Abstract
Ginseng is a traditional herbal medicine used for prevention and treatment of various diseases as a tonic. Recent scientific cohort studies on life prolongation with ginseng consumption support this record, as those who consumed ginseng for more than 5 years had reduced mortality and cognitive decline compared to those who did not. Clinical studies have also shown that acute or long-term intake of ginseng total extract improves acute working memory performance or cognitive function in healthy individuals and those with subjective memory impairment (SMI), mild cognitive impairment (MCI), or early Alzheimer's disease (AD) dementia who are taking AD medication(s). Ginseng contains various components ranging from classical ginsenosides and polysaccharides to more recently described gintonin. However, it is unclear which ginseng component(s) might be the main candidate that contribute to memory or cognitive improvements or prevent cognitive decline in older individuals. This review describes recent clinical contributors to ginseng components in clinical tests and introduces emerging evidence that ginseng components could be novel candidates for cognitive improvement in older individuals, as ginseng components improve SMI cognition and exhibits add-on effects when co-administered with early AD dementia drugs. The mechanism behind the beneficial effects of ginseng components and how it improves cognition are presented. Additionally, this review shows how ginseng components can contribute to SMI, MCI, or early AD dementia when used as a supplementary food and/or medicine, and proposes a novel combination therapy of current AD medicines with ginseng component(s).
Keywords: Alzheimer's disease, MCI, SMI, Ginseng components
Graphical abstract
1. Introduction
Ginseng (Panax ginseng Meyer) root has been traditionally used as a tonic in China, Japan, and Korea for over 2000 years. The traditional tonic effects of ginseng extract include energizing the body, clearing brain and mood elevation, and finally brain health and longevity [1]. Currently, ginseng is used as a functional food or alternative and/or a complementary medicine for health worldwide. There are several ginseng components, which are reponsible for tonic such as ginsenosides, ginseng polysaccharides and gintonin [1]. Accumulating evidences show that ginseng components exhibit beneficial effects against cognitive impairments [2].
On the other hand, dementia is a brain disease including AD, in which normal cognitive functions are maintained during the growth period, but cognitive dysfunctions with accompanying personality changes occur in old age [2,3]. Besides cognitive decline, other common symptoms of AD include apathy (reduced interest in others), emotional changes (e.g., becoming easily angered), behavioral changes (e.g., repeating questions, leaving the house unconditionally), difficulties with language fluency, and weak will [[4], [5], [6], [7]]. But dementia does not affect the patient's consciousness [7]. AD dementia has various causes. Recent studies have identified that it is caused by a decrease in the ability to remove neurotoxic waste products [e.g., β-amyloid (Aβ) and tau protein] that accumulate in the brain with aging. If the waste product called Aβ is efficiently removed from the brain, there is no problem. Otherwise, Aβ molecules form aggregates and become water-insoluble Aβ polymers. Thus, Aβ polymers can lead to AD through brain inflammation and oxidative stresses [8, 9]. As these Aβ polymers spread to other brain areas, they cause toxicity to neurons, resulting in the death of nerve cells around β-amyloid plaques, which are large Aβ polymer aggregates [8]. In addition, β-amyloid plaques-induced neuron deaths, observed in most patients with AD dementia, are closely associated with the brain acetylcholine deficiency. β-amyloid (Aβ) and tau protein also induce glutamate-mediated excitatory neurotoxicity via NMDA receptor over-activation, which results in neurodegeneration [10].
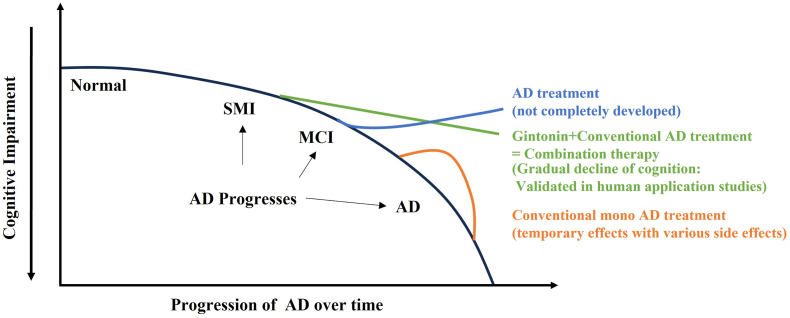
There are at least three stages through which normal individuals may progress to AD dementia due to aging or other causes such as brain microvessel infarctions [11,12]. The first stage is subjective memory impairment or subjective cognitive impairment (SMI or SCI). In this stage, SMI performance on objective memory tests falls within the normal range. However, individuals with SMI typically complain that their memory have subjectively deteriorated [13]. In mild cognitive impairment (MCI), cognitive functions, especially memory, are reduced compared to those of the same age group in the normal population. However, the ability to carry out daily activities remains intact, resulting in a condition that has not yet progressed to dementia severity, and is thus referred to as MCI. MCI is an intermediate stage between normal aging and dementia (Fig. 1). If SMI and MCI are not corrected or improved with appropriate treatment or medication, individuals may exhibit the early symptoms of AD dementia over time. These symptoms include memory impairment, difficulty in remembering names and phone numbers, language disorders, decreased visual and spatial abilities (disorientation), diminished computational ability, changes in personality and emotions, and abnormal behavior [13] (Fig. 1).
Fig. 1.
Summary of strategies for preventing cognitive decline in human beings. Since Alzheimer's disease (AD) is incurable and AD therapy has not been completely developed, combination therapy using conventional AD medicines and ginseng components, such as gintonin, may be beneficial for improving AD symptoms and preventing the dramatic decline of cognitive functions in AD progression. SMI, subjective memory impairment. MCI, mild cognitive impairment.
This review article mainly focuses clinical reports rather than in vitro and in vivo studies on ginseng-induced improvement of cognitive functions from normal people to early Alzheimer's disease (AD) patients. This review article also shows the merits of ginseng utilization for human applications, since ginseng exhibits fewer side effects than current cognitive improvement-related drugs.
2. Associations of ginseng intake and life prolongation in human
Long-term ginseng intake could prolong life span without mental and physical health risks [1]. Modern epidemiology has demonstrated that this belief regarding ginseng may be true. Yi et al. [14] conducted the first cohort study with a long-term follow-up on the association between ginseng intake and mortality in the Kangwha area of Korea, a representative ginseng cultivation area. The study followed 6282 subjects over 55 years for 18.8 years, from March 1985 to December 2003. They found that, after adjusting for all parameters using Cox proportional hazards models, the all-cause mortality for male—but not female—ginseng users was significantly lower than that of non-users [14]. However, they did not clearly explain why a sex difference in ginseng intake-induced reduction of mortality in older individuals may exist. Pradhan et al. [15] reported a cohort study on the association between ginseng intake and mortality in 40–70 year-old women in Shanghai, China. They followed approximately 56,000 female participants with an average follow-up of 14.7 years and used Cox proportional hazards models for statistical analysis, similar to the previously mentioned Korean study. They found that regular ginseng users aged >6 years showed significant reductions in all-cause mortality compared with non-user. Regular ginseng consumption, particularly over long durations, is associated with a decreased risk of all-cause death, death due to cardiovascular diseases, and death due to other diseases [15]. However, they found that the mortality risk did not decrease if ginseng was taken to treat existing illnesses [15], raising the possibility that ginseng intake might be useful for the prevention of diseases rather than disease therapy. Unfortunately, these cohort studies did not showed direct co-relationship between ginseng intake and keeping brain healthy or brain diseases like dementia in prolonging life span [14,15]. They only showed that ginseng intake reduced all-cause mortality. However, this article will show you that the keeping a healthy brain through long-term ginseng intake may contribute to the longevity of long-term ginseng users.
3. Long-term follow-up (cohort) studies show the contribution of long-term ginseng intake for cognitive improvement in older individuals
Lho et al. [16] conducted a study on the effect of long-term ginseng extract intake on cognitive improvement in healthy individuals older than 60 years in Korea. A long-term follow-up study (cohort) analysis was performed on 6422 elderly subjects at 2-year intervals from 2010 to 2016, excluding all factors that could affect the analysis (n = 6422; mean age = 70.2 ± 6.9 years, education = 8.0 ± 5.3 years, female = 56.8%). The research team divided the participants into three groups based on the duration of their use of ginseng: “Subjects who do not take ginseng,” “Subjects who take ginseng but for less than 5 years,” and “Subjects who have taken ginseng extract for more than 5 years.” In addition to group categorization, the research team used two screening tools to determine the general cognitive abilities of older individuals according to whether they were taking ginseng for the medium or long term. The first was the Consortium to Establish a Registry for Alzheimer's Disease (CERAD), a neurocognitive questionnaire used to determine the presence of MCI. The other was the Mini-Mental State Examination (MMSE), a simple test for AD dementia. used. The researchers collected and analyzed the data on cognitive functional ability [16].
They found that individuals who took ginseng for ≥5 years after the age of 60 exhibited higher CERAD and MMSE scores than older individuals who did not take ginseng for ≥5 years. They reported notable cognitive maintenance and a decrease in AD dementia proportion by approximately 8% when ginseng was taken for over 5 years compared to the group that did not take ginseng for 5 years. The dementia incidence rate also decreased substantially, from 4.6 to 0.5% (Table 1). They concluded that their study objectively proved that long-term use of ginseng could delay the onset of MCI and AD dementia due to age (Table 1, p < 0.001) [16].
Table 1.
Summary of a cohort study on cognitive decline and AD dementia prevention effects after long-term ginseng intake.
| Survey year | Number of participants | Age | Cognitive function assessment methods | Long-term ginseng intake or not | MCI or cognitive dysfunction rate (%) | Dementia incidence rate (%) | Reference |
|---|---|---|---|---|---|---|---|
| 2012–2016 | 6422 | >60 | CERAD and MMSE | Did not take ginseng | 32.6 | 4.6 | [16] |
| Have been taking ginseng for less than 5 years | 27.1 (p < 0.001) | 1.8 | |||||
| Taking ginseng for more than 5 years | 24.7 (p < 0.001) | 0.5 |
Recently, Lee et al. [17] recruited 160 healthy individuals between 65 and 90 years and divided them into 2 groups: one with long-term ginseng intake and the other without any ginseng intake. They studied the possible beneficial effects of long-term ginseng extract intake on AD-related cognitive function and found that the long-term ginseng intake group showed higher scores in delayed episodic memory than the group that did not take ginseng; however, the non-memory-related score did not differ between the 2 groups. The effect of ginseng extract intake only appeared prominent over a long period of ginseng intake (>5 years). Since the decline in delayed episodic memory is one of the indicators of the earliest cognitive changes in patients who will develop AD dementia, ginseng intake might be beneficial for preventing the decline of delayed episodic memory, possibly delaying the conversion from MCI to AD dementia in older individuals (Table 2) [17].
Table 2.
Comparison of ginseng gintonin and ginsenosides in cognition-related functions.
| Ginseng components | Gintonin | Ginseng total extract (including ginsenosides/polysaccharides | References |
|---|---|---|---|
| Major effects in in vitro and preclinical in vivo tests |
|
|
[9,[45], [46], [47],[49], [50], [51], [57], [58], [61], [63], [65]] |
| Mode of action |
|
|
[9,40,55] |
| Effects for cognitive improvements in clinical tests |
|
|
[[23], [24], [25], [26], [27], [28], [29], [30],34] |
| Standardized or non-standardized and daily dosage in clinical tests |
|
|
[[23], [24], [25], [26], [27], [28], [29], [30], [31], [32],43,44] |
| Adverse effects |
|
|
[[23], [24], [25], [26], [27], [28], [29], [30], [31], [32]] |
Although it is known that the apolipoprotein ε4 (APOE4) gene increases AD dementia risk, the reason it does so is not well understood [18]. Lee et al. [17] also compared delayed episodic memory scores between older individuals with APOE4-negative and -positive genes in their ginseng intake group. Interestingly, they found that delayed episodic memory scores were significantly higher in the APOE4-negative than in the APOE4-positive subgroup. This seems to indicate that ginseng extract intake over a longer duration is beneficial for delayed memory function in old age, but these effects might be limited to APOE4-negative individuals. Currently, how APOE4-positive genes might negatively affect ginseng intake-induced delayed memory improvement is unknown, and further studies are required to elucidate the effects of positive APOE4 allele gene in humans.
4. Effects of ginseng total extract on normal young adults or older individuals with SMI, MCI, and AD dementia
One of the physiological and pharmacological actions of ginseng is nootropic [19]. There are two types of memory or cognition studies that have explored the effect of ginseng use on humans. The first involves a single dose and acute or immediate memory improvement tests. In these studies, ginseng extract enhanced moment-related performance, including immediate word recall. A single dose of ginseng extract demonstrated prolonged effects on memory function, even after 2 weeks [[20], [21], [22]]. Another set of studies aimed to examine the effects of long-term treatment with ginseng extract, mainly on SMI, MCI, and early AD dementia [[23], [24], [25], [26], [27], [28], [29], [30]]. These studies include the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog), Mini-Mental State Examination (MMSE), and other cognition-related neuropsychological tests, as described below.
5. Acute adiministration of ginseng total extract to young healthy adults enhances acute or immediate memory functions
Following long-term cohort studies on the relationship between ginseng intake and cognitive changes, several studies have demonstrated the acute effects of ginseng on cognitive or memory improvements in young, healthy adults. Kennedy et al. demonstrated that the intake of G115, which contains standardized 4% ginsenosides, improved immediate working memory performance compared to the placebo group. Interestingly, the memory-enhancing effects of G115 were not dosage-dependent [[20], [21], [22]]. In 2022, using 61 healthy individuals (30 Cereboost group and 31 placebo) with average age of 20.5 years in UK, Bell et al. reported that American ginseng extract, known as Cereboost™, improved working memory and attention during the immediate postprandial period, since Cereboost intake redudced error scores of rapid visual information processing task (RVIP) and increased accuracy of attention network task (ANT) scores ([F(1414.98) = 20.50, p < 0.001) [31]. These effects were amplified following two weeks of treatment (acute-on-chronic) compared to acute testing alone [31]. Contrastingly, Yeo et al. [32] demonstrated that the administration of non-standardized Korean red ginseng extract to healthy young male adults (aged 19–25 years; 8 red ginseng and 7 placebo group) decreased central area latency time in the P300 event-related potential (ERP) test compared placebo group [Cz (p < 0.008), C3 (p < 0.005), C4 (p < 0.002), and C mean (p < 0.003)]. As the P300 ERP test is a type of computerized cognitive function test, the P300 latency serves as an index of the processing time required before response generation. It represents a sensitive temporal measure of the neural activity underlying the processes of attention allocation and immediate memory [11]. Additionally, P300 latency is negatively correlated with mental function in healthy individuals, with shorter latencies and amplitudes associated with superior cognitive performance [11]. Collectively, these studies indicate that a single dose of standardized or non-standardized ginseng extract can enhance memory function and reduce ERP latency time, which is the time required for response generation processes.
6. Long-term adiministration of ginseng total extract to healthy adults and older individuals with SMI or MCI improves cognitive function
6.1. Cognitive improvement in normal, SMI, and MCI individuals following long-term ginseng intake
Namgung et al. [33] examined the effects of non-standardized Korean red ginseng total extract on brain volume changes and cognitive improvement in healthy adults aged 18–65 years (8 weeks, 1 g/day). They were divided into two groups: 19 placebo and 18 total ginseng extract intake group. After 8 weeks, they measured the volume changes in the gray matter area of the left parahippocampal gyrus, which plays an important role in memory encoding and retrieval. They found a significant increase in the ginseng total extract intake group compared with the placebo group (z = 2.20, p < 0.03) [33]. Next, they assessed improvements in cognition-related domains such as executive function, attention, and memory between the two groups. Although there was no significant difference between the placebo and ginseng intake groups in each cognition-related domain such as executive functions, attention and memory, the cognitive domain composite scores of three domains exhibited significant intergroup differences. This indicates that long-term ginseng intake affects brain volume, which is closely related to cognitive function, and that an increase in parahippocampal brain volume may contribute to enhanced cognitive function [33].
The effects of ginseng compopnent, gintonin, or non-standardized Korean red ginseng total extract on human memory and cognition can be categorized into three types. The first category includes the cognition-enhancing effects of standardized gintonin-enriched fraction (GEF) targeting the SMI. In an assessor- and participant-blinded placebo-controlled study, Lee et al. [23] demonstrated that after 8 weeks with 76 participants, which was devided into 39 GEF and 37 placebo, the GEF group exhibited significant improvement in K-MMSE scores and the number of correct answers in both word reading and color reading scores in the Korean Color Word Stroop Test (K-CWST), although there was no significant intergroup difference between the placebo and GEF groups. A subsequent study by Lee et al. (2023), also using the GEF, revealed that 136 participants completed the study, which was devided into 61 GEF and 55 placebo group. After 8 weeks, intergroup differences in changes in primary or secondary outcome scores were analyzed. When comparing the GEF and placebo intake groups, they found significant improvements in the K-ADAS and K-SCWT scores (90.96 ± 42.24, p < 0.011 and 89.33 ± 40.95, p < 0.001, with GEF 300 mg/day). The GEF group did not show a significant improvement in K-MMSE scores compared to the placebo group. Interestingly, the effects of GEF on ADAS improvement were dose-dependent, as 600 mg intake led to rapid ADAS improvement after 4 weeks compared to 300 mg administration, which showed ADAS improvement after 8 weeks. These reports indicate that the number of participants involved in clinical tests could be an important factor in GEF-mediated cognitive improvement [24].
Park et al. [25] investigated the effects of non-standardized Korean red ginseng total extract (3 g/day, 6 month intake) in individuals with MCI (aged 50–75 years) in a randomized double-blind placebo-controlled clinical trial. They were divided into ginseng intake group (n = 45) or placebo group (n = 45). The K-MMSE, Korean Instrumental Activities of Daily Living (K-IADL) scale, and Seoul Neuropsychological Screening Battery (SNSB) were used to assess changes in cognitive function. They found that ginseng had no significant effects on MMSE and K-IADL scores compared to the placebo group. However, MCI subjects in the ginseng intake group compared to placebo group exhibited significant improvements in the Rey Complex Figure Test (RCFT) immediate recall scores (p < 0.0405 and p < 0.0342 in per-protocol (PP) and intention-to-treat (ITT) analyses, respectively), as well as in the RCFT 20-min delayed recall (p < 0.0396 and p < 0.0355 in PP and ITT analyses, respectively), throughout over 6 months. These results suggest that ginseng extract and ginseng component may improve cognition in SMI and MCI [25].
6.2. Older individuals with early AD dementia shows cognitive improvement after long-term ginseng intake
There are direct application studies of non-standardized ginseng total extract or standardized GEF as a supplement for patients with early AD dementia who are taking AD-related medicine(s) [23,[26], [27], [28], [29], [30]]. Patients are aged 50–80 years and were diagnosed with Alzheimer's disease and they had been treated with either donepezil, galantamine, memantine or rivastigmine. The number of patients in these studies was 10–15. The period of ginseng total extract intake typically ranges from 8 to 24 weeks, with a dosage of non-standardized Korean red ginseng total extract raging from 4.5 to 9.0 g/day [[26], [27], [28], [29], [30]]. In a study using GEF, patients took 300 mg/day gintonin for 8 weeks [23]. These studies primarily employed cognitive parameters such as the MMSE and ADAS-Cog tests. Long-term administration of non-standardized Korean red ginseng total extract or GEF, in combination with conventional AD drugs, gradually improved cognitive function compared to baseline MMSE or ADAS-cog scores measured before ginseng intake, with minimal adverse effects [[26], [27], [28], [29], [30],34]. Long-term treatment with relatively high doses of non-standardized ginseng total extract for more than 12 weeks also improved the frontal assessment battery, an indication of frontal cortical activity encompassing the right temporal, parietal, and occipital areas in elderly patients with AD [30]. Unfortunately, all of these studies were randomized open-label trials without a double-blind placebo group, with small study populations.
Interestingly, two types of studies have examined whether the long-lasting effects of ginseng on cognitive function persist after ginseng discontinuation of ginseng intake. The results of the 12-week and 24-week follow-up studies yielded different conclusions. The 24-week follow-up studies after ginseng discontinuation showed that the improvements in ADAS and MMSE scores achieved through long-term ginseng intake (4.5 and 9.0 g/day for 24 weeks) were maintained. Conversely, intake of ginseng (4.5 g/day for 12 weeks) led to a decline in cognitive function, reverting to baseline scores after 12 weeks of ginseng discontinuation. These findings imply that a ginseng intake duration of longer than 12 weeks is necessary to maintain these effects, even after stopping ginseng consumption. Although these studies were conducted without a proper placebo group, they aligned well with previous cohort studies, indicating that long-term ginseng intake spanning several years (>5 years) results in less cognitive decline in older people and lower mortality rates compared to non-ginseng users [26,28,29].
7. What are the active molecules of ginseng extract responsible for improvements of human cognitive functions?
7.1. The main ginseng components
Ginseng contains at least three active components: ginseng ginsenosides, ginseng polysaccharides, and gintonin [[35], [36], [37]]. Ginsenosides were the first compounds isolated from ginseng and belong to a class of plant glycosides called ginseng saponins. Saponins are present in most plants. Ginseng saponins (or ginsenosides) are unique saponins found exclusively in ginseng. Although ginsenosides display a wide range of effects in vitro, the specific types or individual pure ginsenosides responsible for these effects have not been clearly defined. Because ginsenosides do not have their own target receptor(s) on the plasma membrane of animal and human cells, their roles as ligands for physiological and pharmacological actions remain uncertain [35]. Ginseng polysaccharides, which consist of low and high molecular weight glucose polymers, can be classified into two groups. One group comprises neutral ginseng neutral polysaccharides, whereas the other consists of acidic polysaccharides rich in galacturonic acid and glucuronic acid. Recent animal studies have demonstrated that acidic polysaccharides are effective in boosting the immune system and providing neuroprotections [38]. The most recent discovery is gintonin, a novel glycolipoprotein. Its ative ingredients are lysophosphatidic acids (LPAs). LPAs are recognized as ligands that specifically bind to LPA receptors in the plasma membranes of animal and human cells. Thus, gintonin functions as a ginseng-derived G-protein-coupled LPA receptor ligand. This is supported by the fact that the action of gintonin can be blocked by an LPA receptor antagonist [39]. LPA and LPA1/6 receptor subtypes are abundant in the mouse brain, among six LPA receptor subtypes [40]. Thus, ginseng gintonin exerts physiological and pharmacological effects on various LPA receptor subtypes in brain [39].
7.2. Ginseng components, dosages, and compositions that have been used to clinically study human cognitive improvements
Clinical studies have employed three types of ginseng preparations to investigate human cognitive improvement. The first type consists of ginseng extracts that contain standardized 4% ginsenosides from Panax ginseng and standardized 10% ginsenosides from American ginseng, Cereboost™ [[20], [21], [22],31,41,42]. The second type encompasses straightforward non-standardized ginseng extracts derived from Korean white ginseng or Korean red ginseng [[26], [27], [28], [29], [30]]. The third type involves the utilization of standardized gintonin (GEF), which contains approximately 0.2% LPAs as the active ingredient [23,24]. For Korean and American ginseng (Panax quinquifolius L.) preparations, dosages range from 100 to 600 mg/day, primarily targeting acute effects on cognitive function [[20], [21], [22],31,41,42]. The dosages of non-standardized ginseng extracts obtained from Korean white and red ginseng range from 4.5 to 9.0 g/day and were administered over a period of 12–24 weeks [[26], [27], [28], [29], [30]]. In the case of ginseng GEF, dosages vary from 300 to 600 mg/day and are administered for a duration of 8 weeks [23,24] (Table 2).
7.3. The working principles of ginseng components for improvements of human cognitive functions
In humans, both standardized and non-standardized ginseng total extracts have been shown to enhance immediate cognitive performance after acute single dosages, as well as to improve cognitive functions in the elderly and individuals with early AD dementia through long-term intake [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Although these studies have established the effects of standardized and non-standardized ginseng total extracts on neuropsychological parameters linked to human cognition, it remains unclear whether long-term ginseng intake can reduce the accumulation of senile amyloid plaques in the AD brain, similarly to recent AD medicines [43,44]. Our understanding of the molecular mechanisms underlying cognitive improvement by ginseng in humans is largely drawn from in vitro and in vivo transgenic AD animal studies. In vitro studies have shown that ginseng components such as gintonin and ginsenosides exhibit antioxidative effects. Consequently, these components inhibit the production of reactive oxygen species (ROS) and free radicals within neuronal mitochondria, thereby promoting neuronal health. Excessive ROS and free radicals in the brain can disrupt calcium homeostasis, as well as nuclear and mitochondrial DNA integrity, leading to alterations in neurotransmission and synaptic activity [9].
Ginseng components, such as ginsenosides and gintonin, have also been shown to attenuate neuroinflammations. Thus, they suppress the formation of inflammation-related products like Cox-2, IL-1β, IL-8 and TNF-α as well as overexpressions of astrocyte GFAP and microglia Iba-1. Additionally, these components inhibit Aβ formation and induced neuronal cell death [9]. In in vivo preclinical transgenic AD animal model studies, oral administration of ginseng components inhibited amyloid plaque accumulation in the hippocampus and cortex. It also increases the brain acetylcholine concentration by elevating choline acetyltransferase activity, which is responsible for acetylcholine synthesis, while reducing acetylcholinesterase activity, which is responsible for acetylcholine breakdown [45]. Furthermore, oral administration of ginseng components such as gintonin enhances the expression of brain-derived neurotrophic factor (BDNF) in the hippocampus [[46], [47], [48]]. Ultimately, the diverse physiological and pharmacological benefits associated with ginseng components contribute to significant improvements in cognition-related behavioral tests, such as the Morris water maze, passive avoidance, and Y-maze tests [49]. These preclinical assessments can serve as substitutes for human cognitive function tests conducted before clinical testing.
7.4. The roles of each ginseng component in improvement of human cognitive functions
Ginsenosides and ginseng polysaccharides are not endogenously present in animals or humans. Its primary role in maintaining brain health involves reducing oxidative stress by increasing the activities of glutathione peroxidase and superoxide dismutase, while decreasing lactate dehydrogenase and malondialdehyde levels [[50], [51], [52]]. Ginsenosides play a pivotal role in inhibiting neuroinflammation stemming from Aβ production and brain aging. They also activate α-secretase while inhibiting β-secretase and γ-secretase, resulting in the reduced production of Aβ and increased production of sAPPα, which are beneficial neurotrophic factors in the nervous system [9,52]. Thus, ginseng ginsenosides and polysaccharides exert beneficial effects through non-receptor-mediated mechanisms, offering neuroprotection against induced neurotoxicity caused by aging (Table 2).
Conversely, LPAs are endogenously present in animals and humans and their physiological and/or pharmacological effects are mediated by LPA receptors [53]. The primary functions of LPA/LPA receptors include cell proliferation, migration, and survival, highlighting their pluripotent nature [54]. Ginseng gintonin, which contains LPAs as its functional components, activates LPA receptors [55]. The activation of LPA receptors by gintonin transmits extracellular information to the cytosol through the initiation of primary signaling pathways from the cell membrane to the nucleus in neuronal cells. This sequence unfolds as follows: gintonin→LPA receptor subtypes→Gαq/11→phospholipase C→IP3→IP3 receptor binding on endoplasmic reticulum (ER)→transient mobilization of cytosolic Ca2+ from ER with a clear mode of action [55]. Free cytosolic Ca2+ is a secondary mediator of various cellular events. For instance, gintonin stimulates glutamate release via Ca2+-mediated exocytosis from hippocampal cells; induces synaptic facilitation and long-term potentials, which are essential for learning and memory via LPA receptors; promotes enhancement of hippocampal-dependent memory; and triggers hippocampal neurogenesis [46,56,57]. Moreover, gintonin stimulates in vitro and in vivo the release of acetylcholine and BDNF in the brain, and induces the synthesis of acetylcholine and BDNF in vivo via LPA receptors [[45], [46], [47], [48]].
7.5. Additive or synergistic effects of combining different ginseng components on cognitive improvements
When various ginseng components are combined, they may complement one another, leading to additive or synergistic effects. Although ginsenosides and ginseng polysaccharides lack their own inherent membrane receptors, they exert beneficial effects through non-receptor-mediated mechanisms, such as non-specific antioxidative and anti-neuroinflammatory effects within the nervous system [9]. Contrastingly, ginseng gintonin-containing LPAs have high affinity for membrane LPA receptors and exert specific effects through these receptors. As previously mentioned, its primary action via LPA receptors is to mobilize cytosolic Ca2+ transiently [2,36]. This mobilized Ca2+ triggers a cascade of processes that enhance cognitive function, including acetylcholine and glutamate release/synthesis, ion channel regulation, hippocampal BDNF release, and neurogenesis [2,36]. Thus, the combination of non-receptor-mediated anti-oxidative stress and anti-neuroinflammatory effects of ginsenosides and ginseng polysaccharides, along with the diverse gintonin-LPA receptor-mediated benefits within the nervous system, can work in tandem. The effects of gintonin, which are achieved through LPA receptor regulation, distinguish it from those of ginsenosides and polysaccharides. Hence, their effects are likely additive or synergistic in enhancing human cognitive function (Fig. 1 and Table 2).
7.6. Ginseng gintonin as a novel candidate for improvement of cognitive functions in older individuals with SMI or early AD dementia
Ginseng gintonin is a promising ginseng-derived nootropic agent for older individuals due to its numerous merits [23,24]. Nevertheless, before considering its industrial applications, two challenges must be addressed. First, gintonin production requires several steps to obtain a gintonin-enriched fraction [58]. Second, the prices of white and red ginseng are considerably higher than those of other herbal medicines. Consequently, fractionation of the gintonin-enriched fraction could significantly increase manufacturing costs, adding to the complexity. This is a key reason why most commercial ginseng products are concentrated hot water extracts reduced to 65% to lower water content. A recent study offered a solution using Korean red ginseng marc (KRGM). Large amounts of KRGM are produced annually after Korean red ginseng water extraction for product manufacturing. Thus, KRGM is considered a type of leftover. Approximately 20% of KRGM is repurposed as animal feed or crop compost, with the remaining 80% discarded as waste. Lee et al. [59] found that a simple ethanol extraction of KRGM could produce KRGM-derived gintonin containing an LPA C18:2 content comparable to the gintonin-enriched fraction from white ginseng [59]. The KRGM gintonin has multiple advantages. First, KRGM is relatively economical, with thousands of tons produced annually, ensuring a consistent supply. KRGM gintonin production can be efficiently scaled up through one-step ethanol extraction at low cost and high efficiency [59]. Additionally, the mode of action of KRGM gintonin is the same as that of gintonin-enriched fraction, as both harness their effects via LPA receptors [59]. Korea's Ministry of Food and Drug Safety has approved its use as a food additive [59]. If KRGM gintonin could be utilized industrially after demonstrating the improvement of cognitive function in older individuals, it would exemplify the transformation and upcycling of KRGM from a simple leftover or waste to a functional food or herbal medicinal product (HMP).
7.7. Comparisons of current AD medicines with ginseng components includng gintonin in improvements of human cgonitive improvements: A possible utilization of ginseng components like gintonin as an adjuvant of AD dementia therapy to obtain maximal efficacy of AD medicines
Currently, three categories of medicines have been approved by the FDA for conventional treatment of AD dementia. One is an acetylcholine degradation inhibitor or NMDA receptor antagonist [10,60], while another is a recently approved antibody treatment designed to eliminate Aβ peptides or inhibits Aβ polymerization, otherwise resulting in brain amyloid plaque accumulations [43,44]. Acetylcholine degradation inhibitors or NMDA receptor antagonists only offer symptomatic relief for AD dementia but do not represent a comprehensive cure. Antibody-based therapeutics that inhibit Aβ aggregates or amyloid plaque accumulation in the brain face the challenge of traversing the BBB because of their high molecular weight [43,44]. Approximately 1% of antibody-based drugs permeate the brain. However, these treatments have considerable adverse effects such as brain edema and hemorrhage, making them both medically problematic and cost-intensive for prolonged use (Table 3).
Table 3.
Comparison of AD medicine and ginseng components including gintonin.
| AD medicines | Ginseng components (gintonin, standardized or non-standardized ginseng extracts) | References | |
|---|---|---|---|
| The main mode of actions in brain |
|
|
[[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21],43,44,47,50,51,57,58,60] |
| Clinical efficacy |
|
|
[[10], [11], [12], [13],[19], [20], [21],[27], [28], [29], [30], [31], [32],36,37] |
| Duration of treatment (week) |
|
|
|
| Current status |
|
|
|
| Adverse effects (%) |
|
|
[[10], [11], [12],19,[23], [24], [25], [26], [27],[29], [30], [31], [32]] |
| Future directions |
|
|
[10,59] |
| Purchase costs |
|
|
Contrastingly, ginseng gintonin, isolated from ginseng, suppressed the accumulation of brain amyloid plaques in an AD mouse model and had no side effects, even when taken for a long time [45,49,61]. Furthermore, if we consider the use of KRGM gintonin, the treatment would be both cost-effective and economical [59]. Additionally, ginseng gintonin opens BBB transiently via LPA1/3 receptors and increases brain permeability in vitro and in vivo studies [[62], [63], [64]], with gintonin demonstrating the capacity to maintain BBB integrity disrupted by Aβ in AD animal mouse models [65]. Ginseng gintonin transiently opens the BBB and enhances the permeability of donepezil, an AD medicine, by 30% in rats, along with 70 kDa high molecular weight dextrin [62]. Notably, in a clinical study employing dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI), Lee et al. [66] found that gintonin-mediated cognitive enhancement is closely linked to a gintonin-mediated increase in BBB permeability. Although ginseng extract or gintonin is currently not approved as HMP, it is worthwhile to test whether the combination therapy of current AD medicines with ginseng extract or gintonin shows add-on effects compared to AD medicine alone. If clinical tests demonstrate enhanced efficacy when gintonin is used as an add-on therapy with AD drugs, it will pave a new avenue for targeted combination therapies for AD with ginseng components, such as gintonin (Fig. 1).
8. Safety of long-term ginseng component intake
Numerous clinical studies related to cognitive improvements have shown that acute and long-term ginseng extract or ginseng components, such as G115, Cereboost™, or gintonin intake, do not cause adverse events [22,31,44,60]. The frequency of adverse effects (nasopharyngitis, upper respiratory tract infection, headache, diarrhea, dizziness, and pruritus) was comparable with that in the placebo group. In cases where adverse effects were observed, the symptoms tended to be mild rather than severe [24,[26], [27], [28], [29], [30],43]. Therefore, ginseng extract and ginseng components are considered safe for individuals of all ages, ranging from young to older adults (Table 2).
9. Perspective and conclusion
Recent cohort and clinical studies have shown that ginseng extract intake at a single dose improves acute cognitive performance, and that long-term ginseng intake improves cognitive function in older individuals with SMI and MCI. Additionally, co-administration of ginseng components, such as gintonin, with AD drugs in patients with early AD dementia exhibited additive effects on cognitive improvement. However, although the clinical efficacy of non-standardized ginseng total extract or standardized ginseng components, such as gintonin, for cognitive or memory improvements has been accumulated in clinical trials, standardized or non-standardized ginseng total extract or ginseng components are not fully established for clinical use as HMP. This is mainly because most previous clinical trials using ginseng were pilot studies with a small number of study participants without the mass assessment of the efficacy, safety, and tolerability.
In conclusion, in order for ginseng to be recognized as HMP rather than a current functional food for cognitive improvement in the future, it might be necessary to provide additional biological markers or evidence that link neuropsychological improvements (e.g., ADAS, MMSE) to the alleviation of AD brain pathology. This could potentially be assessed through advanced imaging techniques such as amyloid-positron emission tomography (PET) or tau-PET, within the context of a comprehensive and large-scale investigations with ginseng component standardization.
Acknowledgements
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (2023R1A2C1003481) awarded to S. Y. Nah.
References
- 1.What is the dongui bogam? Korean Culture and Information Service (KOCIS); 2014-04-29. [Google Scholar]
- 2.Choi S.H., Lee R., Nam S.M., Kim D.G., Cho I.H., Kim H.C., Cho Y., Rhim H., Nah S.Y. Ginseng gintonin, aging societies, and geriatric brain diseases. Integr Med Res. 2021;10 doi: 10.1016/j.imr.2020.100450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J., Kwon S., Jin C., Cho S.Y., Park S.U., Jung W.S., Moon S.K., Park J.M., Ko C.N., Cho K.H. Traditional east asian herbal medicine treatment for Alzheimer's disease: a systematic review and meta-analysis. Pharmaceuticals. 2022;15:174. doi: 10.3390/ph15020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jung S.H., Kim H.R., Chun M.Y., Jang H., Cho M., Kim B., Kim S., Jeong J.H., Yoon S.J., Park K.W., et al. Transferability of alzheimer disease polygenic risk score across populations and its association with alzheimer disease-related phenotypes. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.47162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek M.S., Kim H.K., Han K., Kwon H.S., Na H.K., Lyoo C.H., Cho H. Annual trends in the incidence and prevalence of Alzheimer's disease in South Korea: a nationwide cohort study. Front Neurol. 2022;13 doi: 10.3389/fneur.2022.883549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee D.W., Seong S.J. Korean national dementia plans: from 1st to 3rd. J Korean Med Assoc. 2018;61:298–303. [Google Scholar]
- 7.Galvin J.E., Aisen P., Langbaum J.B., Rodriguez E., Sabbagh M., Stefanacci R., Stern R.A., Vassey E.A., de Wilde A., West N., et al. Early stages of alzheimer's disease: evolving the care team for optimal patient management. Front Neurol. 2021;11 doi: 10.3389/fneur.2020.592302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hampel H., Hardy J., Blennow K., Chen C., Perry G., Kim S.H., Villemagne V.L., Aisen P., Vendruscolo M., Iwatsubo T., et al. The amyloid-β pathway in Alzheimer's disease. Mol Psychiatr. 2021;26:5481–5503. doi: 10.1038/s41380-021-01249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li J., Huang Q., Chen J., Qi H., Liu J., Chen Z., Zhao D., Wang Z., Li X. Neuroprotective potentials of panax ginseng against Alzheimer's Disease: a review of preclinical and clinal evidences. Front Pharmacol. 2021;12:1–15. doi: 10.3389/fphar.2021.688490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siddiqui A.J., Badraoui R., Jahan S., Alshahrani M.M., Siddiqui M.A., Khan A., Adnan M. Targeting NMDA receptor in Alzheimer's disease: identifying novel inhibitors using computational approaches. Front Pharmacol. 2023;21 doi: 10.3389/fphar.2023.1208968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns A. Diagnosis and management of Alzheimer's disease. Dialogues Clin Neurosci. 2000;2:129–138. doi: 10.31887/DCNS.2000.2.2/aburns. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeTure M.A., Dickson D.W. The neuropathological diagnosis of Alzheimer's disease. Mol Neurodegener. 2019;14:32. doi: 10.1186/s13024-019-0333-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack C.R., Jr., Knopman D.S., Weigand S.D., Wiste H.J., Vemuri P., Lowe V., Kantarci K., Gunter J.L., Senjem M.L., Ivnik R.J., et al. An operational approach to national institute on aging-Alzheimer's association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71:765–775. doi: 10.1002/ana.22628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi S.W., Sull J.W., Hong J.S., Linton J.A., Ohrr H. Association between ginseng intake and mortality: Kangwha cohort study. J Alternative Compl Med. 2009;15:921–928. doi: 10.1089/acm.2008.0296. [DOI] [PubMed] [Google Scholar]
- 15.Pradhan P., Wen W., Cai H., Gao Y.T., Yang G., Shu X.O., Zheng W. Association of ginseng consumption with all-cause and cause-specific mortality: Shanghai women's health study. J Epidemiol. 2022;32:469–475. doi: 10.2188/jea.JE20210393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lho S.K., Kim T.H., Kwak K.P., Kim K., Kim B.J., Kim S.G., Kim J.L., Kim T.H., Moon S.W., Park J.Y., et al. Effects of lifetime cumulative ginseng intake on cognitive function in late life. Alzheimer's Res Ther. 2018;10:50. doi: 10.1186/s13195-018-0380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee B.C., Choe Y.M., Suh G.H., Choi I.G., Kim H.S., Hwang J., Yi D., Jhoo J.H., Kim J.W. Ginseng intake and Alzheimer disease-specific cognition in older adults according to apolipoprotein ε4 allele status. Front Aging Neurosci. 2023;15 doi: 10.3389/fnagi.2023.1152626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamazaki Y., Zhao N., Caulfield T.R., Liu C.C., Bu G. Apolipoprotein E and Alzheimer disease: pathobiology and targeting strategies. Nat Rev Neurol. 2019;15:501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malík M., Tlustoš P. Nootropic herbs, shrubs, and trees as potential cognitive enhancers. Plants. 2023;12:1364. doi: 10.3390/plants12061364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reay J.L., Scholey A.B., Kennedy D.O. Panax ginseng (G115) improves aspects of working memory performance and subjective ratings of calmness in healthy young adults. Hum Psychopharmacol. 2010;25:462–471. doi: 10.1002/hup.1138. [DOI] [PubMed] [Google Scholar]
- 21.Reay J.L., Kennedy D.O., Scholey A.B. Single doses of Panax ginseng (G115) reduce blood glucose levels and improve cognitive performance during sustained mental activity. J Psychopharmacol. 2005;19:357–365. doi: 10.1177/0269881105053286. [DOI] [PubMed] [Google Scholar]
- 22.Scholey A.B., Kennedy D.O. Acute, dose-dependent cognitive effects of Ginkgo biloba, Panax ginseng and their combination in healthy young volunteers: differential interactions with cognitive demand. Hum Psychopharmacol. 2002;17:35–44. doi: 10.1002/hup.352. [DOI] [PubMed] [Google Scholar]
- 23.Lee W.J., Shin Y.W., Chang H., Shin H.R., Kim W.W., Jung S.W., Kim M., Nah S.Y. Safety and efficacy of dietary supplement (gintonin-enriched fraction from ginseng) in subjective memory impairment: a randomized placebo-controlled trial. Integr Med Res. 2022;11 doi: 10.1016/j.imr.2021.100773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee R., Lee H., Kim W.W., Kim M.H., Nah S.Y. Cognitive function improvement effects of gintonin enriched fraction in subjective memory impairment: an assessor- and participant-blinded placebo controlled study. J Ginseng Res. 2023;47:735–742. doi: 10.1016/j.jgr.2023.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park K.C., Jin H., Zheng R., Kim S., Lee S.E., Kim B.H., Yim S.V. Cognition enhancing effect of Panax ginseng in Korean volunteers with mild cognitive impairment: a randomized, double-blind, placebo-controlled clinical trial. Transl Clin Pharmacol. 2019;27:92–97. doi: 10.12793/tcp.2019.27.3.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee S.T., Chu K., Sim J.Y., Heo J.H., Kim M. Panax ginseng enhances cognitive performance in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22:222–226. doi: 10.1097/WAD.0b013e31816c92e6. [DOI] [PubMed] [Google Scholar]
- 27.Heo J.H., Lee S.T., Chu K., Oh M.J., Park H.J., Shim J.Y., Kim M. An open-label trial of Korean red ginseng as an adjuvant treatment for cognitive impairment in patients with Alzheimer's disease. Eur J Neurol. 2008;15:865–868. doi: 10.1111/j.1468-1331.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- 28.Heo J.H., Lee S.T., Oh M.J., Park H.J., Shim J.Y., Chu K., Kim M. Improvement of cognitive deficit in Alzheimer's disease patients by long term treatment with Korean red ginseng. J Ginseng Res. 2011;35:457–461. doi: 10.5142/jgr.2011.35.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heo J.H., Lee S.T., Chu K., Oh M.J., Park H.J., Shim J.Y., Kim M. Heat-processed ginseng enhances the cognitive function in patients with moderately severe Alzheimer's disease. Nutr Neurosci. 2012;15:278–282. doi: 10.1179/1476830512Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 30.Heo J.H., Park M.H., Lee J.H. Effect of Korean red ginseng on cognitive function and quantitative eeg in patients with Alzheimer's disease: a preliminary study. J Alternative Compl Med. 2016;22:280–285. doi: 10.1089/acm.2015.0265. [DOI] [PubMed] [Google Scholar]
- 31.Bell L., Whyte A., Duysburgh C., Marzorati M., Van den Abbeele P., Le Cozannet R., Fança-Berthon P., Fromentin E., Williams C. A randomized, placebo-controlled trial investigating the acute and chronic benefits of American Ginseng (Cereboost®) on mood and cognition in healthy young adults, including in vitro investigation of gut microbiota changes as a possible mechanism of action. Eur J Nutr. 2022;61:413–428. doi: 10.1007/s00394-021-02654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeo H.B., Yoon H.K., Lee H.J., Kang S.G., Jung K.Y., Kim L. Effects of Korean red ginseng on cognitive and motor function: a double-blind, randomized, placebo-controlled trial. J Ginseng Res. 2012;36:190–197. doi: 10.5142/jgr.2012.36.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namgung E., Kim J., Jeong H., Hong G., Kim M., Kim R.Y., Kim S., Lyoo I.K. Effects of Korean red ginseng on human gray matter volume and cognitive function: a voxel-based morphometry study. Hum Psychopharmacol. 2021;36 doi: 10.1002/hup.2767. [DOI] [PubMed] [Google Scholar]
- 34.Moon J., Choi S.H., Shim J.Y., Park H.J., Oh M.J., Kim M., Nah S.Y. Gintonin administration is safe and potentially beneficial in cognitively impaired elderly. Alzheimer Dis Assoc Disord. 2018;32:85–87. doi: 10.1097/WAD.0000000000000213. [DOI] [PubMed] [Google Scholar]
- 35.Nah S.Y. Ginseng ginsenoside pharmacology in the nervous system: involvement in the regulation of ion channels and receptors. Front Physiol. 2014;19:98. doi: 10.3389/fphys.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Choi S.H., Jung S.W., Lee B.H., Kim H.J., Hwang S.H., Kim H.K., Nah S.Y. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Zhang Z., Liu J., Guo M., Li H. Panax Ginseng in the treatment of Alzheimer's disease and vascular dementia. J Ginseng Res. 2023;47:506–514. doi: 10.1016/j.jgr.2023.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao R., Lu K., Zong G., Xia Y., Han H., Zhao Y., Wei Z., Lu Y. Ginseng polysaccharides: potential antitumor agents. J Ginseng Res. 2023;47:9–22. doi: 10.1016/j.jgr.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Choi S.H., Lee R.M., Cho H.S., Hwang S.H., Hwang H.I., Rhim H., Kim H.C., Kim D.G., Cho I.H., Nah S.Y. Visualization of the binding between gintonin, a Panax ginseng-derived LPA receptor ligand, and the LPA receptor subtypes and transactivation of the EGF receptor. J Ginseng Res. 2022;46:348–356. doi: 10.1016/j.jgr.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee R., Lee B.H., Choi S.H., Cho Y.J., Cho H.S., Kim H.C., Rhim H., Cho I.H., Rhee M.H., Nah S.Y. Effects of Gintonin-enriched fraction on the gene expression of six lysophosphatidic receptor subtypes. J Ginseng Res. 2021;45:583–590. doi: 10.1016/j.jgr.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White D.J., Camfield D.A., Ossoukhova A., Savage K., Le Cozannet R., Fança-Berthon P., Scholey A. Effects of Panax quinquefolius (American ginseng) on the steady state visually evoked potential during cognitive performance. Hum Psychopharmacol. 2020;35:1–6. doi: 10.1002/hup.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scholey A., Ossoukhova A., Owen L., Ibarra A., Pipingas A., He K., Roller M., Stough C. Effects of American ginseng (Panax quinquefolius) on neurocognitive function: an acute, randomized, double-blind, placebo-controlled, crossover study. Psychopharmacology (Berl) 2010;212:345–356. doi: 10.1007/s00213-010-1964-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sims J.R., Zimmer J.A., Evans C.D., Evans C.D., Lu M., Ardayfio P., Sparks J., Wessels A.M., Shcherbinin S., Wang H., Nery E.S., Sokovronsky M.D. Donanemab in early symptomatic alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330:512–527. doi: 10.1001/jama.2023.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiss A.B., Muhieddine D., Jacob B., Mesbah M., Pinkhasov A., Gomolin I.H., Stecker M.M., Wisniewski T., De Leon J. Alzheimer's disease treatment: the search for a breakthrough. Medicina (kaunas) 2023;59:1084. doi: 10.3390/medicina59061084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim H.J., Shin E.J., Lee B.H., Choi S.H., Jung S.W., Cho I.H., Hwang S.H., Kim J.Y., Han J.S., Chung C., Jang C.G., Rhim H., Kim H.C., Nah S.Y. Oral administration of gintonin attenuates cholinergic impairments by scopolamine, amyloid-β protein, and mouse model of Alzheimer's disease. Mol Cell. 2015;38:796–805. doi: 10.14348/molcells.2015.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim S., Kim M.S., Park K., Kim H.J., Jung S.W., Nah S.Y., Han J.S., Chung C. Hippocampus-dependent cognitive enhancement induced by systemic gintonin administration. J Ginseng Res. 2016;40:55–61. doi: 10.1016/j.jgr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cho Y.J., Choi S.H., Lee R.M., Cho H.S., Rhim H., Kim H.C., Kim B.J., Kim J.H., Nah S.Y. Protective effects of gintonin on reactive oxygen species-induced ht22 cell damages: involvement of LPA1 receptor-BDNF-Akt signaling pathway. Molecules. 2021;26:4138. doi: 10.3390/molecules26144138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim H.K., Kim K., Son Y.K., Nah S.Y., Ahn S.M., Song M. Gintonin stimulates dendritic growth in striatal neurons by activating Akt and CREB. Front Mol Neurosci. 2022;15 doi: 10.3389/fnmol.2022.1014497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang S.H., Shin E.J., Shin T.J., Lee B.H., Choi S.H., Kang J., Kim H.J., Kwon S.H., Jang C.G., Lee J.H., Kim H.C., Nah S.Y. Gintonin, a ginseng-derived lysophosphatidic acid receptor ligand, attenuates Alzheimer's disease-related neuropathies: involvement of non-amyloidogenic processing. J Alzheimers Dis. 2012;31:207–223. doi: 10.3233/JAD-2012-120439. [DOI] [PubMed] [Google Scholar]
- 50.Shin S.J., Nam Y., Park Y.H., Kim M.J., Lee E., Jeon S.G., Bae B.S., Seo J., Shim S.L., Kim J.S., Han C.K., Kim S., Lee Y.Y., Moon M. Therapeutic effects of non-saponin fraction with rich polysaccharide from Korean red ginseng on aging and Alzheimer's disease. Free Radic Biol Med. 2021;164:233–248. doi: 10.1016/j.freeradbiomed.2020.12.454. [DOI] [PubMed] [Google Scholar]
- 51.Wang N., Wang X., He M., Zheng W., Qi D., Zhang Y., Han C.C. Ginseng polysaccharides: a potential neuroprotective agent. J Ginseng Res. 2021;45:211–217. doi: 10.1016/j.jgr.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Z., Chen H., Zhou X., Yang W., Lin Y. Pharmacological effects of natural medicine ginsenosides against Alzheimer's disease. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.952332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanagida K., Shimizu T. Lysophosphatidic acid, a simple phospholipid with myriad functions. Pharmacol Ther. 2023;246 doi: 10.1016/j.pharmthera.2023.108421. [DOI] [PubMed] [Google Scholar]
- 54.Yung Y.C., Stoddard N.C., Chun J. LPA receptor signaling: pharmacology, physiology, and pathophysiology. J Lipid Res. 2014;55:1192–1214. doi: 10.1194/jlr.R046458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Choi S.H., Jung S.W., Lee B.H., Kim H.J., Hwang S.H., Kim H.K., Nah S.Y. Ginseng pharmacology: a new paradigm based on gintonin-lysophosphatidic acid receptor interactions. Front Pharmacol. 2015;6:245. doi: 10.3389/fphar.2015.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin T.J., Kim H.J., Kwon B.J., Choi S.H., Kim H.B., Hwang S.H., Lee B.H., Lee S.M., Zukin R.S., Park J.H., et al. Gintonin, a ginseng-derived novel ingredient, evokes long-term potentiation through N-methyl-D-aspartic acid receptor activation: involvement of LPA receptors. Mol Cell. 2012;34:563–572. doi: 10.1007/s10059-012-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park H., Kim S., Rhee J., Kim H.J., Han J.S., Nah S.Y., Chung C. Synaptic enhancement induced by gintonin via lysophosphatidic acid receptor activation in central synapses. J Neurophysiol. 2015;113:1493–1500. doi: 10.1152/jn.00667.2014. [DOI] [PubMed] [Google Scholar]
- 58.Cho H.J., Choi S.H., Kim H.J., Lee B.H., Rhim H., Kim H.C., Hwang S.H., Nah S.Y. Bioactive lipids in gintonin-enriched fraction from ginseng. J Ginseng Res. 2019;43:209–217. doi: 10.1016/j.jgr.2017.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee R., Kim J.H., Hwang H., Rhim H., Hwang S.H., Cho I.H., Kim D.G., Kim H.C., Nah S.Y. Preparation of red ginseng marc-derived gintonin and its application as a skin nutrient. Nutrients. 2023;15:2574. doi: 10.3390/nu15112574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li R., Xu C., Zhong P., Wang K., Luo Y., Xiao L., Dai X., Han J., Zhang X. Efficacy of acupuncture and pharmacological therapies for vascular cognitive impairment with no dementia: a network meta-analysis. Front Aging Neurosci. 2023;15 doi: 10.3389/fnagi.2023.1181160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ikram M., Jo M.G., Park T.J., Kim M.W., Khan I., Jo M.H., Kim M.O. Oral administration of gintonin protects the brains of mice against aβ-induced Alzheimer disease pathology: antioxidant and anti-inflammatory effects. Oxid Med Cell Longev. 2021;2021 doi: 10.1155/2021/6635552. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 62.Kim D.G., Jang M., Choi S.H., Kim H.J., Jhun H., Kim H.C., Rhim H., Cho I.H., Nah S.Y. Gintonin, a ginseng-derived exogenous lysophosphatidic acid receptor ligand, enhances blood-brain barrier permeability and brain delivery. Int J Biol Macromol. 2018;14:1325–1337. doi: 10.1016/j.ijbiomac.2018.03.158. [DOI] [PubMed] [Google Scholar]
- 63.Choi S.H., Lee N.E., Cho H.J., Lee R.M., Rhim H., Kim H.C., Han M., Lee E.H., Park J., Nah S.Y. Gintonin facilitates brain delivery of donepezil, a therapeutic drug for Alzheimer disease, through lysophosphatidic acid 1/3 and vascular endothelial growth factor receptors. J Ginseng Res. 2021;45:264–272. doi: 10.1016/j.jgr.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo S., Nah S.Y., Lee K., Choi N., Kim H.N. Triculture model of in vitro BBB and its application to study BBB-associated chemosensitivity and drug delivery in glioblastoma. Adv Funct Mater. 2021;32 [Google Scholar]
- 65.Jang M., Choi S.H., Choi J.H., Oh J., Lee R.M., Lee N.E., Cho Y.J., Rhim H., Kim H.C., Cho I.H., et al. Ginseng gintonin attenuates the disruptions of brain microvascular permeability and microvascular endothelium junctional proteins in an APPswe/PSEN-1 double-transgenic mouse model of Αlzheimer's disease. Exp Ther Med. 2021;21:310. doi: 10.3892/etm.2021.9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee W.J., Shin Y.W., Chang H., Shin H.R., Kim W.W., Jung S.W., Choi S.H., Kim M., Nah S.Y. Cognitive improvement effect of gintonin might be associated with blood-brain barrier permeability enhancement: dynamic contrast-enhanced MRI pilot study. Transl Clin Pharmacol. 2021;29:21–32. doi: 10.12793/tcp.2021.29.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]