Abstract
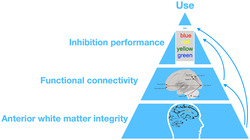
Substance use disorders are characterized by inhibition deficits related to disrupted connectivity in white matter pathways, leading via interaction to difficulties in resisting substance use. By combining neuroimaging with smartphone‐based ecological momentary assessment (EMA), we questioned how biomarkers moderate inhibition deficits to predict use. Thus, we aimed to assess white matter integrity interaction with everyday inhibition deficits and related resting‐state network connectivity to identify multi‐dimensional predictors of substance use. Thirty‐eight patients treated for alcohol, cannabis or tobacco use disorder completed 1 week of EMA to report substance use five times and complete Stroop inhibition testing twice daily. Before EMA tracking, participants underwent resting state functional MRI and diffusion tensor imaging (DTI) scanning. Regression analyses were conducted between mean Stroop performances and whole‐brain fractional anisotropy (FA) in white matter. Moderation testing was conducted between mean FA within significant clusters as moderator and the link between momentary Stroop performance and use as outcome. Predictions between FA and resting‐state connectivity strength in known inhibition‐related networks were assessed using mixed modelling. Higher FA values in the anterior corpus callosum and bilateral anterior corona radiata predicted higher mean Stroop performance during the EMA week and stronger functional connectivity in occipital–frontal–cerebellar regions. Integrity in these regions moderated the link between inhibitory control and substance use, whereby stronger inhibition was predictive of the lowest probability of use for the highest FA values. In conclusion, compromised white matter structural integrity in anterior brain systems appears to underlie impairment in inhibitory control functional networks and compromised ability to refrain from substance use.
Keywords: EMA, inhibition, resting state, Stroop, SUD, white matter
Fiber tract integrity in anterior brain systems was predictive of better inhibitory control within an EMA week and of stronger functional connectivity in occipital‐frontal‐cerebellar regions. Additionally, integrity in these selective white matter tracts moderated the temporal link between inhibitory control and substance use, whereby greater inhibitory control and high tract integrity were predictive of a low probability of drug use. By contrast, impairment in structural integrity in anterior brain systems underwrote functional impairment in executive inhibitory control networks and compromise in everyday expression of inhibition, resulting in a weakened ability to refrain from substance use.
1. INTRODUCTION
Substance use disorders (SUDs) are characterized by complex interactions between cognitive and cerebral alterations that contribute to an unhealthy use pattern. Among the spectrum of executive deficits relevant to SUD, 1 , 2 inhibitory control has been highlighted as a core factor in the development and maintenance of addictive behaviours. 3 , 4 Disturbed connectivity of neural systems subserving inhibitory control involves white matter bundles that link the frontal cortex 5 to other cortical and subcortical regions. 6 While numerous white matter pathways have been identified as underlying inhibitory control, little is known about the mechanisms by which anatomical connectivity disruption contributes to substance use expression in everyday functioning. Ecological momentary assessment (EMA) allows a fine‐grain characterization of the interplay and fluctuations of cognitive processes, mood and behaviours (e.g. substance use) by assessing them multiple times over the course of several days via smartphones. 7 Combining neuroimaging with EMA to identify complex brain/behaviour interactions, 8 we address for the first time, when, in addition to which, white matter connectivity influences decisions to engage in or refrain from substance use by individuals in treatment.
Inhibition reflects the ability to withhold dominant, automatic or prepotent responses to avert an undesirable outcome. 9 Impairment in inhibitory control has been widely described in SUD populations as powerful predictors of several indices of substance use severity such as initiation of substance use, 10 treatment progress in cocaine use disorders, 11 development of alcohol dependence 12 and alcohol craving intensity. 13 These behavioural signals could act as proxies for frontal system integrity, a crucial biomarker of SUD, for which impairments directly mediate inhibition deficits. 14 Exemplary of its predictive validity, longitudinal studies of ‘at risk’ populations, such as individuals with a family history of SUD, demonstrate frontal predisposing alterations prior to the onset of substance abuse. 15 , 16 Furthermore, substance use induces long‐lasting changes in frontal regions that partially explain compromise of inhibition function, leading to impaired control over use. 17 Combining behavioural and cerebral markers, we previously demonstrated that functional connectivity within inhibition‐related resting‐state networks could prospectively predict the frequency of substance use when both inhibition and use are assessed throughout the day via EMA (Chirokoff et al. submitted). Having demonstrated the value of combining an executive functional network with a behavioural measure of inhibition, the current study employed MR diffusion tensor imaging (DTI) to extend our search for anatomical biomarkers that underly inhibition dysfunction and enhance substance use prediction. The integrity of the anatomical connections within the brain, namely, white matter, is crucial for enabling efficient functional connectivity 18 and cognitive functioning. 19 Compromise of the white matter bundles between the frontal cortex and other subcortical and cortical sites has already been linked to tobacco, 20 cannabis 21 and alcohol 22 use. Hence, white matter impairments could underlie both the pattern of functional connectivity disruption resulting from SUD and in turn disturb inhibitory control assessed in daily life, each leading to substance use. Accordingly, we now aim to determine whether and when white matter integrity can predict drug use.
We indexed white matter microstructural integrity with DTI‐derived fractional anisotropy (FA) to test cerebral regions underlying inhibitory performance measured with EMA in SUD. These measures enabled testing the hypothesis of white matter fibre integrity predicting daily use, moderating momentary inhibition performance, and predicting previously identified resting‐state connections relevant to predicting later substance use. To test for specificity of brain–behaviour relations in patients with SUD, we included a sample of participants without a history of SUD to compare measures of inhibition and white matter integrity between the groups.
2. METHOD
2.1. Participants
Volunteers provided written informed consent to participate in this study. Forty‐one individuals with SUD were recruited from Centre Hospitalier Charles Perrens, where they were initiating their first month of regular outpatient treatment for addiction to substance use. All patients fulfilled the DSM‐5 criteria for a current primary use disorder related to alcohol, tobacco or cannabis. These patients received comprehensive care, including pharmacotherapy, individual behavioural treatments (such as relapse prevention and psychosocial support) or a combination of both. Abstinence was encouraged but with no consequences for patient care or study participation if this goal was not reached. Patients with multiple SUDs were eligible for inclusion if one substance (alcohol, tobacco or cannabis) was prioritized for treatment, as determined by the patient and the psychiatrist. Hence, the primary substance was defined as the substance targeted for treatment and used to constitute the alcohol, tobacco, and cannabis groups. To evaluate substance‐related data, a validated French version of the Addiction Severity Index (ASI), which had been modified for tobacco addiction, was employed. 23 , 24 , 25 The Interviewer Severity Ratings from the ASI sections were used to assess the severity of addiction. This rating generates a score from 0 to 9, representing mild severity from 2 to 3, moderate from 4 to 5, severe from 6 to 7 and very severe from 8 to 9.
Participants with a history or current diagnosis of bipolar or schizophrenia disorder, as assessed using the Mini International Neuropsychiatric Interview 5.0.0 (MINI), 26 were excluded. However, SUD patients presenting with a current comorbid depressive disorder were included. Thirty‐six healthy control participants were recruited through community announcement and had to have no past or current psychiatric or neurological disorders. Additionally, all participants needed to be free from conditions that would hinder the use of a smartphone and MRI scanning. This study adhered to the ethical standards outlined in the Helsinki Declaration and received approval from the ethical committee ‘Comité de Protection des Personnes de Sud‐Ouest et Outre‐Mer III’ (no. 2014‐A01668‐39).
2.2. Procedure
Prior investigations have demonstrated the feasibility and validity of EMA methodology in studies related to SUD. 27 Before commencing the experimental phase, all participants underwent a training session to ensure the successful use of a designated smartphone (Samsung Galaxy S with a 10.6 cm screen, 12‐point font size) employed in the study. Each participant carried the smartphone for a duration of 7 days and responded to five electronic surveys per day. These surveys were randomly distributed across five equally spaced time intervals between the participant's self‐determined ‘start’ and ‘end’ of the day. Full completion was encouraged, and participants who completed more than 75% of the assessments received 50€ in‐store vouchers.
For a subsample of participants, magnetic resonance imaging (MRI), including DTI, resting‐state functional MRI and anatomical scans, was conducted 48 h prior to the EMA phase. Subjects who completed both the EMA assessments and the MRI scans were eligible to receive store vouchers with a value of up to 100€ as compensation. The sample size for the MRI analyses was estimated using the package pwr available on R. 28 For a linear regression with 1 predictor (inhibition performance) with a minimal power of 0.80, an alpha level of 0.05 to detect a medium effect size (R 2 = 0.20), we estimated that 32 subjects would be needed.
2.3. EMA assessments
2.3.1. Questionnaires assessments
At each assessment, participants reported if they used any substance or the substance they are treated for (named primary substance) since the last survey and rated their current craving level on a scale from one (no desire or urge) to 7 (extreme desire or urge to use a substance). Both any substance and the primary substance were later used in the analysis as outcomes.
2.3.2. Cognitive mobile tests: Stroop task
Mobile neuropsychological tests were randomly implemented in two out of the five daily surveys to assess executive functioning via a validated mobile colour‐word interference test similar to the Stroop test. 29 This test included the interference condition of a traditional Stroop task where participants had to name as quickly as possible the ink colours of 16 words of colour names that were incongruent with the ink colour (i.e. the word ‘blue’ written in red). The resulting audio files recorded through the smartphone were analysed for precise response times, with longer time indicating poorer inhibition abilities.
2.4. MRI acquisition
Brain imaging data were collected on a 3.0 Tesla GE MRI system using a 32‐channel head coil. Anatomical volumes were acquired using a sagittal 3D T1‐weighted (repetition time (RT) = 8.5 ms, echo time (ET) = 3.2 ms, flip angle = 11°, field of view (FOV) = 256 mm × 256 mm, voxel size = 1 mm3, 176 slices, 9.58 mn). Following the anatomical scan, resting‐state functional images were collected using a single‐shot echo‐planar sequence (RT = 2.2 s, ET = 27 ms, flip angle = 80°, FOV = 192 mm × 192 mm, voxel size = 3 mm × 3 mm × 3.5 mm, 42 axial slices, 11.07 mn), during which participants were instructed to keep their eyes closed, remain awake and not think about anything in particular. Lastly, the DTI images were collected following a spin echo 2D axial echo‐planar diffusion weighting protocol, with 5 b = 0/1500 mm2/s, 64 gradients directions, TR = 16.025 mm, TE = 86.8 mm, number of slices = 64, FOV = 256 mm, phase = A/P, resolution = 2 × 2 × 2 mm, 17.54 mn).
2.4.1. Preprocessing
The structural and diffusion MRI were preprocessed using the publicly available Scalable Informatics for Biomedical Imaging Studies (SIBIS) pipeline. 30 All MRIs were inspected by a radiologist for anatomical anomalies that could interfere with image analysis, resulting in the exclusion of 4 controls and 2 patients with SUD. For each subject, skull stripping and aligning with the SRI 24 atlas 31 were performed by non‐rigidly registering the T1w to the atlas with advanced normalization tools (ANTs). 32 The b = 0 scan of the DTI sequence was aligned to the structural MRI by non‐rigidly aligning the b0 to the T1w via computational morphometry toolkit (CMTK) (https://www.nitrc.org/projects/cmtk/). After realignment and skull‐striping, the pipeline performed removal of bad single shots, echo‐planar structural distortion and eddy‐current distortion correction via FMRIB Software Library (FSL), 33 and FA skeleton estimation by Tract‐Based Spatial Statistic (TBSS). 34 The computed FA skeletons were later used in the whole brain analysis. Images underwent automatic and visual quality control, resulting in the exclusion of 1 control and 1 patient with SUD.
2.4.2. Resting‐state fMRI
Analyses were described previously (Chirokoff et al. submitted) and summarized here. Preprocessing steps included bias field correction, skull stripping, MNI normalization, segmentation, slice timing, motion correction, distortion correction, coregistration, regression of motion parameters and bandpass filtering were conducted using fMRIPrep. 35 After preprocessing, resting‐state networks were computed via dictionary learning and decomposed into 84 regions of interest. Regression analyses were conducted in the SUD group using the Conn toolbox to identify connections in which connectivity strength correlated with mean Stroop performance in the SUD groups while correcting for age. The results were corrected for multiple comparisons using a false discovery rate (FDR) with an alpha level of 0.05.
2.5. Statistical analyses
2.5.1. EMA variables of interest
We calculated each participant's mean Stroop performance (in ms) across the entire week of EMA assessments to serve as the metric for correlation in neuroimaging analyses.
2.5.2. Rs‐fMRI connections of interest
Regions of interest were based on activations studies citing the involvement of the right middle occipital gyrus, right middle and orbital frontal areas, right parietal inferior areas, 36 angular gyrus 37 and the cerebellum 38 in Stroop tasks and previous work conducted in our laboratory for which connections are illustrated in Figure S1.
Using the same sample, our previous work revealed that mean Stroop time in the SUD group was significantly linked to rs‐connectivity strength in seven connections: the right angular ‐ right superior occipital area, right middle occipital area and ‐ the interior parietal, ‐ middle orbital frontal, ‐ middle frontal, ‐ angular, ‐ cerebellar lobule IX and ‐ vermis 10 (Chirokoff et al. submitted). For the current study, connectivity strength within these connections was extracted both in the SUD and control group to be analysed conjointly with white matter integrity.
2.5.3. Whole brain analysis of TBSS skeletons in the SUD group
General linear modelling (GLM) in the SUD group was used to assess the link between the mean Stroop performance and whole‐brain FA skeletons derived from TBSS. Mean Stroop performance across the week was entered into a regression model to predict whole‐brain FA values within the mean FA skeletons masks using the function randomise from FSL. 39 Results were tested against null distributions using 500 permutations, corrected for multiple comparisons using threshold free cluster enhancement (TFCE), and considered significant at p < 0.05. Resulting clusters were automatically labelled via Atlasquery from FSL using Johns Hopkins University (JHU) atlas 40 in the SRI24 space. Mean FA values in the surviving clusters were then extracted in both the SUD and control groups to be analysed conjointly with demographics and EMA‐derived variables.
2.5.4. Conjoint analysis of FA values and EMA‐derived variables
We compared FA values within clusters between SUD and control groups using analysis of covariance (ANCOVA) to control for the interaction age * group effect. Similarly, in the SUD group, these indexes were also compared between sex, comorbidity and type of primary substances using ANCOVA while controlling for age.
2.5.5. Hierarchical modelling analysis of FA values and EMA variables
To assess a potential indirect relation between white matter integrity and substance use in the SUD group, we conducted generalized linear mixed‐effects models (for binomial outcomes) using the lmer4 packages available on R. 41
EMA enables the assessment of target variables repeatedly and intensively in real time, resulting in numerous successive observations of a variable at different time points t. We modelled a time lag in our raw EMA measures to predict substance use at time t + 1 from momentary Stroop performance and substance use at time t. In other words, each observation of the Stroop performance at the given time predicted substance use at the following assessment time, and this prediction was repeated for every successive time point (Time 1 predicts Time 2 that predicts Time 3, …). To avoid contamination of night‐time effects, this time lag excluded all predictions of the first assessment of a new day by the last assessment of the previous days.
We entered Stroop performance at time t into our model centred around the subject's own mean for the week as a first‐level predictor of substance use at t + 1 while controlling for previous use at time t. We then entered the interaction between FA values in the significant clusters from the whole brain analysis and each time point assessment of Stroop (FA values * Stroop time t) as a predictor of subsequent use at time t + 1 while controlling for previous use at time t, age, sex, primary substance type and addiction severity as control variables. This iterative interaction model (FA values * Stroop) is equivalent to moderation testing where white matter FA values could modify the relation between Stroop and subsequent use. To ensure that the moderation effect was specific to the Stroop, we tested for a potential interaction between FA values and craving at time t (FA values * craving time t). In each model, control and independent variables were entered as fixed effects, and random effects on the first‐level slope equations were added. First‐level continuous predictors were centred around the participant's own level, and second‐level continuous predictors were centred around the group mean. Dichotomous predictors at each level were entered uncentred. Missing data at the first level were discarded from the analyses. All analyses were considered significant at p < 0.05 uncorrected. Illustrations of the procedure, from the raw data to the mixed model, are presented in Figure 1.
FIGURE 1.
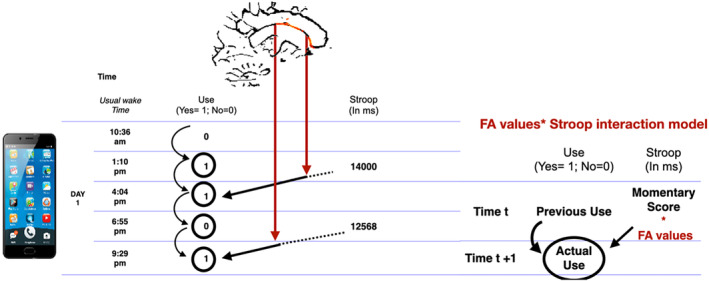
Mixed model procedure from the raw data. Legend: During a typical day of EMA assessment, participants had to report if they used anysubstance and their primary substance (for the SUD sample) 5 times and had to complete theStroop mobile testing twice. The week of assessment hence results in 35‐time point assessmentsof substance use and 14 assessments of inhibition functioning that can be lagged in time topredict the next time point's assessments (Time t+1) from the immediately preceding time pointassessments (Time t). By treating each time point as a repetition, we modeled the prediction offuture use (at time t+1) by the current Stroop performance (at time t) while controlling fromprevious use (at time t). FA values within each cluster were then entered as a moderator of theStroop / use link. To do so the Stroop scores at each time point is multiplied by FA values ofeach cluster, resulting in an interaction term FA value * Stroop for each time t. This interactionterm is entered as predictor of subsequent use (at time t +1) while correcting for previous use(at time t). This is similar to a moderation analysis where FA values (indicated by the red arrow)modified the strength of the prediction between inhibition and use (indicated by the blackarrow).
2.5.6. Conjoint analysis of DTI and rs connectivity with Stroop‐related connection variables
Entering data from all participants (that is, SUD and controls), we conducted a linear model using lmer4 packages available on R 41 to predict FA value within our clusters from connectivity strength in the connections linked to Stroop performance highlighted in our previous studies. Group (control vs. SUD), age and sex were entered as control variables, and results were considered significant at p < 0.05 uncorrected.
3. RESULTS
3.1. Sample characteristics
The SUD group was composed of 38 individuals (19 men, mean age 43.16 ± 11.86), including 19 treated for primary alcohol, 13 for tobacco and 6 for cannabis use disorders. The control group included 31 healthy participants (16 men, mean age 34.35 ± 7.73). Table 1 presents the descriptive statistics for each group.
TABLE 1.
Descriptive statistics of the control and SUD sample (n = 69).
| Control (N = 31) | Any SUD (N = 38) | Alcohol (N = 19) | Tobacco (N = 13) | Cannabis (N = 6) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SD | % | M | SD | % | M | SD | % | M | SD | % | M | SD | % | |
| Age | 34.35*** | 7.73 | 43.16 | 11.86 | 44.32 | 11.36 | 46.46 C | 10.15 | 32.33 | 12.45 | |||||
| Sex (% male) | 51.42 | 50 | 62.50 | 20 | 81.81 | ||||||||||
| Education (years) | 14.42 | 3.06 | 13.18 | 2.44 | 13.53 | 2.27 | 13.69 | 2.56 | 11 | 1.67 | |||||
| Current comorbidity | ‐ | 18.75 | 35.71 | 8.33 | 0 | ||||||||||
| Addiction severity (ASI) | ‐ | ‐ | 6.39 | 0.82 | 6.53 | 0.61 | 6.15 | 1.07 | 6.50 | 0.84 | |||||
| Craving level | 1.01 | 0.04 | 3.03 | 1.30 | 2.84 | 0.86 | 2.67 | 1.50 | 4.09 | 1.60 | |||||
| Any substance use (occasions per week) | 2.05 | 2.74 | 22.05 | 8.46 | 24.90 | 7.92 | 18.80 | 9.58 | 21.70 | 6.65 | |||||
| Primary substance use (occasions per week) | ‐ | ‐ | 13.20 | 8.78 | 11.30 | 8.62 | 17.00 | 9.34 | 12.60 | 7.50 | |||||
| Mean Stroop time (s) | 10.39* | 2.90 | 14.56 | 6.38 | 16.32 | 7.97 | 13.06 | 3.52 | 12.55 | 4.18 | |||||
| Mean FA left anterior corona radiata and corpus callosum | 0.53 | 0.04 | 0.50 | 0.06 | 0.49 | 0.06 | 0.50 | 0.06 | 0.53 | 0.04 | |||||
| Mean FA right anterior corona radiata and corpus callosum | 0.52 | 0.04 | 0.50 | 0.06 | 0.49 | 0.06 | 0.51 | 0.06 | 0.54 | 0.05 | |||||
Abbreviations: A, alcohol ≠ tobacco; ASI, Addiction Severity Index; B, alcohol ≠ cannabis; C, tobacco ≠ cannabis; FA, fractional anisotropy; M, mean; Max, maximum; Min, minimum; s, second; SD, standard deviation; SUD, substance use disorder.
p < 0.05.
p < 0.01.
p < 0.001.
3.2. Whole brain analysis of TBSS skeletons
FA values within two clusters were significantly and negatively linked to the mean Stroop times in the SUD group, that is, higher FA values predicted faster Stroop times. Both clusters encompass the left (for Cluster 1) and right (for Cluster 2) anterior corona radiata and the genu and body of the corpus callosum (Table 2 and Figure 2).
TABLE 2.
Clusters and associated probabilities of belonging to each label.
| Cluster 1 | Cluster 2 | |
|---|---|---|
| ROI | ||
| Anterior corona radiata | 46.18% (left) | 52.41% (right) |
| Superior corona radiata | 0.17% (left) | 4.23% (right) |
| Body of corpus callosum | 19.35% | 16.81% |
| Genu of corpus callosum | 14.43% | 14.75% |
| Unclassified | 19.86% | 11.80% |
FIGURE 2.
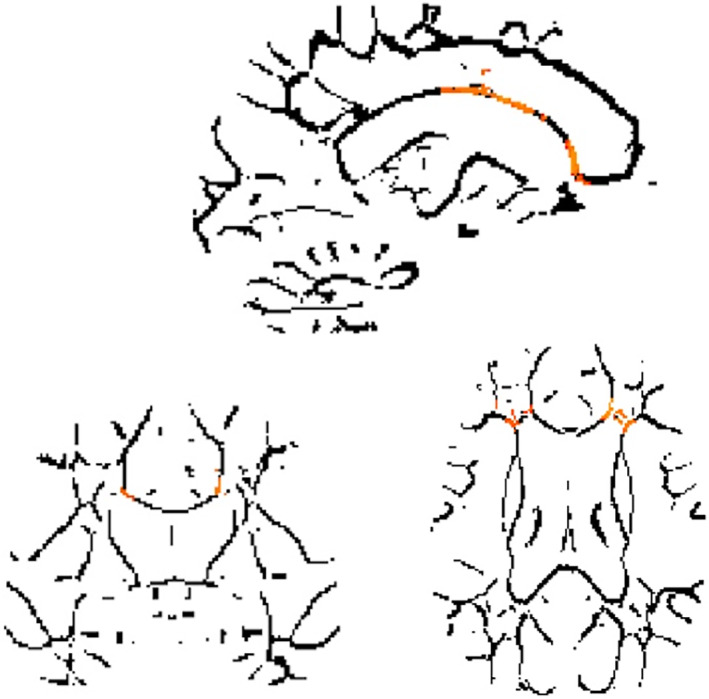
Voxels in which fractional anisotropy (FA) values are significantly linked to shorter mean Stroop times, indicating better performances, in the substance use disorder (SUD) group. Legend: Results of the GLM whole brain analysis using the function randomize from FSL toevaluate the link between FA values in participant's skeletons and their mean Stroop Timeduring the week. Significant voxels are highlighted in orange and negatively linked to theStroop Time, indicating that higher FA values is linked to better inhibition performance.
3.3. Group comparisons
The results of all group comparisons are presented in Table 1. Comparison of the SUD versus control group did not reveal any significant differences in FA values between groups while adjusting for an age * group interaction. Within the SUD group, the FA values did not differ significantly between the alcohol, tobacco and cannabis subgroups for either cluster (left and right left anterior corona radiata and corpus callosum)
3.4. Mixed models in the SUD group: use, inhibition, FA value and resting‐state networks
3.4.1. Associations between FA values, Stroop performance and substance use
As presented in Table 3, higher FA in the right anterior corona radiata and corpus callosum significantly predicted a lower probability of any substance use at time t + 1. FA in the left anterior corona radiata and corpus callosum significantly moderated the link between Stroop performance at time t and the use of any substance at time t + 1, indicating that higher FA significantly enhanced the link between greater inhibition at a given time and subsequent use. As illustrated in Figure 3A, a lower probability of any substance use at time t + 1 was associated with higher FA value within left anterior corona radiata and corpus callosum and shorter Stroop time compared with the rest of the week at time t (−1 standard deviation). Lower FA value within the same cluster was associated with a high probability of use regardless of Stroop functioning at time t.
TABLE 3.
Results of the substance use as outcomes models: prediction of substance use at time t + 1 by fractional anisotropy (FA) values and their interaction with Stroop and craving at time t.
| Outcome at time t + 1 related to use of … | ||||||||
|---|---|---|---|---|---|---|---|---|
| Primary substance | Any substance | |||||||
| Predictors | γ | SE | Z‐value | p‐value | γ | SE | Z‐value | p‐value |
| Left anterior corona radiata and corpus callosum | −0.4310 | 0.2974 | −1.449 | 0.147 | −0.6712 | 0.3912 | −1.716 | 0.086 |
| Right anterior corona radiata and corpus callosum | −0.3363 | 0.3099 | −1.085 | 0.278 | −0.8237 | 0.3991 | −2.064 | 0.039 b |
| Left anterior corona radiata and corpus callosum * Stroop time t | 0.4295 | 0.1596 | 2.691 | 0.007 a | 0.4675 | 0.2300 | 2.032 | 0.042 a |
| Right anterior corona radiata and corpus callosum * Stroop time t | 0.3358 | 0.1609 | 2.087 | 0.036 a | 0.4165 | 0.2342 | 1.778 | 0.075 |
| Left anterior corona radiata and corpus callosum * craving time t | 0.0909 | 0.0880 | 1.032 | 0.302 | 0.1025 | 0.1220 | 0.840 | 0.401 |
| Right anterior corona radiata and corpus callosum * craving time t | 0.0425 | 0.0929 | 0.457 | 0.647 | 0.0276 | 0.1300 | 0.213 | 0.832 |
Surviving correction for previous use at time t, age, sex, addiction severity and primary substance type.
Surviving correction for previous use at time t, age, addiction severity but not sex and primary substance type.
FIGURE 3.

(A–C) Association between use of substance at time t + 1 for any (A) and primary substance (B) and fractional anisotropy (FA) values within left anterior corona radiata and corpus callosum and right anterior corona radiata and corpus callosum (C) for shorter, mean, and longer Stroop time centred around the week at time t. Legend: Illustration of the probability of use associated with FA value in our clusters dependingon Stroop Time performance at time t: shorter time compared to the rest of the week (‐1 standarddeviation) indicating better performance in red, habitual mean time for the week in blue andlonger time compared to the rest of the week (+1 standard deviation) indicating worstperformance in green. S.d.: standard deviation (centered about each individual's mean across the week).
The interaction between FA values in both clusters and Stroop performance at time t significantly predicted the use of the primary substance at time t + 1. As illustrated in Figure 3B,C, higher FA values within both clusters and shorter Stroop time compared with the rest of the week (−1 standard deviation) were associated with the lowest probability of use. However, for patients with the lowest FA values, shorter Stroop Time was inversely associated with a high probability of use. No significant interactions were found between FA values in either cluster and craving.
3.4.2. Associations between FA values and resting‐state networks
As a potential explanation for the interplay between white matter and inhibition, higher FA values within left anterior corona radiata and corpus callosum in the overall sample significantly predicted stronger rs‐fMRI connectivity between the right middle occipital cortex and two regions: right frontal middle orbital and vermis lobule 10. Higher FA value within the right anterior corona radiata and corpus callosum significantly predicted weaker resting‐state connectivity between the right superior occipital and the right angular region and stronger connectivity between the right middle occipital and the right frontal middle orbital regions. These results are displayed in Table 4.
TABLE 4.
Prediction of rs connectivity strength within the seven previously highlighted connections by FA values in left anterior corona radiata and corpus callosum and right anterior corona radiata and corpus callosum.
| Predictors: FA value within | ||||||||
|---|---|---|---|---|---|---|---|---|
| Left anterior corona radiata and corpus callosum | Right anterior corona radiata and corpus callosum | |||||||
| Outcomes: resting‐state connectivity strength between | γ | SE | Z‐value | p‐value | γ | SE | Z‐value | p‐value |
| Occ. Sup R–Angular R | −0.0275 | 0.0167 | −1.642 | 0.106 | −0.0439 | 0.0177 | −2.483 | 0.0162 b |
| Occ. Mid R–Par. Inf. R | 0.0086 | 0.0169 | 0.503 | 0.617 | 0.0071 | 0.0185 | 0.383 | 0.703 |
| Occ. Mid R–Fron. Mid. Orb. R | 0.0363 | 0.0165 | 2.197 | 0.032 a | 0.0347 | 0.0167 | 2.079 | 0.043 a |
| Occ. Mid R–Vermis 10 | 0.0516 | 0.0236 | 2.191 | 0.033 a | 0.0493 | 0.0259 | 1.904 | 0.062 |
| Occ. Mid R–Angular R. | −0.0006 | 0.0167 | −0.037 | 0.971 | −0.0037 | 0.0182 | −0.20 | 0.843 |
| Occ. Mid R–Fron. Mid. R | 0.0020 | 0.0174 | 0.116 | 0.908 | 0.0024 | 0.0189 | 0.128 | 0.899 |
| Occ. Mid R–Cereb. 9R | 0.0279 | 0.0231 | 1.207 | 0.233 | 0.0104 | 0.0255 | 0.409 | 0.684 |
Abbreviations: FA, fractional anisotropy; SUD, substance use disorder.
Surviving correction for control versus SUD, age and sex.
Surviving correction for control versus SUD, sex but not for age.
4. DISCUSSION
Our study is the first to integrate white matter microstructural integrity into the multiple levels of impairment that SUD patients exhibit in real time as they decide to use or to refrain from the use of a substance. Neuroimaging measures of functional and structural connectivity were predictive of the degree of inhibitory control exerted to anticipate the time and type of substance use recorded daily, thereby identifying neural and behavioural mechanisms that contribute both to enabling and to inhibiting substance use.
The results herein highlight the well‐defined role of frontal cortical connections in sustaining inhibition functioning and acting as a risk or resilience factor for substance use. We expanded our knowledge about the constellation of mechanisms contributing to the timing of substance use by demonstrating that FA fibre integrity of the corpus callosum and corona radiata moderated the immediate protective effect of inhibition function on use assessed in real life via EMA. As a possible underlying mechanism, we also demonstrated that FA values in these clusters were linked to the functional resting‐state connections that subserve efficient inhibition abilities. Hence, our study argues for a multiple level of impairments contributing to use, whereby impaired white matter structural integrity in anterior brain systems underlies functional impairment in executive inhibitory control networks and compromised everyday‐life expression of inhibition, leading to a weakened ability to refrain from using a substance.
Low FA values typically reflect low coherence of the linear microstructure of white matter tracts. 42 As presumed outcomes of the toxic effect of substance use, studies demonstrated that FA values decrease with heavy 43 or long‐term drinking, 44 continued smoking 45 and cannabis use 46 generally with lower scores being linked to poorer clinical outcomes. In our study, lower FA values in clusters encompassing the callosal genu and body and the bilateral anterior corona radiata were linked to poorer mean inhibition performance assessed via mobile Stroop testing in the SUD sample. The corpus callosum is the largest white matter tract of the brain 47 in which fibres originating from the genu cross to connect frontal areas, and fibres from the body form the major interhemispheric brain tracts, including the corona radiata. 48 Microstructural differences in the corpus callosum between the SUD and the controls are usually observed across all types of substances (for an extensive review of similarities across substances, see Hampton et al. 49 ). Studies in non‐SUD individuals additionally revealed the high sensitivity and specificity of the corpus callosum integrity to predict inhibition functioning compared with grey matter indexes. 50 Our clusters linked to mean inhibition functioning additionally encompassed the anterior region of the bilateral corona radiata, a region known for its projection from and to prefrontal areas 51 that form part of the limbic–thalamo–cortical circuitry. 52 Similarly, lower FA values in this tract have previously been linked to greater tobacco, 53 alcohol 22 and polydrug use compared with controls. 54 In addition to its compromise in the SUD population, integrity within the anterior corona radiata also predicts better inhibition functioning assessed via classic Stroop testing in healthy populations. 55 Relevantly, this region was also found to mediate abnormal activation during an inhibition task among individuals with a family history of substance misuse, a population at high risk of developing SUD. 56
In addition to replicating the involvement of the corpus callosum and the corona radiata in inhibition using mobile testing, our study emphasized a strong link between brain anatomy and functioning. In our overall sample, higher FA within both clusters was linked to higher connectivity in right occipital‐frontal connections that we previously highlighted as benefitting inhibition functioning (Chirokoff et al. submitted). Higher FA value within left anterior corona radiata and corpus callosum was also linked to higher connectivity strength between the right occipital area and vermis 10. By contrast, higher FA in our right anterior corona radiata and corpus callosum was linked to weaker connectivity in occipito‐angular connection at rest that was identified as detrimental to inhibition performance (Chirokoff et al. submitted). Indeed, white matter integrity has been observed to constrain resting‐state network organization, strength 57 and even adaptation. 58 Notably, it has been demonstrated that chronic substance use such as alcohol exposure affects white matter microstructural integrity, leading through disturbed functional connectivity to decreased cognitive flexibility in rats. 59 A possible explanation for the involvement of our highlighted white matter regions in sustaining inhibition abilities in SUD populations could reside in its links to functional connectivity, as previously suggested in patients with hypertension. 60
Our most striking result was the relation between white matter integrity and the momentary expression of inhibition abilities, sustaining its impact on subsequent use. We previously demonstrated in our SUD sample that the efficiency of everyday‐life inhibition functioning was a strong predictor, and protector, of immediate use. In the current study, this protective effect was found to be moderated by white matter integrity within the corpus callosum and corona radiata, whereby higher integrity appeared to enable inhibition to protect against the use of any substance. This moderation effect was more nuanced for the primary substance, whereby high FA values were associated with a low probability of use. Yet, patients with lower FA values exhibit a surprising effect, whereby better inhibition was associated with higher use risk. Further indication that the moderation effect was specific to inhibition was our observation that interactions denoting moderating effects did not occur with craving. As such, we speculate that our results tend toward a dissociation of craving and inhibition in the prediction of use. We previously suggested that the regulation of craving and inhibition in real life could operate independently in non‐overlapping functional networks (Chirokoff et al. submitted). The current study could lend additional credence to the view that craving and inhibition could be operating in parallel 61 and on independent processes themselves fluctuating in time. 3 Temporal fluctuations in the interactions between both processes could explain the discrepancy between the overlap 62 or the independence (Chirokoff et al. submitted) that craving and inhibition can display and highlight the necessity of assessing the fluctuations of these real‐time predictors of use.
4.1. Limitations
This exploratory study offers preliminary leads of white matter impact on everyday use that necessitates future replications, with a larger sample size, hence our findings must be taken with caution. Regarding further limitations, it should be noted that our study did not replicate the previously observed differences between SUD and the control group concerning FA values in our clusters. 49 However, our SUD sample was significantly older than the control group, and our comparison analyses included correction for an interaction between age and group (control vs. SUD). The potential differential effect of age in both populations has previously been highlighted 63 and emphasizes the need for further investigations with age‐matched groups. Similarly, and in response to the need to identify common mechanisms across different types of SUD, 64 we conducted our analysis in a mixed sample of patients treated for primary alcohol, tobacco and cannabis use disorders, recognizing the need for replication in larger samples.
Whereas we hypothesized a possible dissociation between craving and inhibition regulation in real life, this study did not aim to assess the specific links that both predictors shared in ecological settings. Additional studies are needed to investigate the overlap between the cerebral markers of real‐time predictors of use related to craving and inhibitory control. Furthermore, the relationship between stronger inhibition in real time and use in the patients with the lowest FA values calls for a better understanding of the moderator explaining this unexpected relation if real or dispelling it if a chance event. As a possible confounder, future studies could also incorporate objective biomarkers of use before and after EMA assessments.
Finally, readers should consider the observed effects as modest as our EMA analyses did not include corrections for multiple comparisons.
4.2. Conclusion
This study is the first to translate the involvement of white matter integrity in inhibition and use into ecological conditions in a patient's life, outside of classical laboratory testing, and demonstrate the necessity of investigating how addiction‐related impairments interact in real time. As a possible interpretation, our results could indicate that relatively preserved inhibitory functioning despite white matter alterations in frontal connections would not enable efficient prevention from use. In this context, targeting white matter plasticity and recovery 65 with sustained alcohol sobriety, brain stimulation 66 or even cognitive training 67 could be crucial to maximize the benefits associated with traditional addiction treatments.
AUTHOR CONTRIBUTIONS
Sandra Chanraud, Marc Auriacombe, Fuschia Serre, David Misdrahi and Melina Fatseas contributed to the design, funding of the project and data collection. Valentine Chirokoff contributed to the data preprocessing, analysis, interpretation and writing of the manuscript. Kilian M. Pohl contributed to the data preprocessing. Adolf Pfefferbaum, Edith V. Sullivan, Sylvie Berthoz and Sandra Chanraud contributed to the supervision of Valentine Chirokoff, the interpretation of the results and the writing of the manuscript. All authors contributed to the proofreading of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors report no conflicts of interest for this investigation.
ETHICS STATEMENT
This study adhered to the ethical standards outlined in the Helsinki Declaration and received approval from the ethical committee ‘Comité de Protection des Personnes de Sud‐Ouest et Outre‐Mer III’ (no. 2014‐A01668‐39), and all participants provided written informed consent.
Supporting information
Figure S1. Resting‐state connectivity strength correlating negatively (in red) and positively (in blue) with mean Stroop Times in the SUD sample. Adapted from Chirokoff et al. Submitted.
ACKNOWLEDGEMENTS
This paper is dedicated to the memory of our dear colleague, Joel Swendsen, who passed away suddenly on 14 July 2022. We thank him deeply for initiating, conducting and supervising this study that resulted in this manuscript.
This investigation was supported by funding obtained from the French Agence Nationale de la Recherche (J. Swendsen), the Fondation pour la Recherche Médicale (J. Swendsen), NIAAA grants (AA10723, E.V. Sullivan; AA005965, A. Pfefferbaum), NIDA (DA057567, K.M. Pohl), Fulbright Fellowship (V. Chirokoff), France‐Stanford Fellowship Award (V. Chirokoff), EPHE doctoral school grant (V.Chirokoff) and from the French government in the framework of the University of Bordeaux's France 2030 program/RRI ‘IMPACT’ (S. Chanraud). The Charles Perrens Hospital is acknowledged for staff and office support for this study (M. Auriacombe).
Chirokoff V, Pohl KM, Berthoz S, et al. Multi‐level prediction of substance use: Interaction of white matter integrity, resting‐state connectivity and inhibitory control measured repeatedly in every‐day life. Addiction Biology. 2024;29(5):e13400. doi: 10.1111/adb.13400
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Bechara A, Dolan S, Hindes A. Decision‐making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40(10):1690‐1705. doi: 10.1016/S0028-3932(02)00016-7 [DOI] [PubMed] [Google Scholar]
- 2. Verdejo‐Garcia A, Garcia‐Fernandez G, Dom G. Cognition and addiction. Dialogues Clin Neurosci. 2019;21(3):281‐290. doi: 10.31887/DCNS.2019.21.3/gdom [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flaudias V, Heeren A, Brousse G, Maurage P. Toward a triadic approach to craving in addictive disorders: the metacognitive hub model. Harv Rev Psychiatry. 2019;27(5):326‐331. doi: 10.1097/HRP.0000000000000225 [DOI] [PubMed] [Google Scholar]
- 4. Hofmann W, Friese M, Wiers RW. Impulsive versus reflective influences on health behavior: a theoretical framework and empirical review. Health Psychol Rev. 2008;2(2):111‐137. doi: 10.1080/17437190802617668 [DOI] [Google Scholar]
- 5. Collette F, Van Der Linden M, Laureys S, et al. Exploring the unity and diversity of the neural substrates of executive functioning. Hum Brain Mapp. 2005;25(4):409‐423. doi: 10.1002/hbm.20118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pando‐Naude V, Toxto S, Fernandez‐Lozano S, Parsons CE, Alcauter S, Garza‐Villarreal EA. Gray and white matter morphology in substance use disorders: a neuroimaging systematic review and meta‐analysis. Transl Psychiatry. 2021;11(1):29. doi: 10.1038/s41398-020-01128-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stone AA, Shiffman S. Ecological momentary assessment (EMA) in behavioral medicine. Ann Behav Med. 1994;16(3):199‐202. doi: 10.1093/abm/16.3.199 [DOI] [Google Scholar]
- 8. Gadassi Polack R, Paganini G, Winschel J, Benisty H, Joormann J, Kober H, Mishne G.. Better together: a systematic review of studies combining magnetic resonance imaging with ecological momentary assessment [preprint]. PsyArXiv. 2021. doi: 10.31234/osf.io/mxznb [DOI] [PMC free article] [PubMed]
- 9. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41(1):49‐100. doi: 10.1006/cogp.1999.0734 [DOI] [PubMed] [Google Scholar]
- 10. Nigg JT, Wong MM, Martel MM, et al. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J am Acad Child Adolesc Psychiatry. 2006;45(4):468‐475. doi: 10.1097/01.chi.0000199028.76452.a9 [DOI] [PubMed] [Google Scholar]
- 11. Streeter C, Terhune D, Whitfield T, et al. Performance on the Stroop predicts treatment compliance in cocaine‐dependent individuals. Neuropsychopharmacology. 2008;33(4):827‐836. doi: 10.1038/sj.npp.1301465 [DOI] [PubMed] [Google Scholar]
- 12. Rubio G, Jiménez M, Rodríguez‐Jiménez R, et al. The role of behavioral impulsivity in the development of alcohol dependence: a 4‐year follow‐up study. Alcohol Clin Exp Res. 2008;32(9):1681‐1687. doi: 10.1111/j.1530-0277.2008.00746.x [DOI] [PubMed] [Google Scholar]
- 13. Bernard L, Cyr L, Bonnet‐Suard A, Cutarella C, Bréjard V. Drawing alcohol craving process: a systematic review of its association with thought suppression, inhibition and impulsivity. Heliyon. 2021;7(1):e05868. doi: 10.1016/j.heliyon.2020.e05868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Everitt BJ. Neural and psychological mechanisms underlying compulsive drug seeking habits and drug memories—indications for novel treatments of addiction. Eur J Neurosci. 2014;40(1):2163‐2182. doi: 10.1111/ejn.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monti PM, Miranda R, Nixon K, et al. Adolescence: booze, brains, and behavior. Alcohol: Clin Exp Res. 2005;29(2):207‐220. doi: 10.1097/01.ALC.0000153551.11000.F3 [DOI] [PubMed] [Google Scholar]
- 16. Tarter RE, Kirisci L, Mezzich A, et al. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160(6):1078‐1085. doi: 10.1176/appi.ajp.160.6.1078 [DOI] [PubMed] [Google Scholar]
- 17. Cadet JL, Bisagno V. The primacy of cognition in the manifestations of substance use disorders. Front Neurol. 2013;4:189. doi: 10.3389/fneur.2013.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nozais V, Forkel SJ, Petit L, et al. Atlasing white matter and grey matter joint contributions to resting‐state networks in the human brain [preprint]. Neuroscience. 2022. doi: 10.1101/2022.01.10.475690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Forkel SJ, Friedrich P, Thiebaut de Schotten M, Howells H. White matter variability, cognition, and disorders: a systematic review. Brain Struct Funct. 2022;227(2):529‐544. doi: 10.1007/s00429-021-02382-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baeza‐Loya S, Velasquez KM, Molfese DL, et al. Anterior cingulum white matter is altered in tobacco smokers: anterior cingulum white matter in tobacco smokers. Am J Addict. 2016;25(3):210‐214. doi: 10.1111/ajad.12362 [DOI] [PubMed] [Google Scholar]
- 21. Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci. 2015;16:23‐35. doi: 10.1016/j.dcn.2015.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Daviet R, Aydogan G, Jagannathan K, et al. Associations between alcohol consumption and gray and white matter volumes in the UK Biobank. Nat Commun. 2022;13(1):1175. doi: 10.1038/s41467-022-28735-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brisseau S, Auriacombe M, Franques P, Daulouède J‐P, Tignol J. L'addiction severity index. Le Courrier Des Addictions. 1999;1:200‐203. [Google Scholar]
- 24. Denis C, Fatséas M, Beltran V, et al. Usefulness and validity of the modified addiction severity index: a focus on alcohol, drugs, tobacco, and gambling. Subst Abus. 2016;37(1):168‐175. doi: 10.1080/08897077.2015.1036334 [DOI] [PubMed] [Google Scholar]
- 25. McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9(3):199‐213. doi: 10.1016/0740-5472(92)90062-S [DOI] [PubMed] [Google Scholar]
- 26. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini‐International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM‐IV and ICD‐10. J Clin Psychiatry. 1998;59(Suppl 20):22‐33. quiz 34‐57 [PubMed] [Google Scholar]
- 27. Serre F, Fatseas M, Debrabant R, Alexandre J‐M, Auriacombe M, Swendsen J. Ecological momentary assessment in alcohol, tobacco, cannabis and opiate dependence: a comparison of feasibility and validity. Drug Alcohol Depend. 2012;126(1):118‐123. doi: 10.1016/j.drugalcdep.2012.04.025 [DOI] [PubMed] [Google Scholar]
- 28. R Core Team . (2020). R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- 29. Bouvard A, Dupuy M, Schweitzer P, et al. Feasibility and validity of mobile cognitive testing in patients with substance use disorders and healthy controls. Am J Addict. 2018;27(7):553‐556. doi: 10.1111/ajad.12804 [DOI] [PubMed] [Google Scholar]
- 30. Zhao Q, Sullivan EV, Honnorat N, et al. Association of heavy drinking with deviant fiber tract development in frontal brain systems in adolescents. JAMA Psychiatry. 2021;78(4):407‐415. doi: 10.1001/jamapsychiatry.2020.4064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rohlfing T, Zahr NM, Sullivan EV, Pfefferbaum A. The SRI24 multichannel atlas of normal adult human brain structure. Hum Brain Mapp. 2009;31(5):798‐819. doi: 10.1002/hbm.20906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Avants B, Epstein C, Grossman M, Gee J. Symmetric diffeomorphic image registration with cross‐correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26‐41. doi: 10.1016/j.media.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woolrich MW, Jbabdi S, Patenaude B, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1):S173‐S186. doi: 10.1016/j.neuroimage.2008.10.055 [DOI] [PubMed] [Google Scholar]
- 34. Smith SM, Jenkinson M, Johansen‐Berg H, et al. Tract‐based spatial statistics: voxelwise analysis of multi‐subject diffusion data. Neuroimage. 2006;31(4):1487‐1505. doi: 10.1016/j.neuroimage.2006.02.024 [DOI] [PubMed] [Google Scholar]
- 35. Esteban O, Blair R, Markiewicz CJ, Berleant SL, Moodie C, Ma F, Isik AI, Erramuzpe A, Goncalves M, Poldrack RA, Gorgolewski KJ. poldracklab/fmriprep: 1.0.0‐rc5. 2017. doi: 10.5281/zenodo.996169 [DOI]
- 36. Song Y, Hakoda Y. An fMRI study of the functional mechanisms of Stroop/reverse‐Stroop effects. Behav Brain Res. 2015;290:187‐196. doi: 10.1016/j.bbr.2015.04.047 [DOI] [PubMed] [Google Scholar]
- 37. Coderre EL, van Heuven WJB. Modulations of the executive control network by stimulus onset asynchrony in a Stroop task. BMC Neurosci. 2013;14(1):79. doi: 10.1186/1471-2202-14-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Okayasu M, Inukai T, Tanaka D, Tsumura K, Hosono M, Shintaki R, Takeda M, Nakahara K, Jimura K. An excitatory‐inhibitory fronto‐cerebellar loop resolves the Stroop effect (p. 2022.01.18.476551). bioRxiv. 2022. doi: 10.1101/2022.01.18.476551 [DOI] [PMC free article] [PubMed]
- 39. Winkler AM, Ridgway GR, Webster MA, Smith SM, Nichols TE. Permutation inference for the general linear model. Neuroimage. 2014;92(100):381‐397. doi: 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oishi K, Faria A, Jiang H, et al. Atlas‐based whole brain white matter analysis using large deformation diffeomorphic metric mapping: application to normal elderly and Alzheimer's disease participants. Neuroimage. 2009;46(2):486‐499. doi: 10.1016/j.neuroimage.2009.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed‐effects models using lme4. J Stat Softw. 2015;67(1):1‐48. doi: 10.18637/jss.v067.i01 [DOI] [Google Scholar]
- 42. Ceceli AO, Bradberry CW, Goldstein RZ. The neurobiology of drug addiction: cross‐species insights into the dysfunction and recovery of the prefrontal cortex. Neuropsychopharmacology. 2022;47(1):276‐291. doi: 10.1038/s41386-021-01153-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McEvoy LK, Fennema‐Notestine C, Elman JA, et al. Alcohol intake and brain white matter in middle aged men: microscopic and macroscopic differences. NeuroImage: Clin. 2018;18:390‐398. doi: 10.1016/j.nicl.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. De Santis S, Bach P, Pérez‐Cervera L, et al. Microstructural white matter alterations in men with alcohol use disorder and rats with excessive alcohol consumption during early abstinence. JAMA Psychiatry. 2019;76(7):749‐758. doi: 10.1001/jamapsychiatry.2019.0318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huang H, Zhang Y, Cheng J, Wang W, Wen M. Evaluating the changes of white matter microstructures in tobacco addicts based on diffusion tensor imaging. Med Sci Monit. 2020;26:e919105. doi: 10.12659/MSM.919105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Robinson EA, Gleeson J, Arun AH, et al. Measuring white matter microstructure in 1,457 cannabis users and 1,441 controls: a systematic review of diffusion‐weighted MRI studies. Frontiers Neuroimaging. 2023;2:1129587. doi: 10.3389/fnimg.2023.1129587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Phillips O, Sanchez‐Castaneda C, Elifani F, et al. Tractography of the corpus callosum in Huntington's disease. PLoS ONE. 2013;8(9):e73280. doi: 10.1371/journal.pone.0073280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goldstein A, Covington BP, Mahabadi N, Mesfin FB. Neuroanatomy, corpus callosum. In StatPearls. StatPearls Publishing. 2023. http://www.ncbi.nlm.nih.gov/books/NBK448209/ [PubMed] [Google Scholar]
- 49. Hampton WH, Hanik IM, Olson IR. Substance abuse and white matter: findings, limitations, and future of diffusion tensor imaging research. Drug Alcohol Depend. 2019;197:288‐298. doi: 10.1016/j.drugalcdep.2019.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bettcher BM, Mungas D, Patel N, et al. Neuroanatomical substrates of executive functions: beyond prefrontal structures. Neuropsychologia. 2016;85:100‐109. doi: 10.1016/j.neuropsychologia.2016.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seghete KLM, Herting MM, Nagel BJ. White matter microstructure correlates of inhibition and task‐switching in adolescents. Brain Res. 2013;1527:15‐28. doi: 10.1016/j.brainres.2013.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xin J, Zhang Y, Tang Y, Yang Y. Brain differences between men and women: evidence from deep learning. Front Neurosci. 2019;13:185. doi: 10.3389/fnins.2019.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hudkins M, O'Neill J, Tobias MC, Bartzokis G, London ED. Cigarette smoking and white matter microstructure. Psychopharmacology (Berl). 2012;221(2):285‐295. doi: 10.1007/s00213-011-2621-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Unterrainer HF, Hiebler M, Ragger K, et al. White matter integrity in polydrug users in relation to attachment and personality: a controlled diffusion tensor imaging study. Brain Imaging Behav. 2016;10(4):1096‐1107. doi: 10.1007/s11682-015-9475-4 [DOI] [PubMed] [Google Scholar]
- 55. Takeuchi H, Taki Y, Sassa Y, et al. Regional gray and white matter volume associated with Stroop interference: evidence from voxel‐based morphometry. Neuroimage. 2012;59(3):2899‐2907. doi: 10.1016/j.neuroimage.2011.09.064 [DOI] [PubMed] [Google Scholar]
- 56. Acheson A, Tagamets MA, Winkler A, et al. Striatal activity and reduced white matter increase frontal activity in youths with family histories of alcohol and other substance‐use disorders performing a go/no‐go task. Brain and Behavior. 2015;5(7):e00352. doi: 10.1002/brb3.352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Honey CJ, Sporns O, Cammoun L, et al. Predicting human resting‐state functional connectivity from structural connectivity. Proc Natl Acad Sci. 2009;106(6):2035‐2040. doi: 10.1073/pnas.0811168106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hermundstad AM, Bassett DS, Brown KS, et al. Structural foundations of resting‐state and task‐based functional connectivity in the human brain. Proc Natl Acad Sci. 2013;110(15):6169‐6174. doi: 10.1073/pnas.1219562110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pérez‐Cervera L, De Santis S, Marcos E, et al. Alcohol‐induced damage to the fimbria/fornix reduces hippocampal‐prefrontal cortex connection during early abstinence. Acta Neuropathol Commun. 2023;11(1):101. doi: 10.1186/s40478-023-01597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Li X, Liang Y, Chen Y, et al. Disrupted frontoparietal network mediates white matter structure dysfunction associated with cognitive decline in hypertension patients. J Neurosci. 2015;35(27):10015‐10024. doi: 10.1523/JNEUROSCI.5113-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Krishna A, Strack F. Reflection and impulse as determinants of human behavior. Knowl Action. 2017;145‐167. doi: 10.1007/978-3-319-44588-5_9 [DOI] [Google Scholar]
- 62. Berkman ET, Falk EB, Lieberman MD. In the trenches of real‐world self‐control: neural correlates of breaking the link between craving and smoking. Psychol Sci. 2011;22(4):498‐506. doi: 10.1177/0956797611400918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, Sullivan EV. Accelerated and premature aging characterizing regional cortical volume loss in human immunodeficiency virus infection: contributions from alcohol, substance use, and hepatitis C coinfection. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2018;3(10):844‐859. doi: 10.1016/j.bpsc.2018.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kwako LE, Momenan R, Litten RZ, Koob GF, Goldman D. Addictions neuroclinical assessment: a neuroscience‐based framework for addictive disorders. Biol Psychiatry. 2016;80(3):179‐189. doi: 10.1016/j.biopsych.2015.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Pfefferbaum A, Rosenbloom MJ, Chu W, et al. White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry. 2014;1(3):202‐212. doi: 10.1016/S2215-0366(14)70301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Selim MK, Harel M, De Santis S, et al. Repetitive deep tms in alcohol dependent patients halts progression of white matter changes in early abstinence. Psychiatry Clin Neurosci. 2024;78(3):176‐185. doi: 10.1111/pcn.13624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Filley CM. Cognitive dysfunction in white matter disorders: new perspectives in treatment and recovery. J Neuropsychiatry Clin Neurosci. 2021;33(4):349‐355. doi: 10.1176/appi.neuropsych.21030080 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Resting‐state connectivity strength correlating negatively (in red) and positively (in blue) with mean Stroop Times in the SUD sample. Adapted from Chirokoff et al. Submitted.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


