Abstract
Introduction
Focal segmental glomerulosclerosis (FSGS), the most common primary glomerular disease leading to end-stage kidney disease (ESKD), is characterized by podocyte injury and depletion, whereas minimal change disease (MCD) has better outcomes despite podocyte injury. Identifying mechanisms capable of preventing podocytopenia during injury could transform FSGS to an "MCD-like" state. Preclinical data have reported conversion of an MCD-like injury to one with podocytopenia and FSGS by inhibition of AMP-kinase (AMPK) in podocytes. Conversely, in FSGS, AMPK-activation using metformin (MF) mitigated podocytopenia and azotemia. Observational studies also support beneficial effects of MF on proteinuria and chronic kidney disease (CKD) outcomes in diabetes. A randomized controlled trial (RCT) to test MF in podocyte injury with FSGS has not yet been conducted.
Methods
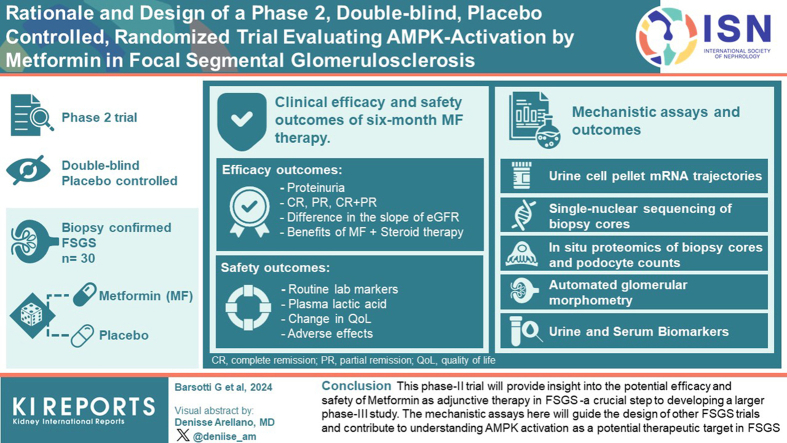
We report the rationale and design of phase 2, double-blind, placebo-controlled RCT evaluating the efficacy and safety of MF as adjunctive therapy in FSGS. By randomizing 30 patients with biopsy-confirmed FSGS to MF or placebo (along with standard immunosuppression), we will study mechanistic biomarkers that correlate with podocyte injury or depletion and evaluate outcomes after 6 months. We specifically integrate novel urine, blood, and tissue markers as surrogates for FSGS progression along with unbiased profiling strategies.
Results and Conclusion
Our phase 2 trial will provide insight into the potential efficacy and safety of MF as adjunctive therapy in FSGS—a crucial step to developing a larger phase 3 study. The mechanistic assays here will guide the design of other FSGS trials and contribute to understanding AMPK activation as a potential therapeutic target in FSGS. By repurposing an inexpensive agent, our results will have implications for FSGS treatment in resource-poor settings.
Keywords: adjunctive therapy, AMP-activated protein kinase, focal segmental glomerulosclerosis, metformin, phase 2, randomized trial
Graphical abstract
FSGS is characterized ultrastructurally by podocyte foot process effacement (FPE) and podocytopenia.1 FSGS causes proteinuria and nephrotic syndrome, and when accompanied by podocytopenia exceeding a critical threshold (∼40% per glomerulus), is sufficient to cause progressive CKD.2 Overall, 40% to 50% of patients with FSGS will progress to ESKD, representing the most common primary glomerular disease associated with ESKD in the US.3 In contrast to FSGS, MCD exhibits FPE and nephrotic syndrome but is associated with less podocytopenia, a treatment-responsive course, and lower ESKD progression to ESKD. Current treatments for FSGS involve immune modulation or hemodynamic interventions.4 Identifying and addressing the mechanisms of podocyte preservation during injury (exemplified by MCD-like injury) could lead to a transformation of FSGS into an "MCD-like" state, mitigating podocytopenia and progressive CKD. To our knowledge, no current therapeutic specifically targets signaling mechanisms preserving podocytes, highlighting a gap in the field.
We previously reported that in young mice, knockdown of Shroom35 in podocytes, induced diffuse podocyte FPE and albuminuria.6, 7, 8 Surprisingly, podocyte FPE was not associated with podocytopenia or FSGS in these animals—suggesting an “MCD-like” pathology. These MCD-like glomeruli had lower glomerular volumes (Vglom)7,8 and showed inactivation of Fyn in podocytes.6 Fyn is a nonreceptor tyrosine kinase known to phosphorylate tyrosine residues on nephrin and regulate the podocyte cytoskeleton.9 Interestingly, Fyn inactivation has also been identified in human MCD.10 Indeed, larger glomeruli are associated with FSGS;7 and in the NEPTUNE cohort, MCD biopsies had reduced Vgloms11 versus FSGS. Given these similarities between human MCD and our MCD-like model, we investigated collateral pathways in Fyn-inactivated podocytes, and identified increased activation of AMPK via translocation of LKB1.7 As further corroboration, genes related to the Fyn-LKB1-Ampk axis were significantly enriched in human MCD versus FSGS glomeruli in a cohort within NEPTUNE.7
AMPK is a serine-threonine kinase activated during cellular stress that suppresses anabolism, promotes autophagy, while supporting cell survival. Mice with diffuse FPE and an MCD-like pathology related to Fyn-inactivation “switched” to podocyte loss, glomerulomegaly, and FSGS when AMPK was inhibited7 pharmacologically or by ageing.7 Conversely, in murine FSGS models, we observed significantly mitigated glomerulomegaly, podocytopenia, azotemia, and FSGS using AMPK activators including MF.7,8,12 Renal protection with MF has also been observed in numerous experimental models ranging from diabetic-associated or obesity-associated kidney disease (∼300 indexed citations), other proteinuric kidney diseases (lupus nephritis,13,14 Alport’s syndrome,15 and subtotal nephrectomy16,17), as well as nonproteinuric kidney diseases.18, 19, 20, 21, 22, 23 Therefore, extensive preclinical data demonstrate the benefit of MF in renal disease, and our work supports a specific role for AMPK in podocyte survival after injury.
In clinic, MF is among the most widely prescribed drugs with known pharmacokinetics and established safety. Specific evaluation in mild-to-moderate CKD (estimated glomerular filtration rate [eGFR] >30 ml/min) has demonstrated no significant increases in lactic acidosis with MF.12,24, 25, 26, 27 Importantly, in numerous observational data and post hoc analyses in diabetes,24,25,28 MF use is repeatedly associated with reduced proteinuria, improved renal survival, and overall patient survival.
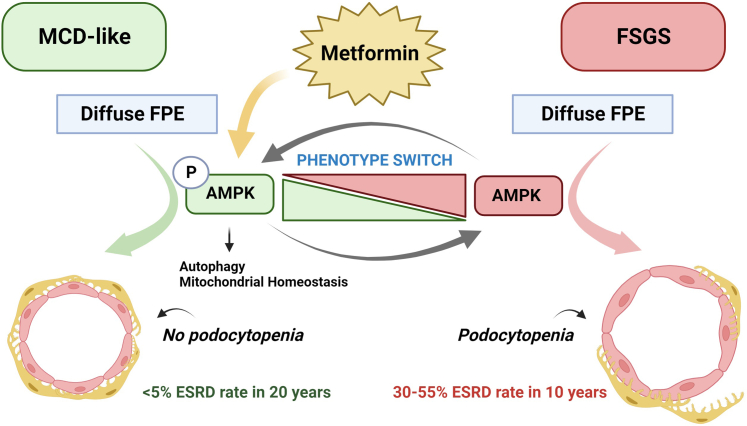
Taken together, extensive preclinical and clinical data point to AMPK-activation by MF in glomerular podocytes as an intervention in need of testing in an RCT in the context of podocyte injury and FPE. This may reveal a therapeutically exploitable strategy to promote podocyte survival, limit glomerulomegaly and mitigate FSGS, shift toward an MCD-like pathology, and direct the development of MCD-like pathology (Figure 1). Here, we report our rationale and study design for the AMP-FSGS trial—a pilot, placebo-controlled RCT to test the utility of MF in biopsy-confirmed FSGS.
Figure 1.
Outlines the mechanistic hypothesis underlying the AMP-FSGS trial. Inhibited AMPK-signaling in the context of FPE promotes glomerulomegaly, podocytopenia, and FSGS, whereas activation of the AMPK signaling pathway by metformin in injured podocytes mitigates podocytopenia and restricts glomerulomegaly promoting an “MCD-like” pathology with better outcomes. Increased AMPK activation enhances prosurvival pathways, including autophagy and improves mitochondrial homeostasis. Therefore, the trial hypothesizes that metformin will activate AMPK, which may serve as a "switch" in injured podocytes, regulating cell survival, and mitigating podocytopenia and FSGS.
AMPK, AMP kinase; MCD, minimal change disease; FPE, foot process effacement; FSGS, focal segmental glomerulosclerosis.
Trial Design
Objectives
The AMP-FSGS trial will investigate whether and how MF therapy will improve biopsy-confirmed FSGS using integrated mechanistic and clinical data.
Objective 1
Here, we will test the hypothesis that MF, with standard-of-care therapy mitigates podocyte loss by inducing AMPK activation and promoting an “MCD-like” pathology. We will examine multiparametric mechanistic correlates of improved outcomes using serial urine, blood, and biopsies by applying conventional and novel approaches, including biomarkers, automated morphometry, podocyte numbers, in situ proteomics, and single-cell transcriptomics.
Objective 2
Clinical efficacy and safety outcomes of 6-month MF therapy will be evaluated. MF-related adverse events (AEs) will be monitored (below) and coincidental anthropometric and metabolic benefits will be captured.
Preenrollment Screening
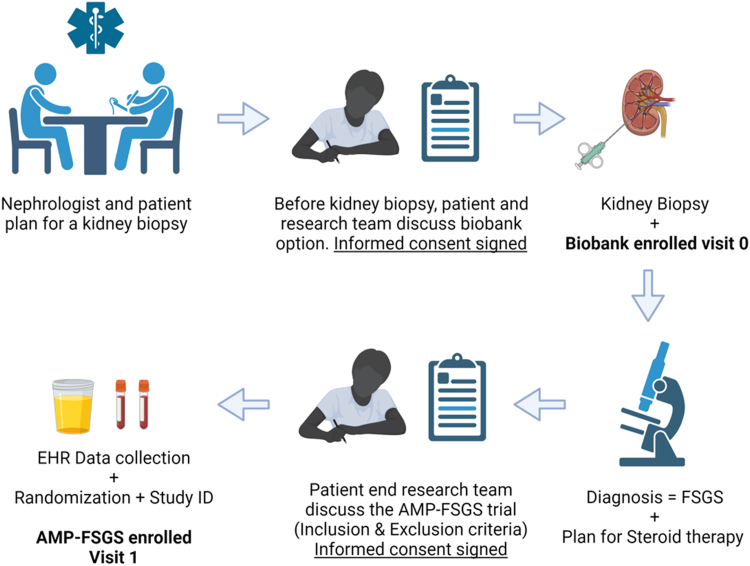
Patients at both sites, Yale University School of Medicine and Icahn School of Medicine at Mount Sinai, are screened during diagnostic kidney biopsies (details of screening, enrollment, are in Supplementary Methods). There is a standard workflow that includes the identification of all patients referred for biopsy via electronic health record reports. At the time of biopsy, all adult patients at both sites are approached for consent in kidney biobanks. To optimize the enrollment process for AMP-FSGS, patients showing specific risk factors for FSGS such as nephrotic syndrome or a urine protein-to-creatinine ratio >1.5 g/g and without a diagnosis of diabetes will be flagged for potential enrollment in the AMP-FSGS trial pending biopsy diagnoses. The enrollment workflow is depicted in Figure 2.
Figure 2.
Enrollment workflow. Schematic describes the enrollment workflow plan from the identification of patients after referral for kidney biopsy by the research team, the approach and consent for biobank enrollment, and sample collection (visit 0). Biobank consent includes optional extra research core for transcriptome evaluation. Once biopsy diagnosis of FSGS (with diffuse FPE) is confirmed and both the treating nephrologist and patient decide on steroid therapy for FSGS (other glomerular diseases are excluded), the research team will approach the patient during clinic visit for enrollment in AMP-FSGS (after evaluating inclusion and exclusion criteria). After AMP-FSGS informed consent is obtained, unique identity generation and randomization will be followed by visit-1 sample collection. FPE, foot process effacement; FSGS, focal segmental glomerulosclerosis.
Enrollment and Randomization
Adults without contraindications to MF with biopsy-confirmed FSGS will be approached for AMP-FSGS enrollment.
Inclusion/Exclusion Criteria and Informed Consent
Given that our goal is to identify patients with “primary” FSGS, we focus on candidates selected for corticosteroid therapy with diffuse FPE on biopsy (inclusion/exclusion criteria in Table 1). Informed consent is obtained during the first postbiopsy clinic visit. The optional repeat biopsy at 6 months will also be discussed.
Table 1.
Study inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| -Liver disease | |
| Participant must be 18 years of age or older, but <80 years age at the time of signing the informed consent. | -eGFR <32 ml/min |
| - Diabetes mellitus diagnosis at the time of biopsy or need for oral hypoglycemic agents/insulin with a HbA1c level >6.5% | |
| AND | -Kidney biopsy with non-FSGS glomerulonephritis, or secondary FSGS. |
| -Dementia | |
| Biopsy-confirmed FSGS as defined by expert renal pathology at either institutions. For homogeneity of diagnoses, demonstrable segmental or global sclerosis lesions (>1 glomerulus) with diffuse podocyte foot process effacement by electron microscopy (>50% of examined glomerular tufts) | -Allergy or sensitivity to metformin |
| -Platelet count <100,000/μl; INR >1.5; bleeding diathesis or blood thinner use contraindicating biopsy. | |
| Therapeutic plan by treating physician for immunomodulatory treatment using Glucocorticoids. | -Current pregnancy or desire to become pregnant during the study period |
| -Under hospice care | |
| -Incarceration | |
| -History of alcohol abuse | |
| -Homelessness | |
| -Inability to consent | |
| -eGFR (calculated using the CKD-Epi equation) of ≥32 ml/min/m2 | -Life expectancy of less than 6 months as determined by the clinical judgement of the patient’s physician |
| - Participants may or may not be receiving an ACE-inhibitor, angiotensin-receptor blocker, or renin inhibitor or SGLT2 inhibitors or other BP medications. | -Simultaneous use of carbonic anhydrase inhibitor agents |
| -Incident FSGS cases or cases identified upon rebiopsy will be included as long as immunosuppressive therapy is planned. | - Currently enrolled in (or completed within the past 30 days) a study of an investigational drug or device. |
ACE, angiotensin-converting enzyme; BP, blood pressure; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; SGLT2, sodium-glucose transport protein 2.
Randomization
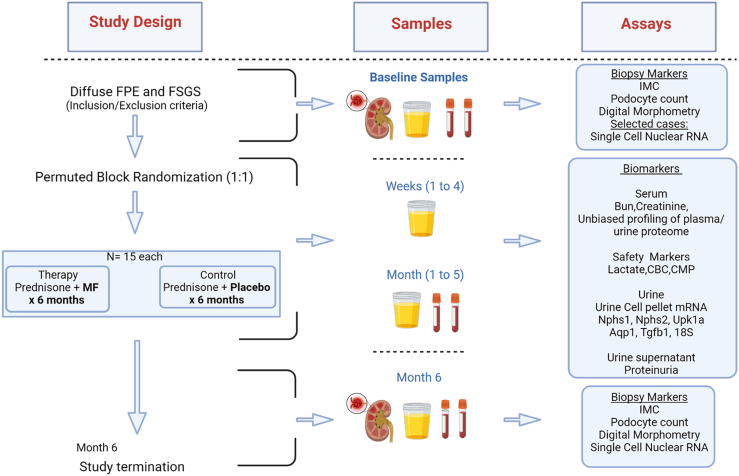
The study will employ a double-blinded design, where both the research team and treating physicians will be blinded to study limb. A permuted block randomization scheme is implemented via REDCap, and randomization is stratified based on the study site. Our first subject has been enrolled and randomized. In Figure 3, we depict the trial schedule, planned visits, and procedures for enrollees.
Figure 3.
Study plan overview. Study design, samples planned, and assays to be performed at prespecified time points are summarized. The study will include 10-visits and will be terminated at 6 months where a repeat research biopsy will be performed in subjects who consent. This “paired-biopsy” cohort will be the subset where IMC, digital morphometry and single nuclear RNA-sequencing will be performed. IMC, imaging mass cytometry.
Interventions
The Investigational Drug Pharmacy will assign participants to therapy (prednisone and MF) or control (prednisone and placebo) and will remain unblinded.
MF Dosing in AMP-FSGS
MF, a US Food and Drug Administration-approved oral antihyperglycemic agent, has well-documented pharmacokinetics and safety data.28, 29, 30 An identical placebo formulation is generated by the Investigational Drug Pharmacy at both sites.
Determinants of initial MF Dosage
MF, is primarily excreted unchanged by the kidneys and is minimally protein-bound, relying on eGFR to establish plasma levels.29 With an eGFR >30 ml/min, studies have not shown an elevated risk of lactic acidosis with MF.24, 25, 26 MF dosing will be based on eGFR at enrollment as follows: (i) if eGFR is between 32 and 45 ml/min, MF will be initiated at 500 mg once a day and (ii) if eGFR >45 ml/min, MF will be initiated at 1000 mg once a day (equivalent to 2 tablets). These dosages align with previous studies.31,32
Dose Modifications and discontinuation of study drug
If eGFR falls to ≤45 ml/min during the study, the MF/placebo dose will be reduced to 500 mg/day. MF/placebo will be discontinued (i.e., guidance for discontinuation of study drug) in cases of the following: (i) eGFR decreasing <32 ml/min in 2 successive readings, (ii) plasma lactate >5 mmol/l in 2 tests or persistently >2.5 mmol/l, (iii) elevated liver function tests without other etiology (e.g., >2 times upper limit of normal liver enzymes, hyperbilirubinemia, or coagulopathy), (iv) hypoglycemic episodes, or (v) gastrointestinal symptom scores indicating intolerance to MF. MF extended-release formulation will be used to minimize gastrointestinal intolerance.
Study Visits
A total of 10 visits are planned for each participant. The visits (Table 2) are designed to optimally capture data points and biosamples to detect early responses to intervention and ensure patient safety while minimizing patient burden.
Table 2.
Study schedule of activities
| Schedule of activities | Enrollment |
Month 1 |
Month 2–5 |
Final visit |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline visit 1 | Wk 1–visit 2 | Wk2–visit 3 | Wk3–visit 4 | Wk4–visit 5 | Mo 2–visit 6 | Mo 3–visit 7 | Mo 4–visit 8 | Mo 5–visit 9 | Mo 6–visit 10 | |
| Procedures | ||||||||||
| Informed consent | X | X (Follow up Bx | ||||||||
| Chart Review and documentation | ||||||||||
| Physical examination | X | X | ||||||||
| Concomitant medication review | X | X | X | X | X | X | X | |||
| Medical history | X | |||||||||
| Study-specific assessments | ||||||||||
| Anthropometry measures | X | X | X | |||||||
| Adverse Effects/QOL questionnaires | X | X | X | X | X | X | X | |||
| Study Drug | ||||||||||
| Dispensing of 30-day study drugs. | X | X | X | X | X | X | ||||
| Laboratory tests | ||||||||||
| Routine blood test for standard-of-care | ||||||||||
| BMP | X | X | X | X | X | X | X | |||
| CBC | X | X | X | X | X | X | X | |||
| Routine urine studies | ||||||||||
| Urine protein-to-creatinine ratio | X | X | X | X | X | X | X | X | X | X |
| 24-hour urine studies | X | X | X | |||||||
| Safety laboratory tests related to the study | ||||||||||
| Liver function tests | X | X | X | X | ||||||
| Plasma lactate levels | X | X | X | X | X | X | X | |||
| Lipid panel | X | X | X | |||||||
| Hemoglobin A1C | X | X | X | |||||||
| Vitamin B12 | X | X | X | |||||||
| Research Studies | ||||||||||
| Plasma or biomarkers | X | X | X | X | ||||||
| Buffy coat/ PBMC | X | X | X | X | ||||||
| Urine for biomarkers | X | X | X | X | X | X | X | X | X | X |
| Urine pellet mRNA∗∗ | X | X | X | X | X | X | X | X | X | X |
| Research biopsy | X | |||||||||
QOL, quality of life, BMP, basic metabolic panel; CBC, complete blood count; PBMC, peripheral blood mononuclear cells; mRN, messenger RNA.
Repeat Biopsy (Paired-Biopsy Cohort)
Prior to enrollment in AMP-FSGS, all patients will have undergone a clinically indicated baseline biopsy. Around 1 of 3 of enrollees will be reconsented for a follow-up biopsy at 6 months, designated as “paired biopsy cohort”. This cohort will use kidney tissue to test the hypothesis that MF treatment activates cellular AMPK-signaling and improves podocyte survival, glomerular morphometry, and histologic parameters when compared to placebo. Three key assays (described below) will be performed in the paired biopsy cohort: single-nuclear sequencing of biopsy core, in situ proteomics including podocyte counts, and automated glomerular morphometry.
Study Oversight
Trial Oversight
The AMP-FSGS trial oversight has been approved by a single institutional review board and by IRBs at both participating centers (Yale University School of Medicine and Icahn School of Medicine at Mount Sinai). A single institutional review board will oversee study protocol amendments and interim study site monitoring, including working with the data and safety monitoring board for AEs reporting. The trial is registered at ct.gov (NCT06090227).
Monitoring and AE Reporting
Active monitoring through electronic health record and monthly questionnaires will capture AEs, whether related or unrelated to the study. Electronic health record and data tools will track AEs, ensuring study-related AEs are captured and investigators will assess causality or consider alternative causes using clinical judgment. Specific adverse effect profiles related to MF, especially gastrointestinal intolerance, hypoglycemia, liver function, vitamin B12, and lactic acidosis, will be specifically monitored. A data and safety monitoring board has been constituted and includes an endocrinologist, pharmacist, and nephrologists with clinical trial experience. The data and safety monitoring board will meet semiannually for trial oversight and serious AEs during the study will be promptly reported.
Oversight Over Study Drug
MF is a US Food and Drug Administration-approved oral agent for glycemic control and is the fourth most prescribed drug globally. Studies have shown MF’s efficacy in other indications.3,33,34 The AMP-FSGS trial obtained an investigational new drug exemption (PIND: 168933) from the US Food and Drug Administration. Following data analysis in year 4, a new investigational new drug application may be supported based on results.
Study Outcomes
Clinical Efficacy and Safety Outcomes of 6-Month MF Therapy
In our preliminary murine data, AMPK-activation (by MF or other agents) improved podocyte survival, ameliorated glomerulomegaly, improved azotemia and reduced proteinuria. In epidemiologic data, the use of MF was associated with improved clinical outcomes, namely eGFR, proteinuria, need for renal replacement therapy, and overall survival. Previous FSGS trials1,35 have used proteinuria changes as primary outcomes and measured eGFR decline or ESKD. Although our phase 2 trial is not primarily designed to detect differences in these major clinical events, we will evaluate eGFR and proteinuria outcomes as exploratory markers of efficacy and evaluate safety of MF therapy in FSGS.
Efficacy Outcomes
The efficacy outcomes include the following:
-
(i)
Proteinuria (random urine protein-to-creatinine ratios) at all 10 study visits will be compared using mixed-effects modeling to evaluate the slope of change in proteinuria over time (continuous outcome). Twenty-four-hour protein estimations will be obtained at 3-months and 6-months.
-
(ii)
Difference in the slope of eGFR over 6 months in each patient, and within study limb, will be compared. Outcomes (i) and (ii) will provide generalizable endpoints for other studies.36,37
-
(iii)
Exploratory analyses will examine additional immunosuppressive requirement, complete remission (CR) (proteinuria <0.5 g/day), partial remission (PR) (>50% reduction in proteinuria from prerandomization) and CR + PR (at study end, and in time-to-event analyses).
-
(iv)
Exploration of potential benefits of MF combination with steroid therapy: anthropometric measurements (height, weight, waist-to-hip ratio) will be obtained at 0, 3, and 6 months to monitor steroid-related metabolic adverse effects, which we expect will be reduced when MF is combined with steroid therapy (as was described).38 The benefit may likely extend to diabetes risk, and hyperlipidemia in the MF limb (hemoglobin A1C and lipids will be monitored every 3 months).
Safety Outcomes
The safety outcomes include the following:
-
(i)
Routine laboratory markers (comprehensive metabolic panel and complete blood count) will be obtained monthly.
-
(ii)
Plasma lactic acid will be obtained at every visit and serum vitamin B12 levels will be obtained 3-monthly.
-
(iii)
Change in quality of life over time, including fatigue, thirst, day/night reversal, and (as kidney disease becomes more severe) itching, nausea, and anorexia will be monitored. At baseline and at each visit, we will use modified Kidney Disease Quality of Life survey instrument to test quality of life with MF treatment (modified to include potential MF-related symptoms).
-
(iv)
Clinical safety outcomes will include diarrhea, clinical hypoglycemia events, lactic acidosis (leading to MF stoppage or study discontinuation), infections, hospitalizations, and any study deaths.
Mechanistic Assays and Outcomes
Our phase 2 study is mainly focused on mechanistic data and assays (below) which will be correlated with patient-level, and study limb-level outcomes.
Urine Cell Pellet mRNA Trajectories
Primary FSGS is considered a podocytopathy resulting in podocyte FPE, podocyte depletion, and progressive CKD.2,39, 40, 41 Podocyte-specific mRNA quantified in the urine have correlated with podocyte depletion in FSGS.39,42, 43, 44, 45 Increased urine Nphs2:creatinine, that is, “podocinuria,” correlated with active disease, and was lower during remission.39 Correlations between podocinuria and proteinuria were altered in treatment-responsive (MCD-like) disease versus progressive, unresponsive FSGS. Prognostic value was also seen with urine Nphs1:Nphs2 ratio,46 urine Aqp2 and Tgfb1 levels.47 These noninvasive and repeatable assays have never been tested in an interventional trial before. Here, we will use urinary podocyte mRNA excretion trajectories in MF versus control patients to represent potential prognostic signals.
The details of urine pellet RNA isolation have been reported (Drs Wiggins, Naik – consultants39) and are described in Supplementary Methods. Briefly, morning-collected urine samples (∼200 ml) will be centrifuged and the pellet cryostored (Supplementary Figure S1). A simultaneous ∼10 ml aliquot will be sent to the clinical laboratory for urinalysis and protein-to-creatinine ratio. Pellets will be batched for RNA extraction, reverse transcription, and quantitative polymerase chain reaction.
Assay Development
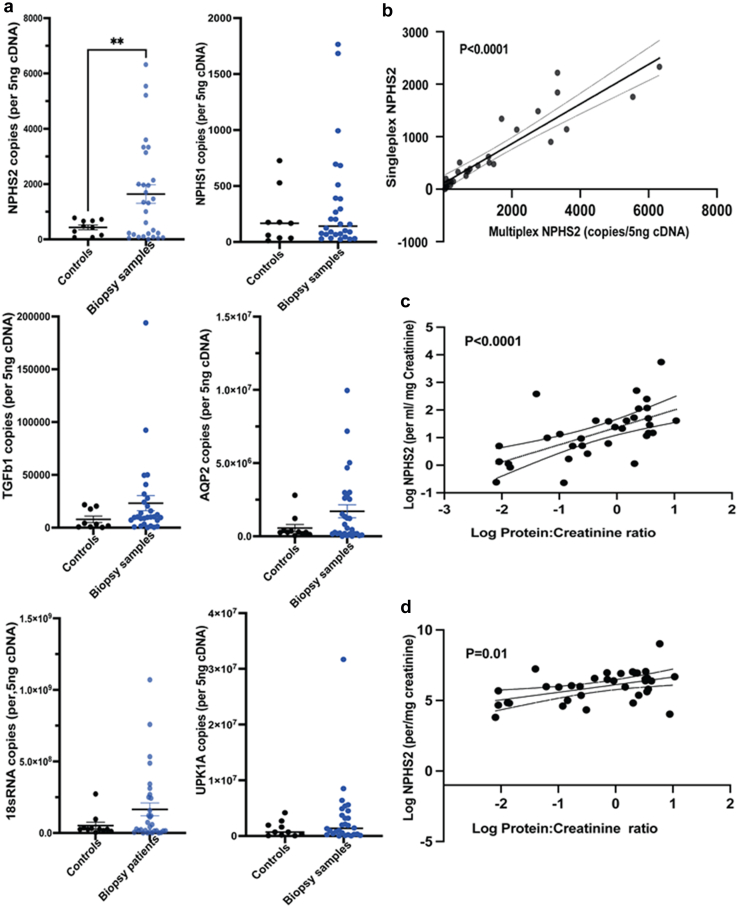
Based on previous observational studies, we will evaluate the following mRNA values in each urine sample: NPHS2, NPHS1, AQP2, TGFB1, 18SRNA, & UPK1A (where each mRNA is expressed as copy numbers/ml urine/mg creatinine). Using a pilot study of 10 pooled control samples (from healthy volunteers) and 30 unselected patients presenting for kidney biopsies (Figure 4, Supplementary Figure S2, Supplementary Table S1, and Supplementary Methods), we established 2 novel multiplex assays in our laboratory to detect and quantify urine mRNA. This minimizes RNA requirement and plate-to-plate variation allowing simultaneous testing of 3-genes in each assay. In our assay, mRNA markers tended to be increased in biopsied patient’s versus healthy controls, although only NPHS2-mRNA was significantly different (Figure 3a). Interestingly, UPK1A representing urothelial cell fraction of urine cell pellet was similar in cases and controls. Copy numbers obtained by singleplex and multiplex panel were highly correlated (log-NPHS2 shown in Figure 3b). We also confirmed that podocinuria (log-NPHS2 mRNA copies/ml or log-copies/mg creatinine47) significantly correlated with simultaneous proteinuria (protein-to-creatinine ratio; Figure 3c and d). Log-NPHS1 mRNA showed weaker correlation with proteinuria in our pilot, and none of the mRNA evaluated correlated with eGFR (not shown).
Figure 4.
Urine pellet mRNA estimation for podocyte markers. (a) Dot plots show urine cell pellet mRNA of NPHS2, NPHS1, TGFb1, AQP2, 18SRNA, UPK1A (per 5 ng cDNA) quantified in controls (n = 10 pooled samples; solid black dots) versus 30 unselected patients needing kidney biopsy (solid blue dots). UPK1A levels notably were similar between the 2 groups (Whiskers/bar = mean±SEM). (b) A correlation line is plotted between Log NPHS2 copy numbers/5ng cDNA obtained by singleplex and these same data obtained by a novel multiplex panel (Spearman R = 0.86; P < 0.0001). (c) Correlation lines of Log NPHS2 copies/ml urine sample versus log protein-to-creatinine ratio (mg/mg) (Spearman R = 0.66; P < 0.0001], and (d) Log NPHS2 copies/mg creatinine is plotted against log protein-to-creatinine ratio (Spearman R = 0.39; P < 0.01).
Analyses
Using this assay in each patient, urine-mRNA values from 10 serial collections (baseline, study visits, and study completion) will be plotted against log protein-to-creatinine ratios obtained simultaneously to obtain trajectories. (i) As reported,39 the correlation lines of log protein-to-log podocin (normalized to creatinine at 1,3, and 6 months) will be evaluated to distinguish treatment-responsive cases from progressive FSGS within each limb. (ii) Urine mRNA trajectories aggregated by study limb will be compared (a) against each other, (b) against clinical outcomes (CR, PR, CR+PR) and, (c) in paired biopsies against histo-morphometric outcomes such as change in podocyte counts and/or Vglom, interstitial fibrosis and tubular atrophy (interstitial fibrosis and tubular atrophy percentage in biopsies, or using digital morphometry – quantitative interstitial area score and composite damage scores).7 (iv) In exploratory analyses, we will evaluate urine mRNA trajectories with single cell transcriptomic signatures of AMPK activation, and proteomic signatures (see below).
Single-Nuclear Sequencing of Biopsy Cores
We will compare podocyte AMPK activation in the MF-therapy limb with prerandomization FSGS biopsies using single nuclear transcriptome profiles. Briefly, both at baseline biopsy (as part of the nephrology biobank in both institutions) and upon follow-up 6-month research biopsy (in consenting enrollees ∼30%), we save a portion of the obtained biopsy core in RNAlater. Subsequently, single nuclear preparation and sequencing will be performed on this “paired-biopsy cohort” (see Supplementary Methods). We will utilize established KPMP protocols developed by the University of Michigan (see Supplementary Data), to ensure comparability of generated data.
Analyses
We will analyze kidney SnRNAseq data using Seurat,48, 49, 50 focusing on glomerular cell types (Supplementary Figure S3). In podocytes, we will identify significantly differentially expressed genes within paired biopsies in each individual, and between treatment groups. Our goal with single nuclear transcriptome analyses is 2-fold as follows: (i) to confirm activation of AMPK signaling in podocytes of patients treated with MF versus placebo. To accomplish this, we will compare podocyte transcriptomes with a novel podocyte-specific AMPK activation mouse model (γ1D316A-transgenic with overactive AMPK γ-subunit),51 using cross-species analyses.52,53 (ii) Our second goal is to evaluate if AMPK activation by MF promotes a gene expression profile in glomerular cells and within podocytes that has significant overlap with human MCD glomerular transcriptome, by utilizing MCD versus FSGS comparisons in microdissected glomerular RNAseq data from NEPTUNE cohort. In each case, significantly differentially expressed genes will be further analyzed for enriched pathways and functional terms. Covariates such as sex, age, and patient status will be considered.
In Situ Proteomics of Biopsy Cores and Podocyte Counts
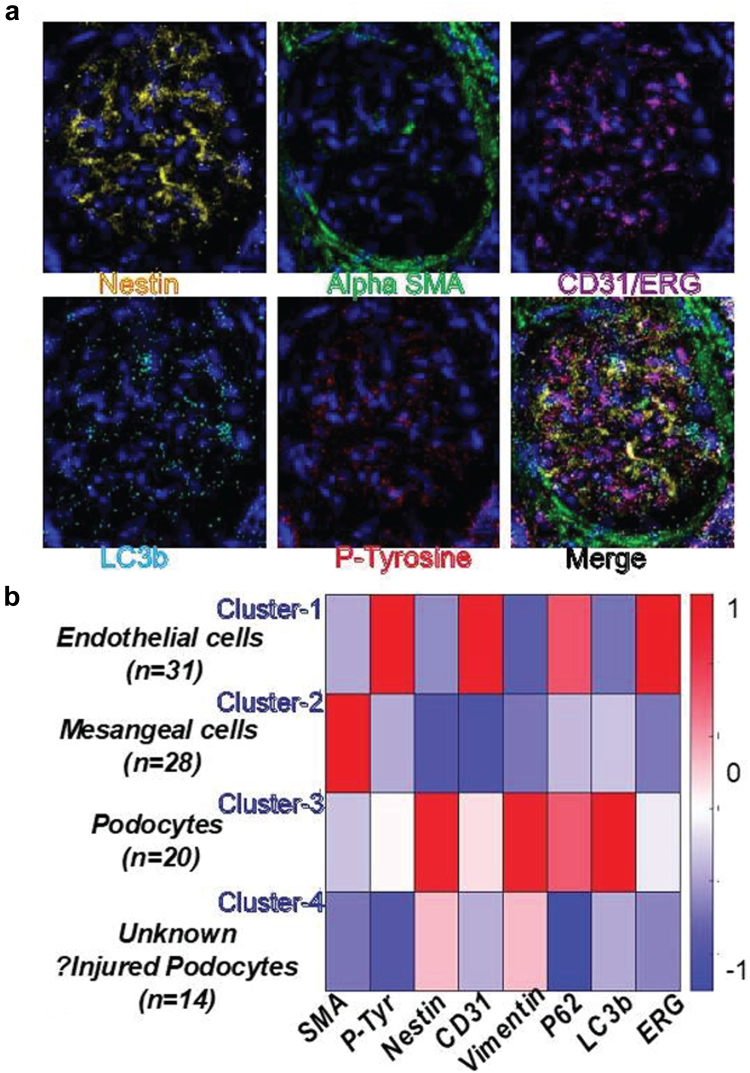
Imaging mass cytometry (Collaborator Dr Cantley)54 will be used to analyze paired biopsies (see Supplementary Methods).54,55 The following comparisons will be made by imaging mass cytometry: (i) final biopsies between MF versus placebo groups, (ii) the initial versus final biopsies within-patient in the MF group, and (iii) in treatment-responsive cases versus others. We aim to identify optimal cell-specific proteomic markers that associate with podocinuria or prognosis and confirm podocyte AMPK activation with MF. In pilot imaging mass cytometry data generated on a nonsclerotic glomerulus in an FSGS biopsy (Figure 5), a focused glomerular pipeline using existing validated antibodies clearly identified normal glomerular cells and cells potentially representing injured podocytes with reduced canonical podocyte markers and lower LC3b, demonstrating quantification of proteins at the single cell level.
Figure 5.
Representative IMC of a nonsclerotic glomerulus from FSGS. (a) Individual channels for Nestin, SMA, CD31/ERG, LC3b, P-Tyrosine, and Merge (P62, DNA intercalator, Vimentin, other markers are not shown). (b) Heatmap identifies cell-type clusters and cell numbers based on colocalized intensities (per pixel) of canonical markers. Cluster 4 cells express lower levels of canonical podocyte markers with reduced autophagy, and likely represent injured podocytes. Thus, intensities of markers-of-interest (LC3b, P62, P-Tyr) can be quantified within single cells. FSGS, focal segmental glomerulosclerosis; IMC, imaging mass cytometry; P-tyr, phosphorylated-tyrosine residues.
Automated Glomerular Morphometry
Our overarching goal with pathologic studies in paired biopsies is to evaluate if MF restricts glomerulomegaly in FSGS and promotes quantitative morphometric features associated with improved outcomes. In addition to conventional histopathology with standard-of-care reporting by renal pathologists at both study centers, we will use our deep-learning model based on U-Net and mask R-CNN digital pathology algorithms which quantify normal tissue compartments in PAS-stained whole-slide biopsy images. In previous work,56 we showed high correlations of digital and conventional pathology, and superior associations of artificial intelligence (AI)-derived continuous variables with clinical outcomes. A modification of this algorithm measures glomerular tuft area and distinguishes sclerotic or nonsclerotic glomeruli. “AI-Vglom” will then be calculated based on the mean AI-area using the Weibel-Gomez equation.7 Mean Vglom will be compared between (i) individuals on serial biopsies by study group, (ii) between final study groups, and (iii) using initial biopsies to correlate with clinical outcomes, agnostic of study groups (N = 30). Additional glomerular parameters such as area density, as well as nonglomerular parameters, (% of image area with interstitial fibrosis or interstitial infiltrates) are evaluated concurrently by our AI algorithm56 reported previously as composite scores (interstitial area score and composite damage score). We anticipate that AI-Vglom will be lower after MF and that nonglomerular parameters will have independent associations with outcomes.
Urine and Serum Biomarkers
Classical illustrative studies using patients with recurrent FSGS after transplantation identified a role for 1 or more circulating protein factors in primary FSGS.1,57, 58, 59 The Olink platform multiplexes 384 inflammatory protein assays and critically includes previously reported candidate plasma markers. This could provide relatively unbiased information regarding FSGS outcomes. In addition, several groups including ours have evaluated and identified the role of plasma markers60,61 and urine markers62, 63, 64 of CKD progression.65, 66, 67 Evaluation of these known urine and plasma markers of tubular injury, CKD progression, as well as a relatively unbiased interrogation of inflammatory signals, to develop prognostic markers in the setting of an FSGS clinical trial has not been performed before. Plasma and urine proteomics will be obtained at baseline, at 3 months and at 6 months. Among patients showing improved clinical outcomes (e.g., CR/PR), we will identify proteins whose Olink levels normalize between month-0 and month-3 and/or month-6. These will serve as biomarkers indicating treatment responsiveness.61,62,64,68 Biomarkers identified at baseline can enrich participation, help risk stratification, whereas those that associate with treatment responsiveness can reduce duration or costs in subsequent FSGS trials.64,69,70
Statistical Analyses
Details of the statistical analyses are described in the Supplementary Methods. Data are entered into a HIPAA-safe REDCap platform created for AMP-FSGS (Yale Redcap).
Baseline Data
Summary statistics will be used to describe participants. Analyses will follow the intention-to-treat principle, grouping participants regardless of adherence to the treatment regimen. Per-protocol analyses will also be performed to include all participants who demonstrate ≥75% adherence to study drug (as assessed via pill counts at study visits) and complete the final study visit.
Genetic FSGS and Sensitivity Analyses
We anticipate genetic testing to be uniformly done in AMP-FSGS enrollees as is usual clinical practice at both study sites. In sensitivity analyses, we will evaluate outcomes after excluding individuals with a confirmed genetic etiology of FSGS and where immunosuppressants were stopped by treating physicians. The study drug will be continued until completion, with the patient’s consent. Heterogeneity of treatment effect, as determined by genetic cause of FSGS, will be evaluated by examining the interaction between genetic FSGS and MF on the outcomes of interest.
Outcomes
(a) Categorical clinical outcomes: Fisher exact test or chi-square test (based on data sparsity) will be used for CR, PR, and similar outcomes; Wilcoxon rank-sum test for continuous outcomes. (b) Within-individual change outcomes: mixed-effects models with time-interaction terms will be used to assess slope outcomes (e.g., proteinuria, eGFR, biomarker levels) across groups. Various covariance structures will be explored based on model fit. (c) Mechanistic outcomes: broad biomarker characterization will be using hypothesis-driven (Mesoscale) and less biased (O-Link) approaches. Dimensionality reduction techniques, including principal component analysis, consensus-based clustering, and PHATE analysis, will be employed. (d) Safety outcomes: measures of association will be calculated for treatment and safety events. Data will be presented in aggregate to the data and safety monitoring board, recognizing the importance of safety concerns beyond statistical significance. The modified Kidney Disease Quality of Life survey responses will be scored based on established criteria, assessing physical function, mental function, kidney disease burden, symptoms, and impact on daily life. Mixed-effects models with a treatment interaction term will analyze treatment impact on adverse effect parts.
Sample Size/Power Analyses
Enrolling 30 individuals (15 per group) will provide 80% power to detect a 1.1 SD difference in continuous outcomes. Our preliminary murine data using AMPK-activation strategies including MF (details in Section 7.8 of Supplementary Methods) suggested that differences in levels of BUN, creatinine, albuminuria, Vglom, and podocyte counts, in multiple FSGS models exceeded this threshold with AMPK-activation. Anticipating a 20% loss-to-follow-up rate, the trial can still detect 1.2 SDs in continuous outcomes. Additional recruitment avenues and collaborations with other medical centers are available if needed.
Statistical Software
Data analyses will be conducted using GraphPad prism (Version 10, La Jolla, California), R (https://www.R-project.org/), Python, Stata (StataCorp LLC, Texas), and SAS.
Discussion
Here, we describe our rationale and design of a phase 2 placebo controlled, double bind, RCT to test MF (in addition to standard-of-care) for FSGS requiring immunosuppressive therapy. If successful, we believe several implications will emerge from the results of our trial. First, any utility of MF in FSGS will suggest an important role for AMPK activation in the survival of injured podocytes. This proof-of-concept step will then provide a foundation for advancing to a larger phase 3 trial aimed at testing the efficacy of MF (or other AMPK-activators) in primary FSGS. The insights gained here are also likely to extend beyond primary FSGS, potentially to secondary FSGS, a more widely prevalent problem with limited therapeutics. It is crucial to emphasize that MF is an affordable, widely manufactured, and safe drug in widespread use. Any utility shown by MF in our study could benefit not only the US population but also have a significant public health impact in resource-poor settings worldwide.
Regardless of the specific outcomes observed with MF in our trial, our study has broader implications for the field. For example, the development of the urine pellet mRNA trajectory as a noninvasive marker of progression of kidney disease represents an advance with implications for trials across other glomerular diseases. The potential utilization and refinement of integrated mechanistic end points embedded in our trial could also serve as surrogates in future clinical trials of glomerular disease. The innovative and unbiased translational tools proposed here pave the way for identifying novel therapeutic targets and prognostic clusters. These tools promise to leverage dynamic multidimensional data encompassing morphometric, transcriptomic, and proteomic information in patients with FSGS while on standard therapy.
Our study also has some limitations. Our sample size could limit inferences on categorical outcomes related to MF, but we anticipate power to support inferences based on continuous outcomes. Variability in urine mRNA within and between patients could pose a challenge. However, our preliminary assay development data including controls and patients with glomerular or nonglomerular pathology, collected in a “real-time” clinic scenario, and the subsequent troubleshooting steps we have undertaken, provide reassurance of reproducibility in trial patients. As a precaution, multiple baseline samples will be collected for each patient to better provide a podocinuria profile for each patient at baseline. Finally, though we aim to restrict enrollment to those who are judged by treating clinicians to have primary FSGS needing immunomodulatory therapy, these patients may also include patients with genetic FSGS, and MF therapy will be evaluated in sensitivity analyses excluding these patients.
Conclusion
This phase 2 trial will provide important insights into the potential efficacy and safety of MF as an adjunctive therapy for FSGS. The findings will inform the design of larger trials and contribute to the understanding of AMPK activation as a potential therapeutic target in FSGS. The results may have implications for the treatment of FSGS and may benefit patient populations beyond the study cohort.
Disclosure
DGM is named coinventor on a pending patent, “Methods and Systems for Diagnosis of Acute Interstitial Nephritis” and is co-founder of the diagnostics company Predict AIN LLC. All the other authors declared no competing interests.
Acknowledgments
This material is based upon work supported by the Department of Defense, Awarding Office: USAMRAA under HT94252310454 number (PI MCM). MCM acknowledges funding from the NIH (grants R01DK122164, R01DK132274, and R21AI178705), and acknowledges Mr Saiprasad Manu, Ms Hongmei Shi for technical assistance. DGM acknowledges grant funds K23DK117065 and R01DK128087. The study team wishes to acknowledge the Michigan Kidney Translational Medicine center (PI Dr Mathias Kretzler), Prof Roger Wiggins for advice on urinary mRNA and Dr Chirag Parikh for guidance with the study’s specimen management system.
Footnotes
GCB, RL, and AK have equal contribution.
Supplementary Methods. Preenrollment screeningEnrollment and randomizationInformed consentInterventionStudy visitsStudy assaysStatistical plan and data analyses
Supplementary References.
Figure S1. Urine processing and pellet quantitative polymerase chain reaction. Step 1: Flowchart showing harmonized urine processing steps starting with urine collection in a sterile, sealed container, followed by centrifugation for cell pellet and storage in RLT-buffer. Step-2: At a later date, total urine pellet RNA is extracted using the RNeasy Mini Kit, and reverse transcription is performed. Initial detection and quantification of urinary mRNA was established using SYBR-green reagents and in-house primers. Absolute standard curves were generated using expression plasmids for each gene. Urinary mRNA detection/ quantification assays were finalized using customized TaqMan assays (Suppl methods). The standard curve shown in step 2 represents R2=0.998 and efficiency of 100.24% to detect NPHS2 copies. Red dots shows standards while blue/green dots represent test samples run in this plate.
Figure S2. Quality control metrics of Urine pellet mRNA. (A) Line diagrams show the mean/standard error of NPHS2- (green dots with error bars) and NPHS1- (blue dots with error bars) shows copies/5ng cDNA of 10 pooled control urine samples to provide an estimate of variability of these urine mRNAs. Individual samples obtained at different times from 7 healthy controls were variably pooled to generate ∼500 ml urine/pooled sample. Pooled samples were utilized to obtain larger cell pellets in each control to allow multiple runs of the same sample across plates. (B and C) Correlation plot of Urine NPHS2 with (B) RNA concentration (range 3–160 ng/mcl) and (C) with 260/280 ratio (range 1.38–2.27) by nanodrop (N = 40), showing absence of significant correlations. Similar evaluation of NPHS1, UPK1A, TGFB1, AQP2 & 18SRNA showed no significant correlations of copy numbers with these quality metrics (not shown).
Figure S3. Transcriptome analyses pipeline for biopsy single nuclear RNAseq. Schematic describes transcriptome analysis pipeline of single nuclear transcriptomes from subset of patients with paired kidney biopsies. Our goals are to evaluate (a) AMPK activation in podocytes with MF treatment using a podocyte-specific AMPK-activation mouse model (b) evaluate significantly differentially expressed genes between known MCD vs FSGS comparisons from the NEPTUNE cohort with MF- vs placebo treatment (c) identify consistently dysregulated genes (>2 fold) that could be tested at the protein level in biopsies (d) identify putative ligand-receptor interactions using our generated proteomic data.
Table S1. Demographics of Cases and Biopsy Controls.
Contributor Information
F. Perry Wilson, Email: francis.p.wilson@yale.edu.
John C. He, Email: cijiang.he@mssm.edu.
Madhav C. Menon, Email: madhav.menon@yale.edu.
Supplementary Material
Supplementary Methods.
Preenrollment screening
Enrollment and randomization
Informed consent
Intervention
Study visits
Study assays
Statistical plan and data analyses
Supplementary References
Figure S1. Urine processing and pellet quantitative polymerase chain reaction. Step 1: Flowchart showing harmonized urine processing steps starting with urine collection in a sterile, sealed container, followed by centrifugation for cell pellet and storage in RLT-buffer. Step-2: At a later date, total urine pellet RNA is extracted using the RNeasy Mini Kit, and reverse transcription is performed. Initial detection and quantification of urinary mRNA was established using SYBR-green reagents and in-house primers. Absolute standard curves were generated using expression plasmids for each gene. Urinary mRNA detection/ quantification assays were finalized using customized TaqMan assays (Suppl methods). The standard curve shown in step 2 represents R2=0.998 and efficiency of 100.24% to detect NPHS2 copies. Red dots shows standards while blue/green dots represent test samples run in this plate.
Figure S2. Quality control metrics of Urine pellet mRNA. (A) Line diagrams show the mean/standard error of NPHS2- (green dots with error bars) and NPHS1- (blue dots with error bars) shows copies/5ng cDNA of 10 pooled control urine samples to provide an estimate of variability of these urine mRNAs. Individual samples obtained at different times from 7 healthy controls were variably pooled to generate ∼500 ml urine/pooled sample. Pooled samples were utilized to obtain larger cell pellets in each control to allow multiple runs of the same sample across plates. (B and C) Correlation plot of Urine NPHS2 with (B) RNA concentration (range 3–160 ng/mcl) and (C) with 260/280 ratio (range 1.38–2.27) by nanodrop (N = 40), showing absence of significant correlations. Similar evaluation of NPHS1, UPK1A, TGFB1, AQP2 & 18SRNA showed no significant correlations of copy numbers with these quality metrics (not shown).
Figure S3. Transcriptome analyses pipeline for biopsy single nuclear RNAseq. Schematic describes transcriptome analysis pipeline of single nuclear transcriptomes from subset of patients with paired kidney biopsies. Our goals are to evaluate (a) AMPK activation in podocytes with MF treatment using a podocyte-specific AMPK-activation mouse model (b) evaluate significantly differentially expressed genes between known MCD vs FSGS comparisons from the NEPTUNE cohort with MF- vs placebo treatment (c) identify consistently dysregulated genes (>2 fold) that could be tested at the protein level in biopsies (d) identify putative ligand-receptor interactions using our generated proteomic data.
Table S1. Demographics of Cases and Biopsy Controls.
References
- 1.De Vriese A.S., Wetzels J.F., Glassock R.J., Sethi S., Fervenza F.C. Therapeutic trials in adult FSGS: lessons learned and the road forward. Nat Rev Nephrol. 2021;17:619–630. doi: 10.1038/s41581-021-00427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wharram B.L., Goyal M., Wiggins J.E., et al. Podocyte depletion causes glomerulosclerosis: diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol. 2005;16:2941–2952. doi: 10.1681/ASN.2005010055. [DOI] [PubMed] [Google Scholar]
- 3.GBD Chronic Kidney Disease Collaboration Collaboration GBDCKD. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vivarelli M., Massella L., Ruggiero B., Emma F. Minimal change disease. Clin J Am Soc Nephrol. 2017;12:332–345. doi: 10.2215/CJN.05000516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hildebrand J.D., Soriano P. Shroom, a PDZ domain-containing actin-binding protein, is required for neural tube morphogenesis in mice. Cell. 1999;99:485–497. doi: 10.1016/s0092-8674(00)81537-8. [DOI] [PubMed] [Google Scholar]
- 6.Wei C., Banu K., Garzon F., et al. SHROOM3-FYN interaction regulates nephrin phosphorylation and affects albuminuria in allografts. J Am Soc Nephrol. 2018;29:2641–2657. doi: 10.1681/ASN.2018060573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banu K., Lin Q., Basgen J.M., et al. AMPK mediates regulation of glomerular volume and podocyte survival. JCI Insight. 2021;6 doi: 10.1172/jci.insight.150004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Agati V.D., Chagnac A., de Vries A.P., et al. Obesity-related glomerulopathy: clinical and pathologic characteristics and pathogenesis. Nat Rev Nephrol. 2016;12:453–471. doi: 10.1038/nrneph.2016.75. [DOI] [PubMed] [Google Scholar]
- 9.Verma R., Wharram B., Kovari I., et al. Fyn binds to and phosphorylates the kidney slit diaphragm component nephrin. J Biol Chem. 2003;278:20716–20723. doi: 10.1074/jbc.M301689200. [DOI] [PubMed] [Google Scholar]
- 10.Zhang S.Y., Kamal M., Dahan K., et al. c-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci Signal. 2010;3:ra39. doi: 10.1126/scisignal.2000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sampson M.G., Robertson C.C., Martini S., et al. Integrative genomics identifies novel associations with APOL1 risk genotypes in black Neptune subjects. J Am Soc Nephrol. 2016;27:814–823. doi: 10.1681/ASN.2014111131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inzucchi S.E., Lipska K.J., Mayo H., Bailey C.J., McGuire D.K. Metformin in patients with type 2 diabetes and kidney disease: a systematic review. JAMA. 2014;312:2668–2675. doi: 10.1001/jama.2014.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jang S.G., Lee J., Hong S.M., Kwok S.K., Cho M.L., Park S.H. Metformin enhances the immunomodulatory potential of adipose-derived mesenchymal stem cells through STAT1 in an animal model of lupus. Rheumatol (Oxf Engl) 2020;59:1426–1438. doi: 10.1093/rheumatology/kez631. [DOI] [PubMed] [Google Scholar]
- 14.Yin Y., Choi S.C., Xu Z., et al. Normalization of CD4+ T cell metabolism reverses lupus. Sci Transl Med. 2015;7:274ra218. doi: 10.1126/scitranslmed.aaa0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Omachi K., Kaseda S., Yokota T., et al. Metformin ameliorates the severity of experimental Alport syndrome. Sci Rep. 2021;11:7053. doi: 10.1038/s41598-021-86109-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borges C.M., Fujihara C.K., Malheiros D., de Ávila V.F., Formigari G.P., Lopes de Faria J.B. Metformin arrests the progression of established kidney disease in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Ren Physiol. 2020;318:F1229–F1236. doi: 10.1152/ajprenal.00539.2019. [DOI] [PubMed] [Google Scholar]
- 17.Satriano J., Sharma K., Blantz R.C., Deng A. Induction of AMPK activity corrects early pathophysiological alterations in the subtotal nephrectomy model of chronic kidney disease. Am J Physiol Ren Physiol. 2013;305:F727–F733. doi: 10.1152/ajprenal.00293.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yi H., Huang C., Shi Y., et al. Metformin attenuates folic-acid induced renal fibrosis in mice. J Cell Physiol. 2018;233:7045–7054. doi: 10.1002/jcp.26505. [DOI] [PubMed] [Google Scholar]
- 19.Takiar V., Nishio S., Seo-Mayer P., et al. Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA. 2011;108:2462–2467. doi: 10.1073/pnas.1011498108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pastor-Soler N.M., Li H., Pham J., et al. Metformin improves relevant disease parameters in an autosomal dominant polycystic kidney disease mouse model. Am J Physiol Ren Physiol. 2022;322:F27–F41. doi: 10.1152/ajprenal.00298.2021. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y., Wang Y., Li Y., et al. Metformin attenuates renal interstitial fibrosis through upregulation of Deptor in unilateral ureteral obstruction in rats. Exp Ther Med. 2020;20:17. doi: 10.3892/etm.2020.9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang M., Weng X., Guo J., Chen Z., Jiang G., Liu X. Metformin alleviated EMT and fibrosis after renal ischemia-reperfusion injury in rats. Ren Fail. 2016;38:614–621. doi: 10.3109/0886022X.2016.1149770. [DOI] [PubMed] [Google Scholar]
- 23.Lin C.X., Li Y., Liang S., et al. Metformin attenuates cyclosporine A-induced renal fibrosis in rats. Transplantation. 2019;103:e285–e296. doi: 10.1097/TP.0000000000002864. [DOI] [PubMed] [Google Scholar]
- 24.Yamada E., Pessin J.E., Kurland I.J., Schwartz G.J., Bastie C.C. Fyn-dependent regulation of energy expenditure and body weight is mediated by tyrosine phosphorylation of LKB1. Cell Metab. 2010;11:113–124. doi: 10.1016/j.cmet.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F., Lu J.X., Tang J.L., et al. Relationship of plasma creatinine and lactic acid in type 2 diabetic patients without renal dysfunction. Chin Med J (Engl) 2009;122:2547–2553. [PubMed] [Google Scholar]
- 26.Ekstrom N., Schioler L., Svensson A.M., et al. Effectiveness and safety of metformin in 51 675 patients with type 2 diabetes and different levels of renal function: a cohort study from the Swedish National Diabetes Register. BMJ Open. 2012;2 doi: 10.1136/bmjopen-2012-001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim V.C., Sum C.F., Chan E.S., Yeoh L.Y., Lee Y.M., Lim S.C. Lactate levels in Asian patients with type 2 diabetes mellitus on metformin and its association with dose of metformin and renal function. Int J Clin Pract. 2007;61:1829–1833. doi: 10.1111/j.1742-1241.2007.01487.x. [DOI] [PubMed] [Google Scholar]
- 28.Eurich D.T., Weir D.L., Majumdar S.R., et al. Comparative safety and effectiveness of metformin in patients with diabetes mellitus and heart failure: systematic review of observational studies involving 34,000 patients. Circ Heart Fail. 2013;6:395–402. doi: 10.1161/CIRCHEARTFAILURE.112.000162. [DOI] [PubMed] [Google Scholar]
- 29.Graham G.G., Punt J., Arora M., et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 30.Lalau J.D., Kajbaf F., Bennis Y., Hurtel-Lemaire A.S., Belpaire F., De Broe M.E. Metformin treatment in patients with Type 2 diabetes and chronic kidney disease stages 3A, 3B, or 4. Diabetes Care. 2018;41:547–553. doi: 10.2337/dc17-2231. [DOI] [PubMed] [Google Scholar]
- 31.Perrone R.D., Abebe K.Z., Watnick T.J., et al. Primary results of the randomized trial of metformin administration in polycystic kidney disease (TAME PKD) Kidney Int. 2021;100:684–696. doi: 10.1016/j.kint.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seliger S.L., Abebe K.Z., Hallows K.R., et al. A randomized clinical trial of metformin to treat autosomal dominant polycystic kidney disease. Am J Nephrol. 2018;47:352–360. doi: 10.1159/000488807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mueller N.T., Differding M.K., Zhang M., et al. Metformin affects gut microbiome composition and function and circulating short-chain fatty acids: a randomized trial. Diabetes Care. 2021;44:1462–1471. doi: 10.2337/dc20-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chu C.D., Powe N.R., McCulloch C.E., et al. Trends in chronic kidney disease care in the US by race and ethnicity, 2012-2019. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.27014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gipson D.S., Trachtman H., Kaskel F.J., et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 2011;80:868–878. doi: 10.1038/ki.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Inker L.A., Heerspink H.J.L., Tighiouart H., et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: a meta-analysis of treatment effects of randomized controlled trials. J Am Soc Nephrol. 2019;30:1735–1745. doi: 10.1681/ASN.2019010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grams M.E., Sang Y., Ballew S.H., et al. Evaluating glomerular filtration rate slope as a surrogate end point for ESKD in clinical trials: an individual participant meta-analysis of observational data. J Am Soc Nephrol. 2019;30:1746–1755. doi: 10.1681/ASN.2019010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stirling C.M., Mathieson P., Boulton-Jones J.M., et al. Treatment and outcome of adult patients with primary focal segmental glomerulosclerosis in five UK renal units. Q J M. 2005;98:443–449. doi: 10.1093/qjmed/hci072. [DOI] [PubMed] [Google Scholar]
- 39.Wickman L., Afshinnia F., Wang S.Q., et al. Urine podocyte mRNAs, proteinuria, and progression in human glomerular diseases. J Am Soc Nephrol. 2013;24:2081–2095. doi: 10.1681/ASN.2013020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang G., Lai F.M., Kwan B.C., et al. Podocyte loss in human hypertensive nephrosclerosis. Am J Hypertens. 2009;22:300–306. doi: 10.1038/ajh.2008.360. [DOI] [PubMed] [Google Scholar]
- 41.Puelles V.G., van der Wolde J.W., Wanner N., et al. mTOR-mediated podocyte hypertrophy regulates glomerular integrity in mice and humans. JCI Insight. 2019;4 doi: 10.1172/jci.insight.99271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuda A., Minakawa A., Sato Y., Shibata H., Hara M., Fujimoto S. Excretion patterns of urinary sediment and supernatant podocyte biomarkers in patients with CKD. Kidney360. 2022;3:63–73. doi: 10.34067/KID.0004772021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuda A., Sato Y., Iwakiri T., et al. Urine podocyte mRNAs mark disease activity in IgA nephropathy. Nephrol Dial Transplant. 2015;30:1140–1150. doi: 10.1093/ndt/gfv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naik A.S., Le D., Aqeel J., et al. Podocyte stress and detachment measured in urine are related to mean arterial pressure in healthy humans. Kidney Int. 2020;98:699–707. doi: 10.1016/j.kint.2020.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding F., Wickman L., Wang S.Q., et al. Accelerated podocyte detachment and progressive podocyte loss from glomeruli with age in Alport syndrome. Kidney Int. 2017;92:1515–1525. doi: 10.1016/j.kint.2017.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y., Hodgin J.B., Afshinnia F., et al. The two kidney to one kidney transition and transplant glomerulopathy: a podocyte perspective. J Am Soc Nephrol. 2015;26:1450–1465. doi: 10.1681/ASN.2014030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fukuda A., Wickman L.T., Venkatareddy M.P., et al. Urine podocin:nephrin mRNA ratio (PNR) as a podocyte stress biomarker. Nephrol Dial Transplant. 2012;27:4079–4087. doi: 10.1093/ndt/gfs313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fu J., Akat K.M., Sun Z., et al. Single-cell RNA profiling of glomerular cells shows dynamic changes in experimental diabetic kidney disease. J Am Soc Nephrol. 2019;30:533–545. doi: 10.1681/ASN.2018090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang Z., Sun Z., Fu J., et al. Recipient APOL1 risk alleles associate with death-censored renal allograft survival and rejection episodes. J Clin Invest. 2021;131 doi: 10.1172/JCI146643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fu J., Wei C., Lee K., et al. Comparison of glomerular and podocyte mRNA profiles in streptozotocin-induced diabetes. J Am Soc Nephrol. 2016;27:1006–1014. doi: 10.1681/ASN.2015040421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Steinberg G.R., Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat Rev Drug Discov. 2019;18:527–551. doi: 10.1038/s41573-019-0019-2. [DOI] [PubMed] [Google Scholar]
- 52.Hodgin J.B., Nair V., Zhang H., et al. Identification of cross-species shared transcriptional networks of diabetic nephropathy in human and mouse glomeruli. Diabetes. 2013;62:299–308. doi: 10.2337/db11-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berthier C.C., Bethunaickan R., Gonzalez-Rivera T., et al. Cross-species transcriptional network analysis defines shared inflammatory responses in murine and human lupus nephritis. J Immunol. 2012;189:988–1001. doi: 10.4049/jimmunol.1103031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh N., Avigan Z.M., Kliegel J.A., et al. Development of a 2-dimensional atlas of the human kidney with imaging mass cytometry. JCI Insight. 2019;4 doi: 10.1172/jci.insight.129477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Avigan Z.M., Singh N., Kliegel J.A., Weiss M., Moeckel G.W., Cantley L.G. Tubular cell dropout in preimplantation deceased donor biopsies as a predictor of delayed graft function. Transplant Direct. 2021;7 doi: 10.1097/TXD.0000000000001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi Z., Salem F., Menon M.C., et al. Deep learning identified pathological abnormalities predictive of graft loss in kidney transplant biopsies. Kidney Int. 2022;101:288–298. doi: 10.1016/j.kint.2021.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savin V.J., Sharma R., Sharma M., et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med. 1996;334:878–883. doi: 10.1056/NEJM199604043341402. [DOI] [PubMed] [Google Scholar]
- 58.Wei C., El Hindi S., Li J., et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. doi: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delville M., Sigdel T.K., Wei C., et al. A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med. 2014;6:256ra136. doi: 10.1126/scitranslmed.3008538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moledina D.G., Hall I.E., Thiessen-Philbrook H., et al. Performance of serum creatinine and kidney injury biomarkers for diagnosing histologic acute tubular injury. Am J Kidney Dis. 2017;70:807–816. doi: 10.1053/j.ajkd.2017.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Menez S., Moledina D.G., Garg A.X., et al. Results from the TRIBE-AKI Study found associations between post-operative blood biomarkers and risk of chronic kidney disease after cardiac surgery. Kidney Int. 2021;99:716–724. doi: 10.1016/j.kint.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Moledina D.G., Wilson F.P., Kukova L., et al. Urine interleukin-9 and tumor necrosis factor-alpha for prognosis of human acute interstitial nephritis. Nephrol Dial Transplant. 2021;36:1851–1858. doi: 10.1093/ndt/gfaa169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Menez S., Moledina D.G., Thiessen-Philbrook H., et al. Prognostic significance of urinary biomarkers in patients hospitalized with COVID-19. Am J Kidney Dis. 2022;79:257–267.e1. doi: 10.1053/j.ajkd.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moledina D.G., Wilson F.P., Pober J.S., et al. Urine TNF-alpha and IL-9 for clinical diagnosis of acute interstitial nephritis. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen T.K., Coca S.G., Estrella M.M., et al. Longitudinal TNFR1 and TNFR2 and kidney outcomes: results from AASK and VA NEPHRON-D. J Am Soc Nephrol. 2022;33:996–1010. doi: 10.1681/ASN.2021060735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Menez S., Ju W., Menon R., et al. Urinary EGF and MCP-1 and risk of CKD after cardiac surgery. JCI Insight. 2021;6 doi: 10.1172/jci.insight.147464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ju W., Nair V., Smith S., et al. Tissue transcriptome-driven identification of epidermal growth factor as a chronic kidney disease biomarker. Sci Transl Med. 2015;7:316ra193. doi: 10.1126/scitranslmed.aac7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun Z., Zhang Z., Banu K., et al. Blood transcriptomes of SARS-CoV-2-Infected kidney transplant recipients associated with immune insufficiency proportionate to severity. J Am Soc Nephrol. 2022;33:2108–2122. doi: 10.1681/ASN.2022010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kerr K.F., Roth J., Zhu K., et al. Evaluating biomarkers for prognostic enrichment of clinical trials. Clin Trials. 2017;14:629–638. doi: 10.1177/1740774517723588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chowdhury F., Williams A., Johnson P. Validation and comparison of two multiplex technologies, Luminex and Mesoscale Discovery, for human cytokine profiling. J Immunol Methods. 2009;340:55–64. doi: 10.1016/j.jim.2008.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.