Abstract
Lenvatinib, a multitarget tyrosine kinase inhibitor for c-Kit and other kinases, has exhibited promising efficacy in treating advanced or metastatic thymic carcinoma (TC). Here, we present the case of a patient with metastatic TC harboring a KIT exon 11 deletion and amplification. The patient exhibited a remarkable response to lenvatinib but experienced rapid disease progression after discontinuation of lenvatinib, referred to as a “disease flare.” This case report indicates that KIT mutations and amplification can predict lenvatinib response in patients with TC. However, in such cases, there might be a risk of disease flares after lenvatinib discontinuation.
Keywords: Case report, Thymic carcinoma, Lenvatinib, Disease flare
Introduction
Thymic carcinoma (TC), a rare cancer with a poor prognosis, is derived from thymic epithelial cells and is treated with systemic chemotherapy for metastatic or recurrent diseases.1 In a phase 2 trial (REMORA),2 lenvatinib, a multitargeted tyrosine kinase inhibitor (TKI) for c-Kit, vascular endothelial growth factor receptor, FGF receptor, and other kinases exhibited an objective response rate of 38% and a median progression-free survival of 9.3 months in patients with previously treated advanced or metastatic TC, which led to the approval of lenvatinib in Japan. However, the biomarkers that predict the response to lenvatinib in patients with TC are not well understood.
A "disease flare" phenomenon, referring to rapid disease progression, has been reported after TKI administration is discontinued in tumors with oncogenic driver mutations such as EGFR mutation.3,4 However, there have been no reports of disease flares in patients treated with lenvatinib for metastatic TC. Here, we report a case of a rapid and remarkable response to lenvatinib as a fourth-line treatment for metastatic TC, followed by a disease flare after discontinuation.
Case Presentation
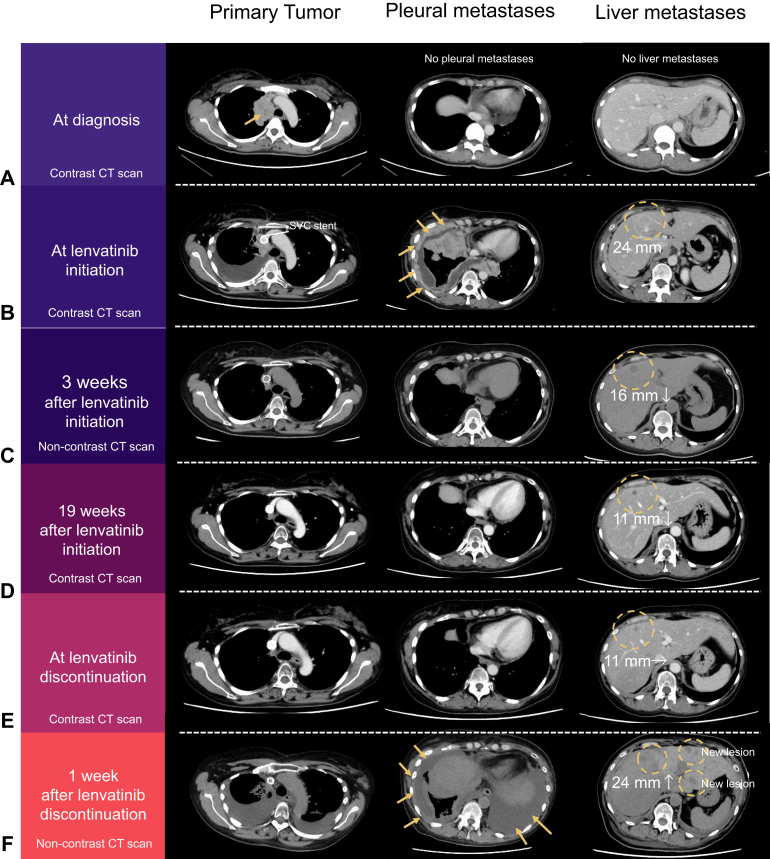
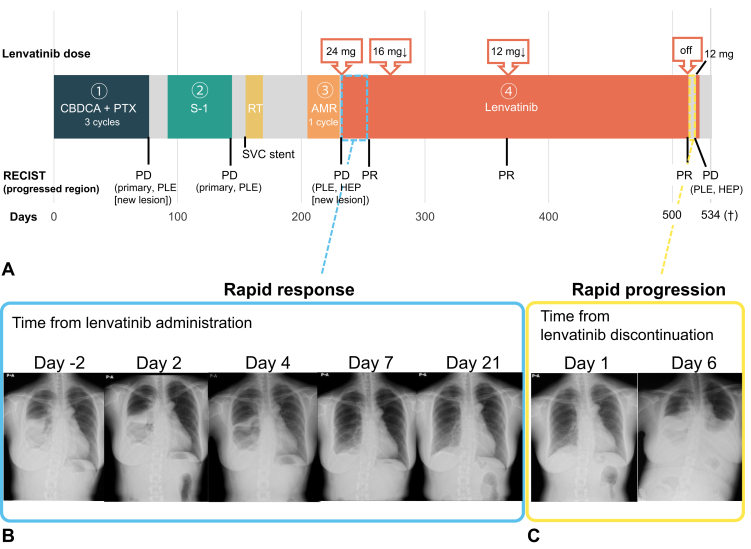
A 48-year-old woman with no history of smoking was diagnosed with stage IVB (Masaoka-Koga classification) squamous cell carcinoma (TC) with multiple bone metastases in 2021 (Fig. 1A). The patient was treated with carboplatin plus paclitaxel as first-line treatment (Fig. 2A). After 11 weeks, primary tumor progression and new pleural dissemination were observed. Although she received S-1 as a second-line treatment, she developed superior vena cava syndrome. After stenting the superior vena cava and palliative irradiation (30 Gray in 10 fractions), she received amrubicin as a third-line treatment. However, the disease progressed rapidly, with worsening pleural dissemination and a new liver metastasis (Fig. 1B). Then, lenvatinib 24 mg oral therapy was initiated as fourth-line treatment. After 4 days of lenvatinib treatment, the malignant pleural effusion and pleural metastases dramatically improved (Fig. 2B). Computed tomography (CT) revealed a partial response to both the pleural and liver metastases at 3 weeks after lenvatinib initiation (Fig. 1C). The dose of lenvatinib was reduced to 12 mg because of adverse events such as increase in alanine aminotransferase/aspartate aminotransferase and peripheral edema. Meanwhile, pleural metastases completely diminished (Fig. 1D).
Figure 1.
CT imaging changes of the primary tumor, pleural metastases, and liver metastases (A) at the time of initial diagnosis, (B) at lenvatinib initiation, (C) 3 weeks and (D) 19 weeks after lenvatinib initiation, (E) at lenvatinib discontinuation (40 weeks after initiation), and (F) 1 week after lenvatinib discontinuation. CT, computed tomography.
Figure 2.
(A) Timeline of the patient’s clinical course. Temporal changes in chest X-ray images after (B) the initiation and (C) the discontinuation of lenvatinib. †, passed away. AMR, amrubicin; CBDCA, carboplatin; HEP, liver metastases; RECIST, Response Evaluation Criteria in Solid Tumors; PD, progressive disease; PLE, pleural metastases; PR, partial response; PTX, paclitaxel; RT, radiation therapy; SVC, superior vena cava.
After 10 months of treatment, the response to lenvatinib continued; however, treatment was suspended owing to liver dysfunction. At the time of lenvatinib discontinuation, obvious progression of liver metastases was not observed on CT scans (Fig. 1E). Three days after treatment discontinuation, the patient developed dyspnea. On CT scan, a disease flare marked by recurrent pleural dissemination and new liver metastases was observed 6 days after discontinuation (Figs. 2C and 1F). Despite undergoing pleural drainage, which confirmed the presence of malignant pleural effusion, and resumption of lenvatinib, her treatment could not be continued because of deteriorating liver function. The patient passed away 2 weeks after the disease flared.
Mutation detection was performed using RNA sequencing (RNA-seq) (methods are detailed in the Supplementary Methods). This revealed a somatic in-frame deletion in KIT exon 11 (p.Q575_P577delinsH), both at the initial diagnosis and at the time of disease flare (Table 1 and Supplementary Fig. 1). In addition, TP53, and KMT2C mutations were detected at both the time points. Nonsense mutations in BAP1 were not detected by the RNA-seq calling pipeline in the initial sample; however, we confirmed that the mutation was present at the initial diagnosis using the Integrative Genomics Viewer. These four mutations were confirmed using whole-exome sequencing and were accompanied by copy number amplification or loss of heterozygosity.
Table 1.
Gene Alternation at Diagnosis and Disease Flare
| Gene |
At Diagnosisa |
At Disease Flare |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| RNA-Seq |
RNA-Seq |
WES |
CN Analysis |
||||||||
| Gene Name | Variant Classification | Amino Acid Change | Total Depth | VAF (%) | Total Depth | VAF (%) | Total Depth | VAF (%) | Major CN | Minor CN | Type |
| KIT | In-frame deletion | p.Q575_P577delinsH | 3527 | 96.9 | 854 | 97.7 | 249 | 30.1 | 4 | 0 | AMP-LOH |
| TP53 | Missense mutation | p.Y236C | 1263 | 87.6 | 165 | 58.8 | 136 | 30.9 | 3 | 0 | AMP-LOH |
| KMT2C | Frameshift deletion | p.F2313Wfs∗8 | 670 | 39.7 | 58 | 20.7 | 328 | 21.0 | 3 | 3 | AMP |
| BAP1 | Nonsense mutation | p.W5∗ | 39b | 23.1b | 97 | 52.6 | 331 | 30.5 | 3 | 0 | AMP-LOH |
AMP, amplification; CN, copy number; GATK, Genome Analysis Toolkit; IGV, Integrative Genomics Viewer; LOH, loss of heterozygosity; RNA-seq, RNA sequencing; VAF, variant allele frequency; WES, whole-exome sequencing.
We could not carry out whole-exome sequencing because of a lack of DNA quantity.
Although the GATK pipeline did not detect this mutation, we observed it using the IGV.
Discussion
To our knowledge, this is the first report detailing a rapid and dramatic response to lenvatinib in a patient with metastatic TC harboring KIT exon 11 mutation and documents an impressive disease flare after the discontinuation of lenvatinib.
c-Kit is involved in the pathogenesis of TC, and its expression in TC has potential diagnostic utility.5 Despite the high expression rate of c-Kit in TC (≥80%),6 c-Kit expression seems not to be a reliable biomarker for the response to multikinase TKIs that include c-Kit.7 In contrast, the potential benefits of selective c-Kit inhibitors for TCs with KIT mutations have been suggested in previous studies (Supplementary Table 1). Furthermore, it has been reported that Ba/F3 cell lines with KIT exon 11 mutations are sensitive to several selective c-Kit inhibitors.6,8
Gain-of-function mutations in KIT are uncommon in patients with TC (≤10%)6; however, mutations in exon 11, which encodes the intracellular membrane junction section of the protein, are the most prevalent.9 Notably, signaling downstream of KIT in gastrointestinal stromal tumors is more potently activated by mutations in exon 11 than those in other exons.10 In fact, patients with gastrointestinal stromal tumors harboring exon 11 KIT mutations displayed a better response and prognosis to imatinib than those with mutations in exon 9.11
In addition, in this case, copy number analysis revealed amplification of the KIT gene with loss of heterozygosity. RNA-seq–based genetic mutation analysis revealed an extremely high variant allele frequency for an in-frame deletion mutation in KIT exon 11. These findings suggest that the tumor could be highly c-Kit-addicted, and we highlight the significance of c-Kit pathway addiction because of KIT exon 11 mutation and amplification as a predictive biomarker for the efficacy of lenvatinib.
Rapid tumor progression was observed in this patient after the cessation of lenvatinib treatment. Whereas liver dysfunction observed at the time of the withdrawal could have been caused by latent disease progression, the drug discontinuation might have accelerated the disease flare. The causes of disease flares are not fully understood, and this phenomenon has not been previously reported in patients with TC. In this case, mutational analysis of the sample collected during disease flares revealed no additional mutations. In contrast, tumor cells maintained a high rate of KIT mutations during amplification. The rapid regrowth of TKI-sensitive clones causes rapid clinical deterioration when TKIs are discontinued.12 From a clinical perspective, the fact that the patient experienced a disease flare, a unique phenomenon of cancers with driver gene mutations treated with TKIs, is also consistent with the KIT mutation playing a central role in this patient. Further research is crucial to elucidate the relationship between KIT mutations and the efficacy of lenvatinib, and disease flare in patients with TC, which would contribute to appropriate patient selection.
In conclusion, in patients with metastatic TC, the presence of KIT exon 11 mutations with amplification may indicate KIT-addicted tumors and serve as a potential biomarker for predicting the efficacy of lenvatinib. However, in such cases, there might be a risk of disease flares after lenvatinib discontinuation.
CRediT Authorship Contribution Statement
Masahiro Torasawa: Investigation, Resources, Methodology, Formal analysis, Writing—original draft, Writing—review and editing, Visualization.
Tatsuya Yoshida: Investigation, Resources, Methodology, Writing—review and editing, Conceptualization.
Kouya Shiraishi: Investigation, Methodology, Formal analysis, Writing—review and editing.
Naoko Goto: Investigation, Formal analysis, Methodology, Writing—review and editing, Visualization.
Toshihide Ueno: Investigation, Formal analysis, Methodology, Writing—review and editing.
Hitoshi Ichikawa: Investigation, Formal analysis, Methodology, Writing—review and editing.
Shigehiro Yagishita: Investigation, Methodology, Resources, Writing—review and editing.
Shinji Kohsaka: Investigation, Writing—review and editing.
Yasushi Goto: Investigation, Resources, Writing—review and editing.
Yasushi Yatabe: Investigation, Resources, Writing—review and editing.
Akinobu Hamada: Investigation, Writing—review and editing.
Hiroyuki Mano: Investigation, Writing—review and editing.
Yuichiro Ohe: Supervision, Investigation, Writing—review and editing.
Disclosure
Dr. Yoshida reports receiving research funding from Amgen, Bristol-Myers Squibb, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, AbbVie, Daiichi-Sankyo, and Takeda paid to his institution; and honoraria from AstraZeneca, K.K, Amgen, Chugai, Merck Sharp & Dohme, Novartis, Ono Pharmaceutical, ArcherDx, Eli Lilly, Roche, and Taiho Pharmaceutical. Dr. Ichikawa reports receiving research funding from Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., Healios K.K., and Ono Pharmaceutical Co., Ltd. paid to his institution. Dr. Yagishita reports receiving research funding from Nippon Boehringer Ingelheim paid to his institution; and honoraria from LSI medicine. Dr. Kohsaka reports receiving research funding from AstraZeneca, Boehringer Ingelheim, Chordia Therapeutics, CIMIC, Konica Minolta, and TransThera Sciences paid to his institution. Dr. Goto reports receiving research funding from AZK and Pfizer (paid to the clinical trial group), and from AbbVie, Eli Lilly, Pfizer, Bristol-Myers Squibb, Ono Pharmaceutical, Novartis, Kyorin, Daiichi-Sankyo, Novartis, and Preferred Network paid to his institution; honoraria from Eli Lilly, Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Bristol-Myers Squibb, Pfizer, Merck Sharp & Dohme, Novartis, Merck, and ThermoFisher Scientific; participated in the advisory board to AstraZeneca, Chugai, Boehringer Ingelheim, Eli Lilly, Taiho Pharmaceutical, Pfizer, Novartis, Guardant Health Inc., Illumina, Daiichi-Sankyo, Ono Pharmaceutical, Bristol-Myers Squibb, and Merck Sharp & Dohme; and has leadership role in Cancer Net Japan and JAMT. Dr. Yatabe reports receiving research funding from ArcherDx, Chugai Pharma, ThermoFisher Scientific, Konika Minolta REALM, NEC Corporation, AstraZeneca, Merk Biopharma, and Johnson & Jonson paid to his institution; honoraria from AbbVie Inc., Amgen, Bayer, Daiichi-Sankyo, Merck Bio-Pharma, Merck Sharp & Dohme, Novartis, AstraZeneca, Agilent/Dako, Chugai Pharma, Jansen-Pharma, and Takeda; participated in the advisory board to Merck Sharp & Dohme, Chugai pharma, AstraZeneca, Novartis, Amgen, Takeda, Daiichi-Sankyo, Janssen-Pharma, and Merck Biopharma. Dr. Mano reports receiving research funding from Konica Minolta Realm; royalty for a patent license from the University of Tokyo; patent pending for design for probes to detect gene fusions. Dr. Ohe reports receiving research funding from AstraZeneca, Chugai Pharma, Eli Lilly, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Pfizer, Taiho Pharmaceutical, Novartis, Takeda, and Janssen paid to his institution; honoraria from AstraZeneca, Chugai Pharma, Eli Lilly, Ono Pharmaceutical, Bristol-Myers Squibb Japan, Nippon Boehringer Ingelheim, Bayer, Pfizer, Merck Sharp & Dohme K.K., Taiho Pharmaceutical, Kyowa Kirin, Takeda, Celltrion, Amgen, Novartis, Nippon Kayaku, and Eisai; consulting or advisory roles for AstraZeneca, Chugai Pharma, Lilly Japan, Ono Pharmaceutical, Novartis, Kyorin, Takeda, Celltrion, Amgen, and Anheart Therapeutics. The remaining authors declare no conflict of interest.
Acknowledgments
This study was not specifically funded. The authors thank the patient and her family for providing informed consent to the institutional review board (2015-059) for this case report.
Footnotes
Cite this article as: Torasawa M, Yoshida T, Shiraishi K, et al. Rapid Response to lenvatinib and disease flare after discontinuation in a patient with thymic carcinoma harboring KIT exon 11 mutation: a case report. JTO Clin Res Rep. 2024;5:100657.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2024.100657.
Supplementary Data
References
- 1.Girard N., Ruffini E., Marx A., Faivre-Finn C., Peters S., ESMO Guidelines Committee Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2015;26(suppl 5):v40–v55. doi: 10.1093/annonc/mdv277. [DOI] [PubMed] [Google Scholar]
- 2.Sato J., Satouchi M., Itoh S., et al. Lenvatinib in patients with advanced or metastatic thymic carcinoma (REMORA): a multicentre, phase 2 trial. Lancet Oncol. 2020;21:843–850. doi: 10.1016/S1470-2045(20)30162-5. [DOI] [PubMed] [Google Scholar]
- 3.Chaft J.E., Oxnard G.R., Sima C.S., Kris M.G., Miller V.A., Riely G.J. Disease flare after tyrosine kinase inhibitor discontinuation in patients with EGFR-mutant lung cancer and acquired resistance to erlotinib or gefitinib: implications for clinical trial design. Clin Cancer Res. 2011;17:6298–6303. doi: 10.1158/1078-0432.CCR-11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akamatsu H., Ono A., Shukuya T., et al. Disease flare after gefitinib discontinuation. Respir Investig. 2015;53:68–72. doi: 10.1016/j.resinv.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 5.Kelly R.J., Petrini I., Rajan A., Wang Y., Giaccone G. Thymic malignancies: from clinical management to targeted therapies. J Clin Oncol. 2011;29:4820–4827. doi: 10.1200/JCO.2011.36.0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girard N. Thymic tumors: relevant molecular data in the clinic. J Thorac Oncol. 2010;5(suppl 4):S291–S295. doi: 10.1097/JTO.0b013e3181f209b9. [DOI] [PubMed] [Google Scholar]
- 7.Giaccone G., Rajan A., Ruijter R., Smit E., van Groeningen C., Hogendoorn P.C.W. Imatinib mesylate in patients with WHO B3 thymomas and thymic carcinomas. J Thorac Oncol. 2009;4:1270–1273. doi: 10.1097/JTO.0b013e3181b6be57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Girard N., Shen R., Guo T., et al. Comprehensive genomic analysis reveals clinically relevant molecular distinctions between thymic carcinomas and thymomas. Clin Cancer Res. 2009;15:6790–6799. doi: 10.1158/1078-0432.CCR-09-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schirosi L., Nannini N., Nicoli D., et al. Activating c-KIT mutations in a subset of thymic carcinoma and response to different c-KIT inhibitors. Ann Oncol. 2012;23:2409–2414. doi: 10.1093/annonc/mdr626. [DOI] [PubMed] [Google Scholar]
- 10.Duensing A., Medeiros F., McConarty B., et al. Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs) Oncogene. 2004;23:3999–4006. doi: 10.1038/sj.onc.1207525. [DOI] [PubMed] [Google Scholar]
- 11.Debiec-Rychter M., Sciot R., Le Cesne A., et al. KIT mutations and dose selection for imatinib in patients with advanced gastrointestinal stromal tumours. Eur J Cancer. 2006;42:1093–1103. doi: 10.1016/j.ejca.2006.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Chmielecki J., Foo J., Oxnard G.R., et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.