Abstract
Purpose
Immune checkpoint inhibitors (ICIs) have significantly improved the survival of patients with cancer and provided long-term durable benefit. However, ICI-treated patients develop a range of toxicities known as immune-related adverse events (irAEs), which could compromise clinical benefits from these treatments. As the incidence and spectrum of irAEs differs across cancer types and ICI agents, it is imperative to characterize the incidence and spectrum of irAEs in a pan-cancer cohort to aid clinical management.
Design
We queried >400 000 trials registered at ClinicalTrials.gov and retrieved a comprehensive pan-cancer database of 71 087 ICI-treated participants from 19 cancer types and 7 ICI agents. We performed data harmonization and cleaning of these trial results into 293 harmonized adverse event categories using Medical Dictionary for Regulatory Activities.
Results
We developed irAExplorer (https://irae.tanlab.org), an interactive database that focuses on adverse events in patients administered with ICIs from big data mining. irAExplorer encompasses 71 087 distinct clinical trial participants from 343 clinical trials across 19 cancer types with well-annotated ICI treatment regimens and harmonized adverse event categories. We demonstrated a few of the irAE analyses through irAExplorer and highlighted some associations between treatment- or cancer-specific irAEs.
Conclusion
The irAExplorer is a user-friendly resource that offers exploration, validation, and discovery of treatment- or cancer-specific irAEs across pan-cancer cohorts. We envision that irAExplorer can serve as a valuable resource to cross-validate users’ internal datasets to increase the robustness of their findings.
Keywords: immune checkpoint inhibitor, immune-related adverse events, irAExplorer, data mining
This article reports the development of irAExplorer, an interactive database of adverse events in patients administered immune checkpoint inhibitors.
Implications for Practice.
Immune-related adverse events (irAEs) represent a repertoire of toxicity profile affecting multiple organ systems. This study profiled the full spectrum of severe adverse events from more than 400 000 participants in clinical trials treated by immune checkpoint inhibitor (ICI). Because of the large sample size, we identified cancer- or treatment-specific ICI-induced adverse events. To facilitate data exploration, the pan-cancer analysis of ICI-induced adverse events is available in an interactive data portal. Identification of irAE can lead to early intervention and optimal management of these patients and may guide patient treatment decisions.
Introduction
Immune checkpoint inhibitors (ICIs) have transformed cancer treatment by improving patient survival and clinical outcome. However, the administration of ICIs may lead to treatment toxicities, known as immune-related adverse events (irAEs). The occurrence of irAEs varies in severity and is graded based on the Common Terminology Criteria for Adverse Events. Severe irAEs (grade ≥ 3) require clinical intervention and can be life-threatening. As the clinical application of ICIs expands to include multiple cancer types1 and treatment modalities, it becomes imperative that the landscape of irAEs be characterized to enable better clinical management.
The earliest recorded irAEs associated with an anti-CTLA4 agent are colitis and diarrhea which were initially discovered in patients with melanoma.2 Subsequently, a diverse range of irAEs have been documented, and the incidence and types of irAEs differ among different ICI agents.3-5 These differences in irAE profiles highlight the need for a thorough understanding of the specific adverse events associated with each ICI agent. Moreover, for the treatment of various tumors, ICI can be administered through different approaches, including monotherapy, combination therapy with other ICIs, or in combination with chemotherapy (ICI plus chemotherapy).
The incidence and patterns of irAEs can vary depending on the specific combination of treatments used. It is, therefore, crucial to consider the entire spectrum of irAEs associated with different treatment regimens. Current systematic reviews of irAE are mostly confined to specific cancer types or ICI agents.6,7 Additionally, individual studies often lack the sample size to differentiate irAEs between treatment regimens and in documenting rare irAEs. Here, we report the development of a pan-cancer irAE database, termed irAExplorer. irAExplorer is built on the mining of over 430 000 clinical trial records deposited at ClinicalTrials.gov. Through data curation and harmonization, we have created a comprehensive dataset of 19 cancer types encompassing 74 541 distinct clinical trial participants across distinct ICI treatment regimens. Additionally, we packaged this curated database as a user-friendly web portal accessible to researchers and clinicians to explore the incidence of cancer- and treatment-specific irAEs.
Material and Methods
Extraction and Harmonization of ClinicalTrials.gov Database
We extracted all 434 409 records deposited to ClinicalTrials.gov as of November 28, 2022. The records were filtered (Supplementary Methods) to only include completed trials involving FDA-approved ICI: ipilimumab, nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab, cemiplimab, relatlimab, dostarlimab, and tremelimumab. This resulted in 343 clinical trials covering 71 087 participants (Supplementary Fig. S1). We harmonized the treatment agent, treatment regimens, and cancer types in each arm (Supplementary Methods and Table S1).
Extraction and Harmonization of Severe Adverse Events Terms
We extracted the incidence of serious (severe) adverse events based on 2 hierarchy of adverse event terms. The first was according to the “System Organ Classes” (SOCs), a confined list of 26 high-level adverse event terms based on “etiology, manifestation sites, or purpose,” as defined by Medical Dictionary for Regulatory Activities. The second was hierarchy (termed secondary hierarchy), included an unrestricted set of adverse event terms freely described at the investigator’s discretion. To consolidate differences between individual records, we harmonized the adverse event terms (Supplementary Methods and Table S2). The harmonization steps are similar to our previous published results.8
Data Availability
The source code for processing CliniclTrials.gov data is made available at public repository on GitHub (https://github.com/zakiF/PublishedPapers/tree/master/irAExplorer).
Results
Construction of irAExplorer
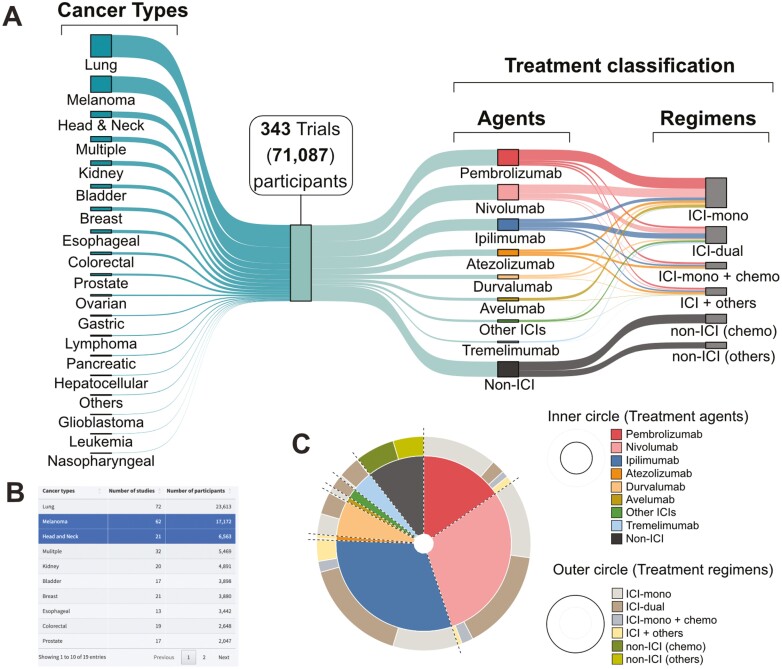
To generate a comprehensive pan-cancer irAE database, we screened the 434 409 clinical trial records deposited at ClinicalTrials.gov. We identified and performed data mining on 343 trials in which ICIs were administered to 71 087 participants (Supplementary Methods; Fig. 1A). This database was analyzed and made available as an interactive web resource called irAExplorer (https://irae.tanlab.org). We captured 19 cancer types including lung cancer (31%, n = 21 994 participants), followed by melanoma (19%, n = 13 704), as the most reported cancer types in clinical trials involving ICI treatment (Fig. 1A, left). A 2-level treatment classification was devised according to the treatment agents and treatment regimens. Trial participants were first classified based on the administered agents and secondly divided into 6 groups based on their treatment regimens (Supplementary Methods; Fig. 1A, right).
Figure 1.
Breakdown of cancer types and treatment classification in the database. (A) Sankey diagram illustrating the proportion of participants across cancer types (left), treatment agents, and treatment regiments (right). Example of the web interface. (B) Table of the cancer types available in the database. User may select cancer(s) of interest as highlighted in blue. (C) Based on the selected cancer(s) in the table, a dual-layer pie chart is generated to show the treatment agents in the inner ring while the treatment regimens are shown in the outer ring. Abbreviations: ICI, immune checkpoint inhibitor; mono, monotherapy; chemo, chemotherapy.
Across all cancer types, the most commonly administered ICI agent was anti-PD1 therapy (pembrolizumab, 21%; nivolumab, 20%) followed by anti-CTLA4 (ipilimumab, 15%). We also identified 12% of participants who received only chemotherapy as part of a control treatment arm in certain ICI clinical trials (Supplementary Fig. S2A). The administration patterns were different between anti-CTLA4 and anti-PD1 agents. The median percentage of anti-PD1 agents administered as dual therapy was 19%, compared to 57% for monotherapy (Supplementary Fig. S2B). Conversely, anti-CTLA4 was administered as dual therapy at 51%, compared to monotherapy at 24%. Through the web portal, users may interactively explore the treatment landscape of both ICI agent(s) and cancer(s) of interest (Fig. 1B, 1C).
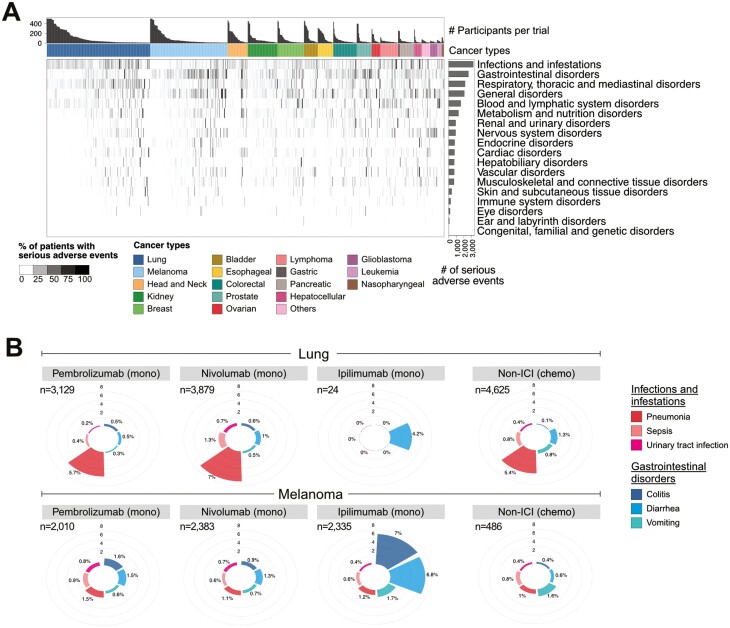
Next, we examined the reported incidence of severe adverse events in all participants. Across all treatment agents or treatment regimens, 44% (31 437 of 71 087 participants) developed severe adverse events (Supplementary Fig. S3). The overall incidence of severe adverse events differed across cancer types, with the highest in leukemia at 75% (Supplementary Fig. S3). In general, combination ICI therapy leads to more severe adverse events, a trend consistent with previous reports and observations in clinical trials.4-6 To obtain further granularity on the adverse event categories, we extracted 2 hierarchies of adverse event categorization: SOCs and their associated secondary-level adverse event terms (Supplementary Table S2). Across cancer types, “infections and infestations” and “gastrointestinal disorders” were the most frequently reported SOC (Fig. 2A).
Figure 2.
Frequency of severe adverse events. (A) Frequency of severe adverse events in each clinical trial as summarized by the SOC hierarchy. The bar graph above the heatmap shows the number of participants recruited in a trial. For the purpose of visualization, the maximum number of participants shown is capped at 500, and trials with less than 50 recruited participants are not shown. The intensity of the heatmap represents the frequency of the reported SOC in each trial. The horizontal bar graph to the right shows the total number of severe adverse events by SOC category. (B) The frequency of secondary-level adverse events terms associated with the SOC “infections and infestation” (shades of red) and “gastrointestinal disorders” (shades of blue) in lung (top panel) and melanoma (bottom panel). The number of participants administered single-agent monotherapy (mono) or chemotherapy (chemo) alone is indicated on the top-left section of the plot.
As SOCs are considered generic terms, we further explored the secondary-level terms associated with these SOCs across the 3 most common ICI agents. The top 3 secondary-level adverse event terms under gastrointestinal disorder SOC included colitis, diarrhea, and vomiting, while pneumonia, sepsis, and urinary tract infection were the most frequent terms under infections and infestation SOC. A side-by-side comparison of these secondary-level adverse event terms between lung and patients with melanoma revealed that pneumonia was more frequent in patients with lung cancer, and the frequency was similar across participants treated with pembrolizumab, nivolumab, or chemotherapy (Fig. 2B). This suggests that pneumonia may be an adverse event that is not associated with ICI therapy. In contrast, we observed that the incidence of colitis and diarrhea was higher in ipilimumab than with pembrolizumab and nivolumab in patients with melanoma (Fig. 2B). Additionally, these were not observed in chemotherapy-treated participants, suggesting that these were adverse events induced by ipilimumab, consistent with previous reports.4,9 Given the low number of patients with lung cancer administered ipilimumab monotherapy (n = 24), it is difficult to draw conclusions on the effects of ipilimumab in this setting. Overall, the construction of irAE explorer with the inclusion of non-ICI agents allows for the identification of cancer- or treatment- specific irAEs.
Pan-Cancer Incidents of Severe Adverse Events
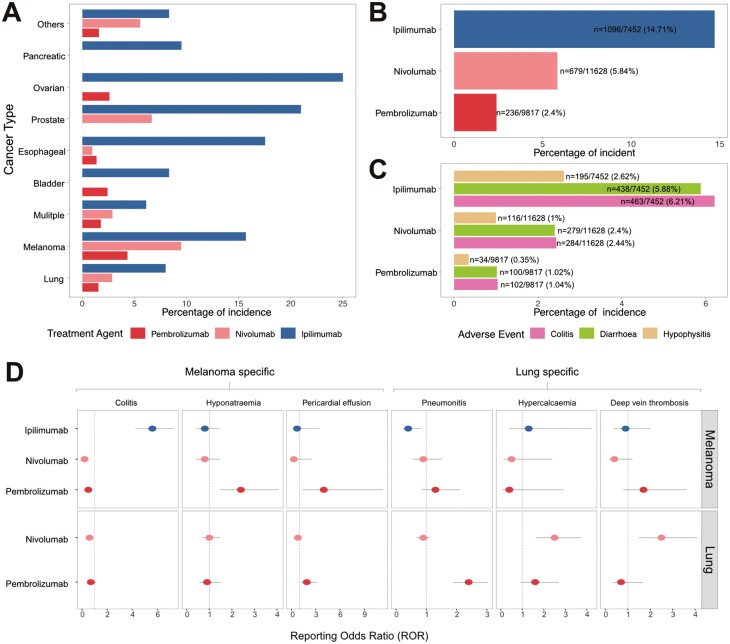
Previous reports on cancer- or treatment-specific irAEs have been limited to a few cancer types and ICI agents.6,7 To demonstrate the use of irAExplorer in pan-cancer investigations, we studied the incidence of irAEs related to anti-CTLA4 treatment, such as colitis, diarrhea, and hypophysitis.9,10 We identified 9 cancer types in which ipilimumab was administered to at least 20 participants as monotherapy or combination therapy. Across these 9 cancer types, the aggregated incidence of these 3 irAEs was higher in anti-CTLA4 treated participants compared with anti-PD1 (Fig. 3A). Moreover, through the interactive irAExplorer portal, users may choose to simplify the representation by aggregating observations across cancer types (Fig. 3B) or a more detailed representation of the individual irAE term (Fig. 3C). Due to the advantage of high number of participants in our database, we also examined the incidence of rare irAEs, such as diabetes and adrenal insufficiency. Incidence of ICI-induced diabetes has been reported at a frequency of 0.2%-1.9% and appear more frequent in anti-PD1 treatment.11-13 Querying all participants treated with ICI monotherapy (n = 31 611), we showed that the highest incidence of diabetes mellitus was in participants treated with the anti-PD1 agent pembrolizumab whilst adrenal insufficiently was more common in ipilimumab-treated participants. (Supplementary Fig. S4A). It is unclear whether, in some clinical trials, primary and secondary adrenal insufficiency are reported similarly, the latter of which can occur in individuals with hypophysitis, which we found also enriched in the anti-CTLA4-treated patients. Finally, we used irAExplorer to query cancer-specific irAEs, such as pneumonitis, which have been reported to have a higher incidence in lung cancer.14 Across all participants administered pembrolizumab monotherapy (n = 20 441), the incidence of pneumonitis is highest in lung and nasopharyngeal cancers at 2.4% (Supplementary Fig. S4B). It is interesting to note that a previous study on pembrolizumab administered to nasopharyngeal also recorded pneumonitis as the most common severe adverse event.15 Taken together, irAExplorer is a powerful resource to facilitate the identification of cancer- or treatment-specific irAEs.
Figure 3.
Comparison of cancer and treatment-specific immune-related adverse events (irAEs). (A) The aggregated frequency of 3 anti-CTLA4 specific irAEs (colitis, diarrhea, and hypophysis) in pembrolizumab, nivolumab, and ipilimumab monotherapy and combination therapy. (B, C) The bar graphs represent the aggregated incident of colitis, diarrhea, and hypophysis across all the cancer types shown in panel A. The numbers on the bar graph indicate the number of participants with the reported irAEs over the total number of participants administered the ICI agent. (D) The reporting odds ratio (ROR) of the top severe adverse events in melanoma and lung cancer participants treated with ipilimumab, pembrolizumab, or nivolumab monotherapy.
Having established that our database was able to generate data consistent with published observations, we calculated proportional reporting ratio (PRR) and reporting odds ratio (ROR) to assess the statistical incidence of the reported “secondary-level adverse event terms” as reported by ClinicalTrial.gov (Supplementary Methods). Overall, there was a high degree of correlation between these 2 measures, indicating the robustness of our dataset (Supplementary Fig. S5). To identify other potential cancer- or treatment-specific irAEs, we compared the ROR of the 2 most recorded cancer types (melanoma and lung cancer) treated with single-agent pembrolizumab, nivolumab, or ipilimumab. Due to the small number of patients with lung cancer administered ipilimumab monotherapy (n = 24), the ROR results were not presented. Overall, we identified 6 adverse events with the highest ROR values. The 3 adverse events considered more frequent in melanoma than in lung cancer were colitis, hyponatremia, and pericardial effusion (Fig. 3D). The incidence of colitis in patients with melanoma is associated with ipilimumab treatment, whereas hyponatremia and pericardial effusion are associated with pembrolizumab treatment. In contrast, the ROR values for pneumonitis, hypercalcemia, and deep vein thrombosis were the highest in lung cancer (Fig. 3D). Pneumonitis is associated with pembrolizumab, whereas hypercalcemia and deep vein thrombosis are more associated with nivolumab than with pembrolizumab in patients with lung cancer.
Overall, the collective information extracted from over 70 000 participants forms the backbone of our database. We demonstrated the reliability of our database in validating previously reported irAEs and its use as a platform to identify potential new cancer- or treatment-specific irAEs. To facilitate public access, our curated database was packaged as an interactive web resource called irAExplorer (https://irae.tanlab.org). Users may interactively explore the landscape of cancer- and treatment-irAEs from extensively curated clinical trials using a range of visualizations and statistical means.
Discussion
The success of ICI treatment in patients with cancer has led to an increase in the number of FDA-approved ICI agents available in clinical trials. However, patients treated with ICIs can develop a myriad of adverse events which are different from toxicities observed with chemotherapies.16 Despite efforts to identify ICI-associated adverse events, large-scale analysis to identify irAEs across cancer types and treatment agents is still lacking. In this study, we generated a comprehensive pan-cancer database of ICI-treated participants encompassing 74 541 participants from 19 cancer types, 7 ICI agents, and 293 harmonized adverse event categories. We made the database available as an interactive web resource termed irAExplorer.
Using colitis as a proof of concept, we demonstrated that irAExplorer data can recapitulate the differences in incidence and unique irAE profiles among patients treated with different ICI agents and regimens. The large number of participants in the irAExplorer database offers further possibilities for examining rare irAE cases specific to a particular ICI treatment or cancer type. We identified a cardiac-related adverse event (pericardial effusion) as an adverse event associated with patients with melanoma treated with pembrolizumab monotherapy. Our observation is in line with a previous publication, which suggests that treatment with anti-PD-1 agent is more associated with pericardial disease than anti-CTLA4 agent.17 However, contrary to our observation that pericardial effusion is more common in melanoma than in lung cancer, the same study reported the opposite. Given that only 2 patients with melanoma were recruited from the study, the discrepancy could be due to the small sample size. This highlights the opportunity offered by irAE explorer to identify irAEs incidence in a large cohort of participants.
At present, there is a lack of standardized methodology to differentiate irAEs from adverse events of other etiologies. The inclusion of chemotherapy comparative cohorts without ICI in our database could serve as a control in determining which adverse events can be considered irAEs. Until recently, ICI-associated hypercalcemia and thrombosis were not well documented.18,19 We reported high ROR for hypercalcemia and deep vein thrombosis in patients with lung cancer treated with nivolumab. Coupling our observation with the recent reports, irAExplorer serves as a useful tool to identify cancer- and treatment-specific irAEs.
Mining a public resource dataset has certain limitations. Primarily, the quality of clinical trial records depends on the individual investigators. As such, we observed a discrepancy in the descriptions of adverse events and cancer types. This was mitigated by our efforts to harmonize the categorization. Moreover, the granularity of adverse event incidence was not reported at the individual level, but rather as an aggregate per clinical trial record. This prevents further comorbidity analysis or association analysis between irAEs and immunotherapy response. Notably, caution should be exercised when extrapolating the incidence rate of adverse events from our database. As the participants in our database were recruited from neoadjuvant and adjuvant settings, the incidence of adverse events is known to differ.20 Moreover, the onset of irAEs varies from a few days to more than 1 year after ICI treatment.21,22 Given the differences in the follow-up periods across these clinical trials, the potential for under-reporting due to delayed adverse event onset is unclear. In addition, the incidence of adverse events is known to differ between low-dose and high-dose ICI.23,24 In our current study, we did not consider the relationship between ICI dosage and irAE which may under- or over-represent some previously documented dose-dependent irAE such as endocrine and dermatological irAEs. Finally, we tried to segregate the combinations of specific ICI + chemotherapy or ICI + targeted therapy in the current study for comparing ICI-related adverse events. However, we do not separate the combination of specific ICI + antibody-drug conjugates (ADC) which represents a limitation of this current study. Moving forward, we plan to add further granularity to the patient cohort such as defining if they were treated with ICIs in the naive setting, or with early-stage disease, which will be important factors to consider on the type of treatment or cancer type.
Overall, we believe that the large number of samples in our dataset coupled with the interactive web portal will facilitate investigations of cancer- and treatment-specific irAEs. In addition, irAExplorer can serve as a valuable resource to cross-validate users’ internal datasets to increase the robustness of their findings.
Supplementary Material
Supplementary material is available at The Oncologist online.
Acknowledgments
We thank the Tan Lab members for their valuable comments on this work.
Contributor Information
Muhammad Zaki Hidayatullah Fadlullah, Department of Oncological Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; Department of Biomedical Informatics, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Ching-Nung Lin, Department of Oncological Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; Department of Biomedical Informatics, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Samuel Coleman, Department of Oncological Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; Department of Biomedical Informatics, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Arabella Young, Department of Pathology, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Abdul Rafeh Naqash, Medical Oncology/TSET Phase 1 Program, Stephenson Cancer Center, The University of Oklahoma, Oklahoma City, OK, USA.
Siwen Hu-Lieskovan, Division of Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Aik Choon Tan, Department of Oncological Sciences, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA; Department of Biomedical Informatics, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Funding
This work was partly supported by the National Institutes of Health (NIH) under Award No. R01DE030508 (to A.C.T.). The content is solely the responsibility of the authors and does not necessarily represent the views of the funders.
Conflict of Interest
Abdul Rafeh Naqash reported serving on the advisory board for Foundation Medicine. The other authors indicated no financial relationships.
Author Contributions
Conception/design: M.Z.H.F. and A.C.T. Collection and/or assembly of data: M.Z.H.F. Methods development: M.Z.H.F. and A.C.T. Web portal implementation: M.Z.H.F. and C.N.L. Data analysis and interpretation: All authors. Final approval of manuscript: All authors.
Data Availability
The source code for processing CliniclTrials.gov data is made available at public repository on GitHub (https://github.com/zakiF/PublishedPapers/tree/master/irAExplorer). irAExplorer is freely available at https://irae.tanlab.org.
References
- 1. Twomey JD, Zhang B.. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. 2021;23(2):39. 10.1208/s12248-021-00574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maker AV, Phan GQ, Attia P, et al. Tumor regression and autoimmunity in patients treated with cytotoxic T lymphocyte–associated antigen 4 blockade and interleukin 2: a phase I/II study. Ann Surg Oncol. 2005;12(12):1005-1016. 10.1245/ASO.2005.03.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ramos-Casals M, Brahmer JR, Callahan MK, et al. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6(1):38. 10.1038/s41572-020-0160-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tawbi HA, Schadendorf D, Lipson EJ, et al. ; RELATIVITY-047 Investigators. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med. 2022;386(1):24-34. 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer. 2019;145(3):639-648. 10.1002/ijc.32132 [DOI] [PubMed] [Google Scholar]
- 7. Khoja L, Day D, Chen TWW, Siu LL, Hansen AR.. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol. 2017;28(10):2377-2385. 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 8. Federer C, Yoo M, Tan AC.. Big data mining and adverse event pattern analysis in clinical drug trials. Assay Drug Dev Technol. 2016;14(10):557-566. 10.1089/adt.2016.742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang ML, Neyaz A, Patil D, et al. Immune-related adverse events in the gastrointestinal tract: diagnostic utility of upper gastrointestinal biopsies. Histopathology. 2020;76(2):233-243. 10.1111/his.13963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Faje AT, Sullivan R, Lawrence D, et al. Ipilimumab-induced hypophysitis: a detailed longitudinal analysis in a large cohort of patients with metastatic melanoma. J Clin Endocrinol Metab. 2014;99(11):4078-4085. 10.1210/jc.2014-2306 [DOI] [PubMed] [Google Scholar]
- 11. Quandt Z, Young A, Perdigoto AL, Herold KC, Anderson MS.. Autoimmune endocrinopathies: an emerging complication of immune checkpoint inhibitors. Annu Rev Med. 2021;72(1):313-330. 10.1146/annurev-med-050219-034237 [DOI] [PubMed] [Google Scholar]
- 12. Stamatouli AM, Quandt Z, Perdigoto AL, et al. Collateral damage: insulin-dependent diabetes induced with checkpoint inhibitors. Diabetes. 2018;67(8):1471-1480. 10.2337/dbi18-0002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marsiglio J, McPherson JP, Kovacsovics-Bankowski M, et al. A single center case series of immune checkpoint inhibitor-induced type 1 diabetes mellitus, patterns of disease onset and long-term clinical outcome. Front Immunol. 2023;14:1229823. 10.3389/fimmu.2023.1229823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271-281. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 15. Hsu C, Lee SH, Ejadi S, et al. Safety and antitumor activity of pembrolizumab in patients with programmed death-ligand 1–positive nasopharyngeal carcinoma: results of the KEYNOTE-028 study. J Clin Oncol. 2017;35(36):4050-4056. 10.1200/JCO.2017.73.3675 [DOI] [PubMed] [Google Scholar]
- 16. Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 17. Inno A, Maurea N, Metro G, et al. Immune checkpoint inhibitors-associated pericardial disease: a systematic review of case reports. Cancer Immunol Immunother. 2021;70(10):3041-3053. 10.1007/s00262-021-02938-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moik F, Chan WSE, Wiedemann S, et al. Incidence, risk factors, and outcomes of venous and arterial thromboembolism in immune checkpoint inhibitor therapy. Blood. 2021;137(12):1669-1678. 10.1182/blood.2020007878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Izzedine H, Chazal T, Wanchoo R, Jhaveri KD.. Immune checkpoint inhibitor–associated hypercalcaemia. Nephrol Dial Transplant. 2022;37(9):1598-1608. 10.1093/ndt/gfaa326 [DOI] [PubMed] [Google Scholar]
- 20. Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med. 2018;24(11):1655-1661. 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 21. Parakh S, Cebon J, Klein O.. Delayed autoimmune toxicity occurring several months after cessation of anti‐PD‐1 therapy. Oncologist. 2018;23(7):849-851. 10.1634/theoncologist.2017-0531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yoest JM. Clinical features, predictive correlates, and pathophysiology of immune-related adverse events in immune checkpoint inhibitor treatments in cancer: a short review. Immuno Targets Ther. 2017;6:73-82. 10.2147/ITT.S126227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hryniewicki AT, Wang C, Shatsky RA, Coyne CJ.. Management of immune checkpoint inhibitor toxicities: a review and clinical guideline for emergency physicians. J Emerg Med. 2018;55(4):489-502. 10.1016/j.jemermed.2018.07.005 [DOI] [PubMed] [Google Scholar]
- 24. Chhabra N, Kennedy J.. A review of cancer immunotherapy toxicity: immune checkpoint inhibitors. J Med Toxicol. 2021;17(4):411-424. 10.1007/s13181-021-00833-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The source code for processing CliniclTrials.gov data is made available at public repository on GitHub (https://github.com/zakiF/PublishedPapers/tree/master/irAExplorer).
The source code for processing CliniclTrials.gov data is made available at public repository on GitHub (https://github.com/zakiF/PublishedPapers/tree/master/irAExplorer). irAExplorer is freely available at https://irae.tanlab.org.