Abstract
Anorexia nervosa (AN) is one of the most common psychiatric disorders among young adults and is associated with a substantial risk of death from suicide and medical complications. Transaminase elevations are common in patients with AN at the time of hospital admission and have been associated with longer lengths of hospital stay. Multiple types of hepatitis may occur in these patients, including two types that occur only in patients with AN: starvation hepatitis and refeeding-induced hepatitis. Starvation hepatitis is characterized by severe transaminase elevation in patients in the advanced phase of protein-energy deprivation and is associated with complications of severe starvation, such as hypoglycaemia, hypothermia, and hypotension. Refeeding-induced hepatitis is characterized by a milder increase in transaminases that occurs in the early refeeding phase and is associated with hypophosphatemia, hypokalemia, and hypomagnesaemia. Among the most common forms of hepatitis, drug-induced liver injury is particularly relevant in this patient cohort, given the frequent use and abuse of methamphetamines, laxatives, antidepressants, and antipsychotics. In this review, we provided an overview of the different forms of anorexic-associated hepatitis, a diagnostic approach that can help the clinician to correctly frame the problem, and indications on their management and treatment.
Keywords: transaminases, hepatitis, refeeding syndrome, starvation, anorexia nervosa, fatty liver
Introduction
Anorexia nervosa (AN) is an eating disorder with onset in adolescence or young adulthood that is characterized by starvation and malnutrition, a high incidence of coexisting psychiatric conditions, treatment resistance, and a substantial risk of death from medical complications and suicide [1]. Two subtypes of AN have been designated: ‘restrictive type’, in which weight loss is achieved by reducing calorie intake; and ‘binge-eating/purging type’, in which the subject is engaged in recurrent episodes of binge eating or purging [1]. AN is associated with a mortality rate that is five to six times higher than that of healthy people of the same age and has an 18-fold increased risk of suicide compared with that in the general population [2].
Transaminase elevations are common in patients with AN at the time of hospital admission, reaching a prevalence of 43% in a retrospective study [3]. Severe transaminase elevation on hospital admission has been associated with a higher risk of developing complications such as hypoglycaemia and hypophosphatemia, and longer lengths of hospital stay [4]. Several independent groups observed that serum transaminase levels inversely correlate with body mass index (BMI), suggesting a role of nutritional status in the liver changes of these patients [3, 5]. Although severe acute hepatitis and acute liver failure associated with AN are not common, there are literature reports showing that they can occur and some attention should be paid to them [6–9].
Multiple types of hepatitis may occur in patients with AN. In particular, two types of hepatitis that occur only in patients with AN have been described: starvation hepatitis and refeeding-induced hepatitis [10]. Starvation hepatitis is characterized by severe transaminase elevation in patients in the advanced phase of protein-energy deprivation and is associated with complications of severe starvation, such as hypoglycaemia, hypothermia, and hypotension. Refeeding-induced hepatitis is characterized by a milder increase in transaminases that occurs in the early refeeding phase and is associated with hypophosphatemia, hypokalemia, and hypomagnesaemia. Table 1 summarizes the main characteristics of these two types of hepatitis. Furthermore, other more common types of hepatitis can occur in patients with AN. In particular, drug-induced liver injury (DILI) takes on some peculiar aspects in patients with AN and deserves specific discussion. In this review, we provide an overview of the different forms of anorexia-associated hepatitis, a diagnostic approach that can help the clinician to correctly frame the problem, and indications on their management and treatment.
Table 1.
Differentiation points between starvation hepatitis and refeeding-induced hepatitis
| Characteristic | Starvation hepatitis | Refeeding-induced hepatitis |
|---|---|---|
| Pathogenesis |
|
Hepatic fat and glycogen deposition |
| Clinical picture | Severe hypoglycaemia, arrhythmia including bradycardia, oedema hypothermia, hypotension, amenorrhea (in females), and coma | Generally asymptomatic, hypophosphatemia, hypokalemia, and hypomagnesaemia. In severe cases, oedema, cardiac dysfunction, and neurological changes |
| Risk of onset and BMI | BMI < 12 kg/m2 | BMI < 16 kg/m2 |
| Serum transaminase levels | Severe increase | Mild increase |
| Imaging studies | The liver can be normal or small | The liver is enlarged and fatty |
| Histopathology of the liver | Mild lobular inflammation and glycogen depletion in the cytoplasm of the oedematous hepatocytes (sign of autophagy). Necrosis and apoptosis are not evident | Fatty liver with moderate portal inflammation, ballooning of hepatocytes, and increased glycogen deposits |
| Principles of treatment | Restorations of adequate caloric replenishment. Careful rehydration | Reduction of nutritional support; gradual rehydration; supplementation with thiamine, potassium, phosphate, and magnesium |
The mechanisms of anorexia-associated hepatitis
Starvation hepatitis
The mechanisms of liver cell injury and death in AN are not completely understood but have been proposed to occur from starvation inducing a process called ‘autophagy’ [11]. Autophagy (literally ‘self-eating’) is a cellular process responsible for the degradation of excess or aberrant long-lived cytosolic proteins and organelles within lysosomes to remove and eventually recycle the resulting macromolecules [5].
In patients with AN, it has been proposed that autophagy in hepatocytes may initially be protective but, when AN-induced starvation worsens and BMI decreases, excessive activation of autophagy leads to increased liver injury [9, 12]. Rautou et al. [11] performed a detailed pathologic examination on liver specimens from 12 patients with severe hepatitis and AN as the only cause. Liver cell glycogen depletion was a constant finding. They found no necrosis or apoptosis of hepatocytes on histology but several autophagosomes—a hallmark of autophagy—in hepatocytes were discovered by using electron microscopy. Immunohistochemical analyses for autophagy-related 5 (ATG5)—a key protein for autophagosome formation—provided similar results. During apoptosis, nuclei are condensed and membrane integrity is saved. Conversely, during autophagic cell death, the permeability of the plasma membrane is altered and transaminases are released. Increased permeability of the hepatocyte membrane that occurs in autophagy could partially explain the marked serum transaminase elevation in the absence of extensive liver cell necrosis or inflammation in these patients [11].
Some authors proposed that, in addition to autophagy, hypovolemia-induced liver hypoxia contributes to the pathogenesis of AN-induced severe hepatitis. Ramsoekh et al. [6] underlined that some patients with AN had a history of hypotensive episodes, while others had signs of left ventricular dysfunction secondary to reduced cardiac mass and volume. They also observed that serum transaminase reduced suddenly after intravenous fluid repletion in many patients before nutritional support was started, similarly to what occurs in hypoxic hepatitis. In these cases, elevated serum lactate dehydrogenase combines with transaminases elevation [10]. Histological signs of hypoxic hepatitis, such as centrilobular changes (necrosis, atrophy, or sinusoidal fibrosis) or venular congestion, can be found in some of these patients [6, 11].
Refeeding-induced hepatitis
Refeeding syndrome (RFS) is a severe metabolic complication associated with an unbalanced reintroduction of nutrition in malnourished patients [13, 14]. As we have summarized in Tables 2 and 3, the early refeeding phase in extremely malnourished patients must be very gradual. If there is a relative excess of dextrose calories during the early stages of refeeding patients with AN, the blood glycaemic value leads to increased insulin secretion and decreased glucagon secretion, which stimulates glycogen, fat, and protein synthesis. This process requires electrolytes, such as phosphate and magnesium, and cofactors, such as thiamine [15]. In malnourished patients with AN, after prolonged periods of starvation, several intracellular electrolytes (including phosphate, potassium, and magnesium) become severely depleted, as well as thiamine, but their serum concentrations may remain normal. In the early phase of refeeding, if there is no careful supplementation of electrolytes and vitamins, RFS may occur, of which severe hypophosphataemia (<0.32 mmol/L), hypokalemia (<2.5 mmol/L), and hypomagnesia (<0.50 mmol/L) are typical diagnostic hallmarks [15, 16].
Table 2.
Refeeding syndrome risk classification
| Minor risk factor | Major risk factor | Very-high risk factor |
|---|---|---|
| BMI < 18.5 kg/m2 | BMI < 16 kg/m2 | BMI < 14 kg/m2 |
| Unintentional weight loss of >10% in the preceding 3–6 months | Unintentional weight loss of >15% in the preceding 3–6 months | Weight loss of >20% in the preceding 3–6 months |
| Very little or no nutritional intake for >5 days | Very little or no nutritional intake for >10 days | Starvation for >15 days |
| History of alcohol or drug abuse | Low levels of serum potassium, phosphate, or magnesium before feeding | |
|
| ||
| Patient classification | ||
|
| ||
| Low risk | High risk | Very-high risk |
|
| ||
| 1 minor risk factor | At least 1 major or 2 minor risk factors | At least 1 very-high risk factor |
Table 3.
Patient management based on refeeding syndrome risk classification
| Low risk | High risk | Very-high risk | |
|---|---|---|---|
| Nutritional support |
|
|
|
| Fluid management | 30–35 mg/kg/day |
|
|
| Sodium restriction | No sodium restriction | Days 1–7: <1 mmol/kg/day | Days 1–10: <1 mmol/kg/day |
| Phosphate |
|
||
| Potassium |
|
||
| Magnesium |
|
||
| Thiamine | 200 or 300 mg/day for 10 days | ||
| Multivitamins | For 10 days | ||
In refeeding-induced hepatitis, a relative excess of dextrose calories during the early stages of refeeding patients with AN, and the subsequent insulin secretion, leads to increased glycogen and triglycerides deposition at the hepatic level. Liver biopsy typically shows a fatty liver with moderate portal inflammation, ballooning of hepatocytes, and increased glycogen deposits [10]. This condition has also been termed ‘refeeding steatosis’ because the histological characteristics, paradoxically, are very similar to those of non-alcoholic steatohepatitis associated with diabetes, obesity, and metabolic syndrome [17]. An enlarged fatty liver can be identified on sonographic imaging and it represents an important diagnostic clue [17, 18].
DILI in patients with AN
Patients with the AN ‘purging type’ tend to take herbal or dietary products that promote meal elimination or weight loss. The term ‘herbal and dietary supplements (HDS)’ defines a broad spectrum of supplements, including vitamins, minerals, dietary elements, food components, natural herbs, herbal preparations, and synthetic compounds, that are used to supplement or replace the diet and could induce liver injury. These products can be purchased without a prescription and taken without medical guidance but, as opposed to conventional drugs, the safety and efficacy of HDS are not always well defined. For dietary supplements, there is a lack of adequate regulatory legislation, such as that governing the market entry of drugs in both the USA (Food and Drug Administration) and Europe (European Medicines Agency). The typical clinical presentation of HDS-induced liver injury is acute hepatitis with latency to onset usually of 1–3 months. Currently, most cases of HDS-induced liver injury are due to multi-ingredient nutritional supplements and the component responsible for the toxicity is usually unknown or can only be suspected [19, 20].
Methamphetamines have appetite-suppressing and exercise-promoting effects that may appeal to young women who are concerned about their weight and body image [21]. It has been observed that the use of several amphetamines can cause clinically evident and sometimes severe or even fatal instances of acute liver injury. The synthetic amphetamine methylenedioxymethamphetamine (familiarly known as ‘ecstasy’) has been implicated in the largest number of cases, many of which were severe and led to acute liver failure and death. The onset of ecstasy-induced hepatitis generally occurs 3–14 days after ingestion and is typically abrupt, with fatigue, weakness, jaundice, and confusion [21].
Another category of substances that are overused by patients affected by AN is laxatives. These patients may initially take laxatives to treat constipation caused by low food intake and dehydration. However, patients with ‘purging-type’ AN mistakenly believe that the laxatives will work to rush food and calories through the gut before they can be absorbed and, thus, will prevent calorie absorption and weight gain [22]. Some types of laxatives (i.e. danthron, docusate sodium, herbal laxatives containing boldo leaf extracts) have a known hepatotoxic potential [23].
Finally, some concomitant antidepressant and antipsychotic agents (i.e. paroxetine, sertraline venlafaxine, amitriptyline, chlorpromazine, olanzapine, and clozapine) have a hepatotoxicity profile that is well described in the literature, ranging from a Likelihood score of A to B according to LiverTox® database [24]. In conclusion, any drug that has been started within the 3 months preceding the transaminase elevation should be considered a potential toxic agent.
Diagnostic approach of liver injury in patients with AN
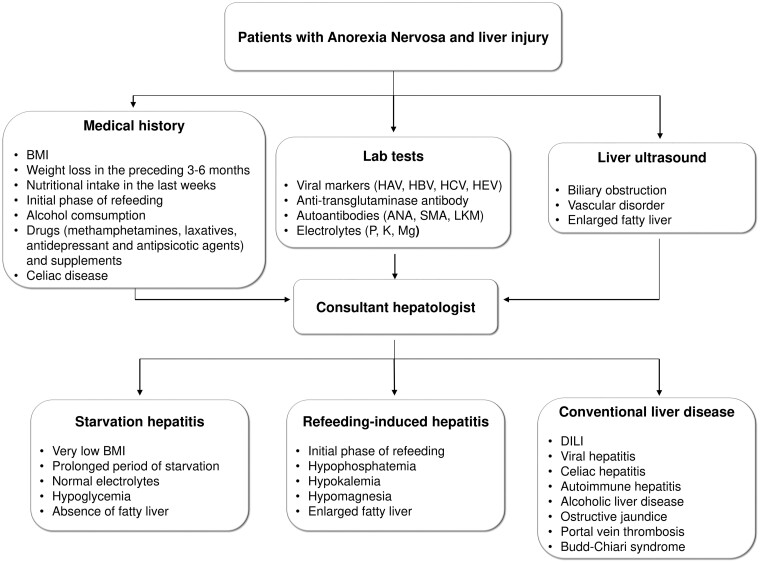
In patients with AN and transaminases elevation, the differential diagnosis of liver damage is not straightforward. Anorexia, vomiting, and unintended weight loss may be onset symptoms of acute viral hepatitis or DILI and can be confused with symptoms of psychiatric disease. The clues to reaching the diagnosis are in the clinical history. It is necessary to disentangle the behaviours that led to the restriction of energy intake. If calorie restriction has been relatively stable for the previous weeks and there has been a sudden worsening of anorexia and weight loss, it is reasonable to suspect a virus or a drug as the cause of hepatitis. Conversely, starvation hepatitis generally occurs in a phase of severe and prolonged calorie restriction, and a very low BMI (<12 kg/m2) represents an important diagnostic clue. For these reasons, a thorough medical history should include questions about the rate and amount of weight loss, compensatory behaviours (fasting, self-induced vomiting, use of laxatives or diuretics), exercise, and menstrual history [25]. In any case, the diagnosis of starvation hepatitis requires the exclusion of other causes of liver damage. A proposed diagnostic approach is depicted in Figure 1.
Figure 1.
Proposed diagnostic flow chart of malnourished patients with AN and liver damage. ANA = anti-nuclear antibodies, SMA = anti-smooth muscle antibodies, LKM = anti-liver–kidney microsome antibodies, BMI = body mass index, DILI = drug-induced liver injury, HAV = hepatitis A virus, HBV = hepatitis B virus, HCV = hepatitis C virus, HEV = hepatitis E virus, M = magnesium, P = phosphorus, K = kalium.
There are other important issues to collect in the clinical history. Celiac disease has a greater prevalence in patients with AN and the possibility of celiac hepatitis (due to non-adherence to the gluten-free diet) must be considered. The alcohol history must also be investigated (amount, type of alcoholic beverages, consumption pattern), and it is also necessary to inquire into drugs and supplements administered within the previous 3 months.
First-line blood tests should include viral markers (IgM antibodies against hepatitis A virus, HBsAg, antibodies against hepatitis C virus, IgM antibodies against hepatitis E virus), even if there is no specific evidence in the literature of an increased incidence of viral hepatitis in patients with AN. Screening for celiac disease (anti-transglutaminase antibodies) and autoimmune hepatitis (anti-nuclear, anti-smooth muscle, anti-liver–kidney microsomal antibodies) is also recommended. Peripheral eosinophilia could be suggestive for DILI. If the patient is in the initial stage of refeeding, it is essential to obtain serum levels of phosphorus, potassium, and magnesium, as they are the cornerstone for the diagnosis of RFS.
Liver ultrasound can be helpful to rule out vascular disorders (i.e. portal vein thrombosis, Budd–Chiari syndrome) and biliary obstruction, and to recognize enlarged fatty liver that is suggestive of refeeding-induced hepatitis [18].
RFS usually occurs within 3–5 days from the start of refeeding [27–29]. According to Friedli et al. [30], we can distinguish imminent and manifested RFS. Imminent RFS is defined by electrolyte shifts only, and specifically by a decrease in serum phosphate levels (<0.6 mmol/L or >30% from baseline) or shifts in the other two electrolytes (potassium and magnesium) below the normal range, occurring within 72 hours after the start of nutrition therapy. Manifested RFS is defined by electrolyte change and clinical symptoms (i.e. weakness, muscle spasms, confusion, arrhythmias, peripheral oedema, and heart failure) [30].
Differentiation points between starvation hepatitis and refeeding-induced hepatitis are summarized in Table 1. The diagnosis of starvation hepatitis is supported by normal electrolytes and hypoglycaemia, and the absence of a fatty liver [10]. A difficult clinical scenario is represented by transaminases elevation with onset during starvation and worsening after the start of nutritional intervention. This situation is still compatible with starvation hepatitis if the components of the RFS are not present [17]. Liver biopsy is usually not necessary and is indicated only in uncertain or chronic cases [31].
Management and treatment of hepatitis in patients with AN
General measures, common to both types of hepatitis, may include glutathione infusion because low glutathione levels have been documented in these patients and N-acetyl cysteine infusion in case of very high transaminase levels (i.e. >2,000 U/L) [6, 18]. In cases of mild–moderate transaminases elevation, S-adenosylmethionine is appealing due to its dual antioxidant and antidepressant activity, but clinical evidence is lacking [32]. In cases of prothrombin time elongation, a single vitamin K infusion is suggested. Serum transaminases can be checked every 2–3 days in inpatients for the first 2 weeks, then every 1–2 weeks until normalization, along with serum electrolytes.
Nutritional support must be agreed with the relevant specialist. For reference, the refeeding programmes based on the guidelines currently ongoing, and stratified according to the risk of RFS, are summarized in Tables 2 and 3 [27, 30, 33–40]. Although similar in several aspects, the nutritional management of the two typical forms of hepatitis in patients with AN is not the same. Patients with starvation hepatitis require adequate caloric replenishment, as summarized in Table 3; however, volume overload should be avoided, as patients affected by AN often present with low cardiac output secondary to reduced cardiac mass and volume. In contrast, patients with RFS require reduced nutritional support (by ∼25%–30%) to avoid the progression of refeeding-induced fatty liver, alongside gradual rehydration by sodium chloride (NaCl) and supplementation with thiamine, potassium, phosphate, and magnesium [10].
Conclusions
In patients affected by AN and transaminase elevation, the consulting hepatologist is tasked with making a differential diagnosis between the classical forms of hepatitis and two specific clinical pictures of patients affected by AN: starvation hepatitis and refeeding-induced hepatitis. Each AN patient should receive nutritional support specific to their form of hepatitis.
Authors’ Contributions
M.B., R.T., and C.P. wrote the paper and prepared tables; A.P., A.G., and A.G. revised the paper for important intellectual content. All authors read and approved the final manuscript.
Acknowledgements
Thanks to Fondazione Roma for the invaluable support for scientific research—FR-CEMAD 21–25.
Contributor Information
Marco Biolato, Department of Medical and Surgical Sciences, CEMAD, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Department of Translational Medicine and Surgery, Catholic University of Sacred Heart, Rome, Italy.
Rosy Terranova, Department of Translational Medicine and Surgery, Catholic University of Sacred Heart, Rome, Italy.
Caterina Policola, Department of Translational Medicine and Surgery, Catholic University of Sacred Heart, Rome, Italy; Unit of Endocrinology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Alfredo Pontecorvi, Department of Translational Medicine and Surgery, Catholic University of Sacred Heart, Rome, Italy; Unit of Endocrinology, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Antonio Gasbarrini, Department of Medical and Surgical Sciences, CEMAD, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Department of Translational Medicine and Surgery, Catholic University of Sacred Heart, Rome, Italy.
Antonio Grieco, Department of Medical and Surgical Sciences, CEMAD, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy; Department of Translational Medicine and Surgery, Catholic University of Sacred Heart, Rome, Italy.
Funding
None.
Conflicts of Interest
Nothing to declare.
References
- 1. Mitchell JE, Peterson CB.. Anorexia nervosa. N Engl J Med 2020;382:1343–51. [DOI] [PubMed] [Google Scholar]
- 2. Ayton A, Ibrahim A, Downs J. et al. From awareness to action: an urgent call to reduce mortality and improve outcomes in eating disorders. Br J Psychiatry 2024;224:3–5. [DOI] [PubMed] [Google Scholar]
- 3. Hanachi M, Melchior JC, Crenn P.. Hypertransaminasemia in severely malnourished adult anorexia nervosa patients: risk factors and evolution under enteral nutrition. Clin Nutr 2013;32:391–5. [DOI] [PubMed] [Google Scholar]
- 4. Rosen E, Sabel AL, Brinton JT. et al. Liver dysfunction in patients with severe anorexia nervosa. Int J Eat Disord 2016;49:151–8. [DOI] [PubMed] [Google Scholar]
- 5. Kheloufi M, Boulanger CM, Durand F. et al. Liver autophagy in anorexia nervosa and acute liver injury. Biomed Res Int 2014;2014:701064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ramsoekh D, Taimr P, Vanwolleghem T.. Reversible severe hepatitis in anorexia nervosa: a case report and overview. Eur J Gastroenterol Hepatol 2014;26:473–7. [DOI] [PubMed] [Google Scholar]
- 7. Sakada M, Tanaka A, Ohta D. et al. Severe steatosis resulted from anorexia nervosa leading to fatal hepatic failure. J Gastroenterol 2006;41:714–5. [DOI] [PubMed] [Google Scholar]
- 8. Dowman J, Arulraj R, Chesner I.. Recurrent acute hepatic dysfunction in severe anorexia nervosa. Int J Eat Disord 2010;43:770–2. [DOI] [PubMed] [Google Scholar]
- 9. Rosen E, Bakshi N, Watters A. et al. Hepatic complications of anorexia nervosa. Dig Dis Sci 2017;62:2977–81. [DOI] [PubMed] [Google Scholar]
- 10. Sakata M, Takaki A, Oyama A. et al. Pathogenesis of severe liver injury in patients with anorexia nervosa: a report of two cases and a literature review. Kurume Med J 2022;67:121–9. [DOI] [PubMed] [Google Scholar]
- 11. Rautou PE, Cazals-Hatem D, Moreau R. et al. Acute liver cell damage in patients with anorexia nervosa: a possible role of starvation-induced hepatocyte autophagy. Gastroenterology 2008;135:840–8. 848.e1-3. [DOI] [PubMed] [Google Scholar]
- 12. Yorimitsu T, Klionsky DJ.. Autophagy: molecular machinery for self-eating. Cell Death Differ 2005;12(Suppl 2):1542–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kraft MD, Btaiche IF, Sacks GS.. Review of the refeeding syndrome. Nutr Clin Pract 2005;20:625–33. [DOI] [PubMed] [Google Scholar]
- 14. McCray S, Walker S, Parrish CR.. Much ado about refeeding. Pract Gastroenterol 2004;XXVIII:26–44. [Google Scholar]
- 15. Mehanna HM, Moledina J, Travis J.. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ 2008;336:1495–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reber E, Friedli N, Vasiloglou MF. et al. Management of refeeding syndrome in medical inpatients. J Clin Med 2019;8:2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Narayanan V, Gaudiani JL, Harris RH. et al. Liver function test abnormalities in anorexia nervosa—cause or effect. Int J Eat Disord 2010;43:378–81. [DOI] [PubMed] [Google Scholar]
- 18. Harris RH, Sasson G, Mehler PS.. Elevation of liver function tests in severe anorexia nervosa. Int J Eat Disord 2013;46:369–74. [DOI] [PubMed] [Google Scholar]
- 19. Navarro VJ, Khan I, Björnsson E. et al. Liver injury from herbal and dietary supplements. Hepatology 2017;65:363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiang W, Xun Y.. Drug reaction with eosinophilia and systemic symptoms syndrome secondary to Chinese oral herbal paste: a case report. Gastroenterol Rep (Oxf) 2023;11:goad047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Amphetamines. https://livertox.nih.gov (28 Novembre 2022, date last accessed).
- 22. Vanin JR, Saylor KE.. Laxative abuse: a hazardous habit for weight control. J Am Coll Health 1989;37:227–30. [DOI] [PubMed] [Google Scholar]
- 23. Gattuso JM, Kamm MA.. Adverse effects of drugs used in the management of constipation and diarrhoea. Drug Saf 1994;n10:47–65. [DOI] [PubMed] [Google Scholar]
- 24. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [Google Scholar]
- 25. Neale J, Hudson LD.. Anorexia nervosa in adolescents. Br J Hosp Med 2020;81:1–8. [DOI] [PubMed] [Google Scholar]
- 26. Hudson LD, Court AJ.. What paediatricians should know about eating disorders in children and young people. J Paediatrics Child Health 2012;48:869–75. [DOI] [PubMed] [Google Scholar]
- 27. Crook MA, Hally V, Panteli JV.. The importance of the refeeding syndrome. Nutrition 2001;17:632–7. [DOI] [PubMed] [Google Scholar]
- 28. Boateng AA, Sriram K, Meguid MM. et al. Refeeding syndrome: treatment considerations based on collective analysis of literature case reports. Nutrition 2010;26:156–67. [DOI] [PubMed] [Google Scholar]
- 29. Marik PE, Bedigian MK.. Refeeding hypophosphatemia in critically ill patients in an intensive care unit. A prospective study. Arch Surg 1996;131:1043–7. [DOI] [PubMed] [Google Scholar]
- 30. Friedli N, Stanga Z, Culkin A. et al. Management and prevention of refeeding syndrome in medical inpatients: an evidence-based and consensus-supported algorithm. Nutrition 2018;47:13–20. [DOI] [PubMed] [Google Scholar]
- 31. Faragalla K, So J, Chan PC. et al. Value of liver biopsy in anorexia nervosa-related transaminitis: a case study and literature review. Hepatol Res 2022;52:652–8. [DOI] [PubMed] [Google Scholar]
- 32. Abou-Saleh MT, Coppen A.. The biology of folate in depression: implications for nutritional hypotheses of the psychoses. J Psychiatr Res 1986;20:91–101. [DOI] [PubMed] [Google Scholar]
- 33. National Institute for Health and Clinical Excellence Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition (Clinical Guidance 32). https://www.nice.org.uk/Guidance/CG32 (27 November 2022, date last accessed). [PubMed]
- 34. Friedli N, Stanga Z, Sobotka L. et al. Revisiting the refeeding syndrome: Results of a systematic review. Nutrition 2017;35:151–60. [DOI] [PubMed] [Google Scholar]
- 35. Brannan PG, Vergne-Marini P, Pak CY. et al. Magnesium absorption in the human small intestine. Results in normal subjects, patients with chronic renal disease, and patients with absorptive hypercalciuria. J Clin Invest 1976;57:1412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gennari FJ. Hypokalemia. N Engl J Med 1998;339:451–8. [DOI] [PubMed] [Google Scholar]
- 37. Marinella MA. Refeeding syndrome in cancer patients. Int J Clin Pract 2008;62:460–5. [DOI] [PubMed] [Google Scholar]
- 38. Thatte L, Oster JR, Singer I. et al. Review of the literature: severe hyperphosphatemia. Am J Med Sci 1995;310:167–74. [DOI] [PubMed] [Google Scholar]
- 39. Weisinger JR, Bellorín-Font E.. Magnesium and phosphorus. Lancet 1998;352:391–6. [DOI] [PubMed] [Google Scholar]
- 40. da Silva JSV, Seres DS, Sabino K. et al. ; Parenteral Nutrition Safety and Clinical Practice Committees, American Society for Parenteral and Enteral Nutrition. ASPEN consensus recommendations for refeeding syndrome. Nutr Clin Pract 2020;35:178–95.32115791 [Google Scholar]