Abstract
Objectives: To evaluate the diagnostic and prognostic value of insulin-like growth factor-1 (IGF-1), galactoagglutinin-3 (GAL-3), and pentamerin-3 (PTX-3) levels in elderly patients with chronic heart failure (CHF). Methods: In this retrospective study, 107 elderly CHF patients treated in Xiangyang Central Hospital were designated as the observation group, and 60 healthy individuals were selected as the control group. The cardiac function indexes and serum IGF-1, Gal-3, and PTX-3 levels were compared between the two groups. Furthermore, the serum IGF-1, Gal-3, and PTX-3 levels in patients across different cardiac function grades were compared, as well as in patients with poor or favorable prognosis. Additionally, receiver operating characteristic (ROC) curve was adopted to explore the diagnostic value of serum IGF-1, Gal-3, and PTX-3 levels for senile CHF; and multivariate logistic regression analysis was used to screen the independent factors affecting patients’ prognosis. Results: The serum IGF-1 level was significantly lower, while the levels of Gal-3 and PTX-3 were significantly higher in the observation group than those of the control group (all P<0.05). The serum IGF-1 level in patients with cardiac function grade IV was lower than that of the patients with cardiac function grade II and III, while the levels of Gal-3 and PTX-3 were higher than those with cardiac function grade II and III (all P<0.05). The serum IGF-1 level in the patients with cardiac function grade III was lower than those with cardiac function grade II, while the levels of Gal-3 and PTX-3 were higher in patients with grade III than those with grade II (all P<0.05). The serum IGF-1 level was lower, while the levels of Gal-3 and PTX-3 were higher in the patients with poor prognosis than those with favorable prognosis (all P<0.05). Conclusion: In elderly CHF patients, IGF-1 level were decreases, while the levels of Gal-3 and PTX-3 were increase. These biomarkers show high sensitivity in diagnosing CHF and are closely linked to the prognosis, indicating their value for clinical assessment and management of CHF.
Keywords: Insulin-like growth factor-1, galactose agglutinin-3, N-pentamer protein-3, elderly chronic heart failure, prognosis
Introduction
Chronic heart failure (CHF) is a prevalent cardiovascular disease characterized by abnormal peripheral blood flow and significant ventricular ejection dysfunction [1]. Representing the end-stage of cardiovascular disease, CHF is associated with high hospitalization and mortality rates, posing a major public health problem [2]. Particularly in the elderly, the incidence and mortality rates of CHF are escalating due to the aging population, severely impacting their life expectancy, health, and quality of life [3,4]. The early clinical manifestations of CHF often lack specificity, and existing diagnostic tools have certain limitations, making the identification of effective biomarkers for diagnosis and prognosis a critical area of clinical research.
Insulin-like growth factor-1 (IGF-1) plays a crucial role in the cardiovascular system, maintaining the normal function and structure of the heart and facilitating the repair of necrotic myocardial cells [5]. Galactosidin-3 (GAL-3), a member of galactosidin-binding agglutinin family, has been recognized for its involvement in oncogenesis and progression of CHF [6]. Pentameric protein-3 (PTX-3), primarily derived from heart and vascular tissues following injury, is involved in inflammatory responses, with its level reflecting the inflammatory state in the human body [7].
This study aims to improve the diagnostic and prognostic assessment of CHF in the elderly by examining the values of serum IGF-1, Gal-3, and PTX-3 levels. Through this investigation, we seek to provide a solid foundation for clinical treatment strategies and ultimately improve patient outcomes by offering insight into the diagnostic and prognostic evaluation of CHF.
Data and methods
Subjects
In this retrospective analysis, a total of 107 elderly patients with CHF admitted to Xiangyang Central Hospital from March 2020 to March 2021 were selected as the observation group. Besides, 60 healthy individuals had physical examination in the same period were selected as the control group.
Inclusion criteria: ① Patients conforming to the diagnostic criteria released by Chinese Guidelines for the Diagnosis and Treatment of Heart Failure (2018) [8]; ② Patients ≥60 years old; ③ Patients with NYHA cardiac function grade II to IV; ④ Patients with complete clinical data.
Exclusion criteria: ① Patients with pulmonary heart disease, congenital heart disease, or other cardiovascular diseases; ② Patients with pulmonary infection; ③ Patients with hepatic or renal insufficiency; ④ Patients with malignant tumor; ⑤ Patients with severe mental illness; ⑥ Patients with hematological diseases or immune system diseases; ⑦ Terminally ill patients; ⑧ Patients who had recent surgery, trauma, or severe infection.
Reagents and instruments
Main reagents
Human IGF-1 kit (Shanghai Zeye Biotechnology Co., Ltd.), human Gal-3 kit (Shanghai Ruipan Biotechnology Co., Ltd.), human PTX-3 kit (Shanghai Zeye Biotechnology Co., Ltd.).
Main instruments
Philips Color Doppler Ultrasonic analyzer (Beckman Kurt Co., LTD.); Automatic Biochemical analyzer (AU5800); Bio-Rad Microplate tester.
Methods
Serum sample collection
5 ml fasting peripheral venous blood of the subject was collected, centrifuged at 10 cm radius and 3000 r/min for 10 min, and then stored at -20°C for subsequent analysis.
Determination of cardiac function
Left ventricular ejection fraction (LVEF) and left ventricular end-diastolic diameter (LVEDD) were tested using color Doppler ultrasound.
Determination of IGF-1, Gal-3 and PTX-3 levels
The serum level of IGF-1 was determined by radioimmunoassay, and the Gal-3 and PTX-3 levels were measured by ELISA in strict accordance with the standard of kit instruction.
Evaluation of prognosis
The subjects were followed up for 12 months by outpatient follow-up or telephone to record their prognosis. Readmission or death due to CHF was considered as the end point. In addition, patients with readmission or death (n=24) were categorized as having a poor prognosis, and the others (n=83) were deemed to have a favorable prognosis.
Observational indices
Primary indicators: (1) The cardiac function indices were observed; (2) Serum levels of IGF-1, Gal-3, and PTX-3 of the two groups were observed; (3) The serums levels of IGF-1, Gal-3, and PTX-3 in patients across different cardiac function grades (II-IV) were observed; (4) The serum levels of IGF-1, Gal-3, and PTX-3 in patients with different prognosis outcomes were compared.
Secondary indicators: (1) The diagnostic value of IGF-1, Gal-3, and PTX-3 for CHF was analyzed by ROC curve; (2) Multivariate logistic regression was adopted for analyzing the independent risk factors affecting patients’ prognosis.
Statistical treatment
Data processing was conducted by using SPSS 26.0. The measured data conforming to normal distribution were represented by (x̅ ± s), the comparison of measurement data among multiple groups was subjected to F test, and the comparison of measured data between two groups was subjected to t-test. The counted data were expressed as n (%), and the comparison of counted data was subjected to x2 test. ROC curve was incorporated to analyze the diagnostic value of IGF-1, Gal-3, and PTX-3 for CHF. Multivariate logistic regression was applied to explore the independent risk factors influencing prognosis. P<0.05 was considered significant.
Results
Clinical data
The observation group comprised 65 males and 42 females, ranging in age from 62 to 79, with an average age of (70.72±5.51) years old; the course of disease ranged from 1 to 15 years, with an average of (8.84±1.95) years; the body weight spanned from 42-87 kg, with an average of (62.41±8.89) kg; the NYHA cardiac function classification identified 24 cases of grade II, 58 cases of grade III, and 25 cases of grade IV. The control group consisted of 37 males and 23 females, aged 61-76 years, with an average age of (69.85±6.62) years old; the course of disease ranged from 1 to 17 years, with an average of (9.09±2.41) years; the body weight varied from 41 to 85 kg, with an average of (61.73±9.34) kg. There were no significant differences in gender, age or body weight between the two groups (all P>0.05).
Comparison of cardiac function indices between the two groups
The observation group displayed a lower LVEF than control group and a higher LVEDD than the control group (both P<0.05, Table 1).
Table 1.
Comparison of cardiac function indexes between the two groups (x̅ ± s)
| Group | Number of Cases | LVEF (%) | LVEDD (mm) |
|---|---|---|---|
| Observation group | 107 | 49.83±5.16 | 51.21±5.57 |
| Control group | 60 | 65.73±4.28 | 34.25±4.09 |
| t | 20.270 | 20.658 | |
| P | <0.001 | <0.001 |
Note: LVEF: left ventricular ejection fraction. LVEDD: left ventricular end diastolic diameter.
Comparison of serum levels of IGF-1, Gal-3, and PTX-3 between the two groups
The serum level of IGF-1 was lower, while the serum levels of Gal-3 and PTX-3 were higher in the observation group than those in the control group (all P<0.05, Table 2).
Table 2.
Comparison of serum IGF-1, Gal-3, and PTX-3 (x̅ ± s)
| Group | Number of Cases | IGF-1 (ng/ml) | Gal-3 (μg/L) | PTX-3 (μg/L) |
|---|---|---|---|---|
| Observation group | 107 | 24.41±5.36 | 36.87±5.23 | 3.81±0.67 |
| Control group | 60 | 38.84±3.24 | 12.28±3.07 | 1.25±0.35 |
| t | 18.985 | 33.316 | 27.540 | |
| P | <0.001 | <0.001 | <0.001 |
Note: IGF-1: insulin-like growth factor-1. GAL-3: galactoagglutinin-3. PTX-3: pentamerin-3.
Comparison of serum levels of IGF-1, Gal-3, and PTX-3 among patient across different cardiac function grades
The serum level of IGF-1 in patients with cardiac function grade IV were significantly lower than those with grades II and III, while the serum levels of Gal-3 and PTX-3 were significantly higher than those with grades II and III (all P<0.05). Furthermore, the serum IGF-1 level in patients with cardiac function grade III was lower than those with grade II, while the levels of Gal-3 and PTX-3 were higher than those with grades II (all P<0.05). See Table 3.
Table 3.
Comparison of serum IGF-1, Gal-3, and PTX-3 levels among patients across different cardiac function grades (x̅ ± s)
| Cardiac function grade | Number of Cases | IGF-1 (ng/ml) | Gal-3 (μg/L) | PTX-3 (μg/L) |
|---|---|---|---|---|
| Grade II | 33 | 32.51±4.87 | 24.94±3.87 | 2.18±0.45 |
| Grade III | 43 | 24.73±5.82* | 37.21±5.61* | 3.76±0.72* |
| Grade IV | 31 | 16.39±5.41*,∆ | 45.69±5.20*,∆ | 4.71±0.74*,∆ |
| F | 70.593 | 139.519 | 122.837 | |
| P | <0.001 | <0.001 | <0.001 |
Note: Compared to grade II;
P<0.05.
Compared to grade III;
P<0.05.
IGF-1: insulin-like growth factor-1. GAL-3: galactoagglutinin-3. PTX-3: pentamerin-3.
Comparison of IGF-1, Gal-3, and PTX-3 levels in patients with different prognosis
The poor prognosis group had lower IGF-1 levels and higher Gal-3 and PTX-3 levels compared to the good prognosis group favorable prognosis group (all P<0.05, Table 4).
Table 4.
Comparison of serum IGF-1, Gal-3, and PTX-3 levels in patients with different prognoses (x̅ ± s)
| Group | Number of Cases | IGF-1 (ng/ml) | Gal-3 (μg/L) | PTX-3 (μg/L) |
|---|---|---|---|---|
| Favorable prognosis | 83 | 30.48±5.46 | 22.22±3.68 | 2.07±0.48 |
| Poor prognosis | 24 | 17.45±4.73 | 50.04±13.39 | 4.93±1.08 |
| t | 10.590 | 17.001 | 18.701 | |
| P | <0.001 | <0.001 | <0.001 |
ROC curve analysis of IGF-1, Gal-3, and PTX-3 in diagnosing CHF
ROC curve analysis indicated high sensitivity and specificity for IGF-1 (92.80% sensitivity and 91.70% specificity), Gal-3 (100.00% sensitivity and 94.00% specificity), and PTX-3 (91.70% sensitivity and 100.00% specificity) in diagnosing CHF (Table 5; Figure 1).
Table 5.
ROC curve analysis of IGF-1, Gal-3, and PTX-3 in diagnosis of CHF
| Indicators | AUC | Cutoff value | Sensitivity (%) | Specificity (%) | 95% CI |
|---|---|---|---|---|---|
| IGF-1 | 0.965 | 21.80 | 92.80 | 91.70 | 0.830~1.000 |
| Gal-3 | 0.996 | 27.15 | 100.00 | 94.00 | 0.989~1.000 |
| PTX-3 | 0.992 | 3.50 | 91.70 | 100.00 | 0.978~1.000 |
Note: AUC represents the area under the curve. CHF: chronic heart failure. IGF-1: insulin-like growth factor-1. GAL-3: galactoagglutinin-3. PTX-3: pentamerin-3.
Figure 1.
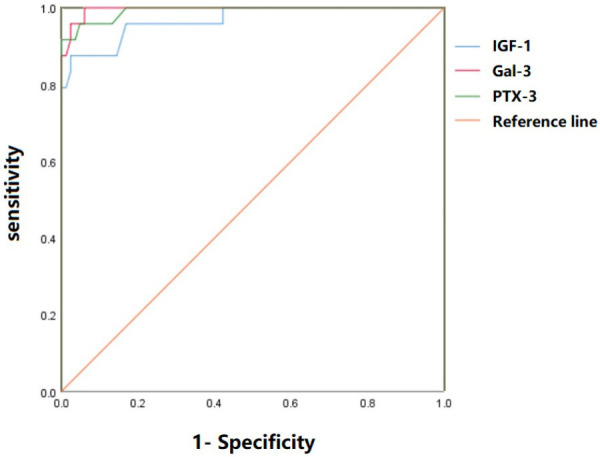
ROC curve analysis of IGF-1, Gal-3 and PTX-3 in the diagnosis of CHF. IGF-1: insulin-like growth factor-1. GAL-3: galactoagglutinin-3. PTX-3: pentamerin-3. CHF: chronic heart failure.
Analysis of independent risk factors
Multivariate logistic regression analysis showed that cardiac function grade, IGF-1, Gal-3, and PTX-3 were independent risk factors affecting the prognosis of CHF (Table 6).
Table 6.
Multivariate logistic regression analysis of independent risk factors affecting prognosis
| Factor | b | S.E | χ2 | P | OR | 95% CI |
|---|---|---|---|---|---|---|
| Gender | 0.736 | 0.486 | 2.293 | 0.130 | 2.088 | 0.805~5.412 |
| Age | 0.586 | 0.402 | 2.125 | 0.145 | 1.797 | 0.817~3.951 |
| Course of disease | 0.942 | 0.604 | 2.432 | 0.119 | 2.565 | 0.785~8.380 |
| BMI | 0.617 | 0.607 | 1.033 | 0.309 | 1.853 | 0.564~6.090 |
| Cardiac Functional Grading | 1.718 | 0.486 | 12.496 | 0.000 | 5.573 | 2.150~14.448 |
| IGF-1 | 1.605 | 0.564 | 8.098 | 0.004 | 4.978 | 1.648~15.036 |
| Gal-3 | 1.491 | 0.437 | 11.641 | 0.001 | 4.442 | 1.886~10.460 |
| PTX-3 | 1.594 | 0.626 | 6.484 | 0.011 | 4.923 | 1.443~15.793 |
Note: IGF-1: insulin-like growth factor-1. Gal-3: galactoagglutinin-3. PTX-3: pentamerin-3.
Discussion
With a quickening pace of life and shifts in lifestyle, the prevalence of chronic heart failure (CHF) has surged, notably emerging as a leading cause of mortality and hospitalization among the elderly [9-11]. Therefore, it is of great value to have timely and accurate diagnosis and prognosis for elderly CHF patients.
IGF-1, a peptide synthesized by endothelial cells and liver cells, shares high homology with insulin and can promote cardiomyocyte hypertrophy and differentiation [12]. As a crucial cardiogenic hormone, IGF-1 is involved in various pathological and physiological processes primarily through paracrine, autocrine, and endocrine mechanisms, and plays a critical role in the occurrence, progression and treatment of CHF [13]. IGF-1 mirrors insulin’s hypoglycemic effects by facilitating the absorption of amino acids and glucose, enhancing the synthesis of lactic acid and collagen, and thus inhibiting the decomposition of glycogen. In addition, IGF-1 can also dilate blood vessels, enhance cardiac output, and reduce vascular resistance [14]. Research [15] has confirmed that serum IGF-1 level of the included 140 CHD sufferers was significantly lower than that of healthy subjects, and IGF-1 was positively connected with LVEF. Our study also indicates that IGF-1 in the observation group was lower than that of the control group, indicating an abnormal decrease in serum IGF-1 in elderly CHF patients. Furthermore, IGF-1 decreased markedly with the worsening of cardiac function, being lowest in Class IV, followed by Class III, and then Class II. IGF-1 level in patients with poor prognosis was lower than those with favorable prognosis, suggesting a significant correlation between declining serum IGF-1 levels and the severity of prognosis in elderly CHF patients. The sensitivity and specificity of IGF-1 were 92.80% and 91.70% respectively, indicating its potential as a valuable biomarker for senile CHF. In addition, IGF-1 was proven to be an independent risk factor affecting the prognosis of CHF.
Galectin-3 (Gal-3), a sugar-binding protein produced by activated macrophages, is widely distributed in the cytoplasm and nucleus. Gal-3 promotes migration of mast cells and macrophages, accelerate formation and deposition of collagen matrix and proliferation of fibroblasts, thereby aggravating heart failure [16]. Meanwhile, GAL-3 is implicated in mediating myocardial fibrosis and inflammation and leading to ventricular remodeling through the promotion of cardiac fibroblast proliferation and collagen deposition. These collectively result in ventricular dysfunction and play a significant role in the occurrence and development of heart failure [17]. Studies have shown that Gal-3, as a new biomarker, is involved in the regulation of cardiac fibrosis and ventricular remodeling, and its abnormally high expression contributes to left ventricular remodeling in chronic heart failure [18]. Research [19] has shown that Gal-3 in patients with NYHA grade II, III and IV was remarkably higher than that in healthy subjects, its level in NYHA patients with good prognosis was notably lower than those with poor prognosis, and Gal-3 was negatively correlated with LVEF and positively correlated with LVEDD. Our study also demonstrated that the serum Gal-3 in observation group was higher than that in control group, reflecting an abnormal increase in serum Gal-3 level in elderly CHF patients. Notably, Gal-3 level was highest in patients with Grade IV cardiac function, followed by Grades III and II, indicating a significant escalation with deteriorating cardiac function. Gal-3 in the poor prognosis group was significantly elevated compared to that in favorable prognosis group, underscoring its association with adverse outcomes in elderly CHF patients. The sensitivity and specificity of Gal-3 were 100.00% and 94.00%, demonstrating good diagnostic value for senile CHF. In addition, Gal-3 was proved as an independent risk factor influencing CHF prognosis.
PTX-3 has emerged as a new inflammatory factor and a newly discovered marker of heart failure. It is produced by various tissue cells, such as vascular endothelial cells, vascular smooth muscle cells and monocyte macrophages, in response to inflammatory stimuli [20,21]. PTX-3 plays a crucial role in major inflammatory signaling pathways, exacerbating the inflammatory response by promoting the expression of endothelial tissue factors and inhibiting the level of fibroblast growth factors, thereby accelerating the development of chronic heart failure patients [22]. Research [23] indicated that PTX-3 level was significantly higher in CHF patients than that in healthy subjects, and patients with poor prognosis had a notably higher PTX-3 level than those with good prognosis; further Pearson linear correlation analysis suggested that PTX-3 was positively correlated with the main adverse cardiac events of CHF, underscoring its utility as an auxiliary indicator to predict the prognosis of CHF patients. Our study showed that PTX-3 level in observation group was significantly higher than that in the control group, indicating an abnormal rise in PTX-3 in elderly CHF patients, which aligns with the previous findings. Notably, the level of PTX-3 was highest in individuals with Grade IV cardiac function, followed by Grades III and II, reflecting a significant increase in elderlies with deteriorating cardiac function. The level of PTX-3 in poor prognosis group was higher than that in favorable prognosis group, suggesting a correlation between elevated PTX-3 level and adverse outcomes in elderly CHF patients. The sensitivity and specificity of Gal-3 were 100.00% and 94.00%, highlighting its promise as a biomarker for clinical diagnosis and prognostic evaluation of CHF.
However, the small sample size of this study is a limitation, and future studies will aim to expand the sample size to obtain more reliable clinical research results.
In summary, IGF-1 levels decrease in elderly CHF patients, whereas Gal-3 and PTX-3 levels increase. These biomarkers demonstrate high sensitivity in diagnosing CHF and have prognostic use, suggesting they are pivotal tools in the clinical management of CHF.
Disclosure of conflict of interest
None.
References
- 1.Borlaug BA, Sharma K, Shah SJ, Ho JE. Heart failure with preserved ejection fraction: JACC scientific statement. J Am Coll Cardiol. 2023;81:1810–1834. doi: 10.1016/j.jacc.2023.01.049. [DOI] [PubMed] [Google Scholar]
- 2.Omote K, Verbrugge FH, Borlaug BA. Heart failure with preserved ejection fraction: mechanisms and treatment strategies. Annu Rev Med. 2022;73:321–337. doi: 10.1146/annurev-med-042220-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones TW, Smith SE, Van Tuyl JS, Newsome AS. Sepsis with preexisting heart failure: management of confounding clinical features. J Intensive Care Med. 2021;36:989–1012. doi: 10.1177/0885066620928299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kosiborod MN, Jhund PS, Docherty KF, Diez M, Petrie MC, Verma S, Nicolau JC, Merkely B, Kitakaze M, DeMets DL, Inzucchi SE, Køber L, Martinez FA, Ponikowski P, Sabatine MS, Solomon SD, Bengtsson O, Lindholm D, Niklasson A, Sjöstrand M, Langkilde AM, McMurray JJV. Effects of Dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141:90–99. doi: 10.1161/CIRCULATIONAHA.119.044138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki K, Claggett B, Minamisawa M, Nochioka K, Mitchell GF, Anand IS, Zannad F, Shah SJ, Lefkowitz M, Shi V, Pfeffer MA, McMurray JJV, Solomon SD. Pulse pressure, prognosis, and influence of Sacubitril/Valsartan in heart failure with preserved ejection fraction. Hypertension. 2021;77:546–556. doi: 10.1161/HYPERTENSIONAHA.120.16277. [DOI] [PubMed] [Google Scholar]
- 6.Ponikowski P, Kirwan BA, Anker SD, McDonagh T, Dorobantu M, Drozdz J, Fabien V, Filippatos G, Göhring UM, Keren A, Khintibidze I, Kragten H, Martinez FA, Metra M, Milicic D, Nicolau JC, Ohlsson M, Parkhomenko A, Pascual-Figal DA, Ruschitzka F, Sim D, Skouri H, van der Meer P, Lewis BS, Comin-Colet J, von Haehling S, Cohen-Solal A, Danchin N, Doehner W, Dargie HJ, Motro M, Butler J, Friede T, Jensen KH, Pocock S, Jankowska EA AFFIRM-AHF investigators. Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: a multicentre, double-blind, randomised, controlled trial. Lancet. 2020;396:1895–1904. doi: 10.1016/S0140-6736(20)32339-4. [DOI] [PubMed] [Google Scholar]
- 7.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368. [DOI] [PubMed] [Google Scholar]
- 8.Authors/Task Force Members; McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2022;24:4–131. doi: 10.1002/ejhf.2333. [DOI] [PubMed] [Google Scholar]
- 9.Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. 2019;21:1306–1325. doi: 10.1002/ejhf.1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hersberger L, Dietz A, Bürgler H, Bargetzi A, Bargetzi L, Kägi-Braun N, Tribolet P, Gomes F, Hoess C, Pavlicek V, Bilz S, Sigrist S, Brändle M, Henzen C, Thomann R, Rutishauser J, Aujesky D, Rodondi N, Donzé J, Stanga Z, Mueller B, Schuetz P. Individualized nutritional support for hospitalized patients with chronic heart failure. J Am Coll Cardiol. 2021;77:2307–2319. doi: 10.1016/j.jacc.2021.03.232. [DOI] [PubMed] [Google Scholar]
- 11.Hua S, Lv B, Qiu Z, Li Z, Wang Z, Chen Y, Han Y, Tucker KL, Wu H, Jin W. Microbial metabolites in chronic heart failure and its common comorbidities. EMBO Mol Med. 2023;15:e16928. doi: 10.15252/emmm.202216928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thorvaldsen T, Ferrannini G, Mellbin L, Benson L, Cosentino F, McMurray JJV, Dahlström U, Lund LH, Savarese G. Eligibility for Dapagliflozin and Empagliflozin in a real-world heart failure population. J Card Fail. 2022;28:1050–1062. doi: 10.1016/j.cardfail.2022.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Khan MS, Rashid AM, Shafi T, Ferreira JP, Butler J. Management of heart failure with reduced ejection fraction in patients with diabetes mellitus and chronic kidney disease. Semin Nephrol. 2023;43:151429. doi: 10.1016/j.semnephrol.2023.151429. [DOI] [PubMed] [Google Scholar]
- 14.Campbell CM, Kahwash R, Abraham WT. Optimizer Smart in the treatment of moderate-to-severe chronic heart failure. Future Cardiol. 2020;16:13–25. doi: 10.2217/fca-2019-0044. [DOI] [PubMed] [Google Scholar]
- 15.Mayne KJ, Shemilt R, Keane DF, Lees JS, Mark PB, Herrington WG. Bioimpedance indices of fluid overload and cardiorenal outcomes in heart failure and chronic kidney disease: a systematic review. J Card Fail. 2022;28:1628–1641. doi: 10.1016/j.cardfail.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altamish M, Samuel VP, Dahiya R, Singh Y, Deb PK, Bakshi HA, Tambuwala MM, Chellappan DK, Collet T, Dua K, Gupta G. Molecular signaling of G-protein-coupled receptor in chronic heart failure and associated complications. Drug Dev Res. 2020;81:23–31. doi: 10.1002/ddr.21627. [DOI] [PubMed] [Google Scholar]
- 17.Bryan Richard S, Huang B, Liu G, Yang Y, Luo S. Impact of ivabradine on the cardiac function of chronic heart failure reduced ejection fraction: meta-analysis of randomized controlled trials. Clin Cardiol. 2021;44:463–471. doi: 10.1002/clc.23581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iyngkaran P, Hanna F, Horowitz JD, Courten MP. Contextualising evidence based medicine in determining the key root for translational effectiveness for chronic disease self-management and heart failure. Rev Cardiovasc Med. 2022;23:37. doi: 10.31083/j.rcm2301037. [DOI] [PubMed] [Google Scholar]
- 19.Williams MT, Kozachik SL, Karlekar M, Wright R. Advance care planning in chronically ill persons diagnosed with heart failure or chronic obstructive pulmonary disease: an integrative review. Am J Hosp Palliat Care. 2020;37:950–956. doi: 10.1177/1049909120909518. [DOI] [PubMed] [Google Scholar]
- 20.Lisco G, Giagulli VA, Iovino M, Zupo R, Guastamacchia E, De Pergola G, Iacoviello M, Triggiani V. Endocrine system dysfunction and chronic heart failure: a clinical perspective. Endocrine. 2022;75:360–376. doi: 10.1007/s12020-021-02912-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsutsui H, Ide T, Ito H, Kihara Y, Kinugawa K, Kinugawa S, Makaya M, Murohara T, Node K, Saito Y, Sakata Y, Shimizu W, Yamamoto K, Bando Y, Iwasaki YK, Kinugasa Y, Mizote I, Nakagawa H, Oishi S, Okada A, Tanaka A, Akasaka T, Ono M, Kimura T, Kosaka S, Kosuge M, Momomura SI. JCS/JHFS 2021 Guideline focused update on diagnosis and treatment of acute and chronic heart failure. J Card Fail. 2021;27:1404–1444. doi: 10.1016/j.cardfail.2021.04.023. [DOI] [PubMed] [Google Scholar]
- 22.Zhou ZF, Wang DJ, Li XM, Zhang CL, Wu CY. Effects of enhanced external counterpulsation on exercise capacity and quality of life in patients with chronic heart failure: a meta-analysis. Medicine (Baltimore) 2021;100:e26536. doi: 10.1097/MD.0000000000026536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Savira F, Wang BH, Kompa AR, Ademi Z, Owen AJ, Zoungas S, Tonkin A, Liew D, Zomer E. Cost-effectiveness of dapagliflozin in chronic heart failure: an analysis from the Australian healthcare perspective. Eur J Prev Cardiol. 2021;28:975–982. doi: 10.1177/2047487320938272. [DOI] [PubMed] [Google Scholar]


