Abstract
Introduction
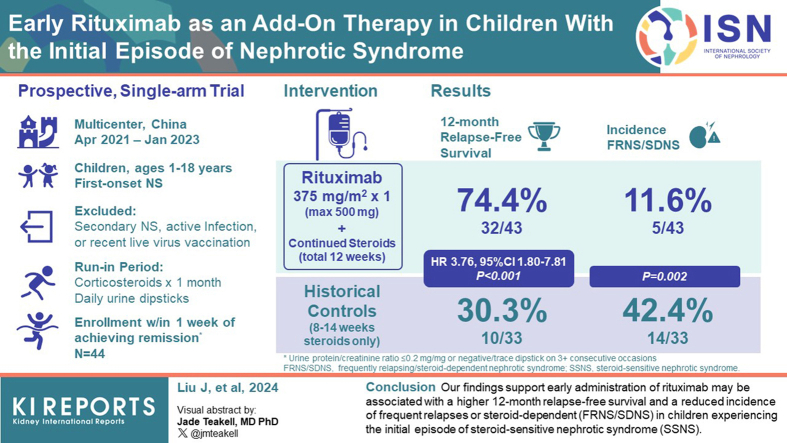
The approximately 70% 12-month relapse in children experiencing the initial episode of steroid-sensitive nephrotic syndrome (SSNS) is a significant concern, with over 50% developing frequent relapses or steroid-dependent nephrotic syndrome (FRNS/SDNS). There is a lack of strategies to reduce relapse after the onset. Whether early administration of rituximab, which effectively reduces relapses in FRNS/SDNS, may be a solution has not been evaluated.
Methods
A prospective, multicenter, open-label, single-arm trial was conducted in China, with a 12-month follow-up. Children aged 1 to 18 years with the first episode of nephrotic syndrome (NS) were screened for eligibility. Proteinuria was evaluated daily using dipsticks. A dose of 375 mg/m2 of rituximab was intravenously infused within 1 week after achieving corticosteroid-induced remission. The main outcome was 12-month relapse-free survival.
Results
Out of the initially 66 children screened, 44 were enrolled and received rituximab, with all but 1 participant completing the 12-month follow-up. The median age at diagnosis was 4.3 years (interquartile range [IQR]: 3.4–5.9), and 33 (77%) of the participants were male. In the rituximab group, the 12-month relapse-free survival was significantly higher compared to historical controls (32 of 43 [74.4%] vs. 10 of 33 [30.3%]; P < 0.001; hazard ratio [HR], 3.76; 95% confidence interval [CI], 1.80–7.81). The post hoc analysis revealed a higher 24-month relapse-free survival and a lower incidence of FRNS/SDNS at the 12-month follow-up. Treatment with rituximab was well-tolerated.
Conclusion
Our findings support that early administration of rituximab may be associated with a higher 12-month relapse-free survival and a reduced incidence of FRNS/SDNS in children experiencing the initial episode of SSNS.
Keywords: children, pediatric nephrology, rituximab, nephrotic syndrome, clinical trial, proteinuria
Graphical abstract
See Commentary on Page 1149
NS is a common glomerular disease in children,1 and corticosteroid therapy serves as the cornerstone for the initial episode.2,3 However, the 12-month relapse-free survival of SSNS after corticosteroid therapy is only 30%,4,5 with approximately half of the patients developing FRNS/SDNS.1 The frequency of relapses during the first 12 months is associated with persistent relapses into adulthood,2 but prevention of SSNS developing into FRNS/SDNS remains a challenge.
Rituximab, an anti-CD20 monoclonal antibody, has shown efficacy in reducing relapses among patients with FRNS/SDNS who may otherwise experience adverse effects from immunosuppressants.2,3 The potential application of rituximab as a first-line treatment to minimize relapse in the initial episode is under investigation. This trial aims to investigate the association between the addition of rituximab to standard care with steroids in initial therapy and reduce the relapse rate during a 12-month period in children with SSNS.
Methods
Study Design
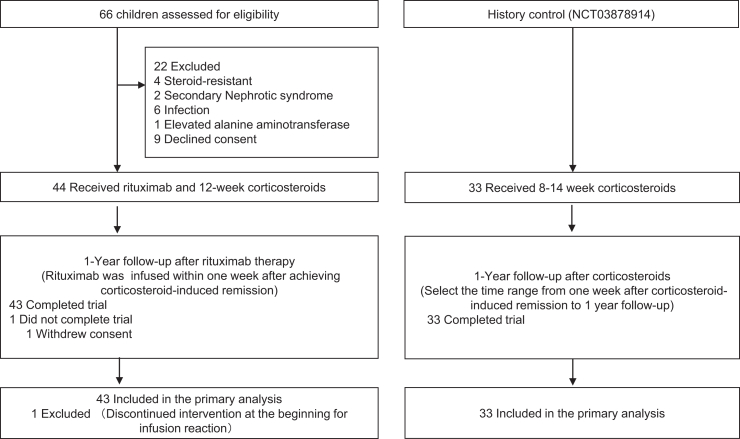
The study, named the Rituximab in the First Episode of Pediatric Idiopathic Nephrotic Syndrome (RTXFIRPedINS), was a prospective, multicenter, open-label, single-arm clinical trial conducted at 8 tertiary hospitals in China from April 13, 2021, to January 17, 2023. The results were compared with historical controls who received standard care with steroids but without RTX treatment.5
This trial was designed, conducted, and managed by the Children’s Hospital of Fudan University. The protocol, which was reported previously,6 is available in Supplement 1. The study adhered to the tenets of the Declaration of Helsinki and was approved by 8 institutional review boards. Written informed consent were obtained from the parents (and assent of patients older than 8 years) after provision of detailed oral and written information concerning the context of the study, potential benefit, and safety aspects. No financial compensation was provided. The study protocol was initially issued on January 5, 2021, and the amendment number is 1.4 (last updated on 5 May 2023).
Patients
Children aged between 1 and 18 years and experiencing the first episode of NS were screened at registered hospitals. Additional key eligibility criteria included a confirmed diagnosis of SSNS, wherein eligible participants underwent corticosteroid therapy and a 1-month run-in period. Participants were enrolled within 1 week of achieving complete remission with proteinuria evaluated daily using dipsticks. Children with a secondary form of NS, active infection, or those who received live vaccination within the last month were excluded from the study. Detailed definitions and inclusion and exclusion criteria are provided in the trial protocol (Supplement 1).
Proteinuria was evaluated daily using dipsticks. Study visits included the screening period, administration of rituximab, visits at 1, 3, 4.5, 6, 9, and 12 months after rituximab administration, as well as at relapse. Urinalysis, complete blood count, biochemistry, immunoglobulins, and lymphocyte subpopulations, were evaluated as outlined in the protocol (Supplement 1).
Control Group
A nonrandomized historical control group (NCT03878914) consisted of 33 patients with the first episode of NS treated with corticosteroids without rituximab between August 6, 2019, and October 31, 2021. These patients received 8 weeks or 12 to 14 weeks of corticosteroid therapy at the Children’s Hospital of Fudan University based on time to remission.
Study Intervention
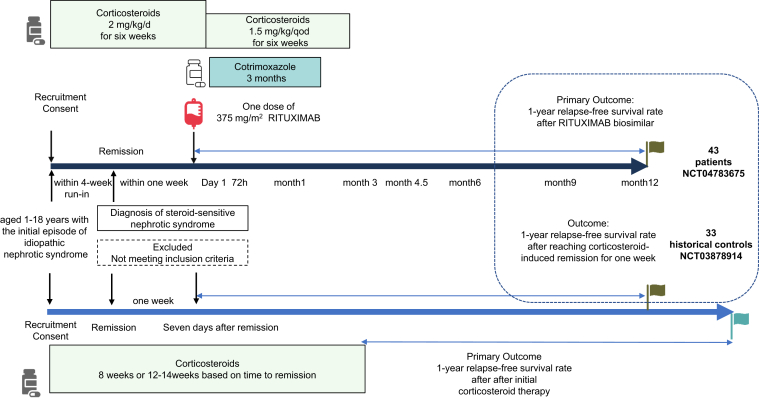
A dose of 375 mg/m2 (maximum dose: 500 mg) of rituximab was intravenously infused within 1 week of complete remission.
The initial treatment for INS consisted of daily steroids at a dose of 2 mg/kg/d or 60 mg/m2/d (maximum 60 mg/d) for 6 weeks, followed by 1.5 mg/kg/d or 40 mg/m2 (maximum 40 mg/d) on alternate days for another 6 weeks.
To prevent infusion reactions, patients received antipyretic and analgesic drugs such as acetaminophen or ibuprofen, once at a regular dose, 30 minutes before the rituximab infusion. Antiallergy medications, cetirizine hydrochloride or cyproheptadine or loratadine, were also administered once at a regular dose. Prior to the rituximab infusion, the patient’s oral corticosteroids were switched to intravenous methylprednisolone at a dose of 1.6 mg/kg, administered once. To prevent Pneumocystis jiroveci, trimethoprim-sulfamethoxazole was administered for 3 months, starting from the beginning of the rituximab treatment (day 1). The prophylactic dose of trimethoprim was 3 mg/kg on alternate days, and the maximum dose of sulfamethoxazole was 960 mg on alternate days.
Response
The primary objective of this exploratory single-arm study was to assess the 12-month relapse-free survival rate, which was defined as the absence of nephrotic-range proteinuria (urine protein-to-creatinine ratio ≥2 mg/mg or dipstick ≥3+ on 3 consecutive days in first morning samples).7 This outcome was compared to historical controls (Figures 1 and 2). As indicated in the Kidney Disease Improving Global Outcomes 2020 guidelines, though there is a lack of data assessing potential variations in the optimal treatment approach based on sex or ethnicity, we acknowledge the significance of considering children at a particularly young age of disease onset (i.e., 1 to 4–6 years old), because they may face a higher risk of progressing to FRNS/SDNS. A delayed response to prednisolone could impact relapse in this age group. Therefore, we have adjusted the results to account for differences among subgroups.
Figure 1.
Trial flowchart between this study and the historical control study. The flow of participants through different stages and interventions.
Figure 2.
Study design between this study and historical control. Overview of the interventions administered during the study and their corresponding outcomes.
The secondary outcomes of this study included the 6-month relapse-free survival rate, the number of days from rituximab infusion to the first relapse. The other secondary end points indicated in Supplements 1 and 2 are not reported.
Exploratory Analyses
For post hoc analysis, relapse-free survival rate at 24-month follow-up, and the incidence of FRNS/SDNS at the 12-month follow-up were compared with standard care, because it is also associated with long-term prognosis, such as the likelihood of relapse into adulthood. Exploratory end points included studying changes in immunologic factors and immune cell subsets in the peripheral blood as predictors of response and relapse.
Safety
The safety end points of this study were the severity of adverse events, which were graded according to the Common Terminology Criteria for Adverse Events, version 5.0.
Statistical Analysis
Predefined statistical analyses are fully detailed in the statistical analysis plan (Supplement 2). This study is a single-arm study. The sample size was based on the expected rate of the primary treatment effect end point and the size of the effect of rituximab treatment. According to the previous literature,4 the 12-month relapse-free survival rate is approximately 30% in children with the first episode of SSNS after corticosteroid therapy. On the basis of that, we estimated that at a 2-sided alpha level of 0.05, with an assumed dropout rate of 10%, a sample size of 44 would provide 80% power to detect a 20% increase in the relapse-free rate in patients receiving rituximab treatment as compared with the traditional treatment.
Data were presented as absolute number (percentage), mean±SD, median and IQR, and HR with a 95% CI, where applicable. The Kaplan-Meier method was used to obtain estimation of 12-month and 24-month relapse-free survival rate. The log-rank tests were used to test the differences between subgroups. The comparison of the intervention versus standard care on the relapse-free survival rate was assessed using Cox proportional hazards regression model, and adjusted for sex, age at onset of diseases, the time of remission induced by corticosteroid. The differences in continuous variables between the 2 treatments was analyzed using the or the t-test or Mann-Whitney U test, and differences in categorical variables was analyzed using the Chi-square test or Fisher exact test.
R software, version 4.1.2 (R Foundation for Statistical Computing) was used to perform all data analyses and generate statistical graphs. Differences were considered to be statistically significant when the P value was 0.05 or less (2-tailed).
This trial is registered at ClinicalTrials.gov (Identifier: NCT04783675). See the Supplementary Information online for complete Methods (Clinical Study Protocol).
Results
Patients
A total of 66 patients were screened between April 13, 2021 and January 12, 2022, of whom 44 were enrolled and received rituximab treatment. The study end date was January 17, 2023. One participant did not complete the 12-month follow-up (Figure 1). The median age of the participants was 4.3 years (IQR: 3.4–5.9), and 33 (77%) of them were male. The median time from remission to rituximab infusion was 5 (IQR: 3–6) days, and the median time from corticosteroids to rituximab infusion was 13 (IQR: 11–17) days. At the time of rituximab infusion, the media level of serum albumin was 2.9 (2.6–3.1) g/dl. There were no statistically significant differences in sex, age at onset of NS, days to remission, or serum biochemistry between the rituximab group and the control group (Table 1). All patients showed B-cell depletion at 72 hour or 1 month after rituximab administration.
Table 1.
Baseline demographic, clinical, and biologic characteristics of the patientsa
| Characteristic | Rituximab (n = 43) | Historical controls (n = 33) | P value |
|---|---|---|---|
| Demographics | |||
| Male, n (%) | 33 (77%) | 24 (73%) | 0.70 |
| Age at onset of disease, yr | 4.3 (2.5) | 3.5 (3.0) | 0.09 |
| Medical history | |||
| Days to remission, d | 8 (4) | 8 (5) | >0.99 |
| Serum biochemistry | |||
| Estimated glomerular filtration rate, ml/min per 1.73 m2 | 140.8 (36.3) | 133.8 (41.7) | 0.22 |
| Prednisolone therapy | |||
| The duration for corticosteroids, wk | 12 | 8 or 12–14 | NA |
NA, not applicable.
Unless otherwise indicated, values are given as median (25th quantile–75th quantile).
Primary Outcome
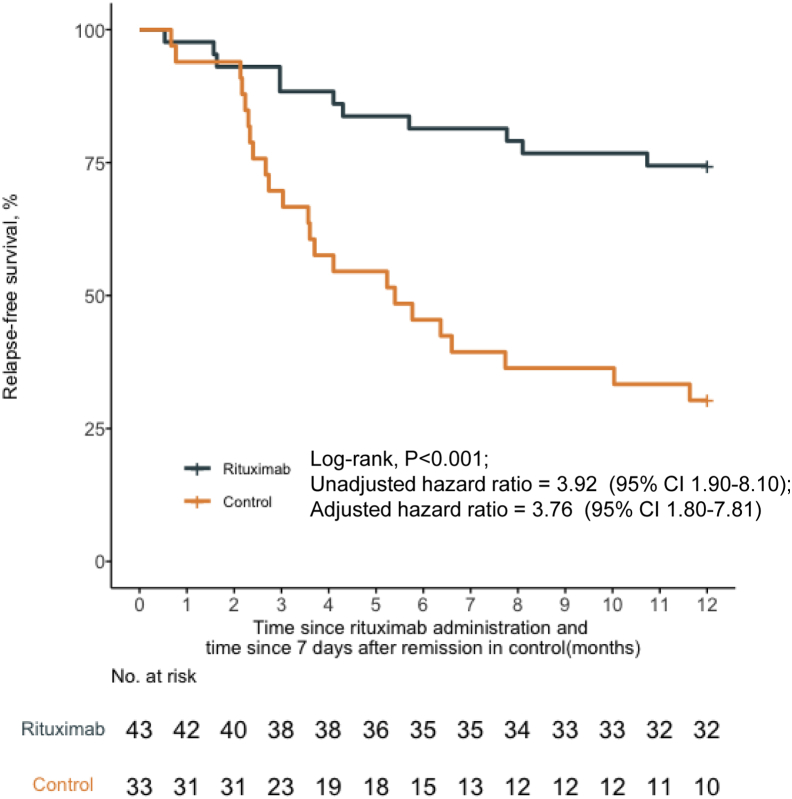
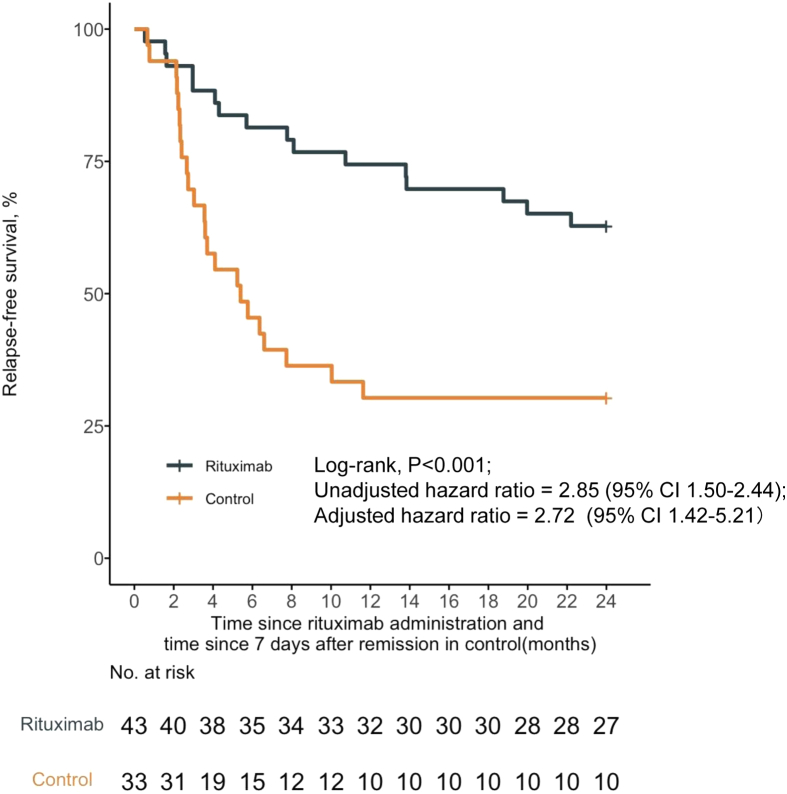
At the 12-month follow-up, 74.4% (32 of 43) of patients in the rituximab group attained remission. There were no statistically significant differences in the response with respect to sex, age at onset of NS (≥4 years or <4 years), or days to remission (≥ 10 days or <10 days) (Figure 3, Supplementary Figure S1). After adjusting for protocol-specified potential prognostic factors using a multivariable Cox regression model, the 12-month relapse-free survival was significantly higher in the rituximab group compared to historical controls (32 of 43 [74.4%] vs. 10 of 33 [30.3%]; P < 0.001; HR, 3.76; 95% CI, 1.80–7.81) (Figure 3).
Figure 3.
Probability of relapse-free survival according to treatment group. The 12-month relapse-free survival rate was significantly higher with rituximab compared with historical controls. CI, confidence interval.
Secondary Outcomes
The 6-Month Relapse-Free Survival Rate
The 6-month relapse-free survival rate in the rituximab therapy was higher than the historical control (35 of 43 [81.4%] vs. 15 of 33[45.5%]; P = 0.005; HR, 3.37; 95% CI, 1.44–7.86).
The Number of Days From Rituximab Infusion to the First Relapse
For patients who experienced relapse, the median time to relapse from the infusion of rituximab was found to be 123 days (95% CI, 73–201 days).
Predictors of Relapse
No significant differences were noted between patients who experienced relapse and those who did not for the following factors: age at onset of NS, serum albumin, estimated glomerular filtration rate, CD19+ percentage within 72 hours after rituximab, days to remission, days from remission to rituximab infusion, days from corticosteroids to rituximab infusion, CD19+ percentage at 1 month after rituximab (Supplementary Figure S2). There was no consistent association between B-cell recovery and relapse. Notably, among patients who relapsed, 5 had less than 1% CD19+ B cells at the relapse onset (Supplementary Figure S3). We also conducted a comprehensive flow cytometric analysis of B, T, NK, and myeloid cells in the patients' peripheral blood mononuclear cells at various time points during the trial, revealing notable differences in T cell subsets.
Post Hoc Analyses
The Incidence of FRNS/SDNS at the 12-Month Follow-Up
All patients who relapsed received corticosteroids according to protocol. The post hoc analysis demonstrated a significantly lower incidence of FRNS/SDNS at the 12-month follow-up compared to historical controls (5 of 43 [11.6%] vs. 14 of 33 [42.4%]; P = 0.002).
The 24-Month Relapse-Free Survival Rate
At the 24-month follow-up, 62.8% (27 of 43) of patients in the rituximab group attained remission. Adjusting for protocol-specified potential prognostic factors using a multivariable Cox regression model revealed a significantly higher 24-month relapse-free survival in the rituximab group compared to historical controls (10 of 33 [30.3%]) (HR, 2.72; 95% CI, 1.42–5.21) (Figure 4).
Figure 4.
Probability of the 24-month relapse-free survival according to treatment group. The 24-month relapse-free survival rate was significantly higher with rituximab compared with historical controls. CI, confidence interval.
Adverse Events
The treatment with rituximab was well-tolerated, and no severe life-threatening events were documented. A total of 46 grade 2 to 4 adverse events were reported in 30 patients, including 34 grade 2 adverse events in 27 patients, 11 grade 3 events in 7 patients, and 1 grade 4 event (Table 2). The most common grade 2 to 4 adverse events were decreased neutrophil count. At the end of the 12-month follow-up, all adverse events, except for 1 grade 3 and 1 grade 2 decreased neutrophil count, were fully resolved. Hypogammaglobulinemia was only reported in 1 patient at the onset of NS (Supplementary Figure S4). Throughout the 1-year follow-up, 3 patients contracted COVID-19, with no reported serious adverse events or relapses.
Table 2.
Adverse events categorized by severity
| Adverse event | Patients (N = 43), no. of adverse events (no. of patients) |
|---|---|
| Adverse events attributed to relapse | 7(7) |
| Adverse events leading to study discontinuation | 1(1) |
| Grade 2 adverse events | |
| Infusion related reactiona | 3(3) |
| Allergic reaction | 1(1) |
| Upper respiratory infection | 9(7) |
| Bronchial infection | 2(2) |
| Mucositis oral | 1(1) |
| Neutrophil count decreased | 16(13) |
| Lymphocyte count decreased | 2(2) |
| Vomiting | 1(1) |
| Grade 3 adverse events | |
| Upper respiratory infection | 1(1) |
| Bronchial infection | 3(3) |
| Neutrophil count decreased | 7(4) |
| Grade 4 adverse events | |
| Neutrophil count decreased | 1(1) |
| Grade 5 adverse events | 0 |
This safety population included all 44 patients who had received after rituximab.
The rate of treatment discontinuation due to adverse events was 2.3% (1 of 44). During rituximab infusion, 6.8% (3 of 44) of participants reported throat irritation. One patient encountered chest discomfort at the seventh minute of infusion; the symptoms promptly disappeared following the administration of steroids, oxygenation, and inhalation. Despite the improvement, the patient’s parents withdrew their consent. The other 2 patients required a rate reduction or temporary discontinuation of infusion and inhalation, but none of these adverse events led to a reduction of rituximab dose.
Almost all cases of neutropenia, except for 1, occurred after 4 weeks of rituximab. The incidence of grade 4 decreased neutrophil count (agranulocytosis) was 2.3% (1 of 43). Two patients with grade 2 to 3 decreased neutrophil count had transient infections (upper respiratory infection and bronchial infection) and received antibiotics. However, the majority of patients with decreased neutrophil counts showed no symptoms. One boy aged 3.5 years experienced a grade 4 decreased neutrophil count during a follow-up 6 months after rituximab, but he did not exhibit any symptoms, and his neutrophil count spontaneously returned to normal levels 1 week later.
Discussion
In this study, we identified a potential positive association between rituximab treatment and improved outcomes in children experiencing an initial episode of SSNS. The percentage of patients maintaining remission during the 1-year follow-up was more than double when compared to historical data on corticosteroids alone (74% vs. 30%). It is noteworthy that the first relapse of SSNS frequently occurs within 12 months of onset, which aligns with the time frame used to define FRNS/SDNS. Up to 42% of children continue to experience relapses into adulthood, especially those with FRNS/SDNS.8,9 The reported cumulative rate of sustained remission after 2 years in children undergoing 8 to 12 weeks of steroid treatment following the initial attack ranged between 20% and 23%.4 Interestingly, this study demonstrated that rituximab treatment may be associated with an elevated 2-year relapse-free rate and a low incidence of FRNS/SDNS at the 12-month follow-up, suggesting potentially positive disease progression for the early rituximab treatment.
Given the high risk of relapse associated with SSNS, there is an urgent need to explore alternative therapies. However, there are no clinical trials investigating the combination of rituximab with corticosteroids as an initial treatment for children with SSNS. Nevertheless, an ongoing French trial in minimal change disease (NCT03970577) is exploring the use of rituximab at the time of initial presentation in adult patients.10 Considering that minimal change disease is the predominant pathologic finding in children with SSNS, this trial will provide valuable insights into the potential benefits in different patient populations.
To date, 3 case series have explored using rituximab as a first-line therapy for SSNS/minimal change disease without concomitant steroid or immunosuppressants. Promising results were seen in 2 studies with 4 weekly doses of 375 mg/m2 in both adult11 and pediatric12 patients. Another study13 suggested that rituximab might not be as effective as corticosteroids as an initial therapy. Among 9 adults, 5 achieved completed remission with no relapse after a single 375 mg/m2 dose. One achieved partial remission, and a second dose of 375 mg/m2 was administered to further reduce proteinuria. Another 1 achieved partial remission but relapsed shortly thereafter. The remaining 2 did not respond but showed a better response to steroids. Notably, all patients in these studies had contraindications to corticosteroids or refused their use. The Kidney Disease Improving Global Outcomes 2020 guidelines recommend rituximab as an initial therapy for adults with minimal change disease when corticosteroids may be relatively contraindicated.2
An important observation was the detection of rituximab in the urine of patients with NS.14 On the basis of the 2023 guidelines3 from the International Pediatric Nephrology Association for FRNS/SDNS, the administration of rituximab is recommended preferably in remission. Various factors contribute to the delayed onset of rituximab's action in treating NS. Considering these investigations and recommendations, our management strategy of combining rituximab with corticosteroids during remission may be more appropriate for children experiencing the initial episode of NS. In this study, we selected a single dose of 375/m2 based on previous reports of its efficacy in preventing relapses among patients with relapsed NS.15,16 It was sufficient to achieve B-cell depletion in our patients. However, it should be noted that in our study, 26% of the patients experienced relapse. We expect to identify some biomarkers that can predict relapse in order to enhance our understanding of this condition and higher dosages of rituximab might be required or other strategies may be needed for patients with certain manifestations.
The most common adverse event observed in this study was rituximab-related neutropenia, which is well-documented in the literature, although its exact mechanism remains unknown.3 Late-onset neutropenia, defined as an absolute neutrophil count of less than 1.5 x 109/L after rituximab treatment, can occur at least 4 weeks after the last infusion and, in some cases, up to 12 months later.17In our cohort, 9.3% and 2.3% of children experienced grade 3 and grade 4 neutropenia, respectively. In comparison, Iijima et al. reported grade 3 and grade 4 neutropenia in 8% of children,18 whereas Kamei et al.19 observed a higher incidence of grade 4 neutropenia at 9.6% in their study involving 114 patients at a median of 66 days after rituximab. The incidence of grade 4 neutropenia was more prevalent in younger children, with a median age of 6.4 years in Kamei et al.’s study.19 In our cohort, patients experiencing grade 2 to 4 neutropenia had a median age of 3.6 years at the time of treatment. In most cases, rituximab-induced late onset grade 1 to 3 neutropenia is self-limiting and resolves without any complications. However, there is a possibility of more severe cases of grade 4 neutropenia. It is crucial for patients and their caregivers to be aware of this potential complication and to seek emergency medical attention promptly if they develop clinical manifestation.
The second most common adverse event was infection, which included upper respiratory infection, bronchial infection, and mucositis oral. Among the patients, 6.8% (3 of 44) experienced infusion-related reactions after taking preventive medication. As part of the study protocol, hepatitis B vaccination and prophylactic sulfamethoxazole were recommended. Throughout the follow-up period, no instances of Hepatitis B virus reactivation or Pneumocystis jiroveci infection were observed. It should be noted that, in order to prevent infusion reaction to rituximab, the oral corticosteroids on the same day were changed to intravenous infusions, which may affect the 12-month relapse rate. Overall, it is crucial to consider these adverse events and the preventive strategies to ensure the safety and well-being of patients undergoing rituximab treatment.
Limitations
This study has several limitations that should be considered when interpreting the results. The main limitation is the single-arm design, which inherently lacks a control group for comparison. This design could introduce bias in outcome evaluation. Various strategies were implemented to improve the quality of the research, such as using historical controls, applying strict eligibility criteria, using objective end points, and ensuring independent assessment of safety data. Due to the predetermined duration of steroids based on the control group's remission days, an analysis of steroid duration and dose interplay in multifactor Cox tests was omitted, potentially influencing relapse associations. Post hoc analyses were conducted to scrutinize data on FRNS/SDNS incidence at the 12-month follow-up and the 24-month relapse-free survival rate. Another limitation involves approximately 30% of pediatric patients receiving rituximab, possibly incurring additional costs and side effects in a cohort already at low relapse risk. It prompts further exploration of rituximab benefits postrelapse and the effective balance of effects, costs, and associated risks. Subsequent research becomes imperative to elucidate rituximab's benefits in specific populations, emphasizing the importance of precise immunophenotyping and targeted therapy selection.
Conclusion
This study offers valuable insights into the addition of rituximab to standard care with steroids in initial therapy; and that may be associated with a higher 12-month relapse-free survival and lower incidence of FRNS/SDNS in children experiencing the initial episode of SSNS. Randomized controlled trials are needed to strengthen the evidence.
Disclosure
HX reported receiving research grants from Shanghai Henlius Biotech, Inc during the conduct of the study. All the other authors declared no competing interests.
Acknowledgments
We thank all our patients, their families, and the site investigators. This work was supported by Key Development Program of Children's Hospital of Fudan University [EK2022ZX01], Clinical Research Plan of Shanghai Hospital Development Center [SHDC2022CRD018], National Key Laboratory of Kidney Diseases, Shanghai Kidney Development and Pediatric Kidney Disease Research Center [2021CRUZDPT03], and a grant from Shanghai Henlius Biotech, Inc [EK00000710].
Footnotes
Supplement 1. Study protocol of the Rituximab in the First Episode of Pediatric Idiopathic Nephrotic Syndrome (RTXFIRPedINS) Trial.
Supplement 2. Statistical Analysis Plan of the Rituximab in the First Episode of Pediatric Idiopathic Nephrotic Syndrome (RTXFIRPedINS) Trial.
Figure S1. Overall relapse-free survival in subgroups.
Figure S2. Characteristics of patients with relapse and nonrelapse.
Figure S3. RTX treatment affects levels of B cells in patients with relapse and nonrelapse.
Figure S4. RTX treatment affects levels of IgG in patients with relapse and nonrelapse.
Contributor Information
Qian Shen, Email: shenqian@shmu.edu.cn.
Hong Xu, Email: hxu@shmu.edu.cn.
Data Availability Statement
Requests for access to data from the RTXFIRPedINS trial should be addressed to the corresponding authors. The individual participant data collected during the trail will be available, after de-identification, when the article has been published with no end date. All proposals requesting data access will need to have a research plan and specify how the data will be used, and all proposals will need the approval of the trial co-investigator team before data release.
Supplementary Material
Supplement 1. Study protocol of the Rituximab in the First Episode of Pediatric Idiopathic Nephrotic Syndrome (RTXFIRPedINS) Trial.
Supplement 2. Statistical Analysis Plan of the Rituximab in the First Episode of Pediatric Idiopathic Nephrotic Syndrome (RTXFIRPedINS) Trial.
Figure S1. Overall relapse-free survival in subgroups.
Figure S2. Characteristics of patients with relapse and nonrelapse.
Figure S3. RTX treatment affects levels of B cells in patients with relapse and nonrelapse.
Figure S4. RTX treatment affects levels of IgG in patients with relapse and nonrelapse.
Supplement 4. Data sharing statement.
References
- 1.Banh T.H., Hussain-Shamsy N., Patel V., et al. Ethnic differences in incidence and outcomes of childhood nephrotic syndrome. Clin J Am Soc Nephrol. 2016;11:1760–1768. doi: 10.2215/CJN.00380116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021;100:S1–S276. doi: 10.1016/j.kint.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Trautmann A., Boyer O., Hodson E., et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2023;38:877–919. doi: 10.1007/s00467-022-05739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schijvens A.M., Teeninga N., Dorresteijn E.M., Teerenstra S., Webb N.J., Schreuder M.F. Steroid treatment for the first episode of childhood nephrotic syndrome: comparison of the 8 and 12 weeks regimen using an individual patient data meta-analysis. Eur J Pediatr. 2021;180:2849–2859. doi: 10.1007/s00431-021-04035-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang X., Shen Q., Rao J., et al. Duration of initial prednisolone therapy for first episode of childhood nephrotic syndrome based on time to response. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.1043285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Shen Q., Xie L., et al. Protocol for an open-label, single-arm, multicentre clinical study to evaluate the efficacy and safety of rituximab in the first episode of paediatric idiopathic nephrotic syndrome. BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-064216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautmann A., Vivarelli M., Samuel S., et al. IPNA clinical practice recommendations for the diagnosis and management of children with steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2020;35:1529–1561. doi: 10.1007/s00467-020-04519-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korsgaard T., Andersen R.F., Joshi S., Hagstrøm S., Rittig S. Childhood onset steroid-sensitive nephrotic syndrome continues into adulthood. Pediatr Nephrol. 2019;34:641–648. doi: 10.1007/s00467-018-4119-8. [DOI] [PubMed] [Google Scholar]
- 9.Fakhouri F., Bocquet N., Taupin P., et al. Steroid-sensitive nephrotic syndrome: from childhood to adulthood. Am J Kidney Dis. 2003;41:550–557. doi: 10.1053/ajkd.2003.50116. [DOI] [PubMed] [Google Scholar]
- 10.Christian M.T., Maxted A.P. Optimizing the corticosteroid dose in steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 2022;37:37–47. doi: 10.1007/s00467-021-04985-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenoglio R., Sciascia S., Beltrame G., et al. Rituximab as a front-line therapy for adult-onset minimal change disease with nephrotic syndrome. Oncotarget. 2018;9:28799–28804. doi: 10.18632/oncotarget.25612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Jin Y., Li Q., et al. Successful treatment of new-onset pediatric nephrotic syndrome with rituximab as a first-line therapy. Kidney Int Rep. 2022;7:2750–2751. doi: 10.1016/j.ekir.2022.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nan Guan M.Z., Zhang M., Chen R., Chen R., Xie Q., Hao C.M. Rituximab as initial therapy in adult patients with minimal change disease. Kidney Int Rep. 2023;8:1102–1104. doi: 10.1016/j.ekir.2023.02.1070. ISSN 2468-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs R., Langer-Jacobus T., Duong M., et al. Detection and quantification of rituximab in the human urine. J Immunol Methods. 2017;451:118–121. doi: 10.1016/j.jim.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Ravani P., Rossi R., Bonanni A., et al. Rituximab in children with steroid-dependent nephrotic syndrome: a multicenter, open-label, noninferiority, randomized controlled trial. J Am Soc Nephrol. 2015;26:2259–2266. doi: 10.1681/ASN.2014080799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ravani P., Magnasco A., Edefonti A., et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1308–1315. doi: 10.2215/CJN.09421010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim Z.R.S., Teh K.L., Das L., Arkachaisri T. Neutropenia following rituximab in paediatric non-malignant diseases: case series and review of the literature. Singapore Med J. 2021 doi: 10.11622/smedj.2021188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iijima K., Sako M., Nozu K., et al. Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384:1273–1281. doi: 10.1016/S0140-6736(14)60541-9. [DOI] [PubMed] [Google Scholar]
- 19.Kamei K., Takahashi M., Fuyama M., et al. Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrol Dial Transplant. 2015;30:91–96. doi: 10.1093/ndt/gfu258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Requests for access to data from the RTXFIRPedINS trial should be addressed to the corresponding authors. The individual participant data collected during the trail will be available, after de-identification, when the article has been published with no end date. All proposals requesting data access will need to have a research plan and specify how the data will be used, and all proposals will need the approval of the trial co-investigator team before data release.