Abstract
Malaria continues to be a significant burden, particularly in Africa, which accounts for 95% of malaria deaths worldwide. Despite advances in malaria treatments, malaria eradication is hampered by insecticide and antimalarial drug resistance. Consequently, the need to discover new antimalarial lead compounds remains urgent. To help address this need, we evaluated the antiplasmodial activity of twenty-two amides and thioamides with pyridine cores and their non-pyridine analogues. Twelve of these compounds showed in vitro anti-proliferative activity against the intraerythrocytic stage of Plasmodium falciparum, the most virulent species of Plasmodium infecting humans. Thiopicolinamide 13i was found to possess submicromolar activity (IC50 = 142 nM) and was >88-fold less active against a human cell line. The compound was equally effective against chloroquine-sensitive and -resistant parasites and did not inhibit β-hematin formation, pH regulation or PfATP4. Compound 13i may therefore possess a novel mechanism of action.
Keywords: Antiplasmodial, Malaria, Pyridine, Amides, Thioamides, P. falciparum
Graphical abstract

Highlights
-
•
Synthesis of 22 compounds including pyridine carboxamides, thioamides and their non-pyridine analogues.
-
•
Synthesized compounds were evaluated for antiplasmodial activity.
-
•
Salt formation and reduction of the pyridine ring increased antiplasmodial activity.
-
•
Thiopicolinamide 13i displayed potent antiplasmodial activity and low toxicity.
-
•
The mechanism of action of 13i is not due to inhibition of hemozoin formation.
1. Introduction
In humans, malaria is caused by parasites of the genus Plasmodium namely P. falciparum, P. vivax, P. malariae, P. knowlesi, P. ovale curtisi and P. ovale wallikeri (Singh et al., 2004; Sutherland et al., 2010). This infectious disease, transmitted by female Anopheles mosquitoes, was responsible for 249 million deaths and 608,000 deaths worldwide in 2022 (WHO, 2023). Africa accounted for 94% of the malaria cases in 2022 with pregnant women and children under five years being the most impacted by the disease (WHO, 2023). P. falciparum, the predominant parasite in this region, is responsible for most of the deaths from malaria (WHO, 2023). Currently, the cornerstone of antimalarial chemotherapy are artemisinin-based combination therapies (ACTs), in which a derivative of artemisinin (1) is paired with a longer-acting antimalarial drug. The spread of parasite resistance to ACTs in the Southeast Asian Western Cambodia region, nonetheless, highlights the continued need to discover and develop antimalarial medicines (Noedl et al., 2008). This is critical in the fight against malaria, which is being undermined both by parasite resistance to antimalarial drugs and mosquito resistance to insecticides (Ranson and Lissenden, 2016). Therefore, new drug leads, preferably with mechanisms of action different from existing drugs, are needed for possible use in combination therapy.
Quinine (2), mefloquine (3) and chloroquine (4) are antimalarials used clinically that contain a quinoline ring (see Fig. 1 for structures). Quinine (2), originally isolated from the bark of Cinchona ledgeriana and Cinchona succirubra, was first shown to possess antimalarial activity and was later modified to create mefloquine (3) and chloroquine (4) (Cragg and Newman, 2013; Christensen, 2015; Jones et al., 2015). Quinine (2), mefloquine (3) and chloroquine (4) kill P. falciparum via inhibition of hemozoin formation (Sullivan et al., 1996).
Fig. 1.
Antimalarial agents: Artemisinin (1), Quinine (2), Mefloquine (3), Chloroquine (4), Methylene blue (5), Proguanil (6), Halofantrine (7), Arterolane (8), 2-acetylpyridine-4-phenyl-3-thiosemicarbazone (9). Quinoline, amide, thioamide and pyridine moieties are shown in red.
The formation of hemozoin permits the malaria parasite to avoid the toxicity of the free heme produced as a consequence of the digestion of host cell hemoglobin (Pandey et al., 2003). Despite the effectiveness of chloroquine and its success as an antimalarial drug, it has become largely useless against P. falciparum as a result of parasite resistance (Duraisingh and Cowman, 2005).
Methylene blue (5), which has a benzothiazine core, also possesses antiplasmodial activity (Vennerstrom et al., 1995; Ehrhardt et al., 2013). Its mechanism of action involves both inhibition of P. falciparum glutathione reductase and, like the quinolines, inhibition of hemozoin formation (Vennerstrom et al., 1995; Färber et al., 1998). Inhibition of hemozoin formation and glutathione reductase results in increased oxidative stress (Becker et al., 2004; Percário et al., 2012). A nitrogen acyclic core is present in antimalarial drugs such as proguanil (6) and halofantrine (7) (Greenwood, 1995). Both continue to be used clinically. Proguanil (6) is effective for both prophylaxis and treatment while halofantrine (7) is used for treatment only (Radloff et al., 1996; McKeage and Scott, 2003). However, the use of both drugs is under threat with the emergence of resistant strains (Høgh et al., 2000). The preparation of new antimalarial agents bearing a quinoline core remains a feasible strategy for the development of new antimalarial compounds for use in combination therapy amidst the widespread quinoline resistance. This is because the process by which the parasite forms hemozoin is presumably unchanged in quinoline-resistant parasites (Wicht et al., 2016). Non-quinoline benzamides have also been shown to inhibit β-hematin (synthetic hemozoin) formation (Wicht et al., 2016). Recently, arterolane (8), which contains the trioxolane skeleton and amide functionality (Fig. 1), has been shown to possess excellent activity against the erythrocytic stages of P. falciparum and P. vivax (Fontaine et al., 2015; Toure et al., 2016). Furthermore, 2-acetylpyridine-4-phenyl-3-thiosemicarbazone (9), which bears a pyridine core, has shown antiplasmodial activity (Marella et al., 2015). Wicht et al. (2016) also reported that electron-deficient and pyridyl-containing amides display antiplasmodial activity. Given the prevalence of the quinoline/pyridine and amide/thioamide moieties in antiplasmodial compounds, we hypothesized that the series of pyridine carboxamides and thio derivatives generated in our lab while exploring electronic and steric effects of picolinamides and related systems on thionations using Lawesson's reagent, could be expanded and evaluated as potential antiplasmodial agents. The objective of this study was therefore to synthesize a series of pyridine carboxamides as well as their thiocarboxamides and to assess their antiplasmodial activity against P. falciparum.
2. Methods
2.1. General information for drug synthesis
Nuclear magnetic resonance spectra were obtained using Bruker Avance 200 and 500 MHz instruments. Unless otherwise stated, the spectra were obtained in CDCl3 and resonances are reported in δ units downfield from tetramethylsilane (TMS), which was used as an internal standard; J values are given in Hz. Multiplicities are described as follows: s = singlet, d = doublet, dd = doublet of doublets, ddd = doublet of doublet of doublets, t = triplet, q = quartet, m = multiplet. FT-IR spectra were obtained on a Vector 22 or Bruker Tensor 37 with Pike Technology MIRacle single reflection ATR instruments. Column chromatography was carried out using silica gel as an adsorbent. Elemental analyses were done at the University of the West Indies, Mona on a PE 2400 CHNS/O Analyzer or on a Thermo Flash EA112 CHN Analyzer at MEDAC Ltd., Surrey, United Kingdom. HR-MS analyses were carried out on a Waters LCT Premier (Es-Tof)/Acquiring i-Class instrument at MEDAC Ltd., Surrey, United Kingdom. Melting points (uncorrected) were determined on a Gallenkamp instrument. Microwave reactions were carried out using an Anton Paar Synthos 3000 reaction system. Specific details about the synthesis of compounds 12a-l and 13a-l are available in the supporting information.
2.2. General considerations for in vitro assays
All compounds were dissolved in DMSO to a concentration of 200 mM, except for compounds 12a, 12d, 12i and 13c, which exhibited lower solubility in DMSO. These compounds were dissolved in DMSO to concentrations between 10 and 100 mM. All compounds were subsequently diluted in culture medium (in some cases after a further dilution in DMSO), with the final concentration of DMSO present in assays never exceeding 0.1% (v/v).
2.3. Cell culture
Synchronous intraerythrocytic stage P. falciparum parasites (strains 3D7 and Dd2) and human foreskin fibroblast (HFF) cells were maintained in vitro as described previously (Jacot et al., 2014; Guan et al., 2021). Briefly, the P. falciparum parasites were maintained within O+ human erythrocytes, suspended in HEPES- and Glutamax™-supplemented RPMI 1640 (Thermo Fisher Scientific, order number 72400120) to which 11 mM D-glucose, 200 μM hypoxanthine, 24 mg/L gentamicin and 0.6% (w/v) Albumax II were added. The HFF cells were maintained in DMEM (Sigma-Aldrich, order number D5638) to which 10% (v/v) bovine calf serum, 50 units/mL penicillin, 50 mg/L streptomycin, 10 mg/L gentamicin, 0.2 mM L-glutamine, and 0.25 mg/L amphotericin B was added.
2.4. In vitro antiplasmodial assays
In vitro antiplasmodial activity against P. falciparum was assessed over 96 h using the malaria SYBR Green I-based fluorescence assay described by Smilkstein et al. (2004) with previously described modifications (Spry et al., 2013). Assays were initiated with parasites in the ring stage at a parasitemia and hematocrit of 0.5 and 1%. Infected erythrocytes incubated with 0.25 μM chloroquine served as zero proliferation controls. Following the 96 h incubation, assay plates were frozen and stored at −80 °C. Subsequent to thawing, the contents of each well were mixed, and a 100 μL aliquot transferred to a second plate containing 100 μL/well SYBR Safe DNA gel stain in 20 mM Tris, pH 7.5, containing 5 mM ethylenediaminetetraacetic acid (EDTA), 0.008% (w/v) saponin and 0.08% (v/v) Triton X-100. The fluorescence in each well was measured as previously described (Spry et al., 2013). Following subtraction of the average fluorescence measured in the zero proliferation control wells, fluorescence in the test-compound-containing wells was expressed as a percentage of the average background-corrected fluorescence measured in the no-inhibitor control wells. To obtain IC50 values, the processed data were fitted with the equation y = Bottom + (Top - Bottom)/(1+(IC50/x)Hill Slope) using GraphPad Prism 9. Where incomplete inhibition was observed at the highest concentration, the ‘bottom’ was constrained to zero. IC50 values were compared following log10 transformation, by performing a one-way ANOVA with Tukey’s multiple comparisons test.
2.5. In vitro HFF cell proliferation assays
Activity against HFF cells was assessed over a period of four days using a SYBR Safe-based assay, essentially as previously reported (Hoegl et al., 2014; Spry et al., 2020). Briefly, assays were performed in 96-well plates, initiated with HFF cells seeded at 1.5–2.4 × 105 cells/mL. HFF cells incubated with the protein synthesis inhibitor cycloheximide (at 10 μM) served as zero proliferation controls. Plates were incubated at 37 °C in a humidified 5% CO2 incubator. After 4 days, an aliquot of the supernatant (150 μL) was carefully removed from each well and discarded, prior to storing the plates at −80 °C. After thawing, the SYBR Safe lysis solution also used for the antiplasmodial assays (150 μL) was added to each well and mixed via pipetting to ensure the HFF cells were detached from the plate and lysed. The plates were then processed as described for the antiplasmodial assay.
2.6. Detergent mediated β-hematin assays
Activity against β-hematin formation was assessed using an assay reported previously (de Sousa et al., 2020). All reagents and buffers were purchased from Sigma-Aldrich (rebranded to Merck). Briefly, compound stocks were made up in either DMSO or MilliQ™ water to a final concentration of 20 mM. A working solution of 70% water/20% NP-40 substitute (305.5 μM)/10% DMSO v/v was prepared, of which 100 μL was added to columns 1 to 11 of a 96-well plate (Greiner). To column 12, 140 μL water, 40 μL NP-40 substitute (305.5 μM) and 20 μL of test compound stock (2 mM final concentration) were added. A 1:2 serial dilution was performed sequentially across the plate from column 12 to column 2, leaving column 1 as a blank. A 0.22 mM working solution of Fe(III)PPIX (bovine haemin, Sigma) was prepared by adding 178.8 μL of a 25 mM Fe(III)PPIX stock in DMSO to 20 mL of a 2 M acetate buffer (pH 4.75–4.9). Of this, 100 μL was added to each well, the plate sealed and incubated for 5 h at 37 °C. Following incubation, 32 μL of a pyridine working solution (20% water/20% acetone/10% 2 M HEPES buffer (pH 7.4)/50% pyridine v/v) was added to each well followed by 60 μL of acetone. The contents of the wells were resuspended and the absorbance of the resultant bis-pyridyl-heme complex measured at 405 nm using a ThermoScientific MultiskanGo plate reader. The data were analysed using GraphPad Prism 9.
2.7. Cytosolic Na+ and pH measurements
Measurements of [Na+]cyt and pHcyt were performed with trophozoite-stage 3D7 parasites that were isolated from their host erythrocytes via brief exposure to saponin (as described in Qiu et al., 2022) and loaded with the fluorescent dye SBFI ([Na+]cyt measurements) or BCECF (pHcyt measurements). [Na+]cyt and pHcyt measurements were carried out in 96-well plates as described in Dennis et al. (2018) and Qiu et al. (2022), respectively. In both cases, the compounds of interest were added to parasites suspended at 37 °C in a saline consisting of 125 mM NaCl, 5 mM KCl, 1 mM MgCl2, 20 mM glucose and 25 mM HEPES (pH 7.1).
3. Results and discussion
3.1. Synthesis
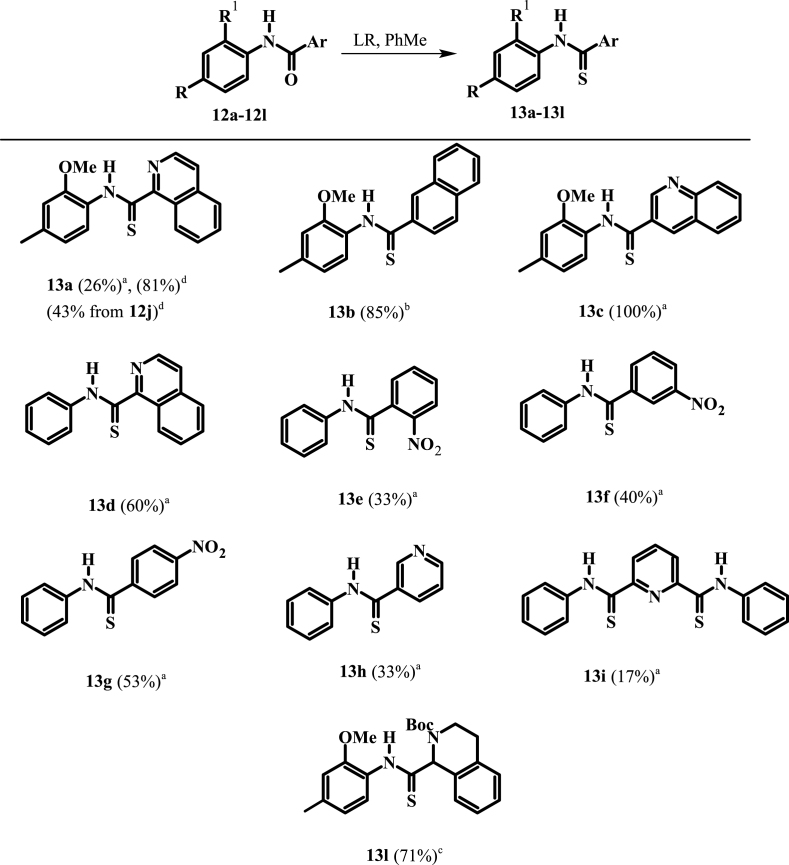
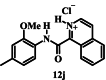
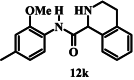
In our study, we chose to synthesize twelve amides (12a-12l), and ten thioamides (13a-13l) including some with pyridine or quinoline rings. Amides 12a-12l were prepared in 32–96% yield from the coupling of 2-methoxy-4-methylaniline (10) and aniline (11) with various acids or acid chlorides (Scheme 1). Salt 12j was prepared in 81% yield from the addition of concentrated hydrochloric acid in tetrahydrofuran (THF) to a cold solution of amide 12a in THF (Scheme 2). On the other hand, amide 12l was prepared in 44% yield over two steps from the reduction of the isoquinoline ring of 12a using 3 mol. equiv. of sodium cyanoborohydride followed by protection using Boc anhydride (Scheme 2).
Scheme 1.
a) EDCI (HOBt), DCM/DMF, rt, 1–6 days; b) (COCl)2, ArCOOH, DMF, 0 °C then amine, DCM, Et3N, rt, 16–18 h; c) Et3N or pyr, DCM/PhMe, ArCOCl, 0 °C then amine rt, 18 h.
Scheme 2.
a) conc. HCl, THF, 12j (81%); b) NaCNBH3, AcOH; c) Boc2O, THF/H2O (1:1), 12l (44% over two steps).
The thionation of amide 12b occurred in 85% yield using only 0.6 mol. equiv. of Lawesson's reagent (LR) (Scheme 3). By contrast, a low yield (26%) was obtained when amide 12a was thionated using excess LR. This implied that the nitrogen of the isoquinoline moiety was either interacting with LR in a side reaction or it was electronically reducing the electron density of the amide functional group. There was a marked increase in the yield to 81% when the reaction was done under microwave conditions using 0.6 mol. equiv. of LR. Surprisingly, only starting material was obtained when we attempted to thionate salt 12j using excess LR, likely owing to the electron deficiency of the isoquinolinium ring. However, 43% of thioamide 13a was obtained when the salt was thionated using 1 mol. equiv. LR under microwave conditions. Thionation of amide 12k using 3 mol. equiv. of LR gave only starting material. This was likely a consequence of the free amine forming adducts with the LR species.
Scheme 3.
Thionation of amides 12a-l using LR. a3 mol. equiv. LR, reflux, PhMe, 24 h; b0.6 mol. equiv. LR, reflux, PhMe, 24 h; c1.2 mol. equiv. LR, reflux, PhMe, 24 h; d0.6-1 mol. equiv. LR, MW, PhMe, 0.5 h.
Thionation of amide 12l was achieved using 1.2 mol. equiv. of LR in refluxing toluene for 24 h. The efficient conversion to thioamide 13l, which has a Boc protecting group installed, suggests that steric hindrance is not a major factor in the reaction (Scheme 3). Thioamides 13c and 13d were obtained in 100 and 60% yields from the respective thionation of their amide precursors using excess (3 mol. equiv.) LR in refluxing toluene for 24 h (Scheme 3). Generally, the yields for thionation of the nitrobenzamides were comparable to those for thionation of amides 12a and 12d. Low yields (17–33%) were obtained when 3-picolinamide 12h and pyridine-2,6-dicarboxamide 12i were thionated using excess LR.
While no evidence was found to support any steric effect from the possible formation of pyridine-LR adduct, the presence of electron withdrawing atoms or groups attached to the carbonyl carbon can reduce the ability of the carbonyl functionality to act as a nucleophile and therefore reduce thionation yields. This effect is most dominant when the standard protocol of 0.6 mol. equiv. of LR is used.
3.2. Antiplasmodial activity
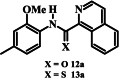
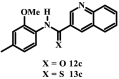
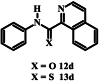
Initially the twenty-two (22) synthesized compounds were tested against intraerythrocytic stage P. falciparum parasites (strain 3D7) at a single concentration of 200 μM, or, where solubility did not permit, at a concentration between 10 and 100 μM. While compounds 12d–12i, 13a, 13d, 13e and 13h showed less than 50% inhibition of parasite proliferation at the single concentration tested, compounds 12a – 12c, 12j – 12l, 13b, 13c, 13f, 13g, 13i and 13l showed greater than 50% inhibition (Fig. S1), and we therefore generated dose-response curves for each to allow determination of the concentration causing 50% inhibition of P. falciparum proliferation (IC50 value; Fig. S2). As shown in Table 1, 2-methoxy-4-methylanilides 12a and 12c which bear isoquinoline and quinoline moieties, respectively, both showed antiplasmodial activity, with IC50 values of 69 and 62 μM, respectively. The corresponding naphthyl analogue (12b), however, showed slightly higher activity (IC50 = 38 μM, p < 0.0079, one-way ANOVA), consistent with the nitrogen-containing heterocycle not being required for activity.
Table 1.
Effect of carboxamides 12a-12l and thioamides 13a-13l on proliferation of intraerythrocytic stage P. falciparum (strain 3D7) in vitro over 96 h.
| Compound | IC50 against P. falciparum (μM)a |
|
|---|---|---|
| X = O | X = S | |
 |
69.2 ± 5.0 | >200 |
 |
37.6 ± 2.1 | 23.3 ± 1.6 |
 |
61.6 ± 1.1 | 61.2 ± 1.6 |
 |
>10 | >50 |
 |
>200 | >200 |
 |
>200 | 109 ± 1 |
 |
>50 | 80.3 ± 6.0 |
 |
>200 | >200 |
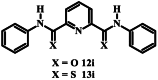 |
>50 | 0.142 ± 0.028 |
 |
136 ± 13 | ND b |
 |
8.81 ± 0.30 | ND |
 |
31.9 ± 1.2 | 172 ± 12 |
Data are averages from two or three independent experiments. IC50 values below the highest concentration tested are presented as mean ± standard error of the mean (SEM, n = 3).
ND, not determined because target compound not obtained.
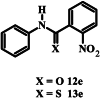
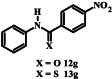
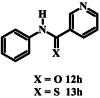
The limited solubility of isoquinoline carboxamide 12d, derived from aniline, made it difficult to assess the importance of the methyl and methoxy substituents on the aniline ring. The more soluble carboxamides without methyl and methoxy substituents and with nitrophenyl (compounds 12e – 12g) and pyridine (compounds 12h and 12i) moieties in place of the isoquinoline moiety also failed to inhibit parasite growth by more than 50% at the highest concentration tested. Although 12f, 12g and 12i were previously tested for blood stage antiplasmodial activity by Wicht et al. (2016) using the NF54 and D10 strains of P. falciparum, they were retested here using the 3D7 strain. This was done to eliminate any strain-specific variations and in order to compare directly their activity with the rest of the compounds in the series which includes quinoline and isoquinoline carbothioamides as well as monopicolinamides.
Introducing a formal positive charge to the nitrogen atom of isoquinoline carboxamide 12a, as in 12j, reduced antiplasmodial activity by about 2-fold (IC50 value of 12j = 136 μM; p = 0.0002, one-way ANOVA), while saturating the nitrogen-containing ring, to generate tetrahydroquinoline 12k, resulted in an ∼ 8-fold increase in activity (IC50 value of 12k = 8.8 μM; p < 0.0001, one-way ANOVA). The corresponding Boc-protected compound (12l, IC50 = 32 µM) also showed improved activity compared with isoquinoline 12a (p < 0.0001, one-way ANOVA) but was less active compared to 12k (p < 0.0001, one-way ANOVA).
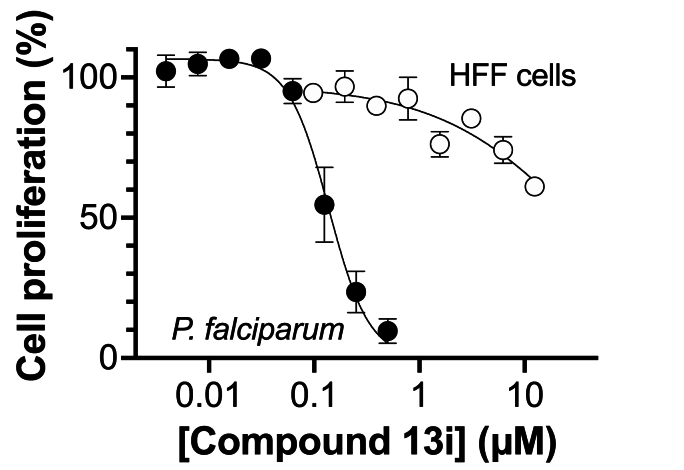
The thiocarboxamide analogue of isoquinoline 12a (compound 13a) did not inhibit proliferation of P. falciparum by >50% at concentrations up to 200 μM. However, the quinoline thiocarboxamide 13c showed similar activity to its carboxamide analogue 12c (IC50 value of compound 13c = 61 μM; p > 0.9999, one-way ANOVA). As for the carboxamides, higher activity was associated with the naphthyl thiocarboxamide derivative (compound 13b, IC50 = 23 μM; p < 0.0001, one-way ANOVA). The Boc-protected tetrahydroquinoline thiocarboxamide (13l) was five-fold less active than its carboxamide equivalent (12l) (p < 0.0001, one-way ANOVA). While the carboxamides without methyl and methoxy substituents lacked activity, three of the corresponding thiocarboxamides showed activity; compounds 13f and 13g, with nitro-substituted phenyl moieties, inhibited parasite growth with IC50 values of 109 and 80 μM, respectively, and pyridine 13i was the most potent of all compounds tested (IC50 = 0.142 μM; p < 0.0001, one-way ANOVA; Fig. 2). The importance of the thiocarboxamide moieties for this activity is demonstrated by compound 12i – the carboxamide analogue - possessing >350-fold lower activity. To assess the selectivity of the potent antiplasmodial effect of compound 13i, the compound was also tested for its activity against human foreskin fibroblast (HFF) cells (Fig. 2), a low-passage number cell line. The compound inhibited proliferation of HFF cells by less than 50% at the highest concentration tested (12.5 μM), corresponding to a selectivity index of >88. To determine whether parasites resistant to chloroquine (4) were cross-resistant to 13i, we tested the compound against Dd2, a chloroquine-resistant strain of P. falciparum (full chloroquine dose-response curves against Dd2 and 3D7 parasite strains are shown in Fig. S3). We found that 13i was equally as effective against Dd2 (IC50 = 0.146 ± 0.018 μM; Fig. S3) as it was against 3D7 (IC50 = 0.142 ± 0.028 μM; p = 0.9, unpaired student's t-test).
Fig. 2.
Effect of thiocarboxamide 13i on the proliferation of intraerythrocytic stage P. falciparum (strain 3D7) and HFF cells in vitro over 96 h. The data are from three independent experiments and error bars represent standard error of the mean (SEM). Where not visible, error bars are smaller than the symbol.
3.3. Mechanism of action studies
In light of the structural similarity between some of our compounds and standard antimalarials known to inhibit parasite proliferation by inhibiting hemozoin formation (Sullivan et al., 1996), we decided to test a selection of the compounds with the highest antiplasmodial activity (12b, 12k, 12l, 13b, 13i) for their ability to inhibit β-hematin formation in a cell-free detergent mediated assay (de Sousa et al., 2020). Whilst chloroquine (included as a control) inhibited β-hematin formation as expected, none of the five compounds tested, including the most potent antiplasmodial derivative identified in this study (13i), had any effect on β-hematin formation (Fig. S4). This suggests that these compounds do not interact with the β-hematin crystal and therefore would be unlikely to interact with hemozoin in the parasite. Given this, the compounds were not taken forward for testing in the cell fractionation assay which can be used to test the ability of a compound to inhibit hemozoin formation within the parasite (Combrinck et al., 2015).
Numerous compounds kill P. falciparum parasites by inhibiting PfATP4 (Rottmann et al., 2010; Spillman et al., 2013; Jiménez-Díaz et al., 2014; Lehane et al., 2014; Vaidya et al., 2014; Flannery et al., 2015; Hewitt et al., 2017; Dennis et al., 2018; Gilson et al., 2019; Ashton et al., 2023), a protein believed to efflux Na+ ions from the parasite cytosol whilst importing H+ equivalents (Spillman et al., 2013). These compounds, which include the clinical candidate cipargamin, formerly known as NITD609 and KAE609 (Rottmann et al., 2010), give rise to a variety of physiological perturbations including an increase in the parasite's cytosolic [Na+] ([Na+]cyt) and pH (pHcyt) (Spillman et al., 2013; Lehane et al., 2014; Vaidya et al., 2014; Hewitt et al., 2017; Dennis et al., 2018). Various other antiplasmodial compounds give rise to a decrease in the parasite's pHcyt. These include inhibitors of the parasite's lactate:H+ transporter PfFNT (e.g. MMV007839 (Golldack et al., 2017; Hapuarachchi et al., 2017)) and V-type H+-ATPase (e.g. concanamycin A (van Schalkwyk et al., 2010)).
We investigated whether 13i had any effect on [Na+]cyt (Fig. 3A and B) or pHcyt (Fig. 3C and D) in isolated trophozoite-stage parasites (3D7 strain). Fig. 3A and C show representative traces from a single Na+ (A) or pH (C) experiment, and Fig. 3B and D show averaged data for the [Na+]cyt (B) and pHcyt (D) reached in the final 5 min of each experiment. Compound 13i had no effect on [Na+]cyt at either of the concentrations tested (1 μM and 10 μM), whereas the PfATP4 inhibitor cipargamin gave rise to a significant increase in [Na+]cyt (Fig. 3A and B), as observed previously (e.g. Lehane et al., 2014). Compound 13i was also without effect on pHcyt (Fig. 3C and D). Consistent with previous results (van Schalkwyk et al., 2010; Lehane et al., 2014; Hapuarachchi et al., 2017), cipargamin gave rise to a small increase in pHcyt while MMV007839 and concanamycin A both gave rise to a significant decrease in pHcyt (Fig. 3C and D). The well-characterised protonophore CCCP also caused a significant decrease in pHcyt, as observed previously (Saliba and Kirk, 2001). Our data therefore suggest that 13i does not kill parasites via inhibition of PfATP4, PfFNT or the V-type H+-ATPase, or by increasing the permeability of membranes to H+. Taken together, although our studies do not establish a mechanism of action for 13i, the fact that it is not one of the frequently encountered mechanisms of inhibition of hemozoin formation or PfATP4 inhibition is an important finding as drugs with novel mechanisms of action are desired.
Fig. 3.
Compound 13i does not affect parasite [Na+]cytor pHcyt. (A,B) Isolated 3D7 parasites loaded with SBFI were exposed to 13i (purple; 1 μM or 10 μM), solvent alone (black; DMSO control), or cipargamin (blue; 50 nM; positive control for PfATP4 inhibition). (C,D) BCECF-loaded 3D7 trophozoites were exposed to 13i (purple; 1 μM or 10 μM), cipargamin (blue; 50 nM; positive control for PfATP4 inhibition), MMV007839 (pink; 2 μM; positive control for PfFNT inhibition), CCCP (grey; 100 nM; protonophore), concanamycin A (green; 100 nM; positive control for V-type H+-ATPase inhibition) or solvent alone (black; DMSO control). Panels A and C show representative traces from a single Na+ (A) or pH (C) experiment. Panels B and D show data averaged from three independent experiments performed on different days in which all compounds/concentrations were tested concurrently. The bars and error bars show the mean + SEM and the symbols show the data from each individual experiment. The data for each compound and concentration were compared to those for the DMSO control using one-way ANOVA with Dunnett’s multiple comparisons test, with the p values shown on the figure. The final [Na+]cyt (B) and pHcyt (D) reached were averaged from the data points obtained 85–90 min and 55–60 min after parasites were first exposed to the compound/solvent, respectively. The DMSO concentration was 0.1% v/v in pH experiments and 0.1–0.2% v/v in Na+ experiments.
Although thiocarboxamide 13i is included in the PubChem database (Kim et al., 2022), the potent antiplasmodial activity we report constitute its first documented biological activity. Given its potency and selectivity, further investigation into its antiplasmodial mechanism of action and structure-activity relationships is warranted. Here we showed that replacement of the two thiocarboxamide moieties with carboxamide groups is not tolerated. Further investigations should focus on preparing analogues of thiocarboxamide 13i for antiplasmodial testing.
In summary, ten new compounds possessing quinoline or isoquinoline scaffolds were synthesized. Additionally, we have identified 13 compounds showing antiplasmodial activity, with two compounds possessing IC50 values below 10 μM, including one with a submicromolar IC50 of 142 nM and greater than 88-fold selectivity over a human cell line. β-Hematin formation was not inhibited in our hands, and as such, 13i may act through a novel mechanism.
CRediT authorship contribution statement
Alexa Redway: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Christina Spry: Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft, Writing – review & editing. Ainka Brown: Conceptualization, Formal analysis, Investigation, Methodology, Validation, Visualization. Ursula Wiedemann: Investigation. Imam Fathoni: Investigation, Writing – review & editing. Larnelle F. Garnie: Investigation, Methodology, Writing – review & editing. Deyun Qiu: Formal analysis, Investigation. Timothy J. Egan: Methodology, Supervision. Adele M. Lehane: Formal analysis, Methodology, Visualization, Writing - review & editing. Yvette Jackson: Conceptualization, Funding acquisition, Project administration, Visualization. Kevin J. Saliba: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing – review & editing. Nadale Downer-Riley: Conceptualization, Data curation, Methodology, Project administration, Resources, Supervision, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgements
We would like to thank the Department of Chemistry and Office of Graduate Studies and Research at the University of the West Indies, Mona for their support. We are grateful to the Canberra Branch of the Australian Red Cross Lifeblood for the provision of red blood cells. Imam Fathoni was supported by a Research Training Program scholarship from the Australian Government. We would also like to thank the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (Award Number 5R01AI143521) for financial support for the consumables and reagents required for the detergent mediated β-hematin inhibition assays. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2024.100536.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- Ashton T.D., Dans M.G., Favuzza P., Ngo A., Lehane A.M., Zhang X., Qiu D., Chandra Maity B., De N., Schindler K.A., Yeo T., Park H., Uhlemann A., Churchyard A., Baum J., Fidock D., Jarman K., Lowes K., Baud D., Brand S., Jackson P., Cowman A., Sleebs B. Optimization of 2,3-dihydroquinazolinone-3-carboxamides as antimalarials targeting PfATP4. J. Med. Chem. 2023;66(5):3540–3565. doi: 10.1021/acs.jmedchem.2c02092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K., Tilley L., Vennerstrom J.L., Roberts D., Rogerson S., Ginsburg H. Oxidative stress in malaria parasite-infected erythrocytes: host–parasite interactions. Int. J. Parasitol. 2004;34(2):163–189. doi: 10.1016/j.ijpara.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Christensen S.B. Drugs and drug leads based on natural products for treatment and prophylaxis of malaria. Evidence-Based Validation of Herbal Medicine. 2015:307–319. doi: 10.1016/B978-0-12-800874-4.00014-3. Elsevier. [DOI] [Google Scholar]
- Combrinck J.M., Fong K.Y., Gibhard L., Smith P.J., Wright D.W., Egan T.J. Optimization of a multi-well colorimetric assay to determine haem species in Plasmodium falciparum in the presence of anti-malarials. Malar. J. 2015;14(1):1–14. doi: 10.1186/s12936-015-0729-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg G.M., Newman D.J. Natural products: a continuing source of novel drug leads. Biochem. Biophys. Acta, Gen. Subj. 2013;1830(6):3670–3695. doi: 10.1016/j.bbagen.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa A.C.C., Combrinck J.M., Maepa K., Egan T.J. Virtual screening as a tool to discover new β-haematin inhibitors with activity against malaria parasites. Sci. Rep. 2020;10(1):1–10. doi: 10.1038/s41598-020-60221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis A.S., Rosling J.E., Lehane A.M., Kirk K. Diverse antimalarials from whole-cell phenotypic screens disrupt malaria parasite ion and volume homeostasis. Sci. Rep. 2018;8(1):8795. doi: 10.1038/s41598-018-26819-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duraisingh M.T., Cowman A.F. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005;94(3):181–190. doi: 10.1016/j.actatropica.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Ehrhardt K., Davioud-Charvet E., Ke H., Vaidya A.B., Lanzer M., Deponte M. The antimalarial activities of methylene blue and the 1,4-naphthoquinone-3-[4-(trifluoromethyl) benzyl]-menadione are not due to inhibition of the mitochondrial electron transport chain. Antimicrob. Agents Chemother. 2013;57(5):2114–2120. doi: 10.1128/AAC.02248-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Färber P., Arscott L., Williams Jr C., Becker K., Schirmer R. Recombinant Plasmodium falciparum glutathione reductase is inhibited by the antimalarial dye methylene blue. FEBS Lett. 1998;422(3):311–314. doi: 10.1016/S0014-5793(98)00031-3. [DOI] [PubMed] [Google Scholar]
- Flannery E.L., McNamara C.W., Kim S.W., Kato T.S., Li F., Teng C.H., Gagaring K., Manary M.J., Barboa R., Meister S., Kuhen K., Vinetz J., Chatterjee A., Winzeler E. Mutations in the P-type cation-transporter ATPase 4, PfATP4, mediate resistance to both aminopyrazole and spiroindolone antimalarials. ACS Chem. Biol. 2015;10(2):413–420. doi: 10.1021/cb500616x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine S.D., Spangler B., Gut J., Lauterwasser E.M., Rosenthal P.J., Renslo A.R. Drug delivery to the malaria parasite using an arterolane‐like scaffold. ChemMedChem. 2015;10(1):47–51. doi: 10.1002/cmdc.201402362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson P.R., Kumarasingha R., Thompson J., Zhang X., Penington J.S., Kalhor R., Bullen H.E., Lehane A.M., Dans M.G., de Koning-Ward T.F., Holien J., da Costa T., Hulett M., Buskes M., Crabb B., Kirk K., Papenfuss A., Cowman A., Abbott B. A 4-cyano-3-methylisoquinoline inhibitor of Plasmodium falciparum growth targets the sodium efflux pump PfATP4. Sci. Rep. 2019;9(1):10292. doi: 10.1038/s41598-019-46500-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golldack A., Henke B., Bergmann B., Wiechert M., Erler H., Blancke Soares A., Spielmann T., Beitz E. Substrate-analogous inhibitors exert antimalarial action by targeting the Plasmodium lactate transporter PfFNT at nanomolar scale. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood D. Conflicts of interest: the genesis of synthetic antimalarial agents in peace and war. J. Antimicrob. Chemother. 1995;36(5):857–872. doi: 10.1093/jac/36.5.857. [DOI] [PubMed] [Google Scholar]
- Guan J., Spry C., Tjhin E.T., Yang P., Kittikool T., Howieson V.M., Ling H., Starrs L., Duncan D., Burgio G., Saliba K.J., Auclair K. Exploring heteroaromatic rings as a replacement for the labile amide of antiplasmodial pantothenamides. J. Med. Chem. 2021;64(8):4478–4497. doi: 10.1021/acs.jmedchem.0c01755. [DOI] [PubMed] [Google Scholar]
- Hapuarachchi S.V., Cobbold S.A., Shafik S.H., Dennis A.S., McConville M.J., Martin R.E., Kirk K., Lehane A.M. The malaria parasite’s lactate transporter PfFNT is the target of antiplasmodial compounds identified in whole cell phenotypic screens. PLoS Pathog. 2017;13(2) doi: 10.1371/journal.ppat.1006180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt S.N., Dranow D.M., Horst B.G., Abendroth J.A., Forte B., Hallyburton I., Jansen C., Baragana B., Choi R., Rivas K.L., Hulverson M., Dumais M., Edwards T., Lorimer D.D., Fairlamb A.H., Gray D.W., Read K.D., Lehane A.M., Kirk K., Myler P.J., Wernimont A., Walpole C., Stacy R., Barrett L., Gilbert I., Van Voorhis Biochemical and structural characterization of selective allosteric inhibitors of the Plasmodium falciparum drug target, prolyl-tRNA-synthetase. ACS Infect. Dis. 2017;3(1):34–44. doi: 10.1021/acsinfecdis.6b00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegl A., Darabi H., Tran E., Awuah E., Kerdo E.S., Habib E., Saliba K.J., Auclair K. Stereochemical modification of geminal dialkyl substituents on pantothenamides alters antimicrobial activity. Bioorg. Med. Chem. Lett. 2014;24(15):3274–3277. doi: 10.1016/j.bmcl.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Høgh B., Clarke P.D., Camus D., Nothdurft H.D., Overbosch D., Günther M., Joubert I., Kain K.C., Shaw D., Roskell N.S., Chulay J. Atovaquone-proguanil versus chloroquine-proguanil for malaria prophylaxis in non-immune travellers: a randomised, double-blind study. Lancet. 2000;356(9245):1888–1894. doi: 10.1016/S0140-6736(00)03260-8. [DOI] [PubMed] [Google Scholar]
- Jacot D., Meissner M., Sheiner L., Soldati-Favre D. In: Toxoplasma gondii: The Model Apicomplexan - Perspectives and Methods. Second ed. Weiss K.K.L.M., editor. Elsevier Academic Press; Burlington: 2014. Genetic Manipulation of Toxoplasma gondii; pp. 577–611. [DOI] [Google Scholar]
- Jiménez-Díaz M.B., Ebert D., Salinas Y., Pradhan A., Lehane A.M., Myrand-Lapierre M.-E., O’Loughlin K.G., Shackleford D.M., Justino de Almeida M., Carrillo A.K., Clark J., Dennis A.S.M., Diep J., Deng X., Duffy S., Endsley A., Fedewa G., Guiguemde W.A., Gómez M.G., Holbrook G., Horst J., Kim C.C., Liu J., Lee M.C.S., Matheny A., Martinez M.S., Miller G., Rodriguez-Alejandre A., Sanz L., Sigal M.S., Spillman N.J., Stein P.D., Wang Z., Zhu F., Waterson D., Knapp S., Shelat A., Avery V.M., Fidock D.A., Gamo F., Charman S.A., Mirsalis J.C., Ma H., Ferrer S., Kirk K., Angulo-Barturen I., Kyle D.E, DeRisi J.L., Floyd D.M., Guy R.K. (+)-SJ733 a clinical candidate for malaria that acts through ATP4 to induce rapid host-mediated clearance of Plasmodium. Proc. Natl. Acad. Sci. USA. 2014;111(50):E5455–E5462. doi: 10.1073/pnas.1414221111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.A., Panda S.S., Hall C.D. Quinine conjugates and quinine analogues as potential antimalarial agents. Eur. J. Med. Chem. 2015;97:335–355. doi: 10.1016/j.ejmech.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Kim S., Cheng T., He S., Thiessen P.A., Li Q., Gindulyte A., Bolton E.E. PubChem protein, gene, pathway, and taxonomy data collections: bridging biology and chemistry through target-centric views of PubChem data. J. Mol. Biol. 2022;167514 doi: 10.1016/j.jmb.2022.167514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehane A.M., Ridgway M.C., Baker E., Kirk K. Diverse chemotypes disrupt ion homeostasis in the malaria parasite. Mol. Microbiol. 2014;94(2):327–339. doi: 10.1111/mmi.12765. [DOI] [PubMed] [Google Scholar]
- Marella A., Shaquiquzzaman M., Akhter M., Verma G., Alam M.M. Novel pyrazole–pyrazoline hybrids endowed with thioamide as antimalarial agents: their synthesis and 3D-QSAR studies. J. Enzym. Inhib. Med. Chem. 2015;30(4):597–606. doi: 10.3109/14756366.2014.958081. [DOI] [PubMed] [Google Scholar]
- McKeage K., Scott L.J. Atovaquone/proguanil. Drugs. 2003;63(6):597–623. doi: 10.2165/00003495-200363060-00006. https://10.2165/00003495-200363060-00006 [DOI] [PubMed] [Google Scholar]
- Noedl H., Se Y., Schaecher K., Smith B.L., Socheat D., Fukuda M.M. Evidence of artemisinin-resistant malaria in western Cambodia. N. Engl. J. Med. 2008;359(24):2619–2620. doi: 10.1056/NEJMc0805011. http://10.1056/NEJMc0805011 [DOI] [PubMed] [Google Scholar]
- Pandey A.V., Babbarwal V.K., Okoyeh J.N., Joshi R.M., Puri S.K., Singh R.L., Chauhan V.S. Hemozoin formation in malaria: a two-step process involving histidine-rich proteins and lipids. Biochem. Biophys. Res. Commun. 2003;308(4):736–743. doi: 10.1016/S0006-291X(03)01465-7. [DOI] [PubMed] [Google Scholar]
- Percário S., Moreira D.R., Gomes B.A., Ferreira M.E., Gonçalves A.C.M., Laurindo P.S., Vilhena T.C., Dolabela M.F., Green M.D. Oxidative stress in malaria. Int. J. Mol. Sci. 2012;13(12):16346–16372. doi: 10.3390/ijms131216346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu D., Pei J.V., Rosling J.E., Thathy V., Li D., Xue Y., Tanner J.D., Penington J.S., Aw Y.T.V., Aw J.Y.H., Xu G., Tripathi A. K, Gnadig N.F., Yeo T., Fairhurst K.J., Stokes B.H., Murithi J.M., Kümpornsin K., Hasemer H., Dennis A.S., Ridgway M.C., Schmitt E.K, Straimer J., Papenfuss A.T., Lee M.C., Corry B., Sinnis P., Fidock D.A., Dooren G.G., Kirk K., Lehane A.M. A G358S mutation in the Plasmodium falciparum Na+ pump PfATP4 confers clinically-relevant resistance to cipargamin. Nat. Commun. 2022;13(1):5746. doi: 10.1038/s41467-022-33403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff P.D., Philips J., Nkeyi M., Kremsner P., Hutchinson D. Atovaquone and proguanil for Plasmodium falciparum malaria. Lancet. 1996;347(9014):1511–1514. doi: 10.1016/S0140-6736(96)90671-6. [DOI] [PubMed] [Google Scholar]
- Ranson H., Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends Parasitol. 2016;32(3):187–196. doi: 10.1016/j.pt.2015.11.010. [DOI] [PubMed] [Google Scholar]
- Rottmann M., McNamara C., Yeung B.K., Lee M.C., Zou B., Russell B., Seitz P., Plouffe D.M., Dharia N.V., Tan J., Cohen S.B., Spencer K.R., González-Páez G.E., Lakshminarayana S.B., Goh A., Suwanarusk R., Jegla T., Schmitt E.K., Beck H., Brun R., Nosten F., Renia L., Dartois V., Keller T.H., Fidock D.A., Winzeler E.A., Diagana T.T. Spiroindolones, a potent compound class for the treatment of malaria. Science. 2010;329(5996):1175–1180. doi: 10.1126/science.1193225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba, K.J., Kirk, K., 2001. H+-coupled pantothenate transport in the intracellular malaria parasite. J. Biol. Chem. 276 (21), 18115–18121. doi: 10.1074/jbc.M010942200. [DOI] [PubMed]
- Singh B., Sung L.K., Matusop A., Radhakrishnan A., Shamsul S.S., Cox-Singh J., Thomas A., Conway D.J. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363(9414):1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- Smilkstein M., Sriwilaijaroen N., Kelly J.X., Wilairat P., Riscoe M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004;48(5):1803–1806. doi: 10.1128/AAC.48.5.1803-1806.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillman N.J., Allen R.J., McNamara C.W., Yeung B.K., Winzeler E.A., Diagana T.T., Kirk K. Na+ regulation in the malaria parasite Plasmodium falciparum involves the cation ATPase PfATP4 and is a target of the spiroindolone antimalarials. Cell Host Microbe. 2013;13(2):227–237. doi: 10.1016/j.chom.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spry C., Barnard L., Kok M., Powell A.K., Mahesh D., Tjhin E.T., Saliba K.J., Strauss E., de Villiers M. Toward a stable and potent coenzyme A-targeting antiplasmodial agent: structure–activity relationship studies of N-Phenethyl-α-methyl-pantothenamide. ACS Infect. Dis. 2020;6(7):1844–1854. doi: 10.1021/acsinfecdis.0c00075. [DOI] [PubMed] [Google Scholar]
- Spry C., Macuamule C., Lin Z., Virga K.G., Lee R.E., Strauss E., Saliba K.J. Pantothenamides are potent, on-target inhibitors of Plasmodium falciparum growth when serum pantetheinase is inactivated. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0054974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan Jr D.J., Gluzman I.Y., Russell D.G., Goldberg D.E. On the molecular mechanism of chloroquine's antimalarial action. Proc. Natl. Acad. Sci. USA. 1996;93(21):11865–11870. doi: 10.1073/pnas.93.21.11865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C.J., Tanomsing N., Nolder D., Oguike M., Jennison C., Pukrittayakamee S., Dolecek C., Hien T.T., do Rosário V.E., Arez A.P., Pinto J., Michon P., Escalante A.A., Nosten F., Burke M., Lee R., Blaze M., Otto T.D., Barnwell J.W., Pain A., Williams J., White N.J., Day N.P., Snounou G., Lockhart P.J., Chiodini P.L., Imwong M., Polley S.D. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 2010;201(10):1544–1550. doi: 10.1086/652240. https://10.1086/652240 [DOI] [PubMed] [Google Scholar]
- Toure, O.A., Valecha, N., Tshefu, A.K., Thompson, R., Krudsood, S., Gaye, O., Rao, B.H.K., Sagara, I., Bose, T.K., Mohanty, S., Rao, B.S., Anvikar, A.R., Mwapasa, V., Noedl, H. Arora, S. Roy, A. Lyer, S.S., Sharma, P., Saha, N., Jalali, R.K., 2016. A phase 3, double-blind, randomized study of arterolane maleate–piperaquine phosphate vs artemether–lumefantrine for falciparum malaria in adolescent and adult patients in Asia and Africa. Clin. Infect. Dis. 62 (8), 964–971. doi: 10.1093/cid/ciw029. [DOI] [PMC free article] [PubMed]
- Vaidya, A.B., Morrisey, J.M., Zhang, Z., Das, S., Daly, T.M., Otto, T.D., Spillman, N.J., Wyvratt, M., Siegl, P., Marfurt, J., Wirijanata, G., Sebayang, B., Price, Ric, P.N., Chatterjee, A., Nagle, A., Stasiak, M., Charman, S., Angulo-Barturen, I., Ferrer, S., Jiménez-Días, M.B., Martínez, S., Gamo, F.J., Avery, V.M., Ruecker, A. Delves, M., Kirk, K., Berriman, M., Kortagere, S., Burrows, J., Fan, E., Bergman, L.W., 2014. Pyrazoleamide compounds are potent antimalarials that target Na+ homeostasis in intraerythrocytic Plasmodium falciparum. Nat. Commun. 5 (1), 5521. doi: 10.1038/ncomms6521. [DOI] [PMC free article] [PubMed]
- van Schalkwyk D.A., Chan X.W., Misiano P., Gagliardi S., Farina C., Saliba K.J. Inhibition of Plasmodium falciparum pH regulation by small molecule indole derivatives results in rapid parasite death. Biochem. Pharmacol. 2010;79(9):1291–1299. doi: 10.1016/j.bcp.2009.12.025. [DOI] [PubMed] [Google Scholar]
- Vennerstrom J.L., Makler M.T., Angerhofer C.K., Williams J.A. Antimalarial dyes revisited: xanthenes, azines, oxazines, and thiazines. Antimicrob. Agents Chemother. 1995;39(12):2671–2677. doi: 10.1128/AAC.39.12.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO World malaria report 2023. 2023. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2023 Retrieved from.
- Wicht K.J., Combrinck J.M., Smith P.J., Hunter R., Egan T.J. Identification and SAR evaluation of hemozoin-inhibiting benzamides active against Plasmodium falciparum. J. Med. Chem. 2016;59(13):6512–6530. doi: 10.1021/acs.jmedchem.6b00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.