Abstract
An increasing number of independent studies indicate that the atypical protein kinase C (PKC) isoforms (aPKCs) are critically involved in the control of cell proliferation and survival. The aPKCs are targets of important lipid mediators such as ceramide and the products of the PI 3-kinase. In addition, the aPKCs have been shown to interact with Ras and with two novel proteins, LIP (lambda-interacting protein; a selective activator of λ/ιPKC) and the product of par-4 (a gene induced during apoptosis), which is an inhibitor of both λ/ιPKC and ζPKC. LIP and Par-4 interact with the zinc finger domain of the aPKCs where the lipid mediators have been shown to bind. Here we report the identification of p62, a previously described phosphotyrosine-independent p56lck SH2-interacting protein, as a molecule that interacts potently with the V1 domain of λ/ιPKC and, albeit with lower affinity, with ζPKC. We also show in this study that ectopically expressed p62 colocalizes perfectly with both λ/ιPKC and ζPKC. Interestingly, the endogenous p62, like the ectopically expressed protein, displays a punctate vesicular pattern and clearly colocalizes with endogenous λ/ιPKC and endogenous ζPKC. P62 colocalizes with Rab7 and partially with lamp-1 and limp-II as well as with the epidermal growth factor (EGF) receptor in activated cells, but not with Rab5 or the transferrin receptor. Of functional relevance, expression of dominant negative λ/ιPKC, but not of the wild-type enzyme, severely impairs the endocytic membrane transport of the EGF receptor with no effect on the transferrin receptor. These findings strongly suggest that the aPKCs are anchored by p62 in the lysosome-targeted endosomal compartment, which seems critical for the control of the growth factor receptor trafficking. This is particularly relevant in light of the role played by the aPKCs in mitogenic cell signaling events.
The generation of lipid second messengers, which target lipid-activated kinases such as the protein kinase C (PKC) family of isozymes (40), is one of the important events during cell signal transduction (18, 24, 29, 32). The different PKC isoforms can be stimulated by distinct lipid mediators. Thus, diacylglycerol is a major cofactor for the classical and novel isotypes (40), whereas ceramide activates the atypical PKC isoforms (aPKCs) (31, 33, 37), which are also stimulated in vitro by PI 3,4,5-P3 (39) and seem to play a critical role downstream of the PI 3-kinase signaling pathway (1, 34, 52). Consistent with this, the aPKCs appear to be involved in a number of important cellular functions. Thus, the inhibition or overexpression of the aPKCs dramatically affects maturation of Xenopus oocytes (14, 15) as well as cell proliferation (4), mitogen-activated protein kinase activation (5, 8, 16, 52, 54), and κB- and AP1-dependent promoter activity (1, 7, 10, 12, 14, 15, 20, 26, 33, 52). Several recent studies have also presented evidence that the aPKCs could be important in other cellular functions such as neuronal (57) and leukemic cell differentiation (55), the maintenance of long-term potentiation (48), interleukin-1β and interleukin-2 signaling (22, 47), α2 integrin gene expression (59, 60), and insulin-activated glucose transport (3). Although critical, ceramide and PI 3,4,5-P3 do not appear to be selective for the aPKCs because they also target other enzymes such as the AKT/PKB kinase, ɛPKC, and ηPKC (in the case of the PI 3-kinase products) (21, 36, 43, 53) or the ceramide-activated protein kinase, Ksr, and the ceramide-activated protein phosphatase (in the case of ceramide) (61, 62). Therefore, the identification of specific modulators of aPKC function and/or subcellular localization should be instrumental in the understanding of their regulation and the role they play in cell signaling. An interesting property of the aPKCs is that they not only bind lipids but can also be targeted by proteins. Thus, we and others have demonstrated that ζPKC, but not αPKC, interacts with Ras (11, 16, 58). Although this is not a peculiarity of ζPKC, because Ras binds to an increasing number of structurally unrelated signaling proteins (28), this observation led us to reason that the existence of substantial differences in the regulatory domain of the different PKC isotypes should permit the isolation of potentially selective protein regulators of the different aPKCs by the two-hybrid system in yeast. Following this experimental approach, we have recently succeeded in identifying two aPKC-interacting proteins: LIP (lambda-interacting protein) and Par-4 (12, 13). LIP is a novel protein that specifically binds to the zinc finger of λ/ιPKC but not to other structurally and functionally related kinases, including ζPKC (12). Interestingly, LIP specifically activates λ/ιPKC but not ζPKC in vitro and in vivo, and its association with λ/ιPKC is dramatically activated following the mitogenic stimulation of quiescent cells (12). Par-4, on the other hand, also binds to the zinc finger of λ/ιPKC, but in contrast to LIP, it interacts with ζPKC and is not an activator but an inhibitor of both aPKCs (13). Par-4 is induced in cells that are committed to undergo apoptosis (50), revealing a novel role for the aPKCs in cell survival (6, 13). Collectively, these results indicate that the selective regulation of the different PKCs could be mediated by protein-protein interactions.
Together with the V3 domain, the V1 region in the regulatory domain of the different PKCs is where the major differences in the amino acid sequence are found (40). Therefore, we carried out a screening by the two-hybrid system, using the V1 region of λ/ιPKC as the bait to isolate potentially novel protein regulators or anchors of λ/ιPKC. Here we report the identification of p62, a previously described phosphotyrosine-independent p56lck SH2-interacting protein (27), as a molecule that interacts potently with λ/ιPKC and, albeit with lower affinity, with ζPKC. While this work was in progress, Puls et al. (44) reported that p62 selectively interacts with ζPKC. These researchers also reported that, in the absence of transfected ζPKC, ectopically expressed p62 “artifactually” forms vesicles called “amorphous aggregates” which do not colocalize with endosomal or lysosomal markers. Puls et al. interpreted these findings as indicating that p62 is a substrate of ζPKC and that the role of ζPKC is to retain p62 in the cytosol, its purported physiological location.
In the present study, we investigated in detail the subcellular location of p62 and its interaction with both aPKCs and found that endogenous as well as ectopically expressed p62 colocalizes with both λ/ιPKC and ζPKC in the lysosome-targeted endosomes. In addition, we demonstrate here that p62 colocalizes with the receptor for the epidermal growth factor (EGF) in activated cells. Of potential functional relevance, expression of a λ/ιPKC dominant negative mutant, but not of the wild-type enzyme, severely impairs the endocytic membrane transport of the EGF receptor with no effect on the transferrin receptor. This study provides further evidence supporting a critical role of the aPKCs in mitogenic signaling and constitutes a significant advance in the understanding of the mechanism of action of these PKC isotypes.
MATERIALS AND METHODS
Two-hybrid screening.
For the yeast two-hybrid screening, pYTH9-λ/ιPKC126 was cotransformed with the human kidney cDNA Matchmaker library in the pGAD10 vector (Clontech Laboratories, Inc.) into the Y190 yeast strain, and the transformants were plated to synthetic medium lacking histidine, leucine, and tryptophan and containing 20 mM 3-amino-1,2,4-triazole. The plates were incubated at 30°C for 5 days. His+ colonies were assayed for β-galactosidase activity by a filter assay, as described below.
β-Galactosidase filter assays.
Yeast strains were patched to synthetic medium lacking leucine and tryptophan, incubated for 3 days at 30°C, and then transferred to a nitrocellulose filter. The filter was placed in aluminum foil atop a sea of liquid nitrogen for 20 s and then immersed in the liquid nitrogen for 1 to 2 s. The filter was allowed to warm to room temperature and then placed on top of Whatman no. 1 paper that had been prewet in Z buffer containing 0.75 mg 5-bromo-4-chloro-3-indolyl-β-d-galactoside per ml. The filters were incubated for 3 h at 30°C. Blue coloration is indicative of β-galactosidase activity.
Plasmids.
pYTH9λ/ιPKC, pYTH9λ/ιPKCREG, pYTH9λ/ιPKC126, pYTH9λ/ιPKCZF, pYTH9λ/ιPKCCAT, pYTH9ζPKC, pYTH9ζPKCREG, pYTH9ζPKC126, pYTH9ζPKCZF, pYTH9αPKCREG, pYTH9ɛPKCREG, pGBT9RafREG, pGBT9Raf, pGBT9RafCAT, pGBT9Mos, pGBT9Lamin, pCDNA3-HA-ζPKC, pCDNA3-HA-λ/ιPKC, pCDNA3-HA-ζPKCMUT, and pCDNA3-HA-λ/ιPKCMUT have previously been described (5, 12, 13). pGAD10-p62, encompassing amino acids 1 to 266 of human p62, was obtained by the two-hybrid screen. An EcoRI/HindIII fragment was excised from pGAD10-p62 and ligated to EcoRI/HindIII-digested pMAL-c2 to obtain pMAL-c2-p621–266. The same fragment was filled in and ligated into the XhoI-filled site of pCDNA3-hemagglutinin (HA) to generate pCDNA3-HA-p621–266. The 3′ end of p62 was isolated by PCR with a human 5′ rapid amplification of cDNA ends-ready cDNA (Clontech Laboratories, Inc.) as the template and the primers 5′-CACGCAGAAGAGGTGGGC-3′ and 5′-CGCTACAAGTGCAGCGTCTG-3′. The conditions of the PCR were as follows: 94°C for 45 s, 60°C for 45 s, and 72°C for 2 min for 30 cycles, with a final extension time of 7 min at 72°C. This PCR product was subcloned into pGEM-T-Easy (Promega). The construct was cut with EcoRI, filled in with Klenow polymerase, and then cut with BamHI. The excised fragment was purified and ligated to pCDNA3-HA-p621–266 previously digested with XbaI, blunt ended, and cut with BamHI to obtain HA-tagged, full-length p62 (pCDNA3-HA-p62). The fragment was also ligated to pMAL-c2-p621–266 cut with HindIII, blunt ended, and digested again with BamHI to make pMAL-c2-p62 (MBP-p62). pCDNA3-myc-p62 was made the same way as pCDNA3-HA-p62. pMAL-c2-LIP(R4), pMAL-c2-par-4, and pMAL-c2-hnRNPA1 have previously been described (12, 13, 38). Expression plasmids for ɛPKC and αPKC were generously provided by F. Überall (2).
p62 interaction studies.
Purified MBP or MBP-p62 (2 μg) was immobilized on amylose beads and incubated with a soluble cell extract (1 mg) of HeLa or 293 cells prepared in lysis buffer (20 mM Tris [pH 7.4], 2 mM EDTA, 2 mM sodium pyrophosphate, 25 mM sodium β-glycerophosphate, 1 mM sodium orthovanadate, 25 mM NaCl, 0.1% Triton X-100, 10% glycerol, 1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, and 10 μg of aprotinin per ml). The binding reaction mixture was incubated at 22°C for 1 h in the absence or presence of phosphatidylserine (50 μg/ml), diacylglycerol (0.8 μg/ml), phorbol myristate acetate (PMA) (10 ng/ml), or C2-ceramide (50 μM). The agarose beads were washed extensively with lysis buffer. Bound maltose binding protein (MBP) fusion proteins and any associated proteins were boiled in sample buffer, fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and immunoblotted with antibodies specific for αPKC, ɛPKC, ζPKC (Life Technologies, Inc.), or λPKC (Transduction Labs). Immune complexes were detected by enhanced chemiluminescence (Amersham).
HeLa or 293 cells were transfected with the CalPhos Maximizer transfection kit (Clontech Laboratories, Inc.) with either control plasmid or an expression vector for HA-tagged p62. Forty hours posttransfection, cell lysates were prepared as described above and immunoprecipitated with an anti-HA monoclonal antibody (12CA5), as described previously (12). In another set of experiments, cells untreated or treated with different stimuli (PMA [10 ng/ml], tumor necrosis factor alpha [50 ng/ml], C2-ceramide [50 μM], or EGF [100 ng/ml]) for different amounts of time (1, 5, 15, and 30 min) were coimmunoprecipitated with an affinity-purified polyclonal anti-p62 antibody or with preimmune serum. Immunoprecipitates were resolved by SDS-PAGE and analyzed by immunoblotting with antibodies specific for αPKC, ɛPKC, ζPKC (Life Technologies, Inc.), or λPKC (Transduction Labs). For p62-EGF receptor coimmunoprecipitation assays, quiescent HeLa cells were stimulated with 100 ng of EGF (Amersham) per ml for different times and cell lysates were immunoprecipitated with an anti-EGFR antibody (151-8 AE4; Developmental Studies Hybroma Bank, University of Iowa), resolved by SDS-PAGE, and analyzed by immunoblotting with an anti-p62 antibody.
Regulation and phosphorylation assays.
Subconfluent cultures of 293 cells in 100-mm-diameter plates were transfected as described above with 20 μg of either pCDNA3-HA, pCDNA3-HAλ/ι-PKC, or pCDNA3-HA-ζPKC. Plasmid DNA was removed 3 h later, and cells were incubated in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum for 16 h. Afterward, the DMEM was replaced for 12 h with medium containing 0.5% fetal calf serum, followed by an additional 12 h with serum-free medium. Cultures were then extracted with lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 1% Triton X-100, 2 mM EDTA, 1 mM EGTA, and protease inhibitors) and immunoprecipitated with 2 μg of anti-HA antibody (12CA5; Boehringer, Mannheim, Germany)/mg of protein extract. Immunoprecipitates were washed seven times with lysis buffer containing 0.5 M NaCl. For in vitro kinase assay, immunocomplexes were incubated with 1 μg of recombinant bacterially produced heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1 [38]) in the presence or absence of 1 μg of either MBP, MBP-LIP(R4), MBP-par-4, or MBP-p62 in the presence or absence of phosphatidylserine as sonicated vesicles (50 μg/ml) and 5 to 10 μCi (100 μM) of [γ-32P]ATP in kinase buffer (35 mM Tris-HCl [pH 7.5], 10 mM MgCl2, 0.5 mM EGTA, 0.1 mM CaCl2, 1 mM phenylphosphate) for 30 min at 30°C in a final volume of 20 μl. Reactions were stopped by the addition of concentrated sample buffer. Samples were boiled for 3 min and separated by SDS-PAGE followed by autoradiography.
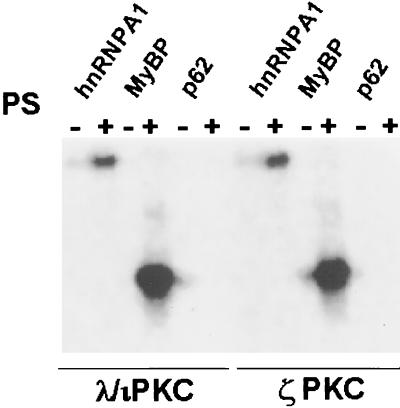
The ability of ζPKC or λ/ιPKC to phosphorylate p62 was measured essentially as described above. Briefly, the immunopurified kinases were incubated in the presence or absence of phosphatidylserine with p62 as a substrate. The myelin basic protein (MyBP) and hnRNPA1 were used as positive controls.
Confocal immunofluorescence microscopy.
HeLa cells were grown on glass coverslips to 80% confluence in DMEM supplemented with fetal calf serum (10%). For experiments in which receptor internalization was analyzed, cells were serum starved for 24 h and incubated for 60 min with EGF (100 ng/ml) (Amersham) at 4°C, washed with DMEM at 4°C, and then incubated at 37°C for the time indicated in each experiment. Cells were rapidly washed twice in ice-cold PBS and fixed in 4% formaldehyde for 15 min at room temperature. Cells were washed four times with PBS and permeabilized with 0.1% Triton X-100 or 0.2% saponin (for lamp-1 and limp-II detection). Free aldehyde groups were quenched with 50 mM NH4Cl, and the fixed cells were incubated with primary antibodies for 1 h at room temperature. Transfected HA-, myc-, or His-tagged proteins were visualized with the monoclonal 12CA5 anti-HA (Boehringer), the monoclonal 9E10 anti-myc (17), the polyclonal anti-HA, or anti-myc antibodies (Santa Cruz Biotechnologies, Inc.) or the monoclonal 13/45/31 anti-His antibody (Dianova). Endogenous p62 was detected with an affinity-purified antibody raised against the MBP-p62 fusion protein. For detection of endogenous λPKC, ζPKC, αPKC, and ɛPKC mouse antibodies were obtained from Transduction Labs. We detected endogenous rab7 with a polyclonal anti-rab7 antibody raised as described previously against the peptide KQETEVELYNNEFPPEPPIK (9) and rab5 with a monoclonal 15 clone from Transduction Labs. The monoclonal H4A3 anti-lamp1 antibody was obtained from the Developmental Studies Hybroma Bank, and the mouse anti-EGFR antibody was from Oncogene Sciences (Mineola, N.Y.). To visualize the human transferrin receptor, we used a monoclonal antibody (B3/25; Boehringer). The secondary antibodies used were a Texas red-conjugated affiniPure goat anti-mouse and anti-rabbit antibody from Jackson Immunoresearch Laboratories, Inc., and a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse and anti-rabbit antibody from Cappel. Glass coverslips were mounted on Mowiol and examined with an MRC 1024 confocal system (Bio-Rad; Richmond, Calif.) mounted on an Axiovert 135 microscope (Zeiss, Oberkochen, Germany).
Cell fractionation and enzymatic markers.
HeLa cells were plated 18 to 36 h before harvesting and allowed to reach a final confluency of 85%. Cells were loaded with horseradish peroxidase (HRP) (Sigma Chemical Co.) (7.5 to 10 mg/ml in DMEM-10% fetal calf serum) at 37°C for 30 min. Cells were chilled on ice, washed four times in ice-cold PBS, and then scraped into homogenization buffer (250 mM [8%] sucrose, 3 mM imidazole [pH 7.4], 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 μg of pepstatin per ml, 1% aprotinin). Cells were homogenized in a 2-ml Dounce homogenizer with an A pestle for 25 strokes. After homogenization, nuclear membranes were pelleted for 10 min at 2,000 × g in an SS-34 rotor at 4°C. The postnuclear supernatant was collected and adjusted to 40.6% sucrose with 62% (wt/wt) sucrose. The postnuclear supernatant was then loaded at the bottom of an SW55 tube and sequentially overlaid with 2 ml of 35% (wt/wt) sucrose and 1 ml of homogenization buffer. The tubes were centrifuged at 35,000 rpm for 60 min at 4°C in an SW55-Ti rotor. Fractions were collected from the top of the tube and assayed for enzymatic markers. The lysosomal marker β-glucuronidase was measured with 4-nitrophenyl-β-d-glucopyranosic acid (Merck) (3a). HRP was used as an endosomal fluid phase marker (23a). The HRP activity was measured at 455 nm with o-dianisidine (Sigma) prepared at 0.1 mg/ml in 50 mM sodium phosphate buffer (pH 5), 0.1% Triton X-100, and 0.003% H2O2. The protein concentration was determined in parallel by the Bradford assay (Bio-Rad).
RESULTS
Yeast two-hybrid screen.
In previous studies in this laboratory, the yeast two-hybrid system has been employed to isolate novel regulators of the aPKCs with the whole regulatory domain of either λ/ιPKC or ζPKC as the bait for screening. This strategy resulted in the isolation of two proteins that selectively interacted with the zinc finger region of both aPKCs but not with other structurally or functionally related kinases (12, 13). However, the V1 region in the regulatory domain of the different PKCs displays the most dissimilar sequence among the different PKC isotypes (40). Therefore, in the study reported here, we fused the V1 domain of λ/ιPKC (amino acids 1 to 126) with the DNA-binding domain of the yeast GAL4 protein (pYTH9-λ/ιPKC126) to make bait to screen a human kidney Matchmaker cDNA library. Colonies that grew on yeast dropout media lacking Leu, Trp, and His but containing 20 mM 3-amino-1,2,4-triazole and that were blue within 20 min when assayed by 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) colony filter assay were selected. Seven positive colonies that stained intensely blue were obtained from 2.5 × 106 screened colonies. The expression of His-3 and LacZ in these colonies was shown to depend on the GAL4 fusion protein by retransformation of the recovered plasmids into yeasts containing the bait construct (Table 1). The sequences of the seven clones revealed that they were identical, corresponding to a partial cDNA coding for the first 266 amino acids of the previously cloned p62 protein (whose full length is 440 amino acids). The protein p62 has been reported to be a phosphotyrosine-independent ligand of the p56lck SH2 domain (27). The p62 full-length clone was obtained by PCR with the appropriate primers and the human kidney Matchmaker cDNA library as a template.
TABLE 1.
Specificity of the interaction between the aPKCs and p62a
| GAL4-BD fusion | Growth on Trp− Leu− His− medium of GAL4-AD fusion
|
|
|---|---|---|
| pGAD10 | pGAD10-p62 | |
| pYTH9 | − | − |
| pYTH9λ/ιPKC126 | − | ++ |
| pYTH9ζPKC126 | − | + |
| pYTH9λ/ιPKCZF | − | − |
| pYTH9ζPKCZF | − | − |
| pYTH9λ/ιPKCREG | − | ++ |
| pYTH9ζPKCREG | − | + |
| pYTH9λ/ιPKC | − | + |
| pYTH9ζPKC | − | + |
| pYTH9λ/ιPKCCAT | − | − |
| pYTH9αPKCREG | − | − |
| pYTH9ɛPKCREG | − | − |
| pGBT9RafCAT | − | − |
| pGBT9RafREG | − | − |
| pGBT9Raf | − | − |
| pGBT9Mos | − | − |
| pGBT9Lamin | − | − |
Y190 cells were cotransformed with expression vectors encoding various GAL4 DNA-binding domain and GAL4 transcriptional activation domain fusion proteins as indicated. Aliquots of the same transformation mixture were plated onto synthetic dextrose plates lacking tryptophan and leucine and plates lacking tryptophan, leucine, and histidine that contained 20 mM 3-aminotriazole. All the transformations grew on the plates lacking tryptophan and leucine (data not shown). Two plus signs indicate growth on the respective plates with very similar numbers of transformants from the same transformation mixture (90 to 100%) obtained on plates lacking tryptophan, leucine, and histidine and on plates lacking tryptophan and leucine. A plus sign indicates that the number of transformants on plates lacking tryptophan, leucine, and histidine was less than 2% of the number of colonies obtained from the same transformation mixture on plates lacking only tryptophan or only leucine. Filter assays for β-galactosidase activity were performed on colonies growing on plates lacking all three amino acids. All colonies developed a blue color (data not shown). Essentially identical results were obtained in three more independent experiments.
The specificity of the interaction between λ/ιPKC126 and the p62 protein was next tested with an unrelated molecule, pGBT9-lamin, which fails to transactivate the reporter constructs (Table 1). Neither the catalytic domains nor the zinc fingers of λ/ιPKC and ζPKC interacted with p62, whereas the full-length λ/ιPKC as well as the V1 region and the whole regulatory domain of ζPKC did interact (Table 1). However, the interaction of p62 with ζPKC was significantly weaker than with λ/ιPKC (Table 1). Interestingly, p62 does not interact with the regulatory domains of αPKC or ɛPKC (Table 1), indicating that it is highly specific for the λ/ιPKC isotype. On the other hand, Raf-1 is a serine-threonine kinase with a structure that is similar overall to that of the aPKCs (41). Both types of kinases are critical components downstream of Ras in mitogenic cascades (4, 7, 25). Interestingly, neither the catalytic domain (Table 1), the full-length (Table 1), nor the regulatory region (Table 1) of Raf-1 interacted with p62. The product of c-mos that is another serine-threonine kinase critically involved, like Raf-1 and the aPKCs, in mitogenic signal transduction in Xenopus oocytes and mammalian cells (49) did not interact with p62 (Table 1). Therefore, these data collectively indicate that p62 binds specifically to the V1 domain of λ/ιPKC and binds with significantly lower affinity to the V1 domain of ζPKC.
PKC-p62 interactions in vitro and in vivo.
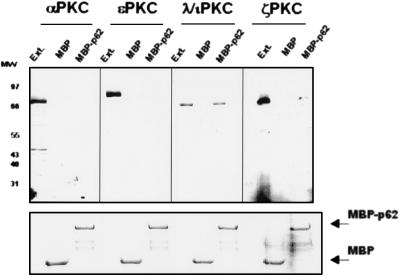
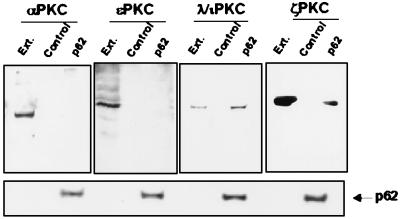
To confirm the interaction observed in yeast, first we expressed p62 as an MBP fusion protein. Afterward, immobilized MBP and MBP-p62 were incubated with extracts of HeLa (Fig. 1) or human 293 cells (data not shown), in the absence or the presence of phosphatidylserine, C2-ceramide, PMA, or diacylglycerol (DAG) and after extensive washing, bound MBPs and any associated proteins were boiled in sample buffer, fractionated by SDS-PAGE, and immunostained with antibodies specific for αPKC, ɛPKC, λ/ιPKC, or ζPKC. These are the major, if not the only, PKC isotypes present in both cell types (49a). Of note, recombinant MBP-p62 but not MBP bound selectively to native λ/ιPKC and ζPKC but not to αPKC or ɛPKC both in the absence (Fig. 1) and in the presence of lipid activators (data not shown). The fraction of ζPKC that associates with MBP-p62 is significantly smaller than that of λ/ιPKC (Fig. 1), consistent with the data from the two-hybrid system (Table 1). Staining of a parallel gel confirmed that all the reactions contained equal molar amounts of MBP fusion proteins (Fig. 1). To determine the binding of p62 to the aPKCs in vivo, HeLa (Fig. 2) or 293 (data not shown) cells were transfected with either control plasmid or HA-tagged p62 expression vectors. Forty hours posttransfection, cell lysates were immunoprecipitated with an anti-HA monoclonal antibody. Immunoprecipitates were resolved by SDS-PAGE and analyzed by immunoblotting with antibodies against αPKC, ɛPKC, λ/ιPKC, or ζPKC. Immunoreactive bands corresponding to λ/ιPKC and ζPKC but not to αPKC or ɛPKC were clearly detected in immunoprecipitates from cells transfected with the HA-p62 vector but not with the empty plasmid (Fig. 2). The amount of ζPKC that coimmunoprecipitates with HA-p62 is significantly lower than that of λ/ιPKC (Fig. 2). Control immunoblots with the anti-HA antibody demonstrate that all lanes contained the same amount of transfected HA-p62 (Fig. 2). The incubation of cell cultures with either EGF, tumor necrosis factor alpha, C2-ceramide, or PMA for different amounts of time did not change the amount of p62 associated with ζPKC or λ/ιPKC and did not allow the interaction of p62 with the non-atypical PKCs (data not shown).
FIG. 1.
p62 interacts with the aPKC in vitro. (Upper panel) Purified MBP or MBP-p62 (400 nM) was immobilized on amylose beads and incubated with 1 mg of protein extracts from HeLa cells at 22°C for 1 h. After extensive washing, the recombinant proteins were fractionated by SDS-PAGE and the associated PKC isotypes were determined with the corresponding isotype-specific antibodies by immunoblotting. Ext., 50 μg of protein extracts was run in parallel as a control. MW, molecular weight (in thousands). (Lower panel) Amounts of MBP fusion proteins in each experiment were the same. Essentially identical results were obtained in two more experiments.
FIG. 2.
p62 interacts with the aPKC in vivo. (Upper panel) Subconfluent cultures of HeLa cells in 100-mm-diameter plates were transfected with 20 μg of either pCDNA3 (control) or pCDNA3-HA-p62 (p62). Plasmid DNA was removed 4 h later, and the cells were incubated in DMEM containing 10% fetal calf serum for 36 h. Cell extracts (200 μg of protein) were immunoprecipitated with anti-HA antibody. Immunoprecipitates were resolved by SDS-PAGE and immunoblotted with isotype-specific anti-PKC antibodies. The extract (Ext.) lane contains 20 μg of cell protein extract. (Lower panel) Immunoblot analysis of the HA-p62 expressed protein in each assay. Essentially identical results were obtained in two more experiments.
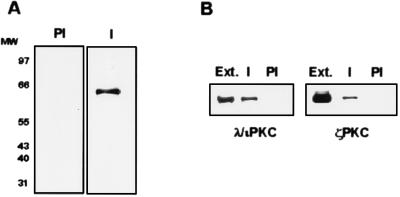
In order to determine whether endogenous p62 and λ/ιPKC can interact in vivo, we next used the MBP-p62 fusion protein to immunize rabbits and generate an antibody against p62. This antiserum specifically recognizes a band of about 62 kDa in immunoblots of extracts from HeLa cells; the band is not seen with the preimmune serum (Fig. 3A). Interestingly, results from Fig. 3B demonstrate the coimmunoprecipitation of endogenous λ/ιPKC and ζPKC with the immune but not the preimmune serum raised against p62. This result indicates the in vivo association of endogenous p62 with both aPKCs. Control immunoblots confirmed the lack of interaction of native p62 with endogenous αPKC or ɛPKC (data not shown). The aPKCs associated with p62 in these immunoprecipitates can be activated by phosphatidylserine in vitro to an extent similar to that of the immunopurified ζPKC or λ/ιPKC (data not shown). The amount of λ/ιPKC or ζPKC that coimmunoprecipitates with p62 does not change when cells are made quiescent and subsequently treated with different stimuli, including EGF (data not shown), strongly suggesting that the in vivo interaction of the aPKCs with p62 is constitutive.
FIG. 3.
Endogenous p62 interacts with the aPKC in vivo. (A) Cell protein extracts from HeLa cells (20 μg) were separated by SDS-PAGE and immunoblotted with either preimmune (PI) or affinity-purified anti-p62 antiserum (I). MW, molecular weight (in thousands). (B) Protein extracts from HeLa cells (200 μg) were immunoprecipitated with either preimmune (PI) or affinity-purified immune anti-p62 antiserum (I), and the associated λ/ιPKC or ζPKC was determined by immunoblotting with isotype-specific antibodies. The extract (Ext.) lane contains 20 μg of cell protein extract. Essentially identical results were obtained in two more experiments.
P62 does not modulate the activity of λ/ιPKC or of ζPKC and is not a substrate for these kinases.
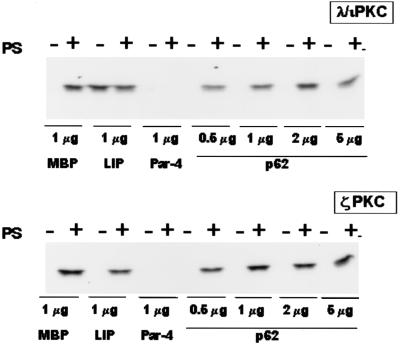
Because λ/ιPKC and ζPKC are regulated by proteins like LIP or Par-4 that bind specifically to their regulatory domains, we sought to determine if the interaction of p62 with λ/ιPKC or ζPKC can regulate their enzymatic activities. Thus, immunopurified λ/ιPKC or ζPKC was incubated in the presence of phosphatidylserine (a classical activator of all PKCs), significantly increasing both λ/ιPKC and ζPKC activities (Fig. 4). Interestingly, the addition of 1 μg of MBP-LIP(R4), but not of MBP alone, produced a dramatic activation of λ/ιPKC (but not of ζPKC) comparable to that produced by phosphatidylserine (Fig. 4), whereas the addition of MBP-Par-4 dramatically inhibited the activities of both kinases. This finding is consistent with previously published results (12, 13). Notably, the addition of up to 5 μg of MBP-p62 produced no effect on the activity of λ/ιPKC or ζPKC (Fig. 4). Therefore, in contrast to LIP and Par-4, p62 is not a regulator of aPKC enzymatic activity.
FIG. 4.
Activities of the aPKC are not regulated by recombinant p62. Immunopurified λ/ιPKC (upper panel) or ζPKC (lower panel) was incubated with 1 μg of either MBP, MBP-LIP (LIP), or MBP-Par-4 (Par-4) or with different amounts of MBP-p62 (p62) either with or without phosphatidylserine (50 μg/ml). Afterward, the activity of both PKCs toward MBP was determined as described in Materials and Methods. Essentially identical results were obtained in two more experiments.
In order to determine whether p62 could serve as a substrate for ζPKC or λ/ιPKC, the immunopurified kinases were incubated in the presence or the absence of phosphatidylserine and the possibility of p62 phosphorylation was investigated. Neither ζPKC nor λ/ιPKC phosphorylated p62, although they potently phosphorylated MyBP and hnRNPA1, two well-established substrates of the aPKCs (Fig. 5).
FIG. 5.
p62 is not a substrate of the aPKC. The ability of immunopurified λ/ιPKC or ζPKC to phosphorylate 1 μg of recombinant hnRNP A1, MyBP, or p62 was determined as described in Materials and Methods either in the absence or in the presence of phosphatidylserine (50 μg/ml). Essentially identical results were obtained in two more experiments.
Subcellular colocalization of p62 with λ/ιPKC and ζPKC.
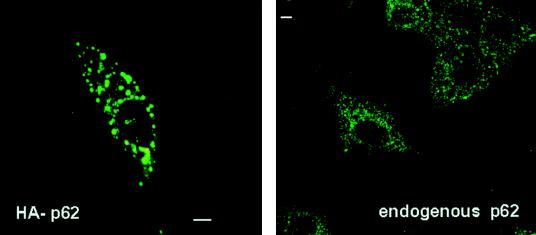
Because p62 is neither a regulator nor a substrate for the aPKCs but displays selective interaction with these kinases, we reasoned that it may act as an anchor, localizing the aPKCs to a specific subcellular region. To begin analyzing this possibility, HeLa cells were transfected with an HA-tagged version of p62, after which the expressed protein was detected by confocal laser scanning microscopy with the monoclonal anti-HA antibody 12CA5. The results shown in Fig. 6 demonstrate that transfected p62 localizes to vesicles of various sizes throughout the cell. In order to determine whether this pattern of expression is physiological, the localization of the endogenous p62 was determined by confocal microscopy with the affinity-purified polyclonal anti-p62 antibody described above. Interestingly, the endogenous p62 displayed a vesicular pattern very similar to that of the transfected construct, but the population of vesicles was more homogeneous, with a smaller size than that produced by the overexpression of p62 (Fig. 6).
FIG. 6.
Cellular localization of ectopically expressed and endogenous p62. HeLa cells were transfected with 5 μg of an expression vector for the HA-tagged p62 (left panel) or left untransfected (right panel). Twenty hours posttransfection, the ectopically expressed HA-p62 (left panel) was immunostained with the monoclonal anti-HA antibody 12CA5, whereas the endogenous protein (right panel) was immunostained with the affinity-purified polyclonal anti-p62 antibody, and both were analyzed by confocal laser scanning microscopy. Bar, 5 μm. Essentially identical results were obtained in three more experiments.
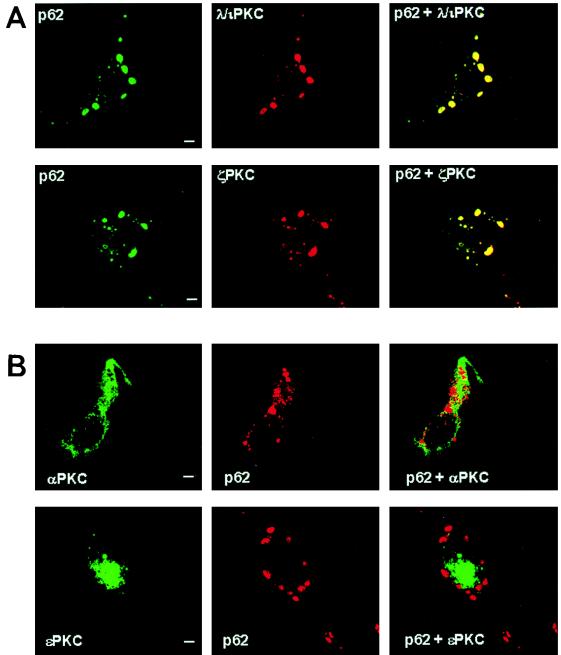
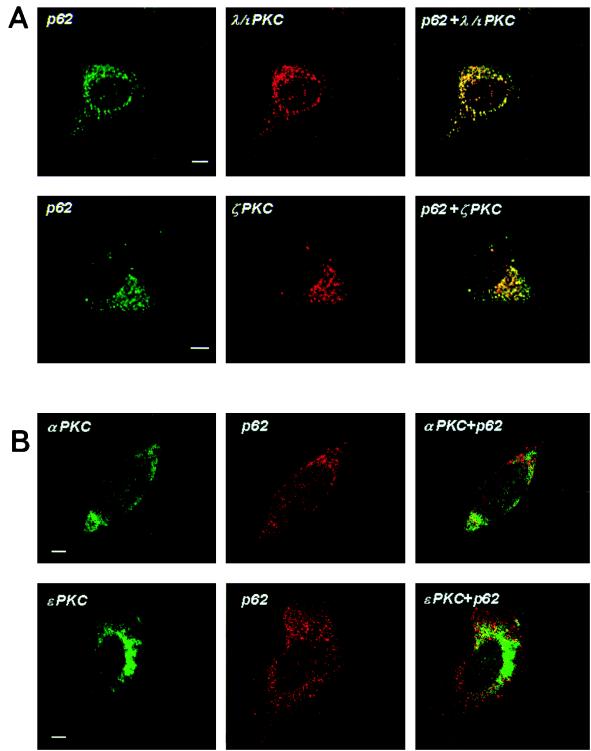
To demonstrate the actual subcellular colocalization of λ/ιPKC and ζPKC with p62, HeLa cells were transfected with myc-tagged p62 along with either HA-λ/ιPKC or HA-ζPKC. Transfected cells were analyzed by confocal laser microscopy with the monoclonal antibody 12CA5 and Texas red-conjugated anti-mouse immunoglobulin G (IgG) (red fluorescence) to detect λ/ιPKC or ζPKC and rabbit polyclonal anti-myc antibody and FITC-conjugated anti-rabbit IgG (green fluorescence) to detect myc-p62. The results shown in Fig. 7 demonstrate that λ/ιPKC and ζPKC both display a punctate vesicular pattern, perfectly colocalizing with p62. Of note, the incubation of these transfectants with different stimuli, including EGF, did not change the localization pattern of p62, λ/ιPKC, or ζPKC (data not shown). To establish the physiological relevance of the aPKC-p62 interaction, it is essential to determine the subcellular localization of endogenous λ/ιPKC and ζPKC and whether they colocalize with endogenous p62. Thus, confocal laser microscopy was utilized in double immunofluorescence experiments with monoclonal antibodies selective for either λ/ιPKC or ζPKC and the affinity-purified anti-p62 antibody. These experiments clearly show that endogenous λ/ιPKC and ζPKC both colocalize with endogenous p62 in vesicles (Fig. 8). Again, cell stimulation did not change the localization of both endogenous proteins (data not shown).
FIG. 7.
Colocalization of ectopically expressed p62 with transfected λ/ιPKC and ζPKC but not with αPKC or ɛPKC. HeLa cells were transfected with 5 μg of an expression vector for myc-tagged p62 along with 5 μg of expression plasmids for either HA-λ/ιPKC or HA-ζPKC (A) or His-αPKC or His-ɛPKC (B). Twenty hours posttransfection, cells were analyzed by confocal laser scanning microscopy with the monoclonal antibody 12CA5 and Texas red-conjugated anti-mouse IgG (red fluorescence) to detect λ/ιPKC or ζPKC or monoclonal anti-His and FITC-conjugated anti-mouse IgG (green fluorescence), αPKC or ɛPKC and rabbit polyclonal anti-myc antibody and either FITC-conjugated (green fluorescence) or Texas red-conjugated (red fluorescence) anti-rabbit IgG, and myc-p62. Bar, 5 μm. Essentially identical results were obtained in three more experiments.
FIG. 8.
Colocalization of endogenous p62 with endogenous λ/ιPKC and ζPKC. HeLa cells were analyzed by confocal laser scanning microscopy with monoclonal anti-λ/ιPKC and monoclonal anti-ζPKC (A) and monoclonal anti-αPKC or anti-ɛPKC (B) and Texas red-conjugated anti-mouse IgG (red fluorescence) and affinity-purified anti-p62 polyclonal antibody and FITC-conjugated anti-rabbit IgG (green fluorescence). Bar, 5 μm. Essentially identical results were obtained in three more experiments.
The following series of experiments demonstrate that αPKC and ɛPKC are not localized in p62-containing vesicles. Thus, HeLa cells were transfected with epitope-tagged αPKC or ɛPKC, and their subcellular localization was compared with that of p62 by confocal microscopy. The results shown in Fig. 7 demonstrate that neither exogenously expressed αPKC nor ɛPKC colocalized with p62. Figure 8 also shows that neither endogenous αPKC nor endogenous ɛPKC colocalized with p62.
Characterization of the p62-containing vesicles.
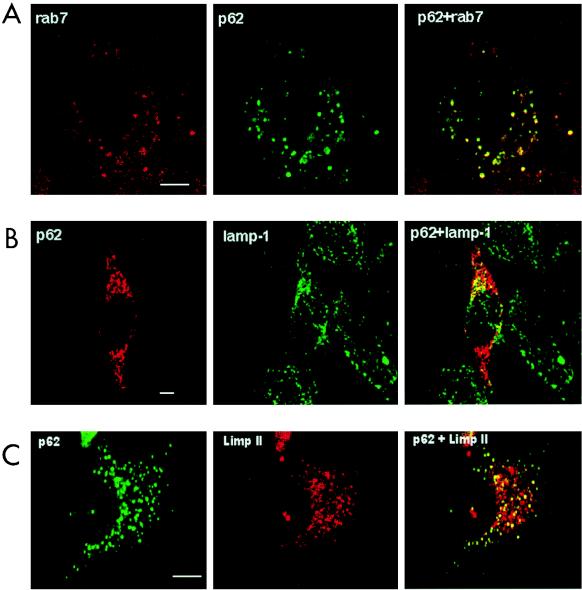
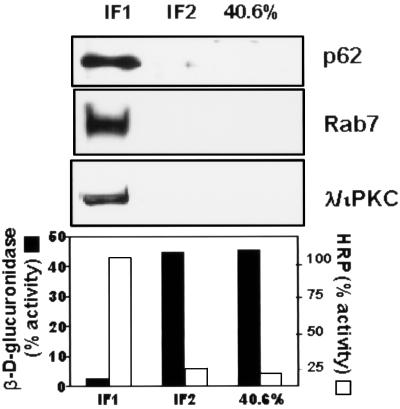
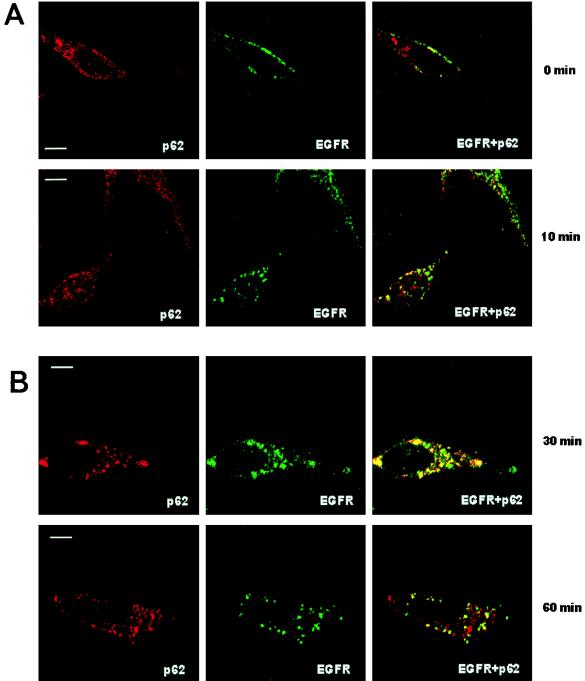
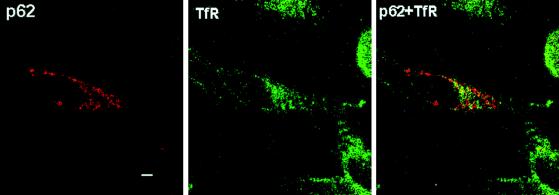
Next we characterized the type of vesicle in which p62 is located. Thus, HeLa cells were transfected with HA-p62, and the colocalization of this protein with endogenous Rab7 (a marker of late endosomes [40a]), Rab5 (a marker of early endosomes [40a]) or lamp-1 and Limp II (both lysosomal markers [21a]) was determined by double immunofluorescence confocal microscopy. Interestingly, the expressed p62 colocalized clearly with Rab7 and partially with lamp-1 and Limp II (Fig. 9) but not with Rab5 (not shown), indicating that the lysosome-targeted late endosomes are the most likely location of p62. The colocalization of exogenous p62 with endosomal markers was observed independently of the size of the vesicles. To obtain independent evidence of the endosomal localization of p62, endosomes were isolated by standard cell fractionation techniques. HeLa cell cultures, which had internalized the fluid phase marker HRP to label the endocytic compartment, were homogenized and fractionated in a sucrose gradient to separate an endosome-enriched fraction. After centrifugation, markers were assayed for the endosomes (with HRP) and lysosomes (with β-glucuronidase) in each of the sucrose layers and interface. We determined that the endocytic marker, HRP, and thus endocytic membranes from these cells were enriched at the 18 to 32% interface of the gradient (IF1), whereas the lysosomal marker, β-glucuronidase, was predominantly localized in the 40.6% sucrose phase and in the 35 to 40.6% interface (IF2). Thus, we examined by Western blot analysis the distribution of p62 and λ/ιPKC throughout the sucrose gradient. Interestingly, and consistent with the immunofluorescence data, p62 has reproducibly been detected at the 18 to 32% interface (IF1) in the same fraction as λ/ιPKC and Rab7 (Fig. 10). The distribution of p62 and λ/ιPKC along the gradient did not change upon cell stimulation (data not shown). Because the aPKCs are activated by growth factors (5, 11), whose receptors are internalized through the lysosome-targeted endosomal pathway, we next determined whether p62 colocalizes with the EGF receptor. Therefore, HeLa cells were made quiescent by 24-h serum starvation, after which they were incubated with 100 ng of EGF per ml for 1 h at 4°C. Afterward, unbound EGF was extensively washed and cells were incubated for different times at 37°C to allow endocytosis. At 0 min of internalization, cells show a pattern of EGF receptors that is predominantly localized in the plasma membrane, with little or no colocalization with p62, which is located in vesicles that are broadly distributed throughout the cell (Fig. 11). Upon incubation at 37°C, the receptors are internalized, forming vesicles that colocalize detectably with p62 at 10 min and maximally at 30 to 60 min (Fig. 11), depending on the kinetics of each experiment. In contrast, the results of Fig. 12 demonstrate that p62 does not colocalize with the endogenous transferrin receptor, a marker of the recycling endosomal pathway.
FIG. 9.
Colocalization of p62 with different endosomal and lysosomal markers. HeLa cells were transfected with 5 μg of an expression vector for HA-tagged p62 and analyzed 20 h posttransfection by confocal laser scanning microscopy with monoclonal anti-HA antibody and FITC-conjugated anti-mouse IgG (green fluorescence) (A) or polyclonal anti-HA antibody (B and C) and Texas red-conjugated anti-rabbit IgG (red fluorescence) (B) or FITC-conjugated anti-rabbit IgG (green fluorescence) (C). Rab7 (A) was detected with an anti-rab 7 polyclonal antibody and Texas red-conjugated anti-rabbit IgG (red fluorescence). Lamp-1 (B) was detected with a monoclonal anti-lamp-1 antibody and FITC-conjugated anti-mouse IgG (green fluorescence). Limp II (C) was detected with a monoclonal anti-Limp II antibody and Texas red-conjugated anti-mouse IgG (red fluorescence). Bar, 5 μm. Essentially identical results were obtained in three more experiments.
FIG. 10.
Association of p62 and λ/ιPKC with endosome-enriched fraction in HeLa cells. HeLa cells loaded with HRP at 37°C for 30 min were homogenized, and the postnuclear supernatant was collected and loaded at the bottom of a sucrose step gradient as described in Materials and Methods. After centrifugation, fractions were analyzed by immunoblotting (with p62, Rab7, and λ/ιPKC) and assayed for enzymatic markers (HRP and β-d-glucuronidase activities). Essentially identical results were obtained in three more experiments.
FIG. 11.
Colocalization of p62 with the EGF receptor. HeLa cells were made quiescent by 24 h of serum starvation, after which they were incubated with 100 ng of EGF per ml for 1 h at 4°C. Afterward, unbound EGF was extensively washed, and the cells were incubated for different times at 37°C and analyzed by confocal laser scanning microscopy with monoclonal anti-EGF receptor and Texas red-conjugated anti-mouse IgG (red fluorescence) and affinity-purified anti-p62 polyclonal antibody and FITC-conjugated anti-rabbit IgG (green fluorescence). Bar, 5 μm. Essentially identical results were obtained in three more experiments.
FIG. 12.
Lack of colocalization of p62 with the transferrin receptor. HeLa cells were transfected with 5 μg of HA-tagged p62 expression vector and analyzed 20 h posttransfection by confocal laser scanning microscopy with a polyclonal anti-HA antibody and Texas red-conjugated anti-rabbit IgG (red fluorescence) to detect the p62 and monoclonal anti-transferrin receptor (TfR) antibody and FITC-conjugated anti-mouse IgG (green fluorescence). Bar, 5 μm. Essentially identical results were obtained in three more experiments.
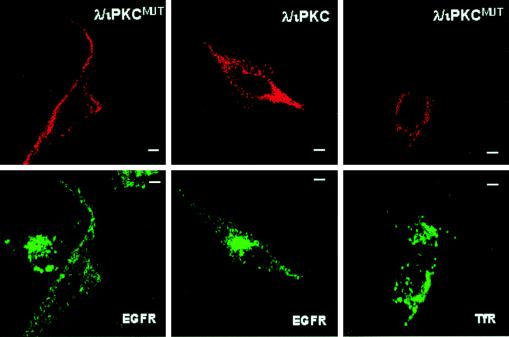
Collectively, these results would be consistent with a model in which p62 encounters the activated EGF receptor in the late endosomal compartment on its way toward the lysosomes. If this model is correct, the aPKCs could be critically involved in regulating the endocytic membrane transport of the EGF receptor. This possibility is particularly attractive, because PI 3-kinase is an upstream modulator of the aPKCs (1, 34) and has been shown to regulate the internalization of growth factor receptors through the lysosome-targeted endosomal pathway (51). We therefore transfected HeLa cells with expression vectors for tagged versions of either wild-type or dominant negative λ/ιPKC, after which cells were made quiescent and incubated with 100 ng of EGF per ml for 1 h at 4°C. After extensive washing of the unbound EGF, cells were incubated at 37°C for 60 min and the vesicular staining of the EGF receptor and that of the transferrin receptor were determined by confocal microscopy. The EGF receptor reaches the juxtanuclear endosomal-lysosomal localization in 30 to 60 min. In the experiments depicted in Fig. 13, the activated receptor was localized in the juxtanuclear region at 60 min in the nonexpressing cells and in those which express wild-type λ/ιPKC. Interestingly, expression of the dominant negative λ/ιPKC severely impaired the transport of internalized EGF receptor to the perinuclear late endosome (Fig. 13). Thus, the receptor remained at the cell surface in scattered peripheral endosomes (Fig. 13). However, the dominant negative mutant of λ/ιPKC showed no effect on the endocytic transport of the transferrin receptor (Fig. 13), consistent with the lack of colocalization of p62 with the recycling endocytic compartment.
FIG. 13.
Role of λ/ιPKC in the endocytic membrane transport of the EGF receptor. HeLa cells were transfected with 5 μg of either HA-tagged λ/ιPKC or HA-tagged λ/ιPKCMUT expression vectors. Twenty hours posttransfection, cells were made quiescent by serum starvation for 24 h. Afterward, cells were incubated for 1 h at 4°C with 100 ng of EGF per ml. After extensive washing of the unbound EGF, cells were incubated for 60 min at 37°C. Cells were analyzed by confocal microscopy with monoclonal antibodies specific for either EGF receptor (EGFR) or transferrin receptor (TfR) and FITC-conjugated anti-mouse IgG (green fluorescence) and a polyclonal anti-HA antibody and Texas red-conjugated anti-rabbit IgG (red fluorescence) to detect the transfected λ/ιPKC constructs. Bar, 5 μm. Essentially identical results were obtained in three more experiments.
DISCUSSION
The regulation and subcellular localization of different PKC isotypes by protein-protein interaction is emerging as a field of intense research. PKCs have traditionally been considered the targets of lipid second messengers, which in most cases, such as DAG or the 3′-phosphoinositides, do not discriminate between the different PKC isotypes. Therefore, the existence of selective protein modulators for the different PKC isoforms may be an elegant way for the distinct isotypes to have a specific mechanism of regulation and/or action. In seminal work, Mochly-Rosen’s group first isolated a family of novel proteins termed RACKs, which serve to localize some classical PKC subspecies to cellular compartments where they may perform specific functions (35, 46). We have recently identified two novel proteins that selectively bind to the aPKCs: LIP and Par-4 (12, 13). Both proteins interact with the zinc finger region of the aPKCs (12, 13), where the lipid second messengers have been shown to bind to regulate the classical isoforms (40, 45). Therefore, it is not surprising that LIP and Par-4 have profound effects on the activation of the aPKCs in vitro and in vivo (12, 13).
Together with the V3 domain, the V1 region in the regulatory domain of the different PKCs is where the major differences in the amino acid sequence are found (40). Kuroda et al. (30) used the regulatory domain of βI-PKC as the bait in a two-hybrid screen and cloned a novel LIM-containing protein, termed ENH, demonstrating that it interacted not only with the V1 domain of βI-PKC but also with that of ζPKC. Therefore, ENH does not appear to be specific for a particular PKC isotype (30). Other LIM proteins, including the LIM kinase, also interacted with different PKC isoforms in a completely unselective manner (30, 42). Mochly-Rosen’s group has recently identified a RACK protein selective for ɛPKC that interacts with the V1 domain of this kinase (9a). Here we show that p62, a previously described phosphotyrosine-independent p56lck SH2-interacting protein (27), potently binds to the V1 domain of λ/ιPKC and, albeit with lower affinity, to ζPKC, but not to αPKC or ɛPKC. Therefore, in contrast to the LIM proteins, p62 shows a clear selectivity for the aPKC isotypes.
While the present study was in preparation, Puls et al. (44) reported the cloning of p62 in a two-hybrid screen with full-length ζPKC as the bait. The mapping of the region in ζPKC where p62 binds is consistent with our observation that p62 interacts with the V1 domain. However, there are a number of differences between the data of Puls et al. (44) and ours. First, in our study p62 is not a substrate for ζPKC. The reasons for this discrepancy are unclear but may be related to differences between the assay conditions employed in the two studies. However, it should be noted that in the experiments shown in Fig. 5 our PKC preparations were shown to be fully capable of phosphorylating two different substrates with no effect on p62. Another difference between the findings of Puls et al. (44) and those reported here concerns the subcellular localization of p62 and its relationship with the aPKCs. Puls et al. (44) claimed that, when ectopically expressed, p62 artifactually forms vesicles (Puls et al. called them “amorphous aggregates”) which do not correlate with endosomal or lysosomal markers. Only the overexpression of ζPKC, according to these researchers, is capable of returning p62 to its purported physiological location, which Puls et al. claim is the cytosol. In another previous study that addressed the subcellular localization of different PKC isotypes ectopically overexpressed in NIH 3T3 cells, diffuse cytosolic staining for ζPKC was also reported (23). However, a more recent report shows a vesicular punctate pattern for endogenous ζPKC in confocal immunofluorescence experiments (56). We show here that exogenously expressed and endogenous p62 localizes, at least in part, in vesicles that contain Rab7 and two lysosomal markers. More importantly, confocal immunofluorescence analysis with an antibody that recognizes the endogenous p62 clearly gives a vesicular staining like that observed with the ectopically expressed protein, strongly suggesting that the physiological location of p62 is not the cytosol but most probably the endosomal compartment. The ectopic expression of tagged versions of λ/ιPKC or ζPKC along with p62 with a different tag demonstrates that both aPKCs colocalize with p62 in the vesicular structures. With regard to the physiological localization of ζPKC and λ/ιPKC, we used monoclonal antibodies selective for each atypical PKC isotype in confocal immunofluorescence experiments to address this issue. Interestingly, and consistent with the punctate vesicular staining of the endogenous p62, these experiments showed that the endogenous λ/ιPKC and ζPKC both give a vesicular pattern that colocalizes with endogenous p62 in the late endosomal compartment.
The demonstration that p62 colocalizes with the EGF receptor in activated cells but not with the transferrin receptor may be physiologically relevant. The aPKCs have been shown to be activated by different growth factors (5, 11, 34, 52) through the PI 3-kinase cascade (1, 34, 39, 52). Interestingly, the inhibition of PI 3-kinase dramatically impairs the internalization of growth factor receptors, which takes place via the lysosome-targeted, but not the recycling, endosomal pathway (51). The fact that the p62-aPKC complex is located in the subcellular compartment that receives the lysosome-targeted growth factor receptor and the reported ability of PI 3-kinase products to activate the aPKCs strongly suggest that these kinases could play a critical role in controlling trafficking of the receptor from the plasma membrane to the lysosomes. In fact, we show here that the expression of dominant negative λ/ιPKC, but not of the wild-type enzyme, severely impairs the endocytic membrane trafficking of the EGF receptor but does not affect the transferrin receptor. This finding is reminiscent of the effect produced by transfection of dominant negative mutants of Rab7 on membrane transport leading from early to late endosomes (19). It is interesting that p62 perfectly colocalizes with Rab7 (see above) but not with Rab5, which controls the uptake from the cell surface to the early endosomes (19). P62 differs from LIP and Par-4 in that it does not modulate aPKC enzymatic activity. However, besides anchoring the aPKCs into the endosomal compartment, p62 could act as a scaffold, allowing the aPKCs to be close to other regulators and/or effectors (44). Understanding how all these molecules assemble in vivo and their relationship with the lipid mediators that target the aPKCs is a challenge for researchers of future studies.
ACKNOWLEDGMENTS
This work was supported by grants SAF96-0216 from CICYT, PM96-0002-C02 from DGICYT, and BIO4-CT97-2071 from the European Union. P.S. is a Fellow of the Comunidad de Madrid. This work was funded in part by Glaxo Wellcome Spain and has benefited from an institutional grant from Fundación Ramón Areces to the CBM.
We are indebted to Esther Garcia, Carmen Ibañez, and Beatriz Ranera for technical assistance. We thank Gonzalo Paris and Isabel Perez for help and enthusiasm.
REFERENCES
- 1.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S-T, Mizuno K, Hirai S-I, Kazlauskas A, Ohno S. EGF or PDGF receptors activate atypical PKCλ through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 2.Baier-Bitterlich G, Überall F, Bauer B, Fresser F, Wachter H, Grunicke H, Utermann G, Altman A, Baier G. Protein kinase C-θ isoenzyme selective stimulation of the transcription factor complex AP-1 in T lymphocytes. Mol Cell Biol. 1996;16:1842–1850. doi: 10.1128/mcb.16.4.1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bandyopadhyay G, Standaert M L, Zhao L, Yu B, Avignon A, Galloway L, Karnam P, Moscat J, Farese R V. Activation of protein kinase C (α, β and ζ) by insulin in 3T3/L1 cells. J Biol Chem. 1997;272:2551–2558. doi: 10.1074/jbc.272.4.2551. [DOI] [PubMed] [Google Scholar]
- 3a.Beaufay H, Amar-Costesec A, Feytmans T. Analytical study of microsomes and isolated subcellular membranes from rat liver. 3. Subfractionation of the microsomal fraction by isopycnic and differential centrifugation in density gradients. J Cell Biol. 1974;61:188–200. doi: 10.1083/jcb.61.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berra E, Diaz-Meco M T, Dominguez I, Municio M M, Sanz L, Lozano J, Chapkin R S, Moscat J. Protein kinase C ζ isoform is critical for mitogenic signal transduction. Cell. 1993;74:555–563. doi: 10.1016/0092-8674(93)80056-k. [DOI] [PubMed] [Google Scholar]
- 5.Berra E, Diaz-Meco M T, Lozano J, Frutos S, Municio M M, Sanchez P, Sanz L, Moscat J. Evidence for a role of MEK and MAPK during signal transduction by protein kinase Cζ. EMBO J. 1995;14:6157–6163. doi: 10.1002/j.1460-2075.1995.tb00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berra E, Municio M M, Sanz L, Frutos S, Diaz-Meco M T, Moscat J. Positioning atypical protein kinase C isoforms in the UV-induced apoptotic signaling cascade. Mol Cell Biol. 1997;17:4346–4354. doi: 10.1128/mcb.17.8.4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bjorkoy G, Overvatn A, Díaz-Meco M T, Moscat J, Johansen T. Evidence for a bifurcation of the mitogenic signaling pathway activated by Ras and phosphatidylcholine-hydrolysing phospholipase C. J Biol Chem. 1995;270:21299–21306. doi: 10.1074/jbc.270.36.21299. [DOI] [PubMed] [Google Scholar]
- 8.Bjorkoy G, Perander M, Overvatn A, Johansen T. Reversion of Ras and phosphatidylcholine-hydrolyzing phospholipase C-mediated transformation of NIH 3T3 cells by a dominant interfering mutant of λPKC is accompanied by the loss of constitutive nuclear MAPK/ERK activity. J Biol Chem. 1997;272:11557–11565. doi: 10.1074/jbc.272.17.11557. [DOI] [PubMed] [Google Scholar]
- 9.Chavrier P, Parton R G, Hauri H P, Simons K, Zerial M. Localization of low molecular weight GTP binding proteins to exocytic and endocytic compartments. Cell. 1990;62:317–329. doi: 10.1016/0092-8674(90)90369-p. [DOI] [PubMed] [Google Scholar]
- 9a.Csukai M, Chen C H, De Matteis M A, Mochly-Rosen D. The coatomer protein β′-COP, a selective binding protein (RACK) for protein kinase C epsilon. J Biol Chem. 1997;272:29200–29206. doi: 10.1074/jbc.272.46.29200. [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Meco M T, Berra E, Municio M M, Sanz L, Lozano J, Dominguez I, Diaz-Golpe V, Lain de Lera M T, Alcami J, Payá C V, Arenzana-Seisdedos F, Virelizier J L, Moscat J. A dominant negative protein kinase C ζ subspecies blocks NF-κB activation. Mol Cell Biol. 1993;13:4770–4775. doi: 10.1128/mcb.13.8.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diaz-Meco M T, Lozano J, Municio M M, Berra E, Frutos S, Sanz L, Moscat J. Evidence for the in vitro and in vivo interaction of Ras with protein kinase C ζ. J Biol Chem. 1994;269:31706–31710. [PubMed] [Google Scholar]
- 12.Diaz-Meco M T, Municio M M, Sanchez P, Lozano J, Moscat J. Lambda-interacting protein, a novel protein that specifically interacts with the zinc finger domain of the atypical protein kinase C isotype λ/ι and stimulates its kinase activity in vitro and in vivo. Mol Cell Biol. 1996;16:105–114. doi: 10.1128/mcb.16.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz-Meco M T, Municio M M, Frutos S, Sanchez P, Lozano J, Sanz L, Moscat J. The product of par-4, a gene induced during apoptosis, interacts selectively with the atypical isoforms of protein kinase C. Cell. 1996;86:777–786. doi: 10.1016/s0092-8674(00)80152-x. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez I, Diaz-Meco M T, Municio M M, Berra E, García de Herreros A, Cornet M E, Sanz L, Moscat J. Evidence for a role of protein kinase C ζ subspecies in maturation of Xenopus laevis oocytes. Mol Cell Biol. 1992;12:3776–3783. doi: 10.1128/mcb.12.9.3776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dominguez I, Sanz L, Arenzana-Seisdedos F, Diaz-Meco M T, Virelizier J L, Moscat J. Inhibition of protein kinase C ζ subspecies blocks the activation of an NF-κB-like activity in Xenopus laevis oocytes. Mol Cell Biol. 1993;13:1290–1295. doi: 10.1128/mcb.13.2.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duang-Fang L, Brett M, Nicholas D, Bradford C B. Protein kinase C-ζ mediates angiotensin II activation of ERK1/2 in vascular smooth muscle cells. J Biol Chem. 1997;279:8146–8160. [Google Scholar]
- 17.Evan G I, Lewis G K, Ramsay G, Bishop J M. Isolation of monoclonal antibodies specific for human c-myc protooncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Exton J H. Phosphatidylcholine breakdown and signal transduction. Biochim Biophys Acta. 1994;1212:26–42. doi: 10.1016/0005-2760(94)90186-4. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Press B, Wandinger-Ness A. Rab 7: an important regulator of late endocytic membrane traffic. J Cell Biol. 1995;131:1435–1452. doi: 10.1083/jcb.131.6.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Folgueira L, McElhinny J A, Bren G D, MacMorran W S, Diaz-Meco M T, Moscat J, Payá C V. Protein kinase C-ζ mediates NF-κB activation in human immunodeficiency virus-infected monocytes. J Virol. 1996;70:223–231. doi: 10.1128/jvi.70.1.223-231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Franke T F, Yang S-H, Chan T O, Datta K, Kazlauskas A, Morrison D K, Kaplan D R, Tsichlis P N. The protein kinase encoded by the proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81:727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 21a.Fukuda M. Lysosomal membrane glycoproteins. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- 22.Gómez J, García A, Borlado L R, Bonay P, Martínez-A C, Silva A, Fresno M, Carrera A C, Eicher-Streiber C, Rebollo A. IL-2 signaling controls actin organization through Rho-like protein family, phosphatidylinositol 3-kinase, and protein kinase C-ζ. J Immunol. 1997;158:1516–1522. [PubMed] [Google Scholar]
- 23.Goodnight J, Mischak H, Kolch W, Mushinski J F. Immunocytochemical localization of eight protein kinase C isozymes overexpressed in NIH 3T3 fibroblasts. J Biol Chem. 1995;270:9991–10001. doi: 10.1074/jbc.270.17.9991. [DOI] [PubMed] [Google Scholar]
- 23a.Gruenberg J, Griffiths G, Howell K E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hannun Y A. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- 25.Howe L R, Leevers S J, Gomez N, Nakielny S, Cohen P, Marshall C J. Activation of MAP kinase pathway by the protein kinase Raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 26.Huang C, Ma W, Bowden G T, Dong Z. Ultraviolet B-induced activated protein-1 activation does not require epidermal growth factor receptor but it is blocked by a dominant negative PKCλ/ι. J Biol Chem. 1996;271:31262–31268. doi: 10.1074/jbc.271.49.31262. [DOI] [PubMed] [Google Scholar]
- 27.Joung I, Strominger J L, Shin J. Molecular cloning of a phosphotyrosine-independent ligand of the p56lckSH2 domain. Proc Natl Acad Sci USA. 1996;93:5991–5995. doi: 10.1073/pnas.93.12.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz M E, McCormick F. Signal transduction from multiple Ras effectors. Curr Opin Genet Dev. 1997;7:75–79. doi: 10.1016/s0959-437x(97)80112-8. [DOI] [PubMed] [Google Scholar]
- 29.Kolesnick R, Golde D W. The sphingomyelin pathway in tumor necrosis factor and interleukin-1 signaling. Cell. 1994;77:325–328. doi: 10.1016/0092-8674(94)90147-3. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda S, Tokunaga C, Kiyohara Y, Higuchi O, Konishi H, Mizuno K, Gill G N, Kikkawa U. Protein-protein interaction of zinc finger LIM domains with protein kinase C. J Biol Chem. 1996;271:31029–31032. doi: 10.1074/jbc.271.49.31029. [DOI] [PubMed] [Google Scholar]
- 31.Limatola C, Barabino B, Nista A, Santoni A. Interleukin 1-β-induced protein kinase C-ζ activation is mimicked by exogenous phospholipase D. Biochem J. 1997;321:497–501. doi: 10.1042/bj3210497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liscovitch M, Cantley L C. Lipid second messengers. Cell. 1994;77:329–334. doi: 10.1016/0092-8674(94)90148-1. [DOI] [PubMed] [Google Scholar]
- 33.Lozano J, Berra E, Municio M M, Diaz-Meco M T, Dominguez I, Sanz L, Moscat J. Protein kinase C isoform is critical for κB-dependent promoter activation by sphingomyelinase. J Biol Chem. 1994;269:19200–19202. [PubMed] [Google Scholar]
- 34.Mendez R, White M F, Myers M G, Rhoads R E. IRS-1-mediated activation of protein kinase C ζ is required for the stimulation of protein synthesis by insulin. Mol Cell Biol. 1997;17:5184–5192. doi: 10.1128/mcb.17.9.5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mochly-Rosen D, Khaner H, López J. Identification of intracellular receptors for activated protein kinase C. Proc Natl Acad Sci USA. 1991;88:3997–4000. doi: 10.1073/pnas.88.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moriya S, Kazlauskas A, Akimoto K, Hirai S-I, Mizuno K, Takenawa T, Fukui Y, Watanabe Y, Ozaki S, Ohno S. Platelet-derived growth factor activates protein kinase Cɛ through redundant and independent signaling pathways involving phospholipase Cγ or phosphatidylinositol 3-kinase. Proc Natl Acad Sci USA. 1996;93:151–155. doi: 10.1073/pnas.93.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muller G, Ayoub M, Storz P, Rennecke J, Fabbro D, Pfizenmaier K. PKCζ is a molecular switch in signal transduction of TNFα, bifunctionally regulated by ceramide and arachidonic acid. EMBO J. 1995;14:1961–1969. doi: 10.1002/j.1460-2075.1995.tb07188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Municio M M, Lozano J, Moscat J, Díaz-Meco M T. Identification of heterogeneous ribonucleoprotein A1 as a novel substrate for protein kinase C ζ. J Biol Chem. 1995;270:15884–15891. doi: 10.1074/jbc.270.26.15884. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi H, Brewer K A, Exton J H. Activation of the isozyme of protein kinase C by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1993;268:13–16. [PubMed] [Google Scholar]
- 40.Nishizuka Y. Intracellular signalling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992;258:607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- 40a.Novick P, Zerial M. The diversity of Rab proteins in vesicle transport. Curr Opin Cell Biol. 1997;9:496–504. doi: 10.1016/s0955-0674(97)80025-7. [DOI] [PubMed] [Google Scholar]
- 41.Ohno Y, Fuji T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Protein kinase C ζ subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci USA. 1989;86:3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okano I, Hiraoka J, Otera H, Nunoue K, Ohashi K, Iwashita S, Hirai M, Mizuno K. Identification and characterization of a novel family of serine/threonine kinases containing two N-terminal LIM motifs. J Biol Chem. 1995;270:31321–31330. doi: 10.1074/jbc.270.52.31321. [DOI] [PubMed] [Google Scholar]
- 43.Palmer R, Dekker L V, Woscholski R, Le Good J A, Gigg R, Parker P. Activation of PRK1 by phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1995;270:22412–22416. doi: 10.1074/jbc.270.38.22412. [DOI] [PubMed] [Google Scholar]
- 44.Puls A, Schmidt S, Grawe F, Stabel S. Interaction of protein kinase C ζ with ZIP, a novel protein kinase C-binding protein. Proc Natl Acad Sci USA. 1997;94:6191–6196. doi: 10.1073/pnas.94.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quest A F G, Bell R M. The regulatory region of protein kinase Cγ. J Biol Chem. 1994;269:20000–20012. [PubMed] [Google Scholar]
- 46.Ron D, Chen C-H, Caldwell J, Jamieson L, Orr E, Mochly-Rosen D. Cloning of an intracellular receptor for protein kinase C: a homolog of the β subunit of G protein. Proc Natl Acad Sci USA. 1994;91:839–843. doi: 10.1073/pnas.91.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rzymkiewicz D M, Tetsuka T, Daphna-Iken D, Srivastava S, Morrison A R. Interleukin-1β activates protein kinase Cζ in renal mesangial cells. J Biol Chem. 1996;271:17241–17246. doi: 10.1074/jbc.271.29.17241. [DOI] [PubMed] [Google Scholar]
- 48.Sacktor T C, Osten P, Valsamis P, Jiang X, Naik M U, Sublette E. Persistent activation of the ζ isoform of protein kinase C in the maintenance of long-term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sagata N, Oskarsson M, Copeland T, Brumbaugh J, Vande Woude F. Function of c-mos protooncogene product in meiotic maturation in Xenopus oocytes. Nature. 1988;335:519–525. doi: 10.1038/335519a0. [DOI] [PubMed] [Google Scholar]
- 49a.Sanchez, P., et al. Unpublished data.
- 50.Sells S F, Wood D P, Joshi-Barve S S, Muthukumar S, Jacob R J, Crist S A, Humphreys S, Rangnekar V M. Commonality of the gene programs induced by effectors of apoptosis in androgen-dependent and -independent prostate cells. Cell Growth Differ. 1994;5:457–466. [PubMed] [Google Scholar]
- 51.Shpetner H, Joly M, Hartley D, Corvera S. Potential sites of PI-3 kinase function in the endocytic pathway revealed by the PI-3 kinase inhibitor, wortmannin. J Cell Biol. 1996;132:595–605. doi: 10.1083/jcb.132.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sontag E, Sontag J M, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C ζ signaling targeted by SV40 small t to promote cell growth and NF-κB activation. EMBO J. 1997;16:5662–5671. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toker A, Meyer M, Reddy K K, Falck J R, Aneja R, Aaneja S, Parra A, Burns D J, Ballas L, M, Cantley L C. Activation of protein kinase C family members by the novel polyphosphoimositides PtdIns-3,4-P2 and PtdIns-3,4,5-P3. J Biol Chem. 1994;269:32358–32367. [PubMed] [Google Scholar]
- 54.Van Dijk M C M, Muriana F J G, van der Hoeven P C J, Widt J, Schaap D, Moolenaar W H, van Blitterswijk W J. Diacylglycerol generated by exogenous phospholipase C activates the mitogen-activated protein kinase pathway independent of Ras- and phorbol ester-sensitive protein kinase C: dependence on protein kinase C-ζ. Biochem J. 1997;323:693–699. doi: 10.1042/bj3230693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ways D K, Posekany K, Devente J, Garris T, Chen J M, Hooker J, Qin W X, Cook P, Fletcher D, Parker P. Overexpression of protein kinase C-ζ stimulates leukemic cell differentiation. Cell Growth Differ. 1994;5:1195–1203. [PubMed] [Google Scholar]
- 56.Westermann P, Knoslich M, Maier O, Lindschan C, Haller H. Protein kinase C bound to the Golgi apparatus supports the formation of constitutive transport vesicles. Biochem J. 1996;320:651–658. doi: 10.1042/bj3200651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wooten M W, Zhou G, Seibenhener M L, Coleman E S. A role for ζ protein kinase C in nerve growth factor induced differentiation of PC12 cells. Cell Growth Differ. 1994;5:395–403. [PubMed] [Google Scholar]
- 58.Wooten M W, Seibenhener M L, Matthews L H, Zhou G, Coleman E S. Modulation of ζ-protein kinase C by cyclic AMP in PC12 cells occurs through phosphorylation by protein kinase A. J Neurochem. 1996;67:1023–1031. doi: 10.1046/j.1471-4159.1996.67031023.x. [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Zutter M M, Santoro S A, Clark R. PDGF-induction of α2 integrin gene expression is mediated by protein kinase C ζ. J Cell Biol. 1996;134:1301–1311. doi: 10.1083/jcb.134.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu J, Clark R. A three-dimensional collagen lattice induces protein kinase C-ζ activity. Role in α2 integrin and collagenase mRNA expression. J Cell Biol. 1997;136:473–483. doi: 10.1083/jcb.136.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao B, Zhang Y, Delikat S, Mathias S, Basu S, Kolesnick R N. Phosphorylation of Raf by ceramide-activated protein kinase. Nature. 1995;378:307–310. doi: 10.1038/378307a0. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Kolesnick R N. Editorial: signalling through the sphingomyelin pathway. Endocrinology. 1995;136:4157–4159. doi: 10.1210/endo.136.10.7664631. [DOI] [PubMed] [Google Scholar]