Abstract
Aging is the largest risk factor for Achilles tendon associated disorders and rupture. Although Achilles tendon macroscale elastic properties are suggested to decline with aging, less is known about the effect of maturity and aging on multiscale viscoelastic properties and their effect on tendon cell behavior. Here, we show dose dependent changes in native multiscale tendon mechanical and structural properties and uncover several nanoindentation properties predicted by tensile mechanics and echogenicity. Alginate hydrogel systems designed to mimic juvenile tendon microscale mechanics revealed that stiffness and viscoelasticity affected Achilles tendon cell aspect ratio and proliferation during aging. This knowledge provides further evidence for the negative impact of maturity and aging on tendon and begins to elucidate how viscoelasticity can control tendon derived cell morphology and expansion.
Keywords: hydrogel, alginate, tendon, collagen, viscoelasticity
Introduction
Aging is the largest risk factor for tendon associated disorders and rupture [1, 2]. Tendon disease increases with age [3] in concert with multiscale property changes spanning the tissue [4–12] and cell levels [10, 13]. Cells derived from human Achilles tendons of aged donors show decreased colony formation, elevated senescence, and decreased migratory ability compared to younger donors [5]. Similar to human studies, rodent animal models have identified age-related deficits in tendon whole joint function, tissue modulus, and cell shape [10, 14]. However, the relationships between these multiscale property changes during aging has remained unknown. Improved understanding for multiscale changes with maturity and aging may elucidate microenvironmental factors influencing tendon cell behavior and allow the development of in vitro systems to further probe the effects of matrix mechanical properties (e.g., elasticity and viscoelasticity).
Matrix elasticity is a contributing regulator of cell migration, apoptosis, proliferation, focal adhesion formation, and spreading. Increased stiffness also alters processes such as neuronal branching [15], breast cancer cell malignancy [16], and stem cell differentiation and stemness [17]. In tendon, increased elasticity is associated with enriched tendon-like markers [18]. In addition to matrix elasticity, the effects of viscoelasticity on cell behavior have been uncovered using hydrogel systems such as those fabricated with alginate [19]. In viscoelastic alginate gels (ionically crosslinked), 3T3 fibroblasts showed enhanced cell spreading compared to gels with no stress relaxation (covalently crosslinked) [19, 20]. Faster relaxing gels synthesized using lower molecular weight alginates result in increased cell spreading and increased proliferation of encapsulated 3T3 cells [19], osteogenic differentiation of encapsulated mesenchymal stem cells [19], and an interconnected cartilage matrix with reduced IL-1β secretion from encapsulated chondrocytes [21]. Viscoelasticity may also have effects on tendon derived cells; however, these effects remain unknown.
The objective of this study was to explore the effects of maturity and aging on multiscale mechanical and structural properties in the Achilles tendon and identify relationships between hierarchies (Figure 1). Using tendon mechanical properties determined during aging, alginate hydrogel systems were designed to mimic matrix mechanics of younger tendons to study whether certain viscoelasticity cues present in younger tendons could induce cell proliferation and remodeling in cells from aged donors. Unlike other hydrogel or synthetic systems, alginate can decouple matrix stiffness and viscoelasticity without affecting mesh size or ligand density [17]. Recapitulating this tendon niche environment within hydrogels utilized for cell culture allows for independent control of physical and chemical properties (e.g., substrate mechanics, ligand density). We hypothesized that maturity and aging would affect tendon properties, and that both stiffness and viscoelasticity would affect cell behavior during aging. Improved understanding for multiscale changes with maturity and aging may elucidate microenvironmental factors influencing tendon cell behavior and allow the development of in vitro systems to understand disease mechanism.
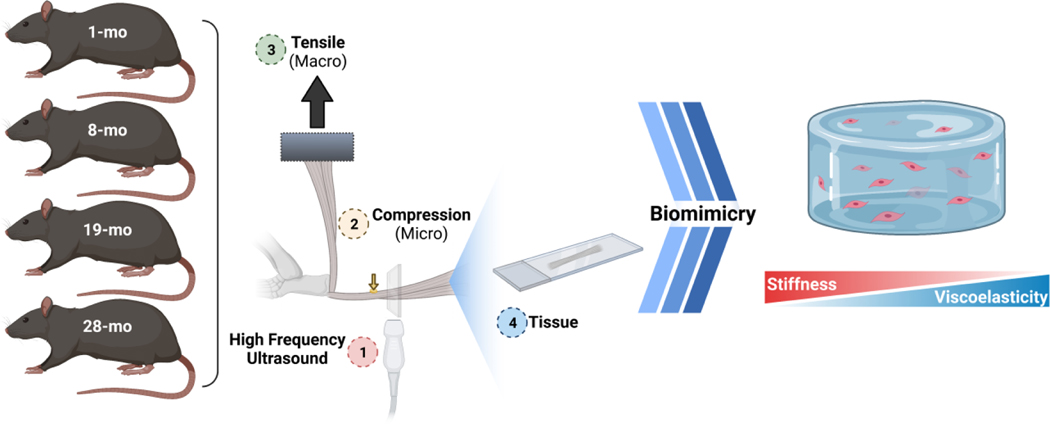
Figure 1 |. Study Design.
Achilles tendons from male F344xBN rats at 1, 8, 19, and 28 months of age were harvested and evaluated for their multiscale properties via high frequency ultrasound (1), nanoindentation (2), tensile mechanical testing (3), and histomorphometric analysis (4). Using microscale properties determined from tendon samples, alginate-based hydrogels were engineered with similar properties to examine the effects of stiffness and viscoelasticity on tendon derived cells during aging.
Methods
Tendon Harvesting
Male Fischer Brown Norway hybrid rats (n = 42) (F344xBN) at 1, 8, 19 and 28 months of age were obtained from the National Institute of Aging (NIA, USA) (Harvard University IACUC approved). The rat model was chosen similar to a previous study [10] and because the F344xBN strain has a lower incidence of age-related pathology than other rat strains [22, 23]. Approximately 80% of male F344xBN rats fed ad libitum survive to 28 month (max life: 36–40 month) [24]. The approximate human-age equivalent for 1, 8, 19, and 28 month old rats in this study is 2, 17, 41, and 61 human years [25]. Following euthanasia with CO2, the weights of the animals were measured and the Achilles tendons (AT) of the right foot, still attached to the insertion at the calcaneus, were harvested from all age groups. During fine dissection of the AT-calcaneus construct, non-tendinous soft tissue was removed. The cross-sectional area of the AT was measured using high frequency ultrasound (HFUS) (Visualsonics VEVO770, 35 MHz transducer), while the tendons were submerged in 1x DPBS during measurement. Transverse images (perpendicular to the longitudinal axis of the AT) were taken every 0.5 mm from the insertion at calcaneus to the myotendinous junction. The cross-section of the tendon was approximated as an elliptical shape with a custom Matlab (R2018b) program and the long- and short-axis diameter was determined to calculate the area of the fitted ellipsoids. The average cross-sectional area of the three central-most images from the midsubstance were evaluated.
Nanoindentation
Nanoindentation was performed using G200 NanoIndenter from Agilent with a flat 98μm in diameter tip and NanoSuite’s built-in method (G-Series DCM CSM Flat Punch Complex Modulus, Gel) as test method. Before measurements, the humidity in the measurement chamber was increased using a humidifier and approximately 500 μl 1x DPBS was placed around the tendon during testing, to prevent drying. For each tendon, eight measurement points, organized in a 2×4 array of 150 μm spacing along the longitudinal axis of the tendon, were performed on the anterior side of the tendons midsubstance (between 3mm and 6mm from the calcaneus insertion). The average of the 5−8 measurement points was calculated to obtain a representative value for each tendon. Data points where the tip and the specimen did not make proper contact during indentations were excluded.
Macroscopic Tensile Testing
Macroscopic mechanical analysis was then completed (Bose ElectroForce 3200, 222 N load cell). Following dissection, the foot was embedded in polymethylmethacrylate (PMMA, Patterson Dental) and the proximal end of the AT was glued with cyanoacrylate between two sandpaper sheets. The gauge length was set to be 12mm for all tendon samples and determined from pictures that were taken from each tendon during testing at preload. The gauge length was measured from the pictures using the measurement tool in ImageJ and an average value was calculated from four measurements. The Achilles-foot constructs were loaded in custom-made fixtures, while submerged in 1x PBS, so that the applied tensile stress to the AT was perpendicular to the calcaneus to mimic physiological loading. The mechanical testing protocol comprised the following parts (Figure S1) including preloading (0.1N), preconditioning (0.5% strain, 30 cycles, sine), stress relaxation (1 and 4% strain) for 600s, frequency sweeps (0.1, 1, 5, and 10Hz at 0.125% strain), and a ramp to failure (0.1% strain/s), similar to previous protocols [26, 27]. Peak forces achieved during preconditioning were very low (approx. 0.1–0.3N).
During testing, force, time, and displacement data were acquired at 100Hz (WinTest v7) and analyzed [28]. The percent relaxation is defined as the ratio to the difference in peak and equilibrium stresses following extension to 1% and 4% strain after 600 seconds of holding [29, 30]. The half time t1/2 of the stress relaxation was defined as the time t needed, so that the measured force f is equal to the half of the difference between the peak force and equilibrium force, after the displacement to 1% and 4% strain, respectively. The dynamic modulus (|E*|) and tan(δ) were computed from dynamic mechanical data, and the toe modulus, linear modulus, and transition strain were determined from ramp-to-failure data [29, 30].
Cell isolation and cell culture
Cells were isolated from ATs at 1 and 28 months (n=6/group). All tendons for cell isolation were minced in DMEM (Glutamax™, ThermoFisher Scientific) using microscissors (Fine Science Tools, Foster City CA, USA). Minced tendons were then incubated in a solution with collagenase (Worthington) for 2–4 h at 37°C on a rocker. After incubation, cells were removed from the collagenase solution, resuspended in DMEM, and plated. The cells were incubated at 37°C and 5% CO2 and the medium containing 10% FBS was changed every 3 days until confluency. Complete DMEM (Glutamax™, ThermoFisher Scientific) was used for all procedures.
Alginate Gels
Ultrapure alginate (UP MVG (>200kDa) and UP VLVG (<75kDa), FMC Biopolymer, Philadelphia PA, USA) was modified with 1500 μM RGD (DS20) using carbodiimide chemistry to enable cell adhesion. Briefly, alginate was dissolved in MES buffer (pH balanced to 6.5 with concentrated NaOH) over night. Under constant stirring, N-hydroxysulfosuccinimide (Thermofisher, #24510), EDC (Sigma, E6383) and GGGGRGDSP (Peptide 2.0) were added sequentially. The reaction proceeded for 20h, before it was quenched with 18 mg of hydroxyl amine per gram of alginate. The coupling efficiency using this protocol was previously characterized using I-labelled RGD peptides [31]. After dialysis and removal of endotoxins, the alginate solution was filtered (0.22 μm), lyophilized for 7 days prior to reconstitution in serum-free DMEM (Glutamax™, Thermofisher) to obtain a 2.85% w/v solution.
To tune the stiffness of the alginate hydrogels, certain amounts of CaSO4 were used (Table S1). CaSO4·2H2O was dissolved in deionized water and autoclaved. 700 μl of RGD modified MVG was cross-linked with 200 μl of CaSO4 stock solutions and 100 μl of HBSS. The different stiffness of the alginate hydrogels was later evaluated using nanoindentation and macroscale testing.
Encapsulation of cells within hydrogels
Tendon derived cells (P1) were expanded for 4 days in complete media (10% FBS and 1% Penicillin-Streptomycin, Glutamax (Thermofisher)). Cells were then trypsinized (0.05% trypsin (Invitrogen)) and resuspended at 1 million cells per 100 μl DMEM. The cells were counted (Countess II, Thermofisher Scientific). Subsequent, 100 μl of the concentrated cell solution were added to the 0.7 ml of the 2.85% (w/t) alginate solution. For 1 ml of a final 2% (w/t) alginate hydrogel, 0.7 ml of the alginate/cell solution was mixed rapidly with 0.2 ml of de-ionized water containing the appropriate CaSO4 concentration, and 0.1ml of cells suspended in DMEM using 3ml syringes connected with a female-female luer lock coupler and then deposited between two glass plates, separated 1 mm apart. The hydrogels were allowed to gel for 30 min, before disks of 8 mm diameter were punched using a biopsy punch and transferred into a 24-well plate with 0.5 ml of complete medium. The encapsulated cells were cultured for three days at 37°C and 5% CO2 without medium change. Three days post encapsulation was selected to minimize potential effects of pericellular matrix production by tendon cells which may confound ligands and mechanics of the surrounding hydrogel [32].
Viability Assay
All images were taken on a confocal microscope (LSM 710 Axio Imager 2, Zeiss, 5x objective). The viability was assessed using a LIVE/DEADTM Viability/Cytotoxicity Kit one day post encapsulation, for mammalian cells from ThermoFisher and the staining was performed according to manufacturer’s protocol (protocol reference number: MP 03224). In brief, the hydrogels were washed with 1x DPBS containing calcium and magnesium ions, incubated with 4μM EthD and 2μM calcein AM solution for 90 minutes at 37°C, followed by a wash step with 1x DPBS containing calcium and magnesium ions and directly imaged after staining. The images were processed using ImageJ and the number of viable cells were counted using analyze particles command within ImageJ, only counting particles that were greater than 150μm2. Over 450 cells were automatically counted per image and the number of dead cells was counted manually.
Proliferation Assay
Cell proliferation (Click-iT™ EdU Alexa Fluor 647 Imaging Kit, ThermoFisher) was evaluated (protocol reference number: MAN0002026), except gels were incubated in 2-hour steps instead of 30 minutes throughout the protocol to allow for full diffusion through the gel. Additionally, 1x DPBS with 50mM calcium chloride was used for the washes to minimize calcium loss during the washes. The cells were labelled with EdU during the last 18h of the 3-day cell culture. Images for the proliferation were taken with a 5x objective, and a 300 μm z-stack starting 100 μm below the surface was taken. To image a great part of the gel (6×6 mm2) a 2×2 tile was taken. The max projection of the z-stacks was analyzed using ImageJ. Approximately 4,000 cells were counted per sample, and the number of proliferating cells were divided by the total count of cells, to calculate the rate of proliferation.
Cell morphology
The actin cytoskeleton was stained (Alexa Fluor 488 Phalloidin, ThermoFisher) and the nuclei were stained with DAPI (ThermoFisher). Both stainings were performed according to manufacturer’s protocol, except 1x DPBS with calcium and magnesium was used. Images for the cell morphology were taken with a 10x water immersed objective and a 2×2 tile scan with a total dimension of 2.8 × 2.8 × 0.1mm was performed. All the images were deconvolved using Huygens Professional (version 9.2.1) software. After deconvoluting the images and correcting for the 3D point spread function by compressing the voxel size in z-direction from 8.84μm down to 3.35μm, the cell aspect ratio was calculated using the Imaris software (version 9.2.1). Only cells that were fully within the 100μm slice were used. Additionally, from the total amount of analyzed cells, 50 cells out of 50–800 cells were chosen randomly per sample, and for each of the four groups (MVG and VLVG with low and high stiffness) six biological replicates were averaged and therefore, the cell aspect ratio of 300 cells per condition were analyzed by dividing the length of the longest axis of the cell, by the length of its shortest axis.
Mechanical properties of alginate gels
Alginate gels were synthesized as described. The gels were equilibrated in complete media for 24 hours at 37° and 5% CO2 prior to mechanical testing. First, the alginate gels were analyzed with the nanoindenter, using the same protocol as for the tendons except the number of measurement points per gel was increased to 16 (4×4 grid), and each measurement point was separated by 400 μm from each other. The mean was then calculated from the 16 measurement points to get an average value for the gel (n=6 gels/group).
Multiple regression analysis
Multiple regression analysis assumed that each dependent and independent parameter was obtained from a single rat. Summary statistics of all variables were examined to ensure assumptions for linear analysis. Pearson’s correlations were calculated between independent variables, with data pooled during aging. A general linear model (GLM) was evaluated to determine if dependent variables were significantly related to the independent parameters.
Backward linear regression was performed to predict microscale mechanical properties (G’, G”, tanδ) from macroscale properties using data pooled during aging. The probability of F to enter was set at 0.05 (and to be removed is set at 0.10) [33, 34] and significance was set at P<0.05, per regression model and variances within the data. The Durbin Watson (DW) statistic was calculated to identify the existence of correlations between independent variables. For each equation, based on established guidelines for interpretation of DW statistic, a DW value lower than 1.08 indicates correlation between the independent variables [33, 34].
Histology and histomorphometry analysis
After fixation and paraffin embedding, the harvested ATs were sectioned in the sagittal plane and stained with hematoxylin and eosin (H&E). The acquired images (Axiozoom Tissue Scanner, Zeiss) were processed with CellProfiler to analyze cellularity and nuclear shape. Regions of interest were selected, and the images were processed with a color deconvolution algorithm to split the histological stains and create two grayscale images corresponding to the hematoxylin and eosin stains. Next, the intensity of the obtained hematoxylin stain image was rescaled to improve the following segmentation. Thereby, a two class Otsu thresholding algorithm was applied to identify and segment the cell nuclei. Lastly, the nuclear shape measurements and segmented nuclei number were extracted to assess cellularity and nuclear aspect ratio.
For multiphoton imaging of collagen in tendon, a Two-Photon Microscope (Zeiss, LSM 980 NLO) was used (Laser: Spectra-Physics X3 InSight IR tuned to 920 nm; Objective: 25× 0.8 NA multi-immersion LD-LCI lens; Condenser: 1.2 NA water immersion). Forward scatter was collected on a Transmitted Light-PMT multi-alkali detector. Reverse scatter was detected with a Non-descanned GaAsP detector behind a CFP (440–480 nm) emission filter. The average collagen fiber organization by computing the angular orientation of fibers in the SHG images with custom code (MATLAB; v2019a; Natick, MA), as previously described [26, 27].
Statistical Analysis
Data normality was assessed with Shapiro Wilk tests (SPSS). One-way (time) or two-way (stiffness and viscoelasticity) ANOVAs with post hoc Student’s t-tests were used. For all the structural and mechanical data, one-way ANOVAs were performed with Tukey’s HSD post hoc tests. For analysis of hydrogel mechanics, cell shape, and proliferation, two-way ANOVAs were performed with Bonferroni post hoc tests. Differences were considered statistically significant when P-values were <0.05 after accounting for multiple comparisons.
Results
Multiscale Tendon Mechanics and Structure are Affected by Aging
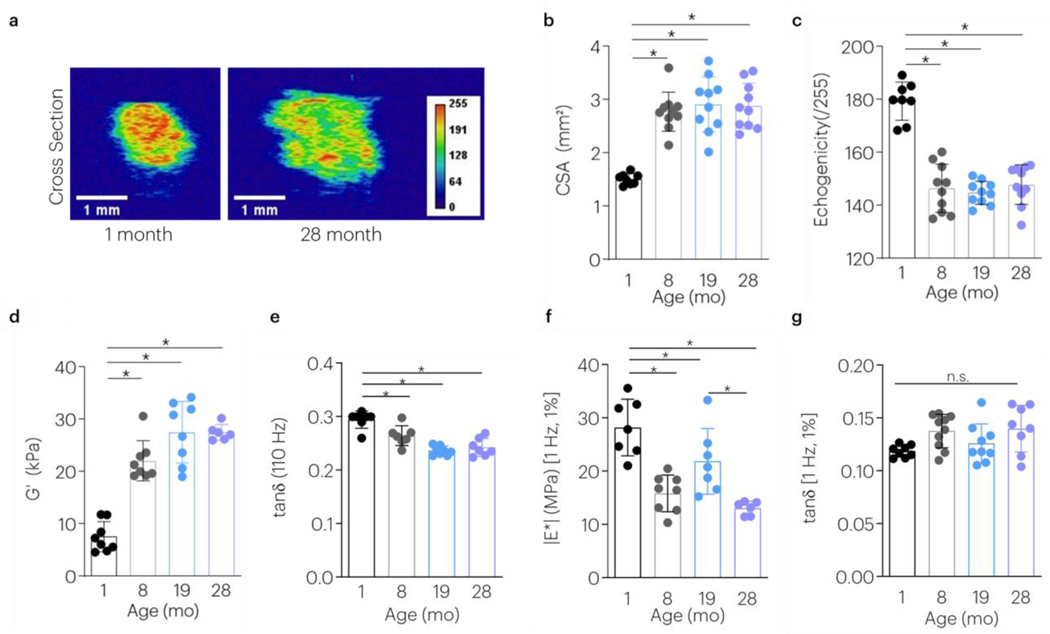
Achilles tendons (AT) from male F344xBN rats throughout aging were harvested for multiscale evaluation. During aging, the cross sectional area (CSA) assessed using high frequency ultrasound (HFUS) increased and achieved its maximal values by 8-months (Figure 2a,b). In contrast, animals continued to grow in body weight until advanced age (Figure S2). During aging, tendon echogenicity first decreased and then remained constant at timepoints beyond 8-months (Figure 2c). At the microscale, younger ATs had decreased storage modulus and higher loss tangent (Figure 2 d,e). Tensile mechanical tests were next used to evaluate viscoelastic, dynamic, and quasi-static mechanical properties at 1 and 4% strain. At the macroscale, the opposite mechanical response from that noted at the microscale was observed; younger ATs showed a higher |E*| (Figure 2f, S4a). No differences with age were detected in the macroscale tan(δ) (Figure 2g, S4b) or percent relaxation (Figure S3a,b,c). The rate of relaxation was slower in 19-month animals compared to 1-month old animals at 4% strain (Figure S3a,d,e). Aging significantly increased the macroscale toe modulus, linear modulus, and transition strain (Figure S4c-e).
Figure 2 |. Maturity and Aging Affects Multiscale Tendon Mechanics and Structure.
The effect of aging on (a,b) tendon cross sectional area, (c) echogenicity, (d) microscale storage modulus (G’), (e) microscale loss tangent (tan(δ)), (f) macroscale dynamic modulus (|E*|), and (g) macroscale tan(δ) was evaluated. Data shown as mean ± s.d. N=7–10 tendons/group. *P < 0.0083 indicates significant differences as evaluated by 1-way ANOVAs with post hoc tests with Bonferroni corrections.
Interrelationships Between Cellularity, Cell Shape, And Tissue Organization with Aging
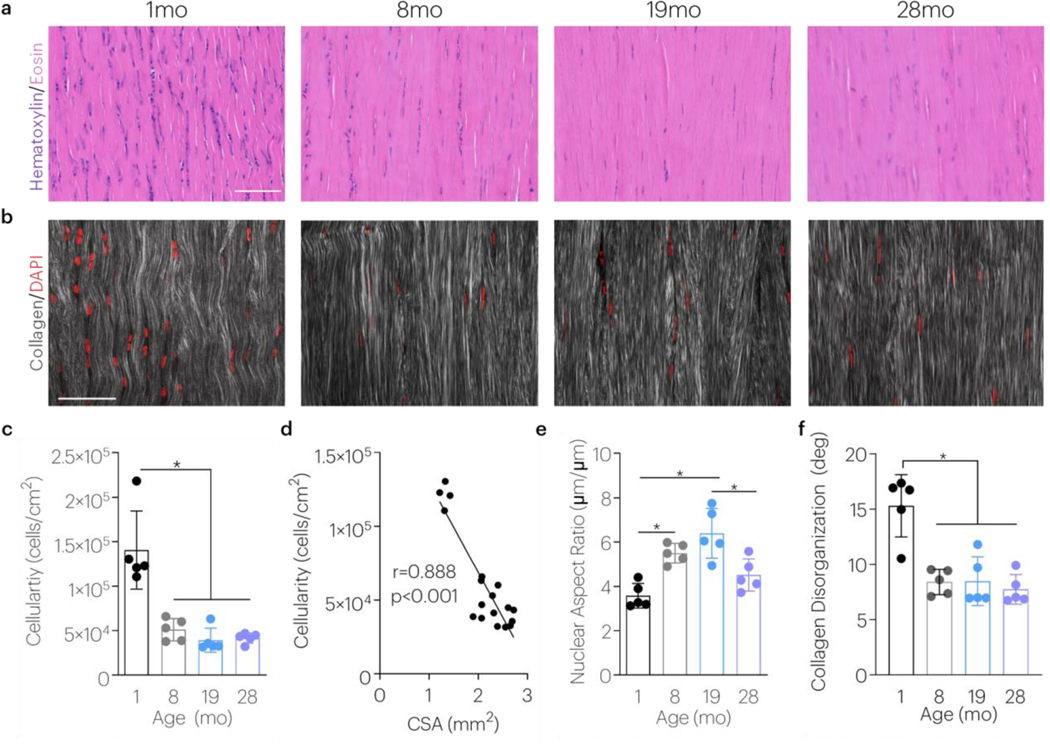
The effect of aging on cellularity and nuclear shape was next evaluated during maturation and aging. Cellularity decreased with maturity, and this finding persisted through aging (Figure 3a,c). Tendon cellularity strongly correlated to tendon size (Figure 3d). The nuclear aspect ratio, however, increased with maturity and aging before declining in advanced age (Figure 3e). Second harmonic imaging revealed that collagen structure varied with aging; tendons became more organized with maturity and this was maintained in later age (Figure 3b,f).
Figure 3 |. Maturity and Aging Affects Tendon Cellularity And Cell Shape.
The effect of aging on tendon histological properties was evaluated using (a) H&E straining (cellularity and nuclear shape) and (b) multiphoton imaging (collagen disorganization). Scale bars = 50μm. (c) Achilles tendon cellularity was evaluated after 1, 8, 19, and 28 months of age. Data shown as mean ± s.d. (N=5 tendons/group). *P < 0.0083 indicates significant differences as evaluated by 1-way ANOVAs with post hoc tests with Bonferroni corrections. (d) The correlation between cellularity and tendon cross sectional area (CSA) was evaluated using a Pearson’s correlation coefficient with data pooled during aging. Achilles tendon (e) nuclear aspect ratio and (f) collagen disorganization were evaluated after 1, 8, 19, and 28 months of age. Data shown as mean ± s.d. (N=5 tendons/group). *P < 0.0083 indicates significant differences as evaluated by 1-way ANOVAs with post hoc tests with Bonferroni corrections.
Multiscale Mechanics and Structure are Related Between Hierarchies
To gain further insight to relationships between length scales, Pearson’s correlations were calculated between macro and microscale properties. Several strong significantly correlated relationships were identified (Figure 4a). In particular, the storage and loss modulus at the microscale was negatively correlated to tendon echogenicity (P<0.01, r = 0.754–0.815) (Figure 4b,c). The microscale storage modulus was positively correlated to the linear modulus acquired during tension (P<0.01, r = 0.733) (Figure 4d). The loss modulus at the microscale was moderately correlated to the loss tangent in tension (p<0.01, r = 0.488) (Figure 4e). Using backward linear regression, microscale mechanical properties were predicted from macroscale elastic, viscoelastic, and structural properties. Regression modeling revealed that macrostructural properties strongly predicted microstructural properties. The microscale storage modulus was predicted by the macroscale |E*| and the transition strain (R2 = 0.76), and the microscale loss modulus was strongly predicted by macroscale echogenicity, the percent relaxation, the relaxation rate, and tan(δ) (R2 = 0.85).
Figure 4 |. Multiscale Mechanics and Structure are Related Between Hierarchies.
(a) Single linear correlations were determined between structural hierarchies, as analyzed with Pearson’s correlation coefficients. Red color gradation indicates strength of positive correlations whereas blue color gradation indicates strength of negative correlations. (b,d) The microscale storage modulus was correlated to tendon echogenicity and the macroscale linear modulus. (c,e) The microscale loss modulus was correlated to tendon echogenicity and the macroscale tan(δ).
Stiffness And Viscoelasticity Affect Cultured Tenocyte Proliferation and Morphology with Aging
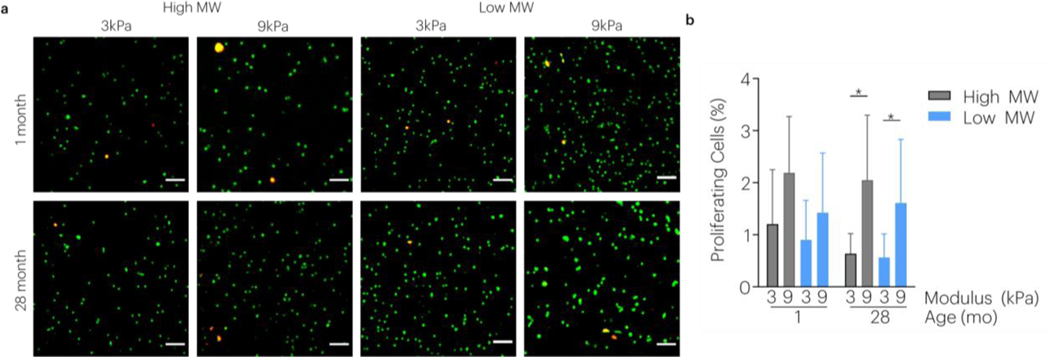
Using these tissue level properties, biomimetic in vitro systems to mimic younger tendon properties were developed to evaluate the effect of matrix mechanics on cell behavior during aging. Calcium crosslinking and alginate molecular weight were independently tuned to create four testable conditions (Figure 5a,b). Nanoindentation revealed that calcium crosslinking and alginate molecular weight independently varied hydrogel modulus and relaxation, respectively (Figure 5c-d). AT cells isolated from rats at 1 and 28 months of age (Figure S5a) were encapsulated into RGD modified gels and maintained viability (Figure S5b,c). Both modulus and viscoelasticity affected the aspect ratio of encapsulated cells from 1-month and 28-month donors (Figure 6). For cells derived from 1-month rats, increased stiffness and relaxation led to increased cell aspect ratio. However, for cells derived from 28-month rats in faster relaxing gels, cells had an increased aspect ratio more in lower stiffness matrices. Aged cells responded to substrate stiffness with increased proliferation regardless of matrix relaxation, but this was only a trend in 1-month donors (Figure 7).
Figure 5 |. Engineering Biomimetic Alginate Hydrogel Systems to Emulate Nanoscale Mechanics of Tendon.
(a) Alginate hydrogels were engineered with independent control over extent of crosslinking and polymer molecular weight. (b) After casting gels, nanoindentation mechanical properties were evaluated. (c,d) The effect of molecular weight and modulus on the storage modulus (G’) and loss tangent (tan(δ)) were evaluated. *P < 0.0125 indicates significant differences, as analyzed by two-way ANOVAs with post hoc tests with Bonferroni corrections. Data shown as mean±s.d. N=5–6 gels/group.
Figure 6 |. Effect of Hydrogel Stiffness and Viscoelasticity on Tendon Cell Shape.
The effect of alginate hydrogel modulus and stress relaxation on cell shape were evaluated and quantified during aging using (a) confocal imaging and (b) image analysis. Cells were stained with phalloidin (green, F-actin) and DAPI (blue, nuclei). *P < 0.0125 indicates significant differences, as analyzed by two-way ANOVAs with post hoc tests with Bonferroni corrections. Data shown as violin plots with median and IQR in dashed lines. N=240cells/group. Scale bar: 100μm; Inset scale bar: 20μm.
Figure 7 |. Effect of Hydrogel Stiffness and Viscoelasticity on Tendon Cell Proliferation.
(a) The effect of alginate hydrogel modulus and stress relaxation on tendon cell proliferation was evaluated during aging using confocal imaging and (b) quantified using image analysis. Cells were stained with EdU (red/yellow, proliferating cells) and Hoechst (green, nuclei). *P < 0.0125 indicates significant differences, as analyzed by two-way ANOVAs with post hoc tests with Bonferroni corrections. Data shown as mean±s.d. in dashed lines. N=6 gels/group. Scale bar: 20μm.
Discussion
Age dependent changes in native multiscale tendon mechanical and structural properties were observed in this study, and local nanoindentation properties were found to be predictable by tensile mechanics and echogenicity. Alginate hydrogels designed to closely mimic juvenile tendon micromechanical properties revealed how elasticity and viscoelasticity affect tendon derived cell behavior in culture.
In agreement with human clinical observation, the rat CSA was elevated during aging, with the most pronounced differences during maturity [12]. Evaluation of echogenicity, which had not been studied during aging in humans, was observed to decrease during maturity, possibly indicating altered fiber packing, organization, and composition [35]. In agreement with our hypothesis, aging affected tendon viscoelasticity at the macro and microscales. Macroscale tensile mechanical evaluation revealed slower relaxation which occurred in concert with decreased tan(δ) at the microscale in compression. Whereas tensile testing probes the properties in the direction of the alignment of the tendon fibers, the compression is perpendicular to the fiber alignment. Changes in mechanics may be attributed to advanced glycation end-products (AGEs) that accumulate throughout life [36]. AGEs could increase the cross-linking of the collagen molecules in the tendon, resulting in reduced viscous properties in tendons in aged individuals. However, other studies evaluating tail tendon during aging suggest that lysine glycation increases, but collagen crosslinks decrease [37]. Rat tails incubated in methylglyoxal, a metabolite that forms AGEs, reduced stress relaxation and fiber sliding, but increased failure stress [38]. Furthermore, new models predict that ribose treatment [36], leads to a viscosity reduction (Maxwell Model) for both tail fascicles and fibers but is more prominent in fibrillar subunits [39]. However, not all tendons may age the same mechanically [40].
Several histomorphometric changes were observed with aging. With maturation, tendon cellularity was decreased, and cells adopted an elongated spindle-shaped morphology in concert with elevated collagen organization. These changes were consistent with previous findings reporting a drop in cellularity at a very early age and an elongation of the cell shape throughout aging [12, 41, 42]. A 67% decrease in cellularity has been reported in rat tail tendon assessed between 1.5 and 9 months of age [42]. We obtained similar results with a 62% decrease in cellularity for ATs between the age of 1 to 8 months. The changes in cellularity may be explained by the large production of matrix proteins leading to the expansion of extracellular matrix [12, 41] and concomitant increase in matrix organization observed here. Moreover, a further decrease in cellularity with aging has been suggested, which is consistent with our results showing a trend in reduced cellularity at later stages of age [42]. In addition to changes in cellularity with aging, previous studies in rat tail tendon have suggested a 4-fold increase in nuclear aspect ratio captured between the age of 1 to 12 months [41]. With aging and elongation of cell shape, the elongated nucleus occupies most of the cell volume and there is decreased presence of synthetically active organelles, suggesting a decrease in cell activity with aging [12, 43].
Predictive models revealed strong correlations between micromechanical properties and tensile mechanics and echogenicity, suggesting further utility in using ultrasound to assess multiscale tendon properties [35]. These models also uncovered the first correlations between compression and tensile elastic and viscoelastic properties in tendon, which we then used to generate biomimetic hydrogel systems. Furthermore, the relationship between tensile mechanics and shear properties provide understanding for measurements obtained from shear wave elastography [44]. This adds to other structure studies that primarily focused on tensile mechanics and tissue composition [27, 45, 46].
Past studies suggest several niche factors may be important for tendon derived cells including the extracellular matrix (ECM) proteins tenomodulin and biglycan [47, 48], hypoxia [49], and substrate mechanics [18]. However, the influence of structural biophysical signals, such as matrix viscoelasticity [19] affecting tendon cell behavior had not yet been thoroughly examined. Although previous work has associated aging with senescence [4], decreased cell metabolism [5], and deficits in self-renewal and clonogenicity [5], this study suggests that the cells, independent of donor age, are still responsive to matrix properties. Similar to 3T3 fibroblasts that showed enhanced cell spreading in faster relaxing gels [19, 20], tendon derived cells showed a similar response. However, unlike 3T3 fibroblasts, proliferation of tendon derived cells was only affected by gel stiffness. Furthermore, cells from younger animals in faster relaxing gels had higher aspect ratios in stiffer gels, but the opposite occurred in cells derived from older animals. We speculate that the storage modulus of tendon derived cells themselves may increase with age [50], and this may compensate for the increased storage modulus observed locally in the present study. Although tenocytes are highly spindle shaped in healthy aligned tendon, a potential limitation of this study is that the cells maintained a generally spherical shape after encapsulation in isotropic hydrogels used in the present study. While this is typical of such non-degradable systems at this early timepoint [19], future studies will examine the effects of matrix degradation, fiber integration into the hydrogels, and longer timepoints on cell morphology and behavior. For example, cell proliferation was increased following incubation with EdU for longer periods (Figure S6). Future studies will also examine effects of gene/protein assays for cells in these systems.
In conclusion, this study found that the elastic and viscoelastic properties of native ATs change throughout maturation and aging. Changes observed in rodents agree with observation in human tendons, such that aging decreased modulus [9, 10], increased tendon cross sectional area [10–12], and decreased cellularity [10, 13]. Notably, the response of cells derived from older animals show that they are still responsive to local mechanical properties. Thus, they may be receptive to therapies designed to modulate local mechanics. This work may also provide benchmarks for successful design of tendon tissued engineered constructs. Future work will further investigate molecular mechanisms of aging in tendon and explore therapies to rejuvenate aged tendon.
Supplementary Material
Statement of Significance.
Aging is the largest risk factor for Achilles tendon associated disorders and rupture. Although Achilles tendon macroscale elastic properties are suggested to decline with aging, less is known about the effect of maturity and aging on multiscale viscoelastic properties and their effect on tendon cell behavior. Here, we show dose dependent changes in native multiscale tendon mechanical and structural properties and uncover several nanoindentation properties predicted by tensile mechanics and echogenicity. Alginate hydrogel systems designed to mimic juvenile tendon microscale mechanics revealed that stiffness and viscoelasticity affected Achilles tendon cell spreading and proliferation during aging. This knowledge provides further evidence for the negative impact of maturity and aging on tendon and begins to elucidate how viscoelasticity can control tendon derived cell morphology and expansion.
Acknowledgments
This work was supported by the Wyss Institute for Biologically Inspired Engineering at Harvard and the National Institute on Aging of the NIH (F32AG057135, K99AG065495).
Grant Support:
This study was supported by the National Institute on Aging at the NIH (F32AG057135, K99AG065495) and the Wyss Institute for Biologically Inspired Engineering.
Footnotes
Competing Interests Statement
The authors receive grant support through the NIH and Wyss Institute. The views and opinions expressed in this article are those of the authors and do not necessarily reflect the position of the Wyss Institute for Biologically Inspired Engineering at Harvard University.
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability
All data in the manuscript is available upon reasonable request.
References
- [1].Suchak AA, Bostick G, Reid D, Blitz S, Jomha N, The incidence of Achilles tendon ruptures in Edmonton, Canada, Foot Ankle Int 26(11) (2005) 932–6. [DOI] [PubMed] [Google Scholar]
- [2].Maffulli N, Wong J, Almekinders LC, Types and epidemiology of tendinopathy, Clin Sports Med 22(4) (2003) 675–92. [DOI] [PubMed] [Google Scholar]
- [3].Teunis T, Lubberts B, Reilly BT, Ring D, A systematic review and pooled analysis of the prevalence of rotator cuff disease with increasing age, J Shoulder Elbow Surg 23(12) (2014) 1913–1921. [DOI] [PubMed] [Google Scholar]
- [4].Zhou Z, Akinbiyi T, Xu L, Ramcharan M, Leong DJ, Ros SJ, Colvin AC, Schaffler MB, Majeska RJ, Flatow EL, Sun HB, Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate, Aging Cell 9(5) (2010) 911–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kohler J, Popov C, Klotz B, Alberton P, Prall WC, Haasters F, Muller-Deubert S, Ebert R, Klein-Hitpass L, Jakob F, Schieker M, Docheva D, Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration, Aging Cell 12(6) (2013) 988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ruzzini L, Abbruzzese F, Rainer A, Longo UG, Trombetta M, Maffulli N, Denaro V, Characterization of age-related changes of tendon stem cells from adult human tendons, Knee Surg Sports Traumatol Arthrosc 22(11) (2014) 2856–66. [DOI] [PubMed] [Google Scholar]
- [7].Slane LC, Thelen DG, Achilles tendon displacement patterns during passive stretch and eccentric loading are altered in middle-aged adults, Med Eng Phys 37(7) (2015) 712–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Slane LC, DeWall R, Martin J, Lee K, Thelen DG, Middle-aged adults exhibit altered spatial variations in Achilles tendon wave speed, Physiol Meas 36(7) (2015) 1485–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dudhia J, Scott CM, Draper ER, Heinegard D, Pitsillides AA, Smith RK, Aging enhances a mechanically-induced reduction in tendon strength by an active process involving matrix metalloproteinase activity, Aging Cell 6(4) (2007) 547–56. [DOI] [PubMed] [Google Scholar]
- [10].Pardes AM, Beach ZM, Raja H, Rodriguez AB, Freedman BR, Soslowsky LJ, Aging leads to inferior Achilles tendon mechanics and altered ankle function in rodents, J Biomech 60 (2017) 30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Stenroth L, Peltonen J, Cronin NJ, Sipila S, Finni T, Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo, J Appl Physiol (1985) 113(10) (2012) 1537–44. [DOI] [PubMed] [Google Scholar]
- [12].Svensson RB, Heinemeier KM, Couppe C, Kjaer M, Magnusson SP, Effect of aging and exercise on the tendon, J Appl Physiol (1985) 121(6) (2016) 1237–1246. [DOI] [PubMed] [Google Scholar]
- [13].Cury DP, Dias FJ, Miglino MA, Watanabe IS, Structural and Ultrastructural Characteristics of Bone-Tendon Junction of the Calcaneal Tendon of Adult and Elderly Wistar Rats, PLoS One 11(4) (2016) e0153568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Williamson PM, Freedman BR, Kwok N, Beeram I, Pennings J, Johnson J, Hamparian D, Cohen E, Galloway JL, Ramappa AJ, DeAngelis JP, Nazarian A, Tendinopathy and tendon material response to load: What we can learn from small Animal studies, Acta Biomater (2021). [DOI] [PMC free article] [PubMed]
- [15].Flanagan LA, Ju YE, Marg B, Osterfield M, Janmey PA, Neurite branching on deformable substrates, Neuroreport 13(18) (2002) 2411–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Paszek MJ, Zahir N, Johnson KR, Lakins JN, Rozenberg GI, Gefen A, Reinhart-King CA, Margulies SS, Dembo M, Boettiger D, Hammer DA, Weaver VM, Tensional homeostasis and the malignant phenotype, Cancer Cell 8(3) (2005) 241–54. [DOI] [PubMed] [Google Scholar]
- [17].Vining KH, Mooney DJ, Mechanical forces direct stem cell behaviour in development and regeneration, Nat Rev Mol Cell Biol 18(12) (2017) 728–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Marturano JE, Schiele NR, Schiller ZA, Galassi TV, Stoppato M, Kuo CK, Embryonically inspired scaffolds regulate tenogenically differentiating cells, J Biomech 49(14) (2016) 3281–3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chaudhuri O, Gu L, Klumpers D, Darnell M, Bencherif SA, Weaver JC, Huebsch N, Lee HP, Lippens E, Duda GN, Mooney DJ, Hydrogels with tunable stress relaxation regulate stem cell fate and activity, Nat Mater 15(3) (2016) 326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chaudhuri O, Gu L, Darnell M, Klumpers D, Bencherif SA, Weaver JC, Huebsch N, Mooney DJ, Substrate stress relaxation regulates cell spreading, Nat Commun 6 (2015) 6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee HP, Gu L, Mooney DJ, Levenston ME, Chaudhuri O, Mechanical confinement regulates cartilage matrix formation by chondrocytes, Nat Mater 16(12) (2017) 1243–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lipman RD, Chrisp CE, Hazzard DG, Bronson RT, Pathologic characterization of brown Norway, brown Norway x Fischer 344, and Fischer 344 x brown Norway rats with relation to age, J Gerontol A Biol Sci Med Sci 51(1) (1996) B54–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wahr PA, Michele DE, Metzger JM, Effects of aging on single cardiac myocyte function in Fischer 344 x Brown Norway rats, Am J Physiol Heart Circ Physiol 279(2) (2000) H559–65. [DOI] [PubMed] [Google Scholar]
- [24].Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW, Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program, J Gerontol A Biol Sci Med Sci 54(11) (1999) B492–501. [DOI] [PubMed] [Google Scholar]
- [25].Sengupta P, The Laboratory Rat: Relating Its Age With Human’s, Int J Prev Med 4(6) (2013) 624–30. [PMC free article] [PubMed] [Google Scholar]
- [26].Freedman BR, Rodriguez AB, Hillin CD, Weiss SN, Han B, Han L, Soslowsky LJ, Tendon healing affects the multiscale mechanical, structural and compositional response of tendon to quasi-static tensile loading, J R Soc Interface 15(139) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Freedman BR, Rodriguez AB, Leiphart RJ, Newton JB, Ban E, Sarver JJ, Mauck RL, Shenoy VB, Soslowsky LJ, Dynamic Loading and Tendon Healing Affect Multiscale Tendon Properties and ECM Stress Transmission, Sci Rep 8(1) (2018) 10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dunkman AA, Buckley MR, Mienaltowski MJ, Adams SM, Thomas SJ, Satchell L, Kumar A, Pathmanathan L, Beason DP, Iozzo RV, Birk DE, Soslowsky LJ, Decorin expression is important for age-related changes in tendon structure and mechanical properties, Matrix Biol 32(1) (2013) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Freedman BR, Salka NS, Morris TR, Bhatt PR, Pardes AM, Gordon JA, Nuss CA, Riggin CN, Fryhofer GW, Farber DC, Soslowsky L, Temporal Healing of Achilles Tendons After Injury in Rodents Depends on Surgical Treatment and Activity, J Am Acad Orthop Surg 25(9) (2017) 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Freedman BR, Gordon JA, Bhatt PR, Pardes AM, Thomas SJ, Sarver JJ, Riggin CN, Tucker JJ, Williams AW, Zanes RC, Hast MW, Farber DC, Silbernagel KG, Soslowsky LJ, Nonsurgical treatment and early return to activity leads to improved Achilles tendon fatigue mechanics and functional outcomes during early healing in an animal model, J Orthop Res 34(12) (2016) 2172–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rowley JA, Madlambayan G, Mooney DJ, Alginate hydrogels as synthetic extracellular matrix materials, Biomaterials 20(1) (1999) 45–53. [DOI] [PubMed] [Google Scholar]
- [32].Loebel C, Mauck RL, Burdick JA, Local nascent protein deposition and remodelling guide mesenchymal stromal cell mechanosensing and fate in three-dimensional hydrogels, Nat Mater 18(8) (2019) 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Neter J, Building the Regression Model i: Selection of Predictor Variables, Applied linear Regression Models., Irwin, Chicago, IL, 1996a. [Google Scholar]
- [34].Neter J, Table B 7 Durbin-Watson Test Bounds. Applied Linear Regression Models, Irwin, Chicago, IL, 1996b. [Google Scholar]
- [35].Riggin CN, Sarver JJ, Freedman BR, Thomas SJ, Soslowsky LJ, Analysis of Collagen Organization in Mouse Achilles Tendon Using High-Frequency Ultrasound Imaging, J Biomech Eng (2013). [DOI] [PMC free article] [PubMed]
- [36].Gautieri A, Passini FS, Silvan U, Guizar-Sicairos M, Carimati G, Volpi P, Moretti M, Schoenhuber H, Redaell A, Berli M, Snedeker JG, Advanced glycation end-products: Mechanics of aged collagen from molecule to tissue, Matrix Biol 59 (2017) 95–108. [DOI] [PubMed] [Google Scholar]
- [37].Stammers M, Ivanova IM, Niewczas IS, Segonds-Pichon A, Streeter M, Spiegel DA, Clark J, Age-related changes in the physical properties, cross-linking, and glycation of collagen from mouse tail tendon, J Biol Chem 295(31) (2020) 10562–10571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Li Y, Fessel G, Georgiadis M, Snedeker JG, Advanced glycation end-products diminish tendon collagen fiber sliding, Matrix Biol 32(3–4) (2013) 169–77. [DOI] [PubMed] [Google Scholar]
- [39].Karathanasopoulos N, Ganghoffer JF, Investigating the Effect of Aging on the Viscosity of Tendon Fascicles and Fibers, Front Bioeng Biotechnol 7 (2019) 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zuskov A, Freedman BR, Gordon JA, Sarver JJ, Buckley MR, Soslowsky LJ, Tendon Biomechanics and Crimp Properties Following Fatigue Loading Are Influenced by Tendon Type and Age in Mice, J Orthop Res (2019). [DOI] [PMC free article] [PubMed]
- [41].Lavagnino M, Gardner K, Arnoczky SP, Age-related changes in the cellular, mechanical, and contractile properties of rat tail tendons, Connect Tissue Res 54(1) (2013) 70–5. [DOI] [PubMed] [Google Scholar]
- [42].Nagy IZ, Von Hahn HP, Verzár F, Age-related alterations in the cell nuclei and the DNA content of rat tail tendon, Gerontologia 15(4) (1969) 258–64. [DOI] [PubMed] [Google Scholar]
- [43].Tuite DJ, Renström PA, O’Brien M, The aging tendon, Scand J Med Sci Sports 7(2) (1997) 72–7. [DOI] [PubMed] [Google Scholar]
- [44].Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM, Continuous Shear Wave Elastography: A New Method to Measure Viscoelastic Properties of Tendons in Vivo, Ultrasound Med Biol 41(6) (2015) 1518–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Robinson PS, Lin TW, Jawad AF, Iozzo RV, Soslowsky LJ, Investigating tendon fascicle structure-function relationships in a transgenic-age mouse model using multiple regression models, Ann Biomed Eng 32(7) (2004) 924–31. [DOI] [PubMed] [Google Scholar]
- [46].Freedman BR, Sarver JJ, Buckley MR, Voleti PB, Soslowsky LJ, Biomechanical and structural response of healing Achilles tendon to fatigue loading following acute injury, J Biomech 47(9) (2014) 2028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bi Y, Ehirchiou D, Kilts TM, Inkson CA, Embree MC, Sonoyama W, Li L, Leet AI, Seo BM, Zhang L, Shi S, Young MF, Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche, Nat Med 13(10) (2007) 1219–27. [DOI] [PubMed] [Google Scholar]
- [48].Alberton P, Dex S, Popov C, Shukunami C, Schieker M, Docheva D, Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells, Stem Cells Dev 24(5) (2015) 597–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zhang J, Wang JH, Human tendon stem cells better maintain their stemness in hypoxic culture conditions, PLoS One 8(4) (2013) e61424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Wu H, Zhao G, Zu H, Wang JH, Wang QM, Aging-related viscoelasticity variation of tendon stem cells (TSCs) characterized by quartz thickness shear mode (TSM) resonators, Sens Actuators (Warrendale Pa) 210 (2015) 369–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in the manuscript is available upon reasonable request.