Abstract
3-Methyladenine (3MeA) DNA glycosylases initiate base excision repair by removing 3MeA. These glycosylases also remove a broad spectrum of spontaneous and environmentally induced base lesions in vitro. Mouse cells lacking the Aag 3MeA DNA glycosylase (also known as the Mpg, APNG or ANPG DNA glycosylase) are susceptible to 3MeA-induced S phase arrest, chromosome aberrations and apoptosis, but it is not known if Aag is solely responsible for repair of 3MeA in vivo. Here we show that in Aag–/– cells, 3MeA lesions disappear from the genome slightly faster than would be expected by spontaneous depurination alone, suggesting that there may be residual repair of 3MeA. However, repair of 3MeA is at least 10 times slower in Aag–/– cells than in Aag+/+ cells. Consequently, 24 h after exposure to [3H]MNU, 30% of the original 3MeA burden is intact in Aag–/– cells, while 3MeA is undetectable in Aag+/+ cells. Thus, Aag is the major DNA glycosylase for 3MeA repair. We also investigated the in vivo repair kinetics of another Aag substrate, 7-methylguanine. Surprisingly, 7-methylguanine is removed equally efficiently in Aag+/+ and Aag–/– cells, suggesting that another DNA glycosylase acts on lesions previously thought to be repaired by Aag.
INTRODUCTION
Maintenance of DNA sequences is essential to life, yet DNA is under constant attack by ubiquitous DNA-damaging agents present both in the environment and within cells. To prevent the potentially deleterious effects of DNA lesions, cells have evolved sophisticated DNA repair systems, including base excision repair (BER), nucleotide excision repair (NER) and DNA repair methyltransferase (MTase) (1).
Alkylating agents comprise one of the broadest classes of DNA-damaging agents and DNA alkylation damage can cause teratogenesis and cancer (1,2). Of particular interest are methylated bases, such as 3-methyladenine (3MeA) and 7-methylguanine (7MeG), that are formed not only by cancer chemotherapeutics and agents in the environment, but also by endogenous cellular processes (3,4). Consequently, even in the absence of exposure to environmental agents, DNA methylation damage can be detected in the genomic DNA of normal mammalian cells (reviewed in 5).
The majority of lesions created by methylating agents are 3MeA (∼10%), 7MeG (∼65–80%) and O6-methylguanine (O6MeG) (∼0.3–7%, depending on the agent) (reviewed in 6). Each of these lesions, if not properly repaired, may have detrimental effects. Of these, 7MeG is considered to be the most benign, since it does not appear to interfere with DNA replication. However, 7MeG lesions may spontaneously depurinate to form potentially mutagenic abasic sites (7–9). 3MeA is cytotoxic and potentially mutagenic in mammalian cells, presumably because it can block DNA replication (10–15). O6MeG, on the other hand, is highly mutagenic, since it mispairs very efficiently with thymine during DNA replication (16,17). Furthermore, O6MeG can also be cytotoxic, presumably as the result of aberrant processing by mismatch repair (18).
MTase, NER and BER are all potentially important for the removal of methylated bases from the genome. MTase repairs O6MeG in a single step by removing the offending methyl group and transfering it to its own active site cysteine. Mammalian NER, on the other hand, is much more complex, involving the interaction of over a dozen proteins that excise an oligonucleotide containing the damage, creating a gap that is then filled by DNA polymerase δ or ɛ and then ligated (reviewed in 1,19). The potential biological relevance of NER activity on methylated bases (20) has not yet been established in vivo. In mammals, the main repair pathway for 3MeA is thought to be BER initiated by the Aag 3MeA DNA glycosylase (alkyladenine DNA glycosylase) (12,21). In addition to 3MeA, Aag is able to remove a broad spectrum of lesions, including 7MeG, hypoxanthine and 1,N6-ethenoadenine (22–24). Following release of the damaged base by Aag, AP endonuclease cleaves the backbone at the abasic site, DNA polymerase β then fills the gap and removes the abasic residue and the resulting nick is ligated (reviewed in 25). An alternative pathway for BER involves the displacement of a short segment of nucleotides that is excised by FEN-1 (flap endonuclease) (26).
3MeA DNA glycosylase activity has been detected in nearly every species examined, suggesting that 3MeA lesions have important biological consequences. Indeed, Aag–/– cells are susceptible to 3MeA-induced sister chromatid exchange, chromosome aberrations, S phase arrest and apoptosis (10). In previous studies, no residual repair of 3MeA was detected in extracts from Aag–/– cells in vitro, suggesting that Aag–/– cells lack the ability to remove toxic 3MeA lesions from the genome (21). However, the conditions for this assay were optimized for detecting bases released by Aag and, therefore, might not detect release by another glycosylase or by NER. Thus, it remained to be determined whether or not 3MeA is actively repaired in Aag–/– cells in vivo.
Here we describe in vivo repair studies in Aag+/+ and Aag–/– cells. Interestingly, the kinetics of 3MeA removal suggest that 3MeA is indeed actively repaired in Aag–/– cells. However, in the absence of Aag, 3MeA lesions are removed much less efficiently. Furthermore, although 3MeA DNA glycosylases have long been thought to be responsible for repair of 7MeG (27), we observed that 7MeG is actively removed from the genome equally well in Aag+/+ and Aag–/– cells, indicating that 7MeG is removed in vivo by an alternative repair system.
MATERIALS AND METHODS
Cells and reagents
N-[3H]methyl-N-nitrosourea (MNU) (2 Ci/mmol) was purchased from Moravek Biochemicals (Brea, CA). Aag+/+ and Aag–/– embryonic stem (ES) cells were described previously (21). For these studies, AB1 and clone 65 were used to study wild-type activity (clone 65 was electroporated simultaneously with Aag–/– clones and is of similar passage number). Four independent Aag–/– clones (clones 21, 29, 38 and 147) were studied. Cells were cultured as previously described (21).
Quantification of methylated bases in genomic DNA
[3H]MNU was shipped in dichloromethane, transferred into EtOH and dried down to a small volume (<5% of the cell suspension volume). Aag+/+ and Aag–/– cells (2.5 × 107) were resuspended in 500 µl phosphate-buffered saline (PBS) and exposed to 200 µM [3H]MNU for 30 min in suspension rotating slowly at 37°C. After exposure, cells were diluted into ES medium and replated into four gelatinized dishes. At 0.5, 1.0, 2.5, 5.5 and 24.5 h after the addition of [3H]MNU, cells were either immediately pelleted (0.5 h) or scraped off the plates and then pelleted (remaining times) and stored at –80°C. The cell pellets were subsequently thawed and incubated at 37°C for 4 h in lysis buffer (10 mM Tris–HCl pH 8.0, 1 mM EDTA, 0.1 M NaCl, 10% SDS, 15 mg/ml RNase A and 20 mg/ml proteinase K). Following phenol extraction, the DNA was ethanol precipitated, resuspended in 1 M HCl and then hydrolyzed at 70°C for 1 h. The mixture was filtered (0.2 cm2 Ultrafree-MC filter; Millipore, Bedford, MA) and the bases separated on an Optisil 10 SCX cation exchange column (starting at 0.1 M ammonium formate, pH 4.0, and 0% acetonitrile for 10 min, followed by a gradient to 0.2 M ammonium formate, pH 4.0, and 5% acetonitrile with a flow rate of 1 ml/min for 60 min). Fractions were collected at 0.5–1 min intervals and the c.p.m. of each was determined by scintillation counting. The total c.p.m. of peaks that eluted at the positions of the 7MeG, 3MeA and O6MeG markers was determined by integration and background c.p.m. was subtracted. For each injection, the quantity of adenine was determined by UV absorbance and the c.p.m./fmol adenine for each adduct was determined to correct for differences in the amount of DNA injected per sample. To calculate the number of unrepaired lesions at 24 h post-exposure, values were corrected according to the extent of DNA replication to compensate for dilution due to replication (see below). For spontaneous depurination of 3MeA, purified calf thymus DNA was treated in neutral PBS and assayed as described above.
DNA replication in MNU-treated Aag+/+ and Aag–/– cells
Aag+/+ and Aag–/– cells (1 × 107) were incubated with or without 200 µM cold MNU in PBS at 37°C exactly as described above for the 3H-labeled MNU. Cell pellets were thawed and resuspended in lysis buffer (see above) for 4 h and sonicated (3 × 20 s pulses on a Braunsonic 1510) to reduce viscosity. An equal amount of each mixture (10 µl) was diluted to 6 ml in buffer (10 mM Tris–HCl pH 8.0, 100 mM NaCl, 1 mM EDTA) and incubated with Hoechst stain for 10 min in the dark. Hoechst fluorescence was measured on a Turner model 450 fluorometer (excitation wavelength 360 nm, emission wavelength 430 nm). The extent of DNA replication that occurred during the course of the experiment was estimated by determining the ratio of fluorescence relative to time 0. The fluorescence of clones AB1, 65, 29 and 38 extracts were determined in duplicate and the results of four independent experiments were then averaged.
MNU toxicity in Aag+/+ and Aag–/– cells
Aag+/+ and Aag–/– cells were plated at various concentrations (100, 300, 500 and 1000 cells/well) onto feeder-coated 24-well plates as previously described (21) and exposed to PBS alone or PBS containing 200 µM cold MNU. After 30 min, wells were rinsed with PBS. Fresh ES medium was added to the wells and the medium was changed every 2 days for 8 days. Colonies were fixed by 15 min incubation in an ethanol/acetic acid solution (3:1) and then stained with crystal violet (0.1 g/l). Percent survival was calculated from colony numbers. The results of four independent experiments were averaged, in which clones 29 and 65 were assayed three times and clones 38 and AB1 once. MNU toxicity was also assayed under the exact conditions used when cells were exposed to [3H]MNU.
RESULTS
MNU toxicity
In order to determine the rate of 3MeA removal in Aag+/+ and Aag–/– mouse ES cells, we planned to expose cells to [3H]MNU and to measure the appearance and subsequent disappearance of 3MeA adducts in the genome. A relatively non-toxic dose of MNU was desired in order to avoid possible indirect effects of toxicity on DNA repair capacity. Aag+/+ and Aag–/– cells were exposed to 200 µM cold MNU, the dose intended for the experiments using [3H]MNU, and the percent survival calculated. After exposing log phase attached ES cells to 200 µM MNU, 92 ± 7% of the Aag+/+ cells survived and 86 ± 11% of the Aag–/– cells survived. In addition, when suspended cells were treated with 200 µM MNU under the same conditions as those planned for exposure to [3H]MNU, MNU had no effect on survival of either Aag+/+ or Aag–/– cells. In other words, the dose of [3H]MNU to be used for measuring in vivo repair rates was low enough that it was not particularly toxic to the cells.
Initial adduct level
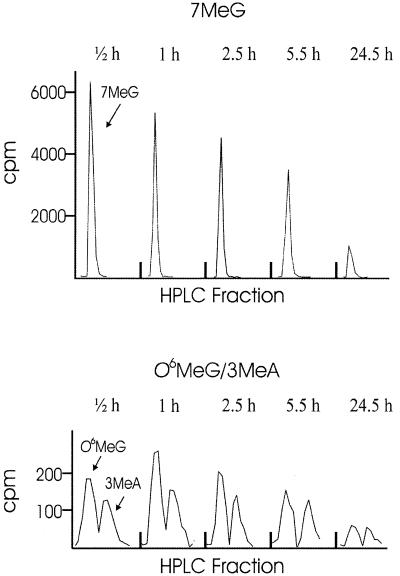
Aag+/+ and Aag–/– cells were exposed to 200 µM [3H]MNU, which creates predominantly three types of lesions: 7MeG (65–70%), 3MeA (8–9%) and O6MeG (5.9–8.2%) (under in vitro conditions; reviewed in 6). After a 30 min incubation in PBS containing 200 µM [3H]MNU, the suspended cells were diluted into normal ES medium and plated. Given that MNU has a half-life of ∼15 min and that the cells were diluted 80-fold, the amount of [3H]MNU remaining in the medium was <0.3% of the initial concentration. Thus, additional tritium labeling or possible toxicity associated with MNU in the medium was essentially non-existent after 30 min. Cells were subsequently harvested either immediately or at various times after MNU exposure. Purified DNA was acid hydrolyzed and the bases were then separated by HPLC. Figure 1 shows an example of c.p.m. associated with 7MeG, O6MeG and 3MeA in a sample from Aag–/– cells. Under these conditions, the quantity of 7MeG, O6MeG and 3MeA remaining in the genome were readily measurable.
Figure 1.
Quantification of damaged bases present in the genome of repair-deficient cells over time. Aag–/– mouse ES cells were exposed to 3H-labeled MNU and genomic DNA was isolated at various times after the start of exposure (t = 0). The first sample was collected immediately following exposure, at 0.5 h. Following HPLC separation of the DNA bases, c.p.m. associated with 7MeG, O6MeG and 3MeA were determined by scintillation counting of individual HPLC fractions. c.p.m. associated with methylated purines is normalized against fmol adenine to correct for variations in the total amount of sample injected.
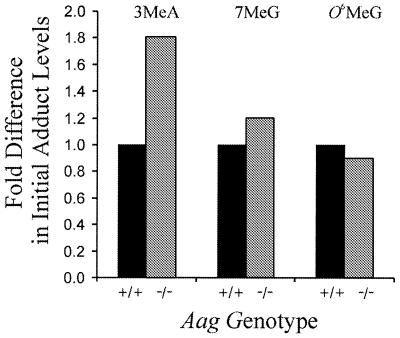
The initial adduct levels for the Aag+/+ and Aag–/– cells were compared immediately following [3H]MNU exposure (Fig. 2 and Table 1). Aag+/+ and Aag–/– cells did not differ significantly in their initial levels of O6MeG, as expected, given that O6MeG is not a substrate of Aag and that Aag status has been shown to be independent of O6MeG repair (12). On the other hand, under identical treatment conditions, 3MeA lesions are 1.8 ± 0.2 fold more abundant in the DNA of the Aag–/– cells compared to Aag+/+ cells. From this result, we conclude that the lower initial levels of 3MeA in the Aag+/+ cells are due to very rapid removal of 3MeA lesions by Aag during the 30 min exposure to [3H]MNU.
Figure 2.
Ratio of initial adduct levels for Aag+/+ and Aag–/– cells quantified immediately after incubation in [3H]MNU for 30 min (t = 0.5; values for Aag+/+ cells are set to 1). Data shown are an average of three or four experiments using more than one independently isolated Aag+/+ and Aag–/– clone. 3MeA levels are significantly higher in Aag–/– cells compared to Aag+/+ cells (P < 0.05, Student’s t-test). Differences between Aag+/+ and Aag–/– cells in the levels of 7MeG and O6MeG are not statistically significant.
Table 1. Initial adduct levels.
| Genotype | Average number of adducts/cell |
||
|---|---|---|---|
| 3MeA | 7MeG | O6MeG | |
| Aag+/+ | 1.2 × 105 | 3.5 × 106 | 2.6 × 105 |
| Aag–/– | 2.2 × 105 | 4.1 × 106 | 2.4 × 105 |
Aag+/+ and Aag–/– mouse ES cells were exposed to [3H]MNU and subsequently assayed for 3MeA, 7MeG and O6MeG as described in Materials and Methods. Calculations are based on an average of 1.5n DNA content. All values are approximated from the average of between three and six experiments.
In addition to 3MeA, Aag is proficient in the removal of 7MeG (28–31), albeit with an ∼25-fold reduction in kcat/KM (32). Nevertheless, the initial 7MeG adduct levels following incubation with MNU are not significantly different between the Aag+/+ and Aag–/– cells (Fig. 2 and Table 1), even when O6MeG is used as an internal control to correct for variations in exposure levels between experiments. Thus, 7MeG is being removed from the genome at similar rates in the Aag+/+ and Aag–/– cells during exposure to [3H]MNU, suggesting that Aag does not contribute significantly to the initial clearance of 7MeG. This result is consistent with the studies of Elder et al., in which similar levels of 7MeG were observed in livers of Aag+/+ and Aag–/– mice 24 h after exposure to MNU (12).
DNA replication
Previous studies of Aag–/– cells have shown that 3MeA lesions inhibit progression through S phase (10). To determine if Aag–/– cells suffered delayed cell cycle progression under the conditions used to study in vivo repair, we measured the amount of DNA present in populations of cells at various times post-exposure to cold MNU (or solvent control) under identical conditions as those used for exposure to [3H]MNU (see Materials and Methods). We found that in the absence of MNU, populations of Aag+/+ and Aag–/– cells doubled after 24 h (the long doubling time is due to an extended lag time, >10 h, caused by trypsinization; data not shown). In contrast, exposure to 200 µM MNU caused a significant reduction in DNA replication. Following MNU exposure, the Aag+/+ and Aag–/– cells showed a 1.7 ± 0.1 and a 1.3 ± 0.1 fold increase in total DNA content, respectively, which is significantly reduced compared to the 2-fold increase in total DNA observed in the absence of MNU (P < 0.05 by Student’s t-test). These results suggest that exposure to 3MeA at ∼1–2 adducts/100 kb (calculated from the values in Table 1) is sufficient to cause a significant delay in DNA replication.
Repair of O6MeG in Aag+/+ and Aag–/– cells
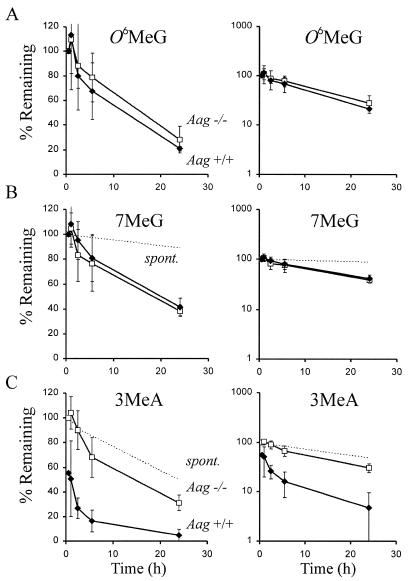
Aag is not expected to effect repair of O6MeG by the repair MTase and this has been confirmed in previous studies (12). Therefore, as an internal control, we monitored repair of O6MeG in Aag+/+ and Aag–/– cells over time following exposure to [3H]MNU (Fig. 3A). As expected, no significant differences were observed between Aag+/+ and Aag–/– cells in the repair kinetics of O6MeG. The observed similarity of O6MeG levels in the Aag+/+ and Aag–/– cells (Figs 2 and 3 and Table 1) indicates that there were no significant differences in the exposure conditions and that percent lesions remaining have been accurately adjusted to correct for dilution due to DNA replication. Furthermore, these results support the interpretation from Figure 2 that the 1.8-fold higher initial level of 3MeA lesions in the Aag–/– cells is due to rapid active repair by Aag in the Aag+/+ cells during the 30 min treatment time.
Figure 3.
Graphs on the left and right are identical, except for the change in scale of the y-axis. Percent of alkylated bases remaining in the genome of Aag–/– (opened squares) and Aag+/+ (closed diamonds) cells relative to t = 0.5 h. Spontaneous depurination of 7MeG and 3MeA at 37°C in PBS are shown as dotted lines that intercept the y-axis at 100% (values for spontaneous depurination of 3MeA were measured as described in Materials and Methods; values for 7MeG were calculated based on published estimates of t1/2; 6,36). The adduct levels at 24 h have been adjusted to correct for dilution due to DNA replication. These data are the average of four or five experiments using AB1 Aag+/+ cells and four independent Aag–/– clones. (A) O6MeG and (B) 7MeG removal from the DNA of Aag–/– and Aag+/+ cells. (C) 3MeA removal from the DNA of Aag+/+ cells and Aag–/– cells. Note that the values plotted for percent 3MeA remaining in Aag+/+ cells are the observed values divided by 1.8, to reflect the 1.8-fold difference in initial 3MeA levels between Aag+/+ and Aag–/– cells (shown in Fig. 2).
It is noteworthy that the number of O6MeG lesions present in the Aag+/+ and Aag–/– cells rises between 0 and 0.5 h post-incubation with [3H]MNU (Fig. 3A, left). A similar rise in the level of methylation damage has been observed by others (33). This initial rise in methylation damage may be due to residual methylating agent that continues to react with chromosomal DNA, as suggested by Ye et al. (33). Most likely, any residual methylating agent is within the cells, rather than in the medium, since cellular exposure was stopped either by dilution (experiments shown here) or by rinsing in PBS (under the conditions of Ye et al.; 33).
Removal of 7MeG lesions in Aag+/+ and Aag–/– cells
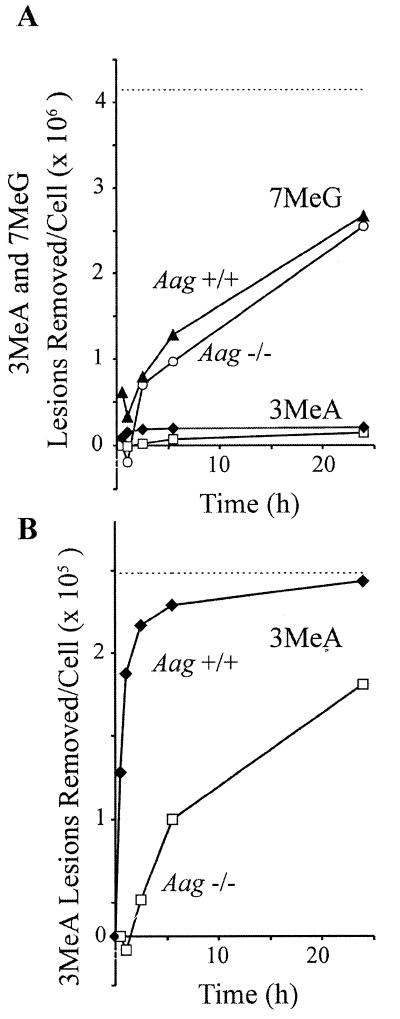
Figure 2 shows that the levels of 7MeG in the DNA of Aag+/+ and Aag–/– cells are similar when measured immediately after the 30 min [3H]MNU exposure, suggesting similar repair kinetics during the first 30 min. Consistent with these results, Aag+/+ and Aag–/– cells show essentially identical rates of 7MeG removal throughout the first 24 h (Fig. 3B). The t1/2 was determined from the slope of the log plot (Fig. 3B, right). We observed that 7MeG is removed from Aag+/+ and Aag–/– cells with a t1/2 = 18 h (r2 = 0.99), which is similar to previously published estimates where t1/2 = ∼30 h in cultured mammalian cells (34,35). Although 7MeG is repaired in Aag+/+ cells much more slowly than 3MeA (where the initial half-life is under 30 min), the quantity of 7MeG lesions repaired is much greater than 3MeA lesions, due to their initial abundance. Figure 4A shows that within the first 5 h, five times more 7MeG than 3MeA was removed.
Figure 4.
(A) Approximate number of 7MeG lesions removed per cell from the DNA of Aag+/+ (filled triangles) and Aag–/– cells (open circles). (A and B) Approximate number of 3MeA lesions removed per cell from the DNA of Aag+/+ (filled diamonds) and Aag–/– cells (open squares) calculated from the data presented in Figure 3 and Table 1. Note the difference in scales in (A) and (B). The initial number of adducts/cell in Aag–/– cells (Table 1) is used as an estimate of the total number of lesions created (dotted lines). The number of adducts observed in Aag–/– cells has been used as an estimate of the starting number of adducts in Aag+/+ cells at 0 h.
After 24 h, 7MeG levels are significantly lower in Aag–/– cells than would be expected due to spontaneous depurination alone (the difference is significant at the 95% confidence interval). In Aag–/– cells, 7MeG is removed with a t1/2 = 17.3 h, which is clearly faster than the rate of spontaneous hydrolysis (t1/2 = ∼147 h, calculated for spontaneous depurination at pH 7.0) (6,36). Furthermore, the kinetics of 7MeG removal are similar for Aag+/+ and Aag–/– cells, as described above. Unexpectedly, these results suggest that there is another repair pathway for 7MeG that is more efficient than Aag.
In vivo repair of 3MeA lesions in Aag+/+ and Aag–/– cells
The glycosylic bond of 3MeA is unstable and readily undergoes spontaneous hydrolysis (2). We measured the rate of spontaneous depurination by treating calf thymus DNA at physiological pH in PBS and quantifying the number of 3MeA lesions remaining in the DNA over time (Fig. 3C, dotted line). We observed that the t1/2 of 3MeA was ∼24 h, which is consistent with previously published studies (2,37).
To determine if 3MeA is actively removed in Aag–/– cells, we measured the amount of 3MeA remaining in the genome of Aag–/– cells at various times after exposure to [3H]MNU and compared the t1/2 in Aag–/– cells to the t1/2 due to spontaneous depurination (Fig. 3C). After 24 h, 31% of the 3MeA lesions in the Aag–/– cells remained in the genome (after correcting for dilution due to DNA replication), which is significantly less than the expected 50% remaining due to spontaneous depurination alone (the difference is significant at the 95% confidence interval). These data suggest that in the absence of Aag, 3MeA is actively removed from the genome by an alternative repair pathway, albeit much less efficiently than by Aag.
Aag+/+ cells harbored 1.8-fold fewer 3MeA lesions than Aag–/– cells immediately following the 30 min exposure to [3H]MNU (Fig. 2 and Table 1). Since the cells were treated simultaneously under conditions that yielded similar levels of other lesions (e.g. O6MeG; Table 1), we conclude that there was very rapid repair of 3MeA in Aag+/+ cells during the initial 30 min exposure time. If one considers that there is residual repair of 3MeA in Aag–/– cells (e.g. that the number of 3MeA lesions created during [3H]MNU exposure is even greater than the level observed in Aag–/– cells immediately following [3H]MNU exposure), then the estimated initial t1/2 of 3MeA in Aag+/+ cells is ∼30 min. Furthermore, adducts are being created not only at the beginning of the exposure, but throughout the exposure time, so that the t1/2 must be far below 30 min for a subset of lesions. In sharp contrast, the t1/2 of 3MeA in Aag–/– cells is >5 h, thus demonstrating that Aag provides a substantial kinetic advantage, so that 3MeA lesions are cleared at least 10 times faster in Aag+/+ cells than in Aag–/– cells. This difference in repair rates is consistent with the model that unrepaired 3MeA lesions are potentially toxic (10), since the vast majority of the 3MeA lesions would be cleared from the genome of wild-type cells well within the time frame of a single cell cycle (∼10 h), whereas Aag–/– cells may be forced to divide in the presence of a substantial number of adducts.
DISCUSSION
Alkylating agents are abundant in our environment and endogenously produced in cells and these agents can react with DNA to create a broad spectrum of alkylated bases (2,10,38). Of particular interest are non-coding potentially toxic lesions, such as 3MeA (10,11,39). Even though 3MeA lesions spontaneously depurinate with a half-life of only 24 h at neutral pH, nearly every species has evolved an efficient 3MeA DNA glycosylase that rapidly removes this adduct from the genome, suggesting that this lesion is of sufficient biological impact to justify a system of very rapid removal.
Historically, our ability to discern the precise biological consequences of 3MeA lesions in mammalian cells has been hampered by both the chemistry of methylating agents, which create a spectrum of lesions (reviewed in 2), and the chemistry of the 3MeA DNA glycosylase enzyme itself, which has been shown to remove a broad spectrum of substrates in vitro, including 7MeG, hypoxanthine and 1,N6-ethenoadenine (23,24,28–30). By combining 3MeA DNA glycosylase-deficient Aag–/– cells with an agent that almost exclusively forms 3MeA, it was revealed that 3MeA lesions induce S-phase arrest, chromosome aberrations, sister chromatid exchanges and apoptosis in mouse ES cells (10). Nevertheless, it was not yet known if Aag is solely responsible for repair of 3MeA in vivo.
If 3MeA is not actively removed in the Aag–/– cells, then we would expect to find 50% of 3MeAs remaining in the DNA of Aag–/– cells 24 h after exposure to [3H]MNU (given t1/2 = 24 h). However, only ∼30% of the 3MeA lesions remained, suggesting that there is active removal of 3MeA in Aag–/– cells. Interestingly, previous in vitro studies of Aag–/– cell and tissue extracts did not reveal any residual 3MeA DNA glycosylase activity (12,21,40). This may have been the case because the in vitro conditions were incombatible with the unknown repair system, such as NER. Another possibility is that the spontaneous depurination rate of 3MeA in vivo is faster than is observed under physiological conditions in vitro. Given that there is genetic evidence in both Saccharomyces cerevisiae and Schizosaccharomyces pombe that NER and 3MeA DNA glycosylases share common substrate(s) (41,42) and given that mammalian NER acts on at least two other methylated bases in vitro (O6MeG and N6-methyladenine) (20), it seems likely that NER is providing residual repair of 3MeA in the absense of Aag.
In 1971, Cleaver showed that methylation damage, induced by agents that predominantly generate 7MeG, was repaired similarly in normal and NER-deficient xeroderma pigmentosum cells. These results suggest that NER does not play a significant role in the removal of methylation damage, presumably because most methylation damage is repaired via BER (43). This result was later strengthened by similar observations in 45 different xeroderma pigmentosum clones (44) and by biochemical evidence that methylation damage induces short patch, rather than long patch repair (45). 3MeA DNA glycosylases were later shown to remove 7MeG in vitro (27) and, since no other enzyme has been shown to remove 7MeG from DNA, it became widely accepted that 7MeG is repaired by 3MeA DNA glycosylase. Furthermore, studies by Ye et al. clearly show that the in vivo repair kinetics of 7MeG in particular sequence contexts are mirrored almost exactly by the repair kinetics of purified Aag in vitro, which is consistent with Aag being responsible for repair of 7MeG in these cells (33). Thus, it is was very surprising to find that 7MeG is removed from the genome similarly in Aag+/+ and Aag–/– cells, indicating that Aag plays a minor role in the repair of 7MeG (at least in these ES cells) and suggesting that another DNA glycosylase initiates BER of 7MeG. Although it is formally possibile that NER acts on 7MeG, this seems unlikely given that NER-deficient cells have normal repair of methylation damage (43,44). One possibility is that 7MeG becomes ring opened to form N5-methyl-N5-formyl-2,5,6-triamino-4-hydroxypyrimidine, which is then removed by the Fpg DNA glycosylase (46). Alternatively, another glycosylase may recognize 7MeG (rather than ring-opened 7MeG). If this is the case, then it will be very interesting to see if other substrates are shared in common with Aag.
While the results presented here suggest that 7MeG is repaired independently of Aag, the results of Ye et al. provide strong evidence that 7MeG is repaired by Aag (the repair kinetics of 7MeG in different sequence contexts in vivo match the in vitro kinetics of Aag) (33). These apparently conflicting results may be due to differential gene expression among cell types. Another possibility is that there may be another repair pathway that acts on 7MeG with similar repair kinetics and sequence context preferences to Aag.
The observation that Aag+/+ and Aag–/– cells have similar levels of 7MeG 24 h after exposure to methylating agent is consistent with the observations of Elder et al. (12). Interestingly, after an additional 6 days, Elder et al. observed essentially complete clearance of 7MeG from the genomic DNA of Aag+/+ cells, while 7MeG lesions persisted in the Aag–/– cells (12). Thus, even though another repair pathway appears to be more efficient than Aag for the majority of 7MeGs, there appears to be a subset of 7MeG adducts that are repaired preferentially by Aag, perhaps due to their sequence context.
Although 7MeG is not thought to inhibit DNA replication or have other serious biological consequences, it does depurinate to form potentially deleterious abasic sites (7–9). Furthermore, it has been estimated that ∼800 000 adducts are formed and repaired each day in each mammalian cell, most of which are thought to be repaired by BER (47). It has been suggested that mutations that arise during the BER repair process may account for a significant portion of the spontaneous mutation rate (47), making the enzyme responsible for repair of 7MeG a potential candidate for promoting mutagenic repair processing.
Given the very broad substrate range of Aag in vitro, many proposals have been put forward as to which of these potential substrates are most biologically relevant. The studies presented here demonstrate that 3MeA, the first substrate identified for this family of enzymes, may after all be one of the most biologically relevant substrates of Aag in mammalian cells. Furthermore, the finding that another repair system is more efficient than Aag in the repair of 7MeG raises the important question as to whether or not Aag is indeed the major repair pathway for other potentially deleterious substrates, such as hypoxanthine and 1,N6-ethenoadenine.
Acknowledgments
ACKNOWLEDGEMENTS
We thank Dr Leona Samson for many helpful suggestions during the course of this study and Drs Barry Gold and Glenn Wilson for their critical comments on the manuscript. The authors also wish to thank Dr John Essigmann for his helpful technical advice. This work was supported by National Institutes of Health Grants R01CA79827-0 and 5-P30-ESO2109-20. S.A.S. was supported by a National Institute of Health Training Grant in Environmental Toxicology, T32 ES07020.
REFERENCES
- 1.Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. ASM Press, Washington, DC.
- 2.Singer B. and Grunberger,D. (1983) Molecular Biology of Mutagens and Carcinogens. Plenum Press, New York, NY.
- 3.Taverna P. and Sedgwick,B. (1996) J. Bacteriol., 178, 5105–5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sedgwick B. (1997) Carcinogenesis, 18, 1561–1567. [DOI] [PubMed] [Google Scholar]
- 5.Marnett L.J. and Burcham,P.C. (1993) Chem. Res. Toxicol., 6, 771–785. [DOI] [PubMed] [Google Scholar]
- 6.Beranek D.T. (1990) Mutat. Res., 231, 11–30. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence C.W., Borden,A., Banerjee,S.K. and LeClerc,J.E. (1990) Nucleic Acids Res., 18, 2153–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gentil A., Cabral-Neto,J.B., Mariage-Samson,R., Margot,A., Imbach,J.L., Rayner,B. and Sarasin,A. (1992) J. Mol. Biol., 227, 981–984. [DOI] [PubMed] [Google Scholar]
- 9.Neto J.B., Gentil,A., Cabral,R.E. and Sarasin,A. (1992) J. Biol. Chem., 267, 19718–19723. [PubMed] [Google Scholar]
- 10.Engelward B.P., Allan,J.M., Dreslin,A.J., Kelly,J.D., Gold,B. and Samson,L.D. (1998) J. Biol. Chem., 273, 5412–5418. [DOI] [PubMed] [Google Scholar]
- 11.Klungland A., Laake,K., Hoff,E. and Seeberg,E. (1995) Carcinogenesis, 16, 1281–1285. [DOI] [PubMed] [Google Scholar]
- 12.Elder R.H., Jansen,J.G., Weeks,R.J., Willington,M.A., Deans,B., Watson,A.J., Mynett,K.J., Bailey,J.A., Cooper,D.P., Rafferty,J.A., Heeran,M.C., Wijnhoven,S.W., van Zeeland,A.A. and Margison,G.P. (1998) Mol. Cell. Biol., 18, 5828–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boiteux S., Huisman,O. and Laval,J. (1984) EMBO J., 3, 2569–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Larson K., Sahm,J., Shenkar,R. and Strauss,B. (1985) Mutat. Res., 150, 77–84. [DOI] [PubMed] [Google Scholar]
- 15.Chaudhuri I. and Essigmann,J.M. (1991) Carcinogenesis, 12, 2283–2289. [DOI] [PubMed] [Google Scholar]
- 16.Loechler E.L., Green,C.L. and Essigmann,J.M. (1984) Proc. Natl Acad. Sci. USA, 81, 6271–6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellison K.S., Dogliotti,E., Connors,T.D., Basu,A.K. and Essigmann,J.M. (1989) Proc. Natl Acad. Sci. USA, 86, 8620–8624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karran P. and Hampson,R. (1996) Cancer Surv., 28, 69–85. [PubMed] [Google Scholar]
- 19.Wood R.D. (1997) J. Biol. Chem., 272, 23465–23468. [DOI] [PubMed] [Google Scholar]
- 20.Huang J.C., Hsu,D.S., Kazantsev,A. and Sancar,A. (1994) Proc. Natl Acad. Sci. USA, 91, 12213–12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelward B., Dreslin,A., Christensen,J., Huszar,D., Kurahara,C. and Samson,L. (1996) EMBO J., 15, 945–952. [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor T.R. and Laval,F. (1990) EMBO J., 9, 3337–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dosanjh M.K., Roy,R., Mitra,S. and Singer,B. (1994) Biochemistry, 33, 1624–1628. [DOI] [PubMed] [Google Scholar]
- 24.Saparbaev M. and Laval,J. (1994) Proc. Natl Acad. Sci. USA, 91, 5873–5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krokan H.E., Standal,R. and Slupphaug,G. (1997) Biochem. J., 325, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klungland A. and Lindahl,T. (1997) EMBO J., 16, 3341–3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas L., Yang,C.H. and Goldthwait,D.A. (1982) Biochemistry, 21, 1162–1169. [DOI] [PubMed] [Google Scholar]
- 28.O’Connor T.R. and Laval,J. (1991) Biochem. Biophys. Res. Commun., 176, 1170–1177. [DOI] [PubMed] [Google Scholar]
- 29.Chakravarti D., Ibeanu,G.C., Tano,K. and Mitra,S. (1991) J. Biol. Chem., 266, 15710–15715. [PubMed] [Google Scholar]
- 30.Samson L., Derfler,B., Boosalis,M. and Call,K. (1991) Proc. Natl Acad. Sci. USA, 88, 9127–9131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engelward B.P., Boosalis,M.S., Chen,B.J., Deng,Z., Siciliano,M.J. and Samson,L.D. (1993) Carcinogenesis, 14, 175–181. [DOI] [PubMed] [Google Scholar]
- 32.Roy R., Brooks,C. and Mitra,S. (1994) Biochemistry, 33, 15131–15140. [DOI] [PubMed] [Google Scholar]
- 33.Ye N., Holmquist,G.P. and O’Connor,T.R. (1998) J. Mol. Biol., 284, 269–285. [DOI] [PubMed] [Google Scholar]
- 34.Thielmann H.W., Schroder,C.H. and Hsie,A.W. (1988) Mutat. Res., 202, 235–250. [DOI] [PubMed] [Google Scholar]
- 35.Shiloh Y. and Becker,Y. (1981) Cancer Res., 41, 5114–5120. [PubMed] [Google Scholar]
- 36.Singer B. (1979) J. Natl Cancer Inst., 62, 1329–1339. [PubMed] [Google Scholar]
- 37.Lawley P.D. and Brookes,P. (1963) Biochem. J., 89, 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao W. and Samson,L. (1993) Proc. Natl Acad. Sci. USA, 90, 2117–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly J.D., Inga,A., Chen,F.-X., Dande,P., Shah,D., Monti,P., Aprile,A., Burns,P.A., Scott,G., Abbondandolo,A., Gold,B. and Fonza,G. (1999) J. Biol. Chem., 274, 18327–18334. [DOI] [PubMed] [Google Scholar]
- 40.Engelward B.P., Weeda,G., Wyatt,M.D., Broekhof,J.L.M., de Wit,J., Douker,I., Allan,J.M., Hoeijmakers,J.H.J. and Samson,L. (1997) Proc. Natl Acad. Sci. USA, 94, 13087–13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xiao W. and Chow,B.L. (1998) Curr. Genet., 33, 92–99. [DOI] [PubMed] [Google Scholar]
- 42.Memisoglu A. and Samson,L. (2000) J. Bacteriol., 182, 2104–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cleaver J.E. (1971) Mutat. Res., 12, 453–462. [DOI] [PubMed] [Google Scholar]
- 44.Thielmann H.W., Edler,L. and Friemel,S. (1986) J. Cancer Res. Clin. Oncol., 112, 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regan J.D. and Setlow,R.B. (1974) Cancer Res., 34, 3318–3325. [PubMed] [Google Scholar]
- 46.Li Q., Laval,J. and Ludlum,D.B. (1997) Carcinogenesis, 18, 1035–1038. [DOI] [PubMed] [Google Scholar]
- 47.Holmquist G.P. (1998) Mutat. Res., 400, 59–68. [DOI] [PubMed] [Google Scholar]