ABSTRACT
CONTEXT:
There are no reports in the literature of massive deep venous thrombosis (DVT) associated with cisplatin, bleomycin and etoposide (BEP) cancer treatment.
CASE REPORT:
The patient was a 18-year-old adolescent with a nonseminomatous germ cell tumor of the right testicle, with the presence of pulmonary, liver, and massive retroperitoneal metastases. Following radical orchiectomy, the patient started chemotherapy according to the BEP protocol (without routine prophylaxis for DVT). On day 4 of the first cycle, massive DVT was diagnosed, extending from both popliteal veins up to the thoracic segment of the inferior vena cava. Thrombolytic therapy with streptokinase was immediately started. On day 2 of thrombolytic therapy, the patient developed acute renal failure, due to extension of the thrombosis to the renal veins. Streptokinase was continued for six days and the outcome was remarkably favorable.
KEY WORDS: Venous thrombosis, Testicular neoplasms, Chemotherapy, Thrombolytic agents, Pulmonary embolism
RESUMO
CONTEXTO:
Não há relatos na literatura de trombose venosa profunda (TVP) extensa associada ao protocolo de quimioterapia cisplatina, bleomicina e etoposite (BEP).
RELATO DO CASO:
O paciente era um adolescente de 18 anos com um tumor germinativo não-seminomatoso no testículo direito, com metástases pulmonares, hepáticas e retroperitoneais. Após orquiectomia radical, o paciente começou a receber quimioterapia de acordo com o protocolo BEP (sem profilaxia rotineira para TVP). No quarto dia do ciclo, TVP massiva foi diagnosticada, estendendo-se das veias poplíteas até o segmento inferior da veia cava torácica. Tratamento trombolítico foi iniciado imediatamente com estreptoquinase. No segundo dia da terapia trombolítica, o paciente desenvolveu insuficiência renal aguda, devido ao acometimento das veias renais pela trombose. Estroptoquinase foi mantida por seis dias e o paciente teve evolução surpreendentemente favorável.
PALAVRAS-CHAVE: Trombose venosa, Câncer do testículo, Quimioterapia, Fibrinolíticos, Embolia pulmonar
INTRODUCTION
Malignant diseases have been associated with a number of vascular phenomena that involve thromboembolism. Such events may result in considerable morbidity, impaired quality of life and, in some instances, may be life-threatening. This is a particularly relevant problem in some malignant diseases such as germ cell tumors (GCT), in which cure or long-term survival is expected for most patients.
The association between anticancer chemotherapy and thromboembolic phenomena was first reported among patients with breast cancer treated with the combination of cyclophosphamide, methotrexate, 5-fluorouracil and tamoxifen (CMF/tam) and among patients with head and neck cancer treated with cisplatin and bleomycin.1-3 Since then, similar events have been reported with a variety of anticancer agents and regimens.4-14 In GCT, local factors may also contribute towards the increased incidence of vascular complications observed in this disease.5,15
We report on the case of a patient with testicular GCT who developed massive deep venous thrombosis (DVT) during chemotherapy using the bleomycin, cisplatin and etoposide (BEP) regimen. This patient had a favorable outcome with the proposed treatment, namely thrombolytic therapy with streptokinase.
CASE REPORT
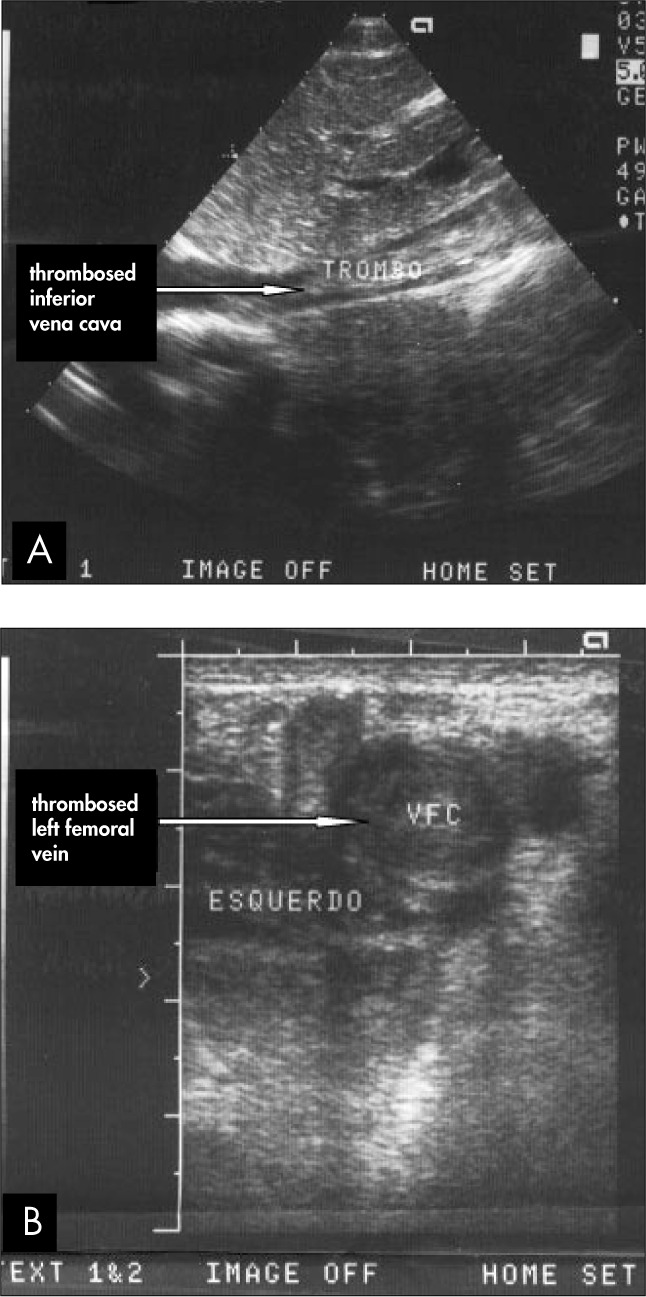
The patient was 18 years old, and had been diagnosed with nonseminomatous GCT of the right testicle, of mixed histology. The computed tomography (CT) scan performed for staging revealed pulmonary and hepatic metastases, as well as massive involvement of retroperitoneal lymph nodes (causing some extrinsic compression of the inferior vena cava and renal veins). High tumor marker levels were found: lactate dehydrogenase (LDH) of 1023 mU/ml; human chorionic gonadotropin β-subunit (βhCG) of 45050 mU/ml; and alpha-fetoprotein (AFP) of 3077 ng/ml (pT3N3M1BS2, AJCC stage IIIC). Radical orchiectomy was performed and eight days postoperatively the patient started chemotherapy according to the BEP protocol (cisplatin 20 mg/m2 intravenously on days 1-5; bleomycin 30 UI intravenously on days 1, 8 and 15; and etoposide 100 mg/m2 intravenously on days 1-5). On day 4 of the first cycle, the patient developed bilateral leg pain and edema. Massive DVT was diagnosed by Doppler ultrasound (Figure 1), extending from both popliteal veins up to the thoracic segment of the inferior vena cava, close to the entrance to the right atrium. Thrombolytic therapy with streptokinase was immediately started (250,000 units intravenously over one hour as a loading dose, followed by 100,000 u/h as a continuous intravenous infusion). On day 2 of thrombolytic therapy, the patient developed acute renal failure, due to extension of the thrombosis to the renal veins (as demonstrated by Doppler echography). Hemodialysis was started, and on day 3 of thrombolytic therapy the patient showed signs of improvement. The recovery of renal function was correlated with improvement in blood flow in the renal veins and inferior vena cava. Streptokinase was stopped on day 6 of thrombolytic therapy.
Figure 1. Doppler ultrasound in a 18-yearold cancer patient showing: A) massive thrombosis of the inferior vena cava; B) massive thrombosis of the left femoral vein.
The patient's symptoms, particularly pain and edema, continued to improve and follow-up Doppler echography performed on days 14 and 60 showed continued venous reperfusion. The patient continued to receive anticoagulation with warfarin (target international normalized ratio [INR] 2-3) until the end of the chemotherapy. Four cycles were given in total, which resulted in a complete clinical and biological response.
DISCUSSION
The pathogenesis of thromboembolic phenomena in cancer patients is probably multifactorial, involving both local factors (endothelial lesions) and systemic factors (coagulation abnormalities). Oberhoff et al. demonstrated the induction of hypercoagulability by means of cyclophosphamide, methotrexate and 5-fluorouracil (CMF) in a group of patients receiving adjuvant chemotherapy for breast cancer.16 Similar data has been reported by other authors.17 Cisplatin is known to induce vasospasm, which may lead to vascular abnormalities such as Raynaud's phenomenon and systemic hypertension or, less commonly, angina, myocardial infarction, mesenteric ischemia, limb ischemia and cerebrovascular accidents.1,5-7,9 However, thrombosis remains the most common cause of such ischemic events. Venous thrombosis is typical, but cases of arterial thromboembolism have also been reported.8,18-20
The increased incidence of vascular complications observed in GCT is an intriguing finding, as these patients tend to be young and fit. Cases of secondary Raynaud's phenomenon, systemic hypertension and thromboembolism were initially reported with the PVB regimen (cisplatin, vinblastine and bleomycin),4,6,8-10 and similar events have also been associated with the BEP regimen.1,20,21 Increased long-term incidence of cardiovascular complications has also been reported in a group of cisplatin-treated GCT patients. The suggested causes were se condary metabolic and hormonal changes, such as hypercholesterolemia, hypertriglyceridemia, obesity and high levels of follicle stimulating hormone (FSH) and luteinizing hormone (LH).22 In a single institutional experience, the incidence of major thromboembolic complications in a group of 179 patients receiving first-line chemotherapy for GCT was as high as 8.4%. Of these, 16.5% were arterial events and 83.3% DVT, with 11 cases of pulmonary thromboembolism and one death. The same authors also suggested that high-dose corti costeroids, presence of liver metastases and an tiemetic therapy were potential risk factors.23
Nevertheless, a number of other predisposing factors may be present in cancer patients. In testicular cancer, for instance, local factors such as retroperitoneal lymph node metastases may cause vascular compression and stasis.15,24 Vascular invasion may result in endothelial damage.25 Even βhCG, which is a GCT marker, has already been suggested as another systemic factor predisposing to thrombosis.4 Recent surgery may sometimes be an additional risk factor. Although at least some of these factors may have contributed towards the development of DVT in our case, there was a clear temporal relationship between the occurrence of the event and the administration of chemotherapy.
In the setting of extensive DVT, early institution of appropriate antithrombotic therapy may prevent complications such as pulmonary thromboembolism and post-thrombotic syndrome.26 In such cases, heparin remains the standard treatment, although there is mounting evidence in the literature in favor of thrombolytic therapy, followed by anticoagulation.12,27-34 There have also been reports of patients with DVT who were successfully treated with tissue plasminogen activator as an alternative to streptokinase, although most of the time this agent has been infused locoregionally (catheter-directed) instead of systemically.35 The placement of a vena cava filter above the level of the thrombosis may occasionally be considered, although in view of the high complication rates observed with most of these devices, their use has most often been reserved for patients experiencing recurrent pulmonary thromboembolism despite optimal anticoagulation.36 In selected cases, novel surgical techniques such as percutaneous thrombectomy may be life-saving.37 In the case of our patient, excellent results were obtained through the administration of thrombolytic therapy with streptokinase, given as an attack dose and followed by continuous, prolonged infusion.
It is worth noting that, in a recent American consensus on the optimal use of anti-thrombotic therapy, the panel supported the use of full doses of low molecular weight heparin (LMWH) or unfractionated heparin as the initial treatment (Grade 1A). The panel also recommended against the routine use of intravenous thrombolytic treatment (Grade 1A), catheter-directed thrombolysis (Grade 1C) or venous thrombectomy (Grade 1C), but also acknowledged that selected patients (such as those with massive ileofemoral DVT and at risk of limb gangrene secondary to venous occlusion) could potentially benefit from the use of intravenous thrombolysis (Grade 2C), catheter-directed thrombolysis (Grade 1C) or venous thrombectomy (Grade 1C).38 Cases of massive DVT, such as the present case, were not specifically addressed by the panel, but it may be assumed they should be managed similarly to patients requiring limb-saving procedures. In view of the extent of the thrombosis, our patient was not suitable for locoregional approaches such as catheter-directed thrombolysis or venous thrombectomy.
In terms of the optimal duration of antithrombotic treatment, the current practice is to keep patients on full doses of LMWH until the end of the chemotherapy. Thereafter, decisions have to be taken on an individual basis. The American panel specifically recommended that cancer patients be kept on anticoagulation with LMWH for an additional 3-6 months after an episode of DVT.38 Patients who achieve cancer remission can be considered for discontinuation, while most others should remain on anticoagulation indefinitely.
CONCLUSION
Many factors may contribute towards the development of DVT in patients with GCT. Cisplatin-based chemotherapy itself should be viewed as a highly effective but potentially thrombogenic treatment. We would point out that simple preventive measures such as prophylactic heparin may be life-saving and should be routinely considered for these patients.
Biographies
Max Senna Mano, MD. Cliniques Universitaires Saint-Luc, Brussels, Belgium.
José Luiz Miranda Guimarães, MD. Hospital Nossa Senhora da Conceição, Porto Alegre, Rio Grande do Sul, Brazil.
Christian Sandor Svend Chicata Sutmöller, MD. Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil.
Sören Franz Marian Chicata Sutmöller, MD. Santa Casa de Misericórdia de Porto Alegre, Porto Alegre, Rio Grande do Sul, Brazil.
Angelo Di Leo, MD. Sandro Pitigliani Medical Oncology Unit, Hospital of Prato, Italy.
Footnotes
Sources of funding: None
Hospital Nossa Senhora da Conceição, Porto Alegre, Rio Grande do Sul, Brazil
REFERENCES
- 1.Doll DC, Yarbro JW. Vascular toxicity associated with antineoplastic agents. Semin Oncol. 1992;19(5):580–596. [PubMed] [Google Scholar]
- 2.Letai A, Kuter DJ. Cancer, coagulation, and anticoagulation. Oncologist. 1999;4(6):443–449. [PubMed] [Google Scholar]
- 3.Sugden EM, Griffiths CL, Greywoode GI, Lewis SM. Acute abdomen during adjuvant chemotherapy: superior mesenteric artery thrombosis associated with CMF chemotherapy. Clin Oncol (R Coll Radiol) 2001;13(6):441–443. doi: 10.1053/clon.2001.9309. [DOI] [PubMed] [Google Scholar]
- 4.Lepidini G, Biancari F, D'Andrea V. Severe thrombosis after chemotherapy for metastatic choriocarcinoma of the testis maintaining complete remission for a long period. Scand J Urol Nephrol. 1997;31(2):221–222. doi: 10.3109/00365599709070337. [DOI] [PubMed] [Google Scholar]
- 5.Icli F, Karaoguz H, Dincol D, et al. Severe vascular toxicity associated with cisplatin-based chemotherapy. Cancer. 1993;72(2):587–593. doi: 10.1002/1097-0142(19930715)72:2<587::aid-cncr2820720242>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Berger CC, Bokemeyer C, Schneider M, Kuczyk MA, Schmoll HJ. Secondary Raynaud's phenomenon and other late vascular complications following chemotherapy for testicular cancer. Eur J Cancer. 1995;31A(13-14):2229–2238. doi: 10.1016/0959-8049(95)00460-2. [DOI] [PubMed] [Google Scholar]
- 7.Czaykowski PM, Moore MJ, Tannock IF. High risk of vascular events in patients with urothelial transitional cell carcinoma treated with cisplatin based chemotherapy. J Urol. 1998;160(6 Pt 1):2021–2024. doi: 10.1097/00005392-199812010-00022. [DOI] [PubMed] [Google Scholar]
- 8.Gerl A. Vascular toxicity associated with chemotherapy for testicular cancer. Anticancer Drugs. 1994;5(6):607–614. doi: 10.1097/00001813-199412000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Stefenelli T, Kuzmits R, Ulrich W, Glogar D. Acute vascular toxicity after combination chemotherapy with cisplatin, vinblastine, and bleomycin for testicular cancer. Eur Heart J. 1988;9(5):552–556. doi: 10.1093/oxfordjournals.eurheartj.a062542. [DOI] [PubMed] [Google Scholar]
- 10.Samuels BL, Vogelzang NJ, Kennedy BJ. Severe vascular toxicity associated with vinblastine, bleomycin, and cisplatin chemotherapy. Cancer Chemother Pharmacol. 1987;19(3):253–256. doi: 10.1007/BF00252982. [DOI] [PubMed] [Google Scholar]
- 11.Cantwell BM, Mannix KA, Roberts JT, Ghani SE, Harris AL. Thromboembolic events during combination chemotherapy for germ cell-malignancy. Lancet. 1988;2(8619):1086–1087. doi: 10.1016/s0140-6736(88)90113-4. [DOI] [PubMed] [Google Scholar]
- 12.Hall MR, Richards MA, Harper PG. Thromboembolic events during combination chemotherapy for germ cell malignancy. Lancet. 1988;2(8622):1259–1259. doi: 10.1016/s0140-6736(88)90858-6. [DOI] [PubMed] [Google Scholar]
- 13.Lederman GS, Garnick MB. Pulmonary emboli as a complication of germ cell cancer treatment. J Urol. 1987;137(6):1236–1237. doi: 10.1016/s0022-5347(17)44466-1. [DOI] [PubMed] [Google Scholar]
- 14.Doll DC, Ringenberg QS, Yarbro JW. Vascular toxicity associated with antineoplastic agents. J Clin Oncol. 1986;4(9):1405–1417. doi: 10.1200/JCO.1986.4.9.1405. [DOI] [PubMed] [Google Scholar]
- 15.Stockler M, Raghavan D. Neoplastic venous involvement and pulmonary embolism in patients with germ cell tumors. Cancer. 1991;68(12):2633–2636. doi: 10.1002/1097-0142(19911215)68:12<2633::aid-cncr2820681221>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Oberhoff C, Winkler UH, Tauchert AM, Schindler AE. Adjuvante CMF-Chemotherapie bei Patientinnen mit Mammakarzinom––Auswirkungen auf Blutgerinnung und Fibrinolyse. [Adjuvant CMF chemotherapy in patients with breast cancer––results on blood coagulation and fibrinolysis] Zentralbl Gynakol. 1997;119(5):211–217. [PubMed] [Google Scholar]
- 17.Taher A, Shamsseddine A, Saghir N, et al. Acquired protein C deficiency following cisplatinum-navelbine administration for locally advanced breast cancer. Case report. Eur J Gynaecol Oncol. 1999;20(4):323–324. [PubMed] [Google Scholar]
- 18.Vos AH, Splinter TA, van der Heul C. Arterial occlusive events during chemotherapy for germ cell cancer. Neth J Med. 2001;59(6):295–299. doi: 10.1016/s0300-2977(01)00173-5. [DOI] [PubMed] [Google Scholar]
- 19.Bachmeyer C, Joly H, Jorest R. Early myocardial infarction during chemotherapy for testicular cancer. Tumori. 2000;86(5):428–430. doi: 10.1177/030089160008600513. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt J, Zenut M, De Marquilly F, Maciejewski C, Plagne R, Lavarenne J. Thrombose artérielle chez un patient traité par chimiothérapie (Protocole BEP) pour un dysgerminome extra-gonadique. [Arterial thrombosis in a patient after chemotherapy (BEP protocol) for extra-gonadal dysgerminoma] Therapie. 1995;50(5):476–478. [PubMed] [Google Scholar]
- 21.Yamada K, Yamashiro S, Itoyama Y, Goto S, Uemura S, Ushio Y. [Sinus thrombosis during CDDP and VP-16 (PE) therapy for suprasellar germ-cell tumor: case report] No Shinkei Geka. 1993;21(11):1025–1029. [PubMed] [Google Scholar]
- 22.Strumberg D, Brügge S, Korn MW, et al. Evaluation of long-term toxicity in patients after cisplatin-based chemotherapy for non-seminomatous testicular cancer. Ann Oncol. 2002;13(2):229–236. doi: 10.1093/annonc/mdf058. [DOI] [PubMed] [Google Scholar]
- 23.Weijl NI, Rutten MF, Zwinderman AH, et al. Thromboembolic events during chemotherapy for germ cell cancer: a cohort study and review of the literature. J Clin Oncol. 2000;18(10):2169–2178. doi: 10.1200/JCO.2000.18.10.2169. [DOI] [PubMed] [Google Scholar]
- 24.Shlebak AA, Smith DB. Incidence of objectively diagnosed thromboembolic disease in cancer patients undergoing cytotoxic chemotherapy and/or hormonal therapy. Cancer Chemother Pharmacol. 1997;39(5):462–466. doi: 10.1007/s002800050599. [DOI] [PubMed] [Google Scholar]
- 25.Dhami MS, Bona RD. Thrombosis in patients with cancer. Postgrad Med. 1993;93(8):131–133. 137–140. doi: 10.1080/00325481.1993.11701721. [DOI] [PubMed] [Google Scholar]
- 26.Marder VJ. Thrombolytic therapy: overview of results in major vascular occlusions. Thromb Haemost. 1995;74(1):101–105. [PubMed] [Google Scholar]
- 27.Doll DC, List AF, Greco FA, Hainsworth JD, Hande KR, Johnson DH. Acute vascular ischemic events after cisplatinbased combination chemotherapy for germ-cell tumors of the testis. Ann Intern Med. 1986;105(1):48–51. doi: 10.7326/0003-4819-105-1-48. [DOI] [PubMed] [Google Scholar]
- 28.Orlando L, Colleoni M, Nole F, et al. Incidence of venous thromboembolism in breast cancer patients during chemotherapy with vinorelbine, cisplatin, 5-fluorouracil as continuous infusion (ViFuP regimen): is prophylaxis required? Ann Oncol. 2000;11(1):117–118. doi: 10.1023/a:1008364801718. [DOI] [PubMed] [Google Scholar]
- 29.Ng CM, Rivera JO. Meta-analysis of streptokinase and heparin in deep vein thrombosis. Am J Health Syst Pharm. 1998;55(19):1995–2001. doi: 10.1093/ajhp/55.19.1995. [DOI] [PubMed] [Google Scholar]
- 30.Gallus AS. Thrombolytic therapy for venous thrombosis and pulmonary embolism. Baillieres Clin Haematol. 1998;11(3):663–673. doi: 10.1016/s0950-3536(98)80088-7. [DOI] [PubMed] [Google Scholar]
- 31.Albuquerque LC, Silveira F, Zago AJ, Bettio J, Petracco JB. Terapia trombolítica em trombose venosa profunda. Experiência clínica inicial. [Thrombolytic therapy in deep venous thrombosis. Initial clinical experience] Arq Bras Cardiol. 1997;68(2):125–128. [PubMed] [Google Scholar]
- 32.Weidmann B, Jansen W, Franzen B, Tauchert M. Lysetherapie bei tiefen Beinvenenthrombosen. [Fibrinolytic therapy of deep vein thombosis] Med Klin (Munich) 1999;94(3):140–149. doi: 10.1007/BF03044844. [DOI] [PubMed] [Google Scholar]
- 33.Rühlmann C, Engelmann L, Scheel H, Siegemund A, Biesold M. Thrombolyse einer ausgedehnten Venenthrombose der unteren Körperhälfte bei Anomalie der Vena cava inferior. [Thrombolysis of an extensive venous thrombosis of the lower body in an anomaly of the vena cava inferior] Dtsch Med Wochenschr. 1996;121(5):124–128. doi: 10.1055/s-2008-1042982. [DOI] [PubMed] [Google Scholar]
- 34.Comerota AJ, Katz ML, White JV. Thrombolytic therapy for acute deep venous thrombosis: how much is enough? Cardiovasc Surg. 1996;4(1):101–104. doi: 10.1016/0967-2109(96)83794-x. [DOI] [PubMed] [Google Scholar]
- 35.Sillesen H, Just S, Jorgensen M, Baekgaard N. Catheter directed thrombolysis for treatment of ilio-femoral deep venous thrombosis is durable, preserves venous valve function and may prevent chronic venous insufficiency. Eur J Vasc Endovasc Surg. 2005;30(5):556–562. doi: 10.1016/j.ejvs.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 36.Chiou AC, Biggs KL, Matsumura JS. Vena cava filters: why, when, what, how? Perspect Vasc Surg Endovasc Ther. 2005;17(4):329–339. doi: 10.1177/153100350501700407. [DOI] [PubMed] [Google Scholar]
- 37.Koga F, Yamada T, Ishimaru H, Sadaoka SI, Mizuo T. Deep vein thrombosis during chemotherapy in a patient with advanced testicular cancer: successful percutaneous thrombectomy under temporary placement of retrievable inferior vena cava filter. Int J Urol. 2001;8(2):90–93. doi: 10.1046/j.1442-2042.2001.00256.x. [DOI] [PubMed] [Google Scholar]
- 38.Büller HR, Agnelli G, Hull RD, Hyers TM, Prins MH, Raskob GE. Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):401S–428S. doi: 10.1378/chest.126.3_suppl.401S. [DOI] [PubMed] [Google Scholar]


