Abstract
Background
Anti‐obesity medications (AOMs) have historically had limited weight‐loss efficacy. However, newer glucagon‐like peptide‐1 receptor agonist (GLP‐1 RA)–based therapies seem to be more effective, including dual agonists of GLP‐1R and the glucagon receptor (GCGR) or glucose‐dependent insulinotropic polypeptide receptor.
Objective
To explore healthcare professionals' (HCPs) experience in obesity treatment and their understanding of agonists of GCGR, glucose‐dependent insulinotropic polypeptide (GIP) RA, and GLP‐1 RA.
Methods
This cross‐sectional online survey of HCPs prescribing AOMs was conducted in the United States in 2023 with a questionnaire designed to evaluate prescribing behavior and understanding of GCGR, GIP RA, and GLP‐1 RA.
Results
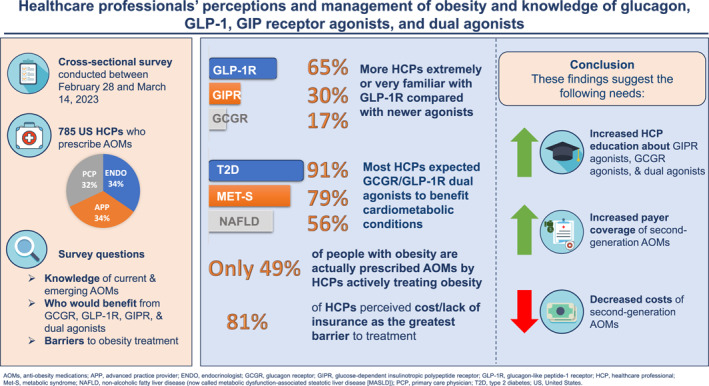
The 785 respondents (251 primary‐care physicians [PCPs], 263 endocrinologists, and 271 advanced practice providers [APPs]) reported 55% of their patients had obesity (body mass index ≥30 kg/m2 or ≥27 with weight‐related complications) and recommended AOMs to 49% overall, significantly more endocrinologists (57% of patients, p < 0.0005) than PCPs (43%) or APPs (46%). The greatest barriers to treatment were medication cost/lack of insurance (mean 4.2 on 1–5 scale [no barrier–extreme barrier]), low patient engagement/adherence (3.3), and inadequate time/staff (3.1). Metformin was the type 2 diabetes (T2D) medication most commonly prescribed to treat obesity in T2D patients (92.5% of respondents). Most HCPs (65%) were very/extremely familiar with GLP‐1 RA, but only 30% with GIP RA and 16% with GCGR. Most HCPs expected dual GCGR/GLP‐1 RA to benefit many obesity‐related conditions; however, only a minority of HCPs perceived that they would benefit non‐cardiometabolic complications of obesity.
Conclusions
Among HCPs prescribing AOMs, gaps exist in the management of people living with obesity as <50% are prescribed AOMs. Barriers to treatment indicate a need to improve access to AOMs. HCPs were less familiar with GCGR or GIP RA than GLP‐1 RA but expect dual GCGR/GLP‐1 RA may offer additional benefits, potentially addressing treatment barriers and access. Thus, there is a need for greater education among HCPs regarding the mechanism of action and therapeutic effects of GCGR agonists, and dual GCGR/GLP‐1 RA, so that the full range of obesity‐related complications can be effectively treated.
Keywords: anti‐obesity agents, glucagon, glucagon‐like peptide‐1, glucose‐dependent insulinotropic polypeptide, health care surveys, obesity
We explored US healthcare professionals' experience in obesity treatment and their understanding of new anti‐obesity medications. The 785 respondents to the survey (251 primary‐care physicians, 263 endocrinologists, 271 advanced practice providers) indicated that they prescribe anti‐obesity medication to less than 50% of their patients with obesity, and reported substantial barriers to treatment, including cost and lack of insurance coverage. Most respondents were familiar with glucagon‐like peptide (GLP)‐1 receptor agonists, but only a minority were familiar with glucose‐dependent insulinotropic polypeptide receptor agonists or glucagon receptor agonists. Most expected investigational dual glucagon/GLP‐1 receptor agonists to potentially benefit many obesity‐related conditions.
1. INTRODUCTION
Obesity is an adiposity‐based chronic disease (ABCD) characterized by excessive fat accumulation that increases risk for cardio‐kidney‐metabolic and biomechanical conditions. 1 , 2 , 3 As of 2020, approximately 988 million people globally had obesity by the World Health Organization criterion of body mass index (BMI) ≥30 kg/m2, and this figure is anticipated to increase to 1914 million people (i.e., 1.914 billion) by 2035. 4 The prevalence of obesity has increased substantially over recent decades in most countries, including the United States (US) where the 30.5% of adults living with obesity in the 1999–2007 period had increased to 41.9% in 2017–2020. 5 During that time, Americans living with severe obesity (BMI ≥40 kg/m2) increased from 4.7% to 9.2% of the adult population. 5 This disease burden is associated with substantial medical costs, estimated to be approximately $173 billion per year in the US (2019 dollars). 6 Per person, annual medical costs for people living with obesity were on average $1861 higher than for those with BMI of 18.5–25 kg/m2. Indirect costs of obesity may be even higher, with one recent analysis reporting annual direct and indirect costs of $280 billion and $396 billion, respectively (2020 dollars). 7
Historically, medications for weight loss have had limited efficacy (average body weight loss typically ≤10% after 1 year of treatment), as well as certain serious safety concerns 8 , 9 (including cardiotoxicity with fenfluramine and sibutramine, 10 neuropsychiatric disorders with rimonabant, 10 and cancer with lorcaserin 11 ). However, medications recently approved by the US Food and Drug Administration (FDA) for chronic weight management that are based on the incretin gut hormone glucagon‐like peptide‐1 (GLP‐1) have demonstrated greater efficacy—namely, liraglutide (Saxenda), semaglutide (Wegovy), and tirzepatide (Zepbound). 12 , 13 Semaglutide, a GLP‐1 mono‐agonist like liraglutide, elicited up to 15% mean weight loss in pivotal clinical trials, in patients with obesity/overweight with and without type 2 diabetes (T2D), 14 , 15 sustained over at least 2 years of treatment. 16 Tirzepatide, a dual agonist of GLP‐1 and glucose‐dependent insulinotropic polypeptide (GIP), the other known incretin hormone in humans, has demonstrated up to 22% mean weight loss after 72 weeks. 17 Tirzepatide also demonstrated improved cardiometabolic risk factors with reductions in systolic and diastolic blood pressure, fasting insulin levels, and lipid levels. 17 These newer agents have been termed second‐generation obesity medications because of the greater health benefits that accompany this degree of weight loss. 9 Furthermore, other investigational agents acting as dual agonists of GLP‐1 and glucagon itself are under clinical investigation for the treatment of obesity. The hope is that their dual receptor activity will elicit substantial weight loss and potentially provide additional weight‐independent reduction of ABCD complications, such as T2D, hypertension, cardiovascular disease, chronic kidney disease, non‐alcoholic fatty liver disease (NAFLD) and non‐alcoholic steatohepatitis (NASH), biomechanical consequences such as obstructive sleep apnea and osteoarthritis, and several types of cancer. 3 , 12 The mechanistic underpinnings for the weight loss associated with these classes of compounds vary: GLP‐1 receptor agonists reduce food intake while glucagon agonism appears to increase energy expenditure 18 , 19 , 20 , 21 ; the mechanism by which GIP agonism elicits weight loss is less clear. 13 , 22 , 23
Previous work on healthcare professionals' (HCP) knowledge of obesity treatment has in general found a lack of familiarity with obesity treatment guidelines, and with anti‐obesity medications (AOMs) themselves. 24 , 25 However, studies focusing on HCP knowledge of second‐generation AOMs, including dual receptor agonists, are lacking. As these compounds approach clinical practice, it is important to establish what HCP involved in obesity management understand about their mechanisms of action and potential therapeutic effects. Consequently, we conducted a survey of US‐based HCP treating people with obesity to investigate their current clinical practice in this field and knowledge of GLP‐1–based mono‐ and dual agonists. Any gaps in knowledge that are identified could be used to develop and target educational efforts among HCP, and ultimately increase access to more effective second‐generation obesity medications for patients.
2. METHODS
2.1. Study design and participants
This was a cross‐sectional study designed by the authors (W.T.G., C.D.M., T.B., R.F.K.) to survey HCP on their management of people living with obesity. It was conducted in the US between 28 February 2023 and 14 March 2023. HCP were identified from online panels and invited by email with a web link to a secure server where they completed a pre‐screening questionnaire for eligibility to participate. Eligible participants were primary‐care physicians (PCP), endocrinologists, and advanced practice providers (APPs) who had been practicing for at least 1 year and were current prescribers of AOMs to patients with BMI ≥30 kg/m2 or BMI ≥27 kg/m2 with weight‐related complications. Participants were ineligible if they were employed by a pharmaceutical company. If they met these inclusion criteria, participants were forwarded to the main questionnaire, which was designed to take 12–15 min to complete. Responses were anonymized, and participants were offered $41 (PCPs, APPs) or $49 (endocrinologists) for completing the survey. The survey adhered to the ICC/ESOMAR international code on market, opinion and social research and data analytics, 26 and the Insights Association code of standards and ethics for market research and data analytics. 27 The market research company dQ&A conducted fieldwork, collated data, and conducted initial analyses.
2.2. Measures
The survey instrument was designed to evaluate AOM‐prescribing HCP understanding of GLP‐1, GIP, and glucagon receptor agonists, including dual agonists, while also evaluating their experiences treating patients living with obesity (with and without T2D) and prescribing patterns of AOMs. The questionnaire included the following topics: HCP demographics; patient demographics; current practices for managing patients with obesity; perceived barriers to obesity treatment; knowledge of current and emerging treatments for obesity; clinical decision‐making and expectations; and the types of patients who would potentially benefit from glucagon, GLP‐1, and glucagon/GLP‐1 receptor dual agonists. Participating AOM‐prescribing HCP were first asked about their familiarity with GLP‐1, GIP, and glucagon receptor agonists, including dual agonists, as well as their therapeutic effects. They were then given more detailed information on the mechanisms of action and therapeutic effects, before being asked what type of patients would potentially benefit from these agonists. A five‐point Likert scale was employed for questions on perceived barriers to obesity treatment, and familiarity with GLP‐1, GIP, and glucagon receptor agonists.
2.3. Statistical analyses
Responses were summarized with descriptive statistics. For inferential analyses, Z tests were conducted with alpha (significance level) pre‐specified as 0.05, without adjustment for multiple testing (as this was an exploratory study). Analyses were performed using MarketSight Premium (MarketSight, Newtown, MA, USA) and SPSS Statistics version 28.0.1.1 (IBM, Armonk, NY, USA).
3. RESULTS
3.1. Demographics
A total of 785 HCP completed the survey (response rate: 49.8% of those eligible after screening): 251 PCPs, 263 endocrinologists, and 271 APPs, all of whom prescribed AOMs. Their demographic characteristics are shown in Table 1. Of the APPs, 146 were physician assistants/associates and 125 were nurse practitioners. PCPs mostly worked in family medicine (n = 204), with some specializing in internal medicine (n = 47). Nearly half of the respondents (45.2%) had been practicing for 10 years or less. Most endocrinologists (56.3%) reported their main clinical practice to be a multi‐specialty private practice or teaching hospital, whereas the most common main practice setting for PCPs and APPs was an office‐based practice or clinic (40.2% of each group). Overall, 46.5% of respondents reported practicing in a suburban setting, 39.5% in an urban setting, and 14.0% in a rural setting.
TABLE 1.
Characteristics of survey respondents.
| Total (N = 785) | Endocrinologists (n = 263) | PCPs (n = 251) | APPs (n = 271) | |
|---|---|---|---|---|
| Years working in current specialty | ||||
| 1–5 | 190 (24.2) | 52 (19.8) | 51 (20.3) | 87 (32.1) |
| 6–10 | 165 (21.0) | 52 (19.8) | 50 (19.9) | 63 (23.2) |
| 11–15 | 147 (18.7) | 50 (19.0) | 40 (15.9) | 57 (21.0) |
| 16–20 | 82 (10.4) | 34 (12.9) | 27 (10.8) | 21 (7.7) |
| >20 | 201 (25.6) | 75 (28.5) | 83 (33.1) | 43 (15.9) |
| Main practice setting a | ||||
| Office‐based practice or clinic | 251 (32.0) | 41 (15.6) | 101 (40.2) | 109 (40.2) |
| Private, multi‐specialty group practice | 145 (18.5) | 78 (29.7) | 39 (15.5) | 28 (10.3) |
| Teaching/academic hospital | 123 (15.7) | 70 (26.6) | 19 (7.6) | 34 (12.5) |
| Private, single specialty group practice | 111 (14.1) | 25 (9.5) | 45 (17.9) | 41 (15.1) |
| Private solo practice | 56 (7.1) | 17 (6.5) | 22 (8.8) | 17 (6.3) |
| Community hospital | 47 (6.0) | 16 (6.1) | 13 (5.2) | 18 (6.6) |
| Large, non‐private healthcare system | 44 (5.6) | 15 (5.7) | 10 (4.0) | 19 (7.0) |
| Private general hospital | 2 (0.3) | 1 (0.4) | 1 (0.4) | 0 |
| Government Veterans Affairs | 1 (0.1) | 0 | 0 | 1 (0.4) |
| Other | 5 (0.6) | 0 | 1 (0.4) | 4 (1.5) |
| Geographical location of practice | ||||
| Urban | 310 (39.5) | 140 (53.2) | 79 (31.5) | 91 (33.6) |
| Suburban | 365 (46.5) | 109 (41.4) | 124 (49.4) | 132 (48.7) |
| Rural | 110 (14.0) | 14 (5.3) | 48 (19.1) | 48 (17.7) |
Note: Data are n (%).
Abbreviations: APPs, advanced practice providers; PCPs, primary care physicians.
Based on the following question: Which of the following best describes your primary practice setting (the setting where you spend 50% or more of your professional time)?
Respondents indicated that an average of 51.9% of their patients were White, 19.7% Black, 17.0% Hispanic, 7.5% Asian, 1.8% Native Hawaiian/Pacific Islander, and 1.7% of other races/ethnicity—these proportions were very similar between endocrinologists, PCPs, and APPs (Table S1). Respondents also reported that an average of 55.3% of their patients had obesity (based on the survey definition of BMI ≥30 or BMI ≥27 with a weight‐related complication), 40.5% had T2D, and 42.6% had both obesity and T2D, with endocrinologists generally reporting higher percentages than PCPs or APPs (Table S1).
3.2. Prescribing behavior
Of the nine potential barriers to treatment of obesity, cost or lack of insurance coverage was identified by the most respondents overall (81.4%) as a substantial or extreme barrier (mean score of 4.2 on Likert scale ranging from 1 [no barrier] to 5 [extreme barrier]). The other barriers were selected by less than 50% of respondents as substantial or extreme, including low patient engagement/adherence with recommendations (43.9%, mean score 3.3), inadequate time and staff to address obesity (38.6%, mean score 3.1), and lack of knowledge of obesity as a disease rather than a lifestyle (31.7%, mean score 2.8) (Figure 1). Responses were generally similar across specialties (Figure S1). The full range of responses overall is shown in Figure S2.
FIGURE 1.
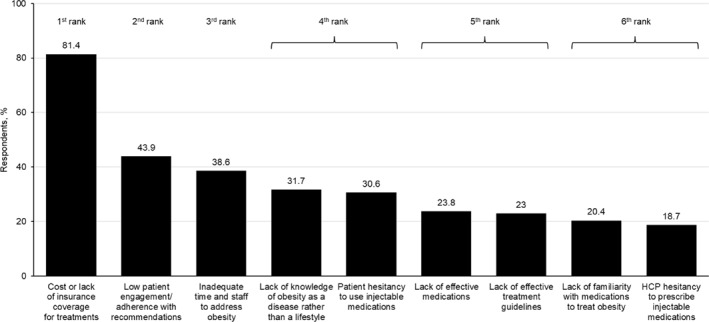
Perceived substantial or extreme barriers to treating obesity. Respondents were asked the following question: In your opinion, how much of a barrier do you find the following items to be toward treating obesity? Response options were not at all a barrier; a slight barrier; a moderate barrier; a substantial barrier; an extreme barrier. Data are the percentage of respondents selecting either a substantial or extreme barrier. Data are ranked based on statistical significance at the 95% confidence level. HCP, healthcare professional.
Significantly more respondents (41.9%, p < 0.05) ranked treatment of complications and comorbidities of obesity as the most desired outcome of prescribing AOMs, compared with body weight reduction (30.2%) and improved quality of life (27.9%). This trend was generally similar across specialties, with the top priority for all being the treatment of complications and comorbidities. However, compared to PCPs and APPs, more endocrinologists ranked body weight reduction as the top priority (endocrinologists: 35.4%; PCPs: 29.9%; APPs: 25.5%), while fewer prioritized improvements to quality of life (endocrinologists: 21.3%; PCPs: 31.1%; APPs: 31.4%).
Of their patients living with obesity, respondents reported recommending AOMs to 48.9% overall and to 62.0% of those with both obesity and T2D (Table S1). Endocrinologists recommended AOMs to significantly more patients (57.2% with obesity, 69.7% with obesity and T2D) than PCPs (43.0%, 55.8%) or APPs (46.4%, 60.4%) (p < 0.0005 for both). Of the six AOMs approved by the FDA, 86.4% of respondents had prescribed the GLP‐1 receptor agonist semaglutide (Wegovy) for the treatment of obesity, followed by the GLP‐1 receptor agonist liraglutide (Saxenda) (74.9%), phentermine (67.9%), phentermine/topiramate (Qsymia) (65.6%), naltrexone/bupropion (Contrave) (60.5%), and orlistat (46.5%) (Table 2). A total of 21.0% of respondents reported they had prescribed all the AOMs above, while only 1.7% indicated they had never prescribed any of the above. Consistently more endocrinologists reported prescribing individual AOMs than PCPs or APPs (Table 2).
TABLE 2.
Medications prescribed for patients with obesity.
| Total (N = 785) | Endocrinologists (n = 263) | PCPs (n = 251) | APPs (n = 271) | |
|---|---|---|---|---|
| Medications prescribed for obesity, a n (%) of HCPs | ||||
| Semaglutide (Wegovy) | 678 (86.4) | 255 (97.0)1st | 224 (89.2)2nd | 199 (73.4)3rd |
| Liraglutide (Saxenda) | 588 (74.9) | 247 (93.9)1st | 178 (70.9)2nd | 163 (60.1)3rd |
| Phentermine (Adipex, Lomaira) | 533 (67.9) | 186 (70.7)1st | 179 (71.3)1st | 168 (62.0)2nd |
| Phentermine/topiramate ER (Qsymia) | 515 (65.6) | 200 (76.0)1st | 157 (62.5)2nd | 158 (58.3)2nd |
| Naltrexone ER/bupropion ER (Contrave) | 475 (60.5) | 170 (64.6)1st | 160 (63.7)1st | 145 (53.5)2nd |
| Orlistat (Xenical, Alli) | 365 (46.5) | 131 (49.8)1st | 140 (55.8)1st | 94 (34.7)2nd |
| All of the above | 165 (21.0) | 78 (29.7) | 53 (21.1) | 34 (12.5) |
| None of the above | 13 (1.7) | 1 (0.4)2nd | 2 (0.8)2nd | 10 (3.7)1st |
| T2D medications prescribed for obesity in T2D patients, b % of HCPs | ||||
| Metformin | 726 (92.5) | 244 (92.8)2nd | 243 (96.8)1st | 239 (88.2)2nd |
| Semaglutide (Ozempic, Rybelsus) | 720 (91.7) | 250 (95.1)1st | 240 (95.6)1st | 230 (84.9)2nd |
| Dulaglutide (Trulicity) | 652 (83.1) | 240 (91.3)1st | 212 (84.5)2nd | 200 (73.8)3rd |
| SGLT2 inhibitors (e.g., Farxiga, Jardiance, Invokana) | 635 (80.9) | 233 (88.6)1st | 214 (85.3)1st | 188 (69.4)2nd |
| Liraglutide (Victoza) | 621 (79.1) | 245 (93.2)1st | 195 (77.7)2nd | 181 (66.8)3rd |
| Tirzepatide (Mounjaro) | 494 (62.9) | 217 (82.5)1st | 140 (55.8)2nd | 137 (50.6)2nd |
| Other | 8 (1.0) | 5 (1.9) | 0 | 3 (1.1) |
| None of the above | 9 (1.1) | 2 (0.8) | 0 | 7 (2.6) |
| Most common T2D medication for first‐line obesity treatment in T2D patients, c % of HCPs | ||||
| Semaglutide (Ozempic, Rybelsus) | 328 (42.3) | 137 (52.5)1st | 89 (35.5)2nd | 102 (38.6)2nd |
| Metformin | 258 (33.2) | 47 (18.0)2nd | 109 (43.4)1st | 102 (38.6)1st |
| Tirzepatide (Mounjaro) | 86 (11.1) | 47 (18.0)1st | 18 (7.2)2nd | 21 (8.0)2nd |
| SGLT2 inhibitors (e.g., Farxiga, Jardiance, Invokana) | 42 (5.4) | 6 (2.3) | 17 (6.8) | 19 (7.2) |
| Dulaglutide (Trulicity) | 40 (5.2) | 14 (5.4) | 11 (4.4) | 15 (5.7) |
| Liraglutide (Victoza) | 22 (2.8) | 10 (3.8) | 7 (2.8) | 5 (1.9) |
Note: Data are % of HCPs and are ranked first, second, or third based on statistical significance at the 95% confidence interval.
Abbreviations: APPs, advanced practice providers; ER, extended release; HCPs, healthcare professionals; PCPs, primary care physicians; SGLT2, sodium‐glucose co‐transporter‐2; T2D, type 2 diabetes.
Based on the following question: Which of the following medications that have been approved by the FDA to treat obesity have you prescribed?
Based on the following question: Have you prescribed any of the following medications approved by the FDA to treat Type 2 diabetes for patients with obesity and T2D?
Based on the following question: Of the medications you selected, which do you prescribe most commonly as a first‐line obesity treatment for patients with Type 2 diabetes?
Metformin and semaglutide (Ozempic, Rybelsus) were the T2D medications most commonly prescribed to treat obesity in T2D patients, with 92.5% and 91.7% of respondents having prescribed these drugs, respectively, for this purpose, followed by the GLP‐1 receptor agonist dulaglutide (83.1%), sodium‐glucose co‐transporter‐2 inhibitors (80.9%), liraglutide (Victoza) (79.1%), and tirzepatide (62.9%) (Table 3). Of the T2D medications prescribed as first‐line treatment of obesity in T2D patients, semaglutide (Ozempic, Rybelsus) was most common (42.3%), followed by metformin (33.2%) and tirzepatide (11.1%) (Table 2).
TABLE 3.
Perceived therapeutic effects of GLP‐1 receptor agonists, GIP receptor agonists, and glucagon receptor agonists.
| n (%) answering yes to the following questions: Do GLP‐1/GIP/glucagon receptor agonists produce the effect below? a | TOTAL (all HCPs surveyed) (N = 785) | ||
|---|---|---|---|
| GLP‐1 receptor agonists | GIP receptor agonists | GCG receptor agonists | |
| Decrease appetite through CNS effects | 550 (70.1) | 366 (46.6) | 218 (27.8) |
| Decrease gastric emptying | 560 (71.3) | 358 (45.6) | 182 (23.2) |
| Increase insulin secretion | 451 (57.5) | 333 (42.4) | 176 (22.4) |
| Decrease glucagon secretion | 445 (56.7) | 304 (38.7) | 202 (25.7) |
| Initial nausea | 640 (81.5) | 415 (52.9) | 224 (28.5) |
| Decrease body weight | 695 (88.5) | 518 (66.0) | 345 (43.9) |
| Decrease blood glucose | 664 (84.6) | 509 (64.8) | 350 (44.6) |
| Increase insulin sensitivity in adipose tissue | 478 (60.9) | 355 (45.2) | 226 (28.8) |
| Increase fatty acid oxidation | 248 (31.6) | 198 (25.2) | 141 (18.0) |
| Increase glycogenolysis and gluconeogenesis | 305 (38.9) | 222 (28.3) | 202 (25.7) |
| Increase energy expenditure | 251 (32.0) | 185 (23.6) | 154 (19.6) |
| Increase lipolysis | 327 (41.7) | 254 (32.4) | 173 (22.0) |
Abbreviations: APPs, advanced practice providers; CNS, central nervous system; GCG, glucagon; GIP, glucose‐dependent insulinotropic polypeptide; GLP‐1, glucagon‐like peptide‐1; HCPs, healthcare professionals; PCPs, primary care physicians.
Each effect was a separate question with a choice of answers of yes, no, and unsure.
3.3. Familiarity with GLP‐1, GIP, and glucagon receptor agonists and knowledge of their effects
Overall, 95.4% of respondents reported at least some familiarity with GLP‐1 receptor agonists, with 64.7% being very or extremely familiar. Mean (SD) familiarity was 3.8 (1.2) on a Likert scale ranging from 1 to 5 (1: not at all familiar; 2: slightly familiar; 3: moderately familiar; 4: very familiar; 5: extremely familiar). Significantly more endocrinologists (87.9%, p < 0.0005) reported being very or extremely familiar with GLP‐1 receptor agonists compared with PCPs (59.8%) and APPs (46.9%) (Figure S3A).
For GIP receptor agonists, 79.6% of respondents reported at least some familiarity, but only 30.3% were very or extremely familiar with them. Mean (SD) familiarity was 2.8 (1.3). Again, there were significant differences (p < 0.0005) between specialties in the proportions reporting being very or extremely familiar with GIP receptor agonists, with endocrinologists (49.8%) more so than PCPs (22.7%) or APPs (18.4%) (Figure S3B).
A total of 70.1% of respondents reported at least some familiarity with glucagon receptor agonists, but only 16.5% were very or extremely familiar with them. Mean (SD) familiarity was 2.3 (1.2), and there were no significant differences between specialties in the percentages of respondents reporting familiarity or lack of familiarity (Figure S3C).
The majority of respondents recognized that GLP‐1 receptor agonists decrease blood glucose (84.6% overall/92.4% of endocrinologists) and body weight (88.5%/94.7%), although slightly fewer perceived them to slow gastric emptying (71.3%/90.5%), decrease appetite via the central nervous system (70.1%/84.4%), and increase insulin secretion (57.5%/76.4%) (Table 3, Table S2). Understanding of the mechanisms and therapeutic effects of GIP receptor agonists was lower overall, but again endocrinologists ranked higher than PCPs and APPs, where only a minority of respondents indicated understanding (Table 3, Table S3). However, knowledge of the mechanisms and effects of glucagon receptor agonists was generally low across all specialties (Table 3, Table S4).
3.4. Expected benefits of GLP‐1 and glucagon receptor agonists, including dual agonists
Overall, the majority of respondents (at least 60%) perceived that GLP‐1 receptor agonists, glucagon receptor agonists, or glucagon receptor/GLP‐1 receptor dual agonists would be particularly beneficial for people with obesity and comorbid T2D, metabolic syndrome, or pre‐diabetes, particularly GLP‐1 receptor agonists (Figure 2). Significantly more respondents perceived that glucagon receptor agonists and dual agonists would benefit those with NASH/NAFLD or dyslipidemia, compared with GLP‐1 receptor mono‐agonists. Generally, less than half of the respondents perceived benefit of these drug classes for non‐cardiometabolic comorbidities of obesity such as sleep apnea, osteoarthritis, and gastroesophageal reflux disease (GERD).
FIGURE 2.
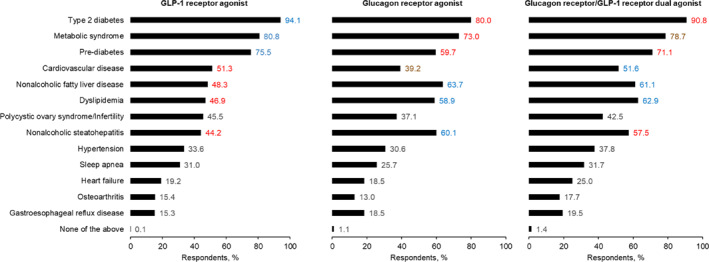
Perceived benefits of GLP‐1 receptor agonists, glucagon receptor agonists, and glucagon receptor/GLP‐1 receptor dual agonists. Respondents were asked the following: GLP‐1 receptor agonists have been shown to increase insulin secretion, decrease gastric emptying, and suppress appetite by acting on feeding centers in the brain. Glucagon receptor agonists have been shown to produce an enhanced effect on body weight reduction through effects on both the liver and adipose tissue. Targeting the liver can result in increased fatty acid oxidation, glycogenolysis, and gluconeogenesis; while targeting adipose tissue can increase lipolysis. Collectively, this can lead to a potential overall increased energy expenditure. Based on the above definition, what patient group(s) with obesity do you think would benefit most from a glucagon‐like peptide‐1 receptor agonist? What patient group(s) with obesity do you think would benefit most from a glucagon receptor agonist? What benefits, in terms of better control , would you expect from the combination of the two receptor agonists compared with the effects of either one alone (for this question, please assume that the medication is considered safe and tolerable in Phase III studies)? Please select all that apply. Data are ranked based on statistical significance at the 95% confidence interval for each patient benefit; first rank is blue, second is red, third is brown. GLP‐1, glucagon‐like peptide‐1.
A consistently high proportion of respondents (>70%) perceived that GLP‐1 receptor agonists would benefit people with obesity who also have metabolic disease (T2D, pre‐diabetes, or metabolic syndrome) across endocrinologists, PCPs, and APPs (Figure S4). However, significantly more endocrinologists perceived this drug class to also benefit those with comorbid NAFLD/NASH (64.6%/62.0%), compared with PCPs (45.0%/41.8%) or APPs (35.4%/29.2%).
The high proportion of respondents who felt that glucagon receptor agonists would benefit those with comorbid T2D was consistent across specialties (79.5%, 79.7%, 80.8% of endocrinologists, PCPs, and APPs, respectively) (Figure S5). However, significantly more endocrinologists perceived that this drug class would benefit people with obesity and comorbid NAFLD/NASH (70.0%/68.8%) than PCPs (59.8%/58.2%) or APPs (61.3%/53.5%). Conversely, compared with endocrinologists, more PCPs and APPs felt that glucagon receptor agonists would benefit people with obesity and other conditions such as dyslipidemia, heart failure and other cardiovascular disease, and GERD—albeit still mostly less than 50% of respondents.
Similarly, significantly more endocrinologists perceived that glucagon receptor/GLP‐1 receptor dual agonists would benefit people with obesity and NAFLD/NASH more than either receptor agonist alone (68.4%/69.2%), compared with PCPs (57.8%/53.0%) and APPs (57.2%/50.2%) (Figure S6). Only a minority of respondents (<50%) across specialties perceived that dual agonists would be more beneficial for people with non‐cardiometabolic comorbidities of obesity than either receptor agonist alone.
4. DISCUSSION
This cross‐sectional survey conducted in early 2023 provides insights into US HCP knowledge of new (second‐generation) AOMs, as well as their current practices in treating obesity. The 785 respondents were relatively evenly split among endocrinologists, PCPs, and APPs, and the reported average racial/ethnic mix of their patients (Table 1) was not substantially different from the US as a whole (White 61.6%, Black 12.4%, Hispanic 18.7%, Asian 6.0%). 28
The main focus of our survey was to investigate the understanding that US AOM‐prescribing HCP treating obesity have of the second‐generation AOMs. 9 Most of these licensed and investigational molecules modulate neurohormonal pathways involving GLP‐1 receptor signaling with or without additional agonist activity at the GIP or glucagon receptors. 9 Members of these drug classes can elicit mean levels of weight loss of 15%–20%, 14 , 17 which approaches levels achieved with bariatric surgery and is sufficient to ameliorate most ABCD complications, including cardiometabolic and biomechanical comorbidities. 9 GLP‐1 receptor agonists stimulate GLP‐1 receptors in the arcuate nucleus of the hypothalamus and brain stem, and projections to other appetite‐modulating brain areas, to reduce feeding, an effect thought to underly the weight loss elicited by compounds in this class, which is particularly efficacious with semaglutide. 18 The involvement of GIP in body weight regulation is less clearly defined, with both agonists and antagonists of the GIP receptor eliciting weight loss in animal models. 13 , 22 , 23 Tirzepatide (a GLP‐1/GIP dual agonist) appears to elicit greater weight loss than semaglutide (a GLP‐1 mono‐agonist), but the role of GIP agonism in this effect is unclear. 29 Glucagon—in addition to counter‐balancing the effects of insulin in glucose homeostasis—also appears to increase energy expenditure and induce satiety, 19 , 20 although the effects of chronic agonism of the glucagon receptor in humans are not yet fully defined, including whether it augments energy expenditure.
In our survey, a high proportion of respondents overall were familiar with GLP‐1 receptor agonists, but fewer with GIP receptor agonists, and fewer still with glucagon receptor agonists. Endocrinologists were more familiar with GLP‐1 and GIP receptor agonists than PCPs and APPs. Knowledge of the therapeutic effects of these compounds followed a similar pattern. When presented with the putative therapeutic effects of GLP‐1 receptor agonists, glucagon receptor agonists, and glucagon receptor/GLP‐1 receptor dual agonists, the majority of respondents felt that all three drug classes may benefit people with obesity and comorbid metabolic disease such as T2D. Dual agonists were felt to be more beneficial than GLP‐1 receptor agonists or glucagon receptor agonists alone for people with NASH/NAFLD and obesity, particularly by endocrinologists. Interestingly, only a minority of respondents perceived that these three drug classes would benefit non‐cardiometabolic complications of obesity, which may reflect an interpretation of a lack of direct effect independent of weight loss.
In other findings from the survey, treating complications and comorbidities of obesity was the most desired outcome of prescribing AOMs (42% of respondents), which is consistent with the American Association of Clinical Endocrinologists clinical guidelines' emphasis on a complications‐centric approach to managing obesity. 2 Cost of treatment, including lack of insurance coverage, was by far the biggest reported barrier to treatment with AOMs, with 81% of respondents reporting it to be a substantial or extreme barrier. Interestingly, cost was also cited as the major limitation to use of AOMs by 72% of participants in a recent survey of endocrinologists in Italy, where anti‐obesity treatment is generally not reimbursed, 30 as well as in the 2021 DocStyles survey of PCPs and APPs in the US. 24 In the US, newer AOMs, like GLP‐1RAs, have large out‐of‐pocket costs for patients, 31 and the majority of US health insurance providers do not cover AOMs, 32 including most state Medicaid programs. 33 , 34 Given that obesity is a chronic disease, and medications therefore need to be used for sustained periods, this cost can be prohibitive for many patients with obesity. In a retrospective study of over 50,000 people with obesity in Florida and Ohio characterizing receipt of AOM prescriptions and prescription fills, only 55% of those with an AOM prescription filled that prescription. 33 Lower rates of prescription fills were associated with Hispanic ethnicity, being a man, Medicaid, traditional Medicare and Medicare Advantage insurance types. In the same study, having a private insurance provider was associated with both the likelihood of receiving an AOM prescription and prescription fill. 33 It is likely that cost and insurance cover contribute to the low rate of AOM prescription fill.
Clearly, efforts are needed to reduce the costs of AOMs and improve patient access. Novel dual and triple agonists that have cardio‐kidney‐metabolic benefits beyond weight loss may influence access. Currently, the most potent weight‐reduction agents, semaglutide and tirzepatide, consistently elicit less weight loss in people with T2D and obesity, than in those with obesity but without T2D. 14 , 15 , 17 Through a presumed increase in energy expenditure and by targeting energy intake, novel glucagon receptor/GLP‐1 receptor dual agonists may bridge this gap and elicit more weight loss in people with T2D, or even reduce complications by other pathways (e.g., selective reduction of liver fat to ameliorate NAFLD/NASH). This could potentially incentivize payer coverage and increase patient access to these medications. The current survey shows that HCP prescribing AOMs view benefits beyond weight loss as important. Furthermore, the advent of small‐molecule oral GLP‐1 receptor agonists 35 will also hopefully result in reduced costs and improve access to AOMs.
Lack of patient engagement was also highlighted as a prominent barrier to treatment in our survey, as it was in the survey of endocrinologists in Italy, 30 the ACTION survey in the US, 36 and the multinational ACTION IO survey. 37 , 38 , 39
Overall, survey respondents reported recommending AOMs to less than half of their patients with obesity (49%), which may reflect the barriers mentioned above. This rate was higher for patients with concomitant T2D (62%) and higher overall by endocrinologists than by PCPs or APPs. The overall rate is much higher than in the general patient population in the US where studies prior to the approval of second‐generation AOMs found that only 1%–3% of adults eligible for AOMs were actually prescribed them. 40 , 41 , 42 This difference probably reflects the eligibility criteria for the current survey (respondents had to be current prescribers of AOMs) as well as the low efficacy of first‐generation medications. A more recent study spanning the period since approval of the second‐generation medication semaglutide found a slightly higher, albeit still very low, AOM prescription rate to people with obesity (8%). 33
Interestingly, almost all respondents (92%) reported prescribing metformin to treat obesity in patients who also had T2D, more than any other T2D medication except semaglutide (also 92%). Furthermore, a third of respondents reported prescribing metformin as a first‐line treatment for obesity in patients who also had T2D, although significantly fewer endocrinologists (18%) than PCPs (43%) or APPs (39%) prescribed metformin as a first‐line treatment of obesity in patients with concomitant T2D. A similar finding emerged from the survey of Italian endocrinologists, where metformin was the most commonly prescribed medication for obesity (30% of respondents). Metformin is not approved for obesity management and any resulting weight loss is quite modest. 43 Thus, this finding raises an alarm since reliance on metformin as an AOM may divert patients and HCP from employing more effective therapy specifically targeted to treat obesity.
In general, APPs' responses were similar to PCPs. This is perhaps unsurprising given that the primary practice settings of APPs and PCPs surveyed were similar with the largest percentage of both (40%) practicing in an office‐based practice or clinic. The large number of APPs surveyed in this study is particularly informative, as according to the US Bureau of Labor Statistics, nurse practitioners are expected to be the fastest growing occupation in the US between 2022 and 2032, with physician assistants also in the top 10 fastest growing occupations in this period. 44 There is a lack of access to primary care in the US and increased numbers of APPs are expected to bridge this gap. 45 Primary care is an important contact point between people living with obesity and the healthcare system. 46 However, obesity is undertreated in this setting. 47 As the role of APPs in primary care, and the treatment of obesity, grows, it will be increasingly important to understand their treatment and prescribing behaviors so that any patient unmet needs can be identified.
This study has certain strengths and limitations. To our knowledge, no other study has recently surveyed the practices and knowledge of US HCP treating obesity, and prescribing AOMs, with a focus on their understanding of the second‐generation AOMs entering clinical practice. Furthermore, the study included more HCP than most other recent single‐country surveys of obesity management. 36 , 38 , 39 Limitations include its cross‐sectional design, exploratory nature, and the potential for recall and sampling biases (as with any retrospective survey using non‐probabilistic purposive sampling). Another limitation is that respondents were asked if they had ever prescribed a given medication; the frequency and timeframe of prescribing was not collected in this analysis. Furthermore, the results reflect the respondents' particular practices and patients; therefore, the findings may not be fully generalizable to other practice settings, or to those HCP treating people with obesity without the use of AOMs (who were ineligible for the survey).
5. CONCLUSIONS
Our survey provides new insights into obesity management by US HCP, including endocrinologists, PCPs, and APPs. As respondents recommend AOMs to nearly half of their patients with obesity, a treatment gap still remains. The reported barriers to treatment, including cost, impact HCP ability to provide appropriate therapy for patients. This finding indicates a need to improve access to these AOMs. In addition, respondents were much more familiar with GLP‐1 receptor agonists than glucagon receptor agonists or GIP receptor agonists. However, they anticipate that the new mechanisms of action of the glucagon receptor agonists, including unimolecular glucagon receptor/GLP‐1 receptor dual agonists, may offer further clinical benefits. These benefits include cardiometabolic benefits beyond weight loss, as well as via other pathways (e.g., selective reduction of liver fat to ameliorate NAFLD/NASH). Since obesity treatment currently focuses on the treatment of complications and comorbidities, this could help to address treatment barriers and patient access.
AUTHOR CONTRIBUTIONS
The authors meet the criteria for authorship as recommended by the International Committee of Medical Journal Editors.
CONFLICT OF INTEREST STATEMENT
W. Timothy Garvey, MD, has served as a consultant on advisory boards for Boehringer Ingelheim, Eli Lilly, Novo Nordisk, Pfizer, Fractyl Health, Alnylam Pharmaceuticals, Inogen, and Merck, and as a site principal investigator for multi‐centered clinical trials sponsored by his university and funded by Novo Nordisk, Eli Lilly, Epitomee, Neurovalens, and Pfizer. Cathy D. Mahle, PhD, is an employee of Boehringer Ingelheim, which is developing a dual glucagon receptor/GLP‐1 receptor agonist. Robert F. Kushner, MD, serves on scientific advisory boards for Novo Nordisk and WW and serves as a consultant for Altimmune, Pfizer, Boehringer Ingelheim and Eli Lilly. Trevor Bell, PhD, is employed by dQ&A, a market research firm whose clients include several pharmaceutical and device companies in the diabetes field.
Supporting information
Supporting Information S1
ACKNOWLEDGMENTS
This study was sponsored by Boehringer Ingelheim Pharmaceuticals, Inc (BIPI). Fieldwork was conducted by dQ&A, which was contracted and funded by BIPI. The authors thank Christianne Pang, BS, MBA, and Emily Lin, BS, of dQ&A for assistance with survey development and data collection. The authors received no payment related to the development of the manuscript. Writing support was provided by Giles Brooke, PhD, CMPP, of Elevate Scientific Solutions LLC, a member of the Envision Pharma Group, which was contracted and funded by BIPI. BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations. Parts of this work were presented at the 41st Annual Meeting of the Obesity Society, 14–17 October 2023, Dallas, TX, USA (ObesityWeek® 2023): poster 136; Garvey WT, Mahle C, Kushner R. Obesity 2023; 31 (Suppl. 2):106.
Garvey WT, Mahle CD, Bell T, Kushner RF. Healthcare professionals' perceptions and management of obesity & knowledge of glucagon, GLP‐1, GIP receptor agonists, and dual agonists. Obes Sci Pract. 2024;e756. 10.1002/osp4.756
REFERENCES
- 1. Fruhbeck G, Busetto L, Dicker D, et al. The ABCD of obesity: an EASO position statement on a diagnostic term with clinical and scientific implications. Obes Facts. 2019;12(2):131‐136. 10.1159/000497124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of Endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(suppl 3):1‐203. 10.4158/ep161356.esgl [DOI] [PubMed] [Google Scholar]
- 3. Mechanick JI, Hurley DL, Garvey WT. Adiposity‐Based Chronic Disease as a new diagnostic term: the American Association of Clinical Endocrinologists and American College of Endocrinology position statement. Endocr Pract. 2017;23(3):372‐378. 10.4158/ep161688.ps [DOI] [PubMed] [Google Scholar]
- 4. World Obesity Federation . World Obesity Atlas 2023. London, UK. Accessed April 28, 2023. https://www.worldobesity.org/resources/resource‐library/world‐obesity‐atlas‐2023
- 5. Centers for Disease Control and Prevention . Adult obesity facts. Accessed April 27, 2023. https://www.cdc.gov/obesity/data/adult.html
- 6. Ward ZJ, Bleich SN, Long MW, Gortmaker SL. Association of body mass index with health care expenditures in the United States by age and sex. PLoS One. 2021;16(3):e0247307. 10.1371/journal.pone.0247307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim N, Estrada J, Chow I, et al. The relative value of anti‐obesity medications compared to similar therapies. Clinicoecon Outcomes Res. 2023;15:51‐62. 10.2147/ceor.s392276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kushner RF. Weight loss strategies for treatment of obesity: lifestyle management and pharmacotherapy. Prog Cardiovasc Dis. 2018;61(2):246‐252. 10.1016/j.pcad.2018.06.001 [DOI] [PubMed] [Google Scholar]
- 9. Garvey WT. New horizons. A new paradigm for treating to target with second‐generation obesity medications. J Clin Endocrinol Metab. 2022;107(4):e1339‐e1347. 10.1210/clinem/dgab848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Onakpoya IJ, Heneghan CJ, Aronson JK. Post‐marketing withdrawal of anti‐obesity medicinal products because of adverse drug reactions: a systematic review. BMC Med. 2016;14(1):191. 10.1186/s12916-016-0735-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sharretts J, Galescu O, Gomatam S, Andraca‐Carrera E, Hampp C, Yanoff L. Cancer risk associated with lorcaserin ‐ the FDA's review of the CAMELLIA‐TIMI 61 trial. N Engl J Med. 2020;383(11):1000‐1002. 10.1056/nejmp2003873 [DOI] [PubMed] [Google Scholar]
- 12. Jastreboff AM, Kushner RF. New frontiers in obesity treatment: GLP‐1 and nascent nutrient‐stimulated hormone‐based therapeutics. Annu Rev Med. 2023;74(1):125‐139. 10.1146/annurev-med-043021-014919 [DOI] [PubMed] [Google Scholar]
- 13. Nauck MA, D'Alessio DA. Tirzepatide, a dual GIP/GLP‐1 receptor co‐agonist for the treatment of type 2 diabetes with unmatched effectiveness regarding glycaemic control and body weight reduction. Cardiovasc Diabetol. 2022;21(1):169. 10.1186/s12933-022-01604-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilding JPH, Batterham RL, Calanna S, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989‐1002. 10.1056/nejmoa2032183 [DOI] [PubMed] [Google Scholar]
- 15. Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3 trial. Lancet. 2021;397(10278):971‐984. 10.1016/s0140-6736(21)00213-0 [DOI] [PubMed] [Google Scholar]
- 16. Garvey WT, Batterham RL, Bhatta M, et al. Two‐year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083‐2091. 10.1038/s41591-022-02026-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205‐216. 10.1056/nejmoa2206038 [DOI] [PubMed] [Google Scholar]
- 18. Bergmann NC, Davies MJ, Lingvay I, Knop FK. Semaglutide for the treatment of overweight and obesity: a review. Diabetes Obes Metab. 2023;25(1):18‐35. 10.1111/dom.14863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Campbell JE, Drucker DJ. Islet alpha cells and glucagon‐‐critical regulators of energy homeostasis. Nat Rev Endocrinol. 2015;11(6):329‐338. 10.1038/nrendo.2015.51 [DOI] [PubMed] [Google Scholar]
- 20. Finan B, Capozzi ME, Campbell JE. Repositioning glucagon action in the physiology and pharmacology of diabetes. Diabetes. 2020;69(4):532‐541. 10.2337/dbi19-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double‐blind trial. Lancet. 2017;389(10077):1399‐1409. 10.1016/s0140-6736(17)30069-7 [DOI] [PubMed] [Google Scholar]
- 22. Guccio N, Gribble FM, Reimann F. Glucose‐dependent insulinotropic polypeptide‐a postprandial hormone with unharnessed metabolic potential. Annu Rev Nutr. 2022;42(1):21‐44. 10.1146/annurev-nutr-062320-113625 [DOI] [PubMed] [Google Scholar]
- 23. Mroz PA, Finan B, Gelfanov V, et al. Optimized GIP analogs promote body weight lowering in mice through GIPR agonism not antagonism. Mol Metab. 2019;20:51‐62. 10.1016/j.molmet.2018.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith M, Gallagher C, Weber D, Dietz WH. Health care providers' attitudes and counseling behaviors related to obesity. Obes Sci Pract. 2023;9(5):501‐507. 10.1002/osp4.686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Turner M, Jannah N, Kahan S, Gallagher C, Dietz W. Current knowledge of obesity treatment guidelines by health care professionals. Obesity. 2018;26(4):665‐671. 10.1002/oby.22142 [DOI] [PubMed] [Google Scholar]
- 26. ICC/ESOMAR . International code on market, opinion and social research and data analytics. 2016. https://esomar.org/uploads/attachments/ckqtawvjq00uukdtrhst5sk9u‐iccesomar‐international‐code‐english.pdf
- 27. The Insights Association . Code of standards and ethics for market research and data analytics. 2022. https://www.insightsassociation.org/Portals/INSIGHTS/IA%20Code_1_6_23_Final.pdf
- 28. Jones N, Marks R, Ramirez R, Ríos‐Vargas M. 2020 Census Illuminates Racial and Ethnic Composition of the Country. United States Census Bureau; 2021. https://www.census.gov/library/stories/2021/08/improved‐race‐ethnicity‐measures‐reveal‐united‐states‐population‐much‐more‐multiracial.html [Google Scholar]
- 29. Gasbjerg LS, Rosenkilde MM, Meier JJ, Holst JJ, Knop FK. The importance of glucose‐dependent insulinotropic polypeptide receptor activation for the effects of tirzepatide. Diabetes Obes Metab. 2023;25(11):3079‐3092. 10.1111/dom.15216 [DOI] [PubMed] [Google Scholar]
- 30. Chianelli M, Busetto L, Attanasio R, et al. Obesity management: attitudes and practice of Italian endocrinologists. Front Endocrinol. 2022;13:1061511. 10.3389/fendo.2022.1061511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levi J, Wang J, Venter F, Hill A. Estimated minimum prices and lowest available national prices for antiobesity medications: improving affordability and access to treatment. Obesity. 2023;31(5):1270‐1279. 10.1002/oby.23725 [DOI] [PubMed] [Google Scholar]
- 32. Schumacher LM, Ard J, Sarwer DB. Promise and unrealized potential: 10 years of the American Medical Association classifying obesity as a disease. Front Public Health. 2023;11:1205880. 10.3389/fpubh.2023.1205880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gasoyan H, Pfoh ER, Schulte R, Sullivan E, Le P, Rothberg MB. Association of patient characteristics and insurance type with anti‐obesity medications prescribing and fills. Diabetes Obes Metab. 2024;26(5):1687‐1696. 10.1111/dom.15473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gomez G, Stanford FC. US health policy and prescription drug coverage of FDA‐approved medications for the treatment of obesity. Int J Obes. 2018;42(3):495‐500. 10.1038/ijo.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wharton S, Blevins T, Connery L, et al. Daily oral GLP‐1 receptor agonist orforglipron for adults with obesity. N Engl J Med. 2023;389(10):877‐888. 10.1056/nejmoa2302392 [DOI] [PubMed] [Google Scholar]
- 36. Kaplan LM, Golden A, Jinnett K, et al. Perceptions of barriers to effective obesity care: results from the national ACTION study. Obesity. 2018;26(1):61‐69. 10.1002/oby.22054 [DOI] [PubMed] [Google Scholar]
- 37. Caterson ID, Alfadda AA, Auerbach P, et al. Gaps to bridge: misalignment between perception, reality and actions in obesity. Diabetes Obes Metab. 2019;21(8):1914‐1924. 10.1111/dom.13752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sharma AM, Belanger A, Carson V, et al. Perceptions of barriers to effective obesity management in Canada: results from the ACTION study. Clin Obes. 2019;9(5):e12329. 10.1111/cob.12329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Iwabu M, Yamauchi T, Shimomura I, Eguchi K, Ogawa Y. Perceptions, attitudes and barriers to obesity management: Japanese data from the ACTION‐IO study. J Diabetes Investig. 2021;12(5):845‐858. 10.1111/jdi.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saxon DR, Iwamoto SJ, Mettenbrink CJ, et al. Antiobesity medication use in 2.2 million adults across eight large health care organizations: 2009‐2015. Obesity. 2019;27(12):1975‐1981. 10.1002/oby.22581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: pharmacotherapy trends in the United States from 1999 to 2010. Obesity. 2015;23(8):1721‐1728. 10.1002/oby.21136 [DOI] [PubMed] [Google Scholar]
- 42. Samaranayake NR, Ong KL, Leung RY, Cheung BM. Management of obesity in the National Health and Nutrition Examination Survey (NHANES), 2007‐2008. Ann Epidemiol. 2012;22(5):349‐353. 10.1016/j.annepidem.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 43. Pu R, Shi D, Gan T, et al. Effects of metformin in obesity treatment in different populations: a meta‐analysis. Ther Adv Endocrinol Metab. 2020;11:2042018820926000. 10.1177/2042018820926000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. US Bureau of Labor Statistics . Fastest growing occupations. Accessed February 19, 2024. https://www.bls.gov/ooh/fastest‐growing.htm
- 45. Ahmed H, Carmody JB. On the looming physician shortage and strategic expansion of graduate medical education. Cureus. 2020;12(7):e9216. 10.7759/cureus.9216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Anderson J, Kushner R, Miller E, Nadglowski J, Still C. Overweight and obesity management for primary care clinicians: executive summary. Clin Diabetes. 2022;41(1):85‐89. 10.2337/cd22-0082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tucker S, Bramante C, Conroy M, et al. The most undertreated chronic disease: addressing obesity in primary care settings. Curr Obes Rep. 2021;10(3):396‐408. 10.1007/s13679-021-00444-y [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


