Abstract
Purpose
Transanal minimally invasive surgery has theoretical advantages for ileal pouch-anal anastomosis surgery. We performed a systematic review assessing technical approaches to transanal IPAA (Ta-IPAA) and meta-analysis comparing outcomes to transabdominal (abd-IPAA) approaches.
Methods
Three databases were searched for articles investigating Ta-IPAA outcomes. Primary outcome was anastomotic leak rate. Secondary outcomes included conversion rate, post operative morbidity, and length of stay (LoS). Staging, plane of dissection, anastomosis, extraction site, operative time, and functional outcomes were also assessed.
Results
Searches identified 13 studies with 404 unique Ta-IPAA and 563 abd-IPAA patients. Anastomotic leak rates were 6.3% and 8.4% (RD 0, 95% CI -0.066 to 0.065, p = 0.989) and conversion rates 2.5% and 12.5% (RD -0.106, 95% CI -0.155 to -0.057, p = 0.104) for Ta-IPAA and abd-IPAA. Average LoS was one day shorter (MD -1, 95% CI -1.876 to 0.302, p = 0.007). A three-stage approach was most common (47.6%), operative time was 261(± 60) mins, and total mesorectal excision and close rectal dissection were equally used (49.5% vs 50.5%). Functional outcomes were similar. Lack of randomised control trials, case-matched series, and significant study heterogeneity limited analysis, resulting in low to very low certainty of evidence.
Conclusions
Analysis demonstrated the feasibility and safety of Ta-IPAA with reduced LoS, trend towards less conversions, and comparable anastomotic leak rates and post operative morbidity. Though results are encouraging, they need to be interpreted with heterogeneity and selection bias in mind. Robust randomised clinical trials are warranted to adequately compare ta-IPAA to transabdominal approaches.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00423-024-03343-7.
Keywords: Transanal, Ileoanal, Ulcerative Colitis, Systematic Review, Meta-analysis
Introduction
Restorative proctocolectomy with ileal pouch-anal anastomosis (IPAA) provides a means of re-establishing intestinal continuity after resection of the colon and rectum[1]. Ulcerative disease and familial adenomatous polyposis (FAP) are the most common indications for proctocolectomy [2–4]. These patients are a relatively young cohort, with the majority undergoing surgery between the 4th-6th decade of life[5–7]. Despite risks of early and late complications, quality of life improves after restorative proctocolectomy in ulcerative colitis [8] and pouch function remains robust over time[6]. Early and late operative outcomes such as pelvic sepsis and anastomotic stricture are independent predictors of pouch failure[9].
Laparoscopic IPAA was introduced 1990s and has shorter hospitalisation, better cosmetic result and improved female fecundity when compared to open surgery[10, 11]. More recently robotic platforms, single incision approaches, and natural orifice specimen extraction have been described and shown to be feasible and safe[12–15]. Transanal surgery offers a unique anatomical perspective to the pelvis. Its application in IPAA was described in 2015 in cadaveric[16] and animal models[17], and then in clinical practice[15, 18, 19] A transanal laparoscopic port is inserted into the anal canal. A purse-string suture is used to close the rectal lumen 3-4 cm above the dentate line, a full thickness rectotomy is then performed circumferentially 1 cm distal to this. Dissection of the distal rectum is continued either in the total mesorectal excision (TME) or close rectal dissection (CRD) plane until it meets the abdominal dissection [20]. The approach provides an alternative means of dividing the distal rectum and performing the stapled anastomosis when compared to the often-challenging cross-stapling of the rectum and creation of the ileoanal anastomosis in the deep pelvis from above.
The technical benefits of Ta-IPAA are 1) direct visualisation of the rectal mucosa to ensure optimum cuff length, 2) single-staple firing, 3) ease of access to the narrow bony pelvis, and 4) two team simultaneous approach, however the significant learning curve of transanal surgery must be considered[20]. If these benefits translate meaningfully into clinical practice, they may result in lower anastomotic leak rates, reduced need for conversion to open surgery, shorter operative time, and better functional outcomes.
This study aims to systematically review the literature on Ta-IPAA to investigate technical characteristics and clinical and functional outcomes after Ta-IPAA and perform a meta-analysis comparing short-term clinical outcomes to established transabdominal (abd-IPAA) approaches.
Materials Methods
Search Strategy
Systematic review and meta-analysis of the literature was performed in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) 2020 statement [21] to identify studies reporting on transanal IPAA outcomes. On March 22nd, 2023 a search of EMBASE, Pubmed, and Cochrane Library databases was performed. The search terms “laparoscopic”, “transabdominal”, “open”, “minimally invasive”, “tapouch” were used in combination with “ipaa”, “tapouch”, “j pouch”, “ileoanal” or “restorative proctocolectomy”. To be eligible for inclusion articles had to be published in English language in peer-reviewed publications. All indications for IPAA were eligible for inclusion. Published abstracts, presentations, case reports, and studies reporting exclusively paediatric cohorts were excluded. Data was accrued and stored in a password protected Microsoft Excel Data Sheet, using a predefined template. Author name, country, year of publication, journal, study design and patient number were extracted for each article. Where applicable operative approach, number of stages, technical characteristics, operative duration, post operative morbidity, anastomotic leak, length of hospital stay (LoS), conversion rates, and functional outcomes were extracted and collected. Number of stages were defined as either single stage (restorative proctectomy without covering ileostomy), 2-stage (restorative proctocolectomy with loop ileostomy followed by reversal of ileostomy), modified 2-stage (Total abdominal colectomy with end ileostomy followed by proctectomy with IPAA formation without covering ileostomy), or 3-stage (total abdominal colectomy with end ileostomy, followed by proctectomy with IPAA formation with loop ileostomy, and finally reversal of loop ileostomy [22]. The study protocol was published on Open Science Foundation Registry (DOI 10.17605/OSF.IO/JAD4S) and registered on the PROSPERO database (ref: CRD42023418322) [23].
Outcome Measures
Primary outcome was anastomotic leak defined as a defect of the intestinal wall integrity at the pouch-anal anastomotic site leading to a communication between the intra and extraluminal compartments, including a perianastomotic abscess, as diagnosed by radiological investigation, reoperation, or examination of the neorectum under anaesthesia or at endoscopy [24]. Secondary outcomes included operative time, LoS, post operative morbidity, and conversion rates. Descriptive outcomes included quality of life and functional indicators. Comparative studies were considered eligible for meta-analysis if they reported on outcomes following Ta-IPAA and abd-IPAA (open, single, or multi-port laparoscopic, and/or robotic).
Study Overlap
Study overlap was addressed on a case-by-case basis for each outcome measure. The cohorts and study period in each article were carefully examined and partial and complete overlaps identified. These were represented graphically by Venn diagram. For a given outcome, the overall aggregated study populations were examined, and papers excluded in a fashion to minimise overall case loss while avoiding double counting. In some cases, this was straightforward while in others it required an examination of all possible inclusion permutations to ensure minimal losses.
Statistical Analysis
Extracted data was transferred into Review Manager 5.4.1 software (The Cochrane Collaboration, 2020) [25] for meta-analysis. Graphs were created using StataSE® 16 [26]. Continuous variables were standardised to mean ± standard deviation using Hozo [27] and Luo and Wan [28, 29] methods. For continuous variables (operative time, LoS) the mean difference and 95% confidence intervals (CI) were calculated. A DerSimonian and Laird method inverse-variance random-effects model was used to determine pooled outcome measures [30]. Heterogeneity between studies was calculated by the inconsistency test (I2). For dichotomous variables (anastomotic leak, conversion rate, morbidity), risk difference (RD) was analysed with its variance and 95% confidence interval. GRADEpro GDT was used to assess certainty of evidence [31]. Statistical analysis was performed by IS and KB.
Results
Study Characteristics
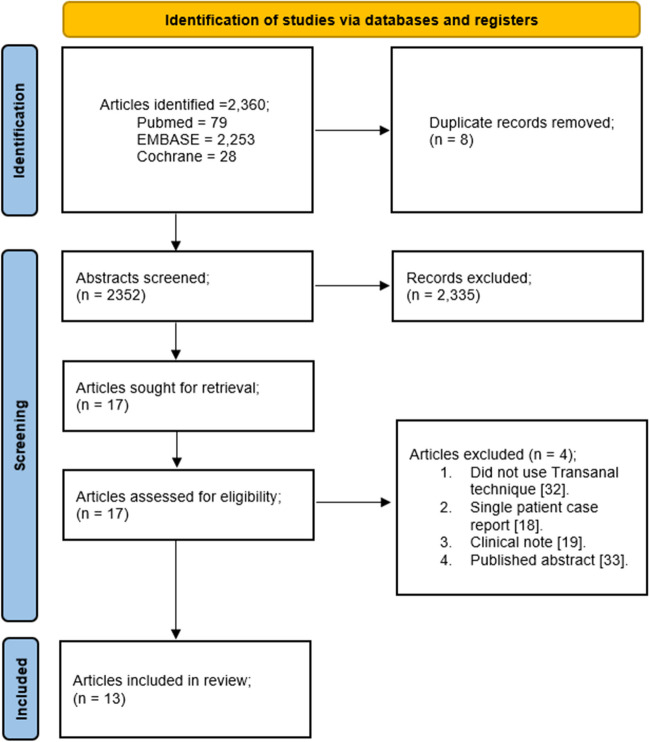
A total of 2,360 articles were identified for consideration. Of these, 2,253 were from EMBASE, 79 from Pubmed, and 28 from Cochrane library. There were 8 duplicates removed, and 2,352 abstracts were screened. A total of 17 papers were taken forward for full assessment, and of these 4 were excluded [18, 19, 32, 33] (Fig. 1).
Fig. 1.
Prisma flowchart of search strategy
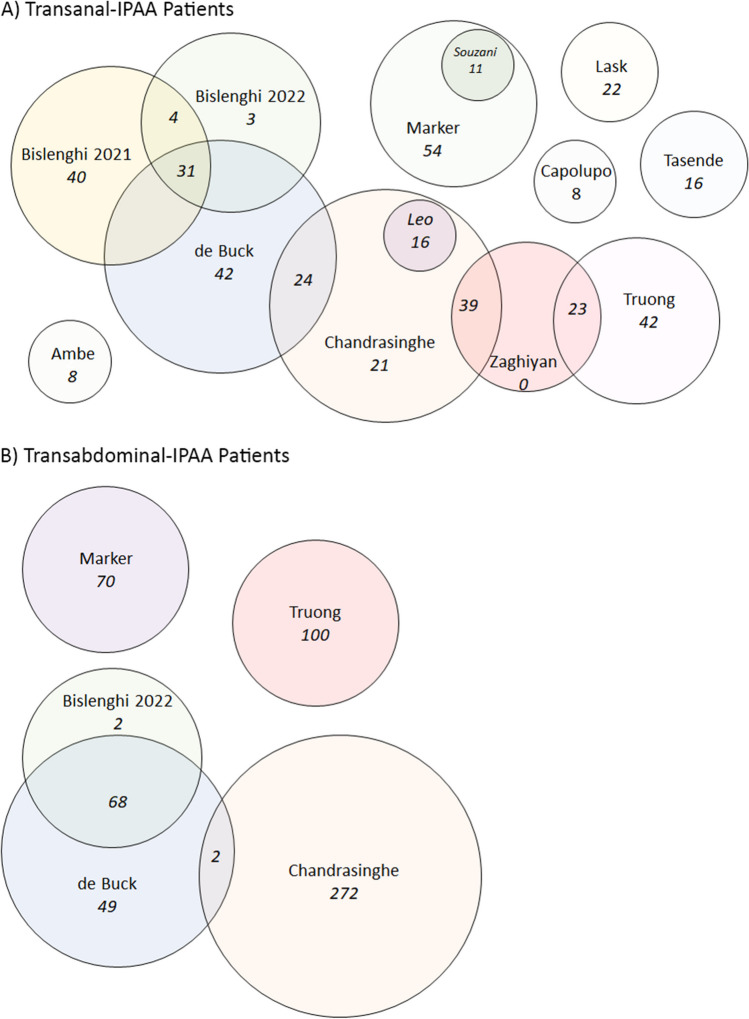
Of the 13 papers identified, all were published after 2015. Of these, five were small (8–16 patients) single centre case series, five were single centre cohort or comparative studies (22–65 Ta-IPAA patients), and three were large multicentre studies (62–100 Ta-IPAA patients) (Table 1). There were no randomised control trials. A gross total of 583 Ta-IPAA and 633 abd-IPAA patients were included, however 179 Ta-IPAA and 70 abd-IPAA patients appeared in two or more studies. This resulted in a total of 404 unique Ta-IPAA and 563 abd-IPAA after overlapping cases were considered (Fig. 2). All patients from Souzani 2019 and Leo 2016 were included in Marker 2022 and Chandrasinghe 2020 respectively, which resulted in their exclusion from analysis unless the outcome of interest was absent from the larger studies. The other overlaps were more complex and resulted in cascading exclusions. For example, when an outcome was present in all studies exclusion of de Buck 2017, Leo 2016, Bislenghi 2022, Souzani 2019, and Zaghiyan 2018 resulted in the minimum patient loss (45) as the remaining patients from these studies were captured in Chandrasinghe 2020, Truong 2022, Marker 2022, and Bislenghi 2021 giving a total of 359 patients for analysis (supplemental information 1).
Table 1.
Summary of included articles
| Author | Country | Publication Year | Study Design | Patient Numbers | Patient Age (mean ± SD) | Journal | Aim |
|---|---|---|---|---|---|---|---|
| Tasende et al. [15] | Spain | 2015 | Single centre, case series | 16 | 40.5 ± 15.7 | Surgical Endoscopy | Initial experience with Ta-IPAA |
| Leo et al. [34] | UK | 2016 | Single centre, case series | 16 | 46 ± 11 | Colorectal Disease | Technical description, Ta-IPAA combined with Single-incision abdominal approach |
| Ambe et al. [35] | Germany | 2017 | Single centre, case series | 8 | 20.5 ± 4.6 | Techniques in Coloproctology | Assess outcomes after Ta-IPAA in FAP |
| de Buck et al. [36] | Belgium, Netherlands, Denmark | 2017 | Multicentre, comparative cohorts |
219 Total 97 Transanal 119 Transabdominal |
Transanal 37 ± 17.8 Transabdominal 39 ± 13.3 | Annals of Surgery | Compare outcomes and technical features of Ta-IPAA and Abd-IPAA |
| Zaghiyan et al. [37] | US, UK, Sri Lanka | 2018 | Multicentre, comparing technical approaches | 62 | 38 ± 13 | Techniques in Coloproctology | Assess technical variations in application, and feasibility of Ta-IPAA in IBD internationally |
| Souzani et al. [38] | Denmark | 2019 | Single centre, case series | 11 | 31 ± 12 | Asian Journal of Endoscopic Surgery | Initial experience with Ta-IPAA |
| Bislenghi et al. [39] | Belgium | 2021 | Single centre, cohort study | 75 | 34 ± 9 | Colorectal Disease | Short term outcomes of Ta-IPAA following modified two-stage approach |
| Chandrasinghe et al. [40] | UK, Netherlands, Italy | 2020 | Multicentre, retrospective comparative cohort |
374 Total 100 Transanal 274 Transabdominal |
Transanal 39.9 ± 12.76 Transabdominal 38.2 ± 13.2 | Journal of Crohn's and Colitis | Compare long-term functional outcomes between Ta-IPAA and abd-IPAA |
| Capolupo et al. [41] | Italy | 2021 | Single centre, case series | 8 | 53.75 ± 14.7 | BMC Surgery | Initial experience with Ta-IPAA |
| Lask et al. [42] | Germany | 2021 | Single centre, retrospective cohort | 22 | 32 ± 12.5 | Patient Safety in Surgery | Determine anastomotic leak rates post Ta-IPAA as well as short + long term pouch function |
| Marker et al. [43] | Denmark | 2022 | Singe centre, retrospective comparative cohort |
135 Total 65 Transanal 70 Transabdominal |
Transanal 32 ± 12 Transabdominal 30 ± 12 | Techniques in Coloproctology | Compare short term outcomes between Ta-IPAA and Lap-IPAA |
| Bislenghi et al. [44] | Belgium | 2022 | Single centre, retrospective comparative cohort |
108 Total 38 Transanal 70 Transabdominal |
Transanal 36.7 ± 14.8 Transabdominal 39.1 ± 13.3 | Langenbeck's Archives of Surgery | Compare functional outcomes and QoL after Ta-IPAA or abd-IPAA for UC |
| Truong et al. [45] | US | 2022 | Single centre, prospective comparative cohort |
165 Total 65 Transanal 100 Transabdominal |
Transanal 37 ± 15 Transabdominal 37.8 ± 18 | Diseases of Colon and Rectum | Initial experience with Ta-IPAA, short term clinical outcomes |
QOL = Quality of Life. UC = Ulcerative Colitis. FAP = Familial adenomatous polyposis
Fig. 2.
Overlap Analysis. Venn diagrams demonstrating (A) Patient overlap for transanal patients between studies, (B) Patient overlap for transabdominal patients. 179 patients overlapped in the Ta-IPAA group, and 70 in the abd-IPAA cohorts, leaving a total of 404 unique Ta-IPAA patients, and 563 abd-IPAA patients across the studies
Technical Aspects and Clinical Outcomes of Ta-IPAA
All 13 papers reported on indication, number of stages, use of close rectal dissection (CRD) or total mesorectal excision (TME), and type of anastomosis. 9 reported on the number of teams used and conversion rates. Eleven papers reported on operative time and extraction site (Table 2). The use of covering ileostomy can be inferred by operative stages, de Buck et al. (3.1% 1-stage, 52.6% modified 2-stage), Bislenghi et al. 2021 and 2022 (100%, 92.11% modified 2-stage), and Chandrasinghe et al. 2022 (54% modified 2-stage) used well studied temporary ileostomy omitting approaches. Ambe et al. 2017 use a “ghost ileostomy”, which has been described elsewhere [46], as an alternative to traditional ostomy formation.
Table 2.
Summary of Ta-IPAA operative characteristics for all studies
| Article | Patient No | Indication | No. of Stages | TME/CRD | 1 or 2 Team Approach | Operative Time (mins—mean ± SD) | Conversion Rate (event no.) |
Extraction Site | Anastomosis |
|---|---|---|---|---|---|---|---|---|---|
| Tasende et al. 2015 | 16 | Ulcerative Colitis | 3-stage | CRD | 2 | 170 ± 50 | 0% (0) | Transanal | Stapled—87.5% Handsewn -12.5% |
| Leo et al. 2016 | 16 | Ulcerative Colitis | 2-stage—18.75% 3-stage—81.25% | TME | 2 | 247 ± 71 | 18.75% (3) | Stoma site |
Stapled—87.5% Handsewn—12.5% |
| Ambe et al. 2017 | 8 | FAP | 1-stage (with "ghost ileostomy") | TME | 1 | 512 ± 104 | 0% (0) | Suprapubic | Stapled |
| de Buck et al. 2017 | 97 | Ulcerative Colitis | 1-stage—3.1% 2-stage—4.1% Modified 2-stage—52.6% 3-stage—40.2% |
TME—45.4% CRD—54.6% |
not specified | 211 ± 54 | 5.2% (5) | Stoma site—7.2%, Transanal—42.3%, Pfannenstiel—20.6% Laparotomy—2.1% Umbilical—1%, Unspecified—26.8% | Stapled—97.9% Handsewn—2.1% |
| Zaghiyan et al. 2018 | 62 | Ulcerative Colitis (97%) Indeterminate Colitis (3%) | 2-stage—11% 3-stage—89% | TME—79% CRD—21% | predominantly 2 team | 266 ± 99 | not reported | Stoma site—56%, Transanal—42% | Stapled—81% Handsewn—19% |
| Souzani et al. 2019 | 11 | Ulcerative Colitis | 3-stage | CRD | 1 | 285 ± 52 | 0% (0) | Transanal | Stapled |
| Bislenghi et al. 2021 | 75 | Ulcerative Colitis | Modified 2 stage | TME—2.7% CRD—97.3% | not specified | 159 ± 23 | 4% (3) | Transanal—88%, Stoma site—12% | Stapled |
| Chandrasinghe et al. 2020 | 100 | Ulcerative Colitis | 1-stage—1% 2-stage—11% modified 2-stage—54% 3-stage—34% |
TME—41.6% CRD—58.3% |
2 | not reported | not reported | not reported | Stapled |
| Capolupo et al. 2021 | 8 | Ulcerative Colitis | 2 -stage—25% 3-stage—75% | CRD | 2 | 326 ± 69 | 0% (0) | Pfannenstiel—87.5% Stoma—12.5% | Stapled |
| Lask et al. 2021 | 22 | Ulcerative Colitis | 2-stage—13.6% 3-stage—86.4% | TME | 2 | 362 ± 163 | not reported | Port site (single port abdominal phase) | Stapled—36.3% Handsewn—63.6% |
| Marker et al. 2022 | 65 | Ulcerative Colitis | 3-stage | CRD | not specified | 277 ± 55 | 0% (0) | 1Stoma site—74% Midline—3% Suprapubic—23% | Stapled |
| Bislenghi et al. 2022 | 38 | Ulcerative Colitis | Modified 2-stage—92.11% 3-stage—7.89% | TME—2.7% CRD—97.3% | not specified | not reported | 0% (0) | not reported | Stapled—97.4% Handsewn—2.6% |
| Truong et al. 2022 | 65 | Ulcerative Colitis (83%) Indeterminate colitis (11%) Crohn’s Disease (6%) |
2-stage – 11% 3-stage – 89% |
TME | 2 | 256 ± 45 | not reported | Stoma site preferred | 2Handsewn—71% Stapled—29% |
CRD = Close Rectal Dissection, TME = Total mesorectal excision. 1 – Marker et al., report site of pouch creation in lieu of specimen extraction site. 2 – These are rates of anastomosis type across all 165 patients, not on Ta−IPAA individually as this is not included in Truong et al.
Ulcerative colitis was the most common indication, accounting for 94.7% of cases. Modified 2-stage (43.9%) and 3-stage (47.6%) were the most employed operative strategies, and CRD (50.5%) and TME (49.5%) were equally used. The stoma site (37%) was the most used extraction site, followed by transanal (28%). Weighted mean operative time was 261 ± 60 min.
The definition of an anastomotic leak varied between papers. Truong 2022, Bislenghi 2020, de Buck 2017, and Marker 2022 included both clinical and radiological leaks defined as identified either by radiological assessment demonstrating contrast enema extravasation at, or a defect in the anastomosis with or without perianastomotic fluid or abscess, or clinically at time of reoperation or rectal examination under anaesthesia, whereas Zaghiyan 2018, Chandrasinghe 2019, and Souzani 2019 also included perianastomotic pelvic abscesses without communication. Lask 2021 exclusively made the diagnosis by endoscopy with evidence of anastomotic defect. The remaining papers did not define anastomotic leakage, with Ambe 2017, Tasende 2015, and Capolupo reporting no leaks.
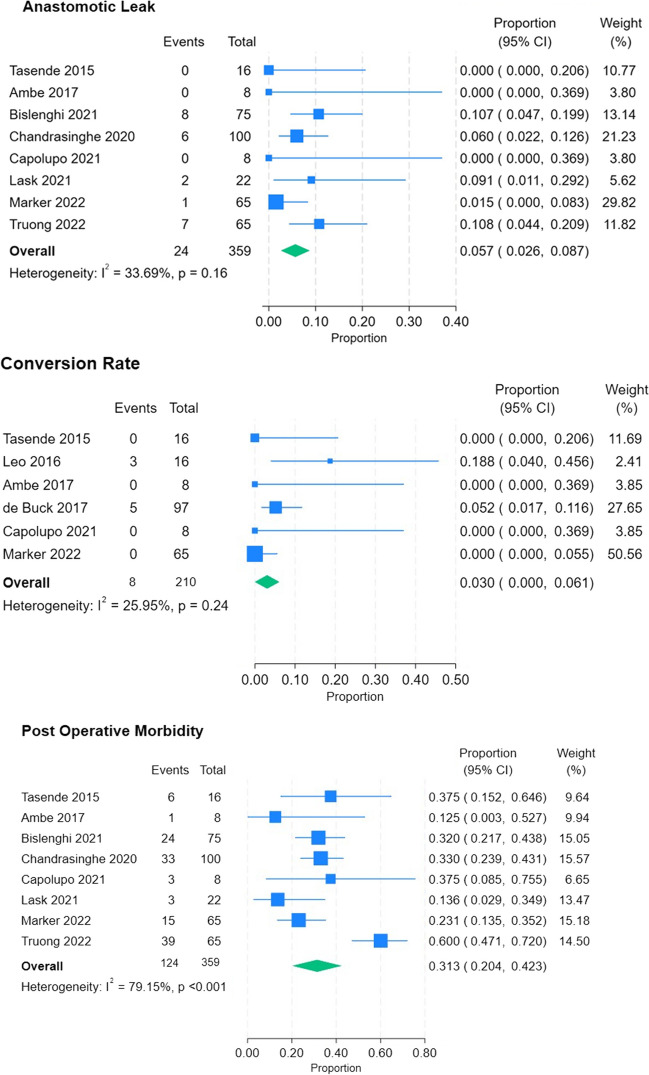
For non-comparative analysis, anastomotic leak rate after Ta-IPAA was 5.7% (CI 2.6–8.7%), conversion rate 3% (CI 0–0.06%), and post operative morbidity 31.3% (CI 20.4–42.3%). Study heterogeneity varied between variables assessed, I2 was 33.69% (p = 0.16), 25.95% (p = 0.24), and 79.15% (p < 0.001) respectively (Fig. 3). Weighted mean LoS was 6.7 days (± 2).
Fig. 3.
Non-comparative outcomes forest plots. Proportion of events as fraction of total population is plotted for each variable. I2 describes study heterogeneity, and p values relate to significance of heterogeneity
Comparative Outcomes
Five studies included comparative Ta-IPAA and abd-IPAA cohorts. Bislenghi 2022, Chandrasinghe, and de Buck include a variety of abd-IPAA techniques (open, single/multiple port laparoscopy, and/or robotic), whereas Truong is an exclusively open cohort, and Marker is exclusively laparoscopic. Not all studies report conversion rates or LoS, and de Buck et al. reports anastomotic leak rate across its total cohort, without a breakdown of Ta-IPAA and abd-IPAA events.
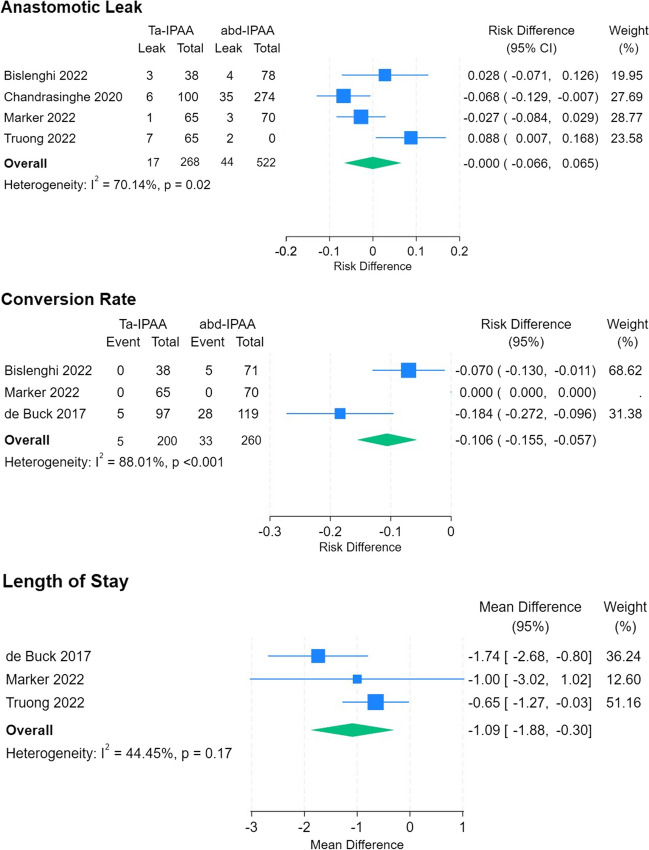
Weighted mean age for Ta-IPAA was 36.7(± 4.15), and 37.5(± 2.6) years for abd-IPAA. There was no significant difference in risk (RD 0, CI -0.066 to 0.065, p = 0.989) of anastomotic leak rate (6.3% Ta-IPAA, 8.4% abd-IPAA), or conversion rate (2.5% Ta-IPAA, 12.7% abd-IPAA, RD -0.106, 95% CI -0.155 to -0.057, p = 0.104) between approaches. Study heterogeneity (I2) was significant in anastomotic leak comparison (70.14%, p = 0.02) and conversion rate analysis (88%, p < 0.001). LoS was one day shorter with Ta-IPAA compared to abd-IPAA (mean difference -1.09, CI -1.88 to -0.3, p = 0.007). Study heterogeneity did not affect analysis (44.44%, p = 0.17) (Fig. 4).
Fig. 4.
Meta-analysis of Ta-IPAA compared to abd-IPAA. Left side of plot favours Ta-IPAA, right side favours abd-IPAA. Risk difference is reported for dichotomous variables, and mean difference for continuous variables. I2 describes study heterogeneity, and p values relates to the significance of heterogeneity
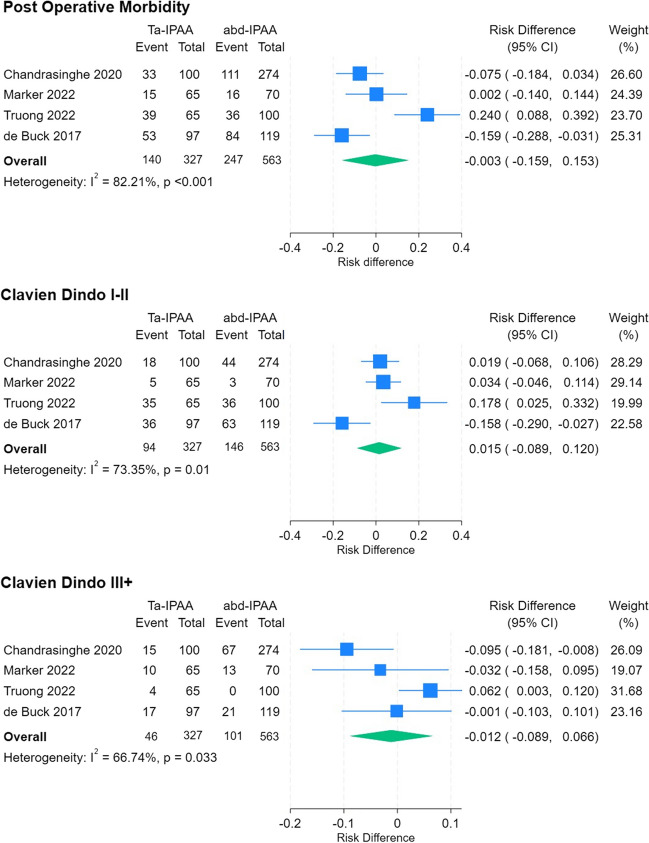
Overall post operative morbidity was comparable between approaches (42.8% Ta-IPAA, 43.9% abd-IPAA, RD -0.003, CI -0.159 to 0.153, p = 0.971). As were rates of Clavien Dindo Class I-II (28.7% Ta-IPAA, 25.9% abd-IPAA, RD 0.015, CI -0.089 to 0.120, p = 0.773), and Class III + (14.1% Ta-IPAA, 17.9% abd-IPAA, RD -0.012, CI -0.089 to 0.066, p = 0.771) complications. There was significant study heterogeneity across morbidity analysis (overall 82.21%, p < 0.001, Clavien Dindo I-II 73.35%, p = 0.010, Clavien Dindo III + 66.74%, p = 0.033) (Fig. 5). There is a small overlap between de Buck and Chandrasinghe (24 ta-IPAA, 2 abd-IPAA). In subgroup analysis with exclusion of de Buck on this basis, the outcomes of comparative meta-analysis (morbidity RD -0.05, CI -0.131 to 0.231, p = 0.537, Clavien Dindo I-II RD 0.055, CI -0.02 to 0.129, p = 0.148, and Clavien Dindo III + RD -0.017, CI -0.124 to 0.090, p = 0.755) still do not reach significance and was comparable to the full analysis and study heterogeneity remains high (I2 = 81.9% p < 0.001, I2 = 39.5% p = 0.19, I2 = 77.8% p = 0.01). Certainty of evidence as per GRADEpro GDT online assessment tool was very low for anastomotic leak, conversion rate, and post operative morbidity, and low for length of stay.
Fig. 5.
Meta-analysis of post operative co-morbidity of Ta-IPAA compared to abd-IPAA. Left side of plot favours Ta-IPAA, right side favours abd-IPAA. I2 describes study heterogeneity, and p values relates to the significance of heterogeneity
Functional outcomes
Two studies compared functional outcomes after transanal and transabdominal IPAA. Chandrasinghe 2020 followed patients at 6 weeks, 3, 6, and 12 months post operatively. Mean Cleveland Global Quality of Life score was similar between Ta-IPAA and abd-IPAA (0.75 ± 0.12, 0.71 ± 14; p = 0.11), but the transanal approach scored better on quality of health (7.30 vs 7.73, p = 0.04) and energy level (6.68 vs 7.17, p = 0.03). 24-h stool frequency was comparable between groups, with similar portions of patients reporting 10 or less stools (Ta-IPAA 78%, abd-IPAA 79%; p = 0.77).
Bislenghi 2022 similarly followed patients at 1, 3, 6, and 12 months. Global Quality of Life scale (GQOL) [47] was higher in transanal group compared to transabdominal (82.7 vs 75.5, p = 0.038). Oresland Score (OS) and Pouch Function Scores (PFS) were used to assess patient functional outcomes. Both scores improved in each group over time, converging towards similar results at 12 months. Ta-IPAA scored better on these measures at 12 months (OS – Ta-IPAA 4.6 vs abd-IPAA 6.2, p = 0.02, PFS – Ta-IPAA 6.1 vs abd-IPAA 7.4, p = 0.32).
Discussion
The 13 papers identified, which describe Ta-IPAA across 9 countries and 3 continents, demonstrate that Ta-IPAA has been adopted across a wide variety of centres, with significant variation in practice regarding the number of operative stages, approach to rectal resection (CRD vs TME), and extraction site. By reviewing and synthesizing the published literature to-date, this analyse provides an overview of current technical nuisances and outcomes in Ta-IPAA and provides a limited comparison against abd-IPAA demonstrating similar post operative outcomes with reduced length of stay.
Several aspects of the operative approach have been hotly debated and examined since its introduction. The stapled J-pouch is now most common due to its simplicity of construction and reliable emptying, whereas the S-pouch is typically reserved for cases where reach is a challenge [48, 49]. Similarly, the stapled ileoanal anastomosis is widely preferred to handsewn with mucosectomy owing to better short-term functional results, reduced rate of stricturing, and ease of creation, except in cases of dysplasia, cancer, or extraintestinal manifestations when complete excision of the rectal mucosa is advised [50–52]. Other aspects such as number of stages, avoidance of defunctioning ileostomy, and plane of dissection remain contested. The single-stage operation is rarely used, while the choice of 2-stage, modified 2-stage, and 3-stage approach is influenced by patient disease processes, physiology, and status of medical management but varies significantly internationally with comparative data primarily from retrospective cohort studies at expert centres [53–57]. Similarly, CRD is preferred in mainland Europe, whereas TME is preferred in the United Kingdom and North America. CRD has been suggested as a means of reducing the risk of nerve and urethral injury and providing a mesorectal “cushion” around the pouch which may help contain small posterior perforations/leaks, but many colorectal surgeons are more accustomed to the TME approach, due to its ubiquity in surgical oncology [58–60]. These international trends are reflected in this pooled analysis. All studies use a J-pouch and only one institution preferred a handsewn anastomosis over stapled. There was varied practice in staging, with a modified 2-stage or 3-stage approach preferred over 1-stage or traditional 2-stage.
While minimally invasive surgery (MIS) is preferable to open surgery, due to the improved female fecundity, reduced adhesions, reduced length of stay and comparable post operative and functional outcomes [7, 61–66], there is still a role for open surgery in this technically demanding procedure, particularly after prior open colectomy due to the increased adhesions, and for resection and transection of the distal rectum and construction of the stapled anastomosis. Many surgeons opt to cross-staple the distal rectum and perform the anastomosis through a small lower midline or pfannenstiel incision when performing MIS. The increased dexterity of robotic instruments may reduce the need for this, and early robotic case series from expert centres demonstrate comparable outcomes to laparoscopic techniques [67–70].
In contrast to transabdominal MIS approaches, transanal MIS provides an alternative approach to the narrow pelvis which in expert hands may overcome the challenges of the abdominal approach, without necessitating conversion to open surgery. Furthermore, the rectum does not need to be cross-stapled as is standard for the double-stapled anastomosis. Instead, the rectal lumen is closed with a purse-string suture and a full thickness rectotomy performed distal to this under direct laparoscopic vision [20]. The specimen can subsequently be extracted through the rectal cuff and anus. Data from RCT and retrospective case matched studies demonstrate reduced post operative patient analgesia requirements and LoS after natural orifice specimen extraction in laparoscopic colectomy and anterior resection [15, 71, 72]. Finally, a single circular stapler fire is used to form the pouch-anal anastomosis, avoiding the need for crossing staple lines. The studies included in this analysis exploited these attributes to varying degrees. Only 3 used transanal extraction preferentially and 2 favoured handsewn anastomosis over single-stapled. It was not possible to perform comparative subgroup analysis examining site of extraction or type of anastomosis due to primary data limitations.
On comparative meta-analysis, anastomotic leak rates between the transanal and transabdominal patient cohorts were comparable. The definition of anastomotic leak varied between papers, but broadly conformed to the principals set out by Rahbari et al., namely disruption of the intestinal wall integrity at the anastomosis with evidence of intra and extraluminal communication, diagnosed by radiological, luminal, or operative findings[24]. Differences in the categorisation of perianastomotic pelvic abscesses without evidence of intraluminal communication, and the diagnostic modalities used between papers may account for the heterogeneity in leak rates. Similarly to our findings, a prior meta-analysis focused on the applicability of Ta-IPAA in the paediatric setting showed outcomes comparable to abd-IPAA (anastomotic leak rate 7.1%, odds ratio 1.36; 95% CI 0.46–4.06, I2 = 68%) however a statistically significant difference between LoS was not demonstrated (mean LoS 7.4 days, 95% CI 6–8.8, odds ratio 0.61 days; 95% CI 2.39–1.17) [73]. Functional outcomes after Ta-IPAA appear to be comparable with a transabdominal approach, in keeping with a narrative review of papers specifically investigating functional outcomes after Ta-IPAA [74] but was limited to 1 year follow-up or less.
The limitations of these analyses are the heterogeneity of studies included and the lack of RCT and case-matched series which introduces selection bias. Much of this heterogeneity is a consequence of the broader variation in approaches to pouch surgery – most particularly in staging, abdominal approach, and plane of dissection. The results, though promising, should be interpreted in this context. The systematic review provided here gives a comprehensive overview of current practices and early outcomes from expert centres, which are comparable to those for transabdominal MIS series (LoS 4–14.3 days, post operative morbidity 25–50%, conversion 1.4–13%) [7]. Furthermore, it highlights operative facets which will need to be standardised for high quality RCT comparing transanal and transabdominal approaches. A head-to-head comparison of ta-IPAA with two-teams, transanal extraction, and single-stapled anastomosis against laparoscopic-IPAA with transabdominal extraction and double-stapled anastomosis in a modified 2-stage or 3-stage approach would be valuable and generalisable. This study design would assess the theoretical advantages of the transanal approach while controlling for other confounding operative variables.
It is well recognised that transanal rectal resection has a significant learning curve [75]. Many of the centres captured in this meta-analysis had well established transanal programmes in place prior to adoption of the transanal IPAA technique, and others have been clear about the improvements in their outcomes over time after adaptation. Subgroup analysis from one centre demonstrated a reduction in anastomotic leak rates between cohort first, second and third tertiles (14% vs 14% vs 5%), suggesting that outcomes improved as familiarity with the approach increased [45]. Transanal programmes for rectal cancer resection have been implemented in many centres, which have resulted in improved outcomes for TaTME compared to early attempts at implementation [76, 77]. It would be reasonable to expect that similar gains could be seen by adaptation of the transanal approach to benign disease.
Conclusion
This review demonstrates the safety, feasibility and reassuring clinical outcomes of Ta-IPAA. Comparative meta-analysis, demonstrates a reduction in length of stay and a trend towards reduced conversion rates compared to abd-IPAA, but was limited by study heterogeneity and a lack of RCTs and case-matched studies, resulting in low to very low certainty of evidence. Robustly designed randomised controlled trials are required to further compare short- and long-term clinical outcomes, as well as quality of life measures. Such trials should take place at centres with established transanal surgery programmes to account for the learning curve associated with these techniques.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
Dr. Ian Stephens and Prof. John Burke conceptualised and designed the study. Literature search and systematic review was performed by Dr. Ian Stephens. Dr. Ian Stephens and Dr. Kevin Byrnes performed the meta-analysis and statistical analysis. All authors were involved in drafting and revision of the manuscript.
Funding
Open Access funding provided by the IReL Consortium.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Parks AG, Nicholls RJ. Proctocolectomy without ileostomy for ulcerative colitis. Br Med J. 1978;2:85. doi: 10.1136/BMJ.2.6130.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee KE, Faye AS, Vermeire S, et al. Perioperative Management of Ulcerative Colitis: A Systematic Review. Dis Colon Rectum. 2022;65(S1):S5–S19. doi: 10.1097/DCR.0000000000002588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fábio GC. Surgical treatment of familial adenomatous polyposis: dilemmas and current recommendations. World J Gastroenterol. 2014;20:16620–16629. doi: 10.3748/WJG.V20.I44.16620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reese GE, Lovegrove RE, Tilney HS, et al. The effect of Crohn’s disease on outcomes after restorative proctocolectomy. Dis Colon Rectum. 2007;50:239–250. doi: 10.1007/s10350-006-0777-x. [DOI] [PubMed] [Google Scholar]
- 5.Aquina C, Fleming F, Becerra A, et al. Who gets a pouch after colectomy in New York state and why? Dis Colon Rectum. 2016;59:e109–e110. doi: 10.1097/01.dcr.0000482708.50838.af. [DOI] [Google Scholar]
- 6.Lightner AL, Mathis KL, Dozois EJ, et al. Results at Up to 30 Years after Ileal Pouch-Anal Anastomosis for Chronic Ulcerative Colitis. Inflamm Bowel Dis. 2017;23:781–790. doi: 10.1097/MIB.0000000000001061. [DOI] [PubMed] [Google Scholar]
- 7.Baek S-J, Dozois EJ, Mathis KL, et al. Safety, feasibility, and short-term outcomes in 588 patients undergoing minimally invasive ileal pouch-anal anastomosis: a single-institution experience. Tech Coloproctol. 2016;20:369–374. doi: 10.1007/s10151-016-1465-z. [DOI] [PubMed] [Google Scholar]
- 8.Spinelli A, Bonovas S, Burisch J, et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Surgical Treatment. J Crohns Colitis. 2022;16:179–189. doi: 10.1093/ecco-jcc/jjab177. [DOI] [PubMed] [Google Scholar]
- 9.Fazio VW, Tekkis PP, Remzi F, et al. Quantification of risk for pouch failure after ileal pouch anal anastomosis surgery. Ann Surg. 2003;238:605–617. doi: 10.1097/01.SLA.0000090940.39838.6A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beyer-Berjot L, Maggiori L, Birnbaum D, et al. A total laparoscopic approach reduces the infertility rate after ileal pouch-anal anastomosis: A 2-center study. Ann Surg. 2013;258:275–282. doi: 10.1097/SLA.0b013e3182813741. [DOI] [PubMed] [Google Scholar]
- 11.Singh P, Bhangu A, Nicholls RJ, Tekkis P. A systematic review and meta-analysis of laparoscopic vs open restorative proctocolectomy. Colorectal Dis. 2013;15(7):e340–351. doi: 10.1111/codi.12231. [DOI] [PubMed] [Google Scholar]
- 12.Deputy M, Pitman F, Sahnan K, et al. An early experience in robotic ileoanal pouch surgery with robotic intracorporeal single-stapled anastomosis (RiSSA) at a tertiary referral centre. Colorectal Dis. 2023;25(6):1169–1175. doi: 10.1111/codi.16528. [DOI] [PubMed] [Google Scholar]
- 13.Khawaja Z, Jamal Z, Zafar N, et al. Role of robotic approach in ileal pouch–anal anastomosis (IPAA): A systematic review of the literature. J Robot Surg. 2022 doi: 10.1007/s11701-022-01490-x. [DOI] [PubMed] [Google Scholar]
- 14.Gash KJ, Goede A, Dixon A. Single incision laparoscopic surgery (SILS) for colorectal resections: the first UK case series of 100 patients. Colorectal Dis. 2011;13:4. doi: 10.1111/j.1463-1318.2011.02660.x. [DOI] [Google Scholar]
- 15.Tasende MM, Delgado S, Jimenez M, et al. Minimal invasive surgery: NOSE and NOTES in ulcerative colitis. Surg Endosc. 2015;29:3313–3318. doi: 10.1007/s00464-015-4087-z. [DOI] [PubMed] [Google Scholar]
- 16.Vahdad MR, Cernaianu G, Semaan A, et al. An experimental study in six fresh human cadavers using a novel approach to avoid abdominal wall incisions in total colectomy: totally transanal laparoendoscopic single-site pull-through colectomy with J-pouch creation. Surg Endosc. 2016;30:3107–3113. doi: 10.1007/s00464-015-4555-5. [DOI] [PubMed] [Google Scholar]
- 17.Vahdad MR, Rahmanian E, Moslemi S, et al. Totally transanal laparo-endoscopic single-site proctocolectomy-lleoanal J-Pouch (TLPC-J): An experimental study of a novel approach. Iran J Med Sci. 2015;40:425–429. [PMC free article] [PubMed] [Google Scholar]
- 18.Coffey JC, Dillon MF, O’Driscoll JS, Faul E. Transanal total mesocolic excision (taTME) as part of ileoanal pouch formation in ulcerative colitis—first report of a case. Int J Colorectal Dis. 2016;31:735–736. doi: 10.1007/s00384-015-2236-4. [DOI] [PubMed] [Google Scholar]
- 19.de Buck van Overstraetan A, Wolthuis AM, D’Hoore A. Transanal completion proctectomy after total colectomy and ileal pouch-anal anastomosis for ulcerative colitis: a modified single stapled technique. Colorectal Dis. 2016;18(4):O141–O144. doi: 10.1111/codi.13292. [DOI] [PubMed] [Google Scholar]
- 20.de Lacy FB, Keller DS, Martin-Perez B, et al. The current state of the transanal approach to the ileal pouch-anal anastomosis. Surg Endosc. 2019;33:1368–1375. doi: 10.1007/S00464-019-06674-5. [DOI] [PubMed] [Google Scholar]
- 21.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372: 10.1136/BMJ.N71 [DOI] [PMC free article] [PubMed]
- 22.Zittan E, Wong-Chong N, Ma GW, et al. Modified two-stage ileal pouch-anal anastomosis results in lower rate of anastomotic leak compared with traditional two-stage surgery for ulcerative colitis. J Crohns Colitis. 2016;10:766–772. doi: 10.1093/ecco-jcc/jjw069. [DOI] [PubMed] [Google Scholar]
- 23.National Institute for Health and Care Research PROSPERO - International prospective register of systematic reviews [Internet]. https://www.crd.york.ac.uk/prospero/. Accessed Sept 2023
- 24.Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: A proposal by the International Study Group of Rectal Cancer. Surgery. 2010;147:339–351. doi: 10.1016/j.surg.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 25.The Cochrane Collaboration (2020) Review Manager (RevMan) [Computer program]. Version 5.4.1. Available at https://www.revman.cochrane.org
- 26.StataCorp (2023) Stata Statistical Software [Computer program]: Release 16. StataCorp LLC, College Station, TX
- 27.Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed]
- 28.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodology. 2014;19(14):135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo D, Wan X, Liu J, Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/J.CCT.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMaster University and Evidence Prime (2022) GRADEpro GDT: GRADEpro Guideline Development Tool [Computer programme]. Hamilton (ON). Available at https://www.gradepro.org
- 32.Kayaalp C, Yagci MA, Soyer V. Laparoscopic and natural orifce transluminal restorative proctocolectomy: No abdominal incision for specimen extraction or ileostomy. Wideochirurgia I Inne Techniki Maloinwazyjne. 2016;11:115–120. doi: 10.5114/wiitm.2016.59578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truong A, Fleshner P, Zaghiyan KN. A single-center experience of transanal ileal pouch-anal anastomosis for inflammatory bowel disease. Dis Colon Rectum. 2019;62:e273–e274. doi: 10.1097/DCR.0000000000001415. [DOI] [Google Scholar]
- 34.Leo CA, Samaranayke S, Maeda Y, et al. Single incision surgery combined with the transanal approach for ileoanal pouch surgery. Colorectal Dis. 2015;17:105. doi: 10.1111/codi.13055. [DOI] [Google Scholar]
- 35.Ambe PC, Zirngibl H, Möslein G. Initial experience with taTME in patients undergoing laparoscopic restorative proctocolectomy for familial adenomatous polyposis. Tech Coloproctol. 2017;21:971–974. doi: 10.1007/S10151-017-1730-9. [DOI] [PubMed] [Google Scholar]
- 36.Overstraeten DBV, Mark-Christensen AA, Wasmann KA, et al. Transanal Versus Transabdominal Minimally Invasive (Completion) Proctectomy with Ileal Pouch-anal Anastomosis in Ulcerative Colitis. Ann Surg. 2017;266:878–883. doi: 10.1097/SLA.0000000000002395. [DOI] [PubMed] [Google Scholar]
- 37.Zaghiyan K, Warusavitarne J, Spinelli A, et al. Technical variations and feasibility of transanal ileal pouch-anal anastomosis for ulcerative colitis and inflammatory bowel disease unclassified across continents. Tech Coloproctol. 2018;22:867–873. doi: 10.1007/s10151-018-1889-8. [DOI] [PubMed] [Google Scholar]
- 38.Levic Souzani K, Nielsen CB, Bulut O. Transanal completion proctectomy with close rectal dissection and ileal pouch-anal anastomosis for ulcerative colitis. Asian J Endosc Surg. 2019;12:281–286. doi: 10.1111/ases.12646. [DOI] [PubMed] [Google Scholar]
- 39.Bislenghi G, Martin-Perez B, Fieuws S, et al. Increasing experience of modified two-stage transanal ileal pouch–anal anastomosis for therapy refractory ulcerative colitis. What have we learned? A retrospective analysis on 75 consecutive cases at a tertiary referral hospital. Colorectal Dis. 2021;23:74–83. doi: 10.1111/codi.15231. [DOI] [PubMed] [Google Scholar]
- 40.Chandrasinghe P, Carvello M, Wasmann K, et al. Long-term function after transanal vs. transabdominal ileal pouch-anal anastomosis for ulcerative colitis: A multi-centre cohort study. J Crohns Colitis. 2019;13:S345. doi: 10.1093/ecco-jcc/jjy222.592. [DOI] [Google Scholar]
- 41.Capolupo GT, Carannante F, Mascianà G, Lauricella S, Mazzotta E, Caricato M (2021) Transanal proctocolectomy and ileal pouch-anal anastomosis (TaIPAA) for ulcerative colitis: medium term functional outcomes in a single centre. BMC Surg 21(1). 10.1186/s12893-020-01007-z [DOI] [PMC free article] [PubMed]
- 42.Lask A, Biebl M, Dittrich L, Fischer A, Adler A, Tacke F, Aigner F, Schmuck R, Chopra S, Knoop M, Pratschke J, Gül-Klein S (2021) Safety of transanal ileal pouch-anal anastomosis for ulcerative colitis: a retrospective observational cohort study. Patient Saf Surg 15(1). 10.1186/s13037-021-00306-5 [DOI] [PMC free article] [PubMed]
- 43.Marker L, Kjær S, Levic-Souzani K, Bulut O. Transanal ileal pouch-anal anastomosis for ulcerative colitis: a single-center comparative study. Tech Coloproctol. 2022;26:875–881. doi: 10.1007/s10151-022-02658-1. [DOI] [PubMed] [Google Scholar]
- 44.Bislenghi G, Denolf M, Fieuws S, Wolthuis A, D’Hoore A. Functional outcomes of transanal versus transabdominal restorative proctectomy with ileal pouch-anal anastomosis in ulcerative colitis—a monocentric retrospective comparative study. Langenbecks Arch Surg. 2022;407(8):3607–3614. doi: 10.1007/s00423-022-02640-3. [DOI] [PubMed] [Google Scholar]
- 45.Truong A, Wood T, Fleshner PR, Zaghiyan KN. A Single-Center Experience of Transanal Proctectomy with IPAA for IBD. Dis Colon Rectum. 2022;65:1121–1128. doi: 10.1097/DCR.0000000000002087. [DOI] [PubMed] [Google Scholar]
- 46.Mari FS, Di Cesare T, Novi L, et al. Does ghost ileostomy have a role in the laparoscopic rectal surgery era? A randomized controlled trial. Surg Endosc. 2015;29:2590–2597. doi: 10.1007/s00464-014-3974-z. [DOI] [PubMed] [Google Scholar]
- 47.Hyland ME, Sodergren SC. Development of a new type of global quality of life scale, and comparison of performance and preference for 12 global scales. Qual Life Res. 1996;5:469–480. doi: 10.1007/BF00540019. [DOI] [PubMed] [Google Scholar]
- 48.Olortegui KS, Graham A, Hyman N. Staging Considerations for the Ileal Pouch-Anal Anastomosis. J Gastrointest Surg. 2022;26:1531–1536. doi: 10.1007/s11605-022-05317-w. [DOI] [PubMed] [Google Scholar]
- 49.Lovegrove RE, Heriot AG, Constantinides V, et al. Meta-analysis of short-term and long-term outcomes of J, W and S ileal reservoirs for restorative proctocolectomy. Colorectal Dis. 2007;9:310–320. doi: 10.1111/j.1463-1318.2006.01093.x. [DOI] [PubMed] [Google Scholar]
- 50.Fazio VW, Kiran RP, Remzi FH, et al. Ileal Pouch Anal Anastomosis. Ann Surg. 2013;257:679–685. doi: 10.1097/SLA.0b013e31827d99a2. [DOI] [PubMed] [Google Scholar]
- 51.Selvaggi F, Pellino G, Canonico S, Sciaudone G. Systematic review of cuff and pouch cancer in patients with Ileal pelvic pouch for ulcerative colitis. Inflamm Bowel Dis. 2014;20:1296–1308. doi: 10.1097/MIB.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 52.Lovegrove RE, Constantinides VA, Heriot AG, et al. A comparison of hand-sewn versus stapled ileal pouch anal anastomosis (IPAA) following proctocolectomy: A meta-analysis of 4183 patients. Ann Surg. 2006;244:18–26. doi: 10.1097/01.sla.0000225031.15405.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peirce C, Remzi FH. Operative strategy, risk factors for leak, and the use of a defunctioning ileostomy with ileal pouch-anal anastomosis: Let’s not divert from diversion and the traditional 3-stage approach for inflammatory bowel disease. J Crohns Colitis. 2016;10:755–757. doi: 10.1093/ecco-jcc/jjw100. [DOI] [PubMed] [Google Scholar]
- 54.Luo WY, Singh S, Cuomo R, Eisenstein S. Modified two-stage restorative proctocolectomy with ileal pouch-anal anastomosis for ulcerative colitis: a systematic review and meta-analysis of observational research. Int J Colorectal Dis. 2020;35:1817–1830. doi: 10.1007/s00384-020-03696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee GC, Deery SE, Kunitake H, et al. Comparable perioperative outcomes, long-term outcomes, and quality of life in a retrospective analysis of ulcerative colitis patients following 2-stage versus 3-stage proctocolectomy with ileal pouch-anal anastomosis. Int J Colorectal Dis. 2019;34:491–499. doi: 10.1007/s00384-018-03221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bikhchandani J, Polites SF, Wagie AE, et al. National trends of 3- Versus 2-stage restorative proctocolectomy for chronic ulcerative colitis. Dis Colon Rectum. 2015;58:199–204. doi: 10.1097/DCR.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 57.Association of Coloproctology of Great Britian and Ireland (2017) Ileoanal Pouch Report. Available at https://www.acpgbi.org.uk/resources/224/ileoanal_pouch_report_2017/
- 58.Nally DM, Kavanagh DO, Winter DC. Close rectal dissection in benign diseases of the rectum: A review. Surgeon. 2019;17:119–126. doi: 10.1016/j.surge.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Reijntjes MA, de Jong DC, Bartels S, et al. Long-term outcomes after close rectal dissection and total mesorectal excision in ileal pouch-anal anastomosis for ulcerative colitis. Tech Coloproctol. 2023;27:297–307. doi: 10.1007/s10151-022-02713-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bartels SAL, Gardenbroek TJ, Aarts M, Ponsioen CY, Tanis PJ, Buskens CJ, Bemelman WA. Short-term morbidity and quality of life from a randomized clinical trial of close rectal dissection and total mesorectal excision in ileal pouch-anal anastomosis. Br J Surg. 2015;102(3):281–287. doi: 10.1002/bjs.9701. [DOI] [PubMed] [Google Scholar]
- 61.Ali UA, Keus F, Heikens JT, et al. Open versus laparoscopic (assisted) ileo pouch anal anastomosis for ulcerative colitis and familial adenomatous polyposis. Cochrane Database Syst Rev. 2009 doi: 10.1002/14651858.CD006267.PUB2. [DOI] [PubMed] [Google Scholar]
- 62.El-Gazzaz GS, Kiran RP, Remzi FH, et al. Outcomes for case-matched laparoscopically assisted versus open restorative proctocolectomy. Br J Surg. 2009;96:522–526. doi: 10.1002/bjs.6578. [DOI] [PubMed] [Google Scholar]
- 63.Fleming FJ, Francone TD, Kim MJ, et al. A laparoscopic approach does reduce short-term complications in patients undergoing ileal pouch-anal anastomosis. Dis Colon Rectum. 2011;54:176–182. doi: 10.1007/DCR.0b013e3181fb4232. [DOI] [PubMed] [Google Scholar]
- 64.Maartense S, Dunker MS, Slors JF, et al. Hand-assisted laparoscopic versus open restorative proctocolectomy with ileal pouch anal anastomosis: A randomized trial. Ann Surg. 2004;240(6):984–992. doi: 10.1097/01.sla.0000145923.03130.1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bartels S, D’Hoore A, Cuesta M, et al. Better fertility preservation after laparoscopic restorative proctocolectomy: A cross-sectional study. Colorectal Dis. 2011;13:6. doi: 10.1111/j.1463-1318.2011.02704.x. [DOI] [Google Scholar]
- 66.Schiessling S, Leowardi C, Kienle P, et al. Laparoscopic versus conventional ileoanal pouch procedure in patients undergoing elective restorative proctocolectomy (LapConPouch Trial) - A randomized controlled trial. Langenbecks Arch Surg. 2013;398:807–816. doi: 10.1007/s00423-013-1088-z. [DOI] [PubMed] [Google Scholar]
- 67.Lightner AL, Grass F, McKenna NP, et al. Short-term postoperative outcomes following robotic versus laparoscopic ileal pouch-anal anastomosis are equivalent. Tech Coloproctol. 2019;23:259–266. doi: 10.1007/s10151-019-01953-8. [DOI] [PubMed] [Google Scholar]
- 68.Lightner AL, Kelley SR, Larson DW. Robotic platform for an IPAA. Dis Colon Rectum. 2018;61:869–874. doi: 10.1097/DCR.0000000000001125. [DOI] [PubMed] [Google Scholar]
- 69.Flynn J, Larach JT, Kong JCH, et al. Robotic versus laparoscopic ileal pouch-anal anastomosis (IPAA): a systematic review and meta-analysis. Int J Colorectal Dis. 2021;36:1345–1356. doi: 10.1007/s00384-021-03868-z. [DOI] [PubMed] [Google Scholar]
- 70.Mark-Christensen A, Pachler FR, Nørager C, et al. Short-term outcome of robot-assisted and open IPAA: An observational single-center study. Dis Colon Rectum. 2015;59:201–207. doi: 10.1097/DCR.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 71.Saurabh B, Chang SC, Ke TW, et al. Natural Orifice Specimen Extraction with Single Stapling Colorectal Anastomosis for Laparoscopic Anterior Resection: Feasibility, Outcomes, and Technical Considerations. Dis Colon Rectum. 2017;60:43–50. doi: 10.1097/DCR.0000000000000739. [DOI] [PubMed] [Google Scholar]
- 72.Wolthuis AM, Fieuws S, Van Den Bosch A, et al. Randomized clinical trial of laparoscopic colectomy with or without natural-orifice specimen extraction. Br J Surg. 2015;102:630–637. doi: 10.1002/bjs.9757. [DOI] [PubMed] [Google Scholar]
- 73.Eshel Fuhrer A, Kammar H, Herzlich J, Sukhotnik I. Transanal ileal pouch anal anastomosis for ulcerative colitis in children and adults: a systematic review and meta-analysis. Pediatr Surg Int. 2022;38:1671–1680. doi: 10.1007/s00383-022-05222-y. [DOI] [PubMed] [Google Scholar]
- 74.Vernon J, Ng D, Khan S, et al. Functional outcomes after transanal ileal pouch–anal anastomosis for ulcerative colitis: narrative review of the current literature. Tech Coloproctol. 2023;27:713–719. doi: 10.1007/s10151-023-02798-y. [DOI] [PubMed] [Google Scholar]
- 75.Larsen SG, Pfeffer F, Kørner H. Norwegian moratorium on transanal total mesorectal excision. Br J Surg. 2019;106:1120–1121. doi: 10.1002/BJS.11287. [DOI] [PubMed] [Google Scholar]
- 76.Zeng Z, Liu Z, Luo S, et al. Three-year outcomes of transanal total mesorectal excision versus standard laparoscopic total mesorectal excision for mid and low rectal cancer. Surg Endosc. 2022;36:3902–3910. doi: 10.1007/s00464-021-08707-4. [DOI] [PubMed] [Google Scholar]
- 77.Serra-Aracil X, Zarate A, Bargalló J, et al. Transanal versus laparoscopic total mesorectal excision for mid and low rectal cancer (Ta-LaTME study): multicentre, randomized, open-label trial. Br J Surg. 2023;110:150–158. doi: 10.1093/bjs/znac324. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.