Keywords: aging, Human Connectome Project, lifespan, subcortex
Abstract
We assessed changes in gray matter volume (GMV) of nine subcortical regions (accumbens, amygdala, brainstem, caudate, cerebellar cortex, pallidum, putamen, thalamus, and ventral diencephalon) across the lifespan in a large sample of participants in the Human Connectome Project (n = 2,458, 5–90 yr old, 1,113 males and 1,345 females). 3T MRI data were acquired using a harmonized protocol and were processed in an identical way for all brains. GMVs of individual regions were adjusted for estimated total intracranial volume and regressed against age. We found highly statistically significant changes in GMV with age (P < 0.001) that were distinct among areas and mostly consistent between sexes, as follows. 1) The GMVs of accumbens, caudate, putamen, and cerebellum decreased with age in a linear fashion. The rate of decrease was steeper in males than in females for all regions. 2) The GMVs of the amygdala, pallidum, thalamus, ventral diencephalon, and brainstem changed with age in a quadratic fashion, i.e., increasing first and decreasing afterward. The estimated age at the peak (vertex) of the parabola was 51.8 yr for the brainstem and 28.0–37.9 yr for the other regions. The peak occurred earlier in males than in females, by an average of 8 yr, with the exception of the brainstem, where the age at the peak was very similar in both sexes. These results confirm previous findings and offer new insights into region-specific age-related changes in subcortical brain GMVs.
NEW & NOTEWORTHY We report mixed effects of age on subcortical grey matter volume (GMV) during lifespan (n = 2458, 5-90 yr old, 1113 male, 1345 female). Striatal and cerebellar GMVs decreased linearly with age, more steeply in males. In contrast, GMVs of the amygdala, pallidum, thalamus, ventral diencephalon, and brainstem changed in a quadratic fashion, increasing first and decreasing afterward, with males peaking earlier than females in all regions but the brainstem where they peaked at nearly the same time.
Listen to this article’s corresponding podcast at https://jneurophysiol.podbean.com/e/jnp-micro-podcasts-aging-and-subcortical-gray-matter-volume/.
INTRODUCTION
Several studies have reported changes in the grey matter volumes (GMVs) of subcortical regions, combining and analyzing data from different studies (1–12). Sample sizes in different studies are moderate; and hardware (magnets), data acquisition protocols, and data processing pipelines typically differ across studies. Although some basic findings have been consistent (e.g., reports of both linear and nonlinear changes with age), comprehensive data from methodologically homogeneous (hardware and software) studies with large sample sizes are lacking. Similarly lacking are data from large samples of male and female brains. Such data have been recently provided by the Human Connectome Project (HCP) (13–15). These data were acquired from a large number of participants (n = 2,458) of ages 5–90 yr, comprising similarly large numbers of male (n = 1,113) and female (n = 1,345) participants using similar Siemens 3T MRI hardware, a harmonized data acquisition protocol, and the same processing pipeline. To our knowledge, this is the first dataset of this magnitude, homogeneity, and sex representation to date. Here, we report on the analysis of changes with age in gray matter GMVs of nine subcortical regions and their comparison between males and females.
MATERIALS AND METHODS
Participants
We analyzed HCP data from 2,458 healthy participants for whom age was given (1,113 male and 1,345 female, age range 5–90 yr), comprising data from HCP-Development (HCP-D; 13), HCP-Young Adult (HCP-YA; 14), and HCP-Aging (HCP-A; 15); all data are publicly available from www.humanconnectome.org. Healthy participants were selected to represent the “typical” population reflecting the ethnic and racial composition of the U.S. and diverse socioeconomic status. Exclusion criteria included premature birth, lifetime history of serious medical or endocrine conditions, neurodevelopmental disorders, and clinical diagnoses of psychiatric and neurological disorders. All participants aged 18 yr and above provided written informed consent. For children under the age of 18 yr, a parent or a legal guardian provided informed, written permission for their child to participate in the study. The research and development committee of the Minneapolis Veterans Affairs Medical Center approved the analysis of these data.
MRI Data Acquisition
For HCP-D and HCP-A projects, a standard Siemens 3T Prisma whole body scanner with an 80 mT/m gradient coil was used (16). The 32-channel head coil enables high acceleration factors via multislice acquisitions. For 5–7 yr old participants, the Ceresensa pediatric 32-channel head coil was used. This advanced scanning technology allows whole brain imaging with a submillimeter resolution of structural MRI. Acquired structural images are T1-weighted (T1w) multiecho MPRAGE and T2-weighted (T2w) SPACE with a volumetric navigator for prospective motion correction. Both structural scans use a sagittal field of view (FOV) 256 × 240 × 166 mm and 0.8 mm isotropic voxels. Other parameters of acquisitions are: for T1w scan, TE = 1.8/3.6/5.4/7.2 ms multiecho, TR/TI = 2,500/1,000, flip angle = 8°; for T2w scan, TR/TE = 3,200/564 ms.
For HCP-YA, the T1w and T2w structural images were acquired using the HCP’s custom 3T Siemens Skyra with a 32-channel head coil (17). The T1w image was acquired using the three-dimensional (3-D) MPRAGE sequence with 0.7-mm isotropic resolution (FOV = 224 mm, matrix = 320, 256 sagittal slices), TR = 2,400 ms, TE = 2.14 ms, TI = 1,000 ms, FA = 8, echo spacing = 7.6 ms. The T2w image was acquired using the variable flip angle turbo spin-echo sequence (Siemens SPACE) with 0.7 mm isotropic resolution (same matrix, FOV, and slices as in the T1w), TR = 3,200 ms, TE = 565 ms.
MRI Data
The HCP structural pipeline processed the high-resolution anatomical T1w and T2w images in three consequent steps: PreFreeSurfer, FreeSurfer, and PostFreeSurfer. It is important that all three datasets be processed identically. The available data through the HCP structural pipeline used FreeSurfer 6.0 for the HCP-D and HCP-A datasets but used FreeSurfer 5.3.0 (17) for the HCP-YA set. To achieve uniformity, we reanalyzed the HCP-YA unprocessed data with the same HCP structural pipeline used for HCP-D and HCP-A using FreeSurfer 6.0.
In addition, single acquisitions of T1w and T2w images of group HCP-YA were used as input, as was done for HCP-D and HCP-A datasets.
The GMV of the brainstem was extracted and the right and left GMVs of cerebellar cortices, nucleus accumbens, amygdala, caudate, pallidum, putamen, thalamus proper, and ventral diencephalon were extracted and averaged.
Statistical Analyses
Standard statistical methods were used to analyze the data, including analysis of covariance (ANCOVA), linear and quadratic regression, t test, etc.
GMVs
To correct for variation in estimated total intracranial volume (eTIV), we computed the fraction of the GMV of individual regions over eTIV and expressed it as a percentage:
| (1) |
Since prior studies have reported nonlinear changes of subcortical GMVs with age (1, 8, 9), we analyzed the relation between GMV and age using linear and quadratic regression to choose the appropriate model for further analyses.
Linear Regression
The linear regression equation was:
| (2) |
where y denotes the year and a and b are regression coefficients. The estimated percent change of per year was:
| (3) |
Quadratic Regression
The quadratic equation was:
| (4) |
where y denotes the year and d, e, and f are regression coefficients. The estimated location (year) of the vertex of the parabola was:
| (5) |
MRI data processing was performed using MATLAB (v. R2016) and statistical analyses using the IBM-SPSS statistical package (v. 27). All P-values reported are two-sided.
RESULTS
The percent of variance explained by linear and quadratic fits for each region is shown in Table 1. It can be seen that for four regions (accumbens, putamen, caudate, and cerebellum) quadratic fits offered a very small improvement over the linear fit (<2%), whereas for the remaining five regions (brainstem, amygdala, pallidum, thalamus, and ventral diencephalon) the quadratic fit offered a substantial improvement (45–300%). Therefore, we analyzed the data for these two groups of regions separately, using linear and quadratic fits.
Table 1.
Percent of variance explained by linear and quadratic fits
| Region | Linear | Quadratic | Quadratic Improvement Over Linear 100 % |
|---|---|---|---|
| Accumbens | 0.405 | 0.405 | 0.0 |
| Caudate | 0.105 | 0.107 | 1.9 |
| Putamen | 0.251 | 0.254 | 1.2 |
| Cerebellum | 0.089 | 0.090 | 1.1 |
| Brainstem | 0.030 | 0.090 | 200.0 |
| Amygdala | 0.180 | 0.334 | 85.5 |
| Pallidum | 0.030 | 0.121 | 303.3 |
| Thalamus | 0.224 | 0.326 | 45.5 |
| Ventral diencephalon | 0.120 | 0.253 | 110.8 |
P < 0.001 for all regression fits; bold type indicates a better fit. See text for details.
Quadratic Dependence of GMV on Age
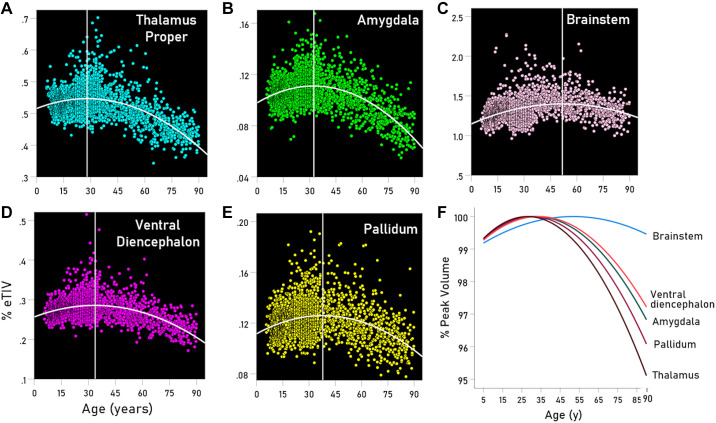
As mentioned earlier, the GMVs of the brainstem, amygdala, pallidum, thalamus, and ventral diencephalon showed a quadratic relation to age, such that GMVs increased up to a certain age (up to the vertex of the quadratic function) and decreased afterward. This is shown in the scatterplots of Fig. 1, A–E and is illustrated in Fig. 1F. The regression fits were highly significant (P < 0.001) for all regions and both sexes. The year (V) corresponding to the vertex (peak of the quadratic function) is given in Table 2 and is indicated by a vertical white line in the scatterplots of Fig. 1, A–E. It is shown as a region-coded colored line in Fig. 1 and summarized for the two sexes in Fig. 2F. We found the following. 1) For the brainstem, the peak occurred late, at 51.8 yr, close to the middle of the lifespan, and it was very similar for males and females (Fig. 2F). 2) For the thalamus, the peak occurred early, at 28 yr, and much earlier in males (at 18.7 yr) than in females (at 31.3 yr) (Table 2, Fig. 2F). 3) For the remaining regions (amygdala, pallidum, and ventral diencephalon) the peak occurred during the third decade but consistently earlier in males than in females (Table 2, Fig. 2F). 4) These results indicate that, with the exception of the brainstem, the first, GMV-increasing period lasts 27.7 yr on the average, whereas the GMV-decreasing period lasts 90–27.7 = 57.3 yr, i.e., twice as long.
Figure 1.
A–E: scatterplots of volume vs. age (years) and fitted quadratic functions for the regions indicated. F: fitted quadratic functions color-coded for the regions indicated and normalized for the function maximum at the parabola peak. See text for details.
Table 2.
Volume changes during aging
| Linear Group | ||||
|---|---|---|---|---|
| %, n = 2,458 | %, n = 1,113 | %, n = 1,345 | ||
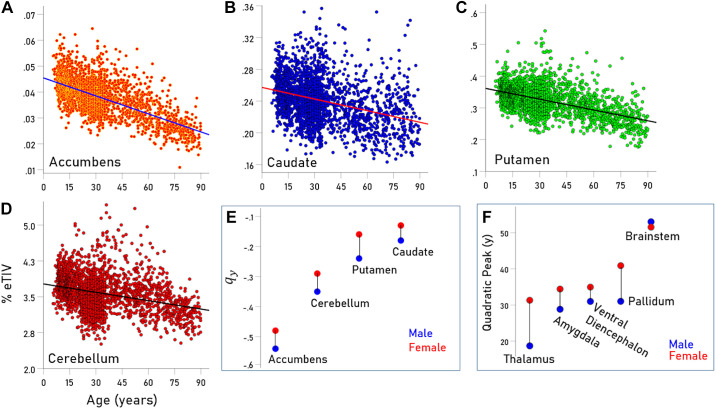
| Accumbens | −0.510 | −0.543 | −0.483 | −0.060 |
| Caudate | −0.190 | −0.183 | −0.134 | −0.049 |
| Cerebellum | −0.155 | −0.347 | −0.288 | −0.059 |
| Putamen | −0.314 | −0.235 | −0.156 | −0.080 |
| Quadratic Group | ||||
|---|---|---|---|---|
| Region | V, yr, n = 2,458 | , n = 1,113 | , n = 1,345 | |
| Amygdala | 32.4 | 28.8 | 34.4 | −5.6 |
| Brainstem | 51.8 | 53.0 | 51.5 | 1.5 |
| Pallidum | 37.9 | 31.0 | 40.9 | −9.9 |
| Thalamus | 28.0 | 18.7 | 31.3 | −12.6 |
| Ventral diencephalon | 32.7 | 31.0 | 35.0 | −4.0 |
Top: linear percent volume change per year for males (M) and females (F). P < 0.001 for all male and female volume vs. age regressions. Bottom: estimated age (year) V at the vertex of the quadratic function (Eq. 5). , estimates for males and females, respectively.
Figure 2.
A–D: scatterplots of volume vs. age (years) and fitted linear functions for the regions indicated. E: rates of volume decrease for males and females for the regions indicated. F: year (V) at peak volume for males and females and regions indicated. See text for details.
Linear Dependence of GMV on Age
The GMVs of accumbens, caudate, putamen, and cerebellum decreased with age in a linear and highly statistically significant fashion (Table 2 and scatterplots in Fig. 2, A–D; P < 0.001 for all regressions). The percent GMV reduction per year was highest for accumbens, followed by putamen, caudate, and cerebellum and was consistently and statistically significantly higher in males than in females (Fig. 2E and Table 2; mean difference ± SE: −0.06175 ± 0.00626, P = 0.002, paired t test).
DISCUSSION
Our results are in keeping with those of other studies (1–12) documenting linear and nonlinear changes of subcortical GMVs with age across the lifespan, depending on the region. The present study was based on HCP MRI data acquired and preprocessed with the harmonized protocol, thus eliminating variability stemming from differences in such protocols, a potential issue shared by studies combining and/or analyzing data collected by various MRI systems and preprocessed in various ways. The homogeneity of data acquisition and preprocessing, combined with the same participant recruitment protocol and the large sample size (total and for both sexes) yielded three clear-cut results, as follows. First, the striatal regions (accumbens, caudate, and putamen) and cerebellar cortex showed a linear decrease of GMV with age ranging from −0.510% per year for accumbens to −0.155% per year for cerebellum (Table 2). Second, all the other regions (thalamus, amygdala, pallidum, ventral diencephalon, and brainstem) showed a quadratic relation (parabola concave downward), i.e., an increase in GMV in younger ages, followed by a decrease later. The first period lasted ∼ 50 yr for the brainstem and ranged from 28 to 38 yr for the rest, depending on the region (Table 2). Third, females showed less steep GMV decrease rates for all regions with linear fits (Fig. 2E) and later quadratic peaks, except for the brainstem (Fig. 2F). We discuss these results briefly.
The study by Bethlehem et al. (1) has provided the most comprehensive account of brain GMV trajectories over the lifespan, based on >100 primary studies. The plot of total subcortical GMV (Fig. 1 in Ref. 1) shows a single peak at 14.4 yr, and that of cortical GMV at 5.9 yr. The data are plotted against a logarithmic time scale and, hence, do not permit evaluation of fit for a quadratic or other function, nor has curve-fitting been described in the paper. Although overall lifespan GMV trajectories are useful, they come at the price of losing information about individual areas that commonly have different GMV trajectories, as evidenced by the results of this and previous studies (4, 18, 19).
With respect to linear GMV lifespan trajectories, all striatal regions (accumbens, caudate, and putamen) showed such trajectories, reflecting comparable GMV decreases in the cortex, which provides massive input to striatum. GMV decrease was steepest in the accumbens, in accord with its major inputs coming from the anterior cingulate and orbitofrontal cortex, areas with similarly steep volume decreases (18). It is possible that striatal changes are secondary to the cortical ones, mediated by anterograde neuronal degeneration, a hypothesis that remains to be investigated. Interestingly, the accumbens GMV trajectory during early childhood shows a ∩-shaped peak at ∼4 yr (20), which suggests that our result (starting from 5 yr onward) could be a continuation of GMV decrease that started earlier, at ∼4 yr; neither caudate nor putamen GMVs showed such a ∩-shaped pattern. Finally, among the remaining subcortical regions, the cerebellum was the only one with a linear volume decrease, as has been reported previously (10, 21).
In contrast to the linear striatal and cerebellar GMV decreases, the thalamus proper and regions connected with it (amygdala, pallidum, and ventral diencephalon) showed a quadratic pattern of GMV trajectory peaking around the 3rd decade (Fig. 1F). The first, ascending leg of the quadratic function lasted ∼25 yr, whereas the second, descending leg lasted ∼50–60 yr, with the result of a net reduction in GMV at the last decade. Remarkably, the ascending leg was very similar for all four regions, whereas the steepness of the second leg differed systematically among regions, with thalamus proper showing the steepest and ventral diencephalon the most shallow GMV decline (thalamus > pallidum > amygdala > ventral diencephalon) (Fig. 1F). It is not clear which are the mechanism(s) underlying this quadratic pattern and its variation among regions. With respect to cortical (linear) GMV decreases, we have hypothesized that they are partly due to low-grade neuroinflammatory processes (18, 19, 22, 23), possibly caused by intermittent reactivation of latent viruses (e.g., human herpes family) (19). Support for this hypothesis was provided by the finding that the Human Leukocyte Antigen (HLA) allele DRB1*13:02 protected against cortical and subcortical atrophy in healthy women (22). Given the known action of HLA molecules in adaptive immunity against viral and other foreign insults, this result suggested that the effective elimination of foreign insults by HLA was the mechanism involved. It is unclear whether such a mechanism may also underlie the decrease in subcortical GMVs observed in the second leg of the quadratic function, as suggested by the protection against subcortical atrophy afforded by HLA DRB1*13:02 (22). It is noteworthy that the neuroinflammatory response of brain regions to systemic administration of lipopolysaccharides (as measured by the activation of microglia) was very similar for cortical and subcortical regions (24), suggesting that the different profiles of GMV decreases [e.g., between accumbens (linear) and thalamus (quadratic)] cannot be explained by the same nonspecific inflammatory response. At face value, the “infectious cause” hypothesis suggests that the detrimental effects of such insults appear earlier in cortical areas and later in subcortical areas, essentially causing the GMV declines following the early cortical peak and late subcortical peak. Since these effects are cumulative and local inflammatory (microglia) responses are comparable across cortical and subcortical regions (24), the different cortical versus subcortical trajectories imply the involvement of the following possible mechanisms, alone or in combination, namely, 1) differential exposure of cortical and (quadratic) subcortical regions, due, e.g., to the differential tropism of insults (e.g., viruses being preferentially localized in cortical areas), 2) differential vulnerability of various regions to the insults due, e.g., to the differential vulnerability of specific neurons, variation in blood supply, etc., and 3) possible anterograde degeneration effects, such as those postulated about the cortico-striatal projection.
Finally, among the five regions with quadratic trajectories, the brainstem stands out as being the only one that ended up with a larger GMV (Fig. 1F). The brainstem comprises the midbrain, the pontine nuclei, and medulla oblongata. Apparently, those regions are resistant to long-term damage, the mechanism(s) of which remain to be investigated.
Limitations
This study used a cross-sectional sample, whereas a longitudinal sample would have been the better choice.
DATA AVAILABILITY
This paper is based on the three Human Connectome Project datasets. The HCP-Development 2.0 Release data used in this report came from https://doi.org/10.15154/1520708. HCP-Young Adult data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657). The HCP-Aging 2.0 Release data used in this report came from https://doi.org/10.15154/1520707.
GRANTS
The study was supported by the University of Minnesota (Kunin Chair in Women’s Healthy Brain Aging and McKnight Presidential Chair in Cognitive Neuroscience); the U.S. Department of Veterans Affairs; the National Institute of Mental Health of the National Institutes of Health (Award U01MH109589); the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657) funded by the 16 NIH Institutes and Centers that support the NIH Blueprint for Neuroscience Research; the National Institute On Aging of the National Institutes of Health under Award Number U01AG052564, and by funds provided by the McDonnell Center for Systems Neuroscience (Washington University, St. Louis, MO).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
DISCLAIMERS
The sponsors had no role in the study design, analysis, interpretation, or writing this paper. The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
AUTHOR CONTRIBUTIONS
P.C. and A.P.G. conceived and designed research; P.C. and A.P.G. analyzed data; P.C. and A.P.G. interpreted results of experiments; A.P.G. prepared figures; P.C. and A.P.G. drafted manuscript; P.C. and A.P.G. edited and revised manuscript; P.C. and A.P.G. approved final version of manuscript.
REFERENCES
- 1. Bethlehem RAI, Seidlitz J, White SR, Vogel JW, Anderson KM, Adamson C, et al. Brain charts for the human lifespan. Nature 604: 525–533, 2022. doi: 10.1038/s41586-022-04554-yr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coupé P, Catheline G, Lanuza E, Manjón JV; Alzheimer’s Disease Neuroimaging Initiative. Towards a unified analysis of brain maturation and aging across the entire lifespan: a MRI analysis. Hum Brain Mapp 38: 5501–5518, 2017. doi: 10.1002/hbm.23743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dima D, Modabbernia A, Papachristou E, Doucet GE, Agartz I, Aghajani M, et al. Subcortical Volumes Across the Lifespan: Data from 18,605 Healthy Individuals Aged 3–90 Years. Hum Brain Mapp 43: 452–469, 2022. doi: 10.1002/hbm.25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, Raz N, Holland D, Dale AM, Walhovd KB; Alzheimer Disease Neuroimaging Initiative. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging 34: 2239–2247, 2013. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goodro M, Sameti M, Patenaude B, Fein G. Age effect on subcortical structures in healthy adults. Psychiatry Res 203: 38–45, 2012. doi: 10.1016/j.pscychresns.2011.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22: 581–594, 2001. doi: 10.1016/S0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 7. MacDonald ME, Pike GB. MRI of healthy brain aging: a review. NMR Biomed 34: e4564, 2021. doi: 10.1002/nbm.4564. [DOI] [PubMed] [Google Scholar]
- 8. Pomponio R, Erus G, Habes M, Doshi J, Srinivasan D, Mamourian E, et al. Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. NeuroImage 208: 116450, 2020. doi: 10.1016/j.neuroimage.2019.116450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Potvin O, Mouiha A, Dieumegarde L, Duchesne S, Alzheimer's Disease Neuroimaging Initiative. Normative data for subcortical regional volumes over the lifetime of the adult human brain. NeuroImage 137: 9–20, 2016. [Erratum in NeuroImage 183: 994–995, 2018]. doi: 10.1016/j.neuroimage.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 10. Romero JE, Coupe P, Lanuza E, Catheline G, Manjón JV; Alzheimer's Disease Neuroimaging Initiative. Toward a unified analysis of cerebellum maturation and aging across the entire lifespan: a MRI analysis. Hum Brain Mapp 42: 1287–1303, 2021. doi: 10.1002/hbm.25293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Serbruyns L, Leunissen I, Huysmans T, Cuypers K, Meesen RL, van Ruitenbeek P, Sijbers J, Swinnen SP. Subcortical volumetric changes across the adult lifespan: subregional thalamic atrophy accounts for age-related sensorimotor performance declines. Cortex 65: 128–138, 2015. doi: 10.1016/j.cortex.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 12. Wang Y, Xu Q, Luo J, Hu M, Zuo C. Effects of age and sex on subcortical volumes. Front Aging Neurosci 11: 259, 2019. doi: 10.3389/fnagi.2019.00259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Somerville LH, Bookheimer SY, Buckner RL, Burgess GC, Curtiss SW, Dapretto M, Elam JS, Gaffrey MS, Harms MP, Hodge C, Kandala S, Kastman EK, Nichols TE, Schlaggar BL, Smith SM, Thomas KM, Yacoub E, Van Essen DC, Barch DM. The Lifespan Human Connectome Project in Development: a large-scale study of brain connectivity development in 5-21 year olds. NeuroImage 183: 456–468, 2018. doi: 10.1016/j.neuroimage.2018.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van Essen DC, Smith SM, Barch DM, Behrens TEJ, Yacoub E, Ugurbil K; WU-Minn HCP Consortium. The WU-Minn Human Connectome Project: an overview. NeuroImage 80: 62–79, 2013. doi: 10.1016/j.neuroimage.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bookheimer SY, Salat DH, Terpstra M, Ances BM, Barch DM, Buckner RL, Burgess GC, Curtiss SW, Diaz-Santos M, Elam JS, Fischl B, Greve DN, Hagy HA, Harms MP, Hatch OM, Hedden T, Hodge C, Japardi KC, Kuhn TP, Ly TK, Smith SM, Somerville LH, Uğurbil K, van der Kouwe A, Van Essen D, Woods RP, Yacoub E. The Lifespan Human Connectome Project in Aging: an overview. NeuroImage 185: 335–348, 2019. doi: 10.1016/j.neuroimage.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harms MP, Somerville LH, Ances BM, Andersson J, Barch DM, Bastiani M, et al. Extending the Human Connectome Project across ages: Imaging protocols for the Lifespan Development and Aging projects. NeuroImage 183: 972–984, 2018. doi: 10.1016/j.neuroimage.2018.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JR, Van Essen DC, Jenkinson M; WU-Minn HCP Consortium. The minimal preprocessing pipelines for the Human Connectome Project. NeuroImage 80: 105–124, 2013. doi: 10.1016/j.neuroimage.2013.04.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Christova P, Georgopoulos AP. Differential reduction of gray matter volume with age in 35 cortical areas in men (more) and women (less). J Neurophysiol 129: 894–899, 2023. doi: 10.1152/jn.00066.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Christova P, Georgopoulos AP. Changes of cortical gray matter volume during development: a Human Connectome Project study. J Neurophysiol 130: 117–122, 2023. doi: 10.1152/jn.00164.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang H, Li J, Su X, Hu Y, Liu T, Ni S, Li H, Zuo X-N, Fu J, Yuan T-F, Yang Z. Growth charts of brain morphometry for preschool children. NeuroImage 255: 119178, 2022. doi: 10.1016/j.neuroimage.2022.119178. [DOI] [PubMed] [Google Scholar]
- 21. Tabatabaei-Jafari H, Walsh E, Shaw ME, Cherbuin N; Alzheimer's Disease Neuroimaging Initiative (ADNI). The cerebellum shrinks faster than normal ageing in Alzheimer’s disease but not in mild cognitive impairment. Hum Brain Mapp 38: 3141–3150, 2017. doi: 10.1002/hbm.23580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. James LM, Christova P, Lewis SM, Engdahl BE, Georgopoulos A, Georgopoulos AP. Protective effect of human leukocyte antigen (HLA) allele DRB1*13:02 on age-related brain gray matter volume reduction in healthy women. eBioMedicine 29: 31–37, 2018. doi: 10.1016/j.ebiom.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James LM, Christova P, Georgopoulos AP. BOLD turnover in task-free state: variation among brain areas and effects of age and human leukocyte antigen (HLA) DRB1*13. Exp Brain Res 240: 1967–1977, 2022. doi: 10.1007/s00221-022-06382-yr. [DOI] [PubMed] [Google Scholar]
- 24. Jung H, Lee H, Kim D, Cheong E, Hyun Y-M, Yu J-W, Um JW. Differential regional vulnerability of the brain to mild neuroinflammation induced by systemic LPS treatment in mice. J Inflamm Res 15: 3053–3063, 2022. doi: 10.2147/JIR.S362006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This paper is based on the three Human Connectome Project datasets. The HCP-Development 2.0 Release data used in this report came from https://doi.org/10.15154/1520708. HCP-Young Adult data were provided by the Human Connectome Project, WU-Minn Consortium (Principal Investigators: David Van Essen and Kamil Ugurbil; 1U54MH091657). The HCP-Aging 2.0 Release data used in this report came from https://doi.org/10.15154/1520707.