Abstract
Aims
Atrial fibrillation (AF) recurs in about one-third of patients after catheter ablation (CA), mostly in the first year. Little is known about the electrophysiological findings and the effect of re-ablation in very late AF recurrences (VLR) after more than 1 year. The aim of this study was to determine the characteristics and outcomes of the first repeat CA after VLR of AF after index CA.
Methods and results
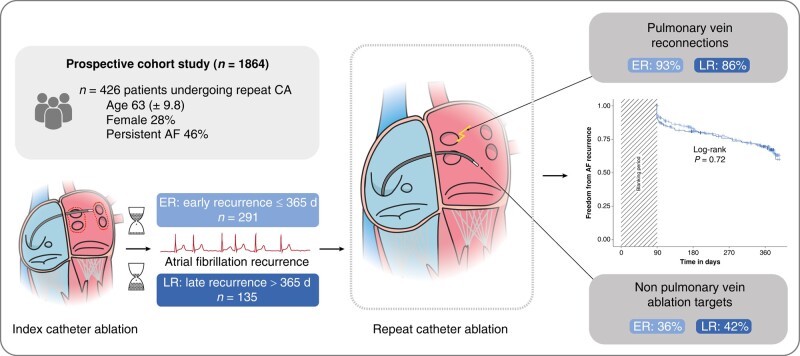
We analysed patients from a prospective Swiss registry that underwent a first repeat ablation procedure. Patients were stratified depending on the time to recurrence after index procedure: early recurrence (ER) for recurrences within the first year and late recurrence (LR) if the recurrence was later. The primary endpoint was freedom from AF in the first year after repeat ablation. Out of 1864 patients included in the registry, 426 patients undergoing a repeat ablation were included in the analysis (28% female, age 63 ± 9.8 years, 46% persistent AF). Two hundred and ninety-one patients (68%) were stratified in the ER group and 135 patients (32%) in the LR group. Pulmonary vein reconnections were a common finding in both groups, with 93% in the ER group compared to 86% in the LR group (P = 0.052). In the LR group, 40 of 135 patients (30%) had a recurrence of AF compared to 90 of 291 patients (31%) in the ER group (log-rank P = 0.72).
Conclusion
There was no association between the time to recurrence of AF after initial CA and the characteristics and outcomes of the repeat procedure.
Keywords: Atrial fibrillation, Repeat ablation, Pulmonary vein isolation, Very late recurrence
Graphical Abstract
Graphical Abstract.
What’s new?
In this large cohort study, there was no association between the time to recurrence of atrial fibrillation (AF) after an initial pulmonary vein isolation and the outcome of the first repeat catheter ablation.
Pulmonary vein reconnections were a common finding, also in patients with a very late recurrence of AF after the index procedure.
The findings were consistent in patients with paroxysmal and persistent AF and in patients undergoing radiofrequency ablation or cryo-ablation during the index procedure.
Introduction
Catheter ablation (CA) is a well-established treatment for atrial fibrillation (AF) improving quality of life and AF-related symptoms.1 When compared with treatment with antiarrhythmic drugs (AADs), CA achieves a substantial reduction in recurrence of atrial arrhythmias and hospitalizations.2,3 Because the pulmonary veins (PVs) are recognized as the major trigger of AF,1,4,5 CA primarily aims at sustained electrical isolation of PV (PVI) from the left atrium. Despite evolving techniques and different sources of energy, arrhythmia recurrences after CA remain a clinical challenge.6–9 Depending on the type of AF (persistent vs. paroxysmal) and patient characteristics, single-procedure success rates at 12 months vary between 52 and 88%10–12 with most of the arrhythmia recurrences occurring in the first year after ablation.13 Pulmonary vein reconnection is thought to be the predominant mechanism of recurrence, with lower rates in later recurrences.13–16 While the clinical relevance of very late recurrences (VLRs) is increasingly recognized,1,17,18 little is known about the electrophysiological findings and the effect of re-ablation in this setting.
The aim of this study was to determine the outcomes and characteristics (including PV reconnections) of the first repeat CA after VLR (>365 days) of AF after index CA procedure in comparison with repeat CA after earlier recurrences within the first year after index CA.
Methods
Study design and population
The study was conducted using the Swiss Atrial Fibrillation Pulmonary Vein Isolation (SWISS-AF PVI) registry (ClinicalTrials.gov identifier: NCT03718364), an ongoing prospective cohort study in Switzerland, enrolling adult patients with paroxysmal or persistent AF undergoing PVI. The study was approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz), and all patients provided their written consent. In our analysis, we included patients that underwent a first repeat ablation procedure between April 2010 and December 2021 at our institution. The index PVI procedure was performed using radiofrequency (RF) energy or cryo-energy. Patients that underwent a surgical index ablation procedure or an ablation procedure at an outside hospital were excluded.
Study procedures and catheter ablation
Repeat ablation procedures were conducted using RF energy and irrigated-tip ablation catheters in combination with a 3D electro-anatomic mapping system (Carto3, Biosense Webster, Inc., Irvine, CA, USA) and a multipolar mapping catheter (Pentaray, Biosense Webster, Inc., Irvine, CA, USA). Before the procedure, transoesophageal echocardiography was performed to rule out thrombus in the left atrial appendage. Antiarrhythmic drugs were stopped at the time of the procedure. To access the left atrium, a fluoroscopy-guided transseptal puncture was performed. The procedures focused on the identification and ablation of PV reconnections. Additional lesions [cavotricuspid isthmus line, mitral isthmus line, roof line, posterior box, superior vena cava isolation, vein of Marshall ethanol ablation, complex fractionated atrial electrogram (CFAE) ablation] were at the discretion of the operator. In the case of peri-mitral flutter, a mitral isthmus line was the standard approach. The position of the mitral isthmus line (antero-medial, antero-lateral, or postero-lateral) was at the discretion of the operator. Re-isolation of the PVs was confirmed by entrance block. If performed, bidirectional block across additional lines was confirmed by differential pacing.
Follow-up
Standard follow-up visits were scheduled at 3, 6, and 12 months after repeat ablation and included a detailed physical examination, a 12-lead electrocardiogram (ECG), and a 7-day Holter ECG recording. Additionally, 12-lead ECGs and 7-day Holter recordings were performed in case of symptoms independent of the follow-up scheme.
Outcomes
The primary endpoint was the efficacy of the first repeat ablation defined as freedom from AF during the first year after repeat PVI procedure. Atrial fibrillation recurrence was defined according to the 2017 expert consensus statement on catheter and surgical ablation of AF1 as any documented episode of AF, atrial flutter, or atrial tachycardia lasting longer than 30 s. According to current guidelines, we applied a blanking period of 90 days1,19 after the ablation procedure.
Statistical analysis
The patients were stratified based on the time to the first recurrence of AF after the index procedure: in this analysis, ER was defined as a recurrence within the first year after index ablation, whereas a recurrence occurring after the first 365 days after index procedure was defined as a LR. These definitions deviate from the expert consensus statement,1 where an ER denotes an AF recurrence within the first 3 months, a LR is defined as a recurrence between 90 days and 1 year after CA, and a VLR is defined as a recurrence >1 year after CA. Categorical variables were expressed as numbers (percentages) and compared using χ2 tests and Fisher’s exact tests as appropriate. Continuous variables were reported as means ± standard deviation (SD) or, if strongly skewed, median (interquartile range) and compared with Student’s t-tests and Wilcoxon’s rank sum tests, respectively. The primary outcome (freedom from AF) was calculated as the time from the repeat procedure in days until the first documented recurrence of AF. In the case of dropout or loss to follow-up, appropriate censoring was applied. The primary outcome was displayed using Kaplan–Meier curves and compared with a log-rank test. Cox proportional hazards models were fitted to identify associations between the outcome of the repeat procedure and the time to recurrence after the first ablation procedure. We used the dichotomized strata ER vs. LR in an unadjusted model (model 1) and in two corrected multivariable models (model 2: age, sex; model 3: age, sex, hypertension, type of AF, left ventricular ejection fraction, indexed left atrial volume). Additionally, we fitted an unadjusted model using the time to AF recurrence after index procedure and the time between index and repeat procedure as a continuous variable. The Cox proportional hazards assumption was verified using the Schoenfeld residuals. The presented P-values are two-sided. A P-value ≤0.05 was regarded as statistically significant. All analyses were performed using the statistical software R version 4.2.3.
Results
Study population
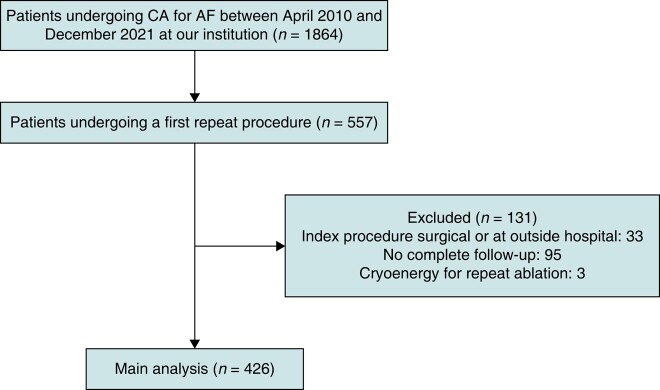
Out of 1864 patients undergoing CA for AF between April 2010 and December 2021, 557 patients underwent a first repeat procedure at our institution. Of these 557 patients, 33 patients were excluded because they underwent an index procedure at an outside hospital or had a prior surgical ablation procedure. Ninety-five patients were excluded because follow-up information was incomplete. Three patients were excluded, because the repeat procedure was performed without a 3D electro-anatomic mapping system using cryo-energy (Figure 1).
Figure 1.
Patient flow diagram. AF, atrial fibrillation; CA, catheter ablation.
Baseline characteristics
Of the 426 patients included in the final analysis, 117 patients (28%) were female, the mean age was 63 (±9.8) years, and 195 patients (46%) had persistent AF. The patients were stratified into two groups: ER (n = 291, 68%) and LR (n = 135, 32%). The median time to AF recurrence after index intervention was 208 days (ER) and 867 days (LR). Except for differences in age and the prevalence of smoking, there were no differences in the baseline parameters (Table 1).
Table 1.
Baseline characteristics, overall and stratified by ER and LR
| Variable | Overall | Early recurrence | Late recurrence | P |
|---|---|---|---|---|
| n | 426 | 291 | 135 | |
| Time to AF recurrence after index ablation [days] [median (IQR)] | 208 (111−452) | 141 (92−218) | 867 (499−1382) | <0.001 |
| Age, years [mean (SD)] | 63 (9.8) | 62 (9.9) | 65 (9) | 0.001 |
| Female, n (%) | 117 (28) | 86 (30) | 31 (23) | 0.193 |
| Smoking | 0.008 | |||
| − current, n (%) | 40 (9.4) | 21 (7.2) | 19 (14) | |
| − past, n (%) | 188 (44) | 122 (42) | 66 (49) | |
| − never, n (%) | 196 (46) | 147 (51) | 49 (37) | |
| Body mass index, kg/m² [median (IQR)] | 26 (24−30) | 26 (24−30) | 27 (25−30) | 0.168 |
| Non-paroxysmal AF, n (%) | 196 (46) | 128 (44) | 68 (50) | 0.260 |
| EHRA score I/II vs. III/IV, n (%) | 126/173 (42/58) | 96/113 (46/54) | 30/60 (33/67) | 0.058 |
| CHA₂DS₂-VASc [median (IQR)] | 2 (1−3) | 2 (1−3) | 2 (1−3) | 0.067 |
| Hypertension, n (%) | 261 (61) | 169 (58) | 92 (68) | 0.060 |
| Diabetes, n (%) | 31 (7.3) | 21 (7.2) | 10 (7.4) | 1.00 |
| Echocardiographic findings | ||||
| LA size (PLAX) (mm) [mean (SD)] | 42 (6.6) | 42 (6.9) | 43 (5.8) | 0.183 |
| LAVI, mL/m² [mean (SD)] | 40 (12) | 39 (12) | 41 (12) | 0.158 |
| LVEF [%, median (IQR)] | 60 (53−63) | 59 (52−62) | 60 (54−64) | 0.177 |
Values are given as mean (±standard deviation), median (interquartile range), or numbers (percentage).
Early recurrence: AF recurrence in the first 365 days after previous procedure. Late recurrence: AF recurrence beyond the first 365 days after previous index intervention.Missing values: smoking (n = 2), EHRA score (n = 127), CHA2DS2-VASc score (n = 11), LA (n = 50), LAVI (n = 108), LVEF (n = 15).
SD, standard deviation; IQR, interquartile range (25–75 percentile); AF, atrial fibrillation; EHRA score, European Heart Rhythm Association score: I = no symptoms, II = mild symptoms, III = severe symptoms, and IV = disabling symptoms; CHA2DS2-VASc score: congestive heart failure, hypertension, age ≥ 75 years (2 points), diabetes mellitus, prior stroke/transient ischaemic attack/thromboembolism (2 points), vascular disease, age 65–74 years, female sex; LA dimension (PLAX), left atrial dimension in the parasternal long axis; LAVI, indexed left atrial volume; LVEF, left ventricular ejection fraction.
Pulmonary vein reconnections
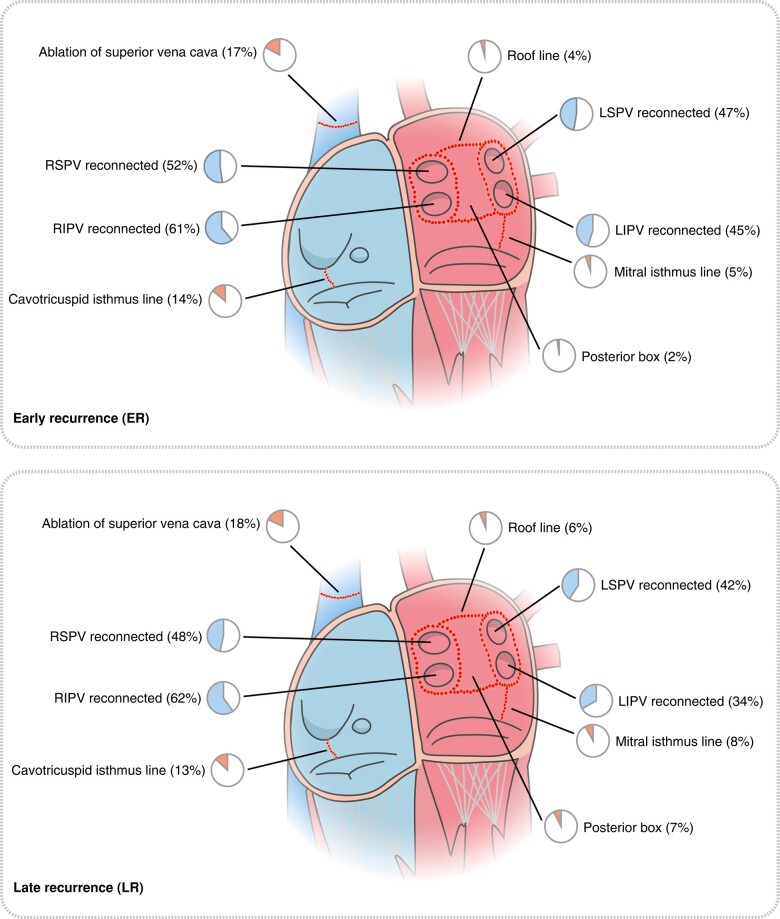
At the repeat procedure, 378 patients (91%) had PV reconnections with a mean of two reconnected PVs (±1.1). In the ER group, 266 patients (93%) had PV reconnections, compared to 112 patients (86%) in the LR group (P = 0.052). Right inferior pulmonary vein was the most common PV to be reconnected in a total of 256 patients (61%, overall), in 175 patients (61%) in the ER group and in 81 patients (62%) in the LR group (Table 2 and Figure 2). Although there was no difference in the number of patients with reconnected PVs after an index ablation using cryo-energy [97/112 patients (89%)] compared to RF energy [281/314 patients (91%)], there were significantly more reconnected PVs in patients after index procedures using RF energy with a mean of 2.1 (±1.1) reconnected PVs compared to a mean of 1.8 (±1.1) PVs after index procedures using cryo-energy (P = 0.024). Also, left superior pulmonary vein (LSPV) was more likely to be reconnected after index procedures using RF energy in 156 patients (50%) compared to 34 patients (31%) after index procedures using cryo-energy (P = 0.001) (see Supplementary material online, Table S1).
Table 2.
Procedural characteristics, overall and stratified by ER and LR
| Variable | Overall | Early recurrence | Late recurrence | P |
|---|---|---|---|---|
| n | 426 | 291 | 135 | |
| Time interval between index and repeat procedure [days] [median (IQR)] | 340 (IQR: 165 − 763) | 218 (IQR: 140 − 357) | 953 (IQR: 625 − 1510) | <0.001 |
| Previous procedure using cryo-energy, n (%) | 112 (26) | 81 (28) | 31 (23) | 0.345 |
| Pulmonary vein reconnections | ||||
| Number of reconnected PVs [mean (SD)] | 2 (1.1) | 2.1 (1.1) | 1.9 (1.2) | 0.143 |
| No pulmonary vein reconnections, n (%) | 39 (9.4) | 21 (7.3) | 18 (14) | 0.052 |
| ≥1 reconnected pulmonary vein(s), n (%) | 378 (91) | 266 (93) | 112 (86) | 0.052 |
| ≥2 reconnected PV, n (%) | 286 (69) | 204 (71) | 82 (63) | 0.129 |
| Left inferior pulmonary vein reconnected, n (%) | 173 (41) | 128 (45) | 45 (34) | 0.055 |
| Left superior pulmonary vein reconnected, n (%) | 190 (45) | 135 (47) | 55 (42) | 0.373 |
| Left common pulmonary vein reconnected, n (%) | 14 (3.3) | 10 (3.4) | 4 (3) | 1.00 |
| Right inferior pulmonary vein reconnected, n (%) | 256 (61) | 175 (61) | 81 (62) | 0.953 |
| Right superior pulmonary vein reconnected, n (%) | 213 (51) | 150 (52) | 63 (48) | 0.539 |
| Non-pulmonary vein ablation targets | ||||
| Non-PV ablation targets, n (%) | 161 (38) | 104 (36) | 57 (42) | 0.239 |
| Cavotricuspid isthmus line, n (%) | 58 (14) | 41 (14) | 17 (13) | 0.789 |
| Mitral isthmus line, n (%) | 26 (6.1) | 15 (5.2) | 11 (8.1) | 0.325 |
| Roof line, n (%) | 19 (4.5) | 11 (3.8) | 8 (5.9) | 0.456 |
| Posterior box, n (%) | 16 (3.8) | 6 (2.1) | 10 (7.4) | 0.015 |
| Ablation of superior vena cava, n (%) | 74 (17) | 49 (17) | 25 (18) | 0.773 |
| Ablation of vein of Marshall, n (%) | 3 (0.7) | 1 (0.3) | 2 (1.5) | 0.494 |
| Ablation of complex fractionated atrial electrograms (CFAE), n (%) | 21 (4.9) | 16 (5.5) | 5 (3.7) | 0.579 |
| Ablation of other arrhythmias, n (%) | 19 (4.5) | 16 (5.5) | 3 (2.2) | 0.203 |
| Electrocardiographic features | ||||
| Rhythm at admission for repeat ablation (%) | 0.267 | |||
| − Sinus rhythm, n (%) | 265 (64) | 181 (64) | 84 (65) | |
| − AF, n (%) | 117 (28) | 77 (27) | 40 (31) | |
| − Typical flutter, n (%) | 7 (1.7) | 7 (2.5) | 0 (0) | |
| − Atypical flutter, n (%) | 13 (3.2) | 11 (3.9) | 2 (1.6) | |
| − Other rhythm, n (%) | 9 (2.2) | 6 (2.1) | 3 (2.3) | |
| PR interval, ms [mean (SD)] | 178 (35) | 178 (33) | 178 (39) | 0.980 |
| QRS duration, ms [median (IQR)] | 99 (IQR: 90 − 110) | 98 (IQR: 88 − 108) | 100 (IQR: 92 − 114) | 0.064 |
Numbers are No. (%) unless otherwise noted. Early recurrence: AF recurrence in the first 365 days after previous procedure.Late recurrence: AF recurrence beyond the first 365 days after previous index intervention.
Other arrhythmias: focal atrial tachycardia (n = 13), atrioventricular-node re-entry tachycardia (n = 3), left atrial micro-re-entry tachycardia (n = 3).
Missing values: pulmonary vein reconnections (n = 9), rhythm at admission for repeat ablation (n = 15), PR interval (n = 169), QRS duration (n = 116).
AF, atrial fibrillation; SD, standard deviation; IQR, interquartile range (25–75 percentile); PV, pulmonary vein; ECG, electrocardiogram.
Figure 2.
Procedural characteristics. Visualization of the rates of pulmonary vein reconnections and the location and rate of the ablation targets performed during the repeat procedures in the group ER and LR. The percentages are visualized by pie charts. For reasons of clarity, vein of Marshall, CFAEs, and other arrhythmia ablations are not shown. RSPV, right superior pulmonary vein; RIPV, right inferior pulmonary vein; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein. See Table 2 for details.
Non-pulmonary vein ablation targets and electrocardiographic features
Overall, 161 patients (38%) underwent ablation of non-PV targets during the repeat procedure: 104 (36%) in the ER group and 57 (42%) in the LR group. The superior vena cava was the most common non-PV target [74 patients (17%)], and the cavotricuspid isthmus line was the second most commonly performed ablation lesion outside the PVs [58 patients (14%)]. For the ablation targets, there was no difference between the two groups except for the posterior box lesion, which was more likely to be performed in the LR group with 10 patients (7.4%) vs. 6 patients (2.1%) in the ER group (P = 0.015). There was also no difference in the electrocardiographic features between the two groups (Table 2).
Procedural complications
For all the repeat procedures, a total of four major complications that required a prolonged hospital stay occurred: two tamponades, one arteriovenous (AV) fistula, and one groin haematoma.
Atrial fibrillation recurrence after repeat procedure
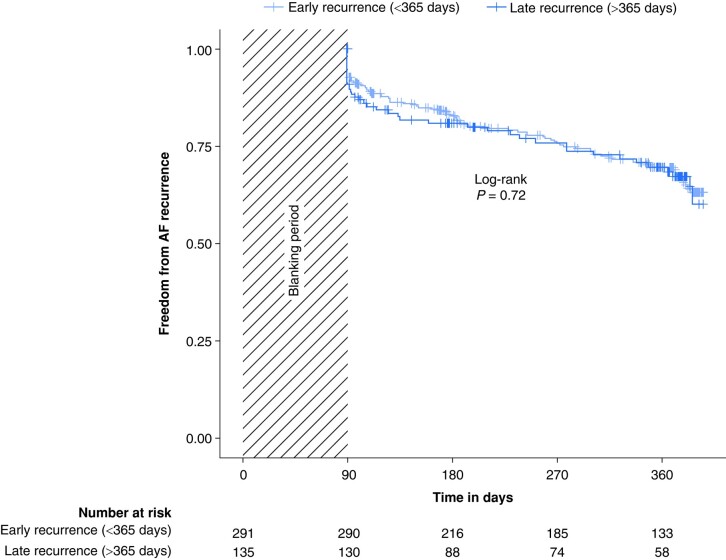
A total of 130 patients (31%) had a recurrence of AF during a follow-up of 12 months after repeat ablation. In the LR group, 40 of 135 patients (30%) had a recurrence of AF compared to 90 of 291 patients (31%) in the ER group. There was no significant difference in freedom from AF recurrence between ER and LR (log-rank P = 0.72, Figure 3). The Cox proportional hazards models did not show an association of the time of the AF recurrence after the index procedure and the outcome of the repeat procedure (Table 3). Freedom from AF recurrence did not differ when comparing patients with paroxysmal to patients with persistent AF (see Supplementary material online, Figure S2) and when comparing patients undergoing RF ablation compared to cryo-ablation during the index procedure (see Supplementary material online, Figure S3). Also, there was no difference in outcomes when comparing patients with AF recurrence within 90 days compared to patients with a recurrence between 91 and 365 days and to patients with a LR (>365 days) (see Supplementary material online, Figure S4). Furthermore, there was no association between the time interval from index procedure to repeat procedure and the outcome of the repeat procedure when analysed as a continuous variable (see Supplementary material online, Table S5).
Figure 3.
Kaplan–Meier plot for freedom from AF recurrence after repeat CA. Freedom from AF recurrence per follow-up days after repeat CA according to time to AF recurrence after index ablation. P-value was calculated by log-rank test. AF, atrial fibrillation.
Table 3.
Association of time to AF recurrence after initial ablation procedure and time to AF recurrence after repeat ablation
| Model 1: unadjusted |
Model 2: age- and sex-adjusted |
Model 3: multivariable adjusted |
|
|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Early recurrence | Reference | Reference | Reference |
| Late recurrence | 1.07 (0.74–1.56) | 1.05 (0.72–1.52) | 1.09 (0.73–1.65) |
| P = 0.7 | P = 0.8 | P = 0.7 |
Model 3 adjusted for age, sex, hypertension, type of AF, left ventricular ejection fraction (LVEF), and indexed left atrial volume (LAVI).CI, confidence interval; HR, hazard ratio.
Discussion
To the best of our knowledge, this is the largest analysis of outcomes and characteristics of the first repeat CA after a VLR (>1 year) of AF after index PVI. Our main findings were as follows: first, the AF recurrence rate after repeat CA after index PVI was approximately 31%; second, the outcomes were independent of the time to AF recurrence after the index PVI with comparable recurrence rates in the ER and LR groups; third, PV reconnections were a common finding, also in patients with a VLR of AF after the index procedure; and fourth, these findings were consistent in patients with paroxysmal and persistent AF and in patients undergoing RF ablation or cryo-ablation during the index procedure.
Few other studies examined the relationship between the time to recurrence after the index procedure and outcomes after repeat CA. Recently, Choi et al.20 found no relationship between the outcomes of repeat CA according to the time to recurrence after the index procedure in an observational study of 198 patients using RF energy including 98 patients after VLR of AF (>12 months). Daimee et al.21 examined the outcomes of 300 repeat ablations and found no association between AF recurrence and the time interval between index and repeat ablation when comparing recurrences within 3 years to recurrences after more than 3 years. In contrast, Gaztañaga et al.22 previously observed an association of the outcomes of the repeat procedure with the time to recurrence after the index procedure with better response to repeat ablation and to AAD treatment after a longer time to AF recurrence after ablation. However, the group undergoing repeat ablation after more than 12 months was relatively small (n = 35), the study was performed before the advent of contact-force sensing catheters, and they used an endpoint of ‘AF control’ defined as ‘no or rare episodes of AF’. In our study, the success of the procedure was approximately 70% 1 year after repeat ablation. We did not observe a difference dependent on the time to recurrence after the index ablation procedure and included a substantially larger number of patients undergoing a repeat ablation after a VLR (n = 135). Our study included cryo- and RF index procedures, and there was no differential effect of the two energy sources on AF recurrence. This is consistent with previous work showing that there is no association of the index procedure modality with the outcome of the repeat procedure.21,23 Due to the non-randomized nature of our comparison, differences in ablation strategies during the repeat procedure may have impacted the outcomes in the two groups in our study. However, differences were minor with a similar rate of ablation of non-PV targets, except for a small but significant difference in the creation of a posterior box lesion (7.4% in the LR vs. 2.1% in the ER group).
The PVs have been reported to contain the triggers of AF mostly in patients with the paroxysmal form of the arrhythmia,4,5 while with disease progression, atrial substrate caused by remodelling is believed to play a major role in maintaining AF.24,25 The comparable procedural findings and ablation outcomes between the two groups in our analysis may suggest that the initial CA was indeed able to slow disease progression and atrial remodelling. Retrospective studies have shown that there is an association between a shorter diagnosis-to-ablation time and better outcomes of an initial CA,26,27 although a recent randomized study did not confirm this finding, at least when comparing direct CA within one month to medical treatment and delayed CA after a time span of 1 year.28 It is conceivable that relevant disease progression and changes in atrial substrate take a longer time to develop. For instance, the recently published 3-year follow-up of the EARLY-AF29 study showed that patients with paroxysmal AF undergoing CA had a lower incidence of persistent AF or atrial tachyarrhythmia when compared with antiarrhythmic therapy.
In our study, we identified a high proportion of PV reconnections in both groups. Hussein et al.13 found PV reconnections in all patients undergoing a repeat procedure after a recurrence within the first year (n = 161) and a recurrence later than 1 year (n = 27) after an initial RF procedure. Similarly, in an analysis by Shah et al.,14 there were PV reconnections in all 18 patients undergoing a repeat ablation for recurrent AF after more than 1 year after an initial RF procedure. In contrast, Sotomi et al.15 found a significant difference between the prevalence of reconnections in their retrospective study comparing patients with a VLR (>12 months, n = 26) to patients with a LR (3–12 months, n = 124) after initial RF ablation. The rate of reconnections in patients with a recurrence in the first year (90%) was comparable to our results (93%) in the ER group. In patients with a VLR (>12 months), the rate of reconnections was lower (69%) than in our corresponding LR group (86%). However, 3D mapping systems were only partly used in their study. It is conceivable that some PV signals may be missed without the systematic use of multipolar catheters for high-resolution mapping of the PVs and the left atrium and a clear voltage cut-off to distinguish between healthy and scar tissues. In our study, we systematically used high-resolution 3D mapping using a multipolar mapping catheter to determine PV reconnections. More recently, Erhard et al.16 found significantly fewer PV reconnections in later recurrences when comparing repeat ablations after 3–24 months (n = 47, PV reconnections 97.9%), 2–5 years (n = 29, PV reconnections 72.4%), and > 5 years (n = 34, PV reconnections 55.9%) after an initial RF procedure. Compared to our study, different cut-offs for time to recurrence after the previous procedure were used. The longer time span may have allowed more subtle differences in atrial remodelling to become apparent. Shah et al.30 examined the prevalence of PV reconnections and additional lesions in patients with a recurrence more than 3 years after the last ablation procedure. Of the 137 patients enrolled in their observational study, 81% had at least one PV reconnection. Additional lesions were much more likely (93%) to be performed than in our study with 36% in the ER group and 42% in the LR group. The analysis was based on a highly selected cohort (137 of 10 378 patients), the time span to the recurrence after the previous procedure was longer, and multiple prior procedures were allowed, which may explain the different findings compared to our study.
In contrast to the studies mentioned, our analysis also included patients that underwent an index procedure using cryo-energy. Although, in our analysis, the rate of patients with reconnected PVs was similar between the types of energy used at the index procedure, the number of reconnected PVs was higher in patients that received an index procedure using RF energy. Specifically, the LSPV was more likely to be reconnected in patients after an index procedure using RF. The presence of reconnected PVs at the repeat procedure in around 90% of patients after cryoballoon (CB) index procedure was higher than in previous studies. Kuck et al.31 reported reconnected PVs in 78% of patients, Heeger et al. reported 7432 and 79%,33 Bordignon et al.34 reported 67%, and Ciconte et al.35 found approximately 50% reconnected PVs. Differences in PV reconnection rates might be explained by heterogeneity of CB generations used and the resolution of electro-anatomic mapping.
Strengths and limitations
The strengths of our analysis include the large cohort size and the standardized follow-up. Limitations of our study include the single centre and the observational nature. Due to methodical reasons, there may be a selection bias because we only included patients undergoing a repeat ablation. For our comparison, we used a definition of ‘ER’ and ‘LR’ with a cut-off between early and late at 1 year after ablation that deviates from the expert consensus statement.1 We believe that this adequately reflects clinical routine. Finally, no findings from patients undergoing pulsed field ablation during the index procedure can be presented because the technology was not available at the time.
Further studies are needed including re-mapping studies in patients with and without AF recurrence and studies including novel energy sources (e.g. pulsed field ablation) to improve the understanding of the temporal relationship between AF diagnosis, index and repeat CA, the mechanisms of AF recurrence and the best ablation strategy at repeat procedure.
Conclusions
In patients undergoing repeat CA for recurrent AF, there was no association between the time to recurrence of AF after initial CA and the characteristics and outcomes of the repeat procedure. Therefore, the decision whether to perform a repeat ablation should not be based on the time to recurrence of AF after initial ablation.
Supplementary Material
Contributor Information
Niklas Stauffer, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Sven Knecht, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Patrick Badertscher, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Philipp Krisai, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Elisa Hennings, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Teodor Serban, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Gian Voellmin, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Stefan Osswald, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Christian Sticherling, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Michael Kühne, Department of Cardiology, University Hospital Basel, Petersgraben 4, 4031 Basel, Switzerland; Cardiovascular Research Institute Basel, University Hospital Basel, Spitalstrasse 2, 4056 Basel, Switzerland.
Supplementary material
Supplementary material is available at Europace online.
Funding
This research did not receive any specific grant.
Data availability
The consent forms, as approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz), do not allow the data to be made publicly available. The data will be shared on reasonable request to the corresponding author.
References
- 1. Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 2017;14:e275–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turagam MK, Musikantow D, Whang W, Koruth JS, Miller MA, Langan MN et al. Assessment of catheter ablation or antiarrhythmic drugs for first-line therapy of atrial fibrillation: a meta-analysis of randomized clinical trials. JAMA Cardiol 2021;6:697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Calkins H, Reynolds MR, Spector P, Sondhi M, Xu Y, Martin A et al. Treatment of atrial fibrillation with antiarrhythmic drugs or radiofrequency ablation: two systematic literature reviews and meta-analyses. Circ Arrhythm Electrophysiol 2009;2:349–61. [DOI] [PubMed] [Google Scholar]
- 4. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med 1998;339:659–66. [DOI] [PubMed] [Google Scholar]
- 5. Nattel S. Paroxysmal atrial fibrillation and pulmonary veins: relationships between clinical forms and automatic versus re-entrant mechanisms. Can J Cardiol 2013;29:1147–9. [DOI] [PubMed] [Google Scholar]
- 6. Knecht S, Sticherling C, Roten L, Badertscher P, Krisai P, Chollet L et al. Efficacy and safety of a novel cryoballoon ablation system: multicentre comparison of 1-year outcome. Europace 2022;24:1926–32. [DOI] [PubMed] [Google Scholar]
- 7. du Fay de Lavallaz J, Badertscher P, Kobori A, Kuck KH, Brugada J, Boveda S et al. Sex-specific efficacy and safety of cryoballoon versus radiofrequency ablation for atrial fibrillation: an individual patient data meta-analysis. Heart Rhythm 2020;17:1232–40. [DOI] [PubMed] [Google Scholar]
- 8. Badertscher P, Weidlich S, Serban T, Krisai P, Voellmin G, Osswald S et al. Pulsed-field ablation versus single-catheter high-power short-duration radiofrequency ablation for atrial fibrillation: procedural characteristics, myocardial injury, and mid-term outcomes. Heart Rhythm 2023;20:1277–8. [DOI] [PubMed] [Google Scholar]
- 9. Badertscher P, Knecht S, Spies F, Völlmin G, Schaer B, Schärli N et al. High-power short-duration ablation index–guided pulmonary vein isolation protocol using a single catheter. J Interv Card Electrophysiol 2022;65:633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kis Z, Muka T, Franco OH, Bramer WM, De Vries LJ, Kardos A et al. The short and long-term efficacy of pulmonary vein isolation as a sole treatment strategy for paroxysmal atrial fibrillation: a systematic review and meta-analysis. Curr Cardiol Rev 2017;13:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Moran D, De Regibus V, De Asmundis C, Takarada K, Mugnai G, Ströker E et al. Second generation cryoballoon ablation for atrial fibrillation in young adults: midterm outcome in patients under 40 years of age. Europace 2018;20:295–300. [DOI] [PubMed] [Google Scholar]
- 12. Ganesan AN, Shipp NJ, Brooks AG, Kuklik P, Lau DH, Lim HS et al. Long-term outcomes of catheter ablation of atrial fibrillation: a systematic review and meta-analysis. J Am Heart Assoc 2013;2:e004549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hussein AA, Saliba WI, Martin DO, Bhargava M, Sherman M, Magnelli-Reyes C et al. Natural history and long-term outcomes of ablated atrial fibrillation. Circ Arrhythm Electrophysiol 2011;4:271–8. [DOI] [PubMed] [Google Scholar]
- 14. Shah AN, Mittal S, Sichrovsky TC, Cotiga D, Arshad A, Maleki K et al. Long-term outcome following successful pulmonary vein isolation: pattern and prediction of very late recurrence. J Cardiovasc Electrophysiol 2008;19:661–7. [DOI] [PubMed] [Google Scholar]
- 15. Sotomi Y, Inoue K, Ito N, Kimura R, Toyoshima Y, Masuda M et al. Cause of very late recurrence of atrial fibrillation or flutter after catheter ablation for atrial fibrillation. Am J Cardiol 2013;111:552–6. [DOI] [PubMed] [Google Scholar]
- 16. Erhard N, Mauer T, Ouyang F, Sciacca V, Rillig A, Reissmann B et al. Mechanisms of late arrhythmia recurrence after initially successful pulmonary vein isolation in patients with atrial fibrillation. PACE Pacing Clin Electrophysiol 2023;46:161–8. [DOI] [PubMed] [Google Scholar]
- 17. Wokhlu A, Hodge DO, Monahan KH, Asirvatham SJ, Friedman PA, Munger TM et al. Long-Term outcome of atrial fibrillation ablation: impact and predictors of very late recurrence. J Cardiovasc Electrophysiol 2010;21:1071–8. [DOI] [PubMed] [Google Scholar]
- 18. Tzou WS, Marchlinski FE, Zado ES, Lin D, Dixit S, Callans DJ et al. Long-term outcome after successful catheter ablation of atrial fibrillation. Circ Arrhythm Electrophysiol 2010;3:237–42. [DOI] [PubMed] [Google Scholar]
- 19. Hindricks G, Potpara T, Dagres N, Bax JJ, Boriani G, Dan GA et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 20. Choi SH, Yu HT, Kim D, Park JW, Kim TH, Uhm JS et al. Late recurrence of atrial fibrillation 5 years after catheter ablation: predictors and outcome. Europace 2023;25:euad113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daimee UA, Akhtar T, Boyle TA, Jager L, Arbab-Zadeh A, Marine JE et al. Repeat catheter ablation for recurrent atrial fibrillation: electrophysiologic findings and clinical outcomes. J Cardiovasc Electrophysiol 2021;32:628–38. [DOI] [PubMed] [Google Scholar]
- 22. Gaztañaga L, Frankel DS, Kohari M, Kondapalli L, Zado ES, Marchlinski FE. Time to recurrence of atrial fibrillation influences outcome following catheter ablation. Heart Rhythm 2013;10:2–9. [DOI] [PubMed] [Google Scholar]
- 23. Zeljkovic I, Knecht S, Spies F, Reichlin T, Osswald S, Kühne M et al. Paroxysmal atrial fibrillation recurrence after redo procedure-ablation modality impact. J Interv Card Electrophysiol 2020;57:77–85. [DOI] [PubMed] [Google Scholar]
- 24. Allessie M, Ausma J, Schotten U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc Res 2002;54:230–46. [DOI] [PubMed] [Google Scholar]
- 25. Heijman J, Voigt N, Nattel S, Dobrev D. Cellular and molecular electrophysiology of atrial fibrillation initiation, maintenance, and progression. Circ Res 2014;114:1483–99. [DOI] [PubMed] [Google Scholar]
- 26. Chew DS, Black-Maier E, Loring Z, Noseworthy PA, Packer DL, Exner DV et al. Diagnosis-to-ablation time and recurrence of atrial fibrillation following catheter ablation: a systematic review and meta-analysis of observational studies. Circ Arrhythm Electrophysiol 2020;13:350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hussein AA, Saliba WI, Barakat A, Bassiouny M, Chamsi-Pasha M, Al-Bawardy R et al. Radiofrequency ablation of persistent atrial fibrillation: diagnosis-to-ablation time, markers of pathways of atrial remodeling, and outcomes. Circ Arrhythm Electrophysiol 2016;9:e003669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kalman JM, Al-Kaisey AM, Parameswaran R, Hawson J, Anderson RD, Lim M et al. Impact of early vs. delayed atrial fibrillation catheter ablation on atrial arrhythmia recurrences. Eur Heart J 2023;44:2447–54. [DOI] [PubMed] [Google Scholar]
- 29. Andrade JG, Deyell MW, Macle L, Wells GA, Bennett M, Essebag V et al. Progression of atrial fibrillation after cryoablation or drug therapy. N Engl J Med 2023;388:105–16. [DOI] [PubMed] [Google Scholar]
- 30. Shah S, Barakat AF, Saliba WI, Abdur Rehman K, Tarakji KG, Rickard J et al. Recurrent atrial fibrillation after initial long-term ablation success: electrophysiological findings and outcomes of repeat ablation procedures. Circ Arrhythm Electrophysiol 2018;11:e005785. [DOI] [PubMed] [Google Scholar]
- 31. Kuck KH, Albenque JP, Chun KRJ, Fürnkranz A, Busch M, Elvan A et al. Repeat ablation for atrial fibrillation recurrence post cryoballoon or radiofrequency ablation in the FIRE AND ICE trial. Circ Arrhythm Electrophysiol 2019;12:e007247. [DOI] [PubMed] [Google Scholar]
- 32. Heeger CH, Wissner E, Mathew S, Deiss S, Lemes C, Rillig A et al. Once isolated, always isolated? Incidence and characteristics of pulmonary vein reconduction after second-generation cryoballoon-based pulmonary vein isolation. Circ Arrhythm Electrophysiol 2015;8:1088–94. [DOI] [PubMed] [Google Scholar]
- 33. Heeger CH, Rexha E, Maack S, Rottner L, Fink T, Mathew S et al. Reconduction after second–generation cryoballoon–based pulmonary vein isolation – impact of different ablation strategies ―. Circ J 2020;84:902–10. [DOI] [PubMed] [Google Scholar]
- 34. Bordignon S, Fürnkranz A, Perrotta L, Dugo D, Konstantinou A, Nowak B et al. High rate of durable pulmonary vein isolation after second-generation cryoballoon ablation: analysis of repeat procedures. Europace 2015;17:725–31. [DOI] [PubMed] [Google Scholar]
- 35. Ciconte G, De Asmundis C, Sieira J, Conte G, Di Giovanni G, Mugnai G et al. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm 2015;12:673–80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The consent forms, as approved by the local ethics committee (Ethikkommission Nordwest- und Zentralschweiz), do not allow the data to be made publicly available. The data will be shared on reasonable request to the corresponding author.