Abstract
Background
Continuous kidney replacement therapy (CKRT) has become an integral part of the care of critically ill children. However, uncertainty exists regarding the current state of how CKRT is prescribed and delivered in children. The main objective of this study was to identify the current practices for pediatric CKRT.
Methods
We conducted a systematic review of the literature from 2012 to 2022 to identify data regarding CKRT timing of initiation, dosing, anticoagulation, fluid removal, and quality monitoring. Using this data, we then performed a two-round modified Delphi process using a multinational internet-assisted survey of prescribers of CKRT.
Results
The survey was constructed using 172 articles that met inclusion criteria (12% of studies were pediatric focused). A total of 147 and 126 practitioners completed the survey in rounds 1 and 2, respectively. Participants represented Europe (9.5–11.6%) and North America including pediatric intensivists, nephrologists, and advance practice providers. Consensus (defined as a ≥ 75% participant response of “sometimes” or “always”) was achieved for 26 statements. There was consensus in the practices of CKRT initiation, dosing, method of anticoagulation, and fluid removal. In contrast, there appears to be greater variability in the methods used for monitoring anticoagulation and the quality of the delivered treatment.
Conclusions
Our study results suggest that the current state of pediatric CKRT practice is reflective of the literature over the last 10 years, which is largely based on the care of adult patients. This data provides a framework to study best practices to further improve outcomes for children receiving CKRT.
Keywords: Continuous kidney replacement therapy, Children, Pediatric intensive care unit, Acute kidney injury
Introduction
Since the introduction of continuous kidney replacement therapy (CKRT) in the 1980s, its use in children has increased substantially, in particular over the past decade [1]. Despite the acceptance of CKRT as a standard therapy for acute kidney failure in critically ill children, there is limited pediatric-specific literature guiding the practice of optimal timing of initiation, delivered dose, anticoagulation strategy, quality monitoring, and fluid removal. Most pediatric CKRT knowledge originates from single-center reports and a 16-year-old registry with selective enrollment [2]. As the use of pediatric CKRT continues to grow and evolve, it is important that we understand the current state of practice.
The Delphi method has been used previously to develop core outcomes for research in a diverse range of clinical conditions [3, 4]. By conducting a systematic review and using a modified Delphi approach, the ultimate goal of this study was to determine the current state of pediatric CKRT prescribing practices in order to inform future research and improvement in clinical care.
Methods
Systematic review and survey development
All study authors conducted a systematic review of the literature to identify current adult and pediatric data regarding CKRT initiation, optimal delivered dose, anticoagulation strategy, fluid removal, and quality monitoring, published in the last 10 years. We limited the timeframe from 2012 to 2022 in order to capture the more current literature on CKRT prescribing practices. We did not limit the search to pediatric studies, given that pediatric CKRT practice is oftentimes extrapolated from studies including adult patients. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [5]. The study was pre-registered on the Prospective Register of Systematic Reviews (CRD42022308911) on March 8, 2022. PubMed, Google Scholar, and Scopus were searched for English-language literature, including human studies of all ages from January 2012 to January 2022. Eligible studies included randomized controlled studies, case–control studies, cohort studies, case series, systematic reviews, meta-analyses, and review articles. The search terms were “continuous renal replacement therapy” or “CRRT” or “continuous kidney replacement therapy” or “CKRT” or “continuous hemofiltration” or “continuous hemodialysis” or “continuous hemodiafiltration.” Studies were included if they included any of the following themes: CKRT initiation, CKRT start, CKRT timing, CKRT dose, CKRT prescription, dialysis dose, solute control, anticoagulation and CKRT, fluid balance and CKRT, fluid removal and CKRT, or CKRT and quality improvement. Studies were excluded if they did not include these themes. The final studies meeting inclusion criteria were agreed upon by all authors.
Survey development and implementation
The full text of the articles meeting inclusion criteria was reviewed by all authors to develop a survey based on the content of the studies. The final survey was approved by the University of Pittsburgh’s Human Research Protection Office in adherence to the Declaration of Helsinki, and study informed consent was obtained from all participants (study ID # 22,010,158). Members of the newly established Worldwide Exploration of Renal Outcomes Collaborative in Kidney Diseases (WE-ROCK) initiative, a multi-national collaborative in kidney replacement therapy, were recruited for participation (all authors are steering committee members) for distribution of the survey to their respective practice groups, excluding trainees. At the time of the distribution of the survey, WE-ROCK members represented eight countries including Australia, Austria, Canada, Italy, Spain, Turkey, the UK, and the USA. The WE-ROCK collaborative includes pediatric prescribers (cardiac intensive care physicians, pediatric intensive care physicians, nephrology physicians, and advance practice providers) of CKRT at differing stages of experience. The survey was self-administered and anonymous using Qualitrics (Provo, USA). We used a two-step modified Delphi method [3]. The survey included demographic questions followed by a series of statements where participants were asked to rate how they practice CKRT by selecting “always” if they always use the stated practice, “sometimes” if they sometimes use the stated practice, or “never” if they never use the stated practice. Responses to free text questions on round 1 of the survey were used to create additional free text questions for round 2 of the survey. It was established a priori that statements with a ≥ 75% response of “sometimes” or “always” in round 1 of the survey would be included in round 2 of the survey. Figure 1 shows the study flow. The 63-item first round of the survey was sent to participants on April 20, 2022 (Supplemental Table 1). The 50-item second survey was sent to participants on June 9, 2022 (Supplemental Table 2). Consensus was defined as those statements with ≥ 75% responses of “sometimes” or “always” on round 1 and round 2 of the survey.
Fig. 1.

Study flow. A systematic review identified studies published in the last 10 years as related to continuous kidney replacement therapy (CKRT) initiation, timing, dose, prescription, solute control, anticoagulation, fluid balance, fluid removal, or quality improvement. Based on the systematic review, 172 studies informed the creation of the first round of the survey. The second round of the survey was created from those statements in the first round of the survey whereby ≥ 75% of respondents answered “sometimes” or “always” and from the responses to free text questions included in the first of the survey
Results
Figure 2 shows the PRISMA flow diagram of retrieved and included studies. The search identified 18,945 articles. A total of 172 publications met the inclusion criteria (84 reviews, 43 cohort studies, 27 systematic reviews/meta-analyses, and 18 randomized control trials). Twenty-one studies included pediatric patients (13 cohort studies and 8 review papers); there were no published randomized controlled trials in children. The survey was disseminated to 248 individuals in 8 countries. Within the USA, the survey was sent to prescribers located in 16 states. A total of 147 prescribers (59.3%) completed round 1 of the survey, and 126 prescribers (50.8%) completed round 2 of the survey. There was a similar distribution of practice locations for both rounds of the survey, with the majority of respondents located in the USA (Table 1). Physicians and nurse practitioners responded to both surveys representing the primary specialties of cardiac critical care medicine, critical care medicine, and nephrology. For both surveys, the majority of respondents identified nephrology as their primary specialty (64.6% for round 1 and 54.8% for round 2). There was a similar representation of differing levels of experience when comparing those who participated in the first and second rounds of the survey (Table 1). For both survey rounds, nephrology was reported as the most common specialty responsible for CKRT order placement (70.1% in round 1 and 61.9% in round 2). Table 2 shows the proportion of responses for both rounds of the survey. For those statements that achieved ≥ 75% responses of “sometimes” or “always” on round 1 of the survey, they were included in round 2. All of the statements that received ≥ 75% responses of “sometimes” or “always” in round 1 of the survey also received ≥ 75% responses of “sometimes” or “always” in round 2 of the survey (Table 2).
Fig. 2.
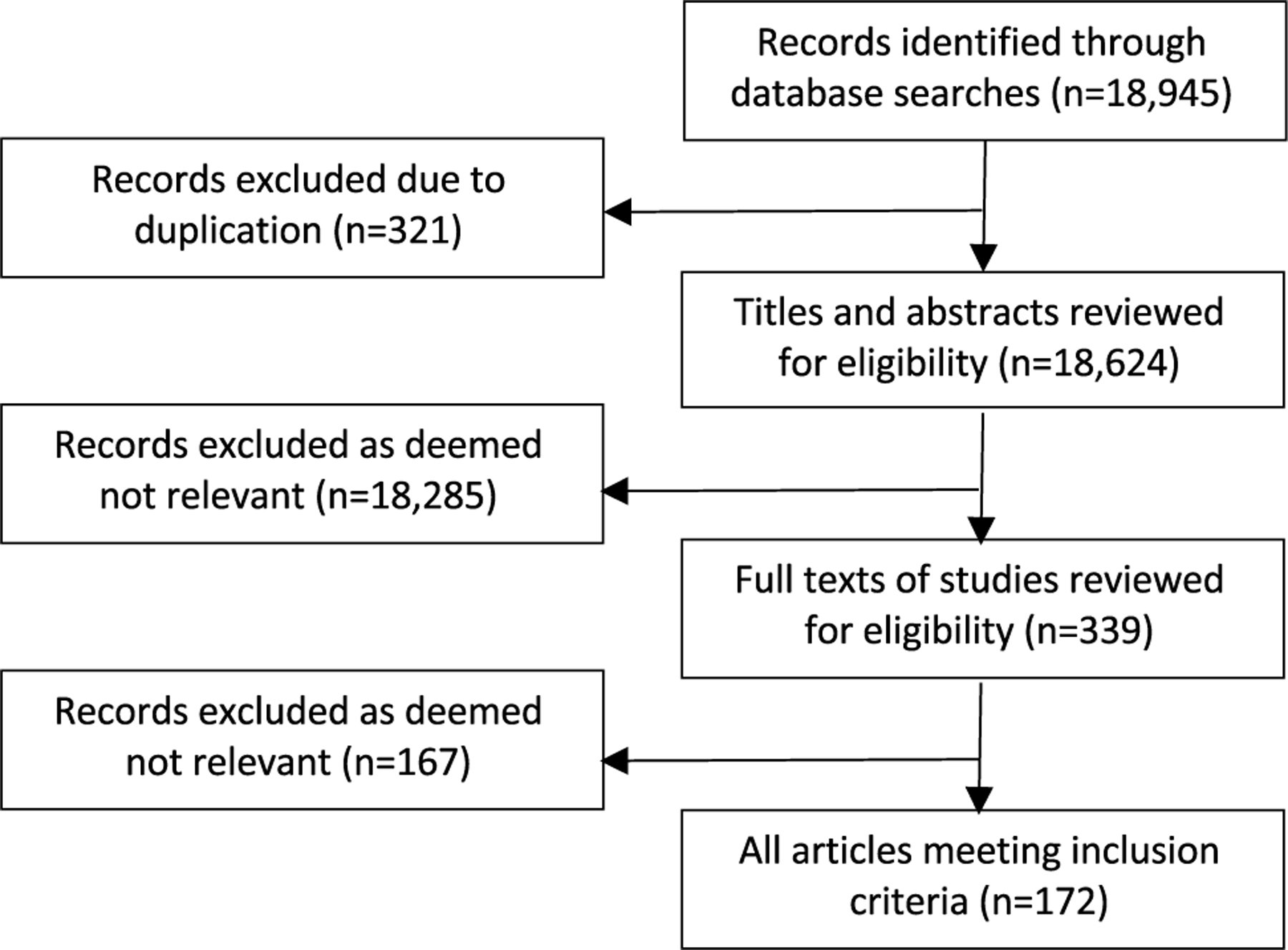
PRISMA flow diagram of retrieved and included records
Table 1.
Prescriber characteristics
| Characteristic | Survey round 1 n = 147 | Survey round 2 n = 126 |
|---|---|---|
| Response rate, n/total (%) | 147/248 (59.3) | 126/248 (50.8) |
| Practice location, n (%) | ||
| Canada | 16 (10.9) | 13 (10.3) |
| Europe | 17 (11.6) | 12 (9.5) |
| USA | 114 (77.6) | 101 (80.2) |
| Primary profession, n (%) | ||
| Physician | 135 (91.8) | 106 (84.1) |
| Nurse practitioner | 8 (5.4) | 20 (15.9) |
| Primary specialty, n (%) | ||
| Cardiac critical care medicine | 15 (10.2) | 15 (11.9) |
| Critical care medicine | 37 (25.2) | 42 (33.3) |
| Nephrology | 95 (64.6) | 69 (54.8) |
| Secondary specialty, n (%) | ||
| Cardiac critical care medicine | 5 (3.4) | 4 (3.2) |
| Critical care medicine | 11 (7.5) | 3 (2.4) |
| Nephrology | 14 (9.5) | 10 (7.9) |
| Not applicable | 117 (79.6) | 109 (86.5) |
| Years in current profession excluding trainee time, n (%) | ||
| 0–5 | 41 (27.9) | 30 (23.8) |
| 6–10 | 32 (21.8) | 28 (22.2) |
| 11–15 | 24 (16.3) | 21 (16.7) |
| 16–20 | 20 (13.6) | 28 (22.2) |
| ≥ 20 | 29 (19.7) | 19 (15.1) |
| Institutional specialty responsible for CKRT order placement | ||
| Critical care | 34 (23.1) | 35 (27.8) |
| Nephrology | 103 (70.1) | 78 (61.9) |
| A combination of the above | 10 (6.8) | 13 (10.3) |
Table 2.
Responses to statements on rounds 1 and 2 of the survey regarding continuous kidney replacement therapy (CKRT) prescribing practices
| Round 1 (n = 147) |
Round 2 (n = 126) |
|||||
|---|---|---|---|---|---|---|
| always% | sometimes% | never% | always% | sometimes% | never% | |
| CKRT initiation | ||||||
| *I start patients on CKRT after they reach a positive fluid balance ≥ 10% in the absence of any other indications | 4.1 | 75.5 | 20.4 | 1.9 | 84.1 | 13.0 |
| *I start patients on CKRT after they reach a positive fluid balance ≥ 15% in the absence of any other indications | 12.2 | 73.5 | 14.3 | 9.5 | 86.5 | 4.0 |
| *I start patients on CKRT after they reach a positive fluid balance ≥ 20% in the absence of any other indications | 41.2 | 48.9 | 9.9 | 27.8 | 68.5 | 3.7 |
| *I start CKRT in a patient with a urine output < 0.3 ml/kg/h for ≥ 24 h in the absence of any other indications | 18.4 | 64.6 | 17.0 | 5.6 | 79.6 | 14.8 |
| I start CKRT in a patient with a serum creatinine 2–2.9 times baseline or greater in the absence of any other indications | 2.7 | 41.5 | 55.8 | |||
| I start CKRT in a patient with urine output < 0.5 ml/kg/h for ≥ 12 h in the absence of any other indications | 2.3 | 65.9 | 31.8 | |||
| I start CKRT in a patient with a serum creatinine that is 3 times baseline or greater in the absence of any other indications | 8.8 | 42.2 | 49.0 | |||
| I start CKRT in a patient with a decrease in eGFR to < 35 ml/min per 1.73 m2 in the absence of any other indications | 4.1 | 47.6 | 48.3 | |||
| CKRT dosing | ||||||
| *I prescribe dialytic dose using body surface area | 56.5 | 21.4 | 22.1 | 68.2 | 16.7 | 15.1 |
| *I use the admission weight of the patient when dosing based on weight or body surface area | 26.7 | 64.9 | 8.4 | 28.6 | 69.8 | 1.59 |
| *I use the ideal body weight of the patient when dosing based on weight or body surface area | 21.1 | 63.9 | 15.0 | 9.5 | 77.8 | 12.7 |
| *If I index dialytic dose to body surface area, I aim for a clearance of 2 L/h/1.73 m2 | 57.9 | 40.0 | 2.1 | 50.8 | 44.4 | 4.8 |
| *I use continuous venovenous hemodiafiltration (CVVHDF) as a mode of CKRT | 43.2 | 53.8 | 3.0 | 64.3 | 33.3 | 2.4 |
| *I use CKRT rather than intermittent hemodialysis for hyperammonemia | 46.9 | 43.2 | 9.9 | 44.4 | 51.6 | 4.0 |
| *I change the hemofilter after 72 h of therapy | 27.3 | 67.4 | 5.3 | 19.9 | 76.2 | 4.0 |
| I prescribe dialytic dose using weight | 25.9 | 26.5 | 47.6 | |||
| I use the current weight of the patient when dosing based on weight or body surface area | 4.6 | 59.5 | 35.9 | |||
| If I index patient dialytic dose to weight, I aim for a clearance of 20–30 ml/kg/h | 40.0 | 49.33 | 10.7 | |||
| If I index patient dialytic dose to weight, I aim for a clearance of 20–45 ml/kg/h | 35.9 | 53.9 | 10.3 | |||
| I use continuous venovenous hemofiltration (CVVH) as a mode of CKRT | 3.4 | 62.6 | 34.0 | |||
| I use continuous venovenous hemodialysis (CVVHD) as a mode of CKRT | 4.1 | 64.6 | 31.3 | |||
| I use slow continuous ultrafiltration (SCUF) as a mode of CKRT | 0 | 39.0 | 61.0 | |||
| I use customizable CKRT solutions | 23.9 | 42.2 | 34.0 | |||
| I modify my standard starting CKRT dialytic dose if I am treating a patient with hyperammonemia | 68.2 | 30.3 | 1.5 | |||
| I modify my standard starting CKRT dialytic dose if I am treating a patient for drug intoxication | 46.8 | 50.8 | 2.4 | |||
| I modify my standard starting CKRT dialytic dose if I am treating a patient with citrate accumulation | 41.0 | 56.1 | 3.0 | |||
| Anticoagulation strategy | ||||||
| *I use citrate as a regional anticoagulant for CKRT | 24.2 | 72.0 | 3.8 | 14.8 | 79.6 | 5.6 |
| *I use systemic heparin as an anticoagulant for CKRT | 5.3 | 86.4 | 8.3 | 3.7 | 92.6 | 3.7 |
| *I use citrate as a regional anticoagulant in patients with liver failure | 22.1 | 56.5 | 21.4 | 18.5 | 61.1 | 20.4 |
| I use prostacyclin as an anticoagulant for CKRT | 0.7 | 23.1 | 76.2 | |||
| When I use heparin for anticoagulation on CKRT I follow ACT values | 29.9 | 37.3 | 32.8 | |||
| When I use heparin for anticoagulation on CKRT I follow PTT values | 30.3 | 48.5 | 21.2 | |||
| When I use heparin for anticoagulation on CKRT I follow Anti-Xa values | 15.2 | 50.0 | 34.9 | |||
| CKRT for patients ≤ 10 kg | ||||||
| *I prescribe CKRT for patients ≤ 10 kg | 51.7 | 48.2 | 0 | 51.8 | 48.2 | 0 |
| *I prescribe a blood prime when an extracorporeal circuit exceeds 10% of a patient’s circulating blood volume | 65.9 | 28.8 | 5.3 | 74.6 | 25.4 | 0 |
| The machine that I use for CKRT in patients ≤ 10 kg is The Cardio-Renal Pediatric Dialysis Emergency Machine (CARPEDIEM) | 8.3 | 39.6 | 52.1 | |||
| The machine that I use for CKRT in patients ≤ 10 kg is The Prismaflex System | 28.3 | 64.2 | 7.6 | |||
| The machine that I use for CKRT in patients ≤ 10 kg is The Aquadex SmartFlow System | 6.1 | 45.0 | 49.0 | |||
| Fluid removal strategy | ||||||
| *I begin removing fluid in the first hour of CKRT | 13.6 | 65.2 | 21.2 | 12.2 | 63.3 | 24.5 |
| *I assess fluid removal goals at least every 24 h | 85.0 | 13.6 | 1.4 | 90.7 | 9.3 | 0 |
| *I assess fluid removal goals at least every 12 h | 51.0 | 47.6 | 1.4 | 46.0 | 52.4 | 1.6 |
| *I assess fluid removal goals at least every 6 h | 22.0 | 65.9 | 12.1 | 7.4 | 74.1 | 18.5 |
| *At my center goals for fluid removal are determined primarily by the critical care medicine team | 43.2 | 42.4 | 14.4 | 18.4 | 57.4 | 24.1 |
| *At my center goals for fluid removal are determined by the critical care and nephrology teams together | 46.9 | 47.6 | 5.4 | 45.2 | 54.8 | 0 |
| *My primary consideration when deciding to initiate net ultrafiltration is hemodynamic status | 40.5 | 58.0 | 1.5 | 38.9 | 61.1 | 0 |
| *My primary consideration when deciding to initiate net ultrafiltration is fluid balance | 29.0 | 66.4 | 4.6 | 16.7 | 77.7 | 5.6 |
| *I achieve my goal net ultrafiltration rate by varying ultrafiltration rate only | 44.2 | 47.6 | 8.2 | 40.7 | 53.7 | 5.6 |
| I assess fluid removal goals at least every 4 h | 14.3 | 53.8 | 31.8 | |||
| I base my maximum fluid removal goal on the patient’s total blood volume | 26.5 | 36.7 | 36.7 | |||
| At my center goals for fluid removal are determined primary by the nephrology team | 3.8 | 53.4 | 42.8 | |||
| I achieve my goal net ultrafiltration rate by varying replacement fluid rate only | 0.8 | 38.5 | 60.1 | |||
| I achieve my goal net ultrafiltration rate by varying both the ultrafiltration rate and the replacement fluid rate | 12.2 | 44.2 | 43.5 | |||
| When calculating net ultrafiltration rate, I account for enteral intake | 58.6 | 35.7 | 5.7 | |||
| When hemodynamic instability occurs on CKRT my first intervention or recommended intervention is to reduce the ultrafiltration rate | 17.1 | 75.7 | 7.1 | |||
| When hemodynamic instability occurs on CKRT my first intervention or recommended intervention is to start a vasoactive agent | 7.1 | 81.4 | 11.4 | |||
| When hemodynamic instability occurs on CKRT my first intervention or recommended intervention is to provide a bolus of fluid | 10.4 | 75.4 | 14.5 | |||
| Quality monitoring | ||||||
| *I monitor filtration fraction | 54.6 | 28.8 | 16.6 | 56.3 | 33.3 | 10.3 |
| I measure delivered CKRT dose based on blood and effluent concentrations of urea nitrogen | 18.8 | 36.2 | 44.9 | |||
Survey statements with ≥ 75% response of “always” or “sometimes” on both rounds of the survey
Timing of CKRT initiation
Respondents indicated that that they used a positive fluid balance of ≥ 10%, ≥ 15%, ≥ 20% at least sometimes in the absence of any other indications to start CKRT on rounds 1 and 2 of the survey (Table 2). In contrast, participants do not use serum creatinine thresholds consistent with Kidney Disease Improving Global Outcomes (KDIGO) criteria for stage 2 or 3 acute kidney injury (AKI) to start CKRT in the absence of any other indications. Participants indicated they “always” or “sometimes” start CKRT in patients with weight-indexed urine output < 0.3 ml/kg/h for 24 h, consistent with KDIGO AKI stage 3, in the absence of any other indications.
CKRT dosing
The majority of providers indicated that they index dialytic dose using body surface area (BSA), aiming for a clearance of 2 L/h/1.73 m2 (Table 2). When prescribers were asked as a free text question their typical dose when prescribing CKRT based on BSA, 38.1% responded with 2 L/h/1.73 m2. The remainder provided a range of 1–2 L/h/1.73 m2. Eighty-nine percent of participants responded on round 1 of the survey that when indexing dialytic dose to weight, they “always” or “sometimes” aim for a clearance of 20–30 ml/kg/h. In the first round of the survey, in response to the free text question asking for a typical dose when dosing by ml/kg/hour, 14.3% responded with 20 ml/kg/h with the remaining participants providing answers ranging from 25 to 60 ml/kg/h. Therefore, in the second round of the survey, the statement was modified to “If I index patient dialytic dose to weight, I aim for a clearance of 20–45 ml/kg/hour.” Ninety percent of prescribers responded to this statement with “always” or “sometimes.” Respondents indicated that when dosing based on weight or BSA, they either use admission weight or ideal body weight, but not current weight (Table 2).
The majority of participants indicated that that they “always” or “sometimes” use continuous venovenous hemodiafiltration (CVVHDF) as a mode of CKRT in rounds 1 and 2 of the survey, 97% and 97.6%, respectively. In contrast, the proportion of responses in round 1 of the survey concerning the use of CVVH and CVVHD did not meet criteria for the second round. When prescribing CVVHDF or CVVH, where prescribers routinely administer replacement fluid, the responses varied: 36.7% pre-dilution, 22.4% post-dilution, 34.7% both, and 6.1% not applicable. Supplemental Tables 3 and 4 show the variety of reported solutions for replacement and dialysate fluid.
In round one of the survey, the most common reasons provided for modifying the dose were hyperammonemia, drug intoxication, and citrate accumulation. Therefore, we included the following statements in round 2 of the survey: “I modify my standard starting CKRT dialytic dose if I am treating a patient with hyperammonemia,” “I modify my standard starting CKRT dialytic dose if I am treating a patient for drug intoxication,” and “I modify my standard starting CKRT dialytic dose if I am treating a patient with citrate accumulation.” In round 2 of the survey, ≥ 75% of prescribers responded with “always” or “sometimes” for all three of these statements.
Anticoagulation strategy
In both rounds of the survey, the majority of prescribers indicated that they use citrate or heparin as an anticoagulant for CKRT. When participants were asked as a free text question the most common anticoagulant used at their center, the responses included: citrate (80.3%), heparin (12.9%), prostacyclin (4.1%), and heparin/citrate equally (2.7%). Prescribers indicated that they use citrate as an anticoagulant in patients with liver failure.
Based on a wide range of responses regarding monitoring parameters while using heparin for anticoagulation, 3 statements were added to the second round of the survey: “When I use heparin for anticoagulation on CKRT I follow ACT values,” “When I use heparin for anticoagulation on CKRT I follow PTT values,” and “When I use heparin for anticoagulation on CKRT I follow Anti-Xa values.” Less than 75% of participants indicated that they “always” or “sometimes” use ACT or anti-Xa values, but 76% responded that they “always” or “sometimes” follow PTT values.
CKRT for patients ≤ 10 kg
All participants indicated that they “sometimes” or “always” prescribe CKRT to patients weighing ≤ 10 kg (Table 2). The majority of survey participants “always” or “sometimes” prescribe a blood prime when a patient’s extracorporeal circuit exceeds 10% of a patient’s circulating blood volume. When asked what machine they use for providing CKRT to patients ≤ 10 kg, the responses included: Prismaflex (51%), Cardio-Renal Pediatric Dialysis Emergency Machine (CARPEDIEM) (29%), and Aquadex (20%). Less than 75% of respondents indicated in round 2 of the survey that they use the CARPEDIEM™ or the Aquadex SmartFlow System™ “always” or “sometimes.” However, 92.5% stated that they use the Prisma Platform™ “always” or “sometimes.”
Fluid removal strategy
Respondents indicated that they assess fluid removal goals at least every 6, 12, or 24 h (Table 2). The majority of prescribers “always” or “sometimes” begin removing fluid in the first hour of CKRT. Fluid removal goals are determined either by the critical care team alone or by the critical care team and nephrology team together. Primarily, considerations when deciding to initiate net ultrafiltration are hemodynamic status and fluid balance. Most participants “always” or “sometimes” achieve net ultrafiltration by varying the ultrafiltration rate only, rather than varying the replacement fluid rate. When asked if they have a maximum net ultrafiltration rate in ml/kg/hour, 62.7% of participants in the first survey responded with “no,” indicating that most centers do not have an established maximum net ultrafiltration rate.
Based on the free text responses regarding participants’ most common intervention for when hemodynamic instability occurs during net ultrafiltration in round 1 of the survey, 3 additional statements were added to the second round of the survey. Greater than 75% of participants responded that when hemodynamic instability occurs on CKRT, their first intervention or recommended intervention is to reduce the ultrafiltration rate, start a vasoactive agent, or provide a fluid bolus.
Quality monitoring
When asked about a quality monitoring program for CKRT, 86.3% responded favorably. The majority of participants “always” or “sometimes” monitor the filtration fraction and target a filtration fraction of 25% or less. Less than 75% of participants “always” or “sometimes” measure delivered CKRT dose based on blood and effluent concentration of urea nitrogen. There were a wide range of responses regarding methods used to monitor dose delivered, including: frequent lab monitoring, nursing specialist charting, chemistry values, looking at the machine, changes in blood urea nitrogen, electrolyte clearance, urea clearance, or ratio of effluent urea nitrogen to blood urea nitrogen.
Discussion
Understanding and addressing the current practice patterns in pediatric CKRT is pivotal to improving outcomes. In contrast to other therapies offered in the pediatric intensive care unit, such as mechanical ventilation or sedation, there are no well-established guidelines for the practice of pediatric CKRT. The KDIGO guidelines provide some recommendations regarding a target effluent dose in ml/kg/h and the use of citrate as the preferred form of anti-coagulation [6]. Despite the survey being voluntary and anonymous with no incentive for participation, just over half of recipients completed the survey. Our study results, including a relatively large number of survey respondents with retention throughout two rounds of surveys, show that there are areas in the delivery of CKRT that prescribers sometimes or always implicate which is indicative of the current state of the literature. The survey results also demonstrate other nuances to prescribing CKRT whereby variability and inconsistency in practice likely exist.
Numerous published pediatric cohort studies have shown a significant association between percent fluid overload thresholds of 10%, 15%, and 20% and poor outcomes [7, 8]. Therefore, it is not surprising that the majority of current prescribers of pediatric CKRT use these values as a threshold to initiate therapy at least sometimes or always. In addition, in both children and adults receiving CKRT, there are study results showing an improvement in survival for patients who have a significant decline in cumulative fluid balance [9] or attain their dry weight [10]. Importantly, however, only 4.1%, 12.2%, and 41.2% of respondents indicated that they always start CKRT in response to a percent fluid overload threshold of 10%, 15%, and 20%, respectively. These results suggest that there is not a fixed, predictable, absolute threshold of fluid overload whereby prescribers begin therapy. The STARRT-AKI trial results in adults and the recently published Bayesian reanalysis of the trial show no survival benefit to an accelerated (initiating dialysis within 12 h of meeting criteria for KDIGO stages 2 or 3) as compared to starting dialysis in response to conventional indications or AKI lasting greater than 72 h [11, 12]. Our survey results suggest that prescribers are not using serum creatinine changes consistent with KDIGO stages 2 or 3 in isolation to determine the need for CKRT. However, 83% and 85.2% of participants in rounds 1 and 2 of our survey, respectively, responded that they “always” or “sometimes” start CKRT based on KDIGO stage 3 urine output criteria in the absence of any other indications.
There are no randomized trials defining the optimal CKRT dose in children. Consistent with dosing regimens discussed in the literature [13, 14], the majority of participants in our survey dosed by BSA as 2 L/h/1.73 m2 or by weight as 20–30 ml/kg/h using admission weight or ideal body weight, but not current weight. There is a non-linear relationship between weight and BSA; the conversion from a weight-based dose in adults matches well with a BSA-based dose in older children, but will result in a disproportionately higher dose in neonates and infants. For example, the same prescription of 2 L/h/1.73 m2 in neonates and infants is equivalent to 70–100 ml/kg/h [15]. Both higher and lower doses can lead to dialysis-related morbidity. The optimal method of determining dialysis dose in children is unknown. There is a critical need to establish the optimal dosing of pediatric CKRT with respect to a weight-based approach as compared to a BSA-based approach.
Our study results show differences in where prescribers routinely administer replacement fluid. Delivering replacement fluid pre-dilution as compared to post-dilution may improve filter lifespan [16]. However, there is also evidence that delivering replacement fluids pre-dilution can decrease daily clearance, requiring a higher prescription dose [17]. It is uncertain how this evidence would extrapolate to the younger population who might be receiving a relatively higher clearance dose due to the nonlinear BSA/weight relationship.
The majority of our survey participants indicated that citrate or heparin is the anticoagulant of choice at their institution. Studies in both children and adults show improved filter life and safety with the use of citrate when compared to heparin [18, 19]. There was consensus regarding the use of citrate anticoagulation in patients with liver failure, consistent with reports that citrate can be used safely in this patient group both in adults and children [20]. Interestingly, the majority of participants indicated that they use CVVHDF as a mode of CKRT. It is possible that the preference for CVVHDF (as opposed to CVVH or CVVHD) is associated with the preference for citrate anticoagulation, given that when using citrate with the Prismaflex System, CVVHDF is the typically the preferred mode. There was wide variability in responses regarding monitoring parameters for the use of heparin with the most agreement in the use of PTT values.
In recent years, there has been an increase in the options for providing CKRT to infants. In both rounds of our surveys, 100% of participants indicated that they at least sometimes prescribe CKRT to patients weighing ≤ 10 kg. Although we found that the majority of prescribers use the Prisma Platform™, published experience with devices like CARPEDIEM™ and the Aquadex SmartFlow System™ will likely increase the use of miniaturized circuits and membranes for neonates in the future [21, 22].
Similar to the results of a survey administered to adult prescribers of CKRT, our survey participants favored early fluid removal [23]. Observational data in children suggest that early fluid removal might be associated with a lower risk of mortality [2, 10]. Fluid removal in CKRT can occur by changing either the amount of ultrafiltration and/or the replacement fluid. As has been shown in a survey of adult practice, most participants in our study achieve fluid removal by varying the ultrafiltration rate rather than the replacement fluid rate [23]. There is a need to establish a safety threshold for fluid removal rates in pediatric CKRT patients.
Most of our survey participants responded that they do have a quality monitoring program for CKRT at their institution. However, there was a wide range of responses for the methods used to monitor dose delivered. The consensus recommendations of the 2016 Acute Dialysis Quality Initiative Conference state that filter efficiency should be monitored using a ratio of effluent fluid to blood urea nitrogen [24]. Forty-five percent of our survey respondents never measure CKRT dose based on blood and effluent concentrations of urea nitrogen. Recently, Mottes and colleagues published a standard for CKRT process measurement [25]. It is likely that the adoption of efforts such as these will create universal standards for tracking the quality of CKRT delivery in the future. As with any high-risk procedure performed in the ICU, the practice of CKRT in children should include clearly defined policies, procedures, and methods to evaluate efficiency and safety at all centers using this therapy [26, 27]. It is essential to establish agreed-upon benchmarks for quality pediatric CKRT that can be integrated into routine clinical practice [28].
Our study has limitations. Since the survey was anonymous, we are unable to determine which individuals participated in both rounds of the survey. Importantly, we sought to preserve the anonymity of the surveys to allow respondents the opportunity to answer in an open and unbiased manner. Given the relatively smaller number of responses from countries outside of the USA, it is possible that the survey results are not generalizable internationally. Inherent in the use of a voluntary web-based survey is the possibility of non-response bias among those who did not respond to the survey.
In conclusion, this study provides data regarding the current state of CKRT prescribing practices as determined from a multidisciplinary group of prescribers of varying levels of experience. Although we found agreement among some areas of how pediatric CKRT is prescribed, there is clearly practice variability. There is a crucial need for further work to understand the drivers of such practice variability. As it currently stands, the majority of pediatric CKRT-related knowledge is based on mainly single-center reports and evidence from randomized control trials in adults. This likely explains in part the practice variability shown in our data. There is a need for multi-center work investigating the practices described in our study results. The information gained in this study can be used to guide future clinical trial design to determine best practices for children receiving CKRT.
Supplementary Material
Acknowledgements
The authors would like to thank the Worldwide Exploration of Renal Replacement Outcomes Collaboration in Kidney Disease (WE-ROCK) Collaborative for helping with recruitment for the study. Importantly, we thank the survey participants for their time in completing the survey.
Funding
NIH K23DK116973 (DYF); Gerber Foundation (KMG), NIH 1RL1HD107780-01 (AAA).
Footnotes
Supplementary Information The online version contains supplementary material available at https://doi.org/10.1007/s00467-022-05864-z.
Declarations
Conflict of interest The authors declare no competing interests.
Data Availability
The datasets generated during and/or analyzed during the current study are presented in the main manuscript. Additional data is available from the corresponding author on reasonable request.
References
- 1.Beltramo F, DiCarlo J, Gruber JB, Taylor T, Totapally BR (2019) Renal replacement therapy modalities in critically Ill children. Pediatr Crit Care Med 20:e1–e9 [DOI] [PubMed] [Google Scholar]
- 2.Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, Hackbarth R, Somers MJ, Baum M, Symons JM, Flores FX, Benfield M, Askenazi D, Chand D, Fortenberry JD, Mahan JD, McBryde K, Blowey D, Goldstein SL (2010) Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis 55:316–325 [DOI] [PubMed] [Google Scholar]
- 3.Dalkey N, Helmer O (1963) An experimental application of the Delphi method to the use of experts. Manag Sci 9:458–467 [Google Scholar]
- 4.Murphy M, Black N, Lamping D, McKee D, Sanderson C, Askham J, Marteau T (1998) Consensus development methods, and their use in clinical guideline development. Health Technol Assess 2:1–88 [PubMed] [Google Scholar]
- 5.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.KDIGO AKI Working Group (2012) KDIGO clinical practice guideline for acute kidney injury. Kidney In Suppl 2:1–138 [Google Scholar]
- 7.Foland JA, Fortenberry JD, Warshaw BL, Pettignano R, Merritt RK, Heard ML, Rogers K, Reid C, Tanner AJ, Easley KA (2004) Fluid overload before continuous hemofiltration and survival in critically ill children: a retrospective analysis. Crit Care Med 32:1771–1776 [DOI] [PubMed] [Google Scholar]
- 8.Hayes LW, Oster RA, Tofil NM, Tolwani AJ (2009) Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24:394–400 [DOI] [PubMed] [Google Scholar]
- 9.Hall A, Crichton S, Dixon A, Skorniakov I, Kellum JA, Ostermann M (2020) Fluid removal associates with better outcomes in critically ill patients receiving continuous renal replacement therapy: a cohort study. Crit Care 24:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein SL, Somers MJ, Baum MA, Symons JM, Brophy PD, Blowey D, Bunchman TE, Baker C, Mottes T, McAfee N, Barnett J, Morrison G, Rogers K, Fortenberry JD (2005) Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int 67:653–658 [DOI] [PubMed] [Google Scholar]
- 11.Wald R, Bagshaw SM; STARRT-AKI Investigators (2020) Timing of initiation of renal-replacement therapy in acute kidney injury. Reply. N Engl J Med 383:1797–1798 [DOI] [PubMed] [Google Scholar]
- 12.Zampieri FG, da Costa BR, Vaara ST, Lamontagne F, Rochwerg B, Nichol AD, McGuinness S, McAuley DF, Ostermann M, Wald R, Bagshaw SM; STARRT-AKI Investigators (2022) A Bayesian reanalysis of the standard versus accelerated initiation of renal-replacement therapy in acute kidney injury (STARRT-AKI) trial. Crit Care 26:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bunchman TE, Donckerwolcke RA (1994) Continuous arterial-venous diahemofiltration and continuous veno-venous diahemofiltration in infants and children. Pediatr Nephrol 8:96–102 [DOI] [PubMed] [Google Scholar]
- 14.Maxvold NJ, Smoyer WE, Custer JR, Bunchman TE (2000) Amino acid loss and nitrogen balance in critically ill children with acute renal failure: a prospective comparison between classic hemofiltration and hemofiltration with dialysis. Crit Care Med 28:1161–1165 [DOI] [PubMed] [Google Scholar]
- 15.Romagnoli S, Clark WR, Ricci Z, Ronco C (2017) Renal replacement therapy for AKI: when? how much? when to stop? Best Pract Res Clin Anaesthesiol 31:371–385 [DOI] [PubMed] [Google Scholar]
- 16.Tsujimoto Y, Miki S, Shimada H, Tsujimoto H, Yasuda H, Kataoka Y, Fujii T (2021) Non-pharmacological interventions for preventing clotting of extracorporeal circuits during continuous renal replacement therapy. Cochrane Database Syst Rev 9:CD013330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bunchman TE, Brophy PD, Goldstein SL (2008) Technical considerations for renal replacement therapy in children. Semin Nephrol 28:488–492 [DOI] [PubMed] [Google Scholar]
- 18.Zaoral T, Hladik M, Zapletalova J, Travnicek B, Gelnarova E (2016) Circuit lifetime with citrate versus heparin in pediatric continuous venovenous hemodialysis. Pediatr Crit Care Med 17:e399–405 [DOI] [PubMed] [Google Scholar]
- 19.Rico MP, Fernandez Sarmiento J, Rojas Velasquez AM, Gonzalez Chaparro LS, Gastelbondo Amaya R, MulettHoyos H, Tibaduiza D, Quintero Gomez AM (2017) Regional citrate anticoagulation for continuous renal replacement therapy in children. Pediatr Nephrol 32:703–711 [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez K, Srivaths PR, Tal L, Watson MN, Riley AA, Himes RW, Desai MS, Braun MC, Akcan Arikan A (2017) Regional citrate anticoagulation for continuous renal replacement therapy in pediatric patients with liver failure. PLoS One 12:e0182134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldstein SL, Vidal E, Ricci Z, Paglialonga F, Peruzzi L, Giordano M, Laforgia N, Ronco C (2022) Survival of infants treated with CKRT: comparing adapted adult platforms with the Carpediem. Pediatr Nephrol 37:667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menon S, Broderick J, Munshi R, Dill L, DePaoli B, Fathallah-Shaykh S, Claes D, Goldstein SL, Askenazi DJ (2019) Kidney support in children using an ultrafiltration device: a multicenter, retrospective study. Clin J Am Soc Nephrol 14:1432–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murugan R, Ostermann M, Peng Z, Kitamura K, Fujitani S, Romagnoli S, Di Lullo L, Srisawat N, Todi S, Ramakrishnan N, Hoste E, Puttarajappa CM, Bagshaw SM, Weisbord S, Palevsky PM, Kellum JA, Bellomo R, Ronco C (2020) Net ultrafiltration prescription and practice among critically Ill patients receiving renal replacement therapy: a multinational survey of critical care practitioners. Crit Care Med 48:e87–e97 [DOI] [PubMed] [Google Scholar]
- 24.Murugan R, Hoste E, Mehta RL, Samoni S, Ding X, Rosner MH, Kellum JA, Ronco C, Acute Disease Quality Initiative (ADQI) Consensus Group (2016) Precision fluid management in continuous renal replacement therapy. Blood Purif 42:266–278 [DOI] [PubMed] [Google Scholar]
- 25.Mottes TA, Goldstein SL, Basu RK (2019) Process based quality improvement using a continuous renal replacement therapy dashboard. BMC Nephrol 20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selewski DT, Askenazi DJ, Kashani K, Basu RK, Gist KM, Harer MW, Jetton JG, Sutherland SM, Zappitelli M, Ronco C, Goldstein SL, Mottes TA (2021) Quality improvement goals for pediatric acute kidney injury: pediatric applications of the 22nd Acute Disease Quality Initiative (ADQI) conference. Pediatr Nephrol 36:733–746 [DOI] [PubMed] [Google Scholar]
- 27.Harer MW, Selewski DT, Kashani K, Basu RK, Gist KM, Jetton JG, Sutherland SM, Zappitelli M, Goldstein SL, Mottes TA, Askenazi DJ (2021) Improving the quality of neonatal acute kidney injury care: neonatal-specific response to the 22nd Acute Disease Quality Initiative (ADQI) conference. J Perinatol 41:185–195 [DOI] [PubMed] [Google Scholar]
- 28.Goldstein SL, Akcan-Arikan A, Alobaidi R, Askenazi DJ, Bagshaw SM, Barhight M, Barreto E, Bayrakci B, Bignall ONR, Bjornstad E, Brophy PD, Chanchlani R, Charlton JR, Conroy AL, Deep A, Devarajan P, Dolan K, Fuhrman DY, Gist KM, Gorga SM, Greenberg JH, Hasson D, Ulrich EH, Iyengar A, Jetton JG, Krawczeski C, Meigs L, Menon S, Morgan J, Morgan CJ, Mottes T, Neumayr TM, Ricci Z, Selewski D, Soranno DE, Starr M, Stanski NL, Sutherland SM, Symons J, Tavares MS, Vega MW, Zappitelli M, Ronco C, Mehta RL, Kellum J, Ostermann M, Basu RK; Pediatric ADQI Collaborative (2022) Consensus-based recommendations on priority activities to address acute kidney injury in children: a modified Delphi consensus statement. JAMA Netw Open 5:e2229442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are presented in the main manuscript. Additional data is available from the corresponding author on reasonable request.


