Abstract
The pasticcino (pas) mutants of Arabidopsis thaliana are a new class of plant developmental mutants; members of this class show ectopic cell proliferation in cotyledons, extra layers of cells in the hypocotyl, and an abnormal apical meristem. This phenotype is correlated with both cell division and cell elongation defects. There are three complementation groups of pas mutants (pas1, pas2, and pas3, with, respectively 2, 1, and 4 alleles). Here we describe in more detail the pas1-1 allele, which was obtained by insertional mutagenesis. The PAS1 gene has been cloned and characterized; it encodes an immunophilin-like protein similar to the p59 FK506-binding protein (FKBP52). PAS1 is characterized by an FKBP-like domain and three tetratricopeptide repeat units. Although the presence of immunophilins in plants has already been demonstrated, the pas1-1 mutant represents the first inactivation of an FKBP-like gene in plants. PAS1 expression is altered in pas1 mutants and in the pas2 and pas3 mutants. The expression of the PAS1 gene is increased in the presence of cytokinins, a class of phytohormones originally discovered because of their ability to stimulate cell division. These results are of particular relevance as they show for the first time that an FKBP-like protein plays an important role in the control of plant development.
In flowering plants, morphogenesis depends on the control of the pattern and numbers of cell divisions and on the control of cell elongation. Although there are many examples of controlled patterns of cell division, we still know very little about how local patterns of cell division are established and maintained (30). In Arabidopsis thaliana, the roles of cell division control in the development of the embryo, the shoot, and the root have been extensively studied (reviewed in references 29 and 30). In the last few years, much progress has been made in this field by the isolation of mutants in which single-gene mutations affect specific modes of cell division control. Some of the corresponding genes have been cloned from A. thaliana (SHOOT MERISTEMLESS [STM] and SCARECROW [SCR]) maize (KNOTTED1), and petunia (NO APICAL MERISTEM) (reviewed in reference 30). These genes do not seem to specify components of the cell division machinery, but they are thought to act upstream in the control of cell division. The elements at the interface between genes like STM and SCR and cell cycle regulators, such as cyclins and the CDC genes, are still unknown.
The growth and differentiation of higher plants is also greatly dependent on environmental stimuli, such as light and temperature, and on endogenous factors, such as phytohormones. Cytokinins (CKs) were originally discovered because of their ability to promote, along with auxins, plant cell division and organogenesis (reviewed in reference 9). Although this discovery initiated a vast amount of fundamental and applied research on the hormonal control of cell proliferation and regeneration, the mechanisms by which auxins and CKs act and interact at the molecular level are unknown. Steroid-like plant growth factors termed brassinosteroids (BR) were first characterized as inducing cell elongation in synergy with auxin, but recently these hormones have also been found to control plant cell divisions and morphogenesis (15; reviewed in reference 11).
The genetic and molecular analysis of hormonal mutants is proving to be a powerful tool for unraveling the mode of action of these molecules. In an attempt to understand the mode of action of CKs and their molecular relationships with auxins in promoting plant cell division, we looked for Arabidopsis mutants with phenotypes which were affected by exogenously applied CKs. We have previously reported the isolation of the pasticcino mutants (pas1, pas2, and pas3) which are affected in both embryonic and vegetative development. Their phenotypes are similar to that of wild-type shoots which have been regenerated in vitro from explants, in the presence of an unbalanced auxin/CK ratio in the medium (12).
The pas1-1 mutant was isolated from the transfer DNA (T-DNA) mutant collection of INRA-Centre de Versailles (2, 12). Here we describe the cloning of the PAS1 gene from the T-DNA-tagged pas1-1 allele. PAS1 codes for an immunophilin-like protein similar to the FK506-binding proteins (FKBP). We also demonstrate that the PAS1 mRNA steady-state level is increased in the presence of CK and that PAS1 gene expression is affected in the other pas mutants.
MATERIALS AND METHODS
Arabidopsis lines and growth conditions.
Seeds from A. thaliana Heynh, ecotype Columbia (Col0) and ecotype Wassilewskija (WS), were kindly provided by J. Giraudat (CNRS, Gif sur Yvette, France) and by K. Feldman (University of Arizona, Tucson, Ariz.), respectively. The mutagenized lines were produced as already described (2, 12). For growth in the greenhouse, seeds were sown on soil and seedlings were transferred into individual pots 10 days after germination. Plants were grown under the following conditions: 16 h of light, 20 to 25°C day temperature, and 10 to 15°C night temperature. For in vitro growth, seeds were sterilized and grown as already described (12).
Benzyladenine (BA), zeatin, 1-(2-chloropyrid-4-yl)-3-phenylurea, and picloram, an auxin analog, were filter sterilized and added to the medium at increasing concentrations from 0 to 10 μM.
Cytological analysis.
For light microscopy, seedlings were fixed in 4% formaldehyde–0.2% glutaraldehyde and then embedded in Historesin (Leica, Rueil Malmaison, France) in accordance with the manufacturer’s instructions. Semithin sections (3 to 5 μm thick) were cut on a Jung RM microtome, stained with 0.05% methylene blue, and examined with a Nikon Microphot FXA microscope.
Isolation of the pas1-1 mutant.
The pasticcino1-1 (pas1-1) mutant was identified in the progeny of T-DNA-mutagenized Arabidopsis ecotype WS lines produced at the Station de Génétique et Amélioration des Plantes (Versailles, France) and had been previously screened for resistance to the Basta herbicide (2, 12). Screening for mutants was based on the early phenotype of 9-day-old plants grown both in the light and in the dark. It was determined that pas1-1 contained a single T-DNA insert, and linkage of the T-DNA insert to the PAS1 gene was tested. More then 100 putative heterozygotes (kanamycin-resistant seedlings with a wild-type phenotype) each produced 25% mutant progeny. Plants heterozygous for the mutation were self-fertilized, and the transmission of the phenotype was confirmed in the M3 generation.
Isolation of genomic DNA and cDNA PAS1 clones.
Seeds harvested from pas1-1 heterozygous plants were grown in vitro as stated above. Fourteen-day-old pas1-1 mutants were harvested for DNA extraction. The BamHI and XbaI genomic DNA fragments adjacent to the right border of the single T-DNA insert were isolated by the strategy of kanamycin rescue (6). pas1-1 genomic DNA was double digested with PstI-BamHI and PstI-XbaI and ligated, respectively, into PstI-BamHI- and PstI-XbaI-digested pResc38 vectors. Transformation of high-efficiency Escherichia coli DH12S competent cells (1010 CFU/μg of DNA) was performed by electroporation, and recombinant clones were checked both by PCR analysis and Southern blot hybridization.
Genomic DNA flanking the left end of the T-DNA insert was isolated by inverse PCR. DNA from homozygous pas1-1 mutants was digested with XbaI and then ligated for 16 h at 16°C in a 200-μl reaction mixture. The ligation mixture was extracted with phenol-CHCl3 and with CHCl3, and DNA was precipitated with ethanol. The DNA was then added to a PCR mixture. Synthetic oligonucleotides corresponding to the left border of the T-DNA were used as primers. The PCR product was digested with XbaI and HindIII restriction enzymes and then cloned into the pBluescript SK+ vector (Stratagene).
Genomic DNA flanking both the right and left borders of the T-DNA insert was used to screen an Arabidopsis Columbia genomic library (EEC-BRIDGE Arabidopsis DNA Stock Center, Cologne, Germany) and an Arabidopsis cDNA library (31). NotI cDNA inserts were cloned into the pBluescript SK+ vector (Stratagene) for restriction analysis, and subclones were used for sequencing. DNA sequencing was performed by using Taq DNA polymerase, dye primers, and an ABI 373A automated DNA sequencer as recommended by the manufacturer (Applied Biosystems). Standard molecular techniques were used throughout (36). The analysis of PAS1 cDNAs and derived protein sequences was performed in part with the GCG and BLAST computer programs and in part by the National Center for Biotechnology Information, Bethesda, Md. RNA from pas1-2 seedlings was reverse transcribed with a poly(dT) primer. Specific primers were used to amplify the pas1-2 cDNA sequence, which was cloned into pBluescript SK+. Clones from several independent PCRs on independent cDNA samples were sequenced.
Southern and Northern blot analysis.
Arabidopsis genomic DNA was isolated as previously described (6). Southern blots on Hybond N membranes were produced as described by the manufacturer (Amersham).
For RNA extraction, 9-day-old wild types (WS and Col0) and pas mutants were harvested and immediately frozen in liquid nitrogen. Total RNA was isolated by grinding the tissues in liquid nitrogen. The samples were then vortexed for 3 min in the presence of an extraction buffer (0.1 M LiCl, 0.1 M Tris-HCl [pH 8], 0.01 M EDTA, 1% sodium dodecyl sulfate-phenol-chloroform mixture (1:1:1). Several phenol-chloroform extractions were then performed. RNA was precipitated overnight at 4°C with 1 volume of 4 M LiCl, followed by a second precipitation with 0.1 volume of sodium acetate, pH 5.2. Northern blots on Hybond N membranes were hybridized to random primer-labeled probes according to the manufacturer’s instructions (Biolabs). Blots were stripped and reprobed with other probes to normalize the amount of RNA loaded.
Mapping of the PAS1 locus.
Restriction fragment length polymorphism (RFLP) analysis was performed on 98 F8 recombinant inbred lines generated from a cross between the ecotypes Landsberg erecta and Col0 (25) and digested with HpaII. The BamHI genomic fragment flanking the T-DNA right border was used as a probe in the Southern blot analysis. The linkage analysis was done with the MapMaker program. Recombination frequencies were calculated as described previously (25) and converted to map distances in centimorgans by using the Kosambi mapping function (21).
Histochemical GUS assays.
Histochemical assays for β-glucuronidase (GUS) expression were performed as described by Mollier et al. (32). The reactions were conducted for 4 to 8 h at 37°C for pas1-1 mutants and for 8 to 16 h for pas1-1 heterozygous plants. Assays of GUS activity were performed as described by Jefferson et al. (19), both on 9-day-old pas1-1 mutants and pas1-1/+ plants.
Functional complementation.
A 2.5-kb XbaI-XhoI fragment corresponding to the PAS1 full-length cDNA was cloned into the pKYLX71 (28) plant binary vector, previously digested with XbaI-XhoI. Agrobacterium tumefaciens C58C1 (pMP90) was transformed by electroporation, and recombinant clones were isolated on kanamycin (20 μg/ml) and checked by Southern blot analysis. Plant transformations were performed by using the in planta transformation system (2). Transformant plants were selected in vitro on kanamycin (100 μg/ml). The F2 and F3 progeny of individual kanamycin-resistant plants were then analyzed for the segregation of pas1 mutants.
Nucleotide sequence accession numbers.
The nucleotide sequences of cDNA-A and cDNA-D have been deposited in GenBank under accession no. U77365 and U77366, respectively.
RESULTS
Isolation of a pleiotropic mutant altered in early development.
The pas1-1 mutant was isolated from the T-DNA collection of INRA-Centre de Versailles as a heterozygous pas1-1/+ line resistant to the Basta herbicide. A pas1 allelic mutant (pas1-2) and several other pas mutants representing three complementation groups were isolated from an ethyl methane sulfonate (EMS) mutant collection based on their abnormal responses to exogenous CKs (12). Mutants grown in the dark had short and wide hypocotyls and lacked apical hooks (Fig. 1A). For pas1 mutants grown in the light, the phenotype was characterized by a very short and thick hypocotyl and an altered cotyledon shape (Fig. 1B). The pas1 mutants could not survive under normal growth conditions but could be maintained in vitro. They often had fused leaves with a vitreous appearance. Both pas1 allelic mutants were characterized by the absence of secondary roots and a primary root shorter than that of the wild type (Fig. 1B and C). After 3 months, pas mutants developed abnormal compact and vitreous rosettes (Fig. 1D). Several mutants produced finger-like structures before growth was arrested. Some pas1 mutants were able to flower but only developed very short stems with abnormal and sterile flowers (data not shown).
FIG. 1.
Phenotypes of pas1 mutants. (A) Seven-day-old plants grown in the dark. Left, pas1-1 plant; right, wild-type plant. (B) Plants 8 days after germination. From left to right, wild type and three pas1-1 mutants. (C) Three-week-old pas1-1 mutants. (D) Three-month-old pas1-1 mutant. (E) Three-week-old wild type. (F) Wild-type plants grown on 5 μM BA for 3 weeks. (G) pas1-1 mutants grown in the absence (left) or in the presence (right) of 5 μM BA. Plants were grown in the light unless otherwise indicated.
While the growth of the wild type was severely inhibited by the presence of 5 μM BA (Fig. 1E and F), the mutant displayed a hypertrophy of the apical part (Fig. 1G). We have previously shown that the response to CK of the pasticcino mutants was characterized by an increase in cell divisions, specifically in the apical part. No significant difference between the pas mutants and the wild type with regard to root growth inhibition in the presence of BA was observed (12). The same results were obtained with other active CK molecules, such as zeatin and 1-(2-chloropyrid-4-yl)-3-phenylurea (data not shown). The growth responses of the pas mutants in the presence of other plant hormones were analyzed in both light and dark growth conditions, but none of the hormones tested (auxin, ethylene, gibberellic acid, abscisic acid, and BR) was able to induce a hypertrophy of the apical parts of pas mutants, indicating that this particular response was specific to CKs (12).
Cytological analysis showed that pas1-1 hypocotyls have extra disorganized cell layers, irregular numbers of cortex cells, a loss of cell adhesion, and ectopic periclinal divisions in the epidermis (Fig. 2A and B). The different meristematic cell layers were never clearly distinguishable. The pas1-1 mutants had meristems with a highly variable structure, ranging from plants with almost no meristem to those with a very large meristem, which filled the entire apical region (Fig. 2C, D, and E).
FIG. 2.
Effects of the pas1 mutation on cell division. Hypocotyl cross sections of the wild type (A) and the pas1 mutant (B) and longitudinal sections of the shoot apical meristems of the wild type (C) and of pas1 mutants (D and E). Bars, 200 μm (A, C, D, and E) and 400 μm (B). Sections were made on 10-day-old seedlings.
After three outcrosses of the pas1-1 mutant with the wild type, segregation was found to be consistent with pas1-1 being a nuclear, recessive, and monogenic mutation. In the progeny of plants heterozygous for pas1-1, the pas1-1 mutation was shown to cosegregate with a single T-DNA insertion carrying the kanamycin resistance gene (see Materials and Methods).
Isolation and molecular characterization of the PAS1 gene.
As genetic analysis of the segregation of the Pas1-1− phenotype and the T-DNA insertion indicated tight linkage between the T-DNA insert and the PAS1 gene, we performed a molecular characterization of the pas1-1 mutation. Southern analysis of DNA extracted from pas1-1 mutants and probed with both the T-DNA right border (data not shown) and left border (Fig. 3A) revealed that the pas1-1 mutation was caused by the insertion of a single T-DNA unit.
FIG. 3.
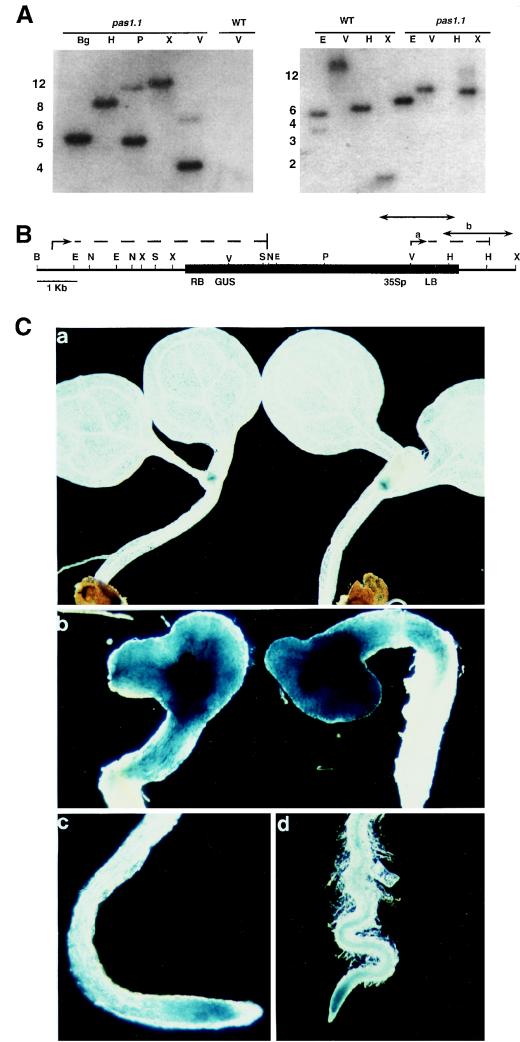
Molecular characterization of the pas1-1 mutation. (A) Southern analysis of pas1-1 and wild-type genomic DNA probed with the left T-DNA border (left) and with the genomic fragment adjacent to the left T-DNA border (right). (B) Schematic map of the T-DNA-tagged pas1-1 allele. The arrows with the dashed lines show the two mRNAs transcribed from the pas1-1 allele. The first transcript is transcribed from the PAS1 promoter through the right T-DNA border giving rise to the translational fusion between the 5′ part of the PAS1 gene and the GUS gene. The second transcript arises due to transcription from the cauliflower mosaic virus 35S promoter (35Sp) through the BAR gene (which confers resistance to Basta) and the 3′ end of the PAS1 gene. The thin black line represents the genomic DNA, and the T-DNA is shown by the black box. B, BamHI; Bg, BglII; E, EcoRI; H, HindIII; N, NsiI; P, PstI; S, SacI; V, EcoRV; X, XbaI. a and b denote the probes used for the Southern analysis. (C) GUS staining of pas1-1/+ plants (a) and pas1-1 mutants (b), and in a pas1-1/+ plant root (c) and in a pas1-1 plant root (d). The plants were grown for 9 days in standard conditions in the light.
We isolated different genomic DNA fragments adjacent to the right and left borders of the T-DNA. BamHI (4 kb) and XbaI (0.4 kb) genomic fragments were cloned as sequences flanking the right border of the T-DNA by using the kanamycin plasmid rescue strategy (6) (see Materials and Methods). A 1.6-kb XbaI genomic fragment adjacent to the T-DNA left border was isolated by an inverse PCR strategy (see Materials and Methods). Synthetic oligonucleotides corresponding to the genomic XbaI fragments, flanking both the T-DNA right and left borders, were used in a PCR on a wild-type genomic-DNA template to verify whether the T-DNA insertion had caused any deletion or rearrangement (data not shown). A sequence analysis of the T-DNA insertion site in the pas1-1 mutant revealed a deletion of 14 bp in the PAS1 gene.
In the pGKB5 binary vector used to generate the T-DNA collection (5), the ATG of the promoterless uidA gene is 40 bp downstream of the T-DNA right border, with no in-frame stop codon. An analysis of the genomic sequence flanking the T-DNA insert revealed an open reading frame (ORF) adjacent to the right border insertion sequence, in frame with the methionine initiation codon of the promoterless uidA (GUS) gene (Fig. 3B). This indicated that a translational fusion of the pas1-1-tagged allele with the uidA coding sequence had occurred, an event which is consistent with the GUS staining of the pas1-1 mutant (Fig. 3C). In further agreement with this, Northern blot analysis of pas1-1 RNA identified a 3.4-kb transcript that hybridized with both a uidA and a PAS1 probe (data not shown).
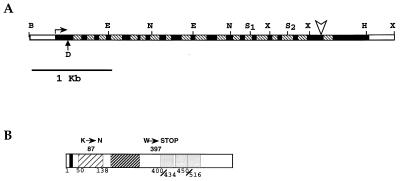
The two XbaI fragments adjacent to the right and left T-DNA borders were used as probes to screen both a wild-type Arabidopsis genomic library (EEC-BRIDGE Arabidopsis DNA Stock Center) and a cDNA Arabidopsis library (31). The genomic sequence of the PAS1 gene was obtained by sequencing both the BamHI and the XbaI clones. Synthetic oligonucleotides corresponding to the genomic sequence allowed us to amplify and sequence the same region from a wild-type (WS) template. The analysis of the genomic sequence and its comparison with the cDNA sequence revealed that the PAS1 gene was interrupted by 18 introns and that the entire gene was 4.2 kb long (Fig. 5A). The T-DNA was inserted before the last intron, 1,747 nucleotides from 5′ end of the cDNA. All the introns identified in the PAS1 gene showed the plant canonical acceptor and donor splice sites (NetPlantGene mail server: www.cbs.dtu.dk/NetPlantGene.html). Several independent cDNA clones were isolated. Among them, cDNA-D was chosen as containing the full-length PAS1 transcript, because Northern blot analysis of RNA from 9-day-old wild-type seedlings showed a transcript of the expected size (data not shown). A sequence analysis of cDNA-D revealed an ORF of 1,902 bp, corresponding to a protein of 634 amino acids (69.7 kDa). The first ATG in the ORF was preceded by several in-frame stop codons, suggesting that this is indeed the start codon. The full-length cDNA and the partial cDNA clones had poly(A) tails, although they differed slightly in the length of the 3′ untranslated region, perhaps due to the presence of different polyadenylation sites (Fig. 4 and 5A). Another class of PAS1 cDNA (cDNA-A), which contained an insert 70 bp longer than that of cDNA-D, was identified. A sequence comparison of the two cDNA classes (A and D) revealed that this difference was due to the lack of splicing of the second intron in the cDNA-A class (Fig. 4). As the possibility exists that the cDNA-A represents an aberrant PAS1 unspliced transcript, we focused our attention on cDNA-D. However, it is possible that the cDNA-A class results from a differential splicing, as more than one independent clone of the cDNA-A type was found in the library. Human FKBP12 is encoded by different mRNAs, varying in abundance and 3′ untranslated region, deriving from the differential splicing of five exons (1, 33).
FIG. 5.
Structure of the PAS1 gene and of the PAS1 protein. (A) Schematic representation of the PAS1 gene. PAS1 contains 19 exons, represented by the black boxes, and 18 introns, shown as hatched boxes. The vertical arrow shows the location of the first ATG codon of cDNA-D. The arrowhead indicates the T-DNA insertion site. The 5′ end of the cDNA is shown by the horizontal arrow. Abbreviations for restriction sites are as defined in the legend for Fig. 3. (B) PAS1 predicted protein. The black box indicates the putative bipartite NLS in the N-terminal region. The two hatched boxes show FKBP-like domains I and II. The three shaded boxes indicate the TPR domains. The positions of the amino acid substitution (K→N) and the stop codon (W→STOP) for the pas1-2 allelic mutant are also indicated.
FIG. 4.
PAS1 cDNA sequence and predicted amino acid sequence. The sequences of both cDNA-A and cDNA-D are shown. The 5′ ends, the start codons, and the 3′ ends of the two classes of cDNAs are indicated by bold face, italic, uppercase letters (cDNA-D) and by underlined letters (cDNA-A). The unspliced intron in cDNA-A (70 nucleotides) is indicated by lowercase letters. The two putative cdc2-type protein kinase C phosphorylation sites are boxed, while the Tyr phosphorylation site is indicated by a boldface broken line. The putative NLSs are underlined. The point mutations in pas1-2 at nucleotides 417 and 1317 are indicated in boldface, and altered residues are circled.
The PAS1 cDNA of the pas1-2 allelic mutant was sequenced. A comparison with the wild-type gene sequence revealed a G-to-A point mutation at nucleotide 1317 (starting from the 5′ end), which creates a translational stop codon (W397STOP), and a G-to-C point mutation at nucleotide 417, which causes a lysine-to-asparagine substitution (K87N) within the first FKBP domain (Fig. 4 and 5B).
The PAS1 gene has been mapped by RFLP analysis with the genomic-DNA fragment adjacent to the T-DNA right border as a probe (Fig. 3B). The PAS1 gene is located on chromosome 3 at 106.6 centimorgans (marker ve042) on the recombinant inbred map (25).
Complementation of the pas1-2 mutant.
We wished to determine whether the mutation in the PAS1 gene was responsible for the Pas1-1− phenotype. The coding region of the full-length PAS1 cDNA-D was cloned into plant binary vector pKYLX71 (28) under the control of the cauliflower mosaic virus promoter (35S2). A. tumefaciens C58C1 (pMP90) transformants were selected on kanamycin and used to transform plants, which were heterozygous for the pas1-2 mutation, by the in planta Agrobacterium-mediated transformation method (2). Kanamycin-resistant T1 plants were allowed to self-pollinate. If the cDNA was able to complement the pas1-2 mutation in the progeny of the heterozygous transformed plant, we expected to obtain only 1 kanamycin-sensitive mutant plant for every 15 wild-type plants (12 kanamycin-resistant and 3 kanamycin-sensitive plants). The segregation analysis was performed in vitro on a kanamycin-supplemented medium and revealed that several independent transformants, heterozygous for the pas1 mutation, segregated with the expected ratio. The segregation analysis of four such transformants is shown in Table 1. Some of the transformants segregating as wild-type kanamycin-resistant plants showed abnormal developmental phenotypes. Whether this is due to the overexpression of PAS1 or to a phenomenon of cosuppression of the PAS1 gene in a wild-type background has yet to be determined. However, we never found any pas1 mutant plant resistant to kanamycin in the progeny of the transgenic plants analyzed.
TABLE 1.
Functional complementation of the pas1-2 mutant as indicated by a segregation analysis for the pas1 mutanta
| Transformant | No. of plants with indicated phenotypeb
|
χ2c | |||
|---|---|---|---|---|---|
| wt Kr | wt Ks | pas1 Ks | Total | ||
| pKY/D1 | 219 | 56 | 18 | 293 | 0.005 |
| pKY/D3 | 315 | 74 | 23 | 412 | 0.312 |
| pKY/D4 | 435 | 91 | 31 | 557 | 0.442 |
| pKY/D13 | 211 | 56 | 18 | 285 | 0.002 |
The segregation analysis was performed for the F2 generation of transgenic plants obtained by transformation of pas1-2 heterozygous plants with PAS1 cDNA-D. The segregation of four independent transformants heterozygous for the mutation is presented, showing a segregation ratio of 1 kanamycin-sensitive pas1 mutant to 15 heterozygous, wild-type plants.
wt, wild type; Kr, kanamycin resistant; Ks, kanamycin sensitive.
Calculated values were based on an expected ratio of 1 mutant plant for every 15 wild-type plants. P < 0.05.
PAS1 has similarities to the immunophilin proteins.
The predicted amino acid sequence of PAS1-D was compared with sequences in current databases (National Center for Biotechnology Information; BLAST network server). The PAS1 protein has significant similarity to FKBPs belonging to the family of immunophilins (reviewed in reference 20). The term FKBP refers to any protein which binds both FK506 and rapamycin immunosuppressive compounds. Several FKBPs have been isolated to date from a broad range of organisms, and they are named according to their molecular masses (13, 42). These proteins also have peptidyl-prolyl cis-trans isomerase (rotamase) activity.
The highest scores for the PAS1 protein were obtained with two plant protein sequences: that of the ROF1 protein from Arabidopsis (FKBP62) (41) and that of wFKBP73 from Triticum aestivum (3), two high-molecular-weight FKBPs. PAS1 has 71% similarity and 31% identity with Arabidopsis ROF1 and 54% similarity and 31.5% identity with wheat wFKBP73. The PAS1 protein also has sequence similarity with several FKBP52 (p59) proteins from mammals (23, 34, 40). The overall degree of sequence similarity of PAS1 with FKBP52 from Homo sapiens, Mus musculus, and Oryctolagus cuniculus (accession no., Q02790, X70887, and M84474, respectively) ranges from 51 to 49% (28 to 24% sequence identity).
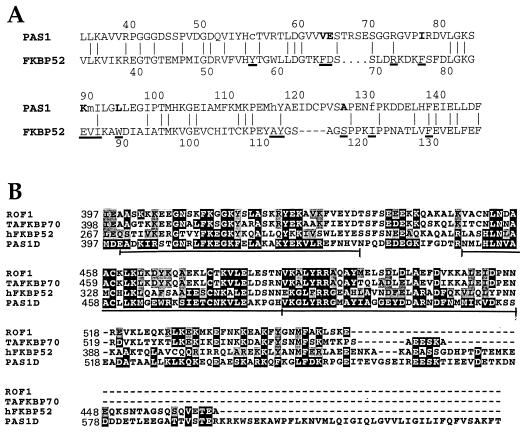
Human FKBP52 contains three FKBP-like domains, but only the N-terminal domain has FKBP-type rotamase activity and the FK506 binding site. Of the 14 FK506-binding residues found in human FKBP12, 13 are conserved in the first FKBP domain of human FKBP52 (20). An alignment of PAS1 with human FKBP52 revealed the presence of an N-terminal FKBP-like domain which showed conservation of 4 of the 14 residues involved in FK506 binding (R77, I91, Y117, and F130) (Fig. 5A). Six residues are identical to those resulting from substitutions at corresponding positions in other FKBPs (i.e., V67 in E. coli FKBP22, E68 in E. coli FKBP16, I81 in Saccharomyces cerevisiae FKBP13, K89 in human FKBP25, L94 in E. coli FKBP22, and A126 in human FKBP13) (20). Some substitutions within the FK506 binding site are conservative substitutions (e.g., E68D and M90V). The C-terminal region of PAS1 is characterized by a tetratricopeptide repeat (TPR) domain: a 34-amino-acid repeat present in multiple arrays. There are three such arrays in PAS1 and in FKBP52 (Fig. 5B and 6B). These domains have been proposed to form amphipathic α-helices that mediate protein-protein interactions (reviewed in references 16 and 22).
FIG. 6.
Alignments of the FK506 binding domain and of the TPR region of PAS1. (A) Comparison of the FK506 binding domain (domain I) of PAS1-D with that of hFKBP52. The first FKBP domain in hFKBP52 spans amino acids 32 to 136. The underlined amino acids are the residues required for FK506 binding in hFKBP12. The corresponding residues in PAS1 are indicated by vertical lines if they are identical, by boldface type if they are identical to corresponding residues in other FKBPs, or by lowercase letters if they are different. The horizontal lines indicate gaps introduced to maximize alignment. (B) Amino acid sequence similarity in the three units of the TPR domain. The sequences compared to PAS1 are ROF1 from A. thaliana (FKBP62), wFKBP73 (TAFKBP70) from T. aestivum, and hFKBP52. Light gray shading indicates similar residues, while identical amino acids are printed in white. The three TPR units are underlined. The alignments were generated with the PILEUP program of the Genetics Computer Group package.
An analysis of the predicted amino acid sequences by the PROSITE method revealed the presence of different putative phosphorylation sites in the PAS1 protein. Among these are two cyclic AMP/cyclic GMP phosphorylation sites, one site for casein kinase, and several protein kinase C phosphorylation sites, two of which correspond to the cdc2 type. One putative tyrosine phosphorylation site is also present in PAS1 (Fig. 4). In the N-terminal region of PAS1-D, a putative bipartite nuclear localization signal (NLS) is present within an uncharged, hydrophobic region (Fig. 4 and 5B).
The PAS1 gene is expressed throughout the plant and is regulated by CK.
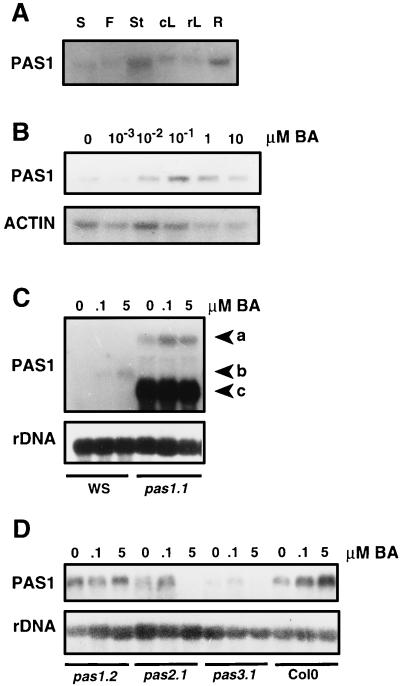
In order to study the mechanism by which the PAS1 protein contributes to development, we analyzed PAS1 gene expression. Total RNA was extracted from different organs of wild-type and pas1-1 heterozygous plants and hybridized with a PAS1 cDNA probe. This revealed that PAS1 was expressed in stems, leaves, flowers, siliques, and roots in wild-type plants (Fig. 7A) and in pas1-1 heterozygous plants (data not shown). As a result of the translational fusion with the uidA gene, the pas1-1 transcript size was approximately 3.4 kb (Fig. 3B). The PAS1-GUS translational fusion allowed us to perform a study of PAS1-1 expression by histological analysis, on both pas1-1 homozygous and heterozygous plants. The pas1-1 mutant showed strong GUS staining in all of the apical part, in the vascular cylinder of the root, and in the root tip (Fig. 3C, panels b and d). This expression was not affected by light or dark growth conditions or by the seedling developmental stage. GUS staining in the pas1-1/+ plants showed a strong staining of the apical and root meristematic regions and no detectable staining in the cotyledons, leaves, hypocotyls, or roots under all conditions tested (Fig. 3C, panels a and c).
FIG. 7.
PAS1 expression analysis. (A) RNAs were isolated from different organs of soil-grown wild-type plants, except for roots, which were prepared from plants grown in vitro. F, flowers; cL, cauline leaves; rL, rosette leaves; S, siliques; St, stem; R, roots. (B) PAS1 induction by BA in the wild type. RNAs were extracted from 9-day-old Col0 BA-treated plants (0, 10−3, 10−2, 10−1, 1, and 10 μM BA). As a control for the loading of RNA samples, the blot was also hybridized with an actin cDNA probe. (C) PAS1 expression in the pas1-1 mutants. RNAs were extracted from 9-day-old mutants and wild-type WS grown on increasing amounts of BA (0, 0.1 and 5 μM). Arrowheads: a, PAS1-GUS chimeric mRNA; b, wild-type PAS1 mRNA; c, chimeric mRNA transcribed from the 35S promoter through the bar gene and the 3′ end of the PAS1 gene (Fig. 3B). (D) PAS1 expression in the pas1-2 allele and in the other two pasticcino mutants, pas2 and pas3-1. Because these mutants are in the Col0 ecotype background, wild-type Col0 was used as the control. RNAs were extracted from 9-day-old BA-treated plants (0, 0.1, and 5 μM BA). In panels C and D the 25S rDNA was used to control the loading of RNA samples. Fifteen micrograms of total RNA was loaded in each lane.
We also investigated CK regulation of PAS1 gene expression in 9-day-old wild-type plants. The steady-state level of PAS1 transcripts was higher in wild-type (ecotype Columbia) plants grown on BA concentrations up to 5 μM than in untreated controls (Fig. 7B). We have previously shown that the pas mutants display a normal response to auxin (12). We analyzed total RNA extracted from 9-day-old wild-type plants grown on 5 μM picloram, an auxin analog. The results revealed that the steady-state level of PAS1 mRNA was weakly increased by auxin, but to a much lesser extent than by CK (data not shown).
The pas mutants show altered expression of the PAS1 gene.
PAS1 gene expression in the two allelic pas1 mutants and in the pas2 and pas3 mutants (12), either in the presence or absence of exogenous CKs (BA) was analyzed.
In pas1-1 mutants two transcripts can be detected due to the insertion of the T-DNA (see above), but the transcript of the wild-type size is absent (Fig. 7C). In heterozygous pas1-1 plants the wild-type transcript is present with the two other transcripts (data not shown). PAS1 mRNA levels were still increased by CK in the pas1-1 mutant, but the response was shifted towards lower BA concentrations compared to the response of the corresponding wild type (ecotype WS) (Fig. 7C). This result was confirmed by a fluorimetric GUS quantitative analysis. PAS1 expression was still induced by BA, both in pas1-1 homozygous and heterozygous plants, but the peak of BA induction of GUS activity was reached at 0.1 μM BA (data not shown). In pas1-2 plants, a transcript of the wild-type size is present and is constitutively expressed (Fig. 7D).
PAS1 expression was affected in pas2-1 and pas3-1 mutants. For pas2-1 mutants, the PAS1 mRNA could be detected in untreated mutants and in mutants grown in the presence of 0.1 μM BA but not in presence of 5 μM BA (Fig. 7D). For pas3-1 mutants, PAS1 mRNA could not be detected unless the mutants were grown at a low BA concentration (0.1 μM) (Fig. 7D). These results suggest that the CK sensitivity of PAS1 expression is modulated by the other two PAS genes, which may be required for its controlled expression.
DISCUSSION
PAS1 is involved in the control of cell proliferation.
We have previously reported the isolation of three classes of mutants with very similar pleiotropic phenotypes characterized by the presence of large, abnormal meristems leading to disorganized rosettes made of fused, vitreous leaves (12). The pas mutants were shown to be particularly altered in their response to CKs (12). The three PAS genes are also involved in the control of embryogenesis. Both pas1-1 and pas1-2 mutants contain mutations (T-DNA insertion and point mutations, respectively) in the coding sequence of the PAS1 gene. The fact that pas1-2 can be complemented with the wild-type PAS1 cDNA confirms that the gene corresponding to the mutant phenotype has indeed been cloned. The expression of the PAS1 gene, which is impaired in pas1 mutants, is also affected in pas2 and pas3 mutants, suggesting a possible molecular basis for the similarities of their phenotypes. These results tend to confirm the previous biochemical analysis of the pas mutants, which demonstrated that pas mutants were biochemically closely linked (12).
The pas mutants are an example of plant mutants which have a general deregulation of the control of cell proliferation. We hypothesize that this deregulation is due to the absence of a functional PAS1 protein, which in the wild type would antagonize cell proliferation. It is proposed that the PAS1 protein accumulates and functions in dividing tissues, such as the meristems, to prevent uncontrolled cell division. In pas1-1/+ plants, the PAS1-GUS protein accumulates preferentially in the meristematic area. In the wild type, posttranscriptional regulation possibly occurs to prevent the production of the protein in all the organs (the PAS1 mRNA is not organ specific). However, in pas1-1 plants, the chimeric PAS1-GUS protein is overproduced not only in the meristematic zones but also in all the tissues undergoing cell division. Possibly the lack of functional PAS1 in the pas1 mutant causes ectopic cell proliferation, which in turn induces ectopic PAS1 expression in a regulatory feedback loop.
The cellular proliferation in pas1-1 plants is enhanced specifically by CKs. In wild-type plants PAS1 expression is up-regulated by CKs, and in pas1-1, pas1-2, and pas2-1 plants, CK regulation of PAS1 expression is altered. Although we previously showed that only CKs have an effect on the pas phenotype (12), the possibility exists that the action of CKs is mediated by other hormones, such as auxins and BR, which are known to interact with CKs in plant development. Therefore, PAS1 may function directly in a CK pathway controlling cell division or in a pathway controlling similar downstream events.
PAS1 is an FKBP-like protein with TPR domains.
The PAS1 protein has significant sequence similarity with the immunophilin family of FKBPs. Two immunophilin families can be distinguished: cyclophilins and FKBPs. The FKBPs, represented by the well-studied FKBP12, bind the immunosuppressants FK506 and rapamycin, whereas the cyclophilins bind cyclosporin A (42). Although the two families do not have structural or sequence homology, all immunophilins identified to date exhibit peptidyl-prolyl cis-trans isomerase (rotamase) activity. Immunophilins are housekeeping proteins, highly conserved throughout evolution, which may mediate critical cellular functions (reviewed in reference 20). Several reports have shown the presence of immunophilins in plants (3, 7, 14, 18, 24). FKBPs with distinct subcellular localizations are known (7, 24, 27; reviewed in reference 20). PAS1 has a nuclear-localization signal, and it is likely, although as yet untested, that PAS1 functions in the nucleus.
Recently, two low-molecular-weight FKBPs were identified in A. thaliana (AtFKBP15-1 and AtFKBP15-2) (26) and two high-molecular-weight FKBPs were identified in both A. thaliana (ROF1 or FKBP62) (41) and wheat (wFKBP73) (3). The PAS1 protein has the most similarity to the large FKBPs such as Arabidopsis ROF1, wheat wFKBP73, and the mammalian FKBP52 and FKBP51. All these proteins have N-terminal FKBP domains and C-terminal TPRs.
Only the most N-terminal of the FKBP domains of human FKBP52 has rotamase activity and a binding site for immunosuppressants FK506 and rapamycin (17, 39). Wheat FKBP73 has rotamase activity, which can be inhibited by FK506 and rapamycin (3). Such binding properties are currently being tested with the PAS1 purified protein. Preliminary tests with FK506 on wild-type Arabidopsis seedlings showed that the drug only affected seedling development at high concentrations and did not induce the Pas1− phenotype. This may be due to a lack of penetration of FK506 into the plants. Further experiments on protoplasts or cell cultures will be performed in order to study the effects of these molecules in plants and to identify their cellular targets.
FKBP52 was identified as a component of unliganded steroid hormone receptor complexes, along with the heat shock proteins hsp90 and hsp70 (4, 8, 34, 40, 43). The TPR domain is necessary for FKBP52 to interact with hsp90 in the steroid receptor complex (35; reviewed in reference 37). The TPR motif is a 34-amino-acid motif of variable sequence which has defined a protein family involved in cell cycle regulation, RNA synthesis, protein transport, Ser-Thr dephosphorylation, and the heat shock response (16, 22). Interestingly, the TPR domains of mammalian FKBP52 have a high degree of similarity, in position and amino acid composition, to the PAS1 TPR domains. We found two mutations in the pas1-2 cDNA sequence. The G-to-A transversion is characteristic of EMS-induced mutations and causes a truncation of the PAS1 protein prior to the TPR domain, similar in position to the alteration caused by the insertion of the T-DNA into pas1-1. The G-to-C mutation causes a single-amino-acid substitution within the FKBP domain. It is likely then that the TPR domain, which is absent from the predicted proteins encoded by both pas1-1 and pas1-2 alleles, is of particular importance in the function of PAS1. Whether the PAS1 protein is able to bind hsp90 and the identities of other potential binding partners have yet to be determined.
As the PAS1 gene encodes an FKBP-like protein similar to steroid receptor-associated FKBPs, it should be noted that the BR, which are plant growth factors, exhibit structural similarity to animal steroid hormones (11). Bioassays on both intact plants and hypocotyl segments have shown that a broad spectrum of cellular responses is elicited by BR. Although the action of BR in the elongation of stem tissues has been well characterized, their effects on cell division and cell differentiation have also been reported. BR are known to act synergistically with auxin in stimulating cell elongation (38), and there is evidence that BR action affects the content of endogenous auxin. BR-induced changes in auxin level may alter the auxin/CK ratio in plant tissues (15).
pas1 mutants represent the first inactivation of a gene encoding an FKBP-like protein in higher eukaryotes. Genetic studies on immunophilin gene disruption in microorganisms have been performed (reviewed in reference 10). Despite the widespread occurrence and strong sequence conservation of immunophilin genes, their inactivation has been shown to have little or no effect on cell viability. This work shows that when the expression of an FKBP-like gene is impaired, as in pas mutants, the control of cell proliferation is affected.
In conclusion, we believe that the pas1 mutants represent a useful tool to understand the role of immunophilin-like proteins in plant development and to unravel their potential functions in plant hormonal signalling pathways.
ACKNOWLEDGMENTS
P.V. was supported by an INRA postdoctoral fellowship from DSPV.
We are grateful to G. Pelletier and his group for providing the T-DNA lines, to B. Courtial for RFLP analysis, and to T. Desprez for technical assistance with software analysis. The A. thaliana genomic and cDNA libraries were kindly provided by J. Mulligan and C. Robaglia, respectively. We also thank E. E. Baulieu and his group for helpful discussions and for the gift of the FK506 and rapamycin molecules and Heather Mckhann and Isabelle Barlier for critical reading of the manuscript.
REFERENCES
- 1.Arakawa H, Nagase H, Hayashi N, Fujiwara T, Ogawa M, Shin S, Nakamura Y. Molecular cloning and expression of a novel human gene that is highly homologous to human FK506-binding protein 12kDa (hFKBP-12) and characterization of two alternatively spliced transcripts. Biochem Biophys Res Commun. 1994;200:836–843. doi: 10.1006/bbrc.1994.1527. [DOI] [PubMed] [Google Scholar]
- 2.Bechtold N, Ellis J, Pelletier G. In planta Agrobacterium mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci Paris. 1993;316:1194–1199. [Google Scholar]
- 3.Blecher O, Erel N, Callebaut I, Aviezer K, Breiman A. A novel plant peptidyl-prolyl-cis-trans-isomerase (PPiase): cDNA cloning, structural analysis, enzymatic activity and expression. Plant Mol Biol. 1996;32:493–504. doi: 10.1007/BF00019101. [DOI] [PubMed] [Google Scholar]
- 4.Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of Hsp90-associated proteins. Science. 1996;274:1715–1717. doi: 10.1126/science.274.5293.1715. [DOI] [PubMed] [Google Scholar]
- 5.Bouchez D, Camilleri C, Caboche M. A binary vector based on Basta resistance for in planta transformation of Arabidopsis thaliana. C R Acad Sci Paris. 1993;316:1188–93. [Google Scholar]
- 6.Bouchez D, Vittorioso P, Courtial B, Camilleri C. Kanamycin rescue: a simple technique for the recovery of T-DNA flanking sequences. Plant Mol Biol Rep. 1996;14:115–123. [Google Scholar]
- 7.Breiman A, Fawcett T W, Ghirardi M L, Mattoo A K. Plant organelles contain distinct peptidylprolyl cis,trans-isomerase. J Biol Chem. 1992;267:21293–21296. [PubMed] [Google Scholar]
- 8.Callebaut I, Renoir J M, Lebeau M C, Massol N, Burny A, Baulieu E E, Mornon J P. An immunophilin that binds Mr 90,000 heat shock protein: main structural features of a mammalian p59. Proc Natl Acad Sci USA. 1992;89:6270–6274. doi: 10.1073/pnas.89.14.6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies P J. Hormones in tissue culture and propagation. In: Davies P J, editor. Plant hormones. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1995. pp. 13–38. [Google Scholar]
- 10.Dhillon N, Thorner J. Immunophilins in the yeast Saccharomyces cerevisiae: a different spin on proline rotamases. Methods Companion Methods Enzymol. 1996;9:165–176. doi: 10.1006/meth.1996.0023. [DOI] [PubMed] [Google Scholar]
- 11.Ecker J R. BRI-ghtening the pathway to steroid hormone signaling events in plants. Cell. 1997;90:825–827. doi: 10.1016/s0092-8674(00)80346-3. [DOI] [PubMed] [Google Scholar]
- 12.Faure J D, Vittorioso P, Santoni V, Fraisier V, Prinsen E, Barlier I, Van Onckelen H, Caboche M, Bellini C. The pasticcino mutants of Arabidopsis thaliana are affected in vegetative development and response to cytokinins. Development. 1998;125:909–918. doi: 10.1242/dev.125.5.909. [DOI] [PubMed] [Google Scholar]
- 13.Fretz H, Albers M A, Galat A, Standaert R F, Lane W S, Burakoff S J, Bierer B E, Schreiber S L. Rapamycin and FK506 binding proteins (immunophilins) J Am Chem Soc. 1991;113:1409–1410. [Google Scholar]
- 14.Gasser C S, Gunning D A, Budelier K A, Brown S M. Structure and expression of cytosolic cyclophilin/peptidyl-prolyl cis-trans isomerase of higher plants and production of active tomato cyclophilin in Escherichia coli. Proc Natl Acad Sci USA. 1990;87:9519–9523. doi: 10.1073/pnas.87.24.9519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaudinová A, Süssenbeková H, Vojéchova M, Kaminek K, Eder J, Kohout L. Different effects of two brassinosteroids on growth, auxin and cytokinin content in tobacco call tissue. Plant Growth Regul. 1995;17:121–126. [Google Scholar]
- 16.Goebl M, Yanagida M. The TPR snap helix: a novel protein repeat motif from mitosis to transcription. Trends Biochem Sci. 1991;16:173–177. doi: 10.1016/0968-0004(91)90070-c. [DOI] [PubMed] [Google Scholar]
- 17.Harding M W, Galat A, Uehling D E, Schreiber S L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- 18.Hayman G T, Miernyk J A. The nucleotide and deduced amino acid sequences of a peptidyl-prolyl cis-trans isomerase from Arabidopsis thaliana. Biochim Biophys Acta. 1994;1219:536–538. doi: 10.1016/0167-4781(94)90083-3. [DOI] [PubMed] [Google Scholar]
- 19.Jefferson R A, Kavanagh T A, Bevan M W. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 1987;6:3901–3907. doi: 10.1002/j.1460-2075.1987.tb02730.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay J E. Structure-function relationships in the FK506-binding protein (FKBP) family of peptidylprolyl cis-trans isomerases. Biochem J. 1996;314:361–385. [PMC free article] [PubMed] [Google Scholar]
- 21.Kosambi D D. The estimation of map distance from recombinant values. Ann Eugen. 1944;12:172–175. [Google Scholar]
- 22.Lamb J R, Tugendreich S, Hieter P. Tetratrico peptide repeat interactions: to TPR or not to TPR? Trends Biol Sci. 1995;20:257–259. doi: 10.1016/s0968-0004(00)89037-4. [DOI] [PubMed] [Google Scholar]
- 23.Lebeau M C, Massol N, Herrick J, Faber L E, Renoir J M, Radanyi C, Baulieu E E. P59, an hsp 90-binding protein: cloning of its cDNA and preparation of a peptide-directed polyclonal antibody. J Biol Chem. 1992;267:4281–4284. [PubMed] [Google Scholar]
- 24.Lippuner V, Chou I T, Scott S V, Ettinger W F, Theg S T, Gasser C S. Cloning and characterization of chloroplast and cytosolic forms of cyclophilin from Arabidopsis thaliana. J Biol Chem. 1994;269:7863–7868. [PubMed] [Google Scholar]
- 25.Lister C, Dean C. Recombinant inbred lines for mapping RFLP and phenotypic markers in Arabidopsis thaliana. Plant J. 1993;4:745–750. doi: 10.1046/j.1365-313x.1996.10040733.x. [DOI] [PubMed] [Google Scholar]
- 26.Luan S, Kudla J, Gruissem W, Schreiber S L. Molecular characterization of a FKBP-type immunophilin from higher plants. Proc Natl Acad Sci USA. 1996;93:6964–6969. doi: 10.1073/pnas.93.14.6964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luan S, Albers M, Schreiber S L. Light-regulated, tissue-specific immunophilins in higher plants. Proc Natl Acad Sci USA. 1994;91:984–988. doi: 10.1073/pnas.91.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiti I B, Murphy J F, Shaw J G, Hunt A G. Plants that express a potyvirus VPg-proteinase gene are resistant to virus infection. Proc Natl Acad Sci USA. 1993;90:6110–6114. doi: 10.1073/pnas.90.13.6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meinke D W. Molecular genetics of plant embryogenesis. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:369–394. [Google Scholar]
- 30.Meyerowitz E M. Genetic control of cell division patterns in developing plants. Cell. 1997;88:299–308. doi: 10.1016/s0092-8674(00)81868-1. [DOI] [PubMed] [Google Scholar]
- 31.Minet M, Dufour M E, Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- 32.Mollier P, Montoro P, Delarue M, Bechtold N, Bellini C, Pelletier G. Promoterless gusA expression in a large number of Arabidopsis thaliana transformants obtained by the in planta infiltration method. C R Acad Sci Paris. 1995;318:465–474. [Google Scholar]
- 33.Peattie D A, Hsiao K, Benasutti M, Lippke J A. Three distinct messenger RNAs can encode the human immunosuppressant-binding protein FKBP12. Gene. 1994;150:251–257. doi: 10.1016/0378-1119(94)90434-0. [DOI] [PubMed] [Google Scholar]
- 34.Peattie D A, Harding M W, Fleming M A, Decenzo M T, Lippke J A, Livingston D J, Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90 kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci USA. 1992;89:10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Radanyi C, Chambraud B, Baulieu E E. The ability of the immunophilin FKBP59-HBI to interact with the 90-kDa heat shock protein is encoded by its tetratricopeptide repeat domain. Proc Natl Acad Sci USA. 1994;91:11197–11201. doi: 10.1073/pnas.91.23.11197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 37.Sánchez E R, Ning Y-M. Immunophilins, heat shock proteins, and glucocorticoid receptor actions in vivo. Methods Companion Methods Enzymol. 1996;9:188–200. doi: 10.1006/meth.1996.0025. [DOI] [PubMed] [Google Scholar]
- 38.Sasse J M. Brassinolide-induced elongation and auxin. Physiol Plant. 1990;80:401–408. [Google Scholar]
- 39.Siekierka J J, Hung S H Y, Poe M, Lin C S, Sigal N H. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989;341:755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- 40.Tai P K K, Albers M W, Chang H, Faber L E, Schreiber S L. Association of a 59-kilodalton immunophilin with the glucocorticoid receptor complex. Science. 1992;256:1315–1318. doi: 10.1126/science.1376003. [DOI] [PubMed] [Google Scholar]
- 41.Vucich V A, Gasser C S. Novel structure of a high molecular weight FK506 binding protein from Arabidopsis thaliana. Mol Gen Genet. 1996;252:510–517. doi: 10.1007/BF02172397. [DOI] [PubMed] [Google Scholar]
- 42.Wiederrecht G, Etzkorn F. Perspectives in drug discovery and design. Amsterdam, The Netherlands: ESCOM Science Publishers, B.V.; 1994. The immunophilins; pp. 57–84. [Google Scholar]
- 43.Yem A W, Tomasselli A G, Heinrikson R L, Zurcher-Neely H, Ruff V A, Johnson R A, Deibel M R. The Hsp56 component of steroid receptor complexes binds to immobilized FK506 and shows homology to FKBP-12 and FKBP-13. J Biol Chem. 1992;267:2868–2871. [PubMed] [Google Scholar]