Key Points
Question
What is the association of polycystic ovary syndrome (PCOS) and irregular cycles (early-life and adulthood) with cardiometabolic conditions?
Findings
In this cross-sectional study of 60 789 participants from the Apple Women’s Health Study, individuals with PCOS had a significantly higher prevalence of cardiometabolic conditions. Longer time to regularity since menarche (adolescence) or irregular cycles (adulthood) were associated with a higher prevalence of several cardiometabolic conditions, even among participants without PCOS, and certain associations were modified by body mass index or physical activity.
Meaning
These findings suggest that early screening of cardiometabolic risk factors and interventions may benefit individuals with longer times to regular menstrual cycles or who experience adulthood irregular cycles, regardless of PCOS diagnosis.
This cross-sectional study evaluates the association of polycystic ovary syndrome, timing to regular menstrual cycles, and irregular cycles with cardiometabolic conditions.
Abstract
Importance
Polycystic ovary syndrome (PCOS), characterized by irregular menstrual cycles and hyperandrogenism, is a common ovulatory disorder. Having an irregular cycle is a potential marker for cardiometabolic conditions, but data are limited on whether the associations differ by PCOS status or potential interventions.
Objective
To evaluate the association of PCOS, time to regularity since menarche (adolescence), and irregular cycles (adulthood) with cardiometabolic conditions.
Design, Setting, and Participants
This cross-sectional study used a large, US-based digital cohort of users of the Apple Research application on their iPhone. Eligibility criteria were having ever menstruated, living in the US, being at age of consent of at least 18 years (or 19 years in Alabama and Nebraska or 21 years in Puerto Rico), and being able to communicate in English. Participants were enrolled between November 14, 2019, and December 13, 2022, and completed relevant surveys.
Exposures
Self-reported PCOS diagnosis, prolonged time to regularity (not spontaneously establishing regularity within 5 years of menarche), and irregular cycles.
Main Outcomes and Measures
The primary outcome was self-reported cardiometabolic conditions, including obesity, prediabetes, type 1 and 2 diabetes, high cholesterol, hypertension, metabolic syndrome, arrhythmia, congestive heart failure, coronary artery disease, heart attack, heart valve disease, stroke, transient ischemic attack (TIA), deep vein thrombosis, and pulmonary embolism measured using descriptive statistics and logistic regression to estimate prevalence odds ratios (PORs) and 95% CIs. Effect modification by lifestyle factors was also estimated.
Results
The study sample (N = 60 789) had a mean (SD) age of 34.5 (11.1) years, with 12.3% having PCOS and 26.3% having prolonged time to regularity. Among a subset of 25 399 participants who completed the hormonal symptoms survey, 25.6% reported irregular cycles. In covariate-adjusted logistic regression models, PCOS was associated with a higher prevalence of all metabolic and several cardiovascular conditions, eg, arrhythmia (POR, 1.37; 95% CI, 1.20-1.55), coronary artery disease (POR, 2.92; 95% CI, 1.95-4.29), heart attack (POR, 1.79; 95% CI, 1.23-2.54), and stroke (POR, 1.66; 95% CI, 1.21-2.24). Among participants without PCOS, prolonged time to regularity was associated with type 2 diabetes (POR, 1.24; 95% CI, 1.05-1.46), hypertension (POR, 1.09; 95% CI, 1.01-1.19), arrhythmia (POR, 1.20; 95% CI, 1.06-1.35), and TIA (POR, 1.33; 95% CI, 1.01-1.73), and having irregular cycles was associated with type 2 diabetes (POR, 1.36; 95% CI, 1.08-1.69), high cholesterol (POR, 1.17; 95% CI, 1.05-1.30), arrhythmia (POR, 1.21; 95% CI, 1.02-1.43), and TIA (POR, 1.56; 95% CI, 1.06-2.26). Some of these associations were modified by high vs low body mass index or low vs high physical activity.
Conclusions and Relevance
These findings suggest that PCOS and irregular cycles may be independent markers for cardiometabolic conditions. Early screening and intervention among individuals with irregular menstrual cycles may be beneficial.
Introduction
The menstrual cycle is an important vital sign beginning in early life.1 Irregular cycles have been associated with adverse health outcomes.2 Polycystic ovary syndrome (PCOS), characterized by irregular cycles and hyperandrogenism, is a common ovulatory disorder affecting 8% to 13% of reproductive-aged females (15-49 years),3,4 but not all individuals with irregular cycles are diagnosed with PCOS (due to underdiagnosis or other etiologies such as hypothalamic amenorrhea).5,6,7,8
Positive associations have been found between PCOS and cardiometabolic conditions, such as the metabolic syndrome, coronary heart disease, and stroke.9,10 Previous population-based studies reported positive associations between irregular cycles and cardiovascular disease (CVD) or premature mortality,11,12,13,14,15,16 while other studies did not find such associations.17,18 These studies generally lacked data on PCOS diagnosis or other ovulatory disorders and therefore did not ascertain whether the associations were solely attributable to underlying PCOS or other etiologies that may confer cardiometabolic risk.19 In addition, most of these studies were limited to menstrual patterns during adulthood. Early-life characteristics, such as time to regularity since menarche,20,21 are important but understudied variables in cardiometabolic health. Furthermore, existing studies were from Europe or of relatively homogeneous or hospital-based populations in the US, and studies on potential early interventions are also lacking. In this study, we evaluated the associations of PCOS, time to regularity (adolescence), and irregular cycles (adulthood) with the prevalence of various cardiometabolic conditions.
Methods
Study Design and Population
This cross-sectional study used data from the Apple Women’s Health Study (AWHS), a prospective, US-based, digital cohort study. This study was approved by the institutional review board at Advarra (CIRB #PRO00037562), and written informed consent was provided at enrollment. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Users of the Apple Research application on their iPhone (Apple Inc) were eligible if they had ever menstruated, were living in the US, were aged at least 18 years (or 19 years in Alabama and Nebraska or 21 years in Puerto Rico), and were able to communicate in English. Eligibility also required sole usage of their iCloud account or iPhone. Enrollment began on November 14, 2019, and is ongoing. Further details were described previously.22
Upon enrollment, participants were asked to complete 5 surveys to collect data on demographics, medical history, reproductive history, hormonal symptoms, and general health and lifestyle (eTable 1 in Supplement 1). We restricted analyses to female participants who enrolled from November 14, 2019, to December 13, 2022, and completed the demographics survey and at least 1 of the 4 remaining surveys. For specific analyses, data were limited to the subset of participants who answered the relevant questions (eFigure 1 in Supplement 1).
PCOS, Time to Cycle Regularity, and Irregular Cycles
Polycystic ovary syndrome (yes or no) was based on self-reported physician diagnosis at enrollment. Using the survey question, “After your first menstrual cycle, how long did it take for your cycle to become regular,” we derived prolonged time to regularity (yes or no), where “yes” included participants who indicated more than 5 years, after using hormones, or not yet regular and “no” included those who reached regularity within 4 years.23 Other derivations are described in the eMethods in Supplement 1. We defined irregular cycles as selection of the response unpredictable periods to the survey question, “Are you troubled by any of the following symptoms” (eTable 1 in Supplement 1). Hereafter, we use the term irregular cycles to reflect the experience of being troubled by unpredictable menstrual periods. For all exposure variables, don’t know, prefer not to answer, and no response were considered missing.
Cardiometabolic Conditions
At enrollment, we collected data on participant self-report of physician diagnosed cardiometabolic conditions, including prediabetes, type 1 diabetes, type 2 diabetes, high cholesterol, hypertension, arrhythmia, congestive heart failure, coronary artery disease (CAD), heart attack, heart valve disease, stroke, transient ischemic attack (TIA), deep vein thrombosis (DVT), and pulmonary embolism. Obesity was defined as a body mass index (BMI) of 30 or greater (as measured by weight in kilograms divided by height in meters squared) using self-reported weight and height. We further defined metabolic syndrome using the World Health Organization 1998 criteria with modification based on data availability (details in the eMethods in Supplement 1).24,25
Covariates
We included covariates in regression models as potential confounders based on a priori knowledge. All covariates were self-reported at enrollment, including age; race and ethnicity (Asian, Hispanic, non-Hispanic Black, non-Hispanic White, multiracial, other [American Indian or Alaska Native, Middle Eastern or North African, Native Hawaiian or Pacific Islander, “none of these fully describe me”]26) because of their known associations with PCOS, irregular cycles, and cardiometabolic conditions27,28,29; education (high school or less, college, graduate school); employment status (employed, unemployed, other); socioeconomic status based on the MacArthur Scale of Subjective Social Status,30 a self-rated rank on a social ladder based on self-perception relative to others (0-3 [low], 4-5 [medium], 6-9 [high]); gravidity (0, 1, ≥2); age at menarche (≤11 years, 12-13 years, 14-15 years, ≥16 years); BMI categories (underweight, <18.5; healthy, 18.5 to <25; overweight, 25 to <30; obesity, ≥30); ever used hormones (yes or no to various forms of hormonal contraception or hormone replacement); and family history of obesity, high cholesterol, hypertension, and diabetes. We acknowledge that some covariates may be downstream factors of the exposures and outcomes with unestablished temporality (eFigure 2 in Supplement 1). Thus, we performed 3 models (unadjusted, adjusted only for age and race and ethnicity, and adjusted for all covariates) to assess the robustness of our findings.
Statistical Analysis
As the AWHS is an ongoing study with currently insufficient data on incident cases during follow-up, this cross-sectional analysis uses baseline data (except for time to regularity that reflects the adolescence period). We examined the distributions of all baseline characteristics with mean (SD) and median (IQR) for continuous variables, or counts and percentages for categorical variables. To understand the associations within the key exposure variables, we first used logistic regression models to analyze associations of prolonged time to regularity with PCOS and irregular cycles, respectively. Then, for the associations of PCOS with each prevalent cardiometabolic condition, we used logistic regressions to estimate the prevalence odds ratios (PORs) and 95% CIs using the following models: (1) unadjusted, (2) minimally adjusted for age and race and ethnicity, and (3) adjusted for all covariates. For the associations of prolonged time to regularity or irregular cycles with cardiometabolic conditions, in addition to the full population, we further performed logistic regression analyses stratified by PCOS status. For each regression model, participants with missing exposure, outcome, or covariates were excluded.
For associations between (1) PCOS and cardiometabolic conditions and (2) irregular cycles and cardiometabolic conditions among participants without PCOS, we evaluated the effect modification by BMI (healthy or underweight, overweight, obesity), physical activity (none or light vs moderate, vigorous, or strenuous), and gravidity (0, 1, or ≥2) as exploratory analyses. For each proposed modifier, we tested for effect modification using the relative excess risk due to interaction (RERI) for additive interaction.31
We performed several exploratory and sensitivity analyses, such as exploring preliminary differences in age at diagnosis of cardiometabolic conditions among participants who reported this information, exploring other ovulatory disorders, such as hypothalamic amenorrhea, based on self-reported characteristics,32,33 and evaluating the validity and stability of our findings. Further details are provided in the eMethods in Supplement 1.
Data processing and analyses were conducted using Python, version 3.6 (Python Software Foundation) and R, version 1.2.5033 (R Foundation for Statistical Computing). All statistical tests were 2-sided with 95% CIs. Significance was defined as an incremental effect for which 95% CI excluded the null and in the case of POR, excluded 1.
Results
Baseline characteristics of the 60 789 participants are shown in Table 1. Overall, participants had a mean (SD) age of 34.5 (11.1) years. Most were non-Hispanic White (71.4%, compared with 3.2% Asian, 7.3% Hispanic, 5.4% non-Hispanic Black, 10.1% multiracial, and 2.6% other), employed (72.0%), and had obtained some college education or higher (82.9%). Among all participants, 26.3% had prolonged time to regularity, and 12.3% reported diagnosed PCOS. Of 25 399 participants who completed the hormonal symptoms survey, 25.6% reported having irregular cycles. The distributions of covariates among the 60 789 vs 25 399 participants were similar (eTable 2 in Supplement 1). Compared with participants without PCOS, those with PCOS had a lower socioeconomic status and gravidity and higher BMI, family history of metabolic conditions, hormone use, and prevalence of cardiometabolic conditions.
Table 1. Participant Baseline Characteristics at Enrollment.
| Characteristic | No. of participants (%) | |||
|---|---|---|---|---|
| Overall (n = 60 789) | PCOS (n = 6484) | No PCOS (n = 46 135) | ||
| Age at enrollment, y | ||||
| Mean (SD) | 34.5 (11.1) | 34.2 (9.0) | 34.7 (11.5) | |
| Median (IQR) | 33 (26-41) | 33 (27-40) | 33 (26-42) | |
| Race and ethnicity | ||||
| Asian | 1942 (3.2) | 195 (3.0) | 1476 (3.2) | |
| Hispanic | 4411 (7.3) | 473 (7.3) | 3255 (7.1) | |
| Non-Hispanic Black | 3294 (5.4) | 266 (4.1) | 2528 (5.5) | |
| Non-Hispanic White | 43 404 (71.4) | 4643 (71.6) | 33 178 (71.9) | |
| Multiraciala | 6131 (10.1) | 736 (11.4) | 4544 (9.9) | |
| Otherb | 1602 (2.6) | 170 (2.6) | 1150 (2.5) | |
| Socioeconomic status | ||||
| 0-3 (low) | 16 284 (26.8) | 2053 (31.7) | 11 966 (25.9) | |
| 4-5 (medium) | 25 590 (42.1) | 2787 (43.0) | 19 373 (42.0) | |
| 6-9 (high) | 18 596 (30.6) | 1612 (24.9) | 14 593 (31.6) | |
| Missing | 319 (0.5) | 32 (0.5) | 203 (0.4) | |
| Employment status | ||||
| Employed | 43 752 (72.0) | 4801 (74.0) | 33 192 (71.9) | |
| Unemployed | 3382 (5.6) | 328 (5.1) | 2526 (5.5) | |
| Other | 13 090 (21.5) | 1304 (20.1) | 10 062 (21.8) | |
| Missing | 565 (0.9) | 51 (0.8) | 355 (0.8) | |
| Education level | ||||
| High school and less | 9923 (16.3) | 874 (13.5) | 7673 (16.6) | |
| Some college or college | 37 399 (61.5) | 4223 (65.1) | 28 141 (61.0) | |
| Graduate school | 13 023 (21.4) | 1354 (20.9) | 10 019 (21.7) | |
| Missing | 444 (0.7) | 33 (0.5) | 302 (0.7) | |
| Body mass indexc | ||||
| Underweight (<18.5) | 1590 (2.6) | 83 (1.3) | 1317 (2.9) | |
| Healthy weight (18.5 to <25) | 20 063 (33.0) | 1144 (17.6) | 16 298 (35.3) | |
| Overweight (25 to <30) | 15 033 (24.7) | 1234 (19.0) | 11 840 (25.7) | |
| Obesity (≥30) | 22 555 (37.1) | 3872 (59.7) | 15 591 (33.8) | |
| Missing | 1548 (2.5) | 151 (2.3) | 1089 (2.4) | |
| Age at menarche, y | ||||
| ≤11 | 17 738 (29.2) | 2219 (34.2) | 13 026 (28.2) | |
| 12-13 | 29 396 (48.4) | 2784 (42.9) | 22 591 (49.0) | |
| 14-15 | 8191 (13.5) | 829 (12.8) | 6246 (13.5) | |
| ≥16 | 1914 (3.1) | 303 (4.7) | 1323 (2.9) | |
| Missing | 3550 (5.8) | 349 (5.4) | 2949 (6.4) | |
| Gravidity | ||||
| 0 | 25 466 (41.9) | 2742 (42.3) | 19 340 (41.9) | |
| 1 | 8028 (13.2) | 1021 (15.7) | 5877 (12.7) | |
| ≥2 | 23 798 (39.1) | 2371 (36.6) | 18 060 (39.1) | |
| Missing | 3497 (5.8) | 350 (5.4) | 2858 (6.2) | |
| Family history of metabolic conditionsd | 35 094 (57.7) | 5024 (77.5) | 29 944 (64.9) | |
| Ever used hormone | 43 781 (72.0) | 5375 (82.9) | 32 332 (70.1) | |
| Time to cycle regularity | ||||
| Regular within 4 y | 34 326 (56.5) | 2443 (37.7) | 27 322 (59.2) | |
| Regular after ≥5 y, after using hormones, or not yet regular (ie, prolonged time to cycle regularity) | 15 977 (26.3) | 3282 (50.6) | 10 385 (22.5) | |
| Missing | 10 486 (17.2) | 759 (11.7) | 8428 (18.3) | |
| Cardiometabolic conditions | ||||
| Prediabetes | 4439 (8.4) | 1538 (23.7) | 2888 (6.3) | |
| Type 1 diabetes | 401 (0.8) | 75 (1.2) | 322 (0.7) | |
| Type 2 diabetes | 1567 (3.0) | 468 (7.2) | 1091 (2.4) | |
| High cholesterol | 6601 (12.5) | 1247 (19.2) | 5327 (11.5) | |
| Hypertension | 6288 (11.9) | 1191 (18.4) | 5071 (11.0) | |
| Metabolic syndrome | 2246 (4.3) | 752 (11.6) | 1487 (3.2) | |
| Arrhythmia | 2138 (4.0) | 344 (5.3) | 1789 (3.9) | |
| Congestive heart failure | 277 (0.5) | 42 (0.6) | 232 (0.5) | |
| Coronary artery disease | 189 (0.4) | 42 (0.6) | 145 (0.3) | |
| Heart attack | 259 (0.5) | 45 (0.7) | 210 (0.5) | |
| Heart valve disease | 462 (0.9) | 65 (1.0) | 395 (0.9) | |
| Stroke | 338 (0.6) | 63 (1.0) | 270 (0.6) | |
| Transient ischemic attack | 461 (0.9) | 95 (1.5) | 363 (0.8) | |
| Deep vein thrombosis | 727 (1.4) | 135 (2.1) | 590 (1.3) | |
| Pulmonary embolism | 476 (0.9) | 109 (1.7) | 365 (0.8) | |
| Among a subset who completed the hormonal symptoms survey | ||||
| No. of participants | 25 399 | 2925 | 21 456 | |
| Irregular cycles | 6508 (25.6) | 1474 (50.4) | 4754 (22.2) | |
Abbreviation: PCOS, polycystic ovary syndrome.
Participants selected more than 1 racial category.
Other includes American Indian or Alaska Native, Middle Eastern or North African, Native Hawaiian or Pacific Islander, or none of these categories.
Measured by weight in kilograms divided by height in meters squared.
Family history of any metabolic conditions included obesity, high cholesterol, hypertension, and type 1 or type 2 diabetes.
Menstrual Cycle Characteristics and PCOS
Table 2 displays the associations of prolonged time to regularity with PCOS and irregular cycles. Longer time to regularity was associated with an increased prevalence of PCOS (unadjusted POR, 3.53; 95% CI, 3.34-3.74) and irregular cycles in adulthood (unadjusted POR, 2.96; 95% CI, 2.78-3.15). The positive association between time to regularity and irregular cycles in adulthood remained among participants without PCOS (unadjusted POR, 2.66; 95% CI, 2.47-2.86). Among participants with PCOS, the associations between time to regularity and irregular cycles were attenuated (unadjusted POR, 2.09; 95% CI, 1.79-2.45) but some remained positive.
Table 2. Association of Prolonged Time to Cycle Regularity With PCOS and Irregular Cycles.
| Association | No. of participants | POR (95% CI) | ||
|---|---|---|---|---|
| Unadjusted | Adjusted model 1a | Adjusted model 2b | ||
| Time to cycle regularity and PCOS | ||||
| Prolonged (yes vs no) | 50 303 | 3.53 (3.34-3.74) | 3.59 (3.39-3.80) | 3.38 (3.17-3.59) |
| Each category, y | 50 303 | NA | NA | NA |
| ≤2 | 31 138 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 3-4 | 3188 | 1.16 (1.01-1.33) | 1.18 (1.02-1.35) | 1.23 (1.06-1.41) |
| ≥5 | 2933 | 2.65 (2.37-2.96) | 2.62 (2.35-2.92) | 2.71 (2.41-3.05) |
| Not yet regular | 5752 | 4.75 (4.41-5.13) | 4.98 (4.60-5.38) | 4.99 (4.59-5.43) |
| Regular after using hormones | 7292 | 3.15 (2.93-3.39) | 3.23 (2.99-3.48) | 2.84 (2.62-3.07) |
| Time to cycle regularity and irregular cycles c | ||||
| Prolonged (yes vs no) | 21 731 | 2.96 (2.78-3.15) | 2.78 (2.61-2.96) | 2.78 (2.60-2.97) |
| Each category, y | 21 731 | NA | NA | NA |
| ≤2 | 13 623 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 3-4 | 1332 | 1.28 (1.12-1.47) | 1.21 (1.06-1.38) | 1.22 (1.05-1.40) |
| ≥5 | 1249 | 1.33 (1.16-1.53) | 1.37 (1.19-1.57) | 1.39 (1.20-1.60) |
| Not yet regular | 2228 | 8.34 (7.56-9.20) | 7.47 (6.77-8.25) | 7.48 (6.73-8.33) |
| Regular after using hormones | 3305 | 1.99 (1.83-2.16) | 1.87 (1.72-2.03) | 1.88 (1.72-2.06) |
| Time to cycle regularity and irregular cycles among participants with PCOS c | ||||
| Prolonged (yes vs no) | 2685 | 2.09 (1.79-2.45) | 1.92 (1.64-2.26) | 2.10 (1.77-2.49) |
| Each category, y | 2685 | NA | NA | NA |
| ≤2 | 1011 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 3-4 | 103 | 0.90 (0.59-1.36) | 0.87 (0.57-1.34) | 0.90 (0.57-1.39) |
| ≥5 | 227 | 0.75 (0.55-1.01) | 0.81 (0.59-1.10) | 0.91 (0.66-1.26) |
| Not yet regular | 670 | 4.65 (3.75-5.80) | 3.99 (3.20-5.01) | 4.39 (3.47-5.58) |
| Regular after using hormones | 674 | 1.45 (1.19-1.76) | 1.35 (1.10-1.65) | 1.44 (1.16-1.78) |
| Time to cycle regularity and irregular cycles among participants without PCOS c | ||||
| Prolonged (yes vs no) | 18 256 | 2.66 (2.47-2.86) | 2.49 (2.31-2.68) | 2.51 (2.32-2.71) |
| Each category, y | 18 256 | NA | NA | NA |
| ≤2 | 12 118 | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| 3-4 | 1183 | 1.34 (1.16-1.55) | 1.27 (1.09-1.46) | 1.27 (1.09-1.47) |
| ≥5 | 964 | 1.27 (1.08-1.49) | 1.30 (1.10-1.53) | 1.35 (1.14-1.59) |
| Not yet regular | 1470 | 7.74 (6.90-8.69) | 6.93 (6.17-7.80) | 7.00 (6.18-7.92) |
| Regular after using hormones | 2521 | 1.82 (1.64-2.00) | 1.70 (1.53-1.87) | 1.72 (1.55-1.91) |
Abbreviations: NA, not applicable; PCOS, polycystic ovary syndrome; POR, prevalence odds ratio.
Adjusted for age and race and ethnicity. Data from participants with missing covariates were excluded from the analysis.
Adjusted for age, race and ethnicity, socioeconomic status, employment, education, age at menarche, gravidity, body mass index (for outcomes other than obesity), family history of metabolic conditions, and ever hormone use. Data from participants with missing covariates were excluded from the analysis.
Among the subset of participants who responded to the survey question used to define irregular cycles in the hormonal symptoms survey.
PCOS and Cardiometabolic Conditions
Table 3 shows the associations between PCOS and the prevalence of each cardiometabolic condition. After adjusting for all covariates, diagnosed PCOS was associated with a higher prevalence of all metabolic conditions, including obesity (POR, 2.94; 95% CI, 2.77-3.12), prediabetes (POR, 3.75; 95% CI, 3.47-4.06), type 1 diabetes (POR, 1.43; 95% CI, 1.07-1.90), type 2 diabetes (POR, 2.76; 95% CI, 2.43-3.15), high cholesterol (POR, 1.68; 95% CI, 1.55-1.81), hypertension (POR, 1.57; 95% CI, 1.45-1.70), and metabolic syndrome (POR, 3.28; 95% CI, 2.94-3.66), as well as cardiovascular conditions, including arrhythmia (POR, 1.37; 95% CI, 1.20-1.55), CAD (POR, 2.92; 95% CI, 1.95-4.29), heart attack (POR, 1.79; 95% CI, 1.23-2.54), stroke (POR, 1.66; 95% CI, 1.21-2.24), TIA (POR, 1.87; 95% CI, 1.44-2.40), DVT (POR, 1.54; 95% CI, 1.24-1.89), and pulmonary embolism (POR, 1.83; 95% CI, 1.43-2.32).
Table 3. Association of Having PCOS With Prevalent Cardiometabolic Conditions (n = 52 619).
| Condition | POR (95% CI) | ||
|---|---|---|---|
| Unadjusted | Adjusted model 1a | Adjusted model 2b | |
| No. of participants | 52 619 | 52 452 | 47 152 |
| Obesity | 2.97 (2.82-3.14) | 3.09 (2.93-3.27) | 2.94 (2.77-3.12) |
| Prediabetes | 4.66 (4.35-4.99) | 5.30 (4.94-5.69) | 3.75 (3.47-4.06) |
| Type 1 diabetes | 1.67 (1.28-2.13) | 1.70 (1.31-2.18) | 1.43 (1.07-1.90) |
| Type 2 diabetes | 3.21 (2.87-3.59) | 4.09 (3.63-4.60) | 2.76 (2.43-3.15) |
| High cholesterol | 1.83 (1.70-1.95) | 2.11 (1.96-2.26) | 1.68 (1.55-1.81) |
| Hypertension | 1.82 (1.70-1.95) | 2.14 (1.99-2.30) | 1.57 (1.45-1.70) |
| Metabolic syndrome | 3.95 (3.60-4.33) | 5.17 (4.69-5.70) | 3.28 (2.94-3.66) |
| Arrhythmia | 1.39 (1.23-1.56) | 1.45 (1.28-1.63) | 1.37 (1.20-1.55) |
| Congestive heart failure | 1.29 (0.92-1.77) | 1.54 (1.09-2.13) | 1.23 (0.83-1.76) |
| Coronary artery disease | 2.07 (1.45-2.89) | 3.20 (2.21-4.56) | 2.92 (1.95-4.29) |
| Heart attack | 1.53 (1.09-2.09) | 1.86 (1.32-2.55) | 1.79 (1.23-2.54) |
| Heart valve disease | 1.17 (0.89-1.52) | 1.28 (0.97-1.66) | 1.21 (0.89-1.62) |
| Stroke | 1.67 (1.26-2.18) | 1.87 (1.40-2.45) | 1.66 (1.21-2.24) |
| Transient ischemic attack | 1.88 (1.49-2.34) | 2.18 (1.72-2.74) | 1.87 (1.44-2.40) |
| Deep vein thrombosis | 1.64 (1.35-1.98) | 1.84 (1.52-2.23) | 1.54 (1.24-1.89) |
| Pulmonary embolism | 2.14 (1.72-2.65) | 2.35 (1.88-2.91) | 1.83 (1.43-2.32) |
Abbreviations: PCOS, polycystic ovary syndrome; POR, prevalence odds ratio.
Adjusted for age and race and ethnicity. Data from participants with missing covariates were excluded from the analysis.
Adjusted for age, race and ethnicity, socioeconomic status, employment, education, age at menarche, gravidity, body mass index (for outcomes other than obesity), family history of metabolic conditions, and ever hormone use. Data from participants with missing covariates were excluded from the analysis.
Time to Regularity and Cardiometabolic Conditions
Figure 1A and B show the covariate-adjusted associations between prolonged time to regularity and the prevalence of cardiometabolic conditions (full estimates are shown in eTable 3 in Supplement 1). In the full study population, prolonged time to regularity was associated with a higher prevalence of all metabolic conditions and several cardiovascular conditions, including arrhythmia, stroke, and TIA. Among the 37 707 participants without PCOS, prolonged time to regularity remained associated with a higher prevalence of prediabetes (POR, 1.20; 95% CI, 1.08-1.33), type 1 diabetes (POR, 1.52; 95% CI, 1.16-1.99), type 2 diabetes (POR, 1.24; 95% CI, 1.05-1.46), hypertension (POR, 1.09; 95% CI, 1.01-1.19), arrythmia (POR, 1.20; 95% CI, 1.06-1.35), and TIA (POR, 1.33; 95% CI, 1.01-1.73).
Figure 1. Covariate-Adjusted Associations of Prolonged Time to Regularity and Irregular Cycles With Prevalent Cardiometabolic Conditions.
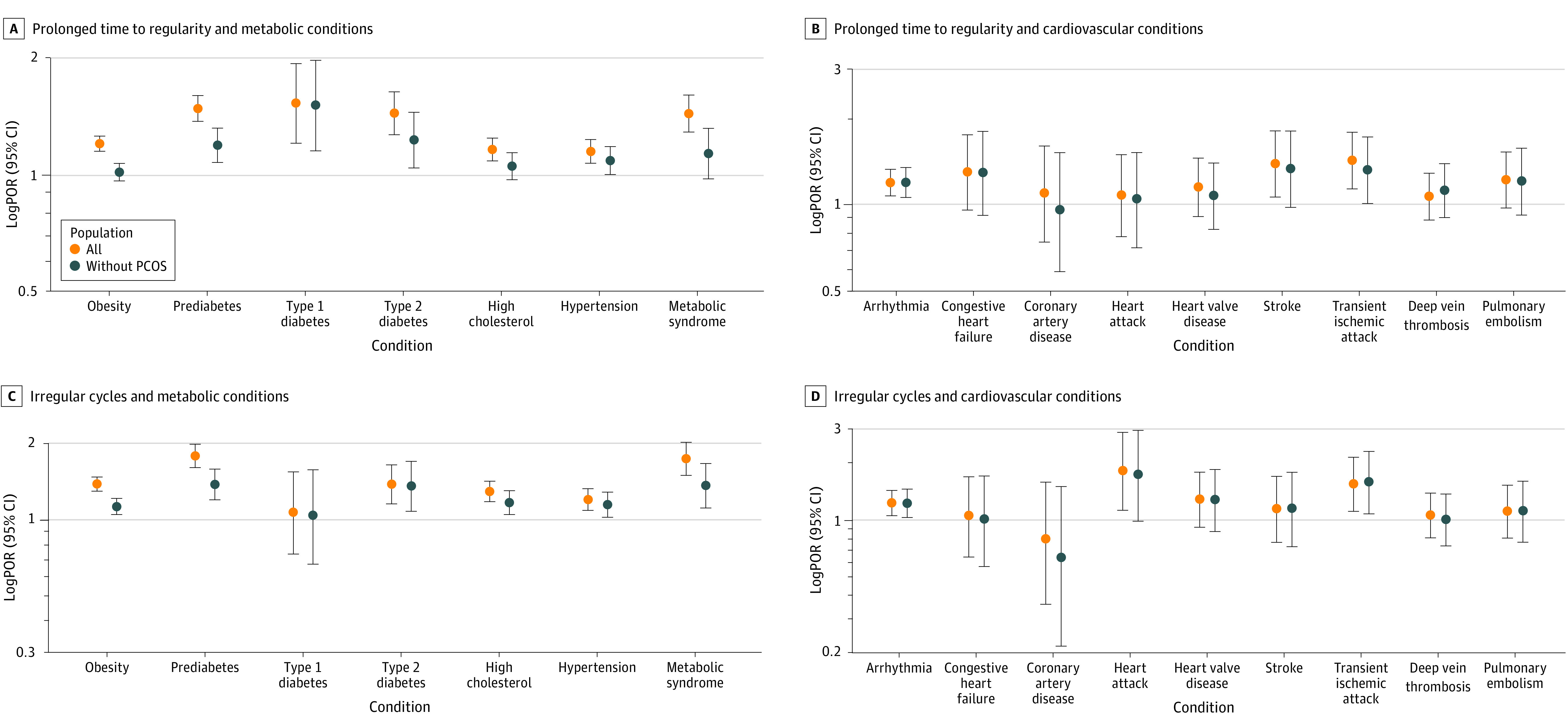
Adjusted for age, race and ethnicity, socioeconomic status, employment, education, age at menarche, gravidity, body mass index (for outcomes other than obesity), family history of metabolic conditions, and ever hormone use. Data from participants with missing covariates were excluded from the analysis. PCOR indicates polycystic ovary syndrome; POR, prevalence odds ratio.
Irregular Cycles and Cardiometabolic Conditions
Figure 1C and D show the covariate-adjusted associations between irregular cycles and the prevalence of cardiometabolic conditions (full estimates are shown in eTable 4 in Supplement 1). Among the 25 115 participants who responded to this survey question, irregular cycles were associated with a higher prevalence of obesity (POR, 1.38; 95% CI, 1.29-1.47), prediabetes (POR, 1.78; 95% CI, 1.60-1.98), type 2 diabetes (POR, 1.38; 95% CI, 1.15-1.64), high cholesterol (POR, 1.29; 95% CI, 1.18-1.41), hypertension (POR, 1.20; 95% CI, 1.09-1.32), metabolic syndrome (POR, 1.73; 95% CI, 1.49-2.01), arrythmia (POR, 1.21; 95% CI, 1.04-1.41), heart attack (POR, 1.80; 95% CI, 1.11-2.85), and TIA (POR, 1.53; 95% CI, 1.09-2.11). These positive associations remained among participants without PCOS (type 2 diabetes: POR, 1.36 [95% CI, 1.08-1.69]; high cholesterol: POR, 1.17 [95% CI, 1.05-1.30]; arrhythmia: POR, 1.21 [95% CI, 1.02-1.43]; TIA: POR, 1.56 [95% CI, 1.06-2.26]).
Exploratory Effect Modification Analyses
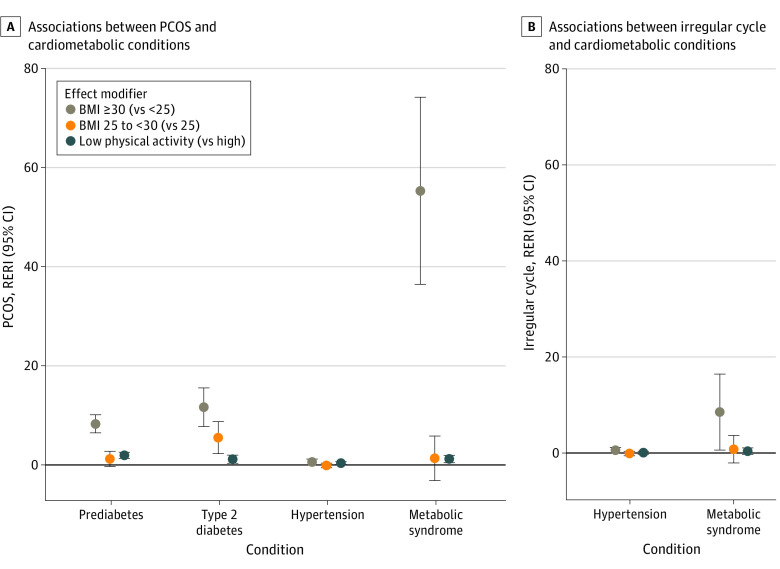
Estimates of RERIs for effect modification by BMI and physical activity are presented in eTables 5 to 8 in Supplement 1, and results with a significant effect modification by either factor are illustrated in Figure 2. There were positive effect modifications by BMI (high vs low) and physical activity (low vs high) for the associations between PCOS and several metabolic conditions (Figure 2A). Additionally, excess risk of arrythmia (RERI, 0.44; 95% CI, 0.08-0.80), stroke (RERI, 1.50; 95% CI, 0.11-2.88), and DVT (RERI, 1.01; 95% CI, 0.15-1.86) were introduced by gravidity (≥2 vs 0). Similar effect modifications by BMI were found for the associations between irregular cycles and certain cardiometabolic conditions among participants without PCOS (Figure 2B).
Figure 2. Exploratory Analyses of Effect Modification by Body Mass Index (BMI) and Physical Activity.
Only results with significant effect modifications by either BMI (as measured by weight in kilograms divided by height in meters squared) or physical activity are shown. Evaluation of effect modification by BMI is not applicable for the outcome of obesity. The full estimates for all conditions are shown in eTables 5 to 8 in Supplement 1. The relative excess risk due to interaction (RERI) indicates the difference between the joint risk ratio and the separate contributions by the exposure and modifier (0, no interaction or exact additivity; >0, positive interaction or more than additivity; <0, negative interaction or less than additivity); the RERI can range to infinity in either direction. The 95% CIs for RERI were calculated based on the delta method. PCOS indicates polycystic ovary syndrome.
Other Exploratory Analyses
Results from other exploratory and sensitivity analyses are shown in eTables 9 to 19 and eFigure 3 in Supplement 1. Within subsets of participants who provided age at diagnosis (eTable 9 in Supplement 1), the mean age at diagnosis for cardiometabolic conditions was 4 to 7 years younger among those with PCOS vs no PCOS or those with prolonged time to regularity vs regular cycles within 4 years. A higher but statistically nonsignificant prevalence was found between possible hypothalamic amenorrhea and arrhythmia (eTable 10 in Supplement 1). Other forms of modeling the time-to-regularity variable, or excluding 409 individuals with potentially misclassified or inaccurate time to regularity, yielded similar conclusions (eTables 11 and 12; eFigure 3 in Supplement 1). Using multiple imputations for missing data resulted in similar findings (eTables 13-15 in Supplement 1). Excluding individuals with possible PCOS based on reported irregularity and hirsutism led to similar conclusions to those previously presented (eTable 16 in Supplement 1). Adjusting for parity instead of gravidity had a minimal impact (eTables 17-19 in Supplement 1).
Discussion
In this cross-sectional study of a large US cohort, we examined associations among PCOS, irregular cycles, and multiple cardiometabolic conditions. Similar to previous studies, we found that PCOS was associated with a higher prevalence of cardiometabolic conditions. Additionally, prolonged time to regularity since menarche or irregular cycles in adulthood was associated with a higher prevalence of certain cardiometabolic conditions, even among participants without PCOS. Some of these associations were potentiated by high BMI or low physical activity. Findings from exploratory analyses suggested a younger age at diagnosis of several cardiometabolic conditions among participants with PCOS or prolonged time to regularity.
While the positive associations between PCOS and cardiometabolic conditions are consistent with prior studies,10,34,35,36,37,38 our findings add to the existing knowledge through the study of a larger population-based cohort with a variety of common or rare (eg, arrythmia, stroke) cardiometabolic conditions. Meta-analyses have reported an increased prevalence of obesity in individuals with PCOS,39 although debate on temporality continues.40,41 Studies have also reported a higher prevalence of impaired glucose tolerance and diabetes among patients with PCOS,42,43 and current international PCOS guidelines recommend diabetes screening.3 Previous studies reported a higher risk of dyslipidemia and hypertension with PCOS, mostly attributable to insulin resistance and hyperandrogenism through lipid metabolism and renin-angiotensin mechanisms.44,45,46 In addition to higher prevalence, our preliminary finding of younger mean age at diagnosis of several metabolic conditions among individuals with PCOS suggests potential earlier onset of metabolic sequelae that may lead to shortening of health and/or life span, warranting future research. For cardiovascular conditions, we found associations between PCOS and higher prevalence of CAD, arrythmia, heart attack, stroke, TIA, DVT, and pulmonary embolism. Existing studies on PCOS and CVD were mostly retrospective or cross-sectional, conducted in small populations or hospital-based populations, and documented mixed findings.9,10,34,35,36,37,38 Further population-based studies followed throughout the life course are needed to provide better insights for causal interpretations.
Existing large cohort studies on cardiac outcomes and other studies of associations between irregular cycles and increased CVD and CVD mortality included limited data on PCOS ascertainment or irregular cycles during adolescence47; therefore, the findings were unable to show whether the observed associations might be explained by underlying PCOS or other etiology or how these conditions may be identified as early as possible. Our study fills in those gaps in several ways. First, we evaluated irregular cycles and cardiometabolic conditions among individuals with and without diagnosed PCOS. We found that having irregular cycles was associated with an elevated prevalence of cardiometabolic conditions, even among participants without PCOS. Second, we observed an elevated prevalence of cardiometabolic conditions among participants with a longer time from menarche to cycle regularity, an early-life marker of menstrual health that has rarely been evaluated in past studies. Third, the findings of our exploratory analyses suggest that individuals with prolonged time to regularity may be younger at diagnosis of several metabolic conditions as well as arrythmia and DVT, posing long-term health consequences. Regular menstruation (and maturation of the reproductive axis) are typically established within 1 to 2 years after menarche,3,48,49 but evidence remains limited on whether irregularity beyond 2 years is harmful.50 Prolonged time to regularity and adulthood irregular cycles may both be vital signs that could serve as markers of metabolic abnormalities, predisposing one to increased CVD prevalence or earlier onset. While future studies are needed to explore the precise time frame, our findings suggest a potentially important time window for early intervention and subsequent cardiometabolic health outcomes.
We also found effect modification by BMI and physical activity for the associations between PCOS or irregular cycles and certain cardiometabolic conditions, which has rarely been reported in population-based studies. While future studies with longitudinal data are warranted, our findings suggest the benefit of early intervention, such as weight management and exercise, that may help to alleviate cardiometabolic conditions among individuals with PCOS and/or irregular cycles. Furthermore, we found that hypothalamic amenorrhea, another etiology of irregular cycles,33,51,52,53,54,55 may contribute to a higher prevalence of arrhythmia, which may be explained by low estrogen levels and stress.33,56,57 Abnormal menstrual patterns may be indicative of a proinflammatory process58 that may result in elevated CVD risk. In summary, irregular cycles may serve as an early vital sign to initiate earlier screening, counseling, and lifestyle interventions for cardiometabolic conditions.
Strengths and Limitations
This study has several strengths. First, a sample size of 60 789 participants allowed for sufficient statistical power to detect even modest associations, including with some less common cardiometabolic conditions. Second, we collected time-to-regularity data as an early-life marker of menstrual health, which could prompt interventions to reduce future cardiometabolic risk. Third, our study was among the first to account for PCOS status when evaluating associations between irregular cycles and cardiometabolic conditions, which could better distinguish the potential pathophysiology of the observed associations. Fourth, we explored preliminary differences in age at diagnosis of cardiometabolic conditions by PCOS status and time to regularity, which may inform future research on early intervention and quality of life over the life course. Finally, the AWHS includes a diverse population by demographics; thus, our findings may be more generalizable than previous studies that evaluated PCOS, irregular cycles, and cardiometabolic conditions.
Our study also has several limitations. First, in this cross-sectional evaluation, all data were self-reported at baseline, so potential misclassification and reverse causation may be present. The cross-sectional design also limited our ability to account for lifetime hormone use in a more refined way that takes into account temporality. Future longitudinal evaluations with incident cases are needed to better understand causal effects and differentiate between increased lifetime risk vs earlier onset so that appropriate interventions over the life course can be explored. Second, the group without PCOS may include unreported and undiagnosed individuals given the complexity of a clinical PCOS diagnosis,59 which may lead to biased estimates. However, some of the associations remained after excluding individuals with possible PCOS based on reported irregular cycles and hirsutism. Third, the survey response of troubled by unpredictable periods to define irregular menstrual cycles is a subjective measure of irregular cycles, but this proxy measure may lead to early self-identification and screening among the general population regardless of health literacy level or access to health care or menstrual tracking technologies. Fourth, unmeasured confounding may exist. Fifth, we were unable to generate commonly used composite CVD end points because we did not have data on cardiovascular death or hospitalization,60 and our definition for metabolic syndrome was modified based on data availability. Additionally, multiple comparisons might increase the type I error rate. However, there is no uniform agreement on whether or when multiple testing adjustments are warranted.61,62,63 Our analysis of PCOS showed large effect sizes and statistical significance, and our analysis on irregular cycles among participants without PCOS are novel and hypothesis generating by nature. Finally, given the criteria and self-selection for enrollment, our findings may not be generalizable to all US individuals or to other populations.
Conclusions
In this cross-sectional study of a large US cohort, we found positive associations between PCOS and cardiometabolic conditions and identified associations between longer time from menarche to cycle regularity or adulthood irregular cycles and certain cardiometabolic conditions, even among individuals without diagnosed PCOS. Some of these associations were modified by BMI and physical activity. Exploratory analyses suggested that individuals with PCOS or prolonged time to regularity may be younger at diagnosis of several cardiometabolic conditions. While further validation is needed, there may be potential benefits to earlier screening and intervention among individuals with irregular cycles, regardless of PCOS diagnosis.
eMethods.
eReference
eTable 1. Survey Questions Relevant to Exposure Variables in This Study
eTable 2. Baseline Characteristics in the Full Study Population for This Analysis vs Participants Who Responded to the Hormonal Symptoms Survey
eTable 3. Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions
eTable 4. Associations Between Having Irregular Cycles at Enrollment and Prevalent Cardiometabolic Conditions Among Participants Who Responded to the Hormonal Symptoms Survey
eTable 5. Covariate-Adjusted Associations of PCOS With Prevalent Cardiometabolic Conditions, Tests for Effect Modification by BMI on the Additive Scale (With BMI <25 as the Referent Group)
eTable 6. Covariate-Adjusted Associations Between Irregular Cycles and Prevalent Cardiometabolic Conditions Among Those Without PCOS, Tests for Effect Modification by BMI on the Additive Scale (With BMI <25 as the Referent Group)
eTable 7. Covariate-Adjusted Associations of PCOS With Prevalent Cardiometabolic Conditions, Tests for Effect Modification by Physical Activity on the Additive Scale (Low vs High)
eTable 8. Covariate-Adjusted Associations Between Irregular Cycles and Prevalent Cardiometabolic Conditions Among Those Without PCOS, Tests for Effect Modification by Physical Activity on the Additive Scale (Low vs High)
eTable 9. Age at Diagnosis for Each Cardiometabolic Condition, Overall and Stratified by PCOS Diagnosis or Time to Regularity, Among a Subset of Participants Who Provided Data of Age at Diagnosis
eTable 10. Covariate-Adjusted Associations of Combined Information on Cycle Irregularity, PCOS, and BMI With Prevalent Cardiometabolic Conditions Among the Subset of Participants Who Responded to the Hormonal Symptoms Survey (n = 25 399)
eTable 11. Covariate-Adjusted Associations of Categorical Time to Cycle Regularity With Prevalent Cardiometabolic Conditions
eTable 12. Covariate-Adjusted Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions, Comparing Results From the Main Analysis to Sensitivity Analyses That Removed 409 Individuals With Potentially Misclassified/Inaccurate Time to Regularity
eTable 13. Associations of Having PCOS With Prevalent Cardiometabolic Conditions, Adjusted for Covariates, Comparing Results From Complete Case Analyses to Pooled Results From Multiple Imputations
eTable 14. Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions, Adjusted for Covariates, Comparing Results From Complete Cases Analyses to Pooled Results From Multiple Imputation
eTable 15. Associations of Having Irregular Cycles With Prevalent Cardiometabolic Conditions Among 25 115 Participants Who Responded to the Relevant Survey Question for Irregular Cycles, Adjusted for Covariates, Comparing Results From Complete Case Analyses to Pooled Results From Multiple Imputation
eTable 16. Associations of Having Irregular Cycles With Prevalent Cardiometabolic Conditions Among 25 115 Participants Who Responded to the Relevant Survey Question for Irregular Cycles, Adjusted for Covariates, Comparing Results From Main Analyses to Sensitivity Analyses Excluding Those With Possible PCOS
eTable 17. Associations of Having PCOS With Prevalent Cardiometabolic Conditions, Comparing Results From Main Analyses Adjusted for Gravidity to Results Adjusted for Parity
eTable 18. Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions, Comparing Results From Main Analyses Adjusted for Gravidity to Results Adjusted for Parity
eTable 19. Associations of Having Irregular Cycles With Prevalent Cardiometabolic Conditions Among 25 115 Participants Who Responded to the Relevant Survey Question for Irregular Cycles, Comparing Results From Main Analyses Adjusted for Gravidity to Results Adjusted for Parity
eFigure 1. Flowchart of Participants in This Study
eFigure 2. Conceptual Model for the Study Questions
eFigure 3. Associations of 1-Year Increase in Time to Cycle Regularity With Prevalent Cardiometabolic Conditions Among a Subset of 37 259 Participants Who Have Reached Cycle Regularity at Enrollment (Not Due to Hormone Use)
Data Sharing Statement
References
- 1.ACOG committee opinion No. 651: menstruation in girls and adolescents: using the menstrual cycle as a vital sign. Obstet Gynecol. 2015;126(6):e143-e146. doi: 10.1097/AOG.0000000000001215 [DOI] [PubMed] [Google Scholar]
- 2.Case AM, Reid RL. Effects of the menstrual cycle on medical disorders. Arch Intern Med. 1998;158(13):1405-1412. doi: 10.1001/archinte.158.13.1405 [DOI] [PubMed] [Google Scholar]
- 3.Teede HJ, Tay CT, Laven JJE, et al. Recommendations from the 2023 international evidence-based guideline for the assessment and management of polycystic ovary syndrome. J Clin Endocrinol Metab. 2023;108(10):2447-2469. doi: 10.1210/clinem/dgad463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tay CT, Garrad R, Mousa A, Bahri M, Joham A, Teede H. Polycystic ovary syndrome (PCOS): international collaboration to translate evidence and guide future research. J Endocrinol. 2023;257(3):e220232. doi: 10.1530/JOE-22-0232 [DOI] [PubMed] [Google Scholar]
- 5.Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855. doi: 10.1093/humrep/dew218 [DOI] [PubMed] [Google Scholar]
- 6.Naz MSG, Tehrani FR, Majd HA, et al. The prevalence of polycystic ovary syndrome in adolescents: a systematic review and meta-analysis. Int J Reprod Biomed. 2019;17(8):533-542. doi: 10.18502/ijrm.v17i8.4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez RC, Moore VM, Rumbold AR, Whitrow MJ, Avery JC, Davies MJ. Diagnosis delayed: health profile differences between women with undiagnosed polycystic ovary syndrome and those with a clinical diagnosis by age 35 years. Hum Reprod. 2021;36(8):2275-2284. doi: 10.1093/humrep/deab101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennett CC, Simon J. The role of polycystic ovary syndrome in reproductive and metabolic health: overview and approaches for treatment. Diabetes Spectr. 2015;28(2):116-120. doi: 10.2337/diaspect.28.2.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Osibogun O, Ogunmoroti O, Michos ED. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30(7):399-404. doi: 10.1016/j.tcm.2019.08.010 [DOI] [PubMed] [Google Scholar]
- 10.Wekker V, van Dammen L, Koning A, et al. Long-term cardiometabolic disease risk in women with PCOS: a systematic review and meta-analysis. Hum Reprod Update. 2020;26(6):942-960. doi: 10.1093/humupd/dmaa029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang ET, Cirillo PM, Vittinghoff E, Bibbins-Domingo K, Cohn BA, Cedars MI. Menstrual irregularity and cardiovascular mortality. J Clin Endocrinol Metab. 2011;96(1):E114-E118. doi: 10.1210/jc.2010-1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gast GCM, Grobbee DE, Smit HA, Bueno-de-Mesquita HB, Samsioe GN, van der Schouw YT. Menstrual cycle characteristics and risk of coronary heart disease and type 2 diabetes. Fertil Steril. 2010;94(6):2379-2381. doi: 10.1016/j.fertnstert.2010.03.044 [DOI] [PubMed] [Google Scholar]
- 13.Solomon CG, Hu FB, Dunaif A, et al. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87(5):2013-2017. doi: 10.1210/jcem.87.5.8471 [DOI] [PubMed] [Google Scholar]
- 14.Wang YX, Stuart JJ, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of cardiovascular disease. JAMA Netw Open. 2022;5(10):e2238513. doi: 10.1001/jamanetworkopen.2022.38513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung HF, Ferreira I, Mishra GD. The association between menstrual symptoms and hypertension among young women: a prospective longitudinal study. Maturitas. 2021;143:17-24. doi: 10.1016/j.maturitas.2020.08.006 [DOI] [PubMed] [Google Scholar]
- 16.Wang YX, Arvizu M, Rich-Edwards JW, et al. Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: prospective cohort study. BMJ. 2020;371:m3464. doi: 10.1136/bmj.m3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper GS, Ephross SA, Weinberg CR, Baird DD, Whelan EA, Sandler DP. Menstrual and reproductive risk factors for ischemic heart disease. Epidemiology. 1999;10(3):255-259. doi: 10.1097/00001648-199905000-00011 [DOI] [PubMed] [Google Scholar]
- 18.Iliodromiti S, Nelson SM. Irregular menstrual cycles are not associated with cardiovascular disease; a cohort study of 40,896 women. Fertil Steril. 2017;108(3):e250. doi: 10.1016/j.fertnstert.2017.07.750 [DOI] [Google Scholar]
- 19.Stuart JJ, Manson JE, Bairey Merz CN, et al. Abstract P110: hypothalamic amenorrhea phenotype and cardiovascular disease risk. Circulation. 2023;147(suppl 1):AP110. doi: 10.1161/circ.147.suppl_1.P110 [DOI] [Google Scholar]
- 20.Mahalingaiah S, Missmer SE, Cheng JJ, Chavarro J, Laden F, Hart JE. Perimenarchal air pollution exposure and menstrual disorders. Hum Reprod. 2018;33(3):512-519. doi: 10.1093/humrep/dey005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wise LA, Mikkelsen EM, Rothman KJ, et al. A prospective cohort study of menstrual characteristics and time to pregnancy. Am J Epidemiol. 2011;174(6):701-709. doi: 10.1093/aje/kwr130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahalingaiah S, Fruh V, Rodriguez E, et al. Design and methods of the Apple Women’s Health Study: a digital longitudinal cohort study. Am J Obstet Gynecol. 2022;226(4):545.e1-545.e29. doi: 10.1016/j.ajog.2021.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valeggia CR, Núñez-de la Mora A. Chapter 21—human reproductive ecology. In: Muehlenbein MP, ed. Basics in Human Evolution. Academic Press; 2015:295-308. doi: 10.1016/B978-0-12-802652-6.00021-9 [DOI] [Google Scholar]
- 24.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539-553. doi: [DOI] [PubMed] [Google Scholar]
- 25.Huang PL. A comprehensive definition for metabolic syndrome. Dis Model Mech. 2009;2(5-6):231-237. doi: 10.1242/dmm.001180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Gibson EA, Jukic AMZ, et al. Menstrual cycle length variation by demographic characteristics from the Apple Women’s Health Study. NPJ Digit Med. 2023;6(1):100. doi: 10.1038/s41746-023-00848-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Engmann L, Jin S, Sun F, et al. ; Reproductive Medicine Network . Racial and ethnic differences in the polycystic ovary syndrome metabolic phenotype. Am J Obstet Gynecol. 2017;216(5):493.e1-493.e13. doi: 10.1016/j.ajog.2017.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nobles J, Cannon L, Wilcox AJ. Menstrual irregularity as a biological limit to early pregnancy awareness. Proc Natl Acad Sci U S A. 2022;119(1):e2113762118. doi: 10.1073/pnas.2113762118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Javed Z, Haisum Maqsood M, Yahya T, et al. Race, racism, and cardiovascular health: applying a social determinants of health framework to racial/ethnic disparities in cardiovascular disease. Circ Cardiovasc Qual Outcomes. 2022;15(1):e007917. doi: 10.1161/CIRCOUTCOMES.121.007917 [DOI] [PubMed] [Google Scholar]
- 30.Moss RH, Kelly B, Bird PK, Pickett KE. Examining individual social status using the MacArthur Scale of Subjective Social Status: Findings from the Born in Bradford study. SSM Popul Health. 2023;23:101463. doi: 10.1016/j.ssmph.2023.101463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathur MB, VanderWeele TJ. R function for additive interaction measures. Epidemiology. 2018;29(1):e5-e6. doi: 10.1097/EDE.0000000000000752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Phylactou M, Clarke SA, Patel B, et al. Clinical and biochemical discriminants between functional hypothalamic amenorrhoea (FHA) and polycystic ovary syndrome (PCOS). Clin Endocrinol (Oxf). 2021;95(2):239-252. doi: 10.1111/cen.14402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shufelt CL, Torbati T, Dutra E. Hypothalamic amenorrhea and the long-term health consequences. Semin Reprod Med. 2017;35(3):256-262. doi: 10.1055/s-0037-1603581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kakoly NS, Khomami MB, Joham AE, et al. Ethnicity, obesity and the prevalence of impaired glucose tolerance and type 2 diabetes in PCOS: a systematic review and meta-regression. Hum Reprod Update. 2018;24(4):455-467. doi: 10.1093/humupd/dmy007 [DOI] [PubMed] [Google Scholar]
- 35.Randeva HS, Tan BK, Weickert MO, et al. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr Rev. 2012;33(5):812-841. doi: 10.1210/er.2012-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003;24(3):302-312. doi: 10.1210/er.2003-0004 [DOI] [PubMed] [Google Scholar]
- 37.Cooney LG, Dokras A. Beyond fertility: polycystic ovary syndrome and long-term health. Fertil Steril. 2018;110(5):794-809. doi: 10.1016/j.fertnstert.2018.08.021 [DOI] [PubMed] [Google Scholar]
- 38.Ollila MM, Arffman RK, Korhonen E, et al. Women with PCOS have an increased risk for cardiovascular disease regardless of diagnostic criteria-a prospective population-based cohort study. Eur J Endocrinol. 2023;189(1):96-105. doi: 10.1093/ejendo/lvad077 [DOI] [PubMed] [Google Scholar]
- 39.Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, obesity and central obesity in women with polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update. 2012;18(6):618-637. doi: 10.1093/humupd/dms030 [DOI] [PubMed] [Google Scholar]
- 40.Motta AB. The role of obesity in the development of polycystic ovary syndrome. Curr Pharm Des. 2012;18(17):2482-2491. doi: 10.2174/13816128112092482 [DOI] [PubMed] [Google Scholar]
- 41.Vilmann LS, Thisted E, Baker JL, Holm JC. Development of obesity and polycystic ovary syndrome in adolescents. Horm Res Paediatr. 2012;78(5-6):269-278. doi: 10.1159/000345310 [DOI] [PubMed] [Google Scholar]
- 42.Joham AE, Ranasinha S, Zoungas S, Moran L, Teede HJ. Gestational diabetes and type 2 diabetes in reproductive-aged women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99(3):E447-E452. doi: 10.1210/jc.2013-2007 [DOI] [PubMed] [Google Scholar]
- 43.Boudreaux MY, Talbott EO, Kip KE, Brooks MM, Witchel SF. Risk of T2DM and impaired fasting glucose among PCOS subjects: results of an 8-year follow-up. Curr Diab Rep. 2006;6(1):77-83. doi: 10.1007/s11892-006-0056-1 [DOI] [PubMed] [Google Scholar]
- 44.Chan DC, Watts GF. Dyslipidaemia in the metabolic syndrome and type 2 diabetes: pathogenesis, priorities, pharmacotherapies. Expert Opin Pharmacother. 2011;12(1):13-30. doi: 10.1517/14656566.2010.502529 [DOI] [PubMed] [Google Scholar]
- 45.Chen MJ, Yang WS, Yang JH, Chen CL, Ho HN, Yang YS. Relationship between androgen levels and blood pressure in young women with polycystic ovary syndrome. Hypertension. 2007;49(6):1442-1447. doi: 10.1161/HYPERTENSIONAHA.106.083972 [DOI] [PubMed] [Google Scholar]
- 46.Dumitrescu R, Mehedintu C, Briceag I, Purcarea VL, Hudita D. The polycystic ovary syndrome: an update on metabolic and hormonal mechanisms. J Med Life. 2015;8(2):142-145. [PMC free article] [PubMed] [Google Scholar]
- 47.Mahalingaiah S, Sun F, Cheng JJ, Chow ET, Lunetta KL, Murabito JM. Cardiovascular risk factors among women with self-reported infertility. Fertil Res Pract. 2017;3:7. doi: 10.1186/s40738-017-0034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legro RS, Lin HM, Demers LM, Lloyd T. Rapid maturation of the reproductive axis during perimenarche independent of body composition. J Clin Endocrinol Metab. 2000;85(3):1021-1025. doi: 10.1210/jc.85.3.1021 [DOI] [PubMed] [Google Scholar]
- 49.Zhang K, Pollack S, Ghods A, et al. Onset of ovulation after menarche in girls: a longitudinal study. J Clin Endocrinol Metab. 2008;93(4):1186-1194. doi: 10.1210/jc.2007-1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlson LJ, Shaw ND. Development of ovulatory menstrual cycles in adolescent girls. J Pediatr Adolesc Gynecol. 2019;32(3):249-253. doi: 10.1016/j.jpag.2019.02.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sweet MG, Schmidt-Dalton TA, Weiss PM, Madsen KP. Evaluation and management of abnormal uterine bleeding in premenopausal women. Am Fam Physician. 2012;85(1):35-43. [PubMed] [Google Scholar]
- 52.Mu F, Rich-Edwards J, Rimm EB, Spiegelman D, Missmer SA. Endometriosis and risk of coronary heart disease. Circ Cardiovasc Qual Outcomes. 2016;9(3):257-264. doi: 10.1161/CIRCOUTCOMES.115.002224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Verit FF, Akyol H, Sakar MN. Low antimullerian hormone levels may be associated with cardiovascular risk markers in women with diminished ovarian reserve. Gynecol Endocrinol. 2016;32(4):302-305. doi: 10.3109/09513590.2015.1116065 [DOI] [PubMed] [Google Scholar]
- 54.Signorello LB, Harlow BL, Cramer DW, Spiegelman D, Hill JA. Epidemiologic determinants of endometriosis: a hospital-based case-control study. Ann Epidemiol. 1997;7(4):267-741. doi: 10.1016/S1047-2797(97)00017-3 [DOI] [PubMed] [Google Scholar]
- 55.Cappola AR, Desai AS, Medici M, et al. Thyroid and cardiovascular disease: research agenda for enhancing knowledge, prevention, and treatment. Circulation. 2019;139(25):2892-2909. doi: 10.1161/CIRCULATIONAHA.118.036859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ganjehei L, Massumi A, Nazeri A, Razavi M. Cardiac arrhythmias in women. Tex Heart Inst J. 2011;38(2):157-159. [PMC free article] [PubMed] [Google Scholar]
- 57.Kuehn BM. Rising heart risks for young women linked to low estrogen. Circulation. 2019;139(4):549-550. doi: 10.1161/CIRCULATIONAHA.118.038754 [DOI] [PubMed] [Google Scholar]
- 58.Kuan KKW, Gibson DA, Whitaker LHR, Horne AW. Menstruation dysregulation and endometriosis development. Front Reprod Health. 2021;3:756704. doi: 10.3389/frph.2021.756704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Christ JP, Cedars MI. Current guidelines for diagnosing PCOS. Diagnostics (Basel). 2023;13(6):1113. doi: 10.3390/diagnostics13061113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marx N, McGuire DK, Perkovic V, et al. Composite primary end points in cardiovascular outcomes trials involving type 2 diabetes patients: should unstable angina be included in the primary end point? Diabetes Care. 2017;40(9):1144-1151. doi: 10.2337/dc17-0068 [DOI] [PubMed] [Google Scholar]
- 61.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43-46. doi: 10.1097/00001648-199001000-00010 [DOI] [PubMed] [Google Scholar]
- 62.Rothman KJ. Six persistent research misconceptions. J Gen Intern Med. 2014;29(7):1060-1064. doi: 10.1007/s11606-013-2755-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sjölander A, Vansteelandt S. Frequentist versus Bayesian approaches to multiple testing. Eur J Epidemiol. 2019;34(9):809-821. doi: 10.1007/s10654-019-00517-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReference
eTable 1. Survey Questions Relevant to Exposure Variables in This Study
eTable 2. Baseline Characteristics in the Full Study Population for This Analysis vs Participants Who Responded to the Hormonal Symptoms Survey
eTable 3. Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions
eTable 4. Associations Between Having Irregular Cycles at Enrollment and Prevalent Cardiometabolic Conditions Among Participants Who Responded to the Hormonal Symptoms Survey
eTable 5. Covariate-Adjusted Associations of PCOS With Prevalent Cardiometabolic Conditions, Tests for Effect Modification by BMI on the Additive Scale (With BMI <25 as the Referent Group)
eTable 6. Covariate-Adjusted Associations Between Irregular Cycles and Prevalent Cardiometabolic Conditions Among Those Without PCOS, Tests for Effect Modification by BMI on the Additive Scale (With BMI <25 as the Referent Group)
eTable 7. Covariate-Adjusted Associations of PCOS With Prevalent Cardiometabolic Conditions, Tests for Effect Modification by Physical Activity on the Additive Scale (Low vs High)
eTable 8. Covariate-Adjusted Associations Between Irregular Cycles and Prevalent Cardiometabolic Conditions Among Those Without PCOS, Tests for Effect Modification by Physical Activity on the Additive Scale (Low vs High)
eTable 9. Age at Diagnosis for Each Cardiometabolic Condition, Overall and Stratified by PCOS Diagnosis or Time to Regularity, Among a Subset of Participants Who Provided Data of Age at Diagnosis
eTable 10. Covariate-Adjusted Associations of Combined Information on Cycle Irregularity, PCOS, and BMI With Prevalent Cardiometabolic Conditions Among the Subset of Participants Who Responded to the Hormonal Symptoms Survey (n = 25 399)
eTable 11. Covariate-Adjusted Associations of Categorical Time to Cycle Regularity With Prevalent Cardiometabolic Conditions
eTable 12. Covariate-Adjusted Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions, Comparing Results From the Main Analysis to Sensitivity Analyses That Removed 409 Individuals With Potentially Misclassified/Inaccurate Time to Regularity
eTable 13. Associations of Having PCOS With Prevalent Cardiometabolic Conditions, Adjusted for Covariates, Comparing Results From Complete Case Analyses to Pooled Results From Multiple Imputations
eTable 14. Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions, Adjusted for Covariates, Comparing Results From Complete Cases Analyses to Pooled Results From Multiple Imputation
eTable 15. Associations of Having Irregular Cycles With Prevalent Cardiometabolic Conditions Among 25 115 Participants Who Responded to the Relevant Survey Question for Irregular Cycles, Adjusted for Covariates, Comparing Results From Complete Case Analyses to Pooled Results From Multiple Imputation
eTable 16. Associations of Having Irregular Cycles With Prevalent Cardiometabolic Conditions Among 25 115 Participants Who Responded to the Relevant Survey Question for Irregular Cycles, Adjusted for Covariates, Comparing Results From Main Analyses to Sensitivity Analyses Excluding Those With Possible PCOS
eTable 17. Associations of Having PCOS With Prevalent Cardiometabolic Conditions, Comparing Results From Main Analyses Adjusted for Gravidity to Results Adjusted for Parity
eTable 18. Associations of Prolonged Time to Cycle Regularity With Prevalent Cardiometabolic Conditions, Comparing Results From Main Analyses Adjusted for Gravidity to Results Adjusted for Parity
eTable 19. Associations of Having Irregular Cycles With Prevalent Cardiometabolic Conditions Among 25 115 Participants Who Responded to the Relevant Survey Question for Irregular Cycles, Comparing Results From Main Analyses Adjusted for Gravidity to Results Adjusted for Parity
eFigure 1. Flowchart of Participants in This Study
eFigure 2. Conceptual Model for the Study Questions
eFigure 3. Associations of 1-Year Increase in Time to Cycle Regularity With Prevalent Cardiometabolic Conditions Among a Subset of 37 259 Participants Who Have Reached Cycle Regularity at Enrollment (Not Due to Hormone Use)
Data Sharing Statement