Abstract
Objective:
To determine if intraoperative near-infrared (NIR) imaging carries benefit in resection of pancreatic neoplasms.
Background:
Resection of pancreatic malignancies is hindered by high rates of local and distant recurrence from positive margins and unrecognized metastases. Improved tumor visualization could improve outcomes. We hypothesized that intraoperative NIR imaging with a clinically approved optical contrast agent could serve as a useful adjunct in assessing margins and extent of disease during pancreatic resections.
Methods:
Twenty patients were enrolled in an open-label clinical trial from July 2016-May 2018. Subjects received second window indocyanine green (ICG) (2.5–5mg/kg) 24 hours prior to pancreatic resection. NIR imaging was performed during staging laparoscopy and after pancreas mobilization in situ and following resection ex vivo. Tumor fluorescence was quantified using tumor-to-background ratio (TBR). Fluorescence at the specimen margin was compared to pathology evaluation.
Results:
Procedures included 9 pancreaticoduodenectomies, 10 distal pancreatectomies, and 1 total pancreatectomy; 21 total specimens were obtained. Three out of 8 non-invasive tumors were fluorescent (mean TBR 2.59±2.57). Twelve out of 13 invasive malignancies (n=12 pancreatic adenocarcinoma, n=1 cholangiocarcinoma) were fluorescent (mean TBR 4.42±2.91). Fluorescence at the transection margin correlated with final pathologic assessment in 12/13 patients. Following neoadjuvant therapy, 4/5 tumors were fluorescent; these 4 tumors showed no treatment response on pathology assessment. One tumor had a significant treatment response and showed no fluorescence.
Conclusions:
Second window ICG reliably accumulates in invasive pancreatic malignancies and provides real-time feedback during pancreatectomy. NIR imaging may help to assess the response to neoadjuvant therapy.
Mini-Abstract
In a prospective open-label clinical trial, twenty patients were given high dose indocyanine green 24 hours prior to pancreatectomy for pancreatic neoplasms. On near-infrared imaging during surgery, invasive pancreatic malignancies were fluorescent, and fluorescence correlated with pathologic margin status. In patients receiving neoadjuvant treatment, fluorescence also correlated with treatment response.
Introduction
Despite improved adjuvant therapy, median survival for patients with resected pancreatic ductal adenocarcinoma (PDAC) is only 20–28 months.1–4 Outcomes following surgical resection are hindered by high rates of local and distant recurrence due to positive surgical margins and occult metastatic disease. The R1/R2 resection rate ranges from 24–42% in several large series but may be as high as 76% with stringent pathologic analysis.5–9 Furthermore, early distant recurrences following margin-negative resection highlight the likelihood of unrecognized metastases at the time of surgery.8, 10
Neoadjuvant therapy is an attractive strategy in the management of PDAC as it may allow for better patient selection. Patients that progress on neoadjuvant therapy are less likely to benefit from resection and may therefore be spared from a morbid procedure. However, neoadjuvant therapy can create confusion in borderline resectable or locally advanced patients that do not progress. Recent data indicate that preoperative imaging in these patients becomes unreliable in predicting resectability.11–13
Adjunctive measures that improve intraoperative assessment of the extent of disease are needed. Intraoperative near-infrared (NIR) imaging is an emerging technology that can improve real-time identification of tumor margins and small nodules during an operation. Indocyanine green (ICG) is the only U.S. Food and Drug Administration (FDA)-approved intraoperative NIR imaging fluorophore. Intraoperative NIR imaging with ICG has traditionally been used to assess vascular perfusion.14, 15 However, an alternative method of ICG administration--the second window ICG technique--has recently demonstrated benefit in tumor imaging.16 Specifically, high dose ICG (5 mg/kg) will accumulate in non-hepatic solid tumors over 24 hours via the enhanced permeability and retention (EPR) effect.17 This technique has demonstrated benefit in identifying pulmonary nodules and gliomas.18, 19
We hypothesized that intraoperative NIR imaging with second window ICG could serve as a useful adjunct in identifying pancreatic neoplasms and assessing margins intraoperatively. The primary outcome was the presence of fluorescence in malignant and pre-malignant neoplasms (TBR ≥ 2.0). Secondary outcomes were the correlation of fluorescence at the resection margin with pathologic margin assessment and correlation of fluorescence with response to neoadjuvant treatment. Here we report on the feasibility and utility of NIR imaging in 20 patients with suspected malignant or pre-malignant pancreatic lesions.
Methods
Study Design
A prospective open-label intraoperative NIR imaging clinical trial was approved by the University of Pennsylvania Institutional Review Board. The primary objective of this study was to determine the feasibility of NIR imaging with second window ICG for pancreatic neoplasms. Twenty patients provided written informed consent and were recruited between July 2016 and May 2018. Included subjects were scheduled for pancreatectomy based on suspicion of a malignant or pre-malignant lesion by preoperative imaging and endoscopic evaluation.
Study Drug
Indocyanine green (ICG) was purchased from the manufacturer (Akorn, Lake Forest, IL). ICG is a water-soluble anionic, amphiphilic NIR fluorophore with a peak excitation wavelength of 805 nm, a peak emission wavelength of 830 nm, and a molecular weight of 775 Da. A 2.5–5 mg/kg ICG dose was given as an intravenous infusion one day prior to pancreatectomy.
Near-Infrared Imaging
Macroscopic surgical fluorescent imaging in situ was performed using the Iridium® system (Visionsense Corps, Philadelphia, PA) or the Stryker 1588AIM (Kalamazoo, MI). All ex vivo imaging was performed using the Iridium®. NIR imaging in situ was performed a minimum of three times during each case. First, the abdomen was triaged by white light and NIR imaging. Any lesions that were suspicious for malignancy on white light imaging were examined for fluorescence and were biopsied. Second, following pancreas mobilization, the primary tumor was analyzed for fluorescence. Third, after pancreas resection, the wound bed was evaluated for residual fluorescence. In each case, the resected pancreatic specimen was imaged on the back-table ex vivo. The pancreatic neck and retroperitoneal margins were assessed for fluorescence. The presence or absence of fluorescence was then compared to both frozen section and final histopathologic analysis. The bile duct and duodenal margins were not assessed for fluorescence because ICG is hepatically excreted which could create background fluorescence at these margins. Importantly, as this was primarily a feasibility trial, the operative approach was not altered based on fluorescence. The use of frozen section, the site of transection, and the decision to resect further were at the surgeon’s discretion based on standard white light imaging and manual palpation, not based on fluorescence.
Fluorescence Microscopy
All resected specimens were examined by a gastrointestinal pathologist. Tumors were formalin fixed and paraffin embedded. Serial sections of tumor and normal tissue were cut for pathology slides. Slides were stained with 4, 6’-diamidino-2-phenylindole (DAPI), and fluorescence microscopy for ICG and DAPI was performed. ICG was pseudo-colored green, and DAPI was pseudo-colored blue. Fluorescence was compared to the corresponding area on hematoxylin and eosin (H&E) stained slides. Positive and negative controls were used for all images. In patients who received neoadjuvant treatment, the entire pancreas was submitted for histologic examination. A treatment response score was given which is an estimated metric reflecting how much tumor was felt to be viable. Poor treatment response was defined as ≥ 90% remaining viable tumor, moderate tumor response was defined as >10 and <90% remaining viable tumor, and good treatment response was defined as ≤ 10% remaining viable tumor.
Statistical Analysis
Descriptive statistics are presented as mean ± standard deviation. Post hoc image analysis was performed using region-of-interest software and HeatMap plugin within ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/). The fluorescence in the tumor and in adjacent uninvolved pancreas was quantified using this technology, and a tumor-to-background fluorescence ratio (TBR) was calculated for each case. A TBR ≥ 2.0 was considered positive for fluorescence based on previous clinical studies using this imaging system.20–22 The association of clinicopathologic features with TBR were assessed using the Pearson’s correlation coefficient for continuous variables and the Wilcoxon rank-sum test for categorical variables.
Results
Patient Characteristics
Twenty patients (11 females, mean age 65.0±15.2 years) were enrolled in the trial. One patient had multifocal PDAC; she presented with jaundice but the dominant mass was in the pancreatic tail. This patient had a total pancreatectomy in which the pancreas was divided at the neck. For analysis purposes, this was treated as a distal pancreatectomy and a pancreaticoduodenectomy. As a result, there are 21 specimens for 20 patients. Fifteen of the 20 patients underwent either a laparoscopic resection or a staging laparoscopy prior to open resection.
Patient characteristics are shown in Table 1. Eighteen patients received 5 mg/kg ICG one day before surgery, and two patients received 2.5 mg/kg ICG one day before surgery. Mean time from drug infusion to imaging was 23.9±3.0 hours. ICG infusion was safe with no serious adverse events.
Table 1:
Characteristics of the study patients.
| N(%) or Mean±SD | ||
|---|---|---|
| Age (years) | 65.0±15.2 | |
| Sex | Male | 11 (55.0) |
| Female | 9 (45.0) | |
| Neoadjuvant Treatment | 4 (20.0) | |
| Laparoscopy Performed | 15 (75.0) | |
| Procedure | Open pancreaticoduodenectomy | 10 (47.6) |
| Laparoscopic distal pancreatectomy Open distal pancreatectomy |
3 (14.3) 8 (38.1) |
|
| Final Pathology | Pancreatic ductal adenocarcinoma | 12 (57.1) |
| Cholangiocarcinoma | 1 (4.8) | |
| IPMN | 3 (14.3) | |
| Neuroendocrine tumor | 2 (9.5) | |
| Serous cystadenoma | 2 (9.5) | |
| Neurofibroma | 1 (4.8) | |
| Mean Hours from Infusion to Imaging | 23.9±3.0 |
Abbreviations: SD standard deviation, IPMN intraductal papillary mucinous neoplasm
NIR Imaging for Benign or Low Grade Malignant Pancreatic Lesions
Eight patients had benign tumors or low-grade malignancies (intraductal papillary mucinous neoplasm (IPMN), serous cystadenoma, neurofibroma, or well-differentiated neuroendocrine tumor) on final pathology. Characteristics of these patients are included in Table 2. Five of the eight lesions were non-fluorescent. Three of these lesions were fluorescent: a main duct IPMN with high grade dysplasia, a microcystic serous cystadenoma, and a mixed-type IPMN with low grade dysplasia. The first two tumors had modest fluorescence (mean fluorescence intensities [MFI] of 63.4 and 85.0 arbitrary units [AU], respectively) but background fluorescence in the normal pancreas was very low (MFI 29.8 and 16.0). Fluorescence was particularly strong in the mixed-type IPMN with low grade dysplasia (MFI 205.3 AU) (Supplemental Figure 1). In this case, there was evidence of ICG pooling around intrapancreatic blood vessels on fluorescence microscopy.
Table 2:
Clinicopathologic characteristics of patients with benign or low-grade malignant tumors.
| Patient | Age | Procedure | Final Pathology | TBR |
|---|---|---|---|---|
| 6 | 75 | DP | Mixed-type IPMN with low grade dysplasia | 7.82 |
| 8 | 71 | PD | Well differentiated pancreatic neuroendocrine tumor | 1.06 |
| 9 | 44 | DP | Microcystic serous cystadenoma | 5.31 |
| 12 | 83 | DP | Main duct IPMN with high grade dysplasia | 2.13 |
| 14 | 22 | DP | Neurofibroma | 1.00 |
| 15 | 61 | PD | Mixed-type IPMN with high grade dysplasia | 1.43 |
| 18 | 72 | PD | Serous cystadenoma | 0.98 |
| 20 | 47 | DP | Well differentiated pancreatic neuroendocrine tumor | 0.99 |
Abbreviations: TBR tumor-to-background ratio, DP distal pancreatectomy, IPMN intraductal papillary mucinous neoplasm, PD pancreaticoduodenectomy
NIR Imaging for Invasive Malignancies
Thirteen specimens were obtained from the 12 patients with invasive malignancies (n=12 PDAC, n=1 cholangiocarcinoma). Twelve out of 13 specimens were fluorescent (mean TBR 4.42±2.91). All fluorescent tumors were fluorescent both in situ and ex vivo. In patients with PDAC, 91.7% (11/12) were fluorescent (mean TBR 4.62±2.95). The one patient with PDAC and a non-fluorescent tumor (TBR 1.25) had preoperative chemoradiotherapy and only 10% remaining viable tumor. Representative images from two patients with PDAC are shown in Figure 1. For patients with PDAC, there was no correlation between TBR and age, BMI, time from infusion to imaging, total ICG dose, tumor size, or procedure (Supplemental Table 1).
Figure 1:
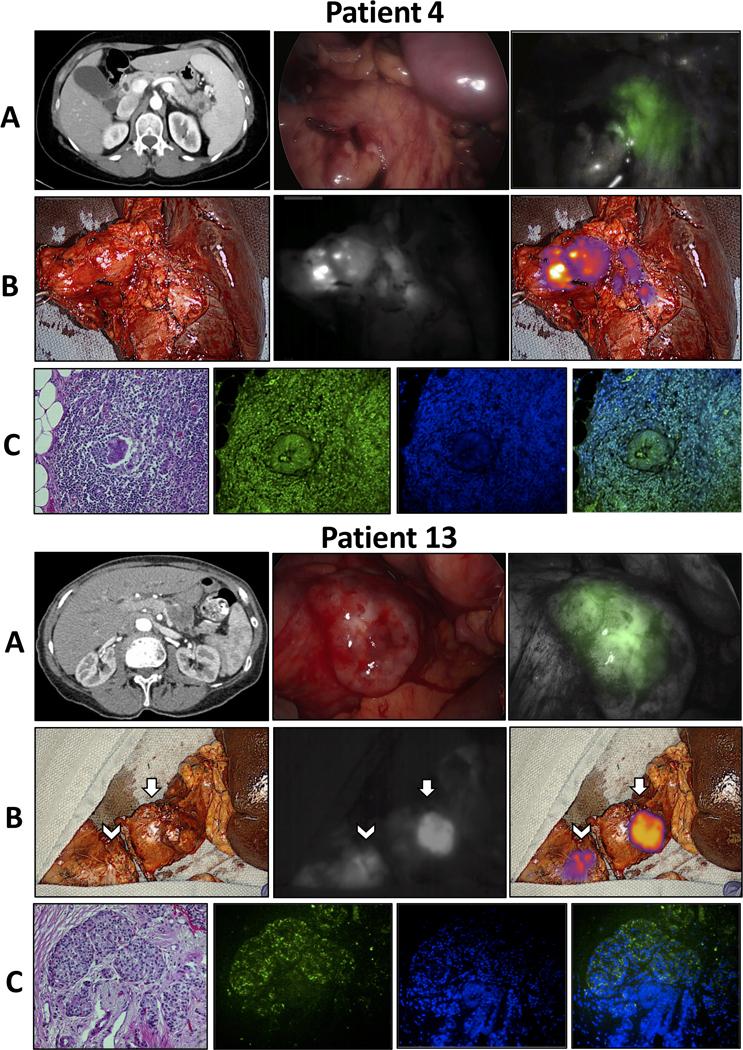
Intraoperative near-infrared (NIR) imaging from two patients with pancreatic adenocarcinoma. Patient 4 underwent a distal pancreatectomy for a pancreatic tail cyst with at least high-grade dysplasia on FNA. Final pathology demonstrated pancreatic ductal adenocarcinoma (PDAC). Patient 13 underwent a total pancreatectomy for multifocal PDAC. Pathology demonstrated a 3.5 cm PDAC in the pancreatic tail (arrows) and multifocal PDAC involving the entire portion of the resected pancreatic head (arrowheads). A) Preoperative CT scan, in situ white light, and NIR images. B) Back table white light, NIR, and overlay images. C) H&E microscopy, ICG fluorescence microscopy, DAPI fluorescence microscopy, and ICG and DAPI overlay fluorescence microscopy.
Ten of the 12 patients with invasive malignancies underwent either a laparoscopic resection or a staging laparoscopy prior to open resection. In every case, the liver had background fluorescence. There were no patients with a lesion that was suspicious visually (i.e. on white light imaging) and also suspicious by fluorescence. Two liver lesions that were suspicious on white light imaging but non-fluorescent were biopsied and sent for frozen section analysis. Pathology showed a benign biliary cyst and a bile duct adenoma.
Correlation between Fluorescence and Pathologic Assessment of Surgical Margins
Pancreatic transection margins were sent for frozen section in five cases. Four of these cases were pancreaticoduodenectomies, and the frozen section that was sent in each case was from the pancreatic neck margin. Frozen sections were not routinely sent in distal pancreatectomies when the transection margin was well away from the mass. Frozen section was also not sent in pancreaticoduodenectomies when the outcome would not change management.
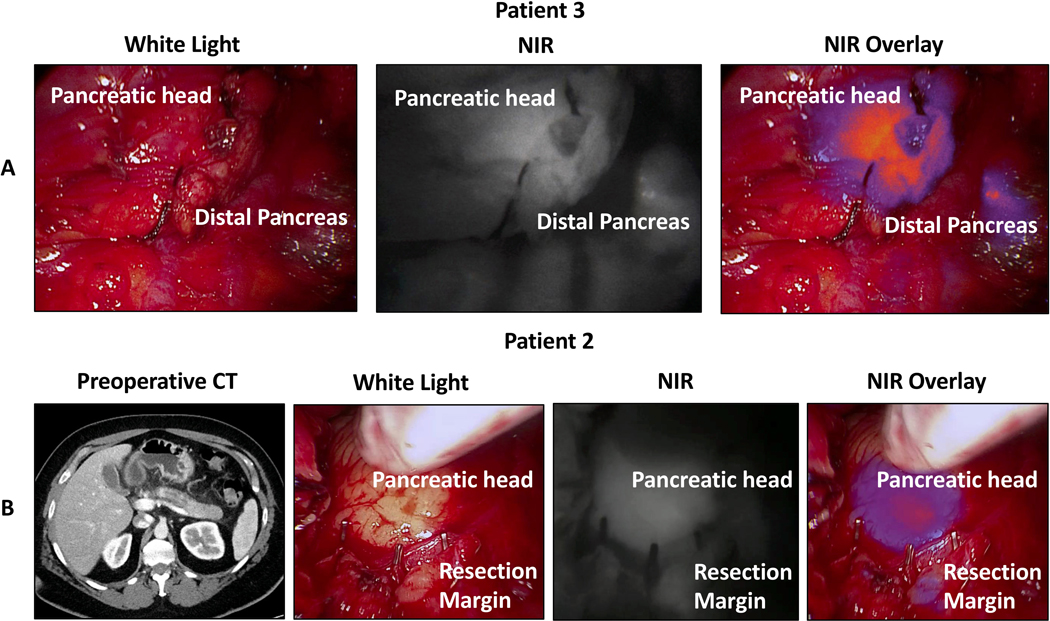
The frozen section was read as adenocarcinoma in 1/5 cases. There was fluorescence at the transection margin in 2/5 cases. In the one instance where both fluorescence and frozen section suggested carcinoma at the margin (Patient 3), the surgeon cut back further to achieve a negative margin (Figure 2A). In the one instance where frozen section was negative but there was fluorescence at the margin, nothing was done. This patient had a negative final margin. Thus, frozen section was 100% accurate in this series.
Figure 2:
Intraoperative near-infrared (NIR) imaging from two patients with fluorescence at the initial resection margin. A) In situ intraoperative white light, near infrared, and overlay images after pancreas transection and prior to specimen removal for Patient 3. The patient underwent a pancreaticoduodenectomy and had both fluorescence at the margin and a positive frozen section. The specimen was cut back further to achieve a negative margin. B) Preoperative CT, in situ intraoperative white light, near infrared, and overlay images after pancreas resection for Patient 2. The patient initially underwent a distal pancreatectomy for duct dilation without a clear tumor. Intraoperative imaging showed diffuse fluorescence throughout the pancreas including in the pancreatic head and at the transection margin. The patient had a positive margin on final pathology and eventually underwent a completion pancreatectomy at which time adenocarcinoma was found throughout the pancreatic head.
There were an additional four cases where fluorescence was identified at the transection margin but no frozen section was sent. In each of these four cases, the surgeon felt that the frozen section outcome would not change management, and in each case, the transection margin was positive for adenocarcinoma on final pathologic assessment. A representative case with fluorescence at the resection margin and carcinoma at the margin on final pathology is shown in Figure 2B. In total, fluorescence at the resection margin correlated with final pathology analysis for 12/13 specimens. The fluorescence status, frozen section diagnosis, and final margin status for malignant tumors are summarized in Table 3.
Table 3:
Comparison of fluorescence at resection margin and margin pathology for patients with invasive malignancies.
| Following Neoadjuvant Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| # | Procedure | Neoadjuvant Treatment | CA19–9 Response* | Tumor Regression on Cross Sectional Imaging | Final Pathology | TBR | Neck Margin Fluorescence | Retroperitoneal Margin Fluorescence | Frozen Section Diagnosis | Final Margin Status |
| 1 | PD | None | -- | -- | PDAC | 7.71 | Yes | No | N/A | (+) neck, (−) uncinate |
| 2 | DP | None | -- | -- | PDAC | 3.77 | Yes | No | N/A | (+) neck |
| 3 | PD | None | -- | -- | Intrapancreatic cholangiocarcinoma | 2.08 | Yes | No | (+) adenocarcinoma |
(−) |
| 4 | DP | None | -- | -- | PDAC | 12.43 | No | No | N/A | (−) |
| 5 | PD | None | -- | -- | PDAC | 2.13 | No | No | (−) | (−) |
| 7 | PD | None | -- | -- | PDAC | 3.29 | Yes | No | N/A | (+) neck, (−) uncinate |
| 10 | DP | CRT | Yes | Yes | PDAC, 10% viable tumor | 1.25 | No | No | N/A | (−) |
| 11 | DP | No | -- | -- | 4.20 | No | No | (−) | (−) | |
| 13 | DP | CO | Yes | No | PDAC, 100% viable tumor | 4.63 | No | No | N/A | (−) |
| 13 | PD | CO | Yes | No | PDAC, 100% viable tumor | 2.76 | Yes | No | N/A | (+) neck, (−) uncinate |
| 16 | DP | CRT | Yes | Yes | PDAC, 100% viable tumor | 4.83 | Yes | No | N/A | (+) neck |
| 17 | PD | No | -- | -- | PDAC | 3.38 | No | No | (+) HGD | (−) |
| 19 | PD | CO | No | No | PDAC, 100% viable tumor | 5.06 | Yes | No | (−) | (−) |
* >50% reduction in CA19–9 immediately prior to surgery
Abbreviations: TBR tumor-to-background ratio, PD pancreaticoduodenectomy, PDAC pancreatic ductal adenocarcinoma, DP distal pancreatectomy, CRT chemoradiotherapy, CO chemotherapy only, HGD high grade dysplasia.
Correlation between Fluorescence and Response to Neoadjuvant Treatment
Four patients had neoadjuvant treatment prior to pancreatic resection (Table 3). Two patients (patients 10 and 16) had neoadjuvant chemoradiation, while two patients (patients 13 and 19) had neoadjuvant chemotherapy only. One was the patient that underwent total pancreatectomy; thus, there were five specimens from these four patients. Four specimens from three of these patients were fluorescent (mean TBR 4.29±1.74) and demonstrated poor treatment response (>90% viable tumor) on pathology. Both specimens were fluorescent in the patient who had a total pancreatectomy (TBR 4.63 and 2.76). One patient had no tumor fluorescence (TBR 1.25) and had a good treatment response with only 10% remaining viable tumor. Representative images from two of these patients are shown in Figure 3.
Figure 3:
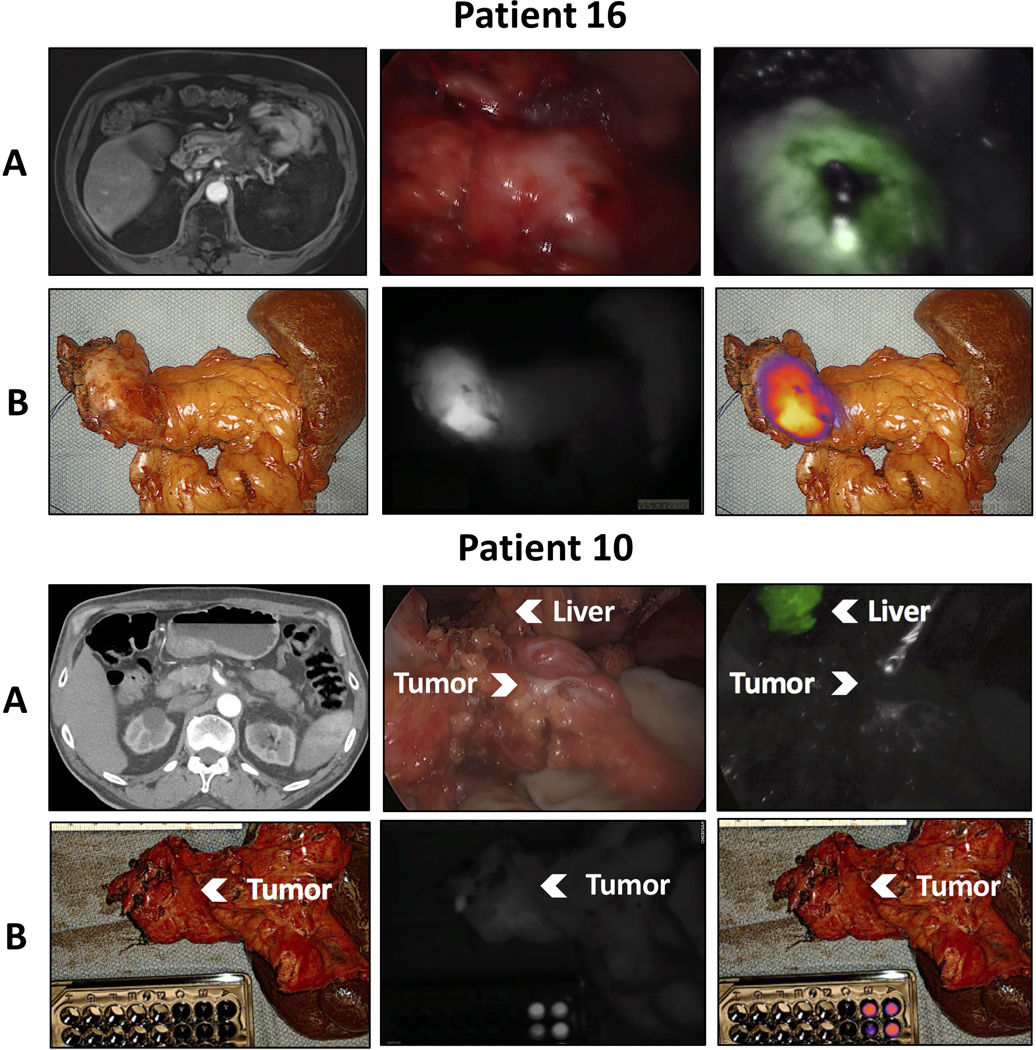
Intraoperative near-infrared (NIR) imaging from two patients following neoadjuvant treatment. Patient 16 underwent an Appleby procedure and had no treatment response on final pathology. Patient 10 underwent a distal pancreatectomy and had good treatment response with only 10% remaining viable tumor. A) Preoperative CT scan, in situ white light, and NIR images. B) Back table white light, near-infrared, and overlay images.
Discussion
In this prospective open-label clinical trial, we demonstrate that intraoperative NIR imaging with a clinically approved fluorophore and imaging system can identify invasive pancreatic malignancies including PDAC. This is the largest clinical trial of intraoperative NIR imaging of PDAC. Intraoperative NIR imaging with second window ICG had 100% sensitivity for viable invasive malignancies. Fluorescence at the resection margin correlated with final pathology in 12/13 cases. Following neoadjuvant therapy, tumor fluorescence correlated with treatment response, suggesting this may be a valuable new tool to assess the response to neoadjuvant treatment.
Although several different fluorescent tracers for intraoperative NIR imaging of PDAC have been studied in preclinical animal models,23–25 successful translation to human clinical trial has been challenging. In a previous clinical trial of NIR imaging with ICG for pancreatic tumors, Hutteman et al. performed NIR imaging immediately after intraoperative infusion of 5–10 mg of ICG in patients undergoing pancreaticoduodenectomy.26 They concluded that ICG provided no useful tumor demarcation. However, the use of perfusion dosing (a small ICG dose given during surgery) rather than the second window ICG technique (a high ICG dose given a day prior to surgery) did not allow for ICG accumulation in tumor via the enhanced permeability and retention (EPR) effect. The EPR effect was first described by Matsumura et al. and suggests that tumor angiogenesis creates excess but leaky capillaries.27 Macromolecules leak out of this defective vasculature and become trapped in tumors due to properties including size, shape, charge, and polarity.28 In this study, the second window technique allowed for reliable ICG accumulation in pancreatic malignancies. These data add to the growing body of evidence that NIR imaging with second window ICG can localize a diverse array of solid tumors.18, 19, 29
This technique could be criticized because 3/8 (37.5%) benign or low-grade malignant tumors were fluorescent. Fluorescence in benign lesions was certainly multifactorial. Intense fluorescence in one benign IPMN was associated with small pockets of ICG pooling around intrapancreatic vasculature on fluorescence microscopy. This may indicate that there was microvascular extravasation from the IPMN as is seen in malignant tumors. Regardless, perhaps the most critical finding here is that the negative predictive value for fluorescence was 100%--tumors with no fluorescence were benign, low grade, or non-viable. Further, for the non-invasive lesions that did fluoresce, intraoperative imaging assisted in visualization, especially in laparoscopic procedures.
The presence or absence of fluorescence at the margin correlated with margin status on final pathology in 12/13 specimens with invasive malignancy. Positive predictive value for fluorescence at the pancreatic neck margin was 83.3% (5/6) while negative predictive value was 100% (7/7). It is unclear why there was fluorescence at the margin in the one patient where fluorescence and pathologic assessment were discordant. This was an advanced tumor requiring a complete portal/superior mesenteric vein resection, but the discordance remains unexplained. Interestingly, fluorescence was seen at the transection margin in four cases where a frozen section was not sent. In one case, the surgeon identified residual tumor in other areas (around the hepatic artery) and knew that this was a margin positive resection. Thus, no frozen section was sent at the transection margin. In a second case (Appleby procedure), the surgeon was at the anatomic limit of resection and thus sent no frozen section. In the two other cases, the preoperative imaging vastly underestimated the extent of the tumor, and the surgeon felt that a positive transection margin would be a marker of advanced disease that was unlikely to be solved by further resection. In each of these four cases the final transection margin was positive for adenocarcinoma. ICG fluorescence predicted the positive margin in each of these four cases, and in three of the four it suggested extensive disease that was underestimated by preoperative imaging. Importantly, gross inspection by the surgeon clearly identified a positive margin in only the first case. The latter two cases were infiltrative tumors in which a positive margin was suggested by ICG but was otherwise uncertain until formal pathologic assessment. This is one potential benefit to NIR imaging - each of these patients might have been spared an extensive, margin-positive resection and instead directed toward neoadjuvant therapy or definitive chemotherapy/chemoradiotherapy.
There are currently no ideal options to assess the response of pancreatic cancer to neoadjuvant therapy. Imaging often does not correlate with response to neoadjuvant treatment or the ability to perform an R0 or R1 resection.12, 13, 30 Tumor markers are useful but cannot be used in isolation to decide upon high-risk resections. In our series, four patients received neoadjuvant therapy. Two of the four patients had evidence of tumor regression on imaging, and three of the four had a significant tumor marker response. However, only one patient had a significant pathologic response. Fluorescence correlated with pathology in all cases. In the patient with good treatment response, the tumor could not be distinguished from surrounding fibrosis intraoperatively with white light and palpation. The surgeon was highly suspicious of a positive margin. However, NIR imaging showed no tumor fluorescence, and in fact the patient had a negative margin. The other three patients required extensive pancreatic resections after neoadjuvant therapy – a total pancreatectomy, an Appleby procedure, and a pancreaticoduodenectomy with portal vein reconstruction. These patients all had highly fluorescent tumors and poor treatment response. In such cases, the ability to confirm poor treatment response prior to resection may have affected the decision to proceed. NIR imaging could therefore be integrated into intraoperative decision-making for borderline resectable or locally advanced PDAC following neoadjuvant therapy.
The results of this study suggest five potential clinical applications for this technology: 1) as an adjunct to intraoperative ultrasound during distal pancreatectomy, 2) for margin assessment, 3) to assess extent of disease, 4) to assess the response to neoadjuvant therapy, and 5) to predict benign disease. When each case was retrospectively assessed using these five criteria, NIR imaging provided valuable information in 18/20 cases (Table 4). The only cases where imaging had no value was for pancreatic neuroendocrine tumors.
Table 4:
Clinical value of intraoperative near-infrared imaging.
| Patient | Procedure | Final Pathology | Benefit of Imaging | Potential Operative Changes Based on NIR Imaging |
|---|---|---|---|---|
| 1 | PD | PDAC | Identified positive margin | Abort resection Consider preoperative therapy |
| 2 | ODP | PDAC | Identified positive margin | Abort resection Consider preoperative therapy |
| 3 | PD | Intrapancreatic cholangiocarcinoma | Identified positive margin | -- |
| 4 | LDP | PDAC | Tumor localization Margin assessment |
-- |
| 5 | PD | PDAC | Margin assessment | -- |
| 6 | ODP | IPMN | Tumor localization | -- |
| 7 | PD | PDAC | Identified positive margin | Abort resection Consider preoperative therapy |
| 8 | PD | Neuroendocrine tumor | -- | -- |
| 9 | LDP | Microcystic serous cystadenoma | Tumor localization | -- |
| 10 | ODP | PDAC, 10% viable tumor | Predicted good response to neoadjuvant therapy | -- |
| 11 | ODP | PDAC | Margin assessment | -- |
| 12 | ODP | IPMN | Tumor localization | -- |
| 13 | TP | PDAC, 100% viable tumor | Predicted poor response to neoadjuvant therapy | Abort high-risk resection entirely |
| 14 | LDP | Neurofibroma | Predicted benign pathology | -- |
| 15 | PD | IPMN | Predicted benign pathology | -- |
| 16 | Appleby | PDAC, 100% viable tumor | Predicted treatment response Identified positive margin |
Abort high-risk resection entirely |
| 17 | PD | PDAC | Margin assessment | -- |
| 18 | PD | Serous cystadenoma | Predicted benign pathology | -- |
| 19 | PD | PDAC, 100% viable tumor | Predicted poor response to neoadjuvant therapy | Abort high-risk resection entirely |
| 20 | ODP | Neuroendocrine tumor | -- | -- |
Abbreviations: NIR near-infrared, PD pancreaticoduodenectomy, PDAC pancreatic ductal adenocarcinoma, ODP open distal pancreatectomy, LDP laparoscopic distal pancreatectomy, IPMN intraductal papillary mucinous neoplasm, TP total pancreatectomy.
There are several limitations to our study. The first is that intraoperative imaging was somewhat limited for pancreaticoduodenectomies due to background fluorescence. ICG is cleared hepatically which creates more fluorescence in the area of the pancreatic head. In an attempt to decrease the background signal, we did decrease the ICG dose to 2.5 mg/kg for two pancreaticoduodenectomies. A pancreatic adenocarcinoma fluoresced at this dose while a cystadenoma did not, but there was no appreciable difference in background fluorescence. We therefore continued with a dose of 5 mg/kg in the remainder of the patients. Background fluorescence was not a limitation during distal pancreatectomy which allowed for more precise tumor localization in this setting. It was especially helpful in laparoscopic procedures, and some minimally invasive platforms including the da Vinci Firefly fluorescence imaging system readily incorporate technology capable of ICG imaging.31, 32 A second limitation is that we retrospectively reviewed the results of imaging and did not use them to change decision making regarding the extent of pancreatic resection. Reviewing what might have been done differently is obviously much different than using the information gained to make real-time intraoperative decisions. Nevertheless, this trial suggested multiple clinical applications that can serve as a foundation when considering relevant clinical endpoints of phase II and III clinical trials. Third, the number of patients who received neoadjuvant treatment was small. Neoadjuvant therapy was used selectively among surgeons in this study and it was therefore difficult to enroll large numbers of treated patients. However, this was one of the more promising findings of the study, and we plan to evaluate the ability of NIR imaging with second window ICG to assess neoadjuvant treatment response in a more sizable future study. Lastly, there were no patients in this series with suspicious liver lesions identified solely by NIR imaging. Another potential benefit to this technology is identification of subclinical metastatic disease not seen with white light. Unfortunately, this could not be evaluated here as there was no patient that presented such a scenario.
In conclusion, this work demonstrates that intraoperative NIR imaging with second window ICG for pancreatic neoplasms is feasible and provides meaningful tumor demarcation that correlates with tumor margins. This technology may also be a promising way to assess the response to neoadjuvant treatment. This study sets a foundation for future intraoperative NIR imaging clinical trials for pancreatic malignancies including the use of targeted fluorescent dyes as they become available.
Supplementary Material
Intraoperative near-infrared (NIR) imaging from a patient with a highly fluorescent intraductal papillary mucinous neoplasm (IPMN). A) Preoperative CT scan, in situ white light, and NIR images. B) Back table white light, NIR, and overlay images. C) H&E microscopy, ICG fluorescence microscopy, DAPI fluorescence microscopy, and ICG and DAPI overlay fluorescence microscopy.
Acknowledgements
The authors would like to acknowledge Ronald P. DeMatteo, MD for his assistance in reviewing the manuscript.
Funding:
SS was supported by the NIH (R01 CA193556).
Footnotes
Type of Study: Original Study
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. [DOI] [PubMed] [Google Scholar]
- 2.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007;297:267–277. [DOI] [PubMed] [Google Scholar]
- 3.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. [DOI] [PubMed] [Google Scholar]
- 4.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011–1024. [DOI] [PubMed] [Google Scholar]
- 5.Wagner M, Redaelli C, Lietz M, et al. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;91:586–594. [DOI] [PubMed] [Google Scholar]
- 6.Howard TJ, Krug JE, Yu J, et al. A margin-negative R0 resection accomplished with minimal postoperative complications is the surgeon’s contribution to long-term survival in pancreatic cancer. J Gastrointest Surg. 2006;10:1338–1345; discussion 1345–1336. [DOI] [PubMed] [Google Scholar]
- 7.Lewis R, Drebin JA, Callery MP, et al. A contemporary analysis of survival for resected pancreatic ductal adenocarcinoma. HPB (Oxford). 2013;15:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winter JM, Cameron JL, Campbell KA, et al. 1423 pancreaticoduodenectomies for pancreatic cancer: A single-institution experience. J Gastrointest Surg. 2006;10:1199–1210; discussion 1210–1191. [DOI] [PubMed] [Google Scholar]
- 9.Esposito I, Kleeff J, Bergmann F, et al. Most pancreatic cancer resections are R1 resections. Ann Surg Oncol. 2008;15:1651–1660. [DOI] [PubMed] [Google Scholar]
- 10.Shin SH, Kim SC, Song KB, et al. A comparative study of laparoscopic vs. open distal pancreatectomy for left-sided ductal adenocarcinoma: a propensity score-matched analysis. J Am Coll Surg. 2015;220:177–185. [DOI] [PubMed] [Google Scholar]
- 11.Xia BT, Fu B, Wang J, et al. Does radiologic response correlate to pathologic response in patients undergoing neoadjuvant therapy for borderline resectable pancreatic malignancy? J Surg Oncol. 2017;115:376–383. [DOI] [PubMed] [Google Scholar]
- 12.Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer. 2012;118:5749–5756. [DOI] [PubMed] [Google Scholar]
- 13.Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg. 2015;261:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keller DS, Ishizawa T, Cohen R, et al. Indocyanine green fluorescence imaging in colorectal surgery: overview, applications, and future directions. Lancet Gastroenterol Hepatol. 2017;2:757–766. [DOI] [PubMed] [Google Scholar]
- 15.Lohman RF, Ozturk CN, Ozturk C, et al. An Analysis of Current Techniques Used for Intraoperative Flap Evaluation. Ann Plast Surg. 2015;75:679–685. [DOI] [PubMed] [Google Scholar]
- 16.Madajewski B, Judy BF, Mouchli A, et al. Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin Cancer Res. 2012;18:5741–5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang JX, Keating JJ, Jesus EM, et al. Optimization of the enhanced permeability and retention effect for near-infrared imaging of solid tumors with indocyanine green. Am J Nucl Med Mol Imaging. 2015;5:390–400. [PMC free article] [PubMed] [Google Scholar]
- 18.Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg. 2014;98:1223–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JY, Thawani JP, Pierce J, et al. Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery. Neurosurgery. 2016;79:856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Predina JD, Newton AD, Corbett C, et al. A Clinical Trial of TumorGlow(R) to Identify Residual Disease during Pleurectomy and Decortication. Ann Thorac Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Predina JD, Newton AD, Xia L, et al. An open label trial of folate receptor-targeted intraoperative molecular imaging to localize pulmonary squamous cell carcinomas. Oncotarget. 2018;9:13517–13529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Predina JD, Newton AD, Keating J, et al. A Phase I Clinical Trial of Targeted Intraoperative Molecular Imaging for Pulmonary Adenocarcinomas. Ann Thorac Surg. 2018;105:901–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tummers WS, Kimura RH, Abou-Elkacem L, et al. Development and preclinical validation of a cysteine knottin peptide targeting Integrin alphavbeta6 for near-infrared fluorescent-guided surgery in pancreatic cancer. Clin Cancer Res. 2018. [DOI] [PubMed] [Google Scholar]
- 24.Lwin TM, Murakami T, Miyake K, et al. Tumor-Specific Labeling of Pancreatic Cancer Using a Humanized Anti-CEA Antibody Conjugated to a Near-Infrared Fluorophore. Ann Surg Oncol. 2018;25:1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park JY, Lee JY, Zhang Y, et al. Targeting the insulin growth factor-1 receptor with fluorescent antibodies enables high resolution imaging of human pancreatic cancer in orthotopic mouse models. Oncotarget. 2016;7:18262–18268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hutteman M, van der Vorst JR, Mieog JS, et al. Near-infrared fluorescence imaging in patients undergoing pancreaticoduodenectomy. Eur Surg Res. 2011;47:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 28.Heneweer C, Holland JP, Divilov V, et al. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: 89Zr-albumin as a model system. J Nucl Med. 2011;52:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keating J, Tchou J, Okusanya O, et al. Identification of breast cancer margins using intraoperative near-infrared imaging. J Surg Oncol. 2016;113:508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Michelakos T, Pergolini I, Castillo CF, et al. Predictors of Resectability and Survival in Patients with Borderline and Locally Advanced Pancreatic Cancer who Underwent Neoadjuvant Treatment with FOLFIRINOX. Ann Surg. 2017. [DOI] [PubMed] [Google Scholar]
- 31.Maker AV, Kunda N. A Technique to Define Extrahepatic Biliary Anatomy Using Robotic Near-Infrared Fluorescent Cholangiography. J Gastrointest Surg. 2017;21:1961–1962. [DOI] [PubMed] [Google Scholar]
- 32.Yu HW, Chung JW, Yi JW, et al. Intraoperative localization of the parathyroid glands with indocyanine green and Firefly(R) technology during BABA robotic thyroidectomy. Surg Endosc. 2017;31:3020–3027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intraoperative near-infrared (NIR) imaging from a patient with a highly fluorescent intraductal papillary mucinous neoplasm (IPMN). A) Preoperative CT scan, in situ white light, and NIR images. B) Back table white light, NIR, and overlay images. C) H&E microscopy, ICG fluorescence microscopy, DAPI fluorescence microscopy, and ICG and DAPI overlay fluorescence microscopy.