Abstract
Background
Atopic dermatitis is a chronic inflammatory disease characterized by increased itch, skin pain, poor sleep quality, and other symptoms that negatively affect patient quality of life. Upadacitinib, an oral selective Janus kinase (JAK) inhibitor with greater inhibitory potency for JAK1 than JAK2, JAK3, or tyrosine kinase 2, is approved to treat moderate-to-severe atopic dermatitis.
Objective
We aimed to evaluate the effect of upadacitinib on patient-reported outcomes over 52 weeks in adults and adolescents with moderate-to-severe atopic dermatitis.
Methods
Data from two phase III monotherapy trials of upadacitinib (Measure Up 1, NCT03569293; Measure Up 2, NCT03607422) were integrated. Changes in pruritus, pain, other skin symptoms, sleep, quality of life, mental health, and patient impression were evaluated. Patient-reported outcome assessments included the Worst Pruritus Numerical Rating Scale, Patient-Oriented Eczema Measure, Dermatology Life Quality Index, Atopic Dermatitis Symptom Scale, Atopic Dermatitis Impact Scale, Hospital Anxiety and Depression Scale, SCORing Atopic Dermatitis index, Patient Global Impression of Severity, Patient Global Impression of Change, and Patient Global Impression of Treatment. Minimal clinically important differences, achievement of scores representing minimal disease burden, and the change from baseline were evaluated in patients who received upadacitinib through week 52 and in patients who received placebo through week 16.
Results
This analysis included 1609 patients (upadacitinib 15 mg, N = 557; upadacitinib 30 mg, N = 567; placebo, N = 485). Baseline demographics and disease characteristics were generally similar across all arms. The proportion of patients treated with upadacitinib reporting improvements in itch increased rapidly by week 1, increased steadily through week 8, and was sustained through week 52. Patients receiving upadacitinib also experienced improvements in pain and other skin symptoms by week 1, which continued through week 16; improvements were maintained through week 52. Patient reports of improved sleep increased rapidly from baseline to week 1, increased steadily through week 32, and were sustained through week 52. Patients experienced quality-of-life improvements through week 8, which were maintained through week 52. By week 1, patients in both upadacitinib groups experienced rapid improvements in emotional state, and by week 12, patients also achieved meaningful improvements in anxiety and depression. Improvements in mental health continued steadily through week 32 and were maintained through week 52. Patients treated with upadacitinib 30 mg generally experienced improvements in patient-reported outcomes earlier than those treated with upadacitinib 15 mg. Through week 16, patients receiving upadacitinib experienced greater improvements versus those receiving placebo in all assessed patient-reported outcomes.
Conclusions
Adults and adolescents with moderate-to-severe atopic dermatitis treated with once-daily upadacitinib 15 or 30 mg experienced early improvements in itch, pain, other skin symptoms, sleep, quality of life, and mental health that were sustained through week 52.
Clinical Trial Registration
ClinicalTrials.gov identifiers NCT03569293 (13 August 2018) and NCT03607422 (27 July 2018).
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-024-00853-4.
Plain Language Summary
Atopic dermatitis, or eczema, is a condition that causes painful itchy dry skin, which is burdensome for patients and has a negative impact on quality of life. These symptoms frequently lead to disruption of daily activities such as school and work, decreased self-confidence, social isolation, anxiety, depression, and sleep disturbance. Symptoms of atopic dermatitis, such as itch and sleep disturbance, can only be assessed by patients. Therefore, it is important to consider patients’ perceptions of their symptoms and the related impact on their quality of life, especially when evaluating treatment benefits. Upadacitinib is an orally administered drug approved to treat moderate-to-severe atopic dermatitis. In two clinical trials (Measure Up 1 and Measure Up 2), we investigated how treatment with upadacitinib (15-mg or 30-mg dose) given once daily to adults and adolescents with moderate-to-severe atopic dermatitis would impact their symptoms and quality of life over a 1-year period. We measured changes over time in patients’ assessments of itch, pain, other skin-related symptoms, sleep, daily activities, emotional state, mental health, and overall quality of life. Patients treated with upadacitinib experienced improvements in symptoms of atopic dermatitis and quality of life within the first 1–2 weeks of treatment. These improvements continued to steadily increase in the following weeks and lasted through 1 year of treatment. In conclusion, once-daily treatment with upadacitinib 15 or 30 mg led to early and lasting improvements in the well-being of patients with atopic dermatitis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40257-024-00853-4.
Key Points
| In this pooled analysis of the Measure Up 1 and Measure Up 2 studies, adults and adolescents with moderate-to-severe atopic dermatitis who received once-daily upadacitinib (15 or 30 mg) treatment experienced early improvements in symptoms and quality of life. |
| Within 1–2 weeks of initiating upadacitinib therapy, improvements were observed in patient-reported outcomes measuring itch, pain, other skin symptoms, sleep, daily activities, emotional state, quality of life, impression of disease severity, impression of treatment efficacy, and treatment satisfaction. |
| Improvements in patient-reported outcomes increased steadily after weeks 1–2 and were maintained through week 52 of upadacitinib treatment. |
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease caused by a combination of genetic, immune, and environmental factors [1–3]. The global prevalence of AD is estimated at 2960 per 100,000 persons, impacting up to 20% of children and 10% of adults [4]. Pruritus, erythema, and dryness/scaling are among the most burdensome signs and symptoms of AD [5–7] and have a negative impact on sleep; academic and occupational performance; and economic, mental, and social well-being [5, 6, 8–13].
Patient-reported outcomes (PROs) are an important complement to clinician-reported outcomes, providing critical insights into patients’ symptom burden and associated quality-of-life impact [14, 15]. Symptoms such as pruritus, skin pain, and sleep disturbance can only be subjectively assessed, underscoring the centrality of PROs in capturing patient perspectives in clinical trials of AD [15]. Accordingly, PROs are core outcome domains for AD trials, as recommended by the Harmonising Outcome Measures for Eczema (HOME) international consensus group [16] and recently reported criteria for minimal disease activity [17]. The inclusion of PRO endpoints in clinical trials permits patient input on treatment impact, as measured by self-reported changes in symptom severity, and facilitates the assessment of treatment satisfaction [15]. Furthermore, PROs enable potential correlations between AD symptoms to be evaluated [7] and, when coupled with clinician-reported outcomes, permit the disease burden and quality-of-life impact of AD to be fully characterized [14].
Upadacitinib is an oral selective Janus kinase (JAK) inhibitor with greater inhibitory potency for JAK1 than JAK2, JAK3, or tyrosine kinase 2 and is currently approved for multiple indications in rheumatology, gastroenterology, and dermatology, including the treatment of patients with moderate-to-severe AD [18, 19]. The efficacy and safety profile of upadacitinib in adults and adolescents with moderate-to-severe AD was previously reported [20–22]. This pooled analysis of findings from the Measure Up 1 and Measure Up 2 studies evaluated the effect of upadacitinib on PROs over 52 weeks in adults and adolescents with moderate-to-severe AD.
Methods
Study Design
Measure Up 1 (NCT03569293) and Measure Up 2 (NCT03607422) are replicate global multicenter, randomized, double-blind, placebo-controlled phase III clinical trials evaluating once-daily oral upadacitinib to treat moderate-to-severe AD. Detailed descriptions of the study design, key eligibility criteria, and methodology for both clinical trials were previously published [20]. Both studies comprised a 35-day screening period followed by a 16-week double-blind period and an ongoing 260-week blinded extension period. Patients were stratified by baseline disease severity (validated Investigator Global Assessment [vIGA-AD] score 3 vs 4), geographic region, and age (adult vs adolescent) and randomized 1:1:1 to receive once-daily orally administered upadacitinib 15 mg, upadacitinib 30 mg, or placebo. After 16 weeks, patients who received placebo were re-randomized 1:1 to receive orally once-daily upadacitinib 15 mg or upadacitinib 30 mg. Topical treatments for AD, including corticosteroids and calcineurin inhibitors, were prohibited through week 16, after which topical treatments could be administered per investigator discretion. Patients, study site investigators, and staff were blinded to treatments throughout the double-blind and blinded extension periods.
Patients
Eligible patients were aged 12–75 years with moderate-to-severe AD, defined as an Eczema Area and Severity Index score ≥ 16, a vIGA-AD score ≥ 3, a weekly average of daily Worst Pruritus Numerical Rating Scale (WP-NRS) score ≥ 4, and ≥ 10% of body surface area affected by AD. Patients had to be candidates for systemic therapy as demonstrated by an inadequate response to topical treatments, a history of use of systemic treatments, or the presence of conditions making topical treatments inadvisable [20]. A detailed description of the patient populations of the Measure Up 1 and Measure Up 2 trials was previously published [20].
Independent ethics committees or institutional review boards at each study site approved the study protocols, informed consent forms, and recruitment materials before patient enrollment. The studies were conducted in accordance with the International Conference for Harmonisation guidelines, applicable regional regulations, and the Declaration of Helsinki. All patients provided written informed consent.
Assessments
We evaluated PROs that included pruritus, pain, and other skin symptoms; quality of sleep; quality of life; mental health; and impression of disease severity, treatment efficacy, and treatment satisfaction. Instruments used to assess PROs included the WP-NRS, Patient-Oriented Eczema Measure (POEM), Dermatology Life Quality Index (DLQI), Children’s DLQI (CDLQI), Atopic Dermatitis Symptom Scale (ADerm-SS) [23], Atopic Dermatitis Impact Scale (ADerm-IS) [23], Hospital Anxiety and Depression Scale (HADS), SCORing Atopic Dermatitis (SCORAD) index itch and sleep disturbance items, Patient Global Impression of Severity (PGIS), Patient Global Impression of Change (PGIC), and Patient Global Impression of Treatment (PGIT). For PRO outcomes, we report achievement of minimal clinically important differences, achievement of an absolute threshold score representative of minimal disease burden, and least squares (LS) mean percent change from baseline. PRO analyses were prespecified endpoints in the individual Measure Up 1 and Measure Up 2 trials, except for absolute threshold scores involving ADerm-IS, ADerm-SS, and POEM (total score) which were assessed post hoc (Table S1 of the Electronic Supplementary Material [ESM]). The percent overlap in patients who achieved minimal disease burden score thresholds for WP-NRS, DLQI, and POEM at week 52 was evaluated in an exploratory analysis.
Statistical Analysis
For this integrated analysis, data from the Measure Up 1 and Measure Up 2 studies were pooled for each treatment group. Calculations for sample size were previously described [20]. Categorical endpoints were summarized using frequency counts, percentages, and 95% confidence interval (CI) of percentages. Continuous endpoints were summarized using LS means, 95% CI, and standard error and were calculated using analysis of covariance, which included baseline measurement, treatment, strata, and study in the model. Scores for daily-assessed PROs (WP-NRS, ADerm-IS Sleep, and ADerm-SS Skin Pain) were based on a 7-day rolling average through week 16 and then assessed at study visits thereafter. Outcomes were reported using the observed case approach, which did not impute missing data and included observed patient data up to treatment discontinuation. The percent overlap for the achievement of minimal disease burden score thresholds for WP-NRS, DLQI, and POEM included only patients with non-missing data for all three outcomes at week 52.
Results
Patients
A total of 1609 patients enrolled in the Measure Up 1 and Measure Up 2 studies were included in this analysis (upadacitinib 15 mg, N = 557; upadacitinib 30 mg, N = 567; placebo, N = 485). Baseline demographics and disease characteristics were generally similar across treatment and placebo groups (Table 1). Patient disposition was previously described in detail [20].
Table 1.
Baseline demographics and disease characteristicsa
| Characteristic | UPA 15 mg (N = 557) | UPA 30 mg (N = 567) | PBO (N = 485) |
|---|---|---|---|
| Sex, n (%) | |||
|
Female Male |
245 (44.0) 312 (56.0) |
250 (44.1) 317 (55.9) |
232 (47.8) 253 (52.2) |
| Age, years, mean (SD) | 33.7 (15.7) | 33.9 (15.9) | 33.8 (15.2) |
| Age group, years, n (%) | |||
|
< 18 ≥ 18 |
75 (13.5) 482 (86.5) |
77 (13.6) 490 (86.4) |
69 (14.2) 416 (85.8) |
| Race, n (%) | |||
|
White Asian Black or African American American Indian/Alaska Native Native Hawaiian/Other Pacific Islander Multiple |
366 (65.7) 128 (23.0) 43 (7.7) 5 (0.9) 3 (0.5) 12 (2.2) |
389 (68.6) 133 (23.5) 25 (4.4) 2 (0.4) 1 (0.2) 17 (3.0) |
328 (67.6) 105 (21.6) 33 (6.8) 7 (1.4) 2 (0.4) 10 (2.1) |
| Hispanic or Latino, n (%) | 58 (10.4) | 63 (11.1) | 58 (12.0) |
| BMI, kg/m2, mean (SD) | 25.8 (5.9) | 25.8 (5.8) | 26.6 (6.0) |
| vIGA-AD score, n (%) | |||
|
3 (moderate) 4 (severe) |
280 (50.3) 277 (49.7) |
280 (49.4) 287 (50.6) |
252 (52.0) 233 (48.0) |
| EASI, mean (SD) | 29.6 (12.3) | 29.3 (11.7) | 28.6 (12.4) |
| Weekly WP-NRS, mean (SD) | 7.2 (1.6) | 7.3 (1.5) | 7.3 (1.6) |
| SCORAD, mean (SD) | 67.4 (12.6) | 67.0 (12.7) | 66.8 (12.6) |
| DLQI, mean (SD) | 16.6 (7.0) | 16.5 (6.9) | 16.8 (7.0) |
| POEM, mean (SD)b | 21.2 (4.9) | 21.6 (5.0) | 21.6 (5.3) |
| ADerm-SS Skin Pain, mean (SD) | 6.3 (2.2) | 6.4 (2.2) | 6.4 (2.3) |
| ADerm-SS TSS-7, mean (SD) | 46.3 (13.6) | 46.3 (13.6) | 46.6 (13.8) |
| ADerm-IS Sleep, mean (SD) | 18.2 (7.4) | 18.5 (7.6) | 19.0 (7.6) |
| ADerm-IS Daily Activities, mean (SD) | 23.1 (10.5) | 22.8 (10.6) | 23.2 (10.6) |
| ADerm-IS Emotional State, mean (SD) | 20.4 (7.9) | 20.1 (8.3) | 20.2 (8.0) |
| HADS, mean (SD) | 12.5 (7.3) | 13.0 (7.7) | 12.5 (7.7) |
ADerm-IS Atopic Dermatitis Impact Scale, ADerm-SS Atopic Dermatitis Symptom Scale, BMI body mass index, DLQI Dermatology Life Quality Index, EASI Eczema Area and Severity Index, HADS Hospital Anxiety and Depression Scale, PBO placebo, POEM Patient-Oriented Eczema Measure, SCORAD SCORing Atopic Dermatitis, TSS-7 7-item total symptom score, SD standard deviation, UPA upadacitinib, vIGA-AD validated Investigator Global Assessment for Atopic Dermatitis, WP-NRS Worst Pruritus Numerical Rating Scale
aMeans and percentages were calculated based on non-missing values
bDLQI was assessed in patients aged ≥ 16 years at baseline
PRO Measures
Pruritus
Patients in the upadacitinib 15- and 30-mg groups experienced rapid and sustained improvements in itch, with more than 10 and 15%, respectively, at week 1 and more than 30 and 40% at week 2 reporting a meaningful improvement in itch (Fig. 1a). Response rates increased steadily thereafter and were generally sustained through week 52. Among patients receiving upadacitinib 15 and 30 mg, 34.3 and 53.0%, respectively, reported no or minimal itch by week 8; response rates then increased steadily or remained sustained thereafter (43.4 and 51.2% at week 52, respectively; Fig. 1b). Across all timepoints, response rates were greater for patients who received upadacitinib 30 mg versus upadacitinib 15 mg. Similar patterns were observed for LS mean percent change from baseline in WP-NRS and SCORAD itch visual analog scale scores (Fig. S1 of the ESM).
Fig. 1.
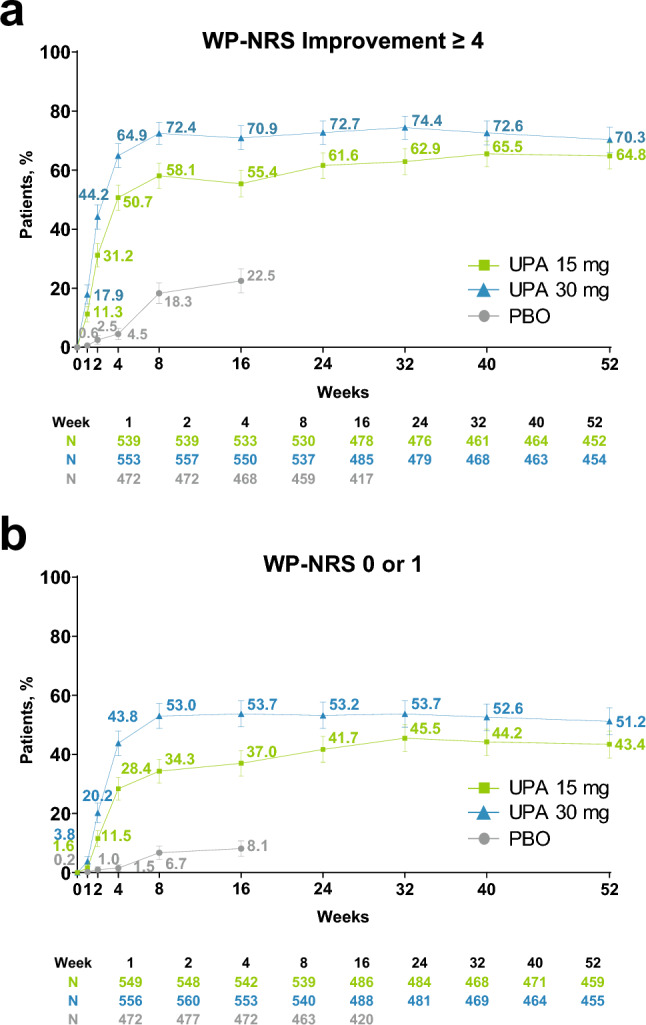
Improvement in itch (observed case [OC]).a a Worst Pruritus Numerical Rating Scale (WP-NRS) improvement ≥ 4.b b WP-NRS 0/1.c Error bars indicate 95% confidence interval. aAssessed daily through week 16, and reported as the weekly average; assessed at scheduled visits thereafter. bAssessed in patients with WP-NRS ≥ 4 at baseline. cAssessed in patients with WP-NRS > 1 at baseline. PBO placebo, UPA upadacitinib
Pain and Other Skin Symptoms
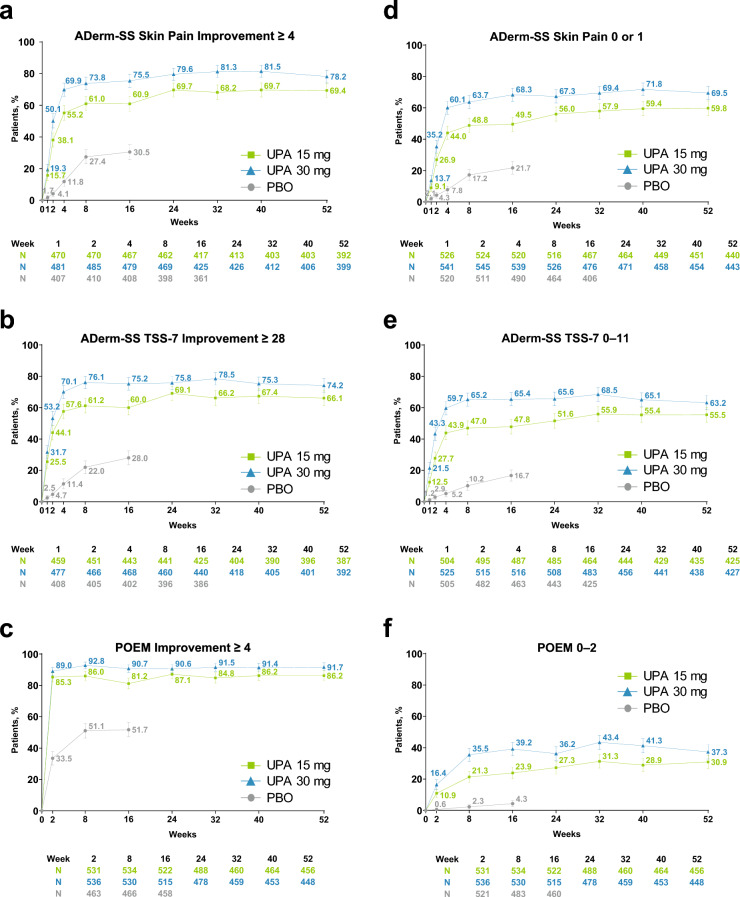
Patients treated with upadacitinib 15 and 30 mg experienced rapid improvements in pain and other skin symptoms in the first few weeks of treatment, with approximately 15 and 20%, respectively, experiencing a meaningful improvement in skin pain (Fig. 2a) and > 25 and >30% reporting a meaningful improvement in skin symptoms by week 1 (Fig. 2b). By week 2, approximately 85 and 90% of patients in the upadacitinib 15- and 30-mg groups, respectively, reported a meaningful improvement in disease severity (Fig. 2c). These improvements generally continued through week 32 and were sustained through week 52, with higher response rates observed for patients receiving upadacitinib 30 mg versus upadacitinib 15 mg at all measured timepoints (Fig. 2a–c).
Fig. 2.
Improvement in pain and other skin symptoms (observed case [OC]). a Atopic Dermatitis Symptom Scale (ADerm-SS) Skin Pain improvement ≥ 4.a b Atopic Dermatitis Symptom Scale 7-item symptom score (ADerm-SS TSS-7) improvement ≥ 28.b c Patient-Oriented Eczema Measure (POEM) improvement ≥ 4.c d ADerm-SS Skin Pain 0/1.d,e e ADerm-SS TSS-7 0–11.f f POEM 0–2.g Error bars indicate 95% confidence interval. aAssessed in patients with ADerm-SS Skin Pain ≥ 4 at baseline. bAssessed in patients with ADerm-SS TSS-7 ≥ 28 at baseline. cAssessed in patients with POEM ≥ 4 at baseline. dAssessed in patients with ADerm-SS Skin Pain ≥ 2 at baseline. eAssessed daily through week 16, and reported as the weekly average; assessed at scheduled visits thereafter. fAssessed in patients with ADerm-SS TSS-7 ≥ 12 at baseline. gAssessed in patients with POEM ≥ 3 at baseline. PBO placebo, UPA upadacitinib
Among patients receiving upadacitinib 15 and 30 mg, 9.1 and 13.7%, respectively, reported no or minimal skin pain by week 1 (Fig. 2d). By week 4, response rates sharply increased to 44.0 and 60.1%, respectively, and then increased steadily through week 16 and were generally sustained through week 52. Similarly, 12.5 and 21.5% of patients in the upadacitinib 15- and 30-mg groups, respectively, reported no or minimal skin symptoms by week 1 (Fig. 2e). Response rates stabilized at approximately week 8 (47.0% and 65.2%), and then were maintained through week 52. At week 2, the proportions of patients treated with upadacitinib 15 and 30 mg who reported clear or almost clear skin was 10.9 and 16.4%, respectively (Fig. 2f). Response rates increased steadily through week 32 and were sustained through week 52. For the upadacitinib 30-mg group, the proportion of patients who reported clear or almost clear skin doubled from week 2 to week 8 (35.5%), with response rates maintained through week 52. Patients receiving upadacitinib 30 mg generally experienced improvements in pain and skin symptoms earlier than those treated with upadacitinib 15 mg, and generally exhibited higher LS mean percent change from baseline in ADerm-SS Skin Pain, ADerm-SS 7-item total symptom score, and POEM (Fig. S2 of the ESM).
Sleep
The proportion of patients in the upadacitinib 15- and 30-mg groups who achieved a meaningful improvement in sleep increased rapidly from baseline, exceeding 15 and 20%, respectively, at week 1 and exceeding 50 and 65%, respectively, at week 4. Improvements increased steadily through week 32 and were sustained through week 52 (Fig. 3a).
Fig. 3.
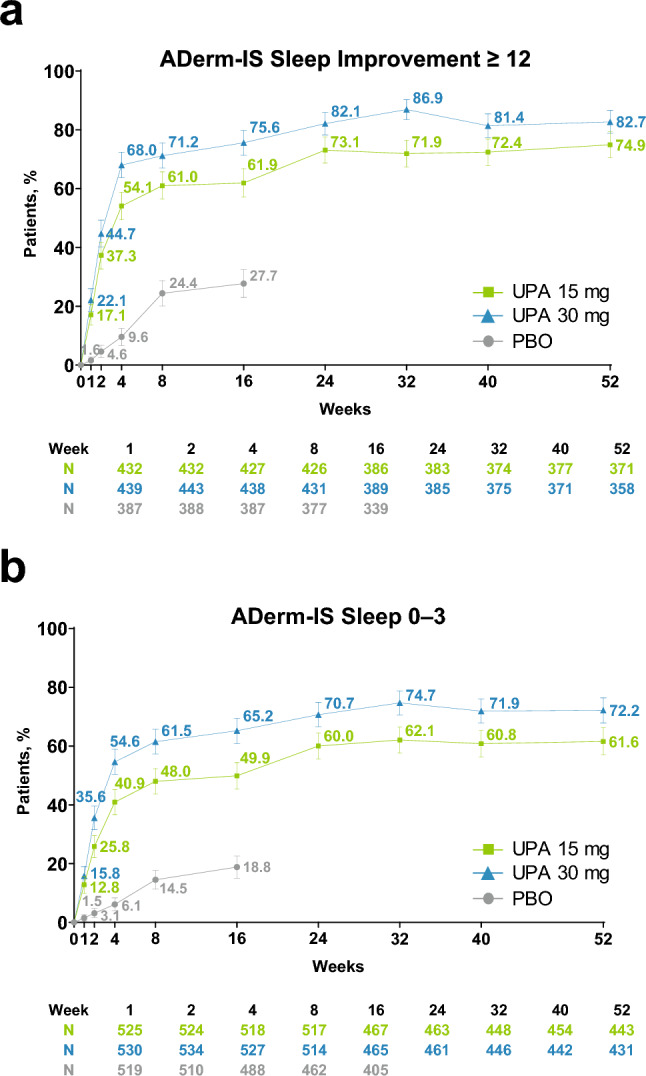
Improvement in sleep (observed case [OC]). a Atopic Dermatitis Impact Scale (ADerm-IS) Sleep improvement ≥ 12.a,b b ADerm-IS Sleep 0–3.b,c Error bars indicate 95% confidence interval. aAssessed in patients with ADerm-IS Sleep ≥ 12 at baseline. bAssessed daily through week 16, and reported as the weekly average; assessed at scheduled visits thereafter. cAssessed in patients with ADerm-IS Sleep ≥ 4 at baseline. PBO placebo, UPA upadacitinib
Among patients receiving upadacitinib 15 and 30 mg, 12.8 and 15.8%, respectively, reported no or minimal sleep disturbance at week 1; response rates increased steadily through week 32 and were maintained through week 52 (Fig. 3b). The LS mean percent change from baseline in ADerm-IS Sleep and SCORAD visual analog scale sleep scores and achievement of zero nights of sleep disturbance were similarly rapid and sustained (Fig. S3a–c of the ESM). Across all timepoints and measures assessed, a higher proportion of patients in the upadacitinib 30-mg group reported improvement in sleep versus those treated with upadacitinib 15 mg (Fig. 3 and Fig. S3 of the ESM).
Quality of Life and Mental Health
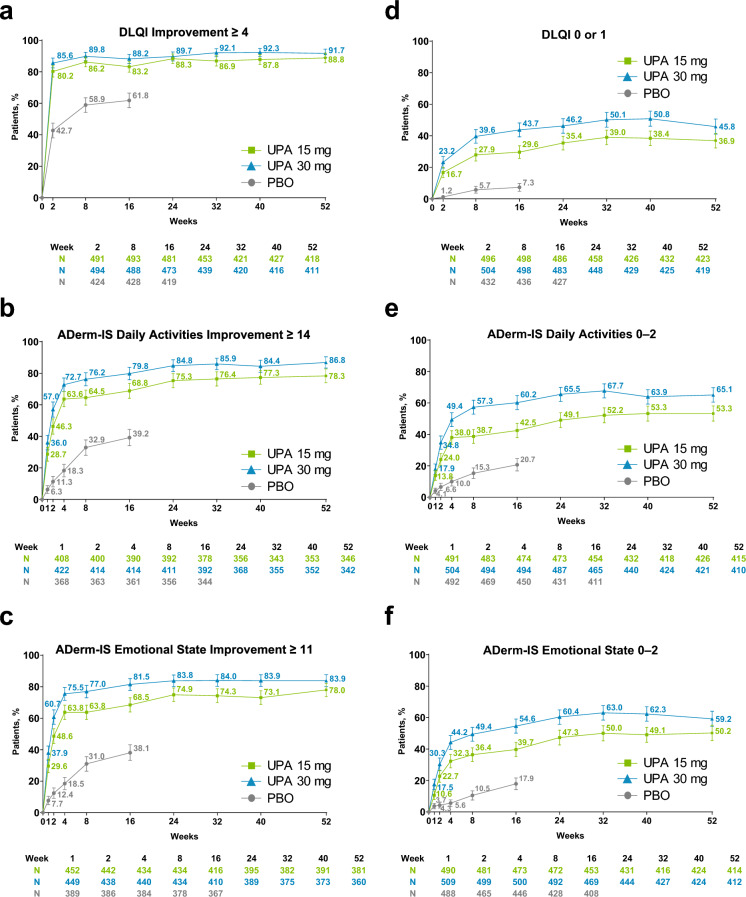
Patients in the upadacitinib groups experienced rapid improvements from baseline in quality of life, with over 80% of patients reporting a meaningful improvement in quality of life by week 2; response rates were maintained through week 52 (Fig. 4a). Similarly, patients who received upadacitinib experienced a meaningful improvement in daily activities (Fig. 4b) and a meaningful improvement in emotional state (Fig. 4c) as early as week 1. Across all measured timepoints, greater proportions of patients receiving upadacitinib 30 mg experienced improvements in quality of life and mental health versus patients receiving upadacitinib 15 mg, and exhibited a higher LS mean percent change from baseline in DLQI, ADerm-IS Daily Activities, and ADerm-IS Emotional State (Fig. S4a, d, e of the ESM). Temporal patterns in CDLQI improvements with upadacitinib were similar to DLQI (Fig. S4b, c of the ESM).
Fig. 4.
Improvement in daily living, daily activities, and emotional state (observed case [OC]). a Dermatology Life Quality Index (DLQI) improvement ≥ 4.a b Atopic Dermatitis Impact Scale (ADerm-IS) Daily Activities improvement ≥ 14.b c ADerm-IS Emotional State improvement ≥ 11.c d DLQI 0/1.d e ADerm-IS Daily Activities 0–2.e f ADerm-IS Emotional State 0–2.f Error bars indicate 95% confidence interval. aAssessed in patients aged ≥ 16 years with DLQI ≥ 4 at baseline. bAssessed in patients with ADerm-IS Daily Activities Domain ≥ 14 at baseline. cAssessed in patients with ADerm-IS Emotional State ≥ 11 at baseline. dAssessed in patients aged ≥ 16 years with DLQI > 1 at baseline. eAssessed in patients with ADerm-IS Daily Activities > 3 at baseline. fAssessed in patients with ADerm-IS Emotional State > 3 at baseline. PBO placebo, UPA upadacitinib
Similarly, the proportion of patients who achieved minimal disease burden scores for minimal impact on quality of life, daily activities, and emotional state increased rapidly from baseline, with steady increases through week 32 (Fig. 4d–f). At week 32, among patients receiving upadacitinib 15 and 30 mg, 39.0% and 50.1%, respectively, reported that AD had no impact on their quality of life (Fig. 4d); 52.2 and 67.7% reported no or minimal impact on their daily activities (Fig. 4e); and 50.0% and 63.0% reported no or minimal impact on their emotional state (Fig. 4f). Response rates were generally maintained through week 52 (Fig. 4d–f). Approximately half the patients in the upadacitinib 15- and 30-mg groups (47.8 and 54.5%) achieved meaningful improvements in anxiety and depression by week 12; this proportion increased through week 32 (54.6 and 61.9%) and was maintained through week 52 (56.7 and 61.3%; Fig. S5 of the ESM)
Achievement of Minimal Disease Burden Thresholds Across PROs Measuring Itch, Disease Severity, and Quality of Life
Among patients treated with upadacitinib 15 or 30 mg who achieved minimal disease burden threshold scores for either itch, disease severity, or quality of life at week 52, 50% of patients achieved the minimal disease burden threshold for all three PROs, with 73% achieving at least two (Fig. 5).
Fig. 5.
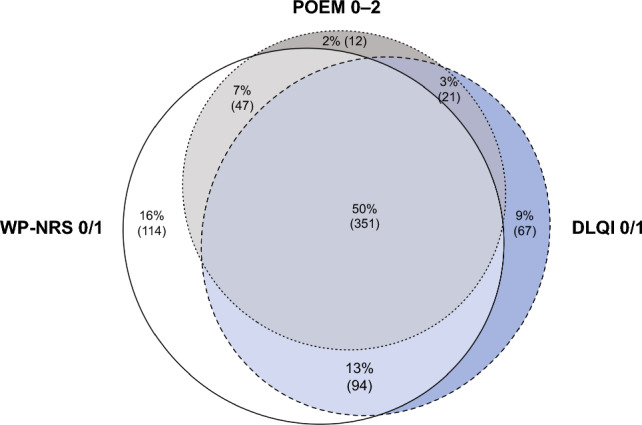
Overlap in achievement of Worst Pruritus Numerical Rating Scale (WP-NRS 0/1), Dermatology Life Quality Index (DLQI) 0/1, and Patient-Oriented Eczema Measure (POEM) 0–2 at week 52. Percentages are calculated among patients who received either upadacitinib 15 or 30 mg or achieved ≥ 1 of the following endpoints at week 52: WP-NRS 0/1, DLQI 0/1, or POEM 0–2. The analysis was based on observed cases, including only patients with non-missing data for all three outcome measures at week 52
Impressions of Disease Severity, Treatment Efficacy, and Treatment Satisfaction
Treatment with upadacitinib led to rapid improvements from baseline in patient impressions regarding disease severity, treatment efficacy, and treatment satisfaction. At week 1, among patients receiving upadacitinib 15 and 30 mg, > 15 and > 25%, respectively, reported “minimal” or “absent” symptoms on the PGIS questionnaire, > 50 and > 60% rated themselves as “very much improved” or “much improved” on the PGIC questionnaire, and > 35 and > 40% were “extremely satisfied” or “very satisfied” as assessed by the PGIT questionnaire. Response rates increased sharply through week 4 and were maintained through week 52 (Fig. S6 of the ESM).
Discussion
In this pooled analysis of findings from the Measure Up 1 and Measure Up 2 studies, adults and adolescents with moderate-to-severe AD treated with once-daily upadacitinib 15 or 30 mg experienced early improvements in multiple PRO domains. A rapid increase in the proportion of patients reporting meaningful improvements was observed by week 1 for itch, pain, other skin symptoms, sleep, daily activities, and emotional well-being and by week 2 for quality of life. Observed improvements were sustained through week 52 for all assessed PROs, with most patients sustaining these improvements without the use of topical medications for AD after week 16 (168/557 [30.2%] and 133/567 [23.5%] patients who received upadacitinib 15 and 30 mg, respectively, used concomitant topical corticosteroids/topical calcineurin inhibitor therapy from weeks 16–52) [21]. Achievement of minimal clinically important differences and minimal disease burden thresholds scores were consistent across the various PRO domains. Improvements were observed with both upadacitinib doses, with numerically greater results with upadacitinib 30 mg compared with upadacitinib 15 mg.
As previously reported, upadacitinib demonstrated a favorable benefit-risk profile through week 52 in the Measure Up 1 and Measure Up 2 clinical trials, with no new safety signals identified. The overall rate of treatment discontinuations because of adverse events was low; discontinuations because of an adverse event occurred more frequently in the upadacitinib 30-mg group than in the upadacitinib 15-mg group [21].
Similar to other advanced therapies (e.g., baricitinib, abrocitinib, tralokinumab, and dupilumab) for the treatment of moderate-to-severe AD [24–28], improvements in PROs with upadacitinib were sustained in the long-term through 52 weeks of treatment. There may be differences in long-term sustainability across therapies; however, an indirect comparison using network meta-analyses for long-term outcomes is not feasible because of study design heterogeneity. This notwithstanding, there appear to be differences in the rapidity of response, with JAK inhibitors providing faster improvement in signs and symptoms of AD [29–32]. Rapid treatment effects have been associated with patients spending a greater time in response with higher cumulative symptom improvement, which may restore patients’ health-related quality of life at earlier timepoints [33]. This present analysis reports on a wide variety of PRO domains and evaluates PROs not well characterized in other analyses, including patient impressions of disease severity, treatment efficacy, and treatment satisfaction, as well as symptom burden and quality of life as measured by the more recently developed ADerm-SS and ADerm-IS questionnaires. Moreover, we evaluated achievement of stringent measure thresholds (e.g., ADerm-SS Skin Pain 0 and ADerm-IS Sleep 0–3) and minimal disease activity optimal targets (e.g., WP-NRS 0 or 1, POEM 0–2, and DLQI 0 or 1) [34] with upadacitinib; the efficacy of other advanced treatments in achieving these stringent endpoints and minimal disease activity optimal targets is not well described.
Limitations of this analysis should be acknowledged. Because of eligibility criteria, patients enrolled in the Measure Up 1 and Measure Up 2 studies may differ from the broad population of patients with moderate-to-severe AD seen in routine clinical practice, which impacts the generalizability of our results. For example, the studies excluded patients with recent cardiovascular conditions (cerebrovascular accident, myocardial infarction, and/or coronary stenting) and uncontrolled hypertension. Patients with a history of eczema herpeticum were also excluded, which although rare among patients with AD [35], has been seen in the clinical setting. Additionally, data presented here combine adult and adolescent populations into a single group, though subgroup analyses have shown similar efficacy of upadacitinib in improving itch, sleep, and quality of life between adults and adolescents at week 16 [36]. Last, all results are based on observed case analyses; however, comparisons between upadacitinib and placebo for the double-blind period (16 weeks) have previously been presented using non-responder imputation [20].
Conclusions
Patients with moderate-to-severe AD who received once-daily treatment with oral upadacitinib 15 or 30 mg experienced improvements in symptoms and health-related quality of life as early as week 1 and week 2, which were sustained through 52 weeks of treatment. No new safety signals were identified. Results of this analysis can help support shared treatment decision making between patients and physicians and may enable more informed treatment strategies for AD.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, reviewing, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship. AbbVie and authors thank all the trial investigators and the patients who participated in this clinical trial. Medical writing support was provided by Akua Adu-Boahene, MD, MPH, Morgan Gingerich, PhD, and Jay Parekh, PharmD, of JB Ashtin, and funded by AbbVie.
Declarations
Funding
AbbVie Inc. provided financial support to conduct this study/analysis, prepare the manuscript, and sponsor the open access fee.
Conflict of interest
Jonathan I. Silverberg receives consulting fees from AbbVie, Anacor Pharmaceuticals, GlaxoSmithKline, Eli Lilly, Regeneron Pharmaceuticals, Pfizer, Procter & Gamble, and MedImmune. He serves as an investigator in trials sponsored by Celgene, GlaxoSmithKline, Eli Lilly, Regeneron Pharmaceuticals, and Roche. Melinda J. Gooderham has been an investigator, speaker and/or advisor for AbbVie, Amgen, Akros, Arcutis, AnaptysBio, Apogee, Aristea, Bausch Health, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Incyte, Janssen, Kyowa Kirin, LEO Pharma, MedImmune, Meiji, Merck, Moonlake, Nimbus, Novartis, Pfizer, Regeneron, Roche, Sanofi Genzyme, Sun Pharma, Tarsus, Takeda, UCB, Union, and Ventyx. Amy S. Paller has been an investigator for AbbVie, Applied Pharma Research, Dermavant, Eli Lilly, Incyte, Janssen, Krystal, Regeneron, and UCB; has been a consultant for Aegerion Pharma, Azitra, BioCryst, Boehringer Ingelheim, Bristol Myers Squibb, Castle Creek, Eli Lilly, Janssen, Krystal, LEO Pharma, Novartis, Regeneron, Sanofi Genzyme, Seanergy, TWI Biotechnology, and UCB; and has served on the data safety monitoring board for AbbVie, Abeona, Catawba, Galderma, and InMed. Mette Deleuran receives consulting, lecturer, and/or investigator fees from AbbVie, Almirall, Arena Pharmaceuticals, ASLAN Pharmaceuticals, Eli Lilly, Incyte, Kymab, La Roche Posay, LEO Pharma, Numab, Pfizer, Pierre Fabre Laboratories, Regeneron Pharmaceuticals, and Sanofi. Christopher G. Bunick has served as an investigator for Almirall, Palvella, and Timber; a consultant for AbbVie, Almirall, Amgen, Apogee, Arcutis, Bristol Myers Squibb, Eli Lilly, LEO Pharma, Novan, Novartis, Ortho-Dermatologics, Pfizer, Sanofi-Regeneron, and UCB; and a speaker for and received honoraria from Allergan, Almirall, LEO Pharma, and UCB. Linda F. Stein Gold has served as an investigator/consultant or speaker for AbbVie, Arcutis, Dermavant, Incyte, LEO Pharma, Lilly, Novartis, Ortho Dermatologics, Pfizer, Sun Pharma, and UCB. DirkJan Hijnen has served as an investigator, consultant, and/or speaker for AbbVie, Almirall, AstraZeneca, Galderma, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, and Sanofi. Brian M. Calimlim, Wan-Ju Lee, Henrique D. Teixeira, Xiaofei Hu, Shiyu Zhang, Yang Yang, Ayman Grada, and Andrew M. Platt are full-time employees of AbbVie, and may hold AbbVie stock or stock options. Diamant Thaçi is an advisor, speaker, or consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celltrion, Galderma, Janssen, Kyowa Kirin, L'Oréal, LEO Pharma, Lilly, Novartis, Pfizer, Regeneron, Sanofi/Genzyme, and UCB.
Ethics approval
The institutional review board and/or independent ethics committee at each study site approved the study protocols, informed consent forms, and recruitment materials before patient enrollment. The studies were conducted in accordance with the International Conference for Harmonisation guidelines, applicable regional regulations, and the Declaration of Helsinki.
Consent to participate
All patients provided written informed consent to participate in this study before screening.
Consent for publication
Not applicable.
Availability of data and material
AbbVie is committed to responsible data sharing regarding the clinical trials we sponsor. This includes access to anonymized individual and trial-level data (analysis data sets), as well as other information (e.g., protocols, clinical study reports, or analysis plans), provided the trials are not part of an ongoing or planned regulatory submission. This includes requests for clinical trial data for unlicensed products and indications. These clinical trial data can be requested by any qualified researchers who engage in rigorous independent scientific research, and will be provided following a review and approval of a research proposal, statistical analysis plan, and execution of a data sharing agreement. Data requests can be submitted at any time after approval in the USA and Europe and after acceptance of this manuscript for publication. The data will be accessible for 12 months, with possible extensions considered. For more information on the process or to submit a request, visit the following link: https://vivli.org/ourmember/abbvie/, then select “Home.”
Code availability
Not applicable.
Author contributions
All authors participated in data interpretation, critically reviewed this manuscript, and provided final approval for publication. JIS, BMC, W-JL, HDT, and XH participated in the study concept/design. ASP, MD, LFSG, HDT, XH, and DT participated in the data acquisition. XH and YY participated in the statistical analysis.
References
- 1.Kantor R, Silverberg JI. Environmental risk factors and their role in the management of atopic dermatitis. Expert Rev Clin Immunol. 2017;13(1):15–26. doi: 10.1080/1744666x.2016.1212660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ständer S. Atopic dermatitis. N Engl J Med. 2021;384(12):1136–1143. doi: 10.1056/NEJMra2023911. [DOI] [PubMed] [Google Scholar]
- 3.Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40(2):84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laughter MR, Maymone MBC, Mashayekhi S, Arents BWM, Karimkhani C, Langan SM, et al. The global burden of atopic dermatitis: lessons from the Global Burden of Disease Study 1990–2017. Br J Dermatol. 2021;184(2):304–309. doi: 10.1111/bjd.19580. [DOI] [PubMed] [Google Scholar]
- 5.Silverberg JI, Gelfand JM, Margolis DJ, Boguniewicz M, Fonacier L, Grayson MH, et al. Patient burden and quality of life in atopic dermatitis in US adults: a population-based cross-sectional study. Ann Allergy Asthma Immunol. 2018;121(3):340–347. doi: 10.1016/j.anai.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Silverberg JI, Chiesa-Fuxench Z, Margolis D, Boguniewicz M, Fonacier L, Grayson M, et al. Epidemiology and burden of sleep disturbances in atopic dermatitis in US adults. Dermatitis. 2022;33(6S):S104–S113. doi: 10.1097/der.0000000000000731. [DOI] [PubMed] [Google Scholar]
- 7.Weisshaar E, Bentz P, Apfelbacher C, Haufe E, Heinrich L, Heratizadeh A, et al. Itching in atopic dermatitis: patient- and physician-reported outcomes in the German atopic dermatitis registry TREATgermany. Acta Derm Venereol. 2023;103:adv00854. doi: 10.2340/actadv.v103.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elsawi R, Dainty K, Smith Begolka W, Barta K, Butler L, Capozza K, et al. The multidimensional burden of atopic dermatitis among adults: results from a large national survey. JAMA Dermatol. 2022;158(8):887–892. doi: 10.1001/jamadermatol.2022.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith Begolka W, Chovatiya R, Thibau IJ, Silverberg JI. Financial burden of atopic dermatitis out-of-pocket health care expenses in the United States. Dermatitis. 2021;32(1s):S62–70. doi: 10.1097/der.0000000000000715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiesa Fuxench ZC, Block JK, Boguniewicz M, Boyle J, Fonacier L, Gelfand JM, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139(3):583–590. doi: 10.1016/j.jid.2018.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Li JC, Fishbein A, Singam V, Patel KR, Zee PC, Attarian H, et al. Sleep disturbance and sleep-related impairment in adults with atopic dermatitis: a cross-sectional study. Dermatitis. 2018;29(5):270–277. doi: 10.1097/der.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drucker AM, Wang AR, Li WQ, Sevetson E, Block JK, Qureshi AA. The burden of atopic dermatitis: summary of a report for the National Eczema Association. J Invest Dermatol. 2017;137(1):26–30. doi: 10.1016/j.jid.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Eckert L, Gupta S, Amand C, Gadkari A, Mahajan P, Gelfand JM. Impact of atopic dermatitis on health-related quality of life and productivity in adults in the United States: an analysis using the National Health and Wellness Survey. J Am Acad Dermatol. 2017;77(2):274–9.e3. doi: 10.1016/j.jaad.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 14.Barrett A, Hahn-Pedersen J, Kragh N, Evans E, Gnanasakthy A. Patient-reported outcome measures in atopic dermatitis and chronic hand eczema in adults. Patient. 2019;12(5):445–459. doi: 10.1007/s40271-019-00373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Copley-Merriman C, Zelt S, Clark M, Gnanasakthy A. Impact of measuring patient-reported outcomes in dermatology drug development. Patient. 2017;10(2):203–213. doi: 10.1007/s40271-016-0196-6. [DOI] [PubMed] [Google Scholar]
- 16.Williams HC, Schmitt J, Thomas KS, Spuls PI, Simpson EL, Apfelbacher CJ, et al. The HOME Core outcome set for clinical trials of atopic dermatitis. J Allergy Clin Immunol. 2022;149(6):1899–1911. doi: 10.1016/j.jaci.2022.03.017. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg J, Gooderham M, Katoh N, Aoki V, Pink A, Binamer Y, et al. Optimizing the management of atopic dermatitis with a new minimal disease activity concept and criteria and consensus-based recommendations for systemic therapy. Br J Dermatol. 2023 doi: 10.1093/bjd/ljac140.022. [DOI] [Google Scholar]
- 18.Rinvoq (upadacitinib). Prescribing information. AbbVie Inc.; 2023.
- 19.Parmentier JM, Voss J, Graff C, Schwartz A, Argiriadi M, Friedman M, et al. In vitro and in vivo characterization of the JAK1 selectivity of upadacitinib (ABT-494) BMC Rheumatol. 2018;2:23. doi: 10.1186/s41927-018-0031-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guttman-Yassky E, Teixeira HD, Simpson EL, Papp KA, Pangan AL, Blauvelt A, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397(10290):2151–2168. doi: 10.1016/s0140-6736(21)00588-2. [DOI] [PubMed] [Google Scholar]
- 21.Simpson EL, Papp KA, Blauvelt A, Chu CY, Hong HC, Katoh N, et al. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the Measure Up 1 and Measure Up 2 randomized clinical trials. JAMA Dermatol. 2022;158(4):404–413. doi: 10.1001/jamadermatol.2022.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reich K, Teixeira HD, de Bruin-Weller M, Bieber T, Soong W, Kabashima K, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397(10290):2169–2181. doi: 10.1016/s0140-6736(21)00589-4. [DOI] [PubMed] [Google Scholar]
- 23.Foley C, Tundia N, Simpson E, Teixeira HD, Litcher-Kelly L, Bodhani A. Development and content validity of new patient-reported outcome questionnaires to assess the signs and symptoms and impact of atopic dermatitis: the Atopic Dermatitis Symptom Scale (ADerm-SS) and the Atopic Dermatitis Impact Scale (ADerm-IS) Curr Med Res Opin. 2019;35(7):1139–1148. doi: 10.1080/03007995.2018.1560222. [DOI] [PubMed] [Google Scholar]
- 24.Simpson E, Armstrong A, Boguniewicz M, Chiesa Fuxench ZC, Feely M, Pierce E, et al. Baricitinib 2 mg for the treatment of atopic dermatitis in North America: long-term efficacy and patient-reported outcomes. Dermatol Ther. 2022;35(12):e15954. doi: 10.1111/dth.15954. [DOI] [PubMed] [Google Scholar]
- 25.Deleuran M, Thaçi D, Beck LA, de Bruin-Weller M, Blauvelt A, Forman S, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82(2):377–388. doi: 10.1016/j.jaad.2019.07.074. [DOI] [PubMed] [Google Scholar]
- 26.Strober B, Mallya UG, Yang M, Ganguli S, Gadkari A, Wang J, et al. Treatment outcomes associated with dupilumab use in patients with atopic dermatitis: 1-year results from the RELIEVE-AD study. JAMA Dermatol. 2022;158(2):142–150. doi: 10.1001/jamadermatol.2021.4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simpson EL, Wollenberg A, Soong W, Steffensen LA, Kurbasic A, Schneider S, et al. Patient-oriented measures for phase 3 studies of tralokinumab for the treatment of atopic dermatitis (ECZTRA 1, 2, and 3) Ann Allergy Asthma Immunol. 2022;129(5):592–604.e5. doi: 10.1016/j.anai.2022.07.007. [DOI] [PubMed] [Google Scholar]
- 28.Reich K, Silverberg JI, Papp KA, Deleuran M, Katoh N, Strober B, et al. Abrocitinib effect on patient-reported outcomes in patients with moderate-to-severe atopic dermatitis: results from phase 3 studies, including the long-term extension JADE EXTEND study. J Eur Acad Dermatol Venereol. 2023 doi: 10.1111/jdv.19254. [DOI] [PubMed] [Google Scholar]
- 29.Munera-Campos M, Carrascosa JM. Janus kinase inhibitors in atopic dermatitis: new perspectives. Actas Dermosifiliogr. 2023;114(8):680–707. doi: 10.1016/j.ad.2023.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Nakashima C, Yanagihara S, Otsuka A. Innovation in the treatment of atopic dermatitis: emerging topical and oral Janus kinase inhibitors. Allergol Int. 2022;71(1):40–46. doi: 10.1016/j.alit.2021.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Nogueira M, Torres T. Janus kinase inhibitors for the treatment of atopic dermatitis: focus on abrocitinib, baricitinib, and upadacitinib. Dermatol Pract Concept. 2021;11(4):e2021145. doi: 10.5826/dpc.1104a145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mikhaylov D, Ungar B, Renert-Yuval Y, Guttman-Yassky E. Oral Janus kinase inhibitors for atopic dermatitis. Ann Allergy Asthma Immunol. 2023;130(5):577–592. doi: 10.1016/j.anai.2023.01.020. [DOI] [PubMed] [Google Scholar]
- 33.Blauvelt A, Silverberg JI, Calimlim Brian M, Liu Y, Platt A, Thyssen JP. 355 Efficacy of upadacitinib for moderate-to-severe atopic dermatitis: analysis of time spent in skin clearance response states from the Measure Up 1, Measure Up 2 and Heads Up studies. Br J Dermatol. 2023 doi: 10.1093/bjd/ljac140.047. [DOI] [Google Scholar]
- 34.Silverberg JI, Gooderham M, Katoh N, Aoki V, Pink AE, Binamer Y, et al. 327 Optimizing the management of atopic dermatitis with a new minimal disease activity concept and criteria and consensus-based recommendations for systemic therapy. Br J Dermatol. 2023 doi: 10.1093/bjd/ljac140.022. [DOI] [Google Scholar]
- 35.Leung DY. Why is eczema herpeticum unexpectedly rare? Antiviral Res. 2013;98(2):153–157. doi: 10.1016/j.antiviral.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lio P, Eichenfield LF, Marcoux D, Lee W-J, Teixeira Henrique D, Raymundo EM, et al. 413. Improvement in itch, symptoms and quality of life with upadacitinib through week 16 in adults and adolescents with atopic dermatitis: results from phase 3 studies (Measure Up 1, Measure Up 2 and AD Up) Br J Dermatol. 2023 doi: 10.1093/bjd/ljad162.033. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.