Abstract
Plumage color is a characteristic trait of ducks that originates as a result of natural and artificial selection. As a conspicuous phenotypic feature, it is a breed characteristic. Previous studies have identified some genes associated with the formation of black and white plumage in ducks. However, studies on the genetic basis underlying the red plumage phenotype in ducks are limited. Here, genome-wide association analysis (GWAS) and selection signal detection (Fst, θπ ratio, and cross-population composite likelihood ratio [XP-CLR]) were conducted to identify candidate regions and genes underlying duck plumage color phenotype. Selection signal detection revealed 29 overlapping genes (including ENPP1 and ULK1) significantly associated with red plumage color in Ji'an Red ducks. ENSAPLG00000012679, ESRRG, and SPATA5 were identified as candidate genes associated with red plumage using GWAS. Selection signal detection revealed that 19 overlapping genes (including GMDS, PDIA6, and ODC1) significantly correlated with light brown plumage in Brown Tsaiya ducks. GWAS to narrow down the significant regions further revealed nine candidate genes (AKT1, ATP6V1C2, GMDS, LRP4, MAML3, PDIA6, PLD5, TMEM63B, and TSPAN8). Notably, in Brown Tsaiya ducks, GMDS, ODC1, and PDIA6 exhibit significantly differentiated allele frequencies among other feather-colored ducks, while in Ji'an Red ducks, ENSAPLG00000012679 has different allele frequency distributions compared with that in other feather-colored ducks. This study offers new insights into the variation and selection of the red plumage phenotype using GWAS and selective signals.
Key words: duck, selection signal detection, genome-wide association analysis, plumage color
INTRODUCTION
Bird plumage exists in various colors. In natural environments, plumage color diversity is usually associated with mating signals and camouflage adaptations to different surroundings. Similarly, human preferences for color have influenced poultry plumage coloration during selective breeding. Plumage color is primarily determined by melanin. Variations in color depths result from differences in the relative distribution and abundance of 2 types of melanin, that is, eumelanin (brown/black) and pheomelanin (yellow/red) (Serra, 1946). Melanin is synthesized within melanocytes. Variations in factors involved in the generation, migration, proliferation, and differentiation of melanocytes can impact melanin production, causing changes in plumage color (Yang et al., 2017; Huang et al., 2020; Hua et al., 2021).
Many genes influencing variation in plumage color phenotype have previously been identified. Extension/melanocortin-1 receptor (MC1R) is crucial in melanin synthesis, influencing various pigment deposition traits in mammals and bird species, including mice (Robbins et al., 1993), pigs (Kijas et al., 1998), sheep (Vage et al., 1999), chickens (Kerje et al., 2003), and ducks (Yu et al., 2013). The dominant white plumage color trait in chickens is associated with PMEL17, whereas the recessive white plumage color trait is related to TYR. In ducks, mutations and large intronic insertions in MITF cause the white plumage phenotype (Zhang et al., 2018; Zhou, et al., 2018). The mottled plumage color trait in chickens is caused by the EDNRB2 mutation (Kinoshita et al., 2014). Similarly, the dark-brown plumage color in chickens is caused by the deletion of a large upstream segment of SOX10 (Gunnarsson et al., 2011).
According to Chinese Poultry Genetic Resources, the Ji'an Red duck is a local specialty breed from Ji'an, Jiangxi, China, known for its red feathers, medium size, beautiful appearance, adaptability, and high egg production, which is cultivated into excellent raw material for plate duck after scientific and systematic selection and breeding. It has wide application prospects. The Brown Tsaiya duck is from Taiwan, China, and is characterized by its light brown feathers, small body size, and high egg production. However, studies on the genetic basis of red/light brown plumage color phenotypes in ducks are limited. Analyzing the reasons for the formation of their plumage color can provide a rational basis for selecting breeds and marking-assisted selection of Ji'an Rd and Brown Tsaiya ducks. Here, we reported the whole genome sequences of 18 red-feathered ducks, including 10 Brown Tsaiya and 8 Ji'an Red. We conducted a genome-wide association study (GWAS) and selection signal detection to identify candidate genes and regions associated with the red plumage color trait in ducks by integrating these data with our previously obtained and 13 publicly available samples. This study contributed to advancing our understanding of the duck plumage color genetics, thereby helping to improve varieties.
MATERIALS AND METHODS
We collected 103 ducks from 12 different breeds. The Brown Tsaiya and Ji'an Red ducks were obtained from the Taiwan and Fujian Provinces (China), respectively. The remaining duck samples were obtained from our previous studies (Zhang et al., 2018; Zhu et al., 2021; Zhang et al., 2023b). We downloaded 13 sets of sequencing data from NCBI. Their detailed information (including breed names, plumage color phenotypes, sampling locations, and primary sequence accession numbers) is presented in Table 1 and Table S1. Blood samples were collected through standard venipuncture. Genomic DNA was extracted using the TIANamp Genomic DNA Kit (TIANGEN Biotech, Beijing, China), according to the manufacturer's instructions. DNA concentration and quality were detected using NanoDrop. For genome sequencing, 2 paired-end libraries with an insert size of 500 bp were constructed and sequenced using the Illumina HiSeq 2500 platform. The average raw read sequence coverage of the duck population is 5x. The animal experimentation protocols were approved by the Animal Welfare Committee of China Agricultural University (permit XK622, Permit 046/2018), and the study was performed following ethical guidelines for animal research.
Table 1.
Summary of individuals used in this study.
| Breed | Abbreviation | Sample size | Geographic origin | Phenotype | Sample origin |
|---|---|---|---|---|---|
| Ji'an Red duck | JAN | 13 | This study; Jiang et al., 2021; Zhou et al., 2018 | red | Jiangxi, China |
| Brown Tsaiya duck | HC | 10 | This study | light brown | Taiwan, China |
| Wendeng Black duck | WD | 8 | Zhang et al., 2023b | black | Shandong, China |
| Putian Black duck | PT | 8 | Zhang et al., 2023b | black | Fujian, China |
| Longsheng-Cui duck | LONGS | 8 | Wang et al., 2021 | black | Guangxi, China |
| Spot-billed duck | BZ | 8 | Zhu et al., 2021 | hemp | Ningxia, China |
| Mallard | MD | 8 | Zhang et al., 2018 | hemp | Zhejiang, China |
| Gaoyou duck | GY | 8 | Zhang et al., 2018 | hemp | Jiangsu, China |
| Jinding duck | JD | 8 | Zhang et al., 2018 | hemp | Fujian, China |
| Mei duck | MEI | 8 | Zhu et al., 2021 | hemp | Anhui, China |
| Shanma duck | SM | 8 | Zhang et al., 2018 | hemp | Fujian, China |
| Shaoxing duck | SX | 8 | Zhang et al., 2018 | hemp | Zhejiang, China |
Alignments and Variant Identification
The raw data were filtered using default parameters using fastp (v0.20.0) (Chen et al., 2018) to avoid low-quality reads. All cleaned reads were aligned to the duck reference genome (cau_duck1.0) using BWA-MEM (v0.7.15) (Li and Durbin, 2009). The Genome Analysis Toolkit (GATK v4.1.8.1) (McKenna et al., 2010) was used for the realignment of reads and SNP calling. The ’SortSam’ command of GATK was used to convert the alignment results to the bam format and read sorting by coordinate. We performed quality control on the generated variant set using the default GATK parameters, retaining only high-quality SNP. Single nucleotide polymorphisms were filtered using the following standards: (a) QUAL > 30.0, (b) QualByDepth (QD) > 5.0, (c) FisherStrand (FS) < 60.0, (d) RMS MappingQuality (MQ) > 40.0, (e) MappingQualityRankSunTest (MQRankSum) > -12.5, and (f) ReadPosRankSum > -8.0. Finally, 11,938,694 SNP were retained for the subsequent association analysis.
Population Structure Analysis
Plink (v1.9) (Chang et al., 2015) was used for principal components analysis (PCA) to generate eigenvectors and eigenvalues. The first 2 principal components were selected as the x and y coordinates, and the data were visualized using the plot function in R.
Genome-Wide Selective Sweep Analysis
Selective sweeps between populations with red and other plumage colors were detected using Fst, θπ ratio, and cross-population composite likelihood ratio (XP-CLR). The Fst value and θπ ratio were calculated with 10-kb windows and slides with 5-kb steps using VCFtools (v0.1.13) (Danecek et al., 2011). XP-CLR was calculated with 10-kb windows and slides with 5-kb steps. In each approach, windows within the top 1% values were considered significant. Overlapping windows within the top 1% values of Fst, θπ ratio, and XP-CLR were simultaneously regarded as candidate selection regions.
GWAS
A univariate mixed linear model in GEMMA (v0.98.1) (Zhou and Stephens, 2012) was used to identify SNPs significantly associated with plumage color phenotype traits. The models are as follows:
where y denotes the vector of phenotypic values for individuals, W is the matrix of covariates, α denotes the vector of corresponding coefficients comprising the intercept, x is the vector of marker genotypes, β is an estimate of the marker/SNP additive effect, u is the vector of random effects, and ϵ is the vector of errors. The Manhattan and Quantile-Quantile plots were drawn using the R package “CMplot” package.
Candidate Gene Analysis
We used Ensembl (http://www.ensembl.org/biomart/martview/) for gene annotation. Metascape (https://metascape.org/gp/index.html) was used for the biological function and relevant pathway analyses. Beagle (v5.4) software (Browning et al., 2018) was used to construct haplotypes within the target regions. Additionally, allele frequencies and π statistics were calculated to verify the candidate sweep regions.
RESULTS
Population Structure
PCA showed a distinct stratification between domesticated breeds and wild populations based on the first principal components (PC1). Additionally, a distinct separation was observed between brown mallards and domestic duck populations based on the second principal components (PC2) (Figure 1).
Figure 1.
Principal components analysis (PCA) plot of duck populations in this study Each color represents a breed, and the abbreviations are as defined in Table 1.
Association Analysis of Red Plumage Phenotype in Ji'an Red Ducks
Selecting Plumage Color Traits
To identify the selection signature associated with red plumage, we searched the duck genome for regions using 3 statistical methods (Fst, π ratio, and XP-CLR) to compare the populations of red (JAN) and non-red (MD, BZ, JD, SX, GY, SM, MEI, WD, PT, and LONGS) plumage ducks based on sliding 10-kb windows with 5-kb steps (Figure 2). The regions within the top 1% values were considered candidate genomic regions. We identified 227, 305, and 538 candidate genes through Fst, π-ratio, and XP-CLR, respectively. On combining Fst, π ratio, and XP-CLR analyses, we identified 29 overlapping selected genes, including DCN, ENPP1, ESRRG, FGD4, GALNT1, GALNTL6, HAPLN1, RASSF6, RIMKLB, SKAP1, SLC25A21, SPAG16, SPATA5, SRGAP1, TPK1, and ULK1 (Table 2). In our list of overlapping selected genes, ENPP1 and ULK1 were notable owing to their association with pigment deposition.
Figure 2.
Selective signals of red plumage color in Ji'an Red duck. (A) Genome wide distribution of Fst. (B) Genome wide distribution of θπ ratio. (C) Genome wide distribution of XP-CLR. (D) Fst, θπ ratio, and XP-CLR screened for overlapping genes.
Table 2.
Overlapping significant regions identified through Fst, pi, and cross-population composite likelihood ratio (XP-CLR) and associated genes in Ji'an red duck.
| Gene | Full Name | Chromosome | Location (bp)1 | Length (bp)1 | Number (SNP)2 |
|---|---|---|---|---|---|
| FLT1 | Fms-related receptor tyrosine kinase 1 | 1 | 140607629-140714774 | 107145 | 0 |
| SRGAP1 | SLIT-ROBO Rho GTPase activating protein 1 | 1 | 34632585-34784998 | 152413 | 0 |
| DCN | Decorin | 1 | 47576720-47618330 | 41610 | 0 |
| FGD4 | FYVE, RhoGEF and PH domain containing 4 | 1 | 61408749-61520984 | 112235 | 0 |
| A2ML1 | alpha-2-macroglobulin like 1 | 1 | 78744053-78759702 | 15649 | 0 |
| RIMKLB | Ribosomal modification protein rimK like family member B | 1 | 78809659-78868404 | 58745 | 0 |
| PIEZO2 | Piezo-type mechanosensitive ion channel component 2 | 2 | 108182771-108436216 | 253445 | 0 |
| TPK1 | Thiamin pyrophosphokinase 1 | 2 | 56610690-56924039 | 313349 | 0 |
| GALNT1 | Glycine receptor alpha 3 | 2 | 88805150-88885411 | 80261 | 0 |
| ESRRG | Estrogen-related receptor gamma | 3 | 19239319-19591403 | 352084 | 2 |
| ENPP1 | Ectonucleotide pyrophosphatase/phosphodiesterase 1 | 3 | 59439252-59495836 | 56584 | 0 |
| APBB2 | Amyloid beta precursor protein binding family B member 2 | 4 | 41121623-41293764 | 172141 | 0 |
| LIMCH1 | LIM and calponin homology domains 1 | 4 | 41347255-41516480 | 169225 | 0 |
| RASSF6 | Ras association domain family member 6 | 4 | 46531424-46545165 | 13741 | 0 |
| SPATA5 | Spermatogenesis associated 5 | 4 | 50364115-50553393 | 189278 | 2 |
| GALNTL6 | Polypeptide N-acetylgalactosaminyltransferase like 6 | 4 | 67885840-68340827 | 454987 | 0 |
| GLRA3 | Glycine receptor alpha 3 | 4 | 69037780-69126058 | 88278 | 0 |
| EHD4 | EH domain containing 4 | 5 | 30785990-30809368 | 23378 | 0 |
| SLC25A21 | Solute carrier family 25 member 21 | 5 | 38842670-39100823 | 258153 | 0 |
| SPAG16 | Sperm-associated antigen 16 | 7 | 7830019-8217436 | 387417 | 0 |
| ULK1 | Unc-51-like autophagy activating kinase 1 | 16 | 2432092-2511157 | 79065 | 0 |
| CA10 | Carbonic anhydrase 10 | 19 | 8107861-8307451 | 199590 | 0 |
| SKAP1 | Src kinase-associated phosphoprotein 1 | 28 | 4928465-5045546 | 117081 | 0 |
| CCBE1 | Collagen and calcium binding EGF domains 1 | Z | 12928073-13020979 | 92906 | 0 |
| CELF4 | CUGBP Elav-like family member 4 | Z | 2270219-2590923 | 320704 | 0 |
| SYK | Spleen-associated tyrosine kinase | Z | 47469844-47525435 | 55591 | 0 |
| HAPLN1 | Hyaluronan and proteoglycan link protein 1 | Z | 75004898-75063923 | 59025 | 0 |
| FAM172A | Family with sequence similarity 172 member A | Z | 79206564-79452504 | 245940 | 0 |
| ADGRV1 | Adhesion G protein-coupled receptor V1 | Z | 80535623-80810901 | 275278 | 0 |
Source: Reference cau_duck1.0 primary assembly (Ensembl).
The number of genomes significant SNP located in gene identified in genome-wide association analysis
GWAS for Plumage Color Traits
Subsequently, we used the red plumage group as the case and the non-red group as the control and performed GWAS to identify candidate genes that influence the plumage color traits in Ji'an Red ducks (Figure 3). We found 3 significant peaks on chromosomes 3, 10, and 21, with 418 significant SNPs. Ensembl was used to annotate significant SNPs. Fifty-five genes were identified around significant peaks. The most significant SNP (chr10: 15076449; −log10p = 16.88) was in ENSAPLG00000012679. ESRRG and SPATA5 overlap within the top 1% windows detected in the selection signal scan of Ji'an Red ducks against other non-red-feathered duck populations, prompting more comprehensive future studies.
Figure 3.
Genome-wide association study for red plumage color in Ji'an Red duck. (A) Manhattan plot of genome-wide association study. (B) Quantile–quantile (Q–Q) plots from the genome-wide association analysis (GWAS).
Analyzing Candidate Genes
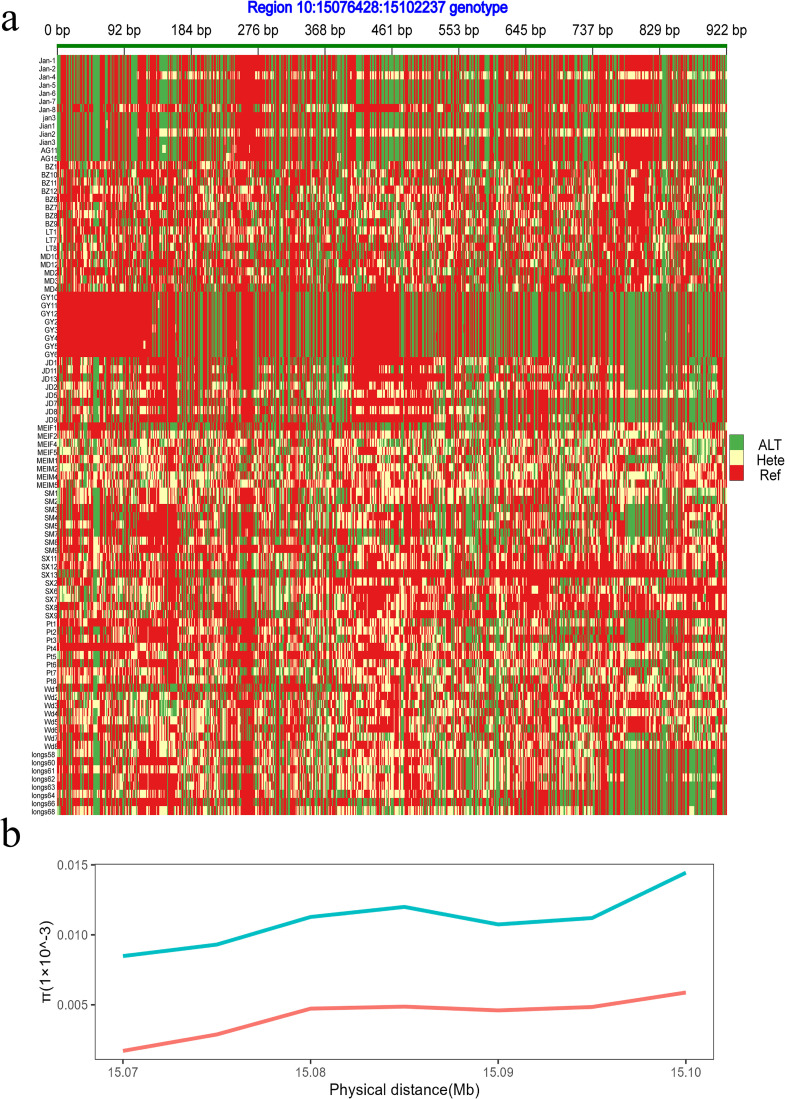
The genomic architecture of the candidate genes in different plumage populations was analyzed by calculating the allele frequency. Notably, ENSAPLG00000012679 exhibited significant differences in allele frequency between Ji'an Red ducks and other plumage populations (Table S2), as verified in the haplotype comparison analysis (Figure 4).
Figure 4.
Haplotype and nucleotide diversities in ENSAPLG00000012679 regions. (A) Heatmap in ENSAPLG00000012679 regions. (B) Nucleotide diversity in ENSAPLG00000012679 regions. Red represents Ji'an Red duck; blue represents ducks with other plumage color.
Association Analysis of Red Plumage Phenotype in Brown Tsaiya Ducks
Selecting Plumage Color Traits
To identify the selection signature associated with red plumage in Brown Tsaiya ducks, we employed the same methods that were used in Ji'an Red ducks (Figure 5). We identified 253, 371, and 490 candidate genes through Fst, π-ratio, and XP-CLR, respectively. On combining Fst, π ratio, and XP-CLR analyses, we identified 19 overlapping selected genes (AKT1, ARHGAP15, ATP6V1C2, CCDC149, FNTA, GMDS, ITGB1BP1, LRFN2, LRP4, LY75, MAML3, ODC1, PDIA6, PEX5L, PLD5, RBPJ, SLC4A7, TMEM63B, and TSPAN8) (Table 3). These genes are potentially associated with light brown plumage color in Brown Tsaiya ducks. In our overlapping selected gene list, GMDS, PDIA6, and ODC1 were notable owing to their association with pigment deposition.
Figure 5.
Selective signals of red plumage color in Brown Tsaiya ducks. (A) Genome wide distribution of Fst. (B) Genome wide distribution of θπ ratio. (C) Genome wide distribution of XP-CLR. d. Fst, θπ ratio, and XP-CLR screened for overlapping genes.
Table 3.
Overlapping significant regions identified through Fst, pi, and cross-population composite likelihood ratio (XP-CLR) and associated genes in Brown Tsaiya ducks.
| Gene | Full name | Chromosome | Location (bp)1 | Length (bp)1 | Number (SNP)2 |
|---|---|---|---|---|---|
| TSPAN8 | Tetraspanin 8 | 1 | 37426355-37505746 | 79391 | 2 |
| SLC4A7 | Solute carrier Family 4 Member 7 | 2 | 40756860-41023690 | 266830 | 0 |
| GMDS | GDP-mannose 4,6-dehydratase | 2 | 79265182-79671011 | 405829 | 67 |
| LRFN2 | Leucine-rich repeat and fibronectin type III domain containing 2 | 3 | 31578187-31799373 | 221186 | 0 |
| TMEM63B | Transmembrane protein 63B | 3 | 33884914-33910150 | 25236 | 9 |
| PLD5 | Phospholipase D family member 5 | 3 | 39264361-39411447 | 147086 | 1 |
| PDIA6 | Protein disulfide isomerase family A member 6 | 3 | 76340827-76361379 | 20552 | 1 |
| ATP6V1C2 | ATPase H+ transporting V1 subunit C2 | 3 | 76352052-76370640 | 18588 | 1 |
| ODC1 | Ornithine decarboxylase 1 | 3 | 76457110-76500422 | 43312 | 0 |
| ITGB1BP1 | Integrin subunit beta 1 binding protein 1 | 3 | 77064448-77071033 | 6585 | 1 |
| CCDC149 | Coiled-coil domain containing 149 | 4 | 35632538-35680171 | 47633 | 0 |
| RBPJ | Recombination signal binding protein for immunoglobulin kappa J region | 4 | 35946940-36092637 | 145697 | 0 |
| MAML3 | Mastermind like transcriptional coactivator 3 | 4 | 56585225-56781405 | 196180 | 10 |
| LRP4 | LDL receptor-related protein 4 | 5 | 32874741-32912588 | 37847 | 27 |
| AKT1 | AKT serine/threonine kinase 1 | 5 | 55272740-55337060 | 64320 | 16 |
| LY75 | Lymphocyte antigen 75 | 7 | 20553853-20598781 | 44928 | 0 |
| ARHGAP15 | Rho GTPase activating protein 15 | 7 | 31076813-31407565 | 330752 | 0 |
| PEX5L | Peroxisomal biogenesis factor 5 like | 9 | 18643390-18680809 | 37419 | 0 |
| FNTA | Farnesyltransferase, CAAX box, alpha | Z | 52676230-52686816 | 10586 | 0 |
Source: Reference cau_duck1.0 primary assembly (Ensembl).
The number of genomes significant SNP located in gene identified in genome-wide association analysis.
GWAS for Plumage Color Traits
GWAS was performed in Brown Tsaiya ducks to further support results from the selection sweep analysis (Figure 6). There were 3 significant peaks on chromosomes 1, 3, and 11, with 2,224 significant SNPs. Among the significant SNPs, 197 candidate genes were annotated. The most significant SNP (chr11: 1844022; −log10p = 57.32) was in AP4E1. Additionally, AKT1, ATP6V1C2, GMDS, LRP4, MAML3, PDIA6, PLD5, TMEM63B, and TSPAN8 were annotated and overlapped within the top 1% windows detected in the selection signal scan of Brown Tsaiya ducks against other non-red-feathered duck populations. Similarly, 2 overlapping genes associated with pigment deposition (PDIA6 and GMDS) were identified using GWAS and selection signal detection and require focus.
Figure 6.
Genome-wide association study for red plumage in Brown Tsaiya ducks. (A) Manhattan plot of genome-wide association study. (B) Quantile–quantile (Q–Q) plots from the genome-wide association analysis (GWAS).
Analyzing Candidate Genes
We calculated the allele frequency differences between Brown Tsaiya and other duck breeds with respect to these significant genes and observed that GMDS, ODC1, and PDIA6 exhibited significant differences between Brown Tsaiya and other duck breeds (Tables S3, S4, and S5); the same was verified in the haplotype comparison analysis (Figure 7; Figures S1 and S2).
Figure 7.
Haplotype and nucleotide diversities in PDIA6 regions. (A) Heatmap in PDIA6 regions. (B) Nucleotide diversity in PDIA6 regions. Red represents the Brown Tsaiya duck; blue represents ducks with other plumage color.
DISCUSSION
During extended domestication and breeding processes, Ji'an Red and Brown Tsaiya ducks exhibit distinct plumage color phenotypes (red/light brown). Ji'an Red and Brown Tsaiya ducks are among the few domestic duck breeds characterized by their red plumage, that holds substantial cultural and economic significance. Therefore, studying candidate genes associated with plumage color traits is essential. Natural and artificial selection significantly influences the genome during animal domestication. Rapidly advancing sequencing technologies provided greater convenience for studying genetic variations associated with phenotypes. In this study, we performed GWAS and selection sweep analysis using SNPs derived from whole genome sequencing on 103 duck samples and identified candidate genes associated with the red plumage phenotype.
In Ji'an Red ducks, we analyzed the genetic basis of the red plumage trait through selection signal analysis and GWAS and identified 19 overlapping genes. Among these genes, ENPP1 (encoding ectonucleotide pyrophosphatase/phosphodiesterase 1) and ULK1 were associated with pigment deposition. The somatomedin-B-like 2 (SMB2) domain of ENPP1 affects epidermal pigmentation and differentiation (Eytan et al., 2013). A missense mutation in ENPP1 can reportedly induce the development of hyperpigmented lesions with hypopigmented macules, causing a hereditary skin disorder (Chourabi et al., 2018). ULK1 regulated melanin levels, where its depletion increased TYR and MITF transcriptions (Ho et al., 2011; Kalie et al., 2013). In addition to the candidate genes associated with pigmentation, ENPP1 and ULK1, 2 other overlapped genes were detected through GWAS, ESRRG, and SPATA5. ESRRG is involved in steroid hormone-mediated signaling pathway and is essential in the development of the renal papilla (Berry et al., 2011). SPATA5 correlates with brain development (Buchert et al., 2016; Tanaka et al., 2015). Other candidate genes were related to growth and development, the nervous system, and fat deposition. The mechanism underlying red plumage is unknown in ducks. We speculated that these genes may influence the development of the red plumage phenotype in Ji'an Red ducks; however, their precise mechanisms of action remain unclear.
We performed selection signal detection (including Fst, θπ ratio, and XP-CLR) on Brown Tsaiya ducks and identified 19 overlapped genes with diverse functions, indicating that plumage color is a complex multifactorial trait. Among the candidate genes, those associated with pigment deposition include GMDS, PDIA6, and OCD1. GMDS is associated with pigmentation traits in chickens (Nie et al., 2016) and is related to the non-white wool trait in sheep (Zhang et al., 2023a). PDIA6 is a member of the protein disulfide isomerase family that activates the Wnt/β-catenin pathway (Gao et al., 2016). The Wnt/β-catenin signaling pathway plays a crucial role in melanogenesis (Liu et al., 2023). PDIA6 is a candidate gene for pigmentation in East Asian populations (Hider et al., 2013). Increased ODC1 levels induce elevated putrescine levels, promoting the generation of TYR (tyrosinase) and facilitating melanin synthesis in human skin (Sridharan et al., 2020). Other candidate genes had multiple functions. TSPAN8, LRFN2, PLD5, ATP6V1C2, ITGB1BP1, CCDC149, MAML3, LY75, and ARHGAP15 correlate with disease (Pan et al., 2015; Onishi et al., 2018; Takagi et al., 2018; Jobst-Schwan et al., 2020; Shah et al., 2020; Verma et al., 2020; Liu et al., 2021; Kessler et al., 2022; Lee et al., 2022; Li et al., 2022; Zhou et al., 2022; Ahmad et al., 2023). SLC4A7 is related to purine and pyrimidine nucleotide biosynthetic processes (Ali et al., 2022). TMEM63B is associated with ion channel activity (Marques et al., 2019; Du et al., 2020). RBPJ is related to angiogenesis (Jiang et al., 2022) and somitogenesis (Shi and Stanley, 2003; Ferjentsik et al., 2009). LRP4 is associated with the development of the nervous system (Shen et al., 2015; DePew and Mosca, 2021). AKT1 is associated with carbohydrate and glucose metabolism (Toda et al., 2020; Wu et al., 2023). PEX5L is related to adipogenesis (Guo et al., 2021). FNTA correlates with protein farnesyltransferase activity (Wang et al., 1996). Among the overlapping genes identified through the selection signal scan analysis, AKT1, ATP6V1C2, GMDS, LRP4, MAML3, PDIA6, PLD5, TMEM63B, and TSPAN8 were detected through GWAS. Notably, GMDS and PDIA6 are markedly associated with pigment deposition in both analysis. Haplotype analysis indicates that the 2 genes allow for a significant differentiation between Brown Tsaiya ducks and those with other plumage colors. Furthermore, there were significant differences in the genotype frequencies for GMDS and PDIA6; however, the SNPs were in the intronic regions. Given the roles of these genes in pigment deposition, it was hypothesized that the genetic basis of the red plumage phenotype is complex, possibly involving multiple genes.
In the Brown Tsaiya and Ji'an Red ducks, distinct significant peaks were not detected in the red/white group of the association analysis. This could be attributed to the influence of interactions among genes or the hierarchical dominance order of feather colors in ducks, with white feathers exhibiting precedence over red feathers. Hence, gene effects may not be detectable at the genomic level.
In this study, we performed selection signal scans (using methods such as Fst, θπ ratio, and XP-CLR) to identify selection signatures. We simultaneously conducted an association mapping analysis to investigate the genetic basis of the red plumage phenotype. GWAS was designed based on a case-control approach, comparing red plumage color ducks to different plumage color ducks. This design facilitated the precise localization of candidate genes influencing plumage color through overlap between multiple methods.
However, there were some limitations that could introduce ambiguity in interpreting the selected signals. The overlapping genes identified in our analysis may be associated with the distinction between red plumage and other phenotypes and with signals of breed-specific selection that occurred during the domestication process, potentially related to other traits. Further studies are required to validate these candidate genes.
The study revealed potential candidate genes involved in the formation of the red plumage phenotype in duck populations. Specifically, ENSAPLG00000012679, ENPP1, ULK1, GMDS, PDIA6, and ODC1 were the candidate genes associated with the formation of the red plumage phenotype in ducks. This study contributes to a deeper understanding of the duck plumage color genetics, highlighting the complexity of the trait's formation and providing new insights into the variation and selection of the red plumage phenotype in Ji'an Red ducks and Brown Tsaiya ducks, which is essential for breeding purposes.
ACKNOWLEDGMENTS
This work was supported by the Beijing Agriculture Innovation Consortium [grant numbers BAIC06-2023] and supported by the High-performance Computing Platform of China Agricultural University.
DISCLOSURES
The authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2024.103694.
Appendix. Supplementary materials
REFERENCES
- Ahmad S.M.S., Nazar H., Rahman M.M., Rusyniak R.S., Ouhtit A. ITGB1BP1, a novel transcriptional target of CD44-downstream signaling promoting cancer cell invasion. Breast Cancer (Dove Med. Press) 2023;15:373–380. doi: 10.2147/BCTT.S404565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali E.S., Liponska A., O'Hara B.P., Amici D.R., Torno M.D., Gao P., Asara J.M., Yap M.F., Mendillo M.L., Ben-Sahra I. The mTORC1-SLC4A7 axis stimulates bicarbonate import to enhance de novo nucleotide synthesis. Mol. Cell. 2022;82:3284–3298. doi: 10.1016/j.molcel.2022.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry R., Harewood L., Pei L., Fisher M., Brownstein D., Ross A., Alaynick W.A., Moss J., Hastie N.D., Hohenstein P., Davies J.A., Evans R.M., FitzPatrick D.R. Esrrg functions in early branch generation of the ureteric bud and is essential for normal development of the renal papilla. Hum. Mol. Genet. 2011;20:917–926. doi: 10.1093/hmg/ddq530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning B.L., Zhou Y., Browning S.R. A one-penny imputed genome from next-generation reference panels. Am. J. Hum. Genet. 2018;103:338–348. doi: 10.1016/j.ajhg.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchert R., Nesbitt A.I., Tawamie H., Krantz I.D., Medne L., Helbig I., Matalon D.R., Reis A., Santani A., Sticht H., Abou Jamra R. SPATA5 mutations cause a distinct autosomal recessive phenotype of intellectual disability, hypotonia and hearing loss. Orphanet J. Rare Dis. 2016;11:130. doi: 10.1186/s13023-016-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.C., Chow C.C., Tellier L.C.A.M., Vattikuti S., Purcell S.M., Lee J.J. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4:7. doi: 10.1186/s13742-015-0047-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.F., Zhou Y.Q., Chen Y.R., Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:884–890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chourabi M., Liew M.S., Lim S., H'Mida-Ben Brahim D., Boussofara L., Dai L., Wong P.M., Foo J.N., Sriha B., Robinson K.S., Denil S., Common J.E., Mamai O., Ben Khalifa Y., Bollen M., Liu J., Denguezli M., Bonnard C., Saad A., Reversade B. ENPP1 mutation causes recessive cole disease by altering melanogenesis. J. Invest. Dermatol. 2018;138:291–300. doi: 10.1016/j.jid.2017.08.045. [DOI] [PubMed] [Google Scholar]
- Danecek P., Auton A., Abecasis G., Albers C.A., Banks E., DePristo M.A., Handsaker R.E., Lunter G., Marth G.T., Sherry S.T., McVean G., Durbin R., Grp G.P.A. The variant call format and VCFtools. Bioinformatics. 2011;27:2156–2158. doi: 10.1093/bioinformatics/btr330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePew A.T., Mosca T.J. Conservation and innovation: versatile roles for LRP4 in nervous system development. J. Dev. Biol. 2021;9:9. doi: 10.3390/jdb9010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H., Ye C., Wu D., Zang Y.Y., Zhang L., Chen C., He X.Y., Yang J.J., Hu P., Xu Z., Wan G., Shi Y.S. The cation channel TMEM63B is an osmosensor required for hearing. Cell Rep. 2020;31 doi: 10.1016/j.celrep.2020.107596. [DOI] [PubMed] [Google Scholar]
- Eytan O., Morice-Picard F., Sarig O., Ezzedine K., Isakov O., Li Q., Ishida-Yamamoto A., Shomron N., Goldsmith T., Fuchs-Telem D., Adir N., Uitto J., Orlow S.J., Taieb A., Sprecher E. Cole disease results from mutations in ENPP1. Am. J. Hum. Genet. 2013;93:752–757. doi: 10.1016/j.ajhg.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferjentsik Z., Hayashi S., Dale J.K., Bessho Y., Herreman A., De Strooper B., Del Monte G., De La Pompa J.L., Maroto M. Notch is a critical component of the mouse somitogenesis oscillator and is essential for the formation of the somites. PLoS Genet. 2009;5 doi: 10.1371/journal.pgen.1000662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Sun B., Fu H., Chi X., Wang F., Qi X., Hu J., Shao S.J.O. PDIA6 promotes the proliferation of HeLa cells through activating the Wnt/β-catenin signaling pathway. Oncotarget. 2016;7:53289. doi: 10.18632/oncotarget.10795. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gunnarsson U., Kerje S., Bed'hom B., Sahlqvist A.S., Ekwall O., Tixier-Boichard M., Kämpe O., Andersson L. The Dark brown plumage color in chickens is caused by an 8.3-kb deletion upstream of SOX10. Pigment Cell Melanoma Res. 2011;24:268–274. doi: 10.1111/j.1755-148X.2011.00825.x. [DOI] [PubMed] [Google Scholar]
- Guo L., Chao X., Huang W., Li Z., Luan K., Ye M., Zhang S., Liu M., Li H., Luo W., Nie Q., Zhang X., Luo Q. Whole transcriptome analysis reveals a potential regulatory mechanism of LncRNA-FNIP2/miR-24-3p/FNIP2 axis in chicken adipogenesis. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.653798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hider J.L., Gittelman R.M., Shah T., Edwards M., Rosenbloom A., Akey J.M., Parra E.J. Exploring signatures of positive selection in pigmentation candidate genes in populations of East Asian ancestry. BMC Evol. Biol. 2013;13:150. doi: 10.1186/1471-2148-13-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H., Kapadia R., Al-Tahan S., Ahmad S., Ganesan A.K. WIPI1 coordinates melanogenic gene transcription and melanosome formation via TORC1 inhibition. J. Biol. Chem. 2011;286:12509–12523. doi: 10.1074/jbc.M110.200543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua G., Chen J., Wang J., Li J., Deng X. Genetic basis of chicken plumage color in artificial population of complex epistasis. Anim. Genet. 2021;52:656–666. doi: 10.1111/age.13094. [DOI] [PubMed] [Google Scholar]
- Huang T., Pu Y., Song C., Sheng Z., Hu X. A quantitative trait locus on chromosome 2 was identified that accounts for a substantial proportion of phenotypic variance of the yellow plumage color in chicken. Poult. Sci. 2020;99:2902–2910. doi: 10.1016/j.psj.2020.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Lin R., Xiao C.Y., Xie T.H., Jiang Y.X., Chen J.H., Ni P., Sung W.K., Han J.L., Du X.Y., Li S.J. Analysis of whole-genome re-sequencing data of ducks reveals a diverse demographic history and extensive gene flow between Southeast/South Asian and Chinese populations. Genet. Sel. Evol. 2021;53:35. doi: 10.1186/s12711-021-00627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q., Ma Y., Zhao Y., Yao M.D., Zhu Y., Zhang Q.Y., Yan B. tRNA-derived fragment tRF-1001: A novel anti-angiogenic factor in pathological ocular angiogenesis. Mol. Ther. Nucleic Acids. 2022;30:407–420. doi: 10.1016/j.omtn.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst-Schwan T., Klämbt V., Tarsio M., Heneghan J.F., Majmundar A.J., Shril S., Buerger F., Ottlewski I., Shmukler B.E., Topaloglu R. Whole exome sequencing identified ATP6V1C2 as a novel candidate gene for recessive distal renal tubular acidosis. Kidney Int. 2020;97:567–579. doi: 10.1016/j.kint.2019.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalie E., Razi M., Tooze S.A. ULK1 regulates melanin levels in MNT-1 cells independently of mTORC1. Plos One. 2013;8:e75313. doi: 10.1371/journal.pone.0075313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerje S., Lind J., Schutz K., Jensen P., Andersson L. Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim. Genet. 2003;34:241–248. doi: 10.1046/j.1365-2052.2003.00991.x. [DOI] [PubMed] [Google Scholar]
- Kessler M.D., Damask A., O'Keeffe S., Banerjee N., Li D., Watanabe K., Marketta A., Van Meter M., Semrau S., Horowitz J., Tang J., Kosmicki J.A., Rajagopal V.M., Zou Y., Houvras Y., Ghosh A., Gillies C., Mbatchou J., White R.R., Verweij N., Bovijn J., Parikshak N.N., LeBlanc M.G., Jones M., Regeneron Genetics C., Collaboration G.-R.D., Glass D.J., Lotta L.A., Cantor M.N., Atwal G.S., Locke A.E., Ferreira M.A.R., Deering R., Paulding C., Shuldiner A.R., Thurston G., Ferrando A.A., Salerno W., Reid J.G., Overton J.D., Marchini J., Kang H.M., Baras A., Abecasis G.R., Jorgenson E. Common and rare variant associations with clonal haematopoiesis phenotypes. Nature. 2022;612:301–309. doi: 10.1038/s41586-022-05448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kijas J.M., Wales R., Tornsten A., Chardon P., Moller M., Andersson L. Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics. 1998;150:1177–1185. doi: 10.1093/genetics/150.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita K., Akiyama T., Mizutani M., Shinomiya A., Ishikawa A., Younis H.H., Tsudzuki M., Namikawa T., Matsuda Y. Endothelin receptor B2 (EDNRB2) is responsible for the tyrosinase-independent recessive white (mow) and mottled (mo) plumage phenotypes in the chicken. PLoS One. 2014;9:e86361. doi: 10.1371/journal.pone.0086361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Krishnan V., Wirth L.J., Nucera C., Venturina M., Sadow P.M., Mita A., Sacks W. Case report of CCDC149-ALK fusion: a novel genetic alteration and a clinically relevant target in metastatic papillary thyroid carcinoma. Thyroid. 2022;32:1580–1585. doi: 10.1089/thy.2022.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Huang J., Chen S., He Y., Wang Z., Peng J. High expression of ATP6V1C2 predicts unfavorable overall survival in patients with colon adenocarcinoma. Front. Genet. 2022;13 doi: 10.3389/fgene.2022.930876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25:1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Li J., Ma Y., Xu C., Wang Y., He Y. MicroRNA miR-145-5p inhibits Phospholipase D 5 (PLD5) to downregulate cell proliferation and metastasis to mitigate prostate cancer. Bioengineered. 2021;12:3240–3251. doi: 10.1080/21655979.2021.1945361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W., Chen Q., Xia Y. New mechanistic insights of melasma. Clin. Cosmet. Investig. Dermatol. 2023;16:429–442. doi: 10.2147/CCID.S396272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques M.C., Albuquerque I.S., Vaz S.H., Bernardes G.J. Overexpression of osmosensitive Ca2+-permeable channel TMEM63B promotes migration in HEK293T cells. Biochemistry. 2019;58:2861–2866. doi: 10.1021/acs.biochem.9b00224. [DOI] [PubMed] [Google Scholar]
- McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., DePristo M.A. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie C., Zhang Z., Zheng J., Sun H., Ning Z., Xu G., Yang N., Qu L. Genome-wide association study revealed genomic regions related to white/red earlobe color trait in the Rhode Island Red chickens. BMC Genet. 2016;17:115. doi: 10.1186/s12863-016-0422-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H., Ichimiya S., Yanai K., Umebayashi M., Nakamura K., Yamasaki A., Imaizumi A., Nagai S., Murahashi M., Ogata H., Morisaki T. RBPJ and MAML3: potential therapeutic targets for small cell lung cancer. Anticancer Res. 2018;38:4543–4547. doi: 10.21873/anticanres.12758. [DOI] [PubMed] [Google Scholar]
- Pan S.J., Wu Y.B., Cai S., Pan Y.X., Liu W., Bian L.G., Sun B., Sun Q.F. Over-expression of tetraspanin 8 in malignant glioma regulates tumor cell progression. Biochem. Biophys. Res. Commun. 2015;458:476–482. doi: 10.1016/j.bbrc.2015.01.128. [DOI] [PubMed] [Google Scholar]
- Robbins L.S., Nadeau J.H., Johnson K.R., Kelly M.A., Roselli-Rehfuss L., Baack E., Mountjoy K.G., Cone R.D. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell. 1993;72:827–834. doi: 10.1016/0092-8674(93)90572-8. [DOI] [PubMed] [Google Scholar]
- Serra J.A. Constitution of hair melanins. Nature. 1946;157:771. doi: 10.1038/157771a0. [DOI] [PubMed] [Google Scholar]
- Shah R., Sharma V., Singh H., Sharma I., Bhat G.A., Shah I.A., Iqbal B., Rafiq R., Nissa N., Muzaffar M., Rasool M.T., Lone G.N., Kaul S., Lone M.M., Rai E., Dar N.A., Sharma S. LRFN2 gene variant rs2494938 provides susceptibility to esophageal cancer in the population of Jammu and Kashmir. J. Cancer Res. Ther. 2020;16:S156–S159. doi: 10.4103/jcrt.JCRT_613_19. [DOI] [PubMed] [Google Scholar]
- Shen C., Xiong W.C., Mei L. LRP4 in neuromuscular junction and bone development and diseases. Bone. 2015;80:101–108. doi: 10.1016/j.bone.2015.05.012. [DOI] [PubMed] [Google Scholar]
- Shi S., Stanley P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl. Acad. Sci. U. S. A. 2003;100:5234–5239. doi: 10.1073/pnas.0831126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan A., Shi M., Leo V.I., Subramaniam N., Lim T.C., Uemura T., Igarashi K., Tien Guan S.T., Tan N.S., Vardy L.A. The polyamine putrescine promotes human epidermal melanogenesis. J. Invest. Dermatol. 2020;140:2032–2040. doi: 10.1016/j.jid.2020.02.009. [DOI] [PubMed] [Google Scholar]
- Takagi K., Miki Y., Onodera Y., Ishida T., Watanabe M., Sasano H., Suzuki T. ARHGAP15 in human breast carcinoma: a potent tumor suppressor regulated by androgens. Int. J. Mol. Sci. 2018;19:804. doi: 10.3390/ijms19030804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A.J., Cho M.T., Millan F., Juusola J., Retterer K., Joshi C., Niyazov D., Garnica A., Gratz E., Deardorff M., Wilkins A., Ortiz-Gonzalez X., Mathews K., Panzer K., Brilstra E., van Gassen K.L., Volker-Touw C.M., van Binsbergen E., Sobreira N., Hamosh A., McKnight D., Monaghan K.G., Chung W.K. Mutations in SPATA5 are associated with microcephaly, intellectual disability, seizures, and hearing loss. Am. J. Hum. Genet. 2015;97:457–464. doi: 10.1016/j.ajhg.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda G., Soeda K., Okazaki Y., Kobayashi N., Masuda Y., Arakawa N., Suwanai H., Masamoto Y., Izumida Y., Kamei N., Sasako T., Suzuki R., Kubota T., Kubota N., Kurokawa M., Tobe K., Noda T., Honda K., Accili D., Yamauchi T., Kadowaki T., Ueki K. Insulin-and lipopolysaccharide-mediated signaling in adipose tissue macrophages regulates postprandial glycemia through Akt-mTOR activation. Mol. Cell. 2020;79:43–53. doi: 10.1016/j.molcel.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vage D.I., Klungland H., Lu D., Cone R.D. Molecular and pharmacological characterization of dominant black coat color in sheep. Mamm. Genome. 1999;10:39–43. doi: 10.1007/s003359900939. [DOI] [PubMed] [Google Scholar]
- Verma S., Bakshi D., Sharma V., Sharma I., Shah R., Bhat A., Bhat G.R., Sharma B., Wakhloo A., Kaul S., Heer V., Bhat A., Abrol D., Verma V., Kumar R. Genetic variants of DNAH 11 and LRFN 2 genes and their association with ovarian and breast cancer. Int. J. Gynecol. Obstet. 2020;148:118–122. doi: 10.1002/ijgo.12997. [DOI] [PubMed] [Google Scholar]
- Wang T., Danielson P.D., Li B.Y., Shah P.C., Kim S.D., Donahoe P.K. The p21(RAS) farnesyltransferase alpha subunit in TGF-beta and activin signaling. Science. 1996;271:1120–1122. doi: 10.1126/science.271.5252.1120. [DOI] [PubMed] [Google Scholar]
- Wang R., Sun J.L., Han H., Huang Y.F., Chen T., Yang M.M., Wei Q., Wan H.F., Liao Y.Y. Whole-genome resequencing reveals genetic characteristics of different duck breeds from the Guangxi region in China. G3 (Bethesda) 2021;11:jkab054. doi: 10.1093/g3journal/jkab054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.X., Xu Y.C., Pantopoulos K., Tan X.Y., Wei X.L., Zheng H., Luo Z. Glycophagy mediated glucose-induced changes of hepatic glycogen metabolism via OGT1-AKT1-FOXO1(Ser238) pathway. J. Nutr. Biochem. 2023;117 doi: 10.1016/j.jnutbio.2023.109337. [DOI] [PubMed] [Google Scholar]
- Yang L., Du X., Wei S., Gu L., Li N., Gong Y., Li S. Genome-wide association analysis identifies potential regulatory genes for eumelanin pigmentation in chicken plumage. Anim. Genet. 2017;48:611–614. doi: 10.1111/age.12573. [DOI] [PubMed] [Google Scholar]
- Yu W., Wang C., Xin Q., Li S., Feng Y., Peng X., Gong Y. Non-synonymous SNPs in MC1R gene are associated with the extended black variant in domestic ducks (Anas platyrhynchos) Anim. Genet. 2013;44:214–216. doi: 10.1111/j.1365-2052.2012.02377.x. [DOI] [PubMed] [Google Scholar]
- Zhang W., Jin M., Lu Z., Li T., Wang H., Yuan Z., Wei C. Whole genome resequencing reveals selection signals related to wool color in sheep. Animals (Basel). 2023;13:3265. doi: 10.3390/ani13203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.Y., Zhu T., Wang L., Lv X.Z., Yang W.F., Qu C.Q., Li H.Y., Wang H.E., Ning Z.H., Qu L.J. Genome-wide association study reveals the genetic basis of duck plumage colors. Genes (Basel) 2023;14:856. doi: 10.3390/genes14040856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Jia Y., Almeida P., Mank J.E., van Tuinen M., Wang Q., Jiang Z., Chen Y., Zhan K., Hou S., Zhou Z., Li H., Yang F., He Y., Ning Z., Yang N., Qu L. Whole-genome resequencing reveals signatures of selection and timing of duck domestication. Gigascience. 2018;7:giy027. doi: 10.1093/gigascience/giy027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Stephens M. Genome-wide efficient mixed-model analysis for association studies. Nat. Genet. 2012;44:821–U136. doi: 10.1038/ng.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xu L., Wang J., Ge B., Wang Q., Wang T., Liu C., Wei B., Wang Q., Gao Y. LRFN2 binding to NMDAR inhibits the progress of ESCC via regulating the Wnt/β-Catenin and NF-κB signaling pathway. Cancer Sci. 2022;113:3566–3578. doi: 10.1111/cas.15510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z.K., Li M., Cheng H., Fan W.L., Yuan Z.R., Gao Q., Xu Y.X., Guo Z.B., Zhang Y.S., Hu J., Liu H.H., Liu D.P., Chen W.H., Zheng Z.Q., Jiang Y., Wen Z.G., Liu Y.M., Chen H., Xie M., Zhang Q., Huang W., Wang W., Hou S.S., Jiang Y. An intercross population study reveals genes associated with body size and plumage color in ducks. Nat. Commun. 2018;9:2648. doi: 10.1038/s41467-018-04868-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Qi X., Chen Y., Wang L., Lv X.Z., Yang W.F., Zhang J.W., Li K.Y., Ning Z.H., Jiang Z.H., Qu L.J. Positive selection of skeleton-related genes during duck domestication revealed by whole genome sequencing. BMC Ecol. Evol. 2021;21:165. doi: 10.1186/s12862-021-01894-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.