu Cancer Research Fund (to T. Yamada) and JSPS KAKENHI (grant no. 23K07607 to T. Yamada).
Contributor Information
Yuki Katayama, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Tadaaki Yamada, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Ryo Sawada, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Hayato Kawachi, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Kenji Morimoto, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Satoshi Watanabe, Department of Respiratory Medicine and Infectious Diseases, Niigata University Graduate School of Medical and Dental Sciences, Niigata, Japan.
Kageaki Watanabe, Department of Thoracic Oncology and Respiratory Medicine, Tokyo Metropolitan Cancer and Infectious Diseases Center Komagome Hospital, Tokyo, Japan.
Takayuki Takeda, Department of Respiratory Medicine, Japanese Red Cross Kyoto Daini Hospital, Kyoto, Japan.
Yusuke Chihara, Department of Respiratory Medicine, Uji-Tokushukai Medical Center, Kyoto, Japan.
Shinsuke Shiotsu, Department of Respiratory Medicine, Japanese Red Cross Kyoto Daiichi Hospital, Kyoto, Japan.
Makoto Hibino, Department of Respiratory Medicine, Shonan Fujisawa Tokushukai Hospital, Kanagawa, Japan.
Taishi Harada, Department of Medical Oncology, Fukuchiyama City Hospital, Kyoto, Japan.
Naoya Nishioka, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Masahiro Iwasaku, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Shinsaku Tokuda, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Koichi Takayama, Department of Pulmonary Medicine, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan.
Conflict of Interest
Tadaaki Yamada reports receiving research grants from Pfizer Inc., Ono Pharmaceutical, Janssen Pharmaceutical, AstraZeneca, and Takeda Pharmaceutical, and personal fees from Eli Lilly. Hayato Kawachi received personal fees from Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca KK, Taiho Pharmaceutical Co. Ltd., Eli Lilly Japan KK, and MSD KK, outside the purview of the submitted work. Satoshi Watanabe reports grants from Boehringer Ingelheim and Nippon Kayaku and lecture fees and speakers bureaus from AstraZeneca, Lilly, Chugai Pharma, Bristol-Myers, Novartis Pharma, Ono Pharmaceutical, Daiichi Sankyo, Taiho Pharmaceutical, Nippon Kayaku, Kyowa Hakko Kirin, Merck, Takeda Pharmaceutical and Celltrion, all outside the scope of the submitted work. Kenji Watanabe reports receiving personal fees from AstraZeneca. Koichi Takayama reports receiving research grants from Chugai-Roche and Ono Pharmaceutical and personal fees from AstraZeneca, Chugai-Roche, MSD-Merck, Eli Lilly, Boehringer Ingelheim, and Daiichi Sankyo. The other authors declare no conflicts of interest.
Ethics Approval
The study protocol was approved by the Ethics Committee of the University Hospital, Kyoto Prefectural University of Medicine (ERB-C-1649) and was conducted in accordance with the tenets of the Declaration of Helsinki. All patients provided written informed consent before enrollment.
Permission to Reproduce Material From Other Sources
We have obtained written permission from the copyright owners to reproduce any material owned by third parties.
Registration
The study protocol has been registered with the University Medical Hospital Information Network (UMIN) Clinical Trials Registry (UMIN 000044807).
Author Contributions
Conception/design: Y.K., T.Y., K.T. Provision of study material or patients: T.Y., S.W., K.W., T.T., Y.C., S.S., M.H., T.H. Collection and/or assembly of data: Y.K., T.Y., R.S., H.K., K.M. Data analysis and interpretation: Y.K., N.N., M.I., S.T. Manuscript writing: Y.K., T.Y. Final approval of manuscript: All authors.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. 10.1016/S0140-6736(15)01281-7 [DOI] [PubMed] [Google Scholar]
- 4. Rittmeyer A, Barlesi F, Waterkamp D, et al. ; OAK Study Group. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265. 10.1016/S0140-6736(16)32517-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. ; KEYNOTE-189 Investigators. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 6. Socinski MA, Jotte RM, Cappuzzo F, et al. ; IMpower150 Study Group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301. 10.1056/NEJMoa1716948 [DOI] [PubMed] [Google Scholar]
- 7. Paz-Ares L, Ciuleanu T-E, Cobo M, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198-211. 10.1016/S1470-2045(20)30641-0 [DOI] [PubMed] [Google Scholar]
- 8. Clarke JM, Hurwitz HI.. Targeted inhibition of VEGF receptor 2: an update on ramucirumab. Expert Opin Biol Ther. 2013;13(8):1187-1196. 10.1517/14712598.2013.810717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet. 2014;384(9944):665-673. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 10. Song Y, Fu Y, Xie Q, et al. Anti-angiogenic agents in combination with immune checkpoint inhibitors: a promising strategy for cancer treatment. Front Immunol. 2020;11:1956. 10.3389/fimmu.2020.01956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Katayama Y, Uchino J, Chihara Y, et al. Tumor neovascularization and developments in therapeutics. Cancers (Basel) 2019;11(3):316. 10.3390/cancers11030316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yoshimura A, Yamada T, Okuma Y, et al. Retrospective analysis of docetaxel in combination with ramucirumab for previously treated non-small cell lung cancer patients. Transl Lung Cancer Res 2019;8(4):450-460. 10.21037/tlcr.2019.08.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harada D, Takata K, Mori S, et al. Previous immune checkpoint inhibitor treatment to increase the efficacy of docetaxel and ramucirumab combination chemotherapy. Anticancer Res. 2019;39(9):4987-4993. 10.21873/anticanres.13688 [DOI] [PubMed] [Google Scholar]
- 14. Kato R, Hayashi H, Chiba Y, et al. Propensity score-weighted analysis of chemotherapy after PD-1 inhibitors versus chemotherapy alone in patients with non-small cell lung cancer (WJOG10217L). J ImmunoTher Cancer. 2020;8(1):e000350. 10.1136/jitc-2019-000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tozuka T, Kitazono S, Sakamoto H, et al. Addition of ramucirumab enhances docetaxel efficacy in patients who had received anti-PD-1/PD-L1 treatment. Lung Cancer. 2020;144:71-75. 10.1016/j.lungcan.2020.04.021 [DOI] [PubMed] [Google Scholar]
- 16. Sundar R, Cho BC, Brahmer JR, Soo RA.. Nivolumab in NSCLC: latest evidence and clinical potential. Ther Adv Med Oncol 2015;7(2):85-96. 10.1177/1758834014567470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Murakami S, Shibaki R, Matsumoto Y, et al. Association between serum level soluble programmed cell death ligand 1 and prognosis in patients with non-small cell lung cancer treated with anti-PD-1 antibody. Thorac Cancer 2020;11(12):3585-3595. 10.1111/1759-7714.13721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nakamura A, Yamaguchi O, Mori K, et al. Multicentre real-world data of ramucirumab plus docetaxel after combined platinum-based chemotherapy with programmed death-1 blockade in advanced non-small cell lung cancer: NEJ051 (REACTIVE study). Eur J Cancer. 2023;184:62-72. 10.1016/j.ejca.2023.01.025 [DOI] [PubMed] [Google Scholar]
- 19. Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matsuzawa R, Morise M, Ito K, et al. 46P Multi-center, phase II study of docetaxel (DTX) plus ramucirumab (RAM) following platinum-based chemotherapy plus ICIs in patients with NSCLC: SCORPION study. J Thoracic Oncol. 2023;18(4):S68. 10.1016/S1556-0864(23)00300-3 [DOI] [Google Scholar]
- 21. Yoh K, Hosomi Y, Kasahara K, et al. A randomized, double-blind, phase II study of ramucirumab plus docetaxel vs placebo plus docetaxel in Japanese patients with stage IV non-small cell lung cancer after disease progression on platinum-based therapy. Lung Cancer. 2016;99:186-193. 10.1016/j.lungcan.2016.07.019 [DOI] [PubMed] [Google Scholar]
- 22. Jung WY, Min KW, Oh YH.. Increased VEGF-A in solid type of lung adenocarcinoma reduces the patients’ survival. Sci Rep. 2021;11(1):1321. 10.1038/s41598-020-79907-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeh CC, Shih L-J, Chang J-L, et al. Synchronous vascular endothelial growth factor protein profiles in both tissue and serum identify metastasis and poor survival in colorectal cancer. Sci Rep. 2019;9(1):4228. 10.1038/s41598-019-40862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sopo M, Anttila M, Hämäläinen K, et al. Expression profiles of VEGF-A, VEGF-D and VEGFR1 are higher in distant metastases than in matched primary high grade epithelial ovarian cancer. BMC Cancer 2019;19(1):584. 10.1186/s12885-019-5757-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Tamimi RM, Collins LC, et al. The association between vascular endothelial growth factor expression in invasive breast cancer and survival varies with intrinsic subtypes and use of adjuvant systemic therapy: results from the Nurses’ Health Study. Breast Cancer Res Treat. 2011;129(1):175-184. 10.1007/s10549-011-1432-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hu Y, Li S, Xiao H, et al. Distinct circulating cytokine/chemokine profiles correlate with clinical benefit of immune checkpoint inhibitor monotherapy and combination therapy in advanced non-small cell lung cancer. Cancer Med 2023;12(11):12234-12252. 10.1002/cam4.5918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ansari MJ, Bokov D, Markov A, et al. Cancer combination therapies by angiogenesis inhibitors; a comprehensive review. Cell Commun Signal 2022;20(1):49. 10.1186/s12964-022-00838-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Santos LV, Cruz MR, Lopes Gde L, Lima JP.. VEGF-A levels in bevacizumab-treated breast cancer patients: a systematic review and meta-analysis. Breast Cancer Res Treat. 2015;151(3):481-489. 10.1007/s10549-015-3410-7 [DOI] [PubMed] [Google Scholar]
- 29. Amano T, Iijima H, Shinzaki S, et al. Vascular endothelial growth factor-A is an Immunohistochemical biomarker for the efficacy of bevacizumab-containing chemotherapy for duodenal and jejunal adenocarcinoma. BMC Cancer 2021;21(1):978. 10.1186/s12885-021-08724-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tabernero J, Hozak RR, Yoshino T, et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann Oncol. 2018;29(3):602-609. 10.1093/annonc/mdx767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang J, Yan J, Liu B.. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. 10.3389/fimmu.2018.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mäkinen T, Veikkola T, Mustjoki S, et al. Isolated lymphatic endothelial cells transduce growth, survival and migratory signals via the VEGF-C/D receptor VEGFR-3. EMBO J. 2001;20(17):4762-4773. 10.1093/emboj/20.17.4762 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.