Abstract
Telomere is a protective structure located at the end of chromosomes of eukaryotes, involved in maintaining the integrity and stability of the genome. Telomeres play an essential role in cancer progression; accordingly, targeting telomere dynamics emerges as an effective approach for the development of cancer therapeutics. Targeting telomere dynamics may work through multifaceted molecular mechanisms; those include the activation of anti-telomerase immune responses, shortening of telomere lengths, induction of telomere dysfunction and constitution of telomerase-responsive drug release systems. In this review, we summarize a wide variety of telomere dynamics-targeted agents in preclinical studies and clinical trials, and reveal their promising therapeutic potential in cancer therapy. As shown, telomere dynamics-active agents are effective as anti-cancer chemotherapeutics and immunotherapeutics. Notably, these agents may display efficacy against cancer stem cells, reducing cancer stem levels. Furthermore, these agents can be integrated with the capability of tumor-specific drug delivery by the constitution of related nanoparticles, antibody drug conjugates and HSA-based drugs.
Keywords: telomere dynamics, telomerase, alternative lengthening of telomeres (ALT), cancer therapy
Graphical Abstract
Introduction
Telomere is a protective structure located at the end of the linear chromosome of eukaryotes, which is made up of tandem repetitive DNA sequences and related proteins. It is essential for maintaining the integrity and stability of the genome. A cancer cell, originated from the aggregates of abnormal mutated cells, is characterized by uncontrollably infinite proliferation, infiltrating into surrounding tissues, further metastasizing to distant organs and ultimately leading to the death of host organisms.1 Remarkable advances in the field of telomere biology have revealed the paramount importance of telomere in cancer progression. However, slightly different from the general hypothesis, several analyses focused on the correlation of telomere lengths and cancer incidence reported that both longer and shorter telomeres were correlated with cancer development.2 A systematic analysis of telomere lengths of 18,430 samples covering tumor and normal tissues from 31 types of cancers revealed that 70% of samples displayed shortening telomere lengths in comparison with matched normal tissues and 30% showed elongating telomere lengths. In detail, elongated telomeres existed in testicular germ cell tumors, low grade glioma and sarcoma, whereas cervical cancer, endometrial cancer, uveal melanoma, lymphoma, kidney papillary and kidney chromophobe carcinoma showed shortened telomeres.3 With the participation of telomerase and alternative lengthening of telomeres (ALT) pathway, longer telomeres indicate more replicative times and provide favorable opportunity for the unlimited proliferation of cancer cells. Meanwhile, shortened telomeres may cause the telomere crisis and genomic instability, which also facilitate tumor progression.4,5 Thus, instead of merely affecting telomere lengths, seeking versatile mechanisms which refer to targeting telomere dynamics may achieve satisfactory results in cancer therapy. The emerging multifaceted strategy as chemotherapeutic, immunotherapeutic and nanomedicine, includes activating anti-telomerase immune responses, shortening telomere lengths, inducing telomere dysfunction and constituting telomerase-responsive drug release system.
In this review, the correlation of telomere dynamics and cancer is illustrated, and current anticancer therapeutics that interfere in telomere dynamics are summarized in detail. Meanwhile, the advantages and disadvantages of those therapeutics and their future directions are also critically discussed to fully exploit their potential in cancer therapy.
Telomere Dynamics as Drug Target
“Telomere” was originally named by Hermann Muller in 1938, based on the Greek words for “end” (telos) and “part” (meros). He discovered that the free end of chromosomes presented a cap-like structure which made it resistant to X-rays.6 The human telomere was first sequenced as tandem 5’-TTAGGG-3’ repeats in 19887 and the same repeated 5’-TTAGGG-3’ sequence exists among 91 vertebrate species.8 As a capping structure at the chromosome termini, telomere consists of tandem repeated 5’-(TTAGGG)n-3’ double stranded DNA sequence (15–20 kb in human telomere) with a terminus of G-rich single stranded 3’-overhang (50–200 nucleotide) and a multiprotein complex that binds to the telomere.9 The 3’-overhang invades and folds back onto the telomeric dsDNA region to form a stable telomeric loop (T-loop) structure with a single-stranded displacement D-loop at the invasion site.10 The multiprotein complex, called shelterin or telosome, is made up of six proteins viz. telomeric repeat factor 1/2 (TRF1/2), TERF1-interacting nuclear factor 2 (TIN2), repressor activator protein 1 (RAP1), protection of telomere 1 (POT1) and tripeptidyl peptidase 1 (TPP1).11 As known, TRF1 and TRF2 bind directly to the double-stranded region of the telomere as homodimers, and subsequently RAP1 is localized to the telomeric DNA duplex by binding to TRF2. In addition, TPP1 forms a heterodimer with POT1, which binds specifically to the single stranded G-rich telomere sequence via POT1. Furthermore, TIN2 binds to TRF1/2 in the telomere double-stranded region and TPP1 in the single-stranded region to stabilize the entire shelterin complex. The shelterin plays an important role in avoiding telomere from being recognized as DNA damage sites, recruiting telomerase to telomere ends, maintaining telomere length, promoting T-loop formation and stabilizing telomere structures.12,13
Telomere becomes shorter by 50–150 bp during each cell division. In normal somatic cells, the ever-worsening telomere erosion ultimately elicits telomere crisis, which is characterized by replicative senescence, genome instability and cell death.14 Therein, scarce cells that accumulate oncogenic mutations can survive in the telomere crisis and become immortalized with malignant phenotypes via the activation of telomere maintaining mechanisms.15 In short, telomere possesses vital biological functions in sustaining the genomic stability; those include protecting chromosome terminus from nuclease degradation or DNA damage repair, ensuring complete end-replication in linear chromosomes, and thus limiting the number of cell divisions and preventing cell canceration.16
Telomere dynamics refer to the shortening and lengthening of telomeres during the process of cell growth. Most human cells display progressive telomere shortening with each division and eventually walk to inevitable senescence and even cell death. Whereas, telomere lengths in embryonic stem cells and germ cells do not shorten obviously due to their strong telomerase activity, ensuring a longer lifespan for these cells. Telomerase activity was first identified in tetrahymena extracts in 1985, which was called telomere terminal transferase at that time.17 Cancer cells featured by infinite proliferation and immortalization counteract the telomere attritions with the assistance of telomerase. As reported, telomerase could be reactivated or upregulated in the majority of cancers (85–90%).18 Moreover, the telomerase negative cancers (10–15%) could employ alternative lengthening telomeres (ALT) mechanisms to elongate the telomeres.19
Telomerase is a ribonucleoprotein complex with reverse transcriptase efficacy, which is composed of a catalytic subunit (telomere reverse transcriptase, TERT, encoded by the hTERT gene located at the human chromosome 5p15.33), an RNA template (telomerase RNA component, TERC, originated from the hTERC gene positioned at the human chromosome 3q26) and a number of accessory protein subunits with regulatory functions. Those accessory protein subunits include dyskerin, TCAB1, NHP2, NOP10, pontin and reptin. Telomerase can use its own RNA as a template to add telomeric DNA repeated sequence to the 3’ end of chromosomes for maintaining the relative stability of telomere length.20,21 Therein, TERT and TERC are core components of the human telomerase holoenzyme, whereas the accessory proteins participate in regulating the assembly and localization of telomerase in vivo.22 Besides maintaining the lengths of telomeres, telomerase also plays a pivotal role in some non-telomeric actions such as promoting the inflammation and immunosuppressive tumor microenvironment through the NF-κB and cGAS-STING pathway,23,24 accelerating tumor angiogenesis with activation of VEGF,25 maintaining the stemness and multipotency of cancer stem cells through upregulating expressions of Oct3/4, NANOG, Sox-2 and LGR5,26 and preventing cells from oxidative stress and DNA damage through the decrease of mitochondrial ROS production.27
The alternative lengthening of telomeres (ALT) mechanism elongates the attrited telomeres based on the DNA homologous recombination (HR) pathways.28 ALT is characterized by highly heterogeneous telomere lengths, frequent exchanges between telomeres and sister chromatids, existence of ALT associated promyelocytic leukemia bodies (APBs) and extra-chromosomal repeated telomeric DNA circles.29 ALT is highly prevalent in cancers derived from mesenchymal tissues, viz neuroendocrine system, soft tissues, and peripheral and central nervous systems.30
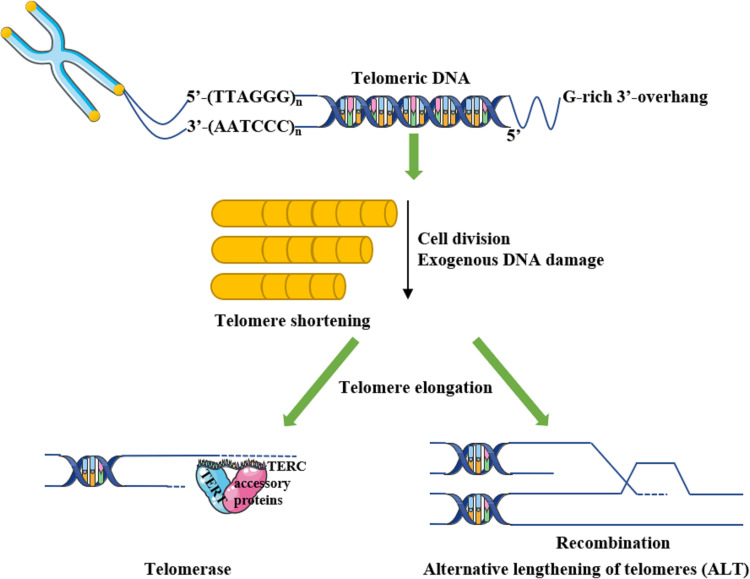
To sum up, as shown in Figure 1, telomerase and ALT are jointly involved in modulating telomere dynamics. However, the interaction between two pathways demands further investigations, on account of the phenomenon that anti-telomerase therapies usually facilitate the generation of ALT.31
Figure 1.
Telomere and telomere dynamics. Telomere, a capping structure at the chromosome termini, consists of tandem repeated 5’-(TTAGGG)n-3’ double stranded DNA sequence (15–20 kb in human telomere) with a terminus of G-rich single stranded 3’-overhang (50–200 nucleotides) and a multiprotein complex that binds to the telomere. Telomere dynamics refer to the shortening and lengthening of telomeres. Specifically, telomeres are shortened during cell division or exogeneous DNA damage, meanwhile elongated with telomerase action or alternative lengthening telomeres (ALT) mechanisms.
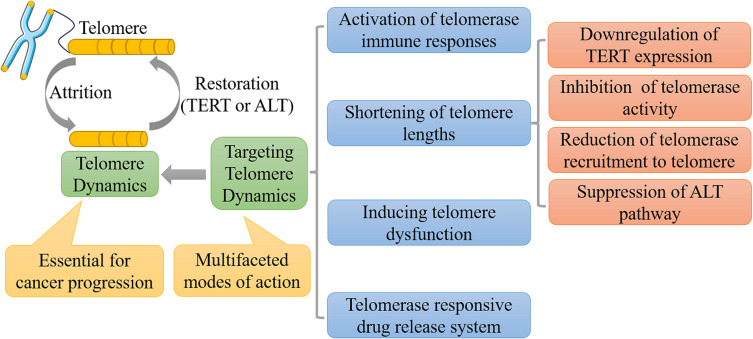
Based on the process of telomere dynamics in cancer cells mentioned above, targeting telomere dynamics has emerged as a promising approach for cancer therapy. As shown in Figure 2, targeting telomere dynamics mainly involves the inhibition of telomerase activity, downregulation of telomerase expression, shortening of telomere lengths, activation of anti-telomerase immune responses, induction of telomere dysfunction, and suppression of the alternative lengthening of telomeres (ALT); as well as the constitution of telomerase-responsive drug release system. Those are elaborated in detail in the following sections on active agents in clinical trials and in preclinical studies.
Figure 2.
Anti-cancer therapeutics targeting telomere dynamics. Considering the pivotal role telomere plays in cancer progression, targeting telomere dynamics may hold great potential in cancer therapy, which covers those of activating anti-telomerase immune responses, shortening telomere lengths, inducing telomere dysfunction and constituting telomerase-responsive drug release system.
Active Agents in Clinical Trials
Currently, telomere has emerged as a promising target in cancer therapy and some telomere-targeted therapeutics have been assessed in the clinical trials for various types of cancers since 2003, as shown in Table 1. Therein, the telomere-targeted drugs approved in the clinical trials mainly fall into two categories: chemotherapeutic and immunotherapeutic agents. Chemotherapeutics are further divided into telomerase inhibitors such as imetelstat.32–38 and KML-001,39 and telomere dysfunction inducers such as nucleoside analogue 6-thio-2’-deoxyguanosine.40 Immunotherapeutics primarily include telomerase-specific oncolytic adenoviruses, such as OBP-30141–44 and KH901;45 hTERT peptide vaccines, such as GV1001,46–56 UCPVax,57–61 VX-001,62–65 UV166–69 and GX301,70 hTERT540–548 peptide,71–73 hTERT572Y peptide74,75 and hTERT and survivin multi-peptide;76,77 hTERT DNA vaccines such as INVAC-1,78 V934/V93579 and INO-1400/1401/5401;80–82 hTERT mRNA vaccines;83 dendritic cell vaccines, such as hTERT RNA transfected DCs84–86 and hTERT peptide pulsed DCs;87–92 as well as transgenic lymphocyte immunization vaccines against telomerase.93,94
Table 1.
Ongoing/Completed Clinical Trials of Telomere-Related Anti-Cancer Therapeutics
| Identifier Code | Intervention/Treatment | Approved Application/Indication | Study Start Year | Phase | Ref | |
|---|---|---|---|---|---|---|
| 1 | NA | Imetelstat | Children with refractory or recurrent solid tumors | 2013 | I | [32] |
| 2 | NA | Imetelstat | Children with recurrent or refractory central nervous system malignancies | 2017 | II | [33] |
| 3 | NCT01273090 | Imetelstat sodium | Children with refractory or recurrent solid tumors or lymphoma | 2011 | I | [34] |
| 4 | NCT01265927 | GRN163L and Trastuzumab | HER2+ breast cancer | 2010 | I | [35] |
| 5 | NCT01137968 | Imetelstat and bevacizumab | Advanced non-small cell lung cancer | 2010 | II | [36] |
| 6 | NCT01256762 | Imetelstat; paclitaxel with or without bevacizumab | Locally recurrent or metastatic breast cancer | 2010 | II | [37] |
| 7 | NCT01242930 | Imetelstat with or without lenalidomide | Multiple myeloma | 2010 | II | [38] |
| 8 | NCT01110226 | KML-001 (sodium metaarsenite) and cisplatin | Advanced solid tumors | 2010 | I | [39] |
| 9 | NCT05208944 | THIO (6-Thio-2’-Deoxyguanosine); Cemiplimab | Advanced non-small cell lung cancer | 2022 | II | [40] |
| 10 | NCT04391049 | OBP-301 (telomerase-specific type 5 adenovirus); carboplatin; paclitaxel and radiation therapy | Locally advanced esophageal and gastroesophageal cancer | 2020 | I | [41] |
| 11 | NCT03190824 | OBP-301 (telomerase specific replication-competent oncolytic adenovirus) | Unresectable metastatic melanoma | 2016 | II | [42] |
| 12 | NCT03213054 | OBP-301 (telomelysin) with radiotherapy | Esophageal cancer | 2017 | I | [43] |
| 13 | NA | Telomelysin (hTERT promoter driven modified oncolytic adenovirus) | Solid tumors | 2010 | I | [44] |
| 14 | NA | KH901(oncolytic adenovirus, replicates in and lyses telomerase-positive tumor cells and expresses GM-CSF) | Head and neck cancers | 2009 | I | [45] |
| 15 | NCT01579188 | GV1001 (after GM-CSF) | Inoperable stage III non-small cell lung cancer | 2012 | III | [46] |
| 16 | NCT00425360 | GV1001; sargramostim; capecitabine and gemcitabine hydrochloride | Locally advanced or metastatic pancreatic cancer | 2007 | III | [47] |
| 17 | NCT00509457 | GV1001 (after radiotherapy and docetaxel) | Locally advanced non-small cell lung cancer | 2007 | II | [48] |
| 18 | NCT00444782 | GV1001; cyclophosphamide and GM-CSF | Advanced hepatocellular carcinoma | 2007 | II | [49] |
| 19 | NA | GV1001 (hTERT: 611–626); HR2822 (hTERT: 540–548) and GM-CSF | Non-small cell lung cancer | 2006 | I/II | [50] |
| 20 | NA | GV1001 and GM-CSF | Inoperable pancreatic cancer | 2006 | I/II | [51] |
| 21 | NCT01247623 | GV1001 and temozolomide | Advanced malignant melanoma | 2010 | I/II | [52] |
| 22 | NA | Temozolomide and GV1001 | Stage IV melanoma | 2011 | I/II | [53] |
| 23 | NA | GV1001 (hTERT: 611–626); p540 (hTERT: 540–548) and GM-CSF or tuberculin | Cutaneous melanoma | 2011 | I | [54] |
| 24 | CTN-2000; CTN-2006 | GV1001 (after radiotherapy and docetaxel) | Non–small cell lung cancer | 2011 | I/II | [55] |
| 25 | ISRCTN4382138 | GV1001 (after gemcitabine and capecitabine) | Advanced pancreatic ductal adenocarcinoma | 2015 | III | [56] |
| 26 | NCT02818426 | UCPVax | Metastatic non-small cell lung cancer | 2016 | I/II | [57] |
| 27 | NCT05528952 | UCPVax (emulsified in montanide ISA-51); atezolizumab and bevacizumab | Unresectable hepatocellular carcinoma | 2022 | II | [58] |
| 28 | NCT04280848 | UCPVax; radiotherapy and temozolomide | Glioblastoma | 2020 | II | [59] |
| 29 | NCT03946358 | UCPVax and atezolizumab | Human papilloma virus positive cancers | 2019 | II | [60] |
| 30 | NCT04263051 | UCPVax and nivolumab | Advanced non-small cell lung cancer | 2020 | II | [61] |
| 31 | NCT01935154 | VX-001 | Stage IV non-small cell lung cancer | 2013 | II | [62] |
| 32 | NA | Vx-001 | Advanced solid tumors | 2012 | II | [63] |
| 33 | NA | Vx-001 (Montanide ISA51) | Advanced non-small cell lung cancer | 2013 | II | [64] |
| 34 | NA | Vx-001 | Advanced non-small cell lung cancer | 2014 | II | [65] |
| 35 | NCT04300244 | Nivolumab and ipilimumab with or without UV1 | Inoperable malignant pleural mesothelioma after first-line platinum-based chemotherapy | 2020 | II | [66] |
| 36 | NCT01789099 | UV1 and GM-CSF (leukine) | Non-small cell lung cancer | 2013 | I/II | [67] |
| 37 | NCT02275416 | UV1; GM-CSF and ipilimumab | Unresectable or metastatic malignant melanoma | 2014 | I/II | [68] |
| 38 | NCT01784913 | UV1 and GM-CSF | Men with metastatic hormone-naive prostate cancer | 2013 | I/II | [69] |
| 39 | NCT02293707 | GX301 (emulsified in montanide ISA-51 and imiquimod 5% cream) | Castration-resistant prostate cancer | 2014 | II | [70] |
| 40 | NCT00079157 | Telomerase: 540–548 peptide vaccine emulsified in montanide ISA-51 and sargramostim (GM-CSF) | Stage IV breast cancer | 2004 | I | [71] |
| 41 | NCT00021164 | Telomerase: 540–548 peptide vaccine (emulsified in montanide ISA-51) and aldesleukin | Metastatic cancer | 2003 | II | [72] |
| 42 | NCT00069940 | Telomerase: 540–548 peptide vaccine and sargramostim (GM-CSF) | Sarcoma or brain tumor | 2003 | I | [73] |
| 43 | NA | TERT572Y peptide vaccine (emulsified in montanide ISA51) | Advanced cancer | 2006 | I | [74] |
| 44 | NA | TERT572Y peptide vaccine | Advanced non-small cell lung cancer | 2007 | I | [75] |
| 45 | NCT00573495 | hTERT/survivin multi-peptide vaccine; daclizumab and prevnar | Metastatic breast cancer | 2007 | I | [76] |
| 46 | NCT00499577 | TERT and surviving multi-peptide vaccine | Myeloma | 2011 | I/II | [77] |
| 47 | NCT04515043 | INVAC-1 | Solid tumor | 2020 | I | [78] |
| 48 | NCT00753415 | V934/V935 hTERT DNA vaccines | Solid tumors | 2008 | I | [79] |
| 49 | NCT02960594 | INO-1400 or INO-1401 (hTERT) with or without INO-9012 (IL-12 DNA) delivered by electroporation | Solid tumors | 2016 | I | [80] |
| 50 | NCT03502785 | INO-5401 (WT1, PSMA and hTERT); INO-9012 (IL-12) and atezolizumab | Locally advanced unresectable or metastatic/recurrent urothelial carcinoma | 2018 | I/II | [81] |
| 51 | NCT03491683 | INO-5401; INO-9012; cemiplimab (REGN2810); radiation and chemotherapy (temozolomide) | Newly-diagnosed glioblastoma | 2018 | I/II | [82] |
| 52 | NA | mRNA (MUC1, CEA, Her-2, telomerase, surviving and MAGE-A1) and GM-CSF | Stage IV renal cell cancer | 2011 | I/II | [83] |
| 53 | NCT00510133 | GRNVAC1 | Acute myelogenous leukemia | 2007 | II | [84] |
| 54 | NCT01153113 | hTERT mRNA DCs | Metastatic prostate cancer | 2010 | I/II | [85] |
| 55 | NA | Tumor RNA-transfected dendritic cells | Renal cancer | 2003 | I | [86] |
| 56 | NCT01410968 | Poly-ICLC and peptide-pulsed dendritic cells | Metastatic, locally advanced, unresectable, or recurrent pancreatic adenocarcinoma | 2011 | I | [87] |
| 57 | NCT00197912 | p53, survivin and telomerase peptide-pulsed dendritic cells | Advanced melanoma | 2005 | I/II | [88] |
| 58 | NCT00197860 | Survivin and telomerase peptides or tumor lysate DCs and low-dose IL-2 | Advanced renal cell carcinoma | 2005 | I/II | [89] |
| 59 | NA | Survivin and telomerase peptide-pulsed dendritic cells and low-dose IL-2 | Metastatic renal cell carcinoma (mRCC) | 2009 | I/II | [90] |
| 60 | NA | P53, survivin and telomerase peptide-pulsed dendritic cells; interleukin (IL)-2 and interferon (IFN)-α2b | Malignant melanoma | 2010 | I/II | [91] |
| 61 | NA | hTERT I540 peptide and keyhole limpet hemocyanin (KLH) autologous DCs | Prostate or breast cancer | 2005 | I | [92] |
| 62 | NCT00061035 | Transgenic lymphocyte immunization vaccine | Prostate adenocarcinoma | 2003 | I | [93] |
| 63 | NCT00925314 | CB-10-01 (transgenic lymphocyte immunization) | Stage III Melanoma | 2009 | II | [94] |
Abbreviation: NA, not available.
Several clinical trials indicated that imetelstat could inhibit telomerase activity in peripheral blood mononuclear cells (PBMCs)32,33 and tumor cells,33 but caused severe side effects in children with recurrent central nervous system (CNS) tumors.33 Meanwhile, imetelstat, as a maintenance therapy with platinum-based chemotherapy, displayed an improvement in overall survival (OS) and median progression-free survival (PFS) in non-small cell lung cancer (NSCLC) patients with short telomere length.36 Similarly, the clinical trial of KML-001 (sodium metaarsenite, a telomerase inhibitor) also demonstrated the potential of combination of KML-001 and platinum agents.39
Clinical trials have shown that OBP-301 (telomelysin, a telomerase specific replication-competent oncolytic adenovirus) possessed favorable antitumor efficacy and tolerated toxicity against various solid tumors,44 and esophageal cancer in combination with radiotherapy.43 Analogously, KH901, an oncolytic adenovirus conditionally replicating in telomerase-positive tumor cells and expressing granulocyte macrophage colony-stimulating factor (GM-CSF), provided clinical benefits in patients with recurrent head and neck cancer, and revealed the possibility of combination with chemotherapy.45
Immune vaccines that have been developed as telomerase targeting agents principally cover hTERT peptide, hTERT DNA, hTERT mRNA, dendritic cell and transgenic lymphocyte immunization vaccines. To speak of peptide vaccines, several clinical trials have examined the safety and efficacy of the combination of GV1001 with radiotherapy or chemotherapy such as gemcitabine, cyclophosphamide, temozolomide, tuberculin and docetaxel.51–61 The results showed that the combinations were well tolerated in patients with non-small cell lung cancer,55–60 inoperable pancreatic cancer56 and melanoma;58,59 and induced GV1001 specific immune responses, which further led to the tumor responses manifested in prolonged survival. Similarly, Vx-001 or UV1 (combination with/without ipilimumab) also developed immunological responses and long-term clinical outcomes without severe side effects in advanced non-small cell lung cancer,67,70,72 unresectable or metastatic malignant melanoma,73 metastatic hormone-naive prostate cancer74 and other solid tumors.68
Referring to DNA and mRNA vaccines, the delivery of hTERT-encoded DNA plasmids with or without interleukin-12 plasmid via intramuscular electroporation could display favorable safety and efficacy in patients with pancreatic cancer, in association with positive immune responses represented by the increased production of hTERT-specific IFN-γ and activation of hTERT-specific T cells.86 Analogously, intradermal injection of naked mRNA coding for tumor-associated antigens such as telomerase, with GM-CSF as adjuvant, could perform well in renal cell cancer patients, with median survival of 24–26 months.89
As to DC vaccines, several clinical trials have demonstrated that the vaccinations with transfected telomerase RNA or the telomerase peptide pulsed DC could be well tolerated and exert beneficial clinical effects in patients with renal cancer,89,90,92 pancreatic adenocarcinoma,93 malignant melanoma91 and prostate or breast cancer.92
Active Agents in Preclinical Studies
In addition to the above clinical trials of telomere-targeted therapeutics, a variety of agents that modulate telomere dynamics have been in preclinical investigations, striving to obtain satisfactory achievements in cancer therapy, as shown in Table 2. As depicted in Figure 2, anti-cancer drugs currently under investigation that target telomere dynamics primarily cover those of activating anti-telomerase immune responses, shortening telomere lengths, inducing telomere dysfunction and constituting telomerase-responsive drug-release system.
Table 2.
Preclinical Researches of Telomere-Related Anti-Cancer Therapeutics
| Drug | Tumor Models | Descriptions | Year | Ref | |
|---|---|---|---|---|---|
| 1 | GV1001; GEM; GM-CSF | Patients with pancreatic adenocarcinomas | Inducing telomerase specific immune responses | 2014 | [95] |
| 2 | GV1001 | MCF7 (human breast adenocarcinoma cell line), Jurkat (human T-cell leukemia cell line), MC38 (murine colon adenocarcinoma) and HeLa (human cervical adenocarcinoma) cells | Reducing HSP70/90, HIF-1α and VEGF | 2014 | [96] |
| 3 | GV1001; GEM | Pancreatic ductal adenocarcinomas (PDACs) | Reducing fibrosis with decreasing TNF-α, interleukin (IL)-6 and IL-1β | 2016 | [97] |
| 4 | GV1001 | Prostate cancer | Binding and antagonizing GnRHR through activation of Gαs/cAMP pathway and downregulating releasing of Gαq-coupled Ca2+ | 2019 | [98] |
| 5 | hTERT peptides | B16F10 melanoma | Inducing high avidity CD4+ TH1 cells, activating dendritic cells, enhancing primary and memory CTL responses | 2012 | [99] |
| 6 | hTERT peptides | Hepatocellular carcinoma | Positive T cell responses | 2018 | [100] |
| 7 | rAAV-/rAdv viral cocktail expressing hTERTC27 | Melanoma | Activation of NK cells | 2010 | [101] |
| 8 | hTERT with a lentiviral vector system | Melanoma | CD8+ T cell responses | 2010 | [102] |
| 9 | hTERT and HER-2/neu multipeptides | Various cancers | Stimulating specific CTLs | 2002 | [103] |
| 10 | TERT and HCV multipeptides; taxanes and alkylating agents | Hepatocellular carcinoma | Enhancing specific T cell responses and reducing Treg frequency | 2015 | [104] |
| 11 | TERT and HCV DNA | Liver cancer | Inducing multi-cytokine response of CD4+ and CD8+ T cells | 2021 | [105] |
| 12 | CCL21-TERT-Fc DNA; anti-4-1BB mAbs | Various cancers | Enhancing the immune responses of NK, CD4+ and CD8+ T cell | 2006 | [106] |
| 13 | TERT DNA and α-CTLA-4/α-PD-1 | TC-1 tumor | Improving antigen-specific immune responses | 2018 | [107] |
| 14 | TERT DNA and CCL21 | Breast cancer | Augmenting antigen-specific immunity | 2007 | [108] |
| 15 | lenti-hTERT vector- transduced DC vaccines | Hepatocellular carcinoma | Stimulating CTLs | 2011 | [109] |
| 16 | rAd-hTERT transduced DCs | Various tumors | Inducing CTLs and increasing IFN-γ | 2006 | [110] |
| 17 | Lipid-mediated transfection of hTERT DNA into DCs | Various tumors | Inducing specific T-cell-mediated tumor immunity | 2003 | [111] |
| 18 | TERT mRNA transfected DCs | Melanoma | Inducing IFN-γ secreting CTLs | 2007 | [112] |
| 19 | hTERT and survivin mRNA, IDO siRNA transfected DCs | Ovarian cancer | Inducing antitumor immunity. | 2013 | [113] |
| 20 | hTERT TCR | Melanoma | Redirecting CD4+ and CD8+ T cells and promoting inflammatory cytokines | 2021 | [114] |
| 21 | TERT expressed with Mannan-modified adenovirus | Melanoma | Inducing antitumor immunity | 2007 | [115] |
| 22 | TERT and VEGFR-2 expressed with mannan-modified adenovirus | Breast and colon cancer | Displaying synergistic antitumor immune responses and reducing angiogenesis | 2015 | [116] |
| 23 | 5-aza-2′-deoxycytidine | Chronic myelogenous leukemia | Decreasing hTERT expression through reducing the binding of c-myc with hTERT promoter | 2014 | [117] |
| 24 | Perylene derivates PM2 and PIPER | Lung cancer | Downregulation of hTERT through inducing G-quadruplex formation in telomere and hTERT promoter regions | 2013 | [118] |
| 25 | A retrovirus vector with full-length hTERT antisense complementary DNA | Ovarian cancer | Decreasing hTERT expression and telomerase activity | 2016 | [119] |
| 26 | ZD55-hTERT (an oncolytic adenovirus-based shRNA delivery system) | Renal cancer | Silencing hTERT expression | 2009 | [120] |
| 27 | PEG-CMCS/CaP nanoparticles loaded with hTERT siRNA | Hepatocellular carcinoma | Silencing hTERT expression | 2014 | [121] |
| 28 | Chelidonine | Breast cancer | Inhibiting telomerase activity | 2018 | [122] |
| 29 | Rhodospirillum rubrum L-asparaginase mutant RrA | Various cancers | Reducing telomerase activity | 2017 | [123] |
| 30 | Suramin | Various cancers | Inhibiting telomerase activity | 2015 | [124] |
| 31 | 2’-O-methyl-RNA delivered with chitosan-coated polylactide-coglycolide (PLGA) nanoparticles | Lung cancer | Inhibiting telomerase activity | 2010 | [125] |
| 32 | Gold nanoparticles (AuNPs) functionalized with 111In-labelled oligonucleotides | Various cancers | Inhibiting telomerase activity | 2021 | [126] |
| 33 | BIBR11532 | Cervical cancer | Evoking TEL mutation and reducing the recruitment of telomerase to telomere | 2013 | [127] |
| 34 | TPP1-OB domain overexpression with lentivirus transduction | Lung cancer | Inhibiting the recruitment of telomerase to telomeres and enhancing the sensitivity to paclitaxel | 2019 | [128] |
| 35 | Cisplatin derivate Tetra-Pt | Various cancers | Inhibiting ALT pathway | 2017 | [129] |
| 36 | Withaferin-A | Various cancers | Blocking ALT pathway | 2017 | [130] |
| 37 | TMPyP4 | Osteosarcoma | Blocking ALT pathway and inducing formation of telomere and FAK G-quadruplex | 2021 | [131] |
| 38 | 6-thio-2’-deoxyguanosine (6-thio-dG) | Lung cancer | Inducing telomere dysfunction | 2015 | [132] |
| 39 | 6-thio-dG | Melanoma | Inducing telomere dysfunction and overcoming therapy resistance | 2018 | [133] |
| 40 | Tolyl terpyridin-Pt complex (Pt-ttpy) | Ovarian carcinoma | Inducing telomere dysfunction through binding to G-quadruplex (G4) structures | 2021 | [134] |
| 41 | Terpyridine platinum (Pt-tpy) | Fibrosarcoma | Inducing telomere dysfunction | 2021 | [135] |
| 42 | RuII-PtII complexes encapsulated with biotin-functionalized DNA cages | Various cancers | Inducing telomere dysfunction through stabilizing G-quadruplex | 2022 | [136] |
| 43 | BRACO-19 | Glioblastoma | Inducing telomere dysfunction through binding to G-quadruplex | 2016 | [137] |
| 44 | Telomestatin | Glioblastoma | Inducing telomere dysfunction through stabilizing G-quadruplex | 2016 | [138] |
| 45 | Schizocommunin derivative | Various cancers | Inducing telomere dysfunction through formation of G-quadruplex | 2018 | [139] |
| 46 | Pyridostatin analogues | Various cancers | Inducing telomere dysfunction through stabilizing G-quadruplex | 2012 | [140] |
| 47 | Lpid nanoparticles (LNPs) containing miR-182-3p | Breast cancer | Inducing telomere dysfunction | 2023 | [141] |
| 48 | 5-azacytidine (5-AZA) | Acute myeloid leukemia | Concomitantly inducing telomere dysfunction and telomere length shortening | 2015 | [142] |
| 49 | Bortezomib | Leukemic and gastric cancer | Concomitantly inducing telomere dysfunction and telomere length shortening | 2015 | [143] |
| 50 | MST-312 | Breast cancer | Concomitantly inducing telomere dysfunction and telomere length shortening | 2014 | [144] |
| 51 | Au@Ag nanorods loaded with DOX | Cervical cancer | Releasing DOX to telomerase positive cells | 2014 | [145] |
| 52 | MSNP-NH2-DOX-DNA system | Various cancers | Slow and sustained releasing of DOX to telomerase positive cells | 2018 | [146] |
| 53 | dCas9-MSNs/DOX/DNA | Cervical cancer | Releasing DOX into the nuclei of telomerase positive cells | 2021 | [147] |
| 54 | PtNPs@DNA | Gastric cancer | Releasing PtNPs to telomerase positive cells and overcoming drug resistance | 2018 | [148] |
| 55 | AS1411/nanotube/RTA | Various cancers | Releasing RTA to telomerase positive cells | 2021 | [149] |
| 56 | Self-assembled DNA polymer | Various cancers | Detecting and in situ monitoring telomerase activity of cancer cells | 2018 | [150] |
| 57 | Dox-AuNP-MB | Various cancers | Detecting the intracellular telomerase activity and releasing DOX to telomerase positive cells | 2016 | [151] |
| 58 | Au-MPs-DOX | Various cancers | Detecting the intracellular telomerase activity and releasing DOX to telomerase positive cells | 2017 | [152] |
Activation of Anti-Telomerase Immune Responses
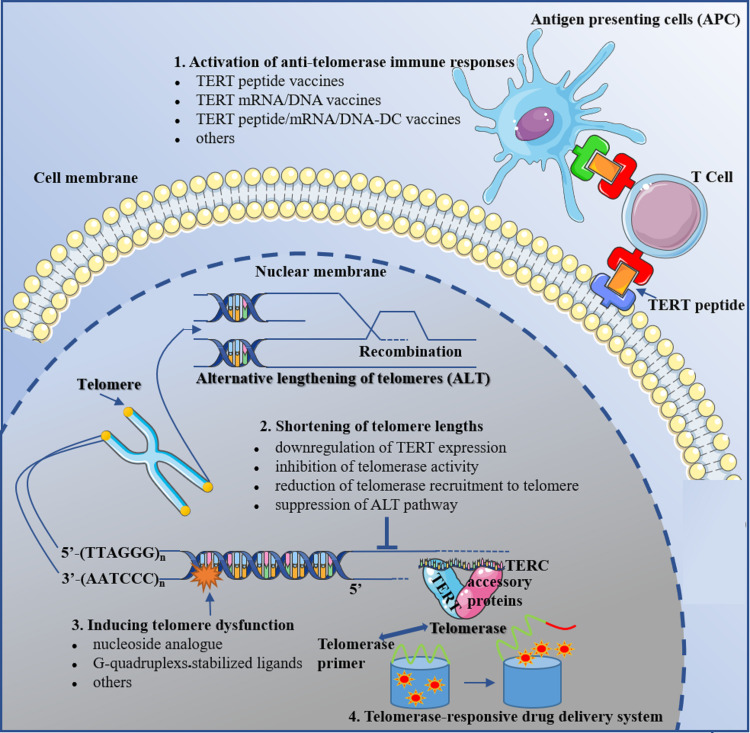
As the majority of telomere dynamics targeted anticancer therapeutics applied in the clinical trial, anti-telomerase immunotherapeutics such as OBP-301, KH901, GV1001, VX-001 and UV1 have achieved impressive efficacy in various tumors with favorable tolerance as illustrated in the previous section. Currently, the agents that activate the anti-telomerase immune responses could be divided into TERT peptide vaccines, TERT mRNA/DNA vaccines, TERT peptide/mRNA/DNA-DC vaccines and others, according to the diverse types of agents and mechanisms of action.
TERT Peptide Vaccines
As described above, several clinical trials reported that combinations of GV1001 (a 16 amino acid hTERT peptide including positions 611–626, EARPALLTSRLRFIPK) with radiotherapy or chemotherapy such as gemcitabine have exhibited superior safety and efficacy in patients with various tumors. Staff et al discovered that GV1001 in combination with gemcitabine and GM-CSF, caused telomerase-specific immune responses and mild adverse effects in 10/17 patients with pancreatic adenocarcinomas.95 In addition to telomerase-related immune responses, GV1001 displayed potential antitumor efficacy, such as inhibiting angiogenesis via the suppression of HSP90/HSP70/HIF-1α/VEGF pathway,96 reducing fibrosis in pancreatic ductal adenocarcinomas (PDACs) via the decrease of TNF-α, interleukin (IL)-6 and IL-1β,97 and antagonizing the gonadotropin-releasing hormone receptor (GnRHR) agonist via the direct binding to GnRHR and activation of Gαs/cAMP pathway.98 Apart from several hTERT peptides,99,100 delivering hTERT peptide vaccines with recombinant adenovirus and adeno-associated virus101 or a lentiviral vector102 led to stronger and more sustained antitumor immune responses and enhanced immunological tolerance. Moreover, multi-peptide vaccines consisting of hTERT and HER-2/neu103 or hepatitis C virus (HCV)104 also exerted prominent anti-tumor effects in antigen-expressing cancer cells.
TERT mRNA/DNA Vaccines
Jansons et al have elucidated that a DNA vaccine derived from rat TERT contributed to the antitumor responses by inducing the release of IFN-γ/IL-2/TNF-α from CD4+ and CD8+ T cells.105 For the purpose of improving the antigen-specific antitumor immunity of DNA vaccines, bringing in the adjuvants has gained extensive attraction. The DNA vaccine (pCCL21-Te-Fc) was based on plasmids and constructed by linking human CCL21 and IgG Fc to two hTERT fragments. As shown, the vaccine could inhibit tumor growth through the CD8+ T cells mediated antitumor immune responses. Furthermore, the combination of pCCL21-Te-Fc and anti-4-1BB monoclonal antibodies (mAbs) could augment immune-responses and significantly prolong the survival of tumor-bearing mice, with 75% by contrast to 25% of mice surviving longer than 120 days.106 Homoplastically, the TERT DNA vaccines combined with immune checkpoint inhibitors107 or CCL21 chemokine108 could prolong survival, enhance immune responses and restrain tumor growth of cervical cancer and breast cancer.
TERT Peptide/mRNA/DNA-DC Vaccines
Besides the free TERT antigen from TERT peptide and the expressed TERT from mRNA or DNA, the primed DCs could also activate specific anti-telomerase immunity. Cui et al constructed a lentivirus which contains hTERT cDNA fragments and transduced the lenti-hTERT vectors into DCs. The lenti-hTERT vector-transduced DC vaccines dramatically evoked anticancer immunity in telomerase positive HepG2 cells.109 Analogously, DC vaccines that expressed hTERT through the transduction with an hTERT recombinant adenoviral vector110 or the transfection with lipid-mediated hTERT plasmid,111 could arouse antitumor immune reactions by the induction of hTERT specific cytotoxic T lymphocytes and the increased production of IFN-γ in hTERT-positive cancer cells.110 Moreover, DCs transfected with TERT mRNA could markedly restrain the TERT-positive tumor growth and improve the survival of tumor-bearing mice, through the induction of antitumor immunity.112 Furthermore, DC vaccines transfected with indoleamine 2, 3-dioxygenase (IDO) siRNA, hTERT, and survivin mRNA also exerted enhanced antitumor effects.113
Others
With the development of T cell receptor (TCR)-engineered T cell anticancer therapy, Radium-4, an hTERT specific TCR sequence derived from the patient who has been vaccinated with hTERT peptide, could suppress tumor growth in hTERT+ melanoma and prolong survival through directing CD4+ and CD8+ T cells, and secreting pro-inflammatory cytokines.114 Considering that the mannan receptors are expressed on the surface of antigen presenting cells (APCs), mannan has been applied to modify the immune-stimulatory viruses, for the sake of inducing antitumor immune responses through activation of APCs. Ding et al constructed a telomerase-expressing adenovirus which was modified with mannan; evidently, the adenovirus successfully delivered the telomerase antigen to DCs and thus activated the CD4+ and CD8+ T cells based antitumor immunity, and remarkably inhibited tumor growth.115 Furthermore, the combination of mannan-modified adenoviruses expressing TERT and vascular endothelial growth factor receptor-2 (VEGFR-2) displayed notably synergistic antitumor immune responses and reduced intratumoral angiogenesis in murine breast and colon cancer models.116
Shortening of Telomere Lengths
In view of the paramount role telomere lengths play in cancer progression, some telomere dynamics-targeted anticancer therapeutics pursue the shortening of telomere lengths. As illustrated in the following section, those anti-cancer therapeutic agents can be divided into direct shortening telomere lengths and indirect shortening telomere lengths. For the latter, the indirect shortening of telomere length may be induced by various mechanisms, such as the downregulation of TERT expression, inhibition of telomerase activity, reduction of telomerase recruitment to telomere, and suppression of ALT pathway. On account of the difficulty to distinguish whether the telomere length shortening was the consequence of the direct drug action, the following section focused on the illustration of the drugs that indirectly affect the telomere lengths through telomerase or ALT pathway.
Downregulation of TERT Expression
Current studies have demonstrated that several signaling pathway inhibitors or gene therapy technology could achieve the downregulation of TERT expression and thus shortening telomere lengths. Grandjenette et al found that 5-aza-2′-deoxycytidine (DAC, a DNA demethylating agent) decreased the lengths of telomeres through the downregulation of TERT expression which was triggered by reducing the binding of c-myc with hTERT promoter.117 Moreover, the perylene derivates PM2 and PIPER evoked the formation of G-quadruplex in regions of both telomere and hTERT promoter in A549 cells, which thereby decreased the hTERT expression. The hTERT downregulation led to significant telomere shortening and ultimately suppressed the proliferation and tumorigenicity of A549 cancer cells.118 Furthermore, Qi et al has created a recombinant retrovirus vector carrying a full-length hTERT antisense complementary DNA. The vector system could obviously downregulate the expression of hTERT gene and telomerase activity, eventually restraining the ovarian tumor growth and prolonging the mice survival time.119 Similarly, the ZD55-hTERT (an adenovirus-based shRNA delivery system)120 and the PEGylated carboxymethyl chitosan/calcium phosphate (PEG-CMCS/CaP) hybrid anionic nanoparticles loaded with hTERT siRNA121 could markedly silence hTERT and thus inhibit tumor growth.
Inhibition of Telomerase Activity
As elaborated above, the telomerase activity inhibitors imetelstat36 and KML-00139 have showed favorable anti-cancer efficacy in combination with platinum-based chemotherapy in the clinical trials. The therapeutics inhibited the activity of telomerase through binding to the telomerase subunits or stabilizing the G-quadruplex, etc. Noureini et al disclosed that the chelidonine (a benzylisoquinoline alkaloid) could make the telomeres shortened to approximately 30% of that in untreated breast cancer MCF7 cells through inhibiting telomerase activity (with an IC50 value of 0.45 ± 0.08 μM at 48 h exposure time).122 Analogously, the Rhodospirillum rubrum L-asparaginase mutant RrA, could gradually decrease the telomere lengths from 10,105 ± 2530 bp to 1233 ± 636 bp after 35 days of treatment; in association, the telomerase activity reduced to less than 29.63 ± 12.3% of control.123 Besides, suramin could enhance the chemosensitivity of cancer cells via inhibiting telomerase (with IC50 values of 1–3 μM at 24 h); and subsequently, induce gradual telomere shortening (by more than 40% reduction with continuous exposure for 6 weeks).124 Apart from the above-mentioned chemotherapeutics, several nanoparticles are also active in the inhibition of telomerase activity. As reported, utilizing chitosan-coated polylactide-coglycolide (PLGA) nanoparticles to deliver 2’-O-methyl-RNA to human lung cancer cells could suppress telomerase with an inhibition rate of about 80% and shorten telomere lengths from 5.9 kb down to 4 kb.125 Similarly, gold nanoparticles (AuNPs) functionalized with 111In-labelled oligonucleotides and cell-penetrating peptide Tat could enhance the uptake of oligonucleotides and inhibit telomerase activity.126
Reducing the Recruitment of Telomerase to Telomere
The TPP1 protein was essential for the recruitment of telomerase to telomeres through the binding of an N-terminal oligonucleotide/oligosaccharide-binding (OB) domain on TPP1 to telomerase. The TPP1 glutamate (E) and leucine (L)-rich (TEL) patch on the surface of TPP1-OB mediates the interaction between TPP1 and telomerase, and thus achieves the recruitment of telomerase to telomeres, leading to telomere elongation.153 Nakashima et al discovered that BIBR1532 could evoke the mutations of TEL patch, shorten the telomere lengths, and thereby induce apoptosis in HeLa cells.127 Considering the crucial role of TPP1-OB, Zhu et al have generated the TPP1-OB domain-overexpressed lung cancer cells via the lentivirus transduction. As shown, TPP1-OB domain overexpression could competitively disrupt the interaction of endogenous TPP1 and telomerase, thus impeding the localization and binding of telomerase to telomeres. The results showed that TPP1-OB could inhibit both lung cancer cell proliferation in vitro and tumor growth in vivo accompanied with shortened telomere lengths.128
Suppression of ALT Pathway
Independent of telomerase, Zheng et al revealed that the derivate of cisplatin Tetra-Pt (bpy) observably could suppress tumor growth through inhibiting ALT pathway in ALT-activated U2OS cancer cells and shorten telomere lengths with a mean length of 943.16 ± 121.65 TFU in treated cells compared with 1396.09 ± 278.16 TFU in untreated cells. Therein, ALT-positive cells treated with Tetra-Pt (bpy) could present fewer ALT-associated promyelocytic leukemia bodies, telomere sister chromatin exchanges and extrachromosomal C-circles due to the reduction of telomeric homologous recombination.129 Similarly, withaferin-A130 and TMPyP4131 also displayed prominent cytotoxicity towards ALT-activated cancer cells through blocking ALT pathway, manifested as fewer extrachromosomal C-circles and ALT-associated promyelocytic leukemia bodies.
Inducing Telomere Dysfunction
Except for the progressive shortening of telomeres, the induction of telomere dysfunction also offers a novel strategy to suppress cancer progress through affecting telomere dynamics. Telomere dysfunction refers to a process of telomere-correlated DNA damages, manifested as chromosomal instability leaded by double-stranded breaks (DSBs) located at telomeres. In the evaluation of drug activity, immune-FISH (fluorescence in situ hybridization) assay was usually utilized to visualize the telomere dysfunction marker (telomere induced DNA damage focis, TIFs or telomere associated DNA damage response focis, TAFs), which was the colocalization of telomeres and DNA damage response proteins such as γH2AX, ATM and 53BP1.154 Currently, anti-cancer chemotherapeutics that induce telomere dysfunction mainly include nucleoside analogues, G-quadruplex ligands and others.
Ilgen et al discovered that 6-thio-2’-deoxyguanosine (6-thio-dG), a nucleoside analogue originated from the approved drug 6-thioguanine, acting as the substrate of telomerase, could be inserted into the newly synthesized telomere sequence, resulting in the disruption and dysfunction of telomeres. As shown, 6-thio-dG could inhibit the proliferation, induce the generation of TIFs and shorten telomeres in various telomerase-positive cancer cells.132 Moreover, 6-thio-dG exhibited ascendant efficacy in impairing cell viability, and inducing telomere dysfunction in telomerase-positive cancer cells and BRAF-mutated or therapy-resistant melanoma cells.133 As elaborated above, 6-thio-dG was applied in a Phase II clinical trial to examine the efficacy and safety of its combination with cemiplimab (a PD-1 inhibitor) on non-small cell lung cancer.40
Telomeres might form four stranded structures named G-quadruplexes (G4s) with the G-rich repeated sequences. The G4s could hinder the recognition and interaction of telomerase and telomere. The ligands of G4s could stabilize the structure of G4s and thus lead to genomic instability.155 Ali et al reported that the tolyl terpyridin-Pt complex (Pt-ttpy) irreversibly bound to G4s with metal coordination features, and thereby brought about the displacement of TRF2 from telomere and telomeric DNA damage and telomere length shortening.134 Similarly, terpyridine platinum (Pt-tpy) and its derivatives increased TIFs and led to chromosomal instability by triggering the formation of micronucleus and chromatin bridges in late mitosis.135 Based on the above-mentioned studies of Pt complex, a chiral RuII-PtII complexes encapsulated with biotin-functionalized DNA cages could enhance the sub-cellular localization and cancer selectivity, meanwhile retaining the activity of G-quadruplex stabilizing.136 Zhou et al demonstrated that BRACO-19 could induce telomeric DNA damage, telomere uncapping and T-loop disassembly characterized by the dissociation of TRF2 and POT1 from telomeres, and simultaneously restrain telomerase activity in human glioblastoma cells.137 Besides, telomestatin, an antibiotic isolated from Streptomyces anulatus 3533-SV4, could stabilize the G4s and contribute to telomere dysfunction and delocalization of TRF2 from telomeres, ultimately inhibiting the growth of glioma stem cells (GSCs) both in vitroandin vivo.138 Furthermore, the novel schizocommunin derivative139 and pyridostatin analogues140 could also cause telomere dysfunction and finally restrain tumor growth through stabilizing G4s.
Besides disrupting the structure of telomeres, impacting on the expression of telomere complex could also lead to telomere dysfunction. Dinami et al discovered that miR-182-3p delivered with lipid nanoparticles (LNPs) could suppress breast cancer through reducing TRF2 expression and inducing DNA damage at telomeric sites. Moreover, the LNPs could cross the blood-brain barrier and reduce the metastatic tumor lesions in the brain.141 Several chemotherapeutics could concomitantly induce telomere dysfunction and telomere length shortening. Zhang et al found that 5-azacytidine (5-AZA, a DNA methyltransferase inhibitor) could trigger telomere dysfunction with the increased number of 53-BP1 and telomere colocalization focis, concurrently downregulating TERT expression and shortening telomeres, and eventually inducing apoptosis of acute myeloid leukemia (AML) cells.142 Analogously, bortezomib (a ubiquitin-proteasome pathway inhibitor143) and MST-312 (a chemically modified analogue from epigallocatechin gallate144) could decrease TERT expression, suppress telomerase activity, shorten telomere lengths; and thus cause telomere dysfunction in leukemia, gastric cancer and breast cancer cells, respectively.
Telomerase-Responsive Drug Release System
Currently, stimuli-activatable design based on endogenous factors (tumor specific features such as low pH and overexpressed enzymes) or exogenous stimuli (light, ultrasound, magnet, and radiation) has emerged as a promising strategy for improving nanomedicines in cancer diagnosis and therapy.156 Considering the general upregulation or activation of telomerase in multifarious cancers, telomerase was chosen as a responsive target for nano-delivery systems of anti-cancer drugs. The telomerase-responsive delivery system could specifically deliver and release drugs to telomerase positive cancers, greatly reduce the toxic and side effects of drugs on normal cells and provide more opportunities for the practical drug application.
Zong et al designed and prepared a telomerase-triggered drug releasing nanocarrier system, in which the nanocarrier displayed a core-shell structure with mesoporous silica nanoparticles as drug loading shell and Au@Ag nanorods (NRs) as the surface enhanced Raman scattering (SERS) active core. The nanocarrier pores were loaded with doxorubicin (DOX) and blocked by an oligonucleotide (CAP1) containing a telomeric repeated complementary sequence and a telomerase substrate primer sequence. The hairpin structure was formed by lengthened telomeric sequences of CAP1, separated from the nanocarrier and thus released DOX from the opened pores. As predicted, the results showed that DOX were only released and diffused into the nuclei of telomerase positive HeLa cells.145 Analogously, Srivastava et al constructed the MSNP-NH2-DOX-DNA system, and discovered that the system displayed slow and sustained release of DOX in telomerase positive MCF-7, K-562, and DL cells, with remarkable inhibition of proliferation and induction of apoptosis; and the efficacy was stronger than that of free DOX.146 Moreover, in order to achieve the active targeting capability of the telomerase-responsive systems, Ma et al generated a CRISPR-dCas9 guided nanosystem dCas9-MSNs/DOX/DNA. This system could release DOX into the nuclei of telomerase overexpressed HeLa cells, and exert enhanced anticancer effects both in vitro and in vivo (with a tumor inhibition rate of 88%).147
Besides the above-mentioned delivery systems based on mesoporous silica nanoparticles, the nanostructures of DNA materials have been applied in the telomerase-responsive delivery system. PtNPs@DNA, a self-assembled DNA icosahedra nanoparticles encapsulated with platinum, could specifically release PtNPs to cancer cells with high telomerase activity, overcome the drug resistance and alleviate systemic toxicity in BCG823/DDP (cisplatin-resistant human gastric cancer) cells.148 Similarly, AS1411/nanotube/RTA, a DNA nanotube modified with targeted aptamer of nucleolin (AS1411) and loaded with ricin A chain (RTA), could accumulate and release RTA more intensively in tumor cells with high expression of telomerase, while showed favorable safety.149
Apart from specifically releasing drugs to telomerase positive cancer cells, the telomerase-responsive delivery system has been utilized as signal probes to dynamically monitor the telomerase activity in living cells, displaying the potential in diagnostic and biological applications. Zhu et al have developed a multivalent self-assembled DNA polymer that was constructed through telomerase primer chain and two hairpin probes functioned with tumor targeting aptamer and signal probe. The DNA polymer performed well in detecting and in situ monitoring the telomerase activity of cancer cells.150 Furthermore, Dox-AuNP-MB, a gold nanoparticle-based molecular beacon conjugated with FITC-labeled telomerase primer hybridized hairpin DNA sequences and loaded with DOX, could detect the intracellular telomerase activity of living cells and precisely release DOX to cancer cells without toxicity to normal cells.151 Similarly, Au-MPs-DOX, a gold nanoparticle-conjugated with carboxyfluorescein (FAM)-fluorescence biopolymer initiated by telomere extension and loaded with DOX, could have superior efficiency for detection of telomerase activity and drug delivery to telomerase-expressed cells.152
Conclusions and Future Directions
Telomeres play a pivotal role in cancer progression; accordingly, targeting telomere dynamics is an effective way for development of novel cancer therapeutics. A variety of telomere-active agents have been extensively investigated; furthermore, the diversity of molecular mechanisms of action are elucidated. The studies of telomere-based cancer therapeutics have achieved substantial progress. Although none of the telomere dynamics-targeted therapeutics has been approved in the clinical application of anticancer therapy, studies have achieved favorable advances in the preclinical studies and early phase of clinical trials, as chemotherapeutics and immunotherapeutics. The main problems impeding the clinical utilization of the telomere dynamics-targeted therapeutic agents include the long lag time between drug administration and the emergence of clinical responses, and related side effects.
Currently, numerous telomere dynamics-targeted therapeutics such as telomerase inhibitors work through shortening telomere lengths. Nevertheless, the tumor suppression effects appear only after the telomeres shortened to the critical lengths, which may demand a long time and depend on the cancer initial telomere length. Meanwhile, the remanent telomerase activity or even the activation of ALT in cancer cells after treatment with telomerase inhibitors may also delay the process of telomere shortening. Hence, telomere dynamics-targeted therapeutics that indirectly shorten telomere lengths may be more suitable for the treatment of cancer patients with short original telomere length, or used as an adjuvant therapy and maintenance therapy to prevent cancer recurrence after conventional surgery, radiotherapy or chemotherapy. In addition to extending the telomere lengths, telomerase may also exert a wide variety of effects, such as maintaining the quantity and multipotency of cancer stem cells through upregulating expressions of CD117, Oct4 and Sox-2,157 promoting cancer cell migration and invasion through increasing MMP9, TGF-β1, integrin β1 (ITGB1), heparinase and VEGF and activating Wnt/β-catenin axis,158 and preventing cell cycle arrest caused by DNA damage through blocking checkpoint signal transduction.159 Therefore, all those mentioned above lay a foundation for the combinations of telomere dynamics-targeted therapeutics and various chemotherapeutics.
Meanwhile, many telomere dynamics-targeted therapeutics may act as immunotherapeutics or immune-modulating agents through eliciting immune responses. As reported, 6-thio-dG could induce telomere stress which activates antitumor immunity via cGAS/STING/IFN-I pathway, and also synergize with immune checkpoint inhibitors.160 What’s more, telomere dysfunction leads to activation of innate immunity through the TERRA-ZBP1 complex induced by the cGAS/STING pathway.161 Furthermore, a prospective cohort of 70 bladder cancer patients reveals the positive correlation between the expression of TERT and PD-L1/2.162 Thus, more and more studies have provided the basis for the combination of telomere dynamics-targeted therapeutics and immunotherapeutics.
Instead of merely shortening telomere lengths, multiple action mechanisms involve in the suppression of telomere dynamics. The diversified strategies for development of telomere-related therapeutics cover the suppression of telomerase activity, downregulation of TERT expression, reduction of telomerase recruitment to telomeres, induction of telomere dysfunction and disruption of alternative lengthening of telomeres (ALT) pathway. The diversity of action mechanisms indicates that a great variety of telomere dynamics-targeted agents including small molecule compounds, ligand-based peptides, recombinant fusion proteins, and monoclonal antibodies could be generated and evaluated.
For the sake of enhancing therapeutic efficacy and reducing toxicity, the telomere targeted agents may be modified and reconstituted. Consequently, the prepared telomere targeted agents are integrated with the capability of tumor-specific drug delivery. These kinds of tumor microenvironment (TME)-oriented therapeutics are generated on the basis of the active targeting delivery systems such as antibody drug conjugates (ADCs) and the passive targeting delivery systems such as human serum albumin (HSA)-based drug conjugates or nanoparticles. In our research, a recombinant EGFR targeted fusion protein conjugate induces telomere length shortening, telomere dysfunction and telomerase downregulation. The conjugate possesses the active targeting capability of Fv fragment of an anti-EGFR monoclonal antibody and the passive targeting capability of the HSA domain.163
Overall, targeting telomere dynamics has emerged as an effective approach for the discovery and development of cancer therapeutics. A wide variety of active agents have been under preclinical investigation and clinical trials. Telomere dynamics-active agents are potentially effective as anti-cancer chemotherapeutics or immunotherapeutics. Therefore, the therapeutics acting through the activation of anti-telomerase immune responses can be developed via the combination with immunotherapeutic agents. The therapeutics working through shortening telomere lengths should be precisely applied in the patients with initial short telomeres, or in the adjuvant and maintenance therapy. Although there are disadvantages, telomere dynamics-targeted therapeutics have shown great potential in cancer therapy.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fan HC, Chang FW, Tsai JD, et al. Telomeres and cancer. Life. 2021;11(12):1405. doi: 10.3390/life11121405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Okamoto K, Seimiya H. Revisiting telomere shortening in cancer. Cells. 2019;8(2):107. doi: 10.3390/cells8020107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthel FP, Wei W, Tang M, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49(3):349–357. doi: 10.1038/ng.3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maciejowski J, de Lange T. Telomeres in cancer: tumour suppression and genome instability. Nat Rev Mol Cell Biol. 2017;18(3):175–186. doi: 10.1038/nrm.2016.171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao J, Pickett HA. Targeting telomeres: advances in telomere maintenance mechanism-specific cancer therapies. Nat Rev Cancer. 2022;22(9):515–532. doi: 10.1038/s41568-022-00490-1 [DOI] [PubMed] [Google Scholar]
- 6.Muller HJ. The remaking of chromosomes. Collect Net. 1938;8:182–195. [Google Scholar]
- 7.Moyzis R, Buckingham J, Cram L, et al. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci U S A. 1988;85(18):6622–6626. doi: 10.1073/pnas.85.18.6622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meyne J, Ratliff R, Moyzis R. Conservation of the human telomere sequence (TTAGGG)n among vertebrates. Proc Natl Acad Sci U S A. 1989;86(18):7049–7053. doi: 10.1073/pnas.86.18.7049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner KJ, Vasu V, Griffin DK. Telomere biology and human phenotype. Cells. 2019;8(1):73. doi: 10.3390/cells8010073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greider C. Telomeres do D-loop-T-loop. Cell. 1999;97(4):419–422. doi: 10.1016/S0092-8674(00)80750-3 [DOI] [PubMed] [Google Scholar]
- 11.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19(18):2100–2110. doi: 10.1101/gad.1346005 [DOI] [PubMed] [Google Scholar]
- 12.Smith EM, Pendlebury DF, Nandakumar J. Structural biology of telomeres and telomerase. Cell Mol Life Sci. 2020;77(1):61–79. doi: 10.1007/s00018-019-03369-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Lange T. How shelterin solves the telomere end-protection problem. Cold Spring Harb Symp Quant Biol. 2010;75:167–177. doi: 10.1101/sqb.2010.75.017 [DOI] [PubMed] [Google Scholar]
- 14.d’Adda Di Fagagna F, Reaper P, Clay-Farrace L, et al. A DNA damage checkpoint response in telomere-initiated senescence. Nature. 2003;426(6963):194–198. doi: 10.1038/nature02118 [DOI] [PubMed] [Google Scholar]
- 15.Shay JW. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016;6(6):584–593. doi: 10.1158/2159-8290.CD-16-0062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vertecchi E, Rizzo A, Salvati E. Telomere targeting approaches in cancer: beyond length maintenance. Int J Mol Sci. 2022;23(7):3784. doi: 10.3390/ijms23073784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greider C, Blackburn E. Identification of a specific telomere terminal transferase activity in tetrahymena extracts. Cell. 1985;43(2):405–413. doi: 10.1016/0092-8674(85)90170-9 [DOI] [PubMed] [Google Scholar]
- 18.Kim N, Piatyszek M, Prowse K, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428 [DOI] [PubMed] [Google Scholar]
- 19.Dilley RL, Greenberg RA. ALTernative telomere maintenance and cancer. Trends Cancer. 2015;1(2):145–156. doi: 10.1016/j.trecan.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang J, Wang Y, Susac L, et al. Structure of telomerase with telomeric DNA. Cell. 2018;173(5):1179–1190 e13. doi: 10.1016/j.cell.2018.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zvereva MI, Shcherbakova DM, Dontsova OA. Telomerase: structure, functions, and activity regulation. Biochemistry. 2010;75(13):1563–1583. doi: 10.1134/s0006297910130055 [DOI] [PubMed] [Google Scholar]
- 22.Podlevsky JD, Chen JJ. It all comes together at the ends: telomerase structure, function, and biogenesis. Mutat Res. 2012;730(1–2):3–11. doi: 10.1016/j.mrfmmm.2011.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu N, Guo XH, Liu JP, Cong YS. Role of telomerase in the tumour microenvironment. Clin Exp Pharmacol Physiol. 2020;47(3):357–364. doi: 10.1111/1440-1681.13223 [DOI] [PubMed] [Google Scholar]
- 24.Ebata H, Loo TM, Takahashi A. Telomere maintenance and the cGAS-STING pathway in cancer. Cells. 2022;11(12):1958. doi: 10.3390/cells11121958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu N, Ding D, Hao W, et al. hTERT promotes tumor angiogenesis by activating VEGF via interactions with the Sp1 transcription factor. Nucleic Acids Res. 2016;44(18):8693–8703. doi: 10.1093/nar/gkw549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walter K, Rodriguez-Aznar E, Ferreira MSV, et al. Telomerase and pluripotency factors jointly regulate stemness in pancreatic cancer stem cells. Cancers. 2021;13(13):3145. doi: 10.3390/cancers13133145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu L, Fidan K, Um JY, Ahn KS. Telomerase: key regulator of inflammation and cancer. Pharmacol Res. 2020;155:104726. doi: 10.1016/j.phrs.2020.104726 [DOI] [PubMed] [Google Scholar]
- 28.Hou K, Yu Y, Li D, et al. Alternative lengthening of telomeres and mediated telomere synthesis. Cancers. 2022;14(9):2194. doi: 10.3390/cancers14092194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cleal K, Norris K, Baird D. Telomere length dynamics and the evolution of cancer genome architecture. Int J Mol Sci. 2018;19(2):482. doi: 10.3390/ijms19020482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Vitis M, Berardinelli F, Sgura A. Telomere length maintenance in cancer: at the crossroad between telomerase and alternative lengthening of telomeres (ALT). Int J Mol Sci. 2018;19(2):606. doi: 10.3390/ijms19020606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hu J, Hwang SS, Liesa M, et al. Antitelomerase therapy provokes ALT and mitochondrial adaptive mechanisms in cancer. Cell. 2012;148(4):651–663. doi: 10.1016/j.cell.2011.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson PA, Drissi R, Muscal JA, et al. A Phase I trial of imetelstat in children with refractory or recurrent solid tumors: a children’s oncology group phase I consortium study (ADVL1112. Clin Cancer Res. 2013;19(23):6578–6584. doi: 10.1158/1078-0432.CCR-13-1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salloum R, Hummel TR, Kumar SS, et al. A molecular biology and Phase II study of imetelstat (GRN163L) in children with recurrent or refractory central nervous system malignancies: a pediatric brain tumor consortium study. J Neurooncol. 2016;129(3):443–451. doi: 10.1007/s11060-016-2189-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.clinicaltrials.gov. Imetelstat sodium in treating young patients with refractory or recurrent solid tumors or lymphoma; 2011. Available from: https://clinicaltrials.gov/ct2/show/NCT01273090?term=NCT01273090&draw=2&rank=1. Accessed April 15, 2024.
- 35.clinicaltrials.gov. A study inhibiting telomerase to reverse trastuzumab resistance in HER2+ breast cancer; 2010. Available from: https://clinicaltrials.gov/ct2/results?cond=&term=NCT01265927&cntry=&state=&city=&dist=. Accessed April 15, 2024.
- 36.Chiappori AA, Kolevska T, Spigel DR, et al. A randomized phase II study of the telomerase inhibitor imetelstat as maintenance therapy for advanced non-small-cell lung cancer. Ann Oncol. 2015;26(2):354–362. doi: 10.1093/annonc/mdu550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.clinicaltrials.gov. Imetelstat in combination with paclitaxel (with or without bevacizumab) in patients with locally recurrent or metastatic breast cancer; 2010. Available from: https://clinicaltrials.gov/ct2/show/NCT01256762?term=NCT01256762&draw=2&rank=1. Accessed April 15, 2024.
- 38.clinicaltrials.gov. Open label study with imetelstat to determine effect of imetelstat in patients w/ previously treated multiple myeloma; 2010. Available from: https://clinicaltrials.gov/ct2/show/NCT01242930?term=NCT01242930&draw=2&rank=1. Accessed April 15, 2024.
- 39.Edelman MJ, Lapidus R, Feliciano J, et al. Phase I and pharmacokinetic evaluation of the anti-telomerase agent KML-001 with cisplatin in advanced solid tumors. Cancer Chemother Pharmacol. 2016;78(5):959–967. doi: 10.1007/s00280-016-3148-x [DOI] [PubMed] [Google Scholar]
- 40.clinicaltrials.gov. THIO sequenced with cemiplimab in advanced NSCLC; 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05208944?term=NCT05208944&draw=2&rank=1. Accessed April 15, 2024.
- 41.clinicaltrials.gov. Testing the addition of the anti-cancer viral therapy telomelysin™ to chemoradiation for patients with advanced esophageal cancer and are not candidates for surgery; 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04391049?term=NCT04391049&draw=2&rank=1. Accessed April 15, 2024.
- 42.clinicaltrials.gov. Evaluate efficacy, immunological response of intratumoral/intralesional oncolytic virus (OBP-301) in metastatic melanoma; 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT03190824?term=NCT03190824&draw=2&rank=1. Accessed April 15, 2024.
- 43.Shirakawa Y, Tazawa H, Tanabe S, et al. Phase I dose-escalation study of endoscopic intratumoral injection of OBP-301 (Telomelysin) with radiotherapy in oesophageal cancer patients unfit for standard treatments. Eur J Cancer. 2021;153:98–108. doi: 10.1016/j.ejca.2021.04.043 [DOI] [PubMed] [Google Scholar]
- 44.Nemunaitis J, Tong AW, Nemunaitis M, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol Ther. 2010;18(2):429–434. doi: 10.1038/mt.2009.262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang J, Zhao X, Wu X, et al. A Phase I study of KH901, a conditionally replicating granulocyte-macrophage colony-stimulating factor: armed oncolytic adenovirus for the treatment of head and neck cancers. Cancer Biol Ther. 2009;8(8):676–682. doi: 10.4161/cbt.8.8.7913 [DOI] [PubMed] [Google Scholar]
- 46.clinicaltrials.gov. Study of the telomerase vaccine GV1001 to treat patients with inoperable stage III non-small cell lung cancer (LucaVax); 2012. Available from: https://clinicaltrials.gov/ct2/show/NCT01579188?term=NCT01579188&draw=2&rank=1. Accessed April 15, 2024.
- 47.clinicaltrials.gov. Gemcitabine and capecitabine with or without vaccine therapy in treating patients with locally advanced or metastatic pancreatic cancer; 2007. Available from: https://clinicaltrials.gov/ct2/show/NCT00425360?term=NCT00425360&draw=2&rank=1. Accessed April 15, 2024.
- 48.clinicaltrials.gov. GV 1001 immunotherapy in patients with non-small cell lung cancer (NSCLC); 2007. Available from: https://clinicaltrials.gov/ct2/show/NCT00509457?term=NCT00509457&draw=2&rank=1. Accessed April 15, 2024.
- 49.Greten TF, Forner A, Korangy F, et al. A phase II open label trial evaluating safety and efficacy of a telomerase peptide vaccination in patients with advanced hepatocellular carcinoma. BMC Cancer. 2010;10(1):209. doi: 10.1186/1471-2407-10-209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brunsvig PF, Aamdal S, Gjertsen MK, et al. Telomerase peptide vaccination: a phase I/II study in patients with non-small cell lung cancer. Cancer Immunol Immunother. 2006;55(12):1553–1564. doi: 10.1007/s00262-006-0145-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernhardt SL, Gjertsen MK, Trachsel S, et al. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: a dose escalating phase I/II study. Br J Cancer. 2006;95(11):1474–1482. doi: 10.1038/sj.bjc.6603437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.clinicaltrials.gov. Trial with telomerase peptide vaccine in combination with temozolomide in patients with advanced malignant melanoma; 2010. Available from: https://clinicaltrials.gov/ct2/show/NCT01247623?term=NCT01247623&draw=2&rank=1. Accessed April 15, 2024.
- 53.Kyte JA, Gaudernack G, Dueland S, Trachsel S, Julsrud L, Aamdal S. Telomerase peptide vaccination combined with temozolomide: a clinical trial in stage IV melanoma patients. Clin Cancer Res. 2011;17(13):4568–4580. doi: 10.1158/1078-0432.CCR-11-0184 [DOI] [PubMed] [Google Scholar]
- 54.Hunger RE, Kernland Lang K, Markowski CJ, et al. Vaccination of patients with cutaneous melanoma with telomerase-specific peptides. Cancer Immunol Immunother. 2011;60(11):1553–1564. doi: 10.1007/s00262-011-1061-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brunsvig PF, Kyte JA, Kersten C, et al. Telomerase peptide vaccination in NSCLC: a phase II trial in stage III patients vaccinated after chemoradiotherapy and an 8-year update on a phase I/II trial. Clin Cancer Res. 2011;17(21):6847–6857. doi: 10.1158/1078-0432.CCR-11-1385 [DOI] [PubMed] [Google Scholar]
- 56.Middleton G, Greenhalf W, Costello E, et al. Immunobiological effects of gemcitabine and capecitabine combination chemotherapy in advanced pancreatic ductal adenocarcinoma. Br J Cancer. 2016;114(5):510–518. doi: 10.1038/bjc.2015.468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.clinicaltrials.gov. Universal cancer peptide-based vaccination in metastatic NSCLC (UCPVax); 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02818426?term=NCT02818426&draw=2&rank=1. Accessed April 15, 2024.
- 58.clinicaltrials.gov. Evaluation of the interest to combine a CD4 Th1-inducer cancer vaccine derived from telomerase and Atezolizumab plus Bevacizumab in unresectable hepatocellular carcinoma (TERTIO); 2022. Available from: https://clinicaltrials.gov/ct2/show/NCT05528952?term=NCT05528952&draw=2&rank=1. Accessed April 15, 2024. [DOI] [PMC free article] [PubMed]
- 59.clinicaltrials.gov. Anticancer therapeutic vaccination using telomerase-derived universal cancer peptides in glioblastoma (UCPVax-Glio); 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04280848?term=NCT04280848&draw=2&rank=1. Accessed April 15, 2024.
- 60.clinicaltrials.gov. Combination of UCPVax vaccine and Atezolizumab for the treatment of human papillomavirus positive cancers (VolATIL) (VolATIL). 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT03946358?term=NCT03946358&draw=2&rank=1. Accessed April 15, 2024.
- 61.clinicaltrials.gov. Evaluation of UCPVax plus Nivolumab as second line therapy in advanced non small cell lung cancer (Optim-UCPVax); 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04263051?term=NCT04263051&draw=2&rank=1. Accessed April 15, 2024.
- 62.Gridelli C, Ciuleanu T, Domine M, et al. Clinical activity of a htert (vx-001) cancer vaccine as post-chemotherapy maintenance immunotherapy in patients with stage IV non-small cell lung cancer: final results of a randomised Phase 2 clinical trial. Br J Cancer. 2020;122(10):1461–1466. doi: 10.1038/s41416-020-0785-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kotsakis A, Vetsika EK, Christou S, et al. Clinical outcome of patients with various advanced cancer types vaccinated with an optimized cryptic human telomerase reverse transcriptase (TERT) peptide: results of an expanded phase II study. Ann Oncol. 2012;23(2):442–449. doi: 10.1093/annonc/mdr396 [DOI] [PubMed] [Google Scholar]
- 64.Georgoulias V, Douillard JY, Khayat D, et al. A multicenter randomized phase IIb efficacy study of Vx-001, a peptide-based cancer vaccine as maintenance treatment in advanced non-small-cell lung cancer: treatment rationale and protocol dynamics. Clin Lung Cancer. 2013;14(4):461–465. doi: 10.1016/j.cllc.2013.02.001 [DOI] [PubMed] [Google Scholar]
- 65.Kotsakis A, Papadimitraki E, Vetsika EK, et al. A phase II trial evaluating the clinical and immunologic response of HLA-A2(+) non-small cell lung cancer patients vaccinated with an hTERT cryptic peptide. Lung Cancer. 2014;86(1):59–66. doi: 10.1016/j.lungcan.2014.07.018 [DOI] [PubMed] [Google Scholar]
- 66.Haakensen VD, Nowak AK, Ellingsen EB, et al. NIPU: a randomised, open-label, phase II study evaluating nivolumab and ipilimumab combined with UV1 vaccination as second line treatment in patients with malignant mesothelioma. J Transl Med. 2021;19(1):232. doi: 10.1186/s12967-021-02905-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brunsvig PF, Guren TK, Nyakas M, et al. Long-term outcomes of a phase I study with UV1, a second generation telomerase based vaccine, in patients with advanced non-small cell lung cancer. Front Immunol. 2020;11:572172. doi: 10.3389/fimmu.2020.572172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ellingsen EB, Bounova G, Kerzeli I, et al. Characterization of the T cell receptor repertoire and melanoma tumor microenvironment upon combined treatment with ipilimumab and hTERT vaccination. J Transl Med. 2022;20(1):419. doi: 10.1186/s12967-022-03624-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lilleby W, Gaudernack G, Brunsvig PF, et al. Phase I/IIa clinical trial of a novel hTERT peptide vaccine in men with metastatic hormone-naive prostate cancer. Cancer Immunol Immunother. 2017;66(7):891–901. doi: 10.1007/s00262-017-1994-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filaci G, Fenoglio D, Nole F, et al. Telomerase-based GX301 cancer vaccine in patients with metastatic castration-resistant prostate cancer: a randomized phase II trial. Cancer Immunol Immunother. 2021;70(12):3679–3692. doi: 10.1007/s00262-021-03024-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.clinicaltrials.gov. Vaccine plus montanide ISA-51 and sargramostim in treating patients with stage IV breast cancer; 2004. Available from: https://clinicaltrials.gov/ct2/show/NCT00079157?term=NCT00079157&draw=2&rank=1. Accessed April 15, 2024.
- 72.clinicaltrials.gov. Vaccine therapy in treating patients with metastatic cancer; 2003. Available from: https://clinicaltrials.gov/ct2/show/NCT00021164?term=NCT00021164&draw=2&rank=1. Accessed April 15, 2024.
- 73.clinicaltrials.gov. Vaccine therapy and sargramostim in treating patients with sarcoma or brain tumor; 2003. Available from: https://clinicaltrials.gov/ct2/show/NCT00069940?term=NCT00069940&draw=2&rank=1. Accessed April 15, 2024.
- 74.Mavroudis D, Bolonakis I, Cornet S, et al. A phase I study of the optimized cryptic peptide TERT(572y) in patients with advanced malignancies. Oncology. 2006;70(4):306–314. doi: 10.1159/000096252 [DOI] [PubMed] [Google Scholar]
- 75.Bolonaki I, Kotsakis A, Papadimitraki E, et al. Vaccination of patients with advanced non-small-cell lung cancer with an optimized cryptic human telomerase reverse transcriptase peptide. J Clin Oncol. 2007;25(19):2727–2734. doi: 10.1200/JCO.2006.10.3465 [DOI] [PubMed] [Google Scholar]
- 76.clinicaltrials.gov. Multipeptide vaccine for advanced breast cancer; 2007. Available from: https://clinicaltrials.gov/ct2/show/NCT00573495?term=NCT00573495&draw=2&rank=1. Accessed April 15, 2024.
- 77.Rapoport AP, Aqui NA, Stadtmauer EA, et al. Combination immunotherapy using adoptive T-cell transfer and tumor antigen vaccination on the basis of hTERT and survivin after ASCT for myeloma. Blood. 2011;117(3):788–797. doi: 10.1182/blood-2010-08-299396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.clinicaltrials.gov. Exploratory study addendum to INVAC1-CT-101 (NCT02301754); 2020. Available from: https://clinicaltrials.gov/ct2/show/NCT04515043?term=NCT04515043&draw=2&rank=1. Accessed April 15, 2024.
- 79.Aurisicchio L, Fridman A, Mauro D, et al. Safety, tolerability and immunogenicity of V934/V935 hTERT vaccination in cancer patients with selected solid tumors: a phase I study. J Transl Med. 2020;18(1):39. doi: 10.1186/s12967-020-02228-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vonderheide RH, Kraynyak KA, Shields AF, et al. Phase 1 study of safety, tolerability and immunogenicity of the human telomerase (hTERT)-encoded DNA plasmids INO-1400 and INO-1401 with or without IL-12 DNA plasmid INO-9012 in adult patients with solid tumors. J Immunother Cancer. 2021;9(7):e003019. doi: 10.1136/jitc-2021-003019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.clinicaltrials.gov. INO-5401 + INO-9012 in combination with atezolizumab in locally advanced unresectable or metastatic/recurrent urothelial carcinoma; 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03502785?term=NCT03502785&draw=2&rank=1. Accessed April 15, 2024.
- 82.clinicaltrials.gov. INO-5401 and INO-9012 delivered by electroporation (EP) in combination with cemiplimab (REGN2810) in newly-diagnosed glioblastoma (GBM); 2018. Available from: https://clinicaltrials.gov/ct2/show/NCT03491683?term=NCT03491683&draw=2&rank=1. Accessed April 15, 2024.
- 83.Rittig SM, Haentschel M, Weimer KJ, et al. Intradermal vaccinations with RNA coding for TAA generate CD8+ and CD4+ immune responses and induce clinical benefit in vaccinated patients. Mol Ther. 2011;19(5):990–999. doi: 10.1038/mt.2010.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khoury HJ, Collins RH, Blum W, et al. Immune responses and long-term disease recurrence status after telomerase-based dendritic cell immunotherapy in patients with acute myeloid leukemia. Cancer. 2017;123(16):3061–3072. doi: 10.1002/cncr.30696 [DOI] [PubMed] [Google Scholar]
- 85.clinicaltrials.gov. Human telomerase reverse transcriptase messenger RNA (hTERT mRNA) transfected dendritic cell vaccines; 2010. Available from: https://clinicaltrials.gov/ct2/show/NCT01153113?term=NCT01153113&draw=2&rank=1. Accessed April 15, 2024.
- 86.Su Z, Dannull J, Heiser A, et al. Immunological and clinical responses in metastatic renal cancer patients vaccinated with tumor RNA-transfected dendritic cells. Cancer Res. 2003;63(9):2127–2133. [PubMed] [Google Scholar]
- 87.Mehrotra S, Britten CD, Chin S, et al. Vaccination with poly(IC:LC) and peptide-pulsed autologous dendritic cells in patients with pancreatic cancer. J Hematol Oncol. 2017;10(1):82. doi: 10.1186/s13045-017-0459-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.clinicaltrials.gov. Dendritic cell based therapy of malignant melanoma; 2005. Available from: https://clinicaltrials.gov/ct2/show/NCT00197912?term=NCT00197912&draw=2&rank=1. Accessed April 15, 2024.
- 89.Berntsen A, Trepiakas R, Wenandy L, et al. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother. 2008;31(8):771–780. doi: 10.1097/CJI.0b013e3181833818 [DOI] [PubMed] [Google Scholar]
- 90.Soleimani A, Berntsen A, Svane IM, Pedersen AE. Immune responses in patients with metastatic renal cell carcinoma treated with dendritic cells pulsed with tumor lysate. Scand J Immunol. 2009;70(5):481–489. doi: 10.1111/j.1365-3083.2009.02322.x [DOI] [PubMed] [Google Scholar]
- 91.Trepiakas R, Berntsen A, Hadrup SR, et al. Vaccination with autologous dendritic cells pulsed with multiple tumor antigens for treatment of patients with malignant melanoma: results from a phase I/II trial. Cytotherapy. 2010;12(6):721–734. doi: 10.3109/14653241003774045 [DOI] [PubMed] [Google Scholar]
- 92.Danet-Desnoyers GA, Luongo JL, Bonnet DA, Domchek SM, Vonderheide RH. Telomerase vaccination has no detectable effect on SCID-repopulating and colony-forming activities in the bone marrow of cancer patients. Exp Hematol. 2005;33(11):1275–1280. doi: 10.1016/j.exphem.2005.07.011 [DOI] [PubMed] [Google Scholar]
- 93.clinicaltrials.gov. Evaluation of transgenic lymphocyte immunization vaccine in subjects with prostate adenocarcinoma; 2003. Available from: https://clinicaltrials.gov/ct2/show/NCT00061035?term=NCT00061035&draw=2&rank=1. Accessed April 15, 2024.
- 94.clinicaltrials.gov. A study of transgenic lymphocyte immunization (TLI) against telomerase in subjects with stage III melanoma; 2009. Available from: https://clinicaltrials.gov/ct2/show/NCT00925314?term=NCT00925314&draw=2&rank=1. Accessed April 15, 2024.
- 95.Staff C, Mozaffari F, Frodin JE, Mellstedt H, Liljefors M. Telomerase (GV1001) vaccination together with gemcitabine in advanced pancreatic cancer patients. Int J Oncol. 2014;45(3):1293–1303. doi: 10.3892/ijo.2014.2496 [DOI] [PubMed] [Google Scholar]
- 96.Kim BK, Kim BR, Lee HJ, et al. Tumor-suppressive effect of a telomerase-derived peptide by inhibiting hypoxia-induced HIF-1alpha-VEGF signaling axis. Biomaterials. 2014;35(9):2924–2933. doi: 10.1016/j.biomaterials.2013.12.077 [DOI] [PubMed] [Google Scholar]
- 97.Park J, Kim Y, Kim H, et al. The anti-fibrotic effect of GV1001 combined with gemcitabine on treatment of pancreatic ductal adenocarcinoma. Oncotarget. 2016;7(46):75081–75093. doi: 10.18632/oncotarget.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim JW, Yadav DK, Kim SJ, et al. Anti-cancer effect of GV1001 for prostate cancer: function as a ligand of GnRHR. Endocr Relat Cancer. 2019;26(2):147–162. doi: 10.1530/ERC-18-0454 [DOI] [PubMed] [Google Scholar]
- 99.Dosset M, Godet Y, Vauchy C, et al. Universal cancer peptide-based therapeutic vaccine breaks tolerance against telomerase and eradicates established tumor. Clin Cancer Res. 2012;18(22):6284–6295. doi: 10.1158/1078-0432.CCR-12-0896 [DOI] [PubMed] [Google Scholar]
- 100.Kumagai M, Mizukoshi E, Tamai T, et al. Immune response to human telomerase reverse transcriptase-derived helper T cell epitopes in hepatocellular carcinoma patients. Liver Int. 2018;38(9):1635–1645. doi: 10.1111/liv.13713 [DOI] [PubMed] [Google Scholar]
- 101.Huo L, Yao H, Wang X, Wong GW, Kung HF, Lin MC. Inhibition of melanoma growth by subcutaneous administration of hTERTC27 viral cocktail in C57BL/6 mice. PLoS One. 2010;5(9):e12705. doi: 10.1371/journal.pone.0012705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adotevi O, Mollier K, Neuveut C, et al. Targeting human telomerase reverse transcriptase with recombinant lentivector is highly effective to stimulate antitumor CD8 T-cell immunity in vivo. Blood. 2010;115(15):3025–3032. doi: 10.1182/blood-2009-11-253641 [DOI] [PubMed] [Google Scholar]
- 103.Scardino A, Gross DA, Alves P, et al. HER-2/neu and hTERT cryptic epitopes as novel targets for broad spectrum tumor immunotherapy. J Immunol. 2002;168(11):5900–5906. doi: 10.4049/jimmunol.168.11.5900 [DOI] [PubMed] [Google Scholar]
- 104.Tagliamonte M, Petrizzo A, Napolitano M, et al. Novel metronomic chemotherapy and cancer vaccine combinatorial strategy for hepatocellular carcinoma in a mouse model. Cancer Immunol Immunother. 2015;64(10):1305–1314. doi: 10.1007/s00262-015-1698-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jansons J, Skrastina D, Kurlanda A, et al. Reciprocal inhibition of immunogenic performance in mice of two potent DNA immunogens targeting HCV-related liver cancer. Microorganisms. 2021;9(5):1073. doi: 10.3390/microorganisms9051073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lin X, Zhou C, Wang S, et al. Enhanced antitumor effect against human telomerase reverse transcriptase (hTERT) by vaccination with chemotactic-hTERT gene-modified tumor cell and the combination with anti-4-1BB monoclonal antibodies. Int J Cancer. 2006;119(8):1886–1896. doi: 10.1002/ijc.22048 [DOI] [PubMed] [Google Scholar]
- 107.Duperret EK, Wise MC, Trautz A, et al. Synergy of immune checkpoint blockade with a novel synthetic consensus DNA vaccine targeting TERT. Mol Ther. 2018;26(2):435–445. doi: 10.1016/j.ymthe.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yamano T, Kaneda Y, Hiramatsu SH, et al. Immunity against breast cancer by TERT DNA vaccine primed with chemokine CCL21. Cancer Gene Ther. 2007;14(5):451–459. doi: 10.1038/sj.cgt.7701035 [DOI] [PubMed] [Google Scholar]
- 109.Cui J, Cui L, Liu Q, Sun Q. Dendritic cells transfected with lentiviral vector-encoding hTERT peptide augment antitumor T cell response in vitro. Mol Med Rep. 2012;5(1):103–107. doi: 10.3892/mmr.2011.610 [DOI] [PubMed] [Google Scholar]
- 110.Chen L, Liang GP, Tang XD, et al. In vitro anti-tumor immune response induced by dendritic cells transfected with hTERT recombinant adenovirus. Biochem Biophys Res Commun. 2006;351(4):927–934. doi: 10.1016/j.bbrc.2006.10.165 [DOI] [PubMed] [Google Scholar]
- 111.Frolkis M, Fischer MB, Wang Z, Lebkowski JS, Chiu CP, Majumdar AS. Dendritic cells reconstituted with human telomerase gene induce potent cytotoxic T-cell response against different types of tumors. Cancer Gene Ther. 2003;10(3):239–249. doi: 10.1038/sj.cgt.7700563 [DOI] [PubMed] [Google Scholar]
- 112.Qiu J, Li GW, Sui YF, et al. Immunization with truncated sequence of telomerase reverse transcriptase induces a specific antitumor response in vivo. Acta Oncol. 2007;46(7):961–968. doi: 10.1080/02841860601166941 [DOI] [PubMed] [Google Scholar]
- 113.Sioud M, Saeboe-Larssen S, Hetland TE, Kaern J, Mobergslien A, Kvalheim G. Silencing of indoleamine 2,3-dioxygenase enhances dendritic cell immunogenicity and antitumour immunity in cancer patients. Int J Oncol. 2013;43(1):280–288. doi: 10.3892/ijo.2013.1922 [DOI] [PubMed] [Google Scholar]
- 114.Dillard P, Koksal H, Maggadottir SM, et al. Targeting telomerase with an HLA Class II-restricted TCR for cancer immunotherapy. Mol Ther. 2021;29(3):1199–1213. doi: 10.1016/j.ymthe.2020.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ding ZY, Wu Y, Luo Y, et al. Mannan-modified adenovirus as a vaccine to induce antitumor immunity. Gene Ther. 2007;14(8):657–663. doi: 10.1038/sj.gt.3302893 [DOI] [PubMed] [Google Scholar]
- 116.Wang Y, Zhang J, Wu Y, et al. Mannan-modified adenovirus targeting TERT and VEGFR-2: a universal tumour vaccine. Sci Rep. 2015;5(1):11275. doi: 10.1038/srep11275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Grandjenette C, Schnekenburger M, Karius T, et al. 5-aza-2’-deoxycytidine-mediated c-myc Down-regulation triggers telomere-dependent senescence by regulating human telomerase reverse transcriptase in chronic myeloid leukemia. Neoplasia. 2014;16(6):511–528. doi: 10.1016/j.neo.2014.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taka T, Huang L, Wongnoppavich A, Tam-Chang SW, Lee TR, Tuntiwechapikul W. Telomere shortening and cell senescence induced by perylene derivatives in A549 human lung cancer cells. Bioorg Med Chem. 2013;21(4):883–890. doi: 10.1016/j.bmc.2012.12.020 [DOI] [PubMed] [Google Scholar]
- 119.Qi Z, Mi R. Inhibition of human telomerase reverse transcriptase in vivo and in vitro for retroviral vector-based antisense oligonucleotide therapy in ovarian cancer. Cancer Gene Ther. 2016;23(1):36–42. doi: 10.1038/cgt.2015.64 [DOI] [PubMed] [Google Scholar]
- 120.Zheng JN, Pei DS, Sun FH, et al. Inhibition of renal cancer cell growth by oncolytic adenovirus armed short hairpin RNA targeting hTERT gene. Cancer Biol Ther. 2009;8(1):84–91. doi: 10.4161/cbt.8.1.7204 [DOI] [PubMed] [Google Scholar]
- 121.Xie Y, Qiao H, Su Z, Chen M, Ping Q, Sun M. PEGylated carboxymethyl chitosan/calcium phosphate hybrid anionic nanoparticles mediated hTERT siRNA delivery for anticancer therapy. Biomaterials. 2014;35(27):7978–7991. doi: 10.1016/j.biomaterials.2014.05.068 [DOI] [PubMed] [Google Scholar]
- 122.Kazemi Noureini S, Fatemi L, Wink M, Lustig AJ. Telomere shortening in breast cancer cells (MCF7) under treatment with low doses of the benzylisoquinoline alkaloid chelidonine. PLoS One. 2018;13(10):e0204901. doi: 10.1371/journal.pone.0204901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhdanov DD, Pokrovsky VS, Pokrovskaya MV, et al. Rhodospirillum rubruml-asparaginase targets tumor growth by a dual mechanism involving telomerase inhibition. Biochem Biophys Res Commun. 2017;492(2):282–288. doi: 10.1016/j.bbrc.2017.08.078 [DOI] [PubMed] [Google Scholar]
- 124.Gan Y, Lu J, Yeung BZ, Cottage CT, Wientjes MG, Au JLS. Pharmacodynamics of telomerase inhibition and telomere shortening by noncytotoxic suramin. AAPS J. 2014;17(1):268–276. doi: 10.1208/s12248-014-9703-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Beisner J, Dong M, Taetz S, et al. Nanoparticle mediated delivery of 2′-O-methyl-RNA leads to efficient telomerase inhibition and telomere shortening in human lung cancer cells. Lung Cancer. 2010;68(3):346–354. doi: 10.1016/j.lungcan.2009.07.010 [DOI] [PubMed] [Google Scholar]
- 126.Bavelaar BM, Song L, Jackson MR, et al. Oligonucleotide-functionalized gold nanoparticles for synchronous telomerase inhibition, radiosensitization, and delivery of theranostic radionuclides. Mol Pharmaceut. 2021;18(10):3820–3831. doi: 10.1021/acs.molpharmaceut.1c00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakashima M, Nandakumar J, Sullivan KD, Espinosa JM, Cech TR. Inhibition of telomerase recruitment and cancer cell death. J Biol Chem. 2013;288(46):33171–33180. doi: 10.1074/jbc.M113.518175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu J, Liu W, Chen C, et al. TPP1 OB-fold domain protein suppresses cell proliferation and induces cell apoptosis by inhibiting telomerase recruitment to telomeres in human lung cancer cells. J Cancer Res Clin Oncol. 2019;145(6):1509–1519. doi: 10.1007/s00432-019-02921-3 [DOI] [PubMed] [Google Scholar]
- 129.Zheng XH, Nie X, Fang Y, et al. A cisplatin derivative Tetra-Pt(bpy) as an oncotherapeutic agent for targeting ALT cancer. J Natl Cancer Inst. 2017;109(10). doi: 10.1093/jnci/djx061 [DOI] [PubMed] [Google Scholar]
- 130.Yu Y, Katiyar SP, Sundar D, et al. Withaferin-A kills cancer cells with and without telomerase: chemical, computational and experimental evidences. Cell Death Dis. 2017;8(4):e2755. doi: 10.1038/cddis.2017.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen J, Jin X, Mei Y, et al. The different biological effects of TMPyP4 and cisplatin in the inflammatory microenvironment of osteosarcoma are attributed to G-quadruplex. Cell Prolif. 2021;54(9):e13101. doi: 10.1111/cpr.13101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mender I, Gryaznov S, Dikmen ZG, Wright WE, Shay JW. Induction of telomere dysfunction mediated by the telomerase substrate precursor 6-thio-2’-deoxyguanosine. Cancer Discov. 2015;5(1):82–95. doi: 10.1158/2159-8290.CD-14-0609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang G, Wu LW, Mender I, et al. Induction of telomere dysfunction prolongs disease control of therapy-resistant melanoma. Clin Cancer Res. 2018;24(19):4771–4784. doi: 10.1158/1078-0432.CCR-17-2773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ali S, Lombardi EP, Ghosh D, et al. Pt-ttpy, a G-quadruplex binding platinum complex, induces telomere dysfunction and G-rich regions DNA damage. Metallomics. 2021;13(6). doi: 10.1093/mtomcs/mfab029 [DOI] [PubMed] [Google Scholar]
- 135.Petrov N, Lee H, Liskovykh M, et al. Terpyridine platinum compounds induce telomere dysfunction and chromosome instability in cancer cells. Oncotarget. 2021;12(15):1444–1456. doi: 10.18632/oncotarget.28020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Xiong K, Ouyang C, Liu J, et al. Chiral RuII‐PtIIComplexes inducing telomere dysfunction against cisplatin‐resistant cancer cells. Angew Chem Int Ed. 2022;61(33). doi: 10.1002/anie.202204866 [DOI] [PubMed] [Google Scholar]
- 137.Zhou G, Liu X, Li Y, et al. Telomere targeting with a novel G-quadruplex-interactive ligand BRACO-19 induces T-loop disassembly and telomerase displacement in human glioblastoma cells. Oncotarget. 2016;7(12):14925–14939. doi: 10.18632/oncotarget.7483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hasegawa D, Okabe S, Okamoto K, Nakano I, Shin-ya K, Seimiya H. G-quadruplex ligand-induced DNA damage response coupled with telomere dysfunction and replication stress in glioma stem cells. Biochem Biophys Res Commun. 2016;471(1):75–81. doi: 10.1016/j.bbrc.2016.01.176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Che T, Chen SB, Tu JL, et al. Discovery of novel schizocommunin derivatives as telomeric G-Quadruplex ligands that trigger telomere dysfunction and the deoxyribonucleic acid (DNA) damage response. J Med Chem. 2018;61(8):3436–3453. doi: 10.1021/acs.jmedchem.7b01615 [DOI] [PubMed] [Google Scholar]
- 140.Muller S, Sanders DA, Di Antonio M, et al. Pyridostatin analogues promote telomere dysfunction and long-term growth inhibition in human cancer cells. Org Biomol Chem. 2012;10(32):6537–6546. doi: 10.1039/c2ob25830g [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dinami R, Pompili L, Petti E, et al. MiR‐182‐3p targets TRF2 and impairs tumor growth of triple‐negative breast cancer. EMBO Mol Med. 2022;15(1):e16033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang X, Li B, de Jonge N, Björkholm M, Xu D. The DNA methylation inhibitor induces telomere dysfunction and apoptosis of leukemia cells that is attenuated by telomerase over-expression. Oncotarget. 2015;6(7):4888–4900. doi: 10.18632/oncotarget.2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Ci X, Li B, Ma X, et al. Bortezomib-mediated down-regulation of telomerase and disruption of telomere homeostasis contributes to apoptosis of malignant cells. Oncotarget. 2015;6(35):38079–38092. doi: 10.18632/oncotarget.5752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gurung RL, Lim SN, Low GK, Hande MP. MST-312 alters telomere dynamics, gene expression profiles and growth in human breast cancer cells. J Nutrigenet Nutrigenomics. 2014;7(4–6):283–298. doi: 10.1159/000381346 [DOI] [PubMed] [Google Scholar]
- 145.Zong S, Wang Z, Chen H, Zhu D, Chen P, Cui Y. Telomerase triggered drug release using a SERS traceable nanocarrier. IEEE Trans NanoBiosci. 2014;13(1):55–60. doi: 10.1109/TNB.2014.2301996 [DOI] [PubMed] [Google Scholar]
- 146.Srivastava P, Hira SK, Sharma A, et al. Telomerase responsive delivery of doxorubicin from mesoporous silica nanoparticles in multiple malignancies: therapeutic efficacies against experimental aggressive murine lymphoma. Bioconjug Chem. 2018;29(6):2107–2119. doi: 10.1021/acs.bioconjchem.8b00342 [DOI] [PubMed] [Google Scholar]
- 147.Ma Y, Mao G, Wu G, Cui Z, Zhang XE, Huang W. CRISPR-dCas9-guided and telomerase-responsive nanosystem for precise anti-cancer drug delivery. ACS Appl Mater Interfaces. 2021;13(7):7890–7896. doi: 10.1021/acsami.0c19217 [DOI] [PubMed] [Google Scholar]
- 148.Ma Y, Wang Z, Ma Y, et al. A telomerase-responsive DNA icosahedron for precise delivery of platinum nanodrugs to cisplatin-resistant cancer. Angew Chem Int Ed Engl. 2018;57(19):5389–5393. doi: 10.1002/anie.201801195 [DOI] [PubMed] [Google Scholar]
- 149.Li CH, Lv WY, Yan Y, Yang FF, Zhen SJ, Huang CZ. Nucleolin-targeted DNA nanotube for precise cancer therapy through forster resonance energy transfer-indicated telomerase responsiveness. Anal Chem. 2021;93(7):3526–3534. doi: 10.1021/acs.analchem.0c04917 [DOI] [PubMed] [Google Scholar]
- 150.Zhu X, Ye H, Liu JW, Yu RQ, Jiang JH. Multivalent self-assembled DNA polymer for tumor-targeted delivery and live cell imaging of telomerase activity. Anal Chem. 2018;90(22):13188–13192. doi: 10.1021/acs.analchem.8b04631 [DOI] [PubMed] [Google Scholar]
- 151.Ma Y, Wang Z, Zhang M, et al. A telomerase-specific doxorubicin-releasing molecular beacon for cancer theranostics. Angew Chem Int Ed Engl. 2016;55(10):3304–3308. doi: 10.1002/anie.201509182 [DOI] [PubMed] [Google Scholar]
- 152.Zhang Z, Jiao Y, Zhu M, Zhang S. Nuclear-shell biopolymers initiated by telomere elongation for individual cancer cell imaging and drug delivery. Anal Chem. 2017;89(7):4320–4327. doi: 10.1021/acs.analchem.7b00591 [DOI] [PubMed] [Google Scholar]
- 153.Nandakumar J, Bell CF, Weidenfeld I, Zaug AJ, Leinwand LA, Cech TR. The TEL patch of telomere protein TPP1 mediates telomerase recruitment and processivity. Nature. 2012;492(7428):285–289. doi: 10.1038/nature11648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rossiello F, Jurk D, Passos JF, d’Adda Di Fagagna F. Telomere dysfunction in ageing and age-related diseases. Nat Cell Biol. 2022;24(2):135–147. doi: 10.1038/s41556-022-00842-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hänsel-Hertsch R, Di Antonio M, Balasubramanian S. DNA G-quadruplexes in the human genome: detection, functions and therapeutic potential. Nat Rev Mol Cell Biol. 2017;18(5):279–284. doi: 10.1038/nrm.2017.3 [DOI] [PubMed] [Google Scholar]
- 156.Li H, Feng Y, Luo Q, et al. Stimuli-activatable nanomedicine meets cancer theranostics. Theranostics. 2023;13(15):5386–5417. doi: 10.7150/thno.87854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hannen R, Bartsch JW. Essential roles of telomerase reverse transcriptase hTERT in cancer stemness and metastasis. FEBS Lett. 2018;592(12):2023–2031. doi: 10.1002/1873-3468.13084 [DOI] [PubMed] [Google Scholar]
- 158.Robinson NJ, Schiemann WP. Means to the ends: the role of telomeres and telomere processing machinery in metastasis. Biochim Biophys Acta. 2016;1866(2):320–329. doi: 10.1016/j.bbcan.2016.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carneiro T, Khair L, Reis CC, et al. Telomeres avoid end detection by severing the checkpoint signal transduction pathway. Nature. 2010;467(7312):228–232. doi: 10.1038/nature09353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mender I, Zhang A, Ren Z, et al. Telomere stress potentiates STING-dependent anti-tumor immunity. Cancer Cell. 2020;38(3):400–411 e6. doi: 10.1016/j.ccell.2020.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nassour J, Aguiar LG, Correia A, et al. Telomere-to-mitochondria signalling by ZBP1 mediates replicative crisis. Nature. 2023;614(7949):767–773. doi: 10.1038/s41586-023-05710-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.El Ahanidi H, El Azzouzi M, Hafidi Alaoui C, et al. Immune checkpoint and telomerase crosstalk is mediated by miRNA-138 in bladder cancer. Front Oncol. 2021;11:795242. doi: 10.3389/fonc.2021.795242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tao H, He S, Zhao C, Wang Y, Sheng W, Zhen Y. Antitumor efficacy of a recombinant EGFR-targeted fusion protein conjugate that induces telomere shortening and telomerase downregulation. Int J Biol Macromol. 2023;226:1088–1099. doi: 10.1016/j.ijbiomac.2022.11.225 [DOI] [PubMed] [Google Scholar]