Abstract
Multidrug-resistant (MDR) pathogens are a rising global health worry that imposes an urgent need for the discovery of novel antibiotics particularly those of natural origin. In this context, we aimed to use the metagenomic nanopore sequence analysis of soil microbiota coupled with the conventional phenotypic screening and genomic analysis for identifying the antimicrobial metabolites produced by promising soil isolate(s). In this study, whole metagenome analysis of the soil sample(s) was performed using MinION™ (Oxford Nanopore Technologies). Aligning and analysis of sequences for probable secondary metabolite gene clusters were extracted and analyzed using the antiSMASH version 2 and DeepBGC. Results of the metagenomic analysis showed the most abundant taxa were Bifidobacterium, Burkholderia, and Nocardiaceae (99.21%, followed by Sphingomonadaceae (82.03%) and B. haynesii (34%). Phenotypic screening of the respective soil samples has resulted in a promising Bacillus isolate that exhibited broad-spectrum antibacterial activities against various MDR pathogens. It was identified using microscopical, cultural, and molecular methods as Bacillus (B.) haynesii isolate MZ922052. The secondary metabolite gene analysis revealed the conservation of seven biosynthetic gene clusters of antibacterial metabolites namely, siderophore lichenicidin VK21-A1/A2 (95% identity), lichenysin (100%), fengycin (53%), terpenes (100%), bacteriocin (100%), Lasso peptide (95%) and bacillibactin (53%). In conclusion, metagenomic nanopore sequence analysis of soil samples coupled with conventional screening helped identify B. haynesii isolate MZ922052 harboring seven biosynthetic gene clusters of promising antimicrobial metabolites. This is the first report for identifying the bacteriocin, lichenysin, and fengycin biosynthetic gene clusters in B. haynesii MZ922052.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13568-024-01701-8.
Keywords: Bacillus haynesii, Metagenomic, Soil, Nanopore sequencing, Multidrug-resistant
Key points
Metagenomic analysis of soil samples coupled with conventional screening is of help to explore the nature of various antimicrobial metabolites.
This study resulted in the isolation of a Bacillus haynesii isolate MZ922052 that exhibited broad-spectrum antibacterial activities against various MDR pathogens.
Secondary metabolite gene analysis revealed the conservation of biosynthetic gene clusters of siderophore lichenicidin VK21-A1/A2, lichenysin, fengycin, terpenes, bacteriocin, Lasso peptide and bacillibactin.
This is the first report for identifying the biosynthetic gene clusters of bacteriocin, lichenysin, and fengycin biosynthetic gene clusters in B. haynesii isolate, MZ922052.
Introduction
Antimicrobial resistance (AMR) is now a worldwide health crisis where antibiotics are increasingly reaching a point where they can no longer effectively treat infections (Hu et al. 2020). Nowadays, there is a decrease in pharmaceutical industry investment in discovering novel antibiotics, triggering, the threat of antibiotic resistance (Chinemerem Nwobodo et al. 2022). There will always be an urgent need for novel antibiotics. Soil harbors many microorganisms that are good sources of antimicrobials and antibiotics. These are shown to be a hopeful source for novel antimicrobials (Polianciuc et al. 2020). Many new antimicrobials have been extracted and fully distinguished from soil bacteria and other diverse natural habitats (Hallaj-Nezhadi et al. 2022). Natural soils are known for their biodiversity and are the leading supplier of possible novel antibiotics (Amin et al. 2015).
Bacillus is a genus characterized by its heterogeneity of bacteria with species producing tremendous antimicrobial metabolites that cure various microbial infections. Bacillus sp. are the most considerable bacterial strains found on earth. They are Gram-positive and endospore-forming bacteria (Rampersad and Ammons 2005; Hallaj-Nezhadi et al. 2022; Vehapi et al. 2023; Caulier et al. 2019). Many studies have been conducted to extract antimicrobial compounds from different strains of Bacillus and characterize these antimicrobials (Berić et al. 2014;Vehapi et al. 2023;.Caulier et al. 2019).
Bacillus genera have many heterogeneous species that produce antimicrobial compounds. Most members of this genus are antibiotic producers. These antibiotics are mostly low-molecular-weight peptides that exhibit antitumor, antibacterial, and antiviral activities Caulier et al. 2019). The antibiotic bacitracin has been known to be synthesized by B. licheniformis and B. subtilis which is known for its efficacy against Gram-positive bacteria (Johnson et al. 1945; Haavik and Froyshov 1975). One of the most important species is B. licheniforms (Saggese et al. 2022). Antimicrobial metabolites that are produced and extracted from Bacillus sp. which are inhabitants of the natural environment, such as soil, provide a substantial role in preventing and curing microbial diseases and are shown to be a leading source of novel antimicrobials (Polianciuc et al. 2020).
Bioinformatics has been a valuable tool for mining genes that produce antimicrobials from soil bacteria (Baltz 2021). Bacterial genome mining is a bioinformatic way to discover the biosynthesis of antimicrobial genes in the genome of bacteria. Computational algorithms approach genome mining to analyze secondary metabolite gene clusters (Baltz 2021). Therefore, it is important to apply metagenomic analysis to secondary metabolite gene clusters for the discovery of novel antimicrobials. Previous studies conducted in our lab where metagenomic nanopore sequencing has been undertaken to determine the biosynthetic gene clusters involved in the biosynthesis of certain functioning metabolites of Alcaligenes faecalis and Paenibacillus ehimensis soil isolates (Eltokhy et al. 2021a, b). Such analysis was coupled with conventional screening and advanced spectroscopic analysis to identify the nature and chemistry of the respective metabolites (Eltokhy et al. 2021a, b). It was found that combining these techniques was found to be helpful and accurate in rapidly identifying the various active metabolites produced by the respective soil isolates (Eltokhy et al. 2021a, b). Therefore, this study aimed to use metagenomic analysis of the soil samples in combination with the conventional phenotypic screening to identify the promising antimicrobial-producing soil isolate(s) and to explore the nature of potential secondary metabolites produced by the respective soil isolate(s).
Materials and methods
Whole metagenome analysis of the soil sample
DNA extraction and quantification
DNA extraction was done by Qiagen DNeasy power-soil kit (Qiagen, Hilden, Germany) according to the producer’s recommendations. DNA concentration was determined by Qubit fluorometer ver. 4.0 (Thermo Fisher Scientific, Waltham, Massachusetts, USA) to ensure there is not less than 55 ng/µL of DNA, as stated in Oxford nanopore Standard operating procedure (Eltokhy et al. 2021a).
Library construction
To 12 µL DNA, 34 µL sequencing buffer, 25.5 µL of loading beads, and 4.5 µL nuclease-free water were included and mixed. Construction of the library was done by a Rapid Sequencing Kit (Oxford Nanopore Technologies, Oxford, UK). Priming and loading onto the FLO-MIN106 (Nanopore Technology, Oxford, England) flow cell were performed after library construction (Eltokhy et al. 2021a).
Sequencing and data analysis
MinION™ (Oxford Nanopore Technologies, Oxford, UK) was applied for running sequences. Twelve hours generate 3.03 M reads with N50 equals 9.29 K. Real-time base calling during sequencing was generated by the Guppy software. The output was in the form of FAST5 and FASTq files, reads below Q7 were excluded. Classification of sequences to taxonomic identifiers was generated by Centrifuge software (Kim et al. 2016)(Kim et al. 2016). Bacterial and viral genomes, as well as human reference genome (GRCh38) downloaded from the National Center of Biotechnology Information (NCBI) RefSeq were used for the construction of the Centrifuge index. Dust masker (v1.0.0, NCBI) was applied for masking low-complexity regions with a dust score greater than 20 in the reference sequences. Re-centrifuged was applied for visualization of results (Martí 2019).
Extraction of secondary metabolites from genome sequences
Aligning and analysis of sequences for probable secondary metabolite gene clusters were extracted by antiSMASH version 2 (Antibiotics and Secondary Metabolite Analysis Shell) (https://antismash.secondarymetabolites.org/#!/start (accessed on 10 December 2023). The genomic sequence was further assembled and analyzed using deepBGC (https://github.com/Merck/deepbgc (accessed on 17 March 2024) (Hannigan et al. 2019). Draft genome comparison was done by applying Mauve software (https://gel.ahabs.wisc.edu/mauve) (accessed on 12 December 2023) (Kapley et al. 2016).
Cheminformatic analysis of the detected secondary metabolites
Cheminformatic analysis of the detected secondary metabolites including the 2D structure and molecular weight analysis was evaluated using PubChem 2.1 database (https://pubchem.ncbi.nlm.nih.gov/ (accessed on 10 January 2024) as previously reported (Kim et al. 2023).
Bacterial isolation and antimicrobial screening
A Bacillus isolate coded SS10 was isolated from the tested soil as previously described (Eltokhy et al. 2021a). Briefly, the collected soil sample was placed in a hot air oven and heated at 80°C for one hour Ince E 2008). About 9 mL saline tube was inoculated with 1 gm soil, and then vortexed for 4 min at 400 rpm Rampersad and Ammons 2005) followed by a 10-fold serial dilution was done within the range of 10− 1 to 10− 6. One mL of each dilution was transferred to the surface of Starch Casein Agar (SCA) and incubated for 7 days (Ranjan and Jadeja 2017). Different colonies were picked from the SCA, and a preliminary screening was performed as previously reported (Eltokhy et al. 2021a, b).
A pure bacterial isolate was screened for antimicrobial activity by agar well diffusion method against three standard strains of E. coli ATCC 25,922, S. aureus ATCC 25,293, and C. albicans ATCC 10,231. The test was also performed against clinical isolates including, three vancomycin-resistance S. aureus (VRSA1, VRSA2, and VRSA3), Staphylococcus (S.) epidermidis (SE1, SE2, SE3), three MDR K. pneumoniae (KP1, KP2, and KP3), two MDR E. coli (EC1 and EC2), Candida albicans, (CA1, CA2) and Candida auris (CS1, CS2). These clinical isolates were provided by the Central Microbiology Lab of Ain Shams Hospital, Cairo, Egypt of anonymous discharged patient samples. The clinical isolates were isolated in the hospital lab for routine checkups of culture and sensitivity. The Faculty of Pharmacy Ain Shams University Ethics Committee Number, ACUC-FP-ASU -REC# 75 approved the study.
The antibiogram of the clinical bacterial isolates was evaluated using the Kirby-Bauer method, against various antibiotic discs (ThermoScientific™ and Oxoid™, MA, USA) according to Clinical Laboratory Standard Institute (CLSI) guidelines 2021 (CLSI 2021). The vancomycin susceptibility test was evaluated using the agar dilution method (resistant isolates, MIC ≥ 16 µg/mL for S. aureus and ≥ 32 µg/mL for other staphylococci) according to CLSI guidelines. Their antibiogram showed that the SE, SE2, and SE3 isolates of SE were resistant to clindamycin, gentamicin, cefoxitin, and ciprofloxacin. The three VRSA isolates were resistant to vancomycin and cefoxitin. The VRSA2 and VRSA3 were resistant to clindamycin, gentamicin, and ciprofloxacin. KP1, KP2, and KP3 were resistant to most of the tested antibiotics according to CLSI guidelines. EC1 was resistant to cefotaxime and imipenem only, while EC2 was resistant to most tested antibiotics. The standard strains, E. coli ATCC® 25,922 and S. aureus ATCC® 25,923 were employed for quality control (CLSI 2021).
Identification of the isolated soil bacteria
The soil bacterial isolate was identified by biochemical reactions and DNA sequencing of 16 S ribosomal RNA. Sequencing and analysis of data for the isolate was done by Sigma Scientific Services Co., Egypt through GATC Biotech Co., Germany. The assembled contig of the 16 S ribosomal RNA was blasted and aligned by BLAST, https://blast.ncbi.nlm.nih.gov/Blast.cgi (accessed on 18 December 2023). The percentage homology between the sequence database and query sequence was provided and determined. The phylogenetic tree was constructed using Log-Expectation through Multiple Sequence Comparisons (MUSCLE, https://www.ebi.ac.uk/Tools/msa/muscle) (accessed on 18 December 2023) (Edgar 2004). Bootstrap analysis (1000 replicates) was applied for inferring phylogenetic trees. The 16 S ribosomal RNA sequence of the selected isolate was deposited in the NCBI GenBank (https://www.ncbi.nlm.nih.gov/).
Deposition of Bacillus isolate SS10 a local culture collection
The molecularly identified Bacillus isolate SS10 was deposited in the Culture Collection Ain Shams University (CCASU), Cairo, Egypt as Bacillus isolate CCASU-SS10-32 (http://ccinfo.wdcm.org/collection/by_id/1186 (accessed on 30 December 2023).
Shake flasks method for production of antimicrobial metabolite(s)
Preparation of the seed culture
Preparation of the seed culture was done as previously mentioned by(Eltokhy et al. 2021a), by transferring three loopfuls of 24 h bacterial culture into starch casein broth (50 mL) and incubated for 24 h at 35°C in a shaking incubator adjusted at 200 rpm. Centrifugation of 1 mL of the culture for 5 min at 16,000 rpm was applied using a microcentrifuge. The sedimented cells were washed twice with 1 mL sterile saline and inoculated in 20 flasks each containing 100 mL of casein starch broth. Incubation of the 20 flasks in a shaking incubator, adjusted at 150 rpm, for 7–10 days at 35°C (Ranjan and Jadeja 2017)
Extraction process
Sequential extraction of cell-free culture medium is carried out by using ethyl acetate and dichloromethane (1:1). Equal volumes of ethyl acetate and cell-free culture medium were added in a separating funnel and shaken thoroughly for 2 h for 10 min of intervals. The separating funnel was left overnight, and the ethyl acetate upper layer was separated and stored at 4°C. The same procedure was repeated for dichloromethane using the culture medium left after extraction with ethyl acetate Ranjan and Jadeja 2017). Ethyl acetate and dichloromethane extracts were dried at 45°C by a rotary evaporator (Buchi R205, Flawil, Switzerland) (Selvin et al. 2009; Ali et al. 2019; Rajaram et al. 2020). The residues left after evaporation were dissolved in 1 mL dimethylsulfoxide (DMSO) (Valan Arasu et al. 2009)and the agar well diffusion method was applied for the examination of the antimicrobial activities of the extracts.
Evaluation of the antimicrobial activities
The residues left after evaporation of either ethyl acetate or dichloromethane extracts were dissolved in 1 mL DMSO (100%). In addition, 0.5 mL of DMSO-residue extract (100%) was taken and diluted with 0.5 mL DMSO to obtain 50% DMSO-residue extract, and both were tested for antimicrobial activity by well agar diffusion where pure DMSO, ethyl acetate and dichloromethane were used as negative controls (Rajaram et al. 2020).
Results
Metagenomics of mud soil from a garden at Luxor, Egypt
The DNA sample was quantified by Qubit fluorometer to ensure it passes the cutoff value of 150% concentration of DNA material and OD 260 nm/280 nm ratio between 1.8 and 2.0 in the sample, as mentioned by the Oxford nanopore manual. A range between 500 and 1080 reads in the sample was obtained. The length of sequences ranged between 250 and 12,000 bp. No duplicate reads were observed or N count (ambiguous). Good quality FastQ files showed a score range between 10 and < 25 per base Phred. The percentage abundance of the taxa showed that the most abundant taxa were Bifidobacterium, Burkholderia and Nocardiaceae (99.21%). Sphingomonadaceae was second showing 82.03%. B. haynesii showed about 34% (Fig. S1). The metagenomics sequences were deposited in the NCBI GenBank sequence Archives under accession number PRJNA1064698 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1064698 (accessed on 16 December 2024).
Antimicrobial preliminary screening
Preliminary screening of the Bacillus isolate SS10 showed positive inhibition of the growth of all the tested bacterial clinical isolates (SE1, SE2, SE2, VRSA1. VRSA2, VRSA3, KP1, KP2, KP3, EC1, EC2 and only one C. albicans isolate CA1 (Table 1). The agar diffusion for evaluating the antimicrobial activities of ethyl acetate extract of B. haynesii isolate MZ922052 against MDR K. pneumoniae clinical isolate (KP1), C. albicans clinical isolate (CA1) and the dichloromethane extract against vancomycin resistant Staphylococcus aureus (VRSA1) and MDR Escherichia coli (EC1) using DMSO, ethyl acetate and dichloromethane as negative controls is depicted in Fig. S2.
Table 1.
The recorded inhibition zones of dichloromethane or ethyl acetate extracts of B. haynesii isolate MZ922052 against various clinical and standard bacterial isolates
| Mean zone of inhibition (mm) ± SD | ||
|---|---|---|
| Tested Microorganisms | Dichloromethane extract | Ethyl acetate extract |
| Clinical isolates | ||
| SE1, SE2, SE3 | 11 ± 0.5 | 16 ± 1.0 |
| VRSA1, VRSA2, VRSA3 | 12 ± 0.5 | 14 ± 1.0 |
| MDR EC1 | 11 ± 0.5 | 11 ± 0.5 |
| MDR EC2 | 12 ± 1.0 | 12 ± 1.0 |
| MDR KP1 | 12 ± 1.0 | 13 ± 0.5 |
| MDR KP2 | 11 ± 1.0 | 14 ± 1.0 |
| MDR KP3 | 11 ± 1.0 | 14 ± 1.0 |
| CA1 | 12 ± 0.5 | 11 ± 0.5 |
| Standard strains | ||
| C. albicans ATCC 10,231 | 12 ± 0.5 | 16 ± 1.0 |
| S. aureus ATCC 25,293 | 11 ± 1.0 | 13 ± 0.5 |
| E. coli ATCC 25,922 | 12 ± 1.0 | 14 ± 0.5 |
MDR, multidrug-resistant; VRSA, vancomycin resistant Staphylococcus aureus; EC, Escherichia coli, KP, Klebsiella pneumoniae, CA, Candida albicans.SE, Streptococcus epidermidis; SD, standard deviation
Identification of Bacillus isolate SS10
Based on microscopical, cultural and 16 S ribosomal DNA sequence alignment, the Bacillus isolate SS10 was identified as B. haynesii isolate MZ922052. The phylogenetic analysis of the B. haynesii isolate MZ922052 (Query) is displayed in Fig. S2. The 16 S ribosomal RNA gene sequence was deposited in the NCBI GenBank database under nucleotide accession number MZ922052.
Antimicrobial evaluation of the extracted metabolite(s)
The ethyl acetate was the optimum solvent for extraction as the mean zone of inhibition of the tested bacteria ranged from 11 to 16 mm ± 1.0 mm, while dichloromethane extract showed weaker inhibition zones (around 11 mm) as presented in Table 1. The antimicrobial activities of either dichloromethane or ethyl acetate extracts of B. haynesii isolate MZ922052 in terms of inhibition zones are displayed in Table 1.
Characterization of the antimicrobial metabolite
Identification of the biosynthetic gene clusters using the antiSMASH
Secondary metabolite gene analysis of B. haynesii isolate MZ922052 revealed the presence of the biosynthetic gene clusters of seven secondary metabolites as follows:
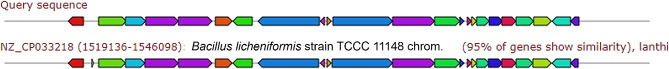
Lantibiotics: belong to a class of polycyclic peptide antibiotics. They are characterized by the presence of methyllanthionine or thioether amino acid lanthionine, as well as 2-aminoisobutyric acid and the unsaturated amino acid dehydroalanine. They are ribosomal synthesized and post-translationally modified peptides. (Gene cluster 100% similar to gene cluster producing the siderophore lichenicidin VK21 A1/A2) (Fig. 1).
Fig. 1.
Biosynthetic gene cluster arrangement of the siderophore lichenicidin VK21-A1/A2 of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (95% identity) biosynthetic gene cluster of B. licheniformis using antiSMASH. Putative biosynthetic genes are presented in blue, additional biosynthetic genes in purple, transport, regulation-related genes in green, and resistance genes in red
-
b)
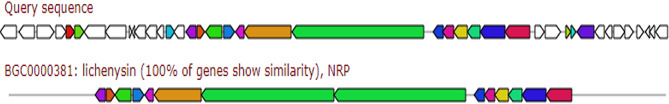
Traditional (multi-)modular non-ribosomal peptide synthases: these peptides are structurally and functionally different peptides that have important medical applications. (Gene cluster 100% similar to gene cluster producing lichenysin) (Fig. 2).
Fig. 2.
Biosynthetic gene cluster arrangement of lichenysin of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (100% identity) biosynthetic gene cluster of B. licheniformis using antiSMASH. Putative biosynthetic genes are presented in green, additional biosynthetic genes in brown, transport, and regulation-related genes in blue, and resistance genes in red
-
c)
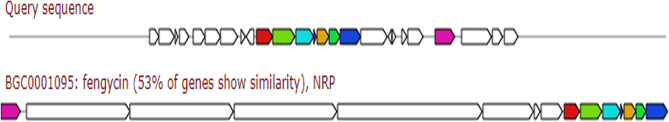
Beta-lactone-containing protease inhibitors: They are efficient biochemical probes and possible leads for new antimicrobial agents. (Gene cluster 53% similar to gene cluster producing the fengycin) (Fig. 3).
Fig. 3.
Biosynthetic gene cluster arrangement of fengycin of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (53% identity) biosynthetic gene cluster of B. licheniformis using antiSMASH. Putative biosynthetic genes are presented in green, additional biosynthetic genes in brown, transport, and regulation-related genes in blue, and resistance genes in red
-
d)
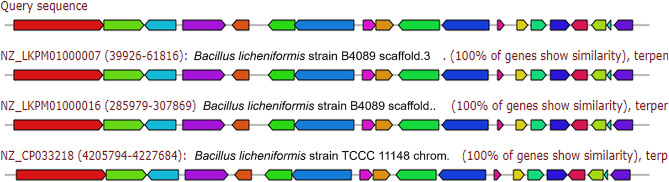
Terpene: Terpenes are a major biosynthetic factory for steroids. They are natural products of essential oils of plentiful flowers and plants. Terpenes have the formula (C5H8) n (Fig. 4).
Fig. 4.
Biosynthetic gene cluster arrangement of terpenes of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (100% identity) biosynthetic gene cluster of three B. licheniformis strains using antiSMASH. Putative biosynthetic genes are presented in green, additional biosynthetic genes in brown, transport, and regulation-related genes in blue, and resistance genes in red
-
e)
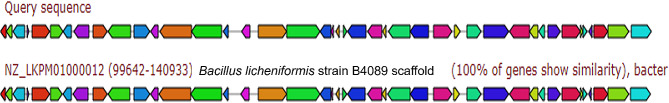
Bacteriocin: Toxin peptide or protein in nature. They inhibit the growth of bacteria that are closely related or similar. They are diverse concerning structure, function, and ecology. Gene cluster showing 100% similarity to B. licheniformis strain B4089 (Fig. 5).
Fig. 5.
Biosynthetic gene cluster arrangement of bacteriocin of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (100% identity) biosynthetic gene cluster of B. licheniformis strain B4089 using antiSMASH. Putative biosynthetic genes are presented in green, additional biosynthetic genes in brown, transport, and regulation-related genes in blue, and resistance genes in red
-
f)
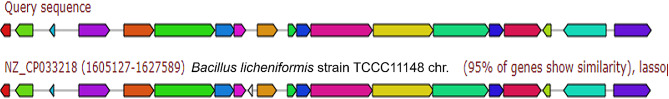
Lasso peptide: The origin of this metabolite is a peptide in nature that is the origin of many compounds used in medicine as receptor blocking action, inhibition of enzymes, and antimicrobial (Fig. 6).
Fig. 6.
Biosynthetic gene cluster arrangement of Lasso peptide of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (95% identity) biosynthetic gene cluster of B. licheniformis strain TCCC 11,148 using antiSMASH. Putative biosynthetic genes are presented in green, additional biosynthetic genes in brown, transport, and regulation-related genes in blue, and resistance genes in red
-
g)
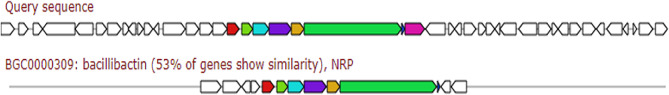
Traditional (multi-)modular non-ribosomal peptide synthases. These are biocatalysts to compile diverse peptides of valuable medicinal relevance. This catalysis occurs by utilization of complex stereospecific and regiospecific reactions (Gene cluster 53% similar to gene cluster producing bacillibactin) (Fig. 7).
Fig. 7.
Biosynthetic gene cluster arrangement of bacillibactin of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (53% identity) biosynthetic gene cluster of B. licheniformis strain TCCC 11,148 using antiSMASH. Putative biosynthetic genes are presented in green, additional biosynthetic genes in brown, transport, and regulation-related genes in blue, and resistance genes in red
-
h)
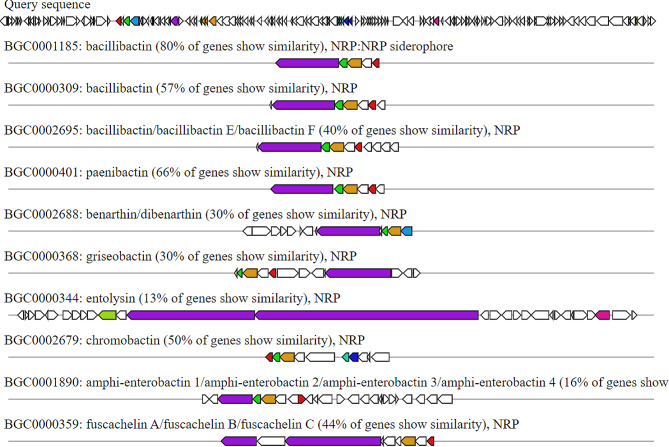
Bacillibactin. This is a catechol-based siderophore with considerable broad-spectrum antimicrobial activity. The gene cluster of B. haynesii isolate MZ922052 was analyzed using deepBGC and showed about 80% similarities to the bacillibactin biosynthetic gene cluster produced by several members of Bacillus species as displayed in Fig. 8.
Fig. 8.
Biosynthetic gene cluster arrangement of bacillibactin of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (80% identity) biosynthetic gene cluster using DeepBGC. Putative biosynthetic genes are presented in purple, additional biosynthetic genes in brown, transport, and regulation-related genes in blue, and resistance genes in red
-
i)
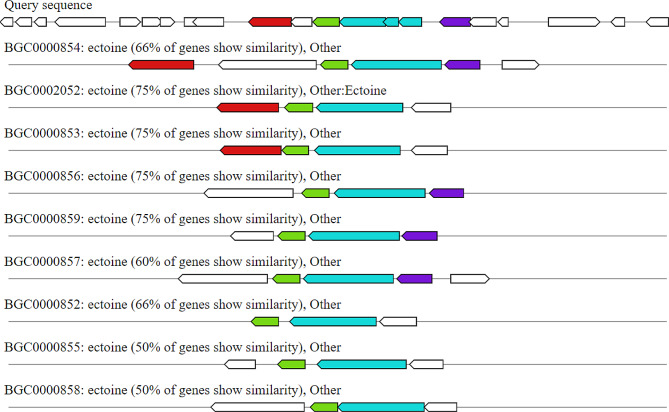
Ectoine. It is a protective substance produced by several bacterial species to allow them to withstand extreme osmotic conditions. The biosynthetic gene cluster of B. haynesii isolate MZ922052 was analyzed using deepBGC and it showed 66% similarity to other ectoine biosynthetic gene clusters produced by various microbial species as shown in Fig. 9.
Fig. 9.
Biosynthetic gene cluster arrangement of ectoine of Bacillus haynesii isolate MZ922052 (query sequence) compared to the homologous (66% identity) biosynthetic gene using DeepBGC. Putative biosynthetic genes are presented in blue, additional biosynthetic genes in purule, transport, and regulation-related genes in green, and resistance genes in red
Cheminformatics
The cheminformatics analysis of the known secondary metabolites produced from the B. haynesii isolate MZ922052 has been carried out and the lacticin 3147 structural peptides Ltnα and Ltnβ were used as templates for structure prediction. Results revealed the conservation of the lanthionine-containing peptide antibiotic (lantibiotic) for the lichenicidin (Fig. S4) The 2D structures of lichenysin and fengycin (a derivative of oxapentanoic acid) were carried out and the molecular weight of each molecule was computed using PubChem 2.1 to be 1021.3 and 1463.7, respectively (Figs. S5 and S6).
Discussion
The continuous evolution of microbes and the accelerated rise in antimicrobial resistance is a pressing need for the discovery of new antimicrobial compounds. Natural resources have always provided solutions to produce several natural products such as antimicrobials(Hutchings et al. 2019). Soil is a habitat for a diverse population of microbes with the potential for new metabolites that have not been discovered yet (Vehapi et al. 2023; Mahjoory et al. 2023). Some of these metabolites are produced as survival mechanisms against microbes present in their environments that we can utilize as antimicrobials. In this study, B. hayensii MZ922052 was isolated from soil Luxor Garden, Egypt, and showed antibacterial activity against MDR K. pneumoniae, S. epidermidis, VRSA and MDR E. coli, and a standard and a clinical isolate of C. albicans. Peng et al. (2023) isolated a novel bacteriocin from B. haynesii which was safe and inhibited the growth of Propionibacterium acne. Peng et al. (2023) stated that the bacteriocin was strongly active against Gram-positive bacteria and to our knowledge Peng et al. study is the only study that was done on B. haynesii as a producer of antimicrobial metabolite (Peng et al. 2023).
Compared to other studies, B. licheniformis collected from Hashemite University Campus area, Jordan, inhibited Streptococcus pneumoniae ATCC 6303, S. aureus ATCC 11,632, Proteus mirabilis ATCC and Enterobacter cloacae ATCC 13,182, but showed no activity against E. coli ATCC 10,145 and Salmonella Typhi ATCC 13,076 (Berić et al. 2014b). A study done on B. licheniformis DSM 13 to detect novel antibiotic gene clusters showed antibacterial action in Gram-positive bacteria, like B. subtilis, S. aureus, Micrococcus luteus, S. simulans, Streptococcus pyogenes, enterococci, but showed no activity against Gram-negative bacteria (Dischinger et al. 2009). Another example that showed the potential of the Bacillus, a study on B. paralicheniformis UBBLi30 that was isolated from traditional fermented food in India showed inhibition against Micrococcus luteus (and its biofilm formation), S. aureus, Streptococcus pyogenes, Propionibacterium acnes and showed no inhibitory effect on C. albicans, E. coli, and P. aeruginosa (Ahire et al. 2020).
Secondary metabolites in the present research were described by applying TLC and LC/MS and the determination of polarities of metabolites and retention factor (RF) were determined by TLC Eltokhy et al. 2021a, b). Wide diverse compounds were found in the cell-free extract of ethyl acetate (results not shown) as reported by LC/MS. AntiSMASH analysis applied to the sequences provided guided us to the nature of the antibacterial secondary metabolites gene clusters. We could not do the correlation between the inhibitory compounds identified by LC/MS and secondary metabolite gene clusters as all peaks of LC/MS were below 1000 m/z mass, while all secondary metabolites identified by antiSMASH had mass above 1000 m/z.
AntiSMASH analysis showed the presence of seven different antimicrobials including licheniciden (polypeptide lanthibiotic), lichenysin, fengycin, bacteriocin, Lasso peptide, and bacillibactin. The lichenicidin showed 100% similarity to lichenicidin VK21 A1/lichenicidin VK21 A2. The lantibiotic is a peptide antibiotic (lanthionine) that is active on Gram-positive bacteria. Depolarization of the bacterial-energized cytoplasmic membrane is the basis for its bactericidal activity, and this is initiated by the formation of aqueous transmembrane pores (Panina et al. 2023). Our results show that ethyl acetate extract of fermentation products of Bacillus sp. have strong growth inhibition against Gram-positive S. aureus and VRSA. In silico studies and whole genome sequencing in addition to microbiological studies have proved the presence of gene clusters for lichenicidin production by our isolated Bacillus strain.
Furthermore, the obtained metagenome sequence was analyzed using DeepBGC software to detect the presence of secondary metabolite biosynthetic gene clusters as previously described (Hannigan et al. 2019) Our results showed the presence of the biosynthetic gene cluster of two important active metabolites namely, bacillibactin (broad-spectrum antibacterial activity) (May et al. 2001) and ectoine (osmolyte substance) (Peters et al. 1990). The action of action is favorable for bacterial growth under extremely unfavorable condition like high salt concentration and accordingly, it can act as a new target for the development of antibacterial agents like the natural antioxidant staphyloxanthin produced by Staphylococcus aureus (Elmesseri et al. 2022). It was previously reported that the bacillibactin class of antibiotics was isolated from marine Bacillus species and biochemically identified to have a promising broad-spectrum antibacterial activity (Chakraborty et al. 2022). To the best of our knowledge, this is the first report about identifying the bacillibactin biosynthetic gene cluster in B. haynesii.
A large multi-modular biocatalyst called lichenysin. This biocatalyst synthesizes structurally and functionally varied peptides with significant medical applications using intricate stereospecific and regiospecific reactions. It is a potent biosurfactant lipopeptide that prevents the formation of bacterial biofilm. Lichenysin is much more potent than surfactin which is produced by B. subtilis (Gudiña and Teixeira 2022). Also, lichenycin has low toxicity which could be used safely. The gene cluster for lichenycin shows 100% similarity to the gene cluster producing lichenysin which predicts our strain to be a source of lichenysin (Coronel-León et al. 2016). A third secondary metabolite which is predicted to be beta-lactone contains protease inhibitors: these are a great biochemical probe and possible source of antibacterial drugs. The predicted compound has 53% similarity to fengycin which is a fungicide used in agriculture (Sur et al. 2018). Fengycin is a cyclic lipopeptide that acts effectively against bacteria and fungi (Sur et al. 2018). The activity of the fermentation ethyl acetate extract or dichloromethane showed very little against Candida. This could be due to fengycin not being extracted by the two solvents used. Extraction of fengycin needs to be precipitated first by hydrochloric acid and then extracted with methanol (Lin et al. 2020).
The fourth secondary metabolite predicted by metagenomic analysis is bacteriocin. These are peptides or proteinaceous toxins secreted by bacteria to prevent the growth of related or similar bacterial strain(s). These compounds vary in function, structure, and ecology. They resemble paramecium and yeast microbicidal factors (Benítez-Chao et al. 2021). The metagenomic analysis shows that it is 100% similar to that of B. licheniformis strain B4089. Guo et al. (2012) isolated a new strain of B. licheniformis from soil that produced a bacteriocin-like substance that was characterized by broad-spectrum antibacterial activity (Guo et al. 2012). The fifth secondary metabolite predicted by metagenomics is the Lasso peptide. Natural products of peptide origin are synthesized by the ribosomes and modified after translation (RiPPs) and identified by having a thread-like structure (Cao et al. 2021). They often are prolific origin of substances with medical relevance. These substances exhibit a variety of intriguing biological actions, including antimicrobial, inhibition of enzyme activity, and blocking receptor activities (Hegemann et al. 2015). Our strain showed 95% similarity to the B. licheniformis strain TCCC 11,148 gene cluster for biosynthesis of Lasso peptide. This aligning result indicates that the present strain acquired different posttranslational modification than that of B. licheniformis strain TCCC 11,148.
The sixth metabolite is a multi-modular non-ribosomal peptide synthase which is a big multi-modular biocatalyst that uses complex stereo and regiospecific reactions to construct functionally and structurally several peptides that possess significant medical uses. Chakraborty et al. isolated four homologous siderophore types of bacillibactin from marine bacteria B. amyloliquefaciens MTCC 12,713 (Chakraborty et al. 2022). P. aeruginosa, K. pneumoniae, vancomycin-resistant Enterococcus faecalis, and MRSA were among the drug-resistant bacteria against which it showed possible inhibitory effects. Gene clusters of the previous bacteria were characterized by sequencing of the entire genome of B. amyloliquefaciens MTCC 12,713. In our study bacillibactin showed 53% similarity to gene cluster producing bacillibactin. This might be a novel derivative of bacillibactin which needs further isolation and characterization. The last gene cluster represents terpenes, with general formula (C5H8)n, these are also compounds of natural origin. Terpenes are also major biosynthetic building blocks for steroids. Terpenes and their derivatives have antibacterial activity against sensitive and MDR bacteria by cell rupture and inhibiting the synthesis of both proteins and DNA (Guimarães et al. 2019). Accordingly, the future perspective of this research is to produce and optimize the production of the respective seven active metabolites and test their activities in more detail. One of the promising approaches to optimize the production of certain microbial metabolites is via the use of various models implemented for statistical optimization. This approach has been successfully used for the production optimization of various secondary antibacterial metabolites such as paromomycin (Ibrahim et al. 2019; El-Housseiny et al. 2021; Ibrahim et al. 2023, antifungal metabolites (El-Sayed et al. 2020), biosurfactants (El-Housseiny et al. 2016, 2020), probiotics against life-threatening pathogens (Mansour et al. 2018), medically-used enzymes such as L-asparaginase (Darnal et al. 2023), enterokinase, (Ebrahimifard et al. 2022), staphylokinase (Shariati et al. 2022), and carbohydrases (Kaur et al. 2021)using the response surface methodology and multifactorial design. Therefore, this approach could be a promising tool for the optimization procedures of the potential antibacterial metabolite produced by B. haynesii MZ922052. In conclusion, metagenomic nanopore sequence analysis of soil coupled with conventional screening methods has been carried out and was shown to be very helpful in identifying new antimicrobial metabolites and their respective biosynthetic gene clusters produced by the soil microbiota. B. haynesii MZ922052 was recovered in this study via conventional screening method coupled with nanopore metagenome screening and showed promising broad-spectrum antibacterial activities against various clinically relevant pathogens. Metagenomic analysis of the respective soil isolate revealed conservation of the biosynthetic gene clusters of seven valuable antibacterial metabolites such as lichenicidin, lichenysin, fengycin. major terpenes, bacteriocin, Lasso peptide, and bacillibactin. This is the first report for identifying the bacteriocin, lichenysin, and fengycin biosynthetic gene clusters in B. haynesii MZ922052. Future studies should be conducted to optimize the production of the respective metabolites and obtain them in pure forms followed by characterization and clinical evaluation for their potential use in humans.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We hereby acknowledge the department of microbiology and Immunology, Faculty of pharmacy, Misr international and Al Azhar University for providing us with all facilities and support required to perform the practical work and conducting this research. The authors also acknowledge the department of Clinical Laboratory Sciences, College of Applied Medical Sciences, King Khalid University for their continuous support and help.
Author contributions
MAE has collected the isolates and performed all experiments incorporated in the manuscript under the supervision and guidance of WNE, KMA, SMR, MSA. WNE, MSA has designed the protocol of this study. KMA, MYA and BTA made the bioinformatic analysis. MAE, WNE, SMR has written the first draft of manuscript. KMA, MYA, BTA and SMR have helped in writing, and revising this manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
and Material.
All data generated or analyzed during this study are included in this published article and supplementary file. The 16 S ribosomal RNA is available at NCBI GenBank database under the accession code, MZ922052 https://www.ncbi.nlm.nih.gov/nuccore/MZ922052.1/ (accessed on 16 December 2024). The metagenomics sequences were deposited in the NCBI GenBank sequence Archives under accession number PRJNA1064698 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1064698 (accessed on 16 December 2024).
Declarations
Ethical approval.
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahire JJ, Kashikar MS, Lakshmi SG, Madempudi R. Identification and characterization of antimicrobial peptide produced by indigenously isolated Bacillus paralicheniformis UBBLi30 strain. 3 Biotech. 2020;10:112. doi: 10.1007/s13205-020-2109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SM, Khan NA, Sagathevan K, Anwar A, Siddiqui R. Biologically active metabolite(s) from haemolymph of red-headed centipede Scolopendra subspinipes possess broad spectrum antibacterial activity. AMB Express. 2019;9:95. doi: 10.1186/s13568-019-0816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin M, Rakhisi Z, Zarei Ahmady A. Isolation and identification of Bacillus species from soil and evaluation of their Antibacterial properties. Avicenna J Clin Microbiol Infect. 2015;2:23233–23233. doi: 10.17795/ajcmi-23233. [DOI] [Google Scholar]
- Baltz RH. Genome mining for drug discovery: progress at the front end. J Ind Microbiol Biotechnol. 2021;48(9–10):1–11. doi: 10.1093/jimb/kuab044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benítez-Chao DF, León-Buitimea A, Lerma-Escalera JA, Morones-Ramírez JR. Bacteriocins: an overview of Antimicrobial, Toxicity, and Biosafety Assessment by in vivo models. Front Microbiol. 2021;12:630695. doi: 10.3389/fmicb.2021.630695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berić T, Stanković S, Draganić V, Kojić M, Lozo J, Fira D. Novel antilisterial bacteriocin licheniocin 50.2 from Bacillus licheniformis VPS50.2 isolated from soil sample. J Appl Microbiol. 2014;116:502–510. doi: 10.1111/jam.12393. [DOI] [PubMed] [Google Scholar]
- Cao L, Beiser M, Koos JD, Orlova M, Elashal HE, Schröder HV, Link AJ. Cellulonodin-2 and Lihuanodin: Lasso peptides with an aspartimide post-translational modification. J Am Chem Soc. 2021;143:11690–11702. doi: 10.1021/jacs.1c05017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulier S, Nannan C, Gillis A, Licciardi F, Bragard C, Mahillon J. Overview of the Antimicrobial compounds produced by members of the Bacillus subtilis Group. Front Microbiol. 2019;10:302. doi: 10.3389/fmicb.2019.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty K, Kizhakkekalam VK, Joy M, Chakraborty RD. Bacillibactin class of siderophore antibiotics from a marine symbiotic Bacillus as promising antibacterial agents. Appl Microbiol Biotechnol. 2022;106:329–340. doi: 10.1007/s00253-021-11632-0. [DOI] [PubMed] [Google Scholar]
- Chinemerem Nwobodo D, Ugwu MC, Oliseloke Anie C, Al-Ouqaili MTS, Chinedu Ikem J, Victor Chigozie U, Saki M. Antibiotic resistance: the challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal. 2022;36(9):e24655. doi: 10.1002/jcla.24655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (2021) CLSI: Performance standards for antimicrobial susceptibility testing. Clinical and Laboratory Standards Institute 2021. In: vol. M100-Ed31 [DOI] [PMC free article] [PubMed]
- Coronel-León J, Marqués AM, Bastida J, Manresa A. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J Appl Microbiol. 2016;120:99–111. doi: 10.1111/jam.12992. [DOI] [PubMed] [Google Scholar]
- Darnal S, Patial V, Kumar V, Kumar S, Kumar V, Padwad YS, Singh D. Biochemical characterization of extremozyme L-asparaginase from Pseudomonas sp. PCH199 for therapeutics. AMB Express. 2023;13:22. doi: 10.1186/s13568-023-01521-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dischinger J, Josten M, Szekat C, Sahl H-G, Bierbaum G. Production of the Novel two-peptide lantibiotic lichenicidin by Bacillus licheniformis DSM 13. PLoS ONE. 2009;4:e6788. doi: 10.1371/journal.pone.0006788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebrahimifard M, Forghanifard MM, Yamchi A, Zarrinpour V, Sharbatkhari M. A simple and efficient method for cytoplasmic production of human enterokinase light chain in E. Coli. AMB Express. 2022;12:160. doi: 10.1186/s13568-022-01504-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Housseiny GS, Aboulwafa MM, Aboshanab KA, Hassouna NAH. Optimization of Rhamnolipid production by P. Aeruginosa isolate P6. J Surfactants Deterg. 2016;19:943–955. doi: 10.1007/s11743-016-1845-4. [DOI] [Google Scholar]
- El-Housseiny GS, Aboshanab KM, Aboulwafa MM, Hassouna NA. Structural and physicochemical characterization of Rhamnolipids produced by Pseudomonas aeruginosa P6. AMB Express. 2020;10:201. doi: 10.1186/s13568-020-01141-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Housseiny GS, Ibrahim AA, Yassien MA, Aboshanab KM. Production and statistical optimization of paromomycin by Streptomyces rimosus NRRL 2455 in solid state fermentation. BMC Microbiol. 2021;21:34. doi: 10.1186/s12866-021-02093-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed SE, Abdelaziz NA, El-Housseiny GS, Aboshanab KM. Octadecyl 3-(3, 5-di-tert-butyl-4-hydroxyphenyl) propanoate, an antifungal metabolite of Alcaligenes faecalis strain MT332429 optimized through response surface methodology. Appl Microbiol Biotechnol. 2020;104:10755–10768. doi: 10.1007/s00253-020-10962-9. [DOI] [PubMed] [Google Scholar]
- Elmesseri RA, Saleh SE, Elsherif HM, Yahia IS, Aboshanab KM. Staphyloxanthin as a potential Novel Target for Deciphering Promising Anti-Staphylococcus Aureus agents. Antibiotics. 2022;11:298. doi: 10.3390/antibiotics11030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltokhy MA, Saad BT, Eltayeb WN, El-Ansary MR, Aboshanab KM, Ashour MSE. A metagenomic nanopore sequence analysis combined with conventional screening and spectroscopic methods for deciphering the Antimicrobial metabolites produced by Alcaligenes faecalis Soil isolate MZ921504. Antibiotics. 2021;10:1382. doi: 10.3390/antibiotics10111382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltokhy MA, Saad BT, Eltayeb WN, Yahia IS, Aboshanab KM, Ashour MSE. Exploring the nature of the Antimicrobial metabolites produced by Paenibacillus ehimensis soil isolate MZ921932 using a metagenomic nanopore sequencing coupled with LC-Mass analysis. Antibiotics. 2021;11:12. doi: 10.3390/antibiotics11010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudiña EJ, Teixeira JA. Bacillus licheniformis: the unexplored alternative for the anaerobic production of lipopeptide biosurfactants? Biotechnol Adv. 2022;60:108013. doi: 10.1016/j.biotechadv.2022.108013. [DOI] [PubMed] [Google Scholar]
- Guimarães AC, Meireles LM, Lemos MF, Guimarães MCC, Endringer DC, Fronza M, Scherer R. Antibacterial activity of terpenes and terpenoids Present in essential oils. Molecules. 2019;24:2471. doi: 10.3390/molecules24132471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Yu Z, Xie J, Zhang R. Identification of a new Bacillus licheniformis strain producing a bacteriocin-like substance. J Microbiol. 2012;50:452–458. doi: 10.1007/s12275-012-2051-3. [DOI] [PubMed] [Google Scholar]
- Haavik HI, Froyshov Ø. Function of peptide antibiotics in producer organisms. Nature. 1975;254:79–82. doi: 10.1038/254079a0. [DOI] [PubMed] [Google Scholar]
- Hallaj-Nezhadi S, Hamdipour R, Shahrvirani M, Zare tin R, Chapeland-leclerc F, Ruprich-Robert G, Esnaashari S, Elyasi Far B, Dilmaghani A. Antimicrobial activity of Bacillus sp. isolated strains of wild honey. BMC Complement Med Ther. 2022;22:78. doi: 10.1186/s12906-022-03551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannigan GD, Prihoda D, Palicka A, Soukup J, Klempir O, Rampula L, Durcak J, Wurst M, Kotowski J, Chang D, Wang R, Piizzi G, Temesi G, Hazuda DJ, Woelk CH, Bitton DA. A deep learning genome-mining strategy for biosynthetic gene cluster prediction. Nucleic Acids Res. 2019;47:e110–e110. doi: 10.1093/nar/gkz654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann JD, Zimmermann M, Xie X, Marahiel MA. Lasso peptides: an Intriguing Class of Bacterial Natural products. Acc Chem Res. 2015;48:1909–1919. doi: 10.1021/acs.accounts.5b00156. [DOI] [PubMed] [Google Scholar]
- Hu X-Y, Logue M, Robinson N. Antimicrobial resistance is a global problem – a UK perspective. Eur J Integr Med. 2020;36:101136. doi: 10.1016/j.eujim.2020.101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings MI, Truman AW, Wilkinson B. Antibiotics: past, present and future. Curr Opin Microbiol. 2019;51:72–80. doi: 10.1016/j.mib.2019.10.008. [DOI] [PubMed] [Google Scholar]
- Ibrahim AA, El-Housseiny GS, Aboshanab KM, Yassien MA, Hassouna NA. Paromomycin production from Streptomyces rimosus NRRL 2455: statistical optimization and new synergistic antibiotic combinations against multidrug resistant pathogens. BMC Microbiol. 2019;19:18. doi: 10.1186/s12866-019-1390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AA, El-Housseiny GS, Aboshanab KM, Startmann A, Yassien MA, Hassouna NA. Statistical optimization and gamma irradiation on cephalosporin C production by Acremonium Chrysogenum W42-I. AMB Express. 2023;13:142. doi: 10.1186/s13568-023-01645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ince EYMKM. Molecular characterization of rhizospheric soil streptomycetes isolated from indigenous Turkish plants and their antimicrobial activity. World J Microbiol Biotechnol. 2008;24:1461–1470. doi: 10.1007/s11274-007-9628-8. [DOI] [Google Scholar]
- Johnson BA, Anker H, Meleney FL (1945) Bacitracin: A New Antibiotic Produced by a Member of the B. subtilis Group. Science (1979) 102:376–377. 10.1126/science.102.2650.376 [DOI] [PubMed]
- Kapley A, Tanksale H, Sagarkar S, Prasad AR, Kumar RA, Sharma N, Qureshi A, Purohit HJ. Antimicrobial activity of Alcaligenes sp. HPC 1271 against multidrug resistant bacteria. Funct Integr Genomics. 2016;16:57–65. doi: 10.1007/s10142-015-0466-8. [DOI] [PubMed] [Google Scholar]
- Kaur A, Soni SK, Vij S, Rishi P. Cocktail of carbohydrases from Aspergillus Niger: an economical and eco-friendly option for biofilm clearance from biopolymer surfaces. AMB Express. 2021;11:22. doi: 10.1186/s13568-021-01183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Song L, Breitwieser FP, Salzberg SL. Centrifuge: rapid and sensitive classification of metagenomic sequences. Genome Res. 2016;26:1721–1729. doi: 10.1101/gr.210641.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L, Zhang J, Bolton EE. PubChem 2023 update. Nucleic Acids Res. 2023;51:D1373–D1380. doi: 10.1093/nar/gkac956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L-Z, Zheng Q-W, Wei T, Zhang Z-Q, Zhao C-F, Zhong H, Xu Q-Y, Lin J-F, Guo L-Q. Isolation and characterization of fengycins produced by Bacillus amyloliquefaciens JFL21 and its broad-spectrum antimicrobial potential against Multidrug-resistant foodborne pathogens. Front Microbiol. 2020;11:579621. doi: 10.3389/fmicb.2020.579621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahjoory Y, Mohammadi R, Hejazi MA, Nami Y. Antifungal activity of potential probiotic limosilactobacillus fermentum strains and their role against toxigenic aflatoxin-producing aspergilli. Sci Rep. 2023;13:388. doi: 10.1038/s41598-023-27721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour NM, Elkhatib WF, Aboshanab KM, Bahr MMA. Inhibition of Clostridium difficile in mice using a mixture of potential probiotic strains Enterococcus faecalis NM815, E. faecalis NM915, and E. faecium NM1015: novel candidates to Control C. difficile infection (CDI) Probiotics Antimicrob Proteins. 2018;10:511–522. doi: 10.1007/s12602-017-9285-7. [DOI] [PubMed] [Google Scholar]
- Martí JM. Recentrifuge: robust comparative analysis and contamination removal for metagenomics. PLoS Comput Biol. 2019;15:e1006967. doi: 10.1371/journal.pcbi.1006967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JJ, Wendrich TM, Marahiel MA. The dhb operon of Bacillus subtilis encodes the Biosynthetic Template for the Catecholic Siderophore 2,3-Dihydroxybenzoate-Glycine-threonine trimeric Ester Bacillibactin. J Biol Chem. 2001;276:7209–7217. doi: 10.1074/jbc.M009140200. [DOI] [PubMed] [Google Scholar]
- Panina IS, Balandin SV, Tsarev AV, Chugunov AO, Tagaev AA, Finkina EI, Antoshina DV, Sheremeteva EV, Paramonov AS, Rickmeyer J, Bierbaum G, Efremov RG, Shenkarev ZO, Ovchinnikova TV. Specific binding of the α-Component of the Lantibiotic Lichenicidin to the Peptidoglycan Precursor lipid II predetermines its antimicrobial activity. Int J Mol Sci. 2023;24:1332. doi: 10.3390/ijms24021332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Z, He M, Yang X, Zhang J. Discovery and characterization of a Novel Bacteriocin HA2-5 that strongly inhibits Propionibacterium acnes. J Agric Food Chem. 2023;71:12741–12748. doi: 10.1021/acs.jafc.3c04617. [DOI] [PubMed] [Google Scholar]
- Peters P, Galinski EA, Trüper HG. The biosynthesis of ectoine. FEMS Microbiol Lett. 1990;71:157–162. doi: 10.1111/j.1574-6968.1990.tb03815.x. [DOI] [Google Scholar]
- Polianciuc SI, Gurzău AE, Kiss B, Ștefan MG, Loghin F. Antibiotics in the environment: causes and consequences. Med Pharm Rep. 2020;93(3):231–240. doi: 10.15386/mpr-1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram SK, Ahmad P, Sujani Sathya Keerthana S, Jeya Cressida P, Ganesh Moorthy I, Suresh RSS. Extraction and purification of an antimicrobial bioactive element from lichen associated Streptomyces olivaceus LEP7 against wound inhabiting microbial pathogens. J King Saud Univ Sci. 2020;32:2009–2015. doi: 10.1016/j.jksus.2020.01.039. [DOI] [Google Scholar]
- Rampersad J, Ammons D. A Bacillus thuringiensis isolation method utilizing a novel stain, low selection and high throughput produced atypical results. BMC Microbiol. 2005;5:52. doi: 10.1186/1471-2180-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan R, Jadeja V. Isolation, characterization and chromatography based purification of antibacterial compound isolated from rare endophytic actinomycetes Micrococcus yunnanensis. J Pharm Anal. 2017;7:343–347. doi: 10.1016/j.jpha.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggese A, De Luca Y, Baccigalupi L, Ricca E. An antimicrobial peptide specifically active against Listeria monocytogenes is secreted by Bacillus pumilus SF214. BMC Microbiol. 2022;22:3. doi: 10.1186/s12866-021-02422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin J, Shanmughapriya S, Gandhimathi R, Seghal Kiran G, Rajeetha Ravji T, Natarajaseenivasan K, Hema TA. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Appl Microbiol Biotechnol. 2009;83:435–445. doi: 10.1007/s00253-009-1878-y. [DOI] [PubMed] [Google Scholar]
- Shariati FS, Keramati M, Cohan RA. Indirect optimization of staphylokinase expression level in dicistronic auto-inducible system. AMB Express. 2022;12:124. doi: 10.1186/s13568-022-01464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sur S, Romo TD, Grossfield A. Selectivity and mechanism of Fengycin, an Antimicrobial Lipopeptide, from Molecular Dynamics. J Phys Chem B. 2018;122:2219–2226. doi: 10.1021/acs.jpcb.7b11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valan Arasu M, Duraipandiyan V, Agastian P, Ignacimuthu S. In vitro antimicrobial activity of Streptomyces Spp. ERI-3 isolated from western ghats rock soil (India) J Mycol Med. 2009;19:22–28. doi: 10.1016/j.mycmed.2008.12.002. [DOI] [Google Scholar]
- Vehapi M, İnan B, Kayacan-Cakmakoglu S, Sagdic O, Özçimen D. Production of Bacillus subtilis soil isolate as biocontrol agent under bioreactor conditions. Arch Microbiol. 2023;205:52. doi: 10.1007/s00203-022-03381-z. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
and Material.
All data generated or analyzed during this study are included in this published article and supplementary file. The 16 S ribosomal RNA is available at NCBI GenBank database under the accession code, MZ922052 https://www.ncbi.nlm.nih.gov/nuccore/MZ922052.1/ (accessed on 16 December 2024). The metagenomics sequences were deposited in the NCBI GenBank sequence Archives under accession number PRJNA1064698 (https://www.ncbi.nlm.nih.gov/sra/PRJNA1064698 (accessed on 16 December 2024).