Abstract
To develop effective PrEP adherence interventions, it is important to understand the interplay between disclosure of pre-exposure prophalxis (PrEP) use, social support, and PrEP adherence. We leveraged the HPTN 082 study conducted among 451 adolescent girls and young women (AGYW) (ages 16 to 25 years, 2016 to 2019) in South Africa and Zimbabwe. Among the 349 who had month three disclosure and PrEP adherence data, 60% (n = 206) felt supported by adults, and 89% (n = 309) disclosed PrEP use to at least one person. PrEP disclosure was not associated with increased adherence, measured by intracellular tenofovir-diphosphate concentrations in dried blood spots. Women who reported having supportive adults, and disclosed to their parents, had higher adherence at 6 months with an increase of 177 fmol/punch (95% CI 12 to 343, t = 2.11, p = 0.04). PrEP interventions that help AGYW identify supportive relationships and effectively communicate the benefits of PrEP may improve PrEP adherence.
Clinicaltrials.gov ID number: NCT02732730.
Keywords: Adherence, HIV prevention, Adolescent girls and young women, Pre-exposure prophylaxis (PrEP), Africa, Disclosure and social support
Introduction
African adolescent girls and young women (AGYW) experience a disproportionate rate of new HIV diagnoses, which account for 25% of all new transmissions globally [1]. Adherence to highly effective oral pre-exposure prophylaxis (PrEP) to prevent HIV acquisition is challenging for many AGYW [2–6]. Factors that negatively impact PrEP adherence for young women comprise low perceived risk of HIV acquisition, intimate partner violence, side effects, depression, stigma related to disclosure of PrEP use, and the burden of taking a daily oral pill [7–14]. AGYW are in critical need of effective intervention programs to mitigate barriers to PrEP adherence. Open-label PrEP demonstration studies aimed at increasing compliance through providing PrEP drug-level feedback, short message service (SMS) reminders, peer-adherence clubs, or conditional cash incentives to motivate AGYW to take PrEP pills persistently did not demonstrate a significant improvement in PrEP adherence compared to the standard of care support [4, 5, 15, 16].
Young women are at least twice as likely to acquire HIV as age-matched African men [17]. Support from social networks (e.g., parents, partners, and friends) has a crucial role in adolescents’ health-promoting behaviours, including adherence to treatment and medication as prescribed [3, 18–20]. For adolescents, their most influential relationships are parental figures, peers, and intimate partners. On the other hand, they have an increased desire for independence from parents during emerging adulthood and are prone to peer pressure and behaviorally vulnerable to HIV (e.g., sexual debut at a younger age, unprotected sex, older sexual partners, concurrent sexual partners, and alcohol abuse) [4, 13, 17–20]. Youth also lack financial autonomy and problem-solving skills, putting them at greater risk of PrEP nonadherence [21]. In addition to social support for health-promoting behaviours, young women may benefit from PrEP adherence support to prevent HIV acquisition. However, to receive support for PrEP adherence, AGYW need to weigh the benefits and risks of disclosure of PrEP use [22, 23]. In the open-label HIV Prevention Trials Network (HPTN) 067/ADAPT study in Cape Town, South Africa, and the multi-site Microbicide Trials Network (MTN) 003/VOICE trial in South Africa, Uganda and Zimbabwe, women who participated in semi-structured interviews highlighted that societal stigma made it difficult to rely on their social network for PrEP adherence support [9, 24]. Qualitative studies indicated that disclosure of PrEP use to their sexual partner can affect PrEP adherence positively (e.g., medication reminders, a place to store pills, and moral support) or negatively (e.g., mistrust, disapproval, and conflicts) due to stigma [7, 23, 25].
Young women have also reported that they consulted older female figures about PrEP because they did not trust the information from their peers and feared gossip among peers and being judged immoral [26, 27]. This suggests that the quality of the social relationship is likely to moderate the effect of disclosure of PrEP use, as is observed with antiretroviral treatment (ART) adherence [28]. Prior research indicates that supportive adult relationships, PrEP disclosure, and PrEP support from trusted members of social groups are essential components for successful PrEP adherence [7, 29, 30]. Given the stigma and gender disparities, a greater understanding of the pathways between disclosure of PrEP use, supportive adult relationships, and PrEP support received from interpersonal relationships is needed to design impactful intervention programs to assist AGYW with improving PrEP adherence.
Based on the Information, Motivation, and Behavioral model [31, 32] situated in the socio-ecological model framework [33, 34], we conceptualized that disclosure to close relationships in their social network and receiving adherence support, such as reminders to take the medication as prescribed, can influence AGYW's ability to take PrEP pills consistently [25, 26]. There is some qualitative evidence on the interplay between disclosure of PrEP use, social support, PrEP support from interpersonal relationships, and PrEP adherence. While qualitative studies provided potential hypotheses for further investigation, it not easy to determine the sequence of effect from disclosure to PrEP support to adherence, and qualitative data do not provide information on the magnitude of the impact at months three and six. We hypothesized that AGYW who disclosed PrEP use would likely have a higher PrEP adherence at months three and six and explored the potential moderating effect of supportive adult relationships. We also hypothesized that the effect of PrEP disclosure on adherence was partially mediated through reminders from those to whom they disclosed PrEP use. Therefore, our study quantitatively investigated: (1) the impact of disclosure of PrEP use on PrEP adherence by relationship type (parents, partners, and friends) at months three and six, (2) whether having supportive adults in their life moderates the magnitude of the effect on AGYW’s PrEP adherence, and (3) if PrEP support in the form of reminders to take PrEP pills mediates the effect of disclosure on adherence.
Methods
Study Design and Population
The HPTN 082 study, an open-label oral PrEP demonstration trial, was conducted from October 2016 to October 2019 among 451 AGYW aged 16 to 25 who are living without HIV. The study participants were recruited from youth-friendly clinics in Harare, Zimbabwe, and Cape Town and Johannesburg, South Africa. The study design, study procedures, and primary findings were published elsewhere [4]. Briefly, all study participants were offered oral PrEP at enrollment and counselled to take it daily. Those who did not initiate PrEP could start PrEP at any time up to 48 weeks during the 52 week follow-up period. The AGYW who accepted PrEP were randomized 1:1 to standard of care or enhanced adherence support. Standard of care adherence support included weekly two-way text messages during the first three months, brief counselling (months one, two, and then quarterly), and offer of participation in peer-led PrEP adherence clubs. The enhanced adherence support included PrEP adherence drug-level feedback at months two and three follow-up visits based on intracellular tenofovir-diphosphate (TFV-DP) concentration in dried blood spots (DBS) for the previous month, In addition to standard of care adherence support. The follow-up visits were at 4, 8, and 12 weeks, and then quarterly. The participants were given 30-day supplies of PrEP pill through the first 12 weeks and then increased to a 3-month supply during the follow-up visits from months three through 12.
Data Collection
The study staff collected demographic data, including age, education, and housing status, on the case report form at enrollment. A self-administered computer-assisted self-interview (CASI) questionnaire was used to obtain sensitive data, including HIV likelihood perception, sexual behavior, and depressive symptoms at enrollment and then quarterly for up to 12 months. PrEP use, disclosure of PrEP use, and PrEP reminder data were collected quarterly by CASI from participants who accepted PrEP.
Measures
PrEP Adherence
The outcome measurement was PrEP adherence at months three and six, assessed using intracellular TFV-DP concentration in DBS, which provides an objective biomarker measure of average PrEP adherence in the prior 4–6 weeks [35]. A DBS concentration of TFV-DP ≥ 700 fmol/punch is associated with taking an average of four or more doses per week, which provided 96% risk reduction among men who have sex with men (MSM), and 200 fmol/punch of TFV-DP is associated with about one dose per week among MSM [36, 37]. The limit of detection for TFV-DP concentration was 33.4 fmol/punch, and non-quantifiable values were set to half of the limit of detection (16.7 fmol/punch).
Disclosure of PrEP Use
The primary exposure disclosure of PrEP use was assessed using the question ‘Have you told anyone that you are taking PrEP?’ asked three months after starting PrEP. If the response was ‘yes’, then relationship-specific (parents, sex partners, and friends) questions were asked. The relationship types were not mutually exclusive. We assigned ‘no’ for the relationship-specific disclosure questions if the AGYW responded ‘no’ to anyone.
Support
To assess if supportive adult relationships modified the effect of disclosure of PrEP use on adherence, we used the question, ‘In general, how supported do you feel by the adults in your life?’ asked at enrollment. ‘In general, how supported do you feel by your close friends in your life?’ was used to evaluate proportion of AGYW who felt supported by close friends. The responses were on a 3-point Likert scale. We dichotomized as well-supported if the response was ‘very well supported’ and not well-supported if it were ‘almost never supported’ or ‘sometimes supported’.
PrEP Support
In this paper, we defined PrEP support as 'a reminder to take PrEP'. We used two questions that were asked three months after initiating PrEP, and they were ‘My family and friends who know I am on PrEP help me remember to take PrEP’ and ‘My household members who know I am on PrEP help me remember to take PrEP’. The responses were on a 5-point Likert scale [0 to 4] from ‘strongly disagree’, ‘disagree’, ‘neither disagree or agree, ‘agree’, to ‘strongly agree’.
HIV Likelihood Perception, Transactional Sex, Condom Use and Depression
We used ‘How would you describe your chances of getting HIV in the next year’ to evaluate HIV likelihood perception. It was dichotomized as perceived HIV likelihood next year if responses were small, moderate, great chance, and prefer not to answer. Transaction sex was defined as having sex with a man in exchange for food, clothes, cosmetics, transportation, items for children, and other items. 'Prefer not to answer' responses were categorized as condomless sex. A brief version (10-item) of the Center for Epidemiologic Studies Depression scale (CESD-10) was used to assess depressive symptoms [38, 39].
Statistical Analysis
Baseline characteristics were summarised by PrEP use disclosure status at month three. The Kruskal–Wallis test was used to test group differences for continuous variables and the Pearson Chi-squared test for categorical variables. We used linear regression to assess the association between PrEP adherence and disclosure of PrEP use to anyone, and each of the social relationships: parents, sex partners, and friends, adjusted for age (adherence increases with age) [40–43] and the three enrollment sites to adjust for social and economic differences among the sites [1]. To evaluate whether the effect of disclosure of PrEP use on adherence was modified by AGYW who had versus did not have supportive adults, we repeated the analysis separately for those who were well-supported and not well-supported.
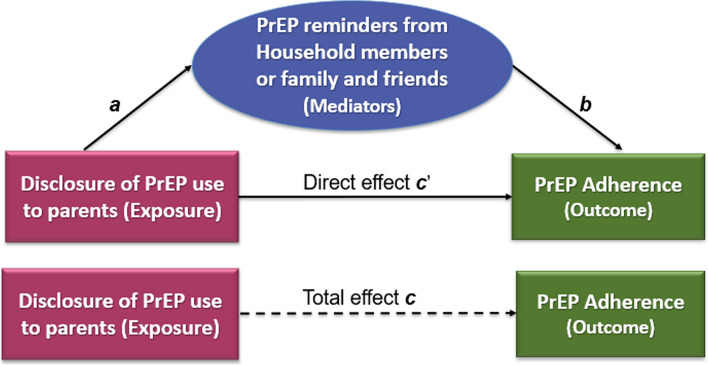
We conducted mediator analyses to assess if PrEP reminders were in the pathway between disclosure of PrEP use and adherence. The total effect (c in Fig. 1) of disclosure of PrEP use on adherence is decomposed as direct effect (c’) and indirect effect (paths a and b). The total effect equals the sum of indirect and direct effects when all three regression analyses were conducted on data with complete cases for exposure, outcome, mediator, and adjusted for covariates (age and sites). First, to assess the effect of disclosure (a) on PrEP reminders (mediator), we regressed reminder to take PrEP on disclosure of PrEP use (exposure). Next, we regressed PrEP adherence (outcome) on disclosure of PrEP use adjusted for the reminders to take PrEP. The effect of the PrEP reminder on adherence is denoted by path b. The product of the coefficients of reminder to take PrEP of the first and the second models (a*b) is the indirect effect, and the direct effect (c’) is the coefficient of the disclosure of PrEP use in the second model [44].
Fig. 1.
Diagram of mediation analysis showing the effect of disclosure of PrEP use on PrEP adherence mediated through PrEP reminders. The product (a*b) of the coefficients of reminder to take PrEP is the indirect effect, and the direct effect is the coefficient of the disclosure of PrEP use adjusted for the reminders to take PrEP
Post-Hoc Analysis: Living with Parents
Based on the results of the initial analysis and recent literature where adherence was significantly higher among AGYW ≤ 18 years old who disclosed PrEP use to their parents, we conducted a post-hoc analysis to investigate if there was differential PrEP support that contributed to higher adherence for AGYW who disclosed to their parents and also lived with them versus those who lived alone or with others. We used the question ‘With whom do you live?’, which had ‘mark all that apply’ response to identify AGYW who lived with their parents. The response choices were partner, parents(s), sibling(s), alone, with own children, roommate(s), and other. AGYW were considered living with their parents regardless of if they also marked other options. We created three levels of disclosure of PrEP use by living status: (1) disclosed to parents and lived with them; (2) disclosed to parents but did not live with them; and (3) did not disclose to parents. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Of the 427 AGYW who accepted PrEP, 371 (87%) had month three PrEP adherence biomarker (DBS TFV-DP) measures and 349 (82%) had data about their disclosure of PrEP use; 343 (80%) had complete data, and were included in the analysis. Overall, the median age was 21 years [Interquartile range (IQR): 19 to 22], 20% were ≤ 18 years of age, 52% lived with their parents, and 23% lived with their partner (Table 1). Eighty-five percent had a primary sex partner, and 50% had a primary partner living with HIV or whose HIV status was unknown. Although the characteristics were similar between those who disclosed PrEP use to someone compared to those who did not, intimate partner violence was higher among AGYW who did not disclose PrEP use to anyone than among AGYW who disclosed PrEP to at least one person (63% versus 46%, χ2 = 3.97, p = 0.05).
Table 1.
Baseline characteristics of the participants by disclosure of PrEP use to anyone
| Baseline characteristics | Overall | Disclosed to anyone by month three | ||
|---|---|---|---|---|
| N (%) or (median, IQR) | N = 349 | Yes N = 309 |
No N = 40 |
χ2 (P value) |
| Age, median (years) | 21 (19, 22) | 21 (19, 22) | 21 (20, 23) | 1.94 (0.16)a |
| Age 18 years | 69 (20%) | 63 (20%) | 6 (15%) | 0.65 (0.42) |
| Secondary school or higher | 204 (58%) | 181 (59%) | 23 (58%) | 0.02 (0.90) |
| Intimate partner violence, past year | 166 (48%) | 141 (46%) | 25 (63%) | 3.97 (0.05) |
| Perceived HIV likelihood in the next yearc | 182 (52%) | 159 (52%) | 24 (60%) | 1.00 (0.32) |
| Primary sexual partner, past 3 months | 296 (85%) | 262 (85%) | 34 (85%) | 0.01 (0.94) |
| Primary partner living with HIV or unknown HIV status | 176 (50%) | 155 (50%) | 21 (53%) | 0.08 (0.78) |
| More than one partner, past year | 44 (13%) | 41 (13%) | 3 (8%) | 0.55 (0.45)b |
| Transaction sex, last 3 monthsd | 85 (24%) | 76 (25%) | 9 (23%) | 0.08 (0.65) |
| Always used condom with vaginal sexe | 62 (18%) | 55 (18%) | 7 (18%) | 0.00 (1.00) |
| Depressive symptom CES-D ≥ 10f | 171 (49%) | 152 (49%) | 19 (48%) | 0.04 (0.84) |
| Living with parents | 180 (52%) | 159 (52%) | 21 (53%) | 0.01 (0.92) |
| Living with partner | 80 (23%) | 71 (23%) | 9 (23%) | 0.00 (0.94) |
aThe median and interquartile range (IQR) are provided for continuous variables
bFisher's exact two-sided p value
c'How would you describe your chances of getting HIV in the next year' was dichotomized as perceived HIV likelihood next year if responses were small, moderate, great chance and prefer not to answer
dTransaction sex is defined as having sex with a man in exchange for food, clothes, cosmetics, transportation, and items for children, and other items
e'Prefer not to answer' responses were categorized as condomless sex
fCenter for Epidemiologic Studies Depression scale is the sum of 10 items (CESD-10), and the range is 0 to 30. CESD-10 score ≥ 10 indicates a likelihood of depressive symptoms
Three months after starting PrEP, 89% (n = 309) of AGYW disclosed PrEP use to at least one person: 72% (n = 251) reported disclosing to their parents, 76% (265) to their sex partners, and 82% (287) to their friends (Table 2). By month six, the cumulative number of AGYW who disclosed PrEP use to anyone increased by only a percent from month three. The average TFV-DP concentration among those who disclosed to anyone, parents, sex partners, or friends were comparable. The DBS TFV-DP ranged from 431 to 441 fmol/punch at month three and 363 to 381 fmol/punch at month six. Drug concentration at month three signals PrEP uptake, and month six indicates persistent adherence. When comparing the impact of disclosure to anyone versus no one on PrEP adherence, we observed a nonsignificant decrease (− 18 fmol/punch, 95% confidence interval (CI) − 153 to 118, t = − 0.28, p = 0.80) in DBS TFV-concentrations of TFV-DP at month three and an increase of 77 fmol/punch, (95% CI − 97 to 251, t = 0.87, p = 0.38) at month six. Even though the association between disclosure to parents, sex partners, or friends and PrEP adherence was nonsignificant, AGYW who disclosed PrEP use to their parents had modestly higher adherence with an increase of TFV-DP concentration of 84 fmol/punch (95% CI − 16 to 184, t = 1.64, p = 0.10) at month three, and 96 fmol/punch (95% CI − 35 to 228, t = 1.46, p = 0.15) at month six compared to those who did not disclose.
Table 2.
Association between disclosure of PrEP use by month three and PrEP adherence at months three and six
| Disclosed PrEP use by 3 months | Increase DBS TFV-DP | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Month three adherencea | Month six adherencea | ||||||||
| N = 349 | Mean (95%CI) | fmol/punch (95% CI) | T test | P value | Mean (95%CI) | fmol/punch (95% CI) | T test | P value | |
| Anyone | 309 (89%) | 431 (387, 474) | − 18 (− 153, 114) | − 0.28 | 0.78 | 368 (307, 429) | 77 (− 97, 251) | 0.87 | 0.38 |
| Parents | 251 (72%) | 441 (401, 497) | 84 (− 16, 184) | 1.64 | 0.10 | 375 (310, 440) | 96 (− 35, 228) | 1.46 | 0.15 |
| Sexual partners | 265 (76%) | 434 (387, 482) | 8 (− 96, 113) | 1.16 | 0.88 | 381 (314,448) | 102 (− 34, 237) | 1.46 | 0.15 |
| Friends | 287 (82%) | 436 (391, 481) | 21 (− 98, 141) | 0.33 | 0.74 | 363 (300, 427) | 56 (− 102, 215) | 0.69 | 0.49 |
DBS TFV-DP tenofovir-diphosphate concentrations in dried blood spots, CI confidence interval
aAdjusted for site and age
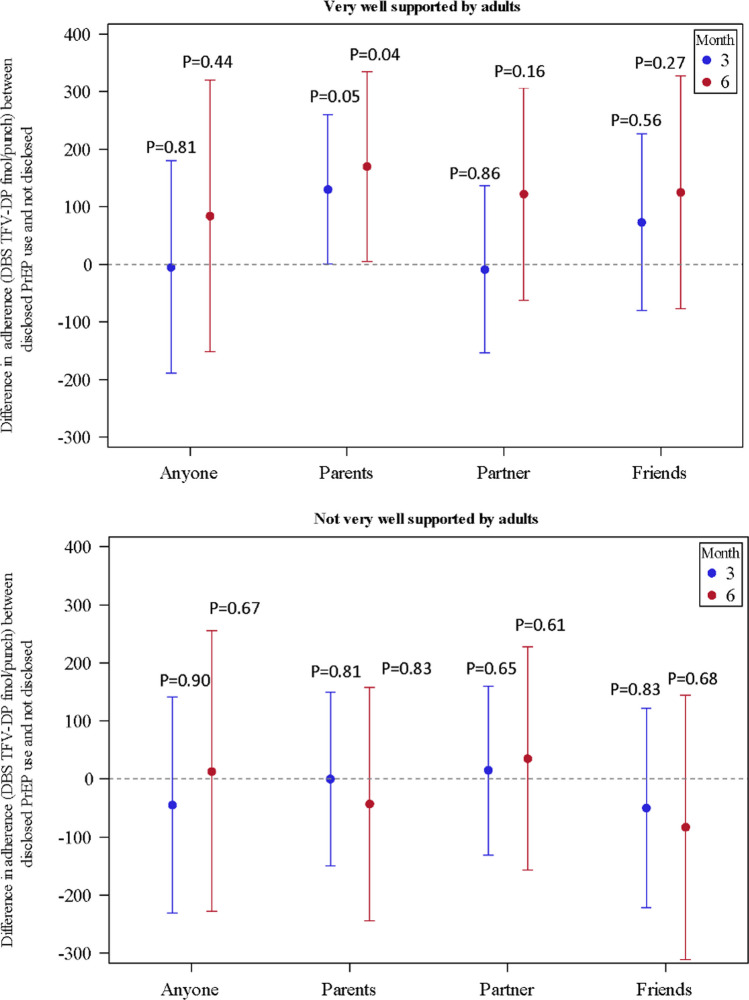
At study entry, 60% (n = 206) of the AGYW reported they felt well supported by the adults in their lives, while 42% (n = 145) felt well supported by their friends. Among the AGYW who had supportive adults, those who disclosed PrEP use to their parents had significantly higher PrEP adherence at month six with an increase of TFV-DP concentration of 177 fmol/punch (95% CI 12 to 343, t = 2.11, p = 0.04), compared to those who did not disclose to their parents (Fig. 2). In contrast, AGYW who did not have supportive adults had slightly lower adherence at month six with a decrease of TFV-DP concentration 22 fmol/punch (95% CI − 232, 187, t = − 0.21, p = 0.83) among those who disclosed to their parents compared to those who did not disclose PrEP use.
Fig. 2.
Differences in adherence between AGYW who disclosed PrEP use compared to non-disclosure and by whether AGYW reported supportive adults. Note that vertical lines represent 95% confidence levels
There was no association between disclosure of PrEP use to anyone and each of the social relationships and reminders to take PrEP by household members or family and friends, except disclosure to parents was significantly associated with an increase in the PrEP reminder score for household members (0.61, 95% CI 0.37 to 0.85, t = 4.93, p < 0.001) and family and friends (0.73, 95% CI 0.50 to 0.96, t = 6.13, p < 0.001). There was no effect of disclosure of PrEP use to parents on PrEP adherence mediated through PrEP reminders from household members or from family and friends (Table 3). The posthoc assessment showed that AGYW who disclosed PrEP use to their parents and lived with them and those who did not live with them did not differ in their PrEP adherence from those who did not disclose PrEP use to their parents, except at month six, those who disclosed to their parents and did not live with them had an increase of TFV-DP concentration of 159 fmol/punch (95% CI 6 to 312, t = 2.04, p = 0.04) compared to those who did not disclose to them (Table 4).
Table 3.
The effect of disclosure of PrEP use on PrEP adherence mediated through PrEP support among AGYW who perceived generally well supported by adults
| Mediators | Increase in DBS TFV-DP concentration | |||||
|---|---|---|---|---|---|---|
| Household members help remember | Family and Friends help remember | |||||
| fmol/punch (95% CI) | Z test | P value | fmol/punch (95% CI) | Z test | P value | |
| Month three adherencea | ||||||
| Indirect effect | 14 (− 24, 53) | 0.73 | 0.47 | 10 (− 32, 52) | 0.49 | 0.63 |
| Direct effect of disclosure | 116 (− 18, 250) | 1.70 | 0.09 | 120 (− 15, 255) | 1.74 | 0.82 |
| Total effect of disclosure to parentsb | 130 (2, 250) | 1.99 | 0.05 | 130 (2, 250) | 1.99 | 0.48 |
| Month six adherencea | ||||||
| Indirect effect | 34 (− 18, 87) | 1.29 | 0.20 | 21 (− 34, 77) | 0.75 | 0.45 |
| Direct effect of disclosure to parents | 143 (− 26, 312) | 1.66 | 0.10 | 156 (− 15, 327) | 1.79 | 0.07 |
| Total effect of disclosure to parentsb | 177 (15, 340) | 2.14 | 0.03 | 177 (15, 340) | 2.14 | 0.03 |
AGYW: Adolescent girls and young women, DBS TFV-DP tenofovir-diphosphate concentrations in dried blood spots, CI confidence interval
aAdjusted for site and age
bThe total effect (the regression coefficient without the mediator in the model) of disclosure of PrEP use on adherence is the sum of direct effect and indirect effect
Table 4.
Disclosure of PrEP use by month three and housing with parents at month three on PrEP adherence at months three and six
| Increase in DBS TFV-DP concentration | ||||||||
|---|---|---|---|---|---|---|---|---|
| Month three adherencea | Month six adherencea | |||||||
| Average (95% CI) |
fmol/punch (95% CI) | T test | P value | Average (95% CI) |
fmol/punch (95% CI) | T test | P value | |
| Disclosed to parents and live with parents (n = 84) | 431 (372, 490) | 90 (− 20, 199) | 1.60 | 0.11 | 308 (238, 379) | 50 (− 92, 193) | 0.69 | 0.49 |
| Disclosed to parents but does not live with parents (n = 143) | 472 (392, 554) | 74 (− 42, 191) | 1.26 | 0.21 | 461 (344, 578) | 159 (6, 312) | 2.04 | 0.04 |
| Did not disclose to parents (n = 108) | 397 (308, 485) | Ref | 333 (223, 442) | Ref | ||||
DBS TFV-DP tenofovir-diphosphate concentrations in dried blood spots, CI confidence interval
aAdjusted for site and age
Discussion
Most AGYW in this open-label PrEP study chose to take oral PrEP and disclosed PrEP use to at least one person, most often parents, sexual partners, and friends. Of note, an AGYW’s decision to disclose PrEP use is not straightforward as she has to weigh the risks and benefits before disclosing [25, 45]. The high disclosure rates suggest the AGYW in this study decided the benefits of PrEP disclosure outweighed the risks, and those who did not disclose may have felt that there was no need to disclose or it posed a risk [25, 45]. One of the strengths of our study was it quantitively evaluated the relationship between PrEP disclosure and adherence at two times points (month three and again at month six). We hypothesized that AGYW would have higher adherence at both months three and six with disclosure but did not find this to be the case; we found no impact of disclosure of PrEP use on adherence at either timepoints. This lack of impact was the same regardless of the relationship type. With regard to support, more than half of the AGYW in our sample reported they felt generally well-supported by adults in their life. In the context of strong general support from adults, we found disclosure of PrEP use to parents was associated with increased adherence compared to AGYW who did not disclose PrEP use to their parents. This association was not dependent on living with parents. The amount of increased adherence was equal to taking about one additional pill per week, on average [46]. Parents have a strong influence from childhood to adolescence and our study provides evidence that PrEP disclosure to parents among AGYW who perceive that adults are generally supportive improves AGYW’s health-promoting PrEP adherence behavior.
Our study findings were similar to a previous PrEP demonstration study in South Africa that found by 6 months after initiating PrEP, 58% of AGYW (ages 16 to 25 years) disclosed PrEP use to their parents, 58% disclosed to partners, and a higher percentage (81%) disclosed to their friends [40]. However, only young women ≤ 18 years of age who disclosed to their parents were 6.8 times more likely to have high adherence at 6 months compared to those who did not disclose to a parent, but the study did not assess the reasons why. Our findings suggest that this difference may be because AGYW ≤ 18 years of age who disclosed to a parent felt well-supported by the adults in their life. In addition, in a study that explored the moderating effect of family dynamics in South Africa, peer adherence support for ART adherence had a positive impact in well-functioning families and a negative impact in dysfunctional families [28].
We found disclosure of PrEP use, especially to parents’,was associated with increased reminders to take PrEP by the household members. This finding is supported by previous research of focus group discussions among AGYW in a PrEP implementation study in Kenya and South Africa, where PrEP disclosure was associated with PrEP support in the form of reminders to take PrEP during the first 3 months after starting PrEP [25]. In particular, the mother’s support was important for integrating PrEP into their daily routine. Similarly, in a PrEP demonstration project in Tanzania and South Africa, AGYW reported disclosure of PrEP use resulted, for the most part, in gaining partner support in the form of reminders to take PrEP daily, but for some, it was a negative experience with having to respond to their partner’s resistance and anger [47]. Further, AGYW tended to initially disclose to supportive maternal figures (mothers, aunts, and older sisters) to gain PrEP support (i.e., explain frequent clinic visits and the PrEP pills) and as a form of respect, consistent with regional social norms. We hypothesized that the effect of PrEP disclosure on adherence was partially mediated through reminders from those to whom they disclosed PrEP use but did not find this to be the case. Despite the positive association between disclosure to parents and reminders, there was no change in the relationship between disclosure and adherence, meaning PrEP reminders were not on the pathway between disclosure and adherence. More research is needed to better understand the relationship between disclosure of PrEP use and adherence to PrEP. Our study had a few limitations. First, questions on reminders to take PrEP were not asked separately for parents, sexual partners, or friends. The lack of specificity by relationship type may have contributed to the lack of evidence of an association between reminders to take PrEP and PrEP adherence despite substantial evidence of disclosure to parents in the context of supportive adults resulting in increased adherence. Similarly, social support questions referred to the general term ‘adults’ and not by relationship types. Consequently, it was not possible to accurately assess whether parental PrEP support (i.e., through PrEP reminders) was in the causal pathway between PrEP disclosure to the parents and adherence. Given the likely differential influence of parents, sexual partners, and friends, it will be important in future research to collect general social support, disclosure, and PrEP support data for each type of relationship (parents, sexual partners, and friends) to tease out the independent contributions by each relationship type. Further, qualitative research strongly suggests that maternal support plays a significant role in PrEP and ART adherence for adolescents [25, 26, 47]. Hence, questions should differentiate between disclosures to maternal and paternal figures and their social and PrEP support.
Conclusions
This open-label PrEP study in South Africa and Zimbabwe underscored that when African AGYW have supportive adults in their lives, disclosure to parents leads to increased PrEP adherence with AGYW taking about one pill more a week, on average compared to those who did not disclose. PrEP interventions that help identify supportive relationships and effectively communicate PrEP use to the parents or guardians, partners, and friends may help normalize PrEP use and improve adherence by African AGYW, and therefore PrEP effectiveness in this important population. Future research is needed to understand the pathways between a supportive environment, disclosure to specific relationships, and PrEP support by specific types of relationships to improve PrEP adherence.
Acknowledgements
The authors thank the individuals who participated in the study, the teams at the Johannesburg, South Africa, Cape Town, South Africa, and Harare, Zimbabwe study sites, and the HIV Prevention Trials Network that supported data collection and management for this work.
Author Contributions
CC, SDM, SH, LGB, DD, PLA, and NM designed and implementation of the parent study. CC and SH were involved in study design and edited the manuscript. GB conducted all analyses, results interpretation and wrote the first draft of the manuscript. DD and KCGC were involved in the study design, statistical oversight, interpretation of the results, and edited the manuscript. BJD was involved in the protocol development and edited the manuscript. PLA was involved in pharmacology oversight and interpretation of the results. All authors reviewed previous versions of the manuscript and approved the final version of the manuscript.
Funding
None.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The HPTN 082 study was supported by award numbers UM1AI068619, UM1AI068613, UM1AI1068617 from the NIH (National Institute of Allergy and Infectious Diseases [NIAID]) to HPTN. This manuscript work was funded by UM1AI1068617. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
Ethical Approval
The HPTN 082 parent study received institutional review boards (IRB) approval from each study site: University of Cape Town Faculty of Health Sciences (reference number: 129/2016), University of Witwatersrand, Human Research Ethics Committee (reference number: 160304), and University of Zimbabwe Joint Research Ethics Committee (reference Number: 27/16). All participants provided written informed consent in English or their local language. Following local regulations, participants below the legal age for consent provided assent, and parent or guardian informed consent was obtained.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.UNAIDS. Joint United Nations Programme on HIV/AIDS. UNAIDS data 2020. Geneva, Switzerland. UNAIDS. 2020;436. https://www.unaids.org/sites/default/files/media_asset/2020_aids-data-book_en.pdf
- 2.Myers L, Scientist B, Town C. Rethinking PrEP for adolescent girls and young women. IAPAC. 2019;(June).
- 3.Gill K, Pidwell T, Dietrich J, Gray G, Bennie T, Kayamba F, et al. A Demonstration open label study to assess the acceptability, safety and use of Truvada pre-exposure prophylaxis in healthy, HIV uninfected adolescents, 15–19 years of age. In: International AIDS society conference (IAS) on HIV science. Paris, France; 2017. https://mtnstopshiv.org/sites/default/files/attachments/KATHERINE-IAS2017MTNplenaryfinalKatherine.pdf
- 4.Celum C, Hosek S, Tsholwana M, Kassim S, Mukaka S, Dye IDBJ, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: results from HPTN 082, a randomized controlled trial. PLoS Med. 2021;18(6):e1003670. doi: 10.1371/journal.pmed.1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celum CL, Gill K, Morton JF, Stein G, Myers L, Thomas KK, et al. Incentives conditioned on tenofovir levels to support PrEP adherence among young South African women: a randomized trial. J Int AIDS Soc. 2020;23(11):e25636. doi: 10.1002/jia2.25636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gill K, Johnson L, Dietrich J, Myer L, Marcus R, Wallace M, et al. Acceptability, safety, and patterns of use of oral tenofovir disoproxil fumarate and emtricitabine for HIV pre-exposure prophylaxis in South African adolescents: an open-label single-arm phase 2 trial. Lancet Child Adolesc Heal. 2020;4(12):875–883. doi: 10.1016/S2352-4642(20)30248-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Velloza J, Khoza N, Scorgie F, Chitukuta M, Mutero P, Mutiti K, et al. The influence of HIV-related stigma on PrEP disclosure and adherence among adolescent girls and young women in HPTN 082: a qualitative study. J Int AIDS Soc. 2020 doi: 10.1002/jia2.25463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery ET, Mensch B, Musara P, Hartmann M, Woeber K, Etima J, et al. Misreporting of product adherence in the MTN-003/VOICE trial for HIV prevention in Africa: participants’ explanations for dishonesty. AIDS Behav. 2017;21(2):481–491. doi: 10.1007/s10461-016-1609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amico KR, Wallace M, Bekker L-G, Roux S, Atujuna M, Sebastian E, et al. Experiences with HPTN 067/ADAPT study-provided open-label PrEP among women in Cape Town: facilitators and barriers within a mutuality framework. AIDS Behav. 2017;21(5):1361–1375. doi: 10.1007/s10461-016-1458-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celum C, Baeten J. PrEP for HIV prevention: evidence, global scale-up, and emerging options. Cell Host Microbe. 2020;27(4):502–506. doi: 10.1016/j.chom.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Patel RC, Stanford-Moore G, Odoyo J, Pyra M, Wakhungu I, Anand K, et al. “Since both of us are using antiretrovirals, we have been supportive to each other”: facilitators and barriers of pre-exposure prophylaxis use in heterosexual HIV serodiscordant couples in Kisumu, Kenya: facilitators. J Int AIDS Soc. 2016;19(1):21134. doi: 10.7448/IAS.19.1.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabral A, Baeten MJ, Ngure K, Velloza J, Odoyo J, Haberer EJ, et al. Intimate partner violence and self-reported pre-exposure prophylaxis interruptions among HIV-negative partners in HIV serodiscordant couples in Kenya and Uganda. J Acquir Immune Defic Syndr. 2018;77(2):154–159. doi: 10.1097/QAI.0000000000001574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts ST, Haberer J, Celum C, Mugo N, Ware NC, Cohen CR, et al. Intimate partner violence and adherence to HIV pre-exposure prophylaxis (PrEP) in African women in HIV serodiscordant relationships: a prospective cohort study. J Acquir Immune Defic Syndr. 2016;73(3):313–322. doi: 10.1097/QAI.0000000000001093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Palanee-Phillips T, Roberts ST, Reddy K, Govender V, Naidoo L, Siva S, et al. Impact of partner-related social harms on women’s adherence to the dapivirine vaginal ring during a phase III trial. J Acquir Immune Defic Syndr. 2018;79(5):580–589. doi: 10.1097/QAI.0000000000001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.4th HIV Research for Prevention conference (HIVR4P//Virtual). J Int AIDS Soc. 10.1002/jia2.25659/full [DOI] [PMC free article] [PubMed]
- 16.Irungu E, Khoza N, Velloza J. Multi-level interventions to promote oral pre-exposure prophylaxis use among adolescent girls and young women: a review of recent research. Curr HIV/AIDS Rep. 2021;18(6):490. doi: 10.1007/s11904-021-00576-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettifor AE, Van Der Straten A, Dunbar MS, Shiboski SC, Padian NS. Early age of first sex: a risk factor for HIV infection among women in Zimbabwe. AIDS 2004;18(10):1435–42. https://journals.lww.com/aidsonline/Fulltext/2004/07020/Early_age_of_first_sex__a_risk_factor_for_HIV.10.aspx [DOI] [PubMed]
- 18.Pettifor A, O’Brien K, MacPhail C, Miller WC, Rees H. Early coital debut and associated HIV risk factors among young women and men in South Africa. Int Perspect Sex Reprod Health 2009;35. https://www.guttmacher.org/journals/ipsrh/2009/early-coital-debut-and-associated-hiv-risk-factors-among-young-women-and [DOI] [PubMed]
- 19.Balkus JE, Brown E, Palanee T, Nair G, Gafoor Z, Zhang J, et al. An empiric HIV risk scoring tool to predict HIV-1 acquisition in African women. Epidemiology and prevention. 2016. www.jaids.com [DOI] [PMC free article] [PubMed]
- 20.Hallett TB, Gregson S, Lewis JJC, Lopman BA, Garnett GP. Behaviour change in generalised HIV epidemics: impact of reducing cross-generational sex and delaying age at sexual debut. Sex Transm Infect. 2007;83(suppl 1):i50–4. https://sti.bmj.com/content/83/suppl_1/i50 [DOI] [PubMed]
- 21.Reif LK, Abrams EJ, Arpadi S, Elul B, McNairy ML, Fitzgerald DW, et al. Interventions to improve antiretroviral therapy adherence among adolescents and youth in low- and middle-income countries: a systematic review 2015–2019. AIDS and behavior. Springer; 2020;1–14. [DOI] [PMC free article] [PubMed]
- 22.Lanham M, Wilcher R, Montgomery ET, Pool R, Schuler S, Lenzi R, et al. Engaging male partners in women’s microbicide use: evidence from clinical trials and implications for future research and microbicide introduction. 2014;10.7448/IAS.17.3.19159 [DOI] [PMC free article] [PubMed]
- 23.Giovenco D, Gill K, Fynn L, Duyver M, O’Rourke S, van der Straten A, et al. Experiences of oral pre-exposure prophylaxis (PrEP) use disclosure among South African adolescent girls and young women and its perceived impact on adherence. PLoS ONE. 2021 doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Straten A, Stadler J, Luecke E, Laborde N, Hartmann M, Montgomery ET. Perspectives on use of oral and vaginal antiretrovirals for HIV prevention: the VOICE-C qualitative study in Johannesburg, South Africa. J Int AIDS Soc. 2014 doi: 10.7448/IAS.17.3.19146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rousseau E, Katz AWK, O’Rourke S, Bekker LG, Delany-Moretlwe S, Bukusi E, et al. Adolescent girls and young women’s PrEP-user journey during an implementation science study in South Africa and Kenya. PLoS ONE. 2021 doi: 10.1371/journal.pone.0258542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meagley K, Schriver B, Geary RS, Fielding-Miller R, Stein AD, Dunkle KL, et al. The gender dimensions of social networks and help-seeking behaviors of young adults in Soweto, South Africa. 10.3402/gha.v9.31138 [DOI] [PMC free article] [PubMed]
- 27.Camlin CS, Koss CA, Getahun M, Owino L, Itiakorit H, Akatukwasa C, et al. Understanding demand for PrEP and early experiences of PrEP use among young adults in rural Kenya and Uganda: a qualitative study. AIDS Behav. 2020;24(7):2149–2162. doi: 10.1007/s10461-020-02780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wouters E, Masquillier C, Ponnet K, le Roux BF. A peer adherence support intervention to improve the antiretroviral treatment outcomes of HIV patients in South Africa: the moderating role of family dynamics. Soc Sci Med. 2014;1(113):145–153. doi: 10.1016/j.socscimed.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 29.Patel RC, Stanford-Moore G, Odoyo J, Pyra M, Wakhungu I, Anand K, et al. “Since both of us are using antiretrovirals, we have been supportive to each other”: Facilitators and barriers of pre-exposure prophylaxis use in heterosexual HIV serodiscordant couples in Kisumu, Kenya: Facilitators. J Int AIDS Soc. 2016 doi: 10.7448/IAS.19.1.21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ajayi AI, Mudefi E, Yusuf MS, Adeniyi OV, Rala N, Goon D Ter. Low awareness and use of pre-exposure prophylaxis among adolescents and young adults in high HIV and sexual violence prevalence settings. Med (United States). 2019;98(43). https://journals.lww.com/md-journal/Fulltext/2019/10250/Low_awareness_and_use_of_pre_exposure_prophylaxis.72.aspx [DOI] [PMC free article] [PubMed]
- 31.Fisher JD, Fisher WA. Changing AIDS-risk behavior. Psychol Bull. 1992;I(3):455–474. doi: 10.1037/0033-2909.111.3.455. [DOI] [PubMed] [Google Scholar]
- 32.Dubov A, Altice FL, Fraenkel L. An information-motivation-behavioral skills model of PrEP uptake. AIDS Behav. 2018;22:3603–3616. doi: 10.1007/s10461-018-2095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kilanowski JF. Breadth of the socio-ecological model. J Agromedicine. 2017;22(4):295–297. doi: 10.1080/1059924X.2017.1358971. [DOI] [PubMed] [Google Scholar]
- 34.Rivet AK. A situated-information motivation behavioral skills model of care initiation and maintenance (sIMB-CIM): an IMB model based approach to understanding and intervening in engagement in care for chronic medical conditions. J Health Psychol. 2011;16(7):1071–1081. doi: 10.1177/1359105311398727. [DOI] [PubMed] [Google Scholar]
- 35.Anderson PL, Liu AY, Castillo-mancilla JR, Gardner EM, Seifert SM, Mchugh C, et al. Intracellular Tenofovir-diphosphate and emtricitabine-triphosphate in dried blood spots following directly observed therapy: the DOT-DBS study. Antimicrob Agents Chemother. 2018;62(1):1–13. doi: 10.1128/AAC.01710-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson PL, Glidden DV, Liu A, Buchbinder S, Lama JR, Guanira JV, et al. Emtricitabine-tenofovir concentrations and pre-exposure prophylaxis efficacy in men who have sex with men. Sci Transl Med. 2012 doi: 10.1126/scitranslmed.3004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grant RM, Anderson PL, McMahan V, Liu A, Amico KR, Mehrotra M, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. doi: 10.1016/S1473-3099(14)70847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baron EC, Davies T, Lund C. Validation of the 10-item centre for epidemiological studies depression scale (CES-D-10) in Zulu, Xhosa and Afrikaans populations in South Africa. BMC Psychiatry. 2017;17(1):1–14. doi: 10.1186/s12888-016-1178-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Velloza J, Hosek S, Donnell D, Anderson PL, Chirenje M, Mgodi N, et al. Assessing longitudinal patterns of depressive symptoms and the influence of symptom trajectories on HIV pre-exposure prophylaxis adherence among adolescent girls in the HPTN 082 randomized controlled trial. J Int AIDS Soc. 2021;24(S2):e25731. doi: 10.1002/jia2.25731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Giovenco D, Pettifor A, Powers KA, et al. The effect of PrEP use disclosure on adherence in a cohort of adolescent girls and young women in South Africa. AIDS Behav. 2022;26:1007–1016. doi: 10.1007/s10461-021-03455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montgomery ET, Van Der Straten A, Chidanyika A, Chipato T, Jaffar S, Padian N. The importance of male partner involvement for women’s acceptability and adherence to female-initiated HIV prevention methods in Zimbabwe. AIDS Behav. 2011;15(5):959–969. doi: 10.1007/s10461-010-9806-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musinguzi N, Kidoguchi L, Mugo NR, Ngure K, Katabira E, Celum CL, et al. Adherence to recommendations for ART and targeted PrEP use among HIV serodiscordant couples in East Africa: the “PrEP as a bridge to ART” strategy. BMC Public Health. 2020;20(1):1621. doi: 10.1186/s12889-020-09712-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pintye J, Kinuthia J, Larsen A, Abuna F, Ochieng B, Ngumbau N, Dettinger J, Gomez L, Haberer JE, Grace John-Stewart JB. PrEP use persistence among Kenyan women who initiated PrEP during pregnancy. CROI. 2022. https://www.croiconference.org/wp-content/uploads/sites/2/posters/2022/CROI2022_Poster_845.pdf
- 44.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037/0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 45.Scorgie F, Khoza N, Baron D, Lees S, Harvey S, Ramskin L, et al. Disclosure of PrEP use by young women in South Africa and Tanzania: qualitative findings from a demonstration project. Culture Health Sex. 2020;23(2):257–272. doi: 10.1080/13691058.2019.1703041. [DOI] [PubMed] [Google Scholar]
- 46.Castillo-Mancilla JR, Zheng J-H, Rower JE, Meditz A, Gardner EM, Predhomme J, et al. Tenofovir, emtricitabine, and tenofovir diphosphate in dried blood spots for determining recent and cumulative drug exposure. AIDS Res Hum Retroviruses. 2012;29(2):384–390. doi: 10.1089/aid.2012.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scorgie F, Khoza N, Baron D, Lees S, Harvey S, Ramskin L, et al. Culture, Health & Sexuality An International Journal for Research, Intervention and Care Disclosure of PrEP use by young women in South Africa and Tanzania: qualitative findings from a demonstration project. Cult Heal Sex An Int J Res Interv Care. 2020;(ISSN: 1369-1058):1369-1058 (Print) 1464-5351 (Online). 10.1080/13691058.2019.1703041 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.