Abstract
We performed a systematic literature review to identify and summarize data from studies reporting clinical efficacy and safety outcomes for trifluridine/tipiracil (FTD/TPI) combined with other antineoplastic agents in advanced cancers, including metastatic colorectal cancer (mCRC). We conducted a systematic search on May 29, 2021, for studies reporting one or more efficacy or safety outcome with FTD/TPI-containing combinations. Our search yielded 1378 publications, with 38 records meeting selection criteria: 35 studies of FTD/TPI-containing combinations in mCRC (31 studies second line or later) and 3 studies in other tumor types. FTD/TPI plus bevacizumab was extensively studied, including 19 studies in chemorefractory mCRC. Median overall survival ranged 8.6-14.4 months and median progression-free survival 3.7-6.8 months with FTD/TPI plus bevacizumab in refractory mCRC. Based on one randomized and several retrospective studies, FTD/TPI plus bevacizumab was associated with improved outcomes compared with FTD/TPI monotherapy. FTD/TPI combinations with chemotherapy or other targeted agents were reported in small early-phase studies; preliminary data indicated higher antitumor activity for certain combinations. Overall, no safety concerns existed with FTD/TPI combinations; most common grade ≥ 3 adverse event was neutropenia, ranging 5%-100% across all studies. In studies comparing FTD/TPI combinations with monotherapy, grade ≥ 3 neutropenia appeared more frequently with combinations (29%-67%) vs. monotherapy (5%-41%). Discontinuation rates due to adverse events ranged 0%-11% for FTD/TPI plus bevacizumab and 0%-17% with other combinations. This systematic review supports feasibility and safety of FTD/TPI plus bevacizumab in refractory mCRC. Data on non-bevacizumab FTD/TPI combinations remain preliminary and need further validation.
Keywords: trifluridine, tipiracil, colorectal neoplasms, antineoplastic agents, antineoplastic drugs, review literature
The data reported here will inform treatment decision-making about the use of combination therapies that include trifluridine/tipiracil in the first-, second-, or third-line setting for the treatment gastrointestinal cancers across tumor types.
Implications for Practice.
Trifluridine/tipiracil is approved as monotherapy in the treatment of metastatic colorectal and gastric cancers and has been explored in combinations with various antineoplastic combinations in clinical trials. This article provides an overview of the evidence for the activity and safety of these combinations across a variety of cancers. The data collected, summarized, and interpreted here will inform treatment of decision-making about the use of combination therapies that include trifluridine/tipiracil in the first-, second-, or third-line setting for the treatment gastrointestinal cancers across tumor types.
Introduction
Fluoropyrimidines, including 5-fluorouracil and capecitabine, alone or as part of combination regimens, have formed the mainstay in treating gastrointestinal cancers.1,2 However, resistance to fluoropyrimidines remains a considerable barrier to effective treatment.2
Trifluridine/tipiracil (FTD/TPI or TAS-102; Taiho Oncology, Inc., Princeton, NJ, USA) is an oral cytotoxic agent comprising trifluridine, a thymidine analog, and tipiracil, a thymidine phosphorylase inhibitor.3 FTD/TPI has a unique mechanism of action distinguishing it from other fluoropyrimidines.2 FTD is incorporated into DNA, causing DNA dysfunction, and tipiracil increases the oral bioavailability of FTD.
In the phase III randomized RECOURSE trial, FTD/TPI monotherapy significantly improved survival versus placebo in patients with chemorefractory metastatic colorectal cancer (mCRC; after ≥ 2 prior systemic regimens). Median overall survival (OS) was 7.1 vs. 5.3 months for FTD/TPI versus placebo (hazard ratio [HR] 0.68 (95% confidence interval [CI], 0.58 − 0.81; P < .001). FTD/TPI also demonstrated a manageable safety profile, with hematologic and gastrointestinal-related adverse events (AEs) being the most common.4 In the phase III randomized TAGS trial, FTD/TPI monotherapy was associated with a significant survival benefit versus placebo (median OS, 5.7 vs. 3.6 months [95% CI, 4.8-6.2]; HR, 0.69 [95% CI, 0.56-0.85]; P < .001) in patients with metastatic gastric or gastroesophageal junction cancer (mGC/GEJC) whose disease progressed after ≥ 2 prior chemotherapy (chemo) regimens.5 As a result, FTD/TPI was approved as third- or later-line treatment for patients with mCRC (in 2015) and mGC/GEJC (in 2019).6
In addition to these two trials, multiple studies over recent years have evaluated the combination of FTD/TPI with targeted therapies, other chemotherapeutic agents, and immunotherapeutic agents, both in mCRC and other cancer types. The combination of FTD/TPI with the anti-vascular endothelial growth factor (VEGF) antibody bevacizumab (BEV) has shown promising results in patients with refractory mCRC in clinical trials,7,8 and FTD/TPI + BEV is now recommended in the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) as a treatment option for patients with this disease.9,10
While there are systematic literature reviews (SLRs) and meta-analyses summarizing FTD/TPI monotherapy in CRC, 11-13 systematic reviews evaluating FTD/TPI-containing combination regimens across tumor types are rare. This SLR’s objective is to identify and summarize data from studies reporting clinical efficacy and safety outcomes for FTD/TPI in combination with other antineoplastic agents in various cancers, including CRC.
Methods
Methods used in this unregistered SLR were prespecified and documented in a study protocol (supporting information). PRISMA reporting guidelines for systematic reviews14,15 and the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 guided reporting.16
Literature Search Strategy
Studies of interest were randomized controlled trials (RCTs), nonrandomized clinical trials, and observational studies in abstract and full paper formats. The SLR was conducted on May 29, 2021, using search terms outlined in the supporting information, Supplementary Tables S1-S3. Data sources included MEDLINE (OvidSP); Embase (OvidSP); Cochrane Library (via Cochrane); conference proceedings (2018-2021) of the American Society of Clinical Oncology, European Society of Medical Oncology, and American Association for Cancer Research; the clinical trial registries: clinicaltrials.gov (https://clinicaltrials.gov/) and UMIN (https://www.umin.ac.jp/ctr/); bibliographies from relevant systematic reviews; grey literature sources; and clinical guidelines.
Following Cochrane guidelines, Population, Intervention, Comparator, Outcome, and Study Design (PICOS) methodology were used to build the search strategies.
Screening and Study Selection
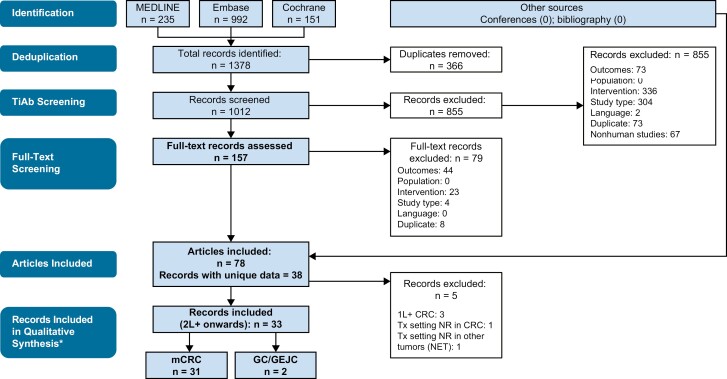
Inclusion and exclusion criteria developed using the PICOS approach were applied to shortlist publications of interest for studies reporting clinical efficacy, safety, and health-related quality of life (HRQoL) outcomes for FTD/TPI combined with other antineoplastic agents to treat patients with cancer (Supplementary Table S4). Using these criteria, 2 researchers independently screened abstracts and then full-text articles in a 2-stage process, with a third reviewer adjudicating any differences. Only studies that fulfilled the inclusion and exclusion criteria and reported one or more defined outcomes were included in the analysis. Identification of records is shown (Fig. 1), aligned with PRISMA statement recommendations.14
Figure 1.
Selection process for studies included in the systematic literature review (PRISMA diagram). *Studies in second-line or later settings were included for qualitative synthesis. Studies based on frontline treatment or where line of treatment was not explicitly reported were summarized separately. Abbreviations: 1L, first line; 2L, second line; GC, gastric cancer; GEJC, gastro-oesophageal junction carcinoma; mCRC, metastatic colorectal cancer; NET, neuroendocrine tumors; NR, not reported; TiAb, title/abstract; Tx, treatment.
Qualitative Synthesis of Data
Findings were tabulated and summarized. One reviewer extracted data from the included records into Microsoft Excel tables, which were then checked and validated by a second reviewer. Outputs included a trial design overview, patient population (tumor type and stage), sample size, follow-up duration, intervention, comparator, primary and secondary endpoints including but not limited to clinical efficacy, safety, and HRQoL. Results were collated and studies were characterized by cancer type, line of treatment, and finally by the type of FTD/TPI combination partner. As this analysis was designed to provide a qualitative review, median values and ranges were provided for survival outcomes.
The study followed a prespecified protocol as a qualitative rather than quantitative analysis. A quantitative meta-analysis was not conducted due to considerable differences between the studies in terms of study design, disease, intervention, and line of treatment. For various combinations of disease, interventions, and lines of treatment, there was an insufficient number of studies for all the groups other than for the FTD/TPI and BEV combination in 2L+ or 3L+ mCRC. Patient characteristics in these individual studies were sporadically reported, and for those that were reported, too diverse to be appropriately combined in a meta-analysis. The outcomes being reported were summarized as median time-to-event endpoints (OS/PFS), which often follow skewed distributions and assumptions valid in meta-analyses of means that may not have been appropriate for medians. For these reasons, a qualitative synthesis of the data was performed.
Risk of Bias Quality Assessment
Criteria from the Cochrane risk-of-bias tool for RCTs16,17 and the Downs and Black checklist for non-RCTs17 were used to assess the risk of bias in RCTs and non-RCTs, respectively. In addition, the Newcastle-Ottawa Scale was used to evaluate single-arm observational studies.18 Two reviewers assessed risk of bias, with disagreement resolved by discussion.
Results
Study Selection
In total, 1378 records were identified across all databases searched (Fig. 1). Of these, 366 were duplicates between sources, and another 934 records were excluded primarily because of interventions or study types not of interest. After screening both abstracts and full-text articles for eligibility, 78 articles were included and yielded 38 records with unique data.
Overall, 31 records included studies of FTD/TPI-containing combinations used in second- or third-line or later (2L+ or 3L+) settings in mCRC, 7,8,19-47 4 records detailed studies of FTD/TPI combinations in the first-line (1L) setting in mCRC (n = 3) 48-50 or where treatment setting was not reported in mCRC (n = 1),51 2 were studies of FTD/TPI-containing combinations used in the 2L + setting in mGC/GEJC,52,53 and one study evaluated FTD/TPI plus temozolamide (TEM) in patients with advanced neuroendocrine tumors (previous treatment unspecified).54
Data in mCRC, and particularly those in previously treated mCRC, were summarized separately for this analysis, as this constituted the largest subset of the results.
FTD/TPI-Containing Combinations in 2L+ or 3L+ mCRC
The designs and the key characteristics of the 31 studies evaluating the use of FTD/TPI combination therapies in 2L+ or 3L+ mCRC identified in the review are presented in Table 1. Ten studies (1 RCT, 2 non-RCTs, and 7 retrospective studies) analyzed FTD/TPI + BEV in 2L+ mCRC, 7,8,20,22-24,27,33,36,46 9 studies (5 non-RCTs and 4 retrospective studies) analyzed FTD/TPI + BEV in 3L+ mCRC,25,28,29,31,34,37,38,43,47 and 4 studies analyzed FTD/TPI in combination with chemotherapeutic agents such as irinotecan (IRI) and oxaliplatin (OXA) (2 in 2L+ and 2 in 3L+).19,26,39,42 Three studies analyzed FTD/TPI + BEV + chemo (OXA or IRI) in mCRC, one reported data from FTD/TPI + nivolumab (NIVO) + OXA32,40,41; and 5 studies (non-RCTs) evaluated FTD/TPI in combination with targeted therapies, such as panitumumab (PAN), nintedanib (NIN), regorafenib (REG), and murlentamab, or immunotherapeutic agents, including NIVO.21,30,35,44,45 One study whose treatment setting was not specified, and therefore not included among these 31 studies, evaluated FTD/TPI with or without ramucirumab in advanced mCRC.51
Table 1.
Studies of FTD/TPI-containing combination regimens in metastatic colorectal cancer.
| Study | Study type/phase | Population specifics | Intervention 1, n | Intervention 2, n | Treatment setting | Reported study endpointsa | Study location | Median follow-up, mo |
|---|---|---|---|---|---|---|---|---|
| FTD/TPI + BEV | ||||||||
| Van Cutsem et al48,50,55 | Phase II RCT |
mCRC | FTD/TPI + BEV n = 77 |
CAP + BEV n = 76 |
1L | PE: PFS SE: OS, QoL, safety |
12 countriesb | NA |
| Oki et al49 | Phase II non-RCT |
mCRC ≥70 years |
FTD/TPI + BEV n = 39 |
— | 1L | PE: PFS SE: OS, ORR, safety |
Japan | 18.9 |
| Pfeiffer et al8 | Phase II RCT | mCRC | FTD/TPI + BEV n = 46 |
FTD/TPI mono n = 47 |
2L+ | PE: PFS SE: OS, ORR, DCR, safety |
Denmark | 10·0 |
| Kuboki et al7 | Phase I/II non-RCT | mCRC | FTD/TPI + BEV n = 25 |
— | 2L+ | PE: PFS at 16 wks SE: PFS, ORR, DCR, TTF, OS, PK, AEs |
Japan | 11·4 |
| Takahashi et al46 | Phase II non-RCT | mCRC | FTD/TPI + BEV n = 97 (safety data set, n = 102) |
— | 2L+ | PE: DCR by RAS status SE: DCR overall, PFS, OS, ORR overall and by RAS status |
Japan | 15.8 |
| Nose et al36 | Retro Obs | mCRC | FTD/TPI + BEV n = 32 |
FTD/TPI mono n = 24 |
2L+ | PE: PFS SE: OS, safety |
Japan | 11.6; 6.3 |
| Fujii et al33 | Retro Obs | mCRC | FTD/TPI + BEV n = 21 |
FTD/TPI mono n = 36 |
2L+ | PE: OS SE: ORR, TTF |
Japan | 14.8 |
| Hisamatsu et al27 | Retro Obs | mCRC | FTD/TPI + BEV n = 24 |
— | 2L+ | PE: PFS SE: ORR, DCR, OS, safety |
Japan | NA |
| Ishikawa et al22 | Retro Obs | mCRC | FTD/TPI + BEV n = 22 |
FTD/TPI mono n = 23 |
2L+ | DCR, PFS, OS, AEs | NA | NA |
| Makiyama et al23 | Retro Obs | mCRC | FTD/TPI + BEV n = 11 |
FTD/TPI mono n = 33 |
2L+ | PFS, OS, safety | Japan | NA |
| Yasuda et al24 | Retro Obs | mCRC | FTD/TPI + BEV n = 33 |
— | 2L+ | Safety | Japan | NA |
| Ota et al20 | Retro Obs | mCRC | FTD/TPI ± BEV n = 14 |
— | 2L+ | PFS, OS, safety | Japan | 10.3 |
| Miano et al34 | Non-RCT | mCRC | FTD/TPI + BEV n = 15 |
— | 3L+ | PE: PFS SE: RR, OS, grade 3 neutropenia |
Italy | 100 |
| Satake et al37 | Phase Ib/II non-RCT | mCRC | FTD/TPI + BEV n = 44 |
— | 3L+ | PE: PFS at 16 wks SE: OS, PFS, TTF, ORR, DCR, safety |
Japan | 15.36 |
| Yoshida et al31 | Phase II non-RCT | mCRC | FTD/TPI + BEV n = 45 |
— | 3L+ | PE: PFS SE: RR, DCR, OS, safety |
Japan | NA |
| Ishizaki et al43 | Phase II non-RCT | mCRC | FTD/TPI + BEV n = 19 |
— | 3L+ | PE: PFS SE: OS, ORR, DCR, AEs, time to ECOG PS ≥ 2 |
Japan | 11.5 |
| Yoshida et al47 | Phase II non-RCT | mCRC | FTD/TPI + BEV n = 32 |
— | 3L+ | PE: PFS SE: TTF, RR, OS, AEs |
Japan | NA |
| Shibutani et al38 | Retro Obs | mCRC | FTD/TPI + BEV n = 36 |
FTD/TPI mono n = 26 |
3L+ | ORR, PFS, OS, safety | Japan | NA |
| Matsuhashi et al29 | Retro Obs | mCRC | FTD/TPI + BEV n = 17 |
— | 3L+ | ORR, DCR, PFS, OS, safety | Japan | NA |
| Kotani et al28 | Retro Obs | mCRC | FTD/TPI + BEV n = 60 |
FTD/TPI mono n = 66 |
3L+ | PFS, ORR, DCR, OS, AEs | Japan | 7.1; 7.2 |
| Yoshida et al25 | Retro Obs | mCRC | FTD/TPI + BEV n = 25 |
FTD/TPI mono n = 16 |
3L+ | ORR, PFS, OS, AEs | Japan | NA |
| FTD/TPI + BEV + Chemo | ||||||||
| Varghese et al40 | Phase I non-RCT | mCRC | FTD/TPI + BEV + IRI n = 24 |
FTD/TPI + IRI n = 26 |
2L+ | PE: Safety, MTD SE: Safety |
NR | NA |
| Yamazaki et al41 | Phase II non-RCT | mCRC | FTD/TPI + BEV + IRI n = 18 |
— | 2L+ | PE: ORR SE: Safety |
Japan | NA |
| Bordonaro et al32 | Phase I non-RCT | mCRC | FTD/TPI + BEV + OXA n = 37 |
FTD/TPI + OXA + NIVO n = 17 |
3L+ | ORR, DCR, PFS, OS, safety | France, Spain, Italy, Germany, Austria, Hungary, UK | NA |
| FTD/TPI + Chemo | ||||||||
| Doi et al19 | Phase I non-RCT | mCRC | FTD/TPI + IRI n = 10 |
— | 2L+ | PE: RD, safety SE: Efficacy, PK |
Japan | 33.7 |
| Argilés et al26 | Phase I non-RCT | mCRC | FTD/TPI + OXA n = 24 |
— | 2L+ | PE: MTD, RD, safety SE: PK, anti-tumor activity |
France, Spain | NA |
| Suenaga et al39 | Phase I non-RCT | mCRC | FTD/TPI + OXA n = 12 |
— | 3L+ | Response, PFS, OS, safety | Japan | 13.8 |
| Cecchini et al42 | Phase Ib/II non-RCT | mCRC | FTD/TPI + OXA n = 41 |
— | 3L+ | PE: ORR SE: PFS, OS, DCR, DOR, safety |
NR | 6.8 |
| FTD/TPI + targeted therapy | ||||||||
| Kato et al44 | Phase I/II non-RCT | mCRC | FTD/TPI + PAN n = 54 (safety population: n = 55) |
— | 2L+ | PE: PFS at 6 mo SE: PFS, OS, ORR, DCR, TTF, safety |
Japan | 16.5 |
| Van Cutsem et al30 | Phase II non-RCT | mCRC | FTD/TPI + MUR n = 15 |
MUR n = 14 |
2L+ | Response, PFS, OS, PD, safety | Belgium, Czech Rep. |
NA |
| Yamazaki et al21 | Phase I/II non-RCT | mCRC | FTD/TPI + NIN n = 52 |
— | 2L+ | PE: PFS at 16 wks SE: OS, DCR, ORR, safety |
Japan | NA |
| Moehler et al35 | Phase I non-RCT | mCRC | FTD/TPI + REG n = 12 |
— | 3L+ | PE: MTD SE: DCR, PFS, OS, safety |
Germany | NA |
| Patel et al45 | Phase II non-RCT | mCRC | FTD/TPI + NIVO n = 18 |
— | 3L+ | PE: irORR SE: ORR, PFS, DCR, OS, safety |
USA | NA |
| Kasper et al51 | Phase IIb RCT |
mCRC | FTD/TPI + RAM n = 40 |
FTD/TPI n = 40 |
NR | PE: OS SE: ORR, DCR, PFS, safety |
Germany | NA |
aWhere PE and SE are not specified in the column, study endpoints were not classified as primary or secondary.
bAustralia, Belgium, Brazil, Denmark, France, Germany, Italy, The Netherlands, Poland, Russia, Spain, and UK.
Abbreviations: 1L, first line; 2L, second line; 3L, third line; AE, adverse event; BEV, bevacizumab; CAP, capecitabine; DCR, disease control rate; DOR, duration of response; ECOG PS, Eastern Cooperative Oncology Group performance status; FTD/TPI, trifluridine/tipiracil; IRI, irinotecan; irORR, immune-related objective response rate; mCRC, metastatic colorectal cancer; mo, months; Mono, monotherapy; MTD, maximum tolerated dose; MUR, murlentamab; NA, not available; NIN, nintedanib; NIVO, nivolumab; Non-RCT, nonrandomized controlled trial; NR, not reported; ORR, overall response rate; OS, overall survival; OXA, oxaliplatin; PAN, panitumumab; PD, pharmacodynamics; PE, primary endpoint; PFS, progression-free survival; PK, pharmacokinetics; QoL, quality of life; RCT, randomized controlled trial; RD, recommended dose; REG, regorafenib; Retro Obs, retrospective observational, RR, response rate; SE, secondary endpoint(s); TTF, time to treatment failure; wks, weeks.
Among the 31 studies, 26 reported one or more efficacy outcomes. Most studies (21/31) were conducted in Japan; all clinical trials were phase I or II, with patient population sizes ranging from 10 to 97 patients. While several retrospective studies (n = 7) evaluated FTD/TPI monotherapy concurrently with FTD/TPI-containing combination regimens, only one RCT8 was powered for statistical comparison of outcomes with FTD/TPI + BEV versus those with FTD/TPI monotherapy.
Efficacy in Patients With Previously Treated mCRC
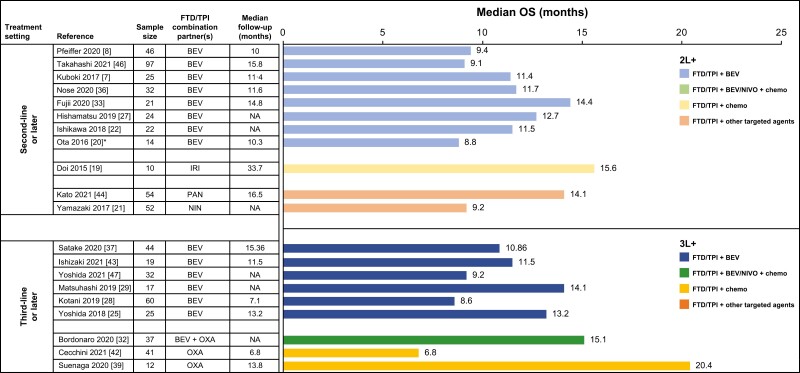
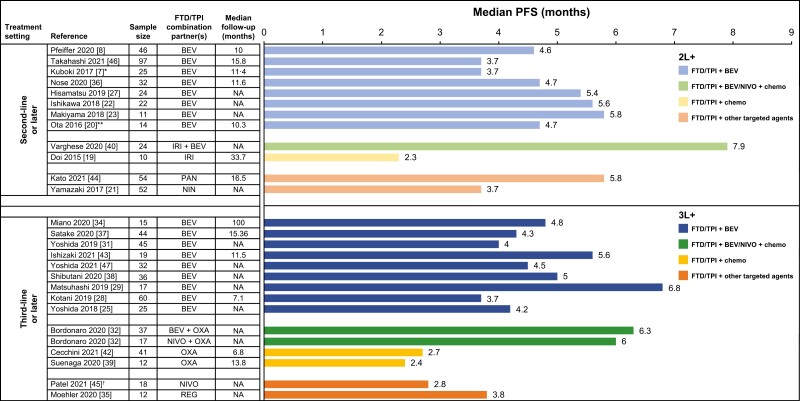
Among patients with mCRC treated with FTD/TPI + BEV in the 2L+ setting, median OS ranged from 8.8 to 14.4 months (Fig. 2; Supplementary Table S5), and median progression-free survival (PFS) ranged from 3.7 to 5.8 months (Fig. 3, Supplementary Table S6).7,8,20,22,23,27,33,36,46 In one RCT, FTD/TPI + BEV treatment was associated with significantly longer OS (HR, 0.55, 95% CI, 0.32-0.94) and PFS (HR, 0.45, 95% CI, 0.29-0.72) compared with FTD/TPI monotherapy.8 In retrospective observational studies in the 2L+ setting, OS HRs for FTD/TPI + BEV versus FTD/TPI monotherapy ranged from 0.24 to 0.30,23,33,36 and PFS HRs ranged from 0.28 to 0.34.
Figure 2.
Median OS with FTD/TPI-containing combination regimens in patients with metastatic colorectal cancer in second- or third-line settings. *Data pertains to patients who received FTD/TPI ± BEV. Abbreviations: 2L, second line; 3L, third line; BEV, bevacizumab; chemo, chemotherapy; FTD/TPI, trifluridine/tipiracil; IRI, irinotecan; NA, not applicable; NIN, nintedanib; NIVO, nivolumab; OS, overall survival; OXA, oxaliplatin; PAN, panitumumab.
Figure 3.
Median PFS with FTD/TPI-containing combination regimens in patients with metastatic colorectal cancer in second- or third-line settings. *Median PFS was 3.7 by central assessment and 5.6 months by investigator assessment. **Data pertain to patients receiving FTD/TPI ± BEV. †Median PFS was 2.2 months per immune-related response criteria and 2.8 months per response evaluation criteria in solid tumors (RECIST). Abbreviations: 2L, second line; 3L, third line; BEV, bevacizumab; chemo, chemotherapy; FTD/TPI, trifluridine/tipiracil; IRI, irinotecan; NA, not applicable; NIN, nintedanib; NIVO, nivolumab; OXA, oxaliplatin; PAN, panitumumab; PFS, progression-free survival; REG, regorafenib.
Ranges of OS and PFS in patients with mCRC treated with FTD/TPI + BEV in the 3L+ setting were similar to that observed in the 2L+ setting (median OS, 8.6 − 14.1 months; median PFS, 3.7 − 6.8 months; Figs. 2 and 3; Supplementary Tables S5 and S6).25,28,29,31,34,37,43,47 A single retrospective observational study showed PFS benefit with FTD/TPI + BEV treatment compared with FTD/TPI monotherapy (HR 0.69) in patients with mCRC treated in the 3L+ setting.28 Together, these data indicated a trend toward OS and PFS benefit with FTD/TPI + BEV compared with FTD/TPI monotherapy in patients with previously treated mCRC. Response rates and disease control rates (DCRs) followed a similar trend, although objective response rates (ORRs) were low overall in patients with previously treated mCRC. In 2L+ mCRC, ORRs with FTD/TPI + BEV ranged from 0% to 3% (Supplementary Fig. S1A) and DCRs ranged from 61% to 76% (Supplementary Fig. S2A).7,8,22,27,33,46 In the 3L+ setting, ORRs ranged from 0% to 8%25,28,29,31,37,38,47 (except for a small study, which reported an ORR of 26%43) (Supplementary Fig. S1A), and DCRs ranged from 53% to 73% (Supplementary Fig. S2A).
The triplet combination of BEV + FTD/TPI + another chemotherapeutic agent was evaluated in three small studies, with two reporting efficacy outcomes. A median OS of 15.1 months and median PFS of 6.3 months were noted with FTD/TPI + BEV + OXA in 3L+ mCRC (Figs. 2 and 3; Supplementary Tables S7 and S8),32 and a median PFS of 7.9 months was noted with FTD/TPI + BEV + IRI in 2L+ mCRC.40 DCRs ranged from 83% to 89% among patients treated with FTD/TPI + BEV + chemo in the 2L+ and 3L+ settings (Supplementary Fig. S2B).
Among FTD/TPI-containing combinations with other targeted therapies, 2 studies (APOLLON evaluating FTD/TPI + PAN and N-task force evaluating FTD/TPI + NIN) enrolled ≈ 50 patients. FTD/TPI + PAN resulted in a median OS of 14.1 months and a median PFS of 5.8 months, whereas FTD/TPI + NIN resulted in a median OS of 9.2 months and median PFS of 3.7 months (Figs. 2 and 3; Supplementary Tables S7 and S8). FTD/TPI + PAN and FTD/TPI + NIN were associated with ORRs of 37% and 8% and DCRs of 82% and 69%, respectively (Supplementary Figs. S1B and S2B).21,44 A small study (REMETY; n = 12), which evaluated FTD/TPI + REG, reported a median PFS of 3.8 months.35
Three of 4 studies evaluating the combination of FTD/TPI with chemotherapeutic agents reported survival data.19,26,39,42 In a small study of FTD/TPI + IRI (n = 10 [9 evaluable patients]) in 2L+ mCRC, median PFS was 2.3 months and median OS was 15.6 months (Figs. 2 and 3; Supplementary Tables S7 and S8).19 In 2 studies of FTD/TPI + OXA, median OS was 6.8 months (n = 41) and 20.4 months (n = 12); median PFS was 2.7 and 2.4 months, respectively.39,42 DCRs ranged from 56% to 68% among patients treated with FTD/TPI + chemo (Supplementary Fig. S2B).
The combination of FTD/TPI + NIVO + OXA was associated with a median PFS of six months, and an ORR of 7%, whereas FTD/TPI + NIVO was associated with a median PFS of 2.8 months and a 0% ORR (Figs. 2 and 3; Supplementary Tables S7 and S8).32,45
Safety in Patients With Previously Treated mCRC
Among patients treated with FTD/TPI + BEV combinations in both 2L+ and 3L+ settings, neutropenia was the most frequently observed grade ≥ 3 AE (Table 2). 7,8,28,29,36-38,43,46,47 In the phase II RCT comparing FTD/TPI + BEV with FTD/TPI monotherapy, the incidence of grade 3 or 4 neutropenia was higher with FTD/TPI + BEV (67%) than with FTD/TPI alone (38%).8 However, in this study, the overall rates of serious AEs were similar in the FTD/TPI and FTD/TPI + BEV groups, and similar numbers of patients discontinued because of AEs. Across retrospective observational studies that evaluated both FTD/TPI and FTD/TPI + BEV in patients with previously treated mCRC,22,28,29,33,36,38 rates of grade 3 or higher neutropenia ranged from 5% to 41% in the FTD/TPI group and 29% to 53% in the FTD/TPI + BEV group. Other key AEs experienced by patients receiving FTD/TPI + BEV included fatigue, nausea, and diarrhea; however, incidences of grade ≥ 3 events of these AEs remained low (Table 2). The proportions of patients discontinuing treatment due to an AE were low, ranging from 0% to 11% across various studies.
Table 2.
Summary of safety in patients with metastatic colorectal cancer treated with FTD/TPI plus bevacizumab in second- or third-line settings.
| Study | Study design | Tx setting | Regimen | Sample size | Grade ≥ 3 AEs, % | % disc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutropenia | FN | Diarrhea | Constipation | Nausea | Vomiting | Asthenia | Fatigue | ||||||
| Pfeiffer et al 8 | RCT | 2L+ | FTD/TPI + BEV | 46 | 67 | 6 | 9 | NA | 2 | 4 | NA | 7 | 2 |
| Takahashi et al46 | Non-RCT | 2L+ | FTD/TPI + BEV | 102 | 54 | 4 | 5 | NA | 5 | NA | NA | 1 | 9 |
| Kuboki et al7 | Non-RCT | 2L+ | FTD/TPI + BEV | 25 | 72 | 16 | 0 | NA | 0 | 0 | NA | 0 | 0 |
| Nose36 | Ret Obs | 2L+ | FTD/TPI + BEV | 32 | 53 | NA | 0 | NA | 0 | NA | NA | 6 | 0 |
| Satake37 | Non-RCT | 3L+ | FTD/TPI + BEV | 44 | 16 | 0 | 5 | NA | 7 | 0 | NA | 0 | 11 |
| Ishizaki43 | Non-RCT | 3L+ | FTD/TPI + BEV | 19 | 5 | NA | 0 | NA | 0 | 0 | NA | NA | 5 |
| Yoshida47 | Non-RCT | 3L+ | FTD/TPI + BEV | 32 | 47 | NA | 0 | NA | 6 | 0 | NA | 3 | 3 |
| Shibutani38 | Ret Obs | 3L+ | FTD/TPI + BEV | 36 | 39 | 0 | 0 | NA | 0 | 0 | NA | 3 | NA |
| Matsuhashi28 | Ret Obs | 3L+ | FTD/TPI + BEV | 17 | 41 | 0 | 0 | NA | 0 | 5 | NA | 0 | NA |
| Kotani27 | Ret Obs | 3L+ | FTD/TPI + BEV | 60 | 50 | 3 | 0 | NA | 0 | 0 | NA | 0 | NA |
Abbreviations: 2L, second line; 3L, third line; AE, adverse event; BEV, bevacizumab; disc, AE-related discontinuation; FN, febrile neutropenia; FTD/TPI, trifluridine/tipiracil; NA, not available; Non-RCT, nonrandomized controlled trial; RCT, randomized controlled trial; Ret Ob, retrospective observational study; Tx, treatment.
Among patients treated with other FTD/TPI combinations in both 2L+ and 3L+ settings, neutropenia was again the most frequent grade ≥ 3 AE observed (incidence ranging from 17% to 100%), and the proportions of patients discontinuing treatment due to an AE ranged from 0% to 17% (Table 3).19,26,40,42,45 Grade ≥ 3 neutropenia was reported at an incidence of 100% with the combination of FTD/TPI with IRI at the highest dose level evaluated in a dose-escalation study.19
Table 3.
Summary of safety in patients with metastatic colorectal cancer treated with other FTD/TPI combinations in second- or third-line settings.
| Study | Study design | Tx setting | Regimen | Sample size | Follow-up (median months) | Grade ≥ 3 AEs, % | % disc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutropenia | FN | Diarrhea | Constipation | Nausea | Vomiting | Asthenia | Fatigue | |||||||
| FTD/TPI + chemo + BEV | ||||||||||||||
| Varghese40 | Non-RCT | 2L+ | FTD/TPI + IRI + BEV | 24 | NR | 42 | NR | 12 | 0 | 4 | 8 | NA | 8 | 0 |
| FTD/TPI + chemo | ||||||||||||||
| Doi19 | Non-RCT | 2L+ | FTD/TPI + IRI | 10 | 33.7 | 100 | 30 | 0 | 0 | 0 | 0 | NA | NA | 0 |
| Varghese40 | Non-RCT | 2L+ | FTD/TPI + IRI | 26 | NA | 23 | NA | 0 | 0 | 12 | 12 | NA | 15 | 4 |
| Argilés26 | Non-RCT | 2L+ | FTD/TPI + OXA | 24 | NA | 17 | NA | 0 | NA | 0 | 4 | 4 | NA | 17 |
| Cecchini42 | Non-RCT | 3L+ | FTD/TPI + OXA | 41 | 6.8 | 20 | NA | 2 | NA | 0 | 0 | NA | 2 | 7 |
| Suenaga39 | Non-RCT | 3L+ | FTD/TPI + OXA | 12 | 13.8 | 25 | 0 | 0 | 0 | 8 | 0 | NA | 0 | NA |
| FTD/TPI + targeted/immunotherapy | ||||||||||||||
| Kato44 | Non-RCT | 2L+ | FTD/TPI + PAN | 55 | 16.5 | 47 | 11 | 2 | NA | 2 | 2 | NA | 4 | 4 |
| Patel45 | Non-RCT | 3L+ | FTD/TPI + NIVO | 18 | NA | 28 | NA | 17 | NA | 11 | 6 | 6 | 11 | 0 |
Abbreviations: 2L, second line; 3L, third line; AE, adverse event; BEV, bevacizumab; chemo, chemotherapy; disc, discontinuation; FN, febrile neutropenia; FTD/TPI, trifluridine/tipiracil; IRI, irinotecan; mCRC, metastatic colorectal cancer; NA, not available; NIVO, nivolumab; Non-RCT, nonrandomized controlled trial; OXA, oxaliplatin; PAN, panitumumab; Tx, treatment.
The standard recommended dose of FTD/TPI requires either no or slight adjustment when combined with other antineoplastic agents. A dose escalation study indicated that FTD/TPI is safe in treating mCRC at the recommended dose of 35 mg/m² bid in combination with 85 mg/m² of OXA Q2W,26 while other studies indicated a lower maximum tolerated dose of FTD/TPI (25 mg/m² bid) when combined with 180 mg/m² IRI Q2W40 or with 120 mg REG daily.35
FTD/TPI + BEV in 1L mCRC
Two studies evaluated FTD/TPI + BEV in the 1L setting in mCRC (Table 1). In the noncomparative phase II RCT TASCO1, in patients with mCRC ineligible for full-dose combination chemotherapy with irinotecan or oxaliplatin or for curative resection of metastatic lesions, FTD/TPI + BEV (n = 77) and capecitabine + BEV (n = 76) were respectively associated with a median PFS of 9.2 and 7.8 months, median OS of 18 and 16.2 months, ORRs of 34% and 30%, and DCRs of 86% and 78%. In the FTD/TPI + BEV and capecitabine + BEV groups, most frequent grade ≥ 3 AEs, were neutropenia (47% and 5%), hand-foot syndrome (0% and 12%) and diarrhea (0% and 8%).48 In extended follow-up, median OS was 22.3 months with FTD/TPI + BEV and 17.7 months with capecitabine + BEV.50,55 Overall, these data indicated clinical activity of the FTD/TPI + BEV regimen in untreated mCRC, with efficacy similar to that of capecitabine + BEV. In a smaller phase II trial in patients with mCRC aged ≥ 70 years, including those ineligible or eligible for (but opted not to receive) oxaliplatin- or irinotecan-containing regimens (n = 37), FTD/TPI + BEV as IL treatment resulted in a median OS of 22.4 months, median PFS of 9.4 months, an ORR of 41%, and a DCR of 87%. Most (72%) patients experienced grade ≥ 3 neutropenia.49
FTD/TPI-Containing Combinations in Other Tumor Types
Two studies evaluated FTD/TPI-containing combination regimens in mGC/GEJC in the 2L+ setting. A phase II clinical trial that evaluated FTD/TPI + ramucirumab in patients with mGC/GEJC who were previously treated (n = 64) resulted in an ORR of 13% and a DCR of 81%. In total, 78% of patients experienced grade ≥ 3 neutropenia. The efficacy and safety data were consistent regardless of previous ramucirumab exposure, and the authors concluded that this regimen had clinical activity in this population.53 Preliminary results from a phase I/II clinical trial assessing FTD/TPI + IRI in patients with previously treated GC (n = 11) indicated a median PFS of 3 months, and a median OS of 10.2 months. Overall, 91% of patients had grade ≥ 3 neutropenia.52
Separately, one phase I dose-escalation trial evaluated FTD/TPI + TEM in patients with advanced neuroendocrine tumors (n = 15; prior treatment unspecified). In this trial, an ORR of 8% and a DCR of 92% was observed; 33% experienced grade ≥ 3 neutropenia.54
Discussion
To our knowledge, this SLR is the first to summarize all published studies of FTD/TPI-containing combination regimens across tumors. Most studies evaluating FTD/TPI-containing combination regimens were conducted in patients with chemorefractory mCRC, with FTD/TPI + BEV being the most extensively studied. This SLR suggests that adding BEV to FTD/TPI yielded a clinically meaningful benefit for disease control 7,8,22,25,27-29,31,33,37,38,43,46,47 and improved survival outcomes versus FTD/TPI alone9 in patients with chemorefractory mCRC. Median PFS was approximately 4 to 6 months, and median OS was around 8 to 14 months with FTD/TPI + BEV. 7,8,20,22,23,25,27-29,33,34,36-38,43,46,47 A recent press release announced positive outcomes in the Phase III randomized SUNLIGHT trial (NCT04737187), which evaluated FTD/TPI + BEV versus FTD/TPI monotherapy in refractory mCRC, although data remain pending (Table 4). These data are in line with recent NCCN Guidelines® of FTD/TPI with or without BEV for patients with chemorefractory mCRC.9,10
Table 4.
Ongoing and recently reported trials of FTD/TPI in combination with other agents in mCRC and other advanced solid tumors (up to date July 2022).
| Study name | Study ID | Setting | Agent(s) | Description and location(s) | Study status |
|---|---|---|---|---|---|
| Advanced mCRC | |||||
| SOLSTICE56 |
NCT03869892 EudraCT: 2017-004059-22 |
1L mCRC | FTD/TPI + BEV versus capecitabine + BEV | Randomized phase III (non-US international) | Active, not recruiting; preliminary results56 |
| TOBACO | NCT05077839 | 1L mCRC | FTD/TPI + oxaliplatin and BEV versus XELOX + BEV | Parallel, randomized, standard-control phase II study (China) | Recruiting |
| TriComB |
NCT04564898 EudraCT: 2020-000923-37 |
1L mCRC | FTD/TPI + Capecitabine and BEV | Single-arm, phase I/II (Italy) | Recruiting |
| FIRE-8 |
NCT05007132 EudraCT: 2019-004223-20 |
1L mCRC | FTD/TPI + panitumumab versus FTD/TPI + BEV | Randomized, open label, multicenter phase II (Germany) | Recruiting |
| TASCO1 | NCT02743221 | 1L mCRC | FTD/TPI + BEV versus capecitabine + BEV | Open-label, randomized phase II | Completed |
| SUNLIGHT |
NCT04737187 EudraCT: 2020-001976-14 |
1L, 2L, or 3L mCRC (refractory mCRC) | FTD/TPI + BEV versus FTD/TPI | Randomized phase III (US/global) | Active, not recruiting |
| (N/A) | UMIN000041621 | mCRC (all lines) | FTD/TPI + BEV | Pooled analysis of 5 trials (Japan) | Preinitiation |
| 3T Study | NCT05356897 | 2L+ mCRC | FTD/TPI + tucatinib + trastuzumab | Single-arm phase II study (US) | Not yet recruiting |
| (N/A) | NCT04294264 | 2L+ mCRC | FTD/TPI + oxaliplatin | Single-arm phase II study (US) | Recruiting |
| (N/A) | NCT02848443 | 2L+ mCRC | FTD/TPI + oxaliplatin (+/− BEV or nivolumab) | Phase I (UK/Europe) | Completed |
| TABAsCO | NCT04109924 | 2L+ mCRC | FTD/TPI + BEV + IRI | Single-arm phase II study (US) | Recruiting |
| WJOG14520G | UMIN000044136 | 2L+ mCRC | FTD/TPI + BEV | Retrospective study (Japan) | No longer recruiting |
| HS-CA102N-101 | NCT03616574 | 2L+ locally advanced/metastatic CRC | FTD/TPI + CA102N | Phase I/II | Enrolling by invitation |
| (N/A) | NCT04511039 | 2L+ locally advanced/mCRC or GEJC | FTD/TPI + Talazoparib | Phase I (US) | Recruiting |
| TASKIN | NCT05201352 | 2L+ mCRC | FTD/TPI + XB2001 versus FTD/TPI + placebo | Randomized (1:1 ratio), double-blind, noncomparative, multi-centre phase II study (France) | Not yet recruiting |
| (N/A) | NCT03317119 | 2L+ mCRC (unresectable) | FTD/TPI + trametinib | Phase I study (US) | Active, not recruiting |
| (N/A) | NCT05130060 | 3L+ mCRC | PolyPEPI1018 vaccine + FTD/TPI | Phase I study (US) | Recruiting |
| COLSTAR |
NCT05223673 EudraCT: 2021-003151-41 |
3L+ KRAS/NRAS and BRAF wt mCRC | Futuximab/modotuximab + FTD/TPI versus FTD/TPI | Randomized, open-label, multicenter, 2-arm phase III safety lead-in study | Recruiting |
| RM-110 | NCT04073615 | 3L+ mCRC | Rivoceranib + FTD/TPI versus monotherapies) | Phase I/II multicenter, open-label, randomized study (US) | Active, not recruiting |
| VELO |
NCT05468892 EudraCT: 2018-001600-12 |
3L+ mCRC (major response to prior 1L; progression on 2L) | FTD/TPI + panitumumab versus FTD/TPI | Open label, phase II randomized study | Completed |
| (N/A) | NCT04868773 | 3L+ mCRC | FTD/TPI + cabozantinib | Phase I study (US) | Recruiting |
| TACTIC | NCT05266820 | 3L+ mCRC | FTD/TPI + thalidomide versus FTD/TPI | Phase II study (China) | Recruiting |
| CT001 | NCT05155124 | 3L mCRC | FTD/TPI + cetuximab | Phase I study (China) | Recruiting |
| Other solid tumors | |||||
| ONC001 | NCT04393298 | 1L+ advanced solid tumors (including mCRC, mGC/mGEJC, others) | UCB6114 ± FTD/TPI | Phase I/II nonrandomized, open-label study (US/UK) (US/UK) | Recruiting |
| (N/A) | NCT04808791 | 1L locally advanced/metastatic GC/GEJ adenocarcinoma | IRI + FTD/TPI + oxaliplatin | Single-arm, phase II study (Canada) | Not yet recruiting |
| (N/A) | NCT04097028 | 1L resectable esophageal/GEJ adenocarcinoma | FTD/TPI + Oxaliplatin | Phase II trial (US) | Recruiting |
| MC1941 | NCT04072445 | 2L+ advanced refractory biliary tract cancer | FTD/TPI + IRI | Single-arm phase II study (US) | Active, not recruiting |
| (N/A) | NCT03368963 | 2L+ advanced GI cancers (dose expansion phase only: pancreatic/CRC) | TAS102 + nanoliposomal IRI | Phases I/II trial (US) | Recruiting |
| ACCRU-GI-1810 | NCT04660760 | 2L+ advanced GC/GEJC | FTD/TPI + ramucirumab versus paclitaxel + ramucirumab | Phase II randomized trial (US) | Recruiting |
| LonGas57 | EudraCT: 2018-004845-18 | Platinum-refractory GEJ adenocarcinoma | FTD/TPI ± BEV | Randomized phase III study (Denmark) | Completed; preliminary results57 |
| RE-ExPEL | EudraCT: 2020-001075-32 | Advanced/metastatic GC/GEJC | Ramucirumab beyond progression plus TAS-102 | Pilot study (Germany) | Ongoing |
| ACOTAS_G098 | EudraCT: 2020-004636-25 | mCRC/mGC/mGEJC | FTD/TPI ± oxaliplatin | Phase II cardiovascular safety study (France) | Ongoing |
| ACE1100-01 | EudraCT: 2021-003799-15 | 3L advanced GC | FTD/TPI + ASC-201 versus FTD/TPI | Randomized, double-blind phase II study (Spain) | Ongoing |
| TRITICC | NCT04059562 | 2L cholangiocarcinoma | FTD/TPI + IRI | Prospective, single arm, open label, exploratory, multi-centre pilot study (Germany) | Recruiting |
| (N/A) | EudraCT: 2018-002936-26 | 2L+ cholangiocarcinoma | FTD/TPI + IRI | Efficacy/safety study (Germany) | Restarted |
Abbreviations: 1L, first line; 2L, second line; 3L, third-line; BEV, bevacizumab; CRC, colorectal cancer; FTD/TPI, trifluridine/tipiracil; GC, gastric cancer; GEJC, gastroesophageal junction carcinoma; GI, gastrointestinal; ID, identifier; IRI, irinotecan; mCRC, metastatic colorectal cancer; N/A, not applicable; wt, wild type.
In three small studies in 2L+/3L+ mCRC, the triplet combination of BEV, FTD/TPI, and chemotherapy (OXA or IRI) resulted in DCRs exceeding 80% and median PFS > 6 months.32,40,41 Although preliminary, these data support further investigation of this triplet combination in previously treated mCRC.
In contrast to data in 2L+ and 3L+ mCRC, FTD/TPI + BEV did not improve outcomes compared with standard of care in untreated mCRC.48 In the noncomparative phase II TASCO1 study, promising clinical activity and tolerability was noted with FTD/TPI + BEV48,50,55; however, preliminary results from the ongoing comparative phase III SOLSTICE trial of FTD/TPI + BEV versus capecitabine + BEV in previously untreated mCRC indicated that FTD/TPI + BEV was not superior to capecitabine + BEV in the 1L setting; median PFS was similar with both regimens (9.3 vs. 9.4 months; HR 0.87, 95% CI, 0.75-1.02).56
Studies evaluating FTD/TPI in combination with targeted agents other than BEV were less common21,30,35,44 and among these, encouraging results were observed with FTD/TPI + PAN, an epidermal growth factor receptor (EGFR) antibody, with a median PFS of ≈ 6 months and median OS of ≈ 14 months.44 Ongoing phase II/III studies are evaluating FTD/TPI + PAN in both 1L (NCT05007132 EudraCT: 2019-004223-20) and 3L+ settings (NCT05468892) in mCRC (Table 4). While results from other studies included in this analysis were less conclusive,21,30,51 the combination of FTD/TPI with non-BEV targeted therapies is an area of active research and ongoing studies are exploring FTD/TPI in combination with agents targeting EGFR, MEK, VEGFR, and HER2 receptors (Table 4).
Other FTD/TPI-containing combinations were less effective and are not being pursued in phase II/III studies. Although phase I studies showed that FTD/TPI combined with OXA or IRI were tolerable in 2L+/3L+ mCRC, preliminary activity reported in 3 studies of FTD/TPI + OXA was not favorable (median PFS, ≈2 months; ORR, 0%-4%).26,39,42 Efficacy data with FTD/TPI + IRI are largely lacking or inconclusive.19 Similarly, combining immunotherapeutic agents with FTD/TPI has not proven to be efficacious: phase II studies of FTD/TPI + NIVO45 and FTD/TPI + NIVO + OXA32 were prematurely halted because of lack of efficacy.
Our analysis identified few published studies of FTD/TPI combinations tumor types other than CRC, such as gastroesophageal cancers, which is not surprising given that FTD/TPI monotherapy was only granted approval for this indication in 2019.6 Following the trend seen in mCRC, newly initiated or ongoing phases I and II studies in mGC/GEJC are exploring several FTD/TPI-containing combinations. Preliminary data from the phase III randomized Danish LonGas trial (EudraCT: 2018-004845-18; Table 4) indicated that adding BEV to FTD/TPI did not improve efficacy outcomes compared with FTD/TPI monotherapy for patients with pretreated metastatic esophagogastric adenocarcinoma, although OS and PFS benefits were seen with both regimens (median PFS, 3.7-3.9 months; OS, 9.0-9.9 months).57
While FTD/TPI combinations in other tumor types are being explored, such as two prospective studies of FTD/TPI + irinotecan in pretreated cholangiocarcinoma (Table 4), these efforts remain relatively rare.
The safety profiles of FTD/TPI-containing combinations mostly showed a higher incidence of grade ≥ 3 hematologic AEs. Neutropenia was the most common grade ≥ 3 AE associated with FTD/TPI-containing combinations, with varying incidence across different regimens and studies. Our analysis indicated that grade ≥ 3 neutropenia may occur in up to 72% of patients in the 2L+ setting,7,8,46 and up to 50% of patients in the 3L+ setting receiving FTD/TPI in combination with BEV.28,29,37,38,43,47 However, while neutropenia was more common with this combination than with FTD/TPI monotherapy,8,38 the difference was not statistically significant,38 and overall, treatment-related discontinuations were low in patients receiving FTD/TPI + BEV. Neutropenia has shown a dose-response relationship with FTD exposure, and higher FTD/TPI plasma levels are associated with improved OS and PFS, and a reduced time to performance status deterioration.36,58 In multiple studies, the presence of FTD/TPI-induced neutropenia—particularly high-grade neutropenia during early cycles—has been shown to be a useful predictive marker for clinical response and survival. 36,58-60
The incidence of non-hematologic grade ≥ 3 events remained low across studies in this analysis. Even in the recently reported phase III SOLSTICE trial, although the incidence of neutropenia was higher with the FTD/TPI + BEV compared with capecitabine + BEV, the non-hematologic safety profile of the former was more favorable.56 Importantly, cardiotoxicity was not a concern with FTD/TPI-containing combinations, unlike with fluoropyrimidines.61 In phase III studies, the overall incidence of grade ≥ 3 cardiac events was rare4,5 and a recent meta-analysis reported no increased cardiotoxicity risk with FTD/TPI compared with placebo.62 These data lend support for FTD/TPI as a drug of choice or as a good backbone for combination therapeutic regimens for patients with cardiac disease or cardiac side effects from previous chemotherapeutic regimens.63 Another patient subset that may benefit from FTD/TPI are those with dihydropyrimidine dehydrogenase (DPD) deficiency, as these patients are at risk of severe life-threatening AEs with 5-FU-containing regimens.64 As FTD/TPI is not metabolized by DPD,65 FTD/TPI is presumably safe for these patients. However, evidence of FTD/TPI’s safety in patients with DPD deficiency is limited to a few case reports66-68 and needs further clinical evaluation.
Quality-of-life (QoL) data were generally not reported in most studies included in this analysis, as these were early feasibility studies. One exception was the TASCO1 study of FTD/TPI + BEV in 1L mCRC. In this study, QoL was generally maintained during treatment, with no clinically relevant changes from baseline observed in global health status, functioning scales, and most symptom scales.48 Further data are needed to more fully establish the QoL impact of different FTD/TPI-based regimens, particularly among patients who were pretreated and/or over 65 years.
Limitations of this review were that the analysis captured phases I and II data only, with all but 2 studies being nonrandomized trials or retrospective observational studies. These studies were heterogeneous for combinations, treatment settings, and patient populations, which, in addition to the relatively small sample sizes (range, 9 to 97 patients), limited our ability to draw definite conclusions. Upcoming data from the phase III randomized SOLSTICE,56 SUNLIGHT (NCT04737187), and COLSTAR (NCT05223673) trials, which will comprise larger datasets, should help further elucidate the role of FTD/TPI combinations in mCRC. Data on non-BEV combinations in mCRC were also limited. This analysis lacked data from other tumor types, particularly mGC/GEJC; however, the bulk of these studies are underway or were recently reported, including the phase III study of FTD/TPI + bevacizumab in platinum-refractory mGC/GEJC (EudraCT: 2018-004845-18). Another possible limitation was that two-thirds (21/31) of the included studies were conducted in Japanese populations, highlighting the need for more data in diverse populations. Fortunately, a good number of ongoing FTD/TPI combination therapy trials are being conducted among populations in the US and Europe (Table 4).
Taken together, this comprehensive SLR consolidates the current body of evidence regarding FTD/TPI combinations in metastatic solid tumors and supports the feasibility and safety of certain FTD/TPI-containing combinations, such as FTD/TPI + BEV, in the guideline-recommended setting of refractory mCRC.
Supplementary Material
Supplementary material is available at The Oncologist online.
Acknowledgments
Professional medical writing and editorial assistance, under the direction of the authors, were provided by Vasupradha Vethantham, PhD, and Kathleen Blake, PhD, of Ashfield MedComms, an Inizio company, funded by Taiho Oncology, Inc.
Contributor Information
Kohei Shitara, National Cancer Center Hospital East, Chiba, Japan; Department of Immunology, Nagoya University Graduate School of Medicine, Nagoya, Japan.
Alfred Falcone, University of Pisa, Pisa, Italy.
Marwan G Fakih, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Ben George, Medical College of Wisconsin, Milwaukee, WI, USA.
Raghav Sundar, Department of Haematology-Oncology, National University Cancer Institute, Singapore, National University Hospital, Singapore; Cancer and Stem Cell Biology Program, Duke-NUS Medical School, Singapore; Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Sandip Ranjan, SmartAnalyst, an Ashfield Advisory Company, Gurugram, Haryana, India.
Eric Van Cutsem, University Hospitals Gasthuisberg Leuven and KU Leuven, Leuven, Belgium.
Funding
This analysis and writing support were funded by Taiho Oncology, Inc.
Conflict of Interest
Kohei Shitara reported research funding from Amgen, Astellas Pharma, Chugai Pharmaceuticals, Daiichi Sankyo, Eisai, MSD, Ono Pharmaceuticals, and Taiho Pharmaceuticals; consulting/advisory relationships with AbbVie Inc., Amgen, Astellas Pharma, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Eli Lilly and Company, GlaxoSmithKline, Guardant Health Japan, Janssen, MSD, Novartis, Pfizer, Inc, Ono Pharmaceuticals, Takeda Pharmaceuticals, and Taiho Pharmaceuticals; and honoraria from Bristol-Myers Squibb, Janssen, and Takeda Pharmaceuticals. Alfred Falcone reported research funding from AstraZeneca, Bayer, Bristol, Lilly, Merck, MSD, Novartis, Roche, Sanofi, and Servier; consulting/advisory relationships with Amgen, Bayer, Bristol, Daiichi Sankyo, Incyte, Lilly, Merck, MSD, Pierre-Fabre, Roche, and Servier; and travel expenses from Roche and Servier. Marwan G. Fakih reported research funding from Amgen, Inc., Bristol-Myers Squibb, Genentech, Novartis Oncology, and Verastem; consulting/advisory relationships with AstraZeneca, Bristol-Myers Squibb, Incyte Corporation, Pfizer, PsiOxus, Taiho Oncology, and Zhuhai Yufan Biotech; honoraria from Guardant 360; and scientific advisory board for Amgen, Inc., Array BioPharm, Bayer Corporation, Eisai, GlaxoSmithKline, Merck, Mirati Therapeutics, Inc., Nouscom, Roche/Genentech, and Xenthera. Ben George reported research funding from Boehringer Ingelheim, CARSgen, Glyconex, Helix, Hoffman La-Roche, Hutchison Medipharma, Mirati Therapeutics, NGM Biopharma, Pfizer, Roche/Genentech, Taiho Oncology, and Toray; consulting/advisory relationships with BMS, Boston Scientific, Exelixis, Foundation Medicine, Ipsen, Taiho Oncology, and Roche/Genentech; honoraria from Ipsen and Taiho Oncology; scientific advisory board for Roche/Genentech; and stock options with XBiotech. Raghav Sundar reported research funding from MSD, Natera, and Paxman Collers; scientific advisory board for Bayer, Bristol Myers Squibb, DKSH, Eisai, GSK, Merck, MSD, Novartis, and Taiho Oncology; honoraria from AstraZeneca, BMS, DKSH, Eli Lilly, Ipsen, MSD, Roche, and Taiho Oncology; and travel expenses from AstraZeneca, DKSH, Eisai, Roche, and Taiho Oncology. Sandip Ranjan reported employment with SmartAnalyst India, Pvt. Ltd.; and consulting/advisory relationships with BMS, Janssen, Pfizer, and Taiho Oncology. Eric Van Cutsem reported institutional research funding/grants from Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier; and consulting/advisory relationships with Abbvie, ALX, Amgen, Array, Astellas, AstraZeneca, Bayer, Beigene, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi, GSK, Incyte, Ipsen, Lilly, Merck Sharp & Dohme, Merck KGaA, Mirati, Novartis, Nordic, Pierre Fabre, Pfizer, Roche, Seattle Genetics, Servier, Takeda, Terumo, Taiho, and Zymeworks. All authors report medical writing support for the submitted work, which was funded by Taiho Oncology, Inc.
Author Contributions
Conception/design: K.S., A.F., M.G.F., B.G., R.S., E.V.C. Supervision: K.S., E.V.C. Provision of study material or patients: All authors. Collection and/or assembly of data: All authors. Data analysis and interpretation: All authors. Manuscript writing: K.S., E.V.C. Final approval of manuscript: All authors.
Data Availability
No new data were generated or analyzed in support of this research.
References
- 1. Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386-1422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 2. Peeters M, Cervantes A, Moreno Vera S, Taieb J.. Trifluridine/tipiracil: an emerging strategy for the management of gastrointestinal cancers. Future Oncol. 2018;14(16):1629-1645. 10.2217/fon-2018-0147 [DOI] [PubMed] [Google Scholar]
- 3. Emura T, Suzuki N, Yamaguchi M, Ohshimo H, Fukushima M.. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. Int J Oncol. 2004;25(3):571-578. [PubMed] [Google Scholar]
- 4. Mayer RJ, Van Cutsem E, Falcone A, et al. ; RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med. 2015;372(20):1909-1919. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 5. Shitara K, Doi T, Dvorkin M, et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19(11):1437-1448. 10.1016/S1470-2045(18)30739-3 [DOI] [PubMed] [Google Scholar]
- 6. Drugs.com. Lonsurf FDA approval history. https://www.drugs.com/history/lonsurf.html. Accessed July 15, 2022.
- 7. Kuboki Y, Nishina T, Shinozaki E, et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): an investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017;18(9):1172-1181. 10.1016/S1470-2045(17)30425-4 [DOI] [PubMed] [Google Scholar]
- 8. Pfeiffer P, Yilmaz M, Möller S, et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(3):412-420. 10.1016/S1470-2045(19)30827-7 [DOI] [PubMed] [Google Scholar]
- 9. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Colon Cancer V.2.2022. ©National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 19, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [Google Scholar]
- 10. Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Rectal Cancer V.3.2022. ©National Comprehensive Cancer Network, Inc. 2022. All rights reserved. Accessed December 19, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [Google Scholar]
- 11. Abrahao ABK, Ko Y-J, Berry S, Chan KKW.. A comparison of regorafenib and TAS-102 for metastatic colorectal cancer: a systematic review and network meta-analysis. Clin Colorectal Cancer. 2018;17(2):113-120. 10.1016/j.clcc.2017.10.016 [DOI] [PubMed] [Google Scholar]
- 12. Sonbol MB, Benkhadra R, Wang Z, et al. A systematic review and network meta-analysis of regorafenib and TAS-102 in refractory metastatic colorectal cancer. Oncologist 2019;24(9):1174-1179. 10.1634/theoncologist.2019-0189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andersen SE, Andersen IB, Jensen BV, et al. A systematic review of observational studies of trifluridine/tipiracil (TAS-102) for metastatic colorectal cancer. Acta Oncol. 2019;58(8):1149-1157. 10.1080/0284186X.2019.1605192 [DOI] [PubMed] [Google Scholar]
- 14. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) 2021;74(9):790-799. 10.1016/j.rec.2021.07.010 [DOI] [PubMed] [Google Scholar]
- 16. Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). Cochrane Collaboration. 2011. Available at: https://handbook-5-1.cochrane.org/. Accessed 18 July 2022. [Google Scholar]
- 17. Downs SH, Black N.. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377-384. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wells GA, Shea B, O’Connell D. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed July 18, 2022.
- 19. Doi T, Yoshino T, Fuse N, et al. Phase I study of TAS-102 and irinotecan combination therapy in Japanese patients with advanced colorectal cancer. Invest New Drugs. 2015;33(5):1068-1077. 10.1007/s10637-015-0271-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ota T, Tsukuda H, Hasegawa Y, et al. Treatment of TAS-102 in patients with metastatic colorectal cancer. Ann Oncol. 2016;27(suppl_7):vii106-vii107. 10.1093/annonc/mdw524.009 [DOI] [Google Scholar]
- 21. Yamazaki K, Kuboki Y, Shinozaki E, et al. A multicentre phase I/II study of TAS-102 with nintedanib in patients with metastatic colorectal cancer refractory to standard therapies (N-task force: EPOC1410). Ann Oncol. 2017;28(suppl_5):v174-v175. 10.1093/annonc/mdx393.042 [DOI] [PubMed] [Google Scholar]
- 22. Ishikawa H, Suzuki K, Kakizawa N, et al. Efficacy, tolerability, and manageability of TAS-102 plus bevacizumab for metastatic colorectal cancer patients in clinical practice. J Clin Oncol. 2018;36(15_suppl):e15535. [Google Scholar]
- 23. Makiyama A, Yamaga S, Hirano G, Makiyama C, Muta T.. A retrospective study to compare TAS-102 with TAS-102+BV in advanced colorectal cancer refractory to standard therapy. Ann Oncol. 2018;29(suppl_7):VII75. [Google Scholar]
- 24. Yasuda K, Kotani D, Kuboki Y, et al. Safety for trifluridine/tipiracil (TAS-102) with bevacizumab combination in patients with refractory metastatic colorectal cancer in real-world clinical practice: the single-institutional experience. J Clin Oncol. 2018;36(4_suppl):865-865. 10.1200/jco.2018.36.4_suppl.865 [DOI] [Google Scholar]
- 25. Yoshida Y, Sakamoto R, Kajitani R, et al. Biweekly administration of TAS-102 for neutropenia prevention in patients with colorectal cancer. Anticancer Res. 2018;38(7):4367-4373. 10.21873/anticanres.12738 [DOI] [PubMed] [Google Scholar]
- 26. Argilés G, André T, Hollebecque A, et al. Phase I dose-escalation of trifluridine/tipiracil in combination with oxaliplatin in patients with metastatic colorectal cancer. Eur J Cancer. 2019;112:12-19. 10.1016/j.ejca.2019.01.101 [DOI] [PubMed] [Google Scholar]
- 27. Hisamatsu A, Ikusue T, Toshima H, Kobayashi K, Shimada K.. Retrospective study of TAS-102 plus bevacizumab for patients with metastatic colorectal cancer in salvage therapy. Ann Oncol. 2019;30(suppl_4):IV93. [Google Scholar]
- 28. Kotani D, Kuboki Y, Horasawa S, et al. Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer 2019;19(1):1253. 10.1186/s12885-019-6475-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsuhashi N, Takahashi T, Fujii H, et al. Combination chemotherapy with TAS-102 plus bevacizumab in salvage-line treatment of metastatic colorectal cancer: A single-center, retrospective study examining the prognostic value of the modified Glasgow Prognostic Score in salvage-line therapy of metastatic colorectal cancer. Mol Clin Oncol 2019;11(4):390-396. 10.3892/mco.2019.1899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Cutsem E, Melichar B, Van den Eynde M, et al. Phase 2 study results of murlentamab, a monoclonal antibody targeting the anti-mullerian-hormone-receptor II (AMHRII), acting through tumor-associated macrophage engagement in advanced/metastatic colorectal cancers. Ann Oncol. 2019;30(suppl_4):iv153-iv154. 10.1093/annonc/mdz183.003 [DOI] [Google Scholar]
- 31. Yoshida Y, Yamada T, Matsuoka H, et al. Biweekly TAS-102 and bevacizumab as a third-line chemotherapy for metastatic colorectal cancer: a phase II multicenter clinical trial (TAS-CC4 study). Ann Oncol. 2019;30(suppl_5):V235. [Google Scholar]
- 32. Bordonaro R, Calvo A, Auriemma A, et al. Trifluridine/tipiracil in combination with oxaliplatin and either bevacizumab or nivolumab: results of the expansion part of a phase I study in patients with metastatic colorectal cancer. J Clin Oncol. 2020;38(4_suppl):140-140. 10.1200/jco.2020.38.4_suppl.140 [DOI] [Google Scholar]
- 33. Fujii H, Matsuhashi N, Kitahora M, et al. Bevacizumab in combination with TAS-102 improves clinical outcomes in patients with refractory metastatic colorectal cancer: a retrospective study. Oncologist 2020;25(3):e469-e476. 10.1634/theoncologist.2019-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miano S, Francini G, Petrioli R, et al. TAS-102 with bevacizumab in patients with chemorefractory metastatic colorectal cancer. Real fife study. Tumori J 2020;106(2 suppl):42-43. [Google Scholar]
- 35. Moehler M, Stein A, Trojan J, et al. PD-8 Regorafenib with TAS-102 in metastatic colorectal cancer patients who progressed after at least two standard therapies: efficacy and safety results of a multicenter phase I study (REMETY). Ann Oncol. 2020;31(suppl_3):S214. [Google Scholar]
- 36. Nose Y, Kagawa Y, Hata T, et al. Neutropenia is an indicator of outcomes in metastatic colorectal cancer patients treated with FTD/TPI plus bevacizumab: a retrospective study. Cancer Chemother Pharmacol. 2020;86(3):427-433. 10.1007/s00280-020-04129-6 [DOI] [PubMed] [Google Scholar]
- 37. Satake H, Kato T, Oba K, et al. Phase Ib/II study of biweekly TAS-102 in combination with bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (BiTS study). Oncologist 2020;25(12):e1855-e1863. 10.1634/theoncologist.2020-0643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Shibutani M, Nagahara H, Fukuoka T, et al. Combining bevacizumab with trifluridine/thymidine phosphorylase inhibitor improves the survival outcomes regardless of the usage history of bevacizumab in front-line treatment of patients with metastatic colorectal cancer. Anticancer Res. 2020;40(7):4157-4163. 10.21873/anticanres.14415 [DOI] [PubMed] [Google Scholar]
- 39. Suenaga M, Wakatsuki T, Mashima T, et al. A phase I study to determine the maximum tolerated dose of trifluridine/tipiracil and oxaliplatin in patients with refractory metastatic colorectal cancer: LUPIN study. Invest New Drugs. 2020;38(1):111-119. 10.1007/s10637-019-00749-9 [DOI] [PubMed] [Google Scholar]
- 40. Varghese AM, Cardin DB, Hersch J, et al. Phase I study of trifluridine/tipiracil plus irinotecan and bevacizumab in advanced gastrointestinal tumors. Clin Cancer Res. 2020;26(7):1555-1562. 10.1158/1078-0432.CCR-19-2743 [DOI] [PubMed] [Google Scholar]
- 41. Yamazaki K, Masuishi T, Tsushima T, et al. P-356 Phase Ib study of irinotecan, bevacizumab and biweekly trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to fluoropyrimidine and oxaliplatin: preliminary report of MODURATE study. Ann Oncol. 2020;31(suppl_3):S205. [Google Scholar]
- 42. Cecchini M, Kortmansky JS, Cui C, et al. A phase 1b expansion study of TAS-102 with oxaliplatin for refractory metastatic colorectal cancer. Cancer. 2021;127(9):1417-1424. 10.1002/cncr.33379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ishizaki T, Mazaki J, Enomoto M, et al. Prospective multicenter phase II study of biweekly TAS-102 and bevacizumab for metastatic colorectal cancer. Anticancer Res. 2021;41(4):2157-2163. 10.21873/anticanres.14988 [DOI] [PubMed] [Google Scholar]
- 44. Kato T, Kagawa Y, Kuboki Y, et al. Safety and efficacy of panitumumab in combination with trifluridine/tipiracil for pre-treated patients with unresectable, metastatic colorectal cancer with wild-type RAS: The phase 1/2 APOLLON study. Int J Clin Oncol. 2021;26(7):1238-1247. 10.1007/s10147-021-01902-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Patel MR, Falchook GS, Hamada K, Makris L, Bendell JC.. A phase 2 trial of trifluridine/tipiracil plus nivolumab in patients with heavily pretreated microsatellite-stable metastatic colorectal cancer. Cancer Med 2021;10(4):1183-1190. 10.1002/cam4.3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Takahashi T, Yamazaki K, Oki E, et al. Phase II study of trifluridine/tipiracil plus bevacizumab by RAS mutation status in patients with metastatic colorectal cancer refractory to standard therapies: JFMC51-1702-C7. ESMO Open 2021;6(2):100093. 10.1016/j.esmoop.2021.100093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yoshida Y, Yamada T, Kamiyama H, et al. ; TAS CC3 Study Group. Combination of TAS-102 and bevacizumab as third-line treatment for metastatic colorectal cancer: TAS-CC3 study. Int J Clin Oncol. 2021;26(1):111-117. 10.1007/s10147-020-01794-8 [DOI] [PubMed] [Google Scholar]
- 48. Van Cutsem E, Danielewicz I, Saunders MP, et al. Trifluridine/tipiracil plus bevacizumab in patients with untreated metastatic colorectal cancer ineligible for intensive therapy: the randomized TASCO1 study. Ann Oncol. 2020;31(9):1160-1168. 10.1016/j.annonc.2020.05.024 [DOI] [PubMed] [Google Scholar]
- 49. Oki E, Makiyama A, Miyamoto Y, et al. Trifluridine/tipiracil plus bevacizumab as a first-line treatment for elderly patients with metastatic colorectal cancer (KSCC1602): A multicenter phase II trial. Cancer Med. 2021;10(2):454-461. 10.1002/cam4.3618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Van Cutsem E, Danielewicz I, Saunders MP, et al. Phase II study evaluating trifluridine/tipiracil + bevacizumab and capecitabine + bevacizumab in first-line unresectable metastatic colorectal cancer (mCRC) patients who are noneligible for intensive therapy (TASCO1): Results of the final analysis on the overall survival. J Clin Oncol. 2021;39(3_suppl):14-14. 10.1200/jco.2021.39.3_suppl.14 [DOI] [Google Scholar]
- 51. Kasper S, Hofheinz RD, Stintzing S, et al. 438P Interim safety analysis of the phase IIb study of ramucirumab in combination with TAS102 vs. TAS102 monotherapy in metastatic colorectal cancer: The RAMTAS trial of the German AIO. Ann Oncol. 2020;31(suppl_4):S427-S428. 10.1016/j.annonc.2020.08.549 [DOI] [Google Scholar]
- 52. Hara H, Mizukami T, Minashi K, et al. A phase I/II trial of trifluridine/tipiracil in combination with irinotecan in patients with advanced gastric cancer refractory to fluoropyrimidine, platinum, and taxane. J Clin Oncol. 2021;39(3_suppl):210-210. 10.1200/jco.2021.39.3_suppl.210 [DOI] [Google Scholar]
- 53. Kawazoe A, Ando T, Hosaka H, et al. Safety and activity of trifluridine/tipiracil and ramucirumab in previously treated advanced gastric cancer: an open-label, single-arm, phase 2 trial. Lancet Gastroenterol Hepatol. 2021;6(3):209-217. 10.1016/S2468-1253(20)30396-4 [DOI] [PubMed] [Google Scholar]
- 54. Uboha NV, Lubner SJ, LoConte NK, et al. Phase 1 dose escalation trial of TAS-102 (trifluridine/tipiracil) and temozolomide in the treatment of advanced neuroendocrine tumors. Invest New Drugs. 2020;38(5):1520-1525. 10.1007/s10637-020-00929-y [DOI] [PubMed] [Google Scholar]
- 55. Van Cutsem E, Danielewicz I, Saunders MP, et al. First-line trifluridine/tipiracil + bevacizumab in patients with unresectable metastatic colorectal cancer: final survival analysis in the TASCO1 study. Br J Cancer. 2022;126(11):1548-1554. 10.1038/s41416-022-01737-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. André T, Falcone A, Shparyk Y, et al. VP11-2021: Trifluridine/tipiracil plus bevacizumab vs capecitabine plus bevacizumab as first line treatment for patients with metastatic colorectal cancer (mCRC) ineligible for intensive therapy: The phase III randomized SOLSTICE study. Ann Oncol. 2021;33(2):229-230. 10.1016/j.annonc.2021.11.006 [DOI] [PubMed] [Google Scholar]
- 57. Pfeiffer P, Yilmaz M, Nordsmark M, et al. O-4 Trifluridine/tipiracil (TAS-102) with or without bevacizumab in patients with pretreated metastatic esophago-gastric adenocarcinoma (mEGA): A Danish randomized trial (LonGas). Ann Oncol. 2022;33(suppl_4):S380. [Google Scholar]
- 58. Yoshino T, Cleary JM, Van Cutsem E, et al. Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann Oncol. 2020;31(1):88-95. 10.1016/j.annonc.2019.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Giuliani J, Bonetti A.. The onset of frade ≥3 neutropenia is associated with longer overall survival in metastatic colorectal cancer patients treated with trifluridine/tipiracil. Anticancer Res. 2019;39(7):3967-3969. 10.21873/anticanres.13551 [DOI] [PubMed] [Google Scholar]
- 60. Kasi PM, Kotani D, Cecchini M, et al. Chemotherapy induced neutropenia at 1-month mark is a predictor of overall survival in patients receiving TAS-102 for refractory metastatic colorectal cancer: a cohort study. BMC Cancer 2016;16:467. 10.1186/s12885-016-2491-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shiga T, Hiraide M.. Cardiotoxicities of 5-fluorouracil and other fluoropyrimidines. Curr Treat Options Oncol. 2020;21(4):27. 10.1007/s11864-020-0719-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lopez CA, Azimi-Nekoo E, Chung SY, et al. Meta-analysis and systematic review of the cardiotoxicity of TAS-102. J Clin Oncol. 2020;38(15_suppl):e16053-e16053. 10.1200/jco.2020.38.15_suppl.e16053 [DOI] [Google Scholar]
- 63. Vaflard P, Ederhy S, Torregrosa C, et al. Fluoropyrimidines cardiac toxicity: 5-fluorouracil, capecitabine, compound S-1 and trifluridine/tipiracil. Bull Cancer. 2018;105(7-8):707-719. 10.1016/j.bulcan.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 64. Wörmann B, Bokemeyer C, Burmeister T, et al. Dihydropyrimidine dehydrogenase testing prior to treatment with 5-fluorouracil, capecitabine, and tegafur: a consensus paper. Oncol Res Treat 2020;43(11):628-636. 10.1159/000510258 [DOI] [PubMed] [Google Scholar]
- 65. Lenz H-J, Stintzing S, Loupakis F.. TAS-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev. 2015;41(9):777-783. 10.1016/j.ctrv.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nakanishi R, Tsutsui A, Tanaka H, et al. A case in which TAS-102 produced disease control without severe adverse events in a patient with recurrent colorectal cancer and dihydropyrimidine dehydrogenase deficiency. Med Clin Res. 2021;6(11):726-729. [Google Scholar]
- 67. Schouten JF, Willems J, Sanders SJWJ, Creemers G-J, Deenen MJ.. Standard-dose trifluridine/tipiracil as safe treatment alternative in metastatic colorectal cancer patients with DPD deficiency. Clin Colorectal Cancer. 2021;20(4):359-363. 10.1016/j.clcc.2021.09.004 [DOI] [PubMed] [Google Scholar]
- 68. Bolzacchini E, Luchena G, Giordano M.. Safety report of TAS-102 in a patient with reduced DPD activity. Clin Colorectal Cancer. 2019;18(4):310-312. 10.1016/j.clcc.2019.07.008 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were generated or analyzed in support of this research.