Abstract
Rationale
The contribution of norepinephrine on the different phases of spatial memory processing remains incompletely understood. To address this gap, this study depleted norepinephrine in the brain and then conducted a spatial learning task with multiple phases.
Methods
Male and female Wistar rats were administered 50 mg/kg/i.p. of DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine) to deplete norepinephrine. After 10 days, rats were trained on a 20-hole Barnes maze spatial navigation task for 5 days. On the fifth day, animals were euthanized and HPLC was used to confirm depletion of norepinephrine in select brain regions. In Experiment 2, rats underwent a similar Barnes maze procedure that continued beyond day 5 to investigate memory retrieval and updating via a single probe trial and two reversal learning periods.
Results
Rats did not differ in Barnes maze acquisition between DSP-4 and saline-injected rats; however, initial acquisition differed between the sexes. HPLC analysis confirmed selective depletion of norepinephrine in dorsal hippocampus and cingulate cortex without impact to other monoamines. When retrieval was tested through a probe trial, DSP-4-improved memory retrieval in males but impaired it in females. Cognitive flexibility was transiently impacted by DSP-4 in males only.
Conclusions
Despite significantly reducing levels of norepinephrine, DSP-4 had only a modest impact on spatial learning and behavioral flexibility. Memory retrieval and early reversal learning were most affected and in a sex-specific manner. These data suggest that norepinephrine has sex-specific neuromodulatory effects on memory retrieval with a lesser effect on cognitive flexibility and no impact on acquisition of learned behavior.
Keywords: Barnes maze, Spatial memory, Retrieval, Behavioral flexibility, Hippocampus, DSP-4, HPLC, Norepinephrine, Sex, Female
Introduction
Although spatial memory is necessary for the survival of all organisms, a static representation of the outside world is insufficient. To be able to adapt, organisms need to “update” their spatial memory representations. By updating and revising the integrated knowledge and rerouting behavior in response to changing circumstances, this flexibility improves chances of survival (Osorio-Gómez et al. 2023). The processing capacity needed for spatial memory updating must be able to compare brand-new interoceptive and extrasensory stimuli with an existing representation and create a new memory if there is a discrepancy. Though the neurophysiological mechanisms necessary for memory updating continue to be studied (Mau et al. 2020), recent work has suggested that spatial memory updating is affected by the neuromodulator norepinephrine (NE) from the locus coeruleus (LC) (Breton-Provencher et al. 2021; Grella et al. 2021). Specifically, some have argued that it is the responsibility of LC-NE to toggle between retrieval of existing information and encoding of new information (Grella et al. 2021).
With only about 3000 neurons, the LC possesses broad and divergent projections throughout the brain, including the hippocampus (Poe et al. 2020). The function of the LC system is often studied in the context of arousal (Chu and Bloom 1973) and stress (Hermans et al. 2011); however, the LC has also been implicated in cognitive behaviors (Arnsten 2000; Aston-Jones and Cohen 2005; Bouret and Sara 2005; Sara and Bouret 2012). Changes in spatial learning task demands require dynamic updating. These changes place the LC in phasic activity mode, demonstrated by a burst firing pattern. However, when cognitive demand necessary to complete spatial learning tasks declines, the LC is placed in tonic impulse mode. (Aston-Jones and Cohen 2005). Therefore, the LC is engaged differentially when there is a need to shift attention or behavioral strategy. Behavioral evidence for this notion has been mixed, however, in a contextual freezing task, Murchison et al. (2004) found that knockout mice for dopamine beta-hydroxylase, a key metabolic enzyme for NE, display impaired memory acquisition. Hamlett et al. (2020) found no deficits in spatial memory acquisition when inhibiting LC-NE release using chemogenetic techniques. Khakpour-Taleghani et al. (2009) found that intra-LC lidocaine immediately before training resulted in impaired spatial memory acquisition. Previous research reports LC perturbation at acquisition (Aston-Jones et al. 1997; Hansen 2017), probe (Hou et al. 2019; Kempadoo et al. 2016), or reversal (Glennon et al. 2019; Kelberman et al. 2022; Uematsu et al. 2015). It’s possible that manipulation of LC NE at only one of these phases allows for compensatory mechanisms to occur that may affect other stages of spatial learning. Therefore, we have effectively knocked down NE transmission throughout all stages of this spatial learning paradigm. Second, there is a lack of studies investigating the role of LC-NE in cognitive tasks in both sexes. The reports available have only examined the sex differences mediated by the LC in acoustic startle (Hormigo et al. 2017), and inflammatory pain (Cardenas et al. 2021). Hormigo et al. (2017), for instance, found that DSP-4 treatment decreases LC neuron density equally in both sexes, but behavioral impairments were more prevalent in males. Taken together, the question of whether the LC’s contribution to spatial memory is sex-dependent remains elusive. As such, it is critical to include female subjects in studies to address this historically neglected area of research.
To address the role of LC-NE in cognitive tasks while considering biological sex, we depleted NE levels in female and male rats using the selective neurotoxin DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine) before subjecting the rats to a Barnes maze spatial learning task. Mechanistically, DSP-4 reduces NE by passing through the blood-brain barrier and cyclizes to form a reactive aziridinium derivative at NE nerve terminals via the NE transporter (Ross and Stenfors 2015). This leads to irreversible inhibition of the NE transporter, which causes NE depletion over time by inhibiting normal recycling and promoting NE metabolism (Ross and Stenfors 2015), effectively removing the primary source of NE that innervates the brain. In two complementary experiments, we studied the role of NE depletion on spatial memory acquisition, retrieval, and reversal learning using the Barnes maze. Additionally, we measured NE, dopamine and serotonin levels concurrently, ensuring that the depletion was NE-specific. We hypothesized that DSP-4 treatment would not affect initial spatial learning (acquisition) but would impair memory during a retrieval task (probe trial) and the use of cognitive flexibility when updating is required (reversal learning), and that this impairment would be less pronounced in females (Hormigo et al. 2017).
Materials and methods
Experiment 1
Subjects and housing conditions
Adult male (300–400 g; n = 16; n = 8 saline; n = 8 DSP-4) and female (200–250 g; n = 16; n = 8 saline; n = 8 DSP-4) Wistar rats (Charles River Laboratories, Wilmington, MA) were purchased, and upon arrival pair-housed by sex for the remainder of this investigation. Rats had ad libitum access to standard rat chow (Lab Rodent Diet 5001, PMI Nutrition International INC., Brentwood, MO) and water throughout the study except while on the Barnes maze. The rats were kept on a reverse 12:12 light/dark cycle upon arrival and given 5 days acclimation before the initiation of experiments. Male and female rats were kept separate from one another in isolator cages in a ventilated and climate-controlled vivarium to limit odor, contact, and interaction between sexes. Two of the females died unexpectedly early in the experiment and were not retained in the analysis (female n = 14 for analyses). This study was approved by the WSU Institutional Animal Care and Use Committee, which adheres to principles, policies, and procedures outlined in the Guide for the Care and Use of Laboratory Animals (Council, N. R. Guide for the Care and Use of Laboratory Animals: Eighth Edition 2011).
Barnes maze apparatus
The Barnes maze, shown in Fig. 1, consisted of a 20-hole custom-made round plastic platform that was elevated 90 cm above the ground (maze diameter 122 cm; hole diameter 11 cm; Formtech Plastics, Oak Park, MI). One of the 20 holes possessed a hidden goal box below the maze where the rat could escape and hide. The rats were motivated to find the hidden platform via aversive stimuli in the forms of a bright overhead light and an 80 dB sound. These stimuli are both anxiogenic and were removed upon successful completion of the Barnes maze procedure. All rats spent 30 s under an opaque start box (21cm length, 21 cm width, 32 cm height) that was placed in the center of the maze prior to trial (and aversive stimuli) initiation.
Fig. 1.
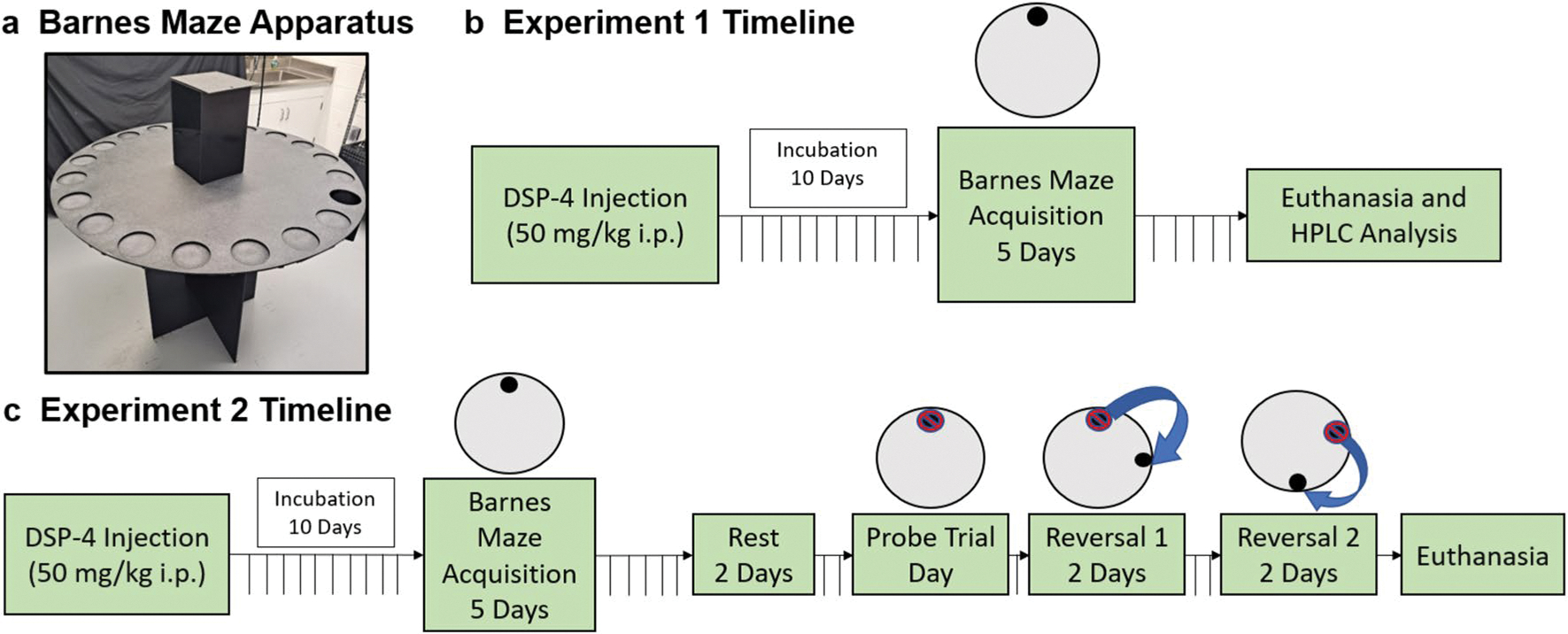
Experimental timelines. Schematic representation of the procedures and timeline used for the Barnes maze behavioral assay and collection of brain tissue for the HPLC analysis. The inset shows the 20-hole Barnes maze apparatus, start box in center of maze, and goal box below the maze (open hole on right)
Barnes maze acquisition protocol
See Fig. 1 for a procedural timeline of Experiments 1 and 2. Ten days before behavioral training, rats were either given a single intraperitoneal injection of 50 mg/kg DSP-4 or 0.9% sterile saline. DSP-4 was obtained from Sigma (CAS No 40416–75–9) and dissolved in 0.9% sterile saline. On each day of the Barnes maze acquisition training, rats were transported one sex at a time from the vivarium to the adjacent behavioral suite where the Barnes maze apparatus was located. Animals were habituated to the behavioral room for ~30 min before testing with males and females kept separate to restrict interaction. The behavioral suite and equipment were cleaned with 70% ethanol in water solution prior to the other sex being tested. Additionally, experimenters changed lab coats between male and female cohorts to avoid sex-specific olfactory cues. In acquisition training, one of the 20 possible escape holes of the Barnes maze harboring a goal box installed beneath it with all other holes covered. On day 1, rats were allowed to freely explore the maze. If a rat did not find the hidden zone before 90 s had elapsed, the experimenter guided the animal to the zone and allowed the remainder of the trial to conclude (total trial time 180 s). This was done so that all rats had some experience with the Barnes maze apparatus and the goal box. On days 2 through 5, an 80 dB white noise and a 120-watt bright light were introduced when the animal was placed under the start box. These aversive stimuli were maintained until the animal entered the goal box then the sound was stopped. The trial was then concluded, and the rat was placed back into its home cage. The maze, the goal box, and the start box were cleaned with 70% ethanol in water solution immediately following each trial.
Each animal underwent 3 trials/day on days 2–5 to find the hidden location and the trial ended on these days when either the hidden zone was entered or 180 s had elapsed. The intertrial interval (ITI) was ~5 min. Ninety minutes following the last trial on day 5, all rats were euthanized via rapid decapitation. Brains were extracted, frozen using dry ice, and stored at −80 °C until preparation for monoamine analysis via high pressure liquid chromatography (HPLC).
HPLC analysis of brain tissue for NE levels
HPLC analysis was performed as described previously in our laboratory to quantify whole tissue levels of NE, dopamine and serotonin (Davidson et al. 2022; Lisieski et al. 2019). Common metabolites (DOPAC, HVA, 3MT, and 5-HIAA) were concurrently quantified alongside their monoamine counterparts. Punches were taken from coronal sections (2 mm) containing the cingulate cortex (CG; 3.2 mm from bregma; 1-mm diameter punch), Dorsal Striatum (dStr; 1.2 mm from bregma; 2-mm diameter punch), and Dorsal Hippocampus (dHip; −2.8 mm from bregma; 2-mm diameter punch) stored in 1.5-mL tubes at −80 °C until all samples were ready for analysis. Each punch was weighed, homogenized by sonication in 50 μL 0.2 N perchloric acid (PCA) for 3–5 s (Misonix XL-2000), centrifuged at ~10,000 g for 10 min at ~4 °C, and the supernatant was collected. A 20-μl aliquot of each sample was collected from the supernatant and placed into a conical HPLC vial, capped with a septum, and placed into an autosampler of the Dionex Ultimate 3000 HPLC system (ThermoFisher Scientific) and held at 5 °C. A series of external standards for NE, dopamine, serotonin and metabolites were prepared fresh daily through serial dilution corresponding to 10, 5, 1, 0.5, or 0.1 ng, or PCA (vehicle). The standards were run prior to and following the unknown samples to ensure consistency of measure across the analysis. Standards and unknown samples (10 μl) were injected onto a reverse-phase column (Hypersil™ BDS C18 column, ThermoFisher Scientific) at 25 °C with a flow rate at 0.6 mL/min (~250 bar). After passing through the column, the sample passed through a guard cell (ESA model 5011A) set to 350 mV (2.1/3.0 mm ID, ThermoFisher Scientific). Electrochemical (coulometric) detection was achieved via an ultra-analytical dual electrode cell (ThermoFisher Scientific) with a reference electrode set at −175 mV and a working electrode at 300 mV (gain = at 100 μA for both).
Values from the detector were captured and analyzed using Chromileon 7.2.10 software (Dionex) to quantify peak height representing levels of monoamines and metabolites. A detection threshold of three times the average baseline values was used, such that only samples with signals exceeding this were included. Standard curve equations using Microsoft Excel for all monoamines were constructed to calculate monoamine levels and verify stability of the instrument. The standard curves for all components indicated sufficient stability for all monoamines (r2 ≥ 0.9995). Tissue levels of monoamines were calculated based on the regression line supplied by standard curves. Absolute values of the samples’ peak heights were adjusted to compensate for differences in tissue weight (ng monoamine/mg wet tissue; ng/mg).
Experiment 2
Subjects and Barnes maze protocol
Thirty-two additional adult male (n = 16; n = 8 saline; n = 8 DSP-4) and female (n = 16; n = 8 saline; n = 8 DSP-4) Wistar rats were obtained and kept in conditions identical to Experiment 1. The Barnes maze protocol conducted for Experiment 2 was identical to Experiment 1 with two exceptions: (1) to increase learning difficulty, rats only received 2 trials per day, and (2) on day 5, rats were placed back into their home cages and remained undisturbed for 2 consecutive days. Following this delay, all rats underwent a single probe trial to test retrieval memory (day 8) and then a first (days 9 and 10) and a second (days 11 and 12) reversal training period (see timeline in Fig. 1). For the probe trial, the original location of the hidden zone was closed, and no escape hole was available. For reversal trials, the hidden zone location was moved 90° (Reversal 1) and 180° (Reversal 2) from the original open-hole location, respectively. All rats were euthanized 90 min following the second reversal’s final trial.
Statistical analyses
Noldus EthoVision XT software was used to quantify behavioral outcome measures, including the number of hidden zone entries (i.e., frequency of visits) and time spent in specified maze locations (e.g., prior trained locations). A camera mounted above the Barnes maze collected the visual data.
Barnes maze Acquisition (Experiments 1 and 2) data were run using a 3-way Mixed Model ANOVA with session (days 1–5) as the repeated factor with condition (DSP-4 vs. saline) and sex (male vs. female) as the between-subject factor. HPLC data (Experiment 1) were analyzed using separate t-tests for each monoamine and region with condition (DSP-4 vs. saline) as the between-subject factor. No corrections were applied, and α = 0.05 was used for all analyses. Memory retrieval (probe trial; Experiment 2) was assessed via duration and frequency of visits to the prior location was analyzed using an ordinary one-way ANOVA with condition (DSP-4 vs. saline) as the independent variable. Reversal trials (Experiment 2) were analyzed separately (Reversal 1 and Reversal 2) using mixed effects analysis with reversal day (days 1 and 2) as the repeated factor and condition (DSP-4 vs. saline) and sex (male and female) as the between-subject factors.
Results
Experiment 1 findings
All rats quickly learn the location of the hidden platform
Over the 5 days of training, both male and female rats learned the location of the hidden platform. A 3-way RM-ANOVA, showed a significant interaction between day and sex [F(4, 232) = 6.62, p < 0.001] with females effectively showing quicker rate of acquisition. DSP-4 was not found to be a significant main effect (p > 0.05) or to interact with the other variables (p > 0.05). Two separate 2-way ANOVAs were performed for female or male rats revealed a main effect of session (days) [F(1.625, 22.75) = 27.37, p < 0.001 (males), F(1.387, 16.64) = 63. 15, p < 0.001 (females)] as presented in Fig.2. DSP-4 did not impair Barnes maze acquisition in females or males relative to saline-controls.
Fig. 2.
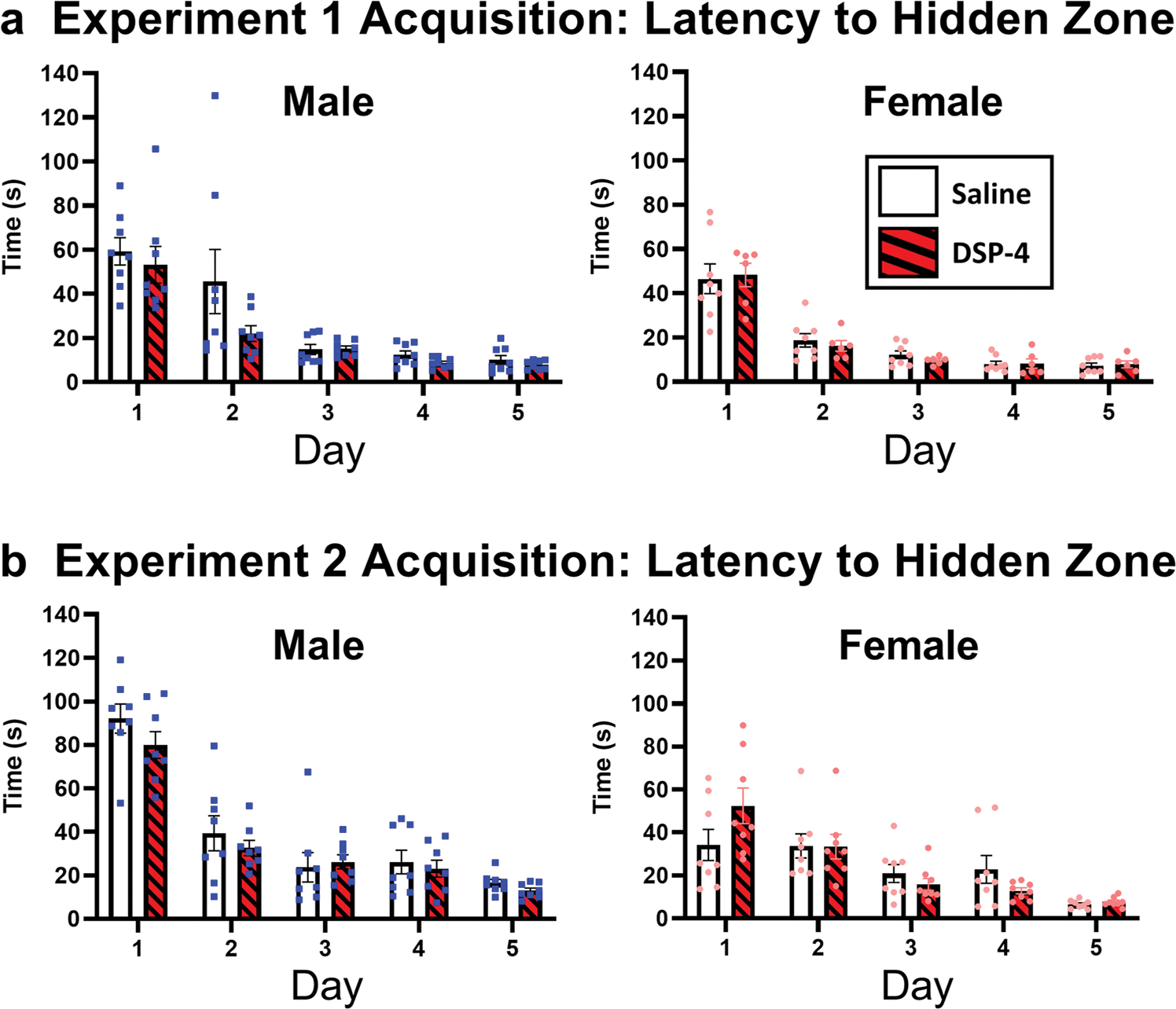
Female and male rats administered DSP-4 acquire spatial learning compared to saline-controls in a Barnes maze task. Both sexes were given 50 mg/kg DSP-4 by i.p.-injection 10 days before Barnes maze testing. Acquisition of Barnes maze spatial learning occurred for 5 consecutive days. A three-way repeated measures mixed model ANOVA found a significant day by sex interaction (p < 0.001) with females effectively acquiring the task quicker. Given this interaction, the current data and future graphs are presented separately by sex with males on the left (represented by blue squares) and females on the right (represented by pink circles)
DSP-4 reduces NE levels in the cingulate cortex and dorsal-hippocampus of female and male rats
Figure 3 shows the amount of NE measured in the CG, dHip and dStr of rats treated with DSP-4 or saline. Male rats treated with DSP-4 had significantly less NE in their CG compared to those injected with saline [t(12) = 2.77, p < 0.01 (Fig. 3a) and dHip t(13) = 2.33, p < 0.01 (Fig. 3b)]. This finding was also seen in females within the CG [t(10) = 2.17, p < 0.05 (Fig. 3d)] and dHip [t(11) = 3.24, p < 0.01 (Fig. 3e)]. Surprisingly, NE was not significantly impacted in the dStr of either sex (p > 0.05; Fig. 3c, f). Dopamine, serotonin, and associated metabolites (DOPAC, HVA, 3-MT, and 5-HIAA) were not different between the treatment groups in any of the 3 regions with one exception that females given DSP-4 had decreased serotonin in the CG (p < 0.01 vs. saline; data not shown).
Fig. 3.
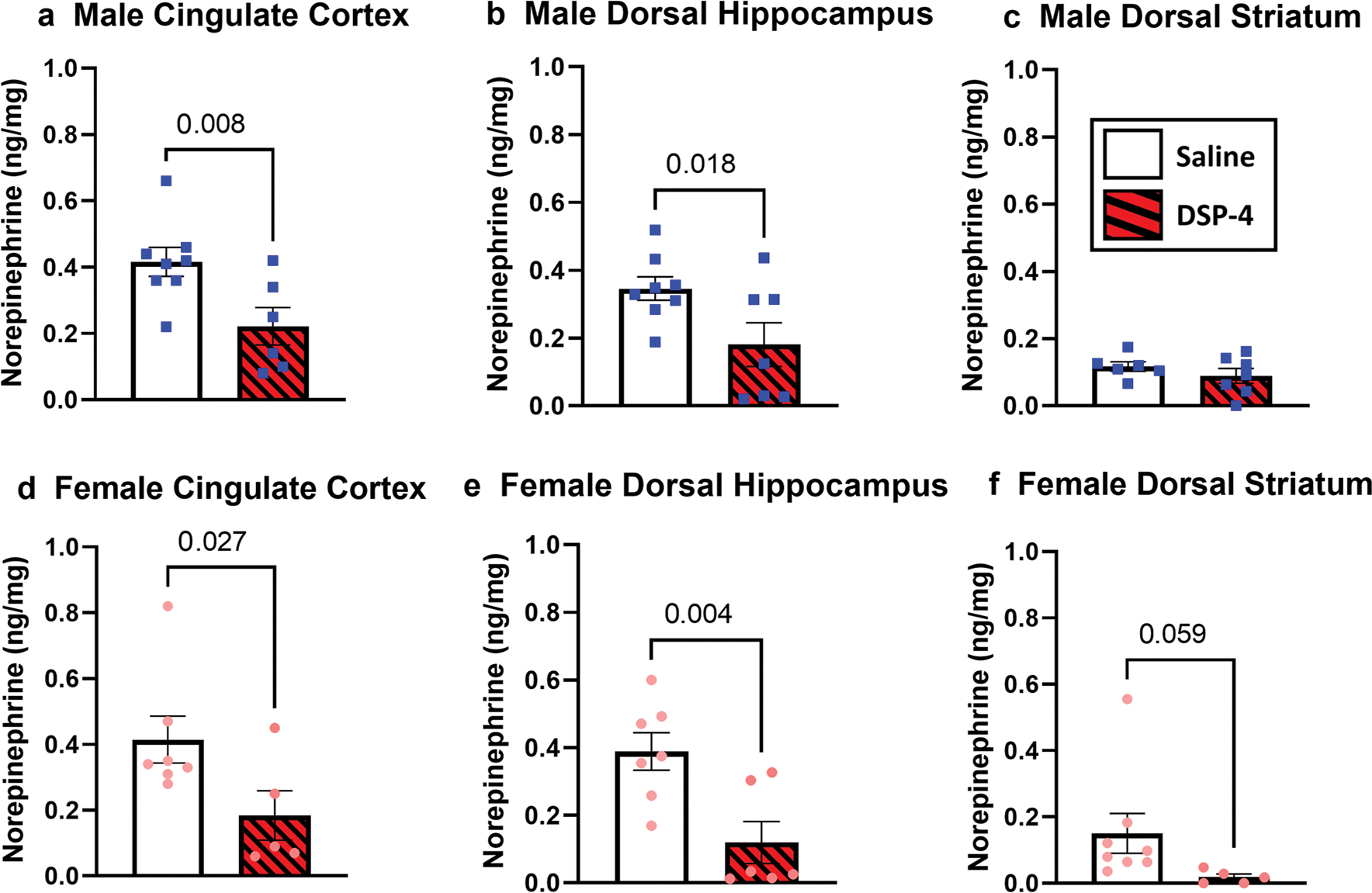
Levels of NE for female and male rats administered DSP-4 or Saline in the Cingulate (CG), Dorsal Hippocampus (dHip), or Dorsal Striatum (dStr): Brains were taken from Experiment 1 to quantify NE levels in particular regions with males presented as blue squares in the top row (Panels a–c) and females presented as pink circles on bottom (Panels d–f). Levels of NE were confirmed to be depleted for both sexes in the CG and dHip, but not in the dStr
Experiment 2 findings
Male rats treated with DSP-4 have augmented memory and females show attenuated retrieval memory on probe day
Figure 1b shows the results for Barnes maze acquisition for Experiment 2, which generally replicated the results demonstrated in Experiment 1 that there was an interaction between day and sex without a significant impact of DSP-4 exposure. The only difference between the two acquisition paradigms were three training sessions per day for Experiment 1 and two sessions per day for Experiment 2. Figure 4 shows the assessment of the probe trial, which focused on retrieval memory as measured by duration and number of visits to the previous hidden zone location from acquisition training. An ordinary one-way ANOVA using sex and condition as factors found a significant impact for frequency of visits [F(3, 28) = 6.67, p < 0.01] and a trending but non-significant impact on duration [F(3, 28) = 2.48, p = 0.082]. Follow up analysis of frequency revealed significant differences between saline and DSP-4 in males (p < 0.05) and females (p < 0.05). Interestingly, the direction of impact between the sexes are in different directions with the DSP-4 group in males, demonstrating an increased number of visits, and the DSP-4 group in females, demonstrating a lower number of visits. Further analysis revealed significant differences between male and female rats that received saline (p < 0.05), and males and females that received DSP-4 (p < 0.01). Planned comparisons between treatment groups within each sex for duration confirmed the omnibus findings with both males (p = 0.059) and females (p > 0.99) failing to demonstrate a significant impact of DSP-4.
Fig. 4.
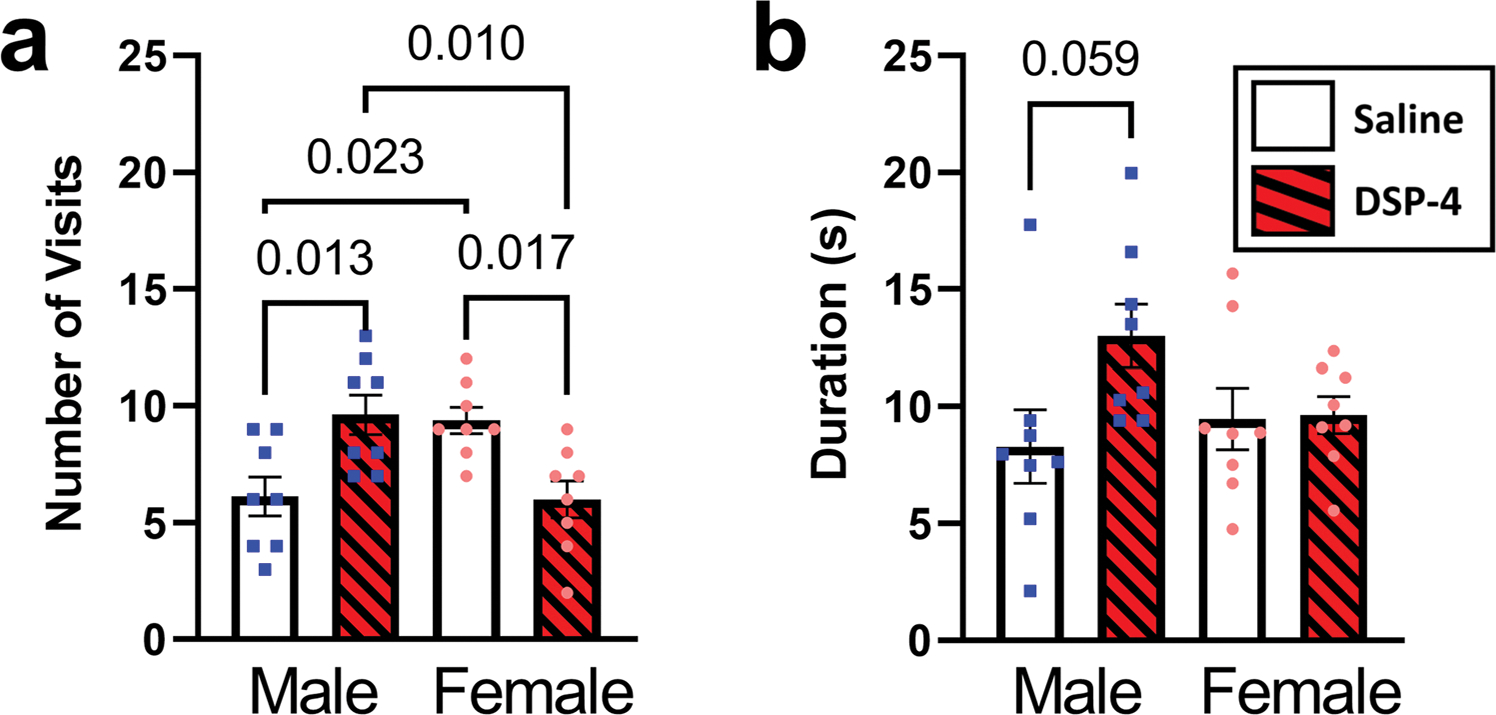
DSP-4 increases memory retrieval in male rats but decreases retrieval in females. Duration of the visits to the hidden platform area and frequency of visits to the hidden platform on probe day are presented with males on the left of each graph (shown as blue squares) and females on the right (shown as pink circles). Post-hoc planned comparisons demonstrated female rats administered DSP-4 showed significantly different number of visits to the previous hidden zone, however, in opposing directions further demonstrating a sex related impact of NE on retrieval memory
Male rats treated with DSP-4 show a deficit in locating the hidden platform on the first reversal test, but not the second reversal test with no discernable impact to female rats
Rats in Experiment 2 were provided with an opportunity to locate the hidden platform in a new location rotated 90° (Reversal 1) and then 180° (Reversal 2) from its original location as shown in Fig. 1 to test the role of NE in behavioral flexibility of spatial learning. Reversal 1 analysis revealed a significant interaction between sex and condition with reversal day [F(3, 26) = 4.27, p < 0.05]. Multiple comparisons revealed significant differences on Reversal 1 Day 1 but not Reversal 1 Day 2. On Reversal Day 1, female and male saline-treated (p < 0.01), but not DSP-4-treated (p = 0.744), rats were significantly different. Within males, there was a significant impact of DSP-4 (p < 0.05), with DSP-4 demonstrating an increased latency compared to saline. This finding was not found in females (p = 0.076). These observations are similar to the retrieval findings with the direction of impact being opposite between the sexes (see Figs. 4 and 5). Analysis of Reversal 2 showed a significant main effect of reversal day [F(1, 27) = 7.38, p < 0.05], but the interaction was not significant (p = 0.270). Planned comparisons confirmed no significant differences between any groups (p > 0.416).
Fig. 5.
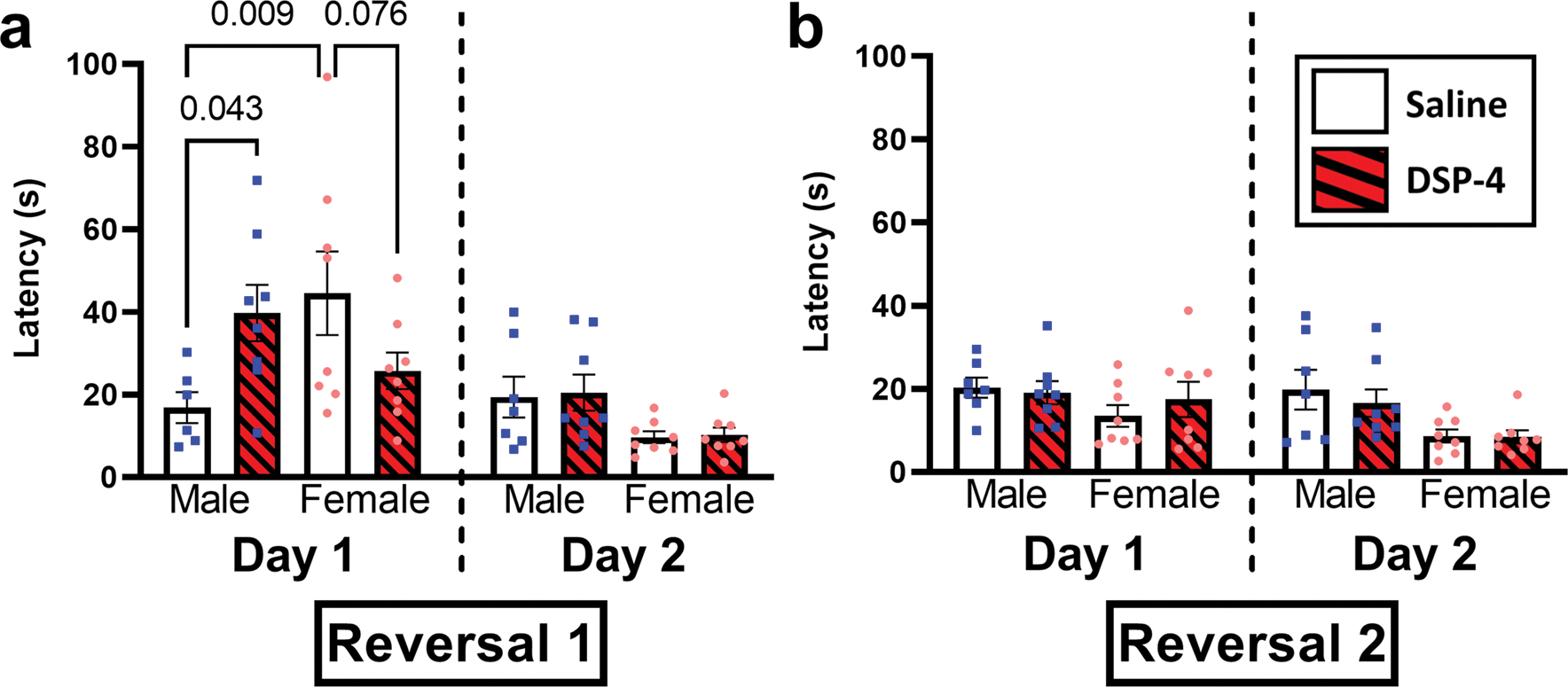
Decreased task performance on Reversal 1 but not Reversal 2 in only male rats given DSP-4. Results from the two reversal periods are presented individually with the males (blue squares) and females (pink circles) presented separately. Planned comparisons demonstrated that female and male saline injected rats had significantly different number of visits to the previous hidden zone and that those administered DSP-4 showed significantly greater number of visits to the previous hidden zone compared to male rats given saline. This effect was transient and only observed on day 1 of Reversal learning. A single male in the saline group was removed from this analysis due to being 3 standard deviations beyond the mean
Discussion
Despite published studies probing the NE system in learning and memory tasks, the role of NE and the implication of potential sex differences in spatial memory acquisition, retrieval, and reversal learning as a measure of behavioral flexibility remains unclear. To elucidate the role of NE on spatial learning and spatial memory updating, female and male Wistar rats were treated with DSP-4, and after 10 days of incubation to allow for NE-depletion, animals were subjected to one of two experimental paradigms. In Experiment 1, rats underwent 5 days of acquisition training on a 20-hole Barnes maze and were euthanized thereafter to quantify brain NE levels. In Experiment 2, animals underwent the same 5 days of acquisition training and returned to their home cages. Two days later, animals were subjected to a single probe trial to test retrieval and to subsequent days of two reversal learning assessments.
Memory acquisition
The hippocampus is a critical brain region putatively thought to be involved in spatial/episodic memory (Moser et al. 2017) and our results here as well as a previous report (Jackisch et al. 2008) show diminished NE in the hippocampus is correlated with neurobehavioral effects. Although we cannot conclude if the diminished NE in the hippocampus caused the behavioral effects, as DSP-4 is known to cause brain-wide reductions in NE (Nowak 2016), two of which we confirmed (CG and dHip) here. HPLC analysis confirmed depletion of NE in both sexes of the DSP-4 treated rats in dHip and CG, and while this treatment did not affect the acquisition of spatial learning, it did affect retrieval and reversal learning. This finding was consistent with our hypothesis and a recent study showing the role of NE in recognition memory and reversal learning in a spatial task (Hamlett et al. 2020). The use of DSP-4 in animals performing spatial memory acquisition tasks have generally provided consistent results (Ross and Stenfors 2015; Sirviö et al. 1991). Using a Cogitat hole board, male rats administered DSP-4 have intact working and reference memory (Hauser et al. 2012; Sontag et al. 2011) and similarly do not have deficits in acquisition learning in the Morris water maze (Sirviö et al. 1991). One study did however report that direct reversible infusion of lidocaine into the LC diminished reference and working memory acquisition on the Morris water maze (Khakpour-Taleghani et al. 2009).
Though all of these studies differed in drug dose, type of administration (systematically vs. CNS) and behavioral tasks used, lidocaine infusions into the LC provide an especially challenging approach given the small size of the nuclei (size), and in the absence of any secondary staining to confirm the LC and the known fact that lidocaine diffuses in the brain from the target area could have resulted in off-target effects of the previous study, leading to an impairment of acquisition. DSP-4 can also have off-target or indirect effects of NE. This is an unlikely occurrence in this study however, as we did not find a reduction in dopamine or serotonin levels in the dHip of rats treated with DSP-4.
It is unclear why females performed better at acquisition. Previous work has reported inconsistent findings related to sex differences on acquisition of spatial memory (Locklear and Kritzer 2014; Saucier et al. 2008). In some of our previous work, our lab has shown that fentanyl conditioned place preference for females can be predicted based on the rat cycle being in non-estrus phases (Gaulden et al. 2021). However, in this study and our lavage analysis from Experiment 1 looking at cytological characteristics of the vaginal epithelium did not suggest that the females were clustered in any given hormonal cycle, rather they were in all categories. Future investigations using intact (naturally cycling) and ovariectomized subjects should be used to further explore, and ultimately, support or refute our findings related to sex differences in spatial memory.
Memory retrieval and reversal
The contribution of the LC-NE system to spatial memory retrieval has received sparse attention with sometimes contradictory results. Pharmacological agonists for β-adrenoreceptors, NE re-uptake inhibitors, as well as optogenetic stimulation of the LC have been shown to impair spatial memory during retrieval (Grella et al. 2021; Swift et al. 2018; Walling et al. 2016). However, lidocaine infusions directly into the LC did not cause deficits in Morris water maze probe test (Khakpour-Taleghani et al. 2009).
Experimental manipulations that augment NE and test for spatial memory have also provided some inconsistent results. A human study using yohimbine (an α2 adrenergic receptor antagonist) in a virtual spatial memory task found no difference compared to the control (Chae et al. 2019). While rats injected with the (non-depleting) NE reuptake inhibitor desipramine showed diminished memory retention when tested 4 h, but importantly not at 24 h, after the last day of acquisition (Walling et al. 2016). Our probe day results testing for memory retrieval in males are consistent with these previous reports but do not extend the findings to females (Fig. 4). In fact, we find that memory retrieval was impaired in female rats injected with DSP-4. It is worth mentioning that the referenced studies exclusively studied male rats, and only a single study measuring spatial learning reported using both males and females administered DSP-4 (Chalermpalanupap et al. 2018). However, that study was not sufficiently powered to detect sex differences. Therefore, our results are the first to indicate a sex-specific regulation of NE during retrieval of spatial learning using DSP-4.
A surprising twist to the story of LC-hippocampus circuity comes from a study where an optogenetic augmentation of the LC-hippocampus pathway resulted in enhanced acquisition and retention of Barnes maze memory (Kempadoo et al. 2016). However, the authors conclusively demonstrate that the effects are being driven by dopamine, not NE. Our HPLC results show that dopamine levels are unaffected by DSP-4 in the dorsal-hippocampus of female or male rats (data not shown). Nevertheless, our results clearly show that normal NE levels are not required for memory acquisition and may enhance retrieval memory in males, but this effect appears to be sex-dependent with females given DSP-4 having a modest deficit in retrieval learning as measured by frequency of visits but not duration.
Consistent with our hypothesis, rats had an initial deficit when required to update their learning (Reversal 1 Day 1). This effect was seen in male, but not female rats treated with DSP-4, and it was transient, as the rats were performing at comparable levels to controls on all other reversal day sessions. It is not clear why this effect was transient. One possibility is that the task we used to measure behavioral flexibility may have been too simple and in a familiar environment such that NE is not required. This idea is consistent with previous work in both rodent olfactory memory (Shakhawat et al. 2015) and human visual memory (Segal et al. 2012) showing that NE is needed for complex pattern separation tasks (e.g., storing similar experiences as separate memories). Thus, future work should use a more difficult reversal task. Interestingly, the pattern of behavior on the day 1 of reversal learning appeared very similar to the pattern of behavior on probe day for retrieval memory. Another possibility is the lack of novelty in the reversal phase; our previous work has shown that novelty can increase both NE levels in several brain regions and the number of Fos-positive cells in the LC (Lisieski et al. 2019). Rats had several days of experience with the Barnes maze; they were tested at the same time of day, required to perform the same task (forage for an escape platform), and presented with the same local/distal cues for acquisition, retrieval and reversal phases of the experiment. Therefore, rotating the location of the escape platform may not have been novel enough or sufficiently difficult to require NE. Lastly, the use of number of visits to the prior location is a proxy measure for recall and may not fully encapsulate the complexity of this feature of memory. Our findings should be tempered in light of these alternative explanations and limitations with further studies conducted that incorporate other measures of recall to support, refute or likely further nuance our interpretations.
Sex difference
In our study, male, but not female, rats treated with DSP-4 surprisingly displayed heightened memory retrieval during the single probe trial on both latency and frequency of visits. Females showed impaired retrieval as measured by the frequency of visits. Though this work is one of the first to identify sex-dependent effects of NE depletion on spatial memory retrieval, previous studies have found sexually dimorphic effects in LC structure and function, NE system modulation, attention, and stress response (Bangasser et al. 2016; Beck and Luine 2010; Mei et al. 2021). The female rat LC has more NE neurons, longer dendrites (Bangasser et al. 2016) but when injected with DSP-4, compensatory receptor upregulation in both α and β-adrenoreceptor numbers have been observed in males more than in females (Hormigo et al. 2017). Future work should explore the mechanistic underpinnings of this sex effect; probing the influence of sex hormones (e.g., estradiol) on spatial learning and retrieval may prove beneficial, as ovarian hormones may impact NE synthesis, release, and metabolism (Berridge and Waterhouse 2003; Pau et al. 2000).
Considerations and conclusions
Despite our best efforts, some potential confounds exist, and some parsimonious explanations could explain our results. For example, we did not directly measure NE levels in Experiment 2, relying on the results from Experiment 1 as confirmatory. Experiment 1 results suggest that NE depletion occurs at least 15 d post-DSP-4 injection. It is unlikely, but possible, for these effects to be reversed 17-day (Experiment 2 probe trial) post-injection; however, several findings exclude this possibility. First, we used a reportedly high dose of DSP-4 (50mg/kg; Ross and Stenfors 2015). Additionally, Wolfman et al. (1994) demonstrated longevity of the DSP-4 effect, with a sustained 50% reduction of NE remaining even after 90 days following injection. We did not conduct a dose-response curve for DSP-4 or test other administration patterns; therefore, our results are not generalizable beyond the current dose and behavioral paradigm and unable to fully explain our observations related the role of NE in spatial learning concomitantly with the impact of sex as a biological variable on this role. Although numerous investigators have shown DSP-4 treatment to downregulate NE as shown here in Fig. 3 (Ross and Stenfors 2015; Sirviö et al. 1991), a recent report (Iannitelli et al. 2022) has shown that DSP-4 may lead to compensatory mechanisms that cause NE hyperactivity via augmentation of NE turnover, which was defined as an increase in MHPG:NE ratio with MHPG being the primary metabolite of NE. Considering this recent report, we cannot conclude that the remaining NE observed in our HPLC analysis was not hyperactive as we did not measure MHPG levels nor calculate a turnover ratio.
In conclusion, despite significantly reducing levels of NE, but not dopamine or serotonin, in dHip and CG, the neuromodulator-depleting effect of DSP-4 only had a modest impact on specific aspects of spatial learning and behavioral flexibility. Memory retrieval was most affected and changed in a sex-specific manner with males showing increased while females displaying decreased retrieval memory after reducing NE in the brain. These data suggest that NE has sex-specific modulator effects on memory retrieval with lesser effect on flexibility and no impact on acquisition of simple learned behavior.
Acknowledgements
We would like to acknowledge Dr. Garry Aston-Jones for his discussions with the authors and intellectual contributions to this manuscript. Additionally, we would like to acknowledge the contribution of the staff of the Wayne State University Department of Laboratory Animals for their maintenance of our subject’s health and wellbeing during these experiments.
Funding
National Institutes of Health: R01-DA-042057 [SAP], R21-DA-052657 [SAP], K01-DA-055068, Mercer University Seed Fund [AG], T32-GM-139807 [ATM, SAP]; Office of Vice President for Research, Wayne State University [CJD, SAP]
Footnotes
Conflict of interest The authors declare no competing interests.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- Arnsten AFT (2000) Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plasticity 7:609619. 10.1155/NP.2000.133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. 10.1146/annurev.neuro.28.061604.135709 [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P (1997) Conditioned responses of monkey locus coeruleus neurons anticipate acquisition of discriminative behavior in a vigilance task. Neuroscience 80(3):697–715. 10.1016/s0306-4522(97)00060-2 [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Wiersielis KR, Khantsis S (2016) Sex differences in the locus coeruleus-norepinephrine system and its regulation by stress. Brain Res 1641(Pt B):177–188. 10.1016/j.brainres.2015.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KD, Luine VN (2010) Evidence for sex-specific shifting of neural processes underlying learning and memory following stress. Physiol Behav 99(2):204–211. 10.1016/j.physbeh.2009.04.011 [DOI] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42(1):33–84. 10.1016/s0165-0173(03)00143-7 [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ (2005) Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28(11):574–582. 10.1016/j.tins.2005.09.002 [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Drummond GT, Sur M (2021) Locus coeruleus norepinephrine in learned behavior: anatomical modularity and spatiotemporal integration in targets. Front Neural Circuits 15:638007. 10.3389/fncir.2021.638007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Papadogiannis A, Dimitrov E (2021) The role of medial prefrontal cortex projections to locus ceruleus in mediating the sex differences in behavior in mice with inflammatory pain. FASEB J 35(7):e21747. 10.1096/fj.202100319RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae WR, Metz S, Weise J, Nowacki J, Piber D, Mueller SC et al. (2019) Effects of glucocorticoid and noradrenergic activity on spatial learning and spatial memory in healthy young adults. Behav Brain Res 373:112072. 10.1016/j.bbr.2019.112072 [DOI] [PubMed] [Google Scholar]
- Chalermpalanupap T, Schroeder JP, Rorabaugh JM, Liles LC, Lah JJ, Levey AI, Weinshenker D (2018) Locus coeruleus ablation exacerbates cognitive deficits, neuropathology, and lethality in P301S tau transgenic mice. J Neurosci 38(1):74–92. 10.1523/jneurosci.1483-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu N, Bloom FE (1973) Norepinephrine-containing neurons: changes in spontaneous discharge patterns during sleeping and waking. Science 179(4076):908–910. 10.1126/science.179.4076.908 [DOI] [PubMed] [Google Scholar]
- Council NR (2011) Guide for the Care and Use of Laboratory Animals: Eighth Edition. (T. N. A. Press; Ed.) [Google Scholar]
- Davidson CJ, Svenson DW, Hannigan JH, Perrine SA, Bowen SE (2022) A novel preclinical model of environment-like combined benzene, toluene, ethylbenzene, and xylenes (BTEX) exposure: Behavioral and neurochemical findings. Neurotoxicol Teratol 91:107076. 10.1016/j.ntt.2022.107076 [DOI] [PubMed] [Google Scholar]
- Gaulden AD, Burson N, Sadik N, Ghosh I, Khan SJ, Brummelte S et al. (2021) Effects of fentanyl on acute locomotor activity, behavioral sensitization, and contextual reward in female and male rats. Drug Alcohol Depend 229(Pt A):109101. 10.1016/j.drugalcdep.2021.109101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon E, Carcea I, Martins ARO, Multani J, Shehu I, Svirsky MA, Froemke RC (2019) Locus coeruleus activation accelerates perceptual learning. Brain Res 1709:39–49. 10.1016/j.brainres.2018.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grella SL, Gomes SM, Lackie RE, Renda B, Marrone DF (2021) Norepinephrine as a spatial memory reset signal. Behav Pharmacol 32(7):531–548. 10.1097/fbp.0000000000000648 [DOI] [PubMed] [Google Scholar]
- Hamlett ED, Ledreux A, Gilmore A, Vazey EM, Aston-Jones G, Boger HA et al. (2020) Inhibitory designer receptors aggravate memory loss in a mouse model of down syndrome. Neurobiol Dis 134:104616. 10.1016/j.nbd.2019.104616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen N (2017) The longevity of hippocampus-dependent memory is orchestrated by the locus coeruleus-noradrenergic system. Neural Plast 2017:2727602. 10.1155/2017/2727602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser J, Sontag TA, Tucha O, Lange KW (2012) The effects of the neurotoxin DSP4 on spatial learning and memory in Wistar rats. Atten Defic Hyperact Disord 4(2):93–99. 10.1007/s12402-012-0076-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans EJ, van Marle HJ, Ossewaarde L, Henckens MJ, Qin S, van Kesteren MT et al. (2011) Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science 334(6059):1151–1153. 10.1126/science.1209603 [DOI] [PubMed] [Google Scholar]
- Hormigo S, Gómez-Nieto R, Sancho C, Herrero-Turrión J, Carro J, López DE, Horta-Júnior J (2017) Morphological correlates of sex differences in acoustic startle response and prepulse inhibition through projections from locus coeruleus to cochlear root neurons. Brain Struct Funct 222(8):3491–3508. 10.1007/s00429-017-1415-1 [DOI] [PubMed] [Google Scholar]
- Hou L, Sun F, Sun W, Zhang L, Wang Q (2019) Lesion of the locus coeruleus damages learning and memory performance in paraquat and maneb-induced pouse Parkinson’s disease model. Neuroscience 419:129–140. 10.1016/j.neuroscience.2019.09.006 [DOI] [PubMed] [Google Scholar]
- Iannitelli AF, Kelberman MA, Lustberg DJ, Korukonda A, McCann KE, Mulvey B,... Weinshenker D (2022) The neurotoxin DSP-4 dysregulates the locus coeruleus-norepinephrine system and recapitulates molecular and behavioral aspects of prodromal neurodegenerative disease. bioRxiv. 10.1101/2022.09.27.509797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackisch R, Gansser S, Cassel JC (2008) Noradrenergic denervation facilitates the release of acetylcholine and serotonin in the hippocampus: towards a mechanism underlying upregulations described in MCI patients? Exp Neurol 213(2):345–353. 10.1016/j.expneurol.2008.06.011 [DOI] [PubMed] [Google Scholar]
- Kelberman MA, Anderson CR, Chlan E, Rorabaugh JM, McCann KE, Weinshenker D (2022) Consequences of hyperphosphorylated tau in the locus coeruleus on behavior and cognition in a rat model of Alzheimer’s disease. J Alzheimers Dis 86(3):1037–1059. 10.3233/jad-215546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV, Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci U S A 113(51):14835–14840. 10.1073/pnas.1616515114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakpour-Taleghani B, Lashgari R, Motamedi F, Naghdi N (2009) Effect of reversible inactivation of locus ceruleus on spatial reference and working memory. Neuroscience 158(4):1284–1291. 10.1016/j.neuroscience.2008.11.001 [DOI] [PubMed] [Google Scholar]
- Lisieski MJ, Karavidha K, Gheidi A, Garibyan RL, Conti AC, Morrow JD, Perrine SA (2019) Divergent effects of repeated cocaine and novel environment exposure on locus coeruleus c-fos expression and brain catecholamine concentrations in rats. Brain Behav 9(3):e01222. 10.1002/brb3.1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locklear MN, Kritzer MF (2014) Assessment of the effects of sex and sex hormones on spatial cognition in adult rats using the Barnes maze. Horm Behav 66(2):298–308. 10.1016/j.yhbeh.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mau W, Hasselmo ME, Cai DJ (2020) The brain in motion: how ensemble fluidity drives memory-updating and flexibility. Elife 9. 10.7554/eLife.63550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei X, Wang L, Yang B, Li X (2021) Sex differences in noradrenergic modulation of attention and impulsivity in rats. Psychopharmacology 238(8):2167–2177. 10.1007/s00213-021-05841-8 [DOI] [PubMed] [Google Scholar]
- Moser EI, Moser MB, McNaughton BL (2017) Spatial representation in the hippocampal formation: a history. Nat Neurosci 20(11):1448–1464. 10.1038/nn.4653 [DOI] [PubMed] [Google Scholar]
- Murchison CF, Zhang XY, Zhang WP, Ouyang M, Lee A, Thomas SA (2004) A distinct role for norepinephrine in memory retrieval. Cell 117(1):131–143. 10.1016/s0092-8674(04)00259-4 [DOI] [PubMed] [Google Scholar]
- Nowak P (2016) Selective lifelong destruction of brain monoaminergic nerves through perinatal DSP-4 treatment. Curr Top Behav Neurosci 29:51–71. 10.1007/7854_2015_398 [DOI] [PubMed] [Google Scholar]
- Osorio-Gómez D, Miranda MI, Guzmán-Ramos K, Bermúdez-Rattoni F (2023) Transforming experiences: neurobiology of memory updating/editing. Front Syst Neurosci 17:1103770. 10.3389/fnsys.2023.1103770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau KY, Hess DL, Kohama S, Bao J, Pau CY, Spies HG (2000) Oestrogen upregulates noradrenaline release in the mediobasal hypothalamus and tyrosine hydroxylase gene expression in the brainstem of ovariectomized rhesus macaques. J Neuroendocrinol 12(9):899–909. 10.1046/j.1365-2826.2000.00549.x [DOI] [PubMed] [Google Scholar]
- Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G et al. (2020) Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 21(11):644–659. 10.1038/s41583-020-0360-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross SB, Stenfors C (2015) DSP4, a selective neurotoxin for the locus coeruleus noradrenergic system. A review of its mode of action. Neurotox Res 27(1):15–30. 10.1007/s12640-014-9482-z [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S (2012) Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron 76(1):130–141. 10.1016/j.neuron.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Saucier DM, Shultz SR, Keller AJ, Cook CM, Binsted G (2008) Sex differences in object location memory and spatial navigation in Long-Evans rats. Anim Cogn 11(1):129–137. 10.1007/s10071-007-0096-1 [DOI] [PubMed] [Google Scholar]
- Segal SK, Stark SM, Kattan D, Stark CE, Yassa MA (2012) Norepinephrine-mediated emotional arousal facilitates subsequent pattern separation. Neurobiol Learn Mem 97(4):465–469. 10.1016/j.nlm.2012.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhawat AM, Gheidi A, MacIntyre IT, Walsh ML, Harley CW, Yuan Q (2015) Arc-expressing neuronal ensembles supporting pattern separation require adrenergic activity in anterior piriform cortex: an exploration of neural constraints on learning. J Neurosci 35(41):14070–14075. 10.1523/jneurosci.2690-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirviö J, Riekkinen P Jr, Valjakka A, Jolkkonen J, Riekkinen PJ (1991) The effects of noradrenergic neurotoxin, DSP-4, on the performance of young and aged rats in spatial navigation task. Brain Res 563(1–2):297–302. 10.1016/0006-8993(91)91550-k [DOI] [PubMed] [Google Scholar]
- Sontag TA, Hauser J, Tucha O, Lange KW (2011) Effects of DSP4 and methylphenidate on spatial memory performance in rats. Atten Defic Hyperact Disord 3(4):351–358. 10.1007/s12402-011-0067-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift KM, Gross BA, Frazer MA, Bauer DS, Clark KJD, Vazey EM et al. (2018) Abnormal locus coeruleus sleep activity alters sleep signatures of memory consolidation and impairs place cell stability and spatial memory. Curr Biol 28(22):3599–3609.e3594. 10.1016/j.cub.2018.09.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Johansen JP (2015) Projection specificity in heterogeneous locus coeruleus cell populations: implications for learning and memory. Learn Mem 22(9):444–451. 10.1101/lm.037283.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling SG, Milway JS, Ingram M, Lau C, Morrison G, Martin GM (2016) The effects of prolonged administration of norepinephrine reuptake inhibitors on long-term potentiation in dentate gyrus, and on tests of spatial and object recognition memory in rats. Neurobiol Learn Mem 128:92–102. 10.1016/j.nlm.2015.12.013 [DOI] [PubMed] [Google Scholar]
- Wolfman C, Abó V, Calvo D, Medina J, Dajas F, Silveira R (1994) Recovery of central noradrenergic neurons one year after the administration of the neurotoxin DSP4. Neurochem Int 25(4):395–400. 10.1016/0197-0186(94)90147-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


