Abstract
Novel therapeutics for the treatment of ischemic stroke remains to be the unmet clinical needs. Previous studies have indicated that salvianolic acid A (SAA) is a promising candidate for the treatment of the brain diseases. However, SAA has poor absolute bioavailability and does not efficiently cross the intact blood-brain barrier (BBB), which limit its efficacy. To this end we developed a brain-targeted liposomes for transporting SAA via the BBB by incorporating the liposomes to a transport receptor, insulin-like growth factor-1 receptor (IGF1R). The liposomes were prepared by ammonium sulfate gradients loading method. The prepared SAA-loaded liposomes (Lipo/SAA) were modified with IGF1R monoclonal antibody to generate IGF1R antibody-conjugated Lipo/SAA (IGF1R-targeted Lipo/SAA). The penetration of IGF1R-targeted Lipo/SAA into the brain was confirmed by labeling with Texas Red, and their efficacy were evaluate using middle cerebral artery occlusion (MCAO) model. The results showed that IGF1R-targeted Lipo/SAA are capable of transporting SAA across the BBB into the brain, accumulation in brain tissue, and sustained releasing SAA for several hours. Administration o IGF1R-targeted Lipo/SAA notably reduced infarct size and neuronal damage, improved neurological function and inhibited cerebral inflammation, which had much higher efficiency than no-targeted SAA.
Keywords: Salvianolic acid A, Brain-targeted liposomes, Ischemic stroke, Blood-brain barrier, Neuroprotective effect
Introduction
Stroke remains to be one of the leading causes of mortality and morbidity worldwide, including hemorrhagic and ischemia strokes, in which ischemic stroke accounted for approximately 87% of all cases [1]. During the past decades, there are no real breakthrough drugs in the treatment of stroke. To date, recombinant tissue plasminogen activator (rtPA), a thrombolytic drug, is still the only one pharmacological treatment approved by FDA for ischemic stroke. Although there are a number of neuroprotective agents in clinic for the treatment of stroke, none of these have shown significant improvement of functional outcome in patients. Furthermore, most putative neuroprotective agents fail to provide any protection in clinic setting, or their therapeutic efficacy are insufficient and have only shown slightly improvement of symptoms [2]. Consequently, the development of novel therapeutical strategies to address the unmet clinical needs is urgent.
Salvianolic acid A (SAA), a most active water-soluble component isolated from a famous traditional Chinese medicine Danshen, has been widely investigated for treatment of many cardiovascular and cerebrovascular diseases. It has been reported that SAA possesses the activities of anti-thrombosis [3], anti-inflammation [4], anti-oxidation [5]. SAA could ameliorate myocardial ischemic [6] and cerebral ischemic injury [7,8], improved impairment of memory [9], and relieve cognitive disorder after chronic cerebral ischemia [10]. Currently, SAA is in a phase II clinical trial for treatment of diabetic microangiopathy (CTR20210544) and in two phase I clinical trials for treatment of angina pectoris and diabetes (CTR20181023, NCT03908242). It is obvious that SAA is a promising candidate for the treatment of the brain diseases. However, SAA has poor absolute bioavailability after oral administration [11]. In our previous study (unpublished data), SAA is rapidly metabolized and eliminated after intravenous injection, and does not efficiently cross the intact blood-brain barrier (BBB) and accumulate in the brain tissue. In the most of previous studies, administration rout of SAA was limited to conventional injection fluid, lacking of targeting effect.
BBB serve as one of the formidable obstacles in delivering therapeutic agents to the central nervous system (CNS). Nanotechnology has come out as an innovative and promising drug delivery platform for delivering neurotherapeutics across BBB. Among various types of nanotechnology, liposomes are the most widely studied carrier systems because of their biocompatibility, low toxicity and easy surface modification [12]. Liposomes can lower the minimum effective dose of drugs by prolonging drug circulation and accumulating drugs at the site of injury.
Although SAA has showed some neuroprotection in pre-clinical investigations, its efficacy in treating brain tissue diseases is limited due to its chemical properties. To this end we developed a brain-targeted liposomes by incorporating the liposomes to an BBB-expressed receptor, insulin-like growth factor-1 receptor (IGF1R), for transporting SAA via the BBB in a safe, controlled, and effective manner.
Materials and Methods
Regents
SAA (purity of 98%) was kindly provided by Professor Guiwu Qu of Binzhou Medical University. Hydrogenated soybean phosphatidylcholine (HSPC) was purchased from Aiweituo Pharmaceutical Technology Co. Ltd. (Shanghai, China). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethylene glycol)-2000] (DSPE-PEG2000-Mal) were products of Pengshuo Biology Co. Ltd. (Shanghai, China). Cholesteryl hemisuccinate (CHEMS) and Texas Red were purchased from Macklin (Shanghai, China). IGF1R antibody (SC-81464) was product of Santa Cruz Biotechnology. NeuN rabbit monoclonal antibody, one step TUNEL apoptosis assay kit, horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG and immunol fluorescence staining kit with FITC-labeled goat anti-rabbit IgG were purchased from Beyotime Biotechnology (Shanghai, China). Anti-CD16 antibody was product of Abcam (ab203883, Abcam). All other chemicals were of analytical grade.
Animals
Male ICR mice (weight, 16–18 g) and Sprague-Dawley (SD) rats (weight, 230–240 g) were purchased from Jinan Pengyue Experimental Animal Breeding Co., Ltd. (NO.370726221101430386, No.370726221101366887 and No.370726230101323358, Jinan, China) and acclimated to laboratory condition for at least 1 week at a temperature of 24 ± 1 °C and 55% ± 5% relative humidity. Mice were kept in cages and were free to take food and tap water. The experiments were performed according to the guidelines specified in the Good Laboratory Practice Regulations by China Food and Drug Administration (CFDA) and recommendations of the National Institutes of Health Guide regarding the Care and Use of Laboratory Animals. The permission of animal use was approved by Office of Experimental Animal Management Committee of Shandong Province, China (License number: SYXK [Lu] 20180028). The Animal Ethics Committee of Yantai University gave consent to all animal protocols (Approval date: March 1, 2023, Dec 15, 2024).
Preparation of SAA-loaded liposomes and IGF1R-targeted Lipo/SAA
The liposomes were prepared by ammonium sulfate gradients loading method. Briefly, HSPC 14.17 mg, CHEMS 4.87 mg, DSPE-PEG2000 3.37 mg and DSPE-PEG2000-Mal 0.87 mg were dissolved and well mixed in 0.5 mL absolute ethanol. The mixture was evaporated to produce lipid film at 65 °C in a water bath and nitrogen atmosphere, then a 4 mL solution of 250 mM was added into the lipid film and stirred for 30 min. The liposome suspension was placed in a glass bottle in an ice bath and sonicated with a probe-type sonicator at 300 W power (sonic & Materials, Inc., 20 kHz) for 5 min. The liposomes were placed in 0.9% saline and then dialyzed overnight. After dialysis, the liposome suspension was incubated with SAA (1.80 mg) for 15 min and then was ultrafiltered and finally eluted three times with PBS, culminating in the production of SAA-loaded liposomes (Lipo/SAA). The obtained Lipo/SAA was stored at 4 °C.
Traut's reagent was used for the thiolation of IGF1R antibody. IGF1R antibody was diluted to a final concentration of 0.2 mg/mL with PBS. IGF1R antibody and 20-times molar excess of Traut's reagent were mixed, and the reaction proceeded at room temperature for 1 h under dark condition and in a nitrogen atmosphere. Residual reagent was removed by ultrafiltration centrifuge. Conjugations of the antibody to the liposomes were performed by incubation of thiolated IGF1R antibody and Lipo/SAA at room temperature for 24 h. The end product of IGF1R antibody-conjugated Lipo/SAA (IGF1R-targeted Lipo/SAA) was obtained. The product was then centrifuged at 3600 rpm for 30 min to remove unconjugated antibody.
Characterization of SAA-loaded liposomes and IGF1R-targeted Lipo/SAA
The particle diameters and diameters distribution, as well as surface zeta potential, were determined by nanoparticle size analyzer (Beckman Coulter, Brea, CA, USA). The surface morphology and nanoparticle shape were observed by transmission electron microscopy (TEM) (JEOL, Tokyo, Japan).
The amount of SAA encapsulated into Lipo/SAA and IGF1R-targeted Lipo/SAA was determined by the high-performance liquid chromatography (HPLC) (Dikma technologies, Foothill Ranch, CA, United States). HPLC was conducted using an C18 column (NeoSphere, 250 mm × 4.6 mm, 5 μm). The mobile phase consisted of acetonitrile and 0.1% phosphoric acid water (24:76 v/v). The flow rate and column oven temperature were respectively set to 1.0 mL/min and 35 °C. The injection volume was 20 μL and the detection wavelength was 286 nm. Drug encapsulation efficiency (EE, %) was calculated by the following equation:
The antibody concentration and coupling efficiency were measured using BCA protein assay kit (Beyotime Biotechnology, Shanghai, China).
In vitro release
The in vitro drug release was analyzed by dialysis method. Free SAA, Lipo/SAA and IGF1R-targeted Lipo/SAA were placed in the dialysis bay, which was immersed in 30 mL of PBS with continuous gentle stirring at 37 °C for 24 h. At selected time points (5 min, 10 min, 20min, 30min, 40min, 60min, 90min, 120min, 180 min for free SAA and 5 min and 30 min, 1, 2, 4, 6, 8, 12 and 24 h for Lipo/SAA and IGF1R-targeted Lipo/SAA), 200 μL of solution was collected from the release medium, and the amount of SAA was detected by HPLC. The cumulative release rate of SAA was calculated.
In Vivo Animal Studies
Assessment of BBB penetration
Intravenous injection of liposomes labeled with Texas Red with or without IGF1R targeter to ICR mice was used for evaluation of liposomal penetration to the brain. Two and 6 h after the injection, mice were sacrificed, and perfused with saline to remove the liposome residues. Brains were removed and cut into slices, the fluorescence images were captured using the PerkinElmer IVIS Spectrum live animal imaging system, with excitation 595 nm and emission 635 nm.
Transient middle cerebral artery occlusion (MCAO) and treatment protocol
The surgical procedure was performed as described in our previous report [13]. Free SAA and IGF1R-targeted Lipo/SAA both at dosage of 20 mg/kg, and edaravone 3 mg/kg were administered intravenously 5 min prior to reperfusion. IGF1R-targeted Lipo/SAA were given only once. Free SAA and edaravone were ministered again at 3 h after reperfusion, and then at 24 h and 48 h after administration. The same volumes of blank liposomes solution were administered in the same manner to rats of the sham and MCAO groups.
Neurological evaluation and infarct analysis
The neurological evaluation was performed at 24 h, 48 h and 72 h after ischemia according to the method described by Yokoo et al. [14]. The total score is 48, which represents worst function. The score was performed by an observer blinded to group assignment. After the final neurological evaluation, animals were weighed, sacrificed with anaesthesia with isoflurane. The brains were removed, immersed in normal saline to clean residual blood. Then the brains were frozen at −20 °C for 15–20 min. Two-mm-thick coronal sections were cut with the aid of a brain slicer matrix, and six coronal slices were obtained. Brain slices were incubated with 2% TTC solution for 10min at 37 °C under dark condition, after which sections were fixed in 4% paraformaldehyde solution for photography. Brain areas were traced and measured using the Image-pro plus 6.0 software. The infarcted regions and the areas of both hemispheres were calculated for each brain slice. The average area of infarction was expressed as the percentage of the whole coronal section.
Immunohistochemical assay
Brain tissues were collected at 72 h after MCAO and fixed in 4% neutral paraformaldehyde. Paraffin-embedded brain tissue sections (5 μm thickness) were regularly dewaxed and hydrated through graded ethanol. Sodium citrated solution was used for heat-induced antigen retrieval. The sections were treated for 10 min with 3% H2O2 followed by 5 % BSA to block non-specific binding for 20 min. After that, sections were incubated with anti-NeuN (at a 1:500 dilution) and anti-CD 16 (at a 1:200 dilution) overnight and then with a horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG secondary antibody for 1 h at 37 °C. The antibody signal was detected using DAB regents. Quantitative analysis was performed by counting the numbers of positively stained cells from 5 randomly selected microscopic regions in the ischemic hemispheres, which was analyzed using Image-Pro Plus. Data were expressed as mean cells numbers per rat.
Immunofluorescence assay
Coronal sections (5 μm thickness) were used. After regular deparaffinization, rehydration and antigen retrieval, sections were incubated for 15 min in proteinase k solution (20 μg/mL) followed by 5% BSA to block non-specific binding for 30 min. Sections were then incubated with anti-NeuN antibody overnight at 4 °C. After washing four times with PBS (pH 7.4), the sections were incubated with TUNEL test regent for 60 min at 37 °C. The FTIC-labeled goat anti-rabbit IgG secondary antibody (1:1000) was added to cover the tissue and incubated at 37 °Cfor 60 min in the dark condition. Then the slides were mounted with antifade polyvinylpyrrolidone mounting medium and coverslip. Fluorescence images were obtained using OLYMPUS fluorescence microscope BX53 (original magnifications × 100). For this TUNEL/NeuN immunofluorescent double-labeled staining, 10 randomly selected microscopic regions per section were imaged and analyze using Image J software. We counted total NeuN-positive cells and TUNEL/NeuN-positive cells, to obtain the percentage of apoptotic neurons.
Fourteen days repeated dose toxicity study in rats
Fifteen rats were randomly divided into three groups: Control, blank liposomes and IGF1R-targeted Lipo/SAA 20 mg/kg, with 5 rats in each group. Animals were infused with IGF1R-targeted Lipo/SAA, once daily for 14 days. The same volumes of blank liposomes were administered in the same manner to rats. Rats in control were given same volumes of normal saline. Animals were observed for changes in clinical signs and toxic reactions. Body weight was measured once daily. At the end of administration, blood was collected for hematology and serum biochemistry from abdominal aorta. Then, animals were dissected and visual observation were performed. Key organs including heart, liver, spleen, lung, kidneys and other organs with obviously abnormal lesions were collected. All samples were fixed in 4% neutral buffered formalin, paraffin embedded, and were sliced into 5 μm sections. Sections were stained with H&E and followed microscopic examination.
Statistical analysis
Statistical analysis was performed using SPSS 17.0 and GraphPad_Prism software 7.0 for Windows. All results were expressed as mean ± standard deviation (SD).
Quantitative data were tested for homogeneity of variance. If the variances were equal (P > 0.05), one-way ANOVA followed by Dunnett test was used. If the variances were unequal (P < 0.05). Nonparametric tests were performed. Comparisons between groups were made using the Kruskal-Wallis test followed by the Mann-Whitney U test. P < 0.05 was considered significant.
Results and Discussion
Characterization of SAA-loaded liposomes and IGF1R-targeted Lipo/SAA
In our previous study (unpublished data), SAA rapidly entered the bloodstream after intravenous given to healthy SD rats, and the plasma drug concentration reaches its peak in an instant (Tmax < 5 min). Intravenous administration of SAA was rapidly distributed to tissues and organs, and then eliminated from plasma, with elimination half-life (t1/2) of approximately 1 h. The concentration of SAA was relatively higher in lung, liver and heart within 5 min to 1 h after the beginning of administration. At 3 h, the drug level in all tissues dropped far below the limit of detection. A limited amount of SAA is capable of entering the central nervous system.
BBB prevents the access of majority of therapeutics to the CNS. Receptor-mediated transcytosis (RMT) is currently concerned BBB-crossing technologies [15]. IGF1R is an BBB-expressed receptor that is highly expressed in brain endothelial cells (BEC) and can transport its ligands (IGF1 and insulin) to specific brain regions [16,17]. Targeting this receptor is considered to be a suitable and promising approach to be developed as carrier for delivering therapeutic molecules into the brain [17].
The present study descried the preparation and efficacy of IGF1R-targeted Lipo/SAA. We created small, stable liposomes utilizing HSPC, CHEMS, DSPE-PEG2000 and DSPE-PEG2000-Mal. Theoretically, these liposomes incorporated anti-IGF1R antibody that can be recognized by IGF1R, mediating transport of SAA across the BBB into the brain via RMT. Our results showed that the encapsulation efficiency (EE) of Lipo/SAA and IGF1R-targeted Lipo/SAA were found to be 81.25% ± 1.24% and 79.25% ± 1.38%, respectively. Drug loading were found to be 6.20% ± 0.47% and 5.16% ± 0.33%. IGF1R-targeted Lipo/SAA possessed 71.39 ± 8.81% coupling rate. The particle size, PDI, and zeta potential of Lipo/SAA and IGF1R-targeted Lipo/SAA were summarized in Table 1. Shape and surface morphology of prepared liposomes were evaluated by TEM. The result showed that the liposomes were spherical in shape, and the particles size distribution were fairly uniform. The antibody conjugation on liposomes did not greatly change the particles size (Fig. 1). This may be attributed to the tight mushroom-like conformation of PEG2000 at a concentration of 4% [18]. Adding monoclonal antibody to its end does not noticeably change the particle size. The average liposome size maintained on the level of 100 nm. It is reported that liposomes with a size of 100 nm crossed the BBB more easily and had much higher accumulation in the brain tissue than large liposomes [19].
Table 1.
The physicochemical properties of liposomes.
| Formulation | Particle size (nm) | Polydispersity Index | Zeta potential (mV) | Encapsulation efficiency (%) | Drug loading (%) |
|---|---|---|---|---|---|
| Lipo/SAA | 106.6 ± 0.35 | 0.161 ± 0.011 | −5.91 ± 0.47 | 81.25 ± 1.24 | 6.20 ± 0.47 |
| IGF1R-targeted Lipo/SAA | 108.2 ± 0.61 | 0.188 ± 0.009 | 5.67 ± 0.79 | 79.25 ± 1.38 | 5.16 ± 0.33 |
Fig. 1.
Transmission electron microscope images of Lipo/SAA (left) and IGF1R-targeted Lipo/SAA (right).
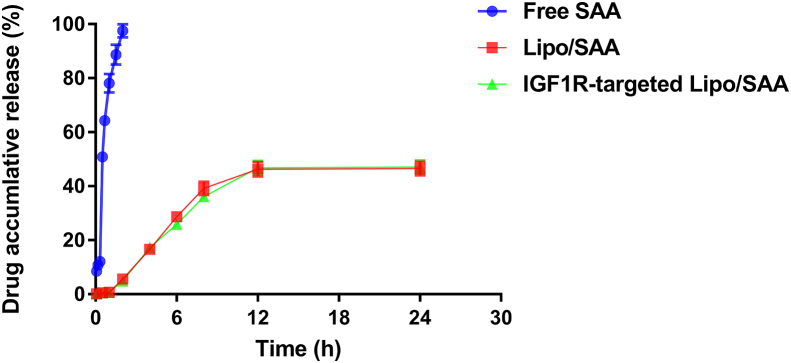
To study the release kinetics, the in vitro release study was performed. The cumulative amount of drug released for free SAA in 3 h was 98%, whereas it was approximately 43% for Lipo/SAA and IGF1R-targeted Lipo/SAA in 24 h, indicating that SAA-loading liposomes exhibited slow-release characteristics (Fig. 2). It is well known that ischemic stroke is followed by the amount production of excitotoxicity, oxidative stress and inflammation, and microvascular injury. These events contribute to brain injury. The onset of oxidative stress and inflammation occur in several minutes and continue for several hours or days [20]. Thus, the sustained release of IGF1R-targeted Lipo/SAA may provide beneficial effect in the treatment of ischemic stroke.
Fig. 2.
Cumulative release curve of drug.
Penetration of Texas Red labeled IGF1R-targeted liposomes into mouse brain
The penetration of IGF1R-targeted liposomes into the brain was confirmed by labeling non-targeted liposomes or IGF1R-targeted liposomes with Texas Red. As shown in Fig. 3, IGF1R-targeted liposomes labeled with Texas Red penetrated the brain and distributed through the brain 2 h and 6 h after administration, yet non-targeted liposomes had only a very small trace. It seems that the current targeted liposomes are capable of transporting SAA across the BBB into the brain, accumulation in brain tissue, and sustained releasing SAA for several hours.
Fig. 3.
Fluorescence images of coronal brain sections of mice. IGF1R-targeted or non-targeted liposomes labeled with Texas Red were administered to ICR mice by intravenous injection. Two or 6 h after the injection, mice were sacrificed, and perfused with saline to remove the liposome residues. Brains were removed and cut into slices, the fluorescence images were captured using the PerkinElmer IVIS Spectrum live animal imaging system, with excitation 595 nm and emission 635 nm.
Neuroprotective of IGF1R-targeted Lipo/SAA in MCAO in rats
In order to evaluate the neuroprotective of IGF1R-targeted Lipo/SAA, MCAO model in rats was used. Representative sections of each group are presented in Fig. 4a. As shown in Fig. 4b, the infarct size of MCAO group was 25.6 ± 2.0% at 72 h after ischemia injury. When treatment with edaravone 3 mg/kg, the infarct size was 17.0 ± 4.1%, which was significantly decreased as compared with MCAO group. Similarly, treatment with free SAA and IGF1R-targeted Lipo/SAA 20 mg/kg inhibited the expansion of the infarct. The infarct sizes were 18.2 ± 8.0% and 13.5 ± 7.0%, respectively. Treatment with IGF1R-targeted Lipo/SAA 20 mg/kg caused approximately 47% reduction in infarct size (Fig. 4b). In addition, administration of IGF1R-targeted Lipo/SAA markedly reduced neurological scores at all selected time points. The effect of free SAA 20 mg/kg was less potent than that of IGF1R-targeted Lipo/SAA in reducing infarct size and neurological scores. Free SAA showed only significant improvement of neurological symptoms on day 2 (Fig. 4c). In this experiment, a single-dose of IGF1R-targeted Lipo/SAA produced favorable outcome, which indicated that the novel drug delivery system allows minimal SAA dose.
Fig. 4.
Neuroprotective of administration of IGF1R-targeted Lipo/SAA in MCAO in rats. Free SAA, IGF1R-targeted Lipo/SAA and edaravone were administered intravenously 5 min prior to reperfusion. IGF1R-targeted Lipo/SAA were given only once. Free SAA and edaravone were ministered again at 3 h after reperfusion, and then at 24 h and 48 h after administration. (a) Representative coronal sections of brain in each group. (b) Infarct area of each group. (c) Neurological scores. Data are represented as mean ± S.D. of 9 animals of each group (n = 5 for Sham group). ∗P < 0.05 and ∗∗P < 0.01 vs. MCAO group.
Effect of IGF1R-targeted Lipo/SAA on ischemic neuronal injury
Seventy-two hours after ischemia-reperfusion brain injury using the intraluminal thread method, the number NeuN-positive cells significantly decreased in brain tissue of MCAO rats, which indicated that neurons were damaged. As compared with MCAO group, administration of IGF1R-targeted Lipo/SAA resulted in markedly higher number of neurons (Fig. 5). Treatment with SAA and edaravone showed similar effect but without statistically significant differences when compared with MCAO group. We further performed immunofluorescence experiment to analyze neuronal apoptosis using a simultaneous NeuN/TUNEL labeling. The number of NeuN- and TUNEL-positive cells notablely increased in brain tissue of MCAO rats. Treatment with edaravone, SAA and IGF1R-targeted Lipo/SAA significantly reduced the number of apoptotic cells, and then inhibited neuronal apoptosis (Fig. 6a and b). IGF1R-targeted Lipo/SAA showed better protective effect against neuronal damage than free SAA.
Fig. 5.
Effect of IGF1R-targeted Lipo/SAA on ischemic neuronal injury. Immunostaining sections of NeuN and NeuN-positive cells. Original magnification, × 100. Data are represented as mean ± S.D. of 5 animals of each group. ##P < 0.01 vs. Sham, ∗P < 0.05 and ∗∗P < 0.01 vs. MCAO group.
Fig. 6.
Effect of IGF1R-targeted Lipo/SAA on neuronal apoptosis. (a) Immunofluorescent pictures of TUNEL/NeuN double-labeled staining of brain tissues. Original magnification, × 100; (b) The percentage of number of TUNEL/NeuN-positive cells. Data are represented as mean ± S.D. of 5 animals of each group. ##P < 0.01 vs. Sham, ∗P < 0.05 and ∗∗P < 0.01 vs. MCAO group.
Effect of IGF1R-targeted Lipo/SAA on cerebral inflammation
Microglia, the resident immune cells of the CNS, play an important role in priming inflammatory response to various brain injuries [21,22]. Following ischemic stroke, microglia increase in number and become activated. Activated microglia become polarized towards M1 or M2 phenotypes. M1-populations have been recognized as detrimental phenotypes. They release proinflammatory factors and free radicals, which can inhibit the repair of CNS and aggravate tissue damage [23]. Thus, inhibition of polarization and function of M1-microglia populations could contribute to temper cerebral inflammation [23,24]. As shown in Fig. 7a and b, 72 h after ischemia-reperfusion brain injury, the amount of CD16-positive cells (M1 phenotype biomarker) significantly increased in brain tissue of MCAO rats. Edaravone and IGF1R-targeted Lipo/SAA markedly reduced the cell numbers. Administration of free SAA 20 mg/kg reduced the number of CD16-positive cells to some degree, but the difference was not statistically significant. Furthermore, IGF1R-targeted Lipo/SAA significantly decreased production of IL-6. The result suggested that IGF1R-targeted Lipo/SAA ameliorated neuroinflammation via inhibition of activation of microglia and release of proinflammatory cytokines. IGF1R-targeted Lipo/SAA exhibited more potent anti-neuroinflammation effect than free SAA.
Fig. 7.
Effect of IGF1R-targeted Lipo/SAA on cerebral inflammation. (a) Immunostaining sections of CD16 and CD16-positive cells. Original magnification, × 100; (b) IL-6 level in brain tissues. Data are represented as mean ± S.D. of 5 animals of each group. ##P < 0.01 vs. Sham, ∗∗P < 0.01 vs. MCAO group.
Fourteen days repeated dose toxicity study of IGF1R-targeted Lipo/SAA in rats
Biosafety of nanomedicines is critically important for clinical applications. To further verify the repeated dose toxicity of IGF1R-targeted Lipo/SAA, we conducted a 14-day repeated dose toxicity test in rats. During the experiment, daily treatment with IGF1R-targeted Lipo/SAA for 14 days had found no clinical abnormalities in animals. Body weight changes showed no obvious discrepancy in all groups. All parameters of hematology and blood chemistry did not significantly differ in animals in IGF1R-targeted Lipo/SAA and control group (Table 2, Table 3). In addition, no macroscopic differences and significant histopathological abnormalities were observed at the end of dosing (Fig. 8). In our previous study of 4-week repeated dose intravenous toxicity, the no observed adverse effect level (NOAEL) of SAA was 20 mg/kg in Beagle dogs following daily intravenous administration [25]. Those results indicate that SAA had good tolerance and safety profiles. Nanoparticles for SAA may not increase its toxicity. Certainly, the safety of long-term administration of higher IGF1R-targeted Lipo/SAA dosage remains to be determined.
Table 2.
Effects of IGF1R-targeted Lipo/SAA on Hematology of rats.
| Parameter | Control | Blank liposomes | IGF1R-targeted Lipo/SAA 20 mg/kg |
|---|---|---|---|
| WBC (109/L) | 6.72 ± 2.28 | 6.91 ± 0.42 | 6.63 ± 3.47 |
| NEU % (%) | 24.98 ± 1.73 | 24.59 ± 3.73 | 24.76 ± 6.69 |
| LYM% (%) | 63.65 ± 2.11 | 62.01 ± 6.03 | 62.88 ± 8.45 |
| MONO% (%) | 10.97 ± 0.68 | 12.62 ± 2.46 | 11.81 ± 1.82 |
| EOS% (%) | 0.76 ± 0.28 | 0.71 ± 0.35 | 0.50 ± 0.39 |
| BASO% (%) | 0.01 ± 0.02 | 0.08 ± 0.16 | 0.05 ± 0.09 |
| RBC (1012/L) | 6.72 ± 0.50 | 6.76 ± 0.39 | 5.80 ± 2.64 |
| HGB (g/dL) | 142.75 ± 9.74 | 145.00 ± 5.23 | 140.75 ± 58.86 |
| HCT (fL) | 53.00 ± 3.26 | 53.65 ± 2.28 | 55.40 ± 20.97 |
| MCV (fL) | 79.00 ± 1.29 | 79.50 ± 4.23 | 77.95 ± 2.72 |
| MCH (pg) | 21.25 ± 0.26 | 21.48 ± 0.90 | 20.20 ± 1.95 |
| MCHC (g/dL) | 269.25 ± 3.40 | 270.25 ± 4.79 | 260.00 ± 17.81 |
| PLT (109/L) | 615.00 ± 151.03 | 683.00 ± 35.15 | 625.25 ± 396.79 |
WBC, White blood cell count; NEU, Neutrophil; LYM, Lymphocyte; MONO, Monocyte; EOS, Eosinophils; BASO, Basophil; RBC, Red blood cell; HGB, Hemoglobin; HCT, Hematocrit; MCV, Mean corpuscular volume; MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; PLT, Platelet; RET, Reticulocyte; PT, Thrombin time.
Table 3.
Effects of IGF1R-targeted Lipo/SAA on clinical chemistry of rats.
| Parameter | Control | Blank liposomes | IGF1R-targeted Lipo/SAA 20 mg/kg |
|---|---|---|---|
| ALT (U/L) | 38.0 ± 2.2 | 33.0 ± 7.2 | 34.5 ± 8.2 |
| AST (U/L) | 108.3 ± 20.4 | 112.3 ± 25.4 | 111.3 ± 25.4 |
| AST/ALT | 2.83 ± 0.44 | 3.13 ± 0.22 | 3.03 ± 0.82 |
| T-BIL (μmol/L) | 0.15 ± 0.06 | 0.17 ± 0.54 | 0.30 ± 0.14 |
| CK (U/L) | 621.8 ± 361.9 | 652.5 ± 298.6 | 679.8 ± 163.8 |
| BUN (mmol/L) | 4.83 ± 0.20 | 4.36 ± 0.35 | 4.95 ± 0.98 |
| CRE (μmol/L) | 22.4 ± 2.8 | 23.1 ± 2.6 | 25.5 ± 1.4 |
| TP (g/L) | 54.35 ± 5.98 | 57.88 ± 1.26 | 48.37 ± 0.35 |
| ALB (g/L) | 29.77 ± 2.96 | 27.22 ± 2.28 | 30.62 ± 1.35 |
| CHOL (mmol/L) | 1.67 ± 0.30 | 1.90 ± 0.97 | 1.73 ± 0.21 |
| TG (mmol/L) | 1.22 ± 0.45 | 2.39 ± 0.49 | 1.53 ± 0.57 |
AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; ALP, Alkaline phosphatase; T-BIL, Total bilirubin; BUN, Blood urea nitrogen; CRE, Creatinine; CK, Creatine kinase; TP, Total protein; ALB, Albumin; CHOL, Cholesterol; TG, Triglyceride;
Fig. 8.
Representative images of histopathological examination showed no significant abnormalities in heart, liver, spleen, lung and kidneys in rats treated with IGF1R-targeted Lipo/SAA 20 mg/kg for 14 days compared with normal control (200× magnification).
In conclusion, the current study demonstrated the ability of the brain-targeted liposomal system to delivery SAA into the brain tissue. IGF1R-targeted Lipo/SAA was capable of crossing the BBB and accumulating in the brain. The prepared brain-targeted liposomes could minimize the dosage of SAA, expand its duration of action and increase activity. IGF1R-targeted Lipo/SAA showed favorable cerebral protection with higher efficiency than free SAA, and had good biosafety. Further preclinical and clinical studies are need before implementation of IGF1R-targeted Lipo/SAA in clinical practice. Future studies will explore the pharmacokinetic characteristics of IGF1R-targeted Lipo/SAA and its minimal effective dose. To achieve better ability to recognize hidden epitopes, high stability, and low immunogenicity, and small size of anti-IGF1R antibodies is expected to be designed and developed.
Author Contributions
YMY: conducted research, analyzed data and wrote original draft.
LY, YYW, GBF, RJ: conducted research and performed statistical analysis.
FHY: designed research and reviewed & edited article. All authors read and approved the final manuscript.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request, pending application and approval.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Hua-Ying Fan reports financial support was provided by Natural Science Foundation of Shandong Province. Hua-Ying Fan reports a relationship with Natural Science Foundation of Shandong Province that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by Natural Science Foundation of Shandong Province (No. ZR202211020017).
References
- 1.Benjamin E.J., Virani S.S., Callaway C.W., Chamberlain A.M., Chang A.R., Cheng S., et al. Heart disease and stroke statistics-2018 update: a report from the American heart association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Goenka L., Uppugunduri Satyanarayana C.R., S S.K., George M. Neuroprotective agents in acute ischemic stroke-A reality check. Biomed Pharmacother. 2019;109:2539–2547. doi: 10.1016/j.biopha.2018.11.041. [DOI] [PubMed] [Google Scholar]
- 3.Fan H.Y., Fu F.H., Yang M.Y., Xu H., Zhang A.H., Liu K. Antiplatelet and antithrombotic activities of salvianolic acid A. Thromb Res. 2010;126:e17–e22. doi: 10.1016/j.thromres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 4.Oh K.S., Oh B.K., Mun J., Seo H.W., Lee B.H. Salvianolic acid A suppress lipopolysaccharide-induced NF-κB signaling pathway by targeting IKKβ. Int Immunopharmacol. 2011;11:1901–1906. doi: 10.1016/j.intimp.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Liu G.T., Zhang T.M., Wang B.E., Wang Y.W. Protective action of seven natural phenolic compounds against peroxidative damage to biomembranes. Biochem Pharmacol. 1992;43:147–152. doi: 10.1016/0006-2952(92)90271-j. [DOI] [PubMed] [Google Scholar]
- 6.Yuan X., Xiang Y., Zhu N., Zhao X., Ye S., Zhong P., et al. Salvianolic acid A protects against myocardial ischemia/reperfusion injury by reducing platelet activation and inflammation. Exp Ther Med. 2017;14:961–966. doi: 10.3892/etm.2017.4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling Y., Jin L., Ma Q., Huang Y., Yang Q., Chen M., et al. Salvianolic acid A alleviated inflammatory response mediated by microglia through inhibiting the activation of TLR2/4 in acute cerebral ischemia-reperfusion. Phytomedicine. 2021;87:153569. doi: 10.1016/j.phymed.2021.153569. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., Song J.K., Yan R., He G.R., Zhang X., Zhou Q.M., et al. Salvianolic acid A alleviate the brain damage in rats after cerebral ischemia-reperfusion through Nrf2/HO-1 pathway. Acta Pharm Sin. 2016;51:1717–1723. [PubMed] [Google Scholar]
- 9.Du G., Zhang J. Protective effects of salvianolic acid A against impairment of memory induced by cerebral ischemia-reperfusion in mice. Chin Med J (Engl). 1997;110:65–68. [PubMed] [Google Scholar]
- 10.Yang Y., Song J., Liu N., Wei G., Liu S., Zhang S., et al. Salvianolic acid A relieves cognitive disorder after chronic cerebral ischemia: involvement of Drd2/Cryab/NF-κB pathway. Pharmacol Res. 2022;175:105989. doi: 10.1016/j.phrs.2021.105989. [DOI] [PubMed] [Google Scholar]
- 11.Sun J., Zhang L., Song J., Tian S., Huang C., Feng Z., et al. Pharmacokinetic study of salvianolic acid A in beagle dog after oral administration by a liquid chromatography-mass spectrometry method: a study on bioavailability and dose proportionality. J Ethnopharmacol. 2013;148:617–623. doi: 10.1016/j.jep.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Bruch G.E., Fernandes L.F., Bassi B.L.T., Alves M.T.R., Pereira I.O., Frézard F., et al. Liposomes for drug delivery in stroke. Brain Res Bull. 2019;152:246–256. doi: 10.1016/j.brainresbull.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Yang M.Y., Yu Q.L., Huang Y.S., Yang G. Neuroprotective effects of andrographolide derivative CX-10 in transient focal ischemia in rat: involvement of Nrf2/AE and TLR/NF-κB signaling. Pharmacol Res. 2019;144:227–234. doi: 10.1016/j.phrs.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Yokoo N., Sheng H., Mixco J., Homi H.M., Pearlstein R.D., Warner D.S. Intraischemic nitrous oxide alters neither neurologic nor histologic outcome: a comparison with dizocilpine. Anesth Analg. 2004;99:896–903. doi: 10.1213/01.ANE.0000132973.32387.8B. [DOI] [PubMed] [Google Scholar]
- 15.Stanimirovic D.B., Sandhu J.K., Costain W.J. Emerging technologies for delivery of biotherapeutics and gene therapy across the blood-brain barrier. BioDrugs. 2018;32:547–559. doi: 10.1007/s40259-018-0309-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werner H., LeRoith D. Insulin and insulin-like growth factor receptors in the brain: physiological and pathological aspects. Eur Neuropsychopharmacol. 2014;24:1947–1953. doi: 10.1016/j.euroneuro.2014.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Alata W., Yogi A., Brunette E., Delaney C.E., van Faassen H., Hussack G., et al. Targeting insulin-like growth factor-1 receptor (IGF1R) for brain delivery of biologics. FASEB J. 2022;36 doi: 10.1096/fj.202101644R. [DOI] [PubMed] [Google Scholar]
- 18.Merino M., Zalba S., Garrido M.J. Immunoliposomes in clinical oncology: state of the art and future perspectives. J Control Release. 2018;275:162–176. doi: 10.1016/j.jconrel.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 19.Fukuta T., Asai T., Sato A., Namba M., Yanagida Y., Kikuchi T., et al. Neuroprotection against cerebral ischemia/reperfusion injury by intravenous administration of liposomal fasudil. Int J Pharm. 2016;506:129–137. doi: 10.1016/j.ijpharm.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 20.Brouns R., De Deyn P.P. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg. 2009;111:483–495. doi: 10.1016/j.clineuro.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Perry V.H., Teeling J. Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 2013;35:601–612. doi: 10.1007/s00281-013-0382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang C.T., Wu W.F., Deng Y.H., Ge J.W. Modulators of microglia activation and polarization in ischemic stroke (Review) Mol Med Rep. 2020;21:2006–2018. doi: 10.3892/mmr.2020.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X., Leak R.K., Shi Y., Suenaga J., Gao Y., Zheng P., et al. Microglial and macrophage polarization—new prospects for brain repair. Nat Rev Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Z.V., Lyu H., Lam S.Y.E., Lam P.K., Poon W.S., Wong G.K.C. The dynamics of microglial polarization reveal the resident neuroinflammatory responses after subarachnoid hemorrhage. Transl Stroke Res. 2020;11:433–449. doi: 10.1007/s12975-019-00728-5. [DOI] [PubMed] [Google Scholar]
- 25.Yang M.Y., Song Z.Y., Gan H.L., Zheng M.H., Liu Q., Meng X.T., et al. Non-clinical safety evaluation of salvianolic acid A: acute, 4-week intravenous toxicities and genotoxicity evaluations. BMC Pharmacol Toxicol. 2022;23:83. doi: 10.1186/s40360-022-00622-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request, pending application and approval.