Abstract
Aggregatibacter actinomycetemcomitans is an opportunistic Gram-negative periodontopathogen strongly associated with periodontitis and infective endocarditis. Recent evidence suggests that periodontopathogens can influence the initiation and progression of oral squamous cell carcinoma (OSCC). Herein we aimed to investigate the effect of A. actinomycetemcomitans-derived extracellular vesicles (EVs) on OSCC cell behavior compared with EVs from periodontopathogens known to associate with carcinogenesis. EVs were isolated from: A. actinomycetemcomitans and its mutant strains lacking the cytolethal distending toxin (CDT) or lipopolysaccharide (LPS) O-antigen; Porphyromonas gingivalis; Fusobacterium nucleatum; and Parvimonas micra. The effect of EVs on primary and metastatic OSCC cells was assessed using cell proliferation, apoptosis, migration, invasion, and tubulogenesis assays. A. actinomycetemcomitans-derived EVs reduced the metastatic cancer cell proliferation, invasion, tubulogenesis, and increased apoptosis, mostly in CDT- and LPS O-antigen-dependent manner. EVs from F. nucleatum impaired the metastatic cancer cell proliferation and induced the apoptosis rates in all OSCC cell lines. EVs enhanced cancer cell migration regardless of bacterial species. In sum, this is the first study demonstrating the influence of A. actinomycetemcomitans-derived EVs on oral cancer in comparison with other periodontopathogens. Our findings revealed a potential antitumorigenic effect of these EVs on metastatic OSCC cells, which warrants further in vivo investigations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00203-024-03976-8.
Keywords: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Fusobacterium nucleatum, Parvimonas micra, Extracellular vesicles, Oral cancer
Background
Oral squamous cell carcinoma (OSCC) is the most common malignancy of the oral cavity (Montero and Patel 2015). In 2020 alone, there were nearly 380,000 new cases and 180,000 deaths from oral cancer globally (Sung et al. 2021). Despite the progress in cancer diagnosis and management, the 5-year survival rate of OSCC remains relatively dismal without significant improvements over the past years (Economopoulou et al. 2019). Therefore, new therapeutic approaches are urgently needed to improve the survival outcomes of patients with OSCC.
Up to 20% of all human cancers are associated with microbial organisms, which can induce tumor-promoting chronic inflammation (Elinav et al. 2013). Recent accumulating evidence suggests that periodontopathogens may contribute to the initiation and progression of cancer (Metsäniitty et al. 2021; Teles et al. 2020; Xiao et al. 2020). The Gram-negative species, Aggregatibacter actinomycetemcomitans is associated with periodontitis and infective endocarditis (Nørskov-Lauritsen 2014). A. actinomycetemcomitans belongs to the HACEK group of bacteria which is a rare cause of infective endocarditis, responsible for 1–3% of all infective endocarditis. About 20% of the HACEK-induced endocarditis is caused by A. actinomycetemcomitans (Revest et al. 2016). The role of A. actinomycetemcomitans in cancer remains elusive. A recent meta-analysis reported that infection with A. actinomycetemcomitans as a single pathogen was not associated with increased risk of cancer (Xiao et al. 2020). On the contrary, A. actinomycetemcomitans showed a strong association with malignancy (Söder et al. 2021) and its virulence factors such as cytolethal distending toxin (CDT) and lipopolysaccharide (LPS) promoted pancreatic cancer (Ungureanu et al. 2023). In OSCC, CDT has been found to mediate anti tumor effects such as growth inhibition (Iwanaga et al. 2007), induction of apoptosis and cell cycle arrest (Yamamoto et al. 2004).
A. actinomycetemcomitans actively releases extracellular vesicles (EVs), also referred to as outer membrane vesicles, containing multiple virulence factors including for example the CDT (Faïs et al. 2016; Oscarsson et al. 2019; Rompikuntal et al. 2012), leukotoxin A (LtxA) (Kato et al. 2002; Kieselbach et al. 2015), outer membrane protein A, outer membrane protein 100, GroEL and peptidoglycan-associated protein (Kieselbach et al. 2015). Of interest, A. actinomycetemcomitans is—to our knowledge—the only known oral bacterial species producing the genotoxin CDT (Belibasakis et al. 2019), which has been implicated in the tumorigenesis of head and neck cancers (Damek-Poprawa et al. 2011; Iwanaga et al. 2007; Teshima et al. 2018; Yamamoto et al. 2004).
Lipopolysaccharide (LPS) is an abundant component of A. actinomycetemcomitans-derived EVs with immunomodulatory properties, hence representing an attractive target in cancer therapy (Jain et al. 2019; Shetab Boushehri and Lamprecht 2018; Song et al. 2018). The serotype-specific polysaccharide determinant of A. actinomycetemcomitans resides in the immunodominant LPS O-antigen, which differentiates the distinct serotypes of this species based on its antigenicity (Lakio et al. 2003; Oscarsson et al. 2019; Page et al. 1991; Sims et al. 1991; Wilson and Schifferle 1991). Lack of LPS O-antigen has been shown to alter both the pathogenic and immunostimulatory traits of A. actinomycetemcomitans (Lindholm et al. 2020; Monasterio et al. 2020). Importantly, LPS and CDT can be delivered into the host cells via EVs (Oscarsson et al. 2019; Rompikuntal et al. 2012; Vanaja et al. 2016).
Bacterial EVs are spherical bilayered proteolipids harboring multiple virulence and immunomodulator factors, which can be fully incorporated into the host cell cytoplasm (Kim et al. 2015; Ñahui Palomino et al. 2021). Therefore, EVs represent a promising target not only as drug delivery vehicles and bacterial vaccines but also in cancer therapeutics (Fazal and Lee 2021; Li et al. 2020). To our knowledge, only two studies explored the effect of bacterial EVs on oral cancer to date (Chen et al. 2023; Liu et al. 2021). In addition to A. actinomycetemcomitans, several EV-producing periodontopathogens have been linked to head and neck carcinogenesis such as Porphyromonas gingivalis, Fusobacterium nucleatum and Parvimonas micra. For instance, EVs from P. gingivalis showed pro-carcinogenic effects on metastatic OSCC cells (Liu et al. 2021). Furthermore, P. gingivalis influenced several tumorigenic events in OSCC including the epithelial-mesenchymal transition (EMT), tumor cell proliferation, invasion, metastasis, and angiogenesis (Lafuente Ibanez de Mendoza et al., 2020; Singh and Singh 2022). While F. nucleatum was associated with better outcomes in OSCC patients (Chen et al. 2020; Neuzillet et al. 2021), P. micra level was progressively increased from OSCC stage 1 to 4, implying a promising prognostic utility (Yang et al. 2018). Research on the role of P. micra in oral cancer is perhaps the most limited of the four periodontopathogens included in this study. In addition to the possible use of P. micra as a prognostic tool (Yang et al. 2018), Parvimonas (W.-H. Lee et al. 2017b; Zhao et al. 2017) and P. micra (Yang et al. 2018) abundance in saliva samples were associated with oral cancer and could differentiate patients with cancer from oral potentially malignant disorders (W.-H. Lee et al. 2017b). Thus bacterial species and their roles in carcinogenesis appear to vary among different individuals (Mager 2006).
Although recent evidence suggests a convincing link between oral dysbiosis and OSCC, the role of bacterial EVs in such association remains, however, unclear. Thus, we investigated whether and how EVs from different A. actinomycetemcomitans strains can influence the behavior of OSCC cells with variable metastatic potentials, compared to EVs from P. gingivalis, F. nucleatum and P. micra.
Materials and methods
Bacterial strains and growth conditions
Four strains of A. actinomycetemcomitans (serotype a) were used: D7SS is a natural genetic competent, smooth-colony derivative of wild-type strain D7S, which was originally isolated from a patient with aggressive periodontitis (Wang et al. 2002); and its cdtABC mutant derivative generated via a knockout approach (Nalbant et al. 2003) (hereafter referred to as D7SS-WT and D7SS-cdt, respectively). The strains SA3138 (Asikainen et al. 1995) and SA3139 (Asikainen et al. 1995; Kanasi et al. 2010) were isolated from a patient with periodontitis, albeit the latter lacks expression of the LPS O-antigen (hereafter referred to as SA3138-WT and SA3139-LPS-O, respectively).
P. micra CCUG 35243 and F. nucleatum CCUG 32989 were purchased from the Culture Collection University of Gothenburg; and P. gingivalis ATCC 33277 from the American Type Culture Collection. A. actinomycetemcomitans strains were cultivated in air supplemented with 5% CO2, at 37 °C on blood agar plates (5% defibrinated horse blood, 5 mg hemin/l, 10 mg Vitamin K/l, Columbia agar base; Oxoid Ltd., Basingstoke, Hampshire, UK) for 4 (D7SS strains) or 5 days (SA3138 and SA3139). A. actinomycetemcomitans strains can be cultured in trypsin soy broth, however, the EV protein pattern is very similar to the one of EVs from agar culture (Rompikuntal et al. 2012). P. micra, F. nucleatum and P. gingivalis were cultured in an anaerobic environment (10% H2, 5% CO2, 85% N2) at 37 °C. P. micra was cultured on blood agar plates for 5 days. F. nucleatum and P. gingivalis were first cultivated on blood agar plates for 2 and 3 days, respectively, and then the culture was continued in liquid broth fastidious anaerobe agar (FAA; Neogen®, Heywood, UK) for 48 h. All procedures were conducted in accordance with the guidelines of the local ethics committee at the Medical Faculty of Umeå University.
Isolation of EVs
The EVs were isolated by ultracentrifugation as described earlier (Lindholm et al. 2020; Rompikuntal et al. 2012). In brief, bacterial cells were harvested from agar plates and suspended in 2 × 25 ml of phosphate-buffered saline (PBS) or liquid broth. The optical density (OD) values of the 25 ml suspensions at 600 nm were: 0.76 (D7SS-WT), 0.56 (D7SS-cdt), 1.12 (SA3138-WT), 1.38 (SA3139-LPS-O), 1.00 (P. gingivalis), 1.36 (F. nucleatum) and 2.96 (P. micra). The number of agar plates used for harvesting the bacterial cells was 5 (D7SS-cdt), 10 (D7SS-WT, SA3138-WT and SA3139-LPS-O), and 30 (P. micra). The suspensions were centrifuged at 12,096 × g for 30 min at 4 °C in a JA-25.50 rotor (Beckman Instruments Inc.). Supernatants were filtered through a 0.45 and 0.2 μm pore-size syringe filters (Filtropur, Sarstedt) and centrifuged at 85,000 × g for 2 h at 4 °C in a 70 Ti rotor (Beckman Instruments Inc.). Pellets were washed twice with PBS (85.000 × g for 2 h at 4 °C) using a Sw60 Ti rotor (Beckman Instruments Inc.), resuspended in PBS, and used as EV preparation without further purification. EVs were tested for the absence of contamination by cultivating small aliquots on blood agar plates in air supplemented with 5% CO2 at 37 °C for 3 days.
Analyses of EV preparation
EV protein concentration was determined using NanoDrop 100 spectrophotometer (Thermo Fisher Scientific) and the preparations were further analyzed using nanoparticle tracking analysis software Zetaview (Particle Metrix, Germany). To visualize proteins in EV samples, we performed a protein gel electrophoresis using Pierce™ Silver Stain Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Samples were separated on Criterion™ TGX™ Precast Gels and Precision Plus Protein™ Standard All Blue (Bio-Rad) was used as a standard. Images were taken with ChemiDoc™ MP imaging system.
Cancer cell lines and EV treatments
Two oral tongue cancer cell lines were used including primary SCC-24A (Department of Otorhinolaryngology, Head and Neck Surgery, Turku University Hospital, Finland) and highly metastatic HSC-3 cells (JCRB Cell Bank, Japan). Cell lines were cultured in 1:1 DMEM/F-12 medium supplemented with 10% heat-inactivated fetal bovine serum (Gibco), penicillin–streptomycin (Gibco), 250 ng/mL amphotericin B (Sigma-Aldrich, St. Louis, MO, USA), 50 µg/mL ascorbic acid (AppliChem, Chicago, IL, USA), and 0.4 µg/mL hydrocortisone (Sigma-Aldrich, St. Louis, MO, USA). For EV treatments, cells were challenged with EVs at the concentration of 5 µg/ml either once (6 h prior to the assay) or twice (6 h earlier and when initiating the assay; hereafter 2 × 5 µg/ml). The used EV concentration was based on recent literatures (Chen et al. 2023; Liu et al. 2021; Zhuang et al. 2023). Cells in the control wells were incubated in the same DMEM medium lacking EVs and referred to as no treatment controls (NTC). All incubations were done at 37 °C unless otherwise indicated.
Real-time cancer cell proliferation and apoptosis assays
Cell proliferation and apoptosis assays were performed as previously described (Almahmoudi et al. 2019). OSCC cells were labelled with CellTrace™ Far Red dye according to the manufacturer’s instructions (Thermo Fisher Scientific) and then seeded in a 96-well plate (Corning) at a density of 2 × 103 cells per well in 100 µl DMEM. The next day, media was replaced with fresh DMEM containing EVs (5 µg/ml) and incubated for 6 h. Then media was replaced with DMEM with or without EVs (5 µg/ml). Finally, the IncuCyte® Caspase-3/7 Green Apoptosis Assay Reagent (Cat. No. 4440) was added. The IncuCyte® Live-Cell Analysis System was used for imaging every 2 h for 2–3 days to assess cell proliferation and apoptosis.
Transwell invasion assay
The effect of EVs on cancer cell invasion was assessed using Myogel-coated Transwell inserts (Corning Incorporated) as previously described (Salo et al. 2015). Briefly, inserts with 8.0 μm pore-size were coated with 50 µl Myogel (2.4 mg/ml) diluted in serum-free DMEM and solidified with rat tail type I collagen (0.8 mg/ml; Corning). Cells (70 × 103/well) were seeded into the upper chambers in 200 µl serum-free DMEM supplemented with 0.5% lactalbumin and 5 µg/ml EVs. The lower chambers contained DMEM (500 µl) supplemented with 10% FBS. After 72 h, the invaded cells were fixed with 4% formaldehyde and stained with 1% toluidine blue in 1% borax. The dye was eluted with 1% SDS solution and absorbance was measured at 650 nm using the FLUOstar® Omega microplate reader (BMG Labtech). The invasion rate was calculated based on the measured absorbance.
Cancer cell migration assay
Cell migration was assessed as recently reported (Karinen et al. 2021). First, a 96-well plate (Essen BioScience) was coated with 50 µl of Myogel (0.5 mg/ml) in serum-free DMEM and incubated overnight. Next, cells in 100 µl DMEM were plated at a density of 25 or 30 × 103 per well (for HSC-3 and SCC-24A, respectively) and incubated overnight. Then DMEM was replaced with DMEM with or without EVs (5 µg/ml) and incubated for 6 h. Next, the WoundMaker™ (Essen BioScience) was used to obtain homogenous and consistent wounds. The wounds were washed 1–2 times with DMEM and after wound inspection DMEM with or without EVs (5 µg/ml) was added. The IncuCyte® Live-Cell Analysis system was used to image the wounds hourly until wound closure. The migration was analyzed using the relative wound density (RWD), which determines the density of the wound region relative to the density of the cell region, based on the initial scratch wound mask.
Tube formation assay
Tube formation assay was performed as previously described (Francescone et al. 2011; Hujanen et al. 2021; Karinen et al. 2021). Briefly, 100 µl Matrigel® (8.9 mg/ml; Corning) was added to a 24-well cell culture plate (Corning) and incubated for 60 min at 37 °C to allow solidification. Cancer cells diluted in 300 µl serum-free DMEM were added on the gel-coated wells at a density of 10 × 104 cells per well and incubated for 4 hours. Cells were then challenged with EVs (5 µg/ml) diluted in serum-free DMEM and incubation continued. Images were taken every 4, 8 and 20 h with ZEISS PrimoVert microscope (AxioCam ERc5s, Zeiss Microscopy) using magnifications 4x and 10x. The ImageJ software with “Angiogenesis Analyzer” plugin was used to measure the different tube formation parameters (Wayne Rasband, National Institute of Health, Bethesda, MD, USA).
Statistical analyses
Statistical analysis was performed using GraphPad Prism Software version 9.4.1 (San Diego, California, USA). The One-way ANOVA with Dunnett`s or Tukey`s multiple comparisons test was used for proliferation, apoptosis, migration, and invasion assays. All experiments were repeated independently three times with triplicates or duplicates for each condition. Statistical significance was set to P ≤ 0.05; * indicates P-values ≤ 0.05; ** indicates P-values ≤ 0.01; *** indicates P-values ≤ 0.001; **** indicates P ≤ 0.0001. Data are represented as mean ± standard error of the mean (SEM).
Results
Characteristics of the EV samples
EVs used in this work were isolated from seven different bacterial strains as previously stated. Protein concentration of the EV samples varied between 0.931 and 9.391 mg/ml, while the particle diameter ranged from 132.96 to 161.16 nm (Table 1). Proteins were detected and visualized with Silver Stain protein gel electrophoresis where the protein sizes were compared to the pre-stained molecular weight marker (Fig. S1).
Table 1.
Characteristics of the bacterial extracellular vesicles
| Bacteria | Strain | Origin | Protein concentration* (mg/ml) | Particle concentration** (particles/ml) |
Particle size** (diameter/nm) |
|---|---|---|---|---|---|
| Aggregatibacter actinomycetemcomitans | D7SS wild type | Patient | 1.987 | 1.20 × 1012 | 143.52 |
| Aggregatibacter actinomycetemcomitans | D7SS cdtABC mutant | Patient | 1.258 | 5.2 × 1011 | 147.08 |
| Aggregatibacter actinomycetemcomitans | SA3138 wild type | Patient | 7.813 | 1.054 × 1012 | 139.22 |
| Aggregatibacter actinomycetemcomitans | SA3139 lacking LPS O-antigen | Patient | 8.732 | 1.6 × 1012 | 138.96 |
| Porphyromonas gingivalis | ATCC 33277 | ATCC | 2.132 | 3.85 × 1011 | 133.16 |
| Fusobacterium nucleatum | CCUG 32989 wild type | CCUG | 0.931 | 3.66 × 1010 | 132.96 |
| Parvimonas micra | CCUG 35243 wild type | CCUG | 9.391 | 4.68 × 1011 | 161.16 |
cdtABC, cytolethal distending toxin subunit A, B and C gene; LPS, lipopolysaccharide; CCUG, Culture Collection University of Gothenburg; ATCC, American Type Culture Collection. *Protein concentration was measured with NanoDrop 100 spectrophotometer (Thermo Fisher Scientific). **Particle concentration and size were analyzed with nanoparticle tracking analysis software Zetaview (Particle Matrix, Germany)
Effect of EVs from A. actinomycetemcomitans lacking CDT on cancer cell behavior
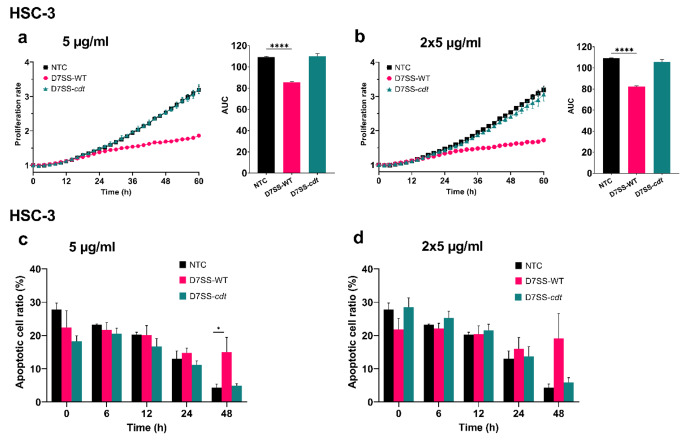
A. actinomycetemcomitans actively produces CDT, which has been shown to induce cell cycle arrest and apoptosis upon transfection into gingival squamous carcinoma cells (Belibasakis et al. 2019; Iwanaga et al. 2007). However, the tumorigenic effect of EVs from strains without this genotoxin is unknown. Thus, we first assessed the effect of EVs isolated from the wild-type A. actinomycetemcomitans (D7SS-WT) and its CDT-lacking derivative (D7SS-cdt) on cell proliferation and apoptosis of primary and metastatic OSCC cell lines. Interestingly, the D7SS-WT-derived EVs (at 5 µg/ml and 2 × 5 µg/ml) remarkably reduced the metastatic HSC-3 cell proliferation (P < 0.0001), while no effect was observed by the D7SS-cdt-derived EVs (Fig. 1a, b). Notably, an increased rate of apoptosis was observed in HSC-3 cells treated with the D7SS-WT-derived EVs (5 µg/ml) compared with the untreated controls (P < 0.05) at 48 h (Fig. 1c). Similarly, the apoptosis ratio was increased in HSC-3 cells at 48 h following treatment with the D7SS-WT-derived EVs (2 × 5 µg/ml), however, the difference was not statistically significant (Fig. 1d). Of note, none of the EVs significantly altered neither the proliferation nor the apoptosis levels of the primary SCC-24A cells (Fig. S2a-d).
Fig. 1.
Effect of A. actinomycetemcomitans D7SS-WT and D7SS-cdt EVs on cancer cell proliferation and apoptosis. Cell proliferation rates are presented as proliferation rate in relation to time with the corresponding area under the curve (AUC). Apoptotic cell ratios are shown from representative time points. (a, b) HSC-3 cell proliferation was significantly inhibited by D7SS-WT EVs. (c, d) HSC-3 cell apoptosis levels were increased in cells treated with EVs from D7SS-WT compared to controls. Values are shown as mean ± SEM. ****P ≤ 0.0001. NTC, no treatment control. Experiments were repeated independently three times with triplicates for each condition
We then evaluated the impact of these EVs on cancer cell migration and invasion. To this end, we first quantified the RWD metric using the IncuCyte® Live-Cell Analysis, which revealed a modest increase in the SCC-24A cell migration upon treatment with the D7SS-WT EVs (2 × 5 µg/ml; P < 0.05) (Fig. 2a). The metastatic HSC-3 cell migration was enhanced by EVs (2 × 5 µg/ml) from both strains (P < 0.05) (Fig. 2b). However, EVs at the concentration of 5 µg/ml showed no statistically significant effect on cancer cell migration (Fig. S2e, f). The Myogel-coated Transwell chambers were then used to study the effect of bacterial EVs on OSCC cell invasion. Interestingly, none of the EVs affected the invasiveness of the primary cells (Fig. 2c), and only those obtained from the D7SS-WT strain significantly blunted the metastatic cell invasion (P < 0.05) (Fig. 2d).
Fig. 2.
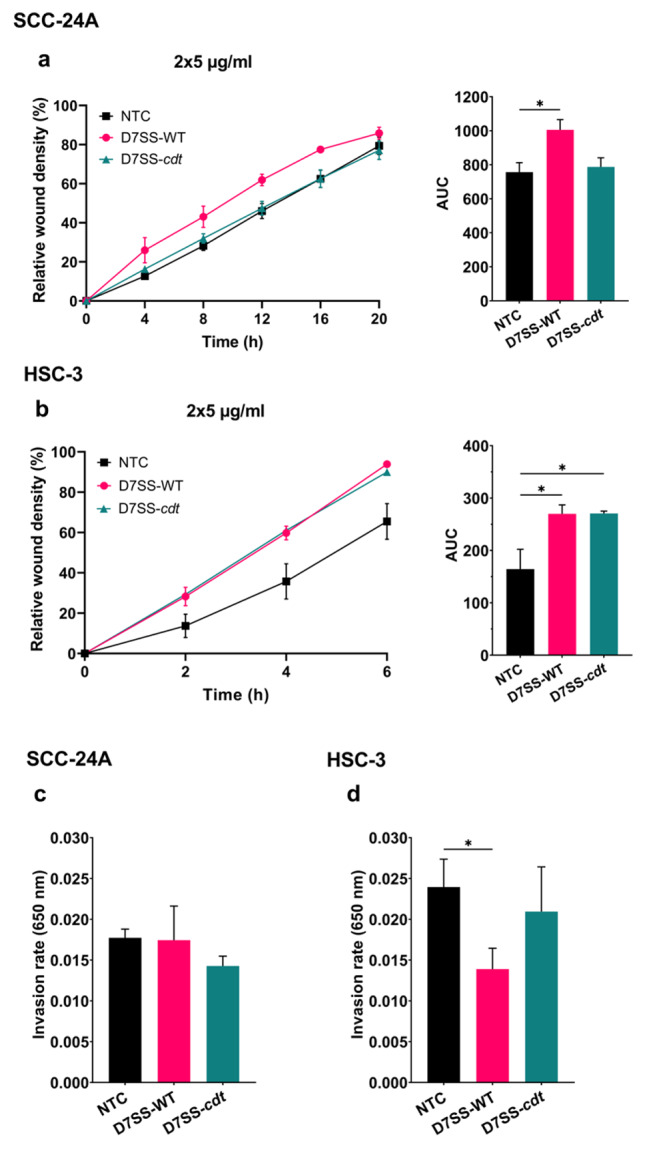
Effect of A. actinomycetemcomitans D7SS-WT and D7SS-cdt EVs on cancer cell migration and invasion. (a, b) Cell migration is presented as the relative wound density over time with the corresponding area under the curve (AUC). (a) SCC-24A cell migration was induced only by EVs (2 × 5 µg/ml) from D7SS-WT. (b) HSC-3 cell migration was increased when cells were treated with D7SS-WT and D7SS-cdt EVs. (c, d) Transwell invasion of SCC-24A and HSC-3 cells treated with EVs (5 µg/ml) from D7SS-WT and D7SS-cdt. (c) SCC-24A invasion was not significantly affected by EVs. (d) HSC-3 cell invasiveness was decreased by D7SS-WT EVs but not by D7SS-cdt EVs. Values are shown as mean ± SEM. *P ≤ 0.05. NTC, no treatment control. Experiments were repeated independently three times with triplicates (migration) or duplicates (invasion) for each condition
Effect of EVs from A. actinomycetemcomitans lacking LPS O-antigen on cancer cell behavior
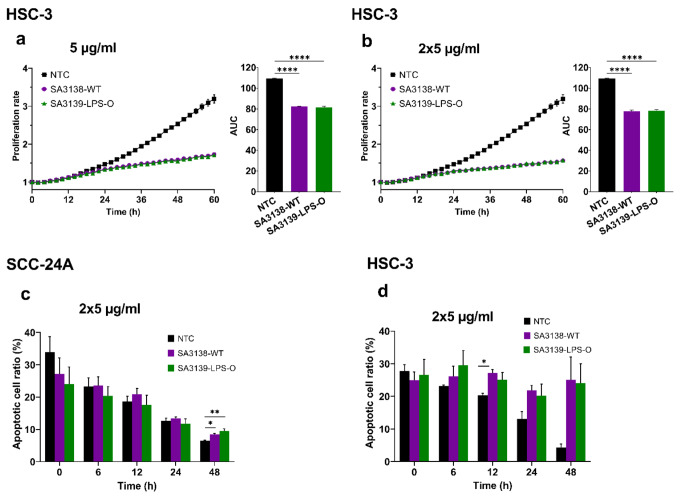
LPS O-antigen plays a key role in A. actinomycetemcomitans virulence (Monasterio et al. 2020). However, it is unknown whether it can also have an impact on cancer cell behavior. Therefore, we next challenged OSCC cells with EVs from the wild-type A. actinomycetemcomitans (SA3138-WT), and a strain lacking the O-antigen polysaccharide (SA3139-LPS-O), as described above. Our results showed that the proliferation of HSC-3 cells was significantly inhibited by EVs from both strains (P < 0.0001; Fig. 3a, b). On the contrary, none of these EVs significantly altered the proliferation of SCC-24A cells (Fig. S3a, b). For the programmed cell death, treatment with these EVs (2 × 5 µg/ml) showed a significantly higher apoptosis levels in SCC-24A cells at 48 h (SA3138-WT, P < 0.05; SA3139-LPS-O, P < 0.01; Fig. 3c), and in HSC-3 cells at 12 h (SA3138-WT, P < 0.05; Fig. 3d). In contrast, EVs (5 µg/ml) from SA3139-LPS-O reduced the apoptosis of SCC-24A cells at 24 h (P < 0.05), but no significant changes were observed in HSC-3 cells (Fig. S3c, d).
Fig. 3.
Effect of EVs from A. actinomycetemcomitans SA3138-WT and SA3139-LPS-O strains on cancer cell proliferation and apoptosis. Cell proliferation rates are presented as proliferation rate in relation to time with the corresponding area under the curve (AUC). Apoptotic cell ratios are shown from representative time points. (a, b) HSC-3 cell proliferation was significantly inhibited by SA3138-WT and SA3139-LPS-O-derived EVs (5 µg/ml and 2 × 5 µg/ml). (c) At 48 h the primary SCC-24A cells treated with SA3138-WT and SA3139-LPS-O-derived EVs (2 × 5 µg/ml) had significantly higher apoptosis rate than control cells. (d) The metastatic HSC-3 cell apoptosis was significantly increased in cells treated with EVs from SA3138-WT at 12 h of treatment. Values are shown as mean ± SEM. ****P ≤ 0.0001. *P ≤ 0.05. NTC, no treatment control. Experiments were repeated independently three times with triplicates for each condition
The migration of SCC-24A cells was significantly enhanced by the wild-type SA3138-WT-derived EVs (2 × 5 µg/ml; P < 0.05; Fig. 4a). EVs (2 × 5 µg/ml) from both strains appeared to promote HSC-3 cell migration, although the differences were not statistically significant (Fig. 4b). Likewise, no significant effect was observed on the migration of OSCC cell lines when treated with the 5 µg/ml EV-dose (Fig. S3e, f). Of interest, the invasion of the primary SCC-24A cells was not affected by EVs from either strain (Fig. 4c). In turn, the invasion of the metastatic HSC-3 cells was significantly reduced only by EVs from the SA3138-WT (P < 0.05) (Fig. 4d).
Fig. 4.

Effect of A. actinomycetemcomitans SA3138-WT and SA3139-LPS-O EVs on cancer cell migration and invasion. (a, b) Cell migration is presented as the relative wound density over time with the corresponding area under the curve (AUC).(a) SA3138-WT EVs increased SCC-24A cell migration compared to control. (b) HSC-3 cell migration was not significantly induced by EVs. (c, d) Transwell invasion of SCC-24A and HSC-3 cells treated with 5 µg/ml of SA3138-WT and SA3139-LPS-O EVs. (c) SCC-24A cell invasion was not affected by EV treatment. (d) SA3138-WT EVs reduced HSC-3 cell invasiveness. Values are shown as mean ± SEM. ** P ≤ 0.01. NTC, no treatment control. Experiments were repeated independently three times with triplicates (migration) or duplicates (invasion) for each condition
Effect of A. actinomycetemcomitans-derived EVs on cancer cell tubulogenesis
Recently, certain aggressive OSCC cells were shown to express the endothelial cell marker CD31 and initiate vascular networks similar to the endothelial cell tubulogenesis when cultured on biological hydrogels (Hujanen et al. 2021). These capillary networks were suggested as a possible mechanism behind metastasis and drug resistance in cancer patients (Williamson et al. 2016). Thus, we aimed to study the effect of EVs from A. actinomycetemcomitans on this process using the tube formation assay. After seeding on Matrigel® for four hours, cancer cells were incubated for two days with or without each of the four A. actinomycetemcomitans-derived EVs at 5 µg/ml. Overall, the primary SCC-24A cells formed fewer tubes on Matrigel® compared to the metastatic HSC-3 cells. Here we show representative images (4x) of tubulogenesis following 20 h of incubation with or without EVs (Fig. 5). All the tube-formation parameters are provided in the supplementary materials (Fig. S4-7).
Fig. 5.
Cancer cell-derived tubulogenesis in SCC-24A and HSC-3 cells treated with A. actinomycetemcomitans EVs. (a) Tube formation in SCC-24A cells was partly inhibited by D7SS-cdt-derived EVs. (b) EVs from the wild-type strains (D7SS-WT and SA3138-WT) decreased tube formation in the metastatic HSC-3 cells. Values are shown as mean ± SEM. NTC, no treatment control. Experiments were repeated independently three times with duplicates for each condition
EVs from the CDT-lacking strain (D7SS-cdt) resulted in less tube formation (i.e., number of meshes) by SCC-24A compared to those from the wild-type and untreated controls, whereas a little effect was observed with EVs from the SA3138-WT and SA3139-LPS-O strains (Fig. 5a). Interestingly, EVs from the wild-type strains (D7SS-WT and SA3138-WT) inhibited the tube formation by the metastatic HSC-3 cells compared to those from their mutant variants (D7SS-cdt or SA3139-LPS-O) and the untreated controls (Fig. 5b). Also, these results possibly suggest that CDT expression may exhibit an opposite effect of on the tubulogenic potential between primary and metastatic cancer cells. Although this finding should be interpreted with caution, it warrants further detailed investigation on the role of CDT.
Effect of EVs from P. Gingivalis, F. Nucleatum and P. micra on cancer cell behavior
The periodontopathogens P. gingivalis, F. nucleatum and P. micra have been gaining attention for their association with multiple cancers including OSCC (Metsäniitty et al. 2021; Yang et al. 2018). However, the contribution of their EVs to the specific processes involved in oral carcinogenesis remain partly unknown. Thus, by employing the same OSCC in vitro model, we next aimed to explore how EVs from these bacteria can affect cancer cell behavior, compared to the findings from the A. actinomycetemcomitans strains.
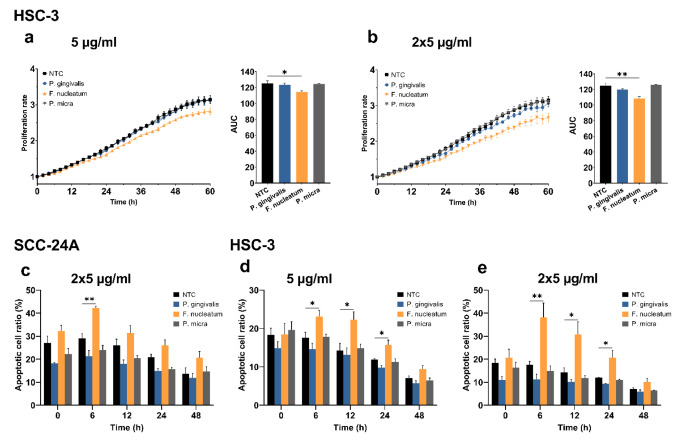
We report that only EVs from the F. nucleatum significantly inhibited the proliferation of HSC-3 cells (5 µg/ml, P < 0.05; 2 × 5 µg/ml, P < 0.01; Fig. 6a, b). None of the EVs affected the proliferation of SCC-24A cells (Fig. S8a, b). The F. nucleatum-derived EVs induced more cancer cell apoptosis, with the peak effect observed as follows: SCC-24A cells at 6 h (2 × 5 µg/ml EVs, P < 0.01; Fig. 6c); HSC-3 cells (5 µg/ml EVs at 6, 12 and 24 h, P < 0.05; Fig. 6d), and (2 × 5 µg/ml EVs at 6 h, P < 0.01; 12 and 24 h, P < 0.05; Fig. 6e). EVs from P. gingivalis showed a modest tendency to resist cell apoptosis although it was not statistically significant (Fig. 6c-e).
Fig. 6.
Effect of EVs from P. gingivalis, F. nucleatum and P. micra on cancer cell proliferation and apoptosis. Cell proliferation rates are presented as proliferation rate in relation to time with the corresponding area under the curve (AUC). Apoptotic cell ratios are shown from representative time points. (a, b)F. nucleatum-derived EVs reduced HSC-3 cell proliferation at both doses. (c) SCC-24A cell apoptosis was significantly higher in cells treated with F. nucleatum EVs (2 × 5 µg/ml) at 6 h. (d, e) Both doses of F. nucleatum EVs significantly increased apoptotic cell ratio of HSC-3 cells at 6, 12, and 24 h. Values are shown as mean ± SEM. *P ≤ 0.05. **P ≤ 0.01. NTC, no treatment control. Experiments were repeated independently three times with triplicates for each condition
The IncuCyte® Live-Cell Analysis revealed a marginally significant increase in the migration of SCC-24A by EVs (2 × 5 µg/mg) from F. nucleatum compared to control cells (P = 0.055; Fig. 7a). HSC-3 cell migration was enhanced by EVs from P. gingivalis (both 5 and 2 × 5 µg/mg; P < 0.05), F. nucleatum and P. micra (5 µg/mg; P < 0.01) (Fig. 7b, c). Finally, we evaluated the effect of these pathogens on OSCC cell invasion using the Transwell assay. Although statistically significant differences were not observed, EVs from P. gingivalis and P. micra seemed to promote SCC-24A cell invasion (Fig. 7d), while P. gingivalis slightly enhanced the invasiveness of HSC-3 cells (Fig. 7e).
Fig. 7.
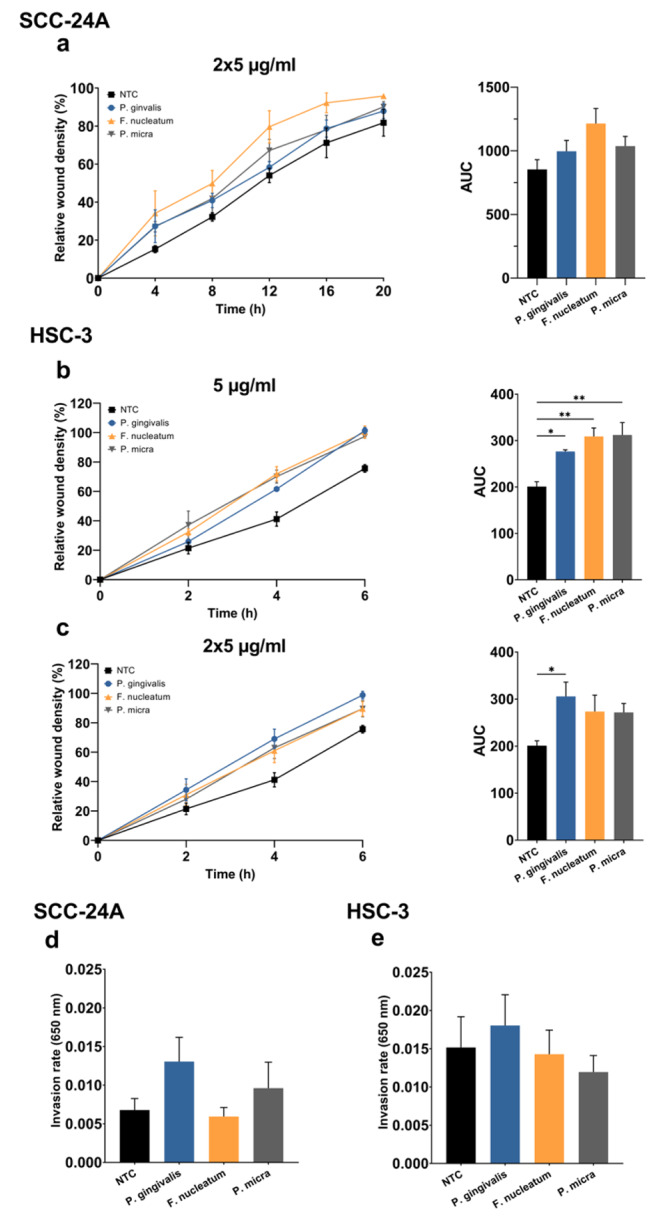
Effect of EVs from P. gingivalis, F. nucleatum and P. micra-derived on cancer cell migration and invasion. (a, b, c) Cell migration is presented as the relative wound density over time with the corresponding area under the curve (AUC). (a)F. nucleatum EVs (2 × 5 µg/ml) slightly promote SCC-24A cell migration, though the difference is not statistically significant (P = 0.055). (b)P. gingivalis, F. nucleatum and P. micra EVs (5 µg/ml) increase HSC-3 cell migration. (c)P. gingivalis EVs (2 × 5 µg/ml) significantly promoted HSC-3 migration. (d, e) Transwell invasion of SCC-24A and HSC-3 cells treated with 5 µg/ml EVs derived from P. gingivalis, F. nucleatum and P. micra did not show any statistically significant differences. Values are shown as mean ± SEM. *P ≤ 0.05. NTC, no treatment control. Experiments were repeated independently three times with triplicates (migration) or duplicates (invasion) for each condition
Discussion
The present work is one of the first studies to investigate the interactions between bacterial EVs and OSCC cells in vitro. We showed that A. actinomycetemcomitans-derived EVs inhibited the proliferation and invasion of the highly metastatic HSC-3 cells and blunted their tubulogenic potential, mostly in CDT and LPS O-antigen dependent manners. Further, our analysis revealed that EVs from F. nucleatum suppressed the proliferation of OSCC cells and increased their apoptosis rate. All EVs tested in this work promoted the migration of cancer cells.
Previously, it was reported that incubation with A. actinomycetemcomitans caused up to 50% decrease in OSCC cell proliferation (Hoppe et al. 2016). Moreover, Teshima et al. showed that OSCC cell infection with A. actinomycetemcomitans induced CDT-dependent DNA double-strand breaks, which occurred independently of apoptosis (Teshima et al. 2018). Although such DNA breaks suggest a pro-carcinogenic activity, several studies have reported CDT-mediated antitumorigenic effects in oral cancer. For instance, transfection of a cdtB-expressing plasmid to OSCC cells enhanced cell cycle arrest and apoptosis in vitro and in vivo (Iwanaga et al. 2007; Yamamoto et al. 2004). Furthermore, a combination of A. actinomycetemcomitans-derived CDT with CD133 monoclonal antibody inhibited the proliferation of the aggressive CD133+ ve oral cancer stem cells (Damek-Poprawa et al. 2011). Consistently, our findings revealed a potential anticancer effect of A. actinomycetemcomitans-derived EVs on metastatic OSCC cells in a CDT-dependent manner.
The activity of LPS on cancer cells is mostly mediated by host-dependent mechanisms (Lundin and Checkoway 2009). LPS activates toll-like receptors (TLRs) on cancer cells leading to a tumor-promoting environment or, alternatively, antitumor immune responses (Basith et al. 2012; Hasnat et al. 2020). LPS can also be delivered into the host cell cytosol via EVs causing pyroptosis and subsequent release of inflammatory cytokines (Vanaja et al. 2016). Here we showed that EVs from A. actinomycetemcomitans strains with and without LPS O-antigen impacted cancer cell behavior. These findings suggest that such structural change of LPS may, at least in part, influence oral tumorigenesis. Interestingly, cancer cell migration was the only process enhanced by all EVs, regardless of the bacterial species. The exact reason is not clear, however, a recent study showed that OSCC cell migration was enhanced by key periodontopathogens, including P. gingivalis and F. nucleatum, via integrin alpha V and FAK activation (Kamarajan et al. 2020).
Cancer cell-derived capillaries or vasculogenic mimicry (VM) have been linked with metastasis and poor survival of OSCC patients (Hujanen et al. 2020). In this context, it is interesting that A. actinomycetemcomitans-derived EVs attenuated the formation of these capillaries by HSC-3 cells, given their high tubulogenic potential across different matrices (Hujanen et al. 2021). Nevertheless, it remains to be elucidated why strains with and without CDT had an opposite effect on the tubulogenesis of primary and metastatic cancer cells, which warrants further mechanistic insights.
A. actinomycetemcomitans produces LtxA that is specific for human white blood cells by interacting with lymphocyte function antigen-1 on susceptible cells. Though LtxA was not included in this study it is noteworthy to mention that it might expose anti-cancer effects. For example, LtxA has been shown to kill malignant white blood cell lines and primary cells isolated from acute myeloid leukemia patients. Healthy peripheral blood mononuclear cells in turn were relatively resistant to LtxA (Kachlany et al. 2010). Even anti-lymphoma activity of LtxA was reported as it caused regression of B-cell tumors in mice (DiFranco et al. 2015). This demonstrates that A. actinomycetemcomitans has also other virulence factors aside from CDT and LPS that interact with cancer and possess therapeutic utility.
The effect of F. nucleatum on OSCC cell proliferation is conflicting, showing both stimulatory (Binder Gallimidi et al. 2015) and non-stimulatory effects (Kamarajan et al. 2020). Herein, we reported anticancer activities of F. nucleatum EVs by suppressing the proliferation and inducing the apoptosis of OSCC cells. Our findings support recent observations that higher tumoral levels of F. nucleatum were associated with better clinical outcomes in patients with head and neck cancers (Chen et al. 2020; Neuzillet et al. 2021). In contrast, one study showed that a higher abundance of F. nucleatum predicted recurrence and shorter disease-free survival in patients with laryngeal cancer (Hsueh et al. 2022). This disagreement between studies may, however, result from variations in the tumor microenvironment between the larynx and oral cavity. Nevertheless, the capacity of F. nucleatum EVs to induce cancer cell migration is consistent with previous studies. F. nucleatum promoted OSCC cell migration by downregulating p53 and E-cadherin (Kamarajan et al. 2020) and nucleatum-derived EVs promoted migration and invasion of OSCC in vitro and metastasis in mice (Chen et al. 2023). Also, another study found that F. nucleatum promoted OSCC cell migration and additionally they described a change in the cell morphology of OSCC cells after a 48-hour treatment with F. nucleatum (Da et al. 2021). Changes in OSCC cell morphology by bacterial EVs was not covered by this study but in the future, it would be of importance to know if such changes can be caused by EVs too.
One important pro-tumorigenic effect of P. gingivalis is the ability to inhibit apoptosis in oral epithelial cells (Lee et al. 2018; Mao et al. 2007). We observed a consistent, though non-statistically significant, trend towards lower apoptosis rates in cancer cells treated with EVs from P. gingivalis. In addition, P. gingivalis has been shown to induce OSCC cell proliferation (Binder Gallimidi et al. 2015; Chang et al. 2019; Hoppe et al. 2016; Kamarajan et al. 2020), although such effect was not observed in this study. Cancer cell migration and invasion were promoted by P. gingivalis (Abdulkareem et al. 2018; Cho et al. 2018; Ha et al. 2016; Inaba et al. 2014; Kamarajan et al. 2020). To date, only one study has explored the effect of P. gingivalis-derived EVs on oral cancer, which markedly induced metastatic HSC-3 cell invasion and migration in vitro (Liu et al. 2021). We reported similar results regarding HSC-3 cell migration, but we did not observe such significant effect on cell invasion.
The oral pathogen P. micra has been linked to gastric (Coker et al. 2018) and colorectal cancers (Löwenmark et al. 2020; Zhao et al. 2021). Our findings support a stimulatory effect of P. micra EVs on the migration of metastatic cancer cells. In this regard, P. micra was enriched in OSCC tumor lesions, wherein the amount of P. micra in oral rinse sample was significantly increased from OSCC stage 1 through stage 4 patients. Thus, P. micra and Parvimonas spp. were suggested as possible parameters in biomarker panels in oral cancer (Yang et al. 2018) and for differentiating patients with oral potentially malignant disorders such as dysplasia from OSCC (W.-H. Lee et al. 2017b).
Overall, OSCC lines responded differently to the bacterial EVs. Although interesting, this is not surprising given the genetic and phenotypic differences between primary and metastatic cancer cells, including their stemness and plasticity (Salem and Salo 2023). For instance, the metastatic cancer cells exhibit high potential to acquire transitional phenotypic states, mediate drug resistance, and initiate endothelial-like capillaries (Hujanen et al. 2021; Salem and Salo 2023). However, studies on the bacterial EVs and cancer are limited, which reflects the newness of this interesting field. Therefore, the exact reason behind this behavior remains unclear and warrants further investigation. Nevertheless, it is noteworthy that bacterial species and stimulation time may play a significant role. For example, colon epithelial cells showed signs of malignant transformation when treated with CDT for 30 weeks (Guidi et al. 2013). In turn, the inducive effect of P. gingivalis on OSCC invasion has been reported after 30 h only (Cho et al. 2018). In two other studies, P. gingivalis induced a pro-malignant phenotype (i.e. EMT) in oral epithelial cells after 120 h (J. Lee et al. 2017b) and promoted primary OSCC cell invasion after 8 days (Abdulkareem et al. 2018).
We acknowledge some limitations of our study including the lack of in-depth mechanistic insight with regards to the molecular basis of EV-cancer cell interactions. Although this work lacked an in vivo model, using human-derived extracellular matrix provided some physiological relevance. In addition, challenging cancer cells with EVs, instead of bacteria, was deemed reasonable since they provide more controllable and predictable conditions compared to actual bacteria. Our EV samples had variable protein concentrations, partly due to the inherent differences across bacterial strains and their capacity to produce EVs and the variation in number of bacterial cells used during EV isolation. The particle size of the studied EVs showed modest variation (132.96–161.16 nm). It is unlikely that the variation would have affected our results since influence on particle uptake is associated with greater particle size differences, and generally spherical particles such as EVs are permissive for uptake by host cells (Baranov et al. 2020). Also, the effect of EV dose- and time-dependency and LPS detoxification warrant more studies in the future. Utilizing EVs as therapeutic agents represents a promising and rapidly emerging area in cancer research (Li et al. 2020).
Conclusion
The present comparative study demonstrates that EVs from periodontopathogens might have the potential to influence the behavior of oral cancer cells with either inhibitory or stimulatory effects. In particular, our in vitro findings reveal a possible antitumorigenic effect of A. actinomycetemcomitans-derived EVs on metastatic OSCC, which encourage further in vivo studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Marjut Metsäniitty, Kari K. Eklund, Tuula Salo, Jan Oscarsson and Abdelhakim Salem contributed to the study’s design and funding. Marjut Metsäniitty performed the experiments and collected data. Shrabon Hasnat and Carina Öhman assisted with the experiments and data collection. Marjut Metsäniitty, Jan Oscarsson and Abdelhakim Salem performed data analysis and interpretation. Marjut Metsäniitty and Abdelhakim Salem wrote the manuscript. Abdelhakim Salem is the corresponding author and main supervisor of this work. All authors read, commented and approved the final manuscript.
Funding
This research was funded by the Doctoral Program in Oral Sciences (FINDOS), University of Helsinki; Suomen Naishammaslääkärit ry; Minerva Foundation, Selma and Maja-Lisa Selander`s Fund; Ida Montinin Säätiö; The Medicine Fund, University of Helsinki; Päivikki and Sakari Sohlberg Foundation; Odontologiska Samfundet i Finland r.f; The Finnish Dental Society, Apollonia; TUA grants (7003193 and 7003766) from the County Council of Västerbotten, Sweden; Insamlingsstiftelsen, Medical Faculty, Umeå University.
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital).
Data availability
Data is provided within the manuscript or supplementary information files and available from the lead authors upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulkareem AA, Shelton RM, Landini G, Cooper PR, Milward MR. Periodontal pathogens promote epithelial-mesenchymal transition in oral squamous carcinoma cells in vitro. Cell Adh Migr. 2018;12(2):127–137. doi: 10.1080/19336918.2017.1322253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almahmoudi R, Salem A, Murshid S, Dourado MR, Apu EH, Salo T, Al-Samadi A (2019) Interleukin-17F has Anti-tumor effects in oral Tongue Cancer. Cancers (Basel) 11(5). 10.3390/cancers11050650 [DOI] [PMC free article] [PubMed]
- American Type Culture Collection Porphyromonas gingivalis (Coykendall Shah and Collins 33277™. https://www.atcc.org/products/33277?nt=wobj-20-q
- Asikainen S, Chen C, Slots J. Actinobacillus actinomycetemcomitans genotypes in relation to serotypes and periodontal status. Oral Microbiol Immunol. 1995;10(2):65–68. doi: 10.1111/j.1399-302x.1995.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Baranov MV, Kumar M, Sacanna S, Thutupalli S, van den Bogaart G. Modulation of Immune responses by particle size and shape. Front Immunol. 2020;11:607945. doi: 10.3389/fimmu.2020.607945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basith S, Manavalan B, Yoo TH, Kim SG, Choi S. Roles of toll-like receptors in cancer: a double-edged sword for defense and offense. Arch Pharm Res. 2012;35(8):1297–1316. doi: 10.1007/s12272-012-0802-7. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Maula T, Bao K, Lindholm M, Bostanci N, Oscarsson J, Ihalin R, Johansson A (2019) Virulence and pathogenicity properties of Aggregatibacter actinomycetemcomitans. Pathogens 8(4). 10.3390/pathogens8040222 [DOI] [PMC free article] [PubMed]
- Binder Gallimidi A, Fischman S, Revach B, Bulvik R, Maliutina A, Rubinstein AM, Nussbaum G, Elkin M. Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model. Oncotarget. 2015;6(26):22613–22623. doi: 10.18632/oncotarget.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Wang H, Liu J, Pan C, Zhang D, Li X, Pan Y. Porphyromonas gingivalis infection promoted the proliferation of oral squamous cell carcinoma cells through the miR-21/PDCD4/AP-1 negative signaling pathway. ACS Infect Dis. 2019;5(8):1336–1347. doi: 10.1021/acsinfecdis.9b00032. [DOI] [PubMed] [Google Scholar]
- Chen Z, Wong PY, Ng CWK, Lan L, Fung S, Li JW, Cai L, Lei P, Mou Q, Wong SH, Wu WKK, Li RJ, Meehan K, Lui VWY, Chow C, Lo KW, Chan ABW, Boon SS, Lau EHL, Chan JYK (2020) The intersection between oral microbiota, Host Gene Methylation and patient outcomes in Head and carcinoma. Cancers, 12(11). https://ovidsp.ovid.com/ovidweb.cgi?T=JS_CSC=Y_NEWS=N_PAGE=fulltext_D=pmnm_AN=33218162 [DOI] [PMC free article] [PubMed]
- Chen G, Gao C, Jiang S, Cai Q, Li R, Sun Q, Xiao C, Xu Y, Wu B, Zhou H. Fusobacterium nucleatum outer membrane vesicles activate autophagy to promote oral cancer metastasis. J Adv Res. 2023 doi: 10.1016/j.jare.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho BH, Jung YH, Kim DJ, Woo BH, Jung JE, Lee JH, Choi YW, Park HR. Acetylshikonin suppresses invasion of Porphyromonas gingivalis–infected YD10B oral cancer cells by modulating the interleukin-8/matrix metalloproteinase axis. Mol Med Rep. 2018;17(2):2327–2334. doi: 10.3892/mmr.2017.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker OO, Dai Z, Nie Y, Zhao G, Cao L, Nakatsu G, Wu WK, Wong SH, Chen Z, Sung JJY, Yu J. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut. 2018;67(6):1024–1032. doi: 10.1136/gutjnl-2017-314281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culture Collection University of Gothenburg. CCUG 35243 - Parvimonas micra. https://ccug.se/strain?id=35243_s=9350p=436sort=cc_collection=entire_records=50_t=
- Culture Collection University of Gothenburg. CCUG 32989T - Fusobacterium nucleatum subsp. nucleatum. https://www.ccug.se/strain?id=32989_s=0_p=1_sort=rel_collection=entire_records=25_t=fusobacterium+nucleatum+32989
- Da J, Wang X, Li L, Xu Y. Fusobacterium nucleatum promotes Cisplatin-Resistance and Migration of oral squamous carcinoma cells by Up-Regulating Wnt5a-Mediated NFATc3 expression. Tohoku J Exp Med. 2021;253(4):249–259. doi: 10.1620/tjem.253.249. [DOI] [PubMed] [Google Scholar]
- Damek-Poprawa M, Volgina A, Korostoff J, Sollecito TP, Brose MS, O’Malley BW, Jr, Akintoye SO, DiRienzo JM. Targeted inhibition of CD133 + cells in oral cancer cell lines. J Dent Res. 2011;90(5):638–645. doi: 10.1177/0022034510393511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranco KM, Johnson-Farley N, Bertino JR, Elson D, Vega BA, Belinka BA, Jr, Kachlany SC. LFA-1-targeting Leukotoxin (LtxA; Leukothera®) causes lymphoma tumor regression in a humanized mouse model and requires caspase-8 and Fas to kill malignant lymphocytes. Leuk Res. 2015;39(6):649–656. doi: 10.1016/j.leukres.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Economopoulou P, de Bree R, Kotsantis I, Psyrri A. Diagnostic tumor markers in Head and Neck squamous cell carcinoma (HNSCC) in the clinical setting. Front Oncol. 2019;9:827. doi: 10.3389/fonc.2019.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- Faïs T, Delmas J, Serres A, Bonnet R, Dalmasso G (2016) Impact of CDT Toxin on Human diseases. Toxins (Basel) 8(7). 10.3390/toxins8070220 [DOI] [PMC free article] [PubMed]
- Fazal S, Lee R (2021) Biomimetic bacterial membrane vesicles for drug delivery applications. Pharmaceutics 13(9). 10.3390/pharmaceutics13091430 [DOI] [PMC free article] [PubMed]
- Francescone RA, Faibish M, Shao R. A Matrigel-based tube formation assay to assess the vasculogenic activity of tumor cells. J Vis Exp. 2011 doi: 10.3791/3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi R, Guerra L, Levi L, Stenerlöw B, Fox JG, Josenhans C, Masucci MG, Frisan T. Chronic exposure to the cytolethal distending toxins of Gram-negative bacteria promotes genomic instability and altered DNA damage response. Cell Microbiol. 2013;15(1):98–113. doi: 10.1111/cmi.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha NH, Park DG, Woo BH, Kim DJ, Choi JI, Park BS, Kim YD, Lee JH, Park HR. Porphyromonas gingivalis increases the invasiveness of oral cancer cells by upregulating IL-8 and MMPs. Cytokine. 2016;86:64–72. doi: 10.1016/j.cyto.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Hasnat S, Hujanen R, Nwaru BI, Salo T, Salem A (2020) The Prognostic Value of Toll-Like receptors in Head and Neck squamous cell carcinoma: a systematic review and Meta-analysis. Int J Mol Sci 21(19). 10.3390/ijms21197255 [DOI] [PMC free article] [PubMed]
- Hoppe T, Kraus D, Novak N, Probstmeier R, Frentzen M, Wenghoefer M, Jepsen S, Winter J. Oral pathogens change proliferation properties of oral tumor cells by affecting gene expression of human defensins. Tumour Biol. 2016;37(10):13789–13798. doi: 10.1007/s13277-016-5281-x. [DOI] [PubMed] [Google Scholar]
- Hsueh CY, Lau HC, Huang Q, Gong H, Sun J, Cao P, Hu C, Zhang M, Tao L, Zhou L. Fusobacterium nucleatum impairs DNA mismatch repair and stability in patients with squamous cell carcinoma of the head and neck. Cancer. 2022;128(17):3170–3184. doi: 10.1002/cncr.34338. [DOI] [PubMed] [Google Scholar]
- Hujanen R, Almahmoudi R, Karinen S, Nwaru BI, Salo T, Salem A. Vasculogenic mimicry: a promising prognosticator in Head and Neck squamous cell carcinoma and esophageal Cancer? A systematic review and Meta-analysis. Cells. 2020;9(2):507. doi: 10.3390/cells9020507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hujanen R, Almahmoudi R, Salo T, Salem A (2021) Comparative analysis of vascular mimicry in Head and Neck squamous cell carcinoma: in Vitro and in vivo approaches. Cancers (Basel) 13(19). 10.3390/cancers13194747 [DOI] [PMC free article] [PubMed]
- Inaba H, Sugita H, Kuboniwa M, Iwai S, Hamada M, Noda T, Morisaki I, Lamont RJ, Amano A. Porphyromonas gingivalis promotes invasion of oral squamous cell carcinoma through induction of proMMP9 and its activation. Cell Microbiol. 2014;16(1):131–145. doi: 10.1111/cmi.12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwanaga K, Tominaga K, Yamamoto K, Habu M, Maeda H, Akifusa S, Tsujisawa T, Okinaga T, Fukuda J, Nishihara T. Local delivery system of cytotoxic agents to tumors by focused sonoporation. Cancer Gene Ther. 2007;14(4):354–363. doi: 10.1038/sj.cgt.7701026. [DOI] [PubMed] [Google Scholar]
- Jain S, Dash P, Minz AP, Satpathi S, Samal AG, Behera PK, Satpathi PS, Senapati S. Lipopolysaccharide (LPS) enhances prostate cancer metastasis potentially through NF-κB activation and recurrent dexamethasone administration fails to suppress it in vivo. Prostate. 2019;79(2):168–182. doi: 10.1002/pros.23722. [DOI] [PubMed] [Google Scholar]
- Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, Kaur M, Mei Y, Rao J. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leuk Res. 2010;34(6):777–785. doi: 10.1016/j.leukres.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan P, Ateia I, Shin JM, Fenno JC, Le C, Zhan L, Chang A, Darveau R, Kapila YL. Periodontal pathogens promote cancer aggressivity via TLR/MyD88 triggered activation of Integrin/FAK signaling that is therapeutically reversible by a probiotic bacteriocin. PLoS Pathog. 2020;16(10):e1008881. doi: 10.1371/journal.ppat.1008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanasi E, Doğan B, Karched M, Thay B, Oscarsson J, Asikainen S. Lack of serotype antigen in A. actinomycetemcomitans. J Dent Res. 2010;89(3):292–296. doi: 10.1177/0022034509358865. [DOI] [PubMed] [Google Scholar]
- Karinen S, Juurikka K, Hujanen R, Wahbi W, Hadler-Olsen E, Svineng G, Eklund KK, Salo T, Åström P, Salem A (2021) Tumour cells express functional lymphatic endothelium-specific hyaluronan receptor in vitro and in vivo: Lymphatic mimicry promotes oral oncogenesis? Oncogenesis, 10(3), 23–23. 10.1038/s41389-021-00312-3 [doi] [DOI] [PMC free article] [PubMed]
- Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32(1):1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- Kieselbach T, Zijnge V, Granström E, Oscarsson J. Proteomics of Aggregatibacter actinomycetemcomitans outer membrane vesicles. PLoS ONE. 2015;10(9):e0138591. doi: 10.1371/journal.pone.0138591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee J, Park J, Gho YS. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin Cell Dev Biol. 2015;40:97–104. doi: 10.1016/j.semcdb.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Lafuente I, de Mendoza I, Mendia M, Garcia de la Fuente X, Andres AMQ, Aguirre Urizar JM. Role of Porphyromonas gingivalis in oral squamous cell carcinoma development: a systematic review. J Periodontal Res. 2020;55(1):13–22. doi: 10.1111/jre.12691. [DOI] [PubMed] [Google Scholar]
- Lakio L, Paju S, Alfthan G, Tiirola T, Asikainen S, Pussinen PJ. Actinobacillus actinomycetemcomitans serotype d-specific antigen contains the O antigen of lipopolysaccharide. Infect Immun. 2003;71(9):5005–5011. doi: 10.1128/iai.71.9.5005-5011.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Roberts JS, Atanasova KR, Chowdhury N, Han K, Yilmaz Ö. Human primary epithelial cells acquire an epithelial-mesenchymal-transition phenotype during long-term infection by the oral opportunistic Pathogen, Porphyromonas gingivalis. Front Cell Infect Microbiol. 2017;7:493. doi: 10.3389/fcimb.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W-H, Chen H-M, Yang S-F, Liang C, Peng C-Y, Lin F-M, Tsai L-L, Wu B-C, Hsin C-H, Chuang C-Y, Yang T, Yang T-L, Ho S-Y, Chen W-L, Ueng K-C, Huang H-D, Huang C-N, Jong Y-J. Bacterial alterations in salivary microbiota and their association in oral cancer. Sci Rep. 2017;7(1):16540. doi: 10.1038/s41598-017-16418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Roberts JS, Atanasova KR, Chowdhury N, Yilmaz Ö. A novel kinase function of a nucleoside-diphosphate-kinase homologue in Porphyromonas gingivalis is critical in subversion of host cell apoptosis by targeting heat-shock protein 27. Cell Microbiol. 2018;20(5):e12825. doi: 10.1111/cmi.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Zhou H, Yang C, Wu Y, Zhou X, Liu H, Wang Y. Bacterial outer membrane vesicles as a platform for biomedical applications: an update. J Controlled Release: Official J Controlled Release Soc. 2020;323:253–268. doi: 10.1016/j.jconrel.2020.04.031. [DOI] [PubMed] [Google Scholar]
- Lindholm M, Metsäniitty M, Granström E, Oscarsson J. Outer membrane vesicle-mediated serum protection in Aggregatibacter actinomycetemcomitans. J Oral Microbiol. 2020;12(1):1747857. doi: 10.1080/20002297.2020.1747857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Liu S, Liu J, Miao L, Zhang S, Pan Y. sRNA23392 packaged by Porphyromonas gingivalis outer membrane vesicles promotes oral squamous cell carcinomas migration and invasion by targeting desmocollin-2. Mol oral Microbiol. 2021;36(3):182–191. doi: 10.1111/omi.12334. [DOI] [PubMed] [Google Scholar]
- Löwenmark T, Löfgren-Burström A, Zingmark C, Eklöf V, Dahlberg M, Wai SN, Larsson P, Ljuslinder I, Edin S, Palmqvist R. Parvimonas micra as a putative non-invasive faecal biomarker for colorectal cancer. Sci Rep. 2020;10(1):15250. doi: 10.1038/s41598-020-72132-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin JI, Checkoway H. Endotoxin and cancer. Environ Health Perspect. 2009;117(9):1344–1350. doi: 10.1289/ehp.0800439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager DL. Bacteria and cancer: cause, coincidence or cure? A review. J Transl Med. 2006;4:14. doi: 10.1186/1479-5876-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Park Y, Hasegawa Y, Tribble GD, James CE, Handfield M, Stavropoulos MF, Yilmaz O, Lamont RJ. Intrinsic apoptotic pathways of gingival epithelial cells modulated by Porphyromonas gingivalis. Cell Microbiol. 2007;9(8):1997–2007. doi: 10.1111/j.1462-5822.2007.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsäniitty M, Hasnat S, Salo T, Salem A (2021) Oral Microbiota-A New Frontier in the Pathogenesis and management of Head and Neck cancers. Cancers (Basel) 14(1). 10.3390/cancers14010046 [DOI] [PMC free article] [PubMed]
- Monasterio G, Castillo F, Astorga J, Hoare A, Terraza-Aguirre C, Cafferata EA, Villablanca EJ, Vernal R. O-Polysaccharide plays a major role on the virulence and immunostimulatory potential of Aggregatibacter actinomycetemcomitans during Periodontal infection. Front Immunol. 2020;11:591240. doi: 10.3389/fimmu.2020.591240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491–508. doi: 10.1016/j.soc.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ñahui Palomino RA, Vanpouille C, Costantini PE, Margolis L. Microbiota-host communications: bacterial extracellular vesicles as a common language. PLoS Pathog. 2021;17(5):e1009508. doi: 10.1371/journal.ppat.1009508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbant A, Chen C, Wang Y, Zadeh HH. Induction of T-cell apoptosis by Actinobacillus actinomycetemcomitans mutants with deletion of ltxA and cdtABC genes: possible activity of GroEL-like molecule. Oral Microbiol Immunol. 2003;18(6):339–349. doi: 10.1046/j.0902-0055.2003.00082.x. [DOI] [PubMed] [Google Scholar]
- Neuzillet C, Marchais M, Vacher S, Hilmi M, Schnitzler A, Meseure D, Leclere R, Lecerf C, Dubot C, Jeannot E, Klijanienko J, Mariani O, Calugaru V, Hoffmann C, Lesnik M, Badois N, Borcoman E, Piaggio E, Kamal M, Bieche I. Prognostic value of intratumoral Fusobacterium nucleatum and association with immune-related gene expression in oral squamous cell carcinoma patients. Sci Rep. 2021;11(1):7870. doi: 10.1038/s41598-021-86816-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nørskov-Lauritsen N. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin Microbiol Rev. 2014;27(2):214–240. doi: 10.1128/cmr.00103-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscarsson J, Claesson R, Lindholm M, Höglund Åberg C, Johansson A (2019) Tools of Aggregatibacter actinomycetemcomitans to evade the host response. J Clin Med 8(7). 10.3390/jcm8071079 [DOI] [PMC free article] [PubMed]
- Page RC, Sims TJ, Engel LD, Moncla BJ, Bainbridge B, Stray J, Darveau RP. The immunodominant outer membrane antigen of Actinobacillus actinomycetemcomitans is located in the serotype-specific high-molecular-mass carbohydrate moiety of lipopolysaccharide. Infect Immun. 1991;59(10):3451–3462. doi: 10.1128/iai.59.10.3451-3462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revest M, Egmann G, Cattoir V, Tattevin P. HACEK endocarditis: state-of-the-art. Expert Rev Anti Infect Ther. 2016;14(5):523–530. doi: 10.1586/14787210.2016.1164032. [DOI] [PubMed] [Google Scholar]
- Rompikuntal PK, Thay B, Khan MK, Alanko J, Penttinen AM, Asikainen S, Wai SN, Oscarsson J. Perinuclear localization of internalized outer membrane vesicles carrying active cytolethal distending toxin from Aggregatibacter actinomycetemcomitans. Infect Immun. 2012;80(1):31–42. doi: 10.1128/IAI.06069-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salem A, Salo T. Identity matters: cancer stem cells and tumour plasticity in head and neck squamous cell carcinoma. Expert Rev Mol Med. 2023;25:e8. doi: 10.1017/erm.2023.4. [DOI] [PubMed] [Google Scholar]
- Salo T, Sutinen M, Hoque Apu E, Sundquist E, Cervigne NK, de Oliveira CE, Akram SU, Ohlmeier S, Suomi F, Eklund L, Juusela P, Åström P, Bitu CC, Santala M, Savolainen K, Korvala J, Paes Leme AF, Coletta RD. A novel human leiomyoma tissue derived matrix for cell culture studies. BMC Cancer. 2015;15:981. doi: 10.1186/s12885-015-1944-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetab Boushehri MA, Lamprecht A. TLR4-Based immunotherapeutics in Cancer: a review of the achievements and shortcomings. Mol Pharm. 2018;15(11):4777–4800. doi: 10.1021/acs.molpharmaceut.8b00691. [DOI] [PubMed] [Google Scholar]
- Sims TJ, Moncla BJ, Darveau RP, Page RC. Antigens of Actinobacillus actinomycetemcomitans recognized by patients with juvenile periodontitis and periodontally normal subjects. Infect Immun. 1991;59(3):913–924. doi: 10.1128/iai.59.3.913-924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Singh AK. Porphyromonas gingivalis in oral squamous cell carcinoma: a review. Microbes Infect. 2022;24(3):104925. doi: 10.1016/j.micinf.2021.104925. [DOI] [PubMed] [Google Scholar]
- Söder B, Källmén H, Yucel-Lindberg T, Meurman JH. Periodontal microorganisms and diagnosis of malignancy: a cross-sectional study. Tumour Biol. 2021;43(1):1–9. doi: 10.3233/tub-200066. [DOI] [PubMed] [Google Scholar]
- Song W, Tiruthani K, Wang Y, Shen L, Hu M, Dorosheva O, Qiu K, Kinghorn KA, Liu R, Huang L. Trapping of Lipopolysaccharide to Promote Immunotherapy against Colorectal Cancer and Attenuate Liver Metastasis. Adv Mater. 2018;30(52):e1805007. doi: 10.1002/adma.201805007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Teles FRF, Alawi F, Castilho RM, Wang Y. Association or Causation? Exploring the oral Microbiome and Cancer Links. J Dent Res. 2020;99(13):1411–1424. doi: 10.1177/0022034520945242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima R, Hanada K, Akada J, Kawano K, Yamaoka Y. Aggregatibacter actinomycetemcomitans infection causes DNA double-strand breaks in host cells. Genes Cells. 2018;23(4):264–273. doi: 10.1111/gtc.12570. [DOI] [PubMed] [Google Scholar]
- Ungureanu BS, Gheorghe DN, Nicolae FM, Râmboiu S, Radu PA, Șurlin VM, Strâmbu VDE, Gheonea DI, Roman A, Șurlin P. Could there be an interplay between periodontal changes and pancreatic malignancies? World J Clin Cases. 2023;11(3):545–555. doi: 10.12998/wjcc.v11.i3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanaja SK, Russo AJ, Behl B, Banerjee I, Yankova M, Deshmukh SD, Rathinam VAK. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and Caspase-11 activation. Cell. 2016;165(5):1106–1119. doi: 10.1016/j.cell.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Goodman SD, Redfield RJ, Chen C. Natural transformation and DNA uptake signal sequences in Actinobacillus actinomycetemcomitans. J Bacteriol. 2002;184(13):3442–3449. doi: 10.1128/jb.184.13.3442-3449.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson SC, Metcalf RL, Trapani F, Mohan S, Antonello J, Abbott B, Leong HS, Chester CP, Simms N, Polanski R, Nonaka D, Priest L, Fusi A, Carlsson F, Carlsson A, Hendrix MJ, Seftor RE, Seftor EA, Rothwell DG, Dive C. Vasculogenic mimicry in small cell lung cancer. Nat Commun. 2016;7:13322. doi: 10.1038/ncomms13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Schifferle RE. Evidence that the serotype b antigenic determinant of Actinobacillus actinomycetemcomitans Y4 resides in the polysaccharide moiety of lipopolysaccharide. Infect Immun. 1991;59(4):1544–1551. doi: 10.1128/iai.59.4.1544-1551.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Zhang Q, Peng Y, Wang D, Liu Y. The effect of periodontal bacteria infection on incidence and prognosis of cancer: a systematic review and meta-analysis. Medicine. 2020;99(15):e19698. doi: 10.1097/MD.0000000000019698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Tominaga K, Sukedai M, Okinaga T, Iwanaga K, Nishihara T, Fukuda J. Delivery of cytolethal distending toxin B induces cell cycle arrest and apoptosis in gingival squamous cell carcinoma in vitro. Eur J Oral Sci. 2004;112(5):445–451. doi: 10.1111/j.1600-0722.2004.00157.x. [DOI] [PubMed] [Google Scholar]
- Yang C-Y, Yeh Y-M, Yu H-Y, Chin C-Y, Hsu C-W, Liu H, Huang P-J, Hu S-N, Liao C-T, Chang K-P, Chang Y-L. Oral Microbiota Community Dynamics Associated with oral squamous cell carcinoma staging. Front Microbiol. 2018;9:862. doi: 10.3389/fmicb.2018.00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Chu M, Huang Z, Yang X, Ran S, Hu B, Zhang C, Liang J. Variations in oral microbiota associated with oral cancer. Sci Rep. 2017;7(1):11773. doi: 10.1038/s41598-017-11779-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Cho WC, Nicolls MR. Colorectal Cancer-Associated Microbiome patterns and signatures. Front Genet. 2021;12:787176. doi: 10.3389/fgene.2021.787176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang WR, Wang Y, Nie W, Lei Y, Liang C, He J, Zuo L, Huang LL, Xie HY. Bacterial outer membrane vesicle based versatile nanosystem boosts the efferocytosis blockade triggered tumor-specific immunity. Nat Commun. 2023;14(1):1675. doi: 10.1038/s41467-023-37369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files and available from the lead authors upon reasonable request.