Abstract
Insulin, insulin-like growth factors (IGF) and their receptors are highly expressed in the adult hippocampus. Thus, disturbances in the insulin-IGF signalling pathway may account for the selective vulnerability of the hippocampus to nascent Alzheimer's disease (AD) pathology. In the present study, we examined the predominant IGF-binding protein in the CSF, IGFBP2.
CSF was collected from 109 asymptomatic members of the parental history-positive PREVENT-AD cohort. CSF levels of IGFBP2, core AD and synaptic biomarkers were measured using proximity extension assay, ELISA and mass spectrometry. Cortical amyloid-beta (Aβ) and tau deposition were examined using 18F-NAV4694 and flortaucipir. Cognitive assessments were performed during up to 8 years of follow-up, using the Repeatable Battery for the Assessment of Neuropsychological Status. T1-weighted structural MRI scans were acquired, and neuroimaging analyses were performed on pre-specified temporal and parietal brain regions. Next, in an independent cohort, we allocated 241 dementia-free ADNI-1 participants into four stages of AD progression based on the biomarkers CSF Aβ42 and total-tau (t-tau). In this analysis, differences in CSF and plasma IGFBP2 levels were examined across the pathological stages. Finally, IGFBP2 mRNA and protein levels were examined in the frontal cortex of 55 autopsy-confirmed AD and 31 control brains from the Quebec Founder Population (QFP) cohort, a unique population isolated from Eastern Canada.
CSF IGFBP2 progressively increased over 5 years in asymptomatic PREVENT-AD participants. Baseline CSF IGFBP2 was positively correlated with CSF AD biomarkers and synaptic biomarkers, and negatively correlated with longitudinal changes in delayed memory (P = 0.024) and visuospatial abilities (P = 0.019). CSF IGFBP2 was negatively correlated at a trend-level with entorhinal cortex volume (P = 0.082) and cortical thickness in the piriform (P = 0.039), inferior temporal (P = 0.008), middle temporal (P = 0.014) and precuneus (P = 0.033) regions. In ADNI-1, CSF (P = 0.009) and plasma (P = 0.001) IGFBP2 were significantly elevated in Stage 2 [CSF Aβ(+)/t-tau(+)]. In survival analyses in ADNI-1, elevated plasma IGFBP2 was associated with a greater rate of AD conversion (hazard ratio = 1.62, P = 0.021). In the QFP cohort, IGFBP2 mRNA was reduced (P = 0.049); however, IGFBP2 protein levels did not differ in the frontal cortex of autopsy-confirmed AD brains (P = 0.462).
Nascent AD pathology may induce an upregulation in IGFBP2 in asymptomatic individuals. CSF and plasma IGFBP2 may be valuable markers for identifying CSF Aβ(+)/t-tau(+) individuals and those with a greater risk of AD conversion.
Keywords: insulin-like growth factor-binding protein-2, insulin-like growth factor, Alzheimer's disease, cerebrospinal fluid, post-mortem brain tissue, RBANS
Brain changes associated with Alzheimer's disease can begin up to 20 years or more before the onset of symptoms. Quesnel et al. find that a protein called IGFBP2 may be a valuable marker for identifying at-risk individuals and could serve as a drug target to restore deficient insulin signalling in the Alzheimer's disease brain.
Introduction
By 2050, is it estimated that 152.8 million individuals worldwide will be affected by dementia.1 Furthermore, the cost of dementia care is projected to reach $16.9 trillion in 2050.2 Alzheimer's disease (AD) remains one of the most challenging medical mysteries, as it is the most common cause of dementia—accounting for 60–80% of all cases.3 The neuropathological hallmarks of AD include the accumulation of extracellular amyloid-beta (Aβ) plaques, intracellular neurofibrillary tangles, as well as the loss of synapses and neurons.4 These brain changes are believed to begin up to 20 years or more before the onset of symptoms.5
It has been well established that insulin resistance and diabetes are risk factors for developing AD.6,7 Indeed, impaired insulin and insulin-like growth factor (IGF) signalling plays a critical role in the pathogenesis of AD.8-10 Post-mortem studies have demonstrated that insulin and IGFs, as well as their receptors and downstream signalling molecules, are decreased in the AD brain.8-11 Furthermore, the insulin-IGF system has been shown to directly modulate Aβ degradation12 and clearance,13 phospho-tau production,14,15 synaptic integrity16 and neuronal survival.17 It is also known that insulin, IGFs and their receptors are highly expressed in the hippocampus, relative to the frontal cortex in the human brain.8 Overall, these findings suggest that impairments in insulin-IGF signalling may account for the selective vulnerability of the hippocampus to nascent AD pathology, and therefore, account for early impairments in episodic memory. Similarly, deficiencies in insulin-IGF signalling may contribute to the reductions in glucose metabolism that are seen in patients with AD and individuals at risk for AD.18 The importance of insulin and IGFs has been emphasized in pilot clinical trials in which the administration of intranasal insulin improved memory, caregiver-rated functional abilities and glucose metabolism in individuals with mild cognitive impairment (MCI) or mild AD.19,20
The insulin-IGF system encompasses a complex collection of proteins that play pivotal roles in glucose metabolism, neurogenesis, synaptogenesis and cell survival.17,21,22 Insulin, IGF-I and IGF-II are the key proteins, which bind to their cell surface receptors.22 The actions of IGF-I and IGF-II are modulated by six IGF-binding proteins (IGFBP), which can bind to IGFs with an equal or greater affinity than the IGF receptors.23,24 Indeed, in the circulation, CSF and local tissues, most extracellular IGFs are bound to so-called IGFBPs, which prolong the half-life of IGFs.22-24 For instance, it has been proposed that IGFBPs may prevent the degradation of IGFs during transport and mobilization, and target IGFs to their receptors.22-24 The latter may be achieved through IGFBPs binding to cell-surface proteoglycans25 and integrins,26 or proteolytic cleavage,27 both of which reduce the binding affinity of IGFBPs for IGFs and promote IGF release.22
The main objective of the current study was to examine a less studied member of the IGF molecular cascade, insulin-like growth factor binding protein-2 (IGFBP2), in both the pre-symptomatic and symptomatic stages of AD. Since IGFBP2 is the most abundant IGFBP in the CSF,28,29 we hypothesized that IGFBP2 plays a critical role in the neurodegenerative process, most likely at the level of neuroprotection and resilience.22 IGFBP2 is increased in the CSF and plasma of patients with a clinical diagnosis of AD,30-35 and is associated with longitudinal atrophy in entorhinal, parahippocampal and inferior temporal regions.36 Moreover, elevated circulating levels of IGFBP2 have been associated with an increased risk of developing AD.35,37,38 Finally, in a pilot study, IGFBP2 has been found to be decreased in the temporal cortex of AD patients.9 These and other findings were verified in the pre-symptomatic and symptomatic stages of the disease, as well as in autopsy-confirmed AD brains.
Materials and methods
PREVENT-AD cohort
Study participants
The PRe-symptomatic EValuation of Experimental or Novel Treatments for Alzheimer's Disease (PREVENT-AD) cohort consists of asymptomatic, ‘at-risk’, individuals with a parental or multi-sibling history of sporadic AD.39 The majority of participants were over the age of 60; however, individuals aged 55–59 years were included if they were within 15 years of the onset of their youngest-affected relative's symptoms. To confirm normal cognition, the Clinical Dementia Rating and Montreal Cognitive Assessment were used at the study eligibility visit. Active PREVENT-AD participants (n = 386) have been followed longitudinally, as annual visits include cognitive assessments, neurosensory tests, blood and (for a subset of individuals) CSF collections, structural and functional MRI scans, as well as PET scans. Each participant and their study partner provided written informed consent. All procedures were approved by the McGill University Faculty of Medicine Institutional Review Board and complied with the ethical principles of the Declaration of Helsinki. A detailed description of the PREVENT-AD cohort is available elsewhere.39
CSF measurements
A Sprotte 24-gauge atraumatic needle was used to perform lumbar punctures in PREVENT-AD participants, following an overnight fast. To exclude cells and insoluble material, CSF samples were centrifuged (∼2000g) within 4 h, for 10 min at room temperature. Finally, the CSF samples were aliquoted (0.5 ml) into polypropylene cryotubes and stored at −80°C.
CSF IGFBP2 levels were measured in a subset of PREVENT-AD participants (n = 109) using the Olink Cardiovascular III panel, which employs proximity extension assay technology. Olink measurements are expressed in arbitrary Normalized Protein eXpression units (NPX), which are on a log2 scale.
CSF AD biomarkers amyloid-beta (Aβ42), phosphorylated tau (p181-tau) and total tau (t-tau) were measured in a subset (n = 101) of PREVENT-AD participants, using the validated Innotest ELISA kit (Fujirebio) following the standardized protocols established by the Biomarkers for Alzheimer's and Parkinson's Disease (BIOMARKAPD) consortium (Aβ42, Cat. No. 81583; p181-tau, Cat. No. 81581; and t-tau, Cat. No. 81579).
Of the 109 PREVENT-AD participants that had CSF IGFBP2 measurements, 106 individuals had the synaptic proteins synaptosomal-associated protein 25 (SNAP25) and synaptotagmin-1 (SYT1) assayed. CSF SNAP25 and SYT1 were immunoprecipitated and their concentrations were determined by mass spectrometry, as previously described.40,41,42 Mass spectrometry results are expressed in arbitrary units.43 As previously reported, CSF levels of growth-associated protein 43 (GAP43) and neurogranin (NRGN) were assessed by validated ELISAs in a subset of PREVENT-AD individuals (n = 46).44,45
Neuroimaging acquisition and processing
In vivo cortical Aβ and phosphorylated tau pathologies were determined using PET tracers 18F-NAV4694 (Navidea Biopharmaceuticals) and flortaucipir (18F-AV1451; Eli Lilly and Co.) in a subset of PREVENT-AD participants that also had CSF IGFBP2 measurements (n = 46 and n = 49, respectively). Aβ and tau PET scans were performed 40–70 min and 80–100 min post-injection, respectively. A 3T Siemens Trio scanner was used to acquire T1-weighted structural MRI scans at the Douglas Mental Health University Institute (Montreal). A Siemens standard 12- or 32-channel coil was used (Siemens Medical Solutions). FreeSurfer 5.3 was used to process the MRI scans, and the Desikan–Killiany atlas was used for parcellation. The preprocessing pipeline for PET images has previously been described.46 Briefly, standardized uptake value ratios (SUVRs) were generated by dividing the signal in the regions of interest (ROIs) by the signal in the reference region. Thus, cerebellar grey matter was used as a reference region for 18F-NAV4694, whilst the inferior cerebellar grey matter was used for flortaucipir. A global cortical ROI was computed to evaluate Aβ deposition, whilst tau deposition was assessed by averaging flortaucipir SUVRs in the entorhinal cortex and lingual gyrus. The imaging processing pipeline CIVET 1.1.12 was used to estimate cortical thickness from T1-weighted images (n = 104).47 Brain volumes were computed using a volumetric pipeline that has been previously described.48
Apolipoprotein E genotyping
The QIAsymphony apparatus and DNA Blood Mini QIA Kit were used to isolate DNA from 200 µl whole blood (Qiagen). The standard QIASymphony isolation program was used following the manufacturer's instructions. The PyroMark Q96 pyrosequencer (Qiagen) was used to determine apolipoprotein E (APOE) genotype in PREVENT-AD. Quantitative PCR was used to amplify DNA, with primers rs429358 amplification forward 5′-ACGGCTGTCCAAGGAGCTG-3′, rs429358 amplification reverse biotinylated 5′-CACCTCGCCGCGGTACTG-3′, rs429358 sequencing 5′CGGACATGGAGGACG-3′, rs7412 amplification forward 5′-CTCCGCGATGCCGATGAC-3′, rs7412 amplification reverse biotinylated 5′-CCCCGGCCTGGTACACTG-3′ and rs7412 sequencing 5′-CGATGACCTGCAGAAG-3′.
Cognitive testing
At annual visits, the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) was used to assess the cognitive performance of PREVENT-AD participants. The RBANS possesses an excellent sensitivity in differentiating normal cognition from MCI.49 Five cognitive domains are evaluated, which include immediate memory, delayed memory, attention, language and visuospatial abilities.49 A total summary score is included as well. Each participant's score is standardized by their age, such that a score of 100 represents the expected cognitive performance for a given age.49 To reduce practice effects in longitudinal assessment, the RBANS was available in four equivalent versions. Furthermore, the battery was administered in English or French depending on the participants’ preferred language. Cognitive measurements are available for up to 8 years of follow-up.
ADNI-1 cohort
Study participants
Led by Principal Investigator Michael W. Weiner, MD, the primary objective of the Alzheimer's Disease Neuroimaging Initiative (ADNI) has been to detect the earliest changes associated with AD, and to track the progression of AD pathology. Given our interest in the earliest possible stages of AD, we restricted our primary analyses to 241 ADNI-1 participants, with CSF data available from 92 cognitively unaffected individuals and 149 individuals with MCI. For analyses involving plasma samples, we restricted our analyses to 58 cognitively unaffected individuals and 396 individuals with MCI that had available data. Two individuals with ambiguous diagnoses were excluded from analyses.
CSF measurements
Lumbar punctures were performed with a 20- or 24-gauge spinal needle, following an overnight fast. CSF samples were frozen within 1 h after collection and shipped on dry ice to the ADNI Biomarker Core laboratory. Following thawing (1 h) with gentle mixing at room temperature, the samples were aliquoted (0.5 ml) into polypropylene vials and stored at −80°C. CSF AD biomarkers Aβ42, p181-tau and t-tau were measured in ADNI-1 samples using the INNO-BIA AlzBio3 immunoassay kits (Fujirebio) and the xMap Luminex platform.
CSF levels of 159 inflammatory, metabolic and lipid analytes, including IGFBP2 had been assessed with the Human Discovery Map panel, a multiplex immunoassay panel developed by Rules Based Medicine. In the case of IGFBP2, eight (imputed) samples exceeded the detectable analyte concentration range and were omitted from subsequent analyses. Finally, in supplementary analyses, we further analysed multiple reaction monitoring mass spectrometry measurements of CSF IGFBP2.
Pathological staging of participants
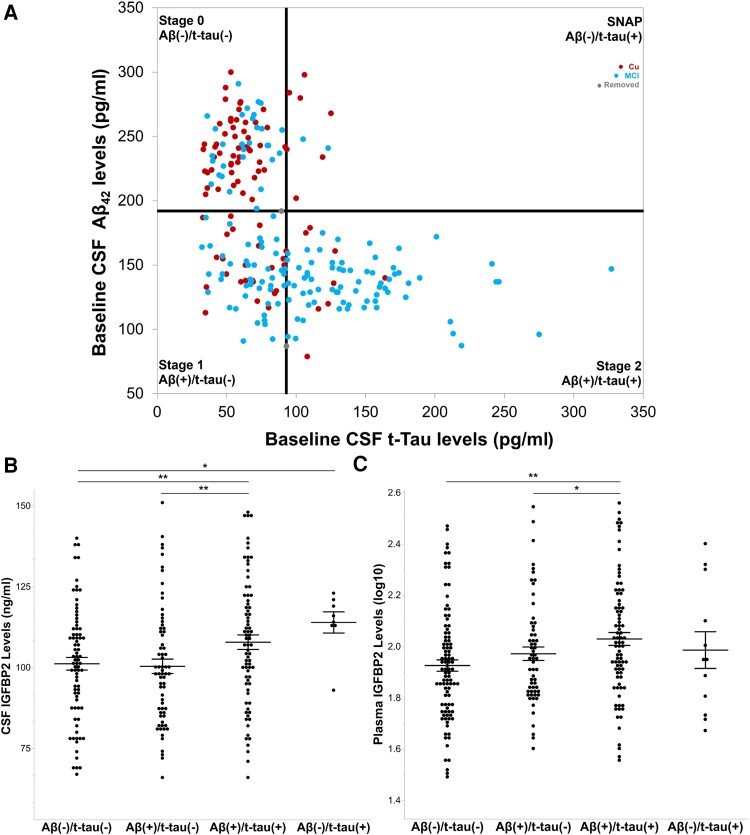
Following the recent emergence of biological frameworks for defining AD,50 we used baseline CSF Aβ42 and CSF t-tau measurements to stage 90 cognitively unaffected individuals and 145 individuals with MCI. We applied the recommended CSF Aβ42 and CSF t-tau thresholds of <192 pg/ml and >93 pg/ml, respectively.51 These cut-off values were generated from autopsy-based AD CSF samples and have been reported to detect mild AD and predict the conversion from MCI to AD.51 Two individuals with biomarker measurements equivalent to the threshold values were removed.
ADNI-1 participants were assigned to Stage 0, Aβ(−)/t-tau(−), if they had normal levels of CSF Aβ42 and CSF t-tau. Participants in Stage 1, Aβ(+)/t-tau(−), exhibited early amyloid pathology, as reflected by reduced levels of CSF Aβ42. However, individuals in Stage 1 did not display significant levels of neuronal loss, as reflected by low levels of CSF t-tau. In Stage 2, Aβ(+)/t-tau(+), participants exhibited low levels of CSF Aβ42 and elevated levels of CSF t-tau. Finally, the Suspected Non-Alzheimer Pathology (SNAP) group, Aβ(−)/t-tau(+), exhibited normal levels of CSF Aβ42 and elevated levels of CSF t-tau, thus suggesting other causes of neurodegeneration and/or dementia.
Plasma measurements
At the baseline visit, plasma samples were drawn following an overnight fast, and 192 analytes that have been reported to be altered in cancer, cardiovascular disease, metabolic disorders, inflammation and AD were analysed with the Human Discovery Map panel, a multiplex immunoassay panel developed on the Luminex xMAP platform by Rules Based Medicine. To meet model assumptions, plasma IGFBP2 levels were log10 transformed by the ADNI investigators.
Apolipoprotein E genotyping
The ABI 7900 real-time thermo-cycler (Applied Biosystems) was used to determine the APOE genotype of ADNI participants. TaqMan quantitative PCR was applied to DNA prepared from EDTA whole blood.
Quebec Founder Population cohort
Study participants
The Quebec Founder Population (QFP) is composed of the descendants of a few thousand French settlers that colonized Nouvelle France in the 17th and 18th centuries.52 The migration and isolated nature of settlements created a founder effect, which resulted in a population with less genetic heterogeneity.52 Genealogical information for this population, for almost four centuries, is available in the BALSAC database. In the present study, we analysed the brains of 55 autopsy-confirmed AD cases and 31 autopsy-confirmed elderly controls, which were obtained from the Douglas-Bell Canada Brain Bank. According to medical record reviews, neuropsychological examinations and caregiver interviews, there was no evidence of memory problems, neurological or neuropsychiatric diseases in the elderly control group. Furthermore, controls only exhibited neuropathology that is associated with healthy ageing (plaque and tangle densities <10/mm3 and <20/mm3 in at least one hippocampal and neocortical section). AD cases had to fulfill the histopathological NINCDS-ADRDA (National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer's Disease and Related Disorders Association) criteria for definite AD.53 This study conforms to the Code of Ethics of the World Medical Association and was approved by the Ethics Board of the Douglas Mental Health University Institute. This study complies with the ethical principles of the Declaration of Helsinki. Each participant provided written informed consent.
IGFBP2 gene expression in the frontal cortex
In the frontal cortex of 31 autopsy-confirmed controls and 55 autopsy-confirmed AD cases, transcriptome-wide gene expression was measured using the human Clariom D Assay, by Génome Québec. Briefly, the Nanodrop Spectrophotometer ND-1000 was used to measure total RNA (NanoDrop Technologies Inc.). RNA integrity was evaluated with the Agilent 2100 Bioanalyzer. Total RNA (10 ng) was used to synthesize sense-strand cDNA. The GeneChip™ WT Terminal Labeling Kit was used to fragment and label single-stranded cDNA, following the manufacturer's instructions. Five micrograms of cDNA were hybridized on the GeneChip™ cartridge array and incubated at 45°C for 17 h in the GeneChip™ Hybridization Oven 640 at 60 rpm. The microarrays were washed in the GeneChip Fluidics Station 450 using the GeneChip™ Hybridization, Wash and Stain Kit, according to the manufacturer’s instructions. Finally, microarrays were scanned in the GeneChip™ Scanner 3000. IGFBP2 mRNA levels are presented on a log2 scale.
IGFBP2 protein levels in the frontal cortex
Of the 86 brains with measured levels of IGFBP2 mRNA, IGFBP2 protein levels were measured in 78 (n = 25 Controls, n = 53 AD). Frontal cortex brain samples were placed in pre-filled tubes containing 2.8 mm ceramic beads (Omni International). One tablet of protease inhibitor was dissolved in 50 ml of cold PBS. Protease inhibitor solution (1 ml) was added to each tube. The Bead Ruptor 24 (Omni International) was used to mechanically homogenize the brain samples, by running twice at 5.65 m/s for 30 s, with a 15-s pause between runs. Following homogenization, the samples were stored overnight at −20°C. To break the cell membranes, two freeze-thaw cycles were performed. Finally, the homogenates were centrifuged for 5 min at 5000 rpm and 4°C. The supernatant was collected and stored for future use at −80°C.
Frontal cortex IGFBP2 protein levels were measured using a commercially available ELISA kit (Cat. No. OKEH00084, Aviva Systems Biology). Protocols were performed according to the manufacturer’s instructions and results were obtained using the BioTek Synergy H1 microplate reader. Sample replicates had a coefficient of variability of <20%. Finally, to normalize IGFBP2 protein levels, total protein concentration was measured using a commercially available bicinchoninic acid assay developed by Pierce (Cat. No. 23225). Finally, normalized IGFBP2 protein ratios were log2 transformed in order to meet model assumptions.
Apolipoprotein E genotyping
DNA was extracted from brain tissue with the DNeasy Tissue Kit (Qiagen). As previously described in PREVENT-AD,39 the PyroMark Q96 pyrosequencer was used to determine APOE genotype.
Statistical analyses
Annual changes in CSF IGFBP2 levels were assessed in a subset of PREVENT-AD participants (n = 27). Each participant's trajectory was analysed using a linear mixed model with a random intercept and slope. Age, sex and APOE ɛ4 carrier status-adjusted linear regression models were used to examine the associations between baseline CSF IGFBP2 and baseline measurements of CSF AD biomarkers (Aβ42, p181-tau, t-tau), CSF synaptic proteins (SYT1, SNAP25, GAP43, NRGN) as well as PET and structural (volumetric, cortical thickness) neuroimaging data. For each PREVENT-AD participant that was followed for 5–8 years (n = 89), an estimated cognitive performance trajectory slope was calculated for each of the five cognitive domains of the RBANS. Thus, linear regression models adjusted for age, sex, APOE ɛ4 carrier status and years of education were used to examine the relationship between rate of change in cognition and baseline CSF IGFBP2 levels. Across all analyses, IGFBP2 was assigned as a dependent variable, except for the association with CSF Aβ42. Finally, given the critical role of the insulin-IGF system in diverse metabolic processes, we further controlled for clinical covariates such as body mass index (BMI), systolic blood pressure and haemoglobin A1c levels (HbA1c) in the supplementary analyses. However, the results of these analyses were similar to those of the original model. Therefore, the following results are presented according to the original model.
In the ADNI-1 cohort, analysis of covariance (ANCOVA) was used to assess the relationship between baseline CSF and baseline plasma IGFBP2 with pathological stage as determined by CSF Aβ42 and CSF t-tau positivity.50,51 To correct for multiple planned comparisons between pathological stages, statistical significance was considered at P ≤ 0.01. Finally, Cox proportional hazards models examined the association between baseline plasma IGFBP2 levels and rate of conversion to AD. Cognitively unaffected participants and individuals with MCI were followed from the baseline visit to the time of diagnosis (of AD) or to the time the participant was last confirmed to be free of AD. Cox models were adjusted for age, gender and APOE ɛ4 carrier status.
In the QFP cohort, the relationship between frontal cortex mRNA, protein levels and diagnosis was evaluated with ANCOVA adjusted for age at death, sex, APOE ɛ4 carrier status and post-mortem delay. Statistical significance was considered at P ≤ 0.05. R2 values are presented as adjusted R2. All analyses were two-tailed and performed in SPSS 23 (IBM) and JMP Pro 16 (SAS).
Results
Demographics
Table 1 summarizes the demographic characteristics of the three cohorts that were used to analyse the role of IGFBP2 in the CSF of asymptomatic (PREVENT-AD) and symptomatic individuals (ADNI-1), as well as in the frontal cortex of autopsy-confirmed, age-matched control and AD cases (QFP).
Table 1.
Baseline participant demographics
| PREVENT-AD | ADNI-1 | QFP | ||||
|---|---|---|---|---|---|---|
| CU | CU | MCI | AD | CU | AD | |
| Sample size, n | 109 | 58 | 395 | 111 | 31 | 55 |
| Mean age, years (SD) | 62.60 (5.43) | 75.11 (5.77) | 74.73 (7.40) | 74.73 (8.08) | 77.39 (11.37) | 80.71 (6.39) |
| Females, n (%) | 76 (69.72) | 28 (48.28) | 140 (35.44) | 47 (42.34) | 11 (35.48) | 23 (41.81) |
| APOE ɛ4+, n (%) | 43 (39.44) | 5 (8.62) | 210 (53.16) | 75 (67.57) | 9 (29.03) | 32 (58.18) |
| Mean BMI, kg/m2 (SD) | 27.11 (4.47) | 27.02 (4.12) | 26.09 (3.97) | 25.59 (3.82) | – | – |
| Mean HbA1c, % (SD) | 5.40 (0.40)a | – | – | – | – | – |
| Mean systolic BP, mmHg (SD) | 120.20 (13.85) | 131.41 (17.65) | 132.79 (18.14) | 135.05 (17.11) | – | – |
| Mean education, years (SD) | 14.88 (2.94)b | 15.67 (2.78) | 15.64 (3.04) | 15.09 (3.21) | – | – |
| Amyloid-positive, n (%) | 37 (33.94) | 21 (36.21) | 205 (51.90) | 91 (81.98) | 0 (0) | 55 (100) |
| Mean CSF Aβ42, pg/ml (SD) | 1145.73 (277.62)c,d | 250.85 (21.08) | 163.48 (52.90) | 142.56 (39.32) | – | – |
| Mean CSF p181-tau, pg/ml (SD) | 46.83 (18.00)c,d | 21.07 (8.43) | 36.15 (19.32) | 42.05 (19.96) | – | – |
| Mean CSF t-tau, pg/ml (SD) | 273.09 (129.97)c,d | 63.62 (21.76) | 102.33 (59.78) | 120.47 (56.58) | – | – |
| Mean CSF IGFBP2, NPX (SD) | 6.89 (0.67) | – | – | – | – | – |
| Mean CSF IGFBP2, ng/ml (SD) | – | 100.85 (15.85) | 104.93 (18.87) | 103.02 (18.76) | – | – |
| Mean plasma IGFBP2, log10 (SD) | – | 1.88 (0.20) | 1.99 (0.23) | 1.91 (0.12) | – | – |
| Mean global Aβ, SUVR (SD) | 1.30 (0.27)e | – | – | – | – | – |
| Mean tau metaROI, SUVR (SD) | 1.17 (0.07)f | – | – | – | – | – |
| Mean cortical IGFBP2, log2 (SD) | – | – | – | – | 2.43 (0.56)g | 2.50 (0.83)g |
| Mean post-mortem interval, h (SD) | – | – | – | – | 30.03 (19.85) | 21.07 (10.36) |
AD = Alzheimer's disease; ADNI = Alzheimer's Disease Neuroimaging Initiative; APOE ɛ4+ = apolipoprotein ɛ4 carriers; Aβ42 = amyloid-beta 42; BMI = body mass index; BP = blood pressure; CU = cognitively unaffected; HbA1c = haemoglobin A1c; IGFBP2 = insulin-like growth factor binding protein-2; MCI = mild cognitive impairment; NPX = Normalized Protein eXpression; p181-tau = phosphorylated tau 181; PREVENT-AD = PRe-symptomatic EValuation of Experiment or Novel Treatments for Alzheimer's disease; QFP = Quebec Founder Population (autopsy confirmed cases only, amyloid positivity dependent on plaque density); RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; ROI = region of interest; SD = standard deviation; SUVR = standardized uptake value ratio; t-tau = total tau.
aOne hundred and seven participants had HbA1c values available.
bOne hundred and six participants had RBANS (Total Score) values available.
cOne hundred and one PREVENT-AD participants had CSF Aβ42, p181-tau and t-tau (pg/ml) values available.
dPREVENT-AD (Fujirebio Innotest ELISA) and ADNI (INNO-BIA AlzBio3 Immunoassay) used different assays to measure the core CSF AD biomarkers, which explains the differences.
eForty-six PREVENT-AD participants had Global Aβ SUVR values available.
fForty-nine PREVENT-AD participants had Tau metaROI SUVR values available.
gSeventy-eight QFP participants had cortical IGFBP2 protein values available.
PREVENT-AD cohort
CSF IGFBP2 increases annually and is associated with CSF and PET biomarkers in asymptomatic AD
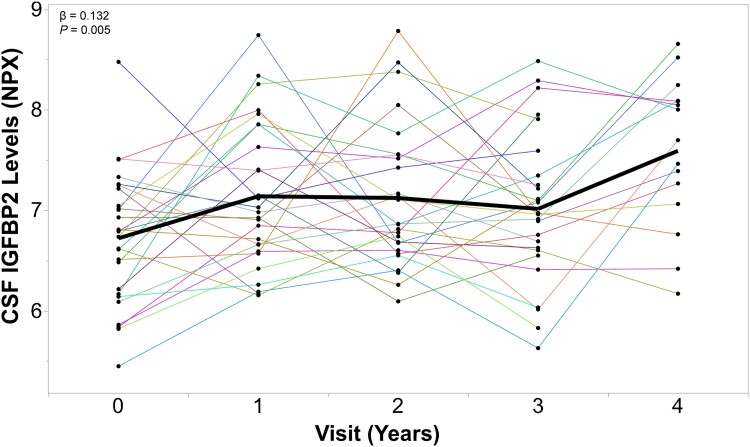
In a subset of cognitively unaffected PREVENT-AD participants that had longitudinal CSF IGFBP2 measurements available (n = 27), a random intercept and random slope linear mixed model revealed that CSF IGFBP2 levels progressively increase over the course of 5 years (β = 0.132, P = 0.005; Fig. 1).
Figure 1.
CSF IGFBP2 levels progressively increase over 5 years in asymptomatic PREVENT-AD participants. IGFBP2 was measured in the CSF of a subset of PREVENT-AD participants (n = 27) at baseline and at follow-up visits, using the Olink Proximity Extension Assay. Linear mixed models accounting for participant-specific trajectories demonstrate CSF IGFBP2 levels increase in a subset of at-risk individuals that have been followed for 5 years. β- and P-values are located in the top left corner. IGFBP2 = insulin-like growth factor binding protein-2; PREVENT-AD = PRe-symptomatic EValuation of Experimental or Novel Treatments for Alzheimer's Disease; NPX = Normalized Protein eXpression.
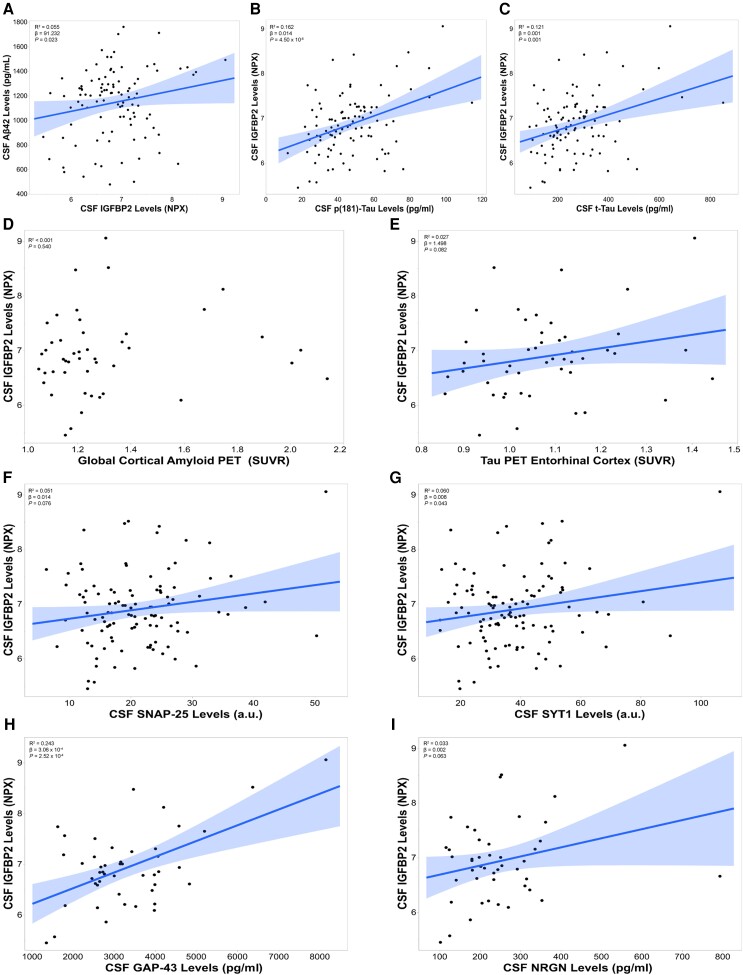
In PREVENT-AD participants (n = 101) that had CSF AD pathological biomarker measurements, baseline CSF IGFBP2 levels were positively correlated with CSF Aβ42 (R2 = 0.055, β = 91.232, P = 0.023; Fig. 2A), CSF p181-tau (R2 = 0.162, β = 0.014, P = 4.50 × 10−5; Fig. 2B) and CSF t-tau (R2 = 0.121, β = 0.001, P = 0.001; Fig. 2C). In a subset of PREVENT-AD participants that underwent Aβ and tau PET scans (n = 46 and n = 49), CSF IGFBP2 was not associated with global cortical Aβ deposition (P = 0.540; Fig. 2D). However, CSF IGFBP2 was positively correlated (trend-level) with tau deposition in the entorhinal cortex (R2 = 0.027, β = 1.498, P = 0.082; Fig. 2E) and lingual gyrus (R2 = 0.034, β = 2.226, P = 0.067; Supplementary Fig. 1).
Figure 2.
CSF IGFBP2 is associated with CSF and PET Alzheimer’s disease biomarkers in the asymptomatic PREVENT-AD cohort. CSF IGFBP2 levels were measured using the Olink Proximity Extension Assay (n = 109). CSF Alzheimer’s disease (AD) biomarkers (A) amyloid-beta 42 (Aβ42) (B) phosphorylated tau 181 (p181-tau) and (C) total tau (t-tau) were measured using validated Innotest ELISA kits, following the standardized protocols established by the BIOMARKAPD consortium (n = 101). (D) The global cortical amyloid standardized uptake value ratio (SUVR) was measured using 18F-NAV4694 (n = 46). (E) Tau deposition in the entorhinal cortex was measured with flortaucipir (n = 49). The synaptic markers (F) SNAP25 (n = 106), (G) SYT1 (n = 106), (H) GAP43 (n = 46) and (I) NRGN (n = 46) were quantified using immunoprecipitation followed by mass spectrometry. Significant or trend-level linear regressions are represented with a confidence region of the fitted line. R2 , β- and P-values are located in the top left corners of each panel. Analyses were adjusted for age, sex and APOE ɛ4 carrier status. a.u. = arbitrary units; BIOMARKAPD = Biomarkers for Alzheimer's and Parkinson's disease; IGFBP2 = insulin-like growth factor binding protein-2; NPX = Normalized Protein eXpression; PREVENT-AD = PRe-symptomatic EValuation of Experimental or Novel Treatments for Alzheimer's Disease.
Finally, CSF IGFBP2 was positively associated with synaptic proteins in the CSF, including SNAP25 (trend-level, R2 = 0.051, β = 0.014, P = 0.076; Fig. 2F), SYT1 (R2 = 0.060, β = 0.008, P = 0.043; Fig. 2G), GAP43 (R2 = 0.243, β = 3.06 × 10−4, P = 2.52 × 10−4; Fig. 2H) and NRGN (trend-level, R2 = 0.033, β = 0.002, P = 0.063; Fig. 2I).
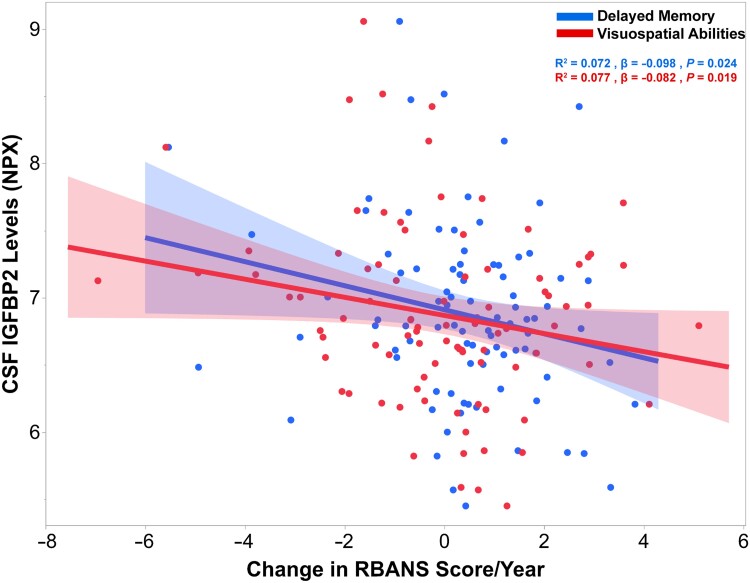
CSF IGFBP2 is linked to changes in delayed memory and visuospatial abilities in asymptomatic AD
Upon computing RBANS cognitive performance trajectory slopes estimated over the course of 5 to 8 years in a subset of PREVENT-AD participants (n = 89), baseline CSF IGFBP2 levels were negatively correlated with estimated rates of change in delayed memory scores (R2 = 0.072, β = −0.098, P = 0.024; Fig. 3). Furthermore, baseline CSF IGFBP2 levels were negatively correlated with rates of change in visuospatial constructional abilities (R2 = 0.077, β = −0.082, P = 0.019; Fig. 3). However, baseline IGFBP2 levels were not associated with changes in immediate memory (P = 0.191), language (P = 0.332) or attention (P = 0.679) (data not shown).
Figure 3.
CSF IGFBP2 is associated with longitudinal changes in delayed memory and visuospatial abilities over 5–8 years in PREVENT-AD. CSF IGFBP2 levels were measured using the Olink Proximity Extension Assay (n = 109). Cognitive performance trajectory slopes were computed for each cognitive domain of the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; delayed memory, visuospatial abilities, language, immediate memory and attention) in a subset of PREVENT-AD participants that were followed for 5–8 years (n = 89). Significant linear regressions are represented with a confidence region of the fitted line. R2, β- and P-values are located in the top right corner. Analyses were adjusted for age, sex, APOE ɛ4 carrier status and years of education. IGFBP2 = insulin-like growth factor binding protein-2; NPX = Normalized Protein eXpression; PREVENT-AD = PRe-symptomatic EValuation of Experimental or Novel Treatments for Alzheimer's Disease.
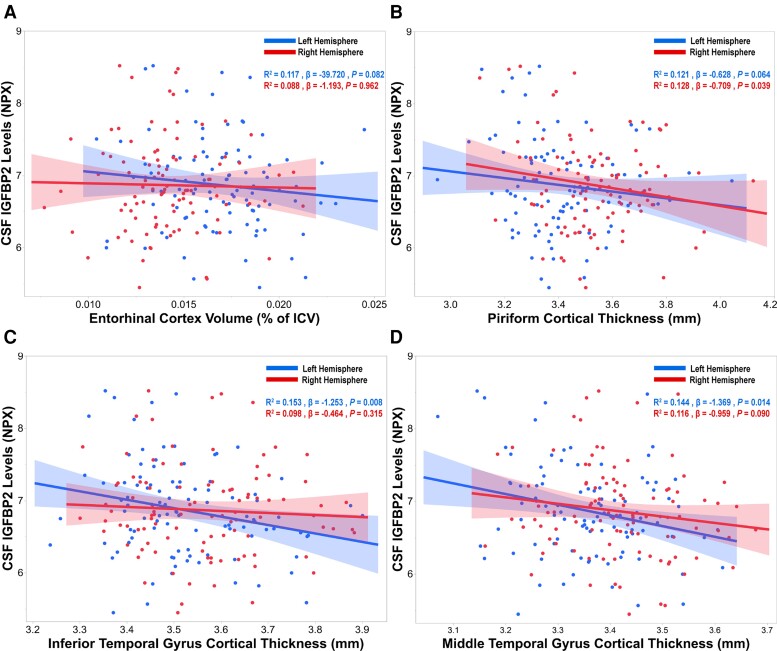
CSF IGFBP2 is associated with cortical atrophy in AD-specific brain regions in asymptomatic AD
We analysed baseline structural neuroimaging data collected from a subset of PREVENT-AD individuals (n = 104) in a cross-sectional fashion. Four individuals were omitted from analyses due to failed quality control regarding subject-specific stereotaxic registration and/or brain masking. After adjusting for total intracranial volume (ICV), baseline CSF IGFBP2 was negatively correlated with entorhinal cortex volumes in the left hemisphere at a trend-level (R2 = 0.117, β = −39.720, P = 0.082; Fig. 4A). However, CSF IGFBP2 was not associated with entorhinal cortex volume in the right hemisphere (P = 0.962).
Figure 4.
CSF IGFBP2 is associated with atrophy in Alzheimer’s disease-related brain regions in PREVENT-AD. CSF IGFBP2 levels were measured using the Olink Proximity Extension Assay (n = 109). T1-weighted structural MRI scans were performed on a subset of PREVENT-AD participants (n = 104). The imaging processing pipeline CIVET 1.1.12 was used to analyse neuroimaging data. (A) Entorhinal cortex volumes were normalized by total intracranial volumes (ICV). Cortical thickness measurements were acquired from Alzheimer’s disease (AD)-related brain regions, such as the (B) piriform cortex, (C) inferior temporal gyrus and (D) middle temporal gyrus. Significant or trend-level linear regressions are represented with a confidence region of the fitted line. R2 , β- and P-values are located in the top right corner of each panel. Analyses were adjusted for age, sex and APOE ɛ4 carrier status. IGFBP2 = insulin-like growth factor binding protein-2; NPX = Normalized Protein eXpression; PREVENT-AD = PRe-symptomatic EValuation of Experimental or Novel Treatments for Alzheimer's Disease.
Next, we analysed baseline cortical thickness in pre-specified temporal and parietal brain regions that are vulnerable to early AD pathology. Baseline CSF IGFBP2 was found to be negatively correlated with cortical thickness in the piriform cortex (trend, left hemisphere: R2 = 0.121, β = −0.628, P = 0.064; right hemisphere: R2 = 0.128, β = −0.709, P = 0.039; Fig. 4B), inferior temporal gyrus (left hemisphere: R2 = 0.153, β = −1.253, P = 0.008; right hemisphere: P = 0.315; Fig. 4C), middle temporal gyrus (left hemisphere: R2 = 0.144, β = −1.369, P = 0.014; right hemisphere: R2 = 0.116, β = −0.959, P = 0.090; Fig. 4D) and precuneus (left hemisphere: P = 0.123; right hemisphere: R2 = 0.131, β = −1.353, P = 0.033; Supplementary Fig. 2).
CSF IGFBP2 changes not caused by alterations to blood–brain barrier integrity in asymptomatic subjects
To examine possible blood–brain barrier dysfunction and possible peripheral vascular contributions to CSF IGFBP2 levels, we measured microprotein levels, red blood cell count and white blood cell count in the CSF. We did not find any associations between these vascular factors and CSF IGFBP2 (Supplementary Fig. 3), consistent with a relatively intact blood–brain barrier in asymptomatic PREVENT-AD participants.54
ADNI-1 cohort
CSF and plasma IGFBP2 concentrations are elevated in CSF Aβ(+)/t-tau(+) individuals
Eighty-nine cognitively unaffected individuals and 144 individuals with MCI from ADNI were staged as Aβ- and/or tau-positive according to recommended CSF Aβ42 and CSF t-tau thresholds of 192 pg/ml and 93 pg/ml, respectively (Fig. 5A).50,51 The results from the CSF multiplex immunoassay (Fig. 5B) revealed that baseline CSF IGFBP2 levels did not differ between Stages 0 (n = 80) Aβ(−)/t-tau(−) and Stage 1 (n = 68) Aβ(+)/t-tau(−), P = 0.541. However, CSF IGFBP2 was significantly elevated at Stage 2 (n = 77) Aβ(+)/t-tau(+) relative to Stage 0 (P = 0.009) and Stage 1 (P = 0.001). Finally, CSF IGFBP2 was significantly increased in SNAP (n = 8) Aβ(−)/t-tau(+), relative to Stage 0 (P = 0.010).
Figure 5.
CSF and plasma IGFBP2 is elevated in CSF Aβ(+)/t-tau(+) individuals from the ADNI-1 cohort. (A) Cognitively unaffected participants (n = 92) and participants with mild cognitive impairment (MCI; n = 149) from the ADNI-1 cohort were staged as CSF amyloid-β and/or CSF total tau-positive according to the recommended thresholds of 192 pg/ml and 93 pg/ml, respectively. Linear models, adjusted for age, sex and APOE ɛ4 carrier status were used to examine mean differences in IGFBP2 protein levels across stages. (B) CSF IGFBP2 was elevated at Stage 2 (n = 77) relative to Stage 0 (n = 80) and Stage 1 (n = 68). Furthermore, CSF IGFBP2 was elevated in suspected non-Alzheimer pathology (SNAP, n = 8) compared with Stage 0. (C) Plasma IGFBP2 was elevated at Stage 2 (n = 84) relative to Stage 0 (n = 98) and Stage 1 (n = 60). However, plasma IGFBP2 did not significantly differ between SNAP (n = 12) and Stage 0. The data are represented as mean ± standard error of the mean. *P < 0.05, **P < 0.01. Aβ42 = amyloid-beta 42; ADNI = Alzheimer's Disease Neuroimaging Initiative; IGFBP2 = insulin-like growth factor binding protein-2; MCI = mild cognitive impairment; SNAP = suspected non-Alzheimer pathology; t-tau = total tau.
To reproduce our findings, we performed replication analyses using CSF mass spectrometry data acquired from a subset of the same ADNI participants. These supplementary analyses revealed that both CSF IGFBP2 peptides, HGLYNLK (Supplementary Fig. 4) and LIQGAPTIR (Supplementary Fig. 5) were significantly reduced at Stage 1 (n = 62) relative to Stage 0 (n = 75), P = 0.005 and P = 0.051 (trend). Although a similar reduction at Stage 1 was observed with the multiplex immunoassay data, it was not statistically significant. However, similar to the multiplex immunoassay data, both IGFBP2 peptides were significantly elevated at Stage 2 (n = 72) relative to Stage 1, P = 1.50 × 10−5 and P = 2.21 × 10−4. Likewise, both IGFBP2 peptides were markedly increased in SNAP (n = 9) relative to Stage 0, P = 4.90 × 10−5 and P = 0.002, consistent with the immunoassay results.
Finally, we staged 58 cognitively unaffected individuals and 196 individuals with MCI from ADNI that had both baseline plasma IGFBP2 measurements and CSF Aβ42 and CSF t-tau measurements (Fig. 5C). Baseline plasma IGFBP2 levels were significantly elevated in Stage 2 (n = 84) relative to Stage 0 (n = 98), P = 0.001, and to Stage 1 (n = 60), P = 0.041 (trend). However, plasma IGFBP2 did not differ between Stage 0 and Stage 1 (P = 0.208), or between Stage 0 and SNAP (n = 12), P = 0.874.
Elevated plasma IGFBP2 is associated with a faster rate of conversion to AD
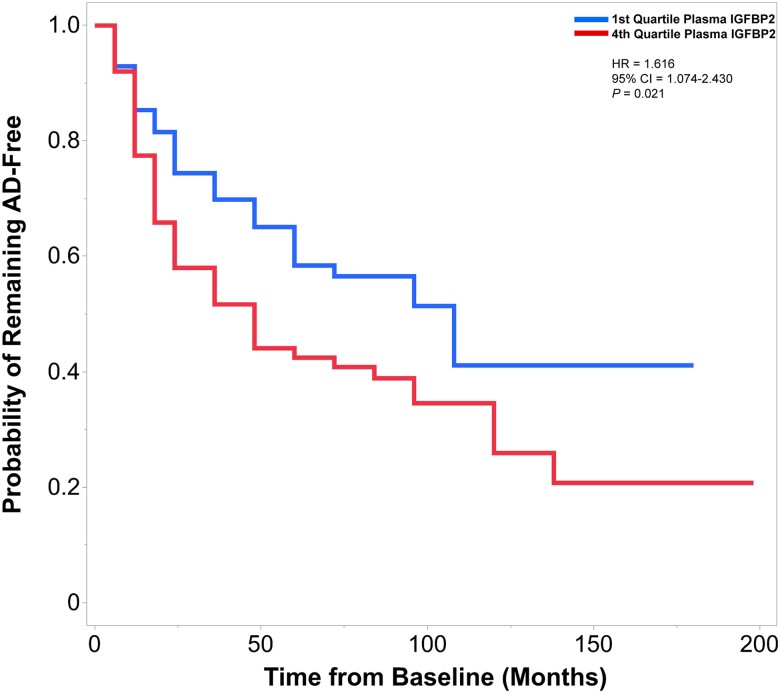
In the primary analysis for conversion to AD in ADNI, we established baseline plasma IGFBP2 threshold values at the 25th percentile (≤1.83251, first quartile, log10 transformed) and above the 75th percentile (≥2.09342, fourth quartile, log10 transformed). A total of 226 individuals that were either cognitively unaffected or had MCI were included in these analyses. Of these dementia-free participants, 107 individuals eventually met the clinical criteria for a diagnosis of AD (mean follow-up, 3.8 years; range, 0.5–16.5 years). Cox proportional hazards models revealed that individuals with plasma IGFBP2 values greater than the 75th percentile exhibited a faster rate of conversion to AD, than individuals with plasma IGFBP2 values less than the 25th percentile [hazard ratio (HR) 1.616, 95% confidence interval (CI) 1.074–2.430, P = 0.021; Fig. 6].
Figure 6.
Elevated plasma IGFBP2 is associated with a greater rate of conversion to Alzheimer’s disease in individuals from the ADNI-1 cohort. Cox proportional hazards models examined the association between baseline plasma IGFBP2 levels and rate of conversion to Alzheimer’s disease (AD). The first quartile and fourth values of plasma IGFBP2 were contrasted. Participants were followed from the baseline visit to the time of diagnosis (of AD), or to the time the participant was last confirmed to be free of AD (mean follow-up, 3.8 years; range, 0.5–16.5 years). Of the 226 individuals that were followed longitudinally, 107 individuals progressed to AD. Individuals with plasma IGFBP2 values in the fourth quartile exhibited a greater rate of conversion to AD, compared with the first quartile. Hazard ratio (HR) and P-values are located in the top right corner. Cox models were adjusted for age, gender and APOE ɛ4 carrier status. ADNI = Alzheimer's Disease Neuroimaging Initiative; IGFBP2 = insulin-like growth factor binding protein-2.
In the secondary analysis, baseline IGFBP2 plasma levels were kept as continuous, and 439 ADNI-1 dementia-free participants with plasma IGFBP2 measurements were included. Of these individuals, 214 were eventually diagnosed with AD (mean follow-up, 3.6 years; range, 0.5–16.5 years). Similar to the first model, elevated plasma IGFBP2 was associated with a greater rate of conversion to AD (HR 1.857, 95%CI 1.054–3.270, P = 0.032).
Quebec Founder Population cohort
Despite reductions in IGFBP2 mRNA, protein levels do not differ in the frontal cortex of AD brains
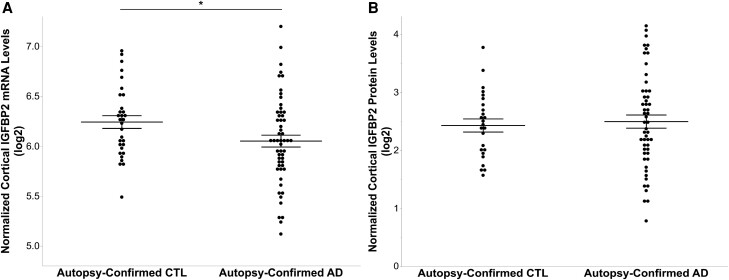
IGFBP2 gene expression was assessed by DNA microarray in the QFP cohort, and demonstrated to be significantly reduced in the frontal cortex of autopsy-confirmed AD brains (n = 55), compared with elderly controls (n = 31, P = 0.049; Fig. 7A). However, as demonstrated through ELISA, IGFBP2 protein levels did not differ in the frontal cortex of AD cases (n = 53) after controlling for total protein levels, relative to controls (n = 25, P = 0.462; Fig. 7B).
Figure 7.
Frontal cortex IGFBP2 gene expression is reduced in autopsy-confirmed Alzheimer’s disease brains, however protein levels do not differ from elderly controls. (A) Microarray technology was used to measure IGFBP2 mRNA levels in the frontal cortex of autopsy-confirmed Alzheimer’s disease brains (AD; n = 55) and elderly controls (CTL; n = 31) from the Quebec Founder Population (QFP) cohort. (B) IGFBP2 protein levels in the frontal cortex were measured in AD (n = 53) and control (n = 25) brains using a commercially available ELISA kit. Analyses were adjusted for age, sex, APOE ɛ4 carrier status and post-mortem interval. The data are represented as mean ± standard error of the mean. *P < 0.05. IGFBP2 = insulin-like growth factor binding protein-2.
Discussion
Our results suggest that nascent AD pathology induces a marked upregulation in IGFBP2, in asymptomatic individuals. CSF and plasma IGFBP2 behave as valuable biomarkers for identifying pre-clinical CSF Aβ(+)/t-tau(+) individuals, and those with a greater risk of AD conversion.
It has been well established that impaired insulin-IGF signalling plays a critical role in AD.8-10 Furthermore, insulin and IGF proteins are highly expressed in the hippocampus.8 Therefore, it is possible that impairments in insulin-IGF signalling may account for the selective vulnerability of the hippocampal formation to nascent AD pathology. Hence, targeting the insulin-IGF system may offer a promising solution to delay, slow down and/or prevent AD, either alone or in combination therapies. However, to develop these therapies, unraveling the molecular intricacies of the insulin-IGF system is necessary. To this end, we investigated the role of the most abundant IGFBP in the CSF, IGFBP2,28,29 during the earliest possible asymptomatic stage of AD, in ‘at-risk’, parental history-positive PREVENT-AD participants.
We observed a positive relationship between IGFBP2 and CSF Aβ42 (Fig. 2A), which is consistent with the proposed IGF-mediated clearance of Aβ.12,13 This notion is in agreement with the finding that current Aβ-lowering therapies induce an increase in CSF Aβ42 in participants with MCI or mild AD.55 Furthermore, our data suggest IGFBP2 may be upregulated as a result of early neuronal loss in the asymptomatic stage of the disease (Fig. 2C), which is consistent with the extensive literature regarding the upregulation of IGFs and IGFBPs following several rodent models of brain damage and recovery.56-63 Furthermore, the administration of des-IGF-I, an analogue of IGF-1 with a low affinity for IGFBPs, failed to attenuate neuronal cell death in mice with experimental hypoxic ischaemic injuries.64 However, the administration of IGFBP-compatible IGF-I significantly reduced the observed neuronal loss.64
Our findings also suggest that IGFBP2 may be modulated by early CSF p181-tau production (Fig. 2B) and early deposition in asymptomatic individuals. PET imaging analyses revealed that IGFBP2 is positively associated (at a trend-level) with significant tau deposition in the entorhinal cortex (Fig. 2E) and lingual gyrus (Supplementary Fig. 1), features that are typically associated with early Braak Stages 2–3.65 These findings are certainly consistent with the fact that insulin and IGFs normally inhibit glycogen synthase kinase-3 (GSK3) activity and phospho-tau production in human neurons, through the phosphoinositide 3-kinase-protein kinase B (PI3K-AKT) signalling pathway.14,15 For instance, the pharmacological inhibition of GSK3 has been linked to increases in IGF-I in the rodent brain,66 whereas conditional transgenic mice overexpressing GSK3 in the cortex and hippocampus display increased tau phosphorylation in AD relevant epitopes,67 degeneration of the dentate gyrus68 and spatial memory impairments.69 Finally, mice overexpressing IGFBP2 display an increase in AKT activity, a GSK3 inhibitor, in the brain.70 Thus, it is tempting to postulate that IGF regulation of GSK3 activity in turn modulates IGFBP2 production via a phospho-tau mediated process that ensures some form of local autoregulation and/or resilience.
Given the prominent loss of synapses in AD, we examined the relationship between IGFBP2 and synaptic proteins in the CSF, namely SNAP25 (Fig. 2F), SYT1 (Fig. 2G), GAP43 (Fig. 2H) and NRGN (Fig. 2I).40-45 The present study's results suggest that synaptic dysfunction and loss may trigger an increase in IGFBP2 synthesis and secretion. This finding is in agreement with the upregulation and regenerative abilities of IGFs to grow axons during the development of the CNS and regrow axons during repair following injury to the CNS and/or peripheral nervous system.21,71-76
Considering the relationship between CSF synaptic markers and CSF IGFBP2, we were interested in examining the relationship between changes in cognition and baseline CSF IGFBP2 in PREVENT-AD. We have demonstrated that CSF IGFBP2 levels are associated with cognitive decline in RBANS delayed memory and visuospatial abilities over a 5–8-year period, in a subset of PREVENT-AD participants (Fig. 3). This finding is consistent with a report that plasma levels of IGFBP2 were negatively correlated with episodic memory performances in participants from the ADNI cohort.77 Thus, given the critical role of the hippocampus in the consolidation of declarative memory and in spatial processing, our data provide compelling evidence that IGFBP2 plays a pivotal role in the integrity of the hippocampal formation, which displays elevated expression levels of insulin, IGFs and their receptors—compared with other brain regions such as the frontal cortex.8 Indeed, plasma IGFBP2 levels have been demonstrated to be negatively correlated with hippocampal volumes in amyloid-negative individuals from the ADNI cohort.77 Consistent with this view, IGFBP2 has been demonstrated to be expressed by neurons and astrocytes in the hippocampus during development and following CNS injury.57-59,62,78,79 Furthermore, the relationship between IGFBP2, delayed memory and hippocampal structure is consistent with the finding that the administration of IGFBP2 has been shown to increase the number of dendritic spines in the dentate gyrus of rodent models of post-traumatic stress disorder.80 In a similar fashion, in cell culture experiments, antibodies targeted against IGFBP2 have been demonstrated to inhibit neurogenesis—a phenomenon that occurs in the dentate gyrus.81 Moreover, IGFBP2 knockout mice exhibit deficits in long-term potentiation as well as impaired performances on the Morris water maze, which heavily relies on the hippocampus.79 Finally, the administration of an IGFBP2-derived peptide has been demonstrated to rescue deficits in synaptic plasticity, memory and learning in a mouse model of SHANK3-mediated postsynaptic deficits.82 Overall, these findings suggest that IGFBP2 in certain circumstances is linked to neuroprotection and hippocampal-mediated cognitive abilities.
Indeed, it is important to take into consideration the early involvement of IGFBP2 in the presymptomatic phase of AD, when presumably resilience is a major player in the brain response to early neurodegeneration. For instance, several neuroprotective genes have been demonstrated to be upregulated by the (soluble) α-secretase cleaved fragment of amyloid precursor protein (sAPPα), amongst them, IGFBP2 and IGF2.83 Thus, it is possible that an increase in binding of IGFBP2 to IGFs may attenuate IGF degradation and/or promote the targeting of IGFs to their receptors, thus enhancing synaptic and terminal resilience in face of the emerging neurodegenerative process. This receptor targeting hypothesis is notably supported by evidence that transgenic mice overexpressing IGFBP2 lacking a proteoglycan-binding domain exhibit severe reductions in synaptic markers and hippocampal weight.84 Finally, in a pilot study conducted on AD brains, temporal cortex IGFBP2 levels were negatively correlated with senile plaque levels—suggesting a role for IGFBP2 in neuroprotection and resilience.9
Given the negative relationship between IGFBP2 and cognitive abilities, we were prompted to examine whether baseline levels of CSF IGFBP2 were associated with anatomical changes in the brain. Interestingly, in asymptomatic PREVENT-AD participants, IGFBP2 was associated with atrophy in the left entorhinal cortex, at a trend-level (Fig. 4A).This is a critical finding, as the entorhinal cortex is the first region that is affected in AD.65,85,86 Furthermore, IGFBP2 was associated with cortical thinning in several pre-specified temporal and parietal brain regions that are known to be affected early on in AD, such as the piriform cortex (Fig. 4B), inferior (Fig. 4C) and middle temporal gyri (Fig. 4D) and precuneus (Supplementary Fig. 2).87,88 Overall, the structural neuroimaging results are in agreement with previous reports of IGFBP2 being associated with atrophy in AD-associated brain regions.36,89,90
Finally, in the PREVENT-AD cohort, changes in CSF IGFBP2 appear to be mostly specific to changes in the CNS, as we did not detect any significant peripheral vascular contributions or blood–brain barrier dysfunction in these asymptomatic individuals (Supplementary Fig. 3).54
To independently validate our observations, we further analysed data from a well-characterized cohort of cognitively unaffected individuals and individuals with MCI from ADNI-1 (Fig. 5A). Our results suggest that elevated CSF (Fig. 5B and Supplementary Figs 4 and 5) and plasma IGFBP2 (Fig. 5C) may be valuable biomarkers of CSF Aβ(+)/t-tau(+) individuals and thus facilitate screening for suitable patients for clinical trials.50,51 This is not the first time that we identified a strong biphasic response as individuals progress from the CSF Aβ(+)/t-tau(−) stage to the CSF Aβ+/t-tau(+) stage (Supplementary Figs 4 and 5). Certain inflammatory proteins such as IL-8, IL-12 and IL-15 display the same initial reduction in the CSF Aβ(+)/t-tau(−) stage followed by marked increases in the CSF Aβ(+)/t-tau(+) stage.91 These results support recent observations that immune activation may become apparent only after the onset of both amyloid and tau pathologies. Unexpectedly, these results also suggest that immune marker activity and IGFBP2 may diminish upon the earliest appearance of amyloid plaque pathology. However, as IGFBP2 regulates IGF signalling in neurons, one might speculate that an increase in binding of IGFBP2 to a limited amount of IGFs may block IGF-mediated suppression of tau phosphorylation, leading to increased levels of phospho-tau and ultimately, promoting tau deposition, neuronal damage and death.
Consistent with this working model and upon conducting survival analyses, we found that elevated circulating IGFBP2 levels were associated with a pronounced rate of conversion to AD in the ADNI cohort (Fig. 6). Overall, our results are consistent with the existing literature that plasma IGFBP2 has been associated with a greater risk of developing AD.35,37,38 Finally, in contrast to the PREVENT-AD cohort, CSF IGFBP2 immunoassay measurements have been previously found to correlate with plasma IGFBP2 levels in the ADNI cohort, which incorporates individuals with a disrupted blood–brain barrier.77
Finally, given the regional-specificity of the insulin-IGF system, we further examined IGFBP2 levels in the human brain.8IGFBP2 mRNA levels were reduced in the frontal cortex of autopsy-confirmed AD brains from the QFP cohort (Fig. 7A). However, cortical IGFBP2 protein levels did not differ between AD brains and elderly controls (Fig. 7B). Thus, our results provide further evidence that support the (hippocampal) regional specificity of the insulin-IGF system, potentially including the IGFBP2 protein.8 However, due to the scarcity of medial temporal lobe tissues, we were unable to confirm the regional specificity of the IGFBP2 protein in the QFP cohort. Nevertheless, our results differ from those of a pilot study, that found IGFBP2 was decreased in the temporal cortex of AD brains.9 It is possible our results differ since we measured cytoplasmic and extracellular IGFBP2, whilst the pilot study measured membrane-bound IGFBP2.9 Finally, although IGFBP2 has been demonstrated to contain a nuclear localization signal and regulate the expression of several genes such as vascular endothelial growth factor, we were unable to differentiate between IGF-dependent and IGF-independent IGFBP2.92
Overall, our data suggest that IGFBP2 may play a critical role in neuroprotection during the asymptomatic stage of AD. However, as amyloid and tau pathology accumulate, the involvement of IGFBP2 appears to notably change. At that stage, elevated concentrations of IGFPB2 are associated with an accelerated rate of MCI to AD conversion in the ADNI cohort, and with subtle declines in delayed memory and visuospatial abilities in asymptomatic PREVENT-AD participants.
In the larger context of the insulin-IGF system, it will be exciting to follow the results of ongoing phase 3 clinical trials involving the administration of semaglutide (Ozempic), a glucagon-like peptide-1 receptor agonist that is clinically approved for the treatment of type 2 diabetes.93 Considering the role of semaglutide in stimulating insulin secretion and signalling,93 exploring therapeutic strategies that enhance IGF signalling in the brain,94 perhaps through IGFBP2, may also warrant further investigation in presymptomatic AD. At the moment, we favor the hypothesis that an anti-IGFBP2 therapy may be effective in amyloid positive individuals whose tau deposition is minimal. As tau pathology emerges, it may prove difficult to change this complex metabolic process as IGFBP2 activity appears less protective and more reactive, similar to many other immune regulators.
Supplementary Material
Acknowledgements
The authors would like to thank Dr Naguib Mechawar at the Douglas Institute/Bell Canada Brain Bank for providing human brain tissues from the Quebec Founder Population. We also wish to thank Mrs Jennifer Tremblay-Mercier, Marie-Elyse Lafaille-Magnan and Melissa Savard as well as Drs Pedro Rosa-Neto, Daniel Auld and David Lafontaine for their technical expertise. Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at:http://adni.loni.usc.edu/wpcontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf. Data used in preparation of this article were obtained from the PRe-symptomatic EValuation of Experimental or Novel Treatments for Alzheimer's Disease (PREVENT-AD) program at the Centre for Studies on Prevention of Alzheimer's Disease (StoP-AD), Douglas Mental Health University Institute Research Centre (http://douglas.research.mcgill.ca/stop-ad-centre). A complete listing of the PREVENT-AD Research Group can be found at: https://preventad.loris.ca/acknowledgements/acknowledgements.php?date=2023-05-01.
Contributor Information
Marc James Quesnel, McGill University, Montréal, QC H3A 1A1, Canada; Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada.
Anne Labonté, Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada; Centre for the Studies in the Prevention of Alzheimer’s Disease, Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada.
Cynthia Picard, Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada; Centre for the Studies in the Prevention of Alzheimer’s Disease, Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada.
Henrik Zetterberg, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 413 45, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal 431 80, Sweden; Department of Neurodegenerative Disease, UCL Institute of Neurology, London WC1N 3BG, UK; UK Dementia Research Institute at UCL, London WC1E 6BT, UK; Hong Kong Center for Neurodegenerative Diseases, Hong Kong, China; Wisconsin Alzheimer’s Disease Research Center, University of Wisconsin School of Medicine and Public Health, University of Wisconsin-Madison, Madison, WI 53792-2420, USA.
Kaj Blennow, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 413 45, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal 431 80, Sweden; Paris Brain Institute, ICM, Pitié-Salpêtrière Hospital, Sorbonne University, 75646 Cedex 13, Paris, France; Neurodegenerative Disorder Research Center, Division of Life Sciences and Medicine, and Department of Neurology, Institute on Aging and Brain Disorders, University of Science and Technology of China and First Affiliated Hospital of USTC, Hefei 230026, P.R. China.
Ann Brinkmalm, Department of Psychiatry and Neurochemistry, Institute of Neuroscience and Physiology, The Sahlgrenska Academy, University of Gothenburg, Gothenburg 413 45, Sweden; Clinical Neurochemistry Laboratory, Sahlgrenska University Hospital, Mölndal 431 80, Sweden.
Sylvia Villeneuve, McGill University, Montréal, QC H3A 1A1, Canada; Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada; Centre for the Studies in the Prevention of Alzheimer’s Disease, Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada.
Judes Poirier, McGill University, Montréal, QC H3A 1A1, Canada; Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada; Centre for the Studies in the Prevention of Alzheimer’s Disease, Douglas Mental Health University Institute, Montréal, QC H4H 1R3, Canada.
for the Alzheimer’s Disease Neuroimaging Initiative:
Michael W Weiner, Paul Aisen, Ronald Petersen, Michael W Weiner, Paul Aisen, Ronald Petersen, Clifford R Jack, William Jagust, John Q Trojanowki, Arthur W Toga, Laurel Beckett, Robert C Green, Andrew J Saykin, John C Morris, Richard J Perrin, Leslie M Shaw, Zaven Khachaturian, Maria Carrillo, William Potter, Lisa Barnes, Marie Bernard, Hector González, Carole Ho, John K Hsiao, Jonathan Jackson, Eliezer Masliah, Donna Masterman, Ozioma Okonkwo, Richard Perrin, Laurie Ryan, Nina Silverberg, Adam Fleisher, Michael W Weiner, Diana Truran Sacrey, Juliet Fockler, Cat Conti, Dallas Veitch, John Neuhaus, Chengshi Jin, Rachel Nosheny, Miriam Ashford, Derek Flenniken, Adrienne Kormos, Robert C Green, Tom Montine, Cat Conti, Ronald Petersen, Paul Aisen, Michael Rafii, Rema Raman, Gustavo Jimenez, Michael Donohue, Devon Gessert, Jennifer Salazar, Caileigh Zimmerman, Yuliana Cabrera, Sarah Walter, Garrett Miller, Godfrey Coker, Taylor Clanton, Lindsey Hergesheimer, Stephanie Smith, Olusegun Adegoke, Payam Mahboubi, Shelley Moore, Jeremy Pizzola, Elizabeth Shaffer, Brittany Sloan, Laurel Beckett, Danielle Harvey, Michael Donohue, Clifford R Jack, Arvin Forghanian-Arani, Bret Borowski, Chad Ward, Christopher Schwarz, David Jones, Jeff Gunter, Kejal Kantarci, Matthew Senjem, Prashanthi Vemuri, Robert Reid, Nick C Fox, Ian Malone, Paul Thompson, Sophia I Thomopoulos, Talia M Nir, Neda Jahanshad, Charles DeCarli, Alexander Knaack, Evan Fletcher, Danielle Harvey, Duygu Tosun-Turgut, Stephanie Rossi Chen, Mark Choe, Karen Crawford, Paul A Yushkevich, Sandhitsu Das, William Jagust, Robert A Koeppe, Eric M Reiman, Kewei Chen, Chet Mathis, Susan Landau, John C Morris, Richard Perrin MD, Nigel J Cairns, Erin Householder, Erin Franklin, Haley Bernhardt R, Louis Lisa Taylor-Reinwald, Leslie M Shaw, John Q Trojanowki, Magdalena Korecka, Michal Figurski, Arthur W Toga, Karen Crawford, Scott Neu, Andrew J Saykin, Kwangsik Nho, Shannon L Risacher, Liana G Apostolova, Li Shen, Tatiana M Foroud, Kelly Nudelman, Kelley Faber, Kristi Wilmes, Michael W Weiner, Leon Thal, Zaven Khachaturian, John K Hsiao, Lisa C Silbert, Betty Lind, Rachel Crissey, Jeffrey A Kaye, Raina Carter, Sara Dolen, Joseph Quinn, Lon S Schneider, Sonia Pawluczyk, Mauricio Becerra, Liberty Teodoro, Karen Dagerman, Bryan M Spann, James Brewer, Helen Vanderswag, Adam Fleisher, Jaimie Ziolkowski, Judith L Heidebrink, M S Lisa Zbizek-Nulph, Joanne L Lord, Lisa Zbizek-Nulph, Ronald Petersen, Sara S Mason, Colleen S Albers, David Knopman, Kris Johnson, Javier Villanueva-Meyer, Valory Pavlik, Nathaniel Pacini, Ashley Lamb, Joseph S Kass, Rachelle S Doody, Victoria Shibley, Munir Chowdhury, Mimi Dang, Yaakov Stern, Lawrence S Honig, Akiva Mintz, Beau Ances, John C Morris, David Winkfield, Maria Carroll, Georgia Stobbs-Cucchi, Angela Oliver, Mary L Creech, Mark A Mintun, Stacy Schneider, David Geldmacher, Marissa Natelson Love, Randall Griffith, David Clark, John Brockington, Daniel Marson, Hillel Grossman, Martin A Goldstein, Jonathan Greenberg, Effie Mitsis, Raj C Shah, Melissa Lamar, Ranjan Duara, Maria T Greig-Custo, Rosemarie Rodriguez, Marilyn Albert, Chiadi Onyike, Leonie Farrington, Scott Rudow, Rottislav Brichko, Stephanie Kielb, Amanda Smith, Balebail Ashok Raj, Kristin Fargher, Martin Sadowski, Thomas Wisniewski, Melanie Shulman, Arline Faustin, Julia Rao, Karen M Castro, Anaztasia Ulysse, Shannon Chen, Mohammed O Sheikh, Jamika Singleton-Garvin, P Murali Doraiswamy, Jeffrey R Petrella, Olga James, Terence Z Wong, Salvador Borges-Neto, Jason H Karlawish, David A Wolk, Sanjeev Vaishnavi, Christopher M Clark, Steven E Arnold, Charles D Smith, Gregory A Jicha, Riham El Khouli, Flavius D Raslau, Oscar L Lopez, MaryAnn Oakley, Donna M Simpson, Anton P Porsteinsson, Kim Martin, Nancy Kowalski, Melanie Keltz, Bonnie S Goldstein, Kelly M Makino, M Saleem Ismail, Connie Brand, Gaby Thai, Aimee Pierce, Beatriz Yanez, Elizabeth Sosa, Megan Witbracht, Brendan Kelley, Trung Nguyen, Kyle Womack, Dana Mathews, Mary Quiceno, Allan I Levey, James J Lah, Ihab Hajjar, Janet S Cellar, Jeffrey M Burns, Russell H Swerdlow, William M Brooks, Daniel H S Silverman, Sarah Kremen, Liana Apostolova, Kathleen Tingus, H Lu, George Bartzokis, Ellen Woo, Edmond Teng, Neill R Graff-Radford, Francine Parfitt, Kim Poki-Walker, Martin R Farlow, Ann Marie Hake, Brandy R Matthews, Jared R Brosch, Scott Herring, Christopher H van Dyck, Adam P Mecca, Adam P Mecca, Susan P Good, Martha G MacAvoy, Richard E Carson, Pradeep Varma, Howard Chertkow, Susan Vaitekunis, Chris Hosein, Sandra Black, Bojana Stefanovic, Chris (Chinthaka) Heyn, Ging-Yuek Robin Hsiung, Ellen Kim, Benita Mudge, Vesna Sossi, Howard Feldman, Michele Assaly, Elizabeth Finger, Stephen Pasternak, Irina Rachinsky, Andrew Kertesz, Dick Drost, John Rogers, Ian Grant, Brittanie Muse, Emily Rogalski, Jordan Robson, M-Marsel Mesulam, Diana Kerwin, Chuang-Kuo Wu, Nancy Johnson, Kristine Lipowski, Sandra Weintraub, Borna Bonakdarpour, Nunzio Pomara, Raymundo Hernando, Antero Sarrael, Howard J Rosen, Bruce L Miller, David Perry, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Kelly MCCann, Jessica Poe, Reisa A Sperling, Keith A Johnson, Gad A Marshall, Jerome Yesavage, Joy L Taylor, Steven Chao, Jaila Coleman, Jessica D White, Barton Lane, Allyson Rosen, Jared Tinklenberg, Christine M Belden, Alireza Atri, Bryan M Spann, Kelly A Clark, Edward Zamrini, Marwan Sabbagh, Ronald Killiany, Robert Stern, Jesse Mez, Neil Kowall, Andrew E Budson, Thomas O Obisesan, Oyonumo E Ntekim, Saba Wolday, Javed I Khan, Evaristus Nwulia, Sheeba Nadarajah, Alan Lerner, Paula Ogrocki, Curtis Tatsuoka, Parianne Fatica, Evan Fletcher, Pauline Maillard, John Olichney, Charles DeCarli, Owen Carmichael, Vernice Bates, Horacio Capote, Michelle Rainka, Michael Borrie, T-Y Lee, Rob Bartha, Sterling Johnson, Sanjay Asthana, Cynthia M Carlsson, Allison Perrin, Anna Burke, Douglas W Scharre, Maria Kataki, Rawan Tarawneh, Brendan Kelley, David Hart, Earl A Zimmerman, Dzintra Celmins, Delwyn D Miller, Laura L Boles Ponto, Karen Ekstam Smith, Hristina Koleva, Hyungsub Shim, Ki Won Nam, Susan K Schultz, Jeff D Williamson, M H S Suzanne Craft, Jo Cleveland, Mia Yang, Kaycee M Sink, Brian R Ott, Jonathan Drake, Geoffrey Tremont, Lori A Daiello, Jonathan D Drake, Marwan Sabbagh, Aaron Ritter, Charles Bernick, Donna Munic, Akiva Mintz, Abigail O’Connelll, Jacobo Mintzer, Arthur Wiliams, Joseph Masdeu, Jiong Shi, Angelica Garcia, Marwan Sabbagh, Paul Newhouse, Steven Potkin, Stephen Salloway, Paul Malloy, Stephen Correia, Smita Kittur, Godfrey D Pearlson, Karen Blank, Karen Anderson, Laura A Flashman, Marc Seltzer, Mary L Hynes, Robert B Santulli, Norman Relkin, Gloria Chiang, Athena Lee, Michael Lin, Lisa Ravdin, Michael W Weiner, Paul Aisen, Ron Petersen, Michael W Weiner, Paul Aisen, Ronald Petersen, Robert C Green, Danielle Harvey, Clifford R Jack, William Jagust, John C Morris, Andrew J Saykin, Leslie M Shaw, Arthur W Toga, John Q Trojanowki, Thomas Neylan, Jordan Grafman, Robert C Green, Tom Montine, Michael W Weiner, Ronald Petersen, Paul Aisen, Gustavo Jimenez, Michael Donohue, Devon Gessert, Jennifer Salazar, Caileigh Zimmerman, Sarah Walter, Olusegun Adegoke, Payam Mahboubi, Lindsey Hergesheimer, Sarah Danowski, Godfrey Coker, Taylor Clanton, Jeremy Pizzola, Elizabeth Shaffer, Catherine Nguyen-Barrera, Thomas Neylan, Jacqueline Hayes, Shannon Finley, Danielle Harvey, Michael Donohue, Clifford R Jack, Matthew Bernstein, Bret Borowski, Jeff Gunter, Matt Senjem, Kejal Kantarci, Chad Ward, Duygu Tosun-Turgut, Rossi Chen, Susan Landau, Robert A Koeppe, Norm Foster, Eric M Reiman, Kewei Chen, John C Morris, Richard J Perrin, Erin Franklin, Leslie M Shaw, John Q Trojanowki, Magdalena Korecka, Michal Figurski, Arthur W Toga, Karen Crawford, Scott Neu, Andrew J Saykin, Tatiana M Foroud, Steven Potkin, Li Shen, Kelley Faber, Sungeun Kim, Kwangsik Nho, Kristi Wilmes, Lon S Schneider, Sonia Pawluczyk, Mauricio Becerra, Liberty Teodoro, Karen Dagerman, Bryan M Spann, James Brewer, Helen Vanderswag, Adam Fleisher, Yaakov Stern, Lawrence S Honig, Akiva Mintz, Raj C Shah, Ajay Sood, Kimberly S Blanchard, Debra Fleischman, Konstantinos Arfanakis, Ranjan Duara, Daniel Varon, Maria T Greig, P Murali Doraiswamy, Jeffrey R Petrella, Olga James, Salvador Borges-Neto, Terence Z Wong, Anton P Porsteinsson, Bonnie Goldstein, Kimberly S Martin, Gaby Thai, Aimee Pierce, Christopher Reist, Beatriz Yanez, Elizabeth Sosa, Megan Witbracht, Carl Sadowsky, Walter Martinez, Teresa Villena, Howard Rosen, David Perry, Raymond Scott Turner, Kathleen Johnson, Brigid Reynolds, Kelly MCCann, Jessica Poe, Reisa A Sperling, Keith A Johnson, Gad Marshall, Christine M Belden, Alireza Atri, Bryan M Spann, Kelly A Clark, Edward Zamrini, Marwan Sabbagh, Thomas O Obisesan, Oyonumo E Ntekim, Saba Wolday, Evaristus Nwulia, Sheeba Nadarajah, Sterling Johnson, Sanjay Asthana, Cynthia M Carlsson, Elaine R Peskind, Eric C Petrie, Gail Li, Jerome Yesavage, Joy L Taylor, Steven Chao, Jaila Coleman, Jessica D White, Barton Lane, Allyson Rosen, Jared Tinklenberg, Michael Lin, Gloria Chiang, Lisa Ravdin, Norman Relkin, Abigail O’Connelll, Jacobo Mintzer, Arthur Wiliams, Scott Mackin, Paul Aisen, Rema Raman, Gustavo Jimenez-Maggiora, Michael Donohue, Devon Gessert, Jennifer Salazar, Caileigh Zimmerman, Sarah Walter, Olusegun Adegoke, Payam Mahboubi, Scott Mackin, Michael W Weiner, Paul Aisen, Rema Raman, Clifford R Jack, Susan Landau, Andrew J Saykin, Arthur W Toga, Charles DeCarli, Robert A Koeppe, Robert C Green, Erin Drake, Michael W Weiner, Paul Aisen, Rema Raman, Mike Donohue, Scott Mackin, Craig Nelson, David Bickford, Meryl Butters, Michelle Zmuda, Clifford R Jack, Matthew Bernstein, Bret Borowski, Jeff Gunter, Matt Senjem, Kejal Kantarci, Chad Ward, Denise Reyes, Robert A Koeppe, Susan Landau, Arthur W Toga, Karen Crawford, Scott Neu, Andrew J Saykin, Tatiana M Foroud, Kelley M Faber, Kwangsik Nho, Kelly N Nudelman, Scott Mackin, Howard Rosen, Craig Nelson, David Bickford, Yiu Ho Au, Kelly Scherer, Daniel Catalinotto, Samuel Stark, Elise Ong, Dariella Fernandez, Meryl Butters, Michelle Zmuda, Oscar L Lopez, MaryAnn Oakley, and Donna M Simpson
the PREVENT-AD Research Group:
Mohammadali Javanray, Sylvia Villeneuve, Judes Poirier, John C S Breitner, Sylvain Baillet, Pierre Bellec, Véronique Bohbot, Mallar Chakravarty, Louis Collins, Mahsa Dadar, Simon Ducharme, Alan Evans, Maiya R Geddes, Rick Hoge, Gerhard Multhaup, Lisa-Marie Münter, Natasha Rajah, Pedro Rosa-Neto, Jean-Paul Soucy, Nathan Spreng, Christine Tardif, Gabriel Aumont-Rodrigue, Mohamed Badawy, Julie Bailly, Andrée-Ann Baril, Lianne Boisvert, Samir Das, Marina Dauar-Tedeschi, Christine Dery, MarieJosée Élie, Alfonso Fajardo Valdez, Vladimir Fonov, Jonathan Gallago, Claudia Greco, Louise Hudon, Yasser Ituria-Medina, Gabriel Jean, Anne Labonté, Marc Lalancette, Jeannie-Marie Leoutsakos, Bery Mohammediyan, Pierre Orban, Valentin Ourry, Cynthia Picard, Ting Qiu, Marc James Quesnel, Jean-Michel Raoult, Jordana Remz, Frederic St-Onge, Elisabeth Sylvain, Jennifer Tremblay-Mercier, Stephanie Tullo, Etienne Vachon-Presseau, Yara Yakoub, Pierre Etienne, Serge Gauthier, Vasavan Nair, Jens Pruessner, Paul Aisen, Elena Anthal, Melissa Appleby, Gülebru Ayranci, Alan Barkun, Thomas Beaudry, Christophe Bedetti, Fatiha Benbouhoud, Sophie Boutin, Jason Brandt, Leopoldina Carmo, Charles Edouard Carrier, Marianne Chapleau, Laksanun Cheewakriengkrai, Yalin Chen, Blandine Courcot, Doris Couture, Suzanne Craft, Claudio Cuello, Christian Dansereau, Leslie-Ann Daoust, Doris Dea, Clément Debacker, René Desautels, Sylvie Dubuc, Guerda Duclair, Marianne Dufour, Mark Eisenberg, Rana El-Khoury, Sarah Farzin, Anne-Marie Faubert, Fabiola Ferdinand, David Fontaine, Josée Frappier, Joanne Frenette, Guylaine Gagné, Valérie Gervais, Renuka Giles, Julie Gonneaud, Renee Gordon, Clifford R Jack, Justin Kat, Christina Kazazian, Zaven S Khachaturian, David S Knopman, Theresa Köbe, Penelope Kostopoulos, Marie-Elyse Lafaille-Magnan, Gloria LeblondBaccichet, Tanya Lee, Claude Lepage, Illana Leppert, Cécile Madjar, Laura Mahar, David Maillet, Jean-Robert Maltais, Axel Mathieu, Sulantha Mathotaarachchi, Ginette Mayrand, Melissa McSweeney, Pierre-François Meyer, Diane Michaud, Justin Miron, Thomas J Montine, John C Morris, Jamie Near, Holly NewboldFox, Nathalie Nilsson, Hazal Ozlen, Véronique Pagé, Tharick A Pascoal, Sandra Peillieux, Mirela Petkova, Alexa Pichet Binette, Morteza Pishnamazi, Galina Pogossova, Alexandre Poirier, Marie-Josée Richer, Pierre Rioux, Mark A Sager, Eunice Farah Saint-Fort, Alyssa Salaciak, Mélissa Savard, Reisa A Sperling, Cherie Strikwerda-Brown, Shirin Tabrizi, Angela Tam, Pierre N Tariot, Eduard Teigner, Louise Théroux, Ronald G Thomas, Paule-Joanne Toussaint, Miranda Tuwaig, Isabelle Vallée, Vinod Venugopalan, Sander C J Verfaillie, Jacob Vogel, Karen Wan, Seqian Wang, and Elsa Yu
Data availability
Data pertaining to the PREVENT-AD cohort can be downloaded from data release 6.0 at https://openpreventad.loris.ca/. CSF, plasma, genetic and clinical data from the ADNI-1 cohort were downloaded from the ADNI website (http://adni.loni.usc.edu/). Data collected from the QFP cohort are not publicly available; however, the data are available from the corresponding author upon reasonable request.
Funding
J.P. is supported by the Fonds de recherche du Québec-Santé (FRQS), the Canadian Institutes of Health Research (CIHR #PJT 153287) and the J. Louis Levesque Foundation. S.V. is supported by a Canada Research Chair and a Canada Fund for Innovation grant, the FRQS, the CIHR, Brain Canada, McGill University and the Alzheimer’s Association. H.Z. is a Wallenberg Scholar supported by grants from the Swedish Research Council (#2022-01018 and #2019-02397), the European Union’s Horizon Europe research and innovation programme under grant agreement No 101053962, Swedish State Support for Clinical Research (#ALFGBG-71320), the Alzheimer Drug Discovery Foundation (ADDF), USA (#201809-2016862), the AD Strategic Fund and the Alzheimer’s Association (#ADSF-21-831376-C, #ADSF-21-831381-C, and #ADSF-21-831377-C), the Bluefield Project, the Olav Thon Foundation, the Erling-Persson Family Foundation, Stiftelsen för Gamla Tjänarinnor, Hjärnfonden, Sweden (#FO2022-0270), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 860197 (MIRIADE), the EU Joint Programme—Neurodegenerative Disease Research (JPND2021-00694), the National Institute for Health and Care Research University College London Hospitals Biomedical Research Centre, and the UK Dementia Research Institute at UCL (UKDRI-1003). K.B. is supported by the Swedish Research Council (#2017-00915 and #2022-00732), the Swedish Alzheimer Foundation (#AF-930351, #AF-939721 and #AF-968270), Hjärnfonden, Sweden (#FO2017-0243 and #ALZ2022-0006), the Swedish state under the agreement between the Swedish government and the County Councils, the ALF-agreement (#ALFGBG-715986 and #ALFGBG-965240), the European Union Joint Program for Neurodegenerative Disorders (JPND2019-466-236), the Alzheimer’s Association 2021 Zenith Award (ZEN-21-848495), and the Alzheimer's Association 2022-2025 Grant (SG-23-1038904 QC). M.J.Q. is supported by the FRQS.
PREVENT-AD was launched in 2011 as a $13.5 million, 7-year public-private partnership using funds provided by McGill University, FRQS, an unrestricted research grant from Pfizer Canada, the J. Louis Levesque Foundation, the Douglas Hospital Research Centre and Foundation, the Government of Canada, and the Canada Foundation for Innovation. Private sector contributions are facilitated by the Development Office of the McGill University Faculty of Medicine and by the Douglas Hospital Research Centre Foundation (http://www.douglas.qc.ca/).
Data collection and sharing for this project was funded by the ADNI (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The CIHR is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuro Imaging at the University of Southern California.
Competing interests
J.P. serves as a scientific advisor to the Alzheimer Society of France. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZPath, Annexon, Apellis, Artery Therapeutics, AZTherapies, CogRx, Denali, Eisai, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures in symposia sponsored by Cellectricon, Fujirebio, Alzecure, Biogen and Roche, and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is part of the GU Ventures Incubator Program (outside submitted work). K.B. has served as a consultant and at advisory boards for Acumen, ALZPath, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd, Novartis, Ono Pharma, Prothena, Roche Diagnostics and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai and Roche Diagnostics; and is a co-founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. GBD 2019 Dementia Forecasting Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the global burden of disease study 2019. Lancet Public Health. 2022;7:e105–e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nandi A, Counts N, Chen S, et al. Global and regional projections of the economic burden of Alzheimer’s disease and related dementias from 2019 to 2050: A value of statistical life approach. EClinicalMedicine. 2022;51:101580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alzheimer’s Association . 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18:700–789. [DOI] [PubMed] [Google Scholar]
- 4. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect Med. 2011;1:a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jack CR Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010;9:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arvanitakis Z, Wilson RS, Bienias JL, Evans DA, Bennett DA. Diabetes mellitus and risk of Alzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–666. [DOI] [PubMed] [Google Scholar]
- 7. Arnold SE, Arvanitakis Z, Macauley-Rambach SL, et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat Rev Neurol. 2018;14:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Steen E, Terry BM, Rivera JE, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease–is this type 3 diabetes? J Alzheimers Dis. 2005;7:63–80. [DOI] [PubMed] [Google Scholar]
- 9. Moloney AM, Griffin RJ, Timmons S, O’Connor R, Ravid R, O’Neill C. Defects in IGF-1 receptor, insulin receptor and IRS-1/2 in Alzheimer’s disease indicate possible resistance to IGF-1 and insulin signalling. Neurobiol Aging. 2010;31:224–243. [DOI] [PubMed] [Google Scholar]
- 10. Talbot K, Wang HY, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kar S, Poirier J, Guevara J, et al. Cellular distribution of insulin-like growth factor-II/mannose-6-phosphate receptor in normal human brain and its alteration in Alzheimer’s disease pathology. Neurobiol Aging. 2006;27:199–210. [DOI] [PubMed] [Google Scholar]
- 12. Farris W, Mansourian S, Chang Y, et al. Insulin-degrading enzyme regulates the levels of insulin, amyloid β-protein, and the β-amyloid precursor protein intracellular domain in vivo. Proc Natl Acad Sci U S A. 2003;100:4162–4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carro E, Trejo JL, Gomez-Isla T, LeRoith D, Torres-Aleman I. Serum insulin-like growth factor I regulates brain amyloid-β levels. Nat Med. 2002;8:1390–1397. [DOI] [PubMed] [Google Scholar]
- 14. Hong M, Lee VM. Insulin and insulin-like growth factor-1 regulate tau phosphorylation in cultured human neurons. J Biol Chem. 1997;272:19547–19553. [DOI] [PubMed] [Google Scholar]
- 15. Schubert M, Brazil DP, Burks DJ, et al. Insulin receptor substrate-2 deficiency impairs brain growth and promotes tau phosphorylation. J Neurosci. 2003;23:7084–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. De Felice FG, Vieira MN, Bomfim TR, et al. Protection of synapses against Alzheimer’s-linked toxins: Insulin signaling prevents the pathogenic binding of aβ oligomers. Proc Natl Acad Sci U S A. 2009;106:1971–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Doré S, Kar S, Quirion R. Insulin-like growth factor I protects and rescues hippocampal neurons against beta-amyloid- and human amylin-induced toxicity. Proc Natl Acad Sci U S A. 1997;94:4772–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willette AA, Bendlin BB, Starks EJ, et al. Association of insulin resistance with cerebral glucose uptake in late middle-aged adults at risk for Alzheimer disease. JAMA Neurol. 2015;72:1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reger MA, Watson GS, Green PS, et al. Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology. 2008;70:440–448. [DOI] [PubMed] [Google Scholar]
- 20. Craft S, Baker LD, Montine TJ, et al. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: A pilot clinical trial. Arch Neurol. 2012;69:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Kusky JR, Ye P, D'Ercole AJ. Insulin-like growth factor-I promotes neurogenesis and synaptogenesis in the hippocampal dentate gyrus during postnatal development. J Neurosci. 2000;20:8435–8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russo VC, Gluckman PD, Feldman EL, Werther GA. The insulin-like growth factor system and its pleiotropic functions in brain. Endocr Rev. 2005;26:916–943. [DOI] [PubMed] [Google Scholar]
- 23. Allard JB, Duan C. IGF-binding proteins: Why do they exist and why are there so many? Front Endocrinol (Lausanne). 2018;9:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hwa V, Oh Y, Rosenfeld RG. The insulin-like growth factor-binding protein (IGFBP) superfamily. Endocr Rev. 1999;20:761–787. [DOI] [PubMed] [Google Scholar]
- 25. Russo VC, Bach LA, Fosang AJ, Baker NL, Werther GA. Insulin-like growth factor binding protein-2 binds to cell surface proteoglycans in the rat brain olfactory bulb. Endocrinology. 1997;138:4858–4867. [DOI] [PubMed] [Google Scholar]
- 26. Schütt BS, Langkamp M, Rauschnabel U, Ranke MB, Elmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32:859–868. [DOI] [PubMed] [Google Scholar]
- 27. Russo VC, Rekaris G, Baker NL, Bach LA, Werther GA. Basic fibroblast growth factor induces proteolysis of secreted and cell membrane-associated insulin-like growth factor binding protein-2 in human neuroblastoma cells. Endocrinology. 1999;140:3082–3090. [DOI] [PubMed] [Google Scholar]
- 28. Roghani M, Lassarre C, Zapf J, Povoa G, Binoux M. Two insulin-like growth factor (IGF)-binding proteins are responsible for the selective affinity for IGF-II of cerebrospinal fluid binding proteins. J Clin Endocrinol Metab. 1991;73:658–666. [DOI] [PubMed] [Google Scholar]
- 29. Ocrant I, Fay CT, Parmelee JT. Characterization of insulin-like growth factor binding proteins produced in the rat central nervous system. Endocrinology. 1990;127:1260–1267. [DOI] [PubMed] [Google Scholar]
- 30. Tham A, Nordberg A, Grissom FE, Carlsson-Skwirut C, Viitanen M, Sara VR. Insulin-like growth factors and insulin-like growth factor binding proteins in cerebrospinal fluid and serum of patients with dementia of the Alzheimer type. J Neural Transm Park Dis Dement Sect. 1993;5:165–176. [DOI] [PubMed] [Google Scholar]
- 31. Salehi Z, Mashayekhi F, Naji M. Insulin like growth factor-1 and insulin like growth factor binding proteins in the cerebrospinal fluid and serum from patients with Alzheimer’s disease. Biofactors. 2008;33:99–106. [DOI] [PubMed] [Google Scholar]
- 32. Hertze J, Nägga K, Minthon L, Hansson O. Changes in cerebrospinal fluid and blood plasma levels of IGF-II and its binding proteins in Alzheimer’s disease: An observational study. BMC Neurol. 2014;14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Åberg D, Johansson P, Isgaard J, et al. Increased cerebrospinal fluid level of insulin-like growth factor-II in male patients with Alzheimer’s disease. J Alzheimers Dis. 2015;48:637–646. [DOI] [PubMed] [Google Scholar]
- 34. O'Bryant SE, Xiao G, Barber R, et al. A serum protein-based algorithm for the detection of Alzheimer disease. Arch Neurol. 2010;67:1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Doecke JD, Laws SM, Faux NG, et al. Blood-based protein biomarkers for diagnosis of Alzheimer disease. Arch Neurol. 2012;69:1318–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bonham LW, Geier EG, Steele NZ, et al. Insulin-like growth factor binding protein 2 is associated with biomarkers of Alzheimer’s disease pathology and shows differential expression in transgenic mice. Front Neurosci. 2018;12:476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGrath ER, Himali JJ, Levy D, et al. Circulating IGFBP-2: A novel biomarker for incident dementia. Ann Clin Transl Neurol. 2019;6:1659–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rocha de Paula M, Gómez Ravetti M, Berretta R, Moscato P. Differences in abundances of cell-signalling proteins in blood reveal novel biomarkers for early detection of clinical Alzheimer’s disease. PLoS One. 2011;6:e17481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tremblay-Mercier J, Madjar C, Das S, et al. Open science datasets from PREVENT-AD, a longitudinal cohort of pre-symptomatic Alzheimer’s disease. Neuroimage Clin. 2021;31:102733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brinkmalm A, Brinkmalm G, Honer WG, et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol Neurodegener. 2014;9:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Öhrfelt A, Brinkmalm A, Dumurgier J, et al. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimers Res Ther. 2016;8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tible M, Sandelius Å, Höglund K, et al. Dissection of synaptic pathways through the CSF biomarkers for predicting Alzheimer disease. Neurology. 2020;95:e953–e961. [DOI] [PubMed] [Google Scholar]
- 43. Xu C, Sellgren CM, Fatouros-Bergman H, et al. CSF levels of synaptosomal-associated protein 25 and synaptotagmin-1 in first-episode psychosis subjects. IBRO Rep. 2020;8:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sandelius Å, Portelius E, Källén Å, et al. Elevated CSF GAP-43 is Alzheimer’s disease specific and associated with tau and amyloid pathology. Alzheimers Dement. 2019;15:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Portelius E, Olsson B, Höglund K, et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: Relation to clinical phenotypes and neuropathology. Acta Neuropathol. 2018;136:363–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. McSweeney M, Binette AP, Meyer PF, et al. Intermediate flortaucipir uptake is associated with Aβ-PET and CSF tau in asymptomatic adults. Neurology. 2020;94:e1190-1200. [DOI] [PubMed] [Google Scholar]
- 47. Zijdenbos AP, Forghani R, Evans AC. Automatic ‘pipeline’ analysis of 3-D MRI data for clinical trials: Application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–1291. [DOI] [PubMed] [Google Scholar]
- 48. Aubert-Broche B, Fonov VS, García-Lorenzo D, et al. A new method for structural volume analysis of longitudinal brain MRI data and its application in studying the growth trajectories of anatomical brain structures in childhood. Neuroimage. 2013;82:393–402. [DOI] [PubMed] [Google Scholar]
- 49. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 50. Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement. 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shaw LM, Vanderstichele H, Knapik-Czajka M, et al. Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Scriver CR. Human genetics: Lessons from Quebec populations. Annu Rev Genomics Hum Genet. 2001;2:69–101. [DOI] [PubMed] [Google Scholar]
- 53. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA work group* under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- 54. Picard C, Nilsson N, Labonté A, et al. Apolipoprotein B is a novel marker for early tau pathology in Alzheimer’s disease. Alzheimers Dement. 2022;18:875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in early Alzheimer’s disease. N Engl J Med. 2023;388:9–21. [DOI] [PubMed] [Google Scholar]
- 56. Gluckman P, Klempt N, Guan J, et al. A role for IGF-1 in the rescue of CNS neurons following hypoxic-ischemic injury. Biochem Biophys Res Commun. 1992;182:593–599. [DOI] [PubMed] [Google Scholar]
- 57. Klempt ND, Klempt M, Gunn AJ, Singh K, Gluckman PD. Expression of insulin-like growth factor-binding protein 2 (IGF-BP 2) following transient hypoxia-ischemia in the infant rat brain. Brain Res Mol Brain Res. 1992;15(1-2):55–61. [DOI] [PubMed] [Google Scholar]
- 58. Beilharz EJ, Russo VC, Butler G, et al. Co-ordinated and cellular specific induction of the components of the IGF/IGFBP axis in the rat brain following hypoxic–ischemic injury. Brain Res Mol Brain Res. 1998;59:119–134. [DOI] [PubMed] [Google Scholar]
- 59. Sandberg Nordqvist AC, Von Holst H, Holmin S, Sara VR, Bellander BM, Schalling M. Increase of insulin-like growth factor (IGF)-1, IGF binding protein-2 and− 4 mRNAs following cerebral contusion. Brain Res Mol Brain Res. 1996;38:285–293. [DOI] [PubMed] [Google Scholar]
- 60. Walter HJ, Berry M, Hill DJ, Logan A. Spatial and temporal changes in the insulin-like growth factor (IGF) axis indicate autocrine/paracrine actions of IGF-I within wounds of the rat brain. Endocrinology. 1997;138:3024–3034. [DOI] [PubMed] [Google Scholar]
- 61. Fletcher L, Isgor E, Sprague S, et al. Spatial distribution of insulin-like growth factor binding protein-2 following hypoxic-ischemic injury. BMC Neurosci. 2013;14:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Breese CR, D'Costa A, Rollins YD, et al. Expression of insulin-like growth factor-1 (IGF-1) and IGF-binding protein 2 (IGF-BP2) in the hippocampus following cytotoxic lesion of the dentate gyrus. J Comp Neurol. 1996;369:388–404. [DOI] [PubMed] [Google Scholar]
- 63. Kar S, Baccichet A, Quirion R, Poirier J. Entorhinal cortex lesion induces differential responses in [125I]insulin-like growth factor I, [125I]insulin-like growth factor II and [125I]insulin receptor binding sites in the rat hippocampal formation. Neuroscience. 1993;55:69–80. [DOI] [PubMed] [Google Scholar]
- 64. Guan J, Williams CE, Skinner SJ, Mallard EC, Gluckman PD. The effects of insulin-like growth factor (IGF)-1, IGF-2, and des-IGF-1 on neuronal loss after hypoxic-ischemic brain injury in adult rats: Evidence for a role for IGF binding proteins. Endocrinology. 1996;137:893–898. [DOI] [PubMed] [Google Scholar]
- 65. Braak H, Braak E. Staging of Alzheimer’s disease-related neurofibrillary changes. Neurobiol Aging. 1995;16:271–284. [DOI] [PubMed] [Google Scholar]
- 66. Bolós M, Fernandez S, Torres-Aleman I. Oral administration of a GSK3 inhibitor increases brain insulin-like growth factor I levels. J Biol Chem. 2010;285:17693–17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lucas JJ, Hernández F, Gómez-Ramos P, Morán MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20(1-2):27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Engel T, Lucas JJ, Gómez-Ramos P, Moran MA, Avila J, Hernández F. Cooexpression of FTDP-17 tau and GSK-3beta in transgenic mice induce tau polymerization and neurodegeneration. Neurobiol Aging. 2006;27:1258–1268. [DOI] [PubMed] [Google Scholar]
- 69. Hernández F, Borrell J, Guaza C, Avila J, Lucas JJ. Spatial learning deficit in transgenic mice that conditionally over-express GSK-3beta in the brain but do not form tau filaments. J Neurochem. 2002;83:1529–1533. [DOI] [PubMed] [Google Scholar]
- 70. Hoeflich A, Reyer A, Ohde D, et al. Dissociation of somatic growth, time of sexual maturity, and life expectancy by overexpression of an RGD-deficient IGFBP-2 variant in female transgenic mice. Aging Cell. 2016;15:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kanje M, Skottner A, Sjöberg J, Lundborg G. Insulin-like growth factor I (IGF-I) stimulates regeneration of the rat sciatic nerve. Brain Res. 1989;486:396–398. [DOI] [PubMed] [Google Scholar]
- 72. Near SL, Whalen LR, Miller JA, Ishii DN. Insulin-like growth factor II stimulates motor nerve regeneration. Proc Natl Acad Sci U S A. 1992;89:11716–11720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Guthrie KM, Nguyen T, Gall CM. Insulin-like growth factor-1 mRNA is increased in deafferented hippocampus: Spatiotemporal correspondence of a trophic event with axon sprouting. J Comp Neurol. 1995;352:147–160. [DOI] [PubMed] [Google Scholar]
- 74. Duan X, Qiao M, Bei F, Kim IJ, He Z, Sanes JR. Subtype-specific regeneration of retinal ganglion cells following axotomy: Effects of osteopontin and mTOR signaling. Neuron. 2015;85:1244–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Anderson MA, O’Shea TM, Burda JE, et al. Required growth facilitators propel axon regeneration across complete spinal cord injury. Nature. 2018;561:396–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Liu Y, Wang X, Li W, et al. A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron. 2017;95:817–833.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Lane EM, Hohman TJ, Jefferson AL. Alzheimer’s disease neuroimaging initiative. Insulin-like growth factor binding protein-2 interactions with Alzheimer’s disease biomarkers. Brain Imaging Behav. 2017;11:1779–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lee WH, Michels KM, Bondy CA. Localization of insulin-like growth factor binding protein-2 messenger RNA during postnatal brain development: Correlation with insulin-like growth factors I and II. Neuroscience. 1993;53:251–265. [DOI] [PubMed] [Google Scholar]
- 79. Khan S, Lu X, Huang Q, et al. IGFBP2 plays an essential role in cognitive development during early life. Adv Sci (Weinh). 2019;6:1901152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Burgdorf J, Colechio EM, Ghoreishi-Haack N, et al. IGFBP2 produces rapid-acting and long-lasting effects in rat models of posttraumatic stress disorder via a novel mechanism associated with structural plasticity. Int J Neuropsychopharmacol. 2017;20:476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Brooker GJ, Kalloniatis M, Russo VC, Murphy M, Werther GA, Bartlett PF. Endogenous IGF-1 regulates the neuronal differentiation of adult stem cells. J Neurosci Res. 2000;59:332–341. [DOI] [PubMed] [Google Scholar]
- 82. Burgdorf JS, Yoon S, Dos Santos M, Lammert CR, Moskal JR, Penzes P. An IGFBP2-derived peptide promotes neuroplasticity and rescues deficits in a mouse model of Phelan-McDermid syndrome. Mol Psychiatry. 2023;28:1101–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stein TD, Anders NJ, DeCarli C, Chan SL, Mattson MP, Johnson JA. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: Support for the amyloid hypothesis. J Neurosci. 2004;24:7707–7717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Schindler N, Mayer J, Saenger S, et al. Phenotype analysis of male transgenic mice overexpressing mutant IGFBP-2 lacking the Cardin-Weintraub sequence motif: Reduced expression of synaptic markers and myelin basic protein in the brain and a lower degree of anxiety-like behaviour. Growth Horm IGF Res. 2017;33:1–8. [DOI] [PubMed] [Google Scholar]
- 85. Gómez-Isla T, Price JL, Mckeel DW Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dickerson BC, Goncharova I, Sullivan MP, et al. MRI-derived entorhinal and hippocampal atrophy in incipient and very mild Alzheimer’s disease. Neurobiol Aging. 2001;22:747–754. [DOI] [PubMed] [Google Scholar]
- 87. Dickerson BC, Bakkour A, Salat DH, et al. The cortical signature of Alzheimer’s disease: Regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Schwarz CG, Gunter JL, Wiste HJ, et al. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. Neuroimage Clin. 2016;11:802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Toledo JB, Da X, Bhatt P, et al. Relationship between plasma analytes and SPARE-AD defined brain atrophy patterns in ADNI. PLoS One. 2013;8:e55531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McLimans KE, Webb JL, Anantharam V, Kanthasamy A, Willette AA. Alzheimer’s disease neuroimaging initiative. Peripheral versus central Index of metabolic dysfunction and associations with clinical and pathological outcomes in Alzheimer’s disease. J Alzheimers Dis. 2017;60:1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Meyer PF, Savard M, Poirier J, et al. Bi-directional association of cerebrospinal fluid immune markers with stage of Alzheimer’s disease pathogenesis. J Alzheimers Dis. 2018;63:577–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Azar WJ, Zivkovic S, Werther GA, Russo VC. IGFBP-2 nuclear translocation is mediated by a functional NLS sequence and is essential for its pro-tumorigenic actions in cancer cells. Oncogene. 2014;33:578–588. [DOI] [PubMed] [Google Scholar]
- 93. Mahapatra MK, Karuppasamy M, Sahoo BM. Therapeutic potential of semaglutide, a newer GLP-1 receptor agonist, in abating obesity, non-alcoholic steatohepatitis and neurodegenerative diseases: A narrative review. Pharm Res. 2022;39:1233–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Doré S, Kar S, Quirion R. Rediscovering an old friend, IGF-I: Potential use in the treatment of neurodegenerative diseases. Trends Neurosci. 1997;20:326–331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data pertaining to the PREVENT-AD cohort can be downloaded from data release 6.0 at https://openpreventad.loris.ca/. CSF, plasma, genetic and clinical data from the ADNI-1 cohort were downloaded from the ADNI website (http://adni.loni.usc.edu/). Data collected from the QFP cohort are not publicly available; however, the data are available from the corresponding author upon reasonable request.